The Extracytoplasmic Protein Quality Control System in Pathogenic Campylobacterota: Its Role in Bacterial Virulence and Maintaining Cellular Envelope Proteostasis
Abstract
1. Introduction
2. Protein Folding in the Cellular Envelope
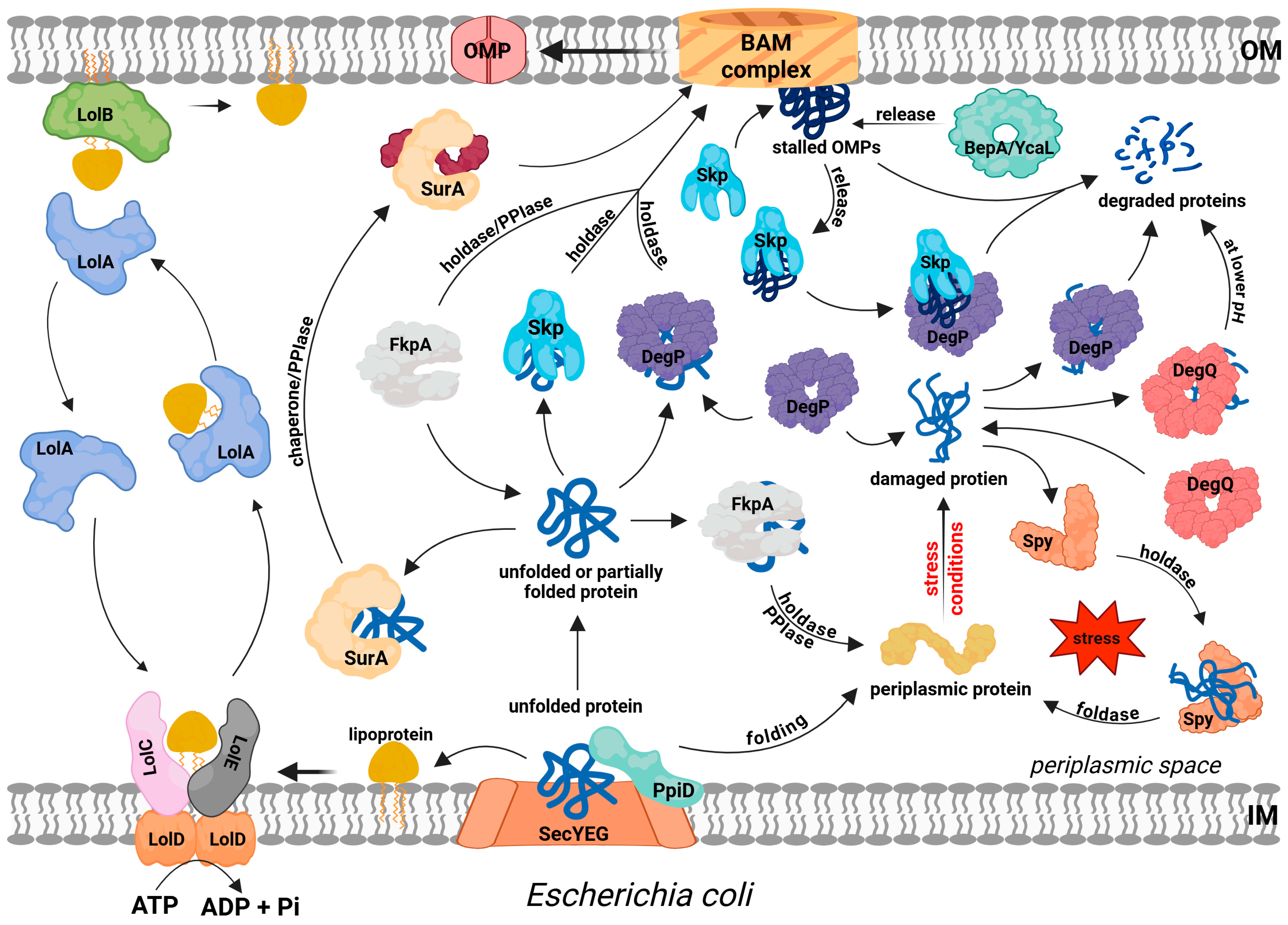
3. Extracytoplasmic Protein Quality Control System in the Model Gram-Negative Bacterium, E. coli
3.1. Folding Catalysts
3.2. General Chaperones
3.3. Proteases
3.4. Lipoprotein Dedicated Maturation/Transportation System
3.5. Folding Factors Networks
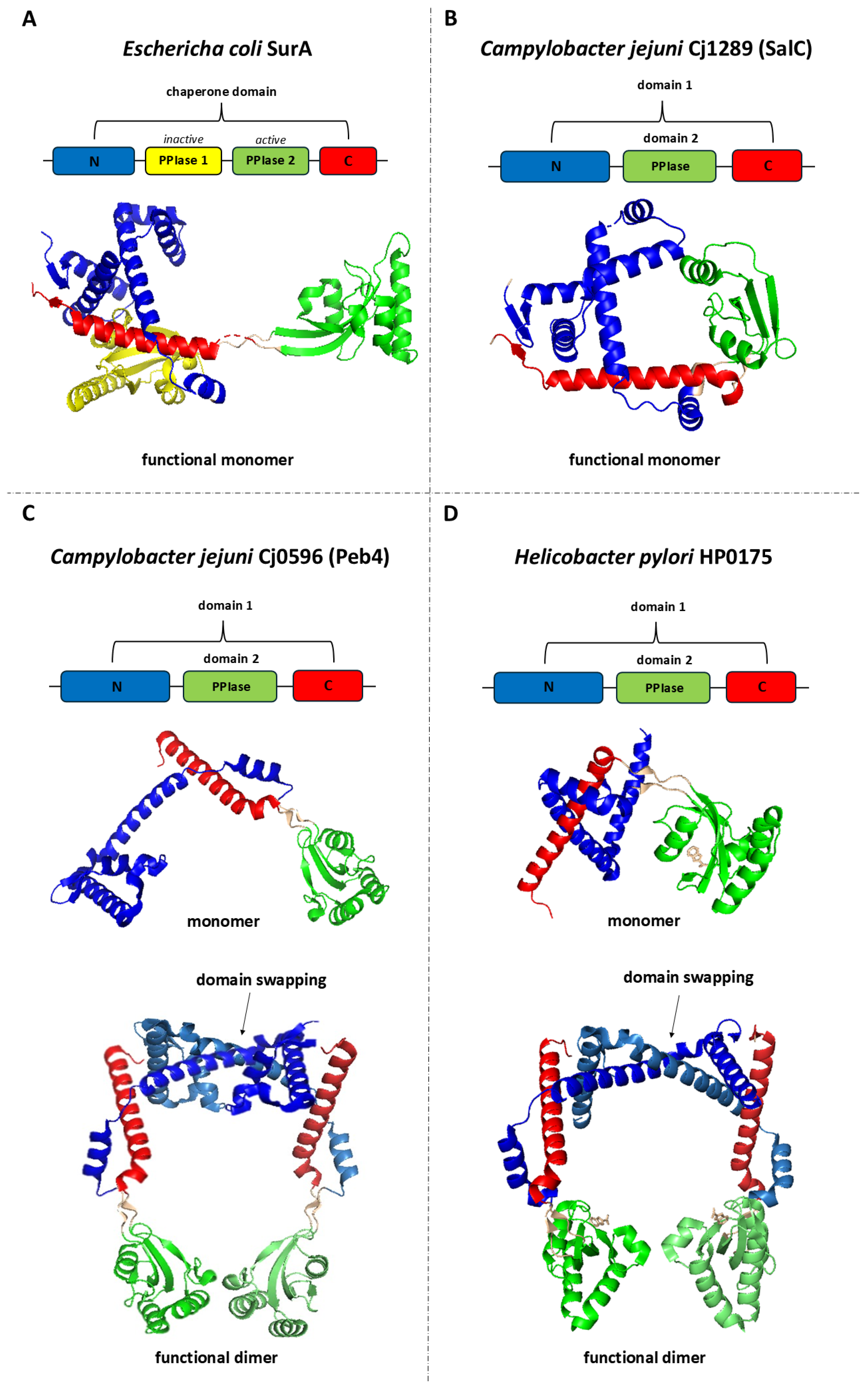
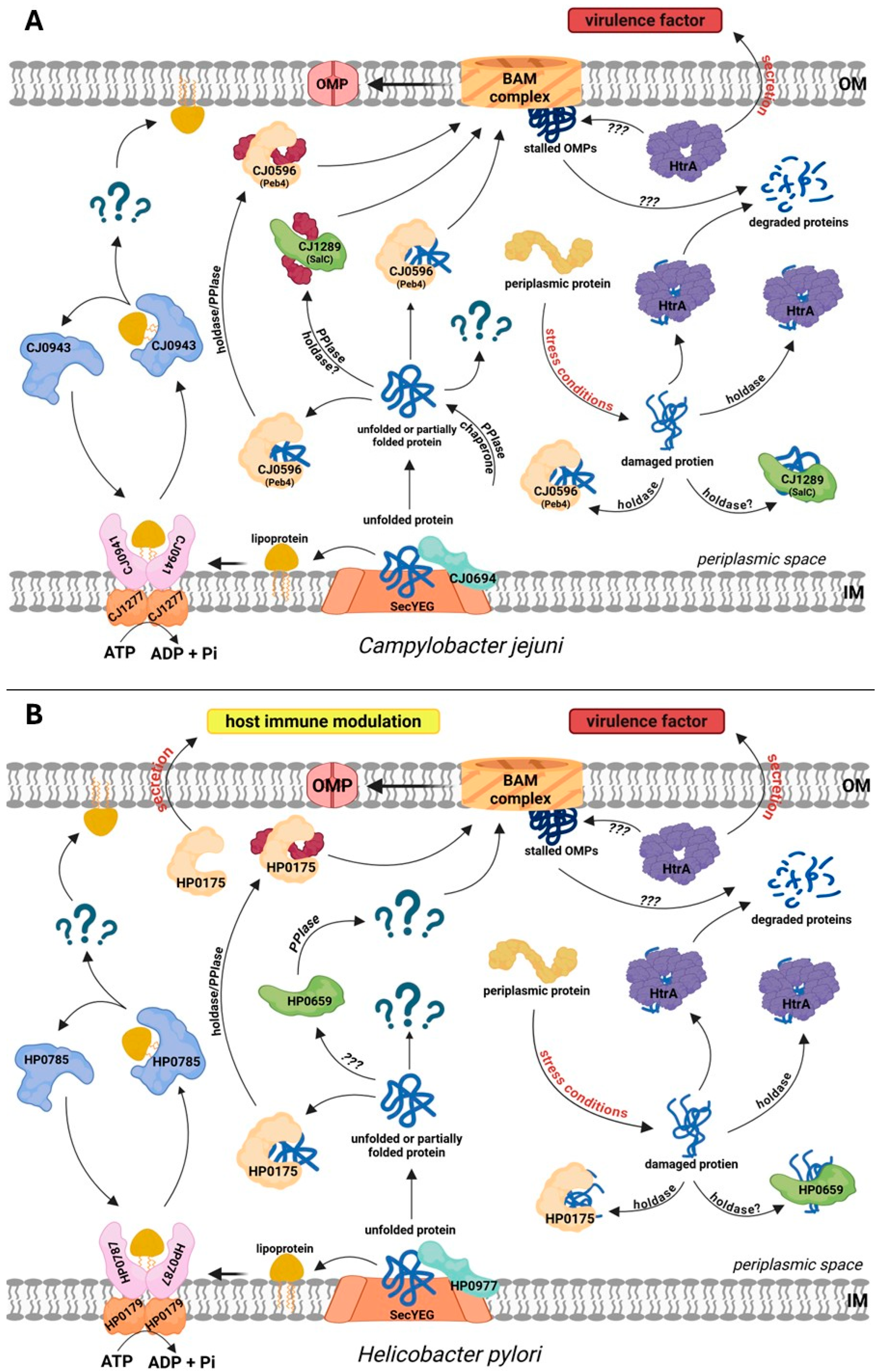
4. Proteins Involved in Protein Folding and Outer Membrane Biogenesis in the Model Campylobacterota, C. jejuni and H. pylori
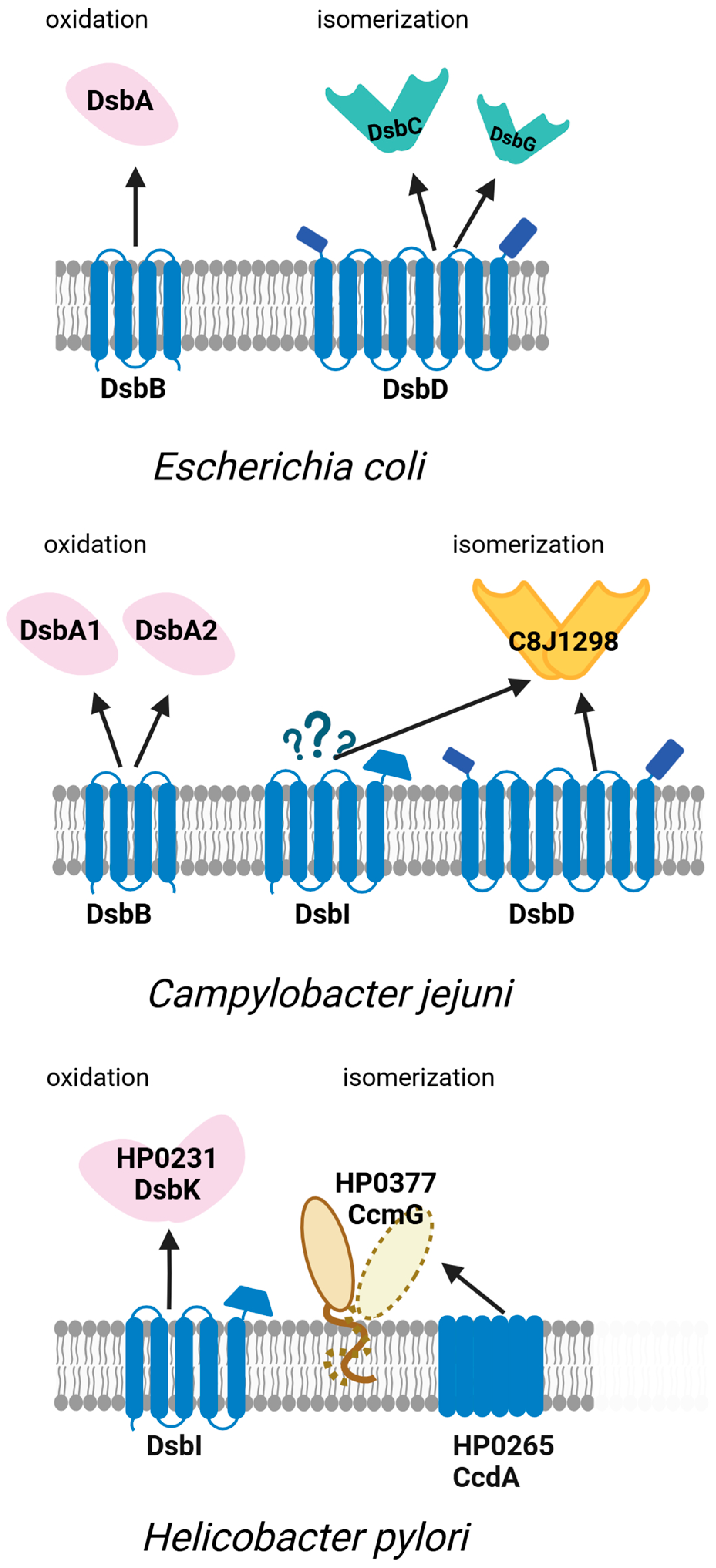
4.1. Formation of Appropriate Disulphide Bridges in the Model Campylobacterota
4.2. Outer Membrane Biogenesis in the Model Campylobacterota
4.3. Role of Proteases in Protein Quality Control in Model Campylobacterota
4.4. Biogenesis of Lipoproteins
5. Extracellular Functions of the EPQCS Components—The Moonlighting Functions
5.1. H. pylori HP0175
5.2. HtrA Proteins
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, J.W.; Schurr, M.J.; LeBlanc, C.L.; Ramamurthy, R.; Buchanan, K.L.; Nickerson, C.A. Mechanisms of Bacterial Pathogenicity. Postgrad. Med. J. 2002, 78, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, N.; Kahne, D.; Silhavy, T.J. Advances in Understanding Bacterial Outer-Membrane Biogenesis. Nat. Rev. Microbiol. 2006, 4, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Klemm, P.; Schembri, M.A. Bacterial Adhesins: Function and Structure. Int. J. Med. Microbiol. 2000, 290, 27–35. [Google Scholar] [CrossRef]
- Davies, J.S.; Currie, M.J.; Wright, J.D.; Newton-Vesty, M.C.; North, R.A.; Mace, P.D.; Allison, J.R.; Dobson, R.C.J. Selective Nutrient Transport in Bacteria: Multicomponent Transporter Systems Reign Supreme. Front. Mol. Biosci. 2021, 8, 699222. [Google Scholar] [CrossRef] [PubMed]
- Merdanovic, M.; Clausen, T.; Kaiser, M.; Huber, R.; Ehrmann, M. Protein Quality Control in the Bacterial Periplasm. Annu. Rev. Microbiol. 2011, 65, 149–168. [Google Scholar] [CrossRef]
- Miot, M.; Betton, J.-M. Protein Quality Control in the Bacterial Periplasm. Microb. Cell Fact. 2004, 3, 4. [Google Scholar] [CrossRef]
- Devlin, T.; Fleming, K.G. A Team of Chaperones Play to Win in the Bacterial Periplasm. Trends Biochem. Sci. 2024, 49, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Waite, D.W.; Chuvochina, M.S.; Hugenholtz, P. Road Map of the Phylum Campylobacterota. In Bergey’s Manual of Systematics of Archaea and Bacteria; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019; pp. 1–11. ISBN 978-1-118-96060-8. [Google Scholar]
- Waite, D.W.; Vanwonterghem, I.; Rinke, C.; Parks, D.H.; Zhang, Y.; Takai, K.; Sievert, S.M.; Simon, J.; Campbell, B.J.; Hanson, T.E.; et al. Comparative Genomic Analysis of the Class Epsilonproteobacteria and Proposed Reclassification to Epsilonbacteraeota (Phyl. Nov.). Front. Microbiol. 2017, 8, 682. [Google Scholar] [CrossRef]
- Nakagawa, S.; Takaki, Y. Nonpathogenic Epsilonproteobacteria. In eLS; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; ISBN 978-0-470-01590-2. [Google Scholar]
- Heimesaat, M.M.; Backert, S.; Alter, T.; Bereswill, S. Human Campylobacteriosis-A Serious Infectious Threat in a One Health Perspective. Curr. Top. Microbiol. Immunol. 2021, 431, 1–23. [Google Scholar] [CrossRef]
- Eusebi, L.H.; Zagari, R.M.; Bazzoli, F. Epidemiology of Helicobacter pylori Infection. Helicobacter 2014, 19 (Suppl. S1), 1–5. [Google Scholar] [CrossRef]
- Myrou, A. Molecular Mechanisms and Treatment Strategies for Helicobacter pylori-Induced Gastric Carcinogenesis and Mucosa-Associated Lymphoid Tissue (MALT) Lymphoma. Cureus 2024, 16, e60326. [Google Scholar] [CrossRef]
- De Geyter, J.; Tsirigotaki, A.; Orfanoudaki, G.; Zorzini, V.; Economou, A.; Karamanou, S. Protein Folding in the Cell Envelope of Escherichia coli. Nat. Microbiol. 2016, 1, 16107. [Google Scholar] [CrossRef]
- Natale, P.; Brüser, T.; Driessen, A.J.M. Sec- and Tat-Mediated Protein Secretion across the Bacterial Cytoplasmic Membrane—Distinct Translocases and Mechanisms. Biochim. Biophys. Acta 2008, 1778, 1735–1756. [Google Scholar] [CrossRef] [PubMed]
- Walton, T.A.; Sandoval, C.M.; Fowler, C.A.; Pardi, A.; Sousa, M.C. The Cavity-Chaperone Skp Protects Its Substrate from Aggregation but Allows Independent Folding of Substrate Domains. Proc. Natl. Acad. Sci. USA 2009, 106, 1772–1777. [Google Scholar] [CrossRef] [PubMed]
- Rollauer, S.E.; Sooreshjani, M.A.; Noinaj, N.; Buchanan, S.K. Outer Membrane Protein Biogenesis in Gram-Negative Bacteria. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20150023. [Google Scholar] [CrossRef] [PubMed]
- Combs, A.N.; Silhavy, T.J. Periplasmic Chaperones: Outer Membrane Biogenesis and Envelope Stress. Annu. Rev. Microbiol. 2024, 78, 191–211. [Google Scholar] [CrossRef]
- Tomasek, D.; Kahne, D. The Assembly of β-Barrel Outer Membrane Proteins. Curr. Opin. Microbiol. 2021, 60, 16–23. [Google Scholar] [CrossRef]
- Messens, J.; Collet, J.-F. Pathways of Disulfide Bond Formation in Escherichia coli. Int. J. Biochem. Cell Biol. 2006, 38, 1050–1062. [Google Scholar] [CrossRef]
- Shouldice, S.R.; Heras, B.; Walden, P.M.; Totsika, M.; Schembri, M.A.; Martin, J.L. Structure and Function of DsbA, a Key Bacterial Oxidative Folding Catalyst. Antioxid. Redox Signal. 2011, 14, 1729–1760. [Google Scholar] [CrossRef]
- Bocian-Ostrzycka, K.M.; Grzeszczuk, M.J.; Banaś, A.M.; Jagusztyn-Krynicka, E.K. Bacterial Thiol Oxidoreductases—From Basic Research to New Antibacterial Strategies. Appl. Microbiol. Biotechnol. 2017, 101, 3977–3989. [Google Scholar] [CrossRef]
- Berkmen, M. Production of Disulfide-Bonded Proteins in Escherichia coli. Protein Expr. Purif. 2012, 82, 240–251. [Google Scholar] [CrossRef]
- Ünal, C.M.; Steinert, M. Microbial Peptidyl-Prolyl Cis/Trans Isomerases (PPIases): Virulence Factors and Potential Alternative Drug Targets. Microbiol. Mol. Biol. Rev. 2014, 78, 544–571. [Google Scholar] [CrossRef]
- Figaj, D.; Ambroziak, P.; Rzepka, I.; Skórko-Glonek, J. SurA-like and Skp-like Proteins as Important Virulence Determinants of the Gram Negative Bacterial Pathogens. Int. J. Mol. Sci. 2022, 24, 295. [Google Scholar] [CrossRef]
- Behrens, S.; Maier, R.; de Cock, H.; Schmid, F.X.; Gross, C.A. The SurA Periplasmic PPIase Lacking Its Parvulin Domains Functions In Vivo and Has Chaperone Activity. EMBO J. 2001, 20, 285–294. [Google Scholar] [CrossRef]
- Justice, S.S.; Hunstad, D.A.; Harper, J.R.; Duguay, A.R.; Pinkner, J.S.; Bann, J.; Frieden, C.; Silhavy, T.J.; Hultgren, S.J. Periplasmic Peptidyl Prolyl Cis-Trans Isomerases Are Not Essential for Viability, but SurA Is Required for Pilus Biogenesis in Escherichia coli. J. Bacteriol. 2005, 187, 7680–7686. [Google Scholar] [CrossRef]
- Gao, M.; Nakajima An, D.; Skolnick, J. Deep Learning-Driven Insights into Super Protein Complexes for Outer Membrane Protein Biogenesis in Bacteria. eLife 2022, 11, e82885. [Google Scholar] [CrossRef] [PubMed]
- Walton, T.A.; Sousa, M.C. Crystal Structure of Skp, a Prefoldin-like Chaperone That Protects Soluble and Membrane Proteins from Aggregation. Mol. Cell 2004, 15, 367–374. [Google Scholar] [CrossRef]
- Combs, A.N.; Silhavy, T.J. The Sacrificial Adaptor Protein Skp Functions to Remove Stalled Substrates from the β-Barrel Assembly Machine. Proc. Natl. Acad. Sci. USA 2022, 119, e2114997119. [Google Scholar] [CrossRef]
- Quan, S.; Koldewey, P.; Tapley, T.; Kirsch, N.; Ruane, K.M.; Pfizenmaier, J.; Shi, R.; Hofmann, S.; Foit, L.; Ren, G.; et al. Genetic Selection Designed to Stabilize Proteins Uncovers a Chaperone Called Spy. Nat. Struct. Mol. Biol. 2011, 18, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Koldewey, P.; Stull, F.; Horowitz, S.; Martin, R.; Bardwell, J.C.A. Forces Driving Chaperone Action. Cell 2016, 166, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Stull, F.; Koldewey, P.; Humes, J.R.; Radford, S.E.; Bardwell, J.C.A. Substrate Protein Folds While It Is Bound to the ATP-Independent Chaperone Spy. Nat. Struct. Mol. Biol. 2016, 23, 53–58. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Sharpe, T.; Mazur, A.; Hiller, S. A Molecular Mechanism of Chaperone-Client Recognition. Sci. Adv. 2016, 2, e1601625. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cheng, Y.; Ma, J.; Wang, Y.; Chang, Z.; Fu, X. Degp Degrades a Wide Range of Substrate Proteins in Escherichia coli under Stress Conditions. Biochem. J. 2019, 476, 3549–3564. [Google Scholar] [CrossRef]
- Ge, X.; Wang, R.; Ma, J.; Liu, Y.; Ezemaduka, A.N.; Chen, P.R.; Fu, X.; Chang, Z. DegP Primarily Functions as a Protease for the Biogenesis of β-Barrel Outer Membrane Proteins in the Gram-Negative Bacterium Escherichia coli. FEBS J. 2014, 281, 1226–1240. [Google Scholar] [CrossRef]
- CastilloKeller, M.; Misra, R. Protease-Deficient DegP Suppresses Lethal Effects of a Mutant OmpC Protein by Its Capture. J. Bacteriol. 2003, 185, 148–154. [Google Scholar] [CrossRef]
- Soltes, G.R.; Martin, N.R.; Park, E.; Sutterlin, H.A.; Silhavy, T.J. Distinctive Roles for Periplasmic Proteases in the Maintenance of Essential Outer Membrane Protein Assembly. J. Bacteriol. 2017, 199, e00418-17. [Google Scholar] [CrossRef][Green Version]
- Skorko-Glonek, J.; Zurawa-Janicka, D.; Koper, T.; Jarzab, M.; Figaj, D.; Glaza, P.; Lipinska, B. HtrA Protease Family as Therapeutic Targets. Curr. Pharm. Des. 2013, 19, 977–1009. [Google Scholar] [CrossRef]
- Harkness, R.W.; Ripstein, Z.A.; Di Trani, J.M.; Kay, L.E. Flexible Client-Dependent Cages in the Assembly Landscape of the Periplasmic Protease-Chaperone DegP. J. Am. Chem. Soc. 2023, 145, 13015–13026. [Google Scholar] [CrossRef] [PubMed]
- Sawa, J.; Malet, H.; Krojer, T.; Canellas, F.; Ehrmann, M.; Clausen, T. Molecular Adaptation of the DegQ Protease to Exert Protein Quality Control in the Bacterial Cell Envelope. J. Biol. Chem. 2011, 286, 30680–30690. [Google Scholar] [CrossRef]
- Waller, P.R.; Sauer, R.T. Characterization of degQ and degS, Escherichia coli Genes Encoding Homologs of the DegP Protease. J. Bacteriol. 1996, 178, 1146–1153. [Google Scholar] [CrossRef]
- Narita, S.; Tokuda, H. Bacterial Lipoproteins; Biogenesis, Sorting and Quality Control. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2017, 1862, 1414–1423. [Google Scholar] [CrossRef]
- El Rayes, J.; Rodríguez-Alonso, R.; Collet, J.-F. Lipoproteins in Gram-Negative Bacteria: New Insights into Their Biogenesis, Subcellular Targeting and Functional Roles. Curr. Opin. Microbiol. 2021, 61, 25–34. [Google Scholar] [CrossRef]
- Rizzitello, A.E.; Harper, J.R.; Silhavy, T.J. Genetic Evidence for Parallel Pathways of Chaperone Activity in the Periplasm of Escherichia coli. J. Bacteriol. 2001, 183, 6794–6800. [Google Scholar] [CrossRef] [PubMed]
- Sklar, J.G.; Wu, T.; Kahne, D.; Silhavy, T.J. Defining the Roles of the Periplasmic Chaperones SurA, Skp, and DegP in Escherichia coli. Genes. Dev. 2007, 21, 2473–2484. [Google Scholar] [CrossRef]
- Alvira, S.; Watkins, D.W.; Troman, L.; Allen, W.J.; Lorriman, J.S.; Degliesposti, G.; Cohen, E.J.; Beeby, M.; Daum, B.; Gold, V.A.; et al. Inter-Membrane Association of the Sec and BAM Translocons for Bacterial Outer-Membrane Biogenesis. eLife 2020, 9, e60669. [Google Scholar] [CrossRef]
- Götzke, H.; Palombo, I.; Muheim, C.; Perrody, E.; Genevaux, P.; Kudva, R.; Müller, M.; Daley, D.O. YfgM Is an Ancillary Subunit of the SecYEG Translocon in Escherichia coli. J. Biol. Chem. 2014, 289, 19089–19097. [Google Scholar] [CrossRef] [PubMed]
- Fürst, M.; Zhou, Y.; Merfort, J.; Müller, M. Involvement of PpiD in Sec-Dependent Protein Translocation. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 273–280. [Google Scholar] [CrossRef]
- Maddalo, G.; Stenberg-Bruzell, F.; Götzke, H.; Toddo, S.; Björkholm, P.; Eriksson, H.; Chovanec, P.; Genevaux, P.; Lehtiö, J.; Ilag, L.L.; et al. Systematic Analysis of Native Membrane Protein Complexes in Escherichia coli. J. Proteome Res. 2011, 10, 1848–1859. [Google Scholar] [CrossRef]
- Miyazaki, R.; Ai, M.; Tanaka, N.; Suzuki, T.; Dhomae, N.; Tsukazaki, T.; Akiyama, Y.; Mori, H. Inner Membrane YfgM-PpiD Heterodimer Acts as a Functional Unit That Associates with the SecY/E/G Translocon and Promotes Protein Translocation. J. Biol. Chem. 2022, 298, 102572. [Google Scholar] [CrossRef]
- Troman, L.A.; Alvira, S.; Daum, B.; Gold, V.A.M.; Collinson, I. Interaction of the Periplasmic Chaperone SurA with the Inner Membrane Protein Secretion (SEC) Machinery. Biochem. J. 2023, 480, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Fenn, K.L.; Horne, J.E.; Crossley, J.A.; Böhringer, N.; Horne, R.J.; Schäberle, T.F.; Calabrese, A.N.; Radford, S.E.; Ranson, N.A. Outer Membrane Protein Assembly Mediated by BAM-SurA Complexes. Nat. Commun. 2024, 15, 7612. [Google Scholar] [CrossRef]
- Lehner, P.A.; Degen, M.; Jakob, R.P.; Modaresi, S.M.; Callon, M.; Burmann, B.M.; Maier, T.; Hiller, S. Architecture and Conformational Dynamics of the BAM-SurA Holo Insertase Complex. Sci. Adv. 2025, 11, eads6094. [Google Scholar] [CrossRef]
- Liechti, G.; Goldberg, J.B. Outer Membrane Biogenesis in Escherichia coli, Neisseria meningitidis, and Helicobacter pylori: Paradigm Deviations in H. pylori. Front. Cell. Infect. Microbiol. 2012, 2, 29. [Google Scholar] [CrossRef] [PubMed]
- Grimshaw, J.P.A.; Stirnimann, C.U.; Brozzo, M.S.; Malojcic, G.; Grütter, M.G.; Capitani, G.; Glockshuber, R. DsbL and DsbI Form a Specific Dithiol Oxidase System for Periplasmic Arylsulfate Sulfotransferase in Uropathogenic Escherichia coli. J. Mol. Biol. 2008, 380, 667–680. [Google Scholar] [CrossRef]
- Lin, D.; Kim, B.; Slauch, J.M. DsbL and DsbI Contribute to Periplasmic Disulfide Bond Formation in Salmonella enterica Serovar Typhimurium. Microbiology 2009, 155, 4014–4024. [Google Scholar] [CrossRef]
- Bocian-Ostrzycka, K.M.; Grzeszczuk, M.J.; Dziewit, L.; Jagusztyn-Krynicka, E.K. Diversity of the Epsilonproteobacteria Dsb (Disulfide Bond) Systems. Front. Microbiol. 2015, 6, 570. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, A.D.; Wywiał, E.; Dunin-Horkawicz, S.; Łasica, A.M.; Wösten, M.M.S.M.; Nagy-Staroń, A.; Godlewska, R.; Bocian-Ostrzycka, K.; Pieńkowska, K.; Łaniewski, P.; et al. Functional and Bioinformatics Analysis of Two Campylobacter jejuni Homologs of the Thiol-Disulfide Oxidoreductase, DsbA. PLoS ONE 2014, 9, e106247. [Google Scholar] [CrossRef] [PubMed]
- Banaś, A.M.; Bocian-Ostrzycka, K.M.; Plichta, M.; Dunin-Horkawicz, S.; Ludwiczak, J.; Płaczkiewicz, J.; Jagusztyn-Krynicka, E.K. C8J_1298, a Bifunctional Thiol Oxidoreductase of Campylobacter jejuni, Affects Dsb (Disulfide Bond) Network Functioning. PLoS ONE 2020, 15, e0230366. [Google Scholar] [CrossRef]
- Raczko, A.M.; Bujnicki, J.M.; Pawłowski, M.; Godlewska, R.; Lewandowska, M.; Jagusztyn-Krynicka, E.K. Characterization of New DsbB-like Thiol-Oxidoreductases of Campylobacter jejuni and Helicobacter pylori and Classification of the DsbB Family Based on Phylogenomic, Structural and Functional Criteria. Microbiology 2005, 151, 219–231. [Google Scholar] [CrossRef]
- Copley, S.D.; Novak, W.R.P.; Babbitt, P.C. Divergence of Function in the Thioredoxin Fold Suprafamily: Evidence for Evolution of Peroxiredoxins from a Thioredoxin-like Ancestor. Biochemistry 2004, 43, 13981–13995. [Google Scholar] [CrossRef]
- Kaakoush, N.O.; Kovach, Z.; Mendz, G.L. Potential Role of Thiol:Disulfide Oxidoreductases in the Pathogenesis of Helicobacter pylori. FEMS Immunol. Med. Microbiol. 2007, 50, 177–183. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Kim, J.; Lee, S.J.; Kim, H.S.; Im, H.N.; Yoon, H.-J.; Kim, K.H.; Kim, S.-J.; Han, B.W.; Suh, S.W. Structural and Functional Characterization of Helicobacter pylori DsbG. FEBS Lett. 2011, 585, 3862–3867. [Google Scholar] [CrossRef]
- Bocian-Ostrzycka, K.M.; Grzeszczuk, M.J.; Banaś, A.M.; Jastrząb, K.; Pisarczyk, K.; Kolarzyk, A.; Łasica, A.M.; Collet, J.-F.; Jagusztyn-Krynicka, E.K. Engineering of Helicobacter pylori Dimeric Oxidoreductase DsbK (HP0231). Front. Microbiol. 2016, 7, 1158. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Kim, J.; An, D.R.; Lee, S.J.; Kim, H.S.; Im, H.N.; Yoon, H.J.; Kim, J.Y.; Kim, S.J.; Han, B.W.; et al. Structural and Functional Characterization of HP0377, a Thioredoxin-Fold Protein from Helicobacter pylori. Acta Crystallogr. D Biol. Crystallogr. 2013, 69, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Roszczenko, P.; Grzeszczuk, M.; Kobierecka, P.; Wywial, E.; Urbanowicz, P.; Wincek, P.; Nowak, E.; Jagusztyn-Krynicka, E.K. Helicobacter pylori HP0377, a Member of the Dsb Family, Is an Untypical Multifunctional CcmG That Cooperates with Dimeric Thioldisulfide Oxidase HP0231. BMC Microbiol. 2015, 15, 135. [Google Scholar] [CrossRef] [PubMed]
- Łasica, A.M.; Jagusztyn-Krynicka, E.K. The Role of Dsb Proteins of Gram-Negative Bacteria in the Process of Pathogenesis. FEMS Microbiol. Rev. 2007, 31, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Heras, B.; Shouldice, S.R.; Totsika, M.; Scanlon, M.J.; Schembri, M.A.; Martin, J.L. DSB Proteins and Bacterial Pathogenicity. Nat. Rev. Microbiol. 2009, 7, 215–225. [Google Scholar] [CrossRef]
- Yu, J.; Kroll, J.S. DsbA: A Protein-Folding Catalyst Contributing to Bacterial Virulence. Microbes Infect. 1999, 1, 1221–1228. [Google Scholar] [CrossRef]
- Stenson, T.H.; Weiss, A.A. DsbA and DsbC Are Required for Secretion of Pertussis Toxin by Bordetella pertussis. Infect. Immun. 2002, 70, 2297–2303. [Google Scholar] [CrossRef]
- Totsika, M.; Heras, B.; Wurpel, D.J.; Schembri, M.A. Characterization of Two Homologous Disulfide Bond Systems Involved in Virulence Factor Biogenesis in Uropathogenic Escherichia coli CFT073. J. Bacteriol. 2009, 191, 3901–3908. [Google Scholar] [CrossRef]
- Ireland, P.M.; McMahon, R.M.; Marshall, L.E.; Halili, M.; Furlong, E.; Tay, S.; Martin, J.L.; Sarkar-Tyson, M. Disarming Burkholderia pseudomallei: Structural and Functional Characterization of a Disulfide Oxidoreductase (DsbA) Required for Virulence In Vivo. Antioxid. Redox Signal. 2014, 20, 606–617. [Google Scholar] [CrossRef]
- Ren, G.; Champion, M.M.; Huntley, J.F. Identification of Disulfide Bond Isomerase Substrates Reveals Bacterial Virulence Factors. Mol. Microbiol. 2014, 94, 926–944. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Anderl, F.; Kruse, T.; Schindele, F.; Jagusztyn-Krynicka, E.K.; Fischer, W.; Gerhard, M.; Mejías-Luque, R. Helicobacter pylori HP0231 Influences Bacterial Virulence and Is Essential for Gastric Colonization. PLoS ONE 2016, 11, e0154643. [Google Scholar] [CrossRef] [PubMed]
- Lasica, A.M.; Wyszynska, A.; Szymanek, K.; Majewski, P.; Jagusztyn-Krynicka, E.K. Campylobacter Protein Oxidation Influences Epithelial Cell Invasion or Intracellular Survival as Well as Intestinal Tract Colonization in Chickens. J. Appl. Genet. 2010, 51, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Banaś, A.M.; Bocian-Ostrzycka, K.M.; Dunin-Horkawicz, S.; Ludwiczak, J.; Wilk, P.; Orlikowska, M.; Wyszyńska, A.; Dąbrowska, M.; Plichta, M.; Spodzieja, M.; et al. Interplay between DsbA1, DsbA2 and C8J_1298 Periplasmic Oxidoreductases of Campylobacter jejuni and Their Impact on Bacterial Physiology and Pathogenesis. Int. J. Mol. Sci. 2021, 22, 13451. [Google Scholar] [CrossRef]
- Grzeszczuk, M.J.; Bocian-Ostrzycka, K.M.; Banaś, A.M.; Roszczenko-Jasinska, P.; Malinowska, A.; Stralova, H.; Haas, R.; Meyer, T.F.; Jagusztyn-Krynicka, E.K. Thioloxidoreductase HP0231 of Helicobacter pylori Impacts HopQ-Dependent CagA Translocation. Int. J. Med. Microbiol. 2018, 308, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Hauck, C.R.; Agerer, F.; Muenzner, P.; Schmitter, T. Cellular Adhesion Molecules as Targets for Bacterial Infection. Eur. J. Cell Biol. 2006, 85, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Godlewska, R.; Dzwonek, A.; Mikuła, M.; Ostrowski, J.; Pawłowski, M.; Bujnicki, J.M.; Jagusztyn-Krynicka, E.K. Helicobacter pylori Protein Oxidation Influences the Colonization Process. Int. J. Med. Microbiol. 2006, 296, 321–324. [Google Scholar] [CrossRef]
- Roszczenko-Jasińska, P.; Giełdoń, A.; Mazur, D.; Spodzieja, M.; Plichta, M.; Czaplewski, C.; Bal, W.; Jagusztyn-Krynicka, E.K.; Bartosik, D. Exploring the Inhibitory Potential of in Silico-Designed Small Peptides on Helicobacter pylori Hp0231 (DsbK), a Periplasmic Oxidoreductase Involved in Disulfide Bond Formation. Front. Mol. Biosci. 2023, 10, 1335704. [Google Scholar] [CrossRef]
- Pei, Z.H.; Ellison, R.T.; Blaser, M.J. Identification, Purification, and Characterization of Major Antigenic Proteins of Campylobacter jejuni. J. Biol. Chem. 1991, 266, 16363–16369. [Google Scholar] [CrossRef]
- Kervella, M.; Pagès, J.M.; Pei, Z.; Grollier, G.; Blaser, M.J.; Fauchère, J.L. Isolation and Characterization of Two Campylobacter Glycine-Extracted Proteins That Bind to HeLa Cell Membranes. Infect. Immun. 1993, 61, 3440–3448. [Google Scholar] [CrossRef]
- Asakura, H.; Yamasaki, M.; Yamamoto, S.; Igimi, S. Deletion of Peb4 Gene Impairs Cell Adhesion and Biofilm Formation in Campylobacter jejuni. FEMS Microbiol. Lett. 2007, 275, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Rathbun, K.M.; Hall, J.E.; Thompson, S.A. Cj0596 Is a Periplasmic Peptidyl Prolyl Cis-Trans Isomerase Involved in Campylobacter jejuni Motility, Invasion, and Colonization. BMC Microbiol. 2009, 9, 160. [Google Scholar] [CrossRef]
- Rathbun, K.M.; Thompson, S.A. Mutation of PEB4 Alters the Outer Membrane Protein Profile of Campylobacter jejuni. FEMS Microbiol. Lett. 2009, 300, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Basak, C.; Pathak, S.K.; Bhattacharyya, A.; Pathak, S.; Basu, J.; Kundu, M. The Secreted Peptidyl Prolyl Cis,Trans-Isomerase HP0175 of Helicobacter pylori Induces Apoptosis of Gastric Epithelial Cells in a TLR4- and Apoptosis Signal-Regulating Kinase 1-Dependent Manner. J. Immunol. 2005, 174, 5672–5680. [Google Scholar] [CrossRef]
- Kale, A.; Phansopa, C.; Suwannachart, C.; Craven, C.J.; Rafferty, J.B.; Kelly, D.J. The Virulence Factor PEB4 (Cj0596) and the Periplasmic Protein Cj1289 Are Two Structurally Related SurA-like Chaperones in the Human Pathogen Campylobacter jejuni. J. Biol. Chem. 2011, 286, 21254–21265. [Google Scholar] [CrossRef] [PubMed]
- Dantu, S.C.; Khavnekar, S.; Kale, A. Conformational Dynamics of Peb4 Exhibit “Mother’s Arms” Chain Model: A Molecular Dynamics Study. J. Biomol. Struct. Dyn. 2017, 35, 2186–2196. [Google Scholar] [CrossRef]
- Naveen, V.; Chu, C.-H.; Chen, B.-W.; Tsai, Y.-C.; Hsiao, C.-D.; Sun, Y.-J. Helicobacter pylori Cell Binding Factor 2: Insights into Domain Motion. J. Struct. Biol. 2016, 194, 90–101. [Google Scholar] [CrossRef]
- Yaseen, A.; Audette, G.F. Structural Flexibility in the Helicobacter pylori Peptidyl-Prolyl Cis,Trans-Isomerase HP0175 Is Achieved through an Extension of the Chaperone Helices. J. Struct. Biol. 2018, 204, 261–269. [Google Scholar] [CrossRef]
- Rouvière, P.E.; Gross, C.A. SurA, a Periplasmic Protein with Peptidyl-Prolyl Isomerase Activity, Participates in the Assembly of Outer Membrane Porins. Genes Dev. 1996, 10, 3170–3182. [Google Scholar] [CrossRef]
- Taylor, A.J.; Zakai, S.A.I.; Kelly, D.J. The Periplasmic Chaperone Network of Campylobacter jejuni: Evidence That SalC (Cj1289) and PpiD (Cj0694) Are Involved in Maintaining Outer Membrane Integrity. Front. Microbiol. 2017, 8, 531. [Google Scholar] [CrossRef]
- Baek, K.T.; Vegge, C.S.; Skórko-Glonek, J.; Brøndsted, L. Different Contributions of HtrA Protease and Chaperone Activities to Campylobacter jejuni Stress Tolerance and Physiology. Appl. Environ. Microbiol. 2011, 77, 57–66. [Google Scholar] [CrossRef]
- Zarzecka, U.; Modrak-Wójcik, A.; Figaj, D.; Apanowicz, M.; Lesner, A.; Bzowska, A.; Lipinska, B.; Zawilak-Pawlik, A.; Backert, S.; Skorko-Glonek, J. Properties of the HtrA Protease from Bacterium Helicobacter pylori Whose Activity Is Indispensable for Growth Under Stress Conditions. Front. Microbiol. 2019, 10, 961. [Google Scholar] [CrossRef]
- Azzam, M.E.; Algranati, I.D. Mechanism of Puromycin Action: Fate of Ribosomes after Release of Nascent Protein Chains from Polysomes. Proc. Natl. Acad. Sci. USA 1973, 70, 3866–3869. [Google Scholar] [CrossRef] [PubMed]
- Zarzecka, U.; Harrer, A.; Zawilak-Pawlik, A.; Skorko-Glonek, J.; Backert, S. Chaperone Activity of Serine Protease HtrA of Helicobacter pylori as a Crucial Survival Factor under Stress Conditions. Cell Commun. Signal. 2019, 17, 161. [Google Scholar] [CrossRef] [PubMed]
- Zarzecka, U.; Grinzato, A.; Kandiah, E.; Cysewski, D.; Berto, P.; Skorko-Glonek, J.; Zanotti, G.; Backert, S. Functional Analysis and Cryo-Electron Microscopy of Campylobacter jejuni Serine Protease HtrA. Gut Microbes 2020, 12, 1810532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, Q.; Tao, X.; Song, G.; Zheng, P.; Li, H.; Sun, H.; Xia, W. The Unique Trimeric Assembly of the Virulence Factor HtrA from Helicobacter pylori Occurs Via N-Terminal Domain Swapping. J. Biol. Chem. 2019, 294, 7990–8000. [Google Scholar] [CrossRef]
- Zarzecka, U.; Skorko-Glonek, J. Intricate Structure-Function Relationships: The Case of the HtrA Family Proteins from Gram-Negative Bacteria. Int. J. Mol. Sci. 2024, 25, 13182. [Google Scholar] [CrossRef]
- Skórko-Glonek, J.; Figaj, D.; Zarzecka, U.; Przepiora, T.; Renke, J.; Lipinska, B. The Extracellular Bacterial HtrA Proteins as Potential Therapeutic Targets and Vaccine Candidates. Curr. Med. Chem. 2017, 24, 2174–2204. [Google Scholar] [CrossRef]
- Brøndsted, L.; Andersen, M.T.; Parker, M.; Jørgensen, K.; Ingmer, H. The HtrA Protease of Campylobacter jejuni Is Required for Heat and Oxygen Tolerance and for Optimal Interaction with Human Epithelial Cells. Appl. Environ. Microbiol. 2005, 71, 3205–3212. [Google Scholar] [CrossRef]
- Bæk, K.T.; Vegge, C.S.; Brøndsted, L. HtrA Chaperone Activity Contributes to Host Cell Binding in Campylobacter jejuni. Gut Pathog. 2011, 3, 13. [Google Scholar] [CrossRef]
- Heimesaat, M.M.; Fischer, A.; Alutis, M.; Grundmann, U.; Boehm, M.; Tegtmeyer, N.; Göbel, U.B.; Kühl, A.A.; Bereswill, S.; Backert, S. The Impact of Serine Protease HtrA in Apoptosis, Intestinal Immune Responses and Extra-Intestinal Histopathology during Campylobacter jejuni Infection of Infant Mice. Gut Pathog. 2014, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Heimesaat, M.M.; Alutis, M.; Grundmann, U.; Fischer, A.; Tegtmeyer, N.; Böhm, M.; Kühl, A.A.; Göbel, U.B.; Backert, S.; Bereswill, S. The Role of Serine Protease HtrA in Acute Ulcerative Enterocolitis and Extra-Intestinal Immune Responses during Campylobacter jejuni Infection of Gnotobiotic IL-10 Deficient Mice. Front. Cell. Infect. Microbiol. 2014, 4, 77. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.-M.; Escher, U.; Mousavi, S.; Boehm, M.; Backert, S.; Bereswill, S.; Heimesaat, M.M. Protease Activity of Campylobacter jejuni HtrA Modulates Distinct Intestinal and Systemic Immune Responses in Infected Secondary Abiotic IL-10 Deficient Mice. Front. Cell. Infect. Microbiol. 2019, 9, 79. [Google Scholar] [CrossRef]
- de Boer, P.; Wagenaar, J.A.; Achterberg, R.P.; van Putten, J.P.M.; Schouls, L.M.; Duim, B. Generation of Campylobacter jejuni Genetic Diversity In Vivo. Mol. Microbiol. 2002, 44, 351–359. [Google Scholar] [CrossRef]
- Tegtmeyer, N.; Moodley, Y.; Yamaoka, Y.; Pernitzsch, S.R.; Schmidt, V.; Traverso, F.R.; Schmidt, T.P.; Rad, R.; Yeoh, K.G.; Bow, H.; et al. Characterisation of Worldwide Helicobacter pylori Strains Reveals Genetic Conservation and Essentiality of Serine Protease HtrA. Mol. Microbiol. 2016, 99, 925–944. [Google Scholar] [CrossRef] [PubMed]
- Zawilak-Pawlik, A.; Zarzecka, U.; Żyła-Uklejewicz, D.; Lach, J.; Strapagiel, D.; Tegtmeyer, N.; Böhm, M.; Backert, S.; Skorko-Glonek, J. Establishment of Serine Protease htrA Mutants in Helicobacter pylori Is Associated with secA Mutations. Sci. Rep. 2019, 9, 11794. [Google Scholar] [CrossRef]
- McClain, M.S.; Voss, B.J.; Cover, T.L. Lipoprotein Processing and Sorting in Helicobacter pylori. mBio 2020, 11, e00911-20. [Google Scholar] [CrossRef]
- Jaiman, D.; Persson, K. Structural and Functional Analysis of the Helicobacter pylori Lipoprotein Chaperone LolA. Front. Microbiol. 2024, 15, 1512451. [Google Scholar] [CrossRef]
- Smith, H.C.; May, K.L.; Grabowicz, M. Teasing Apart the Evolution of Lipoprotein Trafficking in Gram-Negative Bacteria Reveals a Bifunctional LolA. Proc. Natl. Acad. Sci. USA 2023, 120, e2218473120. [Google Scholar] [CrossRef]
- Jeffery, C.J. Protein Moonlighting: What Is It, and Why Is It Important? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20160523. [Google Scholar] [CrossRef]
- Jeffery, C. Intracellular Proteins Moonlighting as Bacterial Adhesion Factors. AIMS Microbiol. 2018, 4, 362–376. [Google Scholar] [CrossRef] [PubMed]
- Atanassov, C.; Pezennec, L.; d’Alayer, J.; Grollier, G.; Picard, B.; Fauchère, J.-L. Novel Antigens of Helicobacter pylori Correspond to Ulcer-Related Antibody Pattern of Sera from Infected Patients. J. Clin. Microbiol. 2002, 40, 547–552. [Google Scholar] [CrossRef]
- Kim, N.; Weeks, D.L.; Shin, J.M.; Scott, D.R.; Young, M.K.; Sachs, G. Proteins Released by Helicobacter pylori In Vitro. J. Bacteriol. 2002, 184, 6155–6162. [Google Scholar] [CrossRef]
- Hattori, K.; Naguro, I.; Runchel, C.; Ichijo, H. The Roles of ASK Family Proteins in Stress Responses and Diseases. Cell Commun. Signal. 2009, 7, 9. [Google Scholar] [CrossRef]
- Duriez, P.J.; Shah, G.M. Cleavage of Poly(ADP-Ribose) Polymerase: A Sensitive Parameter to Study Cell Death. Biochem. Cell Biol. 1997, 75, 337–349. [Google Scholar] [CrossRef]
- Basu, S.; Pathak, S.K.; Chatterjee, G.; Pathak, S.; Basu, J.; Kundu, M. Helicobacter pylori Protein HP0175 Transactivates Epidermal Growth Factor Receptor through TLR4 in Gastric Epithelial Cells. J. Biol. Chem. 2008, 283, 32369–32376. [Google Scholar] [CrossRef] [PubMed]
- Strowski, M.Z.; Cramer, T.; Schäfer, G.; Jüttner, S.; Walduck, A.; Schipani, E.; Kemmner, W.; Wessler, S.; Wunder, C.; Weber, M.; et al. Helicobacter pylori Stimulates Host Vascular Endothelial Growth Factor-A (Vegf-A) Gene Expression Via MEK/ERK-Dependent Activation of Sp1 and Sp3. FASEB J. 2004, 18, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Siregar, G.; Sari, D.; Sungkar, T. Serum VEGF Levels in Helicobacter pylori Infection and Correlation with Helicobacter pylori cagA and vacA Genes. Open Access Maced. J. Med. Sci. 2017, 5, 137–141. [Google Scholar] [CrossRef]
- Liu, N.; Wu, Q.; Wang, Y.; Sui, H.; Liu, X.; Zhou, N.; Zhou, L.; Wang, Y.; Ye, N.; Fu, X.; et al. Helicobacter pylori Promotes VEGF Expression Via the P38 MAPK-mediated COX-2-PGE2 Pathway in MKN45 Cells. Mol. Med. Rep. 2014, 10, 2123–2129. [Google Scholar] [CrossRef]
- Kudaravalli, S.; den Hollander, P.; Mani, S.A. Role of P38 MAP Kinase in Cancer Stem Cells and Metastasis. Oncogene 2022, 41, 3177–3185. [Google Scholar] [CrossRef]
- Zheng, Q.; Li, S.; Wang, A.; Zhe, M.; Yang, P.; Wu, Y.; Zhao, M.; Zhu, Y.; Luo, Y.; Wang, G.; et al. P38 Mitogen-Activated Protein Kinase: Functions and Targeted Therapy in Diseases. MedComm–Oncology 2023, 2, e53. [Google Scholar] [CrossRef]
- Thiel, A.; Mrena, J.; Ristimäki, A. Cyclooxygenase-2 and Gastric Cancer. Cancer Metastasis Rev. 2011, 30, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Chen, C.; Hu, J.; Su, R.; Zhang, J.; Han, Z.; Chen, H.; Li, Y. Molecular Mechanism of Helicobacter pylori-Induced Autophagy in Gastric Cancer. Oncol. Lett. 2019, 18, 6221–6227. [Google Scholar] [CrossRef] [PubMed]
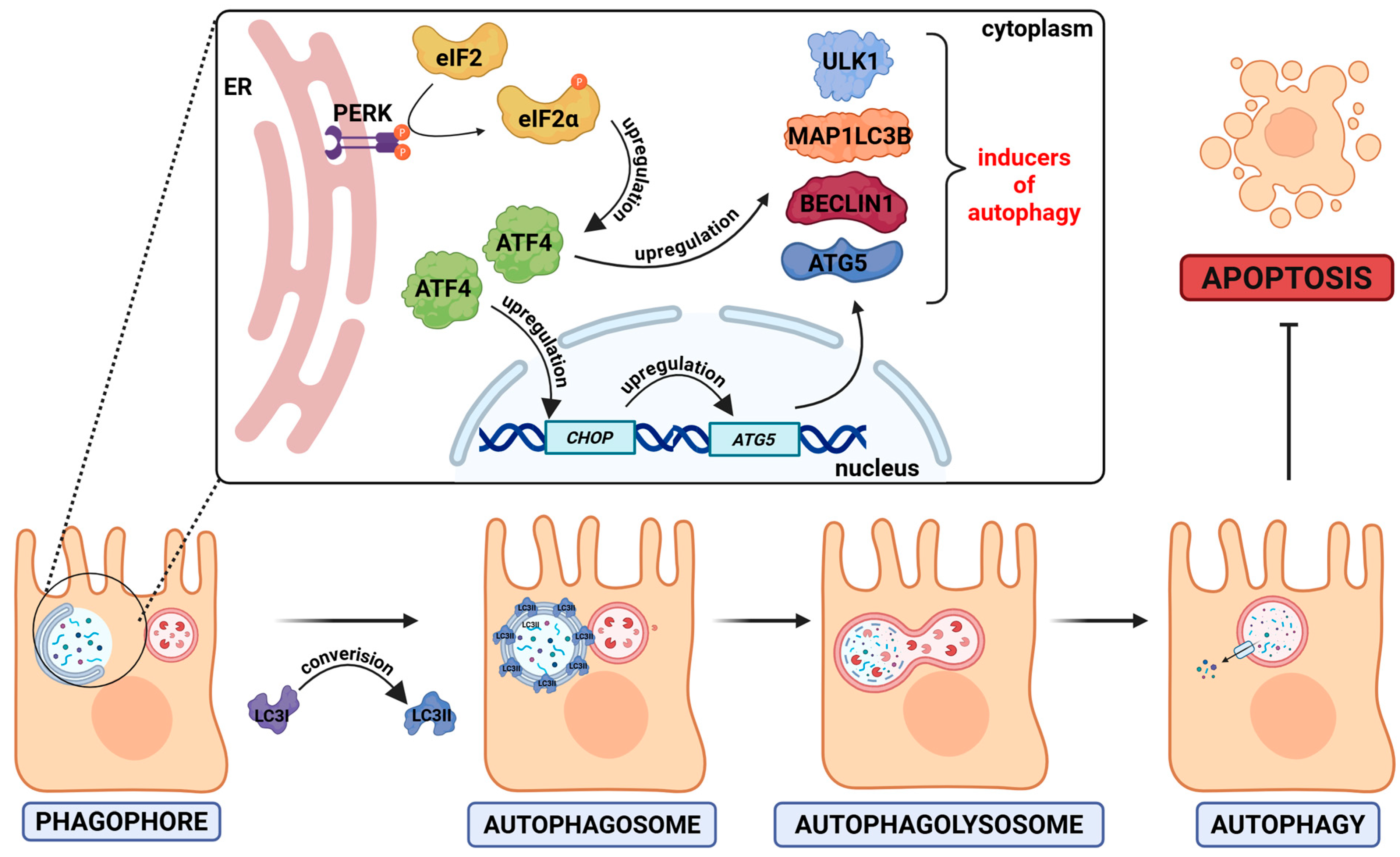
- Halder, P.; Datta, C.; Kumar, R.; Sharma, A.K.; Basu, J.; Kundu, M. The Secreted Antigen, HP0175, of Helicobacter pylori Links the Unfolded Protein Response (UPR) to Autophagy in Gastric Epithelial Cells. Cell. Microbiol. 2015, 17, 714–729. [Google Scholar] [CrossRef]
- Rouschop, K.M.; Dubois, L.J.; Keulers, T.G.; van den Beucken, T.; Lambin, P.; Bussink, J.; van der Kogel, A.J.; Koritzinsky, M.; Wouters, B.G. PERK/eIF2α Signaling Protects Therapy Resistant Hypoxic Cells through Induction of Glutathione Synthesis and Protection against ROS. Proc. Natl. Acad. Sci. USA 2013, 110, 4622–4627. [Google Scholar] [CrossRef]
- Yang, Y.; Shu, X.; Xie, C. An Overview of Autophagy in Helicobacter pylori Infection and Related Gastric Cancer. Front. Cell. Infect. Microbiol. 2022, 12, 847716. [Google Scholar] [CrossRef]
- Akbari, A.; Noorbakhsh Varnosfaderani, S.M.; Haeri, M.S.; Fathi, Z.; Aziziyan, F.; Yousefi Rad, A.; Zalpoor, H.; Nabi-Afjadi, M.; Malekzadegan, Y. Autophagy Induced by Helicobacter pylori Infection Can Lead to Gastric Cancer Dormancy, Metastasis, and Recurrence: New Insights. Hum. Cell 2024, 37, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, A.; Vallström, A.; Petzold, K.; Tegtmeyer, N.; Schleucher, J.; Carlsson, S.; Haas, R.; Backert, S.; Wai, S.N.; Gröbner, G.; et al. Biochemical and Functional Characterization of Helicobacter pylori Vesicles. Mol. Microbiol. 2010, 77, 1539–1555. [Google Scholar] [CrossRef]
- Elmi, A.; Nasher, F.; Jagatia, H.; Gundogdu, O.; Bajaj-Elliott, M.; Wren, B.; Dorrell, N. Campylobacter jejuni Outer Membrane Vesicle-Associated Proteolytic Activity Promotes Bacterial Invasion by Mediating Cleavage of Intestinal Epithelial Cell E-Cadherin and Occludin. Cell. Microbiol. 2016, 18, 561–572. [Google Scholar] [CrossRef]
- Backert, S.; Bernegger, S.; Skórko-Glonek, J.; Wessler, S. Extracellular HtrA Serine Proteases: An Emerging New Strategy in Bacterial Pathogenesis. Cell. Microbiol. 2018, 20, e12845. [Google Scholar] [CrossRef] [PubMed]
- Hoy, B.; Löwer, M.; Weydig, C.; Carra, G.; Tegtmeyer, N.; Geppert, T.; Schröder, P.; Sewald, N.; Backert, S.; Schneider, G.; et al. Helicobacter pylori HtrA Is a New Secreted Virulence Factor That Cleaves E-Cadherin to Disrupt Intercellular Adhesion. EMBO Rep. 2010, 11, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Harrer, A.; Boehm, M.; Backert, S.; Tegtmeyer, N. Overexpression of Serine Protease HtrA Enhances Disruption of Adherens Junctions, Paracellular Transmigration and Type IV Secretion of CagA by Helicobacter pylori. Gut Pathog. 2017, 9, 40. [Google Scholar] [CrossRef]
- Boehm, M.; Hoy, B.; Rohde, M.; Tegtmeyer, N.; Bæk, K.T.; Oyarzabal, O.A.; Brøndsted, L.; Wessler, S.; Backert, S. Rapid Paracellular Transmigration of Campylobacter jejuni across Polarized Epithelial Cells without Affecting TER: Role of Proteolytic-Active HtrA Cleaving E-Cadherin but Not Fibronectin. Gut Pathog. 2012, 4, 3. [Google Scholar] [CrossRef]
- Hoy, B.; Geppert, T.; Boehm, M.; Reisen, F.; Plattner, P.; Gadermaier, G.; Sewald, N.; Ferreira, F.; Briza, P.; Schneider, G.; et al. Distinct Roles of Secreted HtrA Proteases from Gram-Negative Pathogens in Cleaving the Junctional Protein and Tumor Suppressor E-Cadherin. J. Biol. Chem. 2012, 287, 10115–10120. [Google Scholar] [CrossRef]
- Harrer, A.; Bücker, R.; Boehm, M.; Zarzecka, U.; Tegtmeyer, N.; Sticht, H.; Schulzke, J.D.; Backert, S. Campylobacter jejuni Enters Gut Epithelial Cells and Impairs Intestinal Barrier Function through Cleavage of Occludin by Serine Protease HtrA. Gut Pathog. 2019, 11, 4. [Google Scholar] [CrossRef]
- Tegtmeyer, N.; Wessler, S.; Necchi, V.; Rohde, M.; Harrer, A.; Rau, T.T.; Asche, C.I.; Boehm, M.; Loessner, H.; Figueiredo, C.; et al. Helicobacter pylori Employs a Unique Basolateral Type IV Secretion Mechanism for CagA Delivery. Cell Host Microbe 2017, 22, 552–560.e5. [Google Scholar] [CrossRef]
- Bernegger, S.; Vidmar, R.; Fonovic, M.; Posselt, G.; Turk, B.; Wessler, S. Identification of Desmoglein-2 as a Novel Target of Helicobacter pylori HtrA in Epithelial Cells. Cell Commun. Signal. 2021, 19, 108. [Google Scholar] [CrossRef]
- Sharafutdinov, I.; Esmaeili, D.S.; Harrer, A.; Tegtmeyer, N.; Sticht, H.; Backert, S. Campylobacter jejuni Serine Protease HtrA Cleaves the Tight Junction Component Claudin-8. Front. Cell. Infect. Microbiol. 2020, 10, 590186. [Google Scholar] [CrossRef] [PubMed]
- Boehm, M.; Simson, D.; Escher, U.; Schmidt, A.-M.; Bereswill, S.; Tegtmeyer, N.; Backert, S.; Heimesaat, M.M. Function of Serine Protease HtrA in the Lifecycle of the Foodborne Pathogen Campylobacter jejuni. Eur. J. Microbiol. Immunol. 2018, 8, 70–77. [Google Scholar] [CrossRef]

| E. coli Protein | H. pylori Homolog | C. jejuni Homolog | %id./%sim. * | (E-Value) ** | Functions |
|---|---|---|---|---|---|
| Lol COMPLEX | |||||
| LolA | HP0785 | CJ0943 | EC-HP: 30%/43% EC-CJ: –/– HP-CJ: 28%/47% | EC-HP: 2 × 10−6 EC-CJ: – HP-CJ: 1 × 10−16 | Periplasmic chaperone; lipoprotein transport |
| LolB | N/A | N/A | – | – | OM associated lipoprotein; incorporation of lipoproteins into OM |
| LolC | HP0787 (LolF) *** | CJ0941 (LolF) *** | EC-HP: 24%/47% EC-CJ: 22%/46% HP-CJ: 54%/73% | EC-HP: 3 × 10−36 EC-CJ: 6 × 10−28 HP-CJ: 1 × 10−148 | Integral IM protein; part of an ATP-dependent lipoprotein IM sorting system |
| LolD | HP0179 | CJ1277 | EC-HP: 38%/56% EC-CJ: 38%/60% HP-CJ: 29%/58% | EC-HP: 6 × 10−40 EC-CJ: 5 × 10−47 HP-CJ: 2 × 10−30 | IM ABC transporter domain; part of an ATP-dependent lipoprotein IM sorting system |
| LolE | HP0787 (LolF) *** | CJ0941 (LolF) *** | EC-HP: 23%/49% EC-CJ: 22%/46% HP-CJ: 54%/73% | EC-HP: 2 × 10−34 EC-CJ: 3 × 10−14 HP-CJ: 1 × 10−148 | Integral IM protein; part of an ATP-dependent lipoprotein IM sorting system |
| Periplasmic proteases | |||||
| DegP | HP1018/19 (HtrA) | CJ1228 | EC-HP: 42%/62% EC-CJ: 42%/61% HP-CJ: 50%/68% | EC-HP: 5 × 10−90 EC-CJ: 8 × 10−95 HP-CJ: 5 × 10−138 | Periplasmic serine endoprotease/chaperone |
| DegQ | EC-HP: 40%/60% EC-CJ: 38%/59% HP-CJ: 50%/68% | EC-HP: 3 × 10−82 EC-CJ: 2 × 10−81 HP-CJ: 5 × 10−138 | Periplasmic pH-dependent serine endoprotease | ||
| BepA | N/A | N/A | – | – | Periplasmic metalloprotease/chaperone |
| YcaL | N/A | N/A | – | – | Periplasmic metalloprotease |
| Major periplasmic chaperones | |||||
| SurA | HP0175 (HpCBF2) | CJ0596 (PEB4) | EC-HP: 32%/46% EC-CJ: 25%/43% HP-CJ: 35%/55% | EC-HP: 3 × 10−12 EC-CJ: 4 × 10−8 HP-CJ: 4 × 10−42 | Folding and assembly of OMPs; PPIase activity |
| HP0659 | CJ1289 (SalC) | EC-HP: 24%/47% EC-CJ: 21%/51% HP-CJ: 26%/50% | EC-HP: 1 × 10−10 EC-CJ: 2 × 10−8 HP-CJ: 1 × 10−25 | ||
| HP0977 | CJ0694 | EC-HP: –/– EC-CJ: 34%/54% HP-CJ: 32%/54% | EC-HP: – EC-CJ: 2 × 10−4 HP-CJ: 2 × 10−74 | ||
| PpiD | HP0977 | CJ0694 | EC-HP: 25%/48% EC-CJ: 21%/45% HP-CJ: 32%/54% | EC-HP: 1 × 10−18 EC-CJ: 3 × 10−16 HP-CJ: 2 × 10−74 | IM-associated protein; facilitates precursor proteins translocation via SecYEG |
| FkpA (FK506) | N/A | N/A | – | – | PPIase/chaperone |
| Skp | N/A | N/A | – | – | General chaperone |
| Spy | N/A | N/A | – | – | General chaperone; protein refolding |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godlewska, R.; Weltrowski, M.; Skórko-Glonek, J. The Extracytoplasmic Protein Quality Control System in Pathogenic Campylobacterota: Its Role in Bacterial Virulence and Maintaining Cellular Envelope Proteostasis. Int. J. Mol. Sci. 2025, 26, 8371. https://doi.org/10.3390/ijms26178371
Godlewska R, Weltrowski M, Skórko-Glonek J. The Extracytoplasmic Protein Quality Control System in Pathogenic Campylobacterota: Its Role in Bacterial Virulence and Maintaining Cellular Envelope Proteostasis. International Journal of Molecular Sciences. 2025; 26(17):8371. https://doi.org/10.3390/ijms26178371
Chicago/Turabian StyleGodlewska, Renata, Mateusz Weltrowski, and Joanna Skórko-Glonek. 2025. "The Extracytoplasmic Protein Quality Control System in Pathogenic Campylobacterota: Its Role in Bacterial Virulence and Maintaining Cellular Envelope Proteostasis" International Journal of Molecular Sciences 26, no. 17: 8371. https://doi.org/10.3390/ijms26178371
APA StyleGodlewska, R., Weltrowski, M., & Skórko-Glonek, J. (2025). The Extracytoplasmic Protein Quality Control System in Pathogenic Campylobacterota: Its Role in Bacterial Virulence and Maintaining Cellular Envelope Proteostasis. International Journal of Molecular Sciences, 26(17), 8371. https://doi.org/10.3390/ijms26178371






