The Therapeutic Potential of Stem Cells in Depression
Abstract
1. Introduction
1.1. Major Depressive Disorder (MDD): Definition and Pathophysiology
1.2. Stem Cells (SCs)
1.2.1. Classification of Stem Cells
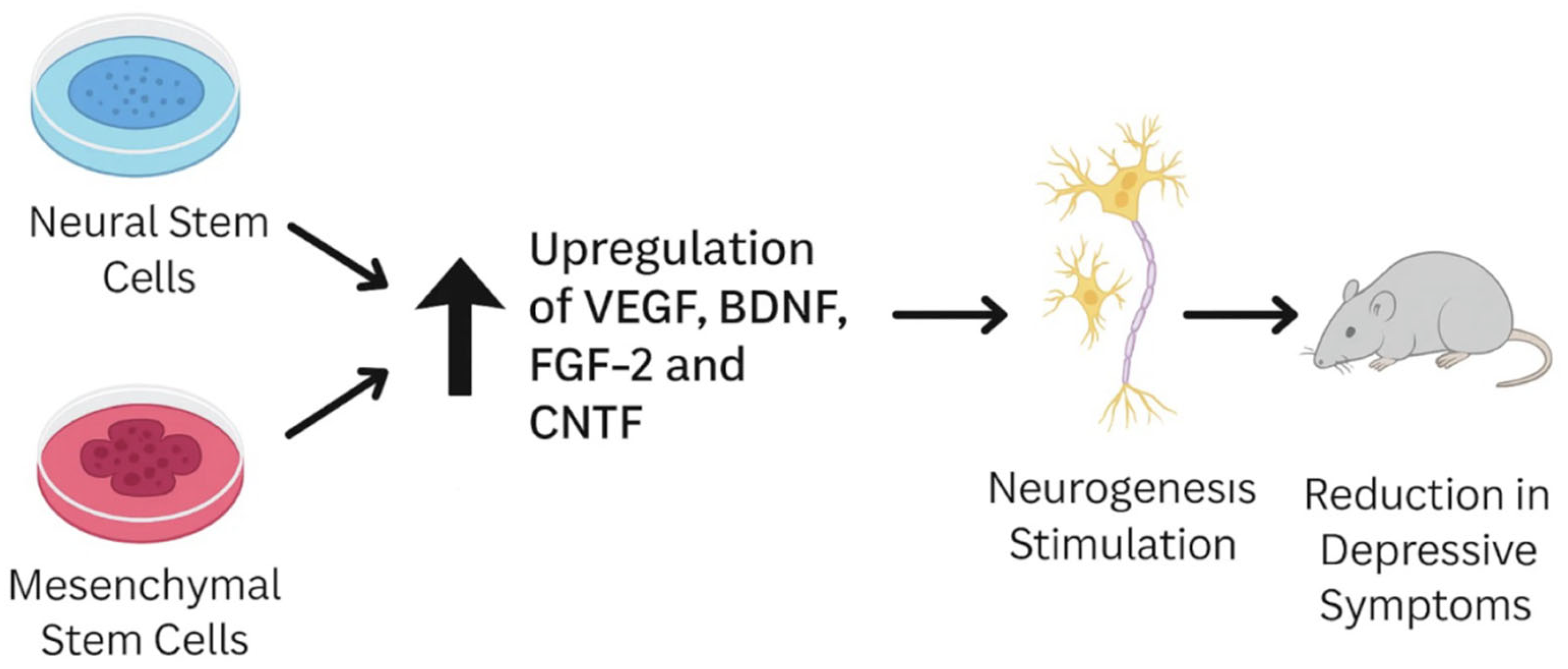
1.2.2. Neural Stem Cells (NSCs)
1.2.3. Mesenchymal Stem Cells (MSCs)
1.2.4. Immunomodulatory and Neurotrophic Functions
1.2.5. Comparative Characteristics: UC-MSCs vs. BM-MSCs
1.2.6. Clinical Relevance and Regulatory Framework
1.2.7. Human Embryonic Stem Cells (hESCs)
1.2.8. Induced Pluripotent Stem Cells (iPSCs)
1.3. Mechanisms of Action of Stem Cell (SC)-Based Therapies in Depression
2. Aim of This Study
3. Results
3.1. Preclinical Efficacy in Animal Models
Delivery Strategies: Intranasal Administration
4. Discussion
Future Directions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MDD | Major depressive disorder |
| SC | Stem cell |
| NSC | Neural stem cell |
| MSC | Mesenchymal stem cell |
| eMSC | Encapsulated mesenchymal stem cell |
| hESC | Human embryonic stem cell |
| iPSC | Induced pluripotent stem cell |
| UC-MSC | Umbilical-cord-derived mesenchymal stem cell |
| BM-MSC | Bone-marrow-derived mesenchymal stem cell |
| BDNF | Brain-derived neurotrophic factor |
| CNTF | Ciliary neurotrophic factor |
| FGF-2 | Fibroblast growth factor 2 |
| VEGF | Vascular endothelial growth factor |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| TNF-α | Tumor necrosis factor alpha |
| TGF-β | Transforming growth factor beta |
| PGE2 | Prostaglandin E2 |
| IDO | Indoleamine 2,3-dioxygenase |
| GABA | Gamma-aminobutyric acid |
| NMDA | N-Methyl-D-aspartate |
| DRN | Dorsal raphe nucleus |
| EVs | Extracellular vesicles |
| HPA | Hypothalamic–pituitary–adrenal (axis) |
| PHQ-9 | Patient Health Questionnaire 9 |
| HAM-D | Hamilton Rating Scale for Depression |
| MADRS | Montgomery–Åsberg Depression Rating Scale |
| GDS | Geriatric Depression Scale |
| HADS | Hospital Anxiety and Depression Scale |
| CNS | Central nervous system |
| INA | Intranasal administration |
| LPS | Lipopolysaccharide |
| FST | Forced Swim Test |
| SPT | Sucrose Preference Test |
| CRH | Corticotropin-releasing hormone |
References
- Institute of Health Metrics and Evaluation. Global Health Data Exchange (GHDx). Available online: https://vizhub.healthdata.org/gbd-results/ (accessed on 3 August 2025).
- Bains, N.; Abdijadid, S. Major Depressive Disorder; StatPearls Publishing: Treasure Island, FL, USA, 2023; Updated 2023 Apr 10; StatPearls [Internet]; 2025 January. Available online: https://www.ncbi.nlm.nih.gov/books/NBK559078 (accessed on 3 August 2025).
- Al-Harbi, K.S. Treatment-resistant depression: Therapeutic trends, challenges, and future directions. Patient Prefer. Adherence 2012, 6, 369–388. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Delva, N.C.; Stanwood, G.D. Dysregulation of brain dopamine systems in major depressive disorder. Exp. Biol. Med. 2021, 246, 1084–1093. [Google Scholar] [CrossRef]
- Park, H.; Poo, M.-M. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 2013, 14, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.F.; Peng, W.; Sweeney, J.A.; Jia, Z.Y.; Gong, Q.Y. Brain structure alterations in depression: Psychoradiological evidence. CNS Neurosci. Ther. 2018, 24, 994–1003. [Google Scholar] [CrossRef]
- Pagnin, D.; de Queiroz, V.; Pini, S.; Cassano, G.B. Efficacy of ECT in depression: A meta-analytic review. J. ECT 2004, 20, 13–20. [Google Scholar] [CrossRef]
- Seri, B.; García-Verdugo, J.M.; McEwen, B.S.; Alvarez-Buylla, A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J. Neurosci. 2001, 21, 7153–7160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McIntyre, R.S.; Alsuwaidan, M.; Baune, B.T.; Berk, M.; Demyttenaere, K.; Goldberg, J.F.; Gorwood, P.; Ho, R.; Kasper, S.; Kennedy, S.H.; et al. Treatment-resistant depression: Definition, prevalence, detection, management, and investigational interventions. World Psychiatry 2023, 22, 394–412. [Google Scholar] [CrossRef] [PubMed]
- Encinas, J.M.; Sierra, A. Neural stem cell deforestation as the main force driving the age-related decline in adult hippocampal neurogenesis. Behav. Brain Res. 2012, 227, 433–439. [Google Scholar] [CrossRef]
- Suh, H.; Consiglio, A.; Ray, J.; Sawai, T.; D’Amour, K.A.; Gage, F.H. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell 2007, 1, 515–528. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lugert, S.; Basak, O.; Knuckles, P.; Haussler, U.; Fabel, K.; Götz, M.; Haas, C.A.; Kempermann, G.; Taylor, V.; Giachino, C. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell 2010, 6, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Y.; Zhang, Y.; Liu, M.; Rong, X.; Jiang, J. Therapeutic potential and mechanisms of stem cells in major depressive disorder: A comprehensive review. Front. Pharmacol. 2024, 15, 1476558. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Galipeau, J.; Sensébé, L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 2018, 22, 824–833. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abdulmalek, O.A.A.Y.; Husain, K.H.; AlKhalifa, H.K.A.A.; Alturani, M.M.A.B.; Butler, A.E.; Moin, A.S.M. Therapeutic Applications of Stem Cell-Derived Exosomes. Int. J. Mol. Sci. 2024, 25, 3562. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shawki, S.; Gaafar, T.; Erfan, H.; El Khateeb, E.; El Sheikhah, A.; El Hawary, R. Immunomodulatory effects of umbilical cord-derived mesenchymal stem cells. Microbiol. Immunol. 2015, 59, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Lim, J.Y.; Lim, T.; Im, K.I.; Kim, N.; Nam, Y.S.; Jeon, Y.W.; Shin, J.C.; Ko, H.S.; Park, I.Y.; et al. Human mesenchymal stem cells derived from umbilical cord and bone marrow exert immunomodulatory effects in different mechanisms. World J. Stem Cells 2020, 12, 1032–1049. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Asari, S.; Itakura, S.; Ferreri, K.; Liu, C.P.; Kuroda, Y.; Kandeel, F.; Mullen, Y. Mesenchymal stem cells suppress B-cell terminal differentiation. Exp. Hematol. 2009, 37, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.J.; Reichardt, L.F. Trk receptors: Roles in neuronal signal transduction. Annu. Rev. Biochem. 2003, 72, 609–642. [Google Scholar] [CrossRef] [PubMed]
- Castrén, E.; Monteggia, L.M. Brain-Derived Neurotrophic Factor Signaling in Depression and Antidepressant Action. Biol. Psychiatry 2021, 90, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Wiese, D.M.; Wood, C.A.; Ford, B.N.; Braid, L.R. Cytokine Activation Reveals Tissue-Imprinted Gene Profiles of Mesenchymal Stromal Cells. Front. Immunol. 2022, 13, 917790. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cui, G.H.; Guo, H.D.; Li, H.; Zhai, Y.; Gong, Z.B.; Wu, J.; Liu, J.S.; Dong, Y.R.; Hou, S.X.; Liu, J.R. RVG-modified exosomes derived from mesenchymal stem cells rescue memory deficits by regulating inflammatory responses in a mouse model of Alzheimer’s disease. Immun. Ageing 2019, 16, 10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Q.; Xu, Y.; Zhu, S.; Jiang, L.; Yao, L.; Yu, X.; Zhang, Y.; Jia, S.; Hong, M.; Zheng, J. Mesenchymal stem cells improve depressive disorder via inhibiting the inflammatory polarization of microglia. J. Psychiatr. Res. 2024, 179, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Qiao, H.; He, D.; Qin, X.; Zhang, Q.; Zhou, Y. Mesenchymal stem cells inhibited the inflammation and oxidative stress in LPS-activated microglial cells through AMPK pathway. J. Neural. Transm. 2019, 126, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Mebarki, M.; Iglicki, N.; Marigny, C.; Abadie, C.; Nicolet, C.; Churlaud, G.; Maheux, C.; Boucher, H.; Monsel, A.; Menasché, P.; et al. Development of a human umbilical cord-derived mesenchymal stromal cell-based advanced therapy medicinal product to treat immune and/or inflammatory diseases. Stem Cell Res. Ther. 2021, 12, 571. [Google Scholar] [CrossRef]
- Iyer, S.; Alsayegh, K.; Abraham, S.; Rao, R.R. Stem cell-based models and therapies for neurodegenerative diseases. Crit. Rev. Biomed. Eng. 2009, 37, 321–353. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mora, C.; Serzanti, M.; Consiglio, A.; Memo, M.; Dell’eRa, P. Clinical potentials of human pluripotent stem cells. Cell Biol. Toxicol. 2017, 33, 351–360. [Google Scholar] [CrossRef]
- Na, L.; Wang, S.; Liu, T.; Zhang, L. Ultrashort wave combined with human umbilical cord mesenchymal stem cell (HUC-MSC) transplantation inhibits NLRP3 inflammasome and improves spinal cord injury via MK2/TTP signalling pathway. Biomed. Res. Int. 2020, 1, 3021750. [Google Scholar] [CrossRef] [PubMed]
- Vadodaria, K.C.; Mertens, J.; Paquola, A.; Bardy, C.; Li, X.; Jappelli, R.; Fung, L.; Marchetto, M.C.; Hamm, M.; Gorris, M.; et al. Generation of functional human serotonergic neurons from fibroblasts. Mol. Psychiatry 2016, 21, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhong, X.; Liu, H.; Hao, L.; Huang, C.T.; Sherafat, M.A.; Jones, J.; Ayala, M.; Li, L.; Zhang, S.C. Generation of serotonin neurons from human pluripotent stem cells. Nat. Biotechnol. 2016, 34, 89–94. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Soliman, M.A.; Aboharb, F.; Zeltner, N.; Studer, L. Pluripotent stem cells in neuropsychiatric disorders. Mol. Psychiatry 2017, 22, 1241–1249. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Costa-Ferro, Z.S.M.; Cunha, R.S.; Rossi, E.A.; Loiola, E.C.; Cipriano, B.P.; Figueiredo, J.C.Q.; da Silva, E.A.; de Lima, A.V.R.; Ribeiro, A.M.d.J.; Junior, V.S.M.; et al. Extracellular vesicles derived from mesenchymal stem cells alleviate depressive-like behavior in a rat model of chronic stress. Life Sci. 2025, 366–367, 123479. [Google Scholar] [CrossRef]
- Kin, K.; Yasuhara, T.; Kameda, M.; Tomita, Y.; Umakoshi, M.; Kuwahara, K.; Kin, I.; Kidani, N.; Morimoto, J.; Okazaki, M.; et al. Cell encapsulation enhances antidepressant effect of the mesenchymal stem cells and counteracts depressive-like behavior of treatment-resistant depressed rats. Mol. Psychiatry 2020, 25, 1202–1214. [Google Scholar] [CrossRef]
- Jiang, W.; Xu, J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2020, 53, e12712. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, Y.J.; Chen, C.N.; Zhan, J.Q.; Liu, Q.S.; Liu, Y.; Jiang, S.Z.; Wei, B. Decreased Plasma Hydrogen Sulfide Level Is Associated with the Severity of Depression in Patients with Depressive Disorder. Front. Psychiatry 2021, 12, 765664. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, X.; Zhang, M.; Liu, H.; Zhu, R.; He, H.; Zhou, Y.; Zhang, Y.; Li, C.; Liang, D.; Zeng, Q.; et al. Bone marrow mesenchymal stem cell-derived exosomes attenuate cerebral ischemia-reperfusion injury-induced neuroinflammation and pyroptosis by modulating microglia M1/M2 phenotypes. Exp. Neurol. 2021, 341, 113700. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Huang, W.; Yi, J.; Deng, Y.; Li, R.; Chen, J.; Shi, J.; Qiu, Y.; Wang, T.; Chen, X.; et al. Mesenchymal stromal cells alleviate depressive and anxiety-like behaviors via a lung vagal-to-brain axis in male mice. Nat. Commun. 2023, 14, 7406. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Costa-Ferro, Z.S.M.; do Prado-Lima, P.A.S.; Onsten, G.A.; Oliveira, G.N.; Brito, G.C.; Ghilardi, I.M.; Dos Santos, P.G.; Bertinatto, R.J.; da Silva, D.V.; Salamoni, S.D.; et al. Bone marrow mononuclear cell transplant prevents rat depression and modulates inflammatory and neurogenic molecules. Prog. Neuropsychopharmacol. Biol. Psychiatry 2022, 113, 110455. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, B.; Yang, J.; Lian, Y.J.; Yu, H.Z.; Wang, Y.X. HMGB1 in depression: An overview of microglial HMBG1 in the pathogenesis of depression. Brain Behav. Immun. Health 2023, 30, 100641. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.-T.; He, K.-J.; Zhang, J.-B.; Ma, Q.-H.; Wang, F.; Liu, C.-F. Advances in intranasal application of stem cells in the treatment of central nervous system diseases. Stem Cell Res. Ther. 2021, 12, 210. [Google Scholar] [CrossRef]
- Nautiyal, K.M.; Dailey, C.A.; Jahn, J.L.; Rodriquez, E.; Son, N.H.; Sweedler, J.V.; Silver, R. Serotonin of mast cell origin contributes to hippocampal function. Eur. J. Neurosci. 2012, 36, 2347–2359. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, X.; Fei, G.-Q.; Liu, W.-J.; Ding, J.; Wang, Y.; Wang, H.; Ji, J.-L.; Wang, X. Adipose-derived mesenchymal stem cells protect against CMS-induced depression-like behaviors in mice via regulating the Nrf2/HO-1 and TLR4/NF-κB signaling pathways. Acta Pharmacol. Sin. 2020, 41, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, Y.; Song, Y.; Gao, Y.; Hao, C.; Zhou, Y.; Bao, S.; Guo, J.; Li, X. Human umbilical cord mesenchymal stem cells reverse depression in rats induced by chronic unpredictable mild stress combined with lipopolysaccharide. CNS Neurosci. Ther. 2024, 30, e14644. [Google Scholar] [CrossRef]
- Kigawa, Y.; Hashimoto, E.; Ukai, W.; Ishii, T.; Furuse, K.; Tsujino, H.; Shirasaka, T.; Saito, T. Stem cell therapy: A new approach to the treatment of refractory depression. J. Neural Transm. 2014, 121, 1221–1232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Li, Y.; Liu, R.; Pan, J.; Tang, X.; Sun, S.; Liu, J.; Ma, W. Human umbilical cord mesenchymal stem cells ameliorate depression by regulating Jmjd3 and microglia polarization in myocardial infarction mice. Psychopharmacology 2021, 238, 2973–2984. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, W.; Xu, J.; Liu, D.; Jiang, H.; Pan, F. Dynamic Effects of Early Adolescent Stress on Depressive-Like Behaviors and Expression of Cytokines and JMJD3 in the Prefrontal Cortex and Hippocampus of Rats. Front. Psychiatry 2018, 9, 471. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shwartz, A.; Betzer, O.; Kronfeld, N.; Kazimirsky, G.; Cazacu, S.; Finniss, S.; Lee, H.K.; Motiei, M.; Dagan, S.Y.; Popovtzer, R.; et al. Therapeutic Effect of Astroglia-like Mesenchymal Stem Cells Expressing Glutamate Transporter in a Genetic Rat Model of Depression. Theranostics 2017, 7, 2690–2703. [Google Scholar] [CrossRef]
- Kot, M.; Neglur, P.K.; Pietraszewska, A.; Buzanska, L. Boosting Neurogenesis in the Adult Hippocampus Using Antidepressants and Mesenchymal Stem Cells. Cells 2022, 11, 3234. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tfilin, M.; Sudai, E.; Merenlender, A.; Gispan, I.; Yadid, G.; Turgeman, G. Mesenchymal Stem Cells Increase Hippocampal Neurogenesis and Counteract Depressive-like Behavior. Mol. Psychiatry 2010, 15, 1164–1175. [Google Scholar] [CrossRef]
- Georgin-Lavialle, S.; Moura, D.S.; Salvador, A.; Chauvet-Gelinier, J.-C.; Launay, J.-M.; Damaj, G.; Côté, F.; Soucié, E.; Chandesris, M.-O.; Barète, S.; et al. Mast cells’ involvement in inflammation pathways linked to depression: Evidence in mastocytosis. Mol. Psychiatry 2016, 21, 1511–1516. [Google Scholar] [CrossRef]
- Sachdeva, P.; Ji, S.; Ghosh, S.; Ghosh, S.; Raghunath, M.; Kim, H.; Bhaskar, R.; Sinha, J.K.; Han, S.S. Plausible Role of Stem Cell Types for Treating and Understanding the Pathophysiology of Depression. Pharmaceutics 2023, 15, 814. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Smulevich, A.B.; Dubnitskaya, E.B.; Voronova, E.I.; Morozova, Y.V.; Radaev, S.M. Efficiency of umbilical cord blood cells in patients with treatment-resistant depression. Bull. Exp. Biol. Med. 2016, 160, 583–588. [Google Scholar] [CrossRef]
- Available online: https://clinicaltrials.gov/ (accessed on 25 July 2025).
- Villar-Gómez, N.; Ojeda-Hernandez, D.D.; López-Muguruza, E.; García-Flores, S.; Bonel-García, N.; Benito-Martín, M.S.; Selma-Calvo, B.; Canales-Aguirre, A.A.; Mateos-Díaz, J.C.; Montero-Escribano, P.; et al. Nose-to-Brain: The Next Step for Stem Cell and Biomaterial Therapy in Neurological Disorders. Cells 2022, 11, 3095. [Google Scholar] [CrossRef]
- Salehi, M.S.; Jurek, B.; Karimi-Haghighi, S.; Nezhad, N.J.; Mousavi, S.M.; Hooshmandi, E.; Safari, A.; Dianatpour, M.; Haerteis, S.; Miyan, J.A.; et al. Intranasal application of stem cells and their derivatives as a new hope in the treatment of cerebral hypoxia/ischemia: A review. Rev. Neurosci. 2022, 33, 583–606. [Google Scholar] [CrossRef]
- Guo, H.; Huang, B.; Wang, Y.; Zhang, Y.; Ma, Q.; Ren, Y. Bone marrow mesenchymal stem cells-derived exosomes improve injury of hippocampal neurons in rats with depression by upregulating microRNA-26a expression. Int. Immunopharmacol. 2020, 82, 106285. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Guo, Z.; Guo, B.; Xie, Y.; Yang, J.; Wang, A. Inhibition of the endogenous CSE/H2S system contributes to hypoxia and serum deprivation-induced apoptosis in mesenchymal stem cells. Mol. Med. Rep. 2014, 9, 2467–2472. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kang, X.; Jiang, L.; Lan, F.; Tang, Y.Y.; Zhang, P.; Zou, W.; Chen, Y.J.; Tang, X.Q. Hydrogen sulfide antagonizes sleep deprivation-induced depression- and anxiety-like behaviors by inhibiting neuroinflammation in a hippocampal Sirt1-dependent manner. Brain Res. Bull. 2021, 177, 194–202. [Google Scholar] [CrossRef] [PubMed]
| Key Property | Description |
|---|---|
| Self-renewal | Ability to proliferate while maintaining an undifferentiated state. |
| Multilineage differentiation | Capacity to generate various specialized cell types. |
| Homing | Directed migration to sites of injury or inflammation. |
| Immunological tolerance | Low immunogenicity that facilitates transplantation. |
| Stem Cell Type | Origin | Differentiation Potential | Immunogenicity | Clinical Use |
|---|---|---|---|---|
| NSC | Neural tissue | Tripotent (neurons, astrocytes, oligodendrocytes) | Low | CNS repair |
| MSC | Bone marrow, adipose tissue, umbilical cord | Multipotent | Very low | Immunomodulation, neurotrophic support |
| hESC | Blastocyst | Pluripotent | High | Experimental applications |
| iPSC | Reprogrammed somatic cells | Pluripotent | Medium | Personalized medicine, disease modeling |
| Study/Authors | Stem Cell Type | Model | Key Outcomes |
|---|---|---|---|
| Kin et al. [33] | Encapsulated MSCs (eMSCs) | Wistar-Kyoto rats (treatment-resistant depression model) | The eMSCs implanted into the lateral ventricle alleviated depressive-like behaviors and boosted neurogenesis in the SVZ and hippocampal dentate gyrus. These effects were not observed with non-encapsulated MSCs or with eMSCs implanted outside neurogenic regions. |
| Costa-Ferro et al. [38] | Bone marrow mononuclear cells | Rats (chronic mild stress model) | Prevented depression/anxiety-like behavior, reduced HMGB1 and IL-1β, increased BDNF |
| Wang et al. [43] | Human umbilical-cord-derived MSCs (hUC-MSCs) | Rats (depression model) | Reversed depressive behavior, normalized hippocampal morphology, reduced cytokines |
| Kigawa et al. [44] | MSCs and sertraline | Rats (prenatal and adolescent induced depression) | Synergistic antidepressant effects |
| Zhang et al. [45] | hUC-MSCs | Myocardial-infarction-induced depression in mice | Improved cardiac and depressive symptoms, downregulated Jmjd3, modulated microglial polarization |
| Shwartz et al. [47] | MSCs overexpressing EAAT1 | FSL rats (genetic depression model) | MSC-EAAT treatment in FSL rats reduced depressive-like behaviors and increased EAAT1 and BDNF expression in the hippocampus. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurczenko, L.; Semeniuk, A.; Leszek, J.W. The Therapeutic Potential of Stem Cells in Depression. Int. J. Mol. Sci. 2025, 26, 8306. https://doi.org/10.3390/ijms26178306
Jurczenko L, Semeniuk A, Leszek JW. The Therapeutic Potential of Stem Cells in Depression. International Journal of Molecular Sciences. 2025; 26(17):8306. https://doi.org/10.3390/ijms26178306
Chicago/Turabian StyleJurczenko, Lidia, Alina Semeniuk, and Jerzy Waldemar Leszek. 2025. "The Therapeutic Potential of Stem Cells in Depression" International Journal of Molecular Sciences 26, no. 17: 8306. https://doi.org/10.3390/ijms26178306
APA StyleJurczenko, L., Semeniuk, A., & Leszek, J. W. (2025). The Therapeutic Potential of Stem Cells in Depression. International Journal of Molecular Sciences, 26(17), 8306. https://doi.org/10.3390/ijms26178306







