Daytime-Dependent Effects of Thiamine on the Thiamine Pool and Pyruvate Dehydrogenase Regulation in the Brain and Heart
Abstract
1. Introduction
2. Results
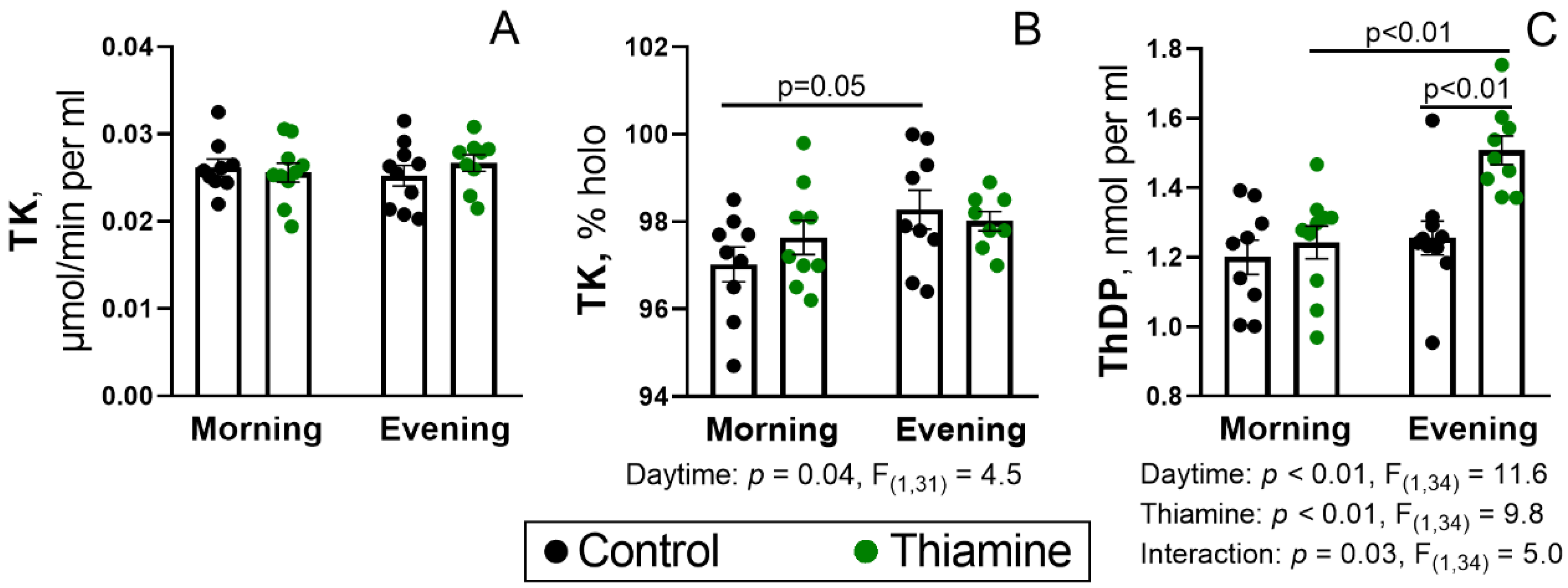
2.1. Status of the Blood TK Activity and ThDP Level During the Day, and Their Regulation by Thiamine Administration
2.2. Daytime Dependence of the Thiamine-Dependent Enzyme Activities and Their Tissue-Specific Changes upon the Thiamine Administration
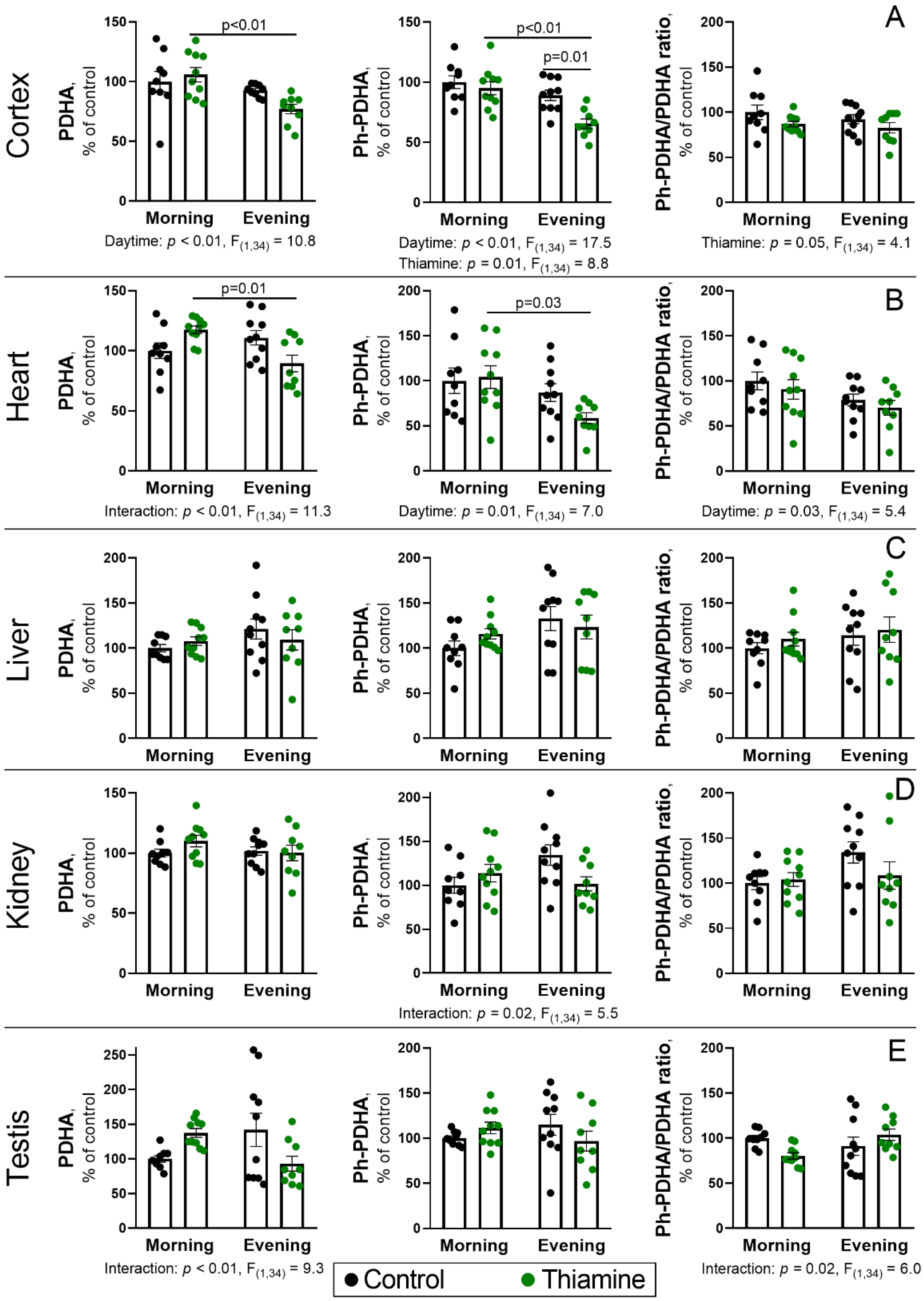
2.3. Daytime Changes in the PDH Protein Level and Phosphorylation, and Its Regulation by Thiamine Administration in Rat Tissues
2.4. Diurnal Changes and Response to Thiamine Administration of PDH Kinases and Phosphatases and the Components of Thiamine Pool in the Cerebral Cortex and Heart
2.5. Daytime Dependence of Physiological Parameters of the Experimental Rats
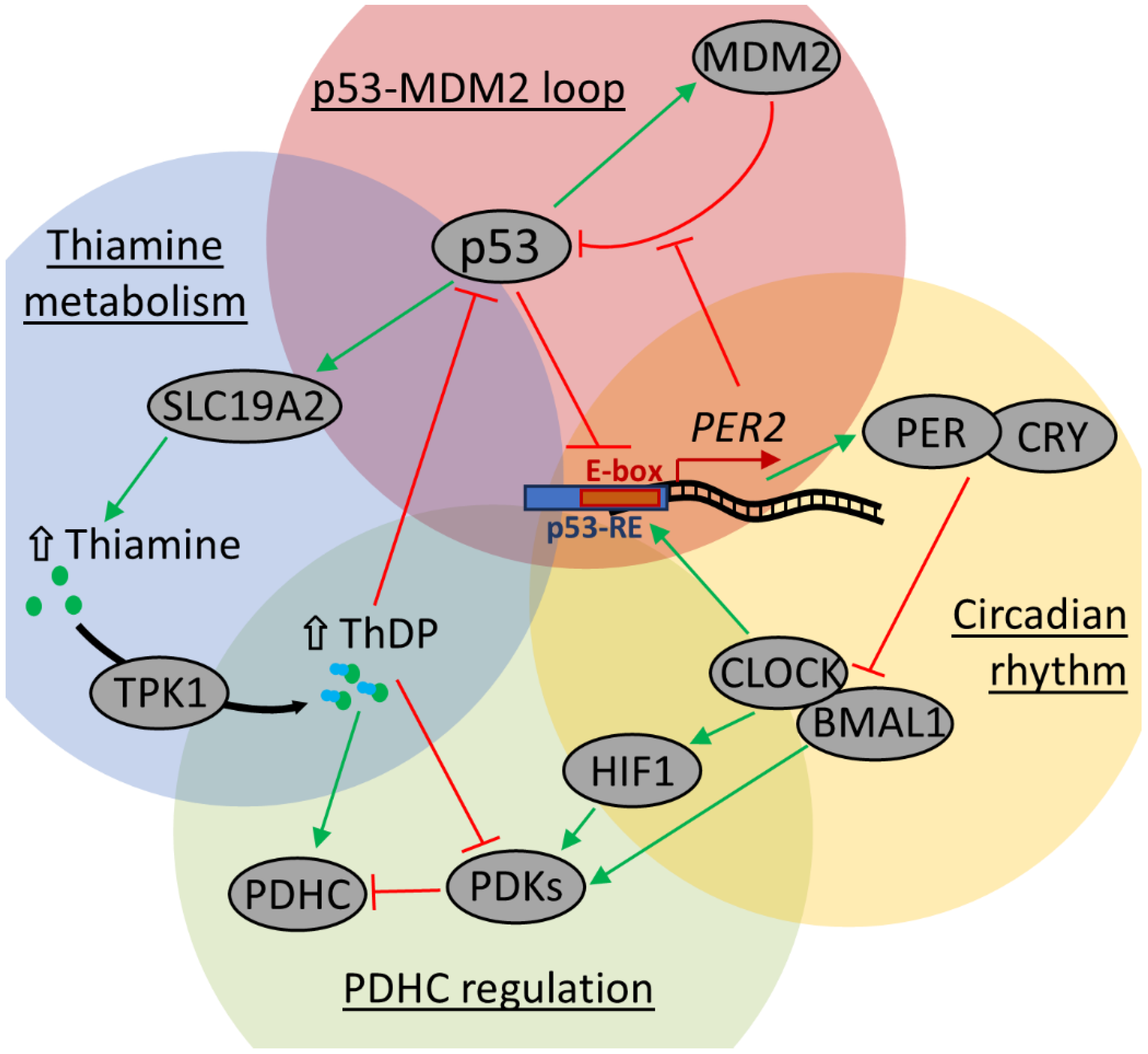
3. Discussion
3.1. Circadian Regulation of Thiamine, ThDP, and Thiamine-Dependent Proteins
3.2. Tissue-Dependent Response of PDHC Regulation and Thiamine Pool to Thiamine Administration
3.3. Potential Clinical Significance of High-Dose Thiamine Supplementation
4. Materials and Methods
4.1. Materials
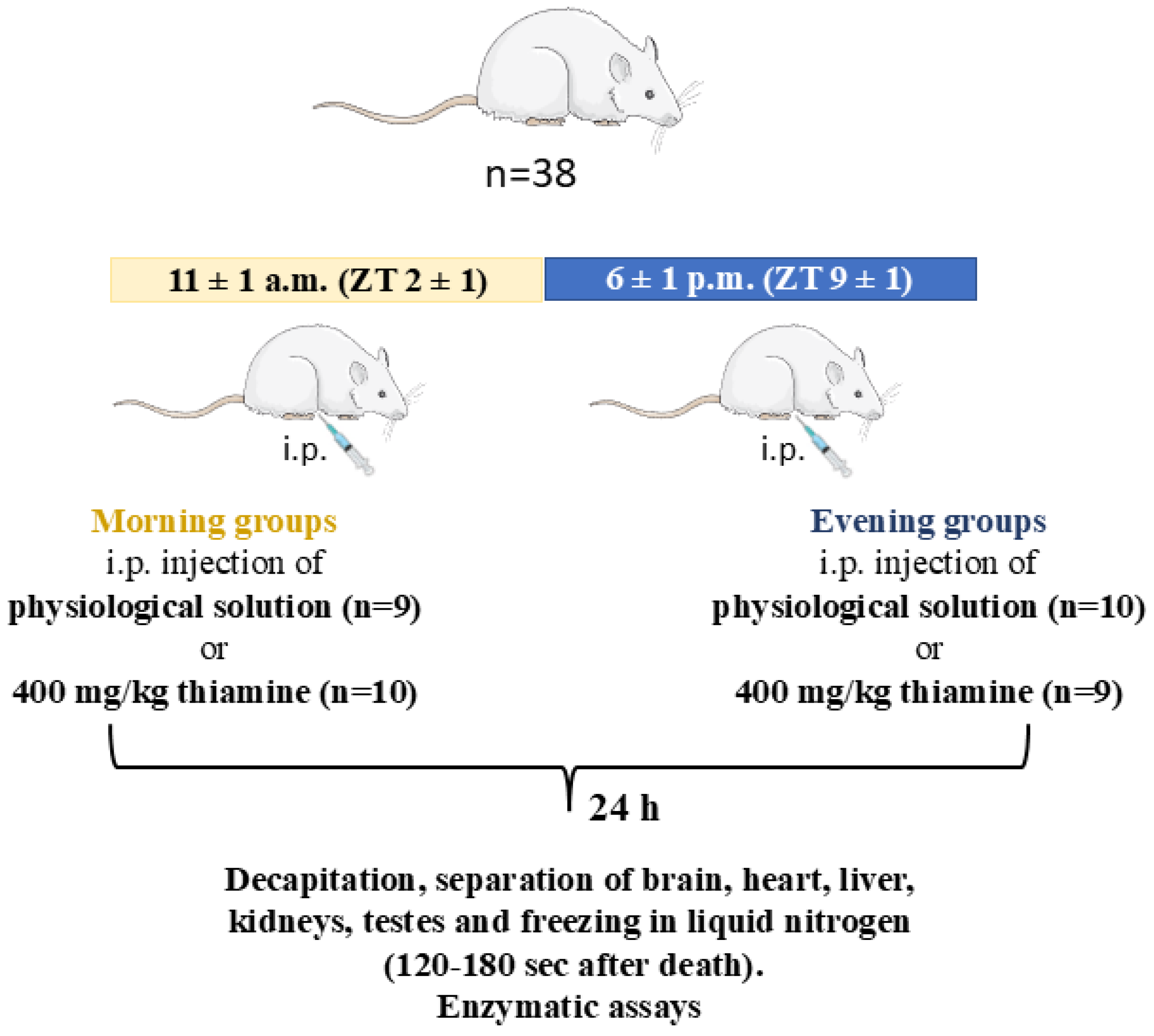
4.2. Animal Experiments and Tissue Sample Collection
4.3. Preparation of the Rat Tissue Homogenates
4.4. Assays of Enzyme Activities in Tissue Homogenates
4.5. Western Blotting
4.6. Quantifications of Thiamine, Thiamine Monophosphate (ThMP), and ThDP in Tissue Extracts
4.7. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ECG | electrocardiography |
| GDH | glutamate dehydrogenase |
| MDH | malate dehydrogenase |
| OGDH | 2-oxoglutarate dehydrogenase or α-ketoglutarate dehydrogenase |
| OGDHC | 2-oxoglutarate dehydrogenase complex or α-ketoglutarate dehydrogenase complex |
| TK | transketolase |
| PDH | pyruvate dehydrogenase |
| PDHC | pyruvate dehydrogenase complex |
| ThDP | thiamine diphosphate, or thiamine pyrophosphate, or cocarboxylase |
| ThMP | thiamine monophosphate |
| TTFD | tetrahydrofurfuryl disulfide |
| ZT | Zeitgeber time |
References
- Makarchikov, A.F.; Wins, P.; Bettendorff, L. Biochemical and medical aspects of vitamin B1 research. Neurochem. Int. 2025, 185, 105962. [Google Scholar] [CrossRef]
- Aleshin, V.A.; Mkrtchyan, G.V.; Bunik, V.I. Mechanisms of Non-coenzyme Action of Thiamine: Protein Targets and Medical Significance. Biochem. Biokhimiia 2019, 84, 829–850. [Google Scholar] [CrossRef]
- Bunik, V.I.; Aleshin, V.A. Analysis of the Protein Binding Sites for Thiamin and Its Derivatives to Elucidate the Molecular Mechanisms of the Noncoenzyme Action of Thiamin (Vitamin B1). Stud. Nat. Prod. Chem. 2017, 53, 375–429. [Google Scholar] [CrossRef]
- Gangolf, M.; Czerniecki, J.; Radermecker, M.; Detry, O.; Nisolle, M.; Jouan, C.; Martin, D.; Chantraine, F.; Lakaye, B.; Wins, P.; et al. Thiamine status in humans and content of phosphorylated thiamine derivatives in biopsies and cultured cells. PLoS ONE 2010, 5, e13616. [Google Scholar] [CrossRef] [PubMed]
- Balaghi, M.; Pearson, W.N. Tissue and Intracellular Distribution of Radioactive Thiamine in Normal and Thiamine-deficient Rats. J. Nutr. 1966, 89, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.; Sarai, K.; Sanemori, H.; Kawasaki, T. Concentrations of thiamine and its phosphate esters in rat tissues determined by high-performance liquid chromatography. J. Nutr. Sci. Vitaminol. 1979, 25, 517–523. [Google Scholar] [CrossRef]
- Mkrtchyan, G.; Aleshin, V.; Parkhomenko, Y.; Kaehne, T.; Di Salvo, M.L.; Parroni, A.; Contestabile, R.; Vovk, A.; Bettendorff, L.; Bunik, V. Molecular mechanisms of the non-coenzyme action of thiamin in brain: Biochemical, structural and pathway analysis. Sci. Rep. 2015, 5, 12583. [Google Scholar] [CrossRef]
- Bunik, V.; Aleshin, V.; Nogues, I.; Kahne, T.; Parroni, A.; Contestabile, R.; Salvo, M.L.; Graf, A.; Tramonti, A. Thiamine-dependent regulation of mammalian brain pyridoxal kinase in vitro and in vivo. J. Neurochem. 2022, 161, 20–39. [Google Scholar] [CrossRef]
- Jonus, H.C.; Byrnes, C.C.; Kim, J.; Valle, M.L.; Bartlett, M.G.; Said, H.M.; Zastre, J.A. Thiamine mimetics sulbutiamine and benfotiamine as a nutraceutical approach to anticancer therapy. Biomed. Pharmacother. 2020, 121, 109648. [Google Scholar] [CrossRef]
- McLure, K.G.; Takagi, M.; Kastan, M.B. NAD+ modulates p53 DNA binding specificity and function. Mol. Cell. Biol. 2004, 24, 9958–9967. [Google Scholar] [CrossRef]
- Pulkkinen, V.; Manson, M.L.; Safholm, J.; Adner, M.; Dahlen, S.E. The bitter taste receptor (TAS2R) agonists denatonium and chloroquine display distinct patterns of relaxation of the guinea pig trachea. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 303, L956–L966. [Google Scholar] [CrossRef]
- Delompré, T.; Belloir, C.; Martin, C.; Salles, C.; Briand, L. Detection of Bitterness in Vitamins Is Mediated by the Activation of Bitter Taste Receptors. Nutrients 2022, 14, 4141. [Google Scholar] [CrossRef]
- Perez-Pineiro, R.; Bjorndahl, T.C.; Berjanskii, M.V.; Hau, D.; Li, L.; Huang, A.; Lee, R.; Gibbs, E.; Ladner, C.; Dong, Y.W.; et al. The prion protein binds thiamine. FEBS J. 2011, 278, 4002–4014. [Google Scholar] [CrossRef] [PubMed]
- Aleshin, V.A.; Mezhenska, O.A.; Parkhomenko, Y.M.; Kaehne, T.; Bunik, V.I. Thiamine Mono- and Diphosphate Phosphatases in Bovine Brain Synaptosomes. Biochem. Biokhimiia 2020, 85, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Yamamoto, D.; Sato, T.; Tanaka, S.; Usui, K.; Manabe, M.; Aoki, Y.; Iwashima, Y.; Saito, Y.; Mino, Y.; et al. Adenosine thiamine triphosphate (AThTP) inhibits poly(ADP-ribose) polymerase-1 (PARP-1) activity. J. Nutr. Sci. Vitaminol. 2011, 57, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Polegato, B.F.; Pereira, A.G.; Azevedo, P.S.; Costa, N.A.; Zornoff, L.A.M.; Paiva, S.A.R.; Minicucci, M.F. Role of Thiamin in Health and Disease. Nutr. Clin. Pract. 2019, 34, 558–564. [Google Scholar] [CrossRef]
- Ott, M.; Werneke, U. Wernicke’s encephalopathy—From basic science to clinical practice. Part 1: Understanding the role of thiamine. Ther. Adv. Psychopharmacol. 2020, 10, 2045125320978106. [Google Scholar] [CrossRef]
- Costantini, A.; Fancellu, R. An open-label pilot study with high-dose thiamine in Parkinson’s disease. Neural Regen. Res. 2016, 11, 406–407. [Google Scholar] [CrossRef]
- Costantini, A.; Pala, M.I.; Grossi, E.; Mondonico, S.; Cardelli, L.E.; Jenner, C.; Proietti, S.; Colangeli, M.; Fancellu, R. Long-Term Treatment with High-Dose Thiamine in Parkinson Disease: An Open-Label Pilot Study. J. Altern. Complement. Med. 2015, 21, 740–747. [Google Scholar] [CrossRef]
- Costantini, A.; Pala, M.I.; Compagnoni, L.; Colangeli, M. High-dose thiamine as initial treatment for Parkinson’s disease. BMJ Case Rep. 2013, 2013, bcr2013009289. [Google Scholar] [CrossRef]
- Costantini, A.; Pala, M.I. Thiamine and Fatigue in Inflammatory Bowel Diseases: An Open-label Pilot Study. J. Altern. Complement. Med. 2013, 19, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Bager, P.; Hvas, C.L.; Rud, C.L.; Dahlerup, J.F. Randomised clinical trial: High-dose oral thiamine versus placebo for chronic fatigue in patients with quiescent inflammatory bowel disease. Aliment. Pharmacol. Ther. 2020, 53, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Costantini, A.; Nappo, A.; Pala, M.I.; Zappone, A. High dose thiamine improves fatigue in multiple sclerosis. BMJ Case Rep. 2013, 2013, bcr2013009144. [Google Scholar] [CrossRef] [PubMed]
- Costantini, A.; Laureti, T.; Pala, M.I.; Colangeli, M.; Cavalieri, S.; Pozzi, E.; Brusco, A.; Salvarani, S.; Serrati, C.; Fancellu, R. Long-term treatment with thiamine as possible medical therapy for Friedreich ataxia. J. Neurol. 2016, 263, 2170–2178. [Google Scholar] [CrossRef]
- Costantini, A.; Pala, M.I.; Tundo, S.; Matteucci, P. High-dose thiamine improves the symptoms of fibromyalgia. BMJ Case Rep. 2013, 2013, bcr2013009019. [Google Scholar] [CrossRef]
- Antonio, C.; Massimo, T.; Gianpaolo, Z.; Immacolata, P.M.; Erika, T. Oral High-Dose Thiamine Improves the Symptoms of Chronic Cluster Headache. Case Rep. Neurol. Med. 2018, 2018, 1–5. [Google Scholar] [CrossRef]
- Coomans, C.P.; van den Berg, S.A.A.; Lucassen, E.A.; Houben, T.; Pronk, A.C.M.; van der Spek, R.D.; Kalsbeek, A.; Biermasz, N.R.; Willems van Dijk, K.; Romijn, J.A.; et al. The Suprachiasmatic Nucleus Controls Circadian Energy Metabolism and Hepatic Insulin Sensitivity. Diabetes 2013, 62, 1102–1108. [Google Scholar] [CrossRef]
- Speksnijder, E.M.; Bisschop, P.H.; Siegelaar, S.E.; Stenvers, D.J.; Kalsbeek, A. Circadian desynchrony and glucose metabolism. J. Pineal Res. 2024, 76, e12956. [Google Scholar] [CrossRef]
- Aleshin, V.A.; Mkrtchyan, G.V.; Kaehne, T.; Graf, A.V.; Maslova, M.V.; Bunik, V.I. Diurnal regulation of the function of the rat brain glutamate dehydrogenase by acetylation and its dependence on thiamine administration. J. Neurochem. 2020, 153, 80–102. [Google Scholar] [CrossRef]
- Aleshin, V.A.; Artiukhov, A.V.; Kaehne, T.; Graf, A.V.; Bunik, V.I. Daytime Dependence of the Activity of the Rat Brain Pyruvate Dehydrogenase Corresponds to the Mitochondrial Sirtuin 3 Level and Acetylation of Brain Proteins, All Regulated by Thiamine Administration Decreasing Phosphorylation of PDHA Ser293. Int. J. Mol. Sci. 2021, 22, 8006. [Google Scholar] [CrossRef]
- Virshup, D.M.; Eide, E.J.; Forger, D.B.; Gallego, M.; Harnish, E.V. Reversible Protein Phosphorylation Regulates Circadian Rhythms. Cold Spring Harb. Symp. Quant. Biol. 2007, 72, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, J.; Sahar, S.; Grimaldi, B.; Tamaru, T.; Takamatsu, K.; Nakahata, Y.; Sassone-Corsi, P. CLOCK-mediated acetylation of BMAL1 controls circadian function. Nature 2007, 450, 1086–1090. [Google Scholar] [CrossRef] [PubMed]
- Stojkovic, K.; Wing, S.S.; Cermakian, N. A central role for ubiquitination within a circadian clock protein modification code. Front. Mol. Neurosci. 2014, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Moseley, A.C.; Almeda-Valdes, P.; Stromsdorfer, K.L.; Franczyk, M.P.; Okunade, A.L.; Patterson, B.W.; Klein, S.; Yoshino, J. Diurnal Variation in PDK4 Expression Is Associated with Plasma Free Fatty Acid Availability in People. J. Clin. Endocrinol. Metab 2018, 103, 1068–1076. [Google Scholar] [CrossRef]
- Wu, P.; Blair, P.V.; Sato, J.; Jaskiewicz, J.; Popov, K.M.; Harris, R.A. Starvation Increases the Amount of Pyruvate Dehydrogenase Kinase in Several Mammalian Tissues. Arch. Biochem. Biophys. 2000, 381, 1–7. [Google Scholar] [CrossRef]
- Neufeld-Cohen, A.; Robles, M.S.; Aviram, R.; Manella, G.; Adamovich, Y.; Ladeuix, B.; Nir, D.; Rousso-Noori, L.; Kuperman, Y.; Golik, M.; et al. Circadian control of oscillations in mitochondrial rate-limiting enzymes and nutrient utilization by PERIOD proteins. Proc. Natl. Acad. Sci. USA 2016, 113, E1673–E1682. [Google Scholar] [CrossRef]
- Artiukhov, A.V.; Solovjeva, O.N.; Balashova, N.V.; Sidorova, O.P.; Graf, A.V.; Bunik, V.I. Pharmacological Doses of Thiamine Benefit Patients with the Charcot–Marie–Tooth Neuropathy by Changing Thiamine Diphosphate Levels and Affecting Regulation of Thiamine-Dependent Enzymes. Biochemistry 2024, 89, 1161–1182. [Google Scholar] [CrossRef]
- Rashid, A.; Iqbal, S.; Bhat, I.; Rashid, J.; Hafeez, I.; Lone, A.; Iqbal, K.; Dar, I. Role of thiamine supplementation in the treatment of patients with heart failure: A double-blind randomized controlled trial. Heart India 2019, 7, 68. [Google Scholar] [CrossRef]
- Meador, K.; Loring, D.; Nichols, M.; Zamrini, E.; Rivner, M.; Posas, H.; Thompson, E.; Moore, E. Preliminary Findings of High-Dose Thiamine in Dementia of Alzheimer’s Type. J. Geriatr. Psychiatry Neurol. 1993, 6, 222–229. [Google Scholar] [CrossRef]
- Gupta, R.K.; Yadav, S.K.; Saraswat, V.A.; Rangan, M.; Srivastava, A.; Yadav, A.; Trivedi, R.; Yachha, S.K.; Rathore, R.K.S. Thiamine deficiency related microstructural brain changes in acute and acute-on-chronic liver failure of non-alcoholic etiology. Clin. Nutr. 2012, 31, 422–428. [Google Scholar] [CrossRef]
- Bémeur, C.; Butterworth, R.F. Nutrition in the Management of Cirrhosis and its Neurological Complications. J. Clin. Exp. Hepatol. 2014, 4, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Tashlitsky, V.N.; Artiukhov, A.V.; Fedorova, N.V.; Sukonnikov, M.A.; Ksenofontov, A.L.; Bunik, V.I.; Baratova, L.A. Analysis of Content of 2-Oxoacids in Rat Brain Extracts Using High-Performance Liquid Chromatography. Biochem. Biokhimiia 2022, 87, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Schwab, M.A.; Kolker, S.; van den Heuvel, L.P.; Sauer, S.; Wolf, N.I.; Rating, D.; Hoffmann, G.F.; Smeitink, J.A.; Okun, J.G. Optimized spectrophotometric assay for the completely activated pyruvate dehydrogenase complex in fibroblasts. Clin. Chem. 2005, 51, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Naito, E.; Ito, M.; Takeda, E.; Yokota, I.; Yoshijima, S.; Kuroda, Y. Molecular Analysis of Abnormal Pyruvate Dehydrogenase in a Patient with Thiamine-Responsive Congenital Lactic Acidemia. Pediatr. Res. 1994, 36, 340–346. [Google Scholar] [CrossRef][Green Version]
- Seifert, F.; Ciszak, E.; Korotchkina, L.; Golbik, R.; Spinka, M.; Dominiak, P.; Sidhu, S.; Brauer, J.; Patel, M.S.; Tittmann, K. Phosphorylation of serine 264 impedes active site accessibility in the E1 component of the human pyruvate dehydrogenase multienzyme complex. Biochemistry 2007, 46, 6277–6287. [Google Scholar] [CrossRef]
- Korotchkina, L.G.; Patel, M.S. Site Specificity of Four Pyruvate Dehydrogenase Kinase Isoenzymes Toward the Three Phosphorylation Sites of Human Pyruvate Dehydrogenase. J. Biol. Chem. 2001, 276, 37223–37229. [Google Scholar] [CrossRef]
- Artiukhov, A.V.; Aleshin, V.A.; Karlina, I.S.; Kazantsev, A.V.; Sibiryakina, D.A.; Ksenofontov, A.L.; Lukashev, N.V.; Graf, A.V.; Bunik, V.I. Phosphonate Inhibitors of Pyruvate Dehydrogenase Perturb Homeostasis of Amino Acids and Protein Succinylation in the Brain. Int. J. Mol. Sci. 2022, 23, 13186. [Google Scholar] [CrossRef]
- Aleshin, V.A.; Graf, A.V.; Artiukhov, A.V.; Boyko, A.I.; Ksenofontov, A.L.; Maslova, M.V.; Nogués, I.; di Salvo, M.L.; Bunik, V.I. Physiological and Biochemical Markers of the Sex-Specific Sensitivity to Epileptogenic Factors, Delayed Consequences of Seizures and Their Response to Vitamins B1 and B6 in a Rat Model. Pharmaceuticals 2021, 14, 737. [Google Scholar] [CrossRef]
- Artiukhov, A.V.; Graf, A.V.; Kazantsev, A.V.; Boyko, A.I.; Aleshin, V.A.; Ksenofontov, A.L.; Bunik, V.I. Increasing Inhibition of the Rat Brain 2-Oxoglutarate Dehydrogenase Decreases Glutathione Redox State, Elevating Anxiety and Perturbing Stress Adaptation. Pharmaceuticals 2022, 15, 182. [Google Scholar] [CrossRef]
- Laforenza, U.; Patrini, C.; Rindi, G. Distribution of Thiamine, Thiamine Phosphates, and Thiamine Metabolizing Enzymes in Neuronal and Glial Cell Enriched Fractions of Rat Brain. J. Neurochem. 2006, 51, 730–735. [Google Scholar] [CrossRef]
- Ronowska, A.; Jankowska-Kulawy, A.; Gul-Hinc, S.; Zyśk, M.; Michno, A.; Szutowicz, A. Effects of Marginal Zn Excess and Thiamine Deficiency on Microglial N9 Cell Metabolism and Their Interactions with Septal SN56 Cholinergic Cells. Int. J. Mol. Sci. 2023, 24, 4465. [Google Scholar] [CrossRef] [PubMed]
- Gul-Hinc, S.; Michno, A.; Zyśk, M.; Szutowicz, A.; Jankowska-Kulawy, A.; Ronowska, A. Protection of Cholinergic Neurons against Zinc Toxicity by Glial Cells in Thiamine-Deficient Media. Int. J. Mol. Sci. 2021, 22, 13337. [Google Scholar] [CrossRef] [PubMed]
- Ke, Z.-J.; Gibson, G.E. Selective response of various brain cell types during neurodegeneration induced by mild impairment of oxidative metabolism. Neurochem. Int. 2004, 45, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Jankowska-Kulawy, A.; Bielarczyk, H.; Pawełczyk, T.; Wróblewska, M.; Szutowicz, A. Acetyl-CoA deficit in brain mitochondria in experimental thiamine deficiency encephalopathy. Neurochem. Int. 2010, 57, 851–856. [Google Scholar] [CrossRef]
- Butterworth, R.F. Thiamin deficiency and brain disorders. Nutr. Res. Rev. 2007, 16, 277–284. [Google Scholar] [CrossRef]
- Graf, A.V.; Artiukhov, A.V.; Solovjeva, O.N.; Ksenofontov, A.L.; Bunik, V.I. Combined Administration of Metformin and Amprolium to Rats Affects Metabolism of Free Amino Acids in the Brain, Altering Behavior, and Heart Rate. Biochemistry 2024, 89, 1692–1710. [Google Scholar] [CrossRef]
- Trebukhina, R.V.; Ostrovsky, Y.M.; Mikhaltsevich, G.N.; Velichko, M.G.; Tumanov, V.N. Transketolase, Pyruvate and Oxoglutarate Dehydrogenase Activities and [14C]Thiamin Turnover in Tissues of Mice Fed Thiamin-Deficient Diet. J. Nutr. 1983, 113, 1285–1291. [Google Scholar] [CrossRef]
- Bocobza, S.E.; Malitsky, S.; Araujo, W.L.; Nunes-Nesi, A.; Meir, S.; Shapira, M.; Fernie, A.R.; Aharoni, A. Orchestration of thiamin biosynthesis and central metabolism by combined action of the thiamin pyrophosphate riboswitch and the circadian clock in Arabidopsis. Plant Cell 2013, 25, 288–307. [Google Scholar] [CrossRef]
- Noordally, Z.B.; Trichtinger, C.; Dalvit, I.; Hofmann, M.; Roux, C.; Zamboni, N.; Pourcel, L.; Gas-Pascual, E.; Gisler, A.; Fitzpatrick, T.B. The coenzyme thiamine diphosphate displays a daily rhythm in the Arabidopsis nucleus. Commun. Biol. 2020, 3, 209. [Google Scholar] [CrossRef]
- Hofmann, M.; Loubéry, S.; Fitzpatrick, T.B. On the nature of thiamine triphosphate in Arabidopsis. Plant Direct 2020, 4, e00258. [Google Scholar] [CrossRef]
- Yan, Z.; Deng, R.; Zhang, H.; Li, J.; Zhu, S. Transcriptome analysis of floret opening and closure both Indica and Japonica rice. 3 Biotech 2022, 12, 188. [Google Scholar] [CrossRef]
- Li, W.; Mi, X.; Jin, X.; Zhang, D.; Zhu, G.; Shang, X.; Zhang, D.; Guo, W. Thiamine functions as a key activator for modulating plant health and broad-spectrum tolerance in cotton. Plant J. 2022, 111, 374–390. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Liu, P.; Zhang, Q.; Zhao, L.; Hao, X.; Liu, L.; Bu, C.; Pan, Y.; Zhang, D.; Song, Y. Dynamic physiological and transcriptome changes reveal a potential relationship between the circadian clock and salt stress response in Ulmus pumila. Mol. Genet. Genom. 2022, 297, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Rosado-Souza, L.; Proost, S.; Moulin, M.; Bergmann, S.; Bocobza, S.E.; Aharoni, A.; Fitzpatrick, T.B.; Mutwil, M.; Fernie, A.R.; Obata, T. Appropriate Thiamin Pyrophosphate Levels Are Required for Acclimation to Changes in Photoperiod. Plant Physiol. 2019, 180, 185–197. [Google Scholar] [CrossRef]
- Uchiyama, Y.; von Mayersbach, H.; Groh, V. Circadian changes in thiamine pyrophosphatase activity of rat hepatocytes—A histochemical study at the electron microscopic level. Cell Mol. Biol. 1982, 28, 245–254. [Google Scholar] [PubMed]
- Sano, S.-i.; Matsuda, Y.; Miyamoto, S.; Nakagawa, H. Thiamine pyrophosphatase and nucleoside diphosphatase in rat brain. Biochem. Biophys. Res. Commun. 1984, 118, 292–298. [Google Scholar] [CrossRef]
- Bennett, M.R.; Schwartz, W.J. Altered circadian rhythmicity is an early sign of murine dietary thiamine deficiency. J. Neurol. Sci. 1999, 163, 6–10. [Google Scholar] [CrossRef]
- Langlais, P.J.; Hall, T. Thiamine deficiency-induced disruptions in the diurnal rhythm and regulation of body temperature in the rat. Metab. Brain Dis. 1998, 13, 225–239. [Google Scholar] [CrossRef]
- Lipton, J.M.; Payne, H.; Garza, H.R.; Rosenberg, R.N. Thermolability in Wernicke’s Encephalopathy. Arch. Neurol. 1978, 35, 750–753. [Google Scholar] [CrossRef]
- Balzamo, E.; Vuillon-Cacciuttolo, G. Facilitation of a state of wakefulness by semi-chronic treatment with sulbutiamin (Arcalion) in Macaca mulatta. Rev. Electroencephalogr. Neurophysiol. Clin. 1982, 12, 373–378. [Google Scholar] [CrossRef]
- Lee, D.; Kim, K.; Lee, Y.; Oh, K.; Jung, S.J. The Relationship Between Thiamine Intake and Long Sleep Duration: Results from the Korea National Health and Nutrition Examination Survey. J. Prev. Med. Public Health 2022, 55, 520–528. [Google Scholar] [CrossRef]
- Reis-Canaan, J.C.; Canaan, M.M.; Costa, P.D.; Rodrigues-Juliatte, T.P.; Pereira, M.C.A.; Castelo, P.M.; Pardi, V.; Murata, R.M.; Pereira, L.J. Association Between Chronotype and Nutritional, Clinical and Sociobehavioral Characteristics of Adults Assisted by a Public Health Care System in Brazil. Nutrients 2021, 13, 2260. [Google Scholar] [CrossRef] [PubMed]
- Hampel, D.; Shahab-Ferdows, S.; Islam, M.M.; Peerson, J.M.; Allen, L.H. Vitamin Concentrations in Human Milk Vary with Time within Feed, Circadian Rhythm, and Single-Dose Supplementation. J. Nutr. 2017, 147, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Ihara, H.; Matsumoto, T.; Kakinoki, T.; Shino, Y.; Hashimoto, R.; Hashizume, N. Estimation of vitamin B1 excretion in 24-hr urine by assay of first-morning urine. J. Clin. Lab. Anal. 2008, 22, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Karabulut, N.P.; Frishman, D. Tissue-specific sequence and structural environments of lysine acetylation sites. J. Struct. Biol. 2015, 191, 39–48. [Google Scholar] [CrossRef]
- Haws, S.A.; Leech, C.M.; Denu, J.M. Metabolism and the Epigenome: A Dynamic Relationship. Trends Biochem. Sci. 2020, 45, 731–747. [Google Scholar] [CrossRef]
- Sato, S.; Solanas, G.; Peixoto, F.O.; Bee, L.; Symeonidi, A.; Schmidt, M.S.; Brenner, C.; Masri, S.; Benitah, S.A.; Sassone-Corsi, P. Circadian Reprogramming in the Liver Identifies Metabolic Pathways of Aging. Cell 2017, 170, 664–677.E11. [Google Scholar] [CrossRef]
- Chiou, Y.-Y.; Lee, C.-Y.; Yang, H.-W.; Cheng, W.-C.; Ji, K.-D. Circadian modulation of glucose utilization via CRY1-mediated repression of Pdk1 expression. J. Biol. Chem. 2024, 300, 105637. [Google Scholar] [CrossRef]
- Scrima, R.; Cela, O.; Agriesti, F.; Piccoli, C.; Tataranni, T.; Pacelli, C.; Mazzoccoli, G.; Capitanio, N. Mitochondrial calcium drives clock gene-dependent activation of pyruvate dehydrogenase and of oxidative phosphorylation. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118815. [Google Scholar] [CrossRef]
- Dyar, K.A.; Ciciliot, S.; Wright, L.E.; Biensø, R.S.; Tagliazucchi, G.M.; Patel, V.R.; Forcato, M.; Paz, M.I.P.; Gudiksen, A.; Solagna, F.; et al. Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Mol. Metab. 2014, 3, 29–41. [Google Scholar] [CrossRef]
- Bryson, J.M.; Cooney, G.J.; Wensley, V.R.; Blair, S.C.; Caterson, I.D. Diurnal patterns of cardiac and hepatic pyruvate dehydrogenase complex activity in gold-thioglucose-obese mice. Biochem. J. 1993, 295, 731–734. [Google Scholar] [CrossRef]
- Gimble, J.M.; Yan, J.; Wang, H.; Liu, Y.; Shao, C. Analysis of Gene Regulatory Networks in the Mammalian Circadian Rhythm. PLoS Comput. Biol. 2008, 4, e1000193. [Google Scholar] [CrossRef]
- Thurley, K.; Herbst, C.; Wesener, F.; Koller, B.; Wallach, T.; Maier, B.; Kramer, A.; Westermark, P.O. Principles for circadian orchestration of metabolic pathways. Proc. Natl. Acad. Sci. USA 2017, 114, 1572–1577. [Google Scholar] [CrossRef]
- Kierans, S.J.; Taylor, C.T. Regulation of glycolysis by the hypoxia-inducible factor (HIF): Implications for cellular physiology. J. Physiol. 2020, 599, 23–37. [Google Scholar] [CrossRef]
- Kim, J.W.; Tchernyshyov, I.; Semenza, G.L.; Dang, C.V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006, 3, 177–185. [Google Scholar] [CrossRef]
- Zhu, Y.; Ma, W.-Q.; Han, X.-Q.; Wang, Y.; Wang, X.; Liu, N.-F. Advanced glycation end products accelerate calcification in VSMCs through HIF-1α/PDK4 activation and suppress glucose metabolism. Sci. Rep. 2018, 8, 13730. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-W.; Lin, S.-C.; Chen, K.-F.; Lai, Y.-Y.; Tsai, S.-J. Induction of Pyruvate Dehydrogenase Kinase-3 by Hypoxia-inducible Factor-1 Promotes Metabolic Switch and Drug Resistance. J. Biol. Chem. 2008, 283, 28106–28114. [Google Scholar] [CrossRef] [PubMed]
- Peek, C.B.; Levine, D.C.; Cedernaes, J.; Taguchi, A.; Kobayashi, Y.; Tsai, S.J.; Bonar, N.A.; McNulty, M.R.; Ramsey, K.M.; Bass, J. Circadian Clock Interaction with HIF1α Mediates Oxygenic Metabolism and Anaerobic Glycolysis in Skeletal Muscle. Cell Metab. 2017, 25, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Tang, D.; Liu, N.; Xiong, W.; Huang, H.; Li, Y.; Ma, Z.; Zhao, H.; Chen, P.; Qi, X.; et al. Reciprocal Regulation between the Circadian Clock and Hypoxia Signaling at the Genome Level in Mammals. Cell Metab. 2017, 25, 73–85. [Google Scholar] [CrossRef]
- Adamovich, Y.; Ladeuix, B.; Golik, M.; Koeners, M.P.; Asher, G. Rhythmic Oxygen Levels Reset Circadian Clocks through HIF1α. Cell Metab. 2017, 25, 93–101. [Google Scholar] [CrossRef]
- McCarthy, J.J.; Andrews, J.L.; McDearmon, E.L.; Campbell, K.S.; Barber, B.K.; Miller, B.H.; Walker, J.R.; Hogenesch, J.B.; Takahashi, J.S.; Esser, K.A. Identification of the circadian transcriptome in adult mouse skeletal muscle. Physiol. Genom. 2007, 31, 86–95. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Y.; Adams, J.A.; Higgins, C.B.; Kelly, S.C.; Zhang, H.; Cho, K.Y.; Johnson, U.G.; Swarts, B.M.; Wada, S.I.; et al. Hepatocyte Period 1 dictates oxidative substrate selection independent of the core circadian clock. Cell Rep. 2024, 43, 114865. [Google Scholar] [CrossRef]
- Miki, T.; Matsumoto, T.; Zhao, Z.; Lee, C.C. p53 regulates Period2 expression and the circadian clock. Nat. Commun. 2013, 4, 2444. [Google Scholar] [CrossRef]
- Lo, P.-K.; Chen, J.-Y.; Tang, P.-P.; Lin, J.; Lin, C.-H.; Su, L.-T.; Wu, C.-H.; Chen, T.-L.; Yang, Y.; Wang, F.-F. Identification of a Mouse Thiamine Transporter Gene as a Direct Transcriptional Target for p53. J. Biol. Chem. 2001, 276, 37186–37193. [Google Scholar] [CrossRef] [PubMed]
- Wijenayake, S.; Tessier, S.N.; Storey, K.B. Regulation of pyruvate dehydrogenase (PDH) in the hibernating ground squirrel, (Ictidomys tridecemlineatus). J. Therm. Biol. 2017, 69, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Aleshina, Y.A.; Aleshin, V.A. Evolutionary Changes in Primate Glutamate Dehydrogenases 1 and 2 Influence the Protein Regulation by Ligands, Targeting and Posttranslational Modifications. Int. J. Mol. Sci. 2024, 25, 4341. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Tian, W.; Cai, L.; Wang, Y.; Zhang, J.; Teng, H.; Du, J.; Sun, Z.S. Expression Profiling Reveals a Positive Regulation Bymper2on Circadian Rhythm of Cytotoxicity Receptors:Ly49candnkg2d. Chronobiol. Int. 2009, 26, 1514–1544. [Google Scholar] [CrossRef]
- Schmutz, I.; Wendt, S.; Schnell, A.; Kramer, A.; Mansuy, I.M.; Albrecht, U. Protein phosphatase 1 (PP1) is a post-translational regulator of the mammalian circadian clock. PLoS ONE 2011, 6, e21325. [Google Scholar] [CrossRef]
- Chornyy, S.; Parkhomenko, Y.; Chorna, N. Thiamine antagonists trigger p53-dependent apoptosis in differentiated SH-SY5Y cells. Sci. Rep. 2017, 7, 10632. [Google Scholar] [CrossRef]
- Aleshin, V.A.; Zhou, X.; Krishnan, S.; Karlsson, A.; Bunik, V.I. Interplay Between Thiamine and p53/p21 Axes Affects Antiproliferative Action of Cisplatin in Lung Adenocarcinoma Cells by Changing Metabolism of 2-Oxoglutarate/Glutamate. Front. Genet. 2021, 12, 658446. [Google Scholar] [CrossRef]
- Bunik, V.I.; Aleshin, V.A.; Zhou, X.; Tabakov, V.Y.; Karlsson, A. Activation of Mitochondrial 2-Oxoglutarate Dehydrogenase by Cocarboxylase in Human Lung Adenocarcinoma Cells A549 Is p53/p21-Dependent and Impairs Cellular Redox State, Mimicking the Cisplatin Action. Int. J. Mol. Sci. 2020, 21, 3759. [Google Scholar] [CrossRef]
- Gotoh, T.; Vila-Caballer, M.; Santos, C.S.; Liu, J.; Yang, J.; Finkielstein, C.V.; Solomon, M.J. The circadian factor Period 2 modulates p53 stability and transcriptional activity in unstressed cells. Mol. Biol. Cell 2014, 25, 3081–3093. [Google Scholar] [CrossRef]
- Gotoh, T.; Kim, J.K.; Liu, J.; Vila-Caballer, M.; Stauffer, P.E.; Tyson, J.J.; Finkielstein, C.V. Model-driven experimental approach reveals the complex regulatory distribution of p53 by the circadian factor Period 2. Proc. Natl. Acad. Sci. USA 2016, 113, 13516–13521. [Google Scholar] [CrossRef]
- Zhang, R.; Lahens, N.F.; Ballance, H.I.; Hughes, M.E.; Hogenesch, J.B. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc. Natl. Acad. Sci. USA 2014, 111, 16219–16224. [Google Scholar] [CrossRef]
- Klyuyeva, A.; Tuganova, A.; Kedishvili, N.; Popov, K.M. Tissue-specific kinase expression and activity regulate flux through the pyruvate dehydrogenase complex. J. Biol. Chem. 2019, 294, 838–851. [Google Scholar] [CrossRef] [PubMed]
- Gudi, R.; Bowker-Kinley, M.M.; Kedishvili, N.Y.; Zhao, Y.; Popov, K.M. Diversity of the pyruvate dehydrogenase kinase gene family in humans. J. Biol. Chem. 1995, 270, 28989–28994. [Google Scholar] [CrossRef] [PubMed]
- Rowles, J.; Scherer, S.W.; Xi, T.; Majer, M.; Nickle, D.C.; Rommens, J.M.; Popov, K.M.; Harris, R.A.; Riebow, N.L.; Xia, J.; et al. Cloning and characterization of PDK4 on 7q21.3 encoding a fourth pyruvate dehydrogenase kinase isoenzyme in human. J. Biol. Chem. 1996, 271, 22376–22382. [Google Scholar] [CrossRef] [PubMed]
- Sugden, M.C.; Holness, M.J. Mechanisms underlying regulation of the expression and activities of the mammalian pyruvate dehydrogenase kinases. Arch. Physiol. Biochem. 2008, 112, 139–149. [Google Scholar] [CrossRef]
- Patnni, C.; Reggiani, C.; Laforenza, U.; Rindi, G. Blood–Brain Transport of Thiamine Monophosphate in the Rat: A Kinetic Study In Vivo. J. Neurochem. 2006, 50, 90–93. [Google Scholar] [CrossRef]
- Geier, E.G.; Chen, E.C.; Webb, A.; Papp, A.C.; Yee, S.W.; Sadee, W.; Giacomini, K.M. Profiling Solute Carrier Transporters in the Human Blood–Brain Barrier. Clin. Pharmacol. Ther. 2013, 94, 636–639. [Google Scholar] [CrossRef]
- Yee, S.W.; Wang, J.; Giacomini, K.M. Rare Diseases Linked to Mutations in Vitamin Transporters Expressed in the Human Blood–Brain Barrier. Clin. Pharmacol. Ther. 2024, 116, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Azimi, M.; Handin, N.; Riselli, A.; Vora, B.; Chun, E.; Yee, S.W.; Artursson, P.; Giacomini, K.M. Proteomic Profiling Reveals Age-Related Changes in Transporter Proteins in the Human Blood-Brain Barrier. bioRxiv 2024. [Google Scholar] [CrossRef]
- Siletti, K.; Hodge, R.; Mossi Albiach, A.; Lee, K.W.; Ding, S.-L.; Hu, L.; Lönnerberg, P.; Bakken, T.; Casper, T.; Clark, M.; et al. Transcriptomic diversity of cell types across the adult human brain. Science 2023, 382. [Google Scholar] [CrossRef] [PubMed]
- Bettendorff, L.; Weekers, L.; Wins, P.; Schoffeniels, E. Injection of sulbutiamine induces an increase in thiamine triphosphate in rat tissues. Biochem. Pharmacol. 1990, 40, 2557–2560. [Google Scholar] [CrossRef]
- Vignisse, J.; Sambon, M.; Gorlova, A.; Pavlov, D.; Caron, N.; Malgrange, B.; Shevtsova, E.; Svistunov, A.; Anthony, D.C.; Markova, N.; et al. Thiamine and benfotiamine prevent stress-induced suppression of hippocampal neurogenesis in mice exposed to predation without affecting brain thiamine diphosphate levels. Mol. Cell. Neurosci. 2017, 82, 126–136. [Google Scholar] [CrossRef]
- Dingwall, K.M.; Delima, J.F.; Binks, P.; Batey, R.; Bowden, S.C. What is the optimum thiamine dose to treat or prevent Wernicke’s encephalopathy or Wernicke–Korsakoff syndrome? Results of a randomized controlled trial. Alcohol. Clin. Exp. Res. 2022, 46, 1133–1147. [Google Scholar] [CrossRef]
- Smith, H.; McCoy, M.; Varughese, K.; Reinert, J.P. Thiamine Dosing for the Treatment of Alcohol-Induced Wernicke’s Encephalopathy: A Review of the Literature. J. Pharm. Technol. 2020, 37, 107–113. [Google Scholar] [CrossRef]
- Babaei-Jadidi, R.; Karachalias, N.; Kupich, C.; Ahmed, N.; Thornalley, P.J. High-dose thiamine therapy counters dyslipidaemia in streptozotocin-induced diabetic rats. Diabetologia 2004, 47, 2235–2246. [Google Scholar] [CrossRef]
- Rad, M.G.; Sharifi, M.; Meamar, R.; Soltani, N. Long term administration of thiamine disulfide improves FOXO1/PEPCK pathway in liver to reduce insulin resistance in type 1 diabetes rat model. Biomed. Pharmacother. 2024, 177, 117053. [Google Scholar] [CrossRef]
- Tanaka, T.; Kono, T.; Terasaki, F.; Yasui, K.; Soyama, A.; Otsuka, K.; Fujita, S.; Yamane, K.; Manabe, M.; Usui, K.; et al. Thiamine Prevents Obesity and Obesity-Associated Metabolic Disorders in OLETF Rats. J. Nutr. Sci. Vitaminol. 2010, 56, 335–346. [Google Scholar] [CrossRef]
- Cantu-Weinstein, A.; Branning, R.; Alamir, M.; Weleff, J.; Do, M.; Nero, N.; Anand, A. Diagnosis and treatment of Wernicke’s encephalopathy: A systematic literature review. Gen. Hosp. Psychiatry 2024, 87, 48–59. [Google Scholar] [CrossRef]
- Strauss, K.A.; Puffenberger, E.G.; Carson, V.J. Maple Syrup Urine Disease. In GeneReviews((R)); Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, DC, USA, 1993. [Google Scholar]
- van Dongen, S.; Brown, R.M.; Brown, G.K.; Thorburn, D.R.; Boneh, A. Thiamine-Responsive and Non-responsive Patients with PDHC-E1 Deficiency: A Retrospective Assessment. JIMD Rep. 2015, 15, 13–27. [Google Scholar] [CrossRef]
- Hata, T.; Grenier, F.; Hiraga, T.; Soya, M.; Okamoto, M.; Soya, H. Promoting arousal associated with physical activity with the vitamin B1 derivative TTFD. J. Physiol. Sci. 2025, 75, 100001. [Google Scholar] [CrossRef]
- Gubler, C.J.; Johnson, L.R.; Wittorf, J.H. Yeast transketolase (sedoheptulose-7-phosphate:d-glyceraldehyde-3-phosphate dihydroxyacetonetransferase, EC 2.2.1.1) assay of thiamine diphosphate. In Methods in Enzymology; Part A: Vitamins and Coenzymes; Elsevier: Amsterdam, The Netherlands, 1970; Volume 18, pp. 120–125. [Google Scholar] [CrossRef]
- Kochetov, G.A. Practice Guidelines on Biochemistry, 2nd ed.; Vysshaya Shkola: Moscow, Russia, 1980. [Google Scholar]
- Tikhomirova, N.K.; Kochetov, G.A. Purification of transketolase from baker’s yeast by an immunoadsorbent. Biochem. Int. 1990, 22, 31–36. [Google Scholar]
- Solovjeva, O.N. Isolation and Properties of Noncovalent Complex of Transketolase with RNA. Biochemistry 2002, 67, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Aleshin, V.A.; Kaehne, T.; Maslova, M.V.; Graf, A.V.; Bunik, V.I. Posttranslational Acylations of the Rat Brain Transketolase Discriminate the Enzyme Responses to Inhibitors of ThDP-Dependent Enzymes or Thiamine Transport. Int. J. Mol. Sci. 2024, 25, 917. [Google Scholar] [CrossRef] [PubMed]
- Solovjeva, O.N.; Selivanov, V.A.; Orlov, V.N.; Kochetov, G.A. Stages of the formation of nonequivalence of active centers of transketolase from baker’s yeast. Mol. Catal. 2019, 466, 122–129. [Google Scholar] [CrossRef]
- Turner, P.V.; Pekow, C.; Vasbinder, M.A.; Brabb, T. Administration of substances to laboratory animals: Equipment considerations, vehicle selection, and solute preparation. J. Am. Assoc. Lab. Anim. Sci. JAALAS 2011, 50, 614–627. [Google Scholar] [PubMed]
- USFDA. Guidance for Industry Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. Available online: https://www.fda.gov/media/72309/download (accessed on 17 July 2025).
- Baevsky, R.M.; Chernikova, A.G. Heart rate variability analysis: Physiological foundations and main methods. Cardiometry 2017, 66–76. [Google Scholar] [CrossRef]
- Graf, A.; Trofimova, L.; Loshinskaja, A.; Mkrtchyan, G.; Strokina, A.; Lovat, M.; Tylicky, A.; Strumilo, S.; Bettendorff, L.; Bunik, V.I. Up-regulation of 2-oxoglutarate dehydrogenase as a stress response. Int. J. Biochem. Cell Biol. 2013, 45, 175–189. [Google Scholar] [CrossRef]
- Müller, C.P.; Pum, M.E.; Amato, D.; Schüttler, J.; Huston, J.P.; De Souza Silva, M.A. The in vivo neurochemistry of the brain during general anesthesia. J. Neurochem. 2011, 119, 419–446. [Google Scholar] [CrossRef]
- Leary, S.; Underwood, W.; Anthony, R.; Cartner, S.; Grandin, T.; Greenacre, C.; Gwaltney-Brant, S.; McCrackin, M.A.; Meyer, R.; Miller, D.; et al. AVMA Guidelines for the Euthanasia of Animals: 2020 Edition; American Veterinary Medical Association: Schaumburg, IL, USA, 2020. [Google Scholar]
- Yatziv, S.L.; Yudco, O.; Vaso, K.; Mizrahi, A.; Devor, M. Anesthesia in mice activates discrete populations of neurons throughout the brain. J. Neurosci. Res. 2021, 99, 3284–3305. [Google Scholar] [CrossRef]
- Tsepkova, P.M.; Artiukhov, A.V.; Boyko, A.I.; Aleshin, V.A.; Mkrtchyan, G.V.; Zvyagintseva, M.A.; Ryabov, S.I.; Ksenofontov, A.L.; Baratova, L.A.; Graf, A.V.; et al. Thiamine Induces Long-Term Changes in Amino Acid Profiles and Activities of 2-Oxoglutarate and 2-Oxoadipate Dehydrogenases in Rat Brain. Biochem. Biokhimiia 2017, 82, 723–736. [Google Scholar] [CrossRef]
- Boyko, A.; Tsepkova, P.; Aleshin, V.; Artiukhov, A.; Mkrtchyan, G.; Ksenofontov, A.; Baratova, L.; Ryabov, S.; Graf, A.; Bunik, V. Severe Spinal Cord Injury in Rats Induces Chronic Changes in the Spinal Cord and Cerebral Cortex Metabolism, Adjusted by Thiamine That Improves Locomotor Performance. Front. Mol. Neurosci. 2021, 14, 620593. [Google Scholar] [CrossRef]
- Artiukhov, A.V.; Grabarska, A.; Gumbarewicz, E.; Aleshin, V.A.; Kahne, T.; Obata, T.; Kazantsev, A.V.; Lukashev, N.V.; Stepulak, A.; Fernie, A.R.; et al. Synthetic analogues of 2-oxo acids discriminate metabolic contribution of the 2-oxoglutarate and 2-oxoadipate dehydrogenases in mammalian cells and tissues. Sci. Rep. 2020, 10, 1886. [Google Scholar] [CrossRef] [PubMed]
- Chretien, D.; Pourrier, M.; Bourgeron, T.; Séné, M.; Rötig, A.; Munnich, A.; Rustin, P. An improved spectrophotometric assay of pyruvate dehydrogenase in lactate dehydrogenase contaminated mitochondrial preparations from human skeletal muscle. Clin. Chim. Acta 1995, 240, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Sánchez, R.; Marín-Hernández, Á.; Del Mazo-Monsalvo, I.; Saavedra, E.; Rodríguez-Enríquez, S. Assessment of the low inhibitory specificity of oxamate, aminooxyacetate and dichloroacetate on cancer energy metabolism. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2017, 1861, 3221–3236. [Google Scholar] [CrossRef]
- Rej, R. Measurement of aspartate aminotransferase activity: Effects of oxamate. Clin. Chem. 1979, 25, 555–559. [Google Scholar] [CrossRef]
- Datta, A.G.; Racker, E. Mechanism of Action of Transketolase. J. Biol. Chem. 1961, 236, 624–628. [Google Scholar] [CrossRef]
- Kochetov, G.A. Transketolase from yeast, rat liver, and pig liver. In Methods in Enzymology; Part E: Carbohydrate Metabolism; Elsevier: Amsterdam, The Netherlands, 1982; Volume 90, pp. 209–223. [Google Scholar] [CrossRef]
- Schellenberger, A.; Hubner, G. On the separation of phosphoric acid esters of thiamine and its analogues by gradient elution. Hoppe. Seylers Z Physiol. Chem. 1965, 343, 189–192. [Google Scholar] [CrossRef]
- Ksenofontov, A.L.; Boyko, A.I.; Mkrtchyan, G.V.; Tashlitsky, V.N.; Timofeeva, A.V.; Graf, A.V.; Bunik, V.I.; Baratova, L.A. Analysis of Free Amino Acids in Mammalian Brain Extracts. Biochem. Biokhimiia 2017, 82, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Körner, R.W.; Vierzig, A.; Roth, B.; Müller, C. Determination of thiamin diphosphate in whole blood samples by high-performance liquid chromatography—A method suitable for pediatric diagnostics. J. Chromatogr. B 2009, 877, 1882–1886. [Google Scholar] [CrossRef] [PubMed]
- Kalambet, Y. Data acquisition and integration. In Gas Chromatography; Elsevier: Amsterdam, The Netherlands, 2021; pp. 505–524. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleshin, V.; Borisova, N.; Artiukhov, A.; Tagirov, K.; Solovjeva, O.; Lavrenteva, E.; Panin, N.; Maslova, M.; Graf, A. Daytime-Dependent Effects of Thiamine on the Thiamine Pool and Pyruvate Dehydrogenase Regulation in the Brain and Heart. Int. J. Mol. Sci. 2025, 26, 8296. https://doi.org/10.3390/ijms26178296
Aleshin V, Borisova N, Artiukhov A, Tagirov K, Solovjeva O, Lavrenteva E, Panin N, Maslova M, Graf A. Daytime-Dependent Effects of Thiamine on the Thiamine Pool and Pyruvate Dehydrogenase Regulation in the Brain and Heart. International Journal of Molecular Sciences. 2025; 26(17):8296. https://doi.org/10.3390/ijms26178296
Chicago/Turabian StyleAleshin, Vasily, Nadejda Borisova, Artem Artiukhov, Kurban Tagirov, Olga Solovjeva, Eva Lavrenteva, Nikolay Panin, Maria Maslova, and Anastasia Graf. 2025. "Daytime-Dependent Effects of Thiamine on the Thiamine Pool and Pyruvate Dehydrogenase Regulation in the Brain and Heart" International Journal of Molecular Sciences 26, no. 17: 8296. https://doi.org/10.3390/ijms26178296
APA StyleAleshin, V., Borisova, N., Artiukhov, A., Tagirov, K., Solovjeva, O., Lavrenteva, E., Panin, N., Maslova, M., & Graf, A. (2025). Daytime-Dependent Effects of Thiamine on the Thiamine Pool and Pyruvate Dehydrogenase Regulation in the Brain and Heart. International Journal of Molecular Sciences, 26(17), 8296. https://doi.org/10.3390/ijms26178296





