Ligand–Enzyme Interaction Modeling of Missense Variants Implicated in Mitochondrial HMG-CoA Synthase Deficiency
Abstract
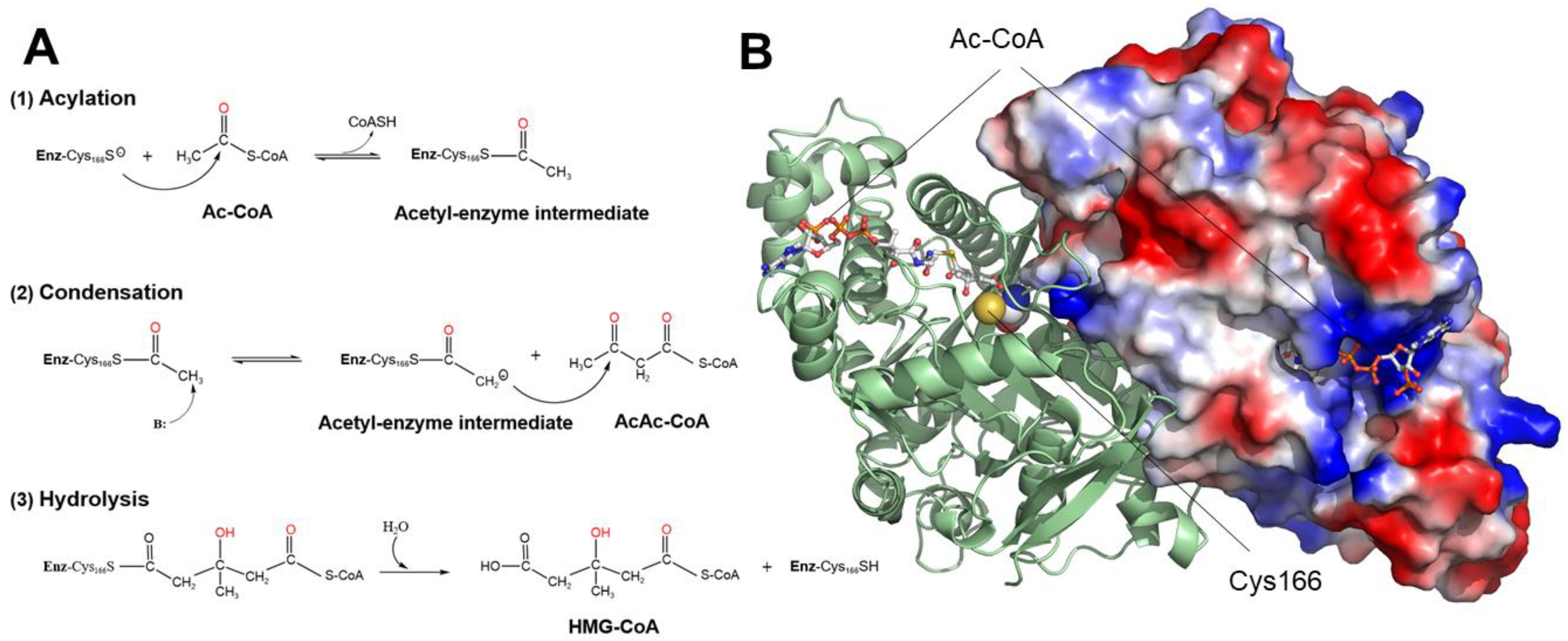
1. Introduction
2. Results and Discussion
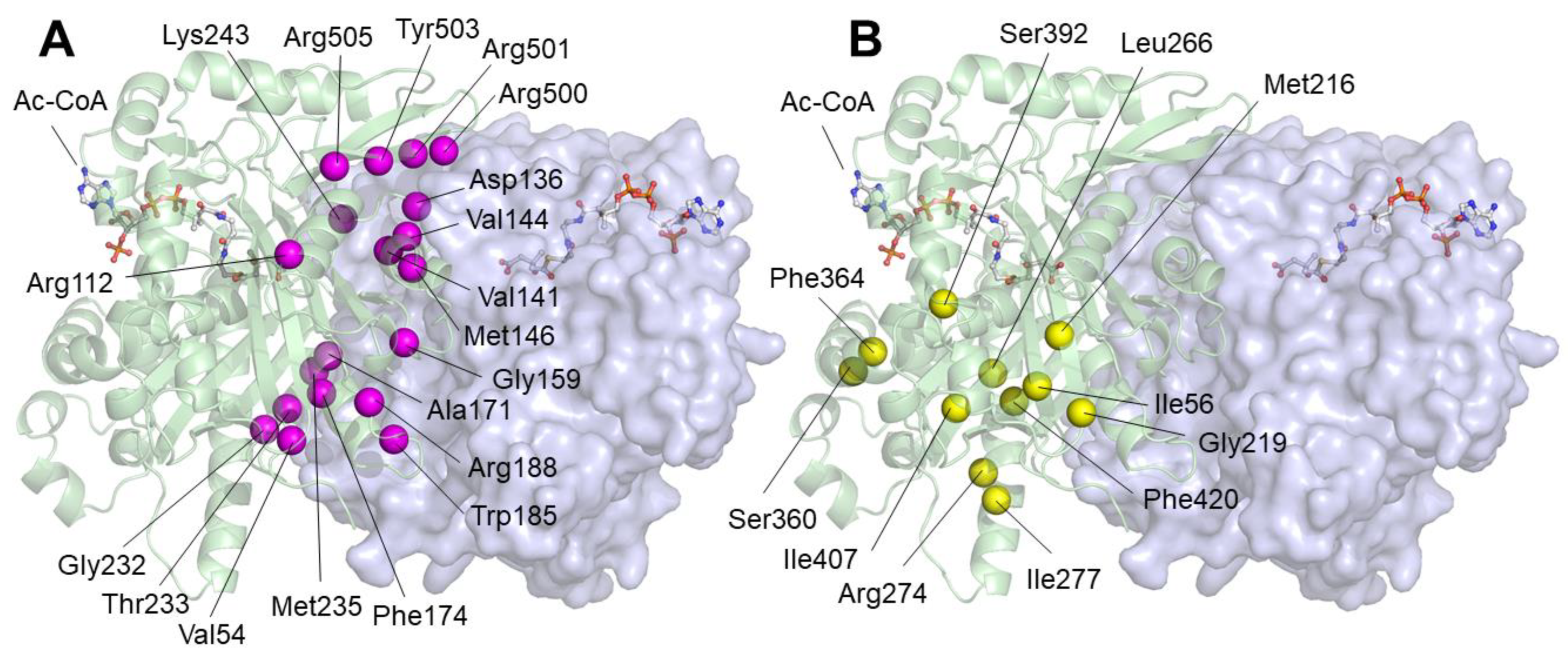
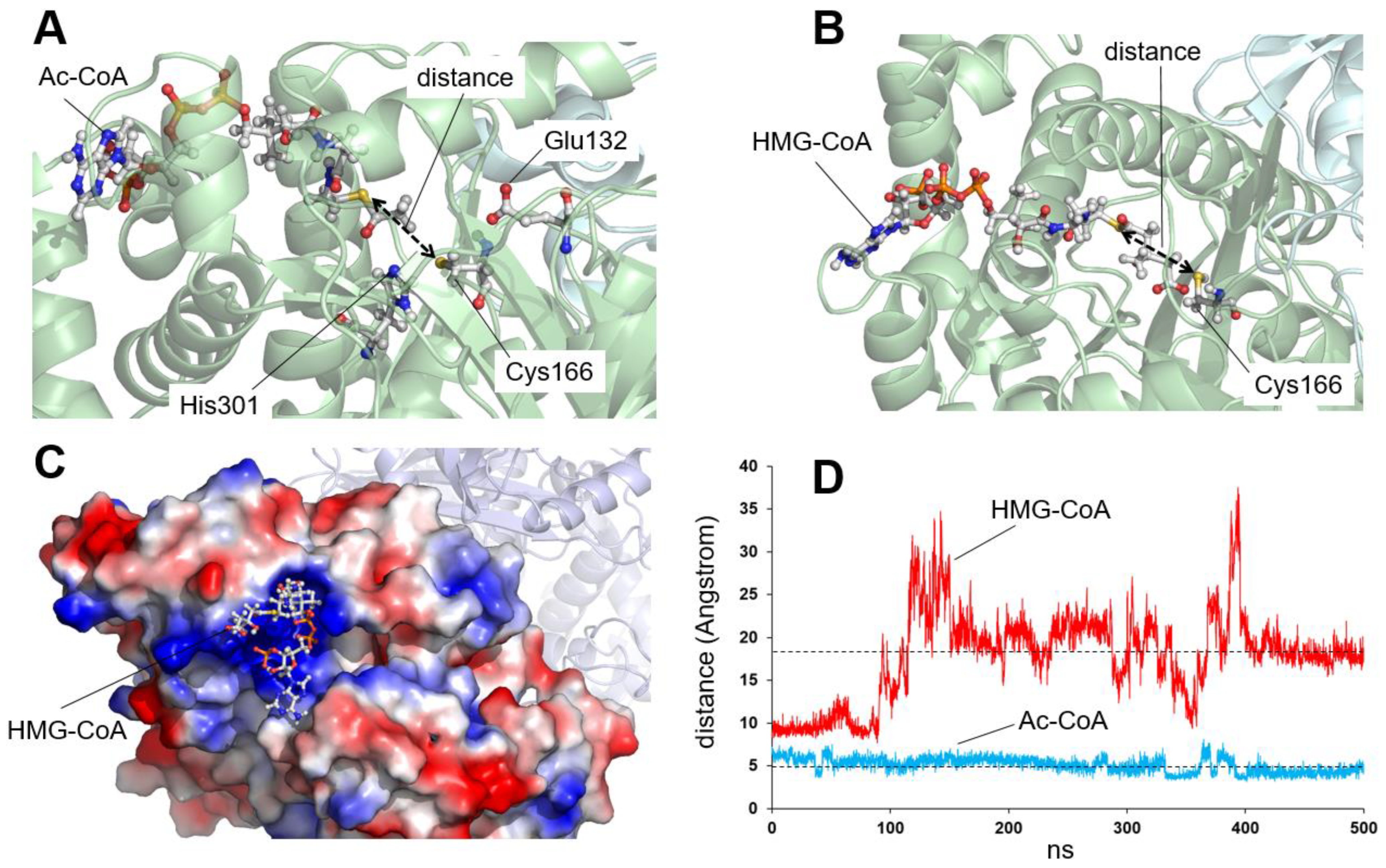
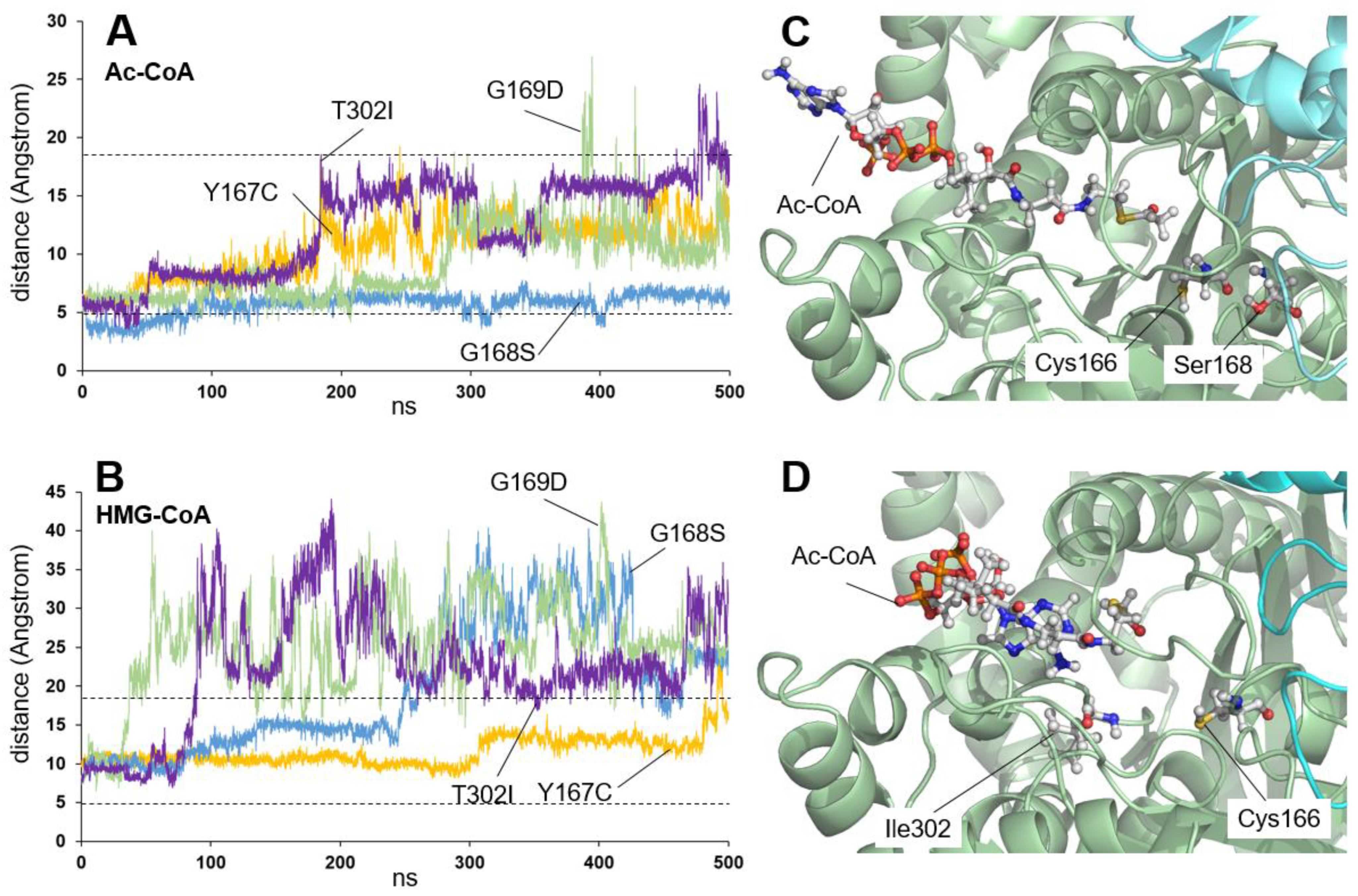
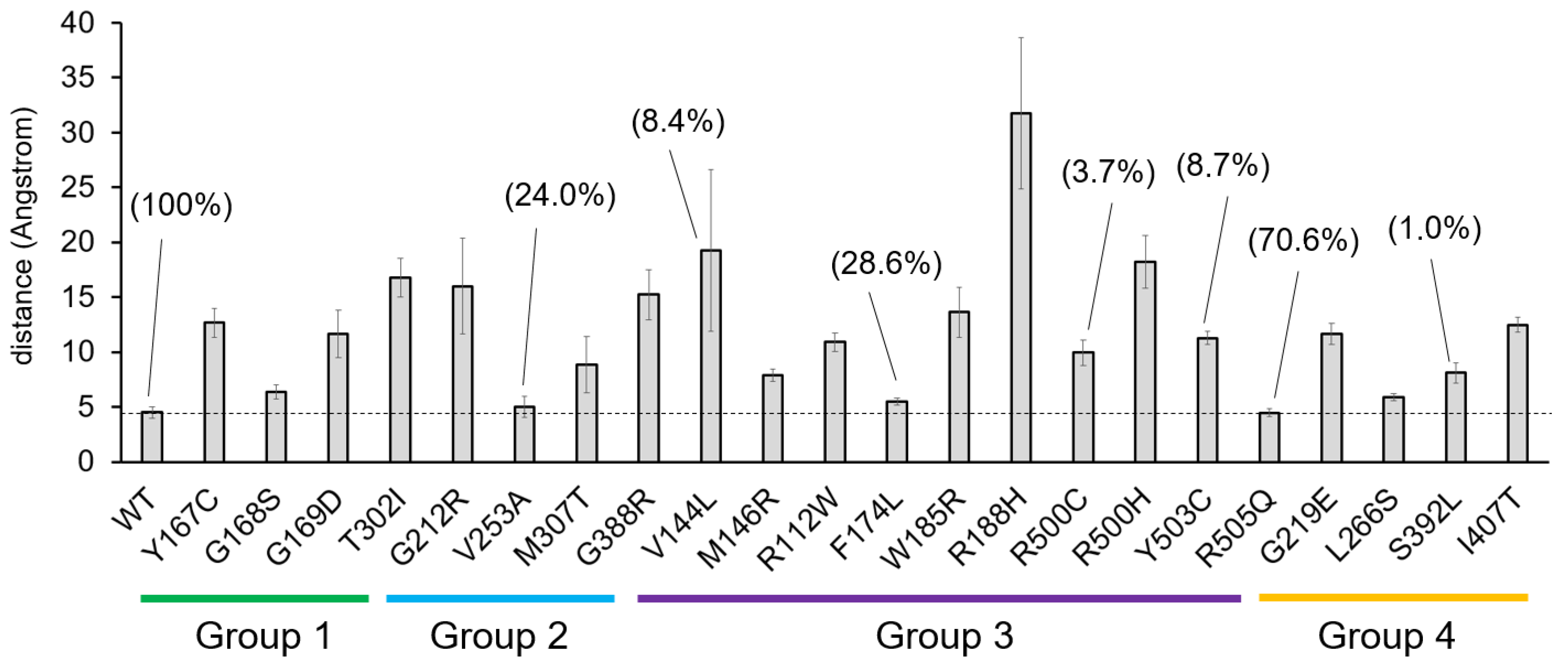
2.1. Residues Located Close to the Active Center
2.2. Residues Located near the Substrate Binding Site
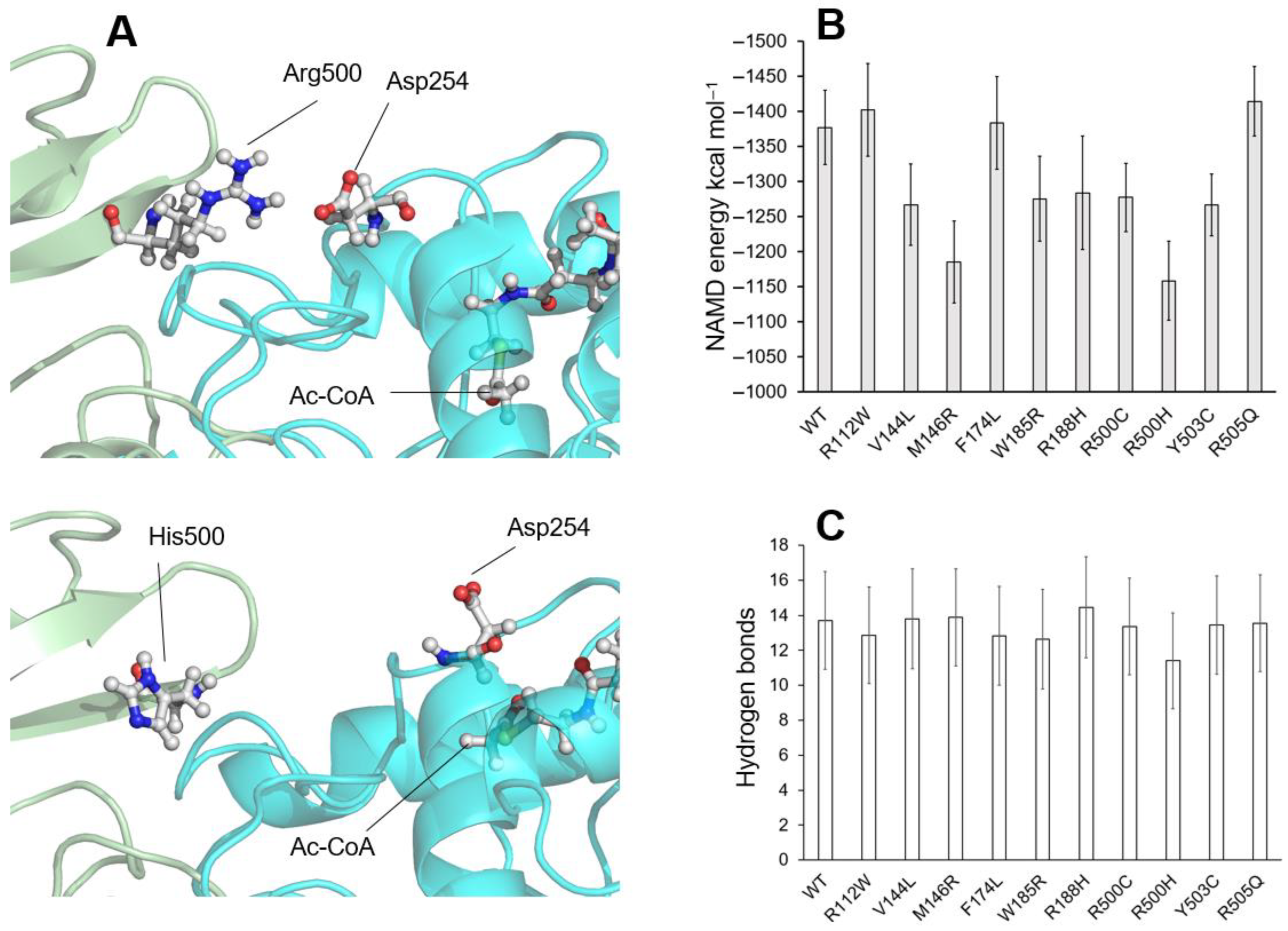
2.3. Residues Located at the Homodimerization Surface
2.4. Residues Located at Other Positions
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hegardt, F.G. Mitochondrial 3-Hydroxy-3-Methylglutaryl-CoA Synthase: A Control Enzyme in Ketogenesis. Biochem. J. 1999, 338, 569–582. [Google Scholar] [CrossRef]
- Fukao, T.; Mitchell, G.; Sass, J.O.; Hori, T.; Orii, K.; Aoyama, Y. Ketone Body Metabolism and Its Defects. J. Inherit. Metab. Dis. 2014, 37, 541–551. [Google Scholar] [CrossRef]
- Suresh, V.V.; Sivaprakasam, S.; Bhutia, Y.D.; Prasad, P.D.; Thangaraju, M.; Ganapathy, V. Not Just an Alternative Energy Source: Diverse Biological Functions of Ketone Bodies and Relevance of HMGCS2 to Health and Disease. Biomolecules 2025, 15, 580. [Google Scholar] [CrossRef]
- Mascaro, C.; Buesa, C.; Ortiz, J.A.; Hare, D.; Hegardt, F.G. Molecular Cloning and Tissue Expression of Human Mitochondrial 3-Hydroxy-3-Methylglutaryl-CoA Synthase. Arch. Biochem. Biophys. 1995, 317, 385–390. [Google Scholar] [CrossRef]
- Bagheri-Fam, S.; Chen, H.; Wilson, S.; Ayers, K.; Hughes, J.; Sloan-Bena, F.; Calvel, P.; Robevska, G.; Puisac, B.; Kusz-Zamelczyk, K.; et al. The Gene Encoding the Ketogenic Enzyme HMGCS2 Displays a Unique Expression during Gonad Development in Mice. PLoS ONE 2020, 15, e0227411. [Google Scholar] [CrossRef]
- Miziorko, H.M.; Lane, M.D. 3-Hydroxy-3-Methylgutaryl-CoA Synthase. Participation of Acetyl-S-Enzyme and Enzyme-S-Hydroxymethylgutaryl-SCoA Intermediates in the Reaction. J. Biol. Chem. 1977, 252, 1414–1420. [Google Scholar] [CrossRef]
- Shafqat, N.; Turnbull, A.; Zschocke, J.; Oppermann, U.; Yue, W.W. Crystal Structures of Human HMG-CoA Synthase Isoforms Provide Insights into Inherited Ketogenesis Disorders and Inhibitor Design. J. Mol. Biol. 2010, 398, 497–506. [Google Scholar] [CrossRef]
- Reed, W.; Clinkenbeard, D.; Lane, M. Molecular and Catalytic Properties of Mitochondrial (Ketogenic) 3-Hydroxy-3-Methylglutaryl Coenzyme A Synthase of Liver. J. Biol. Chem. 1975, 250, 3117–3123. [Google Scholar] [CrossRef]
- Aledo, R.; Zschocke, J.; Pié, J.; Mir, C.; Fiesel, S.; Mayatepek, E.; Hoffmann, G.F.; Casals, N.; Hegardt, F.G. Genetic Basis of Mitochondrial HMG-CoA Synthase Deficiency. Hum. Genet. 2001, 109, 19–23. [Google Scholar] [CrossRef]
- Decru, B.; Lys, M.; Truijens, K.; Mercier, N.; Papadopoulos, J.; Rymen, D.; Roland, D.; Dewulf, J.P.; Vermeersch, P. Mitochondrial HMG-CoA Synthase Deficiency. Mol. Genet. Metab. 2025, 144, 109007. [Google Scholar] [CrossRef]
- Pitt, J.J.; Peters, H.; Boneh, A.; Yaplito-Lee, J.; Wieser, S.; Hinderhofer, K.; Johnson, D.; Zschocke, J. Mitochondrial 3-Hydroxy-3-Methylglutaryl-CoA Synthase Deficiency: Urinary Organic Acid Profiles and Expanded Spectrum of Mutations. J. Inherit. Metab. Dis. 2015, 38, 459–466. [Google Scholar] [CrossRef]
- Ramos, M.; Menao, S.; Arnedo, M.; Puisac, B.; Gil-Rodríguez, M.C.; Teresa-Rodrigo, M.E.; Hernández-Marcos, M.; Pierre, G.; Ramaswami, U.; Baquero-Montoya, C.; et al. New Case of Mitochondrial HMG-CoA Synthase Deficiency. Functional Analysis of Eight Mutations. Eur. J. Med. Genet. 2013, 56, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Puisac, B.; Marcos-Alcalde, I.; Hernández-Marcos, M.; Tobajas Morlana, P.; Levtova, A.; Schwahn, B.C.; DeLaet, C.; Lace, B.; Gómez-Puertas, P.; Pié, J. Human Mitochondrial HMG-CoA Synthase Deficiency: Role of Enzyme Dimerization Surface and Characterization of Three New Patients. Int. J. Mol. Sci. 2018, 19, 1010. [Google Scholar] [CrossRef]
- Ago, Y.; Otsuka, H.; Sasai, H.; Abdelkreem, E.; Nakama, M.; Aoyama, Y.; Matsumoto, H.; Fujiki, R.; Ohara, O.; Akiyama, K.; et al. Japanese Patients with Mitochondrial 3-Hydroxy-3-Methylglutaryl-CoA Synthase Deficiency: In Vitro Functional Analysis of Five Novel HMGCS2 Mutations. Exp. Ther. Med. 2020, 20, 39. [Google Scholar] [CrossRef]
- Niehaus, A.D.; Cooper, H.; Lee, C.U. Mitochondrial HMG-CoA Synthase Deficiency: A Cyclic Vomiting Mimic Without Reliable Biochemical Markers. J. Investig. Med. High. Impact Case Rep. 2024, 12, 23247096241267154. [Google Scholar] [CrossRef]
- Pitt, J.; Peters, H.; Mishra, A.; Eggington, M.; Johnson, D.; Hinderhofer, K.; Kalb, S.; Yaplito-Lee, J.; Cozens, A.; Boneh, A.; et al. Mitochondrial 3-Hydroxy-3-Methylglutaryl Coa Synthase Deficiency May Be Recognised by Increased Concentrations of 4-Hydroxy-6-Methyl-2-Pyrone in Urinary Organic Acid Analysis. Mol. Genet. Metab. 2009, 98, 39–88. [Google Scholar] [CrossRef]
- Wu, S.; Shen, L.; Chen, Q.; Gong, C.; Yang, Y.; Wei, H.; Cao, B.; Chen, Y. Clinical, Biochemical, Molecular, and Outcome Features of Mitochondrial 3-Hydroxy-3-Methylglutaryl-CoA Synthase Deficiency in 10 Chinese Patients. Front. Genet. 2021, 12, 816779. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Miao, J.-K.; Yu, C.-W.; Wan, K.-X.; Zhang, J.; Yuan, Z.-J.; Yang, J.; Wang, D.-J.; Zeng, Y.; Zou, L. Severe Clinical Manifestation of Mitochondrial 3-Hydroxy-3-Methylglutaryl-CoA Synthase Deficiency Associated with Two Novel Mutations: A Case Report. BMC Pediatr. 2019, 19, 344. [Google Scholar] [CrossRef]
- Iossifov, I.; O’Roak, B.J.; Sanders, S.J.; Ronemus, M.; Krumm, N.; Levy, D.; Stessman, H.A.; Witherspoon, K.T.; Vives, L.; Patterson, K.E.; et al. The Contribution of de Novo Coding Mutations to Autism Spectrum Disorder. Nature 2014, 515, 216–221. [Google Scholar] [CrossRef]
- Lee, T.; Takami, Y.; Yamada, K.; Kobayashi, H.; Hasegawa, Y.; Sasai, H.; Otsuka, H.; Takeshima, Y.; Fukao, T. A Japanese Case of Mitochondrial 3-Hydroxy-3-Methylglutaryl-CoA Synthase Deficiency Who Presented with Severe Metabolic Acidosis and Fatty Liver without Hypoglycemia. JIMD Rep. 2019, 48, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Chew, H.B.; Sani, H.A.; Yakob, Y.; Ayob, I.N.M.; Leong, H.Y.; Ting, S.L.; Chan, M.Y.; Abdullah, H.; Ngu, L.H. Phenotypic and Molecular Landscape of Mitochondrial 3-Hydroxy-3-Methylglutaryl-CoA Synthase (HMGCS2) Deficiency in Malaysian Patients. J. Inherit. Metab. Dis. 2024, 47, PO-114. [Google Scholar] [CrossRef]
- Wolf, N.I.; Rahman, S.; Clayton, P.T.; Zschocke, J. Mitochondrial HMG-CoA Synthase Deficiency: Identification of Two Further Patients Carrying Two Novel Mutations. Eur. J. Pediatr. 2003, 162, 279–280. [Google Scholar] [CrossRef]
- Heidari, M.; Soleyman-Nejad, M.; Isazadeh, A.; Shapouri, J.; Taskhiri, M.H.; Ahangari, R.; Mohamadi, A.R.; Ebrahimi, M.; Karimi, H.; Bolhassani, M.; et al. Association of a Novel Homozygous Mutation in the HMGCS2 Gene with an HMGCSD in an Iranian Patient. Mol. Genet. Genom. Med. 2020, 8, e1507. [Google Scholar] [CrossRef]
- Conboy, E.; Vairo, F.; Schultz, M.; Agre, K.; Ridsdale, R.; Deyle, D.; Oglesbee, D.; Gavrilov, D.; Klee, E.W.; Lanpher, B. Mitochondrial 3-Hydroxy-3-Methylglutaryl-CoA Synthase Deficiency: Unique Presenting Laboratory Values and a Review of Biochemical and Clinical Features. JIMD Rep. 2018, 40, 63–69. [Google Scholar] [CrossRef]
- Wang, H.; Li, D.; Song, C.; Yang, Y.; Qian, S.; Cheng, Y. Case report of mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase deficiency. Zhonghua Shi Yong Er Ke Lin Chuang Za Zhi 2020, 35, 1269–1271. [Google Scholar] [CrossRef]
- Australian Genomics Health Alliance Acute Care Flagship; Lunke, S.; Eggers, S.; Wilson, M.; Patel, C.; Barnett, C.P.; Pinner, J.; Sandaradura, S.A.; Buckley, M.F.; Krzesinski, E.I.; et al. Feasibility of Ultra-Rapid Exome Sequencing in Critically Ill Infants and Children with Suspected Monogenic Conditions in the Australian Public Health Care System. JAMA 2020, 323, 2503–2511. [Google Scholar] [CrossRef]
- Rojnueangnit, K.; Maneechai, P.; Thaweekul, P.; Piriyanon, P.; Khositseth, S.; Ittiwut, C.; Chetruengchai, W.; Kamolvisit, W.; Theerapanon, T.; Suphapeetiporn, K.; et al. Expanding Phenotypic and Mutational Spectra of Mitochondrial HMG-CoA Synthase Deficiency. Eur. J. Med. Genet. 2020, 63, 104086. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, L.; Robert, M.F.; Vinarov, D.; Stanley, C.A.; Thompson, G.N.; Morris, A.; Leonard, J.V.; Quant, P.; Hsu, B.Y.; Boneh, A.; et al. Mitochondrial 3-Hydroxy-3-Methylglutaryl-CoA Synthase Deficiency: Clinical Course and Description of Causal Mutations in Two Patients. Pediatr. Res. 2001, 49, 326–331. [Google Scholar] [CrossRef]
- Thompson, G.N.; Hsu, B.Y.; Pitt, J.J.; Treacy, E.; Stanley, C.A. Fasting Hypoketotic Coma in a Child with Deficiency of Mitochondrial 3-Hydroxy-3-Methylglutaryl-CoA Synthase. N. Engl. J. Med. 1997, 337, 1203–1207. [Google Scholar] [CrossRef]
- Sait, H.; Srivastava, S.; Kumar, S.; Varughese, B.; Pandey, M.; Venkatramaiah, M.; Chaudhary, P.; Moirangthem, A.; Mandal, K.; Kapoor, S. Inborn Errors of Ketogenesis: Novel Variants, Clinical Presentation, and Follow-Up in a Series of Four Patients. J. Pediatr. Genet. 2024, 13, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, Y.-L.; Liu, M.; Chen, J.-J.; Li, X.-Q.; Cao, B.-Y.; Gong, C.-X. Clinical, Biochemical, Molecular and Therapeutic Characteristics of Four New Patients of Mitochondrial 3-Hydroxy-3-Methylglutaryl-CoA Synthase Deficiency. Clin. Chim. Acta 2020, 509, 83–90. [Google Scholar] [CrossRef]
- Aledo, R.; Mir, C.; Dalton, R.N.; Turner, C.; Pié, J.; Hegardt, F.G.; Casals, N.; Champion, M.P. Refining the Diagnosis of Mitochondrial HMG-CoA Synthase Deficiency. J. Inherit. Metab. Dis. 2006, 29, 207–211. [Google Scholar] [CrossRef]
- Conlon, T.A.; Fitzsimons, P.E.; Borovickova, I.; Kirby, F.; Murphy, S.; Knerr, I.; Crushell, E. Hypoglycemia Is Not a Defining Feature of Metabolic Crisis in Mitochondrial 3-Hydroxy-3-Methylglutaryl-CoA Synthase Deficiency: Further Evidence of Specific Biochemical Markers Which May Aid Diagnosis. JIMD Rep. 2020, 55, 26–31. [Google Scholar] [CrossRef]
- Zschocke, J.; Penzien, J.M.; Bielen, R.; Casals, N.; Aledo, R.; Pié, J.; Hoffmann, G.F.; Hegardt, F.G.; Mayatepek, E. The Diagnosis of Mitochondrial HMG-CoA Synthase Deficiency. J. Pediatr. 2002, 140, 778–780. [Google Scholar] [CrossRef]
- Wang, M.; Gong, Y.; Ma, X.; Zhu, D.; Zhong, X. Clinical features of a Chinese infant with mitochondrial 3-hydroxy-3-methylglutaryl CoA synthase deficiency and review of the literature. J. Clin. Pediatr. 2020, 37, 858. [Google Scholar] [CrossRef]
- Wu, J.; Lu, A.D.; Zhang, L.P.; Zuo, Y.X.; Jia, Y.P. Study of clinical outcome and prognosis in pediatric core binding factor-acute myeloid leukemia. Zhonghua Xue Ye Xue Za Zhi 2019, 40, 52–57. [Google Scholar] [CrossRef]
- Kılıç, M.; Dorum, S.; Topak, A.; Yazıcı, M.U.; Ezgu, F.S.; Coskun, T. Expanding the Clinical Spectrum of Mitochondrial 3-Hydroxy-3-Methylglutaryl-CoA Synthase Deficiency with Turkish Cases Harboring Novel HMGCS2 Gene Mutations and Literature Review. Am. J. Med. Genet. A 2020, 182, 1608–1614. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, D.; El-Karaksy, H.; Wali, Y.; Youssry, I. Mitochondrial 3-Hydroxymethylglutaryl-CoA Synthase-2 (HMGCS2) Deficiency: A Rare Case with Bicytopenia and Coagulopathy. BMJ Case Rep. 2023, 16, e257011. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.A.; Lascelles, C.V.; Olpin, S.E.; Lake, B.D.; Leonard, J.V.; Quant, P.A. Hepatic Mitochondrial 3-Hydroxy-3-Methylglutaryl-Coenzyme a Synthase Deficiency. Pediatr. Res. 1998, 44, 392–396. [Google Scholar] [CrossRef]
- Williams, M.; Menkovic, I.; Reitnauer, P.; Gilbert, E.; Koeberl, D.; Young, S.P.; Stiles, A.R. Critical Sample Collection Delayed? Urine Organic Acid Analysis Can Still Save the Day! A New Case of HMG-CoA Synthase Deficiency. Mol. Genet. Metab. Rep. 2024, 38, 101062. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Yu, D. Mitochondrial 3-hydroxy-3-methylglutaryl CoA synthase deficiency: A case report and literature review. Zhongguo Dang Dai Er Ke Za Zhi 2018, 20, 930–933. [Google Scholar] [CrossRef] [PubMed]
- Hornbeck, P.V.; Zhang, B.; Murray, B.; Kornhauser, J.M.; Latham, V.; Skrzypek, E. PhosphoSitePlus, 2014: Mutations, PTMs and Recalibrations. Nucleic Acids Res. 2015, 43, D512–D520. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kalé, L.; Schulten, K. Scalable Molecular Dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef]
- Sousa da Silva, A.W.; Vranken, W.F. ACPYPE—AnteChamber PYthon Parser interfacE. BMC Res. Notes 2012, 5, 367. [Google Scholar] [CrossRef] [PubMed]
- Kagami, L.; Wilter, A.; Diaz, A.; Vranken, W. The ACPYPE Web Server for Small-Molecule MD Topology Generation. Bioinformatics 2023, 39, btad350. [Google Scholar] [CrossRef]
- Marcos-Alcalde, Í.; Mendieta-Moreno, J.I.; Puisac, B.; Gil-Rodríguez, M.C.; Hernández-Marcos, M.; Soler-Polo, D.; Ramos, F.J.; Ortega, J.; Pié, J.; Mendieta, J.; et al. Two-Step ATP-Driven Opening of Cohesin Head. Sci. Rep. 2017, 7, 3266. [Google Scholar] [CrossRef]
- Ros-Pardo, D.; Gómez-Puertas, P.; Marcos-Alcalde, Í. STAG2: Computational Analysis of Missense Variants Involved in Disease. Int. J. Mol. Sci. 2024, 25, 1280. [Google Scholar] [CrossRef]
- Roe, D.R.; Cheatham, T.E.I. PTRAJ and CPPTRAJ: Software for Processing and Analysis of Molecular Dynamics Trajectory Data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]




| cDNA | Protein | * Enzyme Activity | Reference (s) |
|---|---|---|---|
| chr1 del. 120, 310, 549-120, 312, 114, GRCh37/hg19 | deletion of exon 1 and part of intron 1 | - | [15] |
| c.1-?_104+?del | deletion of exon 1 | - | [11,16] |
| c.34dupA | p.L14Tfs59 * | - | [17] |
| c.100C>T | p.Q34 * | - | [18] |
| c.104G>T | p.R35M | - | [19] |
| c.130_131insC | p.L44Pfs * 29 | - | [20] |
| c.160G>A | p.V54M | - | [17,21,22] |
| c.167T>A | p.I56N | - | [7] |
| c.181_182ins22 | p.V61Afs * 19 | - | [21] |
| c.220G>A | p.E74K | - | [17] |
| c.252T>G | p.Y84 * | - | [7] |
| c.266G>A | p.G89D | - | [23] |
| c.334C>T | p.R112W | Inactive | [13,15] |
| c.407A>G | p.D136G | - | [15,17] |
| c.409A>T | p.K137 * | - | [24] |
| c.422T>A | P.V141D | - | [17] |
| c.430G>T | p.V144L | 8.4% | [13] |
| c.431_432del | p.V144Sfs * 11 | - | [11] |
| c.437T>C | p.M146R | Inactive | [13] |
| c.476G>T | p.G159V | - | [25] |
| c.500A>G | p.Y167C | Inactive | [9] |
| c.502G>A | p.G168S | - | [11] |
| c.506G>A | p.G169D | Inactive | [11,16] |
| c.512C>T | p.A171V | - | [26] |
| c.520T>C | p.F174L | 28.6% | [11,27,28,29] |
| c.520T>G | p.F174V | - | [30] |
| c.553T>C | p.W185R | Inactive | [11] |
| c.559+1G>T | splicing variant | - | [15,17,21,31] |
| c.563G>A | p.R188H | Inactive | [31,32] |
| c.616C>T | p.R206C | - | [31] |
| c.634G>A | p.G212R | Inactive | [9,11,33,34] |
| c.648G>T | p.M216I | - | [35] |
| c.656G>A | p.G219E | Inactive | [14] |
| c.695G>T | p.G232V | - | [11] |
| c.682C>T | p.R228 * | - | [15] |
| c.697A>G | p.T233A | - | [7] |
| c.704T>C | p.M235T | - | [14] |
| c.717T>G | p.Y239 * | - | [17] |
| c.719A>C | p.D240A | - | [17] |
| c.727A>G | p.K243E | - | [7] |
| c.758T>C | p.V253A | 24.0% | [14,36] |
| c.788delT | p.L263Cfs36 * | - | [17] |
| c.797T>C | p.L266S | Inactive | [11,16] |
| c.821G>A | p.R274H | - | [17] |
| c.830T>A | p.I277D | - | [21] |
| c.847C>T | p.Q283 * | - | [11] |
| c.850+1G>A | splicing variant | - | [15] |
| c.851-2A>C | splicing variant | - | [37] |
| c.862C>T | p.R288 * | - | [37] |
| c.905C>T | p.T302I | - | [26] |
| c.920T>C | p.M307T | Inactive | [32] |
| c.965C>G | p.S322 * | - | [30] |
| c.1016+1G>A | splicing variant | - | [15,33,34] |
| c.1017-2A>G | splicing variant | - | [17] |
| c.1078T>C | p.S360P | - | [11] |
| c.1090T>A | P.F364I | - | [15] |
| c.1118A>T | p.Y373F | - | [38] |
| c.1141A>G | p.M381V | - | [24] |
| c.1156_1157insC | p.L386Pfs * 73 | - | [20] |
| c.1162G>A | p.G388R | Inactive | [11,12,16] |
| c.1175C>T | p.S392L | 1.0% | [14,36] |
| c.1187+1G>C | splicing variant | - | [17,25,35] |
| c.1201G>T | p.E401 * | - | [17,31] |
| c.1220T>C | p.I407T | Inactive | [11,12,16] |
| c.1259T>C | p.F420S | - | [31] |
| c.1270C>T | p.R424 * | - | [28,39] |
| c.1279C>T | p.Q427 * | - | [31] |
| c.1347_1351delAGCCT | p.A450Pfs * 7 | - | [31] |
| c.1349delA | p.N465Tfs1 * | - | [17,40] |
| c.1465delA | p.T489Lfs * 55 | - | [18] |
| c.1480C>T | p.R494 * | - | [27] |
| c.1498C>T | p.R500C | 3.7% | [14] |
| c.1499G>A | p.R500H | Inactive | [9,14] |
| c.1502G>C | p.R501P | - | [21,41] |
| c.1502G>A | p.R501Q | - | [15,17,27,40] |
| c.1508A>G | p.Y503C | 8.7% | [11] |
| c.1514G>A | p.R505Q | 70.6% | [11,13,16] |
| Group | Variant | * Enzyme Activity |
|---|---|---|
| 1. Residues located close to the active center | p.Y167C | Inactive |
| p.G168S | - | |
| p.G169D | Inactive | |
| p.T302I | - | |
| 2. Residues located near the substrate binding site | p.G212R | Inactive |
| p.V253A | 24.0% | |
| p.M307T | Inactive | |
| p.G388R | Inactive | |
| 3. Residues located at the homodimerization surface | p.V144L | 8.4% |
| p.M146R | Inactive | |
| p.R112W | Inactive | |
| p.F174L | 28.6% | |
| p.W185R | Inactive | |
| p.R188H | Inactive | |
| p.R500C | 3.7% | |
| p.R500H | Inactive | |
| p.Y503C | 8.7% | |
| p.R505Q | 70.6% | |
| 4. Residues located at other positions | p.G219E | Inactive |
| p.L266S | Inactive | |
| p.S392L | 1.0% | |
| p.I407T | Inactive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arnedo, M.; Ros-Pardo, D.; Puisac, B.; Lucia-Campos, C.; Gil-Salvador, M.; Latorre-Pellicer, A.; Marcos-Alcalde, Í.; Pié, J.; Gómez-Puertas, P. Ligand–Enzyme Interaction Modeling of Missense Variants Implicated in Mitochondrial HMG-CoA Synthase Deficiency. Int. J. Mol. Sci. 2025, 26, 8266. https://doi.org/10.3390/ijms26178266
Arnedo M, Ros-Pardo D, Puisac B, Lucia-Campos C, Gil-Salvador M, Latorre-Pellicer A, Marcos-Alcalde Í, Pié J, Gómez-Puertas P. Ligand–Enzyme Interaction Modeling of Missense Variants Implicated in Mitochondrial HMG-CoA Synthase Deficiency. International Journal of Molecular Sciences. 2025; 26(17):8266. https://doi.org/10.3390/ijms26178266
Chicago/Turabian StyleArnedo, María, David Ros-Pardo, Beatriz Puisac, Cristina Lucia-Campos, Marta Gil-Salvador, Ana Latorre-Pellicer, Íñigo Marcos-Alcalde, Juan Pié, and Paulino Gómez-Puertas. 2025. "Ligand–Enzyme Interaction Modeling of Missense Variants Implicated in Mitochondrial HMG-CoA Synthase Deficiency" International Journal of Molecular Sciences 26, no. 17: 8266. https://doi.org/10.3390/ijms26178266
APA StyleArnedo, M., Ros-Pardo, D., Puisac, B., Lucia-Campos, C., Gil-Salvador, M., Latorre-Pellicer, A., Marcos-Alcalde, Í., Pié, J., & Gómez-Puertas, P. (2025). Ligand–Enzyme Interaction Modeling of Missense Variants Implicated in Mitochondrial HMG-CoA Synthase Deficiency. International Journal of Molecular Sciences, 26(17), 8266. https://doi.org/10.3390/ijms26178266







