Gas-Binding Studies of Class 1 Sugar Beet Phytoglobin and C86A Mutant Using Isothermal Spectral Shifts in High-Precision Microliter Assay
Abstract
1. Introduction
2. Results
2.1. Initial Protein Modifications
2.2. CO Measurements
2.3. NO Measurements
2.4. Comparison with Prior Binding Data
3. Discussion
4. Materials and Methods
4.1. Protein Expression and Purification
4.2. Protein Labeling and Capillary Loading
4.3. Dual-Emission Spectral Shift Measurements
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BHP | Butyl-sepharose HP, used in aliphatic hydrophobic interaction chromatography |
| BvPgb 1.2 | Class 1 phytoglobin from Beta vulgaris |
| C86A | Mutant of BvPgb 1.2 with cysteine-to-alanine substitution at position 86 |
| CV | Column volume |
| EC50 | Half maximal effective concentration |
| KD | Dissociation constant |
| KH | Hexacoordination equilibrium constant |
| kH | Henry’s law solubility constant |
| LN2 | Liquid nitrogen |
| N/D | Not determined |
| NHS | N-Hydroxysuccinimide |
| PBS-T20 | Phosphate-buffered saline supplemented with 0.05% Tween20 |
| Pgb | Phytoglobin |
| QFF | Q-Sepharose FastFlow, used in anionic exchange chromatography |
| rWT | Recombinant wild type of BvPgb 1.2. |
| SpS | Spectral shift |
| TB | Terrific broth |
References
- Leiva-Eriksson, N.; Pin, P.A.; Kraft, T.; Dohm, J.C.; Minoche, A.E.; Himmelbauer, H.; Bülow, L. Differential Expression Patterns of Non-Symbiotic Hemoglobins in Sugar Beet (Beta vulgaris ssp. vulgaris). Plant Cell Physiol. 2014, 55, 834–844. [Google Scholar] [CrossRef]
- Chammakhi, C.; Pacoud, M.; Boscari, A.; Berger, A.; Mhadhbi, H.; Gharbi, I.; Brouquisse, R. Differential Regulation of the “Phytoglobin-Nitric Oxide Respiration” in Medicago Truncatula Roots and Nodules Submitted to Flooding. Plant Sci. 2025, 352, 112393. [Google Scholar] [CrossRef]
- Fukudome, M.; Uchiumi, T. Regulation of Nitric Oxide by Phytoglobins in Lotus japonicus Is Involved in Mycorrhizal Symbiosis with Rhizophagus irregularis. Plant Sci. 2024, 340, 111984. [Google Scholar] [CrossRef]
- Igamberdiev, A.U.; Bykova, N.V. The Role of Alternative Oxidase in the Maintenance of Cellular Redox Balance under Hypoxia via Participation in Nitric Oxide Turnover. J. Exp. Bot. 2025, eraf021. [Google Scholar] [CrossRef]
- Samant, S.B.; Swain, J.; Yadav, N.; Yadav, R.; Singh, P.; Rai, P.; Sheri, V.; Sreeman, S.; Subramanyam, R.; Pareek, A.; et al. Overexpression of Phytoglobin1 in Rice Leads to Enhanced Nitrogen Use Efficiency via Modulation of Nitric Oxide. Plant Cell Environ. 2025, 48, 2755–2768. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.J.; Hebelstrup, K.H.; Mur, L.A.J.; Igamberdiev, A.U. Plant Hemoglobins: Important Players at the Crossroads between Oxygen and Nitric Oxide. FEBS Lett. 2011, 585, 3843–3849. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.D. Non-Symbiotic Haemoglobins—What’s Happening beyond Nitric Oxide Scavenging? AoB Plants 2012, 2012, pls004. [Google Scholar] [CrossRef]
- Gupta, K.J.; Mur, L.A.J.; Wany, A.; Kumari, A.; Fernie, A.R.; Ratcliffe, R.G. The Role of Nitrite and Nitric Oxide under Low Oxygen Conditions in Plants. New Phytologist 2020, 225, 1143–1151. [Google Scholar] [CrossRef]
- Kundu, S.; Premer, S.A.; Hoy, J.A.; Trent, J.T., III; Hargrove, M.S. Direct Measurement of Equilibrium Constants for High-Affinity Hemoglobins. Biophys. J. 2003, 84, 3931–3940. [Google Scholar] [CrossRef]
- Cochrane, D.W.; Shah, J.K.; Hebelstrup, K.H.; Igamberdiev, A.U. Expression of Phytoglobin Affects Nitric Oxide Metabolism and Energy State of Barley Plants Exposed to Anoxia. Plant Sci. 2017, 265, 124–130. [Google Scholar] [CrossRef]
- Lawson, D.M.; Stevenson, C.E.M.; Andrew, C.R.; Eady, R.R. Unprecedented Proximal Binding of Nitric Oxide to Heme: Implications for Guanylate Cyclase. EMBO J. 2000, 19, 5661–5671. [Google Scholar] [CrossRef]
- Martin, E.; Berka, V.; Bogatenkova, E.; Murad, F.; Tsai, A.-L. Ligand Selectivity of Soluble Guanylyl Cyclase: Effect of the hydrogen-bonding tyrosine in the distal heme pocket on binding of oxygen, nitric oxide, and carbon monoxide.*. J. Biol. Chem. 2006, 281, 27836–27845. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; De La Cruz, L.K.; Yang, X.; Wang, B. Carbon Monoxide Signaling: Examining Its Engagement with Various Molecular Targets in the Context of Binding Affinity, Concentration, and Biologic Responses. Pharmacol. Rev. 2022, 74, 825–875. [Google Scholar] [CrossRef]
- Kuruthukulangarakoola, G.T.; Zhang, J.; Albert, A.; Winkler, B.; Lang, H.; Buegger, F.; Gaupels, F.; Heller, W.; Michalke, B.; Sarioglu, H.; et al. Nitric Oxide-Fixation by Non-Symbiotic Haemoglobin Proteins in under N-Limited Conditions. Plant Cell Environ. 2017, 40, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Durante-Rodríguez, G.; de Francisco-Polanco, S.; García, J.L.; Díaz, E. Characterization of a MHYT Domain-Coupled Transcriptional Regulator That Responds to Carbon Monoxide. Nucleic Acids Res. 2024, 52, 8849–8860. [Google Scholar] [CrossRef]
- Winter, M.B.; Herzik, M.A.; Kuriyan, J.; Marletta, M.A. Tunnels Modulate Ligand Flux in a Heme Nitric Oxide/Oxygen Binding (H-NOX) Domain. Proc. Natl. Acad. Sci. USA 2011, 108, E881–E889. [Google Scholar] [CrossRef]
- Hossain, S.; Boon, E.M. Discovery of a Novel Nitric Oxide Binding Protein and Nitric-Oxide-Responsive Signaling Pathway in Pseudomonas aeruginosa. ACS Infect. Dis. 2017, 3, 454–461. [Google Scholar] [CrossRef]
- Langelaan, D.N.; Ngweniform, P.; Rainey, J.K. Biophysical Characterization of G-Protein Coupled Receptor–Peptide Ligand BindingThis Paper Is One of a Selection of Papers Published in a Special Issue Entitled CSBMCB 53rd Annual Meeting—Membrane Proteins in Health and Disease, and Has Undergone the Journal’s Usual Peer Review Process. Biochem. Cell Biol. 2011, 89, 98–105. [Google Scholar] [CrossRef]
- Orlikowska, M.; Wyciszkiewicz, A.; Węgrzyn, K.; Mehringer, J.; de Souza Paiva, D.; Jurczak, P. Methods for Monitoring Protein-Membrane Binding. Comparison Based on the Interactions between Amyloidogenic Protein Human Cystatin C and Phospholipid Liposomes. Int. J. Biol. Macromol. 2024, 278, 134889. [Google Scholar] [CrossRef]
- Langer, A.; Lüdecke, A.; Bartoschik, T.; Cehlar, O.; Duhr, S.; Baaske, P.; Streicher, W. A New Spectral Shift-Based Method to Characterize Molecular Interactions. ASSAY Drug Dev. Technol. 2022, 20, 83–94, Erratum in ASSAY Drug Dev. Technol. 2022, 20, 136. [Google Scholar] [CrossRef]
- Christensen, S.; Groth, L.; Leiva Eriksson, N.; Nyblom, M.; Bülow, L. Oxidative Implications of Substituting a Conserved Cysteine Residue in Sugar Beet Phytoglobin BvPgb 1.2. Antioxidants 2022, 11, 1615. [Google Scholar] [CrossRef]
- Samaja, M.; Rovida, E.; Niggeler, M.; Perrella, M.; Rossi-Bernardi, L. The Dissociation of Carbon Monoxide from Hemoglobin Intermediate. J. Biol. Chem. 1987, 262, 4528–4533. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Begueria, L.; Cuypers, B.; Van Doorslaer, S.; Abbruzzetti, S.; Bruno, S.; Berghmans, H.; Dewilde, S.; Ramos, J.; Viappiani, C.; Becana, M. Characterization of the Heme Pocket Structure and Ligand Binding Kinetics of Non-Symbiotic Hemoglobins from the Model Legume Lotus japonicus. Front. Plant Sci. 2017, 8, 407. [Google Scholar] [CrossRef] [PubMed]
- Karanicolas, J.; Brooks, C.L. Integrating Folding Kinetics and Protein Function: Biphasic Kinetics and Dual Binding Specificity in a WW Domain. Proc. Natl. Acad. Sci. USA 2004, 101, 3432–3437. [Google Scholar] [CrossRef]
- Bruncsics, B.; Errington, W.; Sarkar, C. MVsim Is a Toolset for Quantifying and Designing Multivalent Interactions. Nat. Commun. 2022, 13, 5029. [Google Scholar] [CrossRef]
- Christensen, S.; Stenström, O.; Akke, M.; Bülow, L. Conformational Dynamics of Phytoglobin BvPgb1. 2 from Beta vulgaris ssp. vulgaris. Int. J. Mol. Sci. 2023, 24, 3973. [Google Scholar] [CrossRef]
- Trent, J.T.; Hvitved, A.N.; Hargrove, M.S. A Model for Ligand Binding to Hexacoordinate Hemoglobins. Biochemistry 2001, 40, 6155–6163. [Google Scholar] [CrossRef]
- Smagghe, B.J.; Sarath, G.; Ross, E.; Hilbert, J.; Hargrove, M.S. Slow Ligand Binding Kinetics Dominate Ferrous Hexacoordinate Hemoglobin Reactivities and Reveal Differences between Plants and Other Species. Biochemistry 2006, 45, 561–570. [Google Scholar] [CrossRef]
- Hargrove, M.S. A Flash Photolysis Method to Characterize Hexacoordinate Hemoglobin Kinetics. Biophys. J. 2000, 79, 2733–2738. [Google Scholar] [CrossRef]
- Leiva Eriksson, N.; Reeder, B.J.; Wilson, M.T.; Bülow, L. Sugar Beet Hemoglobins: Reactions with Nitric Oxide and Nitrite Reveal Differential Roles for Nitrogen Metabolism. Biochem. J. 2019, 476, 2111–2125. [Google Scholar] [CrossRef]
- Perazzolli, M.; Dominici, P.; Romero-Puertas, M.C.; Zago, E.; Zeier, J.; Sonoda, M.; Lamb, C.; Delledonne, M. Arabidopsis Nonsymbiotic Hemoglobin AHb1 Modulates Nitric Oxide Bioactivity. Plant Cell 2004, 16, 2785–2794. [Google Scholar] [CrossRef]
- Hartwell, S.K.; Grudpan, K. Flow-Based Systems for Rapid and High-Precision Enzyme Kinetics Studies. J. Anal. Methods Chem. 2012, 2012, 450716. [Google Scholar] [CrossRef]
- Bleul, R.; Ritzi-Lehnert, M.; Höth, J.; Scharpfenecker, N.; Frese, I.; Düchs, D.; Brunklaus, S.; Hansen-Hagge, T.; Meyer-Almes, F.-J.; Drese, K. Compact, Cost-Efficient Microfluidics-Based Stopped-Flow Device. Anal. Bioanal. Chem. 2011, 399, 1117–1125. [Google Scholar] [CrossRef]
- Gibson, Q. [8] Flash Photolysis Techniques. In Biomembranes—Part E: Biological Oxidations; Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1978; Volume 54, pp. 93–101. [Google Scholar]
- Moerner, W.E. New Directions in Single-Molecule Imaging and Analysis. Proc. Natl. Acad. Sci. USA 2007, 104, 12596–12602. [Google Scholar] [CrossRef] [PubMed]
- Kettisen, K.; Strader, M.B.; Wood, F.; Alayash, A.I.; Bülow, L. Site-Directed Mutagenesis of Cysteine Residues Alters Oxidative Stability of Fetal Hemoglobin. Redox Biol. 2018, 19, 218–225. [Google Scholar] [CrossRef]
- Singh, P.; Kumari, A.; Foyer, C.H.; Gupta, K.J. The Power of the Phytoglobin–NO Cycle in the Regulation of Nodulation and Symbiotic Nitrogen Fixation. New Phytologist 2020, 227, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Mot, A.C.; Puscas, C.; Miclea, P.; Naumova-Letia, G.; Dorneanu, S.; Podar, D.; Dissmeyer, N.; Silaghi-Dumitrescu, R. Redox Control and Autoxidation of Class 1, 2 and 3 Phytoglobins from Arabidopsis thaliana. Sci. Rep. 2018, 8, 13714. [Google Scholar] [CrossRef] [PubMed]
- Timilsina, A.; Dong, W.; Hasanuzzaman, M.; Liu, B.; Hu, C. Nitrate–Nitrite–Nitric Oxide Pathway: A Mechanism of Hypoxia and Anoxia Tolerance in Plants. Int. J. Mol. Sci. 2022, 23, 1522. [Google Scholar] [CrossRef]
- Andrzejczak, O.A.; Havelund, J.F.; Wang, W.-Q.; Kovalchuk, S.; Hagensen, C.E.; Hasler-Sheetal, H.; Jensen, O.N.; Rogowska-Wrzesinska, A.; Møller, I.M.; Hebelstrup, K.H. The Hypoxic Proteome and Metabolome of Barley (Hordeum vulgare L.) with and without Phytoglobin Priming. Int. J. Mol. Sci. 2020, 21, 1546. [Google Scholar] [CrossRef]
- Alexander, C.; Wanner, R.; Johnson, C.; Breitsprecher, D.; Winter, G.; Duhr, S.; Baaske, P.; Ferguson, N. Novel Microscale Approaches for Easy, Rapid Determination of Protein Stability in Academic and Commercial Settings. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2014, 1844, 2241–2250. [Google Scholar] [CrossRef]
- Sander, R. Compilation of Henry’s Law Constants (Version 5.0.0) for Water as Solvent. Atmos. Chem. Phys. 2023, 23, 10901–12440. [Google Scholar] [CrossRef]
- Bevington, P.R.; Robinson, D.K. Error Analysis. In Data Reduction and Error Analysis for the Physical Sciences; McGraw-Hill: Columbus, OH, USA, 2003; pp. 36–51. ISBN 978-0-07-247227-1. [Google Scholar]
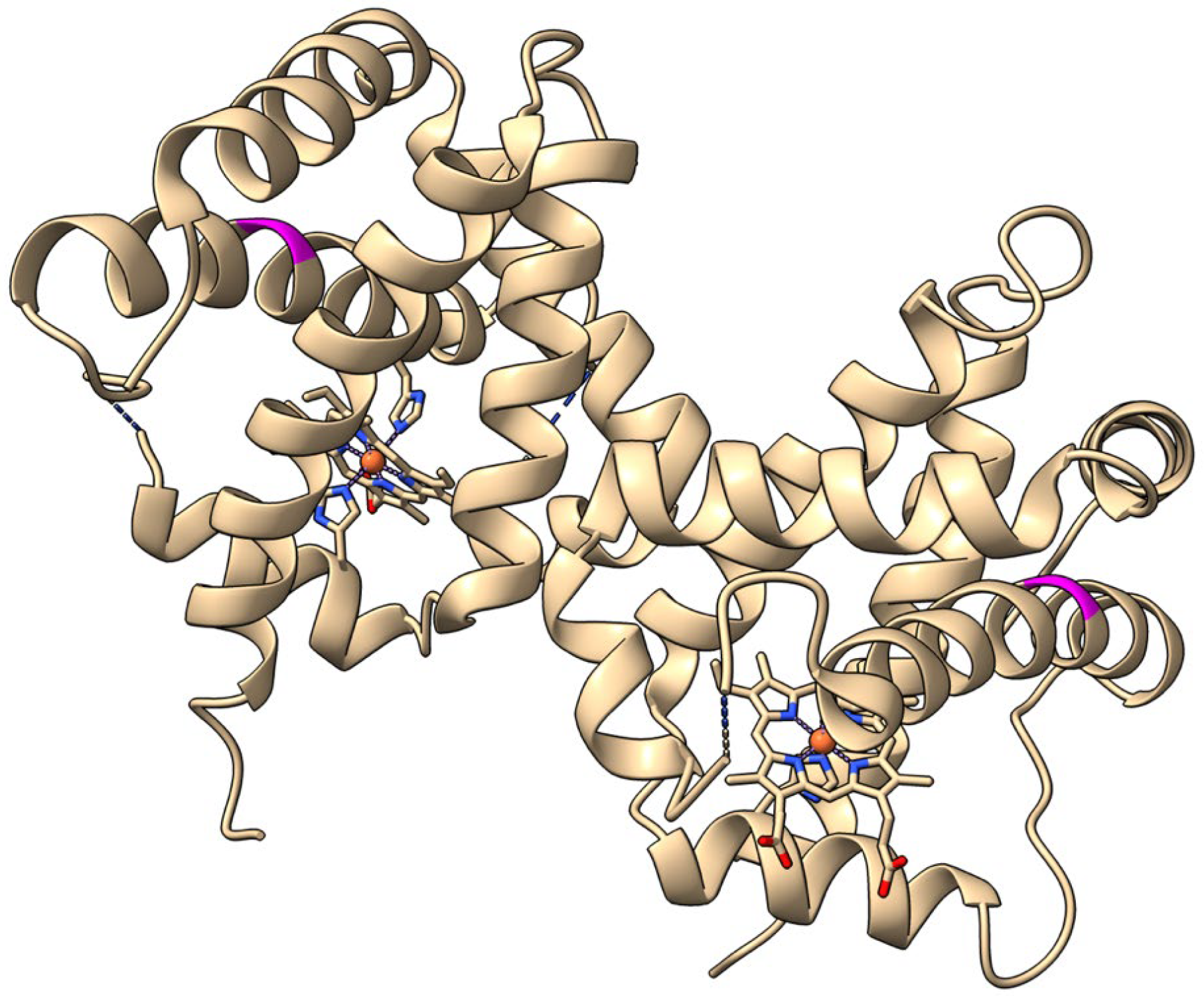
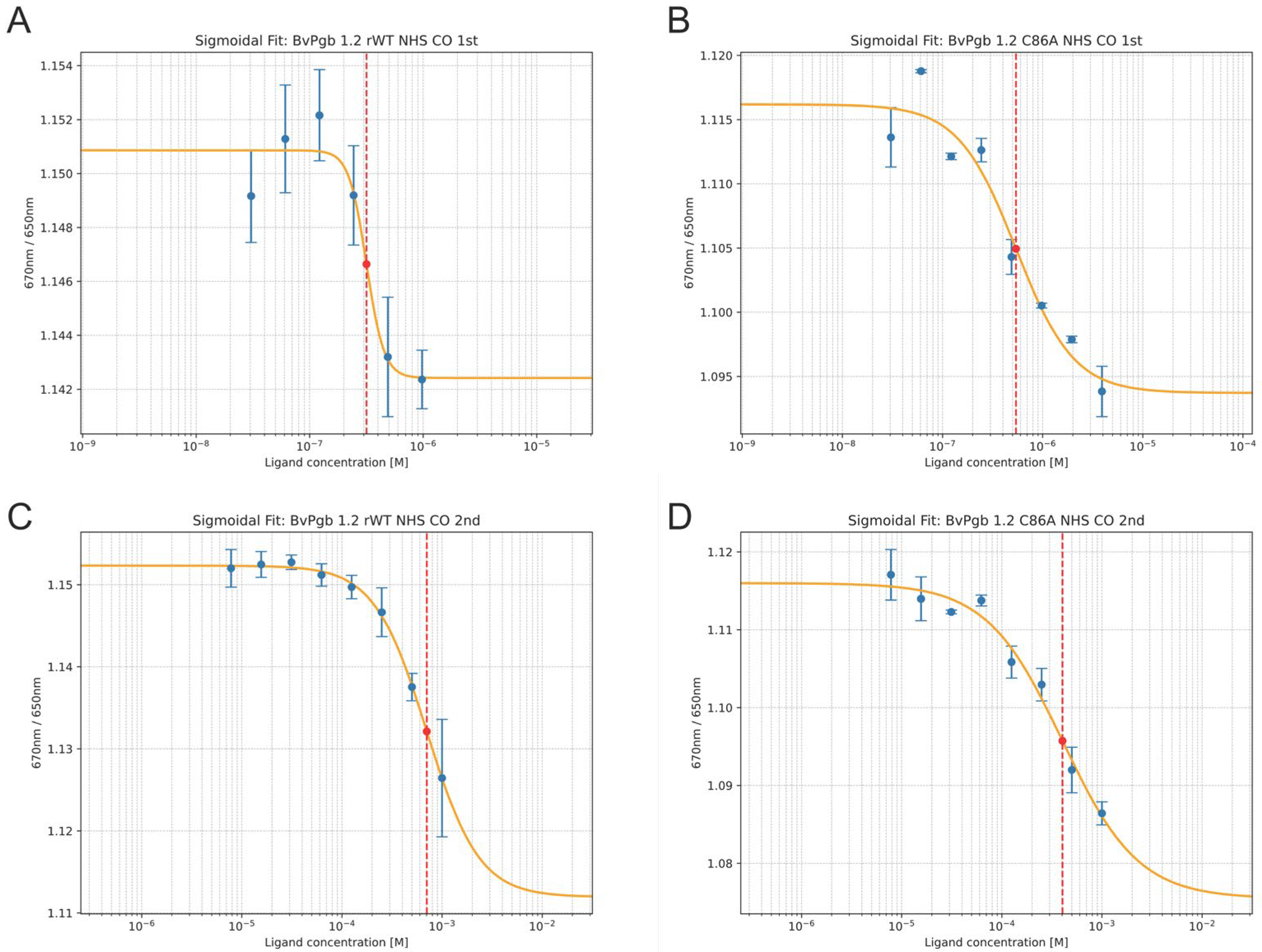
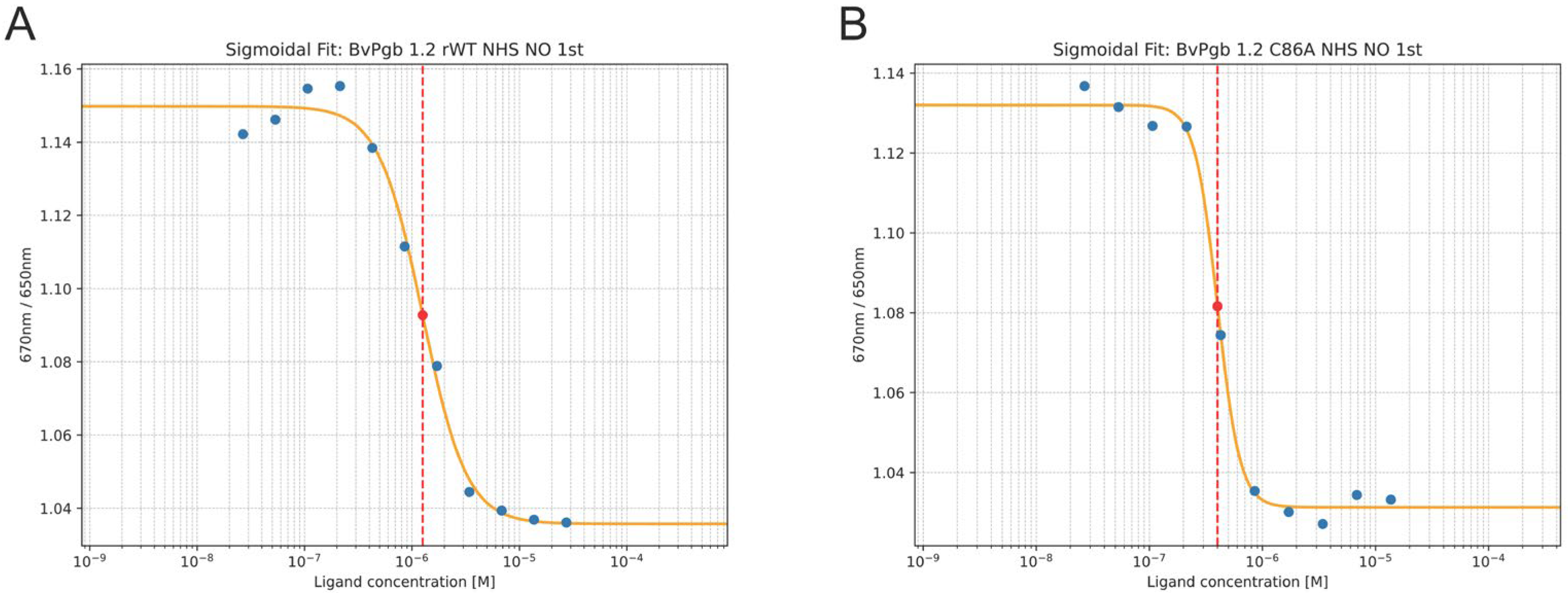
| Isothermal Spectral Shift 1 | Flash Photolysis and Stopped-Flow Rapid Mixing 2 | |||||
|---|---|---|---|---|---|---|
| KD(NO) | KD(CO, open) /EC50,1 | KD(CO, closed) /EC50,2 | KD(NO) | KD(CO, open) | KD(CO, closed) | |
| Protein | [>nM] | [μM] | [nM] | [nM] | [μM] | [nM] |
| rWT | 1600 ± 100 | 700 ± 200 | 400 ± 100 | ~6400 | ~100 | N/D |
| C86A | 400 ± 100 | 400 ± 300 | 500 ± 200 | N/D | N/D | N/D |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Groth, L.; Bülow, L. Gas-Binding Studies of Class 1 Sugar Beet Phytoglobin and C86A Mutant Using Isothermal Spectral Shifts in High-Precision Microliter Assay. Int. J. Mol. Sci. 2025, 26, 8240. https://doi.org/10.3390/ijms26178240
Groth L, Bülow L. Gas-Binding Studies of Class 1 Sugar Beet Phytoglobin and C86A Mutant Using Isothermal Spectral Shifts in High-Precision Microliter Assay. International Journal of Molecular Sciences. 2025; 26(17):8240. https://doi.org/10.3390/ijms26178240
Chicago/Turabian StyleGroth, Leonard, and Leif Bülow. 2025. "Gas-Binding Studies of Class 1 Sugar Beet Phytoglobin and C86A Mutant Using Isothermal Spectral Shifts in High-Precision Microliter Assay" International Journal of Molecular Sciences 26, no. 17: 8240. https://doi.org/10.3390/ijms26178240
APA StyleGroth, L., & Bülow, L. (2025). Gas-Binding Studies of Class 1 Sugar Beet Phytoglobin and C86A Mutant Using Isothermal Spectral Shifts in High-Precision Microliter Assay. International Journal of Molecular Sciences, 26(17), 8240. https://doi.org/10.3390/ijms26178240






