A Perspective on the Role of Mitochondrial Biomolecular Condensates (mtBCs) in Neurodegenerative Diseases and Evolutionary Links to Bacterial BCs
Abstract
1. Introduction
2. Pathological Implications of LLPS and BCs
2.1. TDP-43 and FUS in ALS and FTD
2.2. Tau and α-Synuclein in AD and PD
3. The Role of Mitochondria in Neurodegenerative Diseases
3.1. Role of Mitochondria Dysfunction in Alzheimer’s Disease
3.2. Role of Mitochondria Dysfunction in Parkinson’s Disease
4. Liquid–Liquid Phase Separation (LLPS) in Mitochondria
Correlation Between mtBCs and Neurodegenerative Diseases
5. Intrinsically Disordered Regions in Mitochondrial Proteins
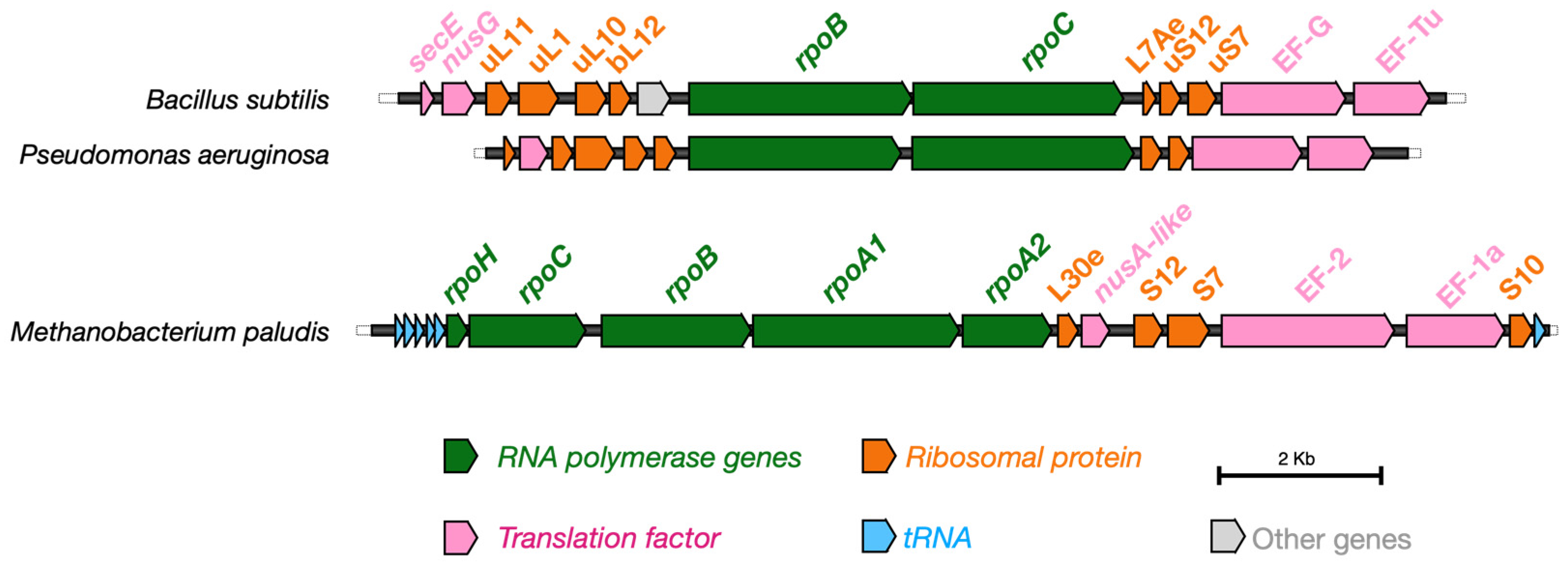
6. Evolutionary Conservation of BCs and the Origin of Life
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| PD | Parkinson’s disease |
| ALS | Amyotrophic Lateral Sclerosis |
| HD | Huntington’s disease |
| FTD | Frontotemporal Dementia |
| LLPS | Liquid–Liquid Phase Separation |
| BCs | Biomolecular Condensates |
| mtBCs | Mitochondrial Biomolecular Condensates |
| MLOs | Membraneless Organelles |
| ER | Endoplasmic Reticulum |
| MRGs | Mitochondrial RNA Granules |
| IDRs | Intrinsically Disordered Regions |
| IDPs | Intrinsically Disordered Proteins |
| OXPHOS | Oxidative Phosphorylation |
| nDNA | Nuclear DNA |
| mtDNA | Mitochondrial DNA |
| PTMs | Post-Translational Modifications |
| RRM | RNA Recognition Motif |
| NLS | Nuclear Localization Signal |
| ROS | Reactive Oxygen Species |
| MFN1/2 | Mitofusin 1/2 |
| DRP1 | Dynamin-Related Protein 1 |
| siRNA | Small Interfering RNA |
| TOMM | Translocase of the Outer Mitochondrial Membrane |
| PINK1 | PTEN-Induced Kinase 1 |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| GO | Gene Ontology |
References
- Xu, Z.; Wang, W.; Cao, Y.; Xue, B. Liquid-Liquid Phase Separation: Fundamental Physical Principles, Biological Implications, and Applications in Supramolecular Materials Engineering. Supramol. Mater. 2023, 2, 100049. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, L.; Dai, T.; Qin, Z.; Lu, H.; Zhang, L.; Zhou, F. Liquid–Liquid Phase Separation in Human Health and Diseases. Signal Transduct. Target. Ther. 2021, 6, 290. [Google Scholar] [CrossRef] [PubMed]
- Hyman, A.A.; Weber, C.A.; Jülicher, F. Liquid-Liquid Phase Separation in Biology. Annu. Rev. Cell Dev. Biol. 2014, 30, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Schäfer, L.V. Thermodynamic Forces from Protein and Water Govern Condensate Formation of an Intrinsically Disordered Protein Domain. Nat. Commun. 2023, 14, 5892. [Google Scholar] [CrossRef]
- Ruff, K.M.; Roberts, S.; Chilkoti, A.; Pappu, R.V. Advances in Understanding Stimulus-Responsive Phase Behavior of Intrinsically Disordered Protein Polymers. J. Mol. Biol. 2018, 430, 4619–4635. [Google Scholar] [CrossRef]
- Alfano, C.; Fichou, Y.; Huber, K.; Weiss, M.; Spruijt, E.; Ebbinghaus, S.; De Luca, G.; Morando, M.A.; Vetri, V.; Temussi, P.A.; et al. Molecular Crowding: The History and Development of a Scientific Paradigm. Chem. Rev. 2024, 124, 3186–3219. [Google Scholar] [CrossRef]
- Das, S.; Lin, Y.-H.; Vernon, R.M.; Forman-Kay, J.D.; Chan, H.S. Comparative Roles of Charge, π, and Hydrophobic Interactions in Sequence-Dependent Phase Separation of Intrinsically Disordered Proteins. Proc. Natl. Acad. Sci. USA 2020, 117, 28795–28805. [Google Scholar] [CrossRef]
- Banani, S.F.; Lee, H.O.; Hyman, A.A.; Rosen, M.K. Biomolecular Condensates: Organizers of Cellular Biochemistry. Nat. Rev. Mol. Cell Biol. 2017, 18, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Yu, X.-Y.; Xu, Y.; Pan, X.; Sun, Y.; Wang, Y.; Song, Y.-H.; Shen, Z. Membraneless Organelles in Health and Disease: Exploring the Molecular Basis, Physiological Roles and Pathological Implications. Signal Transduct. Target. Ther. 2024, 9, 305. [Google Scholar] [CrossRef]
- Orti, F.; Navarro, A.M.; Rabinovich, A.; Wodak, S.J.; Marino-Buslje, C. Insight into Membraneless Organelles and Their Associated Proteins: Drivers, Clients and Regulators. Comput. Struct. Biotechnol. J. 2021, 19, 3964–3977. [Google Scholar] [CrossRef]
- Lafontaine, D.L.J.; Riback, J.A.; Bascetin, R.; Brangwynne, C.P. The Nucleolus as a Multiphase Liquid Condensate. Nat. Rev. Mol. Cell Biol. 2021, 22, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Molliex, A.; Temirov, J.; Lee, J.; Coughlin, M.; Kanagaraj, A.P.; Kim, H.J.; Mittag, T.; Taylor, J.P. Phase Separation by Low Complexity Domains Promotes Stress Granule Assembly and Drives Pathological Fibrillization. Cell 2015, 163, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Zhang, L.; Wu, Y.; Zong, Z.; Wang, B.; Liu, J.; Zhang, L.; Zhou, F. Biomolecular Condensates: Formation Mechanisms, Biological Functions, and Therapeutic Targets. MedComm 2023, 4, e223. [Google Scholar] [CrossRef] [PubMed]
- Moses, D.; Ginell, G.M.; Holehouse, A.S.; Sukenik, S. Intrinsically Disordered Regions Are Poised to Act as Sensors of Cellular Chemistry. Trends Biochem. Sci. 2023, 48, 1019–1034. [Google Scholar] [CrossRef]
- Clerc, I.; Sagar, A.; Barducci, A.; Sibille, N.; Bernadó, P.; Cortés, J. The Diversity of Molecular Interactions Involving Intrinsically Disordered Proteins: A Molecular Modeling Perspective. Comput. Struct. Biotechnol. J. 2021, 19, 3817–3828. [Google Scholar] [CrossRef]
- Uversky, V.N. Biological Liquid–Liquid Phase Separation, Biomolecular Condensates, and Membraneless Organelles: Now You See Me, Now You Don’t. Int. J. Mol. Sci. 2023, 24, 13150. [Google Scholar] [CrossRef]
- Rey, T.; Zaganelli, S.; Cuillery, E.; Vartholomaiou, E.; Croisier, M.; Martinou, J.-C.; Manley, S. Mitochondrial RNA Granules Are Fluid Condensates Positioned by Membrane Dynamics. Nat. Cell Biol. 2020, 22, 1180–1186. [Google Scholar] [CrossRef]
- Uniacke, J.; Zerges, W. Stress Induces the Assembly of RNA Granules in the Chloroplast of Chlamydomonas reinhardtii. J. Cell Biol. 2008, 182, 641–646. [Google Scholar] [CrossRef]
- Liu, H.; Zhuang, H.; Feng, D. Quality Control of Mitochondrial Nucleoids. Trends Cell Biol. 2025; ahead of print. [Google Scholar] [CrossRef]
- Alán, L.; Špaček, T.; Pajuelo Reguera, D.; Jabůrek, M.; Ježek, P. Mitochondrial Nucleoid Clusters Protect Newly Synthesized mtDNA during Doxorubicin- and Ethidium Bromide-Induced Mitochondrial Stress. Toxicol. Appl. Pharmacol. 2016, 302, 31–40. [Google Scholar] [CrossRef]
- Maruri-López, I.; Figueroa, N.E.; Hernández-Sánchez, I.E.; Chodasiewicz, M. Plant Stress Granules: Trends and Beyond. Front. Plant Sci. 2021, 12, 722643. [Google Scholar] [CrossRef] [PubMed]
- Ladouceur, A.-M.; Parmar, B.S.; Biedzinski, S.; Wall, J.; Tope, S.G.; Cohn, D.; Kim, A.; Soubry, N.; Reyes-Lamothe, R.; Weber, S.C. Clusters of Bacterial RNA Polymerase Are Biomolecular Condensates That Assemble through Liquid–Liquid Phase Separation. Proc. Natl. Acad. Sci. USA 2020, 117, 18540–18549. [Google Scholar] [CrossRef] [PubMed]
- Sasazawa, M.; Tomares, D.T.; Childers, W.S.; Saurabh, S. Biomolecular Condensates as Stress Sensors and Modulators of Bacterial Signaling. PLoS Pathog. 2024, 20, e1012413. [Google Scholar] [CrossRef]
- Collins, M.J.; Tomares, D.T.; Nandana, V.; Schrader, J.M.; Childers, W.S. RNase E Biomolecular Condensates Stimulate PNPase Activity. Sci. Rep. 2023, 13, 12937. [Google Scholar] [CrossRef]
- Goldberger, O.; Szoke, T.; Nussbaum-Shochat, A.; Amster-Choder, O. Heterotypic Phase Separation of Hfq Is Linked to Its Roles as an RNA Chaperone. Cell Rep. 2022, 41, 111881. [Google Scholar] [CrossRef]
- Krypotou, E.; Townsend, G.E.; Gao, X.; Tachiyama, S.; Liu, J.; Pokorzynski, N.D.; Goodman, A.L.; Groisman, E.A. Bacteria Require Phase Separation for Fitness in the Mammalian Gut. Science 2023, 379, 1149–1156. [Google Scholar] [CrossRef]
- Janissen, R.; Arens, M.M.A.; Vtyurina, N.N.; Rivai, Z.; Sunday, N.D.; Eslami-Mossallam, B.; Gritsenko, A.A.; Laan, L.; de Ridder, D.; Artsimovitch, I.; et al. Global DNA Compaction in Stationary-Phase Bacteria Does Not Affect Transcription. Cell 2018, 174, 1188–1199.e14. [Google Scholar] [CrossRef]
- Gupta, A.; Joshi, A.; Arora, K.; Mukhopadhyay, S.; Guptasarma, P. The Bacterial Nucleoid-Associated Proteins, HU and Dps, Condense DNA into Context-Dependent Biphasic or Multiphasic Complex Coacervates. J. Biol. Chem. 2023, 299, 104637. [Google Scholar] [CrossRef]
- Zhao, T.; Liu, Y.; Wang, Z.; He, R.; Xiang Zhang, J.; Xu, F.; Lei, M.; Deci, M.B.; Nguyen, J.; Bianco, P.R. Super-resolution Imaging Reveals Changes in Escherichia coli SSB Localization in Response to DNA Damage. Genes Cells 2019, 24, 814–826. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Li, Y.; Jin, X.; Tian, T.; Ma, Q.; Zhao, Z.; Lin, S.; Chen, Z.; Li, B.; Yao, G.; et al. ATP-Dependent Dynamic Protein Aggregation Regulates Bacterial Dormancy Depth Critical for Antibiotic Tolerance. Mol. Cell 2019, 73, 143–156.e4. [Google Scholar] [CrossRef]
- Racki, L.R.; Tocheva, E.I.; Dieterle, M.G.; Sullivan, M.C.; Jensen, G.J.; Newman, D.K. Polyphosphate Granule Biogenesis Is Temporally and Functionally Tied to Cell Cycle Exit during Starvation in Pseudomonas Aeruginosa. Proc. Natl. Acad. Sci. USA 2017, 114, E2440–E2449. [Google Scholar] [CrossRef]
- Saurabh, S.; Chong, T.N.; Bayas, C.; Dahlberg, P.D.; Cartwright, H.N.; Moerner, W.E.; Shapiro, L. ATP-Responsive Biomolecular Condensates Tune Bacterial Kinase Signaling. Sci. Adv. 2022, 8, eabm6570. [Google Scholar] [CrossRef]
- Del Giudice, L.; Alifano, P.; Calcagnile, M.; Di Schiavi, E.; Bertapelle, C.; Aletta, M.; Pontieri, P. Mitochondrial Ribosomal Protein Genes Connected with Alzheimer’s and Tellurite Toxicity. Mitochondrion 2022, 64, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Johri, A.; Beal, M.F. Mitochondrial Dysfunction in Neurodegenerative Diseases. J. Pharmacol. Exp. Ther. 2012, 342, 619–630. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial Dysfunction and Oxidative Stress in Neurodegenerative Diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Monzio Compagnoni, G.; Di Fonzo, A.; Corti, S.; Comi, G.P.; Bresolin, N.; Masliah, E. The Role of Mitochondria in Neurodegenerative Diseases: The Lesson from Alzheimer’s Disease and Parkinson’s Disease. Mol. Neurobiol. 2020, 57, 2959–2980. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, L.; Pontieri, P.; Aletta, M.; Calcagnile, M. Mitochondrial Neurodegenerative Diseases: Three Mitochondrial Ribosomal Proteins as Intermediate Stage in the Pathway That Associates Damaged Genes with Alzheimer’s and Parkinson’s. Biology 2023, 12, 972. [Google Scholar] [CrossRef]
- Nam, J.; Gwon, Y. Neuronal Biomolecular Condensates and Their Implications in Neurodegenerative Diseases. Front. Aging Neurosci. 2023, 15, 1145420. [Google Scholar] [CrossRef]
- Zbinden, A.; Pérez-Berlanga, M.; De Rossi, P.; Polymenidou, M. Phase Separation and Neurodegenerative Diseases: A Disturbance in the Force. Dev. Cell 2020, 55, 45–68. [Google Scholar] [CrossRef]
- Lipiński, W.P.; Visser, B.S.; Robu, I.; Fakhree, M.A.A.; Lindhoud, S.; Claessens, M.M.A.E.; Spruijt, E. Biomolecular Condensates Can Both Accelerate and Suppress Aggregation of α-Synuclein. Sci. Adv. 2022, 8, eabq6495. [Google Scholar] [CrossRef]
- Visser, B.S.; Lipiński, W.P.; Spruijt, E. The Role of Biomolecular Condensates in Protein Aggregation. Nat. Rev. Chem. 2024, 8, 686–700. [Google Scholar] [CrossRef]
- Spannl, S.; Tereshchenko, M.; Mastromarco, G.J.; Ihn, S.J.; Lee, H.O. Biomolecular Condensates in Neurodegeneration and Cancer. Traffic 2019, 20, 890–911. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, L.; Cattin, J.; Knowles, T.P.J.; Bernardes, G.J.L. A Charge-Driven Strategy for Covalent Modification and Modulation of Biomolecular Condensates. J. Am. Chem. Soc. 2025, 147, 28558–28563. [Google Scholar] [CrossRef]
- Qin, C.; Wang, Y.-L.; Zheng, J.; Wan, X.-B.; Fan, X.-J. Current Perspectives in Drug Targeting Intrinsically Disordered Proteins and Biomolecular Condensates. BMC Biol. 2025, 23, 118. [Google Scholar] [CrossRef]
- Jeon, S.; Jeon, Y.; Lim, J.-Y.; Kim, Y.; Cha, B.; Kim, W. Emerging Regulatory Mechanisms and Functions of Biomolecular Condensates: Implications for Therapeutic Targets. Signal Transduct. Target. Ther. 2025, 10, 4. [Google Scholar] [CrossRef]
- Eftekharzadeh, B.; Mayfield, A.; Kauffman, M.G.; Reilly, J.F. Drug Discovery for Diseases with High Unmet Need Through Perturbation of Biomolecular Condensates. J. Mol. Biol. 2024, 436, 168855. [Google Scholar] [CrossRef]
- Biesaga, M.; Frigolé-Vivas, M.; Salvatella, X. Intrinsically Disordered Proteins and Biomolecular Condensates as Drug Targets. Curr. Opin. Chem. Biol. 2021, 62, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Boeynaems, S.; Dorone, Y.; Zhuang, Y.; Shabardina, V.; Huang, G.; Marian, A.; Kim, G.; Sanyal, A.; Şen, N.-E.; Griffith, D.; et al. Poly(A)-Binding Protein Is an Ataxin-2 Chaperone That Regulates Biomolecular Condensates. Mol. Cell 2023, 83, 2020–2034.e6. [Google Scholar] [CrossRef]
- Rhine, K.; Odeh, H.M.; Shorter, J.; Myong, S. Regulation of Biomolecular Condensates by Poly(ADP-Ribose). Chem. Rev. 2023, 123, 9065–9093. [Google Scholar] [CrossRef] [PubMed]
- Loh, D.; Reiter, R.J. Melatonin: Regulation of Biomolecular Condensates in Neurodegenerative Disorders. Antioxidants 2021, 10, 1483. [Google Scholar] [CrossRef] [PubMed]
- Giudice, J.; Jiang, H. Splicing Regulation through Biomolecular Condensates and Membraneless Organelles. Nat. Rev. Mol. Cell Biol. 2024, 25, 683–700. [Google Scholar] [CrossRef]
- Monette, A.; Mouland, A.J. Zinc and Copper Ions Differentially Regulate Prion-Like Phase Separation Dynamics of Pan-Virus Nucleocapsid Biomolecular Condensates. Viruses 2020, 12, 1179. [Google Scholar] [CrossRef]
- Agarwal, A.; Mukhopadhyay, S. Prion Protein Biology Through the Lens of Liquid-Liquid Phase Separation. J. Mol. Biol. 2022, 434, 167368. [Google Scholar] [CrossRef]
- Kamps, J.; Yuste-Checa, P.; Mamashli, F.; Schmitz, M.; Herrera, M.G.; da Silva Correia, S.M.; Gogte, K.; Bader, V.; Zerr, I.; Hartl, F.U.; et al. Regulated Proteolysis Induces Aberrant Phase Transition of Biomolecular Condensates into Aggregates: A Protective Role for the Chaperone Clusterin. J. Mol. Biol. 2024, 436, 168839. [Google Scholar] [CrossRef] [PubMed]
- Hurtle, B.T.; Xie, L.; Donnelly, C.J. Disrupting Pathologic Phase Transitions in Neurodegeneration. J. Clin. Investig. 2023, 133, e168549. [Google Scholar] [CrossRef]
- Fakim, H.; Vande Velde, C. The Implications of Physiological Biomolecular Condensates in Amyotrophic Lateral Sclerosis. Semin. Cell Dev. Biol. 2024, 156, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Ainani, H.; Bouchmaa, N.; Ben Mrid, R.; El Fatimy, R. Liquid-Liquid Phase Separation of Protein Tau: An Emerging Process in Alzheimer’s Disease Pathogenesis. Neurobiol. Dis. 2023, 178, 106011. [Google Scholar] [CrossRef]
- Chandel, I.; Kumari, S.; Bagri, K.; Bhatia, A.; Deshmukh, R. Parkinson’s Disease: Genetics and Neuroinflammatory Insights. Inflammopharmacology 2025, 1–13. [Google Scholar] [CrossRef]
- Linsenmeier, M.; Faltova, L.; Morelli, C.; Capasso Palmiero, U.; Seiffert, C.; Küffner, A.M.; Pinotsi, D.; Zhou, J.; Mezzenga, R.; Arosio, P. The Interface of Condensates of the hnRNPA1 Low-Complexity Domain Promotes Formation of Amyloid Fibrils. Nat. Chem. 2023, 15, 1340–1349. [Google Scholar] [CrossRef]
- Morelli, C.; Faltova, L.; Capasso Palmiero, U.; Makasewicz, K.; Papp, M.; Jacquat, R.P.B.; Pinotsi, D.; Arosio, P. RNA Modulates hnRNPA1A Amyloid Formation Mediated by Biomolecular Condensates. Nat. Chem. 2024, 16, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Siculella, L.; Giannotti, L.; Di Chiara Stanca, B.; Spedicato, F.; Calcagnile, M.; Quarta, S.; Massaro, M.; Damiano, F. A Comprehensive Understanding of hnRNP A1 Role in Cancer: New Perspectives on Binding with Noncoding RNA. Cancer Gene Ther. 2022, 30, 394–403. [Google Scholar] [CrossRef]
- Wood, H. Dual Function of Biomolecular Condensates. Nat. Rev. Neurol. 2023, 19, 67. [Google Scholar] [CrossRef]
- Yang, C.; Tan, W.; Whittle, C.; Qiu, L.; Cao, L.; Akbarian, S.; Xu, Z. The C-Terminal TDP-43 Fragments Have a High Aggregation Propensity and Harm Neurons by a Dominant-Negative Mechanism. PLoS ONE 2010, 5, e15878. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Luo, C.; Jiang, Y.; Hu, T.; Lin, B.; Xie, Y.; Lan, J.; Miao, J. Decoding TDP-43: The Molecular Chameleon of Neurodegenerative Diseases. Acta Neuropathol. Commun. 2024, 12, 205. [Google Scholar] [CrossRef]
- Baloh, R.H. TDP-43: The Relationship between Protein Aggregation and Neurodegeneration in Amyotrophic Lateral Sclerosis and Frontotemporal Lobar Degeneration. FEBS J. 2011, 278, 3539–3549. [Google Scholar] [CrossRef] [PubMed]
- Jo, M.; Lee, S.; Jeon, Y.-M.; Kim, S.; Kwon, Y.; Kim, H.-J. The Role of TDP-43 Propagation in Neurodegenerative Diseases: Integrating Insights from Clinical and Experimental Studies. Exp. Mol. Med. 2020, 52, 1652–1662. [Google Scholar] [CrossRef] [PubMed]
- Romano, R.; De Luca, M.; Del Fiore, V.S.; Pecoraro, M.; Lattante, S.; Sabatelli, M.; La Bella, V.; Bucci, C. Allele-Specific Silencing as Therapy for Familial Amyotrophic Lateral Sclerosis Caused by the p.G376D TARDBP Mutation. Brain Commun. 2022, 4, fcac315. [Google Scholar] [CrossRef]
- Nishimura, A.L.; Shum, C.; Scotter, E.L.; Abdelgany, A.; Sardone, V.; Wright, J.; Lee, Y.-B.; Chen, H.-J.; Bilican, B.; Carrasco, M.; et al. Allele-Specific Knockdown of ALS-Associated Mutant TDP-43 in Neural Stem Cells Derived from Induced Pluripotent Stem Cells. PLoS ONE 2014, 9, e91269. [Google Scholar] [CrossRef]
- Gao, P.; Zhang, Q.; Keely, D.; Cleveland, D.W.; Ye, Y.; Zheng, W.; Shen, M.; Yu, H. Molecular Graph-Based Deep Learning Algorithm Facilitates an Imaging-Based Strategy for Rapid Discovery of Small Molecules Modulating Biomolecular Condensates. J. Med. Chem. 2023, 66, 15084–15093. [Google Scholar] [CrossRef]
- Verde, E.M.; Secco, V.; Ghezzi, A.; Mandrioli, J.; Carra, S. Molecular Mechanisms of Protein Aggregation in ALS-FTD: Focus on TDP-43 and Cellular Protective Responses. Cells 2025, 14, 680. [Google Scholar] [CrossRef]
- Cairns, N.J.; Neumann, M.; Bigio, E.H.; Holm, I.E.; Troost, D.; Hatanpaa, K.J.; Foong, C.; White, C.L.; Schneider, J.A.; Kretzschmar, H.A.; et al. TDP-43 in Familial and Sporadic Frontotemporal Lobar Degeneration with Ubiquitin Inclusions. Am. J. Pathol. 2007, 171, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, E.; Audrain, M.; Ratnam, M.; Ollier, R.; Fuchs, A.; Piorkowska, K.; Pfeifer, A.; Kosco-Vilbois, M.; Seredenina, T.; Afroz, T. Targeting the TDP-43 Low Complexity Domain Blocks Spreading of Pathology in a Mouse Model of ALS/FTD. Acta Neuropathol. Commun. 2024, 12, 156. [Google Scholar] [CrossRef]
- Nonaka, T.; Masuda-Suzukake, M.; Arai, T.; Hasegawa, Y.; Akatsu, H.; Obi, T.; Yoshida, M.; Murayama, S.; Mann, D.M.A.; Akiyama, H.; et al. Prion-like Properties of Pathological TDP-43 Aggregates from Diseased Brains. Cell Rep. 2013, 4, 124–134. [Google Scholar] [CrossRef]
- Chen, C.; Ding, X.; Akram, N.; Xue, S.; Luo, S.-Z. Fused in Sarcoma: Properties, Self-Assembly and Correlation with Neurodegenerative Diseases. Molecules 2019, 24, 1622. [Google Scholar] [CrossRef]
- Trnka, F.; Hoffmann, C.; Wang, H.; Sansevrino, R.; Rankovic, B.; Rost, B.R.; Schmitz, D.; Schmidt, H.B.; Milovanovic, D. Aberrant Phase Separation of FUS Leads to Lysosome Sequestering and Acidification. Front. Cell Dev. Biol. 2021, 9, 716919. [Google Scholar] [CrossRef]
- Niaki, A.G.; Sarkar, J.; Cai, X.; Rhine, K.; Vidaurre, V.; Guy, B.; Hurst, M.; Lee, J.C.; Koh, H.R.; Guo, L.; et al. Loss of Dynamic RNA Interaction and Aberrant Phase Separation Induced by Two Distinct Types of ALS/FTD-Linked FUS Mutations. Mol. Cell 2020, 77, 82–94.e4. [Google Scholar] [CrossRef]
- Shelkovnikova, T.A.; Robinson, H.K.; Southcombe, J.A.; Ninkina, N.; Buchman, V.L. Multistep Process of FUS Aggregation in the Cell Cytoplasm Involves RNA-Dependent and RNA-Independent Mechanisms. Hum. Mol. Genet. 2014, 23, 5211–5226. [Google Scholar] [CrossRef] [PubMed]
- Rhine, K.; Makurath, M.A.; Liu, J.; Skanchy, S.; Lopez, C.; Catalan, K.F.; Ma, Y.; Fare, C.M.; Shorter, J.; Ha, T.; et al. ALS/FTLD-Linked Mutations in FUS Glycine Residues Cause Accelerated Gelation and Reduced Interactions with Wild-Type FUS. Mol. Cell 2020, 80, 666–681.e8. [Google Scholar] [CrossRef]
- Vazquez-Sanchez, S.; Tilkin, B.; Gasset-Rosa, F.; Zhang, S.; Piol, D.; McAlonis-Downes, M.; Artates, J.; Govea-Perez, N.; Verresen, Y.; Guo, L.; et al. Frontotemporal Dementia-like Disease Progression Elicited by Seeded Aggregation and Spread of FUS. Mol. Neurodegener. 2024, 19, 46. [Google Scholar] [CrossRef]
- Abasi, L.S.; Elathram, N.; Movva, M.; Deep, A.; Corbett, K.D.; Debelouchina, G.T. Phosphorylation Regulates Tau’s Phase Separation Behavior and Interactions with Chromatin. Commun. Biol. 2024, 7, 251. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, P.S.; Quan, M.D.; Ferreon, J.C.; Ferreon, A.C.M. Aggregation of Disordered Proteins Associated with Neurodegeneration. Int. J. Mol. Sci. 2023, 24, 3380. [Google Scholar] [CrossRef]
- Longfield, S.F.; Mollazade, M.; Wallis, T.P.; Gormal, R.S.; Joensuu, M.; Wark, J.R.; Van Waardenberg, A.J.; Small, C.; Graham, M.E.; Meunier, F.A.; et al. Tau Forms Synaptic Nano-Biomolecular Condensates Controlling the Dynamic Clustering of Recycling Synaptic Vesicles. Nat. Commun. 2023, 14, 7277. [Google Scholar] [CrossRef]
- Parolini, F.; Tira, R.; Barracchia, C.G.; Munari, F.; Capaldi, S.; D’Onofrio, M.; Assfalg, M. Ubiquitination of Alzheimer’s-Related Tau Protein Affects Liquid-Liquid Phase Separation in a Site- and Cofactor-Dependent Manner. Int. J. Biol. Macromol. 2022, 201, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Villar-Piqué, A.; Lopes Da Fonseca, T.; Outeiro, T.F. Structure, Function and Toxicity of Alpha-synuclein: The Bermuda Triangle in Synucleinopathies. J. Neurochem. 2016, 139, 240–255. [Google Scholar] [CrossRef] [PubMed]
- Zappone, S.; Perego, E.; Rupert, J.; Zacco, E.; Slenders, E.; Tartaglia, G.G.; Vicidomini, G. The Role of RNA in the Nanoscale Organization of α-Synuclein Phase Separation. NAR Mol. Med. 2025, 2, ugaf012. [Google Scholar] [CrossRef]
- Dada, S.T.; Toprakcioglu, Z.; Cali, M.P.; Röntgen, A.; Hardenberg, M.C.; Morris, O.M.; Mrugalla, L.K.; Knowles, T.P.J.; Vendruscolo, M. Pharmacological Inhibition of α-Synuclein Aggregation within Liquid Condensates. Nat. Commun. 2024, 15, 3835. [Google Scholar] [CrossRef]
- Siegert, A.; Rankovic, M.; Favretto, F.; Ukmar-Godec, T.; Strohäker, T.; Becker, S.; Zweckstetter, M. Interplay between Tau and A-synuclein Liquid–Liquid Phase Separation. Protein Sci. 2021, 30, 1326–1336. [Google Scholar] [CrossRef]
- Chinnery, P.F. Primary Mitochondrial Disorders Overview. In GeneReviews; University of Washington: Seattle, WA, USA, 2021. [Google Scholar]
- Pontieri, P.; Hartings, H.; Di Salvo, M.; Massardo, D.R.; De Stefano, M.; Pizzolante, G.; Romano, R.; Troisi, J.; Del Giudice, A.; Alifano, P.; et al. Mitochondrial Ribosomal Proteins Involved in Tellurite Resistance in Yeast Saccharomyces Cerevisiae. Sci. Rep. 2018, 8, 12022. [Google Scholar] [CrossRef]
- Chinnery, P.F.; Howell, N.; Andrews, R.M.; Turnbull, D.M. Clinical Mitochondrial Genetics. J. Med. Genet. 1999, 36, 425–436. [Google Scholar]
- Fields, M.; Marcuzzi, A.; Gonelli, A.; Celeghini, C.; Maximova, N.; Rimondi, E. Mitochondria-Targeted Antioxidants, an Innovative Class of Antioxidant Compounds for Neurodegenerative Diseases: Perspectives and Limitations. Int. J. Mol. Sci. 2023, 24, 3739. [Google Scholar] [CrossRef]
- Gorman, G.S.; Schaefer, A.M.; Ng, Y.; Gomez, N.; Blakely, E.L.; Alston, C.L.; Feeney, C.; Horvath, R.; Yu-Wai-Man, P.; Chinnery, P.F.; et al. Prevalence of Nuclear and Mitochondrial DNA Mutations Related to Adult Mitochondrial Disease. Ann. Neurol. 2015, 77, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.B.; Chinnery, P.F. The Dynamics of Mitochondrial DNA Heteroplasmy: Implications for Human Health and Disease. Nat. Rev. Genet. 2015, 16, 530–542. [Google Scholar] [CrossRef]
- Baker, M.J.; Crameri, J.J.; Thorburn, D.R.; Frazier, A.E.; Stojanovski, D. Mitochondrial Biology and Dysfunction in Secondary Mitochondrial Disease. Open Biol. 2022, 12, 220274. [Google Scholar] [CrossRef]
- Pfanner, N.; Warscheid, B.; Wiedemann, N. Mitochondrial Proteins: From Biogenesis to Functional Networks. Nat. Rev. Mol. Cell Biol. 2019, 20, 267–284. [Google Scholar] [CrossRef]
- Baker, B.M.; Haynes, C.M. Mitochondrial Protein Quality Control during Biogenesis and Aging. Trends Biochem. Sci. 2011, 36, 254–261. [Google Scholar] [CrossRef]
- Moehle, E.A.; Shen, K.; Dillin, A. Mitochondrial Proteostasis in the Context of Cellular and Organismal Health and Aging. J. Biol. Chem. 2019, 294, 5396–5407. [Google Scholar] [CrossRef]
- Islam, M.T. Oxidative Stress and Mitochondrial Dysfunction-Linked Neurodegenerative Disorders. Neurol. Res. 2017, 39, 73–82. [Google Scholar] [CrossRef]
- Burté, F.; Carelli, V.; Chinnery, P.F.; Yu-Wai-Man, P. Disturbed Mitochondrial Dynamics and Neurodegenerative Disorders. Nat. Rev. Neurol. 2015, 11, 11–24. [Google Scholar] [CrossRef]
- Ashleigh, T.; Swerdlow, R.H.; Beal, M.F. The Role of Mitochondrial Dysfunction in Alzheimer’s Disease Pathogenesis. Alzheimer’s Dement. 2023, 19, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Misrani, A.; Tabassum, S.; Yang, L. Mitochondrial Dysfunction and Oxidative Stress in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 617588. [Google Scholar] [CrossRef] [PubMed]
- Reiss, A.B.; Gulkarov, S.; Jacob, B.; Srivastava, A.; Pinkhasov, A.; Gomolin, I.H.; Stecker, M.M.; Wisniewski, T.; De Leon, J. Mitochondria in Alzheimer’s Disease Pathogenesis. Life 2024, 14, 196. [Google Scholar] [CrossRef]
- D’Alessandro, M.C.B.; Kanaan, S.; Geller, M.; Praticò, D.; Daher, J.P.L. Mitochondrial Dysfunction in Alzheimer’s Disease. Ageing Res. Rev. 2025, 107, 102713. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative Stress and the Amyloid Beta Peptide in Alzheimer’s Disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Cardaci, V.; Di Pietro, L.; Zupan, M.C.; Sibbitts, J.; Privitera, A.; Lunte, S.M.; Caraci, F.; Hartley, M.D.; Caruso, G. Characterizing Oxidative Stress Induced by Aβ Oligomers and the Protective Role of Carnosine in Primary Mixed Glia Cultures. Free Radic. Biol. Med. 2025, 229, 213–224. [Google Scholar] [CrossRef]
- Lim, K.-L.; Ng, X.-H.; Grace, L.G.-Y.; Yao, T.-P. Mitochondrial Dynamics and Parkinson’s Disease: Focus on Parkin. Antioxid. Redox Signal. 2012, 16, 935–949. [Google Scholar] [CrossRef]
- Cho, D.-H.; Nakamura, T.; Lipton, S.A. Mitochondrial Dynamics in Cell Death and Neurodegeneration. Cell. Mol. Life Sci. 2010, 67, 3435–3447. [Google Scholar] [CrossRef]
- Mary, A.; Eysert, F.; Checler, F.; Chami, M. Mitophagy in Alzheimer’s Disease: Molecular Defects and Therapeutic Approaches. Mol. Psychiatry 2023, 28, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Moreira, P.I.; Carvalho, C.; Zhu, X.; Smith, M.A.; Perry, G. Mitochondrial Dysfunction is a Trigger of Alzheimer’s Disease Pathophysiology. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2010, 1802, 2–10. [Google Scholar] [CrossRef]
- Swerdlow, R.H.; Khan, S.M. A “Mitochondrial Cascade Hypothesis” for Sporadic Alzheimer’s Disease. Med. Hypotheses 2004, 63, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, R.H.; Burns, J.M.; Khan, S.M. The Alzheimer’s Disease Mitochondrial Cascade Hypothesis: Progress and Perspectives. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2014, 1842, 1219–1231. [Google Scholar] [CrossRef] [PubMed]
- Schon, E.A.; Area-Gomez, E. Mitochondria-Associated ER Membranes in Alzheimer Disease. Mol. Cell. Neurosci. 2013, 55, 26–36. [Google Scholar] [CrossRef]
- Li, X.; Wu, Z.; Si, X.; Li, J.; Wu, G.; Wang, M. The Role of Mitochondrial Dysfunction in the Pathogenesis of Alzheimer’s Disease and Future Strategies for Targeted Therapy. Eur. J. Med. Res. 2025, 30, 434. [Google Scholar] [CrossRef]
- Moon, H.E.; Paek, S.H. Mitochondrial Dysfunction in Parkinson’s Disease. Exp. Neurobiol. 2015, 24, 103–116. [Google Scholar] [CrossRef]
- Larsen, S.B.; Hanss, Z.; Krüger, R. The Genetic Architecture of Mitochondrial Dysfunction in Parkinson’s Disease. Cell Tissue Res. 2018, 373, 21–37. [Google Scholar] [CrossRef]
- Gao, X.-Y.; Yang, T.; Gu, Y.; Sun, X.-H. Mitochondrial Dysfunction in Parkinson’s Disease: From Mechanistic Insights to Therapy. Front. Aging Neurosci. 2022, 14, 885500. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; McDonald, D.; Blain, A.; Mossman, E.; Atkin, K.; Marusich, M.F.; Capaldi, R.; Bone, L.; Smith, A.; Filby, A.; et al. Parkinson’s Disease Neurons Exhibit Alterations in Mitochondrial Quality Control Proteins. npj Park. Dis. 2023, 9, 120. [Google Scholar] [CrossRef]
- Choi, J.; Levey, A.I.; Weintraub, S.T.; Rees, H.D.; Gearing, M.; Chin, L.-S.; Li, L. Oxidative Modifications and Down-Regulation of Ubiquitin Carboxyl-Terminal Hydrolase L1 Associated with Idiopathic Parkinson’s and Alzheimer’s Diseases. J. Biol. Chem. 2004, 279, 13256–13264. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H.V.; Cooper, J.M.; Dexter, D.; Clark, J.B.; Jenner, P.; Marsden, C.D. Mitochondrial Complex I Deficiency in Parkinson’s Disease. J. Neurochem. 1990, 54, 823–827. [Google Scholar] [CrossRef]
- González-Rodríguez, P.; Zampese, E.; Stout, K.A.; Guzman, J.N.; Ilijic, E.; Yang, B.; Tkatch, T.; Stavarache, M.A.; Wokosin, D.L.; Gao, L.; et al. Disruption of Mitochondrial Complex I Induces Progressive Parkinsonism. Nature 2021, 599, 650–656, Erratum in Nature 2022, 603, E1. [Google Scholar] [CrossRef] [PubMed]
- Perier, C.; Bové, J.; Wu, D.-C.; Dehay, B.; Choi, D.-K.; Jackson-Lewis, V.; Rathke-Hartlieb, S.; Bouillet, P.; Strasser, A.; Schulz, J.B.; et al. Two Molecular Pathways Initiate Mitochondria-Dependent Dopaminergic Neurodegeneration in Experimental Parkinson’s Disease. Proc. Natl. Acad. Sci. USA 2007, 104, 8161–8166. [Google Scholar] [CrossRef]
- Haas, R.H.; Nasirian, F.; Nakano, K.; Ward, D.; Pay, M.; Hill, R.; Shults, C.W. Low Platelet Mitochondrial Complex I and Complex II/III Activity in Early Untreated Parkinson’s Disease. Ann. Neurol. 1995, 37, 714–722. [Google Scholar] [CrossRef]
- Bindoff, L.A.; Birch-Machin, M.A.; Cartlidge, N.E.F.; Parker, W.D.; Turnbull, D.M. Respiratory Chain Abnormalities in Skeletal Muscle from Patients with Parkinson’s Disease. J. Neurol. Sci. 1991, 104, 203–208. [Google Scholar] [CrossRef]
- Flønes, I.H.; Toker, L.; Sandnes, D.A.; Castelli, M.; Mostafavi, S.; Lura, N.; Shadad, O.; Fernandez-Vizarra, E.; Painous, C.; Pérez-Soriano, A.; et al. Mitochondrial Complex I Deficiency Stratifies Idiopathic Parkinson’s Disease. Nat. Commun. 2024, 15, 3631. [Google Scholar] [CrossRef]
- Narendra, D.P.; Youle, R.J. The Role of PINK1–Parkin in Mitochondrial Quality Control. Nat. Cell Biol. 2024, 26, 1639–1651. [Google Scholar] [CrossRef]
- Valente, E.M.; Abou-Sleiman, P.M.; Caputo, V.; Muqit, M.M.K.; Harvey, K.; Gispert, S.; Ali, Z.; Del Turco, D.; Bentivoglio, A.R.; Healy, D.G.; et al. Hereditary Early-Onset Parkinson’s Disease Caused by Mutations in PINK1. Science 2004, 304, 1158–1160. [Google Scholar] [CrossRef]
- Kitada, T.; Asakawa, S.; Hattori, N.; Matsumine, H.; Yamamura, Y.; Minoshima, S.; Yokochi, M.; Mizuno, Y.; Shimizu, N. Mutations in the Parkin Gene Cause Autosomal Recessive Juvenile Parkinsonism. Nature 1998, 392, 605–608. [Google Scholar] [CrossRef]
- Wu, W.; Xu, H.; Wang, Z.; Mao, Y.; Yuan, L.; Luo, W.; Cui, Z.; Cui, T.; Wang, X.L.; Shen, Y.H. PINK1-Parkin-Mediated Mitophagy Protects Mitochondrial Integrity and Prevents Metabolic Stress-Induced Endothelial Injury. PLoS ONE 2015, 10, e0132499. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, Q.A. Redox Modulatory Role of DJ-1 in Parkinson’s Disease. Biogerontology 2025, 26, 81. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Wang, Y.; Guo, D.; Wang, G.; Ren, H. Familial Parkinson’s Disease-Associated L166P Mutant DJ-1 Is Cleaved by Mitochondrial Serine Protease Omi/HtrA2. Neurosci. Bull. 2017, 33, 685–694. [Google Scholar] [CrossRef]
- Mullin, S.; Schapira, A. α-Synuclein and Mitochondrial Dysfunction in Parkinson’s Disease. Mol. Neurobiol. 2013, 47, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Pozo Devoto, V.M.; Falzone, T.L. Mitochondrial Dynamics in Parkinson’s Disease: A Role for α-Synuclein? Dis. Models Mech. 2017, 10, 1075–1087. [Google Scholar] [CrossRef] [PubMed]
- Frost, B.; Jacks, R.L.; Diamond, M.I. Propagation of Tau Misfolding from the Outside to the Inside of a Cell. J. Biol. Chem. 2009, 284, 12845–12852. [Google Scholar] [CrossRef]
- Borowski, L.S.; Dziembowski, A.; Hejnowicz, M.S.; Stepien, P.P.; Szczesny, R.J. Human Mitochondrial RNA Decay Mediated by PNPase–hSuv3 Complex Takes Place in Distinct Foci. Nucleic Acids Res. 2013, 41, 1223–1240. [Google Scholar] [CrossRef]
- Santonoceto, G.; Jurkiewicz, A.; Szczesny, R.J. RNA Degradation in Human Mitochondria: The Journey Is Not Finished. Hum. Mol. Genet. 2024, 33, R26–R33. [Google Scholar] [CrossRef]
- Feric, M.; Sarfallah, A.; Dar, F.; Temiakov, D.; Pappu, R.V.; Misteli, T. Mesoscale Structure–Function Relationships in Mitochondrial Transcriptional Condensates. Proc. Natl. Acad. Sci. USA 2022, 119, e2207303119. [Google Scholar] [CrossRef]
- Sasaki, T.; Sato, Y.; Higashiyama, T.; Sasaki, N. Live Imaging Reveals the Dynamics and Regulation of Mitochondrial Nucleoids during the Cell Cycle in Fucci2-HeLa Cells. Sci. Rep. 2017, 7, 11257. [Google Scholar] [CrossRef]
- Rostam, N.; Ghosh, S.; Chow, C.F.W.; Hadarovich, A.; Landerer, C.; Ghosh, R.; Moon, H.; Hersemann, L.; Mitrea, D.M.; Klein, I.A.; et al. CD-CODE: Crowdsourcing Condensate Database and Encyclopedia. Nat. Methods 2023, 20, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Lopez Sanchez, M.I.G.; Krüger, A.; Shiriaev, D.I.; Liu, Y.; Rorbach, J. Human Mitoribosome Biogenesis and Its Emerging Links to Disease. Int. J. Mol. Sci. 2021, 22, 3827. [Google Scholar] [CrossRef]
- Sylvester, J.E.; Fischel-Ghodsian, N.; Mougey, E.B.; O’brien, T.W. Mitochondrial Ribosomal Proteins: Candidate Genes for Mitochondrial Disease. Genet. Med. 2004, 6, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Werner, E.; Gokhale, A.; Ackert, M.; Xu, C.; Wen, Z.; Roberts, A.M.; Roberts, B.R.; Vrailas-Mortimer, A.; Crocker, A.; Faundez, V. The Mitochondrial RNA Granule Modulates Manganese-Dependent Cell Toxicity. Mol. Biol. Cell 2022, 33, ar108. [Google Scholar] [CrossRef]
- Popow, J.; Alleaume, A.-M.; Curk, T.; Schwarzl, T.; Sauer, S.; Hentze, M.W. FASTKD2 Is an RNA-Binding Protein Required for Mitochondrial RNA Processing and Translation. RNA 2015, 21, 1873–1884. [Google Scholar] [CrossRef]
- Antonicka, H.; Shoubridge, E.A. Mitochondrial RNA Granules Are Centers for Posttranscriptional RNA Processing and Ribosome Biogenesis. Cell Rep. 2015, 10, 920–932. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Mao, L.; Chen, C.; Yin, F.; Peng, J. A Novel Homozygous Missense Mutation in the FASTKD2 Gene Leads to Lennox-Gastaut Syndrome. J. Hum. Genet. 2022, 67, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.-T.; Barrientos, A. The Human Mitochondrial DEAD-Box Protein DDX28 Resides in RNA Granules and Functions in Mitoribosome Assembly. Cell Rep. 2015, 10, 854–864. [Google Scholar] [CrossRef]
- Hikiami, R.; Morimura, T.; Ayaki, T.; Tsukiyama, T.; Morimura, N.; Kusui, M.; Wada, H.; Minamiyama, S.; Shodai, A.; Asada-Utsugi, M.; et al. Conformational Change of RNA-Helicase DHX30 by ALS/FTD-Linked FUS Induces Mitochondrial Dysfunction and Cytosolic Aggregates. Sci. Rep. 2022, 12, 16030. [Google Scholar] [CrossRef]
- Zaganelli, S.; Rebelo-Guiomar, P.; Maundrell, K.; Rozanska, A.; Pierredon, S.; Powell, C.A.; Jourdain, A.A.; Hulo, N.; Lightowlers, R.N.; Chrzanowska-Lightowlers, Z.M.; et al. The Pseudouridine Synthase RPUSD4 Is an Essential Component of Mitochondrial RNA Granules. J. Biol. Chem. 2017, 292, 4519–4532. [Google Scholar] [CrossRef] [PubMed]
- Jourdain, A.A.; Koppen, M.; Rodley, C.D.; Maundrell, K.; Gueguen, N.; Reynier, P.; Guaras, A.M.; Enriquez, J.A.; Anderson, P.; Simarro, M.; et al. A Mitochondria-Specific Isoform of FASTK Is Present In Mitochondrial RNA Granules and Regulates Gene Expression and Function. Cell Rep. 2015, 10, 1110–1121. [Google Scholar] [CrossRef]
- Susanto, T.T.; Hung, V.; Levine, A.G.; Chen, Y.; Kerr, C.H.; Yoo, Y.; Oses-Prieto, J.A.; Fromm, L.; Zhang, Z.; Lantz, T.C.; et al. RAPIDASH: Tag-Free Enrichment of Ribosome-Associated Proteins Reveals Composition Dynamics in Embryonic Tissue, Cancer Cells, and Macrophages. Mol. Cell 2024, 84, 3545–3563.e25. [Google Scholar] [CrossRef]
- Lessel, D.; Schob, C.; Küry, S.; Reijnders, M.R.F.; Harel, T.; Eldomery, M.K.; Coban-Akdemir, Z.; Denecke, J.; Edvardson, S.; Colin, E.; et al. De Novo Missense Mutations in DHX30 Impair Global Translation and Cause a Neurodevelopmental Disorder. Am. J. Hum. Genet. 2017, 101, 716–724. [Google Scholar] [CrossRef]
- Alomaim, M.M.; Mushiba, A.M. A Novel De Novo Mutation of the DHX30 Gene in a Patient With Neurodevelopmental Disorder, Severe Motor Impairment, and Absent Language (NEDMIAL). Cureus 2023, 15, e33682. [Google Scholar] [CrossRef]
- Cross, L.A.; McWalter, K.; Keller-Ramey, J.; Henderson, L.B.; Amudhavalli, S.M. A Report of Gonadal Mosaicism in DHX30-Related Neurodevelopmental Disorder. Clin. Dysmorphol. 2020, 29, 161–164. [Google Scholar] [CrossRef]
- Lederbauer, J.; Das, S.; Piton, A.; Lessel, D.; Kreienkamp, H.-J. The Role of DEAD- and DExH-Box RNA Helicases in Neurodevelopmental Disorders. Front. Mol. Neurosci. 2024, 17, 1414949. [Google Scholar] [CrossRef]
- Weis, K. Dead or Alive: DEAD-Box ATPases as Regulators of Ribonucleoprotein Complex Condensation. Biol. Chem. 2021, 402, 653–661. [Google Scholar] [CrossRef]
- Redder, P.; Hausmann, S.; Khemici, V.; Yasrebi, H.; Linder, P. Bacterial Versatility Requires DEAD-Box RNA Helicases. FEMS Microbiol. Rev. 2015, 39, 392–412. [Google Scholar] [CrossRef]
- Nandana, V.; Schrader, J.M. Roles of Liquid–Liquid Phase Separation in Bacterial RNA Metabolism. Curr. Opin. Microbiol. 2021, 61, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Zimorski, V.; Ku, C.; Martin, W.F.; Gould, S.B. Endosymbiotic Theory for Organelle Origins. Curr. Opin. Microbiol. 2014, 22, 38–48. [Google Scholar] [CrossRef]
- Ku, C.; Nelson-Sathi, S.; Roettger, M.; Garg, S.; Hazkani-Covo, E.; Martin, W.F. Endosymbiotic Gene Transfer from Prokaryotic Pangenomes: Inherited Chimerism in Eukaryotes. Proc. Natl. Acad. Sci. USA 2015, 112, 10139–10146. [Google Scholar] [CrossRef] [PubMed]
- Uchiumi, T.; Ohgaki, K.; Yagi, M.; Aoki, Y.; Sakai, A.; Matsumoto, S.; Kang, D. ERAL1 Is Associated with Mitochondrial Ribosome and Elimination of ERAL1 Leads to Mitochondrial Dysfunction and Growth Retardation. Nucleic Acids Res. 2010, 38, 5554–5568. [Google Scholar] [CrossRef]
- Li, S.; Kuang, M.; Chen, L.; Li, Y.; Liu, S.; Du, H.; Cao, L.; You, F. The Mitochondrial Protein ERAL1 Suppresses RNA Virus Infection by Facilitating RIG-I-like Receptor Signaling. Cell Rep. 2021, 34, 108631. [Google Scholar] [CrossRef]
- Chatzispyrou, I.A.; Alders, M.; Guerrero-Castillo, S.; Zapata Perez, R.; Haagmans, M.A.; Mouchiroud, L.; Koster, J.; Ofman, R.; Baas, F.; Waterham, H.R.; et al. A Homozygous Missense Mutation in ERAL1, Encoding a Mitochondrial rRNA Chaperone, Causes Perrault Syndrome. Hum. Mol. Genet. 2017, 26, 2541–2550. [Google Scholar] [CrossRef] [PubMed]
- Fong, K.; Li, Y.; Wang, W.; Ma, W.; Li, K.; Qi, R.Z.; Liu, D.; Songyang, Z.; Chen, J. Whole-Genome Screening Identifies Proteins Localized to Distinct Nuclear Bodies. J. Cell Biol. 2013, 203, 149–164. [Google Scholar] [CrossRef]
- Sayed, A.; Matsuyama, S.; Inouye, M. Era, an Essential Escherichia Coli Small G-Protein, Binds to the 30S Ribosomal Subunit. Biochem. Biophys. Res. Commun. 1999, 264, 51–54. [Google Scholar] [CrossRef]
- Gollop, N.; March, P.E. A GTP-Binding Protein (Era) Has an Essential Role in Growth Rate and Cell Cycle Control in Escherichia Coli. J. Bacteriol. 1991, 173, 2265–2270. [Google Scholar] [CrossRef]
- Gruffaz, C.; Smirnov, A. GTPase Era at the Heart of Ribosome Assembly. Front. Mol. Biosci. 2023, 10, 1263433. [Google Scholar] [CrossRef] [PubMed]
- Jourdain, A.A.; Boehm, E.; Maundrell, K.; Martinou, J.-C. Mitochondrial RNA Granules: Compartmentalizing Mitochondrial Gene Expression. J. Cell Biol. 2016, 212, 611–614. [Google Scholar] [CrossRef]
- Pearce, S.F.; Rebelo-Guiomar, P.; D’Souza, A.R.; Powell, C.A.; Van Haute, L.; Minczuk, M. Regulation of Mammalian Mitochondrial Gene Expression: Recent Advances. Trends Biochem. Sci. 2017, 42, 625–639. [Google Scholar] [CrossRef]
- Rorbach, J.; Boesch, P.; Gammage, P.A.; Nicholls, T.J.J.; Pearce, S.F.; Patel, D.; Hauser, A.; Perocchi, F.; Minczuk, M. MRM2 and MRM3 Are Involved in Biogenesis of the Large Subunit of the Mitochondrial Ribosome. Mol. Biol. Cell 2014, 25, 2542–2555. [Google Scholar] [CrossRef] [PubMed]
- Park, C.B.; Asin-Cayuela, J.; Cámara, Y.; Shi, Y.; Pellegrini, M.; Gaspari, M.; Wibom, R.; Hultenby, K.; Erdjument-Bromage, H.; Tempst, P.; et al. MTERF3 Is a Negative Regulator of Mammalian mtDNA Transcription. Cell 2007, 130, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Wredenberg, A.; Lagouge, M.; Bratic, A.; Metodiev, M.D.; Spåhr, H.; Mourier, A.; Freyer, C.; Ruzzenente, B.; Tain, L.; Grönke, S.; et al. MTERF3 Regulates Mitochondrial Ribosome Biogenesis in Invertebrates and Mammals. PLoS Genet. 2013, 9, e1003178. [Google Scholar] [CrossRef]
- Zi, J.; Wang, W.; Sun, M.; Mei, W.; Li, S.; Li, B.; Xiao, Y.; Fei, Z.; Zhang, R.; Yu, M.; et al. A High Expression of MTERF3 Correlates with Tumor Progression and Predicts Poor Outcomes in Patients with Brain Glioma. Int. J. Clin. Exp. Pathol. 2019, 12, 1909–1920. [Google Scholar]
- Duan, R.; Mahlatsi, R.L.; Wang, Y.; Xu, C.; Wang, M.; Zou, Z.; Liu, Z.; Jiang, H.; Duan, X.; Deng, J.; et al. Novel Mutations in MTERF3: First Report of a New Genetic Cause in Two Chinese Patients with Developmental Delay, Intermittent Hypoglycemia and Metabolic Acidosis. Mitochondrion 2025, 85, 102059. [Google Scholar] [CrossRef]
- Liu, X.; Shen, S.; Wu, P.; Li, F.; Liu, X.; Wang, C.; Gong, Q.; Wu, J.; Yao, X.; Zhang, H.; et al. Structural Insights into Dimethylation of 12S rRNA by TFB1M: Indispensable Role in Translation of Mitochondrial Genes and Mitochondrial Function. Nucleic Acids Res. 2019, 47, 7648–7665. [Google Scholar] [CrossRef]
- Koeck, T.; Olsson, A.H.; Nitert, M.D.; Sharoyko, V.V.; Ladenvall, C.; Kotova, O.; Reiling, E.; Rönn, T.; Parikh, H.; Taneera, J.; et al. A Common Variant in TFB1M Is Associated with Reduced Insulin Secretion and Increased Future Risk of Type 2 Diabetes. Cell Metab. 2011, 13, 80–91. [Google Scholar] [CrossRef]
- Sharoyko, V.V.; Abels, M.; Sun, J.; Nicholas, L.M.; Mollet, I.G.; Stamenkovic, J.A.; Göhring, I.; Malmgren, S.; Storm, P.; Fadista, J.; et al. Loss of TFB1M Results in Mitochondrial Dysfunction That Leads to Impaired Insulin Secretion and Diabetes. Hum. Mol. Genet. 2014, 23, 5733–5749. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Tian, Y.; Liu, F.; Wang, Z.; Tan, R.; Zhang, B.; Quan, P.; Zhang, H.; Yang, J.; Yuan, P. Mitochondrial Transcription Factor B1 Promotes the Progression of Hepatocellular Carcinoma via Enhancing Aerobic Glycolysis. J. Cell Commun. Signal. 2022, 16, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Abyzov, A.; Blackledge, M.; Zweckstetter, M. Conformational Dynamics of Intrinsically Disordered Proteins Regulate Biomolecular Condensate Chemistry. Chem. Rev. 2022, 122, 6719–6748. [Google Scholar] [CrossRef]
- Shi, M.; Wu, Z.; Zhang, Y.; Li, T. Decoding Intrinsically Disordered Regions in Biomolecular Condensates. Fundam. Res. 2025; in press. [Google Scholar] [CrossRef]
- Cascarina, S.M.; Elder, M.R.; Ross, E.D. Atypical Structural Tendencies among Low-Complexity Domains in the Protein Data Bank Proteome. PLoS Comput. Biol. 2020, 16, e1007487. [Google Scholar] [CrossRef]
- Cascarina, S.M.; Ross, E.D. Identification of Low-Complexity Domains by Compositional Signatures Reveals Class-Specific Frequencies and Functions Across the Domains of Life. PLoS Comput. Biol. 2024, 20, e1011372. [Google Scholar] [CrossRef]
- Lee, J.; Cho, H.; Kwon, I. Phase Separation of Low-Complexity Domains in Cellular Function and Disease. Exp. Mol. Med. 2022, 54, 1412–1422. [Google Scholar] [CrossRef]
- Garabedian, M.V.; Su, Z.; Dabdoub, J.; Tong, M.; Deiters, A.; Hammer, D.A.; Good, M.C. Protein Condensate Formation via Controlled Multimerization of Intrinsically Disordered Sequences. Biochemistry 2022, 61, 2470–2481. [Google Scholar] [CrossRef]
- O’Connell, L.C.; Johnson, V.; Otis, J.P.; Hutton, A.K.; Murthy, A.C.; Liang, M.C.; Wang, S.-H.; Fawzi, N.L.; Mowry, K.L. Intrinsically Disordered Regions and RNA Binding Domains Contribute to Protein Enrichment in Biomolecular Condensates in Xenopus Oocytes. Sci. Rep. 2024, 14, 27890. [Google Scholar] [CrossRef] [PubMed]
- Borcherds, W.; Bremer, A.; Borgia, M.B.; Mittag, T. How Do Intrinsically Disordered Protein Regions Encode a Driving Force for Liquid–Liquid Phase Separation? Curr. Opin. Struct. Biol. 2021, 67, 41–50. [Google Scholar] [CrossRef]
- Utami, K.H.; Morimoto, S.; Mitsukura, Y.; Okano, H. The Roles of Intrinsically Disordered Proteins in Neurodegeneration. Biochim. et Biophys. Acta (BBA) Gen. Subj. 2025, 1869, 130772. [Google Scholar] [CrossRef]
- Coskuner, O.; Uversky, V.N. Intrinsically Disordered Proteins in Various Hypotheses on the Pathogenesis of Alzheimer’s and Parkinson’s Diseases. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2019; Volume 166, pp. 145–223. ISBN 978-0-12-816851-6. [Google Scholar]
- Johnson, B.S.; Snead, D.; Lee, J.J.; McCaffery, J.M.; Shorter, J.; Gitler, A.D. TDP-43 Is Intrinsically Aggregation-Prone, and Amyotrophic Lateral Sclerosis-Linked Mutations Accelerate Aggregation and Increase Toxicity. J. Biol. Chem. 2009, 284, 20329–20339. [Google Scholar] [CrossRef]
- Greb-Markiewicz, B.; Zarębski, M.; Ożyhar, A. Multiple Sequences Orchestrate Subcellular Trafficking of Neuronal PAS Domain–Containing Protein 4 (NPAS4). J. Biol. Chem. 2018, 293, 11255–11270. [Google Scholar] [CrossRef]
- Raychaudhuri, S.; Dey, S.; Bhattacharyya, N.P.; Mukhopadhyay, D. The Role of Intrinsically Unstructured Proteins in Neurodegenerative Diseases. PLoS ONE 2009, 4, e5566. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Vicente, M.; Talloczy, Z.; Wong, E.; Tang, G.; Koga, H.; Kaushik, S.; De Vries, R.; Arias, E.; Harris, S.; Sulzer, D.; et al. Cargo Recognition Failure Is Responsible for Inefficient Autophagy in Huntington’s Disease. Nat. Neurosci. 2010, 13, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Ganne, A.; Balasubramaniam, M.; Ayyadevara, S.; Shmookler Reis, R.J. Machine-Learning Analysis of Intrinsically Disordered Proteins Identifies Key Factors That Contribute to Neurodegeneration-Related Aggregation. Front. Aging Neurosci. 2022, 14, 938117. [Google Scholar] [CrossRef]
- Ito, M.; Tohsato, Y.; Sugisawa, H.; Kohara, S.; Fukuchi, S.; Nishikawa, I.; Nishikawa, K. Intrinsically Disordered Proteins in Human Mitochondria. Genes Cells 2012, 17, 817–825. [Google Scholar] [CrossRef]
- Nesterov, S.V.; Ilyinsky, N.S.; Plokhikh, K.S.; Manuylov, V.D.; Chesnokov, Y.M.; Vasilov, R.G.; Kuznetsova, I.M.; Turoverov, K.K.; Gordeliy, V.I.; Fonin, A.V.; et al. Corrigendum to “Order Wrapped in Chaos: On the Roles of Intrinsically Disordered Proteins and RNAs in the Arrangement of the Mitochondrial Enzymatic Machines” [International Journal of Biomacromolecules, 267(2024), 131,455]. Int. J. Biol. Macromol. 2024, 272, 132849. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Z.; He, X.; Pu, S.; Yang, C.; Wu, Q.; Zhou, Z.; Cen, X.; Zhao, H. Mitochondrial Protein Dysfunction in Pathogenesis of Neurological Diseases. Front. Mol. Neurosci. 2022, 15, 974480. [Google Scholar] [CrossRef] [PubMed]
- Rath, S.; Sharma, R.; Gupta, R.; Ast, T.; Chan, C.; Durham, T.J.; Goodman, R.P.; Grabarek, Z.; Haas, M.E.; Hung, W.H.W.; et al. MitoCarta3.0: An Updated Mitochondrial Proteome Now with Sub-Organelle Localization and Pathway Annotations. Nucleic Acids Res. 2021, 49, D1541–D1547. [Google Scholar] [CrossRef]
- Erdős, G.; Dosztányi, Z. Analyzing Protein Disorder with IUPred2A. Curr. Protoc. Bioinform. 2020, 70, e99. [Google Scholar] [CrossRef]
- Mészáros, B.; Erdős, G.; Dosztányi, Z. IUPred2A: Context-Dependent Prediction of Protein Disorder as a Function of Redox State and Protein Binding. Nucleic Acids Res. 2018, 46, W329–W337. [Google Scholar] [CrossRef] [PubMed]
- Bayés, À.; Collins, M.O.; Croning, M.D.R.; Van De Lagemaat, L.N.; Choudhary, J.S.; Grant, S.G.N. Comparative Study of Human and Mouse Postsynaptic Proteomes Finds High Compositional Conservation and Abundance Differences for Key Synaptic Proteins. PLoS ONE 2012, 7, e46683. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.; Muruganujan, A.; Casagrande, J.T.; Thomas, P.D. Large-Scale Gene Function Analysis with the PANTHER Classification System. Nat. Protoc. 2013, 8, 1551–1566. [Google Scholar] [CrossRef]
- Moriya, Y.; Itoh, M.; Okuda, S.; Yoshizawa, A.C.; Kanehisa, M. KAAS: An Automatic Genome Annotation and Pathway Reconstruction Server. Nucleic Acids Res. 2007, 35, W182–W185. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. KEGG for Taxonomy-Based Analysis of Pathways and Genomes. Nucleic Acids Res. 2023, 51, D587–D592. [Google Scholar] [CrossRef]
- Lopez, N.; Camporeale, G.; Salgueiro, M.; Borkosky, S.S.; Visentín, A.; Peralta-Martinez, R.; Loureiro, M.E.; De Prat-Gay, G. Deconstructing Virus Condensation. PLoS Pathog. 2021, 17, e1009926. [Google Scholar] [CrossRef]
- Sagan, S.M.; Weber, S.C. Let’s Phase It: Viruses Are Master Architects of Biomolecular Condensates. Trends Biochem. Sci. 2023, 48, 229–243. [Google Scholar] [CrossRef]
- Kaundal, S.; Anish, R.; Ayyar, B.V.; Shanker, S.; Kaur, G.; Crawford, S.E.; Pollet, J.; Stossi, F.; Estes, M.K.; Prasad, B.V.V. RNA-Dependent RNA Polymerase of Predominant Human Norovirus Forms Liquid-Liquid Phase Condensates as Viral Replication Factories. Sci. Adv. 2024, 10, eadp9333. [Google Scholar] [CrossRef]
- Lu, S.; Ye, Q.; Singh, D.; Cao, Y.; Diedrich, J.K.; Yates, J.R.; Villa, E.; Cleveland, D.W.; Corbett, K.D. The SARS-CoV-2 Nucleocapsid Phosphoprotein Forms Mutually Exclusive Condensates with RNA and the Membrane-Associated M Protein. Nat. Commun. 2021, 12, 502. [Google Scholar] [CrossRef]
- Savastano, A.; Ibáñez De Opakua, A.; Rankovic, M.; Zweckstetter, M. Nucleocapsid Protein of SARS-CoV-2 Phase Separates into RNA-Rich Polymerase-Containing Condensates. Nat. Commun. 2020, 11, 6041. [Google Scholar] [CrossRef]
- Nagies, F.S.P.; Brueckner, J.; Tria, F.D.K.; Martin, W.F. A Spectrum of Verticality across Genes. PLoS Genet. 2020, 16, e1009200. [Google Scholar] [CrossRef]
- Martin, W.F.; Kleinermanns, K. The Geochemical Origin of Microbes, 1st ed.; CRC Press: Boca Raton, FL, USA, 2024; ISBN 978-1-003-37861-7. [Google Scholar]
- Wächtershäuser, G. Towards a Reconstruction of Ancestral Genomes by Gene Cluster Alignment. Syst. Appl. Microbiol. 1998, 21, 473–477. [Google Scholar] [CrossRef]
- Stoebe, B.; Kowallik, K.V. Gene-Cluster Analysis in Chloroplast Genomics. Trends Genet. 1999, 15, 344–347. [Google Scholar] [CrossRef]
- Wang, J.; Dasgupta, I.; Fox, G.E. Many Nonuniversal Archaeal Ribosomal Proteins Are Found in Conserved Gene Clusters. Archaea 2009, 2, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Timmis, J.N.; Ayliffe, M.A.; Huang, C.Y.; Martin, W. Endosymbiotic Gene Transfer: Organelle Genomes Forge Eukaryotic Chromosomes. Nat. Rev. Genet. 2004, 5, 123–135. [Google Scholar] [CrossRef]
- Rangachari, V. Biomolecular Condensates—Extant Relics or Evolving Microcompartments? Commun. Biol. 2023, 6, 656. [Google Scholar] [CrossRef] [PubMed]
- Schnorr, W.E.; Childers, W.S. Biomolecular Condensates: From Bacterial Compartments to Incubator Spaces of Emergent Chemical Systems in Matter-to-Life Transitions. ChemSystemsChem 2024, 6, e202400011. [Google Scholar] [CrossRef]
- Basile, W.; Salvatore, M.; Bassot, C.; Elofsson, A. Why Do Eukaryotic Proteins Contain More Intrinsically Disordered Regions? PLoS Comput. Biol. 2019, 15, e1007186. [Google Scholar] [CrossRef] [PubMed]

| Gene Name (Uniprot ID) | BCs | Associated/Candidate Disorder | Ref. |
|---|---|---|---|
| DHX30 (Q7L2E3) | mtRNA granule Stress granule P-body PCPD 1 | Developmental delay Intellectual disability Muscular hypotonia Gait abnormalities Motor and cognitive delay Congenital clasped thumbs Unilateral undescended testicles | [33,139] |
| DDX28 (Q9NUL7) | mtRNA granule Stress granule | Development and prognosis of colorectal cancer# | [33,139] |
| ERAL1 (O75616) | mtRNA granule | Perrault syndrome | [33,139] |
| FASTKD2 (Q9NYY8) | mtRNA granule | Mitochondrial encephalopathy Late age onset autosomal recessive MELAS-like syndrome with optic atrophy mitochondrial encephalomyopathy and hypertrophic cardiomyopathy # * Pancreatic ductal adenocarcinoma prognosis AD # * | [33,139] |
| MRM2 (Q9UI43) | mtRNA granule | MELAS Non-small cell lung cancer# | [33,139] |
| MRPS31 (Q92665) | mtRNA granule | Moebius syndrome 1 | [33,140] |
| MRPS7 (Q9Y2R9) | mtRNA granule | Neuritis, with brachial predilection Congenital sensorineural deafness Progressive hepatic and renal failure Lactic acidemia | [33,139,140] |
| MRPS9 (P82933) | mtRNA granule | Intellectual disability and dysmorphic features | [33,139] |
| MTERF3 (Q96E29) | mtRNA granule | Multiple cancers * | [33,139] |
| TFB1M (Q8WVM0) | mtRNA granule | Type 2 diabetes risk * | [33,139] |
| MRPS15 (P82914) | Nucleolus | Deafness, autosomal dominant nonsyndromic sensorineural 3rd Stuve-Wiedemann syndrome | [33,140] |
| Genes | Presynaptic Clusters & Postsynaptic Densities | Stress Granule | Nucleolus | Centrosome | P-Body | mtRNA Granule | Other |
|---|---|---|---|---|---|---|---|
| ABCB8 | + 1 | ||||||
| AKAP1 1 | |||||||
| ALDH3A2 | + 2 | + | |||||
| ATAD3A | + | + | + | ||||
| ATAD3B | + | ||||||
| BBC3 | + | ||||||
| BCL2L13 1 | |||||||
| CHCHD6 | + 1 | ||||||
| DLAT | + 1 | + | |||||
| ELAC2 | PcG body | ||||||
| ERAL1 | + | ||||||
| FASTK | + | + | |||||
| GLS | + 1 | ||||||
| GPX4 | + 1 | ||||||
| HSPA9 | + 2 | + | + | + | + | ||
| IDH2 | + 1 | + | |||||
| IMMT | + 1 | ||||||
| LETM1 | + 1 | ||||||
| LIG3 | + | + | |||||
| LONP1 | + 1 | + | |||||
| MTCH1 | + 1 | ||||||
| MTHFD1L | + 1 | + | |||||
| MTX1 | + 1 | + | |||||
| NBR1 | P62 cluster | ||||||
| NOA1 | + | ||||||
| NSUN2 | + | ||||||
| OGG1 | + | + | Nuclear speckle | ||||
| OXR1 | + 1 | ||||||
| PDHX | + 1 | ||||||
| PNKD | + 1 | ||||||
| PTCD1 | + | ||||||
| PUS1 | + | ||||||
| SCO1 | + | ||||||
| SPHK2 | + | ||||||
| SPHKAP 1 | + 3 | ||||||
| SPIRE1 | + 1 | ||||||
| SUPV3L1 | + | ||||||
| TIMM44 | + | ||||||
| TOMM40 | + | ||||||
| TOMM70 | + 1 |
| KEGG Pathway | Genes |
|---|---|
| 4137 Mitophagy–animal (7) 1 | TOMM40 (K11518), BNIP3 (K15464), BNIP3L (K15465), BCL2L13 (K15485), TOMM70 (K17768), MTX1 (K17776), NBR1 (K17987) |
| 04210 Apoptosis (7) 1 | BAD (K02158), BAX (K02159), MCL1 (K02539), CASP9 (K04399), BBC3 (K10132), BAK1 (K14021), BCL2L11 (K16341) |
| 05200 Pathways in cancer (6) 1 | BAD (K02158), BAX (K02159), CASP9 (K04399), BBC3 (K10132), BAK1 (K14021), BCL2L11 (K16341 |
| 05206 MicroRNAs in cancer (4) 1 | GLS, GLS2 (K01425), MCL1 (K02539), BAK1 (K14021), BCL2L11 (K16341) |
| 05210 Colorectal cancer (6) 1 | BAD (K02158), BAX (K02159), CASP9 (K04399), BBC3 (K10132), BAK1 (K14021), BCL2L11 (K16341) |
| 05010 Alzheimer disease (5) 1 05012 Parkinson disease (5) 1 05014 Amyotrophic lateral sclerosis (8) 1 05016 Huntington disease (6) 1 05022 Pathways of neurodegeneration (8) 1 | UQCRH (K00416) AD, PD, ALS, HD, ND, PRI COX6A2 (K02266) AD, PD, ALS, HD, ND, PRI NDUFV3 (K03944) AD, PD, ALS, HD, ND, PRI CASP9 (K04399) AD, PD, ALS, HD, ND, PRI BAD (K02158) AD, ALS, ND, PRI BAX (K02159) PD, ALS, HD, ND, PRI TOMM40 (K11518) ALS, ND CHCHD10 (K22759) ALS BBC3 (K10132) HD BAK1 (K14021) PD |
| KEGG Brite | Genes |
|---|---|
| ko03000 Transcription factors (1) 1 | CHCHD2 (K22758) |
| ko03019 Messenger RNA biogenesis (2) 1 | HSPA9 (K04043), FASTK (K08290) |
| ko03011 Ribosome (3) 1 | MRPL15 (K02876), MRPS18B (K16174), MRPL46 (K17427) |
| ko03009 Ribosome biogenesis (2) 1 | ERAL1 (K03595), NOA1 (K19832) |
| ko03016 Transfer RNA biogenesis (6) 1 | TRMT1 (K00555), ELAC2 (K00784), TRIT1 (K00791), PUS1 (K06173), NSUN2 (K15335), ATP5MF-PTCD1/PTCD1 (K17710) |
| ko03012 Translation factors (1) 1 | MTERF4 (K15031) |
| ko03110 Chaperones and folding catalysts (5) 1 | (CLPB (K03695), HSPA9 (K04043), DNAJC4 (K09524), SPG7 (K09552), DNAJC30 (K19374) |
| ko04131 Membrane trafficking (7) 1 | SNAP29 (K08509), BNIP3 (K15464), BNIP3L (K15465), NBR1 (K17987), VPS13D (K19527), BLOC1S1 (K20185), PNKD (K23864) |
| ko03032 DNA replication proteins (2) 1 | (POLG (K02332), RECQL4 (K10730) |
| ko03400 DNA repair and recombination proteins (6) 1 | POLQ (K02349), UNG (K03648), OGG1 (K03660), RECQL4 (K10730), LIG3 (K10776), EXD2 (K20777) |
| ko03029 Mitochondrial biogenesis (38) 1 | BAX (K02159), COX11 (K02258), POLG (K02332), ERAL1 (K03595), HSPA9 (K04043), ECSIT (K04405), SCO1 (K07152), ATPAF2 (K07556), LONP1 (K08675), TOMM40 (K11518), BAK1 (K14021), MTERF4 (K15031), BNIP3L (K15465), CHCHD6 (K17564), SUPV3L1 (K17675), TWNK (K17680), ATAD3A, ATAD3B (K17681), ATP5MF-PTCD1,PTCD1 (K17710), TOMM70 (K17768), TOMM22 (K17769), MTX1 (K17776), CHCHD4 (K17782), IMMT (K17785), LETM1 (K17800), TIMM44 (K17804), DNLZ (K17808), MTCH1 (K17885), NBR1 (K17987), COA3 (K18175), NOA1 (K19832), CHCHD10 (K22759), NGRN (K23496), MICOS13 (K24624), OXR1 (K25437), ARMCX2 (K26188), ARMCX6 (K26190), MIGA2 (K27289), PRELID3A (K27966) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calcagnile, M.; Alifano, P.; Damiano, F.; Pontieri, P.; Del Giudice, L. A Perspective on the Role of Mitochondrial Biomolecular Condensates (mtBCs) in Neurodegenerative Diseases and Evolutionary Links to Bacterial BCs. Int. J. Mol. Sci. 2025, 26, 8216. https://doi.org/10.3390/ijms26178216
Calcagnile M, Alifano P, Damiano F, Pontieri P, Del Giudice L. A Perspective on the Role of Mitochondrial Biomolecular Condensates (mtBCs) in Neurodegenerative Diseases and Evolutionary Links to Bacterial BCs. International Journal of Molecular Sciences. 2025; 26(17):8216. https://doi.org/10.3390/ijms26178216
Chicago/Turabian StyleCalcagnile, Matteo, Pietro Alifano, Fabrizio Damiano, Paola Pontieri, and Luigi Del Giudice. 2025. "A Perspective on the Role of Mitochondrial Biomolecular Condensates (mtBCs) in Neurodegenerative Diseases and Evolutionary Links to Bacterial BCs" International Journal of Molecular Sciences 26, no. 17: 8216. https://doi.org/10.3390/ijms26178216
APA StyleCalcagnile, M., Alifano, P., Damiano, F., Pontieri, P., & Del Giudice, L. (2025). A Perspective on the Role of Mitochondrial Biomolecular Condensates (mtBCs) in Neurodegenerative Diseases and Evolutionary Links to Bacterial BCs. International Journal of Molecular Sciences, 26(17), 8216. https://doi.org/10.3390/ijms26178216








