1. Introduction
Most pharmaceuticals currently in use are small organic molecules, and, among them, nitrogen-containing heterocyclic scaffolds have shown very useful biological activities against various diseases [
1]. Moreover,
N-heterocyclic structures are widely distributed in nature, including in amino acids, peptides and proteins; nucleotides, nucleosides and nucleic acids; vitamins and many secondary metabolites [
2]. Almost one-third of the best-selling therapeutics contain fused heterocyclic structures, justifying the high scientific interest and research effort towards these compounds.
N-Fused heterocyclic compounds are basic components of several common pharmaceuticals, agrochemicals, plastics, and dyes [
3,
4].
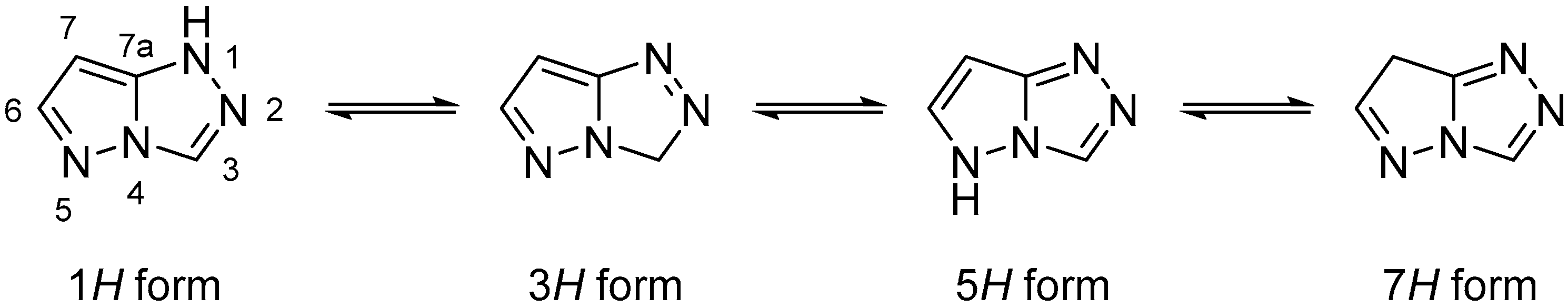
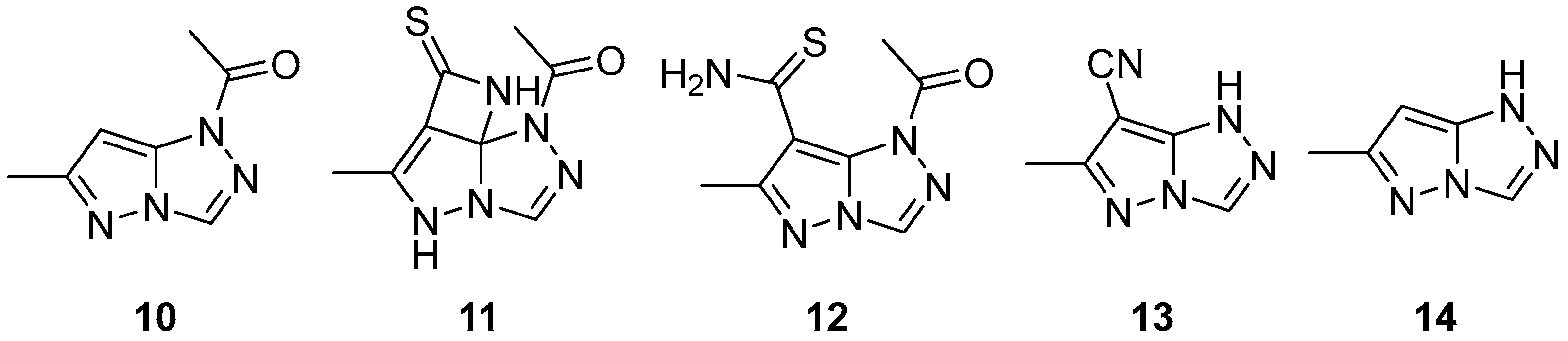
Pyrazolo[5,1-
c][1,2,4]triazoles are a class of N-containing biheterocyclic compounds mainly known for their use in dyes and pigments in a wide variety of fields. The pyrazolo[5,1-
c][1,2,4]triazole system can theoretically exist in four tautomeric forms, namely 1
H, 3
H, 5
H, and 7
H (
Figure 1); the most widely encountered is the 1
H form, while the 5
H form has been reported only in a few compounds, some of which are actually 5-substituted. The remaining two, not fully aromatic forms 3
H and 7
H, have been seen almost exclusively in 3,3- and 7,7-disubstituted compounds, respectively.
The numerous methods reported in the literature for the synthesis of the pyrazolo[5,1-
c][1,2,4]triazole core have been recently reviewed [
5,
6].
The pyrazolo[5,1-c][1,2,4]triazole system combines two frequently used biologically active scaffolds, those of pyrazole and 1,2,4-triazole. Also, as a 5-5 bicyclic system, it is similar in size to other 5-5, 5-6, and 6-6 scaffolds. Not surprisingly, then, a number of biological activity studies have included pyrazolo[5,1-c][1,2,4]triazole derivatives, including several annulated analogs. The aim of the present review is to report all the relevant findings of these studies, in order to highlight the biological potential of this scaffold. Principally, the Reaxys and SciFinder databases were used for the literature search, covering the period between 1988 and 2025, targeting structures containing the pyrazolo[5,1-c][1,2,4]triazole core and the biological activity of these compounds.
Note: This present work is organized based on the biological activity the compounds were tested for, as described below:
Acetylcholinesterase inhibition activity
Anti-inflammatory activity
Analgesic activity
Antidiabetic activity
Antibacterial activity
Antifungal activity
Antiviral activity
Antiprotozoal activity
Anticancer activity
C3a receptor binding activity
Miscellaneous biological activities
Cytotoxicity
Ulcerogenic activity
6. Antibacterial Activity
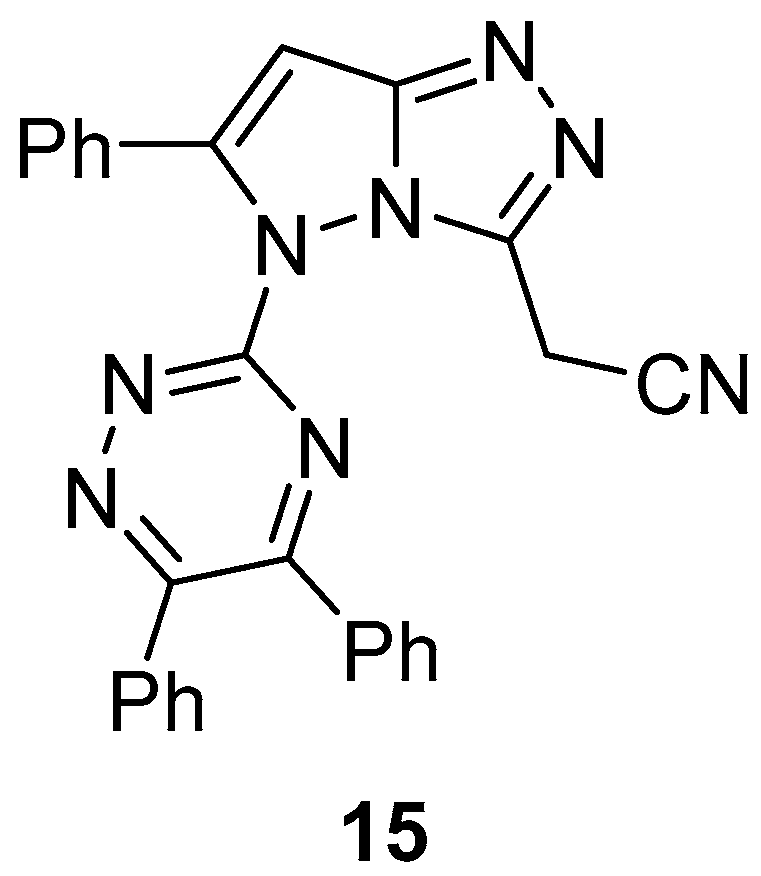
2-[5-(5,6-Diphenyl-1,2,4-triazin-3-yl)-6-phenyl-5
H-pyrazolo[5,1-
c][1,2,4]triazol-3-yl]acetonitrile
15 (
Figure 6) was tested in vitro for antibacterial activity against
Staphylococcus aureus,
Bacillus subtilis,
Escherichia coli, and
Proteus vulgaris and was found to be inactive in all cases [
11].
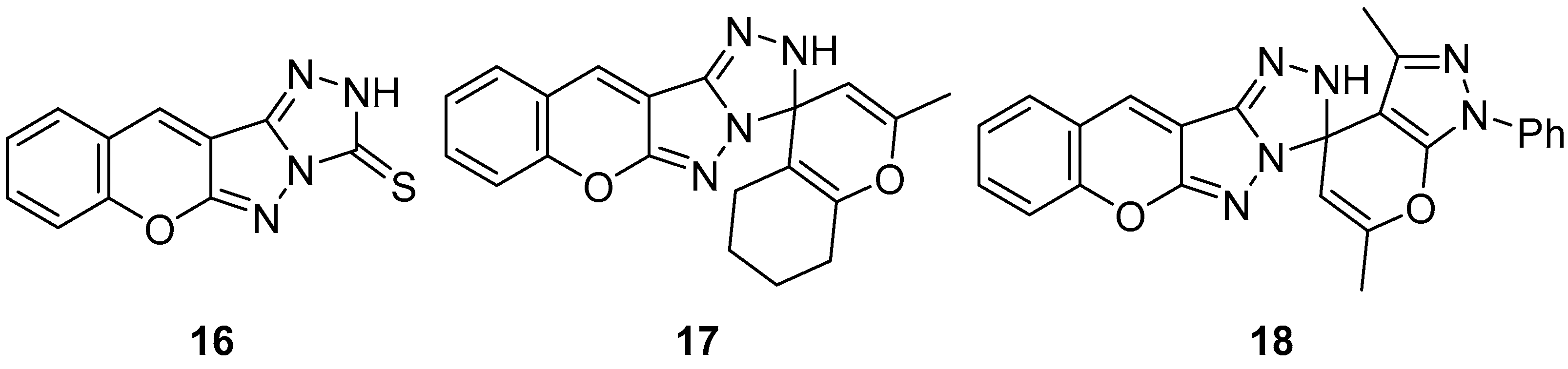
In another study, three chromeno[2′,3′:3,4]pyrazolo[5,1-
c][1,2,4]triazoles
16–
18 (
Figure 7), chromeno[2′,3′:3,4]pyrazolo[5,1-
c][1,2,4]triazole-3(2
H)-thione
16, 2-methyl-5,6,7,8-tetrahydro-2′H-spiro[chromene-4,3′-chromeno[2′,3′:3,4]pyrazolo[5,1-
c][1,2,4]triazole]
17, and 3′,6′-dimethyl-1′-phenyl-1′
H,2
H-spiro[chromeno[2′,3′:3,4]pyrazolo[5,1-
c][1,2,4]triazole-3,4′-pyrano[2,3-
c]pyrazole]
18 were tested in vitro for antibacterial activity against two Gram-positive bacteria,
Bacillus cereus and
Staphylococcus albus, and two Gram-negative bacteria species,
Pseudomonas aeruginosa and
E. coli [
12]. The results are displayed in
Table 7. As can be seen, these compounds showed variable activity against bacteria.
The cytotoxicity of compounds
16–
18 (
Figure 7) was determined on brine shrimp (
Artemia salina) larvae [
12]. The results are shown in
Table 8. Compound
17 has low toxicity, while compounds
16 and
18 are non-toxic.
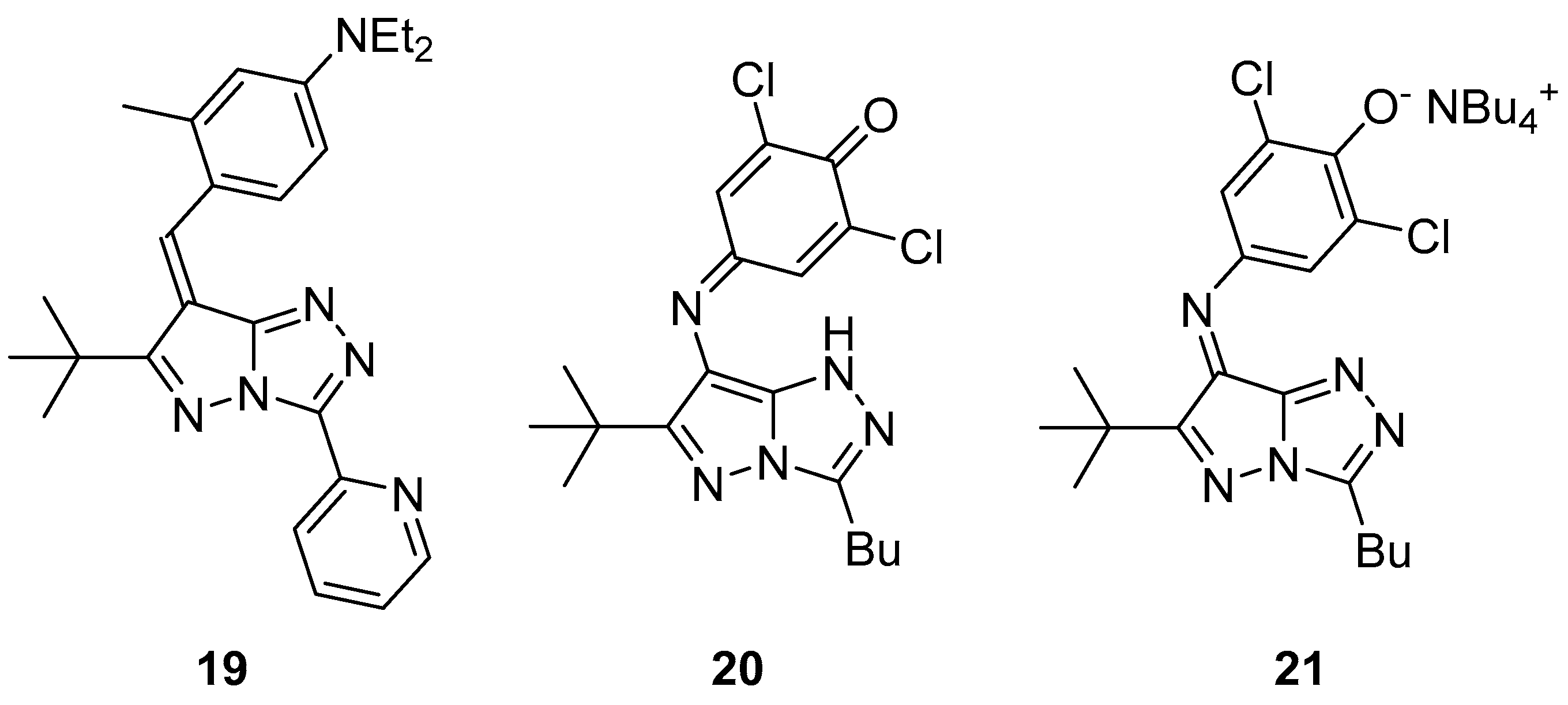
Pyrazolo[5,1-
c][1,2,4]triazoles
19–
21 (
Figure 8), 4-{[6-(1,1-dimethylethyl)-3-(pyridin-2-yl)-7
H-pyrazolo[5,1-
c][1,2,4]triazol-7-ylidene]methyl}-
N,N-diethyl-3-methylaniline
19, 4-{[3-butyl-6-(1,1-dimethylethyl)-1
H-pyrazolo[5,1-
c][1,2,4]triazol-7-yl]imino}-2,6-dichlorocyclohexa-2,5-dien-1-one
20, and tetrabutylammonium 4-{[3-butyl-6-(1,1-dimethylethyl)-7
H-pyrazolo[5,1-
c][1,2,4]triazol-7-ylidene]amino}-2,6-dichlorophenolate
21 were tested for antibacterial activity against
Salmonella typhimurium TA98 and TA100 [
13]. H-1 [Kathon biocide; a 3:1 mixture of 5-chloro-2-methylisothiazol-3(2
H)-one and 2-methylisothiazol-3(2
H)-one)] was used as reference. The results are shown in
Table 9 and
Table 10. It can be seen that compounds
19–
21 have antibacterial activity, while, at the same time, they are less toxic than the reference antibacterial agent (no increase in the number of revertant colonies was observed).
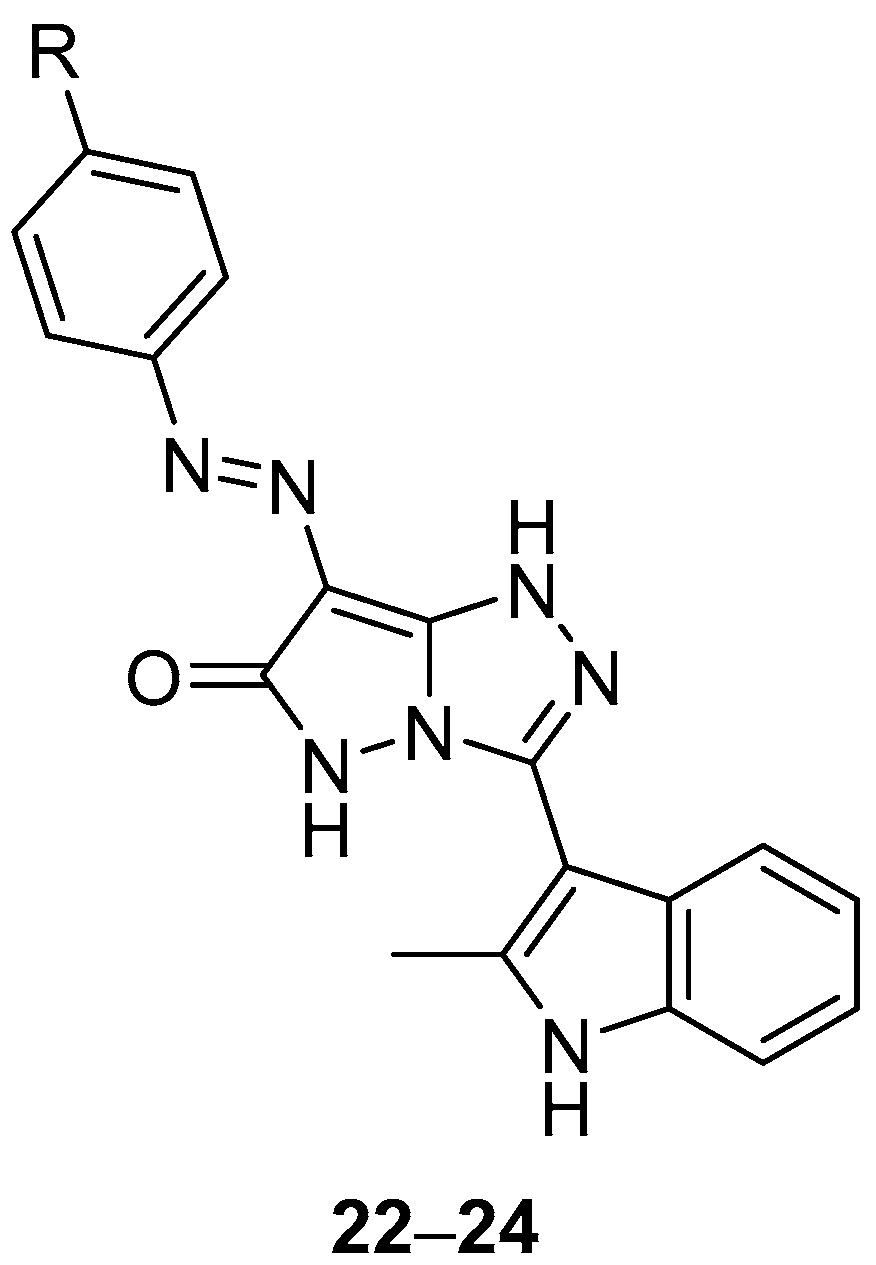
Pyrazolo[5,1-
c][1,2,4]triazol-6(5
H)-ones
22–
24 (
Figure 9), 3-(2-methyl-1
H-indol-3-yl)-7-(phenyldiazenyl)-1
H-pyrazolo[5,1-
c][1,2,4]triazol-6(5
H)-one
22, 3-(2-methyl-1
H-indol-3-yl)-7-(4-methylphenyldiazenyl)-1
H-pyrazolo[5,1-
c][1,2,4]triazol-6(5
H)-one
23 and 7-(4-chlorophenyldiazenyl)-3-(2-methyl-1
H-indol-3-yl)-1
H-pyrazolo[5,1-
c][1,2,4]triazol-6(5
H)-one
24, were also tested in vitro for antibacterial activity against four bacteria,
S. aureus,
P. aeruginosa,
B. subtilis and
E. coli [
14]. Chloramphenicol was used as reference under the same conditions. The results are displayed in
Table 11. Compound
23 exhibited the highest degree of inhibition against
P. aeruginosa and
E. coli, while compound
22 had a high inhibition effect against
P. aeruginosa. Compound
24 showed a high degree of inhibition against
E. coli. However, the activities of the tested compounds are much lower than those of the standard antibacterial agent used.
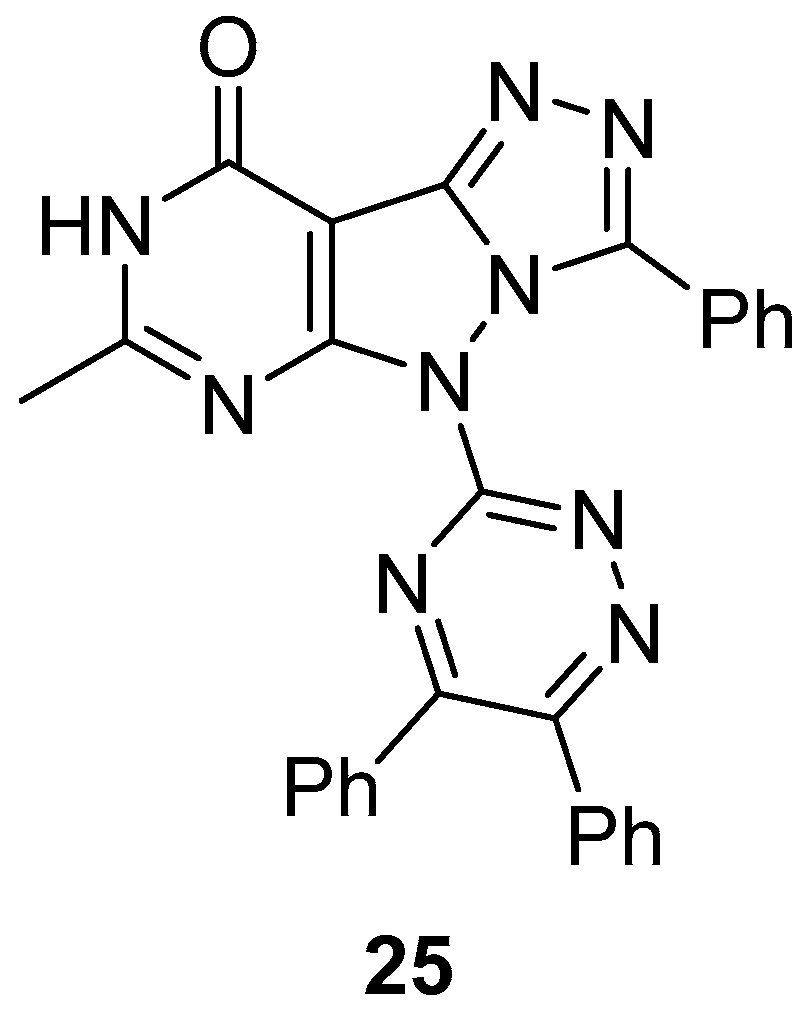
5-(5,6-Diphenyl-1,2,4-triazin-3-yl)-7-methyl-3-phenyl-5H-[1,2,4]triazolo[4′,3′:1,5]pyrazolo[3,4-
d]pyrimidin-9(8
H)-one
25 (
Figure 10) was tested in vitro for antibacterial activity against three bacteria,
S. aureus (MTCCB 737),
Staphylococcus epidermidis (MTCCB 1824), and
E. coli (MTCCB 1652) [
15]. Tetracycline was used as standard drug against bacterial strains at 30 μg/mL concentration. The results are shown in
Table 12. It can be noted that compound
25 showed good inhibition activities against all species of bacterial strains with respect to Tetracycline. The good antibacterial activity was attributed to the presence of the pyrazolo[3,4-
b]pyrimidine scaffold fused with the bioactive heterocyclic moiety of 1,2,4-triazole. The minimum inhibitory concentrations (MIC, μg/mL) of compound
25 against
S. aureus (MTCCB 737) and
E. coli (MTCCB 1652) are shown in
Table 13.
Compound
25 was also tested for cytotoxicity against
A. salina larvae [
15]. Bleomycin and gallic acid were used as standards. As can be seen in
Table 14, compound
25 showed low toxicity against
A. salina larvae.
In another study, compounds
6 and
8 (
Figure 3) were tested in vitro for antibacterial activity against the same three bacteria,
S. aureus,
S. epidermidis, and
E. coli [
8]. The same standard as above was used (Tetracycline at 30 μg/mL concentration). The results are shown in
Table 15. It can be seen that compound
8 showed good inhibition against all the species of bacteria, while compound
6 showed weak inhibition.
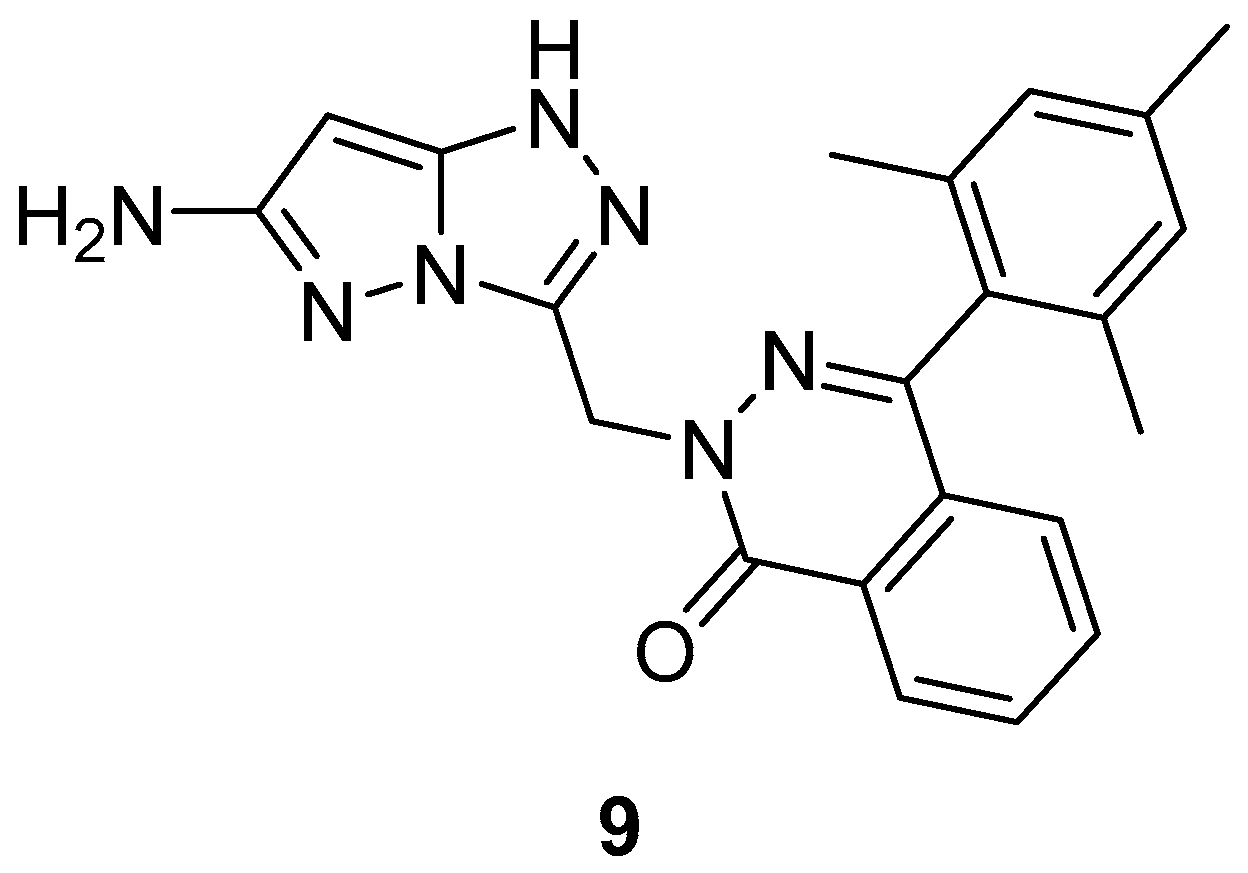
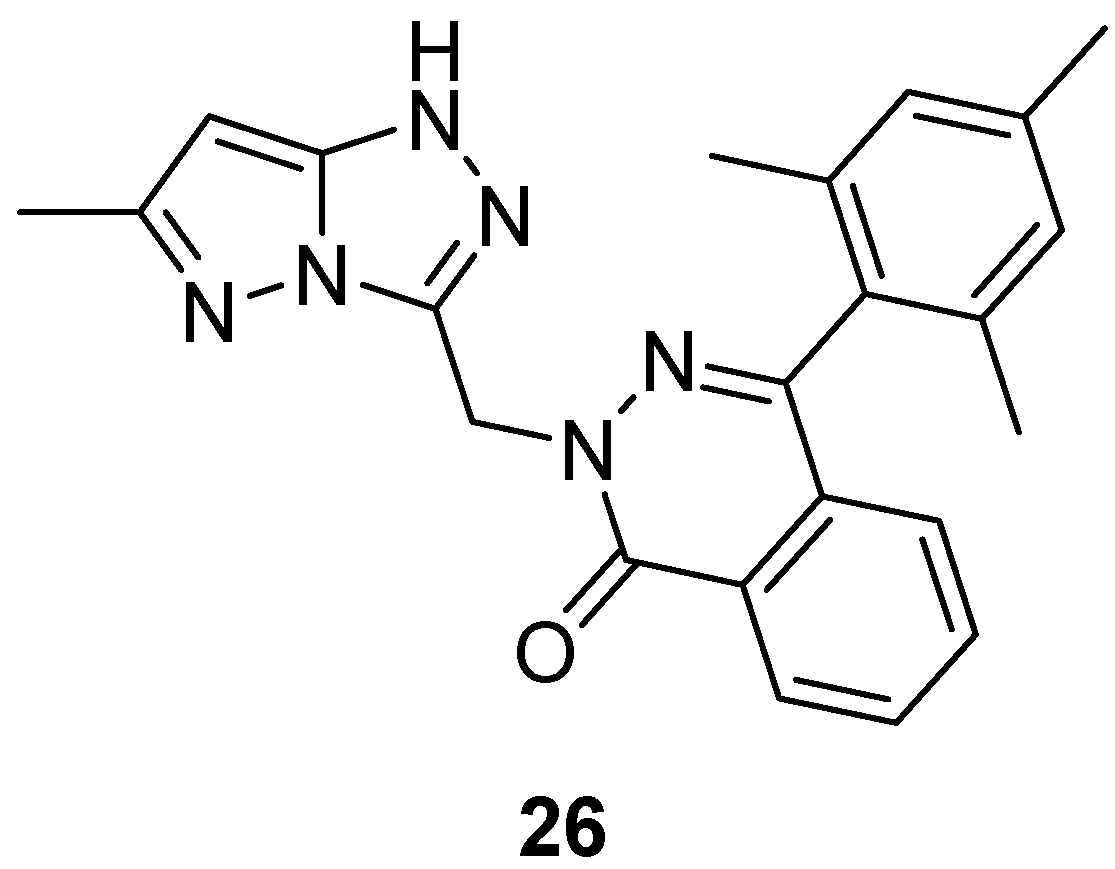
Compound
9 (
Figure 4) and its analog 2-[(6-methyl-1
H-pyrazolo[5,1-
c][1,2,4]triazol-3-yl)methyl]-4-(2,4,6-trimethylphenyl)phthalazin-1(2
H)-one
26 (
Figure 11) were tested in vitro for antibacterial activity against four bacterial strains,
E. coli,
S. aureus,
B. subtilis and
Salmonella typhi [
9,
16]. Amoxicillin was used as standard drug. The observed activities are given in
Table 16. The results indicate that compound
9 exhibited good antibacterial activity against
S. aureus,
B. subtilis, and
S. typhi and lower activity against
E. coli, while compound
26 showed weak activity against all four bacterial species.
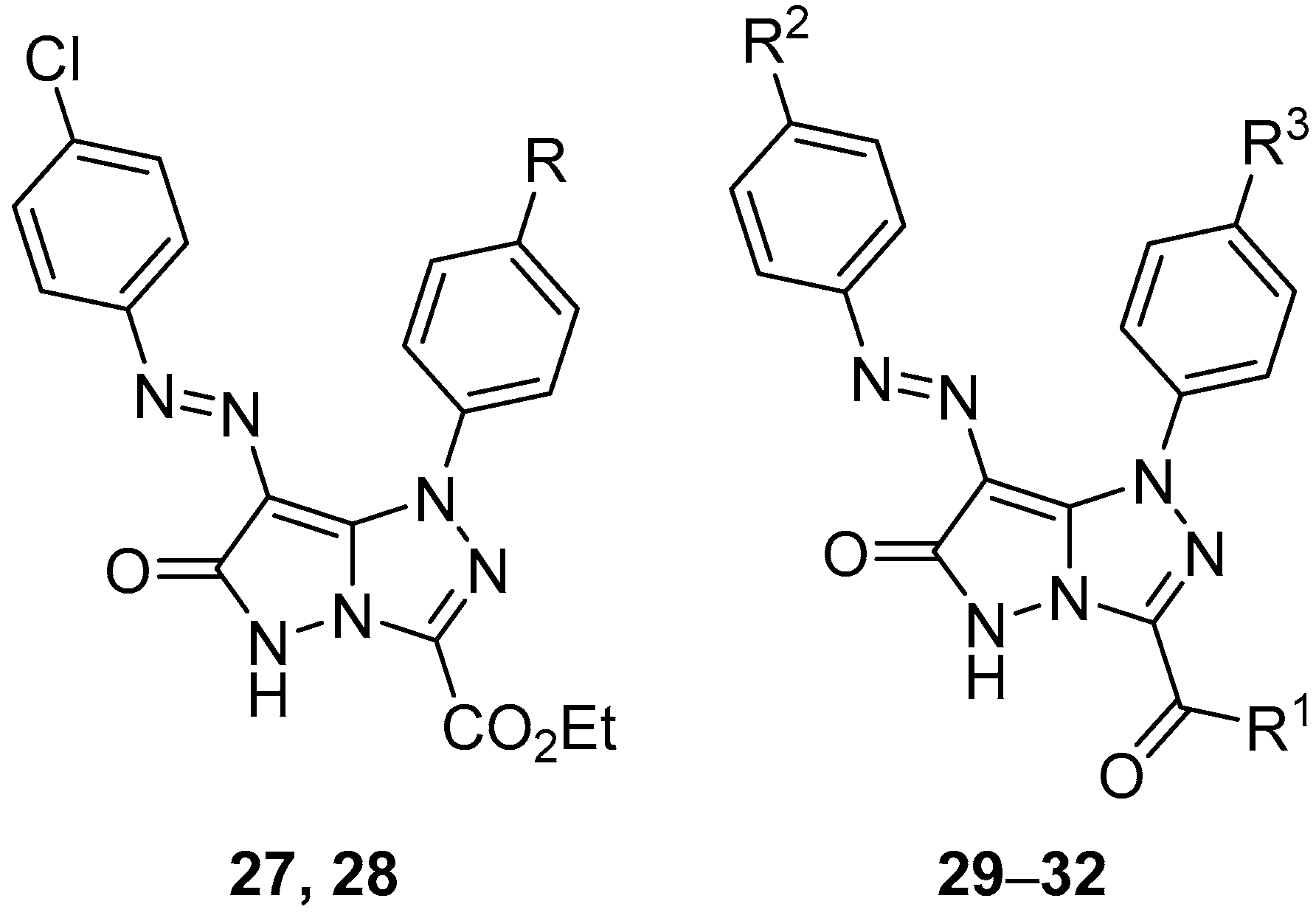
In another study, six pyrazolo[5,1-
c][1,2,4]triazoles
27–
32 (
Figure 12), ethyl 7-[(4-chlorophenyl)diazenyl]-6-oxo-1-phenyl-5,6-dihydro-1
H-pyrazolo[5,1-
c][1,2,4]triazole-3-carboxylate
27, ethyl 7-[(4-chlorophenyl)diazenyl]-1-(4-methylphenyl)-6-oxo-5,6-dihydro-1
H-pyrazolo[5,1-
c][1,2,4]triazole-3-carboxylate
28, 3-acetyl-7-[(4-chlorophenyl)diazenyl]-1-phenyl-1
H-pyrazolo[5,1-
c][1,2,4]triazol-6(5
H)-one
29, 3-acetyl-7-[(4-chlorophenyl)diazenyl]-1-(4-methoxyphenyl)-1
H-pyrazolo[5,1-
c][1,2,4]triazol-6(5
H)-one
30, 3-acetyl-1-(4-methylphenyl)-7-(4-methylphenyldiazenyl)-1
H-pyrazolo[5,1-
c][1,2,4]triazol-6(5
H)-one
31, and 3-benzoyl-7-(4-methylphenyldiazenyl)-1-phenyl-1
H-pyrazolo[5,1-
c][1,2,4]triazol-6(5
H)-one
32 were tested in vitro for antibacterial activity against two Gram-positive bacteria,
Streptococcus pneumoniae and
B. subtilis, and two Gram-negative bacteria species,
P. aeruginosa and
E. coli [
17]. Ampicillin and Gentamicin were used as standard antibacterial agents for Gram-positive and Gram-negative bacteria, respectively. The results are displayed in
Table 17. With the exception of compounds
27,
29, and
30, which showed low activity against
B. subtilis, and compound
28, which revealed low activity against
S. pneumoniae, the compounds tested showed no antibacterial activity.
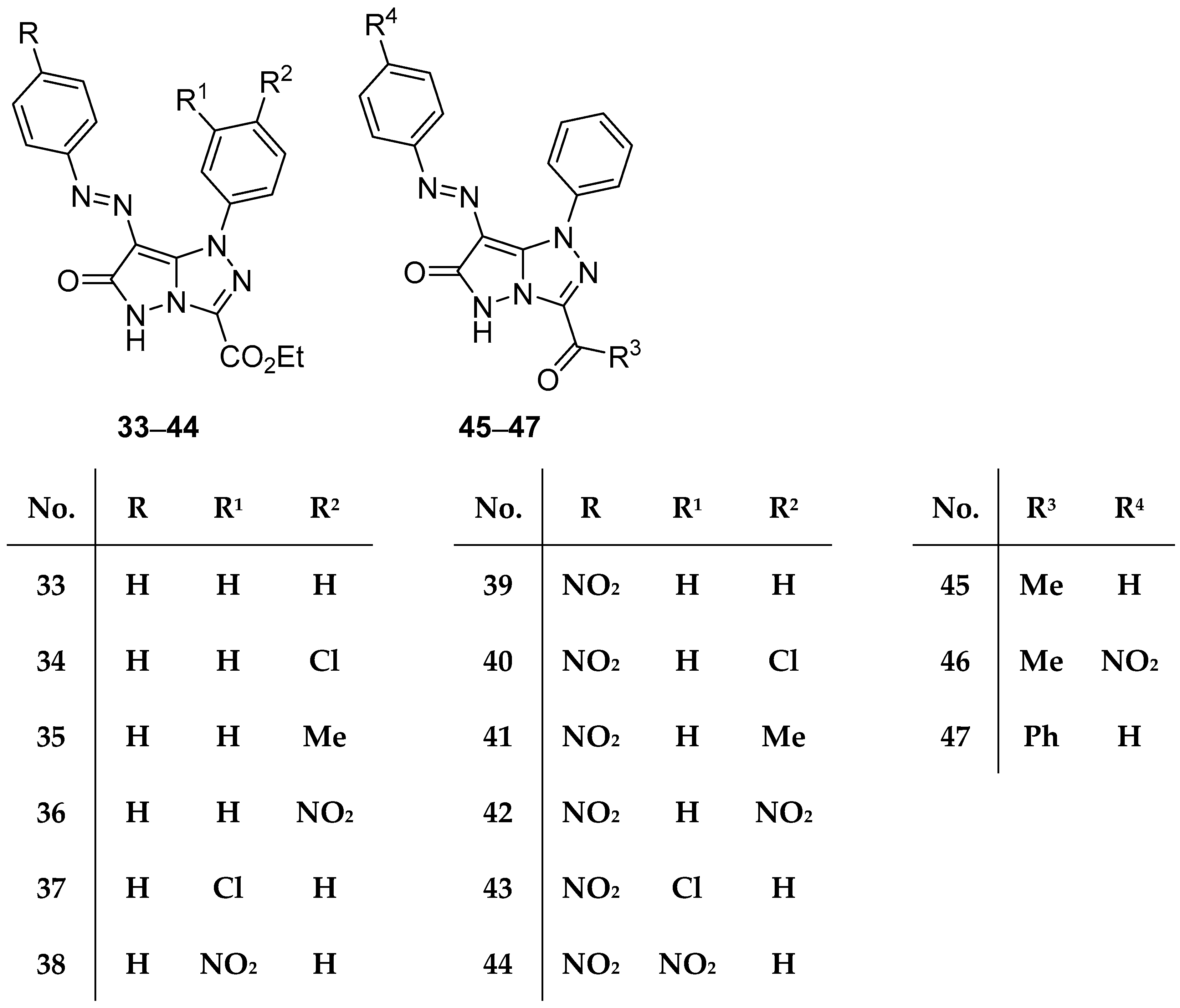
In another study from the same laboratory, fifteen pyrazolo[5,1-
c][1,2,4]triazoles
33–
47 (
Figure 13), ethyl 6-oxo-1-phenyl-7-(phenyldiazenyl)-5,6-dihydro-1
H-pyrazolo[5,1-
c][1,2,4]triazole-3-carboxylate
33, ethyl 1-(4-chlorophenyl)-6-oxo-7-(phenyldiazenyl)-5,6-dihydro-1
H-pyrazolo[5,1-
c][1,2,4]triazole-3-carboxylate
34, ethyl 1-(4-methylphenyl)-6-oxo-7-(phenyldiazenyl)-5,6-dihydro-1
H-pyrazolo[5,1-
c][1,2,4]triazole-3-carboxylate
35, ethyl 1-(4-nitrophenyl)-6-oxo-7-(phenyldiazenyl)-5,6-dihydro-1
H-pyrazolo[5,1-
c][1,2,4]triazole-3-carboxylate
36, ethyl 1-(3-chlorophenyl)-6-oxo-7-(phenyldiazenyl)-5,6-dihydro-1
H-pyrazolo[5,1-
c][1,2,4]triazole-3-carboxylate
37, ethyl 1-(3-nitrophenyl)-6-oxo-7-(phenyldiazenyl)-5,6-dihydro-1
H-pyrazolo[5,1-
c][1,2,4]triazole-3-carboxylate
38, ethyl 7-[(4-nitrophenyl)diazenyl]-6-oxo-1-phenyl-5,6-dihydro-1
H-pyrazolo[5,1-
c][1,2,4]triazole-3-carboxylate
39, ethyl 1-(4-chlorophenyl)-7-[(4-nitrophenyl)diazenyl]-6-oxo-5,6-dihydro-1
H-pyrazolo[5,1-
c][1,2,4]triazole-3-carboxylate
40, ethyl 1-(4-methylphenyl)-7-[(4-nitrophenyl)diazenyl]-6-oxo-5,6-dihydro-1
H-pyrazolo[5,1-
c][1,2,4]triazole-3-carboxylate
41, ethyl 1-(4-nitrophenyl)-7-[(4-nitrophenyl)diazenyl]-6-oxo-5,6-dihydro-1
H-pyrazolo[5,1-
c][1,2,4]triazole-3-carboxylate
42, ethyl 1-(3-chlorophenyl)-7-[(4-nitrophenyl)diazenyl]-6-oxo-5,6-dihydro-1
H-pyrazolo[5,1-
c][1,2,4]triazole-3-carboxylate
43, ethyl 1-(3-nitrophenyl)-7-[(4-nitrophenyl)diazenyl]-6-oxo-5,6-dihydro-1
H-pyrazolo[5,1-
c][1,2,4]triazole-3-carboxylate
44, 3-acetyl-1-phenyl-7-(phenyldiazenyl)-1
H-pyrazolo[5,1-
c][1,2,4]triazol-6(5
H)-one
45, 3-acetyl-7-[(4-nitrophenyl)diazenyl]-1-phenyl-1
H-pyrazolo[5,1-
c][1,2,4]triazol-6(5
H)-one
46, and 3-benzoyl-1-phenyl-7-(phenyldiazenyl)-1
H-pyrazolo[5,1-
c][1,2,4]triazol-6(5
H)-one
47 were tested in vitro for antibacterial activity against the same four bacterial strains, using the same standards for reference [
18]. The determined activities are included in
Table 18. The results reveal that compounds
34,
35,
37,
39,
45, and
46 have medium to weak activity against both Gram-positive bacteria, while compound
47 shows weak activity against
B. subtilis. With the exception of compound
35, which has medium activity against
E. coli, the compounds tested showed no activity against Gram-negative bacteria. A molecular docking study was also included in the work.
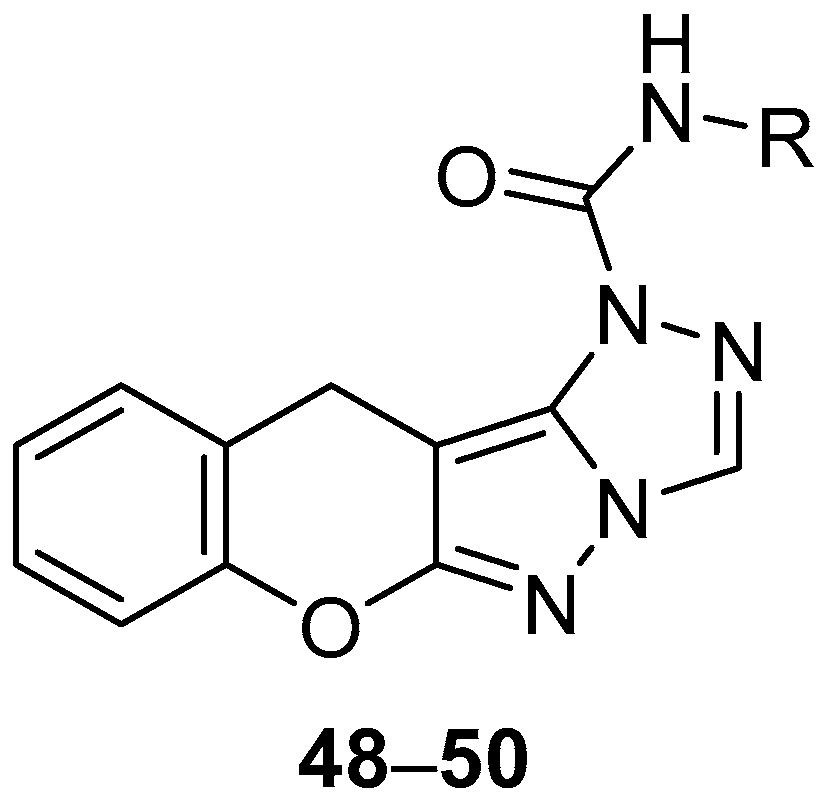
A Chinese patent reports the in vitro testing of chromeno[2′,3′:3,4]pyrazolo[5,1-
c][1,2,4]triazoles
48–
50 (
Figure 14),
N-(2,4-difluorophenyl)chromeno[2′,3′:3,4]pyrazolo[5,1-
c][1,2,4]triazole-1(11
H)-carboxamide
48,
N-ethylchromeno[2′,3′:3,4]pyrazolo[5,1-
c][1,2,4]triazole-1(11
H)-carboxamide
49 and
N-(4-ethyl-2-fluorobenzyl)chromeno[2′,3′:3,4]pyrazolo[5,1-
c][1,2,4]triazole-1(11
H)-carboxamide
50, against
S. aureus, with Penicillin as standard [
19]. The results are shown in
Table 19. Compounds
48 and
50, both containing a fluorine-substituted benzene ring, show good inhibition against
S. aureus, while the activity of compound
49 is significantly lower.
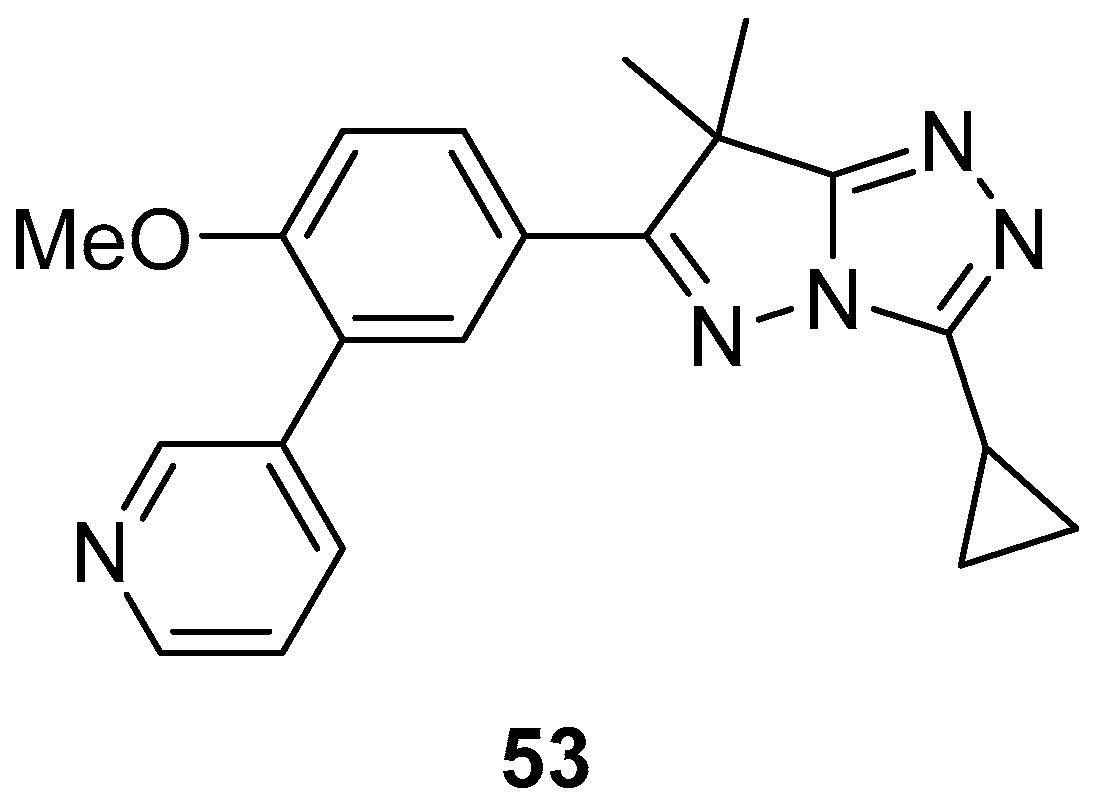
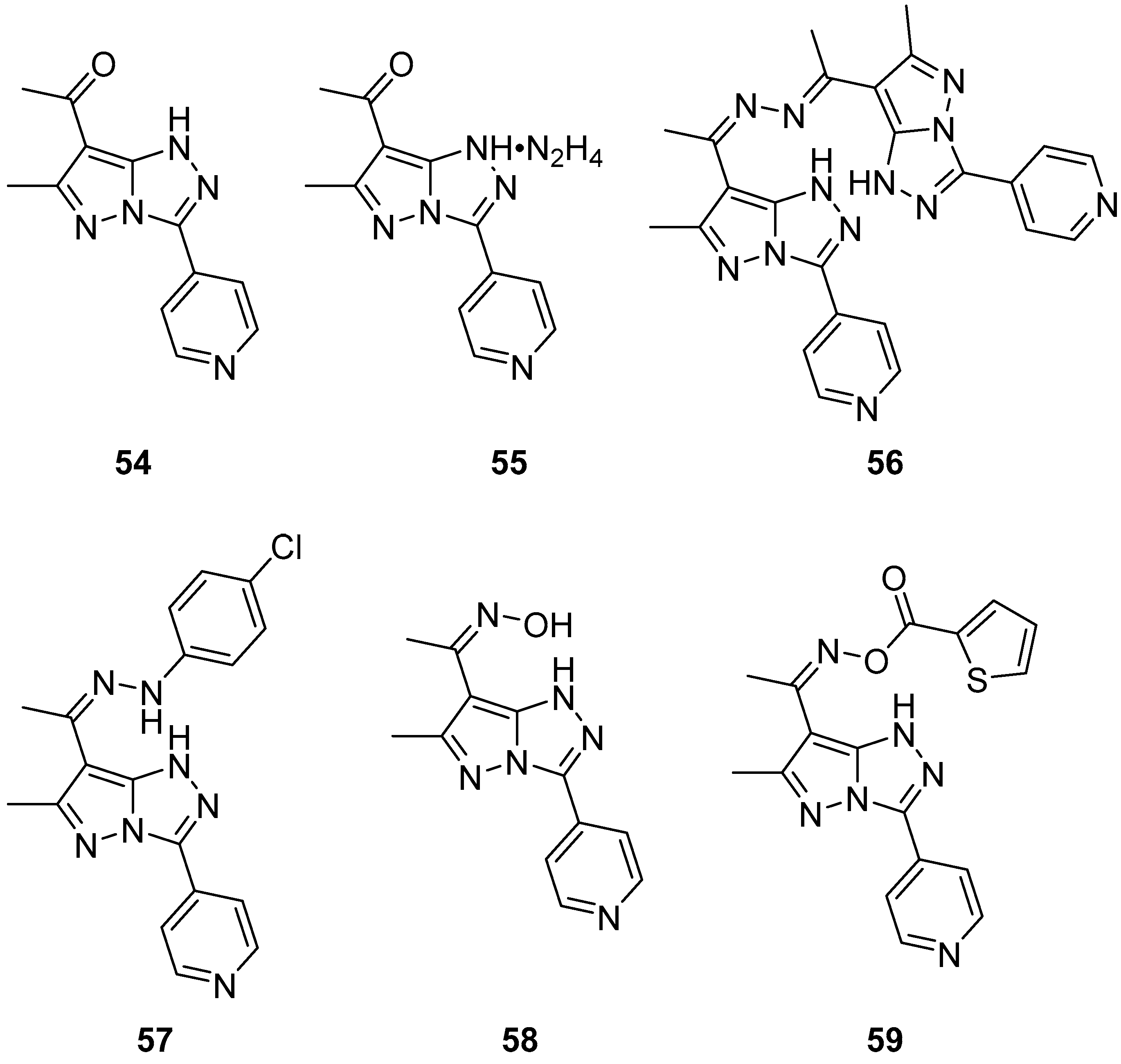
10. Anticancer Activity
3-(Pyridin-4-yl)-1
H-pyrazolo[5,1-
c][1,2,4]triazoles
54–
59, 1-[6-methyl-3-(pyridin-4-yl)-1
H-pyrazolo[5,1-
c][1,2,4]triazol-7-yl]ethan-1-one
54, its hydrazinium salt
55, 1,2-bis{1-[6-methyl-3-(pyridin-4-yl)-1
H-pyrazolo[5,1-
c][1,2,4]triazol-7-yl]ethylidene}hydrazine
56, 7-{1-[2-(4-chlorophenyl)hydrazinylidene]ethyl}-6-methyl-3-(pyridin-4-yl)-1
H-pyrazolo[5,1-
c][1,2,4]triazole
57, 1-[6-methyl-3-(pyridin-4-yl)-1
H-pyrazolo[5,1-
c][1,2,4]triazol-7-yl]ethan-1-one oxime
58, and 1-[6-methyl-3-(pyridin-4-yl)-1
H-pyrazolo[5,1-
c][1,2,4]triazol-7-yl]ethan-1-one
O-thiophene-2-carbonyl oxime
59 (
Figure 18) were tested in vitro for cytotoxicity against Ehrlich–Lettre ascites carcinoma (EAC) tumor cells, with Doxorubicin as reference [
24]. The results are displayed in
Table 29. Compounds
56 and
57 proved to be active toward the used tumor cells; compounds
54,
55, and
58 showed moderate activities, while compound
59 showed no activity, possibly because of its low solubility in the culture medium.
The activity of compounds
56 and
57 against a liver carcinoma cell line (Hep G2) was also examined, again with Doxorubicin as reference [
24]. The results are shown in
Table 30. The low IC
50 value of compound
57 is an indicator of its high inhibitory activity against Hep G2.
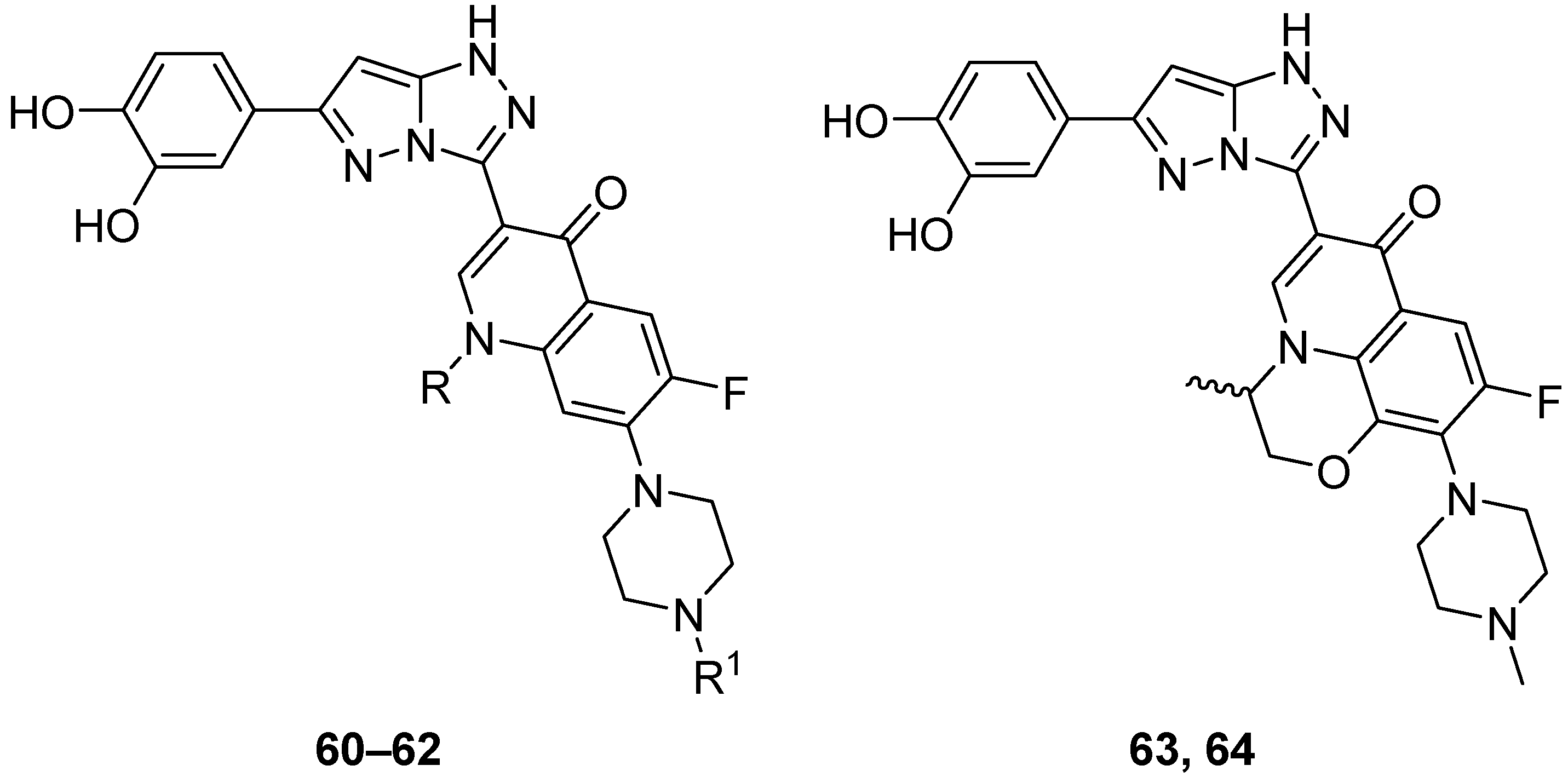
The in vitro antitumor activity for five fluoroquinolones
60–
64 (
Figure 19), 3-[6-(3,4-dihydroxyphenyl)-1
H-pyrazolo[5,1-
c][1,2,4]triazol-3-yl]-6-fluoro-1-methyl-7-(piperazin-1-yl)quinolin-4(1
H)-one
60, 1-cyclopropyl-3-[6-(3,4-dihydroxyphenyl)-1
H-pyrazolo[5,1-
c][1,2,4]triazol-3-yl]-6-fluoro-7-(piperazin-1-yl)quinolin-4(1
H)-one
61, 1-cyclopropyl-3-[6-(3,4-dihydroxyphenyl)-1
H-pyrazolo[5,1-
c][1,2,4]triazol-3-yl]-7-(4-ethylpiperazin-1-yl)-6-fluoroquinolin-4(1
H)-one
62, racemic 6-[6-(3,4-dihydroxyphenyl)-1
H-pyrazolo[5,1-
c][1,2,4]triazol-3-yl]-9-fluoro-3-methyl-10-(4-methylpiperazin-1-yl)-2,3-dihydro-7H-[1,4]oxazino[2,3,4-
ij]quinolin-7-one
63, and its
S enantiomer
64 against L1210 (murine leukemia) and CHO (Chinese hamster ovary) cell lines was evaluated via their respective IC
50 values [
25]. The results are summarized in
Table 31. While the five parent fluoroquinolone antibiotics (norfloxacin, ciprofloxacin, enrofloxacin, ofloxacin, and levofloxacin) had poor inhibitory activities against these cancer line cells (IC
50 > 150 μmol/L), compounds
60–
64 showed IC
50 values < 10 μmol/L, with compounds
61 and
64 being the most active.
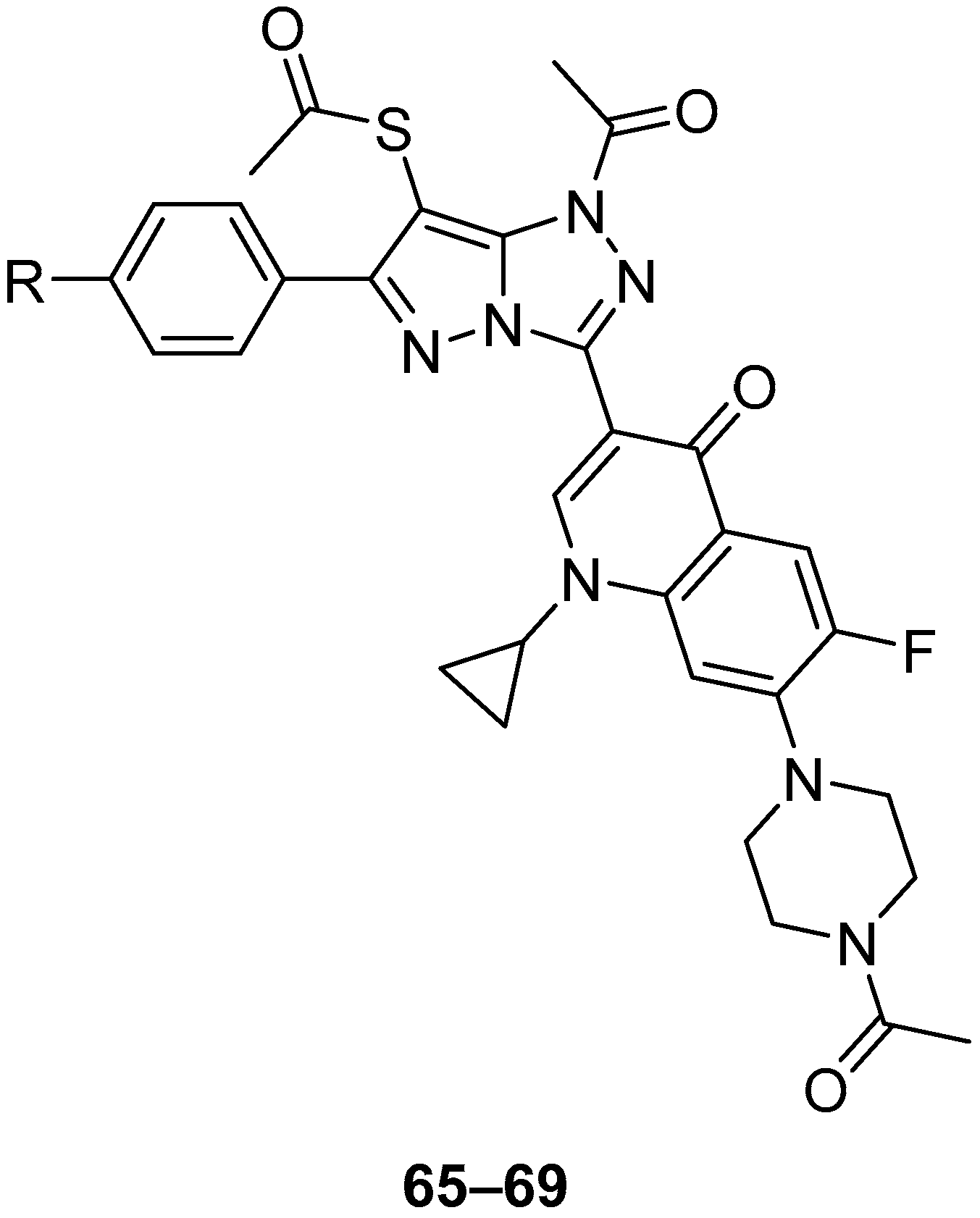
In another study, another five fluoroquinolones
65–
69 (
Figure 20),
S-{1-acetyl-3-[7-(4-acetylpiperazin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinolin-3-yl]-6-phenyl-1
H-pyrazolo[5,1-
c][1,2,4]triazol-7-yl} ethanethioate
65,
S-{1-acetyl-3-[7-(4-acetylpiperazin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinolin-3-yl]-6-(4-methoxyphenyl)-1
H-pyrazolo[5,1-
c][1,2,4]triazol-7-yl} ethanethioate
66,
S-{1-acetyl-3-[7-(4-acetylpiperazin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinolin-3-yl]-6-(4-methylphenyl)-1
H-pyrazolo[5,1-
c][1,2,4]triazol-7-yl} ethanethioate
67,
S-{1-acetyl-3-[7-(C)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinolin-3-yl]-6-(4-chlorophenyl)-1
H-pyrazolo[5,1-
c][1,2,4]triazol-7-yl} ethanethioate
68, and
S-[1-acetyl-3-[7-(4-acetylpiperazin-1-yl)-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinolin-3-yl]-6-(4-nitrophenyl)-1
H-pyrazolo[5,1-
c][1,2,4]triazol-7-yl} ethanethioate
69 were tested in vitro for antitumor activity against L1210 (murine leukemia), HL-60 (human leukemia) and CHO (Chinese hamster ovary) cell lines, with Ciprofloxacin as standard [
26]. The results are given in
Table 32. All these compounds showed IC
50 values in the micromolar range, with compound
69 being the most active against all three cell lines, while Ciprofloxacin showed much weaker activity. The structure–activity relationships from these two studies [
25,
26] show that by substituting the 3-carboxyl group in fluoroquinolones with a fused heterobicycle is conducive to antitumor activity, and that the pyrazolo[5,1-
c][1,2,4]triazoles investigated have greater antitumor activity than their triazolo[3,4-b][1,3,4]thiadiazine precursors and, in the case of compounds
65–
69, the latter’s 4-acetylpiperazinyl analogs.
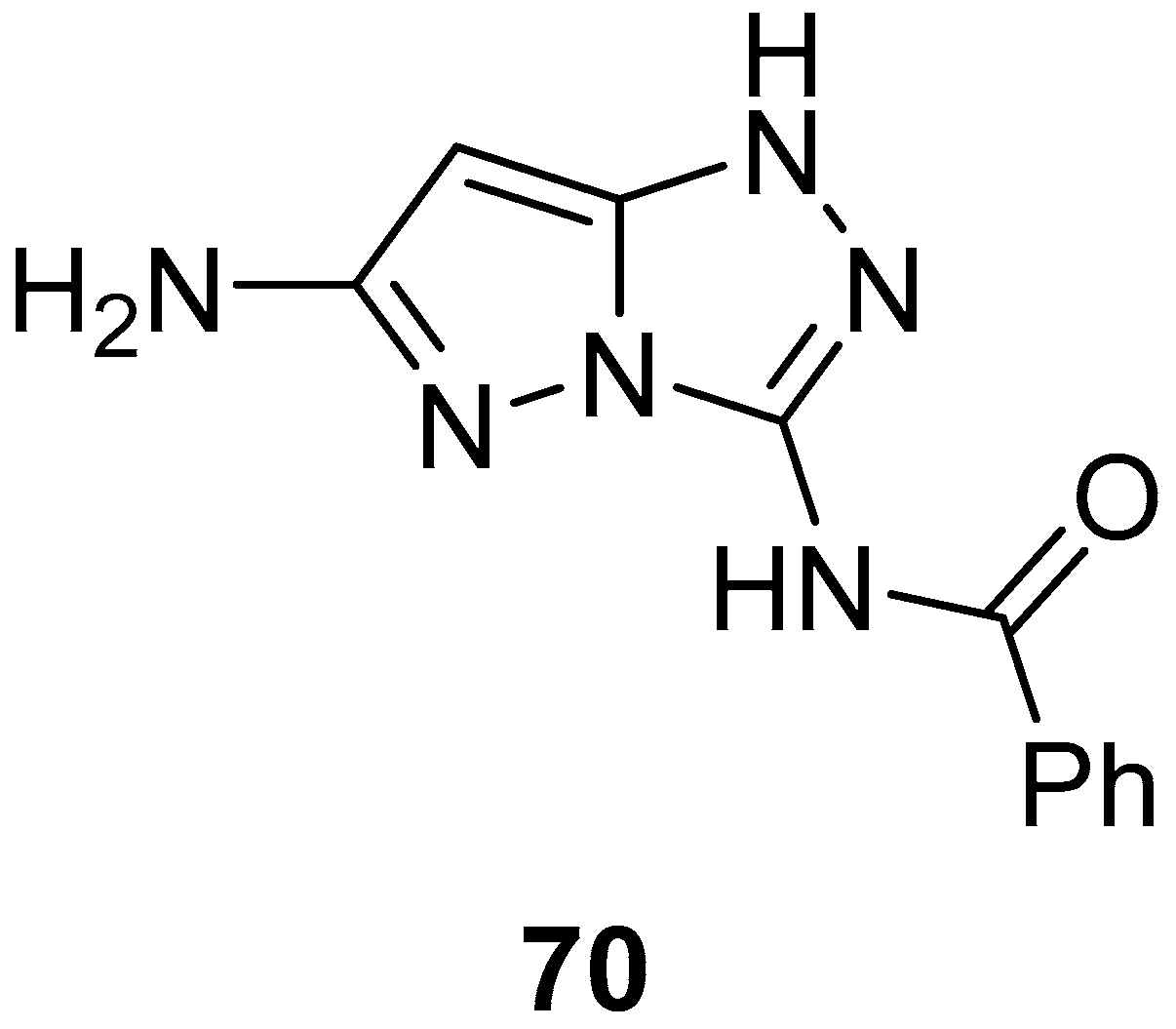
N-(6-Amino-1
H-pyrazolo[5,1-
c][1,2,4]triazol-3-yl)benzamide
70 (
Figure 21) was tested in vitro for antitumor activity against a panel of four human tumor cell lines: hepatocellular carcinoma Hep G2, lung fibroblasts WI 38, kidney of a normal adult African green monkey VERO, and breast cancer MCF-7 [
27]. 5-Fluorouracil was used as reference. The results are reported in
Table 33. As can be observed, compound
70 showed weak inhibitory activity against all four cell lines.
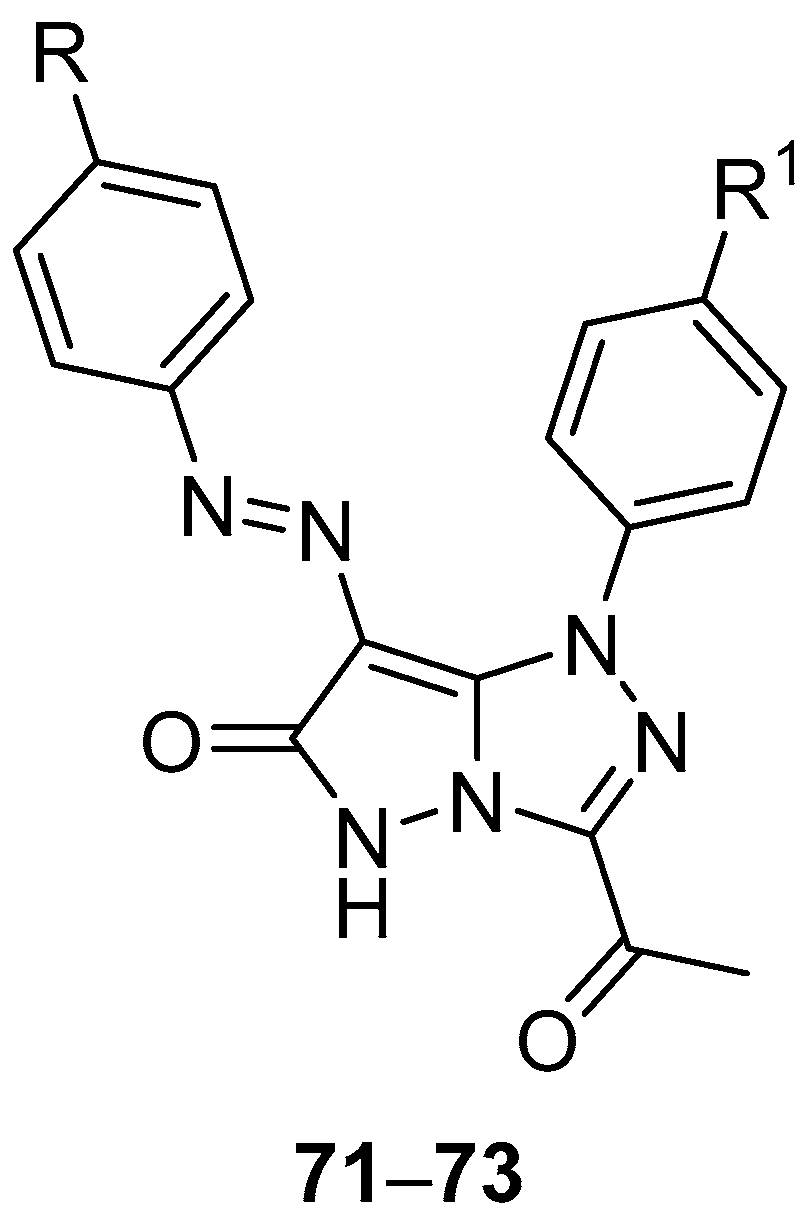
Pyrazolo[5,1-
c][1,2,4]triazoles
27–
32 (
Figure 12) and
71–
73 (
Figure 22), 3-acetyl-1-(4-chlorophenyl)-7-[(4-chlorophenyl)diazenyl]-1
H-pyrazolo[5,1-
c][1,2,4]triazol-6(5
H)-one
71, 3-acetyl-1-(4-chlorophenyl)-7-[(4-methylphenyl)diazenyl]-1
H-pyrazolo[5,1-
c][1,2,4]triazol-6(5
H)-one
72, and 3-acetyl-1-(4-methoxyphenyl)-7-[(4-methylphenyl)diazenyl]-1
H-pyrazolo[5,1-
c][1,2,4]triazol-6(5
H)-one
73 were tested in vitro for antitumor activity against hepatocellular carcinoma Hep G2 and colon cancer HCT116 cell lines, with 5-fluorouracil, Doxorubicin and Imatinib as standard drugs [
17]. The results are summarized in
Table 34. Some of the tested compounds showed good activity against both cancer cell lines: the most active compounds against Hep G2 hepatocellular carcinoma cells were compounds
27,
29, and
72, while compounds
28,
71, and
72 were the most active against colon cancer HCT116 cells.
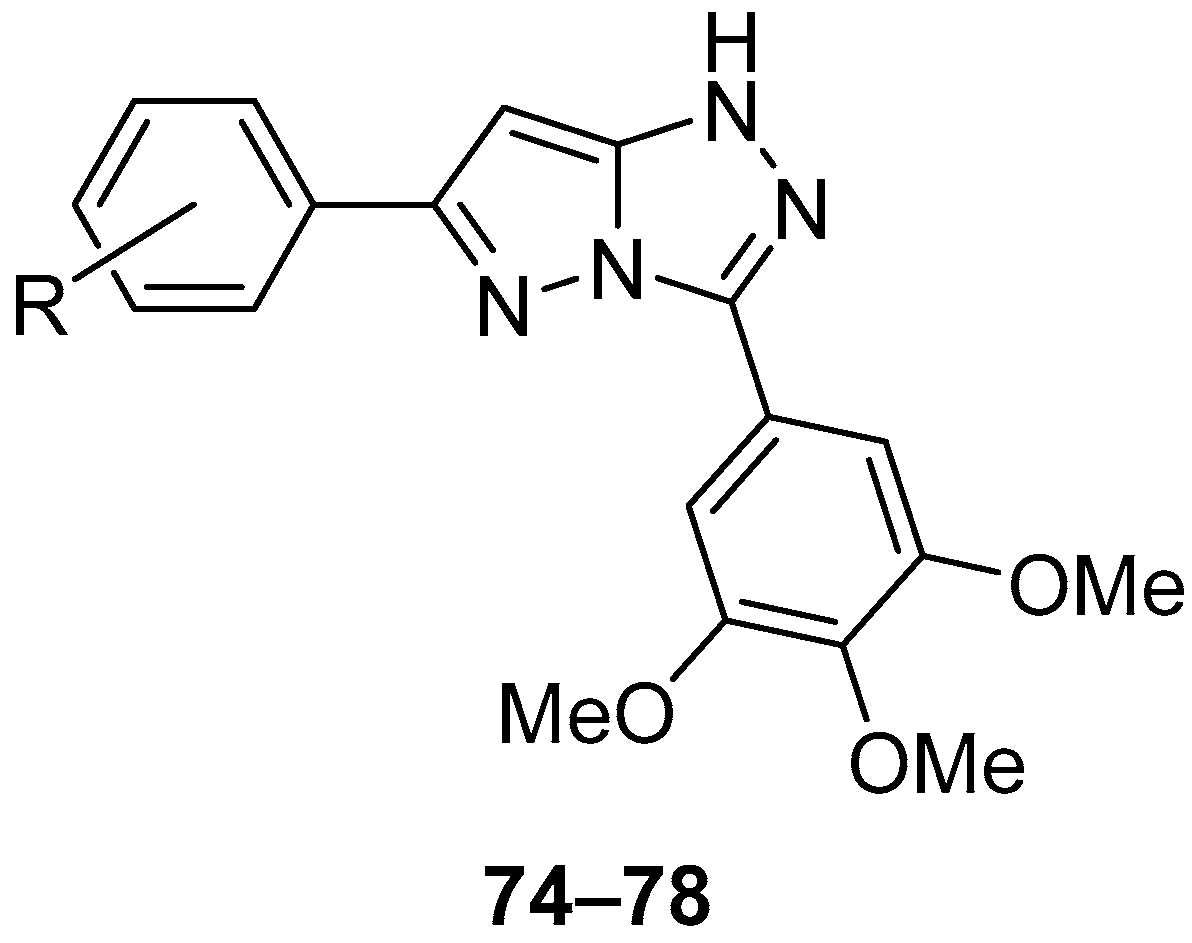
Compounds
74–
78 (
Figure 23), 6-(4-methylphenyl)-3-(3,4,5-trimethoxyphenyl)-1
H-pyrazolo[5,1-
c][1,2,4]triazole
74, 6-(4-chlorophenyl)-3-(3,4,5-trimethoxyphenyl)-1
H-pyrazolo[5,1-
c][1,2,4]triazole
75, 6-(4-methoxyphenyl)-3-(3,4,5-trimethoxyphenyl)-1
H-pyrazolo[5,1-
c][1,2,4]triazole
76, 6-(4-methoxy-3-nitrophenyl)-3-(3,4,5-trimethoxyphenyl)-1
H-pyrazolo[5,1-
c][1,2,4]triazole
77, and 2-methoxy-5-[3-(3,4,5-trimethoxyphenyl)-1
H-pyrazolo[5,1-
c][1,2,4]triazol-6-yl]aniline
78 were tested in vitro for antitumor activity against human gastric adenocarcinoma SGC-7901, human oral epithelial cancer KB, and human fibrosarcoma HT1080 cell lines, with Combretastatin A-4 and Doxorubicin as standard drugs [
28]. The results are presented in
Table 35. All the tested compounds showed good inhibition of all three cancer cell lines studied, with compounds
75 and
78 being the most active.
The acute toxicity of compounds
75 and
76 (
Figure 23) was determined in vivo in mice at a dose of 500 mg/kg [
28]. The compounds were administered by intraperitoneal injection. Since all the mice survived and returned to normal after the administration of this compound stopped, the LD
50 value for intraperitoneal administration was considered greater than 500 mg/kg.
The most active compounds in vitro,
75 and
78, were also investigated in vivo on S-180 sarcoma model mice, using 5-fluorouracil as reference [
28]. The results are presented in
Table 36.
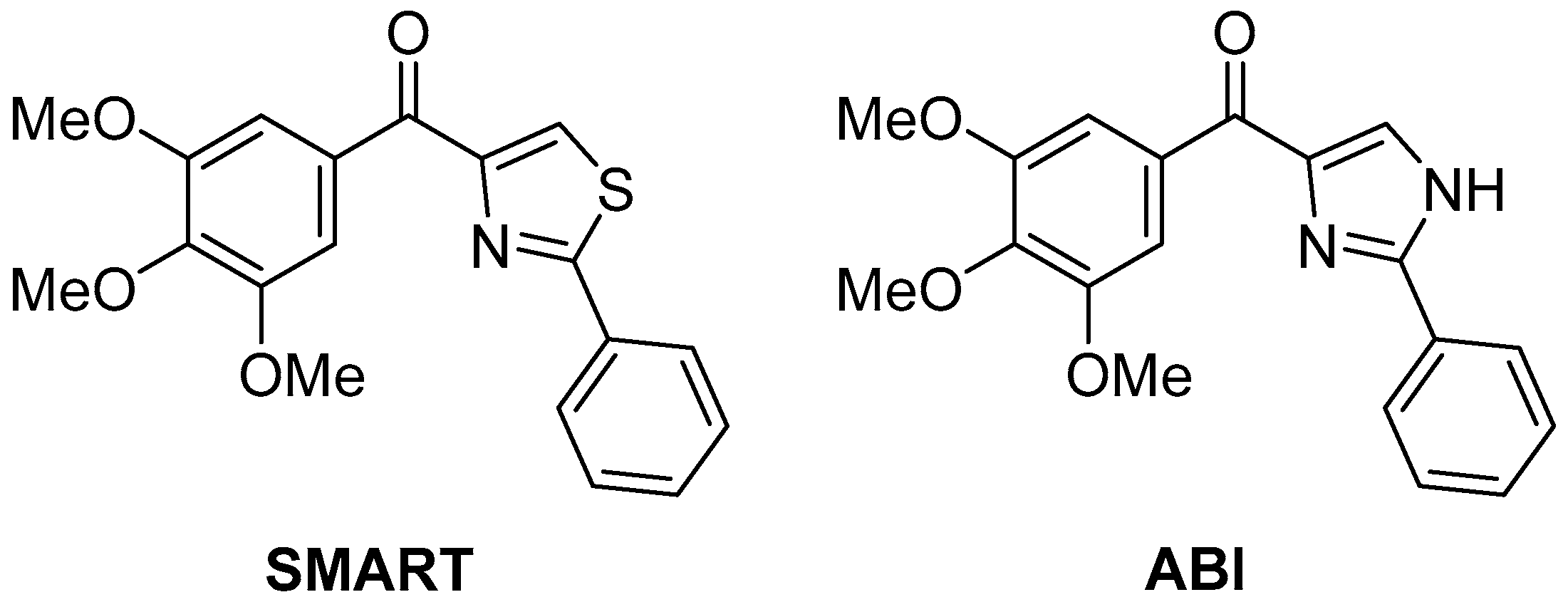
The same compounds
74–
78 were later tested again in vitro for antiproliferative activity against human gastric adenocarcinoma SGC-7901, human lung adenocarcinoma A549, and human fibrosarcoma HT1080 cell lines [
29], with two compounds with potent antiproliferative activity,
SMART [
30] [(2-phenylthiazol-4-yl)(3,4,5-trimethoxyphenyl)methanone] and
ABI [
31] [(2-phenyl-1
H-imidazol-4-yl)(3,4,5-trimethoxyphenyl)methanone] (
Figure 24) as positive controls. The results are displayed in
Table 37. It can be observed that all the pyrazolo[5,1-
c][1,2,4]triazoles tested showed modest antiproliferative activity, especially in comparison with SMART. A number of 1H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazole analogs were also tested in this study, and several of these compounds showed potent antiproliferative activity at sub-micromolar or nanomolar concentrations against the three different cancer cell lines; one derivative in particular, the analog of
78, showed activities close to those of SMART (0.022 ± 0.006, 0.029 ± 0.011, 0.027 ± 0.013, respectively).
Compounds
33–
47 (
Figure 13) were tested in vitro for antitumor activity against Hep G2 and HCT116 cell lines, using 5-fluorouracil, Doxorubicin, and Imatinib as standard drugs [
18]. The results are shown in
Table 38. These compounds showed weak to moderate activity against both cell lines, the most active being compounds
43 and
47.
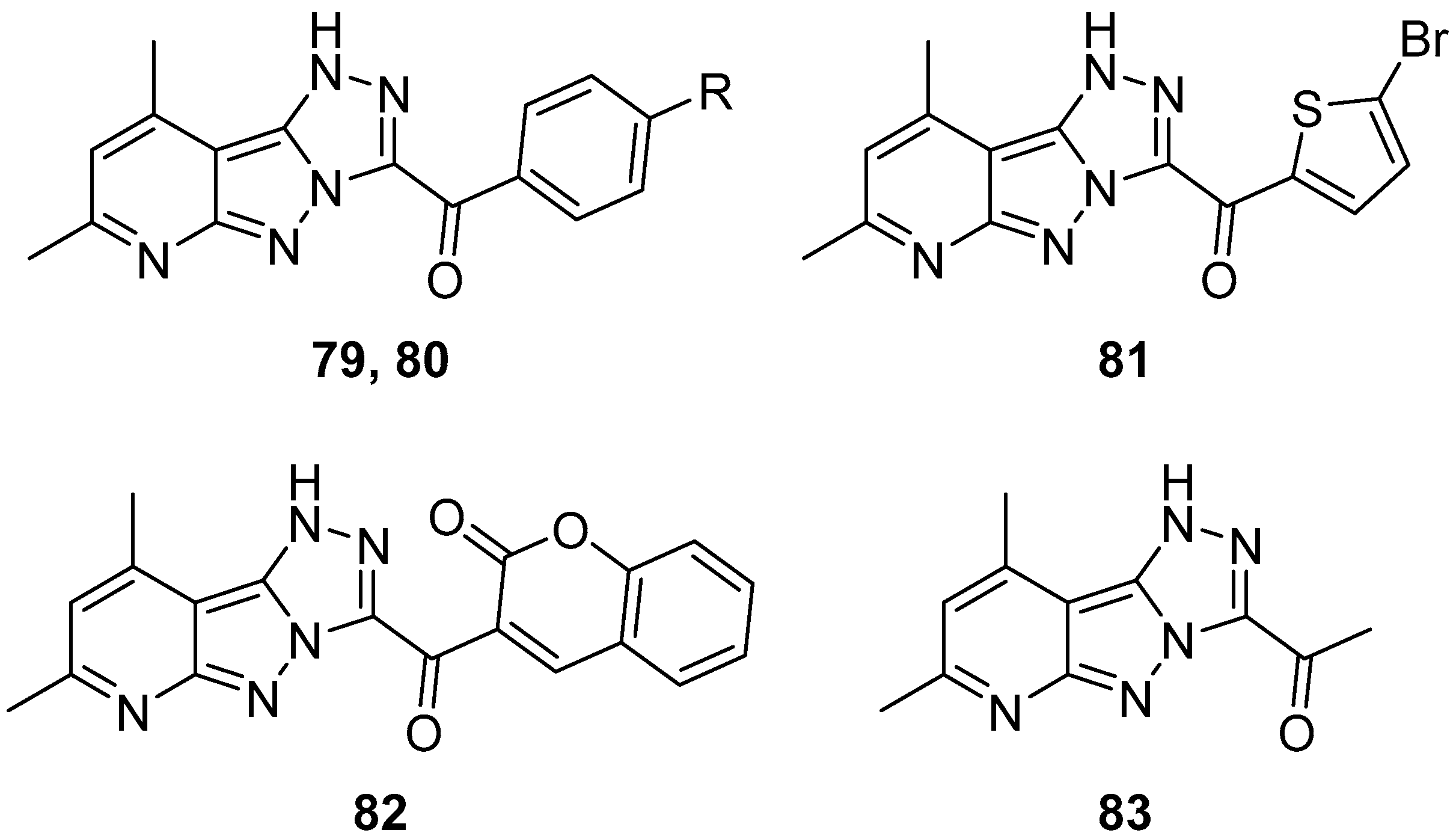
1
H-[1,2,4]Triazolo[4′,3′:1,5]pyrazolo[3,4-
b]pyridines
79–
83: (7,9-dimethyl-1
H-[1,2,4]triazolo[4′,3′:1,5]pyrazolo[3,4-
b]pyridin-3-yl)(4-fluorophenyl)methanone
79, (4-chlorophenyl)(7,9-dimethyl-1
H-[1,2,4]triazolo[4′,3′:1,5]pyrazolo[3,4-
b]pyridin-3-yl)methanone
80, (5-bromothiophen-2-yl)(7,9-dimethyl-1
H-[1,2,4]triazolo[4′,3′:1,5]pyrazolo[3,4-
b]pyridin-3-yl)methanone
81, 3-(7,9-dimethyl-1
H-[1,2,4]triazolo[4′,3′:1,5]pyrazolo[3,4-
b]pyridine-3-carbonyl)-2
H-chromen-2-one
82, and 1-(7,9-dimethyl-1
H-[1,2,4]triazolo[4′,3′:1,5]pyrazolo[3,4-
b]pyridin-3-yl)ethan-1-one
83 (
Figure 25) were tested in vitro for antitumor activity against HCT116, Hep G2, HeLa (cervical cancer) and MCF-7 cell lines, with Doxorubicin as reference [
32]. The results are listed in
Table 39. Compound
82 exhibited very good activity against all cell lines, while all the other compounds demonstrated weak activity in all cases. The remarkable activity of compound
82 could be attributed to the presence of the coumarin fragment in its molecule.
Later, in a study on the discovery of 1,2,4-triazole-based inhibitors of aromatase (CYP19A1), for the treatment of hormone receptor (HR)-positive breast cancer, compounds
79–
82 were among the 78 compounds used to generate the pharmacophore model [
33]. Compounds
80 and
81 were included in the training set (39 compounds), and compounds
79 and
82 in the test set (39 compounds). In the end, this study led to two 1,2,4-triazole-based structures with better estimated activity and fit value than the standard drug Letrozole.
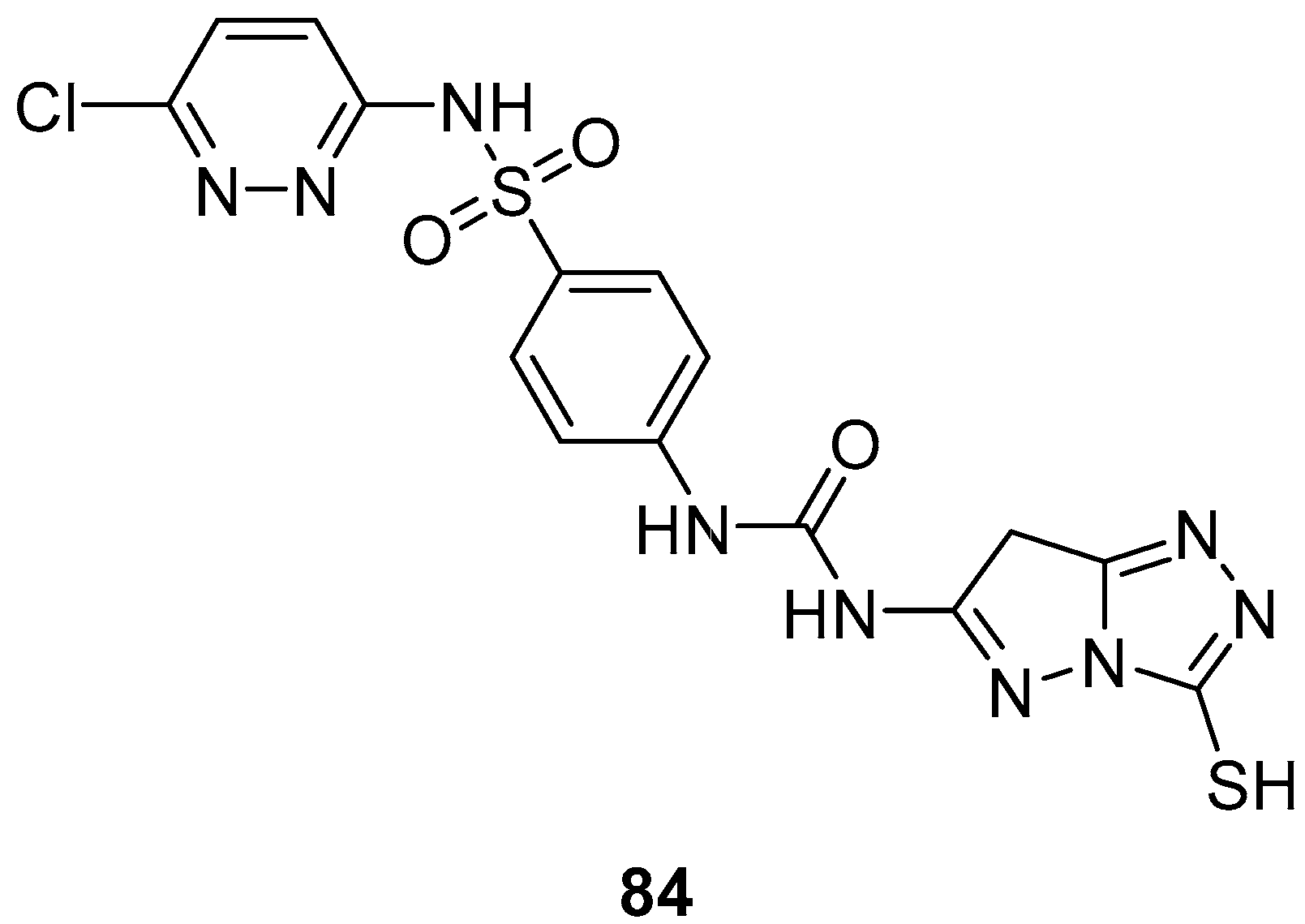
N-(4-{[(6-Chloropyridazin-3-yl)amino]sulfonyl}phenyl)-
N′-(3-sulfanyl-7
H-pyrazolo[5,1-
c][1,2,4]triazol-6-yl)urea
84 (
Figure 26) was subjected to an in vitro cytotoxicity screening against 21 cancer cell lines, representing eight subpanels: leukemia (CCRF-CEM and SR), non-small-cell lung (EKVX, HOP-62, HOP-92 and NCI-H522), central nervous system (SF-268 and SNB-75), melanoma (UACC-62), ovarian (IGROV1 and SK-OV-3), renal (A498, CAK-1 and UO-31), prostate (PC-3), and breast (MCF7, MDA-MB231/ATCC, HS 578T, BT-549, T-47D, and MDA-MB-468), at a single dose of 10
−5 M (10 μM) [
34]. The results were reported as the % growth inhibition (GI) against the cell lines. With the exception of the leukemia SR cell line, against which compound
84 displayed minimal activity (GI = 10%), and renal cancer UO-31 cell line (GI = 14%), no inhibitory activities of this compound were observed (GI < 10%).