Oxidative Stress in Wheat Caused by Ampicillin and Amoxicillin and Their Mixture Applied to the Soil
Abstract
1. Introduction
2. Results and Discussion
2.1. Photosynthetic Pigments
2.2. Chlorophyll Fluorescence
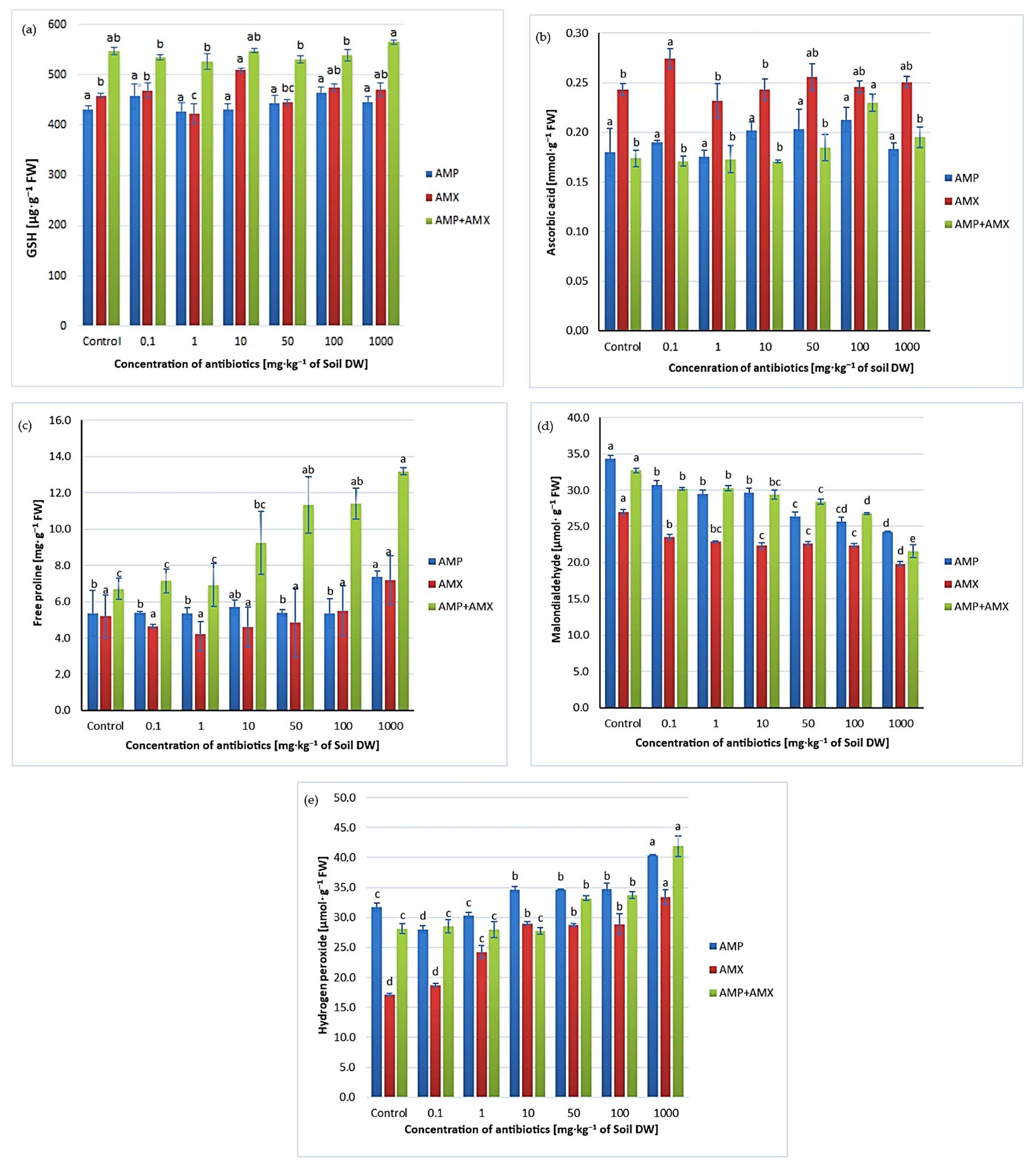
2.3. Ascorbic Acid (AsA) and Glutathione (GSH)
2.4. Free Proline
2.5. Malondialdehyde (MDA) and Hydrogen Peroxide (H2O2)
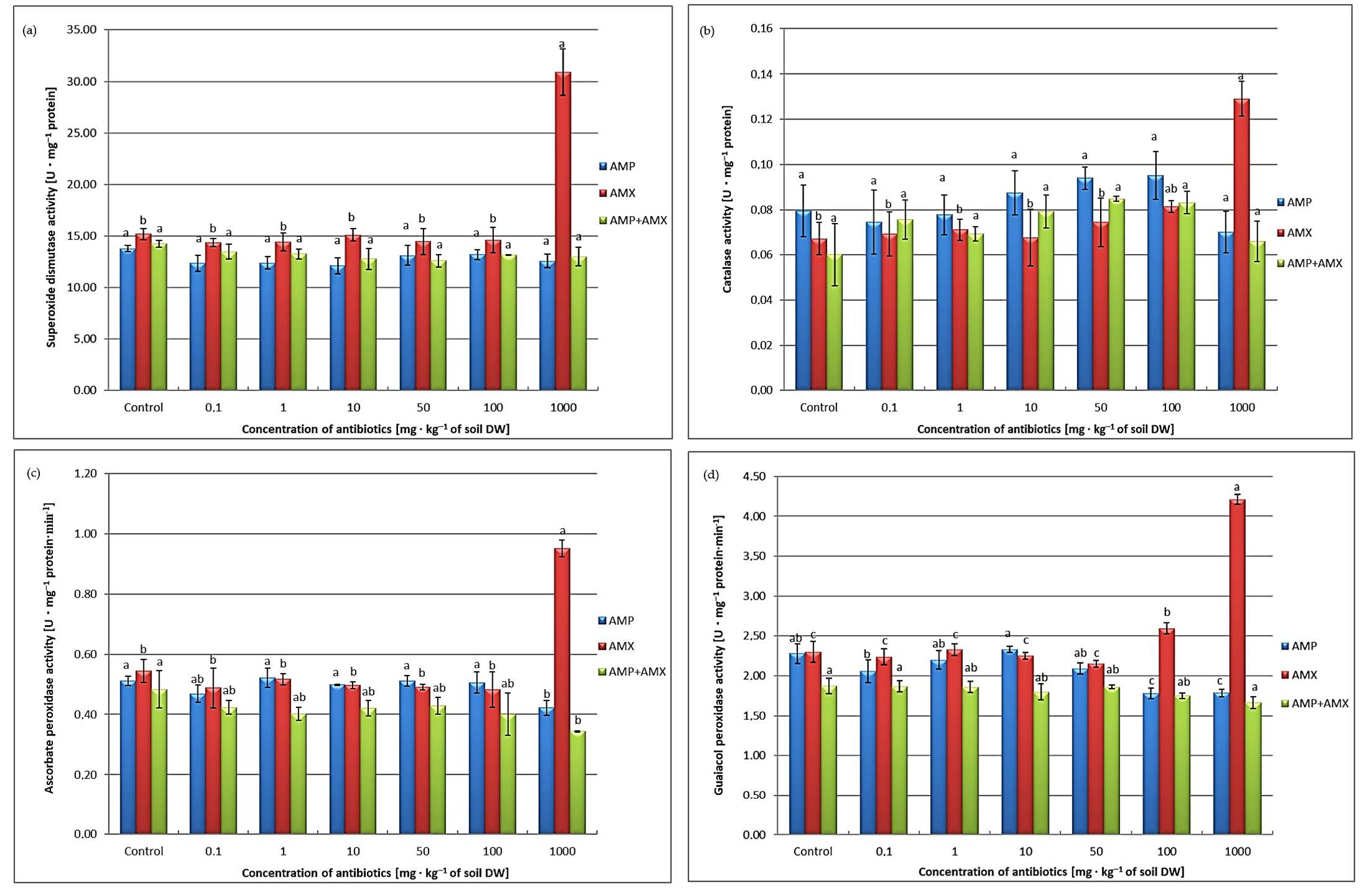
2.6. Antioxidant Enzymes
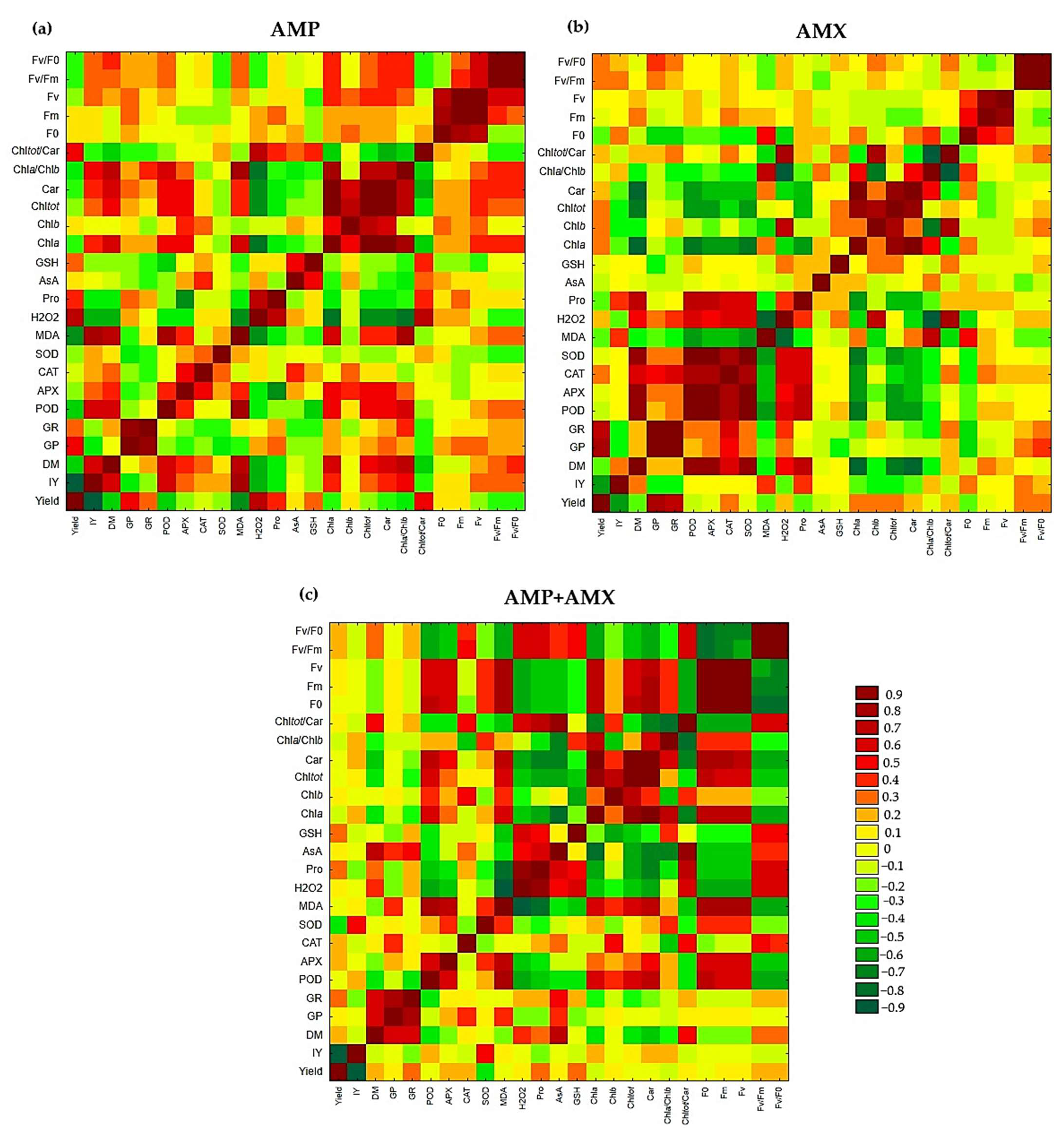
2.7. Pearson Correlation
3. Materials and Methods
3.1. Chemical Reagents
3.2. Plant Cultivation—How the Research Was Conducted
3.3. Contents of Photosynthetic Pigments
3.4. Chlorophyll Fluorescence
3.5. Ascorbic Acid (AsA) and Glutathione (GSH)
3.6. Free Proline
3.7. Malondialdehyde (MDA) and Hydrogen Peroxide (H2O2)
3.8. Antioxidant Enzymes
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMP | Ampicillin |
| AMX | Amoxicillin |
| ROS | Reactive oxygen species |
| H2O2 | Hydrogen peroxide |
| AOX | Complex antioxidant system |
| MDA | Malondialdehyde |
| AsA | Ascorbic acid |
| GSH | Glutathione |
| GSSG | Oxidized glutathione |
| SOD | Superoxide dismutase |
| POD | Guaiacol peroxidase |
| CAT | Catalase |
| APX | Ascorbate peroxidase |
| DW | Dry weight |
| F0 | Initial (minimum) fluorescence |
| Fv | Fluctuating fluorescence |
| Fm | Maximum fluorescence |
References
- Opriş, O.; Copaciu, F.; Soran, M.L.; Ristoiu, D.; Niinemets, U.; Copolovici, L. Influence of nine antibiotics on key secondary metabolites and physiological characteristics in Triticum aestivum: Leaf volatiles as a promising new tool to assess toxicity. Ecotoxicol. Environ. Saf. 2013, 87, 70–79. [Google Scholar] [CrossRef]
- Li, L.; Li, T.; Liu, Y.; Li, L.; Huang, X.; Xie, J. Effects of antibiotics stress on root development, seedling growth, antioxidant status and abscisic acid level in wheat (Triticum aestivum L.). Ecotoxicol. Environ. Saf. 2023, 252, 114621. [Google Scholar] [CrossRef]
- Sousa, B.; Lopes, J.; Leal, A.; Martins, M.; Soares, C.; Valente, I.M.; Rodrigues, J.A.; Fidalgo, F.; Teixeira, J. Response of Solanum lycopersicum L. to diclofenac—Impacts on the plant’s antioxidant mechanisms. Environ. Pollut. 2020, 258, 113762. [Google Scholar] [CrossRef]
- Taschina, M.; Moisa, C.; Lupitu, A.; Copolovici, D.M.; Copolovici, L. Influence of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) on Photosynthetic Parameters and Secondary Metabolites of Plants from Fabaceae Family. Appl. Sci. 2022, 12, 6326. [Google Scholar] [CrossRef]
- Bigott, Y.; Chowdhury, S.P.; Pérez, S.; Montemurro, N.; Manasfi, R.; Schröder, P. Effect of the pharmaceuticals diclofenac and lamotrigine on stress responses and stress gene expression in lettuce (Lactuca sativa) at environmentally relevant concentrations. J. Hazard. Mater. 2021, 403, 123881. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Chen, C.; Li, S.; Ye, P.; Shi, Y.; Sharma, G.; Sarkar, B.; Shaheen, S.M.; Lee, S.S.; Xiao, R.; et al. A comprehensive and global evaluation of residual antibiotics in agricultural soils: Accumulation, potential ecological risks, and attenuation strategies. Ecol. Toxicol. Saf. 2023, 262, 115175. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, J.; Lu, G. Influence of aquatic colloids on the bioaccumulation and biological effects of diclofenac in zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2018, 195, 110470. [Google Scholar] [CrossRef]
- Zhang, T.; Li, N.; Chen, G.; Xu, J.; Ouyang, G.; Zhu, F. Stress symptoms and plant hormone-modulated defense response induced by the uptake of carbamazepine and ibuprofen in Malabar spinach (Basella alba L.). Sci. Total Environ. 2021, 793, 148628. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, M.; Zien-Elabdeen, A.; Hifney, A.F.; Adam, M.S. Phycotoxicity of antibiotics and non-steroidal anti-inflammatory drugs to green algae Chlorella sp. and Desmodesmus spinosus: Assessment of combined toxicity by Box–Behnken experimental design. Environ. Technol. Innov. 2021, 23, 101586. [Google Scholar] [CrossRef]
- Mucsi, Z.; Chass, G.A.; Ábrányi-Balogh, P.; Jójárt, B.; Fang, D.-C.; Ramirez-Cuesta, A.J.; Viskolcz, B.; Csizmadia, I.G. Penicillin’s catalytic mechanism revealed by inelastic neutrons and quantum chemical theory. Phys. Chem. Chem. Phys. 2013, 15, 20447–20455. [Google Scholar] [CrossRef]
- Hirsch, R.; Ternes, T.; Haberer, K.; Kratz, K.-L. Occurrence of antibiotics in the aquatic environment. Sci. Total Environ. 1999, 225, 109–118. [Google Scholar] [CrossRef]
- Rachoski, M.; Gazquez, A.; Calzadilla, P.; Bezus, R.; Rodriguez, A.; Ruiz, O.; Menendez, A.; Maiale, S. Chlorophyll fluorescence and lipid peroxidation changes in rice somaclonal lines subjected to salt stress. Acta Physiol. Plant. 2015, 37, 117. [Google Scholar] [CrossRef]
- Xiong, J.Q.; Kurade, M.B.; Jeon, B.H. Ecotoxicological effects of enrofloxacin and its removal by monoculture of microalgal species and their consortium. Environ. Pollut. 2017, 226, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Alkimin, G.D.; Daniel, D.S.; Frankenbach, S.; Serôdio, J.; Soares, A.M.V.M.; Barata, C.; Nunes, B. Evaluation of pharmaceutical toxic effects of non-standard endpoints on the macrophyte species Lemna minor and Lemna gibba. Sci. Total Environ. 2019, 657, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.Q.; Kurade, M.B.; Kim, J.R.; Roh, H.S.; Jeon, B.H. Ciprofloxacin toxicity and its co-metabolic removal by a freshwater microalga Chlamydomonas Mexicana. J. Hazard. Mater. 2017, 323 Pt A, 212–219. [Google Scholar] [CrossRef]
- Baciak, M.; Sikorski, Ł.; Piotrowicz-Cieślak, A.I.; Adomas, B. Content of biogenic amines in Lemna minor (common duckweed) growing in medium contaminated with tetracycline. Aquat. Toxicol. 2016, 180, 95–102. [Google Scholar] [CrossRef]
- Wang, H.; Jin, M.; Mao, W.; Chen, C.; Fu, L.; Li, Z.; Du, S.; Liu, H. Photosynthetic toxicity of non-steroidal anti-inflammatory drugs (NSAIDs) on green algae Scenedesmus obliquus. Sci. Total Environ. 2020, 707, 136176. [Google Scholar] [CrossRef]
- Wang, H.; Jin, M.; Xu, L.; Xi, H.; Wang, B.; Du, S.; Liu, H.; Wen, Y. Effects of ketoprofen on rice seedlings: Insights from photosynthesis, antioxidative stress, gene expression patterns, and integrated biomarker response analysis. Environ. Pollut. 2020, 263, 114533. [Google Scholar] [CrossRef]
- Pan, X.; Deng, C.; Zhang, D.; Wang, J.; Mu, G.; Chen, Y. Toxic effects of amoxicillin on the photosystem II of Synechocystis sp. characterized by a variety of in vivo chlorophyll fluorescence tests. Aquat. Toxicol. 2008, 89, 207–213. [Google Scholar] [CrossRef]
- Pszczółkowski, P.; Sawicka, B.; Skiba, D.; Barbaś, P.; Noaema, A.H. The Use of Chlorophyll Fluorescence as an Indicator of Predicting Potato Yield, Its Dry Matter and Starch in the Conditions of Using Microbiological Preparations. Appl. Sci. 2023, 13, 10764. [Google Scholar] [CrossRef]
- Qu, J.H. Sensitivity of five kinds of algae to commonly used antibiotics. J. Dalian Inst. Light Ind. 2004, 23, 111–113. [Google Scholar]
- Zhang, D.B.; Sui, Z.H.; Mao, Y.X.; Guo, H.; Zang, X.N.; Zhang, X.C.; Lv, Y.G. Study on the sensitivities of eight antibiotics on Alexandrium tamarense (Lebour) Balech. Acta Oceanol. Sin. 2007, 29, 123–130. [Google Scholar]
- Iori, V.; Zacchini, M.; Pietrini, F. Growth, physiological response and phytoremoval capability of two willow clones exposed to ibuprofen under hydroponic culture. J. Hazard. Mater. 2013, 262, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Kummerová, M.; Zezulka, S.; Babula, P.; Trískaca, J. Possible ecological risk of two pharmaceuticals diclofenac and paracetamol demonstrated on a model plant Lemna minor. J. Hazard. Mater. 2016, 302, 351–361. [Google Scholar] [CrossRef]
- Pawłowska, B.; Telesiński, A.; Sysa, M.; Godela, A.; Ščurek, R.; Biczak, R. Ibuprofen and Ketoprofen—Inert Drugs or Potential Environmental Hazard? Sustainability 2023, 15, 1613. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline Mechanisms of Stress Survival. Antioxid. Redox Signal. 2013, 19, 998–1011. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef]
- Stuchlíková, L.R.; Skálová, L.; Szotáková, B.; Syslová, E.; Vokřál, I.; Vaněk, T.; Podlipná, R. Biotransformation of flubendazole and fenbendazole and their effects in the ribwort plantain (Plantago lanceolata). Ecotoxicol. Environ. Saf. 2018, 147, 681–687. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Q.; Li, J.; Chen, X.; Lang, Q.; Kuang, S. Combined effects of erythromycin and enrofloxacin on antioxidant enzymes and photosynthesis-related gene transcription in Chlorella vulgaris. Aquat. Toxicol. 2019, 212, 138–145. [Google Scholar] [CrossRef]
- Hassan, M.; Israr, M.; Mansoor, S.; Hussain, S.A.; Basheer, F.; Azizullah, A.; Rehman, S.U. Acclimation of cadmium-induced genotoxicity and oxidative stress in mung bean seedlings by priming effect of phytohormones and proline. PLoS ONE 2021, 16, e0257924. [Google Scholar] [CrossRef]
- Hnilickova, H.; Kraus, K.; Vachova, P.; Hnilicka, F. Salinity Stress Affects Photosynthesis, Malondialdehyde Formation, and Proline Content in Portulaca oleracea L. Plants 2021, 10, 845. [Google Scholar] [CrossRef]
- Singh, V.; Pandey, B.; Suthar, S. Phytotoxicity of amoxicillin to the duckweed Spirodela polyrhiza: Growth, oxidative stress, biochemical traits and antibiotic degradation. Chemosphere 2018, 201, 492–502. [Google Scholar] [CrossRef]
- Pawłowska, B.; Feder-Kubis, J.; Telesiński, A.; Biczak, R. Biochemical Responses of Wheat Seedlings on the Introduction of Selected Chiral Ionic Liquids to the Soils. J. Agric. Food Chem. 2019, 67, 3086–3095. [Google Scholar] [CrossRef] [PubMed]
- Christou, A.; Antoniou, C.; Christodoulou, C.; Hapeshi, E.; Stavrou, I.; Michael, C.; Fatta-Kassinos, D.; Fotopoulos, V. Stress-related phenomena and detoxification mechanisms induced by common pharmaceuticals in alfalfa (Medicago sativa L.) plants. Sci. Total Environ. 2016, 557–558, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Biczak, R.; Pawłowska, B. Reaction of spring barley seedlings and H. incongruens crustaceans to the presence of acetylsalicylic acid in soil. J. Environ. Manag. 2022, 302, 113936. [Google Scholar] [CrossRef]
- Guidelines for the Testing of Chemical. Terrestrial Plant: Seedling Test: Seedlings Emergence and Seedling Growth Test; OECD/OCDE 208; OECD: Paris, France, 2006. [Google Scholar]
- Pawłowska, B.; Sysa, M.; Godela, A.; Biczak, R. Antibiotics Amoxicillin, Ampicillin and Their Mixture-Impact on Bacteria, Fungi, Ostracods and Plants. Molecules 2024, 29, 4301. [Google Scholar] [CrossRef]
- Oren, R.; Werk, K.S.; Buchmann, N.; Zimmermann, R. Chlorophyll-nutrient relationships identify nutritionally caused de-cline in Picea abies stands. Can. J. For. Res. 1993, 23, 1187–1195. [Google Scholar] [CrossRef]
- Law, M.Y.; Charles, S.A.; Halliwell, B. Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts. The effect of hydrogen peroxide and of Paraquat. Biochem. J. 1983, 210, 899–903. [Google Scholar] [CrossRef]
- Liu, T.; Zhu, L.; Wang, J.; Wang, J.; Tan, M. Phytotoxicity of imidazolium-based ILs with different anions in soil on Vicia faba seedlings and the influence of anions on toxicity. Chemosphere 2016, 145, 269–276. [Google Scholar] [CrossRef]
- Bates, L.S. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Kohli, R.K.; Arora, K. Arsenic induced root growth inhibition in mung bean (Phaseolus aureus Roxb.) is due to oxidative stress resulting from enhanced lipid peroxidation. Plant Growth Regul. 2007, 53, 65–73. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wu, J.; Zhang, X.; Xia, Y.; Li, Y.; Du, S. Enantioselective oxidative stress caused by chiral ionic liquids forms of 1-alkyl-3-methyl imidazolium tartrate on Scenedesmus obliquus. Sci. Total Environ. 2017, 595, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Abassi, N.A.; Kushad, M.M.; Endress, A.G. Active oxygen scavenging enzymes activities in developing apple flowers and fruits. Sci. Hortic. 1998, 74, 183–194. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutase. I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]

| Concentration of Antibiotics (mg·kg−1 of Soil DW) | Pigments (mg·g−1 DW) | |||||
|---|---|---|---|---|---|---|
| Chla | Chlb | Chla + Chlb | Chla/Chlb | Car | Chl(a + b)/Car | |
| AMP | ||||||
| 0 | 9.045 ± 0.117 c | 4.431 ± 0.113 c | 13.476 ± 0.229 bcd | 2.042 ± 0.027 b | 2.193 ± 0.029 cd | 6.146 ± 0185 a |
| 0.1 | 9.514 ± 0.144 b | 4.652 ± 0.104 bc | 14.156 ± 0.245 b | 2.045 ± 0.017 b | 2.368 ± 0.045 b | 5.984 ± 0.113 a |
| 1 | 10.207 ± 0.091 a | 4.836 ± 0.071 ab | 15.043 ± 0.160 a | 2.111 ± 0.015 a | 2.509 ± 0.028 a | 5.996 ± 0.102 a |
| 10 | 8.787 ± 0.336 cd | 4.534 ± 0.175 c | 13.321 ± 0.508 cde | 1.938 ± 0.014 c | 2.188 ± 0.100 cd | 6.088 ± 0.087 a |
| 50 | 8.976 ± 0.128 c | 5.023 ± 0.093 a | 13.999 ± 0.222 bc | 1.787 ± 0.008 e | 2.271 ± 0.052 bc | 6.164 ± 0.047 a |
| 100 | 8.392 ± 0.071 de | 4.537 ± 0.024 c | 12.930± 0.095 de | 1.850 ± 0.006 d | 2.117 ± 0.011 de | 6.106 ± 0.052 a |
| 1000 | 8.167 ± 0.041 e | 4.527 ± 0.031 c | 12.694 ± 0.069 e | 1.804 ± 0.007 e | 2.039 ± 0.020 e | 6.225 ± 0.077 a |
| AMX | ||||||
| 0 | 8.702 ± 0.078 b | 4.493 ± 0.038 d | 13.195 ± 0.116 c | 1.937 ± 0.0015 a | 2.295 ± 0.014 b | 5.750 ± 0.027 d |
| 0.1 | 9.166 ± 0.041 a | 4.653 ± 0.020 c | 13.818 ± 0.058 b | 1.970 ± 0.005 a | 2.415 ± 0.010 a | 5.723 ± 0.012 d |
| 1 | 9.083 ± 0.256 a | 4.913 ± 0.120 b | 13.996 ± 0.376 ab | 1.849 ± 0.007 b | 2.423 ± 0.072 a | 5.776 ± 0.016 d |
| 10 | 9.149 ± 0.079 a | 5.149 ± 0.034 a | 14.298 ± 0.112 a | 1.777 ± 0.004 c | 2.411 ± 0.017 a | 5.931 ± 0.016 bc |
| 50 | 8.681 ± 0.040 b | 5.006 ± 0.016 ab | 13.687 ± 0.028 b | 1.734 ± 0.013 cd | 2.287 ± 0.013 b | 5.985 ± 0.024 ab |
| 100 | 8.719 ± 0.054 b | 5.059 ± 0.015 ab | 13.779 ± 0.043 b | 1.724 ± 0.015 d | 2.288 ± 0.009 b | 6.023 ± 0.023 a |
| 1000 | 8.270 ± 0.097 c | 4.737 ± 0.056 c | 13.007 ± 0.081 c | 1.746 ± 0.037 cd | 2.215 ± 0.024 b | 5.872 ± 0.034 c |
| AMP + AMX | ||||||
| 0 | 8.812 ± 0.041 b | 5.129 ± 0.017 dc | 13.942 ± 0.053 bc | 1.718 ± 0.006 a | 2.323 ± 0.016 b | 6.002 ± 0.028 e |
| 0.1 | 9.157 ± 0.019 a | 5.501 ± 0.009 a | 14.658 ± 0.026 a | 1.664 ± 0.002 c | 2.406 ± 0.007 a | 6.092 ± 0.017 d |
| 1 | 8.874 ± 0.183 b | 5.321 ± 0.104 b | 14.195 ± 0.286 b | 1.667 ± 0.004 c | 2.326 ± 0.047 b | 6.103 ± 0.005 cd |
| 10 | 8.737 ± 0.075 bc | 5.292 ± 0.048 bc | 14.029 ± 0.122 b | 1.651 ± 0.003 c | 2.299 ± 0.014 b | 6.102 ± 0.019 cd |
| 50 | 8.790 ± 0.048 b | 5.538 ± 0.004 a | 14.329 ± 0.051 ab | 1.587 ± 0.008 d | 2.309 ± 0.016 b | 6.206 ± 0.021 b |
| 100 | 8.135 ± 0.138 d | 5.415 ± 0.107 ab | 13.550 ± 0.241 c | 1.502 ± 0.011 e | 2.136 ± 0.038 c | 6.343 ± 0.056 a |
| 1000 | 8.503 ± 0.028 c | 5.022 ± 0.028 d | 13.526 ± 0.055 c | 1.693 ± 0.004 b | 2.191 ± 0.006 c | 6.174 ± 0.023 bc |
| Concentration of Antibiotics (mg·kg−1 of Soil DW) | Fo | Fm | Fv | Fv/Fm | Fv/Fo |
|---|---|---|---|---|---|
| AMP | |||||
| 0 | 198.3 ± 11.6 a | 990.0 ± 43.9 a | 791.7 ± 33.1 a | 0.799 ± 0.004 a | 3.995 ± 0.110 a |
| 0.1 | 195.2 ± 8.8 a | 982.3 ± 43.4 a | 787.2 ± 35.4 a | 0.801 ± 0.003 a | 4.034 ± 0.079 a |
| 1 | 201.3 ± 10.1 a | 1012.0 ± 50.2 a | 810.7 ± 41.3 a | 0.801 ± 0.004 a | 4.027 ± 0.110 a |
| 10 | 197.5 ± 6.3 a | 999.2 ± 38.3 a | 801.7 ± 33.4 a | 0.802 ± 0.005 a | 4.059 ± 0.111 a |
| 50 | 200.3 ± 14.3 a | 995.7 ± 75.8 a | 795.3 ± 62.1 a | 0.798 ± 0.004 a | 3.969 ± 0.094 a |
| 100 | 200.2 ± 11.6 a | 970.3 ± 43.6 a | 770.2 ± 45.0 a | 0.793 ± 0.015 a | 3.860 ± 0.330 a |
| 1000 | 203.2 ± 5.7 a | 1025.0 ± 39.2 a | 821.8 ± 35.3 a | 0.802 ± 0.005 a | 4.045 ± 0.133 a |
| AMX | |||||
| 0 | 206.5 ± 9.2 a | 1021.5 ± 39.2 a | 815.0 ± 31.6 a | 0.798 ± 0.005 a | 3.948 ± 0.108 a |
| 0.1 | 206.3 ± 7.5 a | 1019.0 ± 40.9 a | 812.7 ± 34.5 a | 0.797 ± 0.004 a | 3.938 ± 0.097 a |
| 1 | 208.2 ± 9.9 a | 1033.3 ± 38.3 a | 825.2 ± 28.5 a | 0.798 ± 0.003 a | 3.966 ± 0.057 a |
| 10 | 202.2 ± 5.8 a | 998.8 ± 24.4 a | 796.7 ± 20.9 a | 0.797 ± 0.005 a | 3.958 ± 0.101 a |
| 50 | 198.3 ± 9.9 a | 997.0 ± 30.3 a | 798.7 ± 21.2 a | 0.801 ± 0.005 a | 4.031 ± 0.117 a |
| 100 | 207.7 ± 7.2 a | 1044.3 ± 38.7 a | 836.7 ± 31.9 a | 0.801 ± 0.002 a | 4.028 ± 0.053 a |
| 1000 | 204.3 ± 10.8 a | 1017.7 ± 52.0 a | 813.3 ± 41.8 a | 0.799 ± 0.003 a | 3.981 ± 0.078 a |
| AMP + AMX | |||||
| 0 | 204.8 ± 9.2 a | 968.7 ± 22.2 a | 763.8 ± 15.5 a | 0.788 ± 0.006 ab | 3.733 ± 0.133 ab |
| 0.1 | 204.5 ± 8.0 a | 961.8 ± 23.3 ab | 757.3 ± 17.2 ab | 0.787 ± 0.005 b | 3.706 ± 0.116 b |
| 1 | 195.8 ± 5.6 ab | 937.7 ± 31.8 abc | 741.8 ± 26.5 abc | 0.791 ± 0.002 ab | 3.787 ± 0.055 ab |
| 10 | 177.2 ± 20.1 bc | 852.0 ± 111.9 bc | 674.8 ± 92.4 bc | 0.791 ± 0.008 ab | 3.803 ± 0.169 ab |
| 50 | 188.7 ± 12.7 abc | 893.5 ± 50.9 abc | 704.8 ± 38.5 abc | 0.789 ± 0.003 ab | 3.738 ± 0.073 ab |
| 100 | 171.5 ± 9.4 c | 841.7 ± 51.5 c | 670.2 ± 42.7 bc | 0.796 ± 0.004 a | 3.907 ± 0.091 a |
| 1000 | 169.7 ± 17.6 c | 833.7 ± 79.0 c | 664.0 ± 61.6 c | 0.796 ± 0.003 a | 3.917 ± 0.079 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biczak, R.; Telesiński, A.; Sysa, M.; Godela, A.; Pawłowska, B. Oxidative Stress in Wheat Caused by Ampicillin and Amoxicillin and Their Mixture Applied to the Soil. Int. J. Mol. Sci. 2025, 26, 8156. https://doi.org/10.3390/ijms26178156
Biczak R, Telesiński A, Sysa M, Godela A, Pawłowska B. Oxidative Stress in Wheat Caused by Ampicillin and Amoxicillin and Their Mixture Applied to the Soil. International Journal of Molecular Sciences. 2025; 26(17):8156. https://doi.org/10.3390/ijms26178156
Chicago/Turabian StyleBiczak, Robert, Arkadiusz Telesiński, Marcin Sysa, Agnieszka Godela, and Barbara Pawłowska. 2025. "Oxidative Stress in Wheat Caused by Ampicillin and Amoxicillin and Their Mixture Applied to the Soil" International Journal of Molecular Sciences 26, no. 17: 8156. https://doi.org/10.3390/ijms26178156
APA StyleBiczak, R., Telesiński, A., Sysa, M., Godela, A., & Pawłowska, B. (2025). Oxidative Stress in Wheat Caused by Ampicillin and Amoxicillin and Their Mixture Applied to the Soil. International Journal of Molecular Sciences, 26(17), 8156. https://doi.org/10.3390/ijms26178156








