RIPK3 Contributes to Thyroid Hormone-Induced Photoreceptor Degeneration †
Abstract
1. Introduction
2. Results
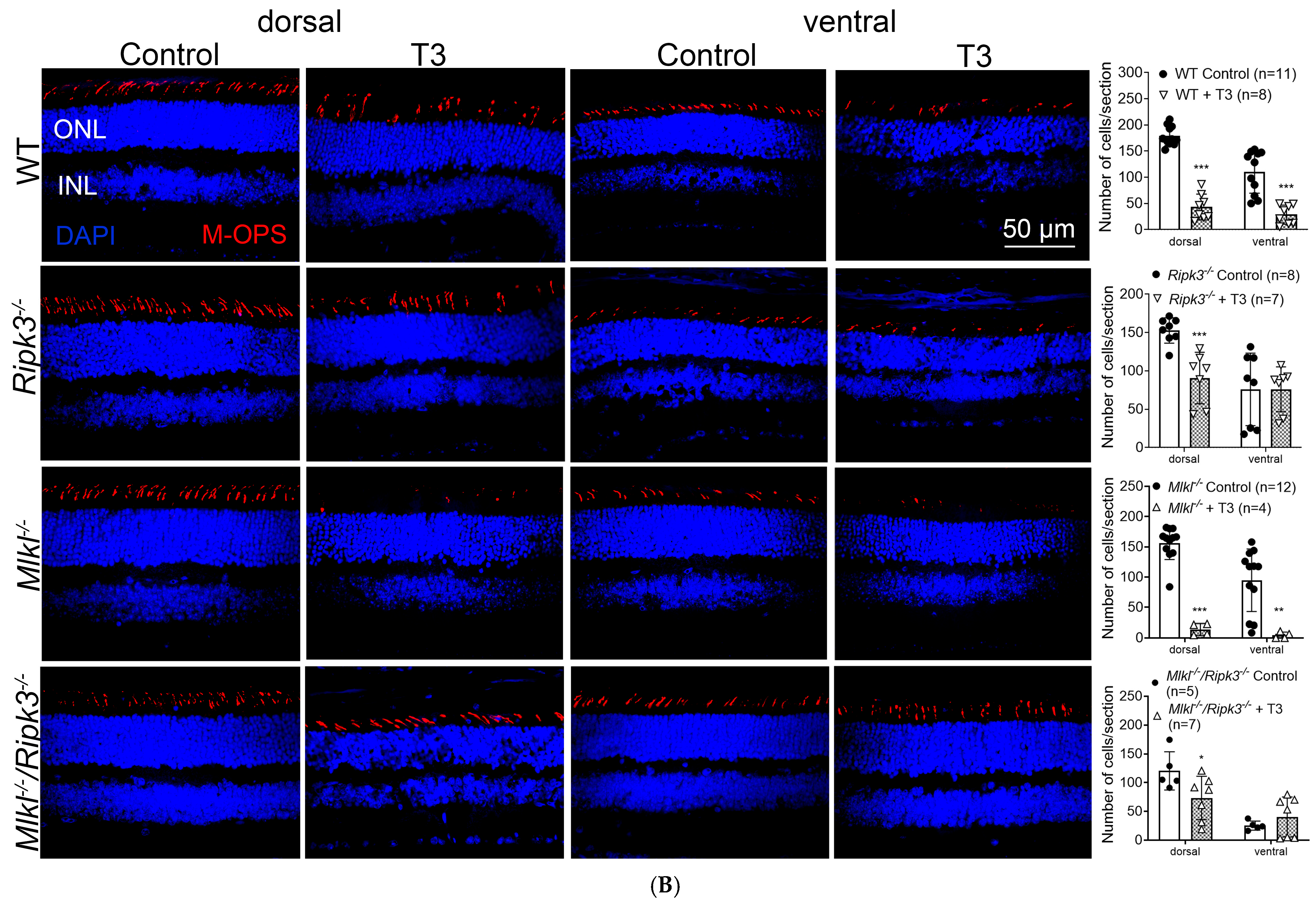
2.1. Deletion of Ripk3 Preserved Retinal/Rod Integrity After T3 Treatment
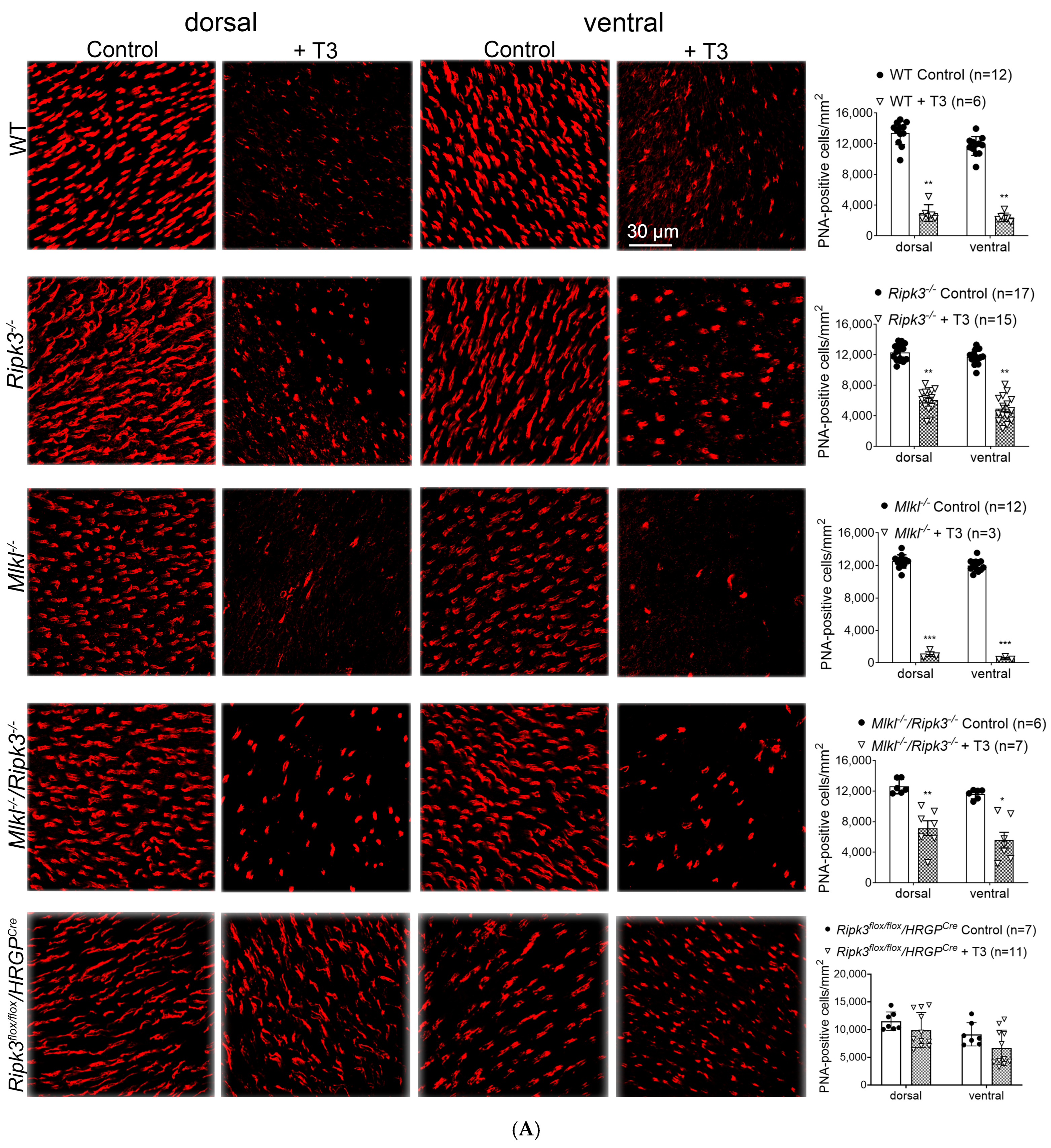
2.2. Deletion of Ripk3 Preserved Cones After T3 Treatment
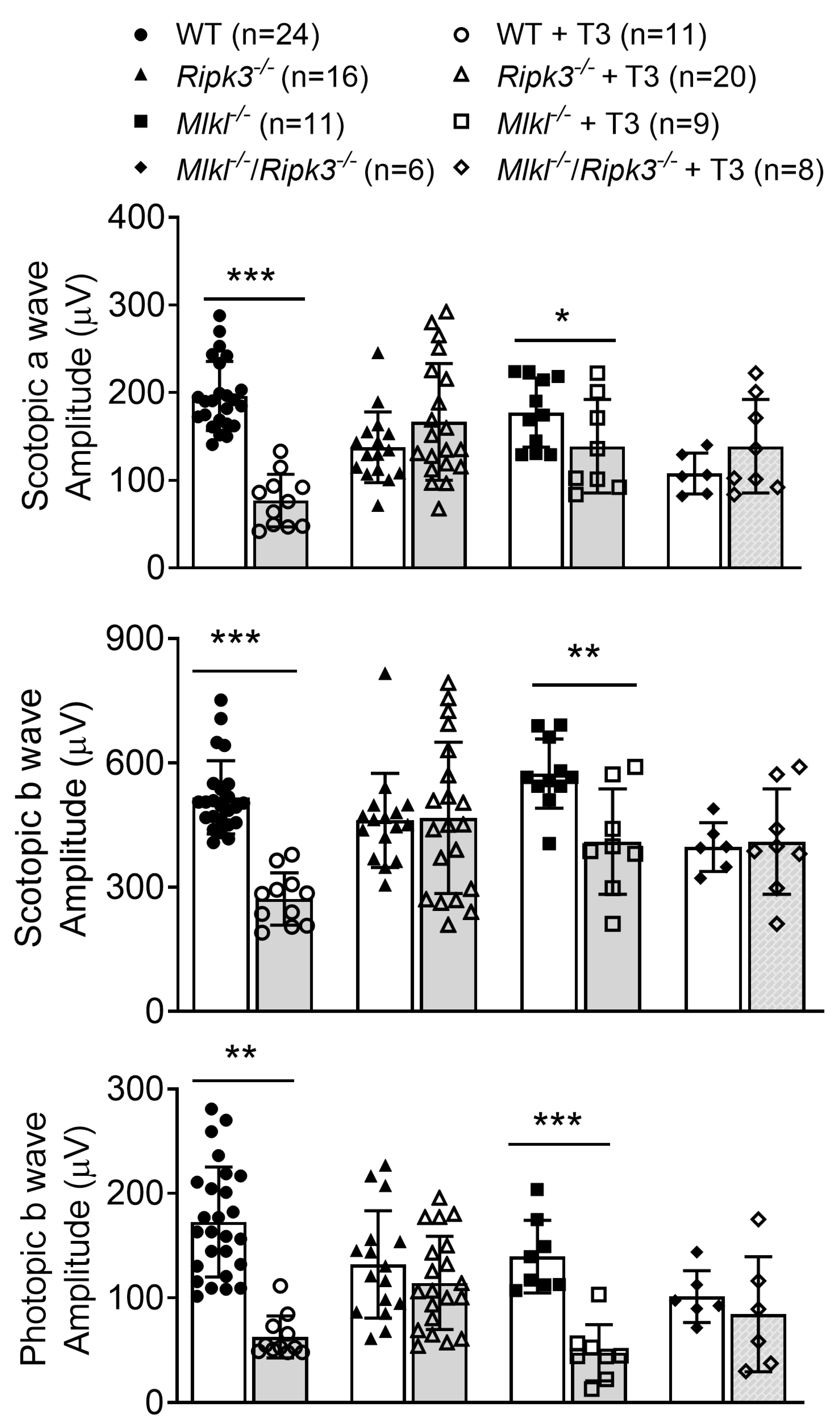
2.3. Deletion of Ripk3 Preserved Retinal Function After T3 Treatment
2.4. Deletion of Ripk3 Reduced Photoreceptor Apoptosis After T3 Treatment
2.5. Deletion of Ripk3 Reduced Photoreceptor DNA Damage/Oxidative Stress After T3 Treatment
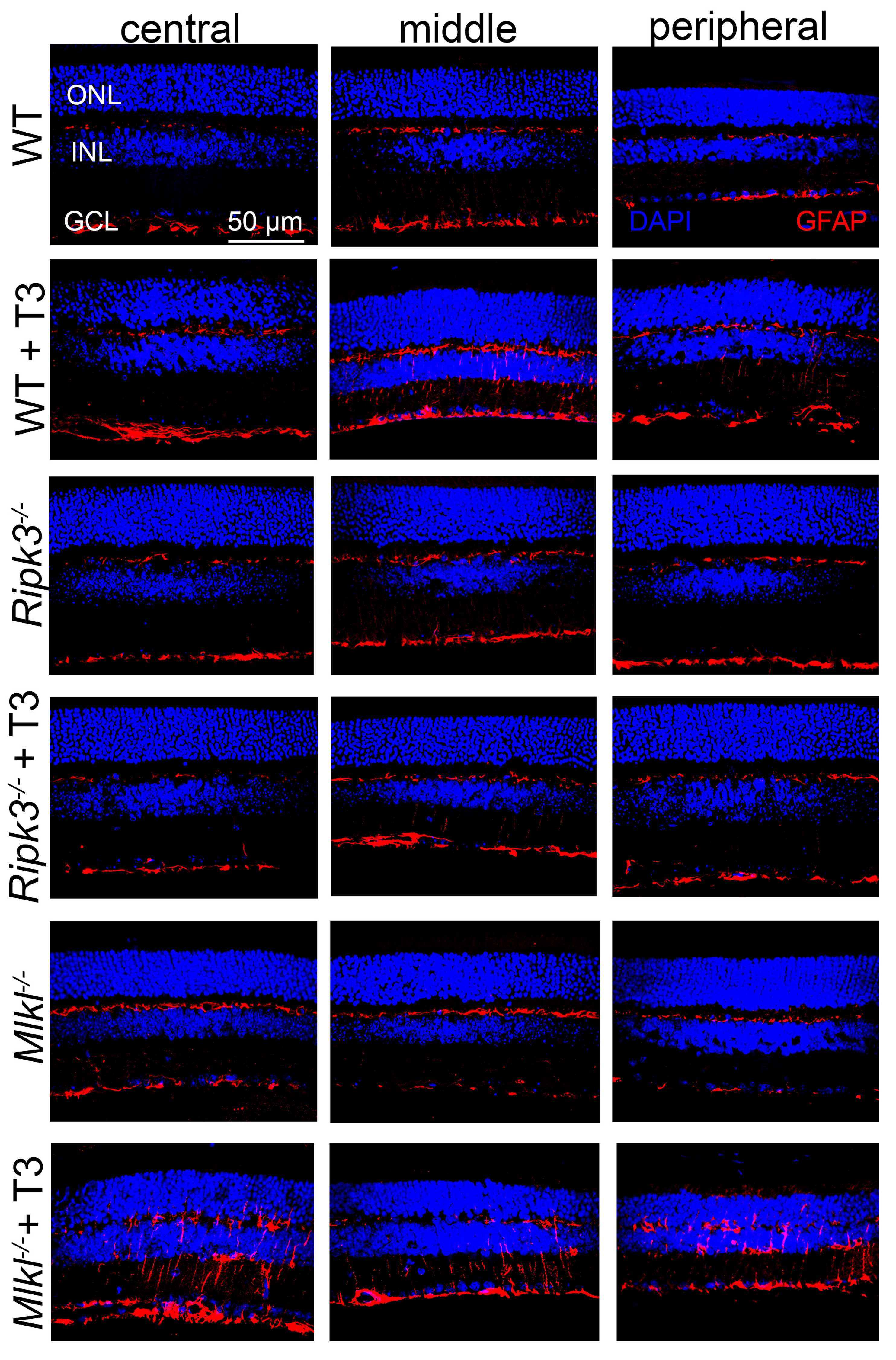
2.6. Deletion of Ripk3 Reduced Retinal Macroglial Cell Activation After T3 Treatment
2.7. Deletion of Ripk3 Diminished the Expression of the Inflammatory Genes After T3 Treatment
3. Discussion
3.1. RIPK3 Contributes to T3-Induced Photoreceptor Degeneration, and This Action Is Likely via a Necrosome-Independent Mechanism
3.2. RIPK3 Plays a Role in T3-Induced Photoreceptor Apoptosis
3.3. RIPK3 Plays a Role in T3-Induced Photoreceptor DNA Damage/Oxidative Stress
3.4. RIPK3 Plays a Role in T3-Induced Upregulation of the Inflammatory Genes in the Retina and Activation of Retinal Macroglial Cells
3.5. Potential Influence of RPE in TH-Induced Photoreceptor Degeneration
4. Materials and Methods
4.1. Mice, Antibodies, and Reagents
4.2. T3 Treatment
4.3. Scotopic and Photopic Electroretinography Recordings
4.4. Eye Preparation, Immunofluorescence Labeling, Confocal Microscopy, and Morphometric Analysis
4.5. TUNEL
4.6. RNA Isolation and Quantitative Real-Time PCR
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, J.; Yang, X.; Dong, A.; Petters, R.M.; Peng, Y.; Wong, F.; Campochiaro, P.A. Oxidative damage is a potential cause of cone cell death in retinitis pigmentosa. J. Cell. Physiol. 2005, 203, 457–464. [Google Scholar] [CrossRef]
- Komeima, K.; Rogers, B.S.; Lu, L.; Campochiaro, P.A. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc. Natl. Acad. Sci. USA 2006, 103, 11300–11305. [Google Scholar] [CrossRef]
- Allocca, M.; Corrigan, J.J.; Mazumder, A.; Fake, K.R.; Samson, L.D. Inflammation, necrosis, and the kinase RIP3 are key mediators of AAG-dependent alkylation-induced retinal degeneration. Sci. Signal. 2019, 12, eaau9216. [Google Scholar] [CrossRef]
- Cruz-Guilloty, F.; Saeed, A.M.; Echegaray, J.J.; Duffort, S.; Ballmick, A.; Tan, Y.; Betancourt, M.; Viteri, E.; Ramkhellawan, G.C.; Ewald, E.; et al. Infiltration of proinflammatory m1 macrophages into the outer retina precedes damage in a mouse model of age-related macular degeneration. Int. J. Inflam. 2013, 2013, 503725. [Google Scholar] [CrossRef]
- Huang, Z.; Zhou, T.; Sun, X.; Zheng, Y.; Cheng, B.; Li, M.; Liu, X.; He, C. Necroptosis in microglia contributes to neuroinflammation and retinal degeneration through TLR4 activation. Cell Death Differ. 2018, 25, 180–189. [Google Scholar] [CrossRef]
- Dunaief, J.L.; Dentchev, T.; Ying, G.-S.; Milam, A.H. The role of apoptosis in age-related macular degeneration. Arch. Ophthalmol. 2002, 120, 1435–1442. [Google Scholar] [CrossRef]
- Sanges, D.; Comitato, A.; Tammaro, R.; Marigo, V. Apoptosis in retinal degeneration involves cross-talk between apoptosis-inducing factor (AIF) and caspase-12 and is blocked by calpain inhibitors. Proc. Natl. Acad. Sci. USA 2006, 103, 17366–17371. [Google Scholar] [CrossRef]
- Viringipurampeer, I.A.; Shan, X.; Gregory-Evans, K.; Zhang, J.P.; Mohammadi, Z.; Gregory-Evans, C.Y. Rip3 knockdown rescues photoreceptor cell death in blind pde6c zebrafish. Cell Death Differ. 2014, 21, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Brent, G.A. Mechanisms of thyroid hormone action. J. Clin. Investig. 2012, 122, 3035–3043. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.Y.; Leonard, J.L.; Davis, P.J. Molecular aspects of thyroid hormone actions. Endocr. Rev. 2010, 31, 139–170. [Google Scholar] [CrossRef] [PubMed]
- Forrest, D.; Reh, T.A.; Rusch, A. Neurodevelopmental control by thyroid hormone receptors. Curr. Opin. Neurobiol. 2002, 12, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Ma, H.; Boye, S.L.; Hauswirth, W.W.; Ding, X.-Q. Overexpression of Type 3 Iodothyronine Deiodinase Reduces Cone Death in the Leber Congenital Amaurosis Model Mice. Adv. Exp. Med. Biol. 2018, 1074, 125–131. [Google Scholar]
- Yang, F.; Ma, H.; Butler, M.R.; Ding, X.-Q. Deficiency of type 2 iodothyronine deiodinase reduces necroptosis activity and oxidative stress responses in retinas of Leber congenital amaurosis model mice. FASEB J. 2018, 32, fj201800484RR. [Google Scholar]
- Ma, H.; Thapa, A.; Morris, L.; Redmond, T.M.; Baehr, W.; Ding, X.-Q. Suppressing thyroid hormone signaling preserves cone photoreceptors in mouse models of retinal degeneration. Proc. Natl. Acad. Sci. USA 2014, 111, 3602–3607. [Google Scholar] [CrossRef]
- Ma, H.W.; Yang, F.; Butler, M.R.; Belcher, J.; Redmond, T.M.; Placzek, A.T.; Scanlan, T.S.; Ding, X. Inhibition of thyroid hormone receptor locally in the retina is a therapeutic strategy for retinal degeneration. FASEB J. 2017, 31, 3425–3438. [Google Scholar] [CrossRef]
- Yang, F.; Ma, H.; Belcher, J.; Butler, M.R.; Redmond, T.M.; Boye, S.L.; Hauswirth, W.W.; Ding, X. Targeting iodothyronine deiodinases locally in the retina is a therapeutic strategy for retinal degeneration. FASEB J. 2016, 30, 4313–4325. [Google Scholar] [CrossRef]
- Ma, H.; Yang, F.; Ding, X.Q. Inhibition of thyroid hormone signaling protects retinal pigment epithelium and photoreceptors from cell death in a mouse model of age-related macular degeneration. Cell Death Dis. 2020, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Yang, F.; Ding, X.Q. Deficiency of thyroid hormone receptor protects retinal pigment epithelium and photoreceptors from cell death in a mouse model of age-related macular degeneration. Cell Death Dis. 2022, 13, 255. [Google Scholar] [CrossRef]
- Ma, H.; Yang, F.; York, L.R.; Li, S.; Ding, X.-Q. Excessive Thyroid Hormone Signaling Induces Photoreceptor Degeneration in Mice. eNeuro 2023, 10, ENEURO.0058-23.2023. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.; Lyubarsky, A.; Nikonov, S.S.; Ma, M.; Srinivas, M.; Kefas, B.; Germain, D.L.S.; Hernandez, A.; Pugh, E.N., Jr.; Forrest, D. Type 3 deiodinase, a thyroid-hormone-inactivating enzyme, controls survival and maturation of cone photoreceptors. J. Neurosci. 2010, 30, 3347–3357. [Google Scholar] [CrossRef]
- Li, S.; Ma, H.; Ding, X.Q. Resveratrol Protects Photoreceptors in Mouse Models of Retinal Degeneration. Antioxidants 2025, 14, 154. [Google Scholar] [CrossRef]
- Chaker, L.; Buitendijk, G.H.; Dehghan, A.; Medici, M.; Hofman, A.; Vingerling, J.R.; Franco, O.H.; Klaver, C.C.; Peeters, R.P. Thyroid function and age-related macular degeneration: A prospective population-based cohort study—The Rotterdam Study. BMC Med. 2015, 13, 94. [Google Scholar] [CrossRef]
- Gopinath, B.; Liew, G.; Kifley, A.; Mitchell, P. Thyroid Dysfunction and Ten-Year Incidence of Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5273–5277. [Google Scholar] [CrossRef] [PubMed]
- Age-Related Eye Disease Study Research Group. Risk factors associated with age-related macular degeneration. A case-control study in the age-related eye disease study: Age-Related Eye Disease Study Report Number 3. Ophthalmology 2000, 107, 2224–2232. [Google Scholar]
- Lin, S.Y.; Hsu, W.-H.; Lin, C.-L.; Lin, C.-C.; Lin, J.-M.; Chang, Y.-L.; Hsu, C.-Y.; Kao, C.-H. Evidence for an Association between Macular Degeneration and Thyroid Cancer in the Aged Population. Int. J. Environ. Res. Public Health 2018, 15, 902. [Google Scholar] [CrossRef]
- Chatziralli, I.; MMitropoulos, P.G.; Niakas, D.; Labiris, G. Thyroidopathy and Age-Related Macular Degeneration: Is There Any Correlation. Biomed. Hub 2017, 2, 54706. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.H.; Xirasagar, S.; Kuang, T.-M.T.; Chang, W.-W.; Cheng, Y.-F.; Kuo, N.-W.; Lin, H.-C. Association of Age-Related Macular Degeneration with Prior Hyperthyroidism and Hypothyroidism: A Case-Control Study. J. Pers. Med. 2022, 12, 602. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, M.; Zhang, Q.; Xu, T.; Tao, L. Thyroid Disease Is Associated with Higher Age-Related Macular Degeneration Risk: Results from a Meta-Analysis of Epidemiologic Studies. Ophthalmic Res. 2021, 64, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Farvardin, M.; Mousavi, S.E.; Zare, K.; Bazdar, S.; Farvardin, Z.; Johari, M. Thyroid Dysfunction as a Modifiable Risk Factor for Wet Type Age-Related Macular Degeneration: A Case-Control Study. J. Curr. Ophthalmol. 2021, 33, 449–452. [Google Scholar] [CrossRef]
- Li, X.; Cheng, J.; Wang, M.; Zhong, Y.; Shi, G.; Yu, A.-Y. Causal Associations of Thyroid Function and Age-Related Macular Degeneration: A Two-Sample Mendelian Randomization Study. Am. J. Ophthalmol. 2022, 239, 108–114. [Google Scholar] [CrossRef]
- Abdelkader, M.; Abass, N. The Relation Between Age Related Macular Degeneration and Thyroid Disorders. Int. J. Ophthalmol. Vis. Sci. 2019, 4, 101–105. [Google Scholar] [CrossRef]
- Blum Meirovitch, S.; Leibovitch, I.; Kesler, A.; Varssano, D.; Rosenblatt, A.; Neudorfer, M. Retina and Nerve Fiber Layer Thickness in Eyes with Thyroid-Associated Ophthalmopathy. Isr. Med. Assoc. J. 2017, 19, 277–281. [Google Scholar] [PubMed]
- Sayin, O.; Yeter, V.; Ariturk, N. Optic Disc, Macula, and Retinal Nerve Fiber Layer Measurements Obtained by OCT in Thyroid-Associated Ophthalmopathy. J. Ophthalmol. 2016, 2016, 9452687. [Google Scholar] [CrossRef]
- Ceresini, G.; Lauretani, F.; Maggio, M.; Ceda, G.P.; Morganti, S.; Usberti, E.; Chezzi, C.; Valcavi, R.; Bandinelli, S.; Guralnik, J.M.; et al. Thyroid function abnormalities and cognitive impairment in elderly people: Results of the Invecchiare in Chianti study. J. Am. Geriatr. Soc. 2009, 57, 89–93. [Google Scholar] [CrossRef]
- Kalmijn, S.; Mehta, K.M.; Pols, H.A.P.; Hofman, A.; Drexhage, H.A.; Breteler, M.M.B. Subclinical hyperthyroidism and the risk of dementia. The Rotterdam study. Clin. Endocrinol. 2000, 53, 733–737. [Google Scholar] [CrossRef]
- Cho, Y.Y.; Kim, B.; Shin, D.W.; Youn, J.; Mok, J.O.; Kim, C.-H.; Kim, S.W.; Chung, J.H.; Han, K.; Kim, T.H. Graves’ disease and the risk of Parkinson’s disease: A Korean population-based study. Brain Commun. 2022, 4, fcac014. [Google Scholar] [CrossRef]
- Vandenabeele, P.; Galluzzi, L.; Vanden Berghe, T.V.; Kroemer, G. Molecular mechanisms of necroptosis: An ordered cellular explosion. Nat. Rev. Mol. Cell Biol. 2010, 11, 700–714. [Google Scholar] [CrossRef]
- Newton, K. RIPK1 and RIPK3: Critical regulators of inflammation and cell death. Trends Cell Biol. 2015, 25, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Paudel, S.; Jeyaseelan, S. Kill Two Birds with One Stone: Role of the RIPK-3 in Necroptosis and Inflammasome Activation. Am. J. Respir. Cell Mol. Biol. 2021, 64, 525–527. [Google Scholar] [CrossRef]
- Moriwaki, K.; Balaji, S.; McQuade, T.; Malhotra, N.; Kang, J.; Chan, F.K.-M. The necroptosis adaptor RIPK3 promotes injury-induced cytokine expression and tissue repair. Immunity 2014, 41, 567–578. [Google Scholar] [CrossRef]
- Afonso, M.B.; Islam, T.; Magusto, J.; Amorim, R.; Lenoir, V.; Simões, R.F.; Teixeira, J.; Silva, L.C.; Wendum, D.; Jéru, I.; et al. RIPK3 dampens mitochondrial bioenergetics and lipid droplet dynamics in metabolic liver disease. Hepatology 2023, 77, 1319–1334. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.; Berger, S.B.; Pillay, S.; Moriwaki, K.; Huang, C.; Guo, H.; Lich, J.D.; Finger, J.; Kasparcova, V.; Votta, B.; et al. RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol. Cell 2014, 56, 481–495. [Google Scholar] [CrossRef] [PubMed]
- York, L.; Ma, H.; Yang, F.; Primeaux, C.; Griffin, C.; Ding, X.Q. The Role of RIPK3 Signaling in Thyroid Hormone-Induced Photoreceptor Degeneration. In Proceedings of the 2023 ARVO Annual Meeting, New Orleans, LA, USA, 23–27 April 2023. [Google Scholar]
- Wang, H.; Sun, L.; Su, L.; Rizo, J.; Liu, L.; Wang, L.-F.; Wang, F.-S.; Wang, X. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol. Cell 2014, 54, 133–146. [Google Scholar] [CrossRef]
- Liu, S.; Liu, H.; Johnston, A.; Hanna-Addams, S.; Reynoso, E.; Xiang, Y.; Wang, Z. MLKL forms disulfide bond-dependent amyloid-like polymers to induce necroptosis. Proc. Natl. Acad. Sci. USA 2017, 114, E7450–E7459. [Google Scholar] [CrossRef]
- Cai, Z.; Jitkaew, S.; Zhao, J.; Chiang, H.-C.; Choksi, S.; Liu, J.; Ward, Y.; Wu, L.-G.; Liu, Z.-G. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat. Cell Biol. 2014, 16, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Huang, Z.; Ren, J.; Zhang, Z.; He, P.; Li, Y.; Ma, J.; Chen, W.; Zhang, Y.; Zhou, X.; et al. Mlkl knockout mice demonstrate the indispensable role of Mlkl in necroptosis. Cell Res. 2013, 23, 994–1006. [Google Scholar] [CrossRef]
- Sun, L.; Wang, H.; Wang, Z.; He, S.; Chen, S.; Liao, D.; Wang, L.; Yan, J.; Liu, W.; Lei, X.; et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 2012, 148, 213–227. [Google Scholar] [CrossRef]
- Murakami, Y.; Matsumoto, H.; Roh, M.; Suzuki, J.; Hisatomi, T.; Ikeda, Y.; Miller, J.W.; Vavvas, D.G. Receptor interacting protein kinase mediates necrotic cone but not rod cell death in a mouse model of inherited degeneration. Proc. Natl. Acad. Sci. USA 2012, 109, 14598–14603. [Google Scholar] [CrossRef]
- Trichonas, G.; Murakami, Y.; Thanos, A.; Morizane, Y.; Kayama, M.; Debouck, C.M.; Hisatomi, T.; Miller, J.W.; Vavvas, D.G. Receptor interacting protein kinases mediate retinal detachment-induced photoreceptor necrosis and compensate for inhibition of apoptosis. Proc. Natl. Acad. Sci. USA 2010, 107, 21695–21700. [Google Scholar] [CrossRef]
- Moriwaki, K.; Chan, F.K. Necrosis-dependent and independent signaling of the RIP kinases in inflammation. Cytokine Growth Factor Rev. 2014, 25, 167–174. [Google Scholar] [CrossRef]
- Li, D.; Chen, J.; Guo, J.; Li, L.; Cai, G.; Chen, S.; Huang, J.; Yang, H.; Zhuang, Y.; Wang, F.; et al. A phosphorylation of RIPK3 kinase initiates an intracellular apoptotic pathway that promotes prostaglandin(2alpha)-induced corpus luteum regression. Elife 2021, 10, e67409. [Google Scholar] [CrossRef]
- Newton, K.; Zhang, J.; Wang, Z.; Li, T.; Liu, C.; Kang, X.; Cui, X.; Yang, J.; Qu, H.; Duanmu, J.; et al. Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science 2014, 343, 1357–1360. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, J.; Wang, Z.; Li, T.; Liu, C.; Kang, X.; Cui, X.; Yang, J.; Qu, H.; Duanmu, J.; et al. Extracellular RIPK3 Acts as a Damage-Associated Molecular Pattern to Exaggerate Cardiac Ischemia/Reperfusion Injury. Circulation 2024, 150, 1791–1811. [Google Scholar] [CrossRef]
- Zhao, X.; Quan, J.; Tan, Y.; Liu, Y.; Liao, C.; Li, Z.; Liao, W.; Liu, J.; Cao, Y.; Luo, X. RIP3 mediates TCN-induced necroptosis through activating mitochondrial metabolism and ROS production in chemotherapy-resistant cancers. Am. J. Cancer Res. 2021, 11, 729–745. [Google Scholar]
- Sureshbabu, A.; Patino, E.; Ma, K.C.; Laursen, K.; Finkelsztein, E.J.; Akchurin, O.; Muthukumar, T.; Ryter, S.W.; Gudas, L.; Choi, A.M.K.; et al. RIPK3 promotes sepsis-induced acute kidney injury via mitochondrial dysfunction. JCI Insight 2018, 3, e98411. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Stanford, D.; Freeman, W.M.; Ding, X.-Q. Transcriptomic Analysis Reveals That Excessive Thyroid Hormone Signaling Impairs Phototransduction and Mitochondrial Bioenergetics and Induces Cellular Stress in Mouse Cone Photoreceptors. Int. J. Mol. Sci. 2024, 25, 7435. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, K.; Bertin, J.; Gough, P.J.; Chan, F.K.-M. A RIPK3-caspase 8 complex mediates atypical pro-IL-1beta processing. J. Immunol. 2015, 194, 1938–1944. [Google Scholar] [CrossRef]
- Orozco, S.L.; Daniels, B.P.; Yatim, N.; Messmer, M.N.; Quarato, G.; Chen-Harris, H.; Cullen, S.P.; Snyder, A.G.; Ralli-Jain, P.; Frase, S.; et al. RIPK3 Activation Leads to Cytokine Synthesis that Continues after Loss of Cell Membrane Integrity. Cell Rep. 2019, 28, 2275–2287.e5. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Malik, A.; Balakrishnan, A.; Malireddi, R.K.S.; Kanneganti, T.-D. RIPK3 Promotes Mefv Expression and Pyrin Inflammasome Activation via Modulation of mTOR Signaling. J. Immunol. 2020, 205, 2778–2785. [Google Scholar] [CrossRef]
- Olivares-Gonzalez, L.; Velasco, S.; Campillo, I.; Rodrigo, R. Retinal Inflammation, Cell Death and Inherited Retinal Dystrophies. Int. J. Mol. Sci. 2021, 22, 2096. [Google Scholar] [CrossRef]
- Lai, D.; Wu, Y.; Shao, C.; Qiu, Q. The Role of Muller Cells in Diabetic Macular Edema. Investig. Ophthalmol. Vis. Sci. 2023, 64, 8. [Google Scholar] [CrossRef]
- Jahnke, L.; Zandi, S.; Elhelbawi, A.; Conedera, F.M.; Enzmann, V. Characterization of Macroglia Response during Tissue Repair in a Laser-Induced Model of Retinal Degeneration. Int. J. Mol. Sci. 2023, 24, 9172. [Google Scholar] [CrossRef]
- Ng, L.; Liu, H.; Liu, Y.; Forrest, D. Biphasic expression of thyroid hormone receptor TRbeta1 in mammalian retina and anterior ocular tissues. Front. Endocrinol. 2023, 14, 1174600. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ng, L.; Liu, H.; Heuer, H.; Forrest, D. Cone photoreceptor differentiation regulated by thyroid hormone transporter MCT8 in the retinal pigment epithelium. Proc. Natl. Acad. Sci. USA 2024, 121, e2402560121. [Google Scholar] [CrossRef]
- Ding, X.-Q.; Yang, F.; Butler, M.; Malek, G.; Ma, H. Thyroid Hormone Regulation of Retinal Pigment Epithelium Morphology and Survival. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1939. [Google Scholar]
- Murphy, J.M.; Czabotar, P.E.; Hildebrand, J.M.; Lucet, I.S.; Zhang, J.-G.; Alvarez-Diaz, S.; Lewis, R.; Lalaoui, N.; Metcalf, D.; Webb, A.I.; et al. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity 2013, 39, 443–453. [Google Scholar] [CrossRef]
- Colijn, S.; Gao, S.; Ingram, K.G.; Menendez, M.; Muthukumar, V.; Silasi-Mansat, R.; Chmielewska, J.J.; Hinsdale, M.; Lupu, F.; Griffin, C.T. The NuRD chromatin-remodeling complex enzyme CHD4 prevents hypoxia-induced endothelial Ripk3 transcription and murine embryonic vascular rupture. Cell Death Differ. 2020, 27, 618–631. [Google Scholar] [CrossRef] [PubMed]
- Le, Y.Z.; Zheng, L.; Zheng, W.; Ash, J.D.; Agbaga, M.-P.; Zhu, M.; E Anderson, R. Mouse opsin promoter-directed Cre recombinase expression in transgenic mice. Mol. Vis. 2006, 12, 389–398. [Google Scholar]
- Le, Y.Z.; Ash, J.D.; Al-Ubaidi, M.R.; Chen, Y.; Ma, J.-X.; Anderson, R.E. Targeted expression of Cre recombinase to cone photoreceptors in transgenic mice. Mol. Vis. 2004, 10, 1011–1018. [Google Scholar]
- Hamidi, S.; Aliesky, H.; Chen, C.-R.; Rapoport, B.; McLachlan, S.M. Variable suppression of serum thyroxine in female mice of different inbred strains by triiodothyronine administered in drinking water. Thyroid 2010, 20, 1157–1162. [Google Scholar] [CrossRef]




| Antibodies/Reagent | Vendor | Catalog Number | Dilutions Used in IF or IB |
|---|---|---|---|
| 3,3′,5-triiodo-L-thyronine | Millipore Sigma (Darmstadt, Germany) | T2877 | |
| DAPI (4,6-Diamidino-2-phenylindole) | Millipore Sigma | D9542 | 1:2000 (IF) |
| biotinylated PNA | Vector Labs (Newark, CA, USA) | B-1075 | 1:200 (IF) |
| Anti-M-opsin | Millipore Sigma | AB5405 | 1:200 (IF) |
| TUNEL | Sigma-Aldrich (Saint Louis, MO, USA) | 11684795910 | 1:10 (IF) |
| anti-γH2AX (p Ser139) | Novus (Centennial, CO, USA) | NB100-2280 | 1:200 (IF) |
| anti-GFAP | DAKO (Glostrup, Denmark) | Z0334 | 1:500 (IF) |
| Alexa Fluor® 555 goat anti-rabbit IgG | ThermoFisher Scientific (Waltham, MA USA) | A21428 | 1:500 (IF) |
| Streptavidin-Cy3 | ThermoFisher Scientific | SA1010 | 1:500 (IF) |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Hprt1 | GCAAACTTTGCTTTCCCTGGTT | CAAGGGCATATCCAACAACA |
| Nlrp3 | CTCCAACCATTCTCTGACCAG | ACAGATTGAAGTAAGGCCGG |
| Il1α | TGCAGTCCATAACCCATGATC | ACAAACTTCTGCCTGACGAG |
| Il1β | ACGGACCCCAAAAGATGAAG | TTCTCCACAGCCACAATGAG |
| Il6 | CAAAGCCAGAGTCCTTCAGAG | GTCCTTAGCCACTCCTTCTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
York, L.R.; Ma, H.; Le, Y.; Griffin, C.T.; Ding, X.-Q. RIPK3 Contributes to Thyroid Hormone-Induced Photoreceptor Degeneration. Int. J. Mol. Sci. 2025, 26, 8154. https://doi.org/10.3390/ijms26178154
York LR, Ma H, Le Y, Griffin CT, Ding X-Q. RIPK3 Contributes to Thyroid Hormone-Induced Photoreceptor Degeneration. International Journal of Molecular Sciences. 2025; 26(17):8154. https://doi.org/10.3390/ijms26178154
Chicago/Turabian StyleYork, Lilliana R., Hongwei Ma, Yun Le, Courtney T. Griffin, and Xi-Qin Ding. 2025. "RIPK3 Contributes to Thyroid Hormone-Induced Photoreceptor Degeneration" International Journal of Molecular Sciences 26, no. 17: 8154. https://doi.org/10.3390/ijms26178154
APA StyleYork, L. R., Ma, H., Le, Y., Griffin, C. T., & Ding, X.-Q. (2025). RIPK3 Contributes to Thyroid Hormone-Induced Photoreceptor Degeneration. International Journal of Molecular Sciences, 26(17), 8154. https://doi.org/10.3390/ijms26178154





