Endothelial-Enriched lncRNA Gm39822 Modulates Inflammation and Dysfunction in Non-Diabetic Endothelial Cells
Abstract
1. Introduction
2. Results
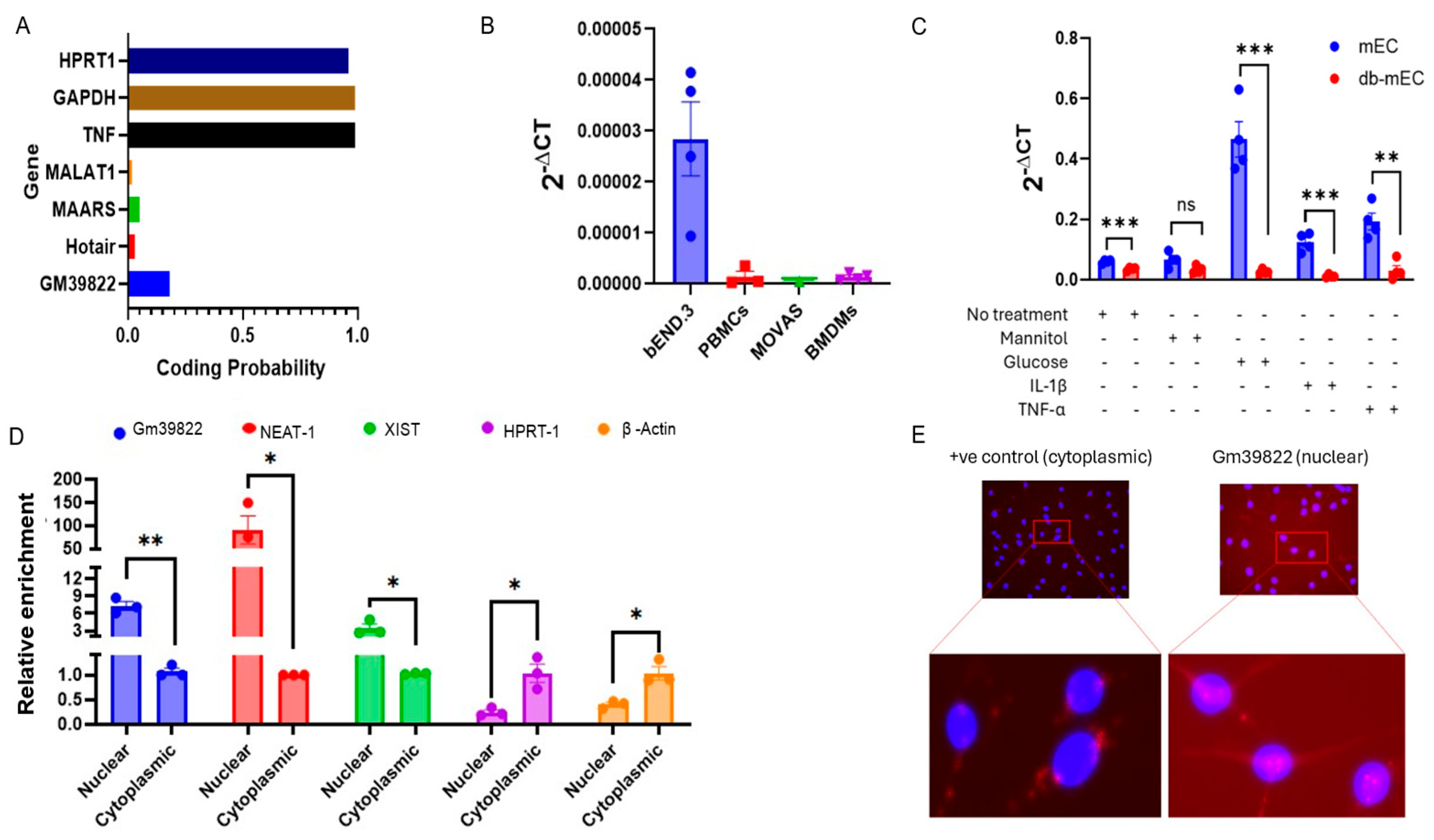
2.1. Gm39822 Is an Endothelial-Enriched lncRNA Localized in the Nucleus
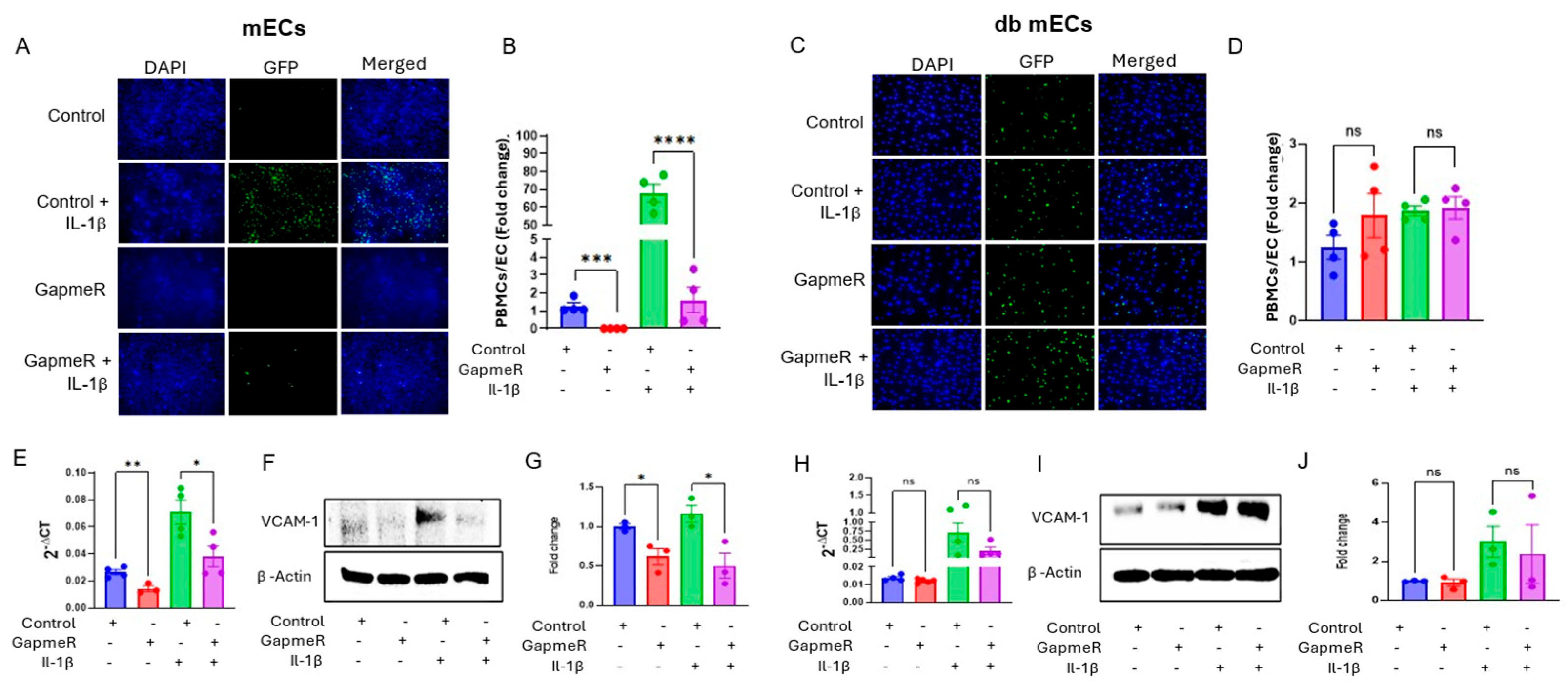
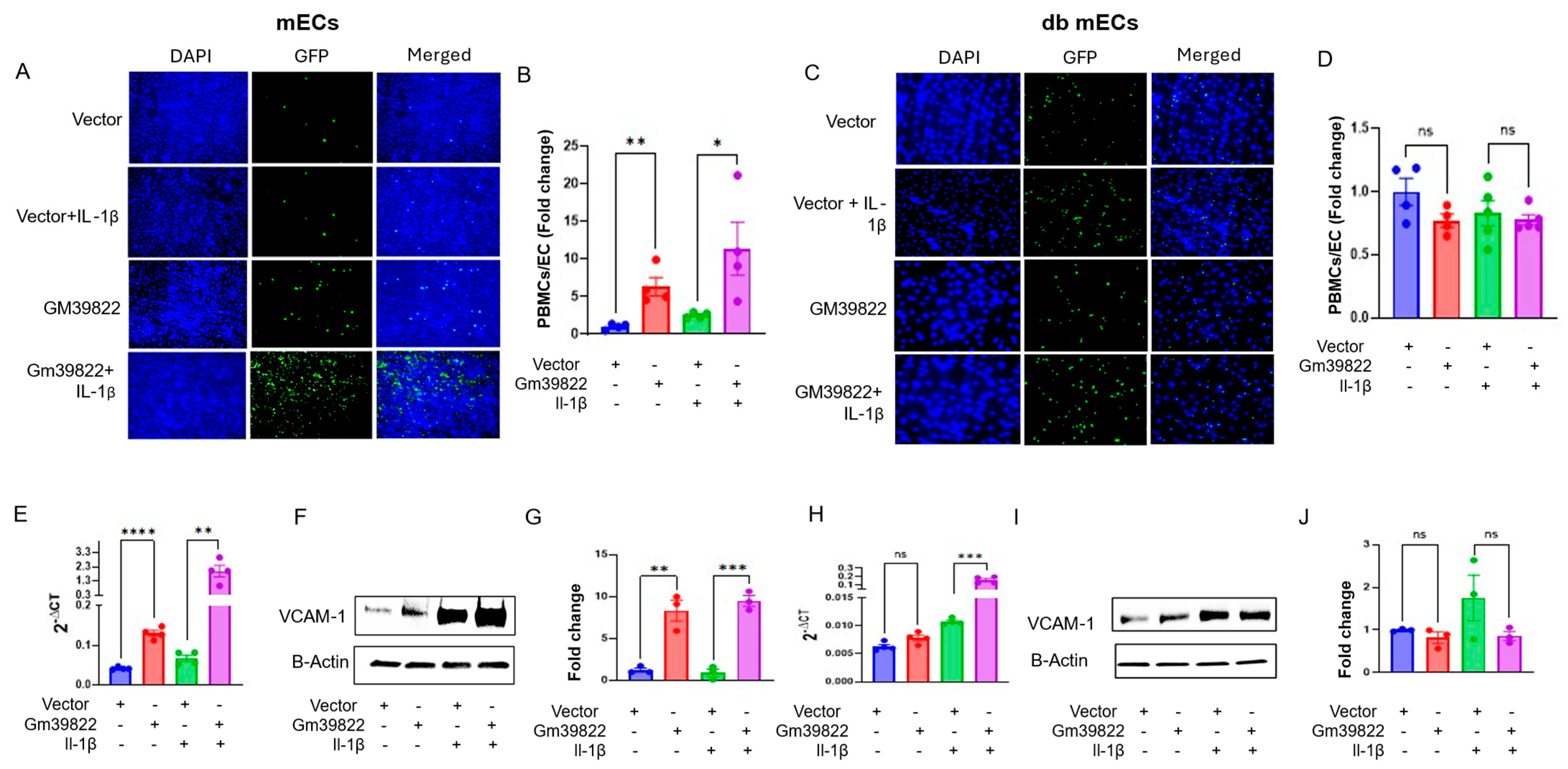
2.2. Gm39822 Promotes the Expression of VCAM-1 and Attachment of Monocytes to Non-Diabetic Endothelial Cells but Not in Diabetic Endothelial Cells
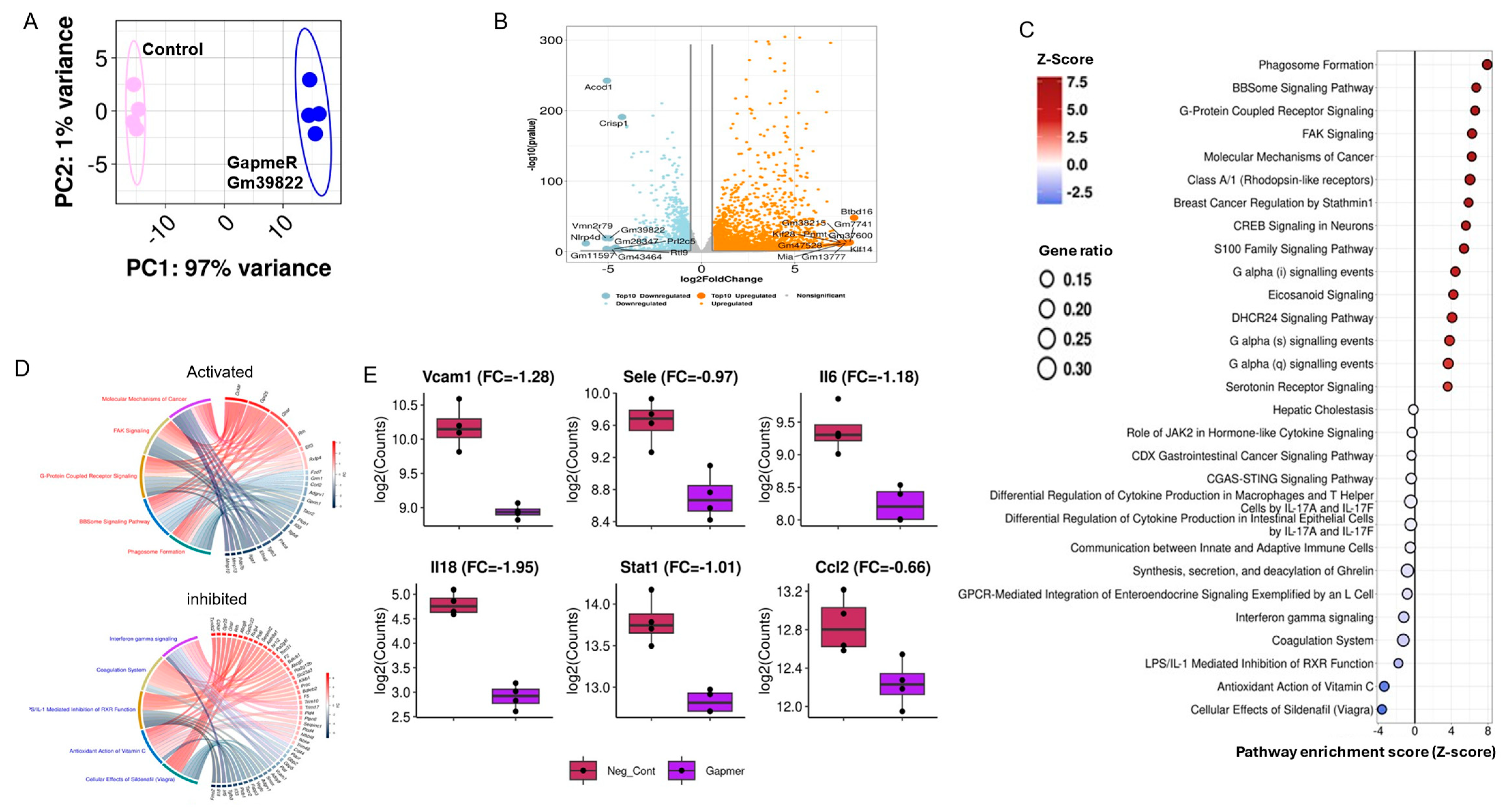
2.3. Gm39822 Regulates the Transcriptomic Network of Cytokine Production in Endothelial Cells
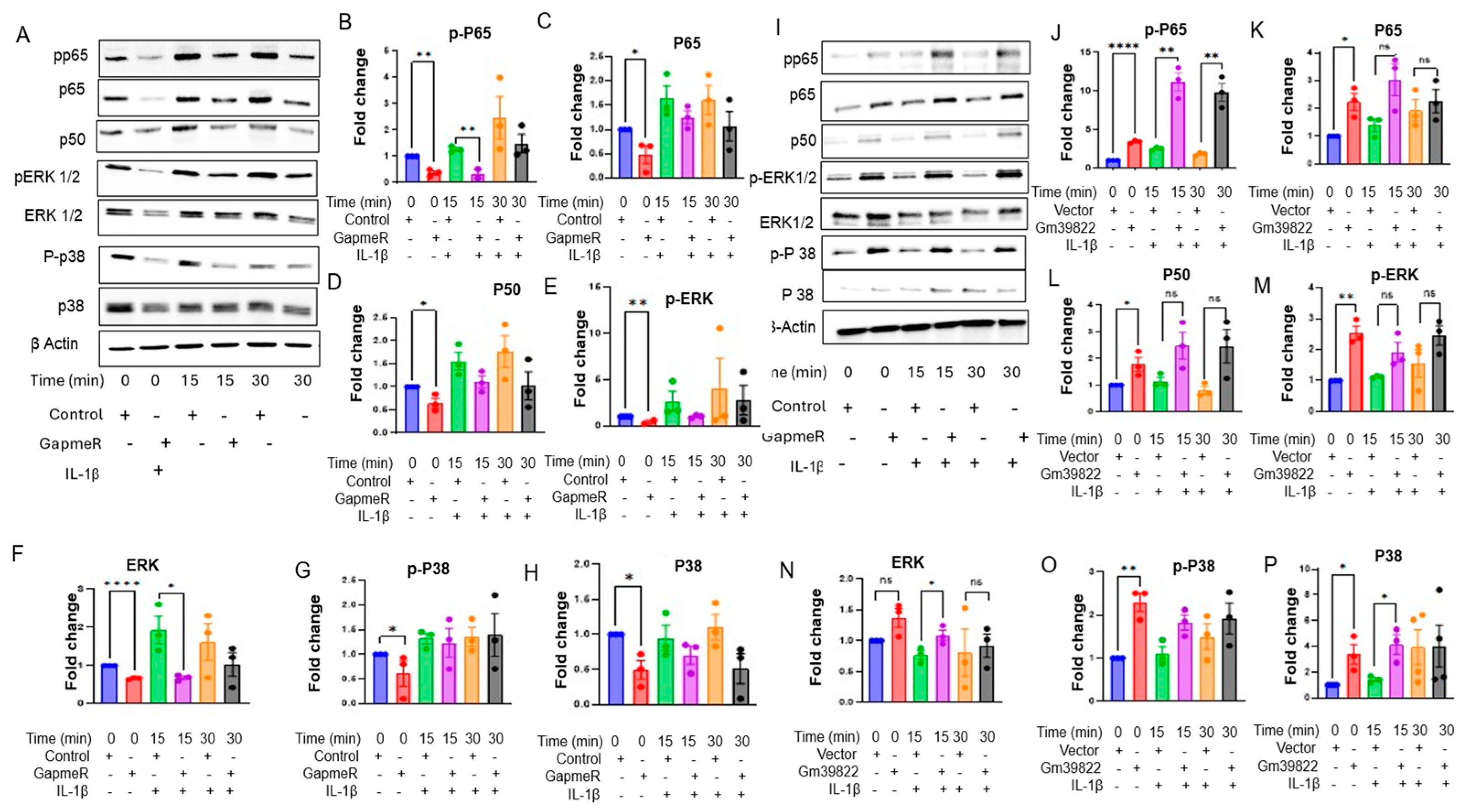
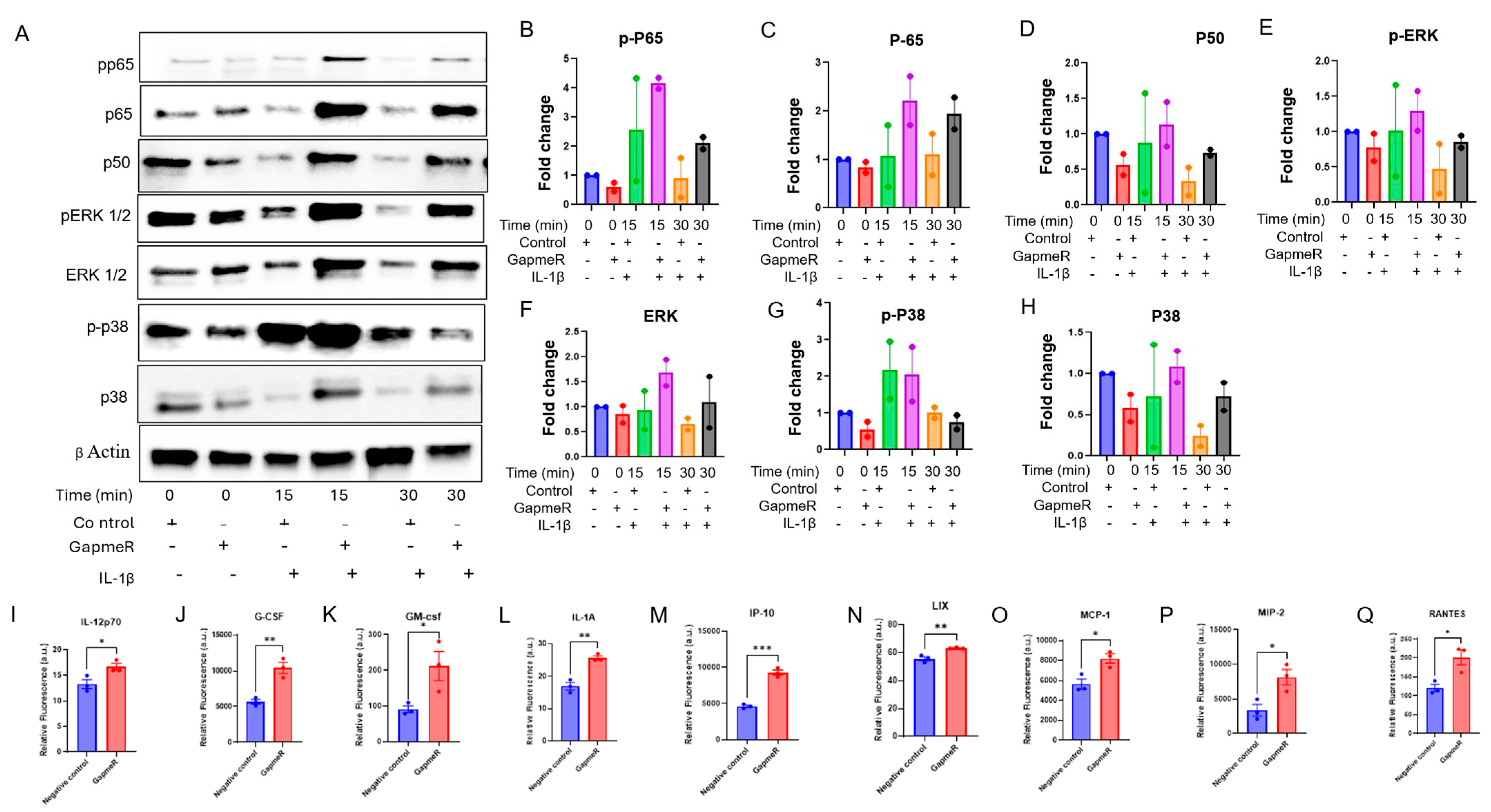
2.4. Knockdown of Gm39822 Suppresses Multiple Pathways of Inflammation in Non-Diabetic Endothelial Cells but Promotes Inflammatory Pathways in Diabetic Endothelial Cells
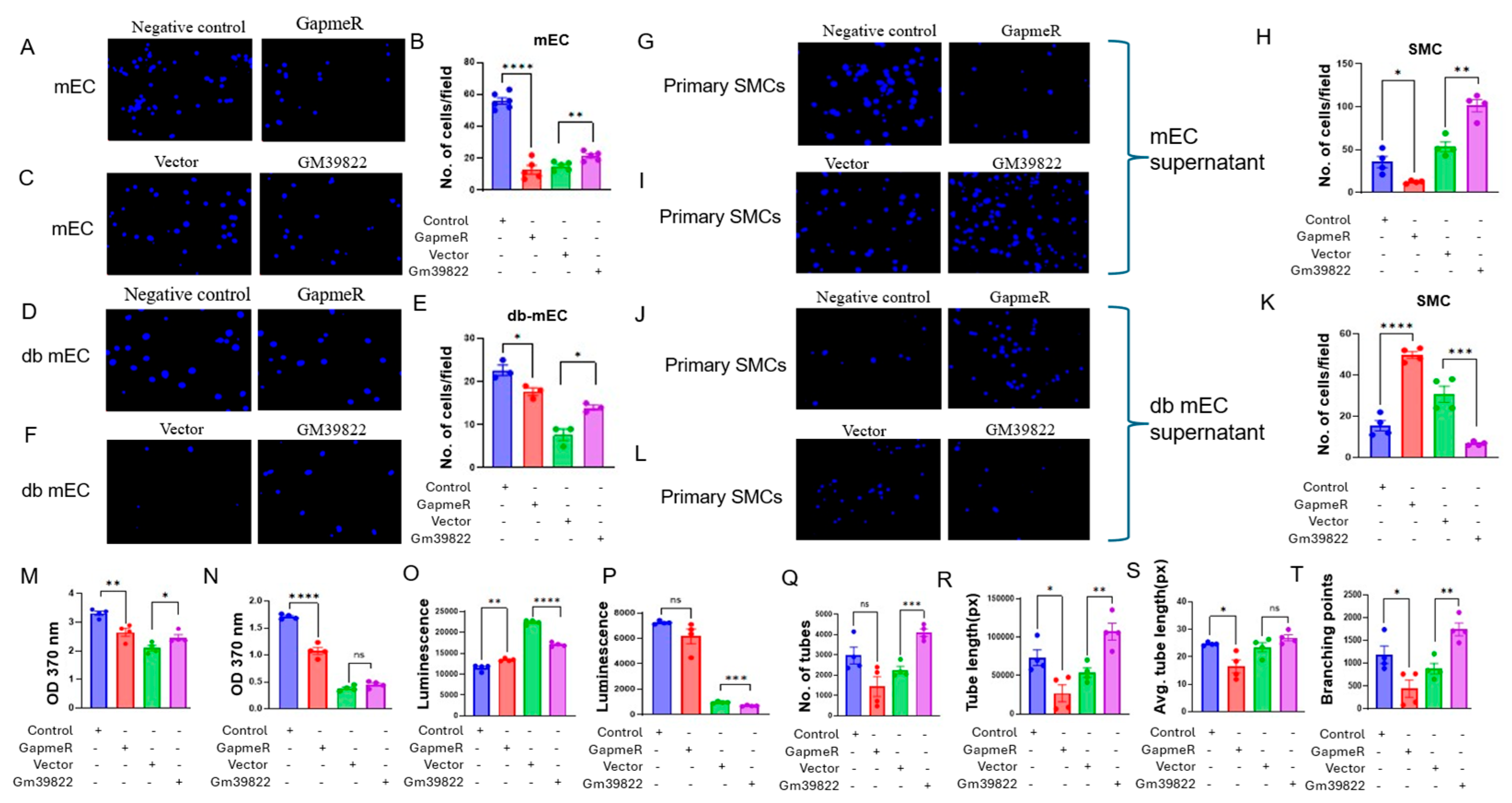
2.5. Endothelial Gm39822 Regulates Migration, Proliferation, Apoptosis, and Angiogenesis in ECs, as Well as the Migration of SMCs
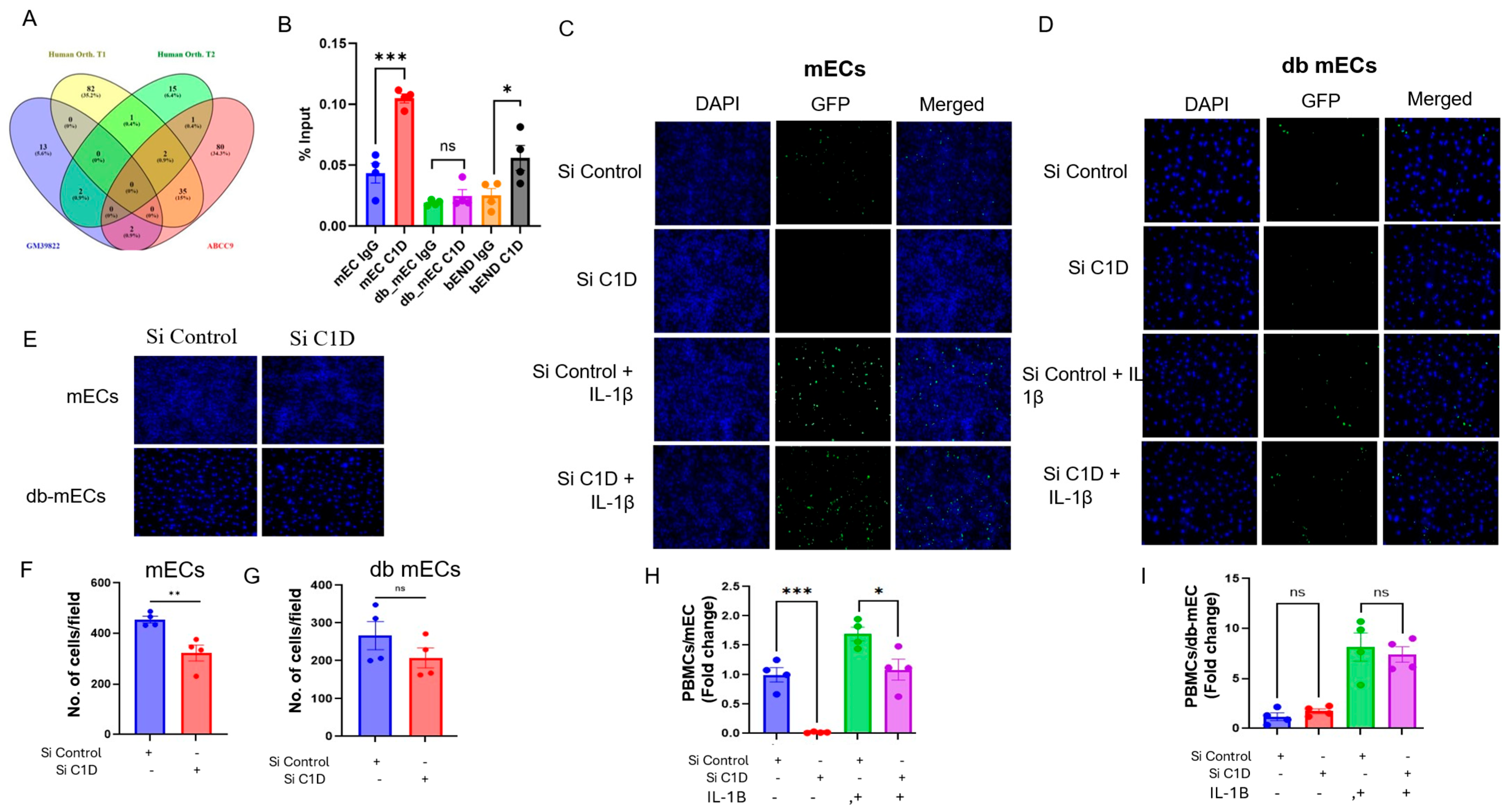
2.6. Gm39822 Interacts with C1D to Regulate Cellular Processes in Non-Diabetic Endothelial Cells
3. Discussion
4. Materials and Methods
4.1. RNA Isolation and qRT-PCR
4.2. Cellular Fractionation
4.3. Protein Coding Potential
4.4. RNA In Situ Hybridization (RNA-ISH)
4.5. Molecular Cloning for Gm39822 Overexpression
4.6. Cell Culture and Transfection
4.7. Cell Adhesion Assay
4.8. Western Blotting and Immunocytochemistry
4.9. Scratch Assay and Transwell Cell Migration Assay
4.10. Bromodeoxyuridine (BrdU) Incorporation Assay
4.11. Ribonucleoprotein Immunoprecipitation (RIP) Assay
4.12. Caspase 3/7 Activity Assay
4.13. Cytokine Profiling in Cell Culture Supernatants
4.14. RNA-Seq Analysis
4.15. Pathway Enrichment Analysis
4.16. Study Approval
4.17. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adhikary, D.; Barman, S.; Ranjan, R.; Stone, H. A Systematic Review of Major Cardiovascular Risk Factors: A Growing Global Health Concern. Cureus 2022, 14, e30119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, W.; Zhu, W.; Dong, J.; Cheng, Y.; Yin, Z.; Shen, F. Mechanisms and Functions of Long Non-Coding RNAs at Multiple Regulatory Levels. Int. J. Mol. Sci. 2019, 20, 5573. [Google Scholar] [CrossRef]
- Tsagakis, I.; Douka, K.; Birds, I.; Aspden, J.L. Long non-coding RNAs in development and disease: Conservation to mechanisms. J. Pathol. 2020, 250, 480–495. [Google Scholar] [CrossRef]
- Michalik, K.M.; You, X.; Manavski, Y.; Doddaballapur, A.; Zornig, M.; Braun, T.; John, D.; Ponomareva, Y.; Chen, W.; Uchida, S.; et al. Long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circ. Res. 2014, 114, 1389–1397. [Google Scholar] [CrossRef]
- Huang, T.; Zhao, H.Y.; Zhang, X.B.; Gao, X.L.; Peng, W.P.; Zhou, Y.; Zhao, W.H.; Yang, H.F. LncRNA ANRIL regulates cell proliferation and migration via sponging miR-339-5p and regulating FRS2 expression in atherosclerosis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1956–1969. [Google Scholar]
- Boulberdaa, M.; Scott, E.; Ballantyne, M.; Garcia, R.; Descamps, B.; Angelini, G.D.; Brittan, M.; Hunter, A.; McBride, M.; McClure, J.; et al. A Role for the Long Noncoding RNA SENCR in Commitment and Function of Endothelial Cells. Mol. Ther. 2016, 24, 978–990. [Google Scholar] [CrossRef]
- Haemmig, S.; Yang, D.; Sun, X.; Das, D.; Ghaffari, S.; Molinaro, R.; Chen, L.; Deng, Y.; Freeman, D.; Moullan, N.; et al. Long noncoding RNA SNHG12 integrates a DNA-PK-mediated DNA damage response and vascular senescence. Sci. Transl. Med. 2020, 12, eaaw1868. [Google Scholar] [CrossRef] [PubMed]
- Simion, V.; Zhou, H.; Pierce, J.B.; Yang, D.; Haemmig, S.; Tesmenitsky, Y.; Sukhova, G.; Stone, P.H.; Libby, P.; Feinberg, M.W. LncRNA VINAS regulates atherosclerosis by modulating NF-kappaB and MAPK signaling. JCI Insight 2020, 5, e140627. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jamaiyar, A.; Wu, W.; Hu, Y.; Zhuang, R.; Sausen, G.; Cheng, H.S.; de Oliveira Vaz, C.; Perez-Cremades, D.; Tzani, A.; et al. Deficiency of lncRNA MERRICAL abrogates macrophage chemotaxis and diabetes-associated atherosclerosis. Cell Rep. 2024, 43, 113815. [Google Scholar] [CrossRef]
- McCoy, M.G.; Jamaiyar, A.; Sausen, G.; Cheng, H.S.; Perez-Cremades, D.; Zhuang, R.; Chen, J.; Goodney, P.P.; Creager, M.A.; Sabatine, M.S.; et al. MicroRNA-375 repression of Kruppel-like factor 5 improves angiogenesis in diabetic critical limb ischemia. Angiogenesis 2023, 26, 107–127. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Rawal, S.; Randhawa, V.; Rizvi, S.H.M.; Sachan, M.; Wara, A.K.; Perez-Cremades, D.; Weisbrod, R.M.; Hamburg, N.M.; Feinberg, M.W. miR-369-3p ameliorates diabetes-associated atherosclerosis by regulating macrophage succinate-GPR91 signalling. Cardiovasc. Res. 2024, 120, 1693–1712. [Google Scholar] [CrossRef]
- Carlevaro-Fita, J.; Johnson, R. Global Positioning System: Understanding Long Noncoding RNAs through Subcellular Localization. Mol. Cell 2019, 73, 869–883. [Google Scholar] [CrossRef]
- Xu, K.; Saaoud, F.; Yu, S.; Drummer, C., IV; Shao, Y.; Sun, Y.; Lu, Y.; Sun, J.; Yu, J.; Jiang, X.; et al. Monocyte Adhesion Assays for Detecting Endothelial Cell Activation in Vascular Inflammation and Atherosclerosis. Methods Mol. Biol. 2022, 2419, 169–182. [Google Scholar]
- Carbone, M.L.; Failla, C.M. Interleukin role in the regulation of endothelial cell pathological activation. Vasc. Biol. 2021, 3, R96–R105. [Google Scholar] [CrossRef]
- Bachstetter, A.D.; Van Eldik, L.J. The p38 MAP Kinase Family as Regulators of Proinflammatory Cytokine Production in Degenerative Diseases of the CNS. Aging Dis. 2010, 1, 199–211. [Google Scholar]
- Pierce, J.B.; Feinberg, M.W. Long Noncoding RNAs in Atherosclerosis and Vascular Injury: Pathobiology, Biomarkers, and Targets for Therapy. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2002–2017. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.A.; Wu, J.S.; Chen, E.S. C1D family proteins in coordinating RNA processing, chromosome condensation and DNA damage response. Cell Div. 2016, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Simion, V.; Zhou, H.; Haemmig, S.; Pierce, J.B.; Mendes, S.; Tesmenitsky, Y.; Perez-Cremades, D.; Lee, J.F.; Chen, A.F.; Ronda, N.; et al. A macrophage-specific lncRNA regulates apoptosis and atherosclerosis by tethering HuR in the nucleus. Nat. Commun. 2020, 11, 6135. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Haemmig, S.; Deng, Y.; Chen, J.; Simion, V.; Yang, D.; Sukhova, G.; Shvartz, E.; Wara, A.; Cheng, H.S.; et al. A Smooth Muscle Cell-Enriched Long Noncoding RNA Regulates Cell Plasticity and Atherosclerosis by Interacting with Serum Response Factor. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2399–2416. [Google Scholar] [CrossRef]
- Bridges, M.C.; Daulagala, A.C.; Kourtidis, A. LNCcation: lncRNA localization and function. J. Cell Biol. 2021, 220, e202009045. [Google Scholar] [CrossRef]
- Grassi, L.; Izuogu, O.G.; Jorge, N.A.N.; Seyres, D.; Bustamante, M.; Burden, F.; Farrow, S.; Farahi, N.; Martin, F.J.; Frankish, A.; et al. Cell type-specific novel long non-coding RNA and circular RNA in the BLUEPRINT hematopoietic transcriptomes atlas. Haematologica 2021, 106, 2613–2623. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.B.; Zhou, H.; Simion, V.; Feinberg, M.W. Long Noncoding RNAs as Therapeutic Targets. Adv. Exp. Med. Biol. 2022, 1363, 161–175. [Google Scholar] [PubMed]
- Perez-Cremades, D.; Chen, J.; Assa, C.; Feinberg, M.W. MicroRNA-mediated control of myocardial infarction in diabetes. Trends Cardiovasc. Med. 2023, 33, 195–201. [Google Scholar] [CrossRef]
- Haemmig, S.; Hashemi Gheinani, A.; Zaromytidou, M.; Siasos, G.; Coskun, A.U.; Cormier, M.A.; Gross, D.A.; Wara, A.; Antoniadis, A.P.; Sun, X.; et al. Novel Lesional Transcriptional Signature Separates Atherosclerosis With and Without Diabetes in Yorkshire Swine and Humans. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1487–1503. [Google Scholar] [CrossRef]
- Malodobra-Mazur, M.; Cierzniak, A.; Kaliszewski, K.; Dobosz, T. PPARG Hypermethylation as the First Epigenetic Modification in Newly Onset Insulin Resistance in Human Adipocytes. Genes 2021, 12, 889. [Google Scholar] [CrossRef]
- Singh, R.; Chandel, S.; Dey, D.; Ghosh, A.; Roy, S.; Ravichandiran, V.; Ghosh, D. Epigenetic modification and therapeutic targets of diabetes mellitus. Biosci. Rep. 2020, 40, BSR20202160. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Sharma, K.; Pratap, K.; Singh, V.; Saini, N. Inhibition of microRNA-128-3p attenuates hypercholesterolemia in mouse model. Life Sci. 2021, 264, 118633. [Google Scholar] [CrossRef]
- Haemmig, S.; Simion, V.; Yang, D.; Deng, Y.; Feinberg, M.W. Long noncoding RNAs in cardiovascular disease, diagnosis, and therapy. Curr. Opin. Cardiol. 2017, 32, 776–783. [Google Scholar] [CrossRef]
- Sun, X.; Icli, B.; Wara, A.K.; Belkin, N.; He, S.; Kobzik, L.; Hunninghake, G.M.; Vera, M.P.; Registry, M.; Blackwell, T.S.; et al. MicroRNA-181b regulates NF-kappaB-mediated vascular inflammation. J. Clin. Investig. 2012, 122, 1973–1990. [Google Scholar]
- Sun, X.; Belkin, N.; Feinberg, M.W. Endothelial microRNAs and atherosclerosis. Curr. Atheroscler. Rep. 2013, 15, 372. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Sit, A.; Feinberg, M.W. Role of miR-181 family in regulating vascular inflammation and immunity. Trends Cardiovasc. Med 2014, 24, 105–112. [Google Scholar] [CrossRef]
- Garcia-Zepeda, E.A.; Rothenberg, M.E.; Ownbey, R.T.; Celestin, J.; Leder, P.; Luster, A.D. Human eotaxin is a specific chemoattractant for eosinophil cells and provides a new mechanism to explain tissue eosinophilia. Nat. Med. 1996, 2, 449–456. [Google Scholar] [CrossRef]
- Fuste, B.; Escolar, G.; Marin, P.; Mazzara, R.; Ordinas, A.; Diaz-Ricart, M. G-CSF increases the expression of VCAM-1 on stromal cells promoting the adhesion of CD34+ hematopoietic cells: Studies under flow conditions. Exp. Hematol. 2004, 32, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Michie, S.A.; Xu, B.; Suzuki, Y. Importance of IFN-gamma-mediated expression of endothelial VCAM-1 on recruitment of CD8+ T cells into the brain during chronic infection with Toxoplasma gondii. J. Interferon Cytokine Res. 2007, 27, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.; Whittaker, S.; Smith, N.; Vora, A.J.; Dumonde, D.C.; Brown, K.A. IL-6 acts on endothelial cells to preferentially increase their adherence for lymphocytes. Clin. Exp. Immunol. 1996, 105, 112–119. [Google Scholar] [CrossRef]
- Schilders, G.; van Dijk, E.; Pruijn, G.J. C1D and hMtr4p associate with the human exosome subunit PM/Scl-100 and are involved in pre-rRNA processing. Nucleic Acids Res. 2007, 35, 2564–2572. [Google Scholar] [CrossRef]
- Tomita, T.; Ieguchi, K.; Takita, M.; Tsukahara, F.; Yamada, M.; Egly, J.M.; Maru, Y. C1D is not directly involved in the repair of UV-damaged DNA but protects cells from oxidative stress by regulating gene expressions in human cell lines. J. Biochem. 2018, 164, 415–426. [Google Scholar] [CrossRef]
- Li, G.; Liu, J.; Abu-Asab, M.; Masabumi, S.; Maru, Y. XPB induces C1D expression to counteract UV-induced apoptosis. Mol. Cancer Res. 2010, 8, 885–895. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandra, A.; Bektik, E.; Randhawa, V.; Feinberg, M.W. Endothelial-Enriched lncRNA Gm39822 Modulates Inflammation and Dysfunction in Non-Diabetic Endothelial Cells. Int. J. Mol. Sci. 2025, 26, 8147. https://doi.org/10.3390/ijms26178147
Chandra A, Bektik E, Randhawa V, Feinberg MW. Endothelial-Enriched lncRNA Gm39822 Modulates Inflammation and Dysfunction in Non-Diabetic Endothelial Cells. International Journal of Molecular Sciences. 2025; 26(17):8147. https://doi.org/10.3390/ijms26178147
Chicago/Turabian StyleChandra, Amit, Emre Bektik, Vinay Randhawa, and Mark W. Feinberg. 2025. "Endothelial-Enriched lncRNA Gm39822 Modulates Inflammation and Dysfunction in Non-Diabetic Endothelial Cells" International Journal of Molecular Sciences 26, no. 17: 8147. https://doi.org/10.3390/ijms26178147
APA StyleChandra, A., Bektik, E., Randhawa, V., & Feinberg, M. W. (2025). Endothelial-Enriched lncRNA Gm39822 Modulates Inflammation and Dysfunction in Non-Diabetic Endothelial Cells. International Journal of Molecular Sciences, 26(17), 8147. https://doi.org/10.3390/ijms26178147








