Antimicrobial Peptides of the Cathelicidin Family: Focus on LL-37 and Its Modifications
Abstract
1. Introduction
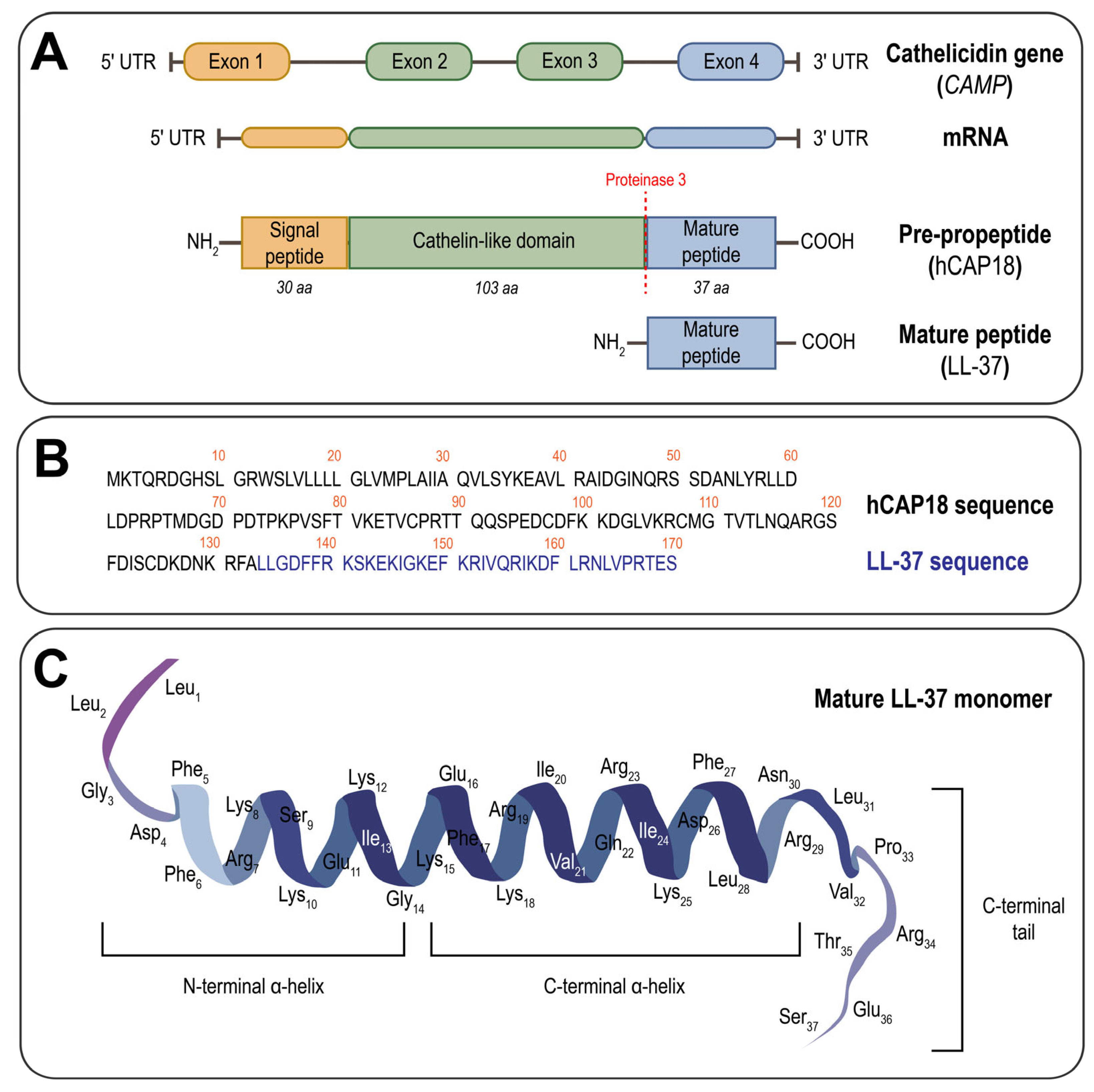
2. Structural and Functional Features of LL-37
3. Endogenous Post-Translational Modifications of LL-37
4. Strategies for LL-37 Functional Optimization
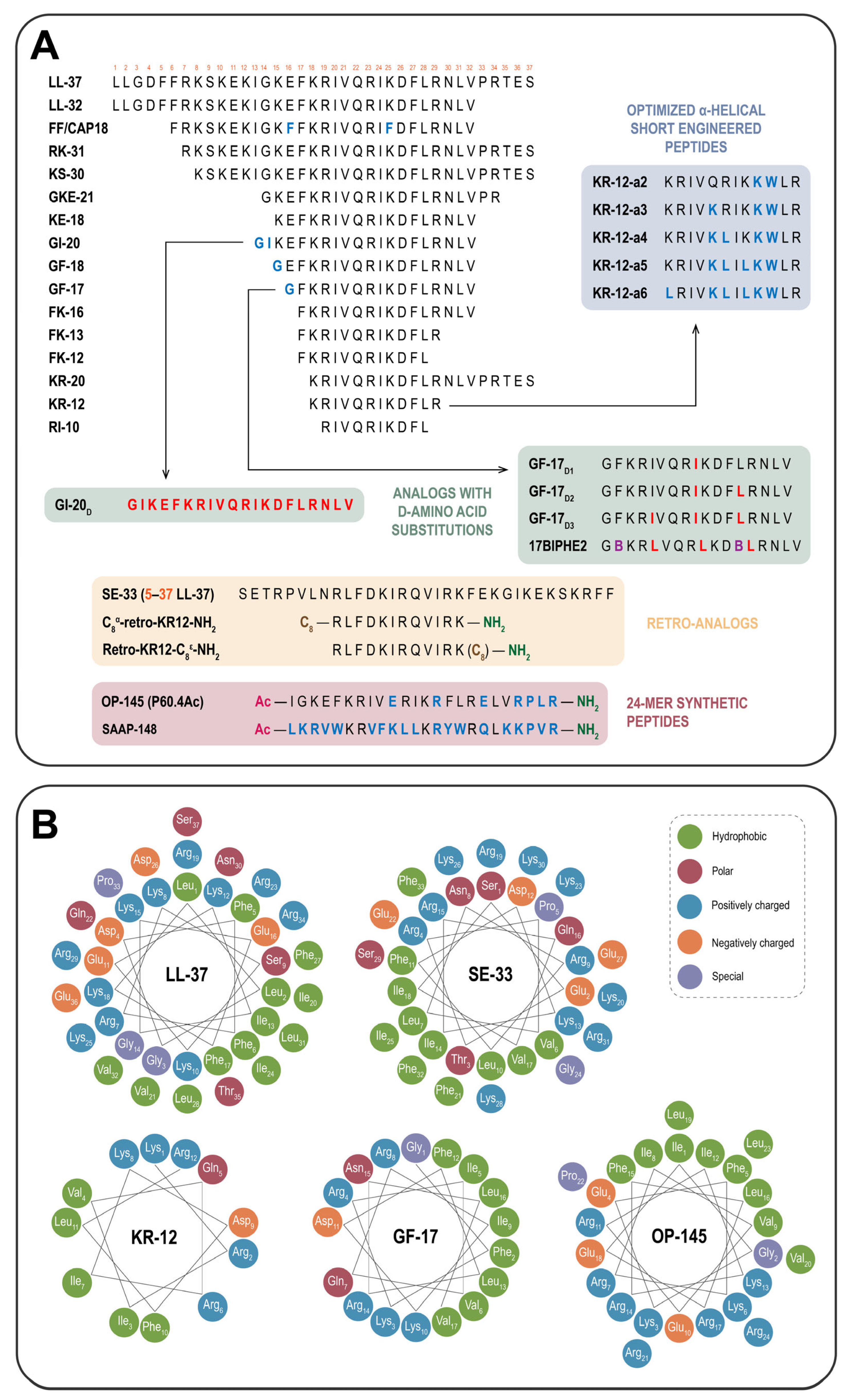
4.1. Shortened Derivatives: Truncated LL-37 Analogs
4.2. Sequence-Reversed Variants: Retro-Analogs of LL-37
4.3. Key Strategies for Modulation of LL-37 and Its Derivatives
4.3.1. Rational Sequence Engineering: Site-Specific Amino Acid Substitutions
4.3.2. Strategies for Modulating Hydrophobicity, Amphipathicity, and Net Charge
4.3.3. Conformational and Structural Modifications: Cyclization, Hybridization and Lipidation
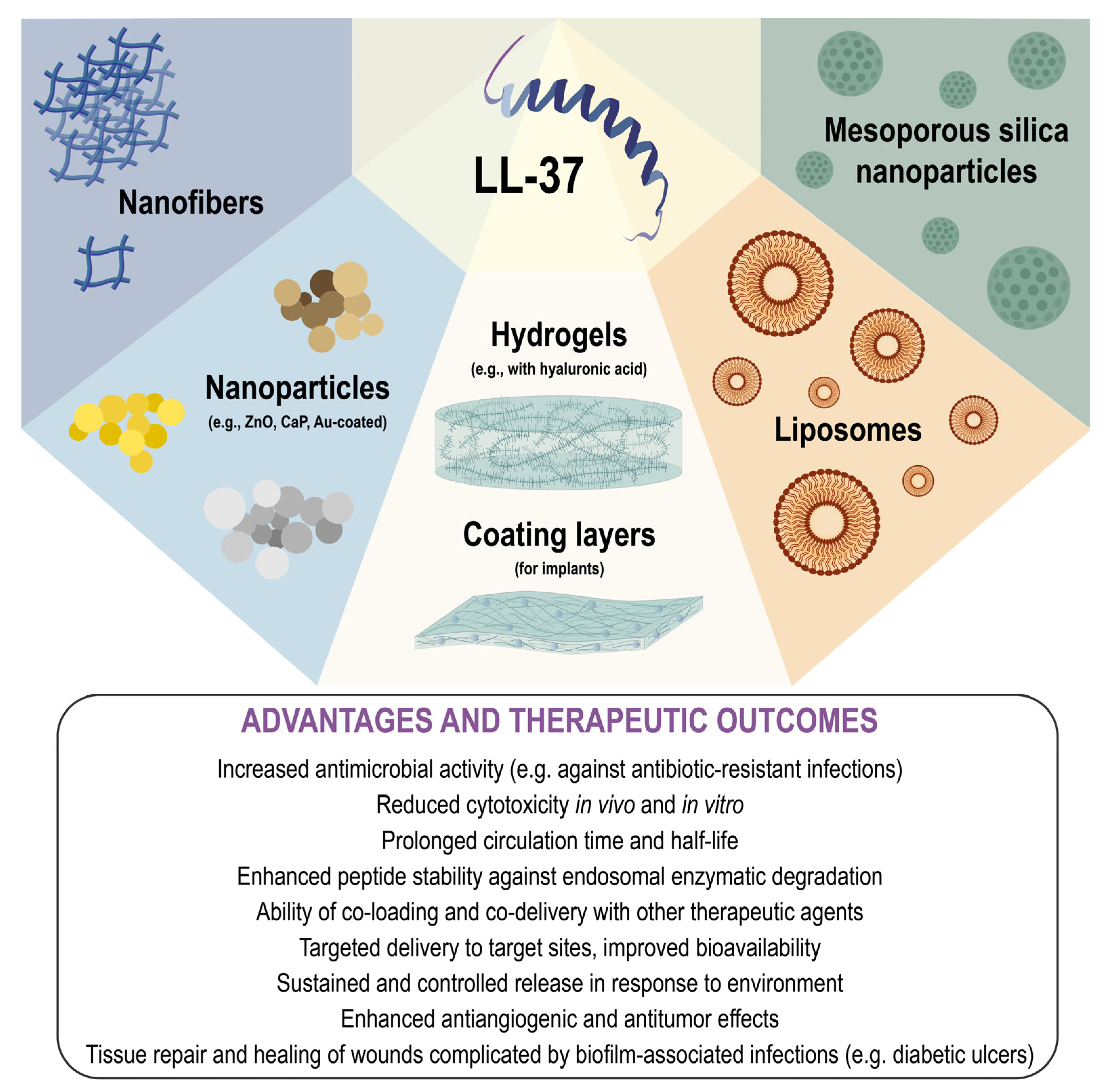
5. Nanoscale Delivery Platforms for LL-37 and Its Derivatives
6. Future Prospects
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Robles Aguilar, G.; Mestrovic, T.; Smith, G.; Han, C.; et al. Global Burden of Bacterial Antimicrobial Resistance 1990–2021: A Systematic Analysis with Forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef] [PubMed]
- de Kraker, M.E.A.; Stewardson, A.J.; Harbarth, S. Will 10 Million People Die a Year Due to Antimicrobial Resistance by 2050? PLoS Med. 2016, 13, e1002184. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Mata, D.I.; Salinas-Carmona, M.C. Antimicrobial Peptides’ Immune Modulation Role in Intracellular Bacterial Infection. Front. Immunol. 2023, 14, 1119574. [Google Scholar] [CrossRef]
- Gawde, U.; Chakraborty, S.; Waghu, F.H.; Barai, R.S.; Khanderkar, A.; Indraguru, R.; Shirsat, T.; Idicula-Thomas, S. CAMPR4: A Database of Natural and Synthetic Antimicrobial Peptides. Nucleic Acids Res. 2023, 51, D377–D383. [Google Scholar] [CrossRef]
- Datta, M.; Rajeev, A.; Chattopadhyay, I. Application of Antimicrobial Peptides as Next-Generation Therapeutics in the Biomedical World. Biotechnol. Genet. Eng. Rev. 2024, 40, 2458–2496. [Google Scholar] [CrossRef]
- Mookherjee, N.; Anderson, M.A.; Haagsman, H.P.; Davidson, D.J. Antimicrobial Host Defence Peptides: Functions and Clinical Potential. Nat. Rev. Drug Discov. 2020, 19, 311–332. [Google Scholar] [CrossRef]
- Scheenstra, M.R.; van Harten, R.M.; Veldhuizen, E.J.A.; Haagsman, H.P.; Coorens, M. Cathelicidins Modulate TLR-Activation and Inflammation. Front. Immunol. 2020, 11, 540919. [Google Scholar] [CrossRef]
- Bhattacharjya, S.; Zhang, Z.; Ramamoorthy, A. LL-37: Structures, Antimicrobial Activity, and Influence on Amyloid-Related Diseases. Biomolecules 2024, 14, 320. [Google Scholar] [CrossRef]
- Bruhn, O.; Grötzinger, J.; Cascorbi, I.; Jung, S. Antimicrobial Peptides and Proteins of the Horse—Insights into a Well-Armed Organism. Vet. Res. 2011, 42, 98. [Google Scholar] [CrossRef]
- Scott, M.G.; Hancock, R.E.W. Cationic Antimicrobial Peptides and Their Multifunctional Role in the Immune System. Crit. Rev. Immunol. 2000, 20, 407–431. [Google Scholar] [CrossRef]
- Kościuczuk, E.M.; Lisowski, P.; Jarczak, J.; Strzałkowska, N.; Jóźwik, A.; Horbańczuk, J.; Krzyżewski, J.; Zwierzchowski, L.; Bagnicka, E. Cathelicidins: Family of Antimicrobial Peptides. A Review. Mol. Biol. Rep. 2012, 39, 10957–10970. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, M. The Role of Cathelicidins in the Innate Host Defenses of Mammals. Curr. Issues Mol. Biol. 2005, 7, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Yoon, G.; Puentes, R.; Tran, J.; Multani, A.; Cobo, E.R. The Role of Cathelicidins in Neutrophil Biology. J. Leukoc. Biol. 2024, 116, 689–705. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.M.; Shi, J.; Ceccarelli, A.; Kim, Y.H.; Park, A.; Ganz, T. Inhibition of Neutrophil Elastase Prevents Cathelicidin Activation and Impairs Clearance of Bacteria from Wounds. Blood 2001, 97, 297–304. [Google Scholar] [CrossRef]
- Lai, Y.; Gallo, R.L. AMPed up Immunity: How Antimicrobial Peptides Have Multiple Roles in Immune Defense. Trends Immunol. 2009, 30, 131–141. [Google Scholar] [CrossRef]
- Nijnik, A.; Hancock, R. Host Defence Peptides: Antimicrobial and Immunomodulatory Activity and Potential Applications for Tackling Antibiotic-Resistant Infections. Emerg. Health Threat. J. 2009, 2, e1. [Google Scholar] [CrossRef]
- Sørensen, O.E.; Gram, L.; Johnsen, A.H.; Andersson, E.; Bangsbøll, S.; Tjabringa, G.S.; Hiemstra, P.S.; Malm, J.; Egesten, A.; Borregaard, N. Processing of Seminal Plasma HCAP-18 to ALL-38 by Gastricsin: A Novel Mechanism of Generating Antimicrobial Peptides in Vagina. J. Biol. Chem. 2003, 278, 28540–28546, Erratum in J. Biol. Chem. 2006, 281, 12999. [Google Scholar] [CrossRef]
- Bevins, C.L. Antimicrobial Peptides as Effector Molecules of Mammalian Host Defense. Contrib. Microbiol. 2002, 10, 106–148. [Google Scholar] [CrossRef]
- Zanetti, M. Cathelicidins, Multifunctional Peptides of the Innate Immunity. J. Leukoc. Biol. 2004, 75, 39–48. [Google Scholar] [CrossRef]
- Dürr, U.H.N.; Sudheendra, U.S.; Ramamoorthy, A. LL-37, the Only Human Member of the Cathelicidin Family of Antimicrobial Peptides. Biochim. Biophys. Acta-Biomembr. 2006, 1758, 1408–1425. [Google Scholar] [CrossRef]
- Tjabringa, G.S.; Ninaber, D.K.; Drijfhout, J.W.; Rabe, K.F.; Hiemstra, P.S. Human Cathelicidin LL-37 Is a Chemoattractant for Eosinophils and Neutrophils That Acts via Formyl-Peptide Receptors. Int. Arch. Allergy Immunol. 2006, 140, 103–112. [Google Scholar] [CrossRef]
- De Yang, B.; Chen, Q.; Schmidt, A.P.; Anderson, G.M.; Wang, J.M.; Wooters, J.; Oppenheim, J.J.; Chertov, O. LL-37, the Neutrophil Granule- and Epithelial Cell-Derived Cathelicidin, Utilizes Formyl Peptide Receptor-like 1 (FPRL1) as a Receptor to Chemoattract Human Peripheral Blood Neutrophils, Monocytes, and T Cells. J. Exp. Med. 2000, 192, 1069–1074. [Google Scholar] [CrossRef]
- Babolewska, E.; Brzezińska-Błaszczyk, E. Human-Derived Cathelicidin LL-37 Directly Activates Mast Cells to Proinflammatory Mediator Synthesis and Migratory Response. Cell. Immunol. 2015, 293, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Vandamme, D.; Landuyt, B.; Luyten, W.; Schoofs, L. A Comprehensive Summary of LL-37, the Factotum Human Cathelicidin Peptide. Cell. Immunol. 2012, 280, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Kahlenberg, J.M.; Kaplan, M.J. Little Peptide, Big Effects: The Role of LL-37 in Inflammation and Autoimmune Disease. J. Immunol. 2013, 191, 4895–4901. [Google Scholar] [CrossRef] [PubMed]
- Zarei-Mehrvarz, E.; Fahimirad, S.; Ghaznavi-rad, E.; Abbasian, S.S.; Abtahi, H. The LL-37 Antimicrobial Peptide as a Treatment for Systematic Infection of Acinetobacter Baumannii in a Mouse Model. Protein Pept. Lett. 2022, 30, 44–53. [Google Scholar] [CrossRef]
- Simonetti, O.; Cirioni, O.; Goteri, G.; Lucarini, G.; Kamysz, E.; Kamysz, W.; Orlando, F.; Rizzetto, G.; Molinelli, E.; Morroni, G.; et al. Efficacy of Cathelicidin LL-37 in an MRSA Wound Infection Mouse Model. Antibiotics 2021, 10, 1210. [Google Scholar] [CrossRef]
- Nagaoka, I.; Tamura, H.; Reich, J. Therapeutic Potential of Cathelicidin Peptide LL-37, an Antimicrobial Agent, in a Murine Sepsis Model. Int. J. Mol. Sci. 2020, 21, 5973. [Google Scholar] [CrossRef]
- Miao, S.; Liu, H.; Yang, Q.; Zhang, Y.; Chen, T.; Chen, S.; Mao, X.; Zhang, Q. Cathelicidin Peptide LL-37: A Multifunctional Peptide Involved in Heart Disease. Pharmacol. Res. 2024, 210, 107529. [Google Scholar] [CrossRef]
- Lu, F.; Zhu, Y.; Zhang, G.; Liu, Z. Renovation as Innovation: Repurposing Human Antibacterial Peptide LL-37 for Cancer Therapy. Front. Pharmacol. 2022, 13, 944147. [Google Scholar] [CrossRef]
- Mohanty, S.; Kamolvit, W.; Zambrana, S.; Gonzales, E.; Tovi, J.; Brismar, K.; Östenson, C.G.; Brauner, A. HIF-1 Mediated Activation of Antimicrobial Peptide LL-37 in Type 2 Diabetic Patients. J. Mol. Med. 2022, 100, 101–113. [Google Scholar] [CrossRef]
- Pahar, B.; Madonna, S.; Das, A.; Albanesi, C.; Girolomoni, G. Immunomodulatory Role of the Antimicrobial LL-37 Peptide in Autoimmune Diseases and Viral Infections. Vaccines 2020, 8, 517. [Google Scholar] [CrossRef] [PubMed]
- Ribon, M.; Seninet, S.; Mussard, J.; Sebbag, M.; Clavel, C.; Serre, G.; Boissier, M.C.; Semerano, L.; Decker, P. Neutrophil Extracellular Traps Exert Both Pro- and Anti-Inflammatory Actions in Rheumatoid Arthritis That Are Modulated by C1q and LL-37. J. Autoimmun. 2019, 98, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Podaza, E.; Palacios, F.; Croci, D.O.; Risnik, D.; Yan, X.J.; Almejún, M.B.; Colado, A.; Elías, E.E.; Borge, M.; Morande, P.E.; et al. Expression and Function of Cathelicidin HCAP18/LL-37 in Chronic Lymphocytic Leukemia. Haematologica 2020, 105, e465–e469. [Google Scholar] [CrossRef] [PubMed]
- Svensson, D.; Nilsson, B.O. Human Antimicrobial/Host Defense Peptide LL-37 May Prevent the Spread of a Local Infection through Multiple Mechanisms: An Update. Inflamm. Res. 2025, 74, 36. [Google Scholar] [CrossRef]
- Guerra, M.E.S.; Vieira, B.; Calazans, A.P.C.T.; Destro, G.V.; Melo, K.; Rodrigues, E.; Waz, N.T.; Girardello, R.; Darrieux, M.; Converso, T.R. Recent Advances in the Therapeutic Potential of Cathelicidins. Front. Microbiol. 2024, 15, 1405760. [Google Scholar] [CrossRef]
- Zeth, K.; Sancho-Vaello, E. Structural Plasticity of LL-37 Indicates Elaborate Functional Adaptation Mechanisms to Bacterial Target Structures. Int. J. Mol. Sci. 2021, 22, 5200. [Google Scholar] [CrossRef]
- Zhu, Y.; Mohapatra, S.; Weisshaar, J.C. Rigidification of the Escherichia Coli Cytoplasm by the Human Antimicrobial Peptide LL-37 Revealed by Superresolution Fluorescence Microscopy. Proc. Natl. Acad. Sci. USA 2019, 116, 1017–1026. [Google Scholar] [CrossRef]
- Sancho-Vaello, E.; François, P.; Bonetti, E.J.; Lilie, H.; Finger, S.; Gil-Ortiz, F.; Gil-Carton, D.; Zeth, K. Structural Remodeling and Oligomerization of Human Cathelicidin on Membranes Suggest Fibril-Like Structures as Active Species. Sci. Rep. 2017, 7, 15371. [Google Scholar] [CrossRef]
- Xhindoli, D.; Pacor, S.; Benincasa, M.; Scocchi, M.; Gennaro, R.; Tossi, A. The Human Cathelicidin LL-37—A Pore-Forming Antibacterial Peptide and Host-Cell Modulator. Biochim. Biophys. Acta-Biomembr. 2016, 1858, 546–566. [Google Scholar] [CrossRef]
- Oren, Z.; Lerman, J.C.; Gudmundsson, G.H.; Agerberth, B.; Shai, Y. Structure and Organization of the Human Antimicrobial Peptide LL-37 in Phospholipid Membranes: Relevance to the Molecular Basis for Its Non-Cell-Selective Activity. Biochem. J. 1999, 341, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Narayana, J.L.; Mishra, B.; Zhang, Y.; Wang, F.; Wang, C.; Zarena, D.; Lushnikova, T.; Wang, X. Design of Antimicrobial Peptides: Progress Made with Human Cathelicidin LL-37. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2019; Volume 1117, pp. 215–240. [Google Scholar]
- Wang, G. Structures of Human Host Defense Cathelicidin LL-37 and Its Smallest Antimicrobial Peptide KR-12 in Lipid Micelles. J. Biol. Chem. 2008, 283, 32637–32643. [Google Scholar] [CrossRef] [PubMed]
- Porcelli, F.; Verardi, R.; Shi, L.; Henzler-Wildman, K.A.; Ramamoorthy, A.; Veglia, G. NMR Structure of the Cathelicidin-Derived Human Antimicrobial Peptide LL-37 in Dodecylphosphocholine Micelles. Biochemistry 2008, 47, 5565–5572. [Google Scholar] [CrossRef] [PubMed]
- Xhindoli, D.; Morgera, F.; Zinth, U.; Rizzo, R.; Pacor, S.; Tossi, A. New Aspects of the Structure and Mode of Action of the Human Cathelicidin LL-37 Revealed by the Intrinsic Probe p-Cyanophenylalanine. Biochem. J. 2015, 465, 443–457. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Han, H.; Miller, D.W.; Wang, G. Solution Structures of Human Ll-37 Fragments and NMR-Based Identification of a Minimal Membrane-Targeting Antimicrobial and Anticancer Region. J. Am. Chem. Soc. 2006, 128, 5776–5785. [Google Scholar] [CrossRef]
- Sancho-Vaello, E.; Gil-Carton, D.; François, P.; Bonetti, E.J.; Kreir, M.; Pothula, K.R.; Kleinekathöfer, U.; Zeth, K. The Structure of the Antimicrobial Human Cathelicidin LL-37 Shows Oligomerization and Channel Formation in the Presence of Membrane Mimics. Sci. Rep. 2020, 10, 17356. [Google Scholar] [CrossRef]
- Johansson, J.; Gudmundsson, G.H.; Rottenberg, M.E.; Berndt, K.D.; Agerberth, B. Conformation-Dependent Antibacterial Activity of the Naturally Occurring Human Peptide LL-37. J. Biol. Chem. 1998, 273, 3718–3724. [Google Scholar] [CrossRef]
- Henzler-Wildman, K.A.; Lee, D.K.; Ramamoorthy, A. Mechanism of Lipid Bilayer Disruption by the Human Antimicrobial Peptide, LL-37. Biochemistry 2003, 42, 6545–6558. [Google Scholar] [CrossRef]
- Henzler-Wildman, K.A.; Martinez, G.V.; Brown, M.F.; Ramamoorthy, A. Perturbation of the Hydrophobic Core of Lipid Bilayers by the Human Antimicrobial Peptide LL-37. Biochemistry 2004, 43, 8459–8469. [Google Scholar] [CrossRef]
- Wang, G.; Mishra, B.; Epand, R.F.; Epand, R.M. High-Quality 3D Structures Shine Light on Antibacterial, Anti-Biofilm and Antiviral Activities of Human Cathelicidin LL-37 and Its Fragments. Biochim. Biophys. Acta-Biomembr. 2014, 1838, 2160–2172. [Google Scholar] [CrossRef]
- Rekha, R.S.; Padhi, A.; Frengen, N.; Hauenstein, J.; Végvári, Á.; Agerberth, B.; Månsson, R.; Guðmundsson, G.H.; Bergman, P. The Di-Leucine Motif in the Host Defense Peptide LL-37 Is Essential for Initiation of Autophagy in Human Macrophages. Cell Rep. 2025, 44, 115031. [Google Scholar] [CrossRef]
- De Miguel Catalina, A.; Forbrig, E.; Kozuch, J.; Nehls, C.; Paulowski, L.; Gutsmann, T.; Hildebrandt, P.; Mroginski, M.A. The C-Terminal VPRTES Tail of LL-37 Influences the Mode of Attachment to a Lipid Bilayer and Antimicrobial Activity. Biochemistry 2019, 58, 2447–2462. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Bryzek, D.; Dobosz, E.; Scavenius, C.; Svoboda, P.; Rapala-Kozik, M.; Lesner, A.; Frydrych, I.; Enghild, J.; Mydel, P.; et al. A Novel Biological Role for Peptidyl-Arginine Deiminases: Citrullination of Cathelicidin LL-37 Controls the Immunostimulatory Potential of Cell-Free DNA. J. Immunol. 2018, 200, 2327–2340. [Google Scholar] [CrossRef] [PubMed]
- Koro, C.; Hellvard, A.; Delaleu, N.; Binder, V.; Scavenius, C.; Bergum, B.; Gówczyk, I.; Roberts, H.M.; Chapple, I.L.C.; Grant, M.M.; et al. Carbamylated LL-37 as a Modulator of the Immune Response. Innate Immun. 2016, 22, 218–229. [Google Scholar] [CrossRef]
- Al-Adwani, S.; Wallin, C.; Balhuizen, M.D.; Veldhuizen, E.J.A.; Coorens, M.; Landreh, M.; Végvári, Á.; Smith, M.E.; Qvarfordt, I.; Lindén, A.; et al. Studies on Citrullinated LL-37: Detection in Human Airways, Antibacterial Effects and Biophysical Properties. Sci. Rep. 2020, 10, 2376. [Google Scholar] [CrossRef]
- Lande, R.; Pietraforte, I.; Mennella, A.; Palazzo, R.; Spinelli, F.R.; Giannakakis, K.; Spadaro, F.; Falchi, M.; Riccieri, V.; Stefanantoni, K.; et al. Complementary Effects of Carbamylated and Citrullinated Ll37 in Autoimmunity and Inflammation in Systemic Lupus Erythematosus. Int. J. Mol. Sci. 2021, 22, 1650. [Google Scholar] [CrossRef]
- Tilvawala, R.; Thompson, P.R. Peptidyl Arginine Deiminases: Detection and Functional Analysis of Protein Citrullination. Curr. Opin. Struct. Biol. 2019, 59, 205. [Google Scholar] [CrossRef]
- Verbrugge, F.H.; Tang, W.H.W.; Hazen, S.L. Protein Carbamylation and Cardiovascular Disease. Kidney Int. 2015, 88, 474–478. [Google Scholar] [CrossRef]
- Delanghe, S.; Delanghe, J.R.; Speeckaert, R.; Van Biesen, W.; Speeckaert, M.M. Mechanisms and Consequences of Carbamoylation. Nat. Rev. Nephrol. 2017, 13, 580–593. [Google Scholar] [CrossRef]
- Sultana Rekha, R.; Rao Muvva, S.J.; Wan, M.; Raqib, R.; Bergman, P.; Brighenti, S.; Gudmundsson, G.H.; Agerberth, B. Phenylbutyrate Induces LL-37-Dependent Autophagy and Intracellular Killing of Mycobacterium Tuberculosis in Human Macrophages. Autophagy 2015, 11, 1688–1699. [Google Scholar] [CrossRef]
- Wang, C.; Feng, L.; Yu, H.; Wang, Y. Relationship Between Structure and Function of Cathelicidins and Their Molecular Design: A Review. Chin. J. Biotechnol. 2017, 33, 27–35. [Google Scholar] [CrossRef]
- Ciornei, C.D.; Sigurdardóttir, T.; Schmidtchen, A.; Bodelsson, M. Antimicrobial and Chemoattractant Activity, Lipopolysaccharide Neutralization, Cytotoxicity, and Inhibition by Serum of Analogs of Human Cathelicidin LL-37. Antimicrob. Agents Chemother. 2005, 49, 2845–2850. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Schauber, J.; Coda, A.; Lin, H.; Dorschner, R.A.; Schechter, N.M.; Bonnart, C.; Descargues, P.; Hovnanian, A.; Gallo, R.L. Kallikrein-Mediated Proteolysis Regulates the Antimicrobial Effects of Cathelicidins in Skin. FASEB J. 2006, 20, 2068–2080. [Google Scholar] [CrossRef] [PubMed]
- Lakshmaiah Narayana, J.; Mechesso, A.F.; Rather, I.I.G.; Zarena, D.; Luo, J.; Xie, J.; Wang, G. Origami of KR-12 Designed Antimicrobial Peptides and Their Potential Applications. Antibiotics 2024, 13, 816. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Banerjee, N.; Roychowdhury, T.; Dutta, A.; Chattopadhyay, S.; Chatterjee, S. Site-Specific Amino Acid Substitution in Dodecameric Peptides Determines the Stability and Unfolding of c-MYC Quadruplex Promoting Apoptosis in Cancer Cells. Nucleic Acids Res. 2018, 46, 9932–9950. [Google Scholar] [CrossRef]
- Luo, Y.; McLean, D.T.F.; Linden, G.J.; McAuley, D.F.; McMullan, R.; Lundy, F.T. The Naturally Occurring Host Defense Peptide, LL-37, and Its Truncated Mimetics KE-18 and KR-12 Have Selected Biocidal and Antibiofilm Activities Against Candida Albicans, Staphylococcus Aureus, and Escherichia Coli In Vitro. Front. Microbiol. 2017, 8, 544. [Google Scholar] [CrossRef]
- Reißer, S.; Prock, S.; Heinzmann, H.; Ulrich, A.S. Protein ORIGAMI: A Program for the Creation of 3D Paper Models of Folded Peptides. Biochem. Mol. Biol. Educ. 2018, 46, 403–409. [Google Scholar] [CrossRef]
- Torres, M.D.T.; Sothiselvam, S.; Lu, T.K.; de la Fuente-Nunez, C. Peptide Design Principles for Antimicrobial Applications. J. Mol. Biol. 2019, 431, 3547–3567. [Google Scholar] [CrossRef]
- Saporito, P.; Vang Mouritzen, M.; Løbner-Olesen, A.; Jenssen, H. LL-37 Fragments Have Antimicrobial Activity Against Staphylococcus Epidermidis Biofilms and Wound Healing Potential in HaCaT Cell Line. J. Pept. Sci. 2018, 24, e3080. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, Y.; Wang, Z.; Yang, J.; Chu, X.; Liu, J.; Zhao, Y. Polypeptide-Engineered DNA Tetrahedrons for Targeting Treatment of Colorectal Cancer via Apoptosis and Autophagy. J. Control. Release 2019, 309, 48–58. [Google Scholar] [CrossRef]
- Ren, S.X.; Shen, J.; Cheng, A.S.L.; Lu, L.; Chan, R.L.Y.; Li, Z.J.; Wang, X.J.; Wong, C.C.M.; Zhang, L.; Ng, S.S.M.; et al. FK-16 Derived from the Anticancer Peptide LL-37 Induces Caspase-Independent Apoptosis and Autophagic Cell Death in Colon Cancer Cells. PLoS ONE 2013, 8, e63641. [Google Scholar] [CrossRef]
- Yu, Y.; Cooper, C.L.; Wang, G.; Morwitzer, M.J.; Kota, K.; Tran, J.P.; Bradfute, S.B.; Liu, Y.; Shao, J.; Zhang, A.K.; et al. Engineered Human Cathelicidin Antimicrobial Peptides Inhibit Ebola Virus Infection. iScience 2020, 23, 100999. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.B.; Seo, J. Antimicrobial Peptides Under Clinical Investigation. Pept. Sci. 2019, 111, e24122. [Google Scholar] [CrossRef]
- Haisma, E.M.; Göblyös, A.; Ravensbergen, B.; Adriaans, A.E.; Cordfunke, R.A.; Schrumpf, J.; Limpens, R.W.A.L.; Schimmel, K.J.M.; Den Hartigh, J.; Hiemstra, P.S.; et al. Antimicrobial Peptide P60.4Ac-Containing Creams and Gel for Eradication of Methicillin-Resistant Staphylococcus Aureus from Cultured Skin and Airway Epithelial Surfaces. Antimicrob. Agents Chemother. 2016, 60, 4063–4072. [Google Scholar] [CrossRef] [PubMed]
- Malanovic, N.; Drijfhout, J.W.; Kriechbaum, M.; Schmuck, M.; de Breij, A.; Nibbering, P.; Lohner, K. Point Mutation in the Hydrophobic Region Drives Selectivity and Activity of OP-145, a Derivative of Human Cathelicidin LL-37. Biophys. J. 2014, 106, 442A. [Google Scholar] [CrossRef]
- De Breij, A.; Riool, M.; Kwakman, P.H.S.; De Boer, L.; Cordfunke, R.A.; Drijfhout, J.W.; Cohen, O.; Emanuel, N.; Zaat, S.A.J.; Nibbering, P.H.; et al. Prevention of Staphylococcus Aureus Biomaterial-Associated Infections Using a Polymer-Lipid Coating Containing the Antimicrobial Peptide OP-145. J. Control. Release 2016, 222, 1–8. [Google Scholar] [CrossRef]
- De Breij, A.; Riool, M.; Cordfunke, R.A.; Malanovic, N.; De Boer, L.; Koning, R.I.; Ravensbergen, E.; Franken, M.; Van Der Heijde, T.; Boekema, B.K.; et al. The Antimicrobial Peptide SAAP-148 Combats Drug-Resistant Bacteria and Biofilms. Sci. Transl. Med. 2018, 10, eaan4044. [Google Scholar] [CrossRef]
- Bouhrour, N.; van der Reijden, T.J.K.; Voet, M.M.; Schonkeren-Ravensbergen, B.; Cordfunke, R.A.; Drijfhout, J.W.; Bendali, F.; Nibbering, P.H. Novel Antibacterial Agents SAAP-148 and Halicin Combat Gram-Negative Bacteria Colonizing Catheters. Antibiotics 2023, 12, 1743. [Google Scholar] [CrossRef]
- Ön, A.; Vejzovic, D.; Jennings, J.; Parigger, L.; Cordfunke, R.A.; Drijfhout, J.W.; Lohner, K.; Malanovic, N. Bactericidal Activity to Escherichia Coli: Different Modes of Action of Two 24-Mer Peptides SAAP-148 and OP-145, Both Derived from Human Cathelicidine LL-37. Antibiotics 2023, 12, 1163. [Google Scholar] [CrossRef]
- Schmitz, M.G.J.; Riool, M.; de Boer, L.; Vrehen, A.F.; Bartels, P.A.A.; Zaat, S.A.J.; Dankers, P.Y.W. Development of an Antimicrobial Peptide SAAP-148-Functionalized Supramolecular Coating on Titanium to Prevent Biomaterial-Associated Infections. Adv. Mater. Technol. 2023, 8, 2201846. [Google Scholar] [CrossRef]
- Piller, P.; Wolinski, H.; Cordfunke, R.A.; Drijfhout, J.W.; Keller, S.; Lohner, K.; Malanovic, N. Membrane Activity of LL-37 Derived Antimicrobial Peptides Against Enterococcus Hirae: Superiority of SAAP-148 over OP-145. Biomolecules 2022, 12, 523. [Google Scholar] [CrossRef]
- Li, X.; Ding, Y.; Xue, J.; Fu, Y.; Yan, F.; Song, N.; Hu, H.; Cong, W.; Lu, Z.; Li, Y. Peptide Double-Stapling and Arginine N-Glycosylation Triggered the Development of Therapeutic Antimicrobial Peptides Capable of Killing Drug-Resistant Bacteria in Mice. J. Med. Chem. 2025, 68, 4511–4526. [Google Scholar] [CrossRef]
- Pinilla, G.; Coronado, Y.T.; Chaves, G.; Muñoz, L.; Navarrete, J.; Salazar, L.M.; Taborda, C.P.; Muñoz, J.E. In Vitro Antifungal Activity of LL-37 Analogue Peptides Against Candida spp. J. Fungi 2022, 8, 1173. [Google Scholar] [CrossRef]
- Rico-Mata, R.; De Leon-Rodriguez, L.M.; Avila, E.E. Effect of Antimicrobial Peptides Derived from Human Cathelicidin LL-37 on Entamoeba Histolytica Trophozoites. Exp. Parasitol. 2013, 133, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Paduszynska, M.A.; Neubauer, D.; Kamysz, W.; Kamysz, E. Anticandidal Activity of Lipopeptides Containing an LL-37-Derived Peptide Fragment KR12. Molecules 2025, 30, 1598. [Google Scholar] [CrossRef] [PubMed]
- White, J.K.; Muhammad, T.; Alsheim, E.; Mohanty, S.; Blasi-Romero, A.; Gunasekera, S.; Strömstedt, A.A.; Ferraz, N.; Göransson, U.; Brauner, A. A Stable Cyclized Antimicrobial Peptide Derived from LL-37 with Host Immunomodulatory Effects and Activity Against Uropathogens. Cell. Mol. Life Sci. 2022, 79, 411. [Google Scholar] [CrossRef] [PubMed]
- Gunasekera, S.; Muhammad, T.; Strömstedt, A.A.; Rosengren, K.J.; Göransson, U. Backbone Cyclization and Dimerization of LL-37-Derived Peptides Enhance Antimicrobial Activity and Proteolytic Stability. Front. Microbiol. 2020, 11, 168. [Google Scholar] [CrossRef]
- Kamysz, E.; Sikorska, E.; Karafova, A.; Dawgul, M. Synthesis, Biological Activity and Conformational Analysis of Head-to-Tail Cyclic Analogues of LL37 and Histatin 5. J. Pept. Sci. 2012, 18, 560–566. [Google Scholar] [CrossRef]
- Neubauer, D.; Jaśkiewicz, M.; Migoń, D.; Bauer, M.; Sikora, K.; Sikorska, E.; Kamysz, E.; Kamysz, W. Retro Analog Concept: Comparative Study on Physico-Chemical and Biological Properties of Selected Antimicrobial Peptides. Amino Acids 2017, 49, 1755–1771. [Google Scholar] [CrossRef]
- Gasanov, V.A.; Vorotelyak, E.A.; Vasiliev, A.V. Production of Antimicrobial Peptides (Cathelicidin Analogues) and Evaluation of Their Biological Properties. Biol. Bull. 2022, 49, S148–S151. [Google Scholar] [CrossRef]
- Gasanov, V.; Vorotelyak, E.; Vasiliev, A. Expression of the Antimicrobial Peptide SE-33-A2P, a Modified Analog of Cathelicidin, and an Analysis of Its Properties. Antibiotics 2024, 13, 190. [Google Scholar] [CrossRef] [PubMed]
- Trenin, A.S.; Arzumanian, V.G.; Zhmak, M.N.; Shelukhina, I.V.; Makarova, Y.V.; Ivanov, I.A.; Bychkova, O.P.; Budikhina, A.S.; Balyasova, L.S.; Tsetlin, V.I. Synthesis and Antimicrobial Activity of a New Drug Based on Retro-Analog Cathelicidin-Polypeptide SE-33. Russ. J. Bioorgan. Chem. 2019, 45, 252–264. [Google Scholar] [CrossRef]
- Kamysz, E.; Sikorska, E.; Bauer, M.; Sikora, K.; Neubauer, D. Influence of Lipidation Pattern of the KR12 Fragment of Peptide LL-37 on Its Antibacterial and Hemolytic Activities. Int. J. Mol. Sci. 2023, 24, 5505. [Google Scholar] [CrossRef] [PubMed]
- Xuan, J.; Feng, W.; Wang, J.; Wang, R.; Zhang, B.; Bo, L.; Chen, Z.S.; Yang, H.; Sun, L. Antimicrobial Peptides for Combating Drug-Resistant Bacterial Infections. Drug Resist. Updat. 2023, 68, 100954. [Google Scholar] [CrossRef]
- Kim, S.; Hyun, S.; Lee, Y.; Lee, Y.; Yu, J. Nonhemolytic Cell-Penetrating Peptides: Site Specific Introduction of Glutamine and Lysine Residues into the α-Helical Peptide Causes Deletion of Its Direct Membrane Disrupting Ability but Retention of Its Cell Penetrating Ability. Biomacromolecules 2016, 17, 3007–3015. [Google Scholar] [CrossRef]
- Gunasekera, S.; Muhammad, T.; Strömstedt, A.A.; Rosengren, K.J.; Göransson, U. Alanine and Lysine Scans of the LL-37-Derived Peptide Fragment KR-12 Reveal Key Residues for Antimicrobial Activity. ChemBioChem 2018, 19, 931–939. [Google Scholar] [CrossRef]
- Kuroda, K.; Fukuda, T.; Krstic-Demonacos, M.; Demonacos, C.; Okumura, K.; Isogai, H.; Hayashi, M.; Saito, K.; Isogai, E. MiR-663a Regulates Growth of Colon Cancer Cells, After Administration of Antimicrobial Peptides, by Targeting CXCR4-P21 Pathway. BMC Cancer 2017, 17, 33. [Google Scholar] [CrossRef]
- Hayashi, M.; Kuroda, K.; Ihara, K.; Iwaya, T.; Isogai, E. Suppressive Effect of an Analog of the Antimicrobial Peptide of LL-37 on Colon Cancer Cells via Exosome-Encapsulated MiRNAs. Int. J. Mol. Med. 2018, 42, 3009–3016. [Google Scholar] [CrossRef]
- Wang, G.; Elliott, M.; Cogen, A.L.; Ezell, E.L.; Gallo, R.L.; Hancock, R.E.W. Structure, Dynamics, and Antimicrobial and Immune Modulatory Activities of Human LL-23 and Its Single-Residue Variants Mutated on the Basis of Homologous Primate Cathelicidins. Biochemistry 2012, 51, 653–664. [Google Scholar] [CrossRef]
- Capozzi, E.; Aureli, S.; Minicozzi, V.; Rossi, G.C.; Stellato, F.; Morante, S. Designing Effective Anticancer-Radiopeptides. A Molecular Dynamics Study of Their Interaction with Model Tumor and Healthy Cell Membranes. Biochim. Biophys. Acta-Biomembr. 2018, 1860, 2348–2355. [Google Scholar] [CrossRef]
- Wang, G.; Hanke, M.L.; Mishra, B.; Lushnikova, T.; Heim, C.E.; Thomas, V.C.; Bayles, K.W.; Kielian, T. Transformation of Human Cathelicidin LL-37 into Selective, Stable, and Potent Antimicrobial Compounds. ACS Chem. Biol. 2014, 9, 1997–2002. [Google Scholar] [CrossRef]
- Moncla, B.J.; Pryke, K.; Rohan, L.C.; Graebing, P.W. Degradation of Naturally Occurring and Engineered Antimicrobial Peptides by Proteases. Adv. Biosci. Biotechnol. 2011, 02, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hao, D.; Chen, Y.; Xu, Y.; Tan, J.; Huang, Y.; Li, F.; Chen, Y. Inhibitory Effects and Mechanisms of Physiological Conditions on the Activity of Enantiomeric Forms of an α-Helical Antibacterial Peptide Against Bacteria. Peptides 2011, 32, 1488–1495. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, D.; Shukla, S.K.; Prakash, O.; Zhang, G. Structural Determinants of Host Defense Peptides for Antimicrobial Activity and Target Cell Selectivity. Biochimie 2010, 92, 1236–1241, Erratum in Biochimie 2011, 93, 631. [Google Scholar] [CrossRef] [PubMed]
- González, R.; Mendive-Tapia, L.; Pastrian, M.B.; Albericio, F.; Lavilla, R.; Cascone, O.; Iannucci, N.B. Enhanced Antimicrobial Activity of a Peptide Derived from Human Lysozyme by Arylation of Its Tryptophan Residues. J. Pept. Sci. 2016, 22, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.W.; Yip, B.S.; Cheng, H.T.; Wang, A.H.; Chen, H.L.; Cheng, J.W.; Lo, H.J. Increased Potency of a Novel D-β-Naphthylalanine-Substituted Antimicrobial Peptide Against Fluconazole-Resistant Fungal Pathogens. FEMS Yeast Res. 2009, 9, 967–970. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front. Cell. Infect. Microbiol. 2016, 6, 235805. [Google Scholar] [CrossRef]
- Andrushchenko, V.V.; Vogel, H.J.; Prenner, E.J. Interactions of Tryptophan-Rich Cathelicidin Antimicrobial Peptides with Model Membranes Studied by Differential Scanning Calorimetry. Biochim. Biophys. Acta-Biomembr. 2007, 1768, 2447–2458. [Google Scholar] [CrossRef]
- Takahashi, T.; Kulkarni, N.N.; Lee, E.Y.; Zhang, L.J.; Wong, G.C.L.; Gallo, R.L. Cathelicidin Promotes Inflammation by Enabling Binding of Self-RNA to Cell Surface Scavenger Receptors. Sci. Rep. 2018, 8, 4032. [Google Scholar] [CrossRef]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of Antimicrobial Peptide Action and Resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef]
- Fernández-Vidal, M.; Jayasinghe, S.; Ladokhin, A.S.; White, S.H. Folding Amphipathic Helices into Membranes: Amphiphilicity Trumps Hydrophobicity. J. Mol. Biol. 2007, 370, 459–470. [Google Scholar] [CrossRef]
- Gagat, P.; Ostrówka, M.; Duda-Madej, A.; Mackiewicz, P. Enhancing Antimicrobial Peptide Activity Through Modifications of Charge, Hydrophobicity, and Structure. Int. J. Mol. Sci. 2024, 25, 10821. [Google Scholar] [CrossRef]
- Ridyard, K.E.; Overhage, J. The Potential of Human Peptide Ll-37 as an Antimicrobial and Anti-Biofilm Agent. Antibiotics 2021, 10, 650. [Google Scholar] [CrossRef] [PubMed]
- Jacob, B.; Park, I.S.; Bang, J.K.; Shin, S.Y. Short KR-12 Analogs Designed from Human Cathelicidin LL-37 Possessing Both Antimicrobial and Antiendotoxic Activities Without Mammalian Cell Toxicity. J. Pept. Sci. 2013, 19, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Rajasekaran, G.; Shin, S.Y. LL-37-Derived Short Antimicrobial Peptide KR-12-A5 and Its d-Amino Acid Substituted Analogs with Cell Selectivity, Anti-Biofilm Activity, Synergistic Effect with Conventional Antibiotics, and Anti-Inflammatory Activity. Eur. J. Med. Chem. 2017, 136, 428–441. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, G.; Kim, E.Y.; Shin, S.Y. LL-37-Derived Membrane-Active FK-13 Analogs Possessing Cell Selectivity, Anti-Biofilm Activity and Synergy with Chloramphenicol and Anti-Inflammatory Activity. Biochim. Biophys. Acta-Biomembr. 2017, 1859, 722–733. [Google Scholar] [CrossRef]
- Jahangiri, S.; Jafari, M.; Arjomand, M.; Mehrnejad, F. Molecular Insights into the Interactions of GF-17 with the Gram-Negative and Gram-Positive Bacterial Lipid Bilayers. J. Cell. Biochem. 2018, 119, 9205–9216. [Google Scholar] [CrossRef]
- Xie, F.; Zan, Y.; Zhang, X.; Zhang, H.; Jin, M.; Zhang, W.; Zhang, Y.; Liu, S. Differential Abilities of Mammalian Cathelicidins to Inhibit Bacterial Biofilm Formation and Promote Multifaceted Immune Functions of Neutrophils. Int. J. Mol. Sci. 2020, 21, 1871. [Google Scholar] [CrossRef]
- Tan, T.; Wu, D.; Li, W.; Zheng, X.; Li, W.; Shan, A. High Specific Selectivity and Membrane-Active Mechanism of Synthetic Cationic Hybrid Antimicrobial Peptides Based on the Peptide FV7. Int. J. Mol. Sci. 2017, 18, 339. [Google Scholar] [CrossRef]
- Wanmakok, M.; Orrapin, S.; Intorasoot, A.; Intorasoot, S. Expression in Escherichia Coli of Novel Recombinant Hybrid Antimicrobial Peptide AL32-P113 with Enhanced Antimicrobial Activity In Vitro. Gene 2018, 671, 1–9. [Google Scholar] [CrossRef]
- Yang, S.T.; Kim, J.I.; Shin, S.Y. Effect of Dimerization of a β-Turn Antimicrobial Peptide, PST13-RK, on Antimicrobial Activity and Mammalian Cell Toxicity. Biotechnol. Lett. 2009, 31, 233–237. [Google Scholar] [CrossRef]
- Yang, H.; Lu, B.; Zhou, D.; Zhao, L.; Song, W.; Wang, L. Identification of the First Cathelicidin Gene from Skin of Chinese Giant Salamanders Andrias Davidianus with Its Potent Antimicrobial Activity. Dev. Comp. Immunol. 2017, 77, 141–149. [Google Scholar] [CrossRef]
- Fox, M.A.; Thwaite, J.E.; Ulaeto, D.O.; Atkins, T.P.; Atkins, H.S. Design and Characterization of Novel Hybrid Antimicrobial Peptides Based on Cecropin A, LL-37 and Magainin II. Peptides 2012, 33, 197–205. [Google Scholar] [CrossRef]
- Chuang, C.M.; Monie, A.; Wu, A.; Mao, C.P.; Hung, C.F. Treatment with LL-37 Peptide Enhances Antitumor Effects Induced by CpG Oligodeoxynucleotides Against Ovarian Cancer. Hum. Gene Ther. 2009, 20, 303–313. [Google Scholar] [CrossRef]
- Rounds, T.; Straus, S.K. Lipidation of Antimicrobial Peptides as a Design Strategy for Future Alternatives to Antibiotics. Int. J. Mol. Sci. 2020, 21, 9692. [Google Scholar] [CrossRef]
- Kapica, M.; Kamysz, E.; Grabowska, O.; Tesmar, A.; Pająk, M.; Chmur, K.; Brzeski, J.; Samsonov, S.A.; Wyrzykowski, D. Interactions of Laurylated and Myristoylated KR12 Fragment of the LL37 Peptide with Polyoxidovanadates. Molecules 2025, 30, 1589. [Google Scholar] [CrossRef] [PubMed]
- Lei, R.; Yang, C.; Sun, Y.; Li, D.; Hao, L.; Li, Y.; Wu, S.; Li, H.; Lan, C.; Fang, X. Turning Cationic Antimicrobial Peptide KR-12 into Self-Assembled Nanobiotics with Potent Bacterial Killing and LPS Neutralizing Activities. Nanoscale 2023, 16, 887–902. [Google Scholar] [CrossRef] [PubMed]
- Dongrui, Z.; Miyamoto, M.; Yokoo, H.; Demizu, Y. Innovative Peptide Architectures: Advancements in Foldamers and Stapled Peptides for Drug Discovery. Expert Opin. Drug Discov. 2024, 19, 699–723. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, M.; Wang, Z.; Liu, Z.; Chen, S.; Li, X.; Shi, Y.; Hu, H. Discovery of Novel Antibacterial Agent for the Infected Wound Treatment: All-Hydrocarbon Stapling Optimization of LL-37. Theranostics 2024, 14, 1181–1194. [Google Scholar] [CrossRef]
- Li, H.; Hu, Y.; Pu, Q.; He, T.; Zhang, Q.; Wu, W.; Xia, X.; Zhang, J. Novel Stapling by Lysine Tethering Provides Stable and Low Hemolytic Cationic Antimicrobial Peptides. J. Med. Chem. 2020, 63, 4081–4089. [Google Scholar] [CrossRef]
- Li, Y.; Wu, M.; Chang, Q.; Zhao, X. Stapling Strategy Enables Improvement of Antitumor Activity and Proteolytic Stability of Host-Defense Peptide Hymenochirin-1B. RSC Adv. 2018, 8, 22268–22275. [Google Scholar] [CrossRef]
- Moradi, S.V.; Hussein, W.M.; Varamini, P.; Simerska, P.; Toth, I. Glycosylation, an Effective Synthetic Strategy to Improve the Bioavailability of Therapeutic Peptides. Chem. Sci. 2016, 7, 2492–2500. [Google Scholar] [CrossRef] [PubMed]
- Radaic, A.; de Jesus, M.B.; Kapila, Y.L. Bacterial Anti-Microbial Peptides and Nano-Sized Drug Delivery Systems: The State of the Art Toward Improved Bacteriocins. J. Control. Release 2020, 321, 100–118. [Google Scholar] [CrossRef] [PubMed]
- Hilchie, A.L.; Hoskin, D.W.; Power Coombs, M.R. Anticancer Activities of Natural and Synthetic Peptides. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2019; Volume 1117, pp. 131–147. [Google Scholar]
- Akkın, S.; Varan, G.; Bilensoy, E. A Review on Cancer Immunotherapy and Applications of Nanotechnology to Chemoimmunotherapy of Different Cancers. Molecules 2021, 26, 3382. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Szalata, M.; Gorczyński, A.; Karczewski, J.; Eder, P.; Severino, P.; Cabeda, J.M.; Souto, E.B.; Słomski, R. Cancer Nanopharmaceuticals: Physicochemical Characterization and In Vitro/In Vivo Applications. Cancers 2021, 13, 1896. [Google Scholar] [CrossRef]
- Delong, R.K.; Comer, J.; Mathew, E.N.; Jaberi-Douraki, M. Comparative Molecular Immunological Activity of Physiological Metal Oxide Nanoparticle and Its Anticancer Peptide and RNA Complexes. Nanomaterials 2019, 9, 1670. [Google Scholar] [CrossRef]
- Niemirowicz, K.; Prokop, I.; Wilczewska, A.Z.; Wnorowska, U.; Piktel, E.; Wątek, M.; Savage, P.B.; Bucki, R. Magnetic Nanoparticles Enhance the Anticancer Activity of Cathelicidin LL-37 Peptide Against Colon Cancer Cells. Int. J. Nanomed. 2015, 10, 3843–3853. [Google Scholar] [CrossRef]
- Wnorowska, U.; Fiedoruk, K.; Piktel, E.; Prasad, S.V.; Sulik, M.; Janion, M.; Daniluk, T.; Savage, P.B.; Bucki, R. Nanoantibiotics Containing Membrane-Active Human Cathelicidin LL-37 or Synthetic Ceragenins Attached to the Surface of Magnetic Nanoparticles as Novel and Innovative Therapeutic Tools: Current Status and Potential Future Applications. J. Nanobiotechnol. 2020, 18, 3. [Google Scholar] [CrossRef]
- Chereddy, K.K.; Her, C.H.; Comune, M.; Moia, C.; Lopes, A.; Porporato, P.E.; Vanacker, J.; Lam, M.C.; Steinstraesser, L.; Sonveaux, P.; et al. PLGA Nanoparticles Loaded with Host Defense Peptide LL37 Promote Wound Healing. J. Control. Release 2014, 194, 138–147. [Google Scholar] [CrossRef]
- Izquierdo-Barba, I.; Vallet-Regí, M.; Kupferschmidt, N.; Terasaki, O.; Schmidtchen, A.; Malmsten, M. Incorporation of Antimicrobial Compounds in Mesoporous Silica Film Monolith. Biomaterials 2009, 30, 5729–5736. [Google Scholar] [CrossRef]
- Tsikourkitoudi, V.; Karlsson, J.; Merkl, P.; Loh, E.; Henriques-Normark, B.; Sotiriou, G.A. Flame-Made Calcium Phosphate Nanoparticles with High Drug Loading for Delivery of Biologics. Molecules 2020, 25, 1747. [Google Scholar] [CrossRef]
- van Gent, M.E.; Kłodzińska, S.N.; Severin, M.; Ali, M.; van Doodewaerd, B.R.; Bos, E.; Koning, R.I.; Drijfhout, J.W.; Nielsen, H.M.; Nibbering, P.H. Encapsulation into Hyaluronic Acid-Based Nanogels Improves the Selectivity Index of the Snake Cathelicidin Ab-Cath. Nanomed. Nanotechnol. Biol. Med. 2023, 52, 102694. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Carvalho, I.F.; Montelaro, R.C.; Gomes, P.; Martins, M.C.L. Covalent Immobilization of Antimicrobial Peptides (AMPs) onto Biomaterial Surfaces. Acta Biomater. 2011, 7, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.; Leal, E.C.; Carvalho, E. Bioactive Antimicrobial Peptides as Therapeutic Agents for Infected Diabetic Foot Ulcers. Biomolecules 2021, 11, 1894. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Tong, A.; Li, X.; Gao, X.; Mei, L.; Zhou, L.; Zhang, X.; You, C.; Guo, G. Enhanced Antitumor Effects by Docetaxel/LL 37-Loaded Thermosensitive Hydrogel Nanoparticles in Peritoneal Carcinomatosis of Colorectal Cancer. Int. J. Nanomed. 2015, 10, 7291–7305. [Google Scholar] [CrossRef]
- van Gent, M.E.; van Baaren, T.; Kłodzińska, S.N.; Ali, M.; Dolezal, N.; van Doodewaerd, B.R.; Bos, E.; de Waal, A.M.; Koning, R.I.; Drijfhout, J.W.; et al. Encapsulation of SAAP-148 in Octenyl Succinic Anhydride-Modified Hyaluronic Acid Nanogels for Treatment of Skin Wound Infections. Pharmaceutics 2023, 15, 429. [Google Scholar] [CrossRef]
- van Gent, M.E.; Klodzinska, S.N.; Drijfhout, J.W.; Nielsen, H.M.; Nibbering, P.H. Encapsulation in Oleyl-Modified Hyaluronic Acid Nanogels Substantially Improves the Clinical Potential of the Antimicrobial Peptides SAAP-148 and Ab-Cath. Eur. J. Pharm. Biopharm. 2023, 193, 254–261. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, J.; Wei, G.; Shen, Z.; Li, B.; Wu, J.; Liu, J. Exploring the Antimicrobial Potential of LL-37 Derivatives: Recent Developments and Challenges. ACS Biomater. Sci. Eng. 2025, 11, 3145–3164. [Google Scholar] [CrossRef]
- Kube, S.; Hersch, N.; Naumovska, E.; Gensch, T.; Hendriks, J.; Franzen, A.; Landvogt, L.; Siebrasse, J.P.; Kubitscheck, U.; Hoffmann, B.; et al. Fusogenic Liposomes as Nanocarriers for the Delivery of Intracellular Proteins. Langmuir 2017, 33, 1051–1059. [Google Scholar] [CrossRef]
- Malam, Y.; Loizidou, M.; Seifalian, A.M. Liposomes and Nanoparticles: Nanosized Vehicles for Drug Delivery in Cancer. Trends Pharmacol. Sci. 2009, 30, 592–599. [Google Scholar] [CrossRef]
- Sevcsik, E.; Pabst, G.; Richter, W.; Danner, S.; Amenitsch, H.; Lohner, K. Interaction of LL-37 with Model Membrane Systems of Different Complexity: Influence of the Lipid Matrix. Biophys. J. 2008, 94, 4688–4699. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Meade, J.; Devine, D.; Sadeghpour, A.; Rappolt, M.; Goycoolea, F.M. Chitosan-Coated Liposomal Systems for Delivery of Antibacterial Peptide LL17-32 to Porphyromonas Gingivalis. Heliyon 2024, 10, e34554. [Google Scholar] [CrossRef] [PubMed]
- Fumakia, M.; Ho, E.A. Nanoparticles Encapsulated with LL37 and Serpin A1 Promotes Wound Healing and Synergistically Enhances Antibacterial Activity. Mol. Pharm. 2016, 13, 2318–2331. [Google Scholar] [CrossRef]
- Niemirowicz, K.; Piktel, E.; Wilczewska, A.Z.; Markiewicz, K.H.; Durnaś, B.; Wątek, M.; Puszkarz, I.; Wróblewska, M.; Niklińska, W.; Savage, P.B.; et al. Core-Shell Magnetic Nanoparticles Display Synergistic Antibacterial Effects Against Pseudomonas Aeruginosa and Staphylococcus Aureus When Combined with Cathelicidin LL-37 or Selected Ceragenins. Int. J. Nanomed. 2016, 11, 5443–5455. [Google Scholar] [CrossRef]
- Su, Y.; Wang, H.; Mishra, B.; Lakshmaiah Narayana, J.; Jiang, J.; Reilly, D.A.; Hollins, R.R.; Carlson, M.A.; Wang, G.; Xie, J. Nanofiber Dressings Topically Delivering Molecularly Engineered Human Cathelicidin Peptides for the Treatment of Biofilms in Chronic Wounds. Mol. Pharm. 2019, 16, 2011–2020. [Google Scholar] [CrossRef]
- Zhang, H.; Dong, M.; Xu, H.; Li, H.; Zheng, A.; Sun, G.; Jin, W. Recombinant Lactococcus Lactis Expressing Human LL-37 Prevents Deaths from Viral Infections in Piglets and Chicken. Probiotics Antimicrob. Proteins 2024, 16, 2150–2160. [Google Scholar] [CrossRef]
- Dlozi, P.N.; Gladchuk, A.; Crutchley, R.D.; Keuler, N.; Coetzee, R.; Dube, A. Cathelicidins and Defensins Antimicrobial Host Defense Peptides in the Treatment of TB and HIV: Pharmacogenomic and Nanomedicine Approaches Towards Improved Therapeutic Outcomes. Biomed. Pharmacother. 2022, 151, 113189. [Google Scholar] [CrossRef]
- Zhou, Y.; Shi, Y.; Yang, L.; Sun, Y.; Han, Y.; Zhao, Z.; Wang, Y.; Liu, Y.; Ma, Y.; Zhang, T.; et al. Genetically Engineered Distal Airway Stem Cell Transplantation Protects Mice from Pulmonary Infection. EMBO Mol. Med. 2020, 12, e10233. [Google Scholar] [CrossRef]
- Li, Z.; Song, Y.; Yuan, P.; Guo, W.; Hu, X.; Xing, W.; Ao, L.; Tan, Y.; Wu, X.; Ao, X.; et al. Antibacterial Fusion Protein BPI21/LL-37 Modification Enhances the Therapeutic Efficacy of HUC-MSCs in Sepsis. Mol. Ther. 2020, 28, 1806. [Google Scholar] [CrossRef]
- Gasanov, V.A.o.; Kashirskikh, D.A.; Khotina, V.A.; Kuzmina, D.M.; Nikitochkina, S.Y.; Mukhina, I.V.; Vorotelyak, E.A.; Vasiliev, A.V. Preclinical Evaluation of the Safety, Toxicity and Efficacy of Genetically Modified Wharton’s Jelly Mesenchymal Stem/Stromal Cells Expressing the Antimicrobial Peptide SE-33. Cells 2025, 14, 341. [Google Scholar] [CrossRef]
- Gasanov, V.A.o.; Kashirskikh, D.A.; Khotina, V.A.; Lee, A.A.; Nikitochkina, S.Y.; Kuzmina, D.M.; Mukhina, I.V.; Vorotelyak, E.A.; Vasiliev, A.V. Genetically Modified Mesenchymal Stromal/Stem Cells as a Delivery Platform for SE-33, a Cathelicidin LL-37 Analogue: Preclinical Pharmacokinetics and Tissue Distribution in C57BL/6 Mice. Antibiotics 2025, 14, 429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ul Ain, Q.; Schulz, C.; Pircher, J. Role of Antimicrobial Peptide Cathelicidin in Thrombosis and Thromboinflammation. Front. Immunol. 2023, 14, 1151926. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, L.; Zhang, R.; Wu, R.; Si, D.; Ahmad, B.; Petitte, J.N.; Mozdziak, P.E.; Li, Z.; Guo, H.; et al. A Highly Efficient Hybrid Peptide Ameliorates Intestinal Inflammation and Mucosal Barrier Damage by Neutralizing Lipopolysaccharides and Antagonizing the Lipopolysaccharide-Receptor Interaction. FASEB J. 2020, 34, 16049–16072. [Google Scholar] [CrossRef]
- Ting, D.S.J.; Goh, E.T.L.; Mayandi, V.; Busoy, J.M.F.; Aung, T.T.; Periayah, M.H.; Nubile, M.; Mastropasqua, L.; Said, D.G.; Htoon, H.M.; et al. Hybrid Derivative of Cathelicidin and Human Beta Defensin-2 Against Gram-Positive Bacteria: A Novel Approach for the Treatment of Bacterial Keratitis. Sci. Rep. 2021, 11, 18304. [Google Scholar] [CrossRef]
| Type of PTM | Mechanism | Functional Consequences | References |
|---|---|---|---|
| Citrullination | Enzymatic conversion of Arg to citrulline by PADs Occurs during inflammation and may contribute to the development of autoimmune diseases | Loss of α-helical structure and antibacterial activity Increased antigen presentation Suppression of IFN-I production and B-cell maturation | [54,56,58] |
| Carbamylation | Non-enzymatic (chemical) reaction with cyanate (MPO-mediated) Lys and Leu convertion to homocitrulline Observed during neutrophilic inflammation | Promotes autoantibody production Maintain innate immune activation (pDCs, B cells) Contributes to SLE pathogenesis | [55,59,60] |
| Acetylation and formylation | Formation of acetyl and formyl groups at the N-terminus of the peptide Observed predominantly in neutrophils | Preserves antimicrobial activity but abrogates autophagy induction Alters immune regulatory functions | [52] |
| Modification | Mechanism/Approach | Examples | Biological Properties | References |
|---|---|---|---|---|
| Truncated analogs | Removal of N-terminal hydrophobic residues, shorter bioactive fragments | GF-17, FK-16, FK-13, KR-12, RI-10, etc. | Reduced toxicity Retained activity Improved selectivity and stability | [43,64,65,70,102] |
| Retro-analog engineering | Sequence reversal while preserving critical residues | SE-33, Retro-KR12-C8ε-NH2, C8α-retro-KR12-NH2 | Increased protease resistance Enhanced selectivity | [86,92,93,94] |
| Point mutations and sequence substitutions | Replacement of hydrophobic residues with hydrophilic amino acids | Positional Q and K mutants of LL-37, derivatives of KR-12 (KR-12-a1 to KR-12-a6) | Reduced toxicity Retained or enhanced antimicrobial activity Decreased hemolytic activity Improved selectivity Increased antitumor activity | [95,97,101] |
| Replacement of positively charged amino acids with hydrophobic residues | Glu and Lys to Phe substitutions in FF/CAP18, Ser9 to Ala or Val in LL-37 (LL-23 generation) | Increased antitumor activity Increased antibacterial and immunosuppressive activity | [98,99] | |
| Substitutions of residues with non-canonical amino acid | Targeted incorporation of non-canonical amino acids (e.g., aromatic or β-amino acids) | β-amino acids in LL-37 | Increased resistance to proteases Enhanced antimicrobial activity Improved peptide translocation across the bacterial membranes | [108] |
| Substitutions of L-amino acids with D-enantiomers | Introduction of D-residues at critical positions (e.g., protease-sensitive sites) | GF-17d1-3, GI-20d, 17BIPHE2 | Increased resistance to proteases Preservation of activity Decreased cytotoxicity | [51,73,103] |
| Cyclization | Formation of disulfide bridges or “head-to-tail” cyclization | CD4-PP | Enhanced stability under physiological conditions | [87,88,89] |
| Hydrocarbon stapling | Introduction of covalent alkyl bridges between side chains of α,α-disubstituted amino acids (e.g., in positions of i, i + 4 or i, i + 7) | SLP-51, KR-12(Q5,D9) | Stabilization of α-helix Increased conformational stability Increased protease resistance | [83,129,130] |
| Terminal modifications | N-terminal acetylation, C-terminal amidation | OP-145, SAAP-148, AC-1, AC-2 | Decreased cytotoxicity Enhanced exopeptidase resistance (prevents aminopeptidase cleavage, reduce carboxypeptidase sensitivity) | [74,75,76,78,79,80,81,84] |
| Charge and hydrophobicity modulation | Reduction in net positive charge, hydrophobic residue substitution | 17F2, positional Q and K mutants of LL-37, KE-18, KR-12 | Reduced hemolysis and cytotoxicity with maintained antimicrobial activity Enhanced affinity for bacterial membranes Improved anti-biofilm activity | [67,96,112] |
| Lipidation | Conjugation with fatty acids (e.g., myristic, lauric, benzoic or octanoic acid) | Laurylated (C12-KR12) and myristoylated (C14-KR12). KR-12 peptide, Myr-KR-12N, Myr-KR-12C, Retro-KR12-C8ε-NH2 | Increased membrane interaction and antimicrobial potency | [86,94,127,128] |
| Hybridization or dimerization | Fusion of functional domains from multiple peptides or bioactive molecules to combine or enhance effects | LL-37/magainin II fusion; LL-37 conjugation with CpG oligodeoxynucleotides | Increased antimicrobial activity compared to the parental peptides Improve selectivity Enhance activation of innate immunity | [124,125] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voronko, O.E.; Khotina, V.A.; Kashirskikh, D.A.; Lee, A.A.; Gasanov, V.A.o. Antimicrobial Peptides of the Cathelicidin Family: Focus on LL-37 and Its Modifications. Int. J. Mol. Sci. 2025, 26, 8103. https://doi.org/10.3390/ijms26168103
Voronko OE, Khotina VA, Kashirskikh DA, Lee AA, Gasanov VAo. Antimicrobial Peptides of the Cathelicidin Family: Focus on LL-37 and Its Modifications. International Journal of Molecular Sciences. 2025; 26(16):8103. https://doi.org/10.3390/ijms26168103
Chicago/Turabian StyleVoronko, Olga Evgenevna, Victoria Alexandrovna Khotina, Dmitry Alexandrovich Kashirskikh, Arthur Anatolievich Lee, and Vagif Ali oglu Gasanov. 2025. "Antimicrobial Peptides of the Cathelicidin Family: Focus on LL-37 and Its Modifications" International Journal of Molecular Sciences 26, no. 16: 8103. https://doi.org/10.3390/ijms26168103
APA StyleVoronko, O. E., Khotina, V. A., Kashirskikh, D. A., Lee, A. A., & Gasanov, V. A. o. (2025). Antimicrobial Peptides of the Cathelicidin Family: Focus on LL-37 and Its Modifications. International Journal of Molecular Sciences, 26(16), 8103. https://doi.org/10.3390/ijms26168103







