Fenofibrate Differently Affects the Heart’s Morphology and Metabolism in Young and Old Rats
Abstract
1. Introduction
2. Results
2.1. Lipids and Biochemical Markers in Blood Serum
2.2. Fenofibrate Effects on the Morphology of Cardiac Muscle
2.3. Impact of Fenofibrate on Lipid Droplets’ Area in Cardiomyocytes
2.4. Fenofibrate Effects on Immunohistochemical Markers of Cardiac Hypertrophy
2.5. Impact of Fenofibrate on the Expression of Genes and Proteins Involved in Cardiac Muscle Energy Metabolism
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Procedures
4.2. Serum Levels of Lipids and Biochemical Markers
4.3. Histological Stains
4.4. Immunohistochemical Analyses
4.5. Reverse Transcription and Quantitative Real-Time PCR
4.6. Western Blotting for Protein Quantification
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chong, B.; Jayabaskaran, J.; Jauhari, S.M.; Chan, S.P.; Goh, R.; Kueh, M.T.W.; Li, H.; Chin, Y.H.; Kong, G.; Anand, V.V.; et al. Global Burden of Cardiovascular Diseases: Projections from 2025 to 2050. Eur. J. Prev. Cardiol. 2024, zwae281. [Google Scholar] [CrossRef]
- Mubarik, S.; Naeem, S.; Shen, H.; Mubarak, R.; Luo, L.; Hussain, S.R.; Hak, E.; Yu, C.; Liu, X. Population-Level Distribution, Risk Factors, and Burden of Mortality and Disability-Adjusted Life Years Attributable to Major Noncommunicable Diseases in Western Europe (1990–2021): Ecological Analysis. JMIR Public Health Surveill. 2024, 10, e57840. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, Z.; Yin, X.; QirongXu; Li, F.; Zhu, F. Global Burden of Ischemic Stroke Attributable to High Body Mass Index in 204 Countries and Territories, 1990–2021. BMC Cardiovasc. Disord. 2024, 24, 584. [Google Scholar] [CrossRef]
- Dhingra, R.; Vasan, R.S. Age as a Risk Factor. Med. Clin. N. Am. 2012, 96, 87–91. [Google Scholar] [CrossRef]
- Liu, H.-H.; Li, J.-J. Aging and Dyslipidemia: A Review of Potential Mechanisms. Ageing Res. Rev. 2015, 19, 43–52. [Google Scholar] [CrossRef]
- Sidhu, G.; Tripp, J. Fenofibrate. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Keech, A.; Simes, R.J.; Barter, P.; Best, J.; Scott, R.; Taskinen, M.R.; Forder, P.; Pillai, A.; Davis, T.; Glasziou, P.; et al. Effects of Long-Term Fenofibrate Therapy on Cardiovascular Events in 9795 People with Type 2 Diabetes Mellitus (the FIELD Study): Randomised Controlled Trial. Lancet 2005, 366, 1849–1861, Erratum in Lancet, 2006, 368, 1415. https://doi.org/10.1016/S0140-6736(06)69594-9. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, H.N.; Elam, M.B.; Lovato, L.C.; Crouse, J.R., 3rd; Leiter, L.A.; Linz, P.; Friedewald, W.T.; Buse, J.B.; Gerstein, H.C.; Probstfield, J.; et al. Effects of Combination Lipid Therapy in Type 2 Diabetes Mellitus. N. Engl. J. Med. 2010, 362, 1563–1574, Erratum in N. Engl. J. Med. 2010, 362, 1748. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.P.; Vasques-Nóvoa, F.; Ferrão, D.; Saraiva, F.; Falcão-Pires, I.; Neves, J.S.; Sharma, A.; Rossignol, P.; Zannad, F.; Leite-Moreira, A. Fenofibrate and Heart Failure Outcomes in Patients With Type 2 Diabetes: Analysis From ACCORD. Diabetes Care 2022, 45, 1584–1591. [Google Scholar] [CrossRef]
- Jo, S.-H.; Nam, H.; Lee, J.; Park, S.; Lee, J.; Kyoung, D.-S. Fenofibrate Use Is Associated With Lower Mortality and Fewer Cardiovascular Events in Patients With Diabetes: Results of 10,114 Patients From the Korean National Health Insurance Service Cohort. Diabetes Care 2021, 44, 1868–1876. [Google Scholar] [CrossRef] [PubMed]
- Malur, P.; Menezes, A.; DiNicolantonio, J.J.; O’Keefe, J.H.; Lavie, C.J. The Microvascular and Macrovascular Benefits of Fibrates in Diabetes and the Metabolic Syndrome: A Review. Mo. Med. 2017, 114, 464–471. [Google Scholar]
- Rakhshandehroo, M.; Knoch, B.; Müller, M.; Kersten, S. Peroxisome Proliferator-Activated Receptor Alpha Target Genes. PPAR Res. 2010, 2010, 612089. [Google Scholar] [CrossRef] [PubMed]
- Ogata, T.; Miyauchi, T.; Sakai, S.; Takanashi, M.; Irukayama-Tomobe, Y.; Yamaguchi, I. Myocardial Fibrosis and Diastolic Dysfunction in Deoxycorticosterone Acetate-Salt Hypertensive Rats Is Ameliorated by the Peroxisome Proliferator-Activated Receptor-Alpha Activator Fenofibrate, Partly by Suppressing Inflammatory Responses Associated with the Nuclear Factor-Kappa-B Pathway. J. Am. Coll. Cardiol. 2004, 43, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Jen, H.-L.; Liu, P.-L.; Chen, Y.-H.; Yin, W.-H.; Chen, J.-W.; Lin, S.-J. Peroxisome Proliferator-Activated Receptor α Reduces Endothelin-1-Caused Cardiomyocyte Hypertrophy by Inhibiting Nuclear Factor-κB and Adiponectin. Mediators Inflamm. 2016, 2016, 5609121. [Google Scholar] [CrossRef]
- Refaie, M.M.M.; Shehata, S.; Bayoumi, A.M.A.; El-Tahawy, N.F.G.; Abdelzaher, W.Y. The IL-6/STAT Signaling Pathway and PPARα Are Involved in Mediating the Dose-Dependent Cardioprotective Effects of Fenofibrate in 5-Fluorouracil-Induced Cardiotoxicity. Cardiovasc. Drugs Ther. 2022, 36, 817–827. [Google Scholar] [CrossRef]
- Ibarra-Lara, L.; Sánchez-Aguilar, M.; Sánchez-Mendoza, A.; Del Valle-Mondragón, L.; Soria-Castro, E.; Carreón-Torres, E.; Díaz-Díaz, E.; Vázquez-Meza, H.; Guarner-Lans, V.; Rubio-Ruiz, M.E. Fenofibrate Therapy Restores Antioxidant Protection and Improves Myocardial Insulin Resistance in a Rat Model of Metabolic Syndrome and Myocardial Ischemia: The Role of Angiotensin II. Molecules 2016, 22, 31. [Google Scholar] [CrossRef]
- Cortes-Lopez, F.; Sanchez-Mendoza, A.; Centurion, D.; Cervantes-Perez, L.G.; Castrejon-Tellez, V.; Del Valle-Mondragon, L.; Soria-Castro, E.; Ramirez, V.; Sanchez-Lopez, A.; Pastelin-Hernandez, G.; et al. Fenofibrate Protects Cardiomyocytes from Hypoxia/Reperfusion- and High Glucose-Induced Detrimental Effects. PPAR Res. 2021, 2021, 8895376. [Google Scholar] [CrossRef] [PubMed]
- Rich, M.W.; Chyun, D.A.; Skolnick, A.H.; Alexander, K.P.; Forman, D.E.; Kitzman, D.W.; Maurer, M.S.; McClurken, J.B.; Resnick, B.M.; Shen, W.K.; et al. Knowledge Gaps in Cardiovascular Care of the Older Adult Population: A Scientific Statement From the American Heart Association, American College of Cardiology, and American Geriatrics Society. Circulation 2016, 133, 2103–2122. [Google Scholar] [CrossRef]
- Ayan, M.; Pothineni, N.V.; Siraj, A.; Mehta, J.L. Cardiac Drug Therapy-Considerations in the Elderly. J. Geriatr. Cardiol. 2016, 13, 992–997. [Google Scholar] [CrossRef]
- Andres, T.M.; McGrane, T.; McEvoy, M.D.; Allen, B.F.S. Geriatric Pharmacology: An Update. Anesthesiol. Clin. 2019, 37, 475–492. [Google Scholar] [CrossRef]
- Zubrzycki, A.; Wrońska, A.; Kotulak-Chrząszcz, A.; Wierzbicki, P.M.; Kmieć, Z. Fenofibrate Impairs Liver Function and Structure More Pronounced in Old than Young Rats. Arch. Gerontol. Geriatr. 2020, 91, 104244. [Google Scholar] [CrossRef]
- Wronska, A.; Zubrzycki, A.; Kotlarz, G.; Kmiec, Z. Fenofibrate Mildly Stimulates Browning-Associated Expression in White Adipose Tissues of Young but Not Old Male Rats. J. Physiol. Pharmacol. 2023, 74, 169–183. [Google Scholar] [CrossRef]
- Wrońska, A.; Kieżun, J.; Kmieć, Z. High-Dose Fenofibrate Stimulates Multiple Cellular Stress Pathways in the Kidney of Old Rats. Int. J. Mol. Sci. 2024, 25, 3038. [Google Scholar] [CrossRef]
- Lazzeroni, D.; Villatore, A.; Souryal, G.; Pili, G.; Peretto, G. The Aging Heart: A Molecular and Clinical Challenge. Int. J. Mol. Sci. 2022, 23, 16033. [Google Scholar] [CrossRef]
- Park, J.; Song, H.; Moon, S.; Kim, Y.; Cho, S.; Han, K.; Park, C.-Y.; Cho, S.W.; Oh, C.-M. Cardiometabolic Benefits of Fenofibrate in Heart Failure Related to Obesity and Diabetes. Cardiovasc. Diabetol. 2024, 23, 343. [Google Scholar] [CrossRef]
- Rius-Pérez, S.; Torres-Cuevas, I.; Millán, I.; Ortega, Á.L.; Pérez, S. PGC-1α, Inflammation, and Oxidative Stress: An Integrative View in Metabolism. Oxid. Med. Cell Longev. 2020, 2020, 1452696. [Google Scholar] [CrossRef]
- Shimano, H. SREBPs: Physiology and Pathophysiology of the SREBP Family. FEBS J. 2009, 276, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Sun, X.; Wang, C.; Wang, F.; Fang, C.; Hu, Z. PFKM Inhibits Doxorubicin-Induced Cardiotoxicity by Enhancing Oxidative Phosphorylation and Glycolysis. Sci. Rep. 2022, 12, 11684. [Google Scholar] [CrossRef] [PubMed]
- Jain, P. Traditional and Novel Non-Statin Lipid-Lowering Drugs. Indian Heart J. 2024, 76 (Suppl. 1), S38–S43. [Google Scholar] [CrossRef] [PubMed]
- Depboylu, B.; Doğru-Abbasoğlu, S.; Aykaç-Toker, G.; Uysal, M. Increased Susceptibility of Serum and Apo-B-Containing Lipoproteins to Peroxidation in Aged Rats. Clin. Exp. Med. 2007, 7, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Gälman, C.; Matasconi, M.; Persson, L.; Parini, P.; Angelin, B.; Rudling, M. Age-Induced Hypercholesterolemia in the Rat Relates to Reduced Elimination but Not Increased Intestinal Absorption of Cholesterol. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E737–E742. [Google Scholar] [CrossRef]
- Pettersen, J.C.; Pruimboom-Brees, I.; Francone, O.L.; Amacher, D.E.; Boldt, S.E.; Kerlin, R.L.; Ballinger, W.E. The PPARα Agonists Fenofibrate and CP-778875 Cause Increased β-Oxidation, Leading to Oxidative Injury in Skeletal and Cardiac Muscle in the Rat. Toxicol. Pathol. 2012, 40, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.H.; Armani, A.; McKenney, J.M.; Jacobson, T.A. Safety Considerations with Fibrate Therapy. Am. J. Cardiol. 2007, 99, 3C–18C. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Ko, K.; Park, S.; Lee, D.R.; Lee, J. Effect of Fenofibrate Medication on Renal Function. Korean J. Fam. Med. 2017, 38, 192–198. [Google Scholar] [CrossRef]
- Naderi-Boldaji, V.; Joukar, S.; Noorafshan, A.; Raji-Amirhasani, A.; Naderi-Boldaji, S.; Bejeshk, M.-A. The Effect of Blood Flow Restriction along with Low-Intensity Exercise on Cardiac Structure and Function in Aging Rat: Role of Angiogenesis. Life Sci. 2018, 209, 202–209. [Google Scholar] [CrossRef]
- Lunde, I.G.; Rypdal, K.B.; Van Linthout, S.; Diez, J.; González, A. Myocardial Fibrosis from the Perspective of the Extracellular Matrix: Mechanisms to Clinical Impact. Matrix Biol. 2024, 134, 1–22. [Google Scholar] [CrossRef]
- Hayashi, H.; Wang, C.; Miyauchi, Y.; Omichi, C.; Pak, H.-N.; Zhou, S.; Ohara, T.; Mandel, W.J.; Lin, S.-F.; Fishbein, M.C.; et al. Aging-Related Increase to Inducible Atrial Fibrillation in the Rat Model. J. Cardiovasc. Electrophysiol. 2002, 13, 801–808. [Google Scholar] [CrossRef]
- Lin, J.; Lopez, E.F.; Jin, Y.; Van Remmen, H.; Bauch, T.; Han, H.-C.; Lindsey, M.L. Age-Related Cardiac Muscle Sarcopenia: Combining Experimental and Mathematical Modeling to Identify Mechanisms. Exp. Gerontol. 2008, 43, 296–306. [Google Scholar] [CrossRef]
- Huet, E.; Gabison, E.; Vallee, B.; Mougenot, N.; Linguet, G.; Riou, B.; Jarosz, C.; Menashi, S.; Besse, S. Deletion of Extracellular Matrix Metalloproteinase Inducer/CD147 Induces Altered Cardiac Extracellular Matrix Remodeling in Aging Mice. J. Physiol. Pharmacol. 2015, 66, 355–366. [Google Scholar]
- Mesquita, T.R.R.; Zhang, R.; de Couto, G.; Valle, J.; Sanchez, L.; Rogers, R.G.; Holm, K.; Liu, W.; Marbán, E.; Cingolani, E. Mechanisms of Atrial Fibrillation in Aged Rats with Heart Failure with Preserved Ejection Fraction. Heart Rhythm. 2020, 17, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Kane, A.E.; Bisset, E.S.; Heinze-Milne, S.; Keller, K.M.; Grandy, S.A.; Howlett, S.E. Maladaptive Changes Associated With Cardiac Aging Are Sex-Specific and Graded by Frailty and Inflammation in C57BL/6 Mice. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 233–243. [Google Scholar] [CrossRef]
- Hafstad, A.D.; Khalid, A.M.; Hagve, M.; Lund, T.; Larsen, T.S.; Severson, D.L.; Clarke, K.; Berge, R.K.; Aasum, E. Cardiac Peroxisome Proliferator-Activated Receptor-Alpha Activation Causes Increased Fatty Acid Oxidation, Reducing Efficiency and Post-Ischaemic Functional Loss. Cardiovasc. Res. 2009, 83, 519–526. [Google Scholar] [CrossRef]
- Lebrasseur, N.K.; Duhaney, T.-A.S.; De Silva, D.S.; Cui, L.; Ip, P.C.; Joseph, L.; Sam, F. Effects of Fenofibrate on Cardiac Remodeling in Aldosterone-Induced Hypertension. Hypertension 2007, 50, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cheng, Y.; Gu, J.; Wang, S.; Zhou, S.; Wang, Y.; Tan, Y.; Feng, W.; Fu, Y.; Mellen, N.; et al. Fenofibrate Increases Cardiac Autophagy via FGF21/SIRT1 and Prevents Fibrosis and Inflammation in the Hearts of Type 1 Diabetic Mice. Clin. Sci. 2016, 130, 625–641. [Google Scholar] [CrossRef] [PubMed]
- Kivelä, R.; Bry, M.; Robciuc, M.R.; Räsänen, M.; Taavitsainen, M.; Silvola, J.M.U.; Saraste, A.; Hulmi, J.J.; Anisimov, A.; Mäyränpää, M.I.; et al. VEGF-B-Induced Vascular Growth Leads to Metabolic Reprogramming and Ischemia Resistance in the Heart. EMBO Mol. Med. 2014, 6, 307–321. [Google Scholar] [CrossRef]
- Manickam, N.; Sultan, I.; Panthel, J.; Kujundzic, H.; Fischer, A.; Schmitz, K.; Ruz Jurado, M.; Morales, D.R.; John, D.; Glaser, S.-F.; et al. Beneficial Effects of Vascular Endothelial Growth Factor B Gene Transfer in the Aged Heart. Cardiovasc. Res. 2025, cvaf046. [Google Scholar] [CrossRef] [PubMed]
- Shang, R.; Lal, N.; Lee, C.S.; Zhai, Y.; Puri, K.; Seira, O.; Boushel, R.C.; Sultan, I.; Räsänen, M.; Alitalo, K.; et al. Cardiac-Specific VEGFB Overexpression Reduces Lipoprotein Lipase Activity and Improves Insulin Action in Rat Heart. Am. J. Physiol. Endocrinol. Metab. 2021, 321, E753–E765. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, F.; Wang, J.; Zhang, D.; Zhao, X. Ketogenic Diet Attenuates Aging-Associated Myocardial Remodeling and Dysfunction in Mice. Exp. Gerontol. 2020, 140, 111058. [Google Scholar] [CrossRef]
- Della Corte, V.; Pacinella, G.; Todaro, F.; Pecoraro, R.; Tuttolomondo, A. The Natriuretic Peptide System: A Single Entity, Pleiotropic Effects. Int. J. Mol. Sci. 2023, 24, 9642. [Google Scholar] [CrossRef]
- Skelly, D.A.; Squiers, G.T.; McLellan, M.A.; Bolisetty, M.T.; Robson, P.; Rosenthal, N.A.; Pinto, A.R. Single-Cell Transcriptional Profiling Reveals Cellular Diversity and Intercommunication in the Mouse Heart. Cell Rep. 2018, 22, 600–610. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, H.; Huang, X.; Wang, S.; Ouyang, X.; Wang, Y.; Cao, Q.; Yang, L.; Tao, Y.; Lai, H. Pirfenidone Attenuates Cardiac Hypertrophy against Isoproterenol by Inhibiting Activation of the Janus Tyrosine Kinase-2/Signal Transducer and Activator of Transcription 3 (JAK-2/STAT3) Signaling Pathway. Bioengineered 2022, 13, 12772–12782. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Li, P.; Han, P.; Kang, Y.J.; Zhang, W. Gene Expression Patterns and Related Pathways in the Hearts of Rhesus Monkeys Subjected to Prolonged Myocardial Ischemia. Exp. Biol. Med. 2023, 248, 350–360. [Google Scholar] [CrossRef]
- Althurwi, H.N.; Elshenawy, O.H.; El-Kadi, A.O.S. Fenofibrate Modulates Cytochrome P450 and Arachidonic Acid Metabolism in the Heart and Protects against Isoproterenol-Induced Cardiac Hypertrophy. J. Cardiovasc. Pharmacol. 2014, 63, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.-Y.; Lu, H.-Q.; Yan, Q.-J.; Zou, J. A Reduction in ADAM17 Expression Is Involved in the Protective Effect of the PPAR-α Activator Fenofibrate on Pressure Overload-Induced Cardiac Hypertrophy. PPAR Res. 2018, 2018, 7916953. [Google Scholar] [CrossRef]
- Castiglioni, L.; Gelosa, P.; Muluhie, M.; Mercuriali, B.; Rzemieniec, J.; Gotti, M.; Fiordaliso, F.; Busca, G.; Sironi, L. Fenofibrate Reduces Cardiac Remodeling by Mitochondrial Dynamics Preservation in a Renovascular Model of Cardiac Hypertrophy. Eur. J. Pharmacol. 2024, 978, 176767. [Google Scholar] [CrossRef]
- Kochansky, C.J.; Lyman, M.J.; Fauty, S.E.; Vlasakova, K.; D’mello, A.P. Administration of Fenofibrate Markedly Elevates Fabp3 in Rat Liver and Plasma and Confounds Its Use as a Preclinical Biomarker of Cardiac and Muscle Toxicity. Lipids 2018, 53, 947–960. [Google Scholar] [CrossRef]
- Yang, J.; Sambandam, N.; Han, X.; Gross, R.W.; Courtois, M.; Kovacs, A.; Febbraio, M.; Finck, B.N.; Kelly, D.P. CD36 Deficiency Rescues Lipotoxic Cardiomyopathy. Circ. Res. 2007, 100, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- Koonen, D.P.Y.; Febbraio, M.; Bonnet, S.; Nagendran, J.; Young, M.E.; Michelakis, E.D.; Dyck, J.R.B. CD36 Expression Contributes to Age-Induced Cardiomyopathy in Mice. Circulation 2007, 116, 2139–2147. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, N.; Gill, J.F.; Brink, M.; Handschin, C. Moderate Modulation of Cardiac PGC-1α Expression Partially Affects Age-Associated Transcriptional Remodeling of the Heart. Front. Physiol. 2018, 9, 242. [Google Scholar] [CrossRef]
- Fannin, S.W.; Lesnefsky, E.J.; Slabe, T.J.; Hassan, M.O.; Hoppel, C.L. Aging Selectively Decreases Oxidative Capacity in Rat Heart Interfibrillar Mitochondria. Arch. Biochem. Biophys. 1999, 372, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Dongil, P.; Pérez-García, A.; Hurtado-Carneiro, V.; Herrero-de-Dios, C.; Álvarez, E.; Sanz, C. PAS Kinase Deficiency Reduces Aging Effects in Mice. Aging 2020, 12, 2275–2301. [Google Scholar] [CrossRef]
- Lee, W.H.; Kim, S.G. AMPK-Dependent Metabolic Regulation by PPAR Agonists. PPAR Res. 2010, 2010, 549101. [Google Scholar] [CrossRef]
- Liu, G.-Z.; Hou, T.-T.; Yuan, Y.; Hang, P.-Z.; Zhao, J.-J.; Sun, L.; Zhao, G.-Q.; Zhao, J.; Dong, J.-M.; Wang, X.-B.; et al. Fenofibrate Inhibits Atrial Metabolic Remodelling in Atrial Fibrillation through PPAR-α/Sirtuin 1/PGC-1α Pathway. Br. J. Pharmacol. 2016, 173, 1095–1109. [Google Scholar] [CrossRef]
- Li, W.-Y.; Yao, C.-X.; Zhang, S.-F.; Wang, S.-L.; Wang, T.-Q.; Xiong, C.-J.; Li, Y.-B.; Zang, M.-X. Improvement of Myocardial Lipid Accumulation and Prevention of PGC-1α Induction by Fenofibrate. Mol. Med. Rep. 2012, 5, 1396–1400. [Google Scholar] [CrossRef]
- Kar, D.; Bandyopadhyay, A. Targeting Peroxisome Proliferator Activated Receptor α (PPAR α) for the Prevention of Mitochondrial Impairment and Hypertrophy in Cardiomyocytes. Cell Physiol. Biochem. 2018, 49, 245–259. [Google Scholar] [CrossRef]
- Zubrzycki, A.; Wrońska, A.; Wierzbicki, P.M.; Kmieć, Z. Age-Related Effects of Fenofibrate on the Hepatic Expression of Sirtuin 1, Sirtuin 3, and Lipid Metabolism-Related Genes. Acta Biochim. Pol. 2023, 70, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.-H.; Chen, T.-P.; Lee, C.-H.; Wu, Y.-C.; Lin, Y.-M.; Lin, P.J. Cardiomyocytic Apoptosis Following Global Cardiac Ischemia and Reperfusion Can Be Attenuated by Peroxisome Proliferator-Activated Receptor Alpha but Not Gamma Activators. Shock 2006, 26, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Yue, T.; Bao, W.; Jucker, B.M.; Gu, J.; Romanic, A.M.; Brown, P.J.; Cui, J.; Thudium, D.T.; Boyce, R.; Burns-Kurtis, C.L.; et al. Activation of Peroxisome Proliferator-Activated Receptor-Alpha Protects the Heart from Ischemia/Reperfusion Injury. Circulation 2003, 108, 2393–2399. [Google Scholar] [CrossRef]
- Kim, S.K.; Zhao, Z.S.; Lee, Y.J.; Lee, K.E.; Kang, S.M.; Choi, D.; Lim, S.-K.; Chung, N.; Lee, H.C.; Cha, B.S. Left-Ventricular Diastolic Dysfunction May Be Prevented by Chronic Treatment with PPAR-Alpha or -Gamma Agonists in a Type 2 Diabetic Animal Model. Diabetes Metab. Res. Rev. 2003, 19, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Ussher, J.R.; Folmes, C.D.L.; Jaswal, J.S.; Stanley, W.C. Myocardial Fatty Acid Metabolism in Health and Disease. Physiol. Rev. 2010, 90, 207–258. [Google Scholar] [CrossRef]
- Li, D.; Zhang, L.; Gong, Q.; Deng, H.; Luo, C.; Zhou, T.; Huang, W.; Xu, Y. The Role of Myocardial Energy Metabolism Perturbations in Diabetic Cardiomyopathy: From the Perspective of Novel Protein Post-Translational Modifications. Clin. Epigenetics 2025, 17, 15. [Google Scholar] [CrossRef]
- Eberlé, D.; Hegarty, B.; Bossard, P.; Ferré, P.; Foufelle, F. SREBP Transcription Factors: Master Regulators of Lipid Homeostasis. Biochimie 2004, 86, 839–848. [Google Scholar] [CrossRef]
- Kumar, R.; Chhillar, N.; Gupta, D.S.; Kaur, G.; Singhal, S.; Chauhan, T. Cholesterol Homeostasis, Mechanisms of Molecular Pathways, and Cardiac Health: A Current Outlook. Curr. Probl. Cardiol. 2024, 49, 102081. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.H.; Wang, Z.V. Glucose Metabolism in Cardiac Hypertrophy and Heart Failure. J. Am. Heart Assoc. 2019, 8, e012673. [Google Scholar] [CrossRef]
- Wang, J.; Qin, L.; Feng, Y.; Zheng, R.; Deng, C.; Xiong, Y.; Zuo, B. Molecular Characterization, Expression Profile, and Association Study with Meat Quality Traits of Porcine PFKM Gene. Appl. Biochem. Biotechnol. 2014, 173, 1640–1651. [Google Scholar] [CrossRef]
- Mhaskar, Y.; Dunaway, G.A. The Subunit Proportions and Kinetic Properties of 6-Phosphofructo-1-Kinase Isozymes from Rat Heart Atria and Ventricle Progressively Change during Aging. Mol. Cell Biochem. 1991, 107, 39–45. [Google Scholar] [CrossRef]
- Gerhart-Hines, Z.; Rodgers, J.T.; Bare, O.; Lerin, C.; Kim, S.-H.; Mostoslavsky, R.; Alt, F.W.; Wu, Z.; Puigserver, P. Metabolic Control of Muscle Mitochondrial Function and Fatty Acid Oxidation through SIRT1/PGC-1alpha. EMBO J. 2007, 26, 1913–1923. [Google Scholar] [CrossRef] [PubMed]
- Murugasamy, K.; Munjal, A.; Sundaresan, N.R. Emerging Roles of SIRT3 in Cardiac Metabolism. Front. Cardiovasc. Med. 2022, 9, 850340. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jiang, S.; Wang, H.; Wang, Y.; Han, Y.; Jiang, J. Berberine Exerts Protective Effects on Cardiac Senescence by Regulating the Klotho/SIRT1 Signaling Pathway. Biomed. Pharmacother. 2022, 151, 113097. [Google Scholar] [CrossRef]
- Zhang, J.; He, Z.; Fedorova, J.; Logan, C.; Bates, L.; Davitt, K.; Le, V.; Murphy, J.; Li, M.; Wang, M.; et al. Alterations in Mitochondrial Dynamics with Age-Related Sirtuin1/Sirtuin3 Deficiency Impair Cardiomyocyte Contractility. Aging Cell 2021, 20, e13419. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Y.; Song, L.; Yu, L.; Zhang, L.; Zhang, Y.; Xing, Y.; Yin, Y.; Ma, H. SIRT3 Deficiency Exacerbates P53/Parkin-mediated Mitophagy Inhibition and Promotes Mitochondrial Dysfunction: Implication for Aged Hearts. Int. J. Mol. Med. 2018, 41, 3517–3526. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Slotabec, L.; Cheng, F.; Tan, Y.; Li, J. Alterations of SIRT1/SIRT3 Subcellular Distribution in Aging Undermine Cardiometabolic Homeostasis during Ischemia and Reperfusion. Aging Cell 2023, 22, e13930. [Google Scholar] [CrossRef]
- Liao, Y.; Ke, B.; Long, X.; Xu, J.; Wu, Y. Abnormalities in the SIRT1-SIRT3 Axis Promote Myocardial Ischemia-Reperfusion Injury through Ferroptosis Caused by Silencing the PINK1/Parkin Signaling Pathway. BMC Cardiovasc. Disord. 2023, 23, 582. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, X.; Shi, L.; Wang, J.; Xu, Q.; Yu, B.; Qu, A. A Time-Series Minimally Invasive Transverse Aortic Constriction Mouse Model for Pressure Overload-Induced Cardiac Remodeling and Heart Failure. Front. Cardiovasc. Med. 2023, 10, 1110032. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, J.A. Histological and Histochemical Methods: Theory and Practice, 5th ed.; Scion Publishing: Banbury, UK, 2015. [Google Scholar]
- Kiezun, J.; Godlewski, J.; Krazinski, B.E.; Kozielec, Z.; Kmiec, Z. Galanin Receptors (GalR1, GalR2, and GalR3) Expression in Colorectal Cancer Tissue and Correlations to the Overall Survival and Poor Prognosis of CRC Patients. Int. J. Mol. Sci. 2022, 23, 3735. [Google Scholar] [CrossRef] [PubMed]
- Silver, N.; Best, S.; Jiang, J.; Thein, S.L. Selection of Housekeeping Genes for Gene Expression Studies in Human Reticulocytes Using Real-Time PCR. BMC Mol. Biol. 2006, 7, 33. [Google Scholar] [CrossRef] [PubMed]

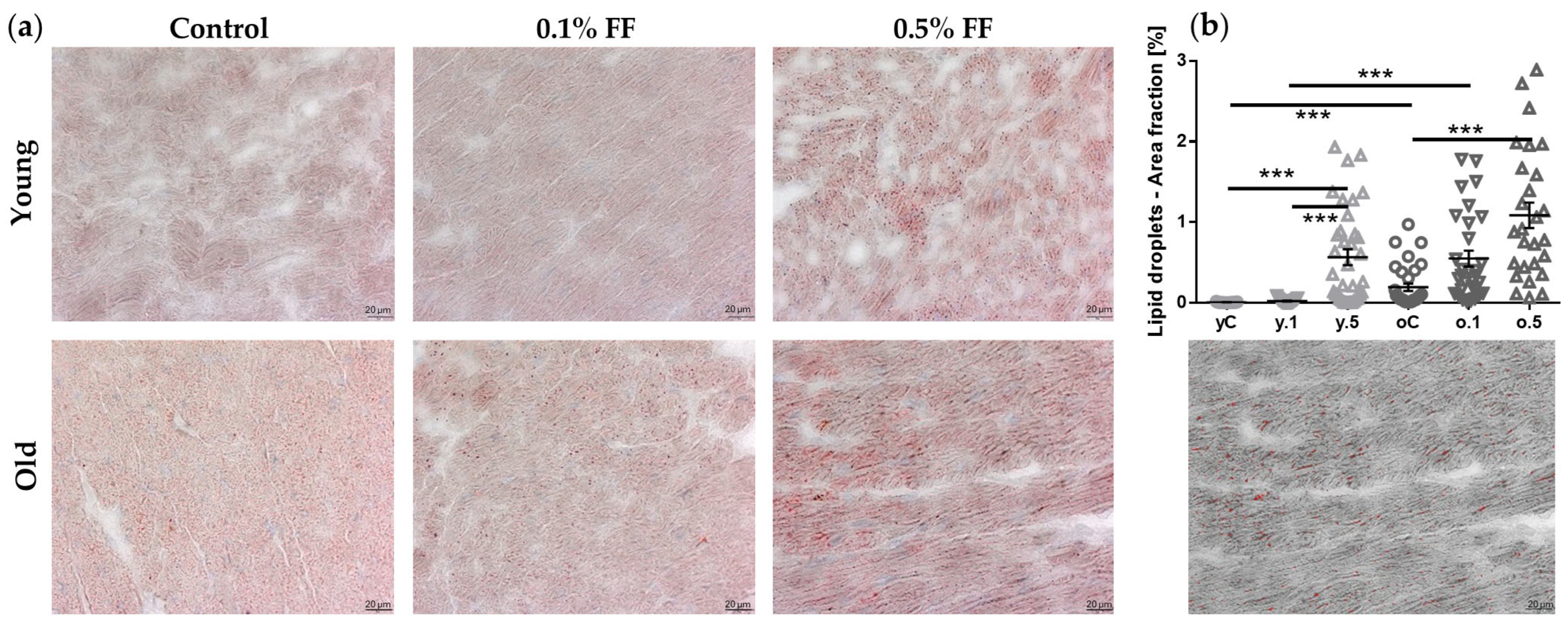
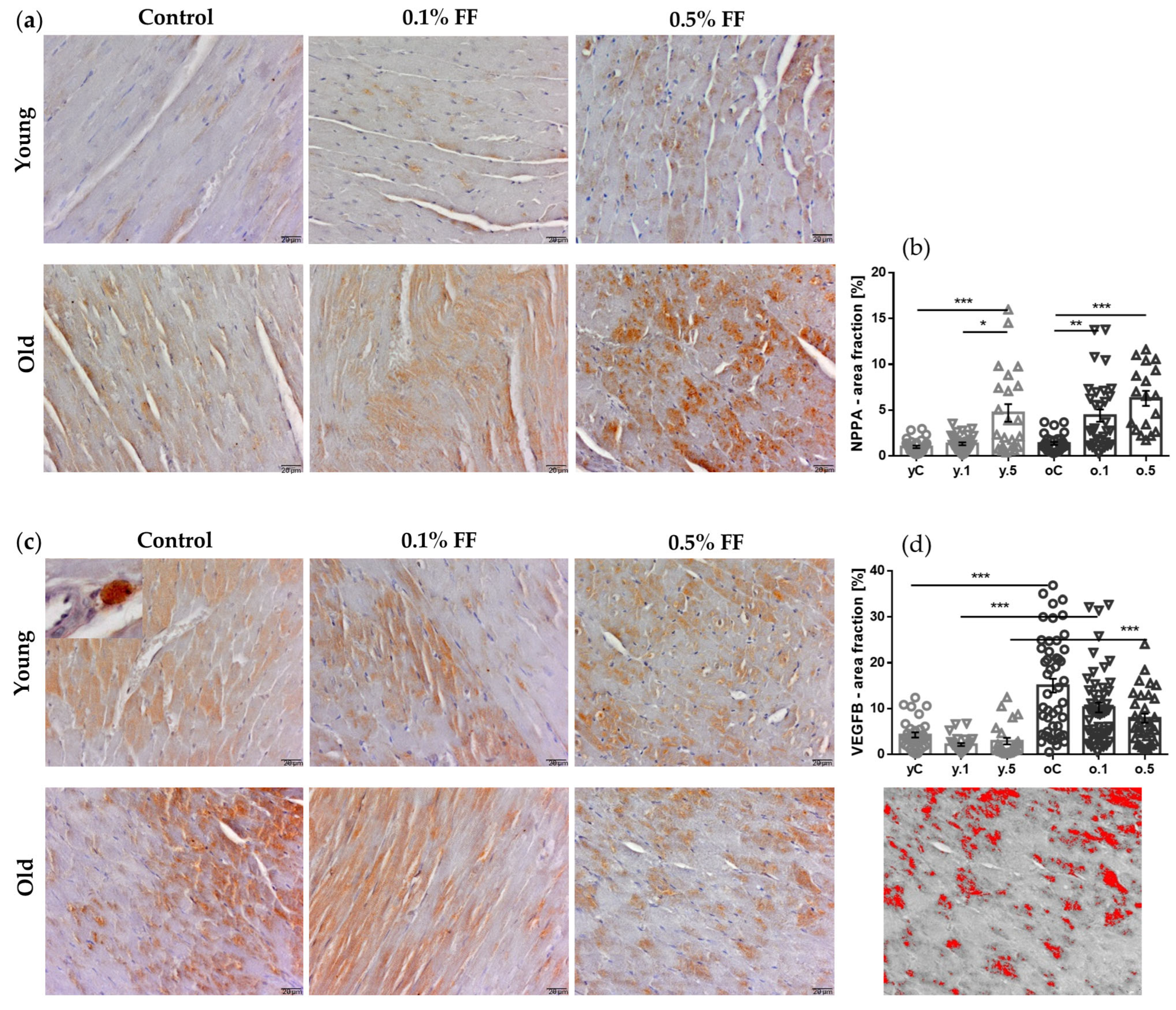
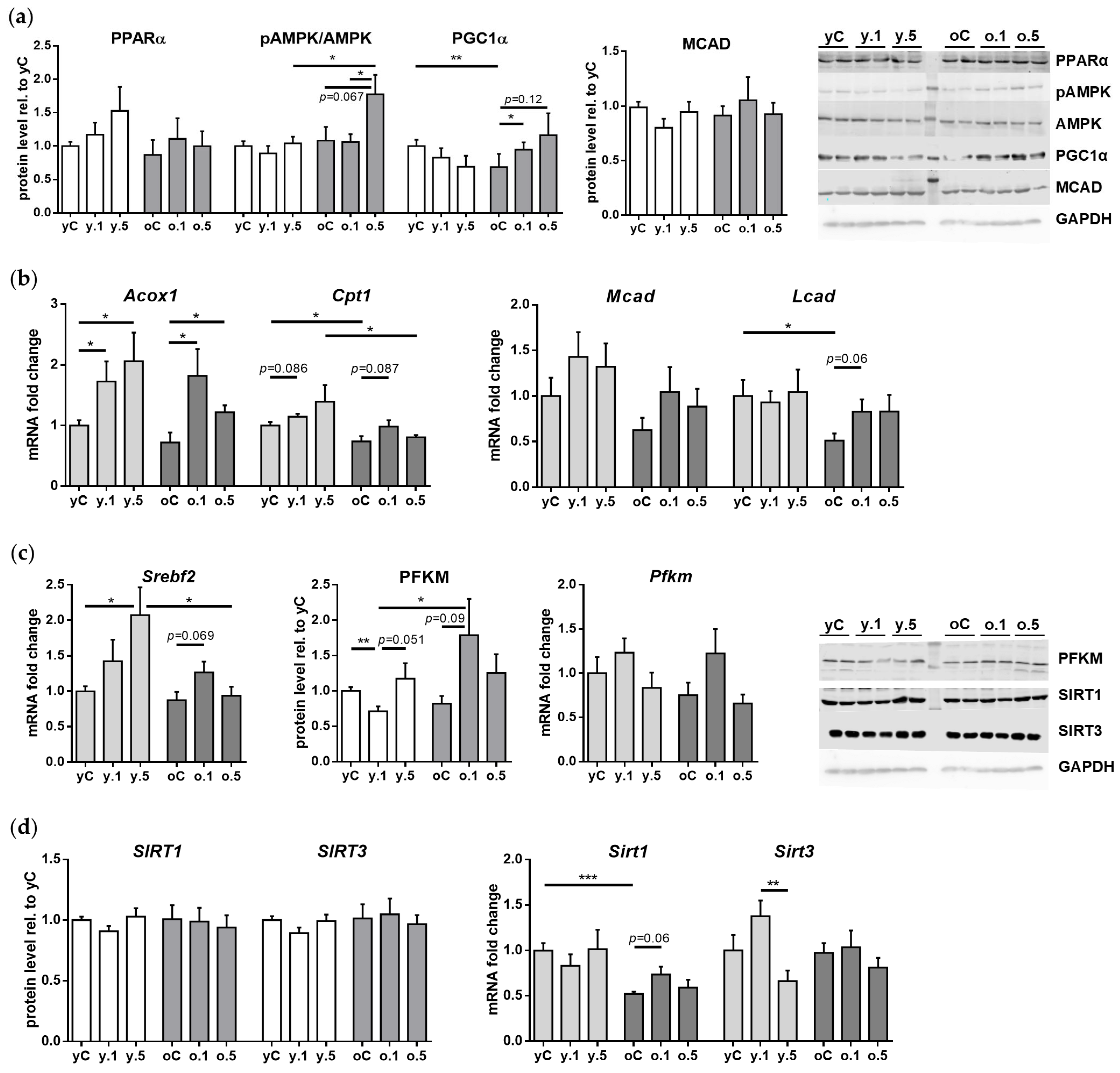
| Control | 0.1% FF | 0.5% FF | Control | 0.1% FF | 0.5% FF | |
|---|---|---|---|---|---|---|
| Young Rats | Old Rats | |||||
| TG | 53.18 ± 5.50 | 40.44 ± 5.13 | 46.50 ± 4.18 | 114.5 ± 10.50 ### | 91.91 ± 9.66 | 52.11 ± 5.57 * |
| Chol | 66.24 ± 4.265 | 26.78 ± 2.697 * | 44.00 ± 4.856 | 110.2 ± 5.754 # | 78.10 ± 3.247 | 53.67 ± 11.84 ** |
| LDH | 795.1 ± 94.12 | 672.3 ± 110.2 | 922 ± 128.6 | 969.8 ± 130.5 | 1052 ± 153.4 | 920.2 ± 193 |
| CK | 1392 ± 163.5 | 1132 ± 320.8 | 3179 ± 1104 | 1113 ± 211 | 1416 ± 219.1 | 863.8 ± 267.6 |
| Creatinine | 0.67 ± 0.02 | 0.63 ± 0.02 | 0.77 ± 0.04 * | 0.74 ± 0.03 | 0.77 ± 0.06 | 0.99 ± 0.04 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wrońska, A.; Kieżun, J.; Kmieć, Z. Fenofibrate Differently Affects the Heart’s Morphology and Metabolism in Young and Old Rats. Int. J. Mol. Sci. 2025, 26, 8038. https://doi.org/10.3390/ijms26168038
Wrońska A, Kieżun J, Kmieć Z. Fenofibrate Differently Affects the Heart’s Morphology and Metabolism in Young and Old Rats. International Journal of Molecular Sciences. 2025; 26(16):8038. https://doi.org/10.3390/ijms26168038
Chicago/Turabian StyleWrońska, Agata, Jacek Kieżun, and Zbigniew Kmieć. 2025. "Fenofibrate Differently Affects the Heart’s Morphology and Metabolism in Young and Old Rats" International Journal of Molecular Sciences 26, no. 16: 8038. https://doi.org/10.3390/ijms26168038
APA StyleWrońska, A., Kieżun, J., & Kmieć, Z. (2025). Fenofibrate Differently Affects the Heart’s Morphology and Metabolism in Young and Old Rats. International Journal of Molecular Sciences, 26(16), 8038. https://doi.org/10.3390/ijms26168038







