Mechanisms and Therapeutic Advances of PXR in Metabolic Diseases and Cancer
Abstract
1. Introduction
2. Molecular Characteristics and Biological Functional Mechanisms of PXR
2.1. Structure and Function of PXR
2.2. PXR Signaling Pathways
2.3. PXR Target Genes
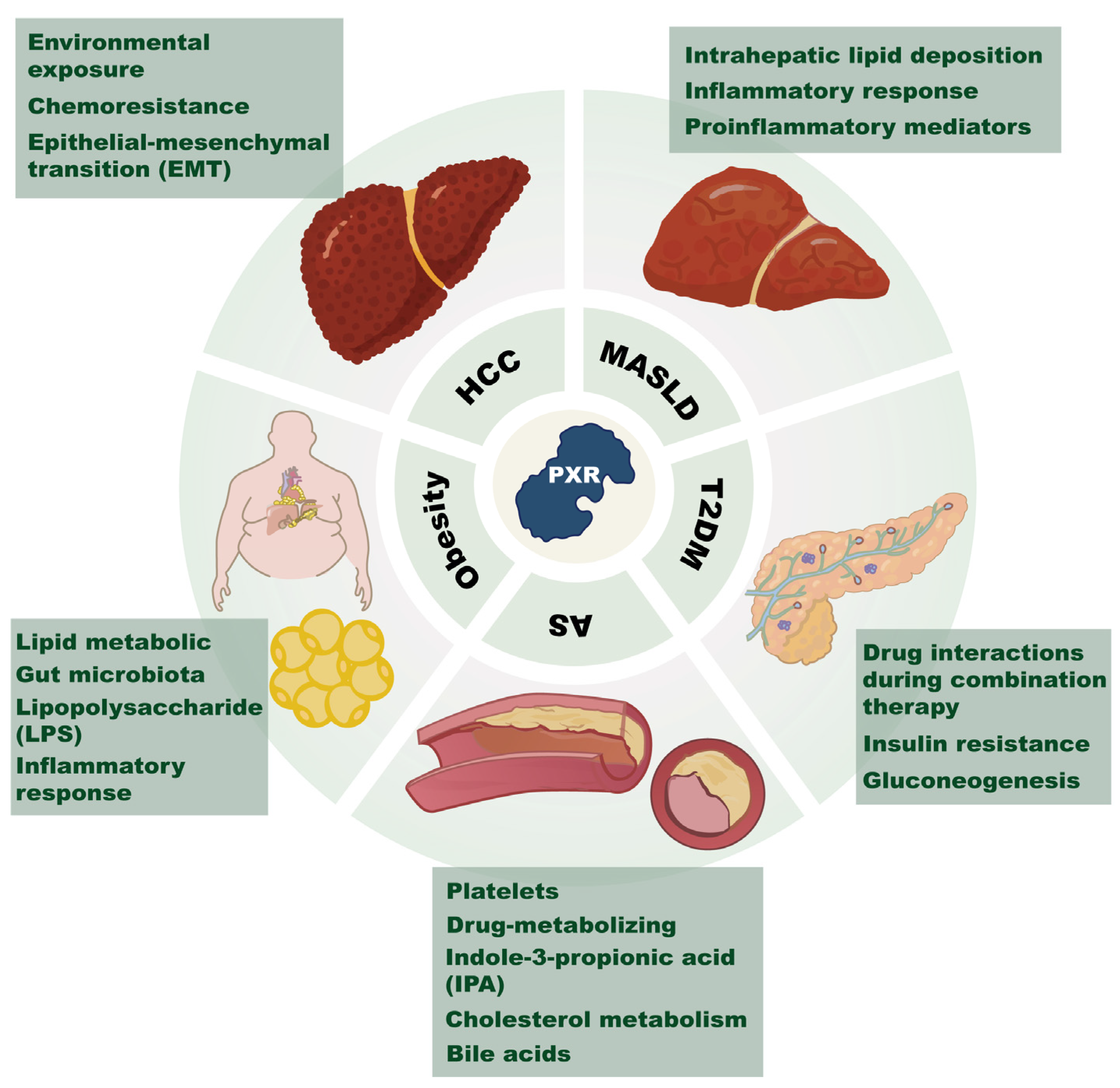
3. PXR in Metabolic Diseases and Cancer
3.1. T2DM
3.2. Obesity
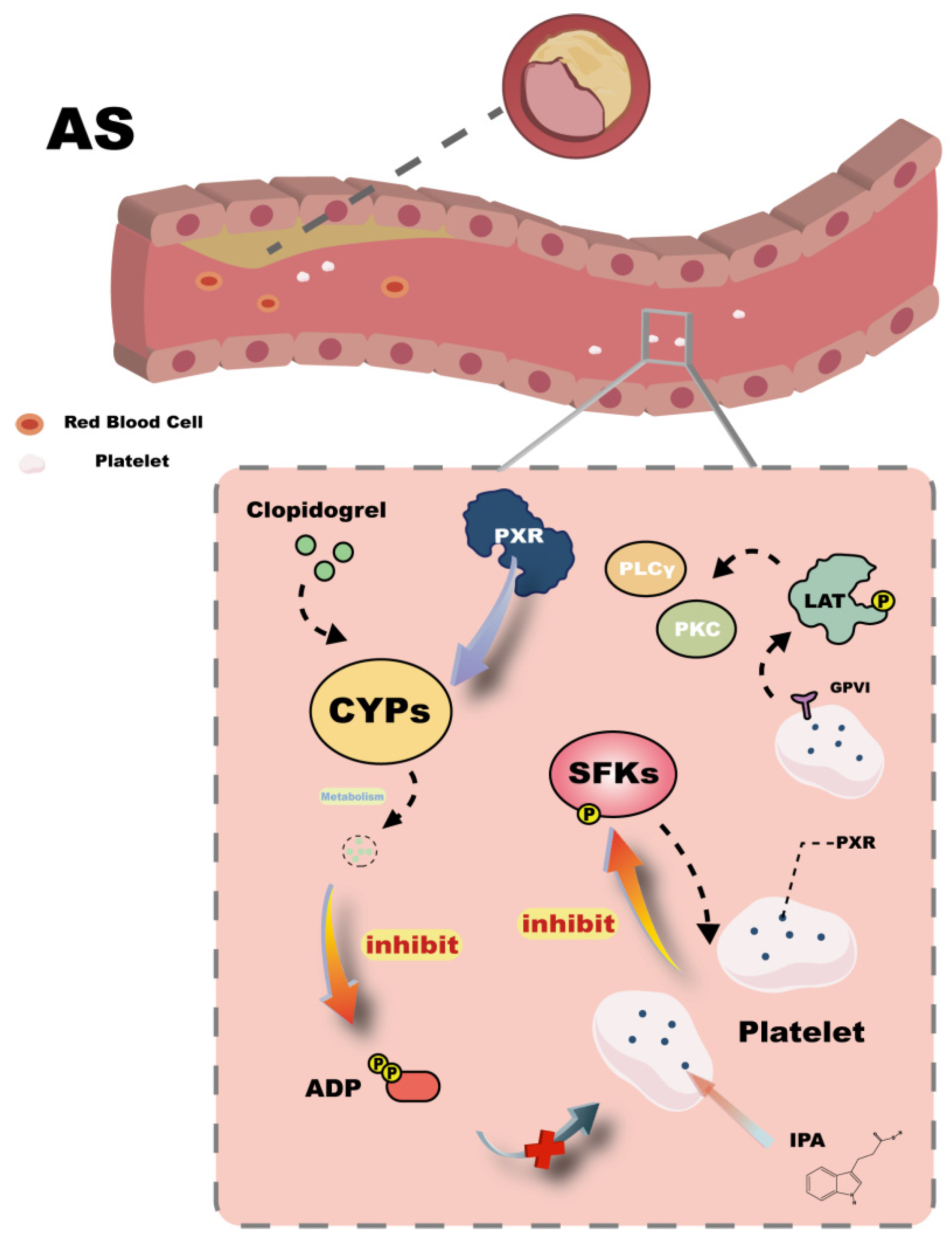
3.3. Atherosclerosis
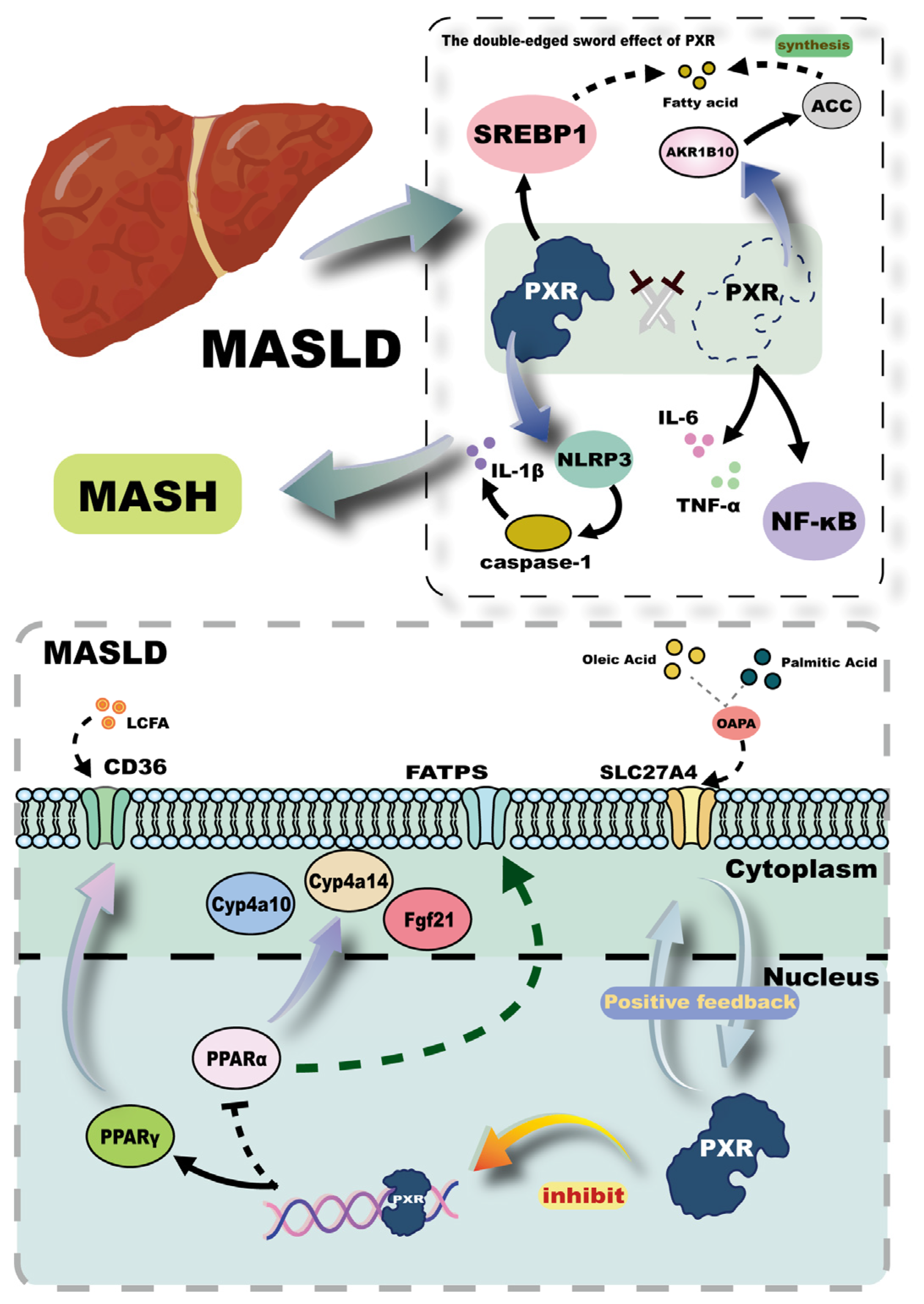
3.4. MASLD
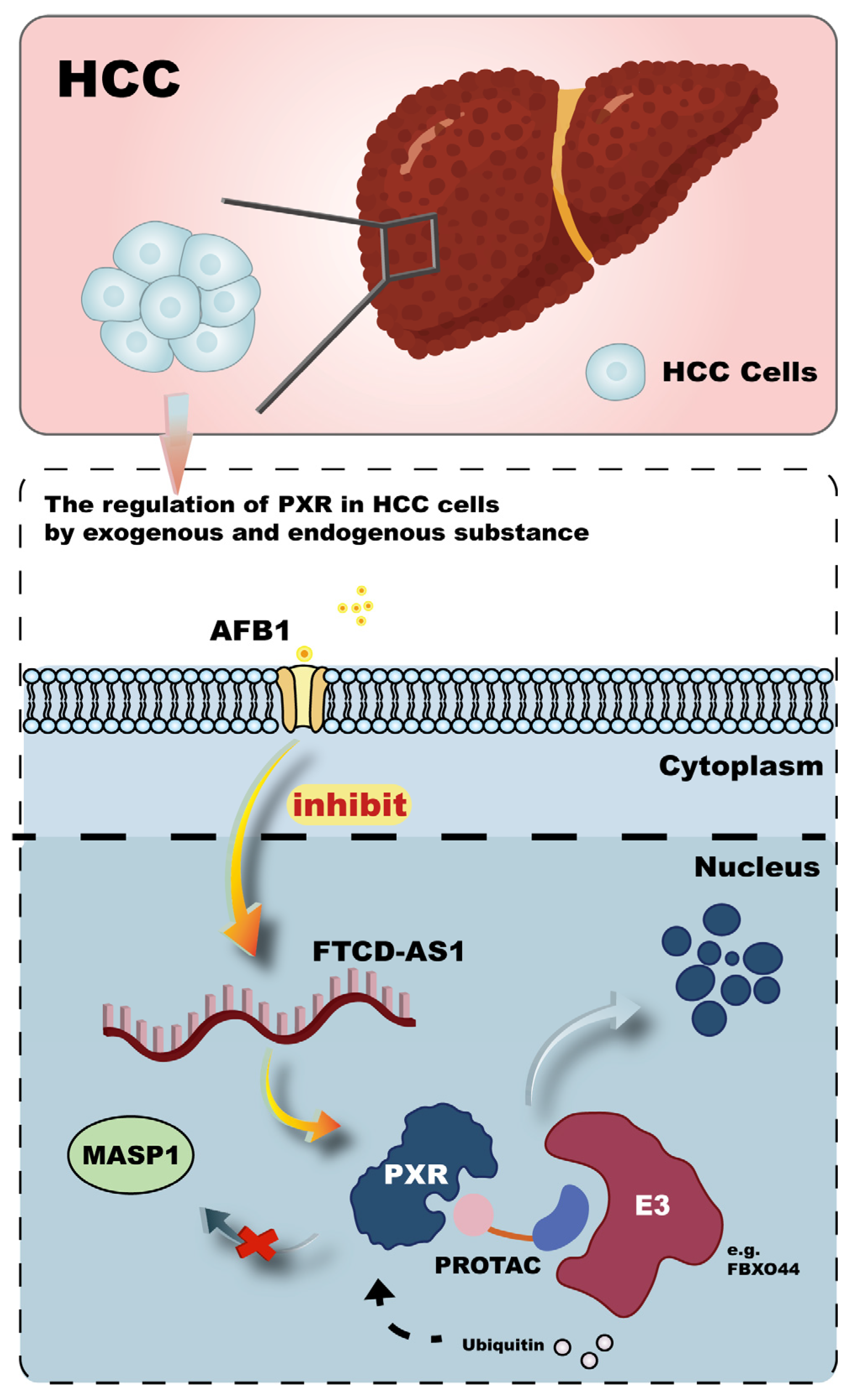
3.5. Cancer
4. The Therapeutic Potential of PXR as a Drug Target
4.1. PXR Agonists and Antagonists
4.2. Challenges in Drug Development
4.3. Novel Therapeutic Strategies and Multi-Omics Approaches
5. Future Research Directions
5.1. Interactions Between PXR and the Gut Microbiota
5.2. Personalized Medicine
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PXR | pregnane X receptor |
| T2DM | type 2 diabetes mellitus |
| AS | atherosclerosis |
| MASLD | metabolic dysfunction-associated steatotic liver disease |
| NRs | nuclear receptors |
| DBD | DNA-binding domain |
| LBD | ligand-binding domain |
| ZF | zinc finger |
| hPXR-LBD | human PXR LBD |
| Trp-Zip | tryptophan zipper |
| RXR | retinoid X receptor |
| CYP | cytochrome P450 |
| PCN | pregnenolone 16α-carbonitrile |
| AF | activation function |
| hRXRα | human RXRα |
| PXRE | ER6-type PXR response element |
| P-gp | P-glycoprotein |
| MRP2 | multidrug resistance-associated protein 2 |
| ABC | ATP-binding cassette |
| SRC1 | steroid receptor coactivator-1 |
| MDR1 | multidrug resistance protein 1 |
| CESs | carboxylesterases |
| JNK | c-Jun N-terminal kinase |
| PXR-KO | PXR-knockout |
| HFD | high-fat diet |
| CAR | constitutive androstane receptor |
| FOXO1 | forkhead box protein O1 |
| G6P | glucose-6-phosphatase |
| PEPCK1 | phosphoenolpyruvate carboxykinase 1 |
| HNF4α | hepatocyte nuclear factor 4 alpha |
| GLUT2 | glucose transporter 2 |
| PCBs | polychlorinated biphenyls |
| NDL-PCBs | non-dioxin-like PCBs |
| PBDEs | polybrominated diphenyl ethers |
| IPA | indole-3-propionic acid |
| B3galt5 | β-1,3-galactosyltransferase 5 |
| LPS | lipopolysaccharide |
| NF-κB | nuclear factor-kappa B |
| AP-1 | activator protein-1 |
| LDL-C | low-density lipoprotein cholesterol |
| DIT | diffuse intimal thickening |
| SFKs | Src family kinases |
| IF | Intermittent fasting |
| HSP90 | heat shock protein 90 |
| SREBP2 | sterol regulatory element-binding protein 2 |
| NAFLD | non-alcoholic fatty liver disease |
| MPHs | primary human hepatocytes |
| SLC27A4 | solute carrier family 27 member 4 |
| PPARs | peroxisome proliferator-activated receptors |
| FATPs | fatty acid transport proteins |
| LCFAs | long-chain fatty acids |
| MASH | Metabolic dysfunction-associated steatohepatitis |
| IL-6 | interleukin-6 |
| TNF-α | tumor necrosis factor-α |
| IL-1β | interleukin-1β |
| SREBP1 | sterol regulatory element-binding protein 1 |
| AKR1B10 | aldo-keto reductase 1B10 |
| ACC | acetyl-CoA carboxylase |
| HCC | Hepatocellular carcinoma |
| HBV | hepatitis B virus |
| AFB1 | aflatoxin B1 |
| FTCD-AS1 | FTCD antisense RNA 1 |
| MASP1 | mannan-binding lectin serine protease 1 |
| PFESA-BP2 | perfluoroether sulfonic acid BP2 |
| TNBC | triple-negative breast cancer |
| NICD | Notch intracellular domain |
| PROTACs | proteolysis-targeting chimeras |
| FBXO44 | F-box protein 44 |
| EMT | Epithelial-mesenchymal transition |
| LASP2 | LIM and SH3 protein 2 |
| 5-FU | 5-fluorouracil |
| CAC | colorectal cancer |
| FLCWK | Fengliang Changweikang |
| TBC | tributyl citrate |
| HTS | High-throughput screening |
| FKK6 | Felix Kopp Kortagere 6 |
| DMEs | drug-metabolizing enzymes |
| UGT1A1 | UDP-glucuronosyltransferase 1A1 |
| DDIs | drug–drug interactions |
| DILI | drug-induced liver injury |
| ERS | endoplasmic reticulum stress |
| MDCs | metabolism-disrupting chemicals |
| DSBs | double-strand breaks |
| DRE | drug-resistant epilepsy |
| NSCLC | non-small cell lung cancer |
| GWAS | genome-wide association studies |
| PTMs | Post-translational modifications |
| DSS | dextran sulfate sodium |
| UC | ulcerative colitis |
| BA | bile acid |
| IBD | inflammatory bowel disease |
| BSH | bacterial bile salt hydrolase |
| ZBBAAs | bacterial bile acid amides |
| SNPs | single nucleotide polymorphisms |
| HN | hypertensive nephropathy |
References
- Frigo, D.E.; Bondesson, M.; Williams, C. Nuclear receptors: From molecular mechanisms to therapeutics. Essays Biochem. 2021, 65, 847–856. [Google Scholar] [CrossRef]
- Weatherman, R.V.; Fletterick, R.J.; Scanlan, T.S. Nuclear-receptor ligands and ligand-binding domains. Annu. Rev. Biochem. 1999, 68, 559–581. [Google Scholar] [CrossRef]
- Kliewer, S.A.; Moore, J.T.; Wade, L.; Staudinger, J.L.; Watson, M.A.; Jones, S.A.; McKee, D.D.; Oliver, B.B.; Willson, T.M.; Zetterström, R.H. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell 1998, 92, 73–82. [Google Scholar] [CrossRef]
- Wu, B.; Li, S.; Dong, D. 3D structures and ligand specificities of nuclear xenobiotic receptors CAR, PXR and VDR. Drug Discov. Today 2013, 18, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Schulman, I.G. Nuclear receptors as drug targets for metabolic disease. Adv. Drug Deliv. Rev. 2010, 62, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, X.-D.; Shapiro, M.D.; Lip, G.Y.; Tilg, H.; Valenti, L.; Somers, V.K.; Byrne, C.D.; Targher, G.; Yang, W. Global burden of metabolic diseases, 1990–2021. Metabolism 2024, 160, 155999. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xie, W. Targeting xenobiotic receptors PXR and CAR for metabolic diseases. Trends Pharmacol. Sci. 2012, 33, 552–558. [Google Scholar] [CrossRef]
- Khan, J.A.; Camac, D.M.; Low, S.; Tebben, A.J.; Wensel, D.L.; Wright, M.C.; Su, J.; Jenny, V.; Gupta, R.D.; Ruzanov, M. Developing Adnectins that target SRC co-activator binding to PXR: A structural approach toward understanding promiscuity of PXR. J. Mol. Biol. 2015, 427, 924–942. [Google Scholar] [CrossRef]
- Wallace, B.D.; Betts, L.; Talmage, G.; Pollet, R.M.; Holman, N.S.; Redinbo, M.R. Structural and functional analysis of the human nuclear xenobiotic receptor PXR in complex with RXRα. J. Mol. Biol. 2013, 425, 2561–2577. [Google Scholar] [CrossRef]
- He, J.; Gao, J.; Xu, M.; Ren, S.; Stefanovic-Racic, M.; O’Doherty, R.M.; Xie, W. PXR ablation alleviates diet-induced and genetic obesity and insulin resistance in mice. Diabetes 2013, 62, 1876–1887. [Google Scholar] [CrossRef]
- Nakae, J.; Kitamura, T.; Silver, D.L.; Accili, D. The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. J. Clin. Investig. 2001, 108, 1359–1367. [Google Scholar] [CrossRef]
- Cheng, J.; Ma, X.; Gonzalez, F.J. Pregnane X receptor-and CYP3A4-humanized mouse models and their applications. Br. J. Pharmacol. 2011, 163, 461–468. [Google Scholar] [CrossRef]
- Carnahan, V.E.; Redinbo, M.R. Structure and function of the human nuclear xenobiotic receptor PXR. Curr. Drug Metab. 2005, 6, 357–367. [Google Scholar] [CrossRef]
- Di Masi, A.; De Marinis, E.; Ascenzi, P.; Marino, M. Nuclear receptors CAR and PXR: Molecular, functional, and biomedical aspects. Mol. Asp. Med. 2009, 30, 297–343. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.; Dash, A.K.; Ponnusamy, K.; Tyagi, R.K. Nuclear localization signal region in nuclear receptor PXR governs the receptor association with mitotic chromatin. Chromosome Res. 2018, 26, 255–276. [Google Scholar] [CrossRef] [PubMed]
- Watkins, R.E.; Wisely, G.B.; Moore, L.B.; Collins, J.L.; Lambert, M.H.; Williams, S.P.; Willson, T.M.; Kliewer, S.A.; Redinbo, M.R. The human nuclear xenobiotic receptor PXR: Structural determinants of directed promiscuity. Science 2001, 292, 2329–2333. [Google Scholar] [CrossRef]
- Watkins, R.E.; Davis-Searles, P.R.; Lambert, M.H.; Redinbo, M.R. Coactivator binding promotes the specific interaction between ligand and the pregnane X receptor. J. Mol. Biol. 2003, 331, 815–828. [Google Scholar] [CrossRef]
- Rashidian, A.; Mustonen, E.-K.; Kronenberger, T.; Schwab, M.; Burk, O.; Laufer, S.A.; Pantsar, T. Discrepancy in interactions and conformational dynamics of pregnane X receptor (PXR) bound to an agonist and a novel competitive antagonist. Comput. Struct. Biotechnol. J. 2022, 20, 3004–3018. [Google Scholar] [CrossRef]
- Noble, S.M.; Carnahan, V.E.; Moore, L.B.; Luntz, T.; Wang, H.; Ittoop, O.R.; Stimmel, J.B.; Davis-Searles, P.R.; Watkins, R.E.; Wisely, G.B. Human PXR forms a tryptophan zipper-mediated homodimer. Biochemistry 2006, 45, 8579–8589. [Google Scholar] [CrossRef]
- Wang, W.; Prosise, W.W.; Chen, J.; Taremi, S.S.; Le, H.V.; Madison, V.; Cui, X.; Thomas, A.; Cheng, K.-C.; Lesburg, C.A. Construction and characterization of a fully active PXR/SRC-1 tethered protein with increased stability. Protein Eng. Des. Sel. 2008, 21, 425–433. [Google Scholar] [CrossRef]
- Lehmann, J.M.; McKee, D.D.; Watson, M.A.; Willson, T.M.; Moore, J.T.; Kliewer, S.A. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J. Clin. Investig. 1998, 102, 1016–1023. [Google Scholar] [CrossRef]
- Orans, J.; Teotico, D.G.; Redinbo, M.R. The nuclear xenobiotic receptor pregnane X receptor: Recent insights and new challenges. Mol. Endocrinol. 2005, 19, 2891–2900. [Google Scholar] [CrossRef]
- Chai, S.C.; Cherian, M.T.; Wang, Y.-M.; Chen, T. Small-molecule modulators of PXR and CAR. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2016, 1859, 1141–1154. [Google Scholar] [CrossRef]
- Ma, X.; Cheung, C.; Krausz, K.W.; Shah, Y.M.; Wang, T.; Idle, J.R.; Gonzalez, F.J. A double transgenic mouse model expressing human pregnane X receptor and cytochrome P450 3A4. Drug Metab. Dispos. 2008, 36, 2506–2512. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Maldonado, E.; Huber, A.D.; Chai, S.C.; Nithianantham, S.; Li, Y.; Wu, J.; Poudel, S.; Miller, D.J.; Seetharaman, J.; Chen, T. Chemical manipulation of an activation/inhibition switch in the nuclear receptor PXR. Nat. Commun. 2024, 15, 4054. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.M.; Mangelsdorf, D.J. Nuclear receptors, RXR, and the big bang. Cell 2014, 157, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Almazroo, O.A.; Miah, M.K.; Venkataramanan, R. Drug metabolism in the liver. Clin. Liver Dis. 2017, 21, 1–20. [Google Scholar] [CrossRef]
- Meng, C.; Zhou, L.; Huang, L.; Gu, Q.; Du, X.; Wang, C.; Liu, F.; Xia, C. Chlorogenic acid regulates the expression of NPC1L1 and HMGCR through PXR and SREBP2 signaling pathways and their interactions with HSP90 to maintain cholesterol homeostasis. Phytomedicine 2024, 123, 155271. [Google Scholar] [CrossRef]
- Wang, H.; Faucette, S.; Sueyoshi, T.; Moore, R.; Ferguson, S.; Negishi, M.; LeCluyse, E.L. A novel distal enhancer module regulated by pregnane X receptor/constitutive androstane receptor is essential for the maximal induction of CYP2B6 gene expression. J. Biol. Chem. 2003, 278, 14146–14152. [Google Scholar] [CrossRef]
- Mahadevan, D.; List, A.F. Targeting the multidrug resistance-1 transporter in AML: Molecular regulation and therapeutic strategies. Blood 2004, 104, 1940–1951. [Google Scholar] [CrossRef]
- Geick, A.; Eichelbaum, M.; Burk, O. Nuclear receptor response elements mediate induction of intestinal MDR1 by rifampin. J. Biol. Chem. 2001, 276, 14581–14587. [Google Scholar] [CrossRef]
- Dietrich, C.G.; Geier, A.; Salein, N.; Lammert, F.; Roeb, E.; Oude Elferink, R.P.; Matern, S.; Gartung, C. Consequences of bile duct obstruction on intestinal expression and function of multidrug resistance-associated protein 2. Gastroenterology 2004, 126, 1044–1053. [Google Scholar] [CrossRef]
- Kast, H.R.; Goodwin, B.; Tarr, P.T.; Jones, S.A.; Anisfeld, A.M.; Stoltz, C.M.; Tontonoz, P.; Kliewer, S.; Willson, T.M.; Edwards, P.A. Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J. Biol. Chem. 2002, 277, 2908–2915. [Google Scholar] [CrossRef] [PubMed]
- Association, A.D. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014, 37, S81–S90. [Google Scholar] [CrossRef] [PubMed]
- LaMoia, T.E.; Shulman, G.I. Cellular and molecular mechanisms of metformin action. Endocr. Rev. 2021, 42, 77–96. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Goodman, A.M.; Group, M.M.S. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. N. Engl. J. Med. 1995, 333, 541–549. [Google Scholar] [CrossRef]
- Krausova, L.; Stejskalova, L.; Wang, H.; Vrzal, R.; Dvorak, Z.; Mani, S.; Pavek, P. Metformin suppresses pregnane X receptor (PXR)-regulated transactivation of CYP3A4 gene. Biochem. Pharmacol. 2011, 82, 1771–1780. [Google Scholar] [CrossRef]
- Shan, E.; Zhu, Z.; He, S.; Chu, D.; Ge, D.; Zhan, Y.; Liu, W.; Yang, J.; Xiong, J. Involvement of pregnane X receptor in the suppression of carboxylesterases by metformin in vivo and in vitro, mediated by the activation of AMPK and JNK signaling pathway. Eur. J. Pharm. Sci. 2017, 102, 14–23. [Google Scholar] [CrossRef]
- Petersen, K.F.; Laurent, D.; Rothman, D.L.; Cline, G.W.; Shulman, G.I. Mechanism by which glucose and insulin inhibit net hepatic glycogenolysis in humans. J. Clin. Investig. 1998, 101, 1203–1209. [Google Scholar] [CrossRef]
- Barthel, A.; Schmoll, D. Novel concepts in insulin regulation of hepatic gluconeogenesis. Am. J. Physiol. -Endocrinol. Metab. 2003, 285, E685–E692. [Google Scholar] [CrossRef]
- Kodama, S.; Koike, C.; Negishi, M.; Yamamoto, Y. Nuclear receptors CAR and PXR cross talk with FOXO1 to regulate genes that encode drug-metabolizing and gluconeogenic enzymes. Mol. Cell. Biol. 2004, 24, 7931–7940. [Google Scholar] [CrossRef]
- Yeagley, D.; Guo, S.; Unterman, T.; Quinn, P.G. Gene-and activation-specific mechanisms for insulin inhibition of basal and glucocorticoid-induced insulin-like growth factor binding protein-1 and phosphoenolpyruvate carboxykinase transcription. J. Biol. Chem. 2001, 276, 33705–33710. [Google Scholar] [CrossRef]
- Puigserver, P.; Rhee, J.; Donovan, J.; Walkey, C.J.; Yoon, J.C.; Oriente, F.; Kitamura, Y.; Altomonte, J.; Dong, H.; Accili, D. Insulin-regulated hepatic gluconeogenesis through FOXO1–PGC-1α interaction. Nature 2003, 423, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Jiang, L.; Kong, W.; Xie, Q.; Li, P.; Liu, X.; Zhang, J.; Liu, M.; Wang, Z.; Zhu, L. PXR activation impairs hepatic glucose metabolism partly via inhibiting the HNF4α–GLUT2 pathway. Acta Pharm. Sin. B 2022, 12, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Hassani-Nezhad-Gashti, F.; Rysä, J.; Kummu, O.; Näpänkangas, J.; Buler, M.; Karpale, M.; Hukkanen, J.; Hakkola, J. Activation of nuclear receptor PXR impairs glucose tolerance and dysregulates GLUT2 expression and subcellular localization in liver. Biochem. Pharmacol. 2018, 148, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.; Liu, J.; Qu, F.; Chen, A.; He, W. Polychlorinated biphenyls exposure and type 2 diabetes: Molecular mechanism that causes insulin resistance and islet damage. Environ. Toxicol. 2024, 39, 2466–2476. [Google Scholar] [CrossRef]
- Kim, S.; Li, H.; Jin, Y.; Armad, J.; Gu, H.; Mani, S.; Cui, J.Y. Maternal PBDE exposure disrupts gut microbiome and promotes hepatic proinflammatory signaling in humanized PXR-transgenic mouse offspring over time. Toxicol. Sci. 2023, 194, 209–225. [Google Scholar] [CrossRef]
- González-Muniesa, P.; Mártinez-González, M.-A.; Hu, F.B.; Després, J.-P.; Matsuzawa, Y.; Loos, R.J.F.; Moreno, L.A.; Bray, G.A.; Martinez, J.A. Obesity. Nat. Rev. Dis. Primers 2017, 3, 17034. [Google Scholar] [CrossRef]
- Piché, M.-E.; Tchernof, A.; Després, J.-P. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ. Res. 2020, 126, 1477–1500, Erratum in Circ. Res. 2020, 127, e107. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, R.; Liu, J.; Yuan, J.; Zhang, S.; Chi, Z.; Yu, W.; Yu, Q.; Wang, Z.; Chen, S. A two-front nutrient supply environment fuels small intestinal physiology through differential regulation of nutrient absorption and host defense. Cell 2024, 187, 6251–6271.e20. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Li, H.; Xu, P.; Liu, Q.; Sun, Y.; Zhang, Z.; Wu, T.; Tang, Q.; Jia, Q. B3galt5 functions as a PXR target gene and regulates obesity and insulin resistance by maintaining intestinal integrity. Nat. Commun. 2024, 15, 5919. [Google Scholar] [CrossRef]
- Zhao, T.; Zhong, G.; Wang, Y.; Cao, R.; Song, S.; Li, Y.; Wan, G.; Sun, H.; Huang, M.; Bi, H. Pregnane X Receptor Activation in Liver Macrophages Protects against Endotoxin-Induced Liver Injury. Adv. Sci. 2024, 11, 2308771. [Google Scholar] [CrossRef] [PubMed]
- Okamura, M.; Shizu, R.; Abe, T.; Kodama, S.; Hosaka, T.; Sasaki, T.; Yoshinari, K. PXR functionally interacts with NF-κB and AP-1 to downregulate the inflammation-induced expression of chemokine CXCL2 in mice. Cells 2020, 9, 2296. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, S.; Dutta, M.; Asubonteng, J.O.; Polunas, M.; Goedken, M.; Gonzalez, F.J.; Cui, J.Y.; Gyamfi, M.A. Pregnane X receptor exacerbates nonalcoholic fatty liver disease accompanied by obesity-and inflammation-prone gut microbiome signature. Biochem. Pharmacol. 2021, 193, 114698. [Google Scholar] [CrossRef] [PubMed]
- Gebreyesus, L.H.; Choi, S.; Neequaye, P.; Mahmoud, M.; Mahmoud, M.; Ofosu-Boateng, M.; Twum, E.; Nnamani, D.O.; Wang, L.; Yadak, N. Pregnane X receptor knockout mitigates weight gain and hepatic metabolic dysregulation in female C57BL/6 J mice on a long-term high-fat diet. Biomed. Pharmacother. 2024, 173, 116341. [Google Scholar] [CrossRef]
- Perdomo, C.M.; Cohen, R.V.; Sumithran, P.; Clément, K.; Frühbeck, G. Contemporary medical, device, and surgical therapies for obesity in adults. Lancet 2023, 401, 1116–1130. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Stary, H.C.; Chandler, A.B.; Glagov, S.; Guyton, J.R.; Insull Jr, W.; Rosenfeld, M.E.; Schaffer, S.A.; Schwartz, C.J.; Wagner, W.D.; Wissler, R.W. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 1994, 89, 2462–2478. [Google Scholar] [CrossRef]
- Ding, Z.; Liu, S.; Wang, X.; Theus, S.; Deng, X.; Fan, Y.; Zhou, S.; Mehta, J.L. PCSK9 regulates expression of scavenger receptors and ox-LDL uptake in macrophages. Cardiovasc. Res. 2018, 114, 1145–1153, Erratum in Cardiovasc. Res. 2022, 118, 1849. [Google Scholar] [CrossRef]
- Virmani, R.; Burke, A.P.; Farb, A.; Kolodgie, F.D. Pathology of the vulnerable plaque. J. Am. Coll. Cardiol. 2006, 47, C13–C18. [Google Scholar] [CrossRef]
- van der Meijden, P.E.; Heemskerk, J.W. Platelet biology and functions: New concepts and clinical perspectives. Nat. Rev. Cardiol. 2019, 16, 166–179. [Google Scholar] [CrossRef]
- Unsworth, A.J.; Flora, G.D.; Gibbins, J.M. Non-genomic effects of nuclear receptors: Insights from the anucleate platelet. Cardiovasc. Res. 2018, 114, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Flora, G.D.; Sahli, K.A.; Sasikumar, P.; Holbrook, L.-M.; Stainer, A.R.; AlOuda, S.K.; Crescente, M.; Sage, T.; Unsworth, A.J.; Gibbins, J.M. Non-genomic effects of the Pregnane X Receptor negatively regulate platelet functions, thrombosis and haemostasis. Sci. Rep. 2019, 9, 17210. [Google Scholar] [CrossRef] [PubMed]
- Swales, K.E.; Moore, R.; Truss, N.J.; Tucker, A.; Warner, T.D.; Negishi, M.; Bishop-Bailey, D. Pregnane X receptor regulates drug metabolism and transport in the vasculature and protects from oxidative stress. Cardiovasc. Res. 2012, 93, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Zhou, L.; Dai, S.; Zhang, P.; Zhong, H.; Zhou, W.; Zhao, X.; Xu, H.; Zhao, G.; Wu, H. Intermittent fasting inhibits platelet activation and thrombosis through the intestinal metabolite indole-3-propionate. Life Metab. 2025, 4, loaf002. [Google Scholar] [CrossRef]
- Miettinen, T.A.; Puska, P.; Gylling, H.; Vanhanen, H.; Vartiainen, E. Reduction of serum cholesterol with sitostanol-ester margarine in a mildly hypercholesterolemic population. N. Engl. J. Med. 1995, 333, 1308–1312. [Google Scholar] [CrossRef]
- Zhou, C.; King, N.; Chen, K.Y.; Breslow, J.L. Activation of PXR induces hypercholesterolemia in wild-type and accelerates atherosclerosis in apoE deficient mice. J. Lipid Res. 2009, 50, 2004–2013. [Google Scholar] [CrossRef]
- Brown, C.; Kariuki, W.; Zhong, H.A.; Kippes, A.; Sui, Y. Cannabidiol promotes intestinal cholesterol uptake mediated by Pregnane X receptor. Front. Endocrinol. 2024, 15, 1398462. [Google Scholar] [CrossRef]
- Sui, Y.; Park, S.H.; Helsley, R.N.; Sunkara, M.; Gonzalez, F.J.; Morris, A.J.; Zhou, C. Bisphenol A increases atherosclerosis in pregnane X receptor-humanized ApoE deficient mice. J. Am. Heart Assoc. 2014, 3, e000492. [Google Scholar] [CrossRef]
- Kuan, Y.-C.; Hashidume, T.; Shibata, T.; Uchida, K.; Shimizu, M.; Inoue, J.; Sato, R. Heat shock protein 90 modulates lipid homeostasis by regulating the stability and function of sterol regulatory element-binding protein (SREBP) and SREBP cleavage-activating protein. J. Biol. Chem. 2017, 292, 3016–3028. [Google Scholar] [CrossRef] [PubMed]
- Itkonen, A.; Hakkola, J.; Rysä, J. Adverse outcome pathway for pregnane X receptor-induced hypercholesterolemia. Arch. Toxicol. 2023, 97, 2861–2877. [Google Scholar] [CrossRef] [PubMed]
- Seidah, N.G.; Awan, Z.; Chrétien, M.; Mbikay, M. PCSK9: A key modulator of cardiovascular health. Circ. Res. 2014, 114, 1022–1036. [Google Scholar] [CrossRef] [PubMed]
- Dusek, J.; Mejdrová, I.; Dohnalová, K.; Smutny, T.; Chalupsky, K.; Krutakova, M.; Skoda, J.; Rashidian, A.; Pavkova, I.; Škach, K. The hypolipidemic effect of MI-883, the combined CAR agonist/PXR antagonist, in diet-induced hypercholesterolemia model. Nat. Commun. 2025, 16, 1418. [Google Scholar] [CrossRef]
- Wu, G.; Wei, X.; Li, D.; Xiao, G.; Jia, C.; Zeng, Z.; Chen, Z. Selection and evaluation of quality markers for the regulation of PXR-CYP3A4/FXR-LXRα by Exocarpium Citri Grandis for the treatment of hyperlipidaemia with dispelling blood stasis and removing phlegm. Biomed. Pharmacother. 2024, 170, 116089. [Google Scholar] [CrossRef]
- Gofton, C.; Upendran, Y.; Zheng, M.-H.; George, J. MAFLD: How is it different from NAFLD? Clin. Mol. Hepatol. 2022, 29, S17. [Google Scholar] [CrossRef]
- Schaffer, J.E.; Lodish, H.F. Expression cloning and characterization of a novel adipocyte long chain fatty acid transport protein. Cell 1994, 79, 427–436. [Google Scholar] [CrossRef]
- Shen, C.; Pan, Z.; Xie, W.; Zhao, J.; Miao, D.; Zhao, L.; Liu, M.; Zhong, Y.; Zhong, C.; Gonzalez, F.J. Hepatocyte-specific SLC27A4 deletion ameliorates nonalcoholic fatty liver disease in mice via suppression of phosphatidylcholine-mediated PXR activation. Metabolism 2025, 162, 156054. [Google Scholar] [CrossRef]
- Martin, G.; Schoonjans, K.; Lefebvre, A.-M.; Staels, B.; Auwerx, J. Coordinate regulation of the expression of the fatty acid transport protein and acyl-CoA synthetase genes by PPARα and PPARγ activators. J. Biol. Chem. 1997, 272, 28210–28217. [Google Scholar] [CrossRef]
- Rakhshandehroo, M.; Hooiveld, G.; Müller, M.; Kersten, S. Comparative analysis of gene regulation by the transcription factor PPARα between mouse and human. PLoS ONE 2009, 4, e6796. [Google Scholar] [CrossRef]
- Yan, L.; Yan, Y.; Yang, K.; Chang, Q.; Zhang, L. Metabolomics reveals dysregulated all-trans retinoic acid and polyunsaturated fatty acid metabolism contribute to PXR-induced hepatic steatosis in mice. Toxicol. Lett. 2024, 398, 150–160. [Google Scholar] [CrossRef]
- Barretto, S.A.; Lasserre, F.; Fougerat, A.; Smith, L.; Fougeray, T.; Lukowicz, C.; Polizzi, A.; Smati, S.; Régnier, M.; Naylies, C. Gene expression profiling reveals that PXR activation inhibits hepatic PPARα activity and decreases FGF21 secretion in male C57Bl6/J mice. Int. J. Mol. Sci. 2019, 20, 3767. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, H.; Li, Y.; Zhang, Y.; Bian, Y.; Zeng, Y.; Yao, X.; Wan, J.; Chen, X.; Li, J. S100A4 enhances protumor macrophage polarization by control of PPAR-γ-dependent induction of fatty acid oxidation. J. Immunother. Cancer 2021, 9, e002548. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Febbraio, M.; Wada, T.; Zhai, Y.; Kuruba, R.; He, J.; Lee, J.H.; Khadem, S.; Ren, S.; Li, S. Hepatic fatty acid transporter Cd36 is a common target of LXR, PXR, and PPARγ in promoting steatosis. Gastroenterology 2008, 134, 556–567.e1. [Google Scholar] [CrossRef]
- Kotiya, D.; Jaiswal, B.; Ghose, S.; Kaul, R.; Datta, K.; Tyagi, R.K. Role of PXR in hepatic cancer: Its influences on liver detoxification capacity and cancer progression. PLoS ONE 2016, 11, e0164087. [Google Scholar] [CrossRef]
- Wang, S.; Lei, T.; Zhang, K.; Zhao, W.; Fang, L.; Lai, B.; Han, J.; Xiao, L.; Wang, N. Xenobiotic pregnane X receptor (PXR) regulates innate immunity via activation of NLRP3 inflammasome in vascular endothelial cells. J. Biol. Chem. 2014, 289, 30075–30081. [Google Scholar] [CrossRef]
- Kayagaki, N.; Warming, S.; Lamkanfi, M.; Walle, L.V.; Louie, S.; Dong, J.; Newton, K.; Qu, Y.; Liu, J.; Heldens, S. Non-canonical inflammasome activation targets caspase-11. Nature 2011, 479, 117–121. [Google Scholar] [CrossRef]
- Bitter, A.; Rümmele, P.; Klein, K.; Kandel, B.A.; Rieger, J.K.; Nüssler, A.K.; Zanger, U.M.; Trauner, M.; Schwab, M.; Burk, O. Pregnane X receptor activation and silencing promote steatosis of human hepatic cells by distinct lipogenic mechanisms. Arch. Toxicol. 2015, 89, 2089–2103. [Google Scholar] [CrossRef]
- Jassim, A.; Rahrmann, E.P.; Simons, B.D.; Gilbertson, R.J. Cancers make their own luck: Theories of cancer origins. Nat. Rev. Cancer 2023, 23, 710–724. [Google Scholar] [CrossRef]
- Couzin-Frankel, J. The bad luck of cancer: Analysis suggests most cases can’t be prevented. Science 2015, 347, 12. [Google Scholar] [CrossRef]
- Vincze, O.; Colchero, F.; Lemaître, J.-F.; Conde, D.A.; Pavard, S.; Bieuville, M.; Urrutia, A.O.; Ujvari, B.; Boddy, A.M.; Maley, C.C. Cancer risk across mammals. Nature 2022, 601, 263–267. [Google Scholar] [CrossRef]
- Nunney, L. The real war on cancer: The evolutionary dynamics of cancer suppression. Evol. Appl. 2013, 6, 11–19. [Google Scholar] [CrossRef]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Zhong, W.; Wu, X.; Ni, Z.; Lv, W.; Fan, Y.; Chen, L.; Lin, H.; Xie, Y.; Lin, J. AFB1 consolidates HBV harm to induce liver injury and carcinogenic risk by inactivating FTCD-AS1-PXR-MASP1 axis. Toxicology 2025, 511, 154057. [Google Scholar] [CrossRef] [PubMed]
- Kostecki, G.; Chuang, K.; Buxton, A.; Dakshanamurthy, S. Dose-Dependent PFESA-BP2 Exposure Increases Risk of Liver Toxicity and Hepatocellular Carcinoma. Curr. Issues Mol. Biol. 2025, 47, 98. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Fan, Q.-Y.; Liu, S.-B.; Zhang, S.-Y. Pregnane X receptor (PXR) deficiency promotes hepatocarcinogenesis via induction of Akr1c18 expression and prostaglandin F2α (PGF2α) levels. Biochem. Pharmacol. 2024, 225, 116309. [Google Scholar] [CrossRef]
- Carter, S.K. Some thoughts on resistance to cancer chemotherapy. Cancer Treat. Rev. 1984, 11, 3–7. [Google Scholar] [CrossRef]
- Wang, Z.-J.; Zhan, X.-Y.; Ma, L.-Y.; Yao, K.; Dai, H.-Y.; Santhanam, R.K.; Zhou, M.-S.; Jia, H. Activation of the γ-secretase/NICD-PXR/Notch pathway induces Taxol resistance in triple-negative breast cancer. Biochem. Pharmacol. 2024, 230, 116577. [Google Scholar] [CrossRef]
- Wang, H.; Chu, F.; Zhang, X.-f.; Zhang, P.; Li, L.-x.; Zhuang, Y.-l.; Niu, X.-f.; He, X.; Li, Z.-j.; Bai, Y. TPX2 enhances the transcription factor activation of PXR and enhances the resistance of hepatocellular carcinoma cells to antitumor drugs. Cell Death Dis. 2023, 14, 64. [Google Scholar] [CrossRef]
- Huber, A.D.; Jung, Y.-H.; Li, Y.; Lin, W.; Wu, J.; Poudel, S.; Carrigan, A.G.; Mishra, A.; High, A.A.; Chen, T. First-in-Class Small Molecule Degrader of Pregnane X Receptor Enhances Chemotherapy Efficacy. J. Med. Chem. 2024, 67, 18549–18575. [Google Scholar] [CrossRef]
- Huber, A.D.; Lin, W.; Poudel, S.; Miller, D.J.; Chen, T. PROTAC-mediated activation, rather than degradation, of a nuclear receptor reveals complex ligand-receptor interaction network. Structure 2024, 32, 2352–2363.e8. [Google Scholar] [CrossRef]
- Gee, R.R.F.; Huber, A.D.; Wu, J.; Bajpai, R.; Loughran, A.J.; Pruett-Miller, S.M.; Chen, T. The F-box-only protein 44 regulates pregnane X receptor protein level by ubiquitination and degradation. Acta Pharm. Sin. B 2023, 13, 4523–4534. [Google Scholar]
- Gerstberger, S.; Jiang, Q.; Ganesh, K. Metastasis. Cell 2023, 186, 1564–1579. [Google Scholar] [CrossRef] [PubMed]
- Steeg, P.S. Targeting metastasis. Nat. Rev. Cancer 2016, 16, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Sengez, B.; Carr, B.I.; Alotaibi, H. EMT and inflammation: Crossroads in HCC. J. Gastrointest. Cancer 2023, 54, 204–212. [Google Scholar] [CrossRef]
- Sato, T.; Shizu, R.; Baba, R.; Ooka, A.; Hosaka, T.; Kanno, Y.; Yoshinari, K. Pregnane X receptor inhibits the transdifferentiation of hepatic stellate cells by down-regulating periostin expression. Biochem. J. 2024, 481, 1173–1186. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, J.; Li, X.; Wu, G. Oxysterols in tumor immune microenvironment (TIME). J. Steroid Biochem. Mol. Biol. 2024, 245, 106634. [Google Scholar] [CrossRef]
- Pacini, C.; Duncan, E.; Gonçalves, E.; Gilbert, J.; Bhosle, S.; Horswell, S.; Karakoc, E.; Lightfoot, H.; Curry, E.; Muyas, F. A comprehensive clinically informed map of dependencies in cancer cells and framework for target prioritization. Cancer Cell 2024, 42, 301–316.e9. [Google Scholar] [CrossRef]
- Konzack, A.; Karpale, M.; Smutny, T.; Hassanen, M.; Lassila, P.; Ahonen, M.H.; Elkhwanky, M.-S.; Kummu, O.; Pavek, P.; Hakkola, J. LIM and SH3 Protein 2 (Lasp2) is a novel PXR target gene in mouse liver. Mol. Pharmacol. 2025, 107, 100019. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, L.; Zhou, H.; Liu, Y.; Fan, C.; Wang, L.; Li, A.; Miao, Y.; Li, Q.; Qiu, X. Lasp2 enhances tumor invasion via facilitating phosphorylation of FAK and predicts poor overall survival of non-small cell lung cancer patients. Mol. Carcinog. 2017, 56, 2558–2565. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L. Knockdown of LASP2 inhibits the proliferation, migration, and invasion of cervical cancer cells. J. Cell. Biochem. 2019, 120, 15389–15396. [Google Scholar] [CrossRef]
- Grunewald, T.G.; Butt, E. The LIM and SH3 domain protein family: Structural proteins or signal transducers or both? Mol. Cancer 2008, 7, 31. [Google Scholar] [CrossRef]
- Li, J.; Hu, S.; Zhang, Z.; Qian, L.; Xue, Q.; Qu, X. LASP2 is downregulated in human liver cancer and contributes to hepatoblastoma cell malignant phenotypes through MAPK/ERK pathway. Biomed. Pharmacother. 2020, 127, 110154. [Google Scholar] [CrossRef]
- Zhong, L.; Wang, Q.; Kou, Z.; Gan, L.; Yang, Z.; Pan, J.; Huang, L.; Chen, Y. The combination of FLCWK with 5-FU inhibits colon cancer and multidrug resistance by activating PXR to suppress the IL-6/STAT3 pathway. J. Cell. Mol. Med. 2024, 28, e70185. [Google Scholar] [CrossRef]
- Mao, K.; Liu, C.; Tang, Z.; Rao, Z.; Wen, J. Advances in drug resistance of osteosarcoma caused by pregnane X receptor. Drug Metab. Rev. 2024, 56, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Barzegar, S.; Pirouzpanah, S. Zinc finger proteins and ATP-binding cassette transporter-dependent multidrug resistance. Eur. J. Clin. Investig. 2024, 54, e14120. [Google Scholar] [CrossRef] [PubMed]
- Staudinger, J.L.; Goodwin, B.; Jones, S.A.; Hawkins-Brown, D.; MacKenzie, K.I.; LaTour, A.; Liu, Y.; Klaassen, C.D.; Brown, K.K.; Reinhard, J. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc. Natl. Acad. Sci. USA 2001, 98, 3369–3374. [Google Scholar] [CrossRef]
- Moore, L.B.; Goodwin, B.; Jones, S.A.; Wisely, G.B.; Serabjit-Singh, C.J.; Willson, T.M.; Collins, J.L.; Kliewer, S.A. St. John’s wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc. Natl. Acad. Sci. USA 2000, 97, 7500–7502. [Google Scholar] [CrossRef]
- Jones, S.A.; Moore, L.B.; Shenk, J.L.; Wisely, G.B.; Hamilton, G.A.; McKee, D.D.; Tomkinson, N.C.; LeCluyse, E.L.; Lambert, M.H.; Willson, T.M. The pregnane X receptor: A promiscuous xenobiotic receptor that has diverged during evolution. Mol. Endocrinol. 2000, 14, 27–39. [Google Scholar] [CrossRef]
- Tabb, M.M.; Kholodovych, V.; Grün, F.; Zhou, C.; Welsh, W.J.; Blumberg, B. Highly chlorinated PCBs inhibit the human xenobiotic response mediated by the steroid and xenobiotic receptor (SXR). Environ. Health Perspect. 2004, 112, 163–169. [Google Scholar] [CrossRef]
- Fuchs, I.; Hafner-Blumenstiel, V.; Markert, C.; Burhenne, J.; Weiss, J.; Haefeli, W.E.; Mikus, G. Effect of the CYP3A inhibitor ketoconazole on the PXR-mediated induction of CYP3A activity. Eur. J. Clin. Pharmacol. 2013, 69, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.-F.; Zhu, L.-X.; Jie, J.; Yang, P.-X.; Chen, X. The intracellular mechanism of berberine-induced inhibition of CYP3A4 activity. Curr. Pharm. Des. 2021, 27, 4179–4185. [Google Scholar] [CrossRef] [PubMed]
- Staudinger, J.L. Clinical applications of small molecule inhibitors of Pregnane X receptor. Mol. Cell. Endocrinol. 2019, 485, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Ekins, S.; Kholodovych, V.; Ai, N.; Sinz, M.; Gal, J.; Gera, L.; Welsh, W.J.; Bachmann, K.; Mani, S. Computational discovery of novel low micromolar human pregnane X receptor antagonists. Mol. Pharmacol. 2008, 74, 662–672. [Google Scholar] [CrossRef]
- Gee, R.R.F.; Huber, A.D.; Chen, T. Regulation of PXR in drug metabolism: Chemical and structural perspectives. Expert Opin. Drug Metab. Toxicol. 2024, 20, 9–23. [Google Scholar] [CrossRef]
- Su, H.; Liang, H.; Tian, J.; Zheng, L.; Li, H.; Yang, X.; Yin, S.; Bi, H. Discovery of PXR agonists from Hypericum japonicum: A class of novel nonaromatic acylphloroglucinol-terpenoid adducts. Bioorganic Chem. 2024, 147, 107354. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, G.; Bian, Z.; Chow, V.; Grimaldi, M.; Carivenc, C.; Sirounian, S.; Li, H.; Sladekova, L.; Mott, S. An abundant ginger compound furanodienone alleviates gut inflammation via the xenobiotic nuclear receptor PXR in mice. Nat. Commun. 2025, 16, 1280, Erratum in Nat. Commun. 2025, 16, 2133. [Google Scholar] [CrossRef]
- Venkatesh, M.; Mukherjee, S.; Wang, H.; Li, H.; Sun, K.; Benechet, A.P.; Qiu, Z.; Maher, L.; Redinbo, M.R.; Phillips, R.S. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity 2014, 41, 296–310. [Google Scholar] [CrossRef]
- Sládeková, L.; Li, H.; DesMarais, V.M.; Beck, A.P.; Guzik, H.; Vyhlídalová, B.; Gu, H.; Mani, S.; Dvořák, Z. Unlocking the potential: FKK6 as a microbial mimicry-based therapy for chronic inflammation-associated colorectal cancer in a murine model. J. Pharmacol. Exp. Ther. 2025, 392, 100059. [Google Scholar] [CrossRef]
- Dvořák, Z.; Vyhlídalová, B.; Pečinková, P.; Li, H.; Anzenbacher, P.; Špičáková, A.; Anzenbacherová, E.; Chow, V.; Liu, J.; Krause, H. In vitro safety signals for potential clinical development of the anti-inflammatory pregnane X receptor agonist FKK6. Bioorganic Chem. 2024, 144, 107137. [Google Scholar] [CrossRef]
- Lynch, C.; Margolis, R.; Niebler, J.; Travers, J.; Sakamuru, S.; Zhao, T.; Klumpp-Thomas, C.; Huang, R.; Xia, M. Identification of human pregnane X receptor antagonists utilizing a high-throughput screening platform. Front. Pharmacol. 2024, 15, 1448744. [Google Scholar] [CrossRef]
- Ekins, S.; Chang, C.; Mani, S.; Krasowski, M.D.; Reschly, E.J.; Iyer, M.; Kholodovych, V.; Ai, N.; Welsh, W.J.; Sinz, M. Human pregnane X receptor antagonists and agonists define molecular requirements for different binding sites. Mol. Pharmacol. 2007, 72, 592–603. [Google Scholar] [CrossRef]
- Kamaraj, R.; Pavek, P. Novel antagonists reveal the mechanism of PXR inhibition. Trends Pharmacol. Sci. 2024, 45, 961–963. [Google Scholar] [CrossRef]
- Tolson, A.H.; Wang, H. Regulation of drug-metabolizing enzymes by xenobiotic receptors: PXR and CAR. Adv. Drug Deliv. Rev. 2010, 62, 1238–1249. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.-F.; Liu, J.-P.; Chowbay, B. Polymorphism of human cytochrome P450 enzymes and its clinical impact. Drug Metab. Rev. 2009, 41, 89–295. [Google Scholar] [CrossRef] [PubMed]
- Anzenbacher, P.; Anzenbacherova, E. Cytochromes P450 and metabolism of xenobiotics. Cell. Mol. Life Sci. CMLS 2001, 58, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Holčapek, M.; Kolářová, L.; Nobilis, M. High-performance liquid chromatography–tandem mass spectrometry in the identification and determination of phase I and phase II drug metabolites. Anal. Bioanal. Chem. 2008, 391, 59–78. [Google Scholar] [CrossRef]
- Yao, N.; Zeng, C.; Zhan, T.; He, F.; Liu, M.; Liu, F.; Zhang, H.; Xiong, Y.; Xia, C. Oleanolic acid and ursolic acid induce UGT1A1 expression in HepG2 cells by activating PXR rather than CAR. Front. Pharmacol. 2019, 10, 1111. [Google Scholar] [CrossRef]
- Elmeliegy, M.; Vourvahis, M.; Guo, C.; Wang, D.D. Effect of P-glycoprotein (P-gp) inducers on exposure of P-gp substrates: Review of clinical drug–drug interaction studies. Clin. Pharmacokinet. 2020, 59, 699–714. [Google Scholar] [CrossRef]
- Hosack, T.; Damry, D.; Biswas, S. Drug-induced liver injury: A comprehensive review. Ther. Adv. Gastroenterol. 2023, 16, 17562848231163410. [Google Scholar] [CrossRef]
- Duintjer Tebbens, J.; Azar, M.; Friedmann, E.; Lanzendörfer, M.; Pávek, P. Mathematical models in the description of pregnane X receptor (PXR)-regulated cytochrome P450 enzyme induction. Int. J. Mol. Sci. 2018, 19, 1785. [Google Scholar] [CrossRef]
- Reschly, E.; Krasowski, M.D. Evolution and function of the NR1I nuclear hormone receptor subfamily (VDR, PXR, and CAR) with respect to metabolism of xenobiotics and endogenous compounds. Curr. Drug Metab. 2006, 7, 349–365. [Google Scholar] [CrossRef]
- Pu, S.; Pan, Y.; Wang, Z.; Liu, H.; Zhang, J.; Zhang, Q.; Wang, M. Forsythiaside A Reduces Acetaminophen Hepatotoxic Metabolism by Inhibiting Pregnane X Receptor. Molecules 2025, 30, 1187. [Google Scholar] [CrossRef]
- Adachi, M.; Kumagai, T.; Hosho, K.; Nagata, K.; Fujiyoshi, M.; Shimada, M. Exploring Acute Liver Damage: Slimming Health Foods and CYP3A4 Induction. Yonago Acta Medica 2024, 67, 124–134. [Google Scholar] [CrossRef]
- Cui, Q.; Jiang, T.; Xie, X.; Wang, H.; Qian, L.; Cheng, Y.; Li, Q.; Lu, T.; Yao, Q.; Liu, J.; et al. S-nitrosylation attenuates pregnane X receptor hyperactivity and acetaminophen-induced liver injury. JCI Insight 2024, 9, e172632. [Google Scholar] [CrossRef]
- Erradhouani, C.; Piccini, B.; Maillot-Marechal, E.; Aït-Aïssa, S.; Balaguer, P.; Coumoul, X.; Brion, F. New insights into the regulation of cyp3a65 expression in transgenic tg (cyp3a65: GFP) zebrafish embryos. Aquat. Toxicol. 2025, 279, 107250. [Google Scholar] [CrossRef]
- Chai, S.C.; Wright, W.C.; Chen, T. Strategies for developing pregnane X receptor antagonists: Implications from metabolism to cancer. Med. Res. Rev. 2020, 40, 1061–1083. [Google Scholar] [CrossRef]
- Hu, J.; Tian, J.; Zhang, F.; Wang, H.; Yin, J. Pxr-and Nrf2-mediated induction of ABC transporters by heavy metal ions in zebrafish embryos. Environ. Pollut. 2019, 255, 113329. [Google Scholar] [CrossRef]
- Wang, S.-W.; Gao, C.; Zheng, Y.-M.; Yi, L.; Lu, J.-C.; Huang, X.-Y.; Cai, J.-B.; Zhang, P.-F.; Cui, Y.-H.; Ke, A.-W. Current applications and future perspective of CRISPR/Cas9 gene editing in cancer. Mol. Cancer 2022, 21, 57. [Google Scholar] [CrossRef]
- George, J.; Chalmers, J.D.; Coquelin, K.-S.; Frame, L.; Henderson, C.J.; Kapelyukh, Y.; Lang, C.C.; Read, K.D.; Stanley, L.A.; Wolf, C.R. Use of an extensively humanized mouse model to predict the risk of drug–drug interactions in patients receiving dexamethasone. J. Pharmacol. Exp. Ther. 2025, 392, 100053. [Google Scholar] [CrossRef]
- Wang, J.; Fu, J.; Sun, W.; Yin, X.; Lv, K.; Zhang, J. Functionalized PEG-PLA nanoparticles for brain targeted delivery of ketoconazole contribute to pregnane X receptor overexpressing in drug-resistant epilepsy. Epilepsy Res. 2022, 186, 107000. [Google Scholar] [CrossRef]
- Niu, X.; Wu, T.; Yin, Q.; Gu, X.; Li, G.; Zhou, C.; Ma, M.; Su, L.; Tang, S.; Tian, Y. Combination of paclitaxel and PXR antagonist SPA70 reverses paclitaxel-resistant non-small cell lung cancer. Cells 2022, 11, 3094. [Google Scholar] [CrossRef]
- Smith, R.P.; Eckalbar, W.L.; Morrissey, K.M.; Luizon, M.R.; Hoffmann, T.J.; Sun, X.; Jones, S.L.; Force Aldred, S.; Ramamoorthy, A.; Desta, Z. Genome-wide discovery of drug-dependent human liver regulatory elements. PLoS Genet. 2014, 10, e1004648. [Google Scholar] [CrossRef]
- Cui, J.Y.; Gunewardena, S.S.; Rockwell, C.E.; Klaassen, C.D. ChIPing the cistrome of PXR in mouse liver. Nucleic Acids Res. 2010, 38, 7943–7963. [Google Scholar] [CrossRef]
- Cui, J.Y.; Klaassen, C.D. RNA-Seq reveals common and unique PXR-and CAR-target gene signatures in the mouse liver transcriptome. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2016, 1859, 1198–1217. [Google Scholar] [CrossRef]
- Xu, Y.; An, Z.; Wang, S.; Ni, Y.; Zhou, M.; Feng, Q.; Gou, X.; Xu, M.; Qi, Y. Dual role of pregnane X receptor in nonalcoholic fatty liver disease. Curr. Mol. Pharmacol. 2024, 17, E18761429259143. [Google Scholar] [CrossRef]
- Niu, B.; Pan, T.; Xiao, Y.; Wang, H.; Zhu, J.; Tian, F.; Lu, W.; Chen, W. The therapeutic potential of dietary intervention: Based on the mechanism of a tryptophan derivative-indole propionic acid on metabolic disorders. Crit. Rev. Food Sci. Nutr. 2025, 65, 1729–1748. [Google Scholar] [CrossRef]
- Flannigan, K.L.; Nieves, K.M.; Szczepanski, H.E.; Serra, A.; Lee, J.W.; Alston, L.A.; Ramay, H.; Mani, S.; Hirota, S.A. The pregnane X receptor and indole-3-propionic acid shape the intestinal mesenchyme to restrain inflammation and fibrosis. Cell. Mol. Gastroenterol. Hepatol. 2023, 15, 765–795. [Google Scholar] [CrossRef]
- Peng, R.; Song, C.; Gou, S.; Liu, H.; Kang, H.; Dong, Y.; Xu, Y.; Hu, P.; Cai, K.; Feng, Q. Gut Clostridium sporogenes-derived indole propionic acid suppresses osteoclast formation by activating pregnane X receptor. Pharmacol. Res. 2024, 202, 107121. [Google Scholar] [CrossRef]
- Ul Ain, N.; Naveed, M.; Aziz, T.; Shabbir, M.A.; Al Asmari, F.; Abdi, G.; Sameeh, M.Y.; Alhhazmi, A.A. Mix-match synthesis of nanosynbiotics from probiotics and prebiotics to counter gut dysbiosis via AI integrated formulation profiling. Sci. Rep. 2024, 14, 18397. [Google Scholar] [CrossRef]
- Gentry, E.C.; Collins, S.L.; Panitchpakdi, M.; Belda-Ferre, P.; Stewart, A.K.; Carrillo Terrazas, M.; Lu, H.-h.; Zuffa, S.; Yan, T.; Avila-Pacheco, J. Reverse metabolomics for the discovery of chemical structures from humans. Nature 2024, 626, 419–426. [Google Scholar] [CrossRef]
- Rimal, B.; Collins, S.L.; Tanes, C.E.; Rocha, E.R.; Granda, M.A.; Solanki, S.; Hoque, N.J.; Gentry, E.C.; Koo, I.; Reilly, E.R. Bile salt hydrolase catalyses formation of amine-conjugated bile acids. Nature 2024, 626, 859–863. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Yang, M.J.; Liao, C.Y.; Taha, R.; Li, Q.Y.; Abdelmotalab, M.I.; Zhao, S.Y.; Xu, Y.; Jiang, Z.Z.; Chu, C.H. Atractylodes macrocephala Koidz polysaccharide ameliorates DSS-induced colitis in mice by regulating the gut microbiota and tryptophan metabolism. Br. J. Pharmacol. 2025, 182, 1508–1527. [Google Scholar] [CrossRef]
- Wu, T.; Li, L.; Zhou, W.; Bi, G.; Jiang, X.; Guo, M.; Yang, X.; Fang, J.; Pang, J.; Fan, S. Gut Microbiota Affects Mouse Pregnane X Receptor Agonist Pregnenolone 16α-Carbonitrile-Induced Hepatomegaly by Regulating Pregnane X Receptor and Yes-Associated Protein Activation. Drug Metab. Dispos. 2024, 52, 597–605. [Google Scholar] [CrossRef]
- Mbatchi, L.C.; Brouillet, J.-P.; Evrard, A. Genetic variations of the xenoreceptors NR1I2 and NR1I3 and their effect on drug disposition and response variability. Pharmacogenomics 2018, 19, 61–77. [Google Scholar] [CrossRef]
- Xie, H.; Zheng, Y.; Zhang, H.; Guo, Y.; Liu, M.; Weng, Q.; Wu, X. Association of NR1I2 Polymorphism with Midazolam Clearance in Mechanically Ventilated ICU Patients: A Population Pharmacokinetic and Pharmacogenetic Study. Drug Des. Dev. Ther. 2025, 19, 1527–1541. [Google Scholar] [CrossRef]
- Chen, Y.-b.; Zhou, Z.-y.; Li, G.-m.; Xiao, C.-x.; Yu, W.-b.; Zhong, S.-l.; Cai, Y.-f.; Jin, J.; Huang, M. Influences of an NR1I2 polymorphism on heterogeneous antiplatelet reactivity responses to clopidogrel and clinical outcomes in acute ischemic stroke patients. Acta Pharmacol. Sin. 2019, 40, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Gouda, M.A.; Buschhorn, L.; Schneeweiss, A.; Wahida, A.; Subbiah, V. N-of-1 trials in cancer drug development. Cancer Discov. 2023, 13, 1301–1309. [Google Scholar] [CrossRef]
- Asim, A.; Wang, F.; Pu, D.; Wang, S.; Wang, D.; Li, W.; Yu, F.; Ji, L. How Uremic Toxins Alter Atorvastatin Disposition: Molecular Mechanisms of Inhibition of the Enzyme CYP3A4. Balk. Med. J. 2025, 42, 37. [Google Scholar] [CrossRef]
- Pan, Y.; Yang, Z.; Wei, M.; Gan, Y.; Liu, M.; Zou, W. Hypertensive Nephropathy Changes the Expression of Drug-Metabolizing Enzymes and Transporters in Spontaneously Hypertensive Rat Liver and Kidney. Eur. J. Drug Metab. Pharmacokinet. 2025, 50, 39–51. [Google Scholar] [CrossRef]


| Compound | Class | Role | Research Stage | Potential Applications | Reference |
|---|---|---|---|---|---|
| Rifampicin | Antibiotic | Agonist | Clinical | Anti-tuberculosis therapy; regulation of drug metabolism | [21] |
| Pregnenolone-16α-carbonitrile (PCN) | Synthetic steroid | Agonist | Preclinical | Species-specific PXR activator commonly used in rodent models | [117] |
| Hyperforin | Natural product | Agonist | Clinical | Treatment of depression; modulation of drug metabolism | [118] |
| SR12813 | Synthetic small molecule | Agonist | Preclinical | Activation of PXR; regulation of cholesterol metabolism | [119] |
| Highly Chlorinated Polychlorinated Biphenyls (PCBs) | Industrial pollutant | Agonist | Preclinical | Activation of PXR-mediated xenobiotic responses | [120] |
| Ketoconazole | Antifungal drug | Antagonist | Clinical (withdrawn due to hepatotoxicity) | Inhibits the PXR–CYP3A4 axis; antifungal agent | [121] |
| Berberine | Natural alkaloid | Antagonist | Preclinical | Sensitizes colorectal cancer to chemotherapy; reverses P-gp/CYP3A4-mediated resistance | [122] |
| SPA70 | Synthetic small molecule | Antagonist | Preclinical | Competitively binds to the PXR ligand-binding domain, preventing agonist-induced activation | [123] |
| SPB03255 | Synthetic small molecule | Antagonist | Preclinical | Inhibits PXR activation | [124] |
| The newly identified components from Hypericum japonicum | Natural products | Agonist | Preclinical | Potential therapeutic agents for cholestasis via PXR activation | [126] |
| Cannabidiol (CBD) | Natural products | Agonist | Preclinical | Selective PXR agonist; exhibits high activity toward hPXR | [69] |
| Resveratrol (RES) | Natural products | Antagonist | Preclinical | Suppresses PXR-mediated downstream gene expression; binds to non-canonical sites on PXR | [69] |
| Furanodienone (FDN) | Natural products | Agonist | Preclinical | Activates PXR via unique interaction with LBD, inducing conformational changes | [127] |
| Paclitaxel | Natural products | Agonist | Preclinical | Anticancer agent; induces PXR activation and contributes to chemoresistance | [100] |
| MI-883 | Synthetic small molecule | Antagonist | Preclinical | Modulates downstream signaling pathways via dual regulation of PXR and CAR | [74] |
| IPA | Indole derivative | Agonist | Preclinical | Microbiota-derived PXR agonist; regulates intestinal barrier function | [128] |
| Felix Kopp Kortagere 6 (FKK6) | Synthetic small molecule | Agonist | Preclinical | Selectively activates intestinal PXR; exhibits anti-inflammatory effects | [129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, Y.; Liu, S.; Wang, L.; Peng, D.; Chen, W.; Zhang, Y.; Wang, Y. Mechanisms and Therapeutic Advances of PXR in Metabolic Diseases and Cancer. Int. J. Mol. Sci. 2025, 26, 8029. https://doi.org/10.3390/ijms26168029
Bi Y, Liu S, Wang L, Peng D, Chen W, Zhang Y, Wang Y. Mechanisms and Therapeutic Advances of PXR in Metabolic Diseases and Cancer. International Journal of Molecular Sciences. 2025; 26(16):8029. https://doi.org/10.3390/ijms26168029
Chicago/Turabian StyleBi, Yuanbo, Sifan Liu, Lei Wang, Daiyin Peng, Weidong Chen, Yue Zhang, and Yanyan Wang. 2025. "Mechanisms and Therapeutic Advances of PXR in Metabolic Diseases and Cancer" International Journal of Molecular Sciences 26, no. 16: 8029. https://doi.org/10.3390/ijms26168029
APA StyleBi, Y., Liu, S., Wang, L., Peng, D., Chen, W., Zhang, Y., & Wang, Y. (2025). Mechanisms and Therapeutic Advances of PXR in Metabolic Diseases and Cancer. International Journal of Molecular Sciences, 26(16), 8029. https://doi.org/10.3390/ijms26168029






