Differential Immune Checkpoint Expression in CD4+ and CD4− NKT Cell Populations During Healthy Pregnancy
Abstract
1. Introduction
2. Results
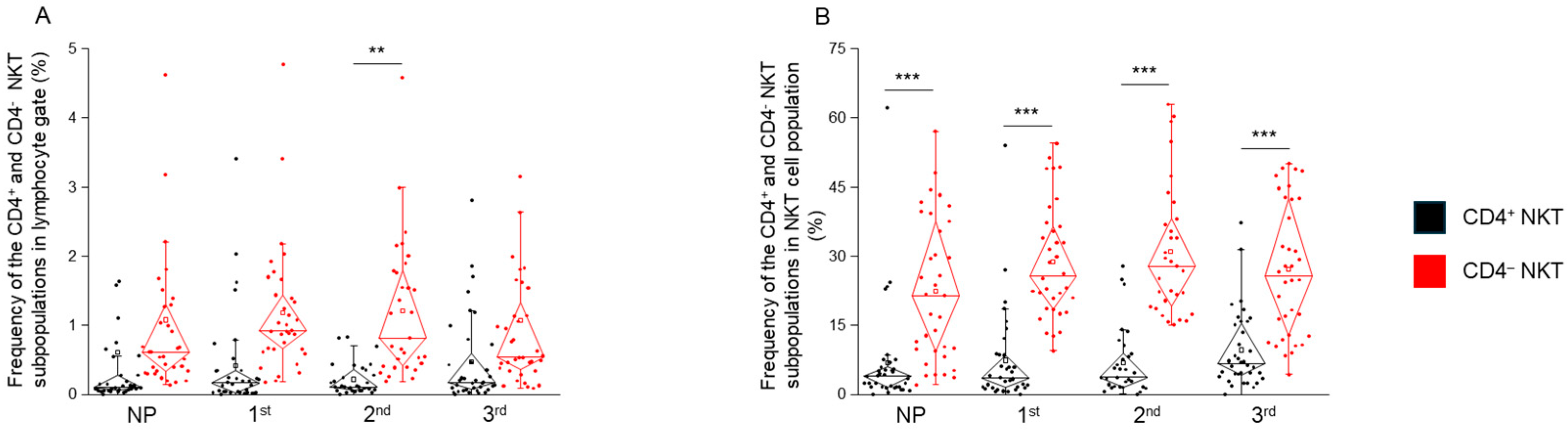
2.1. The Frequency of CD4+ and CD4− NKT Cell Subpopulations in Healthy Pregnancy and Non-Pregnant Women
2.2. PD-1 and PD-L1 Expression by CD4+ and CD4− NKT Cell Subpopulations During Healthy Pregnancy and in Non-Pregnant Women
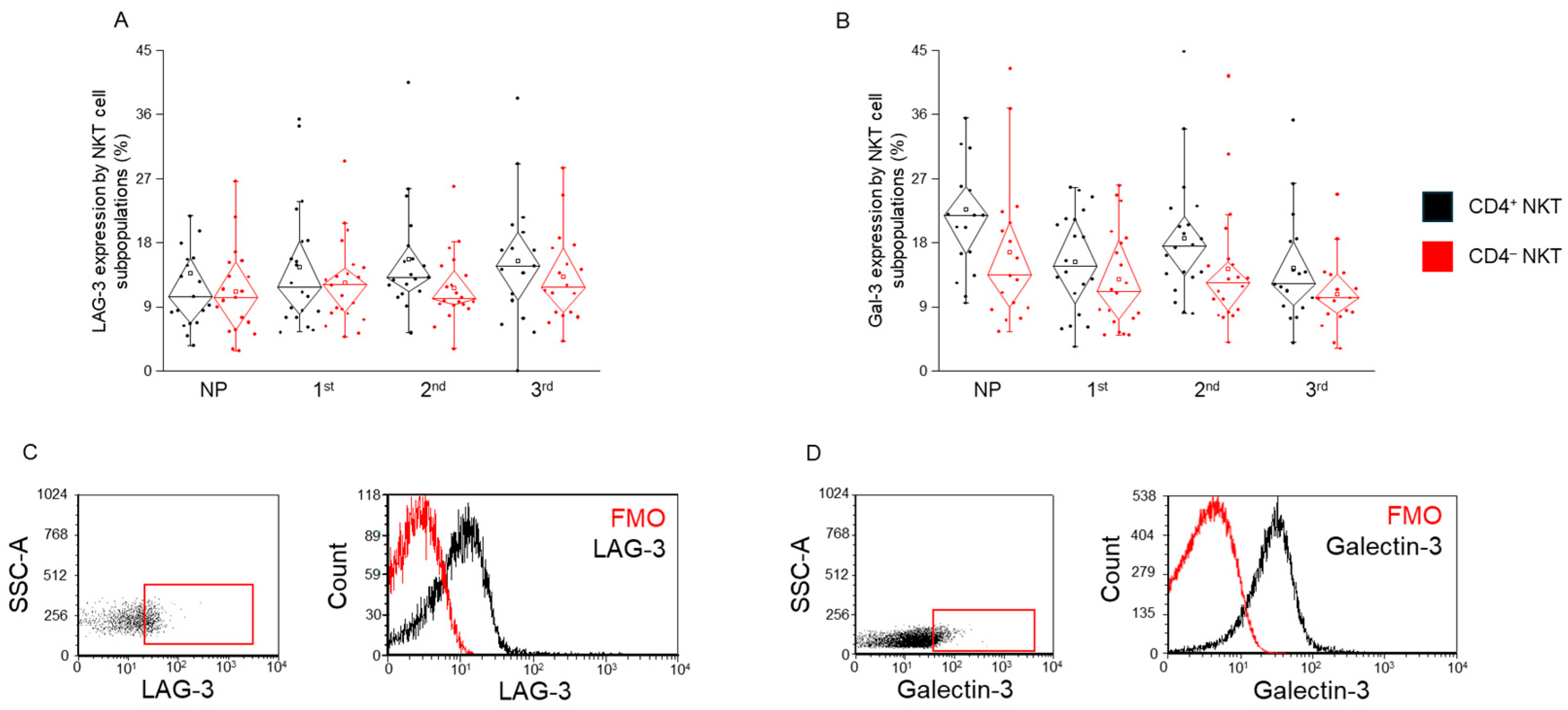
2.3. LAG-3 and Gal-3 Expression by CD4+ and CD4− NKT Cell Subpopulations During Healthy Pregnancy and in Non-Pregnant Women
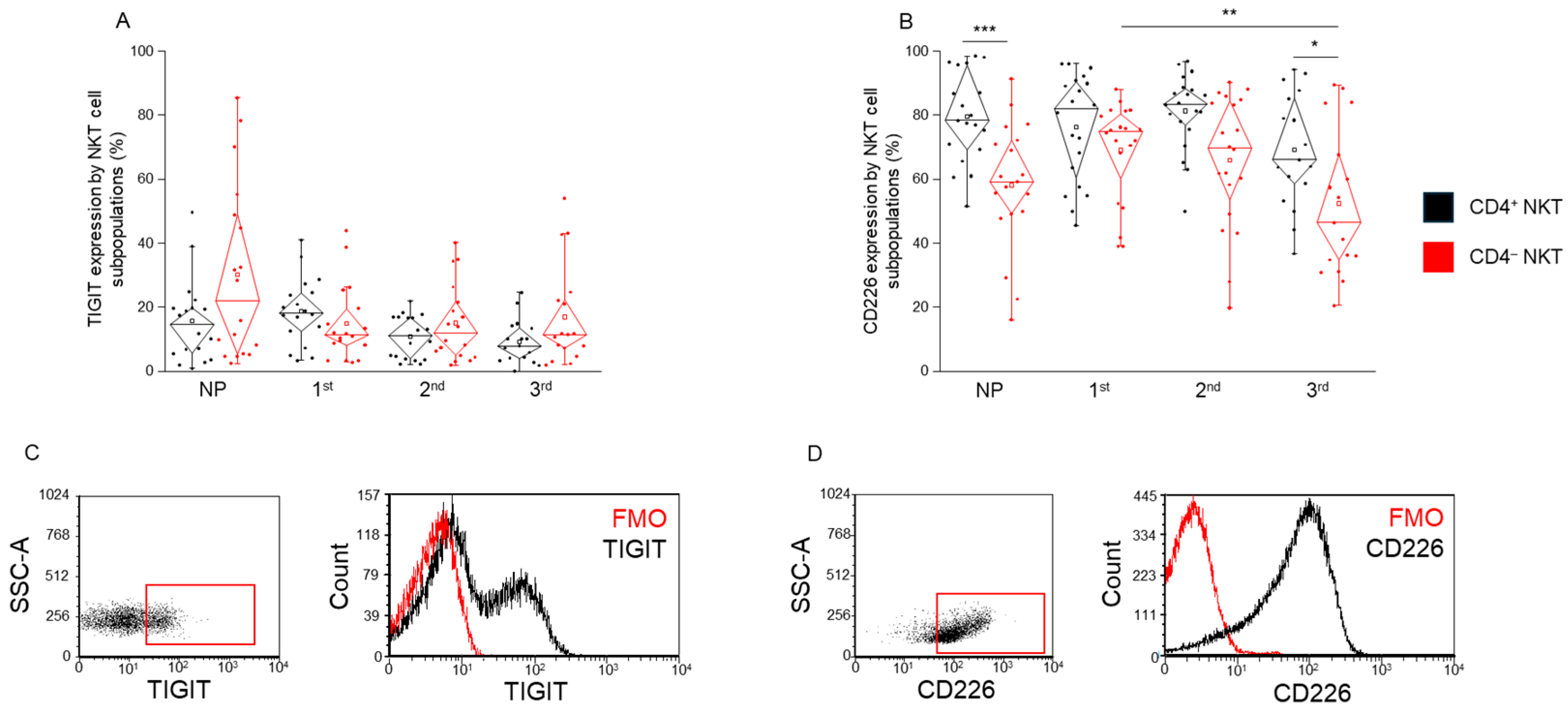
2.4. TIGIT and CD226 Receptor Expression by CD4+ and CD4− NKT Cell Subpopulations During Healthy Pregnancy and in Non-Pregnant Women
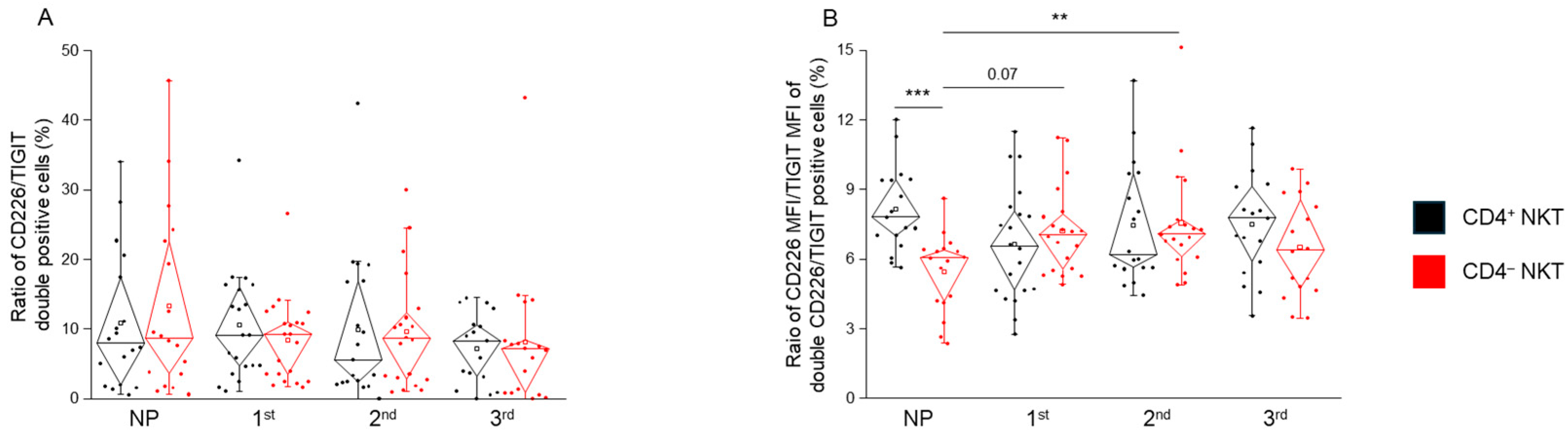
2.5. TIGIT and CD226 MFI and the Frequency of Double-Positive NKT Cell Subpopulations in Healthy Pregnancy and Non-Pregnant Women
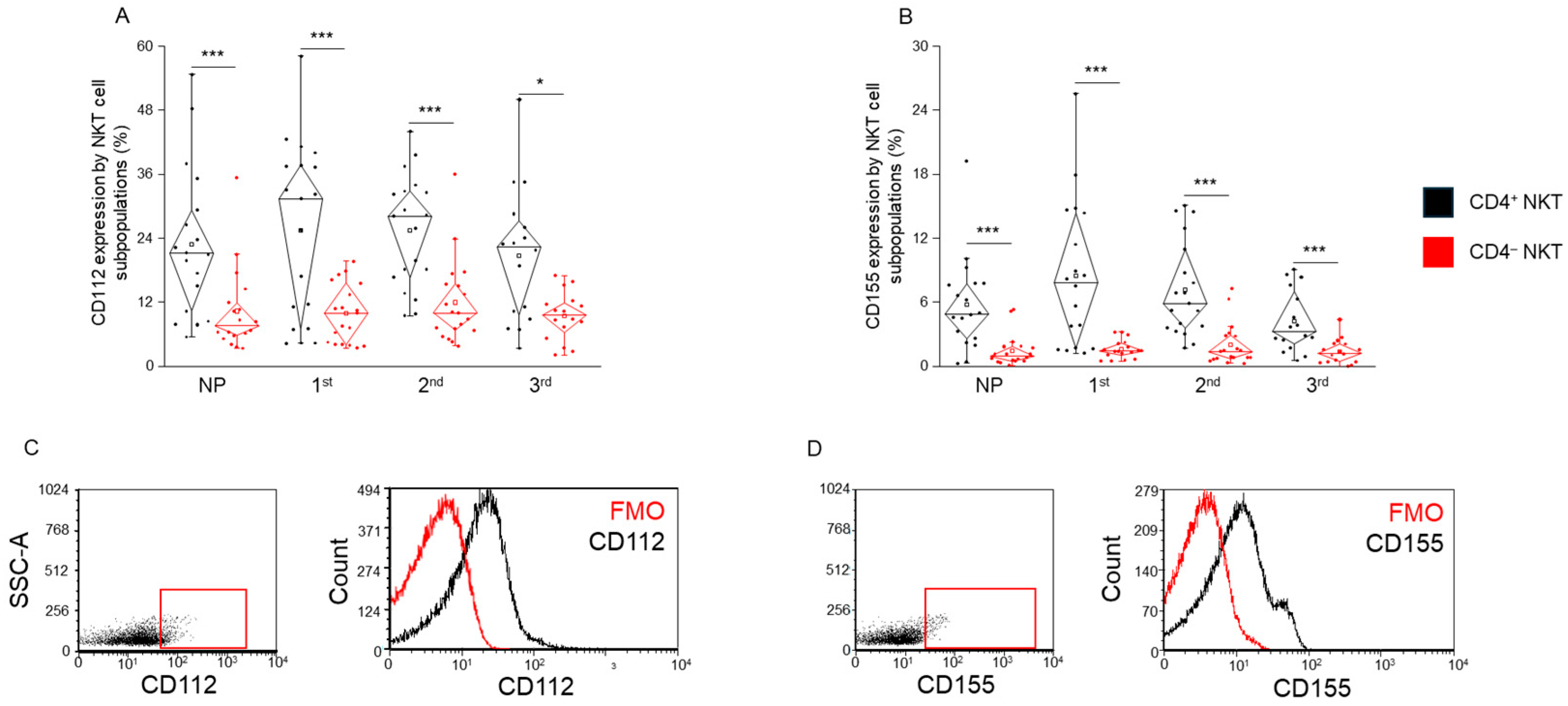
2.6. CD112 and CD155 Ligand Expression by CD4+ and CD4− NKT Cell Subpopulations in Healthy Pregnancy and Non-Pregnant Women
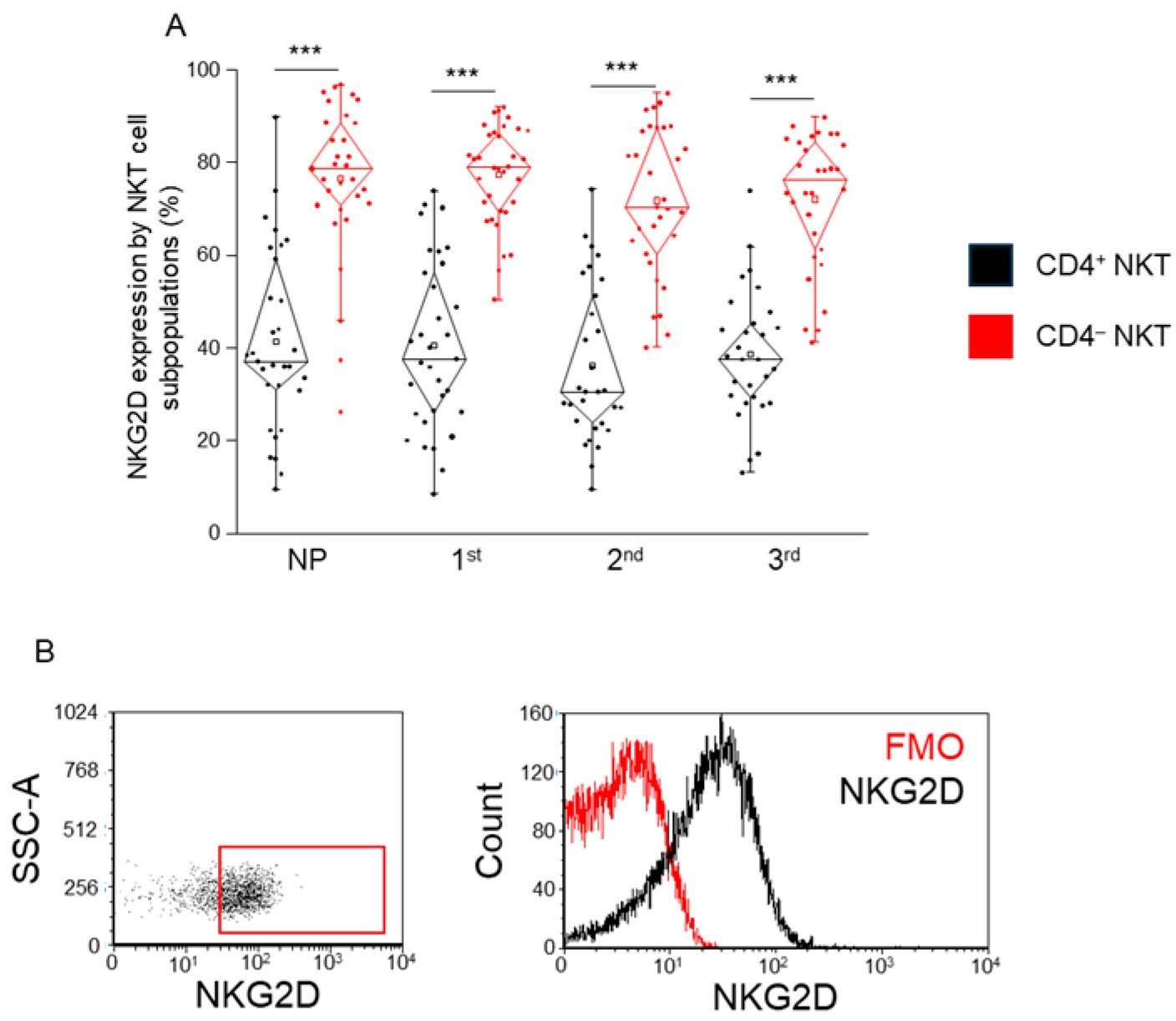
2.7. Expression of the Activation Receptor NKG2D by CD4+ and CD4− NKT Cell Subpopulations During Healthy Pregnancy and in Non-Pregnant Women
3. Discussion
4. Materials and Methods
4.1. Ethical Approval
4.2. Participants
4.3. Sample Collection, PBMC Separation, and Cryopreservation
4.4. Flow Cytometric Measurement
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Exley, M.; Garcia, J.; Balk, S.P.; Porcelli, S. Requirements for CD1d Recognition by Human Invariant Vα24+ CD4−CD8− T Cells. J. Exp. Med. 1997, 186, 109. [Google Scholar] [CrossRef]
- Bendelac, A.; Killeen, N.; Littman, D.R.; Schwartz, R.H. A Subset of CD4 + Thymocytes Selected by MHC Class I Molecules. Science 1994, 263, 1774–1778. [Google Scholar] [CrossRef]
- Liu, J.; Hill, B.J.; Darko, S.; Song, K.; Quigley, M.F.; Asher, T.E.; Morita, Y.; Greenaway, H.Y.; Venturi, V.; Douek, D.C.; et al. The Peripheral Differentiation of Human Natural Killer T Cells. Immunol. Cell Biol. 2019, 97, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, D.I.; Kronenberg, M. Going Both Ways: Immune Regulation via CD1d-Dependent NKT Cells. J. Clin. Investig. 2004, 114, 1379–1388. [Google Scholar] [CrossRef]
- Gumperz, J.E.; Miyake, S.; Yamamura, T.; Brenner, M.B. Functionally Distinct Subsets of CD1d-Restricted Natural Killer T Cells Revealed by CD1d Tetramer Staining. J. Exp. Med. 2002, 195, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.T.; Benlagha, K.; Teyton, L.; Bendelac, A. Distinct Functional Lineages of Human Vα24 Natural Killer T Cells. J. Exp. Med. 2002, 195, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Jelinek, T.; Paiva, B.; Hajek, R. Update on PD-1/PD-L1 Inhibitors in Multiple Myeloma. Front. Immunol. 2018, 9, 2431. [Google Scholar] [CrossRef]
- Miko, E.; Meggyes, M.; Doba, K.; Barakonyi, A.; Szereday, L. Immune Checkpoint Molecules in Reproductive Immunology. Front. Immunol. 2019, 10, 846. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, J.; Kwak-Kim, J.; Wang, W. Immune Checkpoint Inhibitors and Reproductive Failures. J. Reprod. Immunol. 2023, 156, 103799. [Google Scholar] [CrossRef]
- Veras, E.; Kurman, R.J.; Wang, T.L.; Shih, I.M. PD-L1 Expression in Human Placentas and Gestational Trophoblastic Diseases. Int. J. Gynecol. Pathol. 2017, 36, 146–153. [Google Scholar] [CrossRef]
- Hu, X.; Lai, S.; Liao, A. Immune Checkpoint for Pregnancy. Semin. Immunopathol. 2025, 47, 26. [Google Scholar] [CrossRef]
- Okuyama, M.; Mezawa, H.; Kawai, T.; Urashima, M. Elevated Soluble PD-L1 in Pregnant Women’s Serum Suppresses the Immune Reaction. Front. Immunol. 2019, 10, 86. [Google Scholar] [CrossRef]
- Anderson, A.C.; Joller, N.; Kuchroo, V.K. Lag-3, Tim-3, and TIGIT: Co-Inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 2016, 44, 989–1004. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Shi, J.-L.; Zheng, Z.-M.; Lin, Z.; Li, M.-Q.; Shao, J.; Chen, M.; Shi, J.-L.; Zheng, Z.-M.; Lin, Z.; et al. Galectins: Important Regulators in Normal and Pathologic Pregnancies. Int. J. Mol. Sci. 2022, 23, 10110. [Google Scholar] [CrossRef] [PubMed]
- Zych, M.; Roszczyk, A.; Dąbrowski, F.; Kniotek, M.; Zagożdżon, R. Soluble Forms of Immune Checkpoints and Their Ligands as Potential Biomarkers in the Diagnosis of Recurrent Pregnancy Loss—A Preliminary Study. Int. J. Mol. Sci. 2024, 25, 499. [Google Scholar] [CrossRef] [PubMed]
- Yeo, J.; Ko, M.; Lee, D.H.; Park, Y.; Jin, H.S. Tigit/Cd226 Axis Regulates Anti-Tumor Immunity. Pharmaceuticals 2021, 14, 200. [Google Scholar] [CrossRef]
- Jantz-Naeem, N.; Böttcher-Loschinski, R.; Borucki, K.; Mitchell-Flack, M.; Böttcher, M.; Schraven, B.; Mougiakakos, D.; Kahlfuss, S. TIGIT Signaling and Its Influence on T Cell Metabolism and Immune Cell Function in the Tumor Microenvironment. Front. Oncol. 2023, 13, 1060112. [Google Scholar] [CrossRef]
- Fu, W.; Cai, R.; Ma, Z.; Li, T.; Lei, C.; Zhao, J.; Hu, S. TIGIT-Fc as a Potential Therapeutic Agent for Fetomaternal Tolerance. Front. Immunol. 2021, 12, 649135. [Google Scholar] [CrossRef]
- Fu, B.; Wei, H. Decidual Natural Killer Cells and the Immune Microenvironment at the Maternal-Fetal Interface. Sci. China Life Sci. 2016, 59, 1224–1231. [Google Scholar] [CrossRef]
- Kaito, Y.; Sugimoto, E.; Nakamura, F.; Tsukune, Y.; Sasaki, M.; Yui, S.; Yamaguchi, H.; Goyama, S.; Nannya, Y.; Mitani, K.; et al. Immune Checkpoint Molecule DNAM-1/CD112 Axis Is a Novel Target for Natural Killer-Cell Therapy in Acute Myeloid Leukemia. Haematologica 2024, 109, 1107–1120. [Google Scholar] [CrossRef]
- Mor, G.; Cardenas, I.; Abrahams, V.; Guller, S. Inflammation and Pregnancy: The Role of the Immune System at the Implantation Site. Ann. N.Y. Acad. Sci. 2011, 1221, 80–87. [Google Scholar] [CrossRef]
- Meggyes, M.; Miko, E.; Szigeti, B.; Farkas, N.; Szereday, L. The Importance of the PD-1/PD-L1 Pathway at the Maternal-Fetal Interface. BMC Pregnancy Childbirth 2019, 19, 74. [Google Scholar] [CrossRef]
- Meggyes, M.; Nagy, D.U.; Feik, T.; Boros, A.; Polgar, B.; Szereday, L. Examination of the TIGIT-CD226-CD112-CD155 Immune Checkpoint Network during a Healthy Pregnancy. Int. J. Mol. Sci. 2022, 23, 10776. [Google Scholar] [CrossRef] [PubMed]
- Meggyes, M.; Nagy, D.U.; Saad Al Deen, I.; Parkanyi, B.; Szereday, L. CD8+ and CD8− NKT Cells Exhibit Phenotypic Changes During Pregnancy. Immunol. Investig. 2022, 52, 35–51. [Google Scholar] [CrossRef]
- Meggyes, M.; Miko, E.; Polgar, B.; Bogar, B.; Farkas, B.; Illes, Z.; Szereday, L. Peripheral Blood TIM-3 Positive NK and CD8+ T Cells throughout Pregnancy: TIM-3/Galectin-9 Interaction and Its Possible Role during Pregnancy. PLoS ONE 2014, 9, e92371. [Google Scholar] [CrossRef]
- Meggyes, M.; Lajko, A.; Palkovics, T.; Totsimon, A.; Illes, Z.; Szereday, L.; Miko, E. Feto-Maternal Immune Regulation by TIM-3/Galectin-9 Pathway and PD-1 Molecule in Mice at Day 14.5 of Pregnancy. Placenta 2015, 36, 1153–1160. [Google Scholar] [CrossRef]
- Berzins, S.P.; Ritchie, D.S. Natural Killer T Cells: Drivers or Passengers in Preventing Human Disease? Nat. Rev. Immunol. 2014, 14, 640–646. [Google Scholar] [CrossRef]
- Godfrey, D.I.; Stankovic, S.; Baxter, A.G. Raising the NKT Cell Family. Nat. Immunol. 2010, 11, 197–206. [Google Scholar] [CrossRef]
- Godfrey, D.I.; MacDonald, H.R.; Kronenberg, M.; Smyth, M.J.; Van Kaer, L. NKT Cells: What’s in a Name? Nat. Rev. Immunol. 2004, 4, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Game, D.S.; Davies, D.; Lombardi, G.; Lechler, R.I. Activated CD1d-Restricted Natural Killer T Cells Secrete IL-2: Innate Help for CD4+CD25+ Regulatory T Cells? Eur. J. Immunol. 2005, 35, 1193–1200. [Google Scholar] [CrossRef] [PubMed]


| Non-Pregnant | 1st Trimester | 2nd Trimester | 3rd Trimester | |
|---|---|---|---|---|
| No. of women | 20 | 26 | 28 | 29 |
| Age (years) | 29.85 (22–43) | 32.77 (18–41) | 32.54 (18–43) | 32.10 (23–44) |
| Gestation age at sampling (weeks) | - | 13.96 ± 2.44 | 24.96 ± 1.82 | 30.86 ± 3.77 |
| Gestation age at birth (weeks) | - | 39.05 ± 1.39 | 38.65 ± 1.87 | 39.34 ± 0.81 |
| Gravidity | - | 0.76 | 0.77 | 0.89 |
| Parity | - | 1.15 | 1.08 | 1.41 |
| Antigen | Format | Clone | Isotype | Manufacturer | Catalog No |
|---|---|---|---|---|---|
| CD3 | BV510 | UCHT1 | Mouse BALB/c IgG1, κ | BD Biosciences | 563109 |
| CD4 | FITC | RPA-T4 | Mouse IgG1, κ | BD Biosciences | 555346 |
| CD8 | APC-H7 | SK1 | Mouse BALB/c IgG1, κ | BD Biosciences | 560179 |
| CD226 | BV421 | DX11 | Mouse BALB/c IgG1, κ | BD Biosciences | 742493 |
| TIGIT | PE | A1553G | Mouse IgG2a, κ | Biolegend | 372704 |
| CD112 | PE | R2.525 | Mouse IgG1, κ | BD Biosciences | 551057 |
| CD155 | APC | SKII.4 | Mouse IgG1, κ | Biolegend | 337618 |
| PD-1 | PE | PD1.3 | IgG2b Mouse | Beckmann Coult | B30634 |
| PD-L1 | BV421 | MIH1 | Mouse BALB/c IgG1, κ | BD Biosciences | 563738 |
| LAG-3 | PerCp | 11C3C65 | Mouse IgG1, κ | Biolegend | 369312 |
| Galectin-3 | PE | B2C10 | Mouse BALB/c IgG1, κ | BD Biosciences | 565676 |
| NKG2D | PE-Cy7 | 1D11 | Mouse RBF/DnJ IgG1, κ | BD Biosciences | 562365 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meggyes, M.; David, N.U.; Mezosi, L.; Vastag, F.; Kevey, D.; Szereday, L. Differential Immune Checkpoint Expression in CD4+ and CD4− NKT Cell Populations During Healthy Pregnancy. Int. J. Mol. Sci. 2025, 26, 8022. https://doi.org/10.3390/ijms26168022
Meggyes M, David NU, Mezosi L, Vastag F, Kevey D, Szereday L. Differential Immune Checkpoint Expression in CD4+ and CD4− NKT Cell Populations During Healthy Pregnancy. International Journal of Molecular Sciences. 2025; 26(16):8022. https://doi.org/10.3390/ijms26168022
Chicago/Turabian StyleMeggyes, Matyas, Nagy U. David, Livia Mezosi, Fanni Vastag, Dora Kevey, and Laszlo Szereday. 2025. "Differential Immune Checkpoint Expression in CD4+ and CD4− NKT Cell Populations During Healthy Pregnancy" International Journal of Molecular Sciences 26, no. 16: 8022. https://doi.org/10.3390/ijms26168022
APA StyleMeggyes, M., David, N. U., Mezosi, L., Vastag, F., Kevey, D., & Szereday, L. (2025). Differential Immune Checkpoint Expression in CD4+ and CD4− NKT Cell Populations During Healthy Pregnancy. International Journal of Molecular Sciences, 26(16), 8022. https://doi.org/10.3390/ijms26168022






