Natural Biomolecules and Light: Antimicrobial Photodynamic Strategies in the Fight Against Antibiotic Resistance
Abstract
1. Introduction
2. Antimicrobial Photodynamic Therapy
3. Natural Photosensitizers
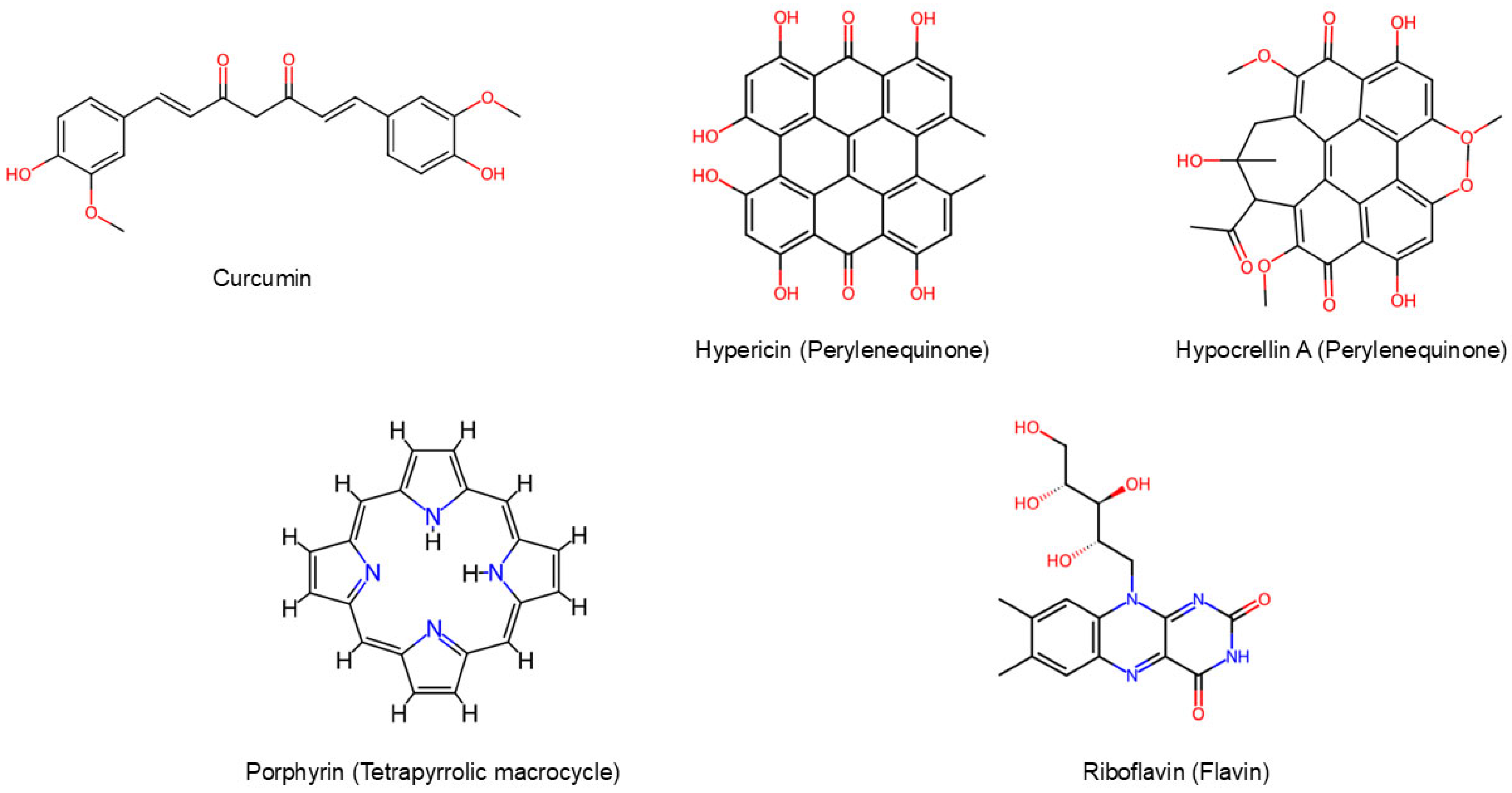
3.1. Curcuminoids
3.1.1. Curcumin in Combination with Nanostructures
3.1.2. In Vivo Studies
3.2. Perylenequinones
3.2.1. Hypericin
3.2.2. Hypericin in Combination with Nanostructures
3.3. Tetrapyrrolic Macrocycles
3.3.1. Endogenous Porphyrins
3.3.2. Exogenous Porphyrin Precursor and Porphyrins
3.4. Flavins
3.4.1. Endogenous Riboflavins
3.4.2. Exogenous Riboflavins
4. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| aPDT | Antimicrobial Photodynamic Therapy |
| PS | Photosensitizer |
| AMR | Antimicrobial Resistance |
| WHO | World Health Organization |
| EPS | Extracellular Polymeric Substances |
| ROS | Reactive Oxygen Species |
| CUR | Curcumin |
| MRSA | Methicillin-Resistant Staphylococcus aureus |
| VRSA | Vancomycin-Resistant Staphylococcus aureus |
| CFU | Colony-Forming Unit |
| DHC | 3,3′-dihydroxycurcumin |
| DAPMA | N,N-di-(3-aminopropyl)-N-methylamine |
| SPD | Spermidine |
| SPM | Spermine |
| NP | Nanoparticle |
| PLGA | Poly (lactic-co-glycolic acid) |
| MIC | Minimum Inhibitory Concentration |
| MBC | Minimum Bactericidal Concentration |
| PTT | Photothermal Therapy |
| AuNPs/CS | Chitosan-coated gold Nanoparticles |
| CD | Carbon Dot Composite |
| AC-Gel@ | Hybrid hydrogel |
| AgTPP | Silver-Porphyrin Cyclodextrin and Phenylboronic acid |
| CurNisNP | Curcumin and Nisin in Nanoparticles |
| aPSDT | Antimicrobial Photo-Sonodynamic Treatment |
| GCDNH | Gold-Curcumin-Polydopamine Nanohybrid |
| HA | Hypocrellin A |
| TEM | Transmission Electron Microscope |
| SEM | Scansion Electron Microscope |
| AnBox·4Cl | Cationic Macrocyclic Carrier |
| HYP | Hypericin |
| aSDT | Antimicrobial Sonodynamic Therapy |
| D-Trp | D-Tryptophan |
| NIR | Near-Infrared |
| DiR | 1,1-dioctadecyl-3,3,3,3-tetramethylindotricarbocyanine iodide |
| Hyp-βCD | Hypericin β-cyclodextrin |
| ALA | Aminolevulinic Acid |
| CPIII | Coproporphyrin III |
| PPIX | Protoporphyrin IX |
| BFO | Bismuth Ferrite |
| SHG | Second Harmonic Generation |
| RPE1 | Human Immortalized Retinal Epithelial (cells) |
| FMN | Flavin Mononucleotide |
| FAD | Flavin Adenine Dinucleotide |
| UVA | Ultraviolet A |
| PBS | Phosphate-Buffered Saline |
| Rf-AuNP | Gold Nanoparticle functionalized with Riboflavin |
| Rf-CD | Riboflavin-based Carbon polymerized Dot |
References
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef]
- Samreen; Ahmad, I.; Malak, H.A.; Abulreesh, H.H. Environmental antimicrobial resistance and its drivers: A potential threat to public health. J. Glob. Antimicrob. Resist. 2021, 27, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.W.K.; Millar, B.C.; Moore, J.E. Antimicrobial Resistance (AMR). Br. J. Biomed. Sci. 2023, 80, 11387. [Google Scholar] [CrossRef] [PubMed]
- Larsson, D.G.J.; Flach, C.F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef]
- Devi, N.S.; Mythili, R.; Cherian, T.; Dineshkumar, R.; Sivaraman, G.K.; Jayakumar, R.; Prathaban, M.; Duraimurugan, M.; Chandrasekar, V.; Peijnenburg, W.J.G.M. Overview of antimicrobial resistance and mechanisms: The relative status of the past and current. Microbe 2024, 3, 100083. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial Life on Surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Keren, I.; Kaldalu, N.; Spoering, A.; Wang, Y.; Lewis, K. Persister cells and tolerance to antimicrobials. FEMS Microbiol. Lett. 2004, 230, 13–18, Erratum in FEMS Microbiol. Lett. 2004, 234, 187. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Passaglia, E.; Sgarbossa, A. Innovative Phosphorene Nanoplatform for Light Antimicrobial Therapy. Pharmaceutics 2023, 15, 2748. [Google Scholar] [CrossRef]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial photodynamic therapy—What we know and what we don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef]
- Delcanale, P.; Abbruzzetti, S.; Viappiani, C. Photodynamic treatment of pathogens. Riv. Nuovo Cim. 2022, 45, 407–459. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, R.; Zaat, S.A.J.; Breukink, E.; Heger, M. Antibacterial photodynamic therapy: Overview of a promising approach to fight antibiotic-resistant bacterial infections. J. Clin. Transl. Res. 2015, 1, 140–167. [Google Scholar] [PubMed]
- Surur, A.K.; Barros de Oliveira, A.; De Annunzio, S.R.; Ferrisse, T.M.; Fontana, C.R. Bacterial resistance to antimicrobial photodynamic therapy: A critical update. J. Photochem. Photobiol. B 2024, 255, 112905. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Sahu, A.; Mukherjee, T.; Mohanty, S.; Das, P.; Nayak, N.; Kumari, S.; Singh, R.P.; Pattnaik, A. Divulging the potency of naturally derived photosensitizers in green PDT: An inclusive review of mechanisms, advantages, and future prospects. Photochem. Photobiol. Sci. 2025, 24, 191–214. [Google Scholar] [CrossRef]
- Polat, E.; Kang, K. Natural Photosensitizers in Antimicrobial Photodynamic Therapy. Biomedicines 2021, 9, 584. [Google Scholar] [CrossRef]
- Fiegler-Rudol, J.; Kapłon, K.; Kotucha, K.; Moś, M.; Skaba, D.; Kawczyk-Krupka, A.; Wiench, R. Hypocrellin-Mediated PDT: A Systematic Review of Its Efficacy, Applications, and Outcomes. Int. J. Mol. Sci. 2025, 26, 4038. [Google Scholar] [CrossRef]
- Youf, R.; Müller, M.; Balasini, A.; Thétiot, F.; Müller, M.; Hascoët, A.; Jonas, U.; Schönherr, H.; Lemercier, G.; Montier, T.; et al. Antimicrobial Photodynamic Therapy: Latest Developments with a Focus on Combinatory Strategies. Pharmaceutics 2021, 13, 1995. [Google Scholar] [CrossRef]
- Hussain, Y.; Alam, W.; Ullah, H.; Dacrema, M.; Daglia, M.; Khan, H.; Arciola, C.R. Antimicrobial Potential of Curcumin: Therapeutic Potential and Challenges to Clinical Applications. Antibiotics 2022, 11, 322. [Google Scholar] [CrossRef]
- Barua, N.; Buragohain, A.K. Therapeutic Potential of Curcumin as an Antimycobacterial Agent. Biomolecules 2021, 11, 1278. [Google Scholar] [CrossRef]
- Minhaco, V.M.T.R.; Maquera Huacho, P.M.; Mancim Imbriani, M.J.; Tonon, C.C.; Chorilli, M.; Rastelli, A.N.S.; Spolidorio, D.M.P. Improving antimicrobial activity against endodontic biofilm after exposure to blue light-activated novel curcumin nanoparticle. Photodiagnosis Photodyn. Ther. 2023, 42, 103322. [Google Scholar] [CrossRef]
- Di Luca, M.; Vittorio, O.; Cirillo, G.; Curcio, M.; Czuban, M.; Voli, F.; Farfalla, A.; Hampel, S.; Nicoletta, F.P.; Iemma, F. Electro-responsive graphene oxide hydrogels for skin bandages: The outcome of gelatin and trypsin immobilization. Int. J. Pharm. 2018, 546, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.Y.; Chang, K.C.; Chen, L.Y.; Hu, A. Low-dose blue light irradiation enhances the antimicrobial activities of curcumin against Propionibacterium acnes. J. Photochem. Photobiol. B 2018, 189, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Crugeira, P.J.L.; Almeida, H.H.S.; Teixeira, L.G.; Barreiro, M.F. Photodynamic inactivation of Staphylococcus aureus by ecological antibacterial solutions associating LED (ʎ 450 ± 10 nm) with curcumin and olive leaf extracts. J. Photochem. Photobiol. B 2023, 238, 112626. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, I.P.; Pinto, J.G.; Souza, B.M.N.; Miñán, A.G.; Ferreira-Strixino, J. Antimicrobial photodynamic therapy with curcumin on methicillin-resistant Staphylococcus aureus biofilm. Photodiagnosis Photodyn. Ther. 2022, 37, 102729. [Google Scholar] [CrossRef]
- Dias, L.D.; Aguiar, A.S.N.; De Melo, N.J.; Inada, N.M.; Borges, L.L.; De Aquino, G.L.B.; Camargo, A.J.; Bagnato, V.S.; Napolitano, H.B. Structural basis of antibacterial photodynamic action of curcumin against S. aureus. Photodiagnosis Photodyn. Ther. 2023, 43, 103654. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, Q.; Huang, Y.; Qi, M.; Yan, H.; Li, W.; Zhuang, H. Antibacterial Efficacy and Mechanisms of Curcumin-Based Photodynamic Treatment against Staphylococcus aureus and Its Application in Juices. Molecules 2022, 27, 7136. [Google Scholar] [CrossRef] [PubMed]
- De Annunzio, S.R.; De Freitas, L.M.; Blanco, A.L.; Da Costa, M.M.; Carmona-Vargas, C.C.; De Oliveira, K.T.; Fontana, C.R. Susceptibility of Enterococcus faecalis and Propionibacterium acnes to antimicrobial photodynamic therapy. J. Photochem. Photobiol. B 2018, 178, 545–550. [Google Scholar] [CrossRef]
- Tonon, C.C.; Paschoal, M.A.; Correia, M.; Spolidório, D.M.; Bagnato, V.S.; Giusti, J.S.; Santos-Pinto, L. Comparative Effects of Photodynamic Therapy mediated by Curcumin on Standard and Clinical Isolate of Streptococcus mutans. J. Contemp. Dent. Pract. 2015, 16, 1–6. [Google Scholar]
- Soares, J.M.; Yakovlev, V.V.; Blanco, K.C.; Bagnato, V.S. Photodynamic inactivation and its effects on the heterogeneity of bacterial resistance. Sci. Rep. 2024, 14, 28268, Erratum in Sci. Rep. 2024, 14, 30124. [Google Scholar] [CrossRef] [PubMed]
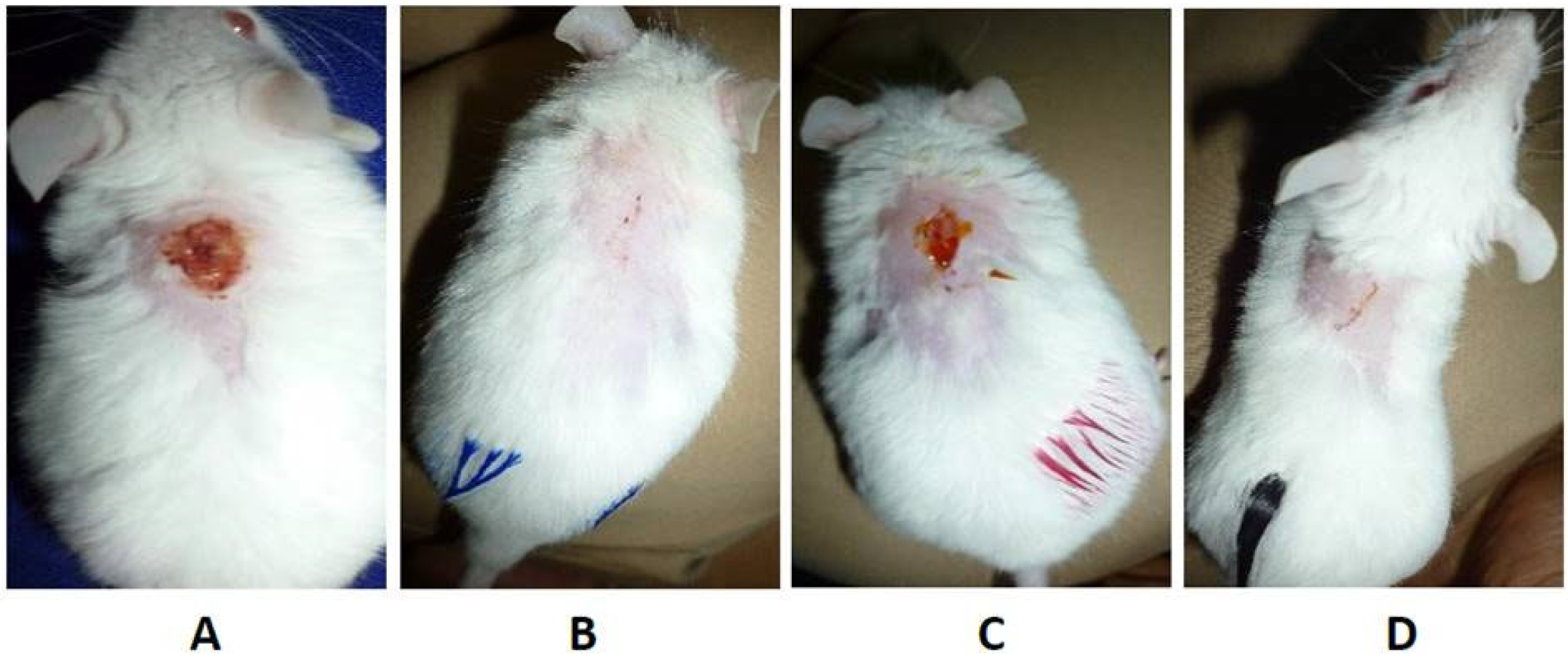
- Akhtar, F.; Khan, A.U.; Misba, L.; Akhtar, K.; Ali, A. Antimicrobial and antibiofilm photodynamic therapy against vancomycin resistant Staphylococcus aureus (VRSA) induced infection in vitro and in vivo. Eur. J. Pharm. Biopharm. 2021, 160, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.P.; Santos, M.S.; Rodrigues, P.L.F.; Araújo, T.S.D.; De Oliveira, J.M.; Rosa, L.P.; Bagnato, V.S.; Da Silva, F.C. Photodynamic therapy with curcumin in the reduction of Enterococcus faecalis biofilm in bone cavity: rMicrobiological and spectral fluorescence analysis. Photodiagnosis Photodyn. Ther. 2021, 33, 102084. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, R.; Li, P.; Fan, P.; Ma, L.; Cai, X.; Hou, Y.; Li, B.; Su, J. Natural borneol improves cellular uptake of curcumin to enhance its photodynamic bactericidal activity against Escherichia coli ATCC 8739. Food Microbiol. 2024, 127, 104686. [Google Scholar] [CrossRef]
- Abdulrahman, H.; Misba, L.; Ahmad, S.; Khan, A.U. Curcumin induced photodynamic therapy mediated suppression of quorum sensing pathway of Pseudomonas aeruginosa: An approach to inhibit biofilm in vitro. Photodiagnosis Photodyn. Ther. 2020, 30, 101645. [Google Scholar] [CrossRef]
- Pereira, J.A.; Polaquini, C.R.; Dos Santos, V.; Caiaffa, K.S.; Rabelo, R.L.; Theodoro, R.D.S.; Theodoro, L.H.; Regasini, L.O.; Duque, C. Antibiofilm and cytotoxic effect of 3,3′-dihydroxycurcumin (DHC) as photosensitizer agent in antimicrobial photodynamic therapy for endodontic purposes. Photodiagnosis Photodyn. Ther. 2021, 36, 102534. [Google Scholar] [CrossRef]
- Li, Z.; Shi, M.; Li, N.; Xu, R. Application of Functional Biocompatible Nanomaterials to Improve Curcumin Bioavailability. Front. Chem. 2020, 8, 589957. [Google Scholar] [CrossRef]
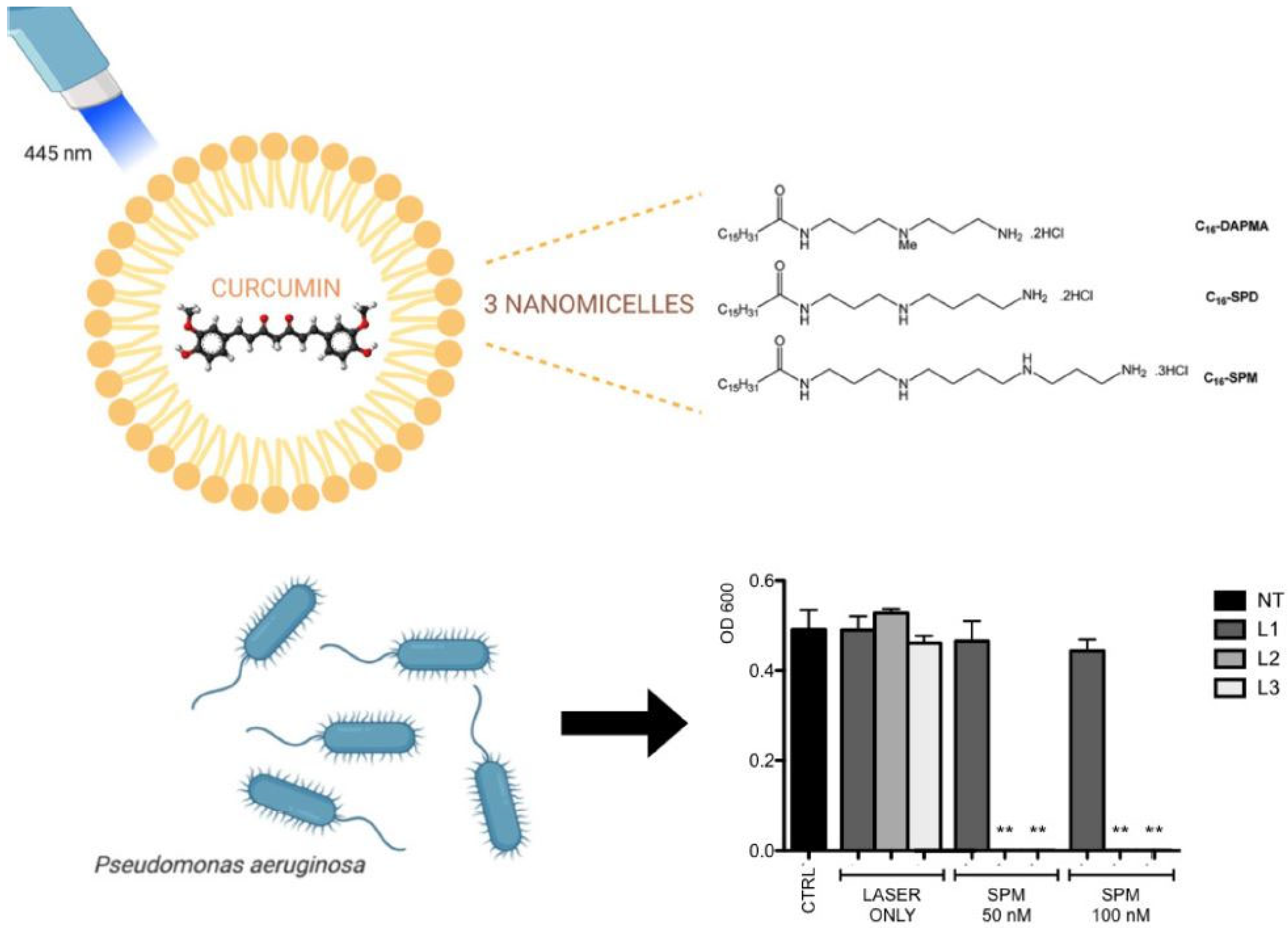
- Rupel, K.; Zupin, L.; Brich, S.; Mardirossian, M.; Ottaviani, G.; Gobbo, M.; Di Lenarda, R.; Pricl, S.; Crovella, S.; Zacchigna, S.; et al. Antimicrobial activity of amphiphilic nanomicelles loaded with curcumin against Pseudomonas aeruginosa alone and activated by blue laser light. J. Biophotonics 2021, 14, e202000350. [Google Scholar] [CrossRef]
- Dediu, V.; Ghitman, J.; Gradisteanu Pircalabioru, G.; Chan, K.H.; Iliescu, F.S.; Iliescu, C. Trends in Photothermal Nanostructures for Antimicrobial Applications. Int. J. Mol. Sci. 2023, 24, 9375. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, P.; Su, R.; Wen, F.; Jia, Z.; Lv, Y.; Cai, J.; Su, W. Curcumin-loaded multifunctional chitosan gold nanoparticles: An enhanced PDT/PTT dual-modal phototherapeutic and pH-responsive antimicrobial agent. Photodiagnosis Photodyn. Ther. 2022, 39, 103011. [Google Scholar] [CrossRef] [PubMed]
- Wen, F.; Li, P.; Meng, H.; Yan, H.; Huang, X.; Cui, H.; Su, W. Nitrogen-doped carbon dots/curcumin nanocomposite for combined Photodynamic/photothermal dual-mode antibacterial therapy. Photodiagnosis Photodyn. Ther. 2022, 39, 103033. [Google Scholar] [CrossRef]
- Su, W.; Zhang, Y.; Chen, Y.; Huo, L.; Wen, F.; Cai, J.; Gong, Q.; Li, P. Intelligent response agarose/chitosan hydrogel wound dressing: Synergistic chemotherapy, PDT and PTT effects against bacterial infection. Int. J. Biol. Macromol. 2025, 298, 139927. [Google Scholar] [CrossRef] [PubMed]
- Trigo-Gutierrez, J.K.; Vega-Chacón, Y.; Soares, A.B.; Mima, E.G.d.O. Antimicrobial Activity of Curcumin in Nanoformulations: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 7130. [Google Scholar] [CrossRef]
- Zheng, B.; McClements, D.J. Formulation of More Efficacious Curcumin Delivery Systems Using Colloid Science: Enhanced Solubility, Stability, and Bioavailability. Molecules 2020, 25, 2791. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Jin, J.; Pu, F.; Bai, Y.; Chen, Y.; Li, Y.; Wang, X. Cardioprotective effects of curcumin against myocardial I/R injury: A systematic review and meta-analysis of preclinical and clinical studies. Front. Pharmacol. 2023, 14, 1111459. [Google Scholar] [CrossRef]
- Ghorbanpour, S.; Pourhajibagher, M.; Noroozian, M.; Ghaffari, H.; Bahador, A. Photoactivation of Curcumin Doped Poly-Lactic-Co-Glycolic Acid Nanoparticles in Rat Model with Fixed Orthodontic Appliances. ScientificWorldJournal 2022, 2022, 1–11. [Google Scholar] [CrossRef] [PubMed]
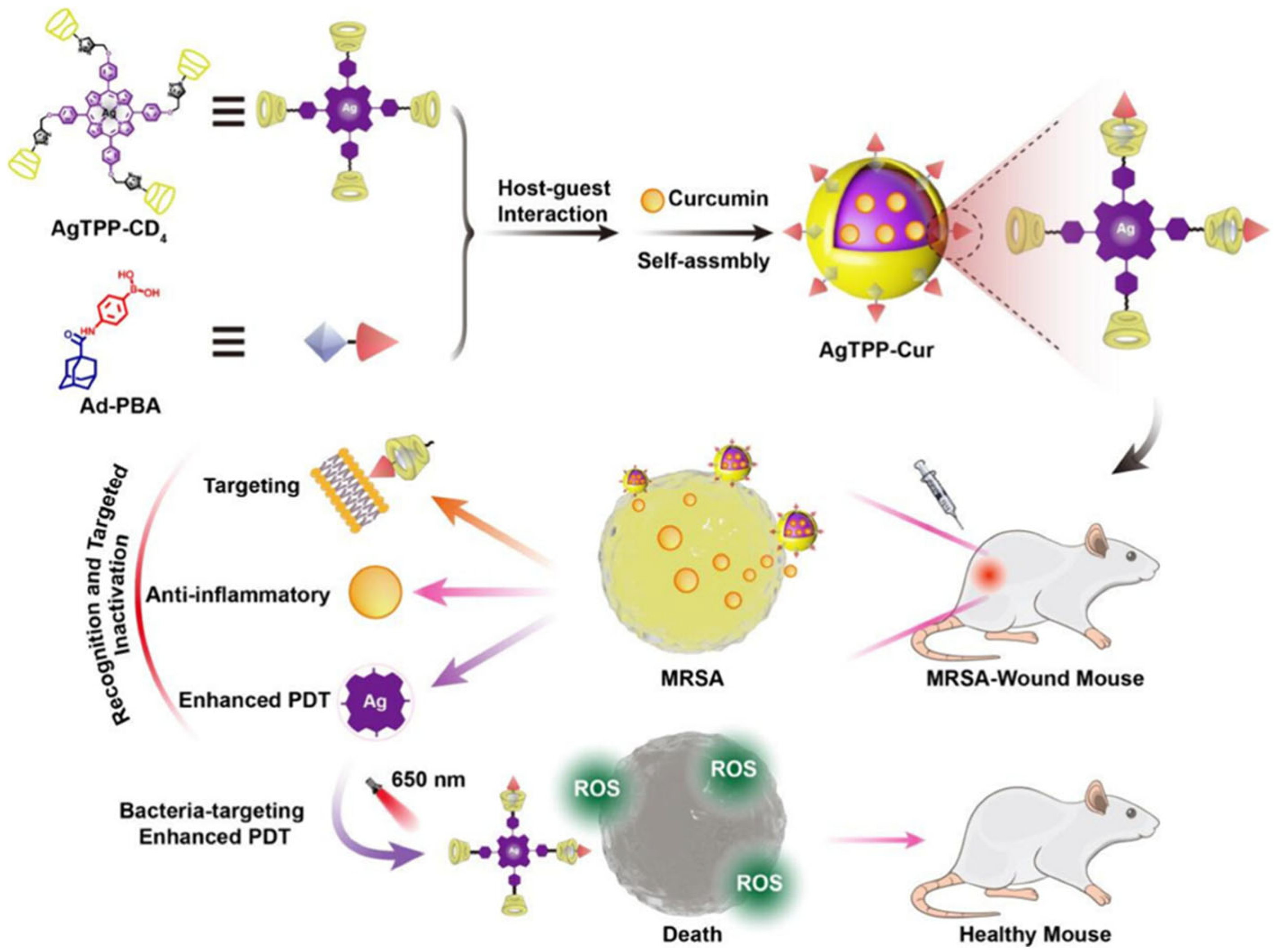
- Huang, W.; Wang, A.; Wang, W.; Lin, L.; Rong, J.; Tian, J.; Zhang, W. A Bacteria-Targeting Supramolecular Nanophotosensitizer for Combating Multidrug Resistant Bacteria. ACS Biomater. Sci. Eng. 2025, 11, 1741–1750. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Pourakbari, B.; Bahador, A. Contribution of antimicrobial photo-sonodynamic therapy in wound healing: An in vivo effect of curcumin-nisin-based poly (L-lactic acid) nanoparticle on Acinetobacter baumannii biofilms. BMC Microbiol. 2022, 22, 28. [Google Scholar] [CrossRef]
- Faghani-Eskandarkolaei, P.; Heli, H.; Akbari, N.; Koohi-Hosseinabadi, O.; Sari Aslani, F.; Sattarahmady, N. Antibacterial and anti-biofilm activities of gold-curcumin nanohybrids and its polydopamine form upon photo-sonotherapy of Staphylococcus aureus infected implants: In vitro and animal model studies. Int. J. Biol. Macromol. 2024, 282, 137430. [Google Scholar] [CrossRef] [PubMed]
- Siewert, B.; Stuppner, H. The photoactivity of natural products—An overlooked potential of phytomedicines? Phytomedicine 2019, 60, 152985. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, X.; Ran, X.; Gao, R.; Sun, J.; Zhuang, K.; You, Z.; Zhang, Z.; Ran, Y. Hypocrellin A-mediated photodynamic antibacterial activity against Cutibacterium acnes: An in vitro study. Photodiagnosis Photodyn. Ther. 2025, 51, 104467. [Google Scholar] [CrossRef]
- Xiao, Q.; Wu, J.; Pang, X.; Jiang, Y.; Wang, P.; Leung, A.W.; Gao, L.; Jiang, S.; Xu, C. Discovery and Development of Natural Products and their Derivatives as Photosensitizers for Photodynamic Therapy. Curr. Med. Chem. 2018, 25, 839–860. [Google Scholar] [CrossRef] [PubMed]
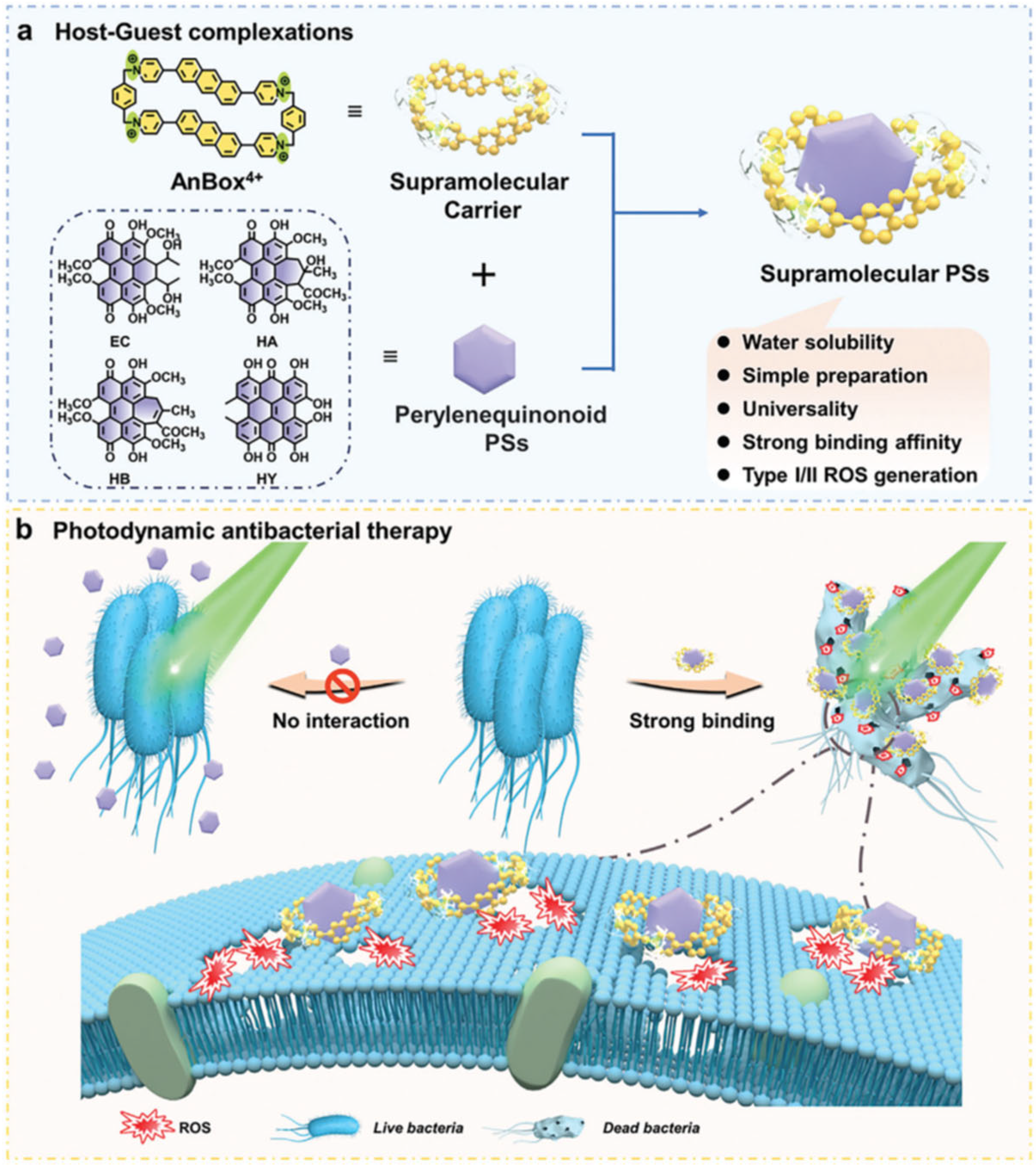
- Gao, Z.; Zheng, X.; Dong, X.; Liu, W.; Sha, J.; Bian, S.; Li, J.; Cong, H.; Lee, C.; Wang, P. A General Strategy for Enhanced Photodynamic Antimicrobial Therapy with Perylenequinonoid Photosensitizers Using a Macrocyclic Supramolecular Carrier. Adv. Healthc. Mater. 2024, 13, 2401778. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Zhang, J.; Xia, C.Y.; Ding, K.; Li, X.X.; Pan, X.G.; Xu, J.K.; He, J.; Zhang, W.K. Hypericin: A natural anthraquinone as promising therapeutic agent. Phytomedicine 2023, 111, 154654. [Google Scholar] [CrossRef] [PubMed]
- Sgarbossa, A.; Youssef, T.; Lenci, F. Photosensitized structural modifications of the lens protein a-crytsallin: Do all modifications impair chaperone-like activity? Photochem. Photobiol. 2003, 77, 567–571. [Google Scholar] [CrossRef]
- Souza Amaral, L.; Orzari Ribeiro, A.; Rodrigues Perussi, J. Evidence of hypericin photoinactivation of E. faecalis: From planktonic culture to mammalian cells selectivity up to biofilm disruption. Photodiagnosis Photodyn. Ther. 2020, 31, 101759. [Google Scholar] [CrossRef]
- Alam, S.T.; Le, T.A.N.; Park, J.S.; Kwon, H.C.; Kang, K. Antimicrobial Biophotonic Treatment of Ampicillin-Resistant Pseudomonas aeruginosa with Hypericin and Ampicillin Cotreatment Followed by Orange Light. Pharmaceutics 2019, 11, 641. [Google Scholar] [CrossRef]
- Barroso, R.A.; Navarro, R.; Tim, C.R.; De Paula Ramos, L.; De Oliveira, L.D.; Araki, Â.T.; Fernandes, K.G.C.; Macedo, D.; Assis, L. Antimicrobial photodynamic therapy against Propionibacterium acnes biofilms using hypericin (Hypericum perforatum) photosensitizer: In vitro study. Lasers Med. Sci. 2021, 36, 1235–1240. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Fekrazad, R.; Zhang, L.; Jiang, X.; He, G.; Wen, X. Polyphenolic natural products as photosensitizers for antimicrobial photodynamic therapy: Recent advances and future prospects. Front. Immunol. 2023, 14, 1275859. [Google Scholar] [CrossRef]
- Angelini, N.; Cubeddu, R.; Lenci, F.; Losi, A.; Pifferi, A.; Sgarbossa, A.; Taroni, P.; Vecli, A.; Viappiani, C. Artificial models of biological photoreceptors: Effect of quenchers on the fluorescence properties of hypericin embedded in liposomes. J. Photochem. Photobiol. B 1997, 38, 245–252. [Google Scholar] [CrossRef]
- Malacrida, A.M.; Dias, V.H.C.; Silva, A.F.; Dos Santos, A.R.; Cesar, G.B.; Bona, E.; Campanerut-Sá, P.A.Z.; Caetano, W.; Mikcha, J.M.G. Hypericin-mediated photoinactivation of polymeric nanoparticles against Staphylococcus aureus. Photodiagnosis Photodyn. Ther. 2020, 30, 101737. [Google Scholar] [CrossRef]
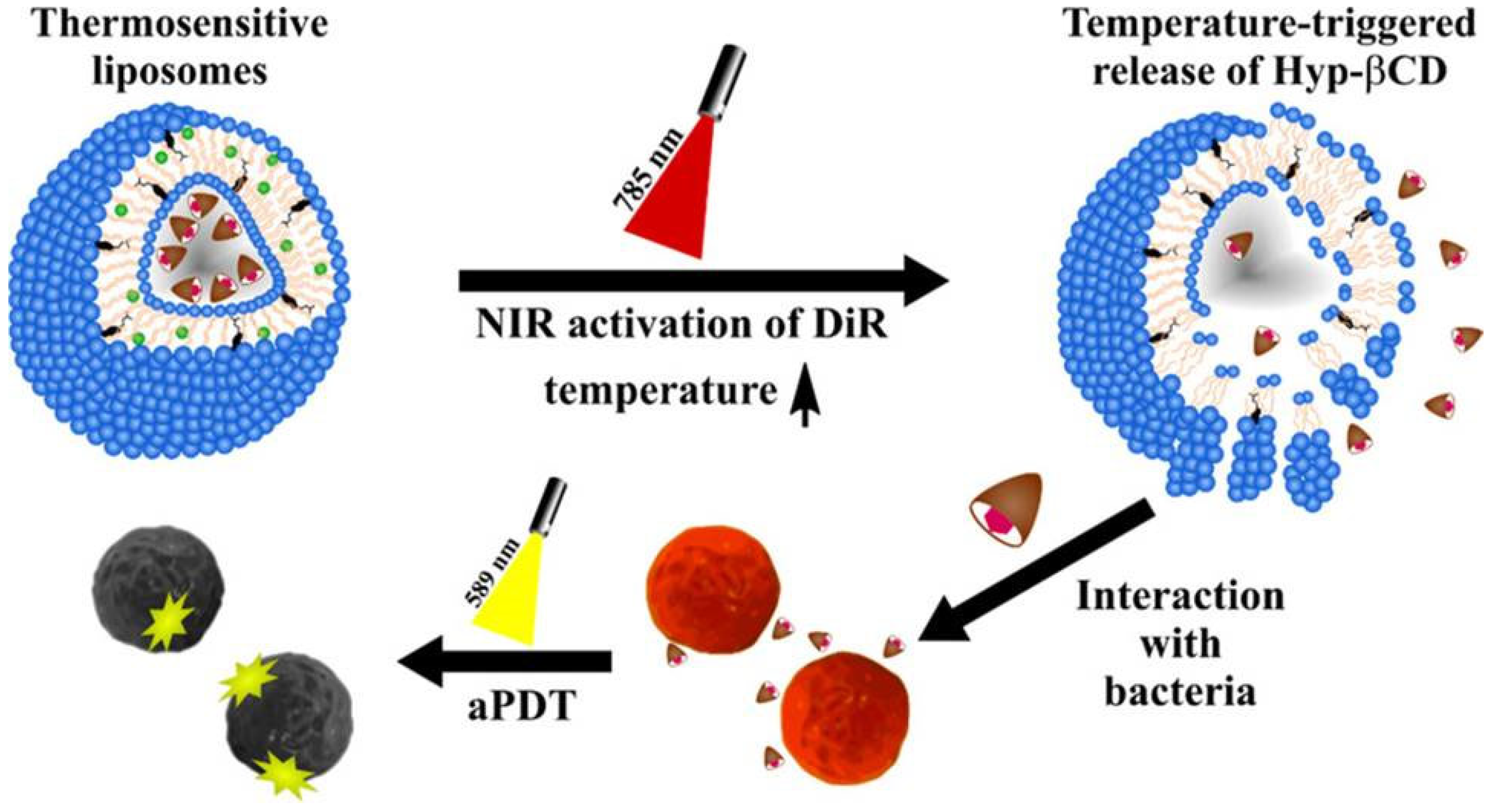
- Dayyih, A.A.; Gutberlet, B.; Preis, E.; Engelhardt, K.H.; Amin, M.U.; Abdelsalam, A.M.; Bonsu, M.; Bakowsky, U. Thermoresponsive Liposomes for Photo-Triggered Release of Hypericin Cyclodextrin Inclusion Complex for Efficient Antimicrobial Photodynamic Therapy. ACS Appl. Mater. Interfaces 2022, 14, 31525–31540. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Hosseini, N.; Bahador, A. Antimicrobial activity of D-amino acid in combination with photo-sonoactivated hypericin nanoparticles against Acinetobacter baumannii. BMC Microbiol. 2023, 23, 23. [Google Scholar] [CrossRef]
- Chen, S.; Huang, B.; Tian, J.; Zhang, W. Advancements of Porphyrin-Derived Nanomaterials for Antibacterial Photodynamic Therapy and Biofilm Eradication. Adv. Healthc. Mater. 2024, 13, 2401211. [Google Scholar] [CrossRef] [PubMed]
- Savelyeva, I.O.; Zhdanova, K.A.; Gradova, M.A.; Gradov, O.V.; Bragina, N.A. Cationic Porphyrins as Antimicrobial and Antiviral Agents in Photodynamic Therapy. Curr. Issues Mol. Biol. 2023, 45, 9793–9822. [Google Scholar] [CrossRef] [PubMed]
- Hadi, J.; Wu, S.; Soni, A.; Gardner, A.; Brightwell, G. Genetic Factors Affect the Survival and Behaviors of Selected Bacteria during Antimicrobial Blue Light Treatment. Int. J. Mol. Sci. 2021, 22, 10452. [Google Scholar] [CrossRef] [PubMed]
- Battisti, A.; Morici, P.; Ghetti, F.; Sgarbossa, A. Spectroscopic characterization and fluorescence imaging of Helicobacter pylori endogenous porphyrins. Biophys. Chem. 2017, 229, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Leanse, L.G.; Goh, X.S.; Cheng, J.X.; Hooper, D.C.; Dai, T. Dual-wavelength photo-killing of methicillin-resistant Staphylococcus aureus. JCI Insight 2020, 5, e134343. [Google Scholar] [CrossRef]
- Woźniak, A.; Grinholc, M. Combined Antimicrobial Blue Light and Antibiotics as a Tool for Eradication of Multidrug-Resistant Isolates of Pseudomonas aeruginosa and Staphylococcus aureus: In Vitro and In Vivo Studies. Antioxidants 2022, 11, 1660. [Google Scholar] [CrossRef]
- Astuti, S.D.; Ardyana, Y.; Arifianto, D.; Winarno; Puspita, P.S.; Yuwana, Y.G.Y.; Pradhana, A.A.S. Antimicrobial Photodynamic Effectiveness of Light Emitting Diode (Led) For Inactivation on Staphylococcus aureus Bacteria and Wound Healing in Infectious Wound Mice. J. Phys. Conf. Ser. 2020, 1505, 012060. [Google Scholar] [CrossRef]
- Shashin, D.M.; Demina, G.R.; Linge, I.A.; Vostroknutova, G.N.; Kaprelyants, A.S.; Savitsky, A.P.; Shleeva, M.O. The Effect of Antimicrobial Photodynamic Inactivation on the Protein Profile of Dormant Mycolicibacterium smegmatis Containing Endogenous Porphyrins. Int. J. Mol. Sci. 2023, 24, 13968. [Google Scholar] [CrossRef]
- Wang, Y.; Ferrer-Espada, R.; Baglo, Y.; Gu, Y.; Dai, T. Antimicrobial Blue Light Inactivation of Neisseria gonorrhoeae: Roles of Wavelength, Endogenous Photosensitizer, Oxygen, and Reactive Oxygen Species. Lasers Surg. Med. 2019, 51, 815–823. [Google Scholar] [CrossRef]
- Fila, G.; Krychowiak, M.; Rychlowski, M.; Bielawski, K.P.; Grinholc, M. Antimicrobial blue light photoinactivation of Pseudomonas aeruginosa: Quorum sensing signaling molecules, biofilm formation and pathogenicity. J. Biophotonics 2018, 11, e201800079. [Google Scholar] [CrossRef]
- Morici, P.; Battisti, A.; Tortora, G.; Menciassi, A.; Checcucci, G.; Ghetti, F.; Sgarbossa, A. The in vitro Photoinactivation of Helicobacter pylori by a Novel LED-Based Device. Front. Microbiol. 2020, 11, 283. [Google Scholar] [CrossRef]
- Salviatto, L.T.C.; Prates, R.A.; Pavani, C.; Bussadori, S.K.; Deana, A.M. The influence of growth medium on the photodynamic susceptibility of Aggregatibacter actinomycetemcomitans to antimicrobial blue light. Lasers Med. Sci. 2023, 38, 274. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, Y.; Zong, Y.; Dong, F.; Zhang, L.; Wang, G.; Dong, H.; Wang, Y. Response of genes related to iron and porphyrin transport in Porphyromonas gingivalis to blue light. J. Photochem. Photobiol. B 2023, 241, 112670. [Google Scholar] [CrossRef]
- Amin, R.M.; Bhayana, B.; Hamblin, M.R.; Dai, T. Antimicrobial blue light inactivation of Pseudomonas aeruginosa by photo-excitation of endogenous porphyrins: In vitro and in vivo studies. Lasers Surg. Med. 2016, 48, 562–568. [Google Scholar] [CrossRef]
- Li, Y.; Wu, M.X. Visualization and elimination of polymicrobial biofilms by a combination of ALA-carvacrol-blue light. J. Photochem. Photobiol. B 2022, 234, 112525. [Google Scholar] [CrossRef]
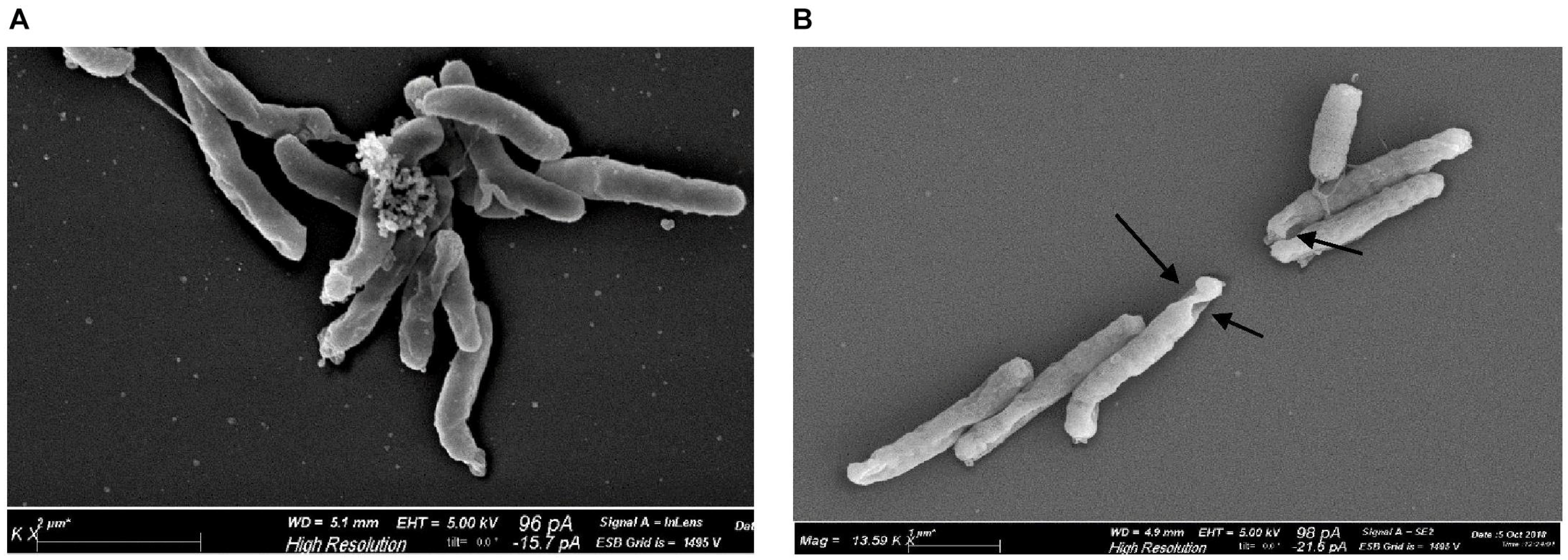
- Walter, A.B.; Simpson, J.; Jenkins, J.L.; Skaar, E.P.; Jansen, E.D. Optimization of optical parameters for improved photodynamic therapy of Staphylococcus aureus using endogenous coproporphyrin III. Photodiagnosis Photodyn. Ther. 2020, 29, 101624. [Google Scholar] [CrossRef]
- Alves, G.B.; Calderari, M.R.D.C.M.; Fonseca, E.N.D.; Sant’anna, L.O.; Santos, L.S.D.; Mattos-Guaraldi, A.L. Photodynamic Inactivation Mediated by Endogenous Porphyrins of Corynebacterium diphtheriae in Planktonic and Biofilm Forms. ACS Omega 2025, 10, 9177–9186. [Google Scholar] [CrossRef]
- Wang, X.; Wan, M.; Zhang, L.; Dai, Y.; Hai, Y.; Yue, C.; Xu, J.; Ding, Y.; Wang, M.; Xie, J.; et al. ALA_PDT Promotes Ferroptosis-Like Death of Mycobacterium abscessus and Antibiotic Sterilization via Oxidative Stress. Antioxidants 2022, 11, 546. [Google Scholar] [CrossRef]
- Sahu, K.; Krishna, H.; Shrivastava, R.; Majumdar, A.; Chowdhury, A.; Chakraborty, S.; Majumder, S.K. Evaluation of the potential of Delta-aminolevulinic acid for simultaneous detection of bioburden and anti-microbial photodynamic therapy of MRSA infected wounds in Swiss albino mice. J. Photochem. Photobiol. B 2024, 254, 112892. [Google Scholar] [CrossRef]
- Bumah, V.V.; Morrow, B.N.; Cortez, P.M.; Bowman, C.R.; Rojas, P.; Masson-Meyers, D.S.; Suprapto, J.; Tong, W.G.; Enwemeka, C.S. The importance of porphyrins in blue light suppression of Streptococcus agalactiae. J. Photochem. Photobiol. B 2020, 212, 111996. [Google Scholar] [CrossRef]
- Anane, Y.A.; Apalata, T.; Vasaikar, S.; Okuthe, G.E.; Songca, S.P. In vitro antimicrobial photodynamic inactivation of multidrug-resistant Acinetobacter baumannii biofilm using Protoporphyrin IX and Methylene blue. Photodiagnosis Photodyn. Ther. 2020, 30, 101752. [Google Scholar] [CrossRef]
- Akbar, A.; Khan, S.; Chatterjee, T.; Ghosh, M. Unleashing the power of porphyrin photosensitizers: Illuminating breakthroughs in photodynamic therapy. J. Photochem. Photobiol. B 2023, 248, 112796. [Google Scholar] [CrossRef]
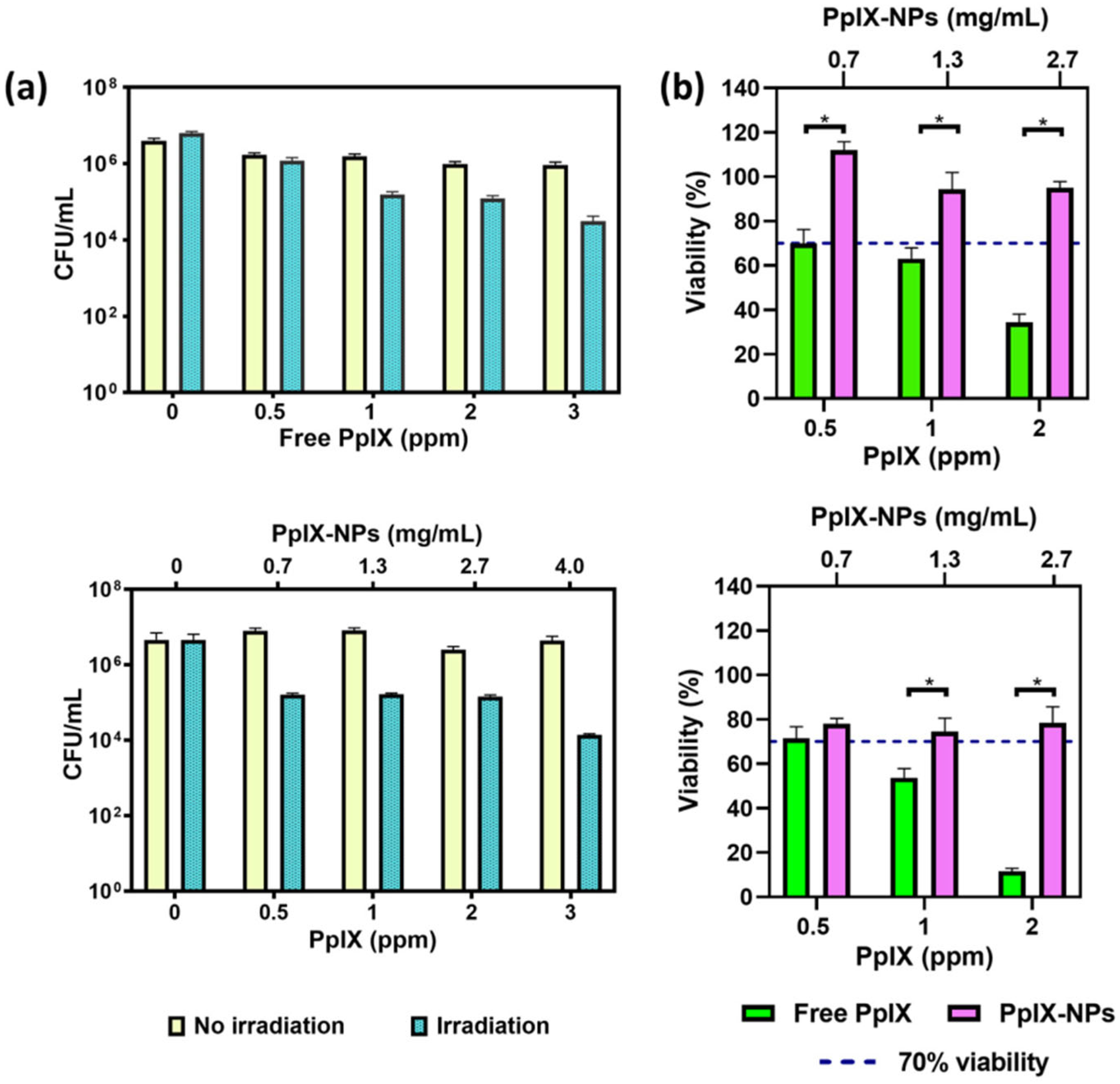
- Izquierdo, N.; Gamez, E.; Alejo, T.; Mendoza, G.; Arruebo, M. Antimicrobial Photodynamic Therapy Using Encapsulated Protoporphyrin IX for the Treatment of Bacterial Pathogens. Materials 2024, 17, 1717. [Google Scholar] [CrossRef]
- Buzzá, H.H.; Alves, F.; Tomé, A.J.B.; Chen, J.; Kassab, G.; Bu, J.; Bagnato, V.S.; Zheng, G.; Kurachi, C. Porphyrin nanoemulsion for antimicrobial photodynamic therapy: Effective delivery to inactivate biofilm-related infections. Proc. Natl. Acad. Sci. USA 2022, 119, e2216239119. [Google Scholar] [CrossRef]
- Kumar, M.; Jukanti, A.; Cahan, R.; Nause, A.; Minnes, R. Second harmonic generation-mediated Photodynamic Therapy for Staphylococcus aureus: A novel approach using Bismuth Ferrite-Protoporphyrin IX conjugates. Photodiagnosis Photodyn. Ther. 2025, 52, 104512. [Google Scholar] [CrossRef]
- AbouAitah, K.; Geioushy, R.A.; Nour, S.A.; Emam, M.T.H.; Zakaria, M.A.; Fouad, O.A.; Shaker, Y.M.; Kim, B.S. A Combined Phyto- and Photodynamic Delivery Nanoplatform Enhances Antimicrobial Therapy: Design, Preparation, In Vitro Evaluation, and Molecular Docking. ACS Appl. Bio Mater. 2024, 7, 6873–6889. [Google Scholar] [CrossRef]
- Glowacka-Sobotta, A.; Czarczynska-Goslinska, B.; Ziental, D.; Wysocki, M.; Michalak, M.; Güzel, E.; Sobotta, L. Versatile Porphyrin Arrangements for Photodynamic Therapy—A Review. Nanomaterials 2024, 14, 1879. [Google Scholar] [CrossRef]
- Cardoso, D.R.; Libardi, S.H.; Skibsted, L.H. Riboflavin as a photosensitizer. Effects on human health and food quality. Food Funct. 2012, 3, 487–502. [Google Scholar] [CrossRef]
- El-Gendy, A.O.; Ezzat, S.; Samad, F.A.; Dabbous, O.A.; Dahm, J.; Hamblin, M.R.; Mohamed, T. Studying the viability and growth kinetics of vancomycin-resistant Enterococcus faecalis V583 following femtosecond laser irradiation (420–465 nm). Lasers Med. Sci. 2024, 39, 144. [Google Scholar] [CrossRef]
- Hintmann, M.; Zimbelmann, S.; Emde, B.; Biedendieck, R.; Jahn, D. Antibiotic Effect of High-Power Blue Laser Radiation. Photonics 2024, 11, 220. [Google Scholar] [CrossRef]
- Hashem, M. Antimicrobial capacity and physico-chemical characteristics of adhesive resin containing riboflavin after photodynamic therapy. Photodiagnosis Photodyn. Ther. 2021, 33, 102145, Erratum in Sci. Rep. 2022, 40, 103165. [Google Scholar] [CrossRef]
- Afrasiabi, S.; Al Gburi, A.Q.K.; Ranjbar Omrani, L.; Chiniforush, N.; Moradi, Z. Evaluation of riboflavin, nanocurcumin, and hydrogen peroxide under light conditions: Reduction of mature dental biofilms and enamel mineral loss. Photodiagnosis Photodyn. Ther. 2024, 50, 104379. [Google Scholar] [CrossRef]
- Afrasiabi, S.; Entezari, S.; Etemadi, A.; Chiniforush, N. The influence of different mode of power density during antimicrobial photodynamic therapy for photokilling of Streptococcus mutans. Photodiagnosis Photodyn. Ther. 2023, 44, 103770. [Google Scholar] [CrossRef]
- Pordel, E.; Ghasemi, T.; Afrasiabi, S.; Benedicenti, S.; Signore, A.; Chiniforush, N. The Effect of Different Output Powers of Blue Diode Laser along with Curcumin and Riboflavin against Streptococcus mutans around Orthodontic Brackets: An In Vitro Study. Biomedicines 2023, 11, 2248. [Google Scholar] [CrossRef]
- Kamran, M.A.; Qasim, M.; Udeabor, S.E.; Hameed, M.S.; Mannakandath, M.L.; Alshahrani, I. Impact of riboflavin mediated photodynamic disinfection around fixed orthodontic system infected with oral bacteria. Photodiagnosis Photodyn. Ther. 2021, 34, 102232. [Google Scholar] [CrossRef]
- Shahi Ardakani, A.; Benedicenti, B.; Solimei, L.; Shahabi, S.; Afrasiabi, S. Reduction of Multispecies Biofilms on an Acrylic Denture Base Model by Antimicrobial Photodynamic Therapy Mediated by Natural Photosensitizers. Pharmaceuticals 2024, 17, 1232. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Ghafari, H.A.; Bahador, A. Postbiotic mediators derived from Lactobacillus species enhance riboflavin-mediated antimicrobial photodynamic therapy for eradication of Streptococcus mutans planktonic and biofilm growth. BMC Oral. Health 2024, 24, 836. [Google Scholar] [CrossRef]
- Tkaczyk, M.; Mertas, A.; Kuśka-Kiełbratowska, A.; Fiegler-Rudol, J.; Bobela, E.; Cisowska, M.; Morawiec, T.; Skaba, D.; Wiench, R. In Vitro Evaluation of Candida spp. and Staphylococcus aureus Sensitivity to 450 nm Diode Laser-Mediated Antimicrobial Photodynamic Therapy with Curcumin and Riboflavin. Int. J. Mol. Sci. 2025, 26, 5645. [Google Scholar] [CrossRef]
- Kim, M.J.; Lianto, D.K.; Koo, G.H.; Yuk, H.G. Antibacterial mechanism of riboflavin-mediated 460 nm light emitting diode illumination against Listeria monocytogenes in phosphate-buffered saline and on smoked salmon. Food Control 2021, 124, 107930. [Google Scholar] [CrossRef]
- Buchovec, I.; Klimkaitė, L.; Sužiedėlienė, E.; Bagdonas, S. Inactivation of Opportunistic Pathogens Acinetobacter baumannii and Stenotrophomonas maltophilia by Antimicrobial Photodynamic Therapy. Microorganisms 2022, 10, 506. [Google Scholar] [CrossRef]
- Zhu, L.; Li, C.; Wang, D. Photodynamic inactivation of antibiotic-resistant bacteria in whole blood using riboflavin photodynamic method. Front. Microbiol. 2024, 15, 1404468. [Google Scholar] [CrossRef]
- Buchovec, I.; Vyčaitė, E.; Badokas, K.; Sužiedelienė, E.; Bagdonas, S. Application of Antimicrobial Photodynamic Therapy for Inactivation of Acinetobacter baumannii Biofilms. Int. J. Mol. Sci. 2022, 24, 722. [Google Scholar] [CrossRef] [PubMed]
- Afrasiabi, S.; Chiniforush, N. Antibacterial potential of riboflavin mediated blue diode laser photodynamic inactivation against Enterococcus faecalis: A laboratory investigation. Photodiagnosis Photodyn. Ther. 2023, 41, 103291. [Google Scholar] [CrossRef]
- Moradi, M.; Fazlyab, M.; Pourhajibagher, M.; Chiniforush, N. Antimicrobial action of photodynamic therapy on Enterococcus faecalis biofilm using curing light, curcumin and riboflavin. Aust. Endod. J. 2022, 48, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Mills, B.; Kiang, A.; Mohanan, S.M.P.C.; Bradley, M.; Klausen, M. Riboflavin-Vancomycin Conjugate Enables Simultaneous Antibiotic Photo-Release and Photodynamic Killing against Resistant Gram-Positive Pathogens. JACS Au 2023, 3, 3014–3023. [Google Scholar] [CrossRef]
- Najari, E.; Zamani, S.; Sheikh Arabi, M.; Ardebili, A. Antimicrobial photodynamic effect of the photosensitizer riboflavin, alone and in combination with colistin, against pandrug-resistant Pseudomonas aeruginosa clinical isolates. J. Infect. Chemother. 2024, 30, 892–898. [Google Scholar] [CrossRef]
- Rivas Aiello, M.B.; Ghilini, F.; Martínez Porcel, J.E.; Giovanetti, L.; Schilardi, P.L.; Mártire, D.O. Riboflavin-Mediated Photooxidation of Gold Nanoparticles and Its Effect on the Inactivation of Bacteria. Langmuir 2020, 36, 8272–8281. [Google Scholar] [CrossRef]
- Marković, Z.M.; Kováčová, M.; Jeremić, S.R.; Nagy, Š.; Milivojević, D.D.; Kubat, P.; Kleinová, A.; Budimir, M.D.; Mojsin, M.M.; Stevanović, M.J.; et al. Highly Efficient Antibacterial Polymer Composites Based on Hydrophobic Riboflavin Carbon Polymerized Dots. Nanomaterials 2022, 12, 4070. [Google Scholar] [CrossRef]
- Soleimani, F.; Karimi, A.R.; Hadizadeh, M. Multifunctional riboflavin/chitosan-based dianhydride crosslinked hydrogels: Photodynamic therapy, antioxidant, and antibacterial properties. Next Res. 2025, 2, 100139. [Google Scholar] [CrossRef]
- Almoammar, S.; Alnazeh, A.A.; Kamran, M.A.; Al Jearah, M.M.; Qasim, M.; Abdulla, A.M. Photoactivated riboflavin-doped hydroxy apatite nanospheres infiltered in orthodontic adhesives. Microsc. Res. Tech. 2025, 88, 213–223. [Google Scholar] [CrossRef]
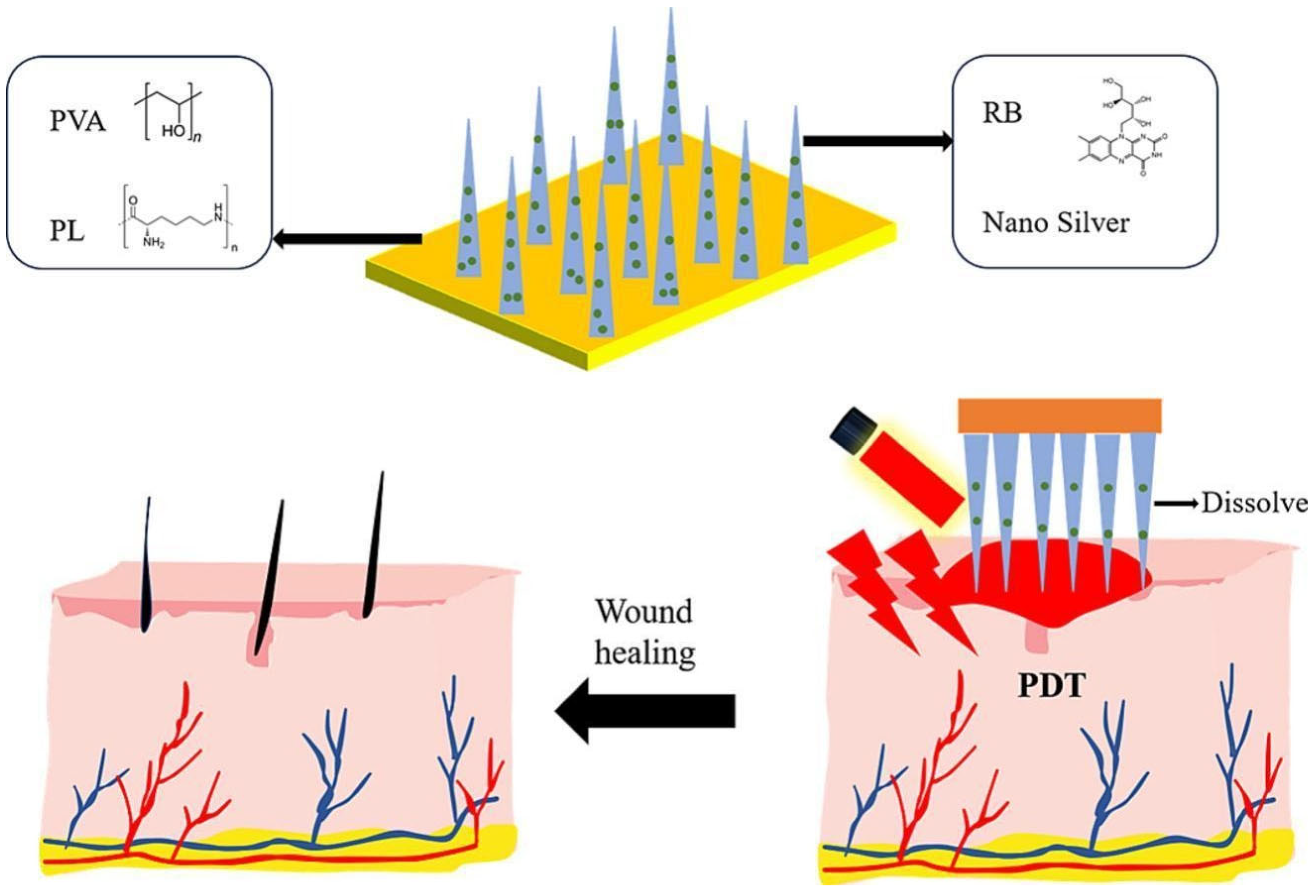
- Zong, Q.; Zhou, R.; Zhao, Z.; Sheng, C.; Li, W.; Li, A.; Liu, Z.; Li, X.; Zheng, Y.; Ning, Y.; et al. Riboflavin-dissolving microneedles for the elimination of photodynamic bacterial biofilms. Int. J. Pharm. 2025, 681, 125858. [Google Scholar] [CrossRef]
- Clardy, J.; Walsh, C. Lessons from natural molecules. Nature 2004, 432, 829–837. [Google Scholar] [CrossRef]

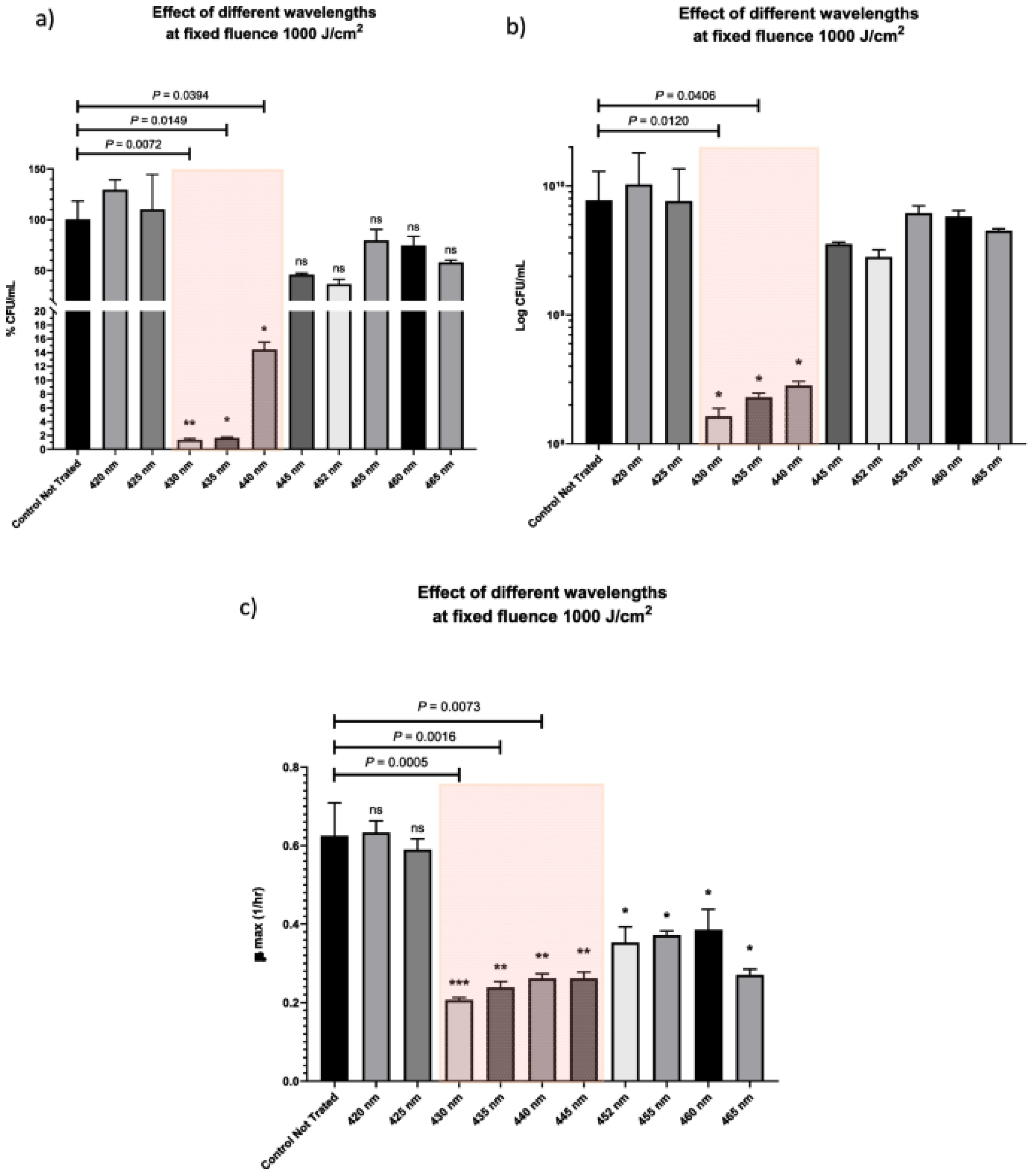
| PS | Absorption Bands (nm) | 3O2 Quantum Yield (ΦΔ) | Solubility (in H2O) | Photostability | PDT Mechanism | Antimicrobial Mechanisms | Clinical Applications | Refs |
|---|---|---|---|---|---|---|---|---|
| Curcumin | 420–430 nm | ~0.36–0.50 | Poor | Low (rapid degradation, light-sensitive) | Mainly Type I | Membrane and protein oxidation | Wound healing, oral infections, and dental caries | [17] |
| Hypocrellin A | 465–470 nm | ~0.85 | Moderate | Moderate (sensitive to light and oxygen) | Type I and II | Membrane disruption and mitochondrial dysfunction | Skin infections | [18] |
| Hypericin | 545–590 nm (visible), 590–610 nm (red edge) | ~0.40–0.60 | Very poor | High | Mainly Type II | Induces apoptosis via ROS only against gram positive bacteria and fungi. | Skin infections, biofilm-associated infections | [17,19] |
| Porphyrin | 410–420 (Soret), 650–670 (Q-bands) | ~0.65–0.80 | Good to moderate | High | Mainly Type II | Membrane and DNA disruption and efflux pumps inactivation | Periodontic diseases, antibiotic-resistant infection | [19] |
| Riboflavin | 440–460 nm | ~0.55–0.60 | Excellent | Moderate (photo-sensitive) | Type I and II | Biofilm disruption and the EPS matrix were damaged | Dental infections, sepsis models, and implant coatings | [16] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amendola, G.; Di Luca, M.; Sgarbossa, A. Natural Biomolecules and Light: Antimicrobial Photodynamic Strategies in the Fight Against Antibiotic Resistance. Int. J. Mol. Sci. 2025, 26, 7993. https://doi.org/10.3390/ijms26167993
Amendola G, Di Luca M, Sgarbossa A. Natural Biomolecules and Light: Antimicrobial Photodynamic Strategies in the Fight Against Antibiotic Resistance. International Journal of Molecular Sciences. 2025; 26(16):7993. https://doi.org/10.3390/ijms26167993
Chicago/Turabian StyleAmendola, Greta, Mariagrazia Di Luca, and Antonella Sgarbossa. 2025. "Natural Biomolecules and Light: Antimicrobial Photodynamic Strategies in the Fight Against Antibiotic Resistance" International Journal of Molecular Sciences 26, no. 16: 7993. https://doi.org/10.3390/ijms26167993
APA StyleAmendola, G., Di Luca, M., & Sgarbossa, A. (2025). Natural Biomolecules and Light: Antimicrobial Photodynamic Strategies in the Fight Against Antibiotic Resistance. International Journal of Molecular Sciences, 26(16), 7993. https://doi.org/10.3390/ijms26167993







