Cathepsins in Neurological Diseases
Abstract
1. Introduction
2. Materials and Methods
3. Cathepsins in Neuroinflammation and Aging
4. Cathepsins and Parkinson’s Disease
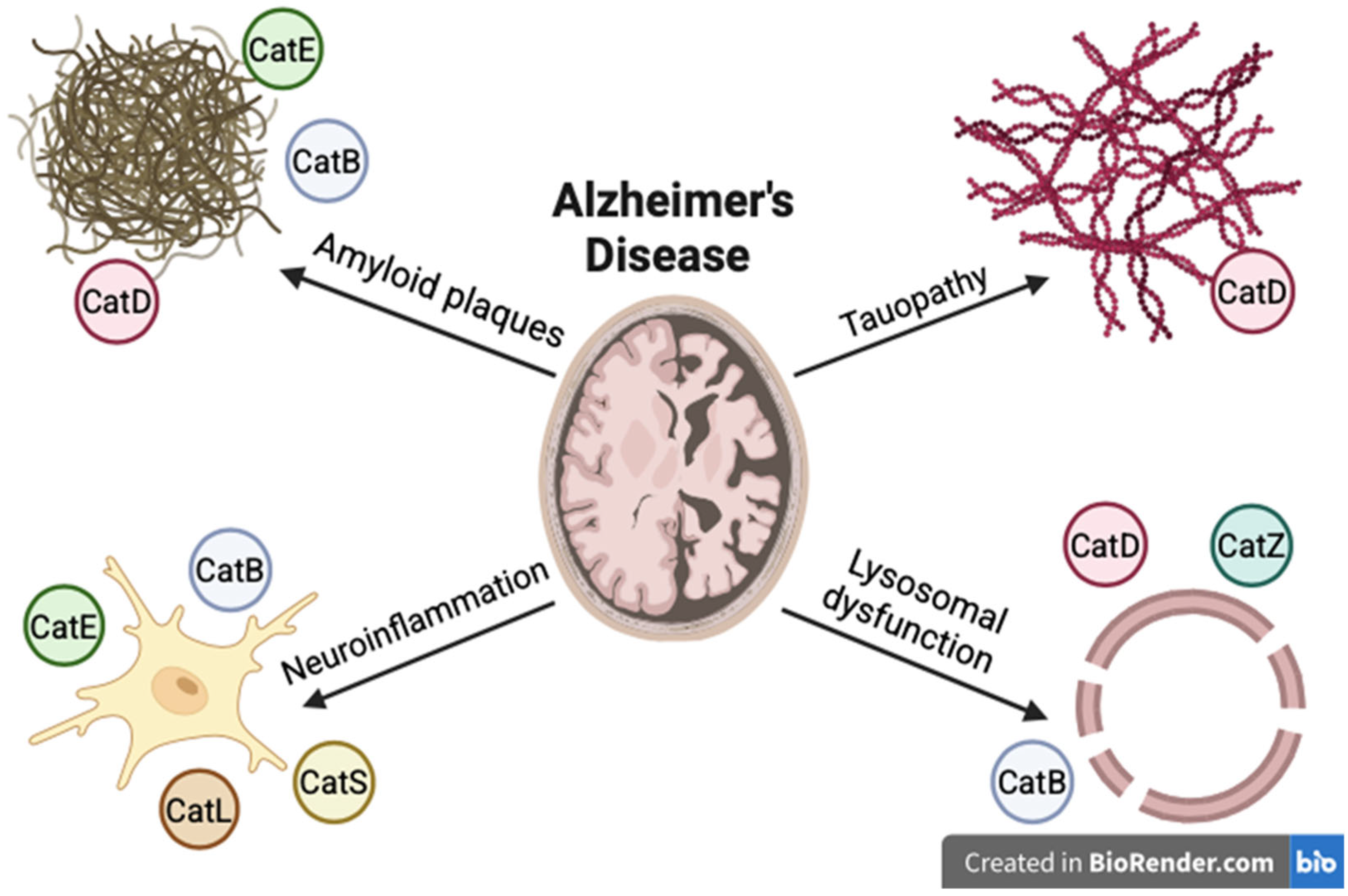
5. Cathepsins and Alzheimer’s Disease
6. Cathepsins and Stroke
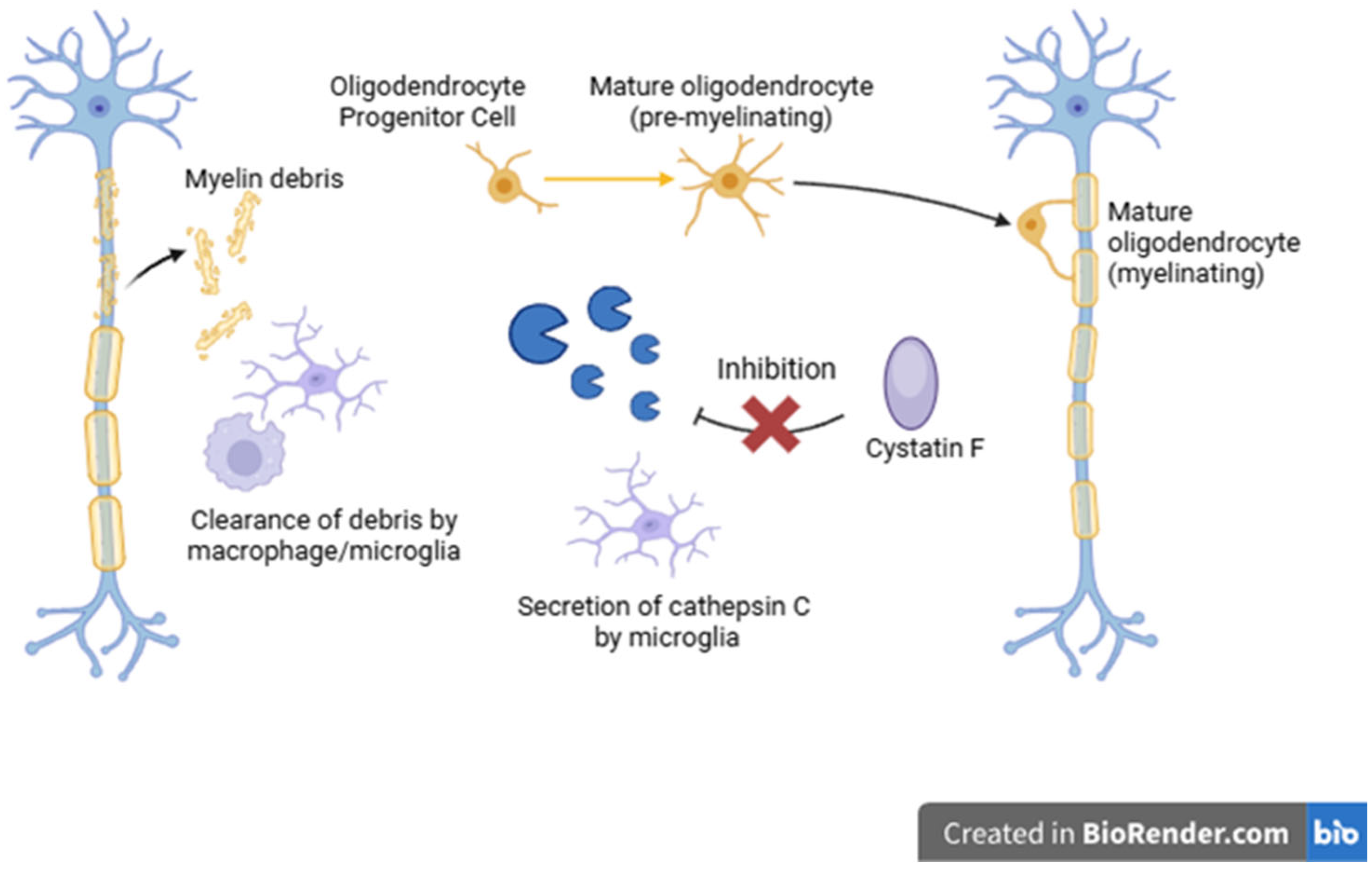
7. Cathepsins and Multiple Sclerosis
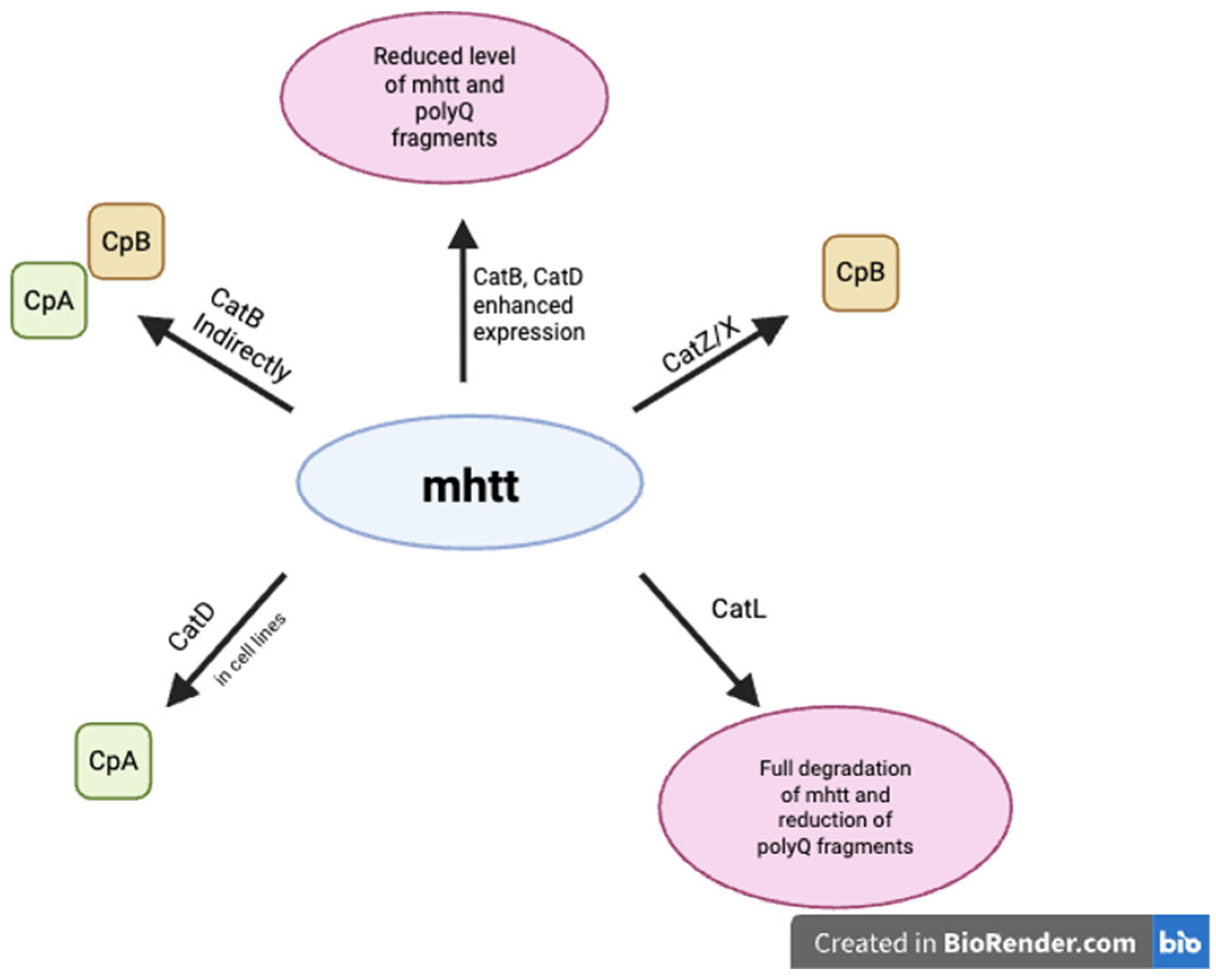
8. Cathepsins and Huntington’s Disease
9. Cathepsins and Epilepsy
10. Cathepsins and Amyotrophic Lateral Sclerosis
11. Clinical Use of Cathepsins
12. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brown, G.C.; Vilalta, A. How microglia kill neurons. Brain Res. 2015, 1628 Pt B, 288–297. [Google Scholar] [CrossRef]
- Glezer, I.; Simard, A.R.; Rivest, S. Neuroprotective role of the innate immune system by microglia. Neuroscience 2007, 147, 867–883. [Google Scholar] [CrossRef]
- Turk, V.; Stoka, V.; Vasiljeva, O.; Renko, M.; Sun, T.; Turk, B.; Turk, D. Cysteine cathepsins: From structure, function and regulation to new frontiers. Biochim. Biophys. Acta 2012, 1824, 68–88. [Google Scholar] [CrossRef] [PubMed]
- Turk, B. Targeting proteases: Successes, failures and future prospects. Nat. Rev. Drug Discov. 2006, 5, 785–799. [Google Scholar] [CrossRef]
- Qiao, L.; Hamamichi, S.; Caldwell, K.A.; Caldwell, G.A.; Yacoubian, T.A.; Wilson, S.; Xie, Z.-L.; Speake, L.D.; Parks, R.; Crabtree, D.; et al. Lysosomal enzyme cathepsin D protects against alpha-synuclein aggregation and toxicity. Mol. Brain 2008, 1, 17. [Google Scholar] [CrossRef]
- Yelamanchili, S.V.; Chaudhuri, A.D.; Flynn, C.T.; Fox, H.S. Upregulation of cathepsin D in the caudate nucleus of primates with experimental parkinsonism. Mol. Neurodegener. 2011, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Li, D.; Zhang, Y.; Liu, J.; Wang, S.; Wang, Z.; Huang, T.; Wang, Y.; Zhang, H.; Chen, G.; et al. The induction of neuronal death by up-regulated microglial cathepsin H in LPS-induced neuroinflammation. J. Neuroinflamm. 2015, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- Felbor, U.; Kessler, B.; Mothes, W.; Goebel, H.H.; Andersson, C.; Hübner, C.A. Neuronal loss and brain atrophy in mice lacking cathepsins B and L. Proc. Natl. Acad. Sci. USA 2002, 99, 7883–7888. [Google Scholar] [CrossRef]
- Cermak, S.; Kosicek, M.; Mladenovic-Djordjevic, A.; Smiljanic, K.; Kanazir, S.; Hecimovic, S. Loss of Cathepsin B and L leads to lysosomal dysfunction, NPC-like cholesterol sequestration and accumulation of the key Alzheimer’s proteins. PLoS ONE 2016, 11, e0167428. [Google Scholar] [CrossRef]
- Bednarski, E.; Lynch, G. Cytosolic proteolysis of tau by cathepsin D in hippocampus following suppression of cathepsins B and L. J. Neurochem. 1996, 67, 1846–1855. [Google Scholar] [CrossRef]
- Pišlar, A.; Božić, B.; Zidar, N.; Kos, J. Inhibition of cathepsin X reduces the strength of microglial-mediated neuroinflammation. Neuropharmacology 2017, 114, 88–100. [Google Scholar] [CrossRef]
- Ritzel, R.M.; Doran, S.J.; Glaser, E.P.; Meadows, V.E.; Faden, A.I.; Stoica, B.A.; Loane, D.J. Old age increases microglial senescence, exacerbates secondary neuroinflammation, and worsens neurological outcomes after acute traumatic brain injury in mice. Neurobiol. Aging 2019, 77, 194–206. [Google Scholar] [CrossRef]
- Fan, K.; Wu, X.; Fan, B.; Li, N.; Lin, Y.; Yao, Y.; Ma, J. Up-regulation of microglial cathepsin C expression and activity in lipopolysaccharide-induced neuroinflammation. J. Neuroinflamm. 2012, 9, 96. [Google Scholar] [CrossRef]
- Liang, J.; Li, N.; Zhang, Y.; Hou, C.; Yang, X.; Shimizu, T.; Wang, X.; Ikenaka, K.; Fan, K.; Ma, J. Disinhibition of Cathepsin C Caused by Cystatin F Deficiency Aggravates the Demyelination in a Cuprizone Model. Front. Mol. Neurosci. 2016, 9, 152. [Google Scholar] [CrossRef]
- Ma, J.; Tanaka, K.F.; Yamada, G.; Ikenaka, K. Induced expression of cathepsins and cystatin C in a murine model of demyelination. Neurochem. Res. 2007, 32, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Wendt, W.; Lübbert, H.; Stichel, C.C. Upregulation of cathepsin S in the aging and pathological nervous system of mice. Brain Res. 2008, 1232, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Dauth, S.; Sîrbulescu, R.F.; Jordans, S.; Rehders, M.; Avena, L.; Oswald, J.; Lerchl, A.; Saftig, P.; Brix, K. Cathepsin K deficiency in mice induces structural and metabolic changes in the central nervous system that are associated with learning and memory deficits. BMC Neurosci. 2011, 12, 74. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Cui, Y.; Zhang, J.; Yan, R.; Su, D.; Zhao, D.; Wang, A.; Feng, T. Temporal trends in the prevalence of Parkinson’s disease from 1980 to 2023: A systematic review and meta-analysis. Lancet Healthy Longev. 2024, 5, e464–e479. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Qiao, L.; Li, M.; Wen, X.; Zhang, W.; Li, X. Global, regional, national epidemiology and trends of Parkinson’s disease from 1990 to 2021: Findings from the Global Burden of Disease Study 2021. Front. Aging Neurosci. 2024, 16, 1498756. [Google Scholar] [CrossRef]
- Su, D.; Cui, Y.; He, C.; Yin, P.; Bai, R.; Zhu, J.; Lam, J.S.T.; Zhang, J.; Yan, R.; Zheng, X.; et al. Projections for prevalence of Parkinson’s disease and its driving factors in 195 countries and territories to 2050: Modelling study of Global Burden of Disease Study 2021. BMJ 2025, 388, e080952. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Okun, M.S. Diagnosis and treatment of Parkinson disease: A review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef]
- Morris, H.R.; Spillantini, M.G.; Sue, C.M.; Williams-Gray, C.H. The pathogenesis of Parkinson’s disease. Lancet 2024, 403, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef] [PubMed]
- Słowikowski, B.; Owecki, W.; Jeske, J.; Jezierski, M.; Draguła, M.; Goutor, U.; Jagodziński, P.P.; Kozubski, W.; Dorszewska, J. Epigenetics and the neurodegenerative process. Epigenomics 2024, 16, 473–491. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Hirst, W.D.; Federoff, H.J.; Harms, A.S.; Stoessl, A.J.; Kordower, J.H. Nigrostriatal tau pathology in parkinsonism and Parkinson’s disease. Brain 2024, 147, 444–457. [Google Scholar] [CrossRef]
- Moussaud, S.; Jones, D.R.; Moussaud-Lamodière, E.L.; Delenclos, M.; Ross, O.A.; McLean, P.J. Alpha-synuclein and tau: Teammates in neurodegeneration? Mol. Neurodegener. 2014, 9, 43. [Google Scholar] [CrossRef]
- Yusufujiang, A.; Zeng, S.; Li, H. Cathepsins and Parkinson’s disease: Insights from Mendelian randomization analyses. Front. Aging Neurosci. 2024, 16, 1380483. [Google Scholar] [CrossRef]
- McGlinchey, R.P.; Lee, J.C. Cysteine cathepsins are essential in lysosomal degradation of α-synuclein. Proc. Natl. Acad. Sci. USA 2015, 112, 9322–9327. [Google Scholar] [CrossRef]
- Senkevich, K.; Gan-Or, Z. Autophagy lysosomal pathway dysfunction in Parkinson’s disease; evidence from human genetics. Parkinsonism Relat. Disord. 2020, 73, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Prieto Huarcaya, S.; Drobny, A.; Marques, A.R.A.; Di Spiezio, A.; Dobert, J.P.; Balta, D.; Werner, C.; Rizo, T.; Gallwitz, L.; Bub, S.; et al. Recombinant pro-CTSD (cathepsin D) enhances SNCA/α-synuclein degradation in α-synucleinopathy models. Autophagy 2022, 18, 1127–1151. [Google Scholar] [CrossRef]
- Kang, J.; Kim, J.W.; Heo, H.; Lee, J.; Park, K.Y.; Yoon, J.H.; Chang, J. Identification of BAG2 and cathepsin D as plasma biomarkers for Parkinson’s disease. Clin. Transl. Sci. 2021, 14, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Parnetti, L.; Paciotti, S.; Eusebi, P.; Dardis, A.; Zampieri, S.; Chiasserini, D.; Tasegian, A.; Tambasco, N.; Bembi, B.; Calabresi, P.; et al. Cerebrospinal fluid β-glucocerebrosidase activity is reduced in Parkinson’s disease patients. Mov. Disord. 2017, 32, 1423–1431. [Google Scholar] [CrossRef]
- van Dijk, K.D.; Persichetti, E.; Chiasserini, D.; Eusebi, P.; Beccari, T.; Calabresi, P.; Berendse, H.W.; Parnetti, L.; van de Berg, W.D. Changes in endolysosomal enzyme activities in cerebrospinal fluid of patients with Parkinson’s disease. Mov. Disord. 2013, 28, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, X.; Fei, X.; Xia, L.; Qin, Z.; Liang, Z. Parkinson’s disease involves autophagy and abnormal distribution of cathepsin L. Neurosci. Lett. 2011, 489, 62–67. [Google Scholar] [CrossRef]
- Jiang, Y.; Fan, W.; Li, Y.; Xue, H. Genetic insights into the role of cathepsins in Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis: Evidence from Mendelian randomization study. Brain Behav. 2025, 15, e70207. [Google Scholar] [CrossRef]
- Lu, C.; Cai, X.; Zhi, S.; Wen, X.; Shen, J.; Ercoli, T.; Simula, E.R.; Masala, C.; Sechi, L.A.; Solla, P. Exploring the association between cathepsin B and Parkinson’s disease. Brain Sci. 2024, 14, 482. [Google Scholar] [CrossRef]
- Sulatsky, M.I.; Stepanenko, O.V.; Mikhailova, E.V.; Sulatskaya, A.I. Cathepsin B prevents cell death by fragmentation and destruction of pathological amyloid fibrils. Cell Death Discov. 2025, 11, 61. [Google Scholar] [CrossRef]
- Jones-Tabah, J.; He, K.; Karpilovsky, N.; Senkevich, K.; Deyab, G.; Pietrantonio, I.; Goiran, T.; Cousineau, Y.; Nikanorova, D.; Goldsmith, T.; et al. The Parkinson’s disease risk gene cathepsin B promotes fibrillar alpha-synuclein clearance, lysosomal function and glucocerebrosidase activity in dopaminergic neurons. Mol. Neurodegener. 2024, 19, 88. [Google Scholar] [CrossRef]
- Aufschnaiter, A.; Habernig, L.; Kohler, V.; Diessl, J.; Carmona-Gutierrez, D.; Eisenberg, T.; Keller, W.; Büttner, S. The coordinated action of calcineurin and cathepsin D protects against α-synuclein toxicity. Front. Mol. Neurosci. 2017, 10, 207. [Google Scholar] [CrossRef]
- Drobny, A.; Boros, F.A.; Balta, D.; Prieto Huarcaya, S.; Caylioglu, D.; Qazi, N.; Vandrey, J.; Schneider, Y.; Dobert, J.P.; Pitcairn, C.; et al. Reciprocal effects of alpha-synuclein aggregation and lysosomal homeostasis in synucleinopathy models. Transl. Neurodegener. 2023, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Mantle, D.; Falkous, G.; Ishiura, S.; Perry, R.H.; Perry, E.K. Comparison of cathepsin protease activities in brain tissue from normal cases and cases with Alzheimer’s disease, Lewy body dementia, Parkinson’s disease and Huntington’s disease. J. Neurol. Sci. 1995, 131, 65–70. [Google Scholar] [CrossRef]
- Nelson, M.P.; Boutin, M.; Tse, T.E.; Lu, H.; Haley, E.D.; Ouyang, X.; Zhang, J.; Auray-Blais, C.; Shacka, J.J. The lysosomal enzyme alpha-galactosidase A is deficient in Parkinson’s disease brain in association with the pathologic accumulation of alpha-synuclein. Neurobiol. Dis. 2018, 110, 68–81. [Google Scholar] [CrossRef]
- Moors, T.E.; Paciotti, S.; Ingrassia, A.; Quadri, M.; Breedveld, G.; Tasegian, A.; Chiasserini, D.; Eusebi, P.; Duran-Pacheco, G.; Kremer, T.; et al. Characterization of brain lysosomal activities in GBA-related and sporadic Parkinson’s disease and dementia with Lewy bodies. Mol. Neurobiol. 2019, 56, 1344–1355. [Google Scholar] [CrossRef]
- Wu, H.C.; Chang, K.H.; Chiang, M.C.; Chen, C.M. Alterations of plasma galectin-3 and C3 levels in patients with Parkinson’s disease. Brain Sci. 2021, 11, 1515. [Google Scholar] [CrossRef]
- Schulte, T.; Böhringer, S.; Schöls, L.; Müller, T.; Fischer, C.; Riess, O.; Przuntek, H.; Berger, K.; Epplen, J.T.; Krüger, R. Modulation of disease risk according to a cathepsin D / apolipoprotein E genotype in Parkinson’s disease. J. Neural Transm. 2003, 110, 749–755. [Google Scholar] [CrossRef]
- Bezrukova, A.I.; Basharova, K.S.; Emelyanov, A.K.; Rybakov, A.V.; Miliukhina, I.V.; Pchelina, S.N.; Usenko, T.S. Autophagy process in Parkinson’s disease depends on mutations in the GBA1 and LRRK2 genes. Biochem. Genet. 2025. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Niu, J.Y.; Xiong, J.; Nie, S.K.; Zeng, F.; Zhang, Z.H. LRRK2 G2019S mutation inhibits degradation of α-synuclein in an in vitro model of Parkinson’s disease. Curr. Med. Sci. 2018, 38, 1012–1017. [Google Scholar] [CrossRef]
- van der Wateren, I.M.; Knowles, T.P.J.; Buell, A.K.; Dobson, C.M.; Galvagnion, C. C-terminal truncation of α-synuclein promotes amyloid fibril amplification at physiological pH. Chem. Sci. 2018, 9, 5506–5516. [Google Scholar] [CrossRef]
- Liu, C.W.; Giasson, B.I.; Lewis, K.A.; Lee, V.M.; Demartino, G.N.; Thomas, P.J. A precipitating role for truncated alpha-synuclein and the proteasome in alpha-synuclein aggregation: Implications for pathogenesis of Parkinson disease. J. Biol. Chem. 2005, 280, 22670–22678. [Google Scholar] [CrossRef] [PubMed]
- McGlinchey, R.P.; Lacy, S.M.; Huffer, K.E.; Tayebi, N.; Sidransky, E.; Lee, J.C. C-terminal α-synuclein truncations are linked to cysteine cathepsin activity in Parkinson’s disease. J. Biol. Chem. 2019, 294, 9973–9984. [Google Scholar] [CrossRef] [PubMed]
- Tsujimura, A.; Taguchi, K.; Watanabe, Y.; Tatebe, H.; Tokuda, T.; Mizuno, T.; Tanaka, M. Lysosomal enzyme cathepsin B enhances the aggregate forming activity of exogenous α-synuclein fibrils. Neurobiol. Dis. 2015, 73, 244–253. [Google Scholar] [CrossRef]
- Ferree, A.W. Cathepsin oxidation alters alpha-synuclein processing. Front. Neurol. 2019, 10, 530. [Google Scholar] [CrossRef]
- Nakanishi, H. Microglial cathepsin B as a key driver of inflammatory brain diseases and brain aging. Neural Regen. Res. 2020, 15, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Koprich, J.B.; Reske-Nielsen, C.; Mithal, P.; Matthies, J.; Hopkins, S.-E.; Campbell, K.M.; Freed, M.; Gearing, F.; Liggins, K.J.; Parmar, M.; et al. Neuroinflammation mediated by IL-1beta increases susceptibility of dopamine neurons to degeneration in an animal model of Parkinson’s disease. J. Neuroinflamm. 2008, 5, 8. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, M.; Du, R.H.; Qiao, C.; Jiang, C.Y.; Zhang, K.Z.; Ding, J.H.; Hu, G. MicroRNA-7 targets Nod-like receptor protein 3 inflammasome to modulate neuroinflammation in the pathogenesis of Parkinson’s disease. Mol. Neurodegener. 2016, 11, 28. [Google Scholar] [CrossRef]
- Pišlar, A.; Tratnjek, L.; Glavan, G.; Živin, M.; Kos, J. Upregulation of cysteine protease cathepsin X in the 6-hydroxydopamine model of Parkinson’s disease. Front. Mol. Neurosci. 2018, 11, 412. [Google Scholar] [CrossRef] [PubMed]
- Pišlar, A.H.; Zidar, N.; Kikelj, D.; Kos, J. Cathepsin X promotes 6-hydroxydopamine-induced apoptosis of PC12 and SH-SY5Y cells. Neuropharmacology 2014, 82, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Gan, P.; Xia, Q.; Hang, G.; Zhou, Y.; Qian, X.; Wang, X.; Ding, L. Knockdown of cathepsin D protects dopaminergic neurons against neuroinflammation-mediated neurotoxicity through inhibition of NF-κB signalling pathway in Parkinson’s disease model. Clin. Exp. Pharmacol. Physiol. 2019, 46, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, S.; Reinheckel, T.; Krainc, D. Inhibition of cysteine protease cathepsin L increases the level and activity of lysosomal glucocerebrosidase. JCI Insight 2024, 9, e169594. [Google Scholar] [CrossRef]
- Lin, L.; Wu, Z.; Luo, H.; Huang, Y. Cathepsin-mediated regulation of alpha-synuclein in Parkinson’s disease: A Mendelian randomization study. Front. Aging Neurosci. 2024, 16, 1394807. [Google Scholar] [CrossRef]
- Sun, H.; Tang, Q.; Yan, X.; Xie, W.; Xu, Y.; Zhang, W. Cathepsins and neurological diseases: A Mendelian randomization study. Front. Neurosci. 2024, 18, 1454369. [Google Scholar] [CrossRef]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Zhang, Y.; Wang, J.Z. Updates in Alzheimer’s disease: From basic research to diagnosis and therapies. Transl. Neurodegener. 2024, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Wang, X. Alzheimer’s disease: Insights into pathology, molecular mechanisms and therapy. Protein Cell 2025, 16, 83–120. [Google Scholar] [CrossRef]
- Chai, Y.L.; Chong, J.R.; Weng, J.; Howlett, D.; Halsey, A.; Lee, J.H.; Attems, J.; Aarsland, D.; Francis, P.T.; Chen, C.P.; et al. Lysosomal cathepsin D is upregulated in Alzheimer’s disease neocortex and may be a marker for neurofibrillary degeneration. Brain Pathol. 2019, 29, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Li, J.; Du, P.; Jin, W.; Gao, G.; Cui, D. Senile plaques in Alzheimer’s disease arise from Aβ- and cathepsin D-enriched mixtures leaking out during intravascular haemolysis and microaneurysm rupture. FEBS Lett. 2023, 597, 1007–1040. [Google Scholar] [CrossRef] [PubMed]
- Suire, C.N.; Leissring, M.A. Cathepsin D: A candidate link between amyloid β-protein and tauopathy in Alzheimer disease. J. Exp. Neurol. 2021, 2, 10–15. [Google Scholar]
- Terron, H.M.; Parikh, S.J.; Abdul-Hay, S.O.; Sahara, T.; Kang, D.; Dickson, D.W.; Saftig, P.; LaFerla, F.M.; Lane, S.; Leissring, M.A. Prominent tauopathy and intracellular β-amyloid accumulation triggered by genetic deletion of cathepsin D: Implications for Alzheimer disease pathogenesis. Alzheimers Res. Ther. 2024, 16, 70. [Google Scholar] [CrossRef]
- Chai, Y.L.; Liang, N.H.P.; Chong, J.R.; Venketasubramanian, N.; Tan, B.Y.; Hilal, S.; Chen, C.P.; Lai, M.K.P. Serum cathepsin D is a potential biomarker for Alzheimer’s disease dementia and cognitive decline. J. Alzheimers Dis. 2023, 91, 989–998. [Google Scholar] [CrossRef]
- Kim, J.W.; Jung, S.Y.; Kim, Y.; Heo, H.; Hong, C.H.; Seo, S.W.; Choi, S.H.; Son, S.J.; Lee, S.; Chang, J. Identification of cathepsin D as a plasma biomarker for Alzheimer’s disease. Cells 2021, 10, 138. [Google Scholar] [CrossRef]
- Crawford, F.C.; Freeman, M.J.; Schinka, J.; Abdullah, L.I.; Richards, D.; Sevush, S.; Duara, R.; Mullan, M.J. The genetic association between cathepsin D and Alzheimer’s disease. Neurosci. Lett. 2000, 289, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Ntais, C.; Polycarpou, A.; Ioannidis, J.P.A. Meta-analysis of the association of the cathepsin D Ala224Val gene polymorphism with the risk of Alzheimer’s disease: A HuGE gene-disease association review. Am. J. Epidemiol. 2004, 159, 527–536. [Google Scholar] [CrossRef]
- Schuur, M.; Ikram, M.A.; van Swieten, J.C.; Isaacs, A.; Vergeer-Drop, J.M.; Hofman, A.; Oostra, B.A.; Breteler, M.M.; van Duijn, C.M. Cathepsin D gene and the risk of Alzheimer’s disease: A population-based study and meta-analysis. Neurobiol. Aging. 2011, 32, 1607–1614. [Google Scholar] [CrossRef]
- Urbanelli, L.; Emiliani, C.; Massini, C.; Persichetti, E.; Orlacchio, A.; Pelicci, G.; Sorbi, S.; Hasilik, A.; Bernardi, G.; Orlacchio, A. Cathepsin D expression is decreased in Alzheimer’s disease fibroblasts. Neurobiol. Aging. 2008, 29, 12–22. [Google Scholar] [CrossRef]
- Chitranshi, N.; Kumar, A.; Sheriff, S.; Gupta, V.; Godinez, A.; Saks, D.; Sarkar, S.; Shen, T.; Mirzaei, M.; Basavarajappa, D.; et al. Identification of novel cathepsin B inhibitors with implications in Alzheimer’s disease: Computational refining and biochemical evaluation. Cells 2021, 10, 1946. [Google Scholar] [CrossRef]
- Mueller-Steiner, S.; Zhou, Y.; Arai, H.; Arai, H.; Roberson, E.D.; Sun, B.; Chen, J.; Wang, X.; Yu, G.; Esposito, L.; et al. Antiamyloidogenic and neuroprotective functions of cathepsin B: Implications for Alzheimer’s disease. Neuron 2006, 51, 703–714. [Google Scholar] [CrossRef]
- Sundelöf, J.; Sundström, J.; Hansson, O.; Eriksdotter-Jönhagen, M.; Giedraitis, V.; Larsson, A.; Degerman-Gunnarsson, M.; Ingelsson, M.; Minthon, L.; Blennow, K.; et al. Higher cathepsin B levels in plasma in Alzheimer’s disease compared to healthy controls. J. Alzheimers Dis. 2010, 22, 1223–1230. [Google Scholar] [CrossRef]
- Wu, Y.; Mumford, P.; Noy, S.; Cleverley, K.; Mrzyglod, A.; Luo, D.; van Dalen, F.; Verdoes, M.; Fisher, E.M.C.; Wiseman, F.K. Cathepsin B abundance, activity and microglial localisation in Alzheimer’s disease–Down syndrome and early onset Alzheimer’s disease; the role of elevated cystatin B. Acta Neuropathol. Commun. 2023, 11, 132. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Zhao, D.; Zhou, Y.; Kong, W.; Xie, Z.; Xiong, Y.; Li, Y.; Zhao, S.; Kou, X.; Zhang, S.; et al. Cathepsin B modulates microglial migration and phagocytosis of amyloid β in Alzheimer’s disease through PI3K-Akt signaling. Neuropsychopharmacology 2025, 50, 640–650. [Google Scholar] [CrossRef]
- Hook, V.; Yoon, M.; Mosier, C.; Ito, G.; Podvin, S.; Head, B.P.; Rissman, R.; O’Donoghue, A.J.; Hook, G. Cathepsin B in neurodegeneration of Alzheimer’s disease, traumatic brain injury, and related brain disorders. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140428. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Meng, J.; Kong, W.; Wu, Z.; Lan, F.; Narengaowa; Hayashi, Y.; Yang, Q.; Bai, Z.; Nakanishi, H.; et al. Microglial cathepsin E plays a role in neuroinflammation and amyloid β production in Alzheimer’s disease. Aging Cell 2022, 21, e13565. [Google Scholar] [CrossRef]
- Islam, M.I.; Nagakannan, P.; Shcholok, T.; Contu, F.; Mai, S.; Albensi, B.C.; Del Bigio, M.R.; Wang, J.F.; Sharoar, M.G.; Yan, R.; et al. Regulatory role of cathepsin L in induction of nuclear laminopathy in Alzheimer’s disease. Aging Cell 2022, 21, e13531. [Google Scholar] [CrossRef]
- Yoshiyama, Y.; Arai, K.; Oki, T.; Hattori, T. Expression of invariant chain and pro-cathepsin L in Alzheimer’s brain. Neurosci. Lett. 2000, 290, 125–128. [Google Scholar] [CrossRef]
- Liu, P.P.; Liu, X.H.; Ren, M.J.; Liu, X.T.; Shi, X.Q.; Li, M.L.; Li, S.A.; Yang, Y.; Wang, D.D.; Wu, Y.; et al. Neuronal cathepsin S increases neuroinflammation and causes cognitive decline via CX3CL1-CX3CR1 axis and JAK2-STAT3 pathway in aging and Alzheimer’s disease. Aging Cell 2025, 24, e14393. [Google Scholar] [CrossRef]
- Hou, Y.; Chu, X.; Park, J.H.; Zhu, Q.; Hussain, M.; Li, Z.; Madsen, H.B.; Yang, B.; Wei, Y.; Wang, Y.; et al. Urolithin A improves Alzheimer’s disease cognition and restores mitophagy and lysosomal functions. Alzheimers Dement. 2024, 20, 4212–4233. [Google Scholar] [CrossRef]
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.O.; Pandian, J.; Lindsay, P.; FGrupper, M.; Rautalin, I. World Stroke Organization: Global Stroke Fact Sheet 2025. Int. J. Stroke 2025, 20, 132–144. [Google Scholar] [CrossRef]
- Ding, R.; Wu, L.; Wei, S.; Lu, H.; Qin, X.; Liu, X.; Wang, Y.; Liu, W.; Li, H.; Luo, B.; et al. Multi-targeted Olink proteomics analyses of cerebrospinal fluid from patients with aneurysmal subarachnoid hemorrhage. Proteome Sci. 2024, 22, 11. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, Y.; Liu, S.; Liu, Y.; Yang, X.; Liu, G.; Shimizu, T.; Ikenaka, K.; Fan, K.; Ma, J. Cathepsin C promotes microglia M1 polarization and aggravates neuroinflammation via activation of Ca2+-dependent PKC/p38MAPK/NF-κB pathway. J. Neuroinflamm. 2019, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.W.; Han, X.S.; Xu, X.T.; Fang, Y.N.; Chen, H.B.; Jiang, T. Acute phase serum cathepsin S level and cathepsin S/cystatin C ratio are the associated factors with cerebral infarction and their diagnostic value for cerebral infarction. Kaohsiung J. Med. Sci. 2019, 35, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Zhang, S.; Huang, L.; Peng, Z.; Lu, H.; He, Q.; Chen, R.; Hu, L.; Wang, B.; Sun, B.; et al. Single-cell RNA sequencing of peripheral blood reveals that monocytes with high cathepsin S expression aggravate cerebral ischemia–reperfusion injury. Brain Behav. Immun. 2023, 107, 330–344. [Google Scholar] [CrossRef]
- Rodgers, K.J.; Watkins, D.J.; Miller, A.L.; Chan, P.Y.; Karanam, S.; Brissette, W.H.; Long, C.J.; Jackson, C.L. Destabilizing role of cathepsin S in murine atherosclerotic plaques. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 851–856. [Google Scholar] [CrossRef]
- Sukhova, G.K.; Zhang, Y.; Pan, J.H.; Wada, Y.; Yamamoto, T.; Naito, M.; Kodama, T.; Tsimikas, S.; Witztum, J.L.; Lu, M.L. Deficiency of cathepsin S reduces atherosclerosis in LDL receptor-deficient mice. J. Clin. Investig. 2003, 111, 897–906. [Google Scholar] [CrossRef]
- Aoki, T.; Kataoka, H.; Ishibashi, R.; Nozaki, K.; Hashimoto, N. Cathepsin B, K, and S are expressed in cerebral aneurysms and promote the progression of cerebral aneurysms. Stroke 2008, 39, 2603–2610. [Google Scholar] [CrossRef]
- Zhao, R.; He, X.W.; Shi, Y.H.; Liu, Y.S.; Liu, F.D.; Hu, Y.; Zhuang, M.T.; Feng, X.Y.; Zhao, L.; Zhao, B.Q.; et al. Cathepsin K knockout exacerbates haemorrhagic transformation induced by recombinant tissue plasminogen activator after focal cerebral ischaemia in mice. Cell. Mol. Neurobiol. 2019, 39, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Li, H.; Yu, Z.; Cao, C.; Xu, Z.; Peng, L.; Zhang, J.H.; Chen, G. Cathepsin B as a key regulator of ferroptosis in microglia following intracerebral hemorrhage. Neurobiol. Dis. 2024, 194, 106468. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Cao, Y. Correlation of cathepsin S with coronary stenosis degree, carotid thickness, blood pressure, glucose and lipid metabolism and vascular endothelial function in atherosclerosis. Exp. Ther. Med. 2020, 19, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, I.; Hultman, K.; Edsfeldt, A.; Hedblad, B.; Fredrikson, G.N.; Björkbacka, H.; Nilsson, J.; Bengtsson, E. High levels of cathepsin D and cystatin B are associated with increased risk of coronary events. Open Heart 2016, 3, e000353. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Jin, J.; Jin, D.; Cui, L.; Li, X.; Rei, Y.; Jiang, H.; Zhao, G.; Yang, G.; et al. Increased serum cathepsin K in patients with coronary artery disease. Yonsei Med. J. 2014, 55, 912–919. [Google Scholar] [CrossRef]
- Faraday, N.; Schunke, K.; Saleem, S.; Fu, J.; Wang, B.; Zhang, J.; Morrell, C.; Dore, S. Cathepsin G-dependent modulation of platelet thrombus formation in vivo by blood neutrophils. PLoS ONE 2013, 8, e71447. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.I.; Marcus, J.M.; Lee, J.H.; Garcia, P.L.; Singh, V.; Shacka, J.J.; Zhang, J.; Gropen, T.I.; Falany, C.N.; Andrabi, S.A. Restoration of CTSD (cathepsin D) and lysosomal function in stroke is neuroprotective. Autophagy 2021, 17, 1330–1348. [Google Scholar] [CrossRef]
- Bersano, A.; Kraemer, M.; Burlina, A.; Mancuso, M.; Finsterer, J.; Sacco, S.; Salvarani, C.; Caputi, L.; Chabriat, H.; Oberstein, S.L.; et al. Heritable and non-heritable uncommon causes of stroke. J. Neurol. 2021, 268, 2780–2797. [Google Scholar] [CrossRef] [PubMed]
- Bugiani, M.; Kevelam, S.H.; Bakels, H.S.; Waisfisz, Q.; Ceuterick-de Groote, C.; Niessen, H.W.; Abbink, T.E.; Lesnik Oberstein, S.A.; van der Knaap, M.S. Cathepsin A-related arteriopathy with strokes and leukoencephalopathy (CARASAL). Neurology 2016, 87, 1777–1786. [Google Scholar] [CrossRef] [PubMed]
- Guey, S.; Chabriat, H. Monogenic causes of cerebral small vessel disease and stroke. Handb. Clin. Neurol. 2024, 204, 273–287. [Google Scholar] [CrossRef]
- Finsterer, J.; Scorza, C.A.; Scorza, F.A.; Wakil, S.M. Update on hereditary, autosomal dominant cathepsin-A-related arteriopathy with strokes and leukoencephalopathy (CARASAL). Acta Neurol. Belg. 2019, 119, 299–303. [Google Scholar] [CrossRef]
- Haki, M.; Al-Biati, H.A.; Al-Tameemi, Z.S.; Ali, I.S.; Al-Hussaniy, H.A. Review of multiple sclerosis: Epidemiology, etiology, pathophysiology, and treatment. Medicine 2024, 103, e37297. [Google Scholar] [CrossRef] [PubMed]
- Doshi, A.; Chataway, J. Multiple sclerosis, a treatable disease. Clin. Med. 2016, 16 (Suppl. 6), s53–s59. [Google Scholar] [CrossRef]
- Hauser, S.L.; Cree, B.A.C. Treatment of multiple sclerosis: A review. Am. J. Med. 2020, 133, 1380–1390.e2. [Google Scholar] [CrossRef]
- Kuhlmann, T.; Moccia, M.; Coetzee, T.; Cohen, J.A.; Correale, J.; Graves, J.; Marrie, R.A.; Montalban, X.; Yong, V.W.; Thompson, A.J.; et al. Multiple sclerosis progression: Time for a new mechanism-driven framework. Lancet Neurol. 2023, 22, 78–88. [Google Scholar] [CrossRef]
- Pišlar, A.; Bolčina, L.; Kos, J. New insights into the role of cysteine cathepsins in neuroinflammation. Biomolecules 2021, 11, 1796. [Google Scholar] [CrossRef]
- Stoka, V.; Vasiljeva, O.; Nakanishi, H.; Turk, V. The role of cysteine protease cathepsins B, H, C, and X/Z in neurodegenerative diseases and cancer. Int. J. Mol. Sci. 2023, 24, 15613. [Google Scholar] [CrossRef]
- Haves-Zburof, D.; Paperna, T.; Gour-Lavie, A.; Mandel, I.; Glass-Marmor, L.; Miller, A. Cathepsins and their endogenous inhibitors cystatins: Expression and modulation in multiple sclerosis. J. Cell. Mol. Med. 2011, 15, 2421–2429. [Google Scholar] [CrossRef]
- Allan, E.R.O.; Campden, R.I.; Ewanchuk, B.W.; Tailor, P.; Balce, D.R.; McKenna, N.T.; Greene, C.J.; Warren, A.L.; Reinheckel, T.; Yates, R.M. A role for cathepsin Z in neuroinflammation provides mechanistic support for an epigenetic risk factor in multiple sclerosis. J. Neuroinflamm. 2017, 14, 103. [Google Scholar] [CrossRef]
- Allan, E.R.; Yates, R.M. Redundancy between cysteine cathepsins in murine experimental autoimmune encephalomyelitis. PLoS ONE 2015, 10, e0128945. [Google Scholar] [CrossRef] [PubMed]
- Holm Nielsen, S.; Karsdal, M.; Manoel, B.; Bay-Jensen, A.C.; Henriksen, K. Diagnostic potential of blood-based biomarkers in multiple sclerosis. Front. Neurol. 2024, 15, 1425046. [Google Scholar] [CrossRef]
- Medina, A.; Mahjoub, Y.; Shaver, L.; Pringsheim, T. Prevalence and incidence of Huntington’s disease: An updated systematic review and meta-analysis. Mov. Disord. 2022, 37, 2327–2335. [Google Scholar] [CrossRef] [PubMed]
- Drobny, A.; Prieto Huarcaya, S.; Dobert, J.; Kluge, A.; Bunk, J.; Schlothauer, T.; Zunke, F. The role of lysosomal cathepsins in neurodegeneration: Mechanistic insights, diagnostic potential and therapeutic approaches. Biochim. Biophys. Acta Mol. Cell Res. 2022, 1869, 119243. [Google Scholar] [CrossRef]
- Waelter, S.; Boeddrich, A.; Lurz, R.; Scherzinger, E.; Lueder, G.; Lehrach, H.; Wanker, E.E. Accumulation of mutant huntingtin fragments in aggresome-like inclusion bodies as a result of insufficient protein degradation. Mol. Biol. Cell 2001, 12, 1393–1407. [Google Scholar] [CrossRef]
- Walker, F.O. Huntington’s disease. Lancet 2007, 369, 218–228. [Google Scholar] [CrossRef]
- Vonsattel, J.P.; Myers, R.H.; Stevens, T.J.; Ferrante, R.J.; Bird, E.D.; Richardson, E.P., Jr. Neuropathological classification of Huntington’s disease. J. Neuropathol. Exp. Neurol. 1985, 44, 559–577. [Google Scholar] [CrossRef] [PubMed]
- Bhutani, N.; Piccirillo, R.; Hourez, R.; Venkatraman, P.; Goldberg, A.L. Cathepsins L and Z are critical in degrading polyglutamine-containing proteins within lysosomes. J. Biol. Chem. 2012, 287, 17471–17482. [Google Scholar] [CrossRef]
- Kim, Y.J.; Sapp, E.; Cuiffo, B.G.; Sobin, L.; Yoder, J.; Kegel, K.B.; Qin, Z.H.; Detloff, P.; Aronin, N.; DiFiglia, M. Lysosomal proteases are involved in generation of N-terminal huntingtin fragments. Neurobiol. Dis. 2006, 22, 346–356. [Google Scholar] [CrossRef]
- Liang, Q.; Ouyang, X.; Schneider, L.; Zhang, J. Reduction of mutant huntingtin accumulation and toxicity by lysosomal cathepsins D and B in neurons. Mol. Neurodegener. 2011, 6, 37. [Google Scholar] [CrossRef]
- Ragunathan, K.; Veeraraghavan, V.; Kaushik, J.S. ILAE 2025 Classification of Epileptic Seizures: Key Revisions and Implications for Clinical Practice. Indian Pediatr. 2025, 62, 623–627. [Google Scholar] [CrossRef]
- Delgado-Escueta, A.V.; Bajorek, J.G. Status epilepticus: Mechanisms of brain damage and rational management. Epilepsia 1982, 23 (Suppl. 1), S29–S41. [Google Scholar] [CrossRef] [PubMed]
- GBD Epilepsy Collaborators. Global, regional, and national burden of epilepsy, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Public Health 2025, 10, e203–e227. [Google Scholar] [CrossRef] [PubMed]
- Joensuu, T.; Lehesjoki, A.E.; Kopra, O. Molecular background of EPM1–Unverricht-Lundborg disease. Epilepsia 2008, 49, 557–563. [Google Scholar] [CrossRef]
- Guerrini, R.; Takahashi, T. Myoclonus and epilepsy. Handb. Clin. Neurol. 2013, 111, 667–679. [Google Scholar] [CrossRef]
- Lehtinen, M.K.; Tegelberg, S.; Schipper, H.; Su, H.; Zukor, H.; Manninen, O.; Kopra, O.; Joensuu, T.; Hakala, P.; Bonni, A.; et al. Cystatin B deficiency sensitizes neurons to oxidative stress in progressive myoclonus epilepsy, EPM1. J. Neurosci. 2009, 29, 5910–5915. [Google Scholar] [CrossRef]
- Hook, G.; Reinheckel, T.; Ni, J.; Wu, Z.; Kindy, M.; Peters, C.; Hook, V. Cathepsin B gene knockout improves behavioral deficits and reduces pathology in models of neurologic disorders. Pharmacol. Rev. 2022, 74, 600–629. [Google Scholar] [CrossRef]
- Houseweart, M.K.; Pennacchio, L.A.; Vilaythong, A.; Peters, C.; Noebels, J.L.; Myers, R.M. Cathepsin B but not cathepsins L or S contributes to the pathogenesis of Unverricht-Lundborg progressive myoclonus epilepsy (EPM1). J. Neurobiol. 2003, 56, 315–327. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, E.; Kokaia, Z.; Nanobashvili, A.; Reeben, M.; Lehesjoki, A.E.; Saarma, M.; Lindvall, O. Seizures induce widespread upregulation of cystatin B, the gene mutated in progressive myoclonus epilepsy, in rat forebrain neurons. Eur. J. Neurosci. 2000, 12, 1687–1695. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Aviles, E.R.; Fujikawa, D.G. Nuclear translocation of mitochondrial cytochrome c, lysosomal cathepsins B and D, and three other death-promoting proteins within the first 60 minutes of generalized seizures. J. Neurosci. Res. 2010, 88, 1727–1737. [Google Scholar] [CrossRef] [PubMed]
- Gorter, J.A.; Van Vliet, E.A.; Rauwerda, H.; Breit, T.; Stad, R.; van Schaik, L.; Vreugdenhil, E.; Redeker, S.; Hendriksen, E.; Aronica, E. Dynamic changes of proteases and protease inhibitors revealed by microarray analysis in CA3 and entorhinal cortex during epileptogenesis in the rat. Epilepsia 2007, 48 (Suppl. 5), 53–64. [Google Scholar] [CrossRef] [PubMed]
- Goutman, S.A.; Hardiman, O.; Al-Chalabi, A.; Chió, A.; Savelieff, M.G.; Kiernan, M.C.; Feldman, E.L. Recent advances in the diagnosis and prognosis of amyotrophic lateral sclerosis. Lancet Neurol. 2022, 21, 480–493. [Google Scholar] [CrossRef]
- Feldman, E.L.; Goutman, S.A.; Petri, S.; Mazzini, L.; Savelieff, M.G.; Shaw, P.J.; Sobue, G. Amyotrophic lateral sclerosis. Lancet 2022, 400, 1363–1380. [Google Scholar] [CrossRef]
- Longinetti, E.; Fang, F. Epidemiology of amyotrophic lateral sclerosis: An update of recent literature. Curr. Opin. Neurol. 2019, 32, 771–776. [Google Scholar] [CrossRef]
- Ilieva, H.; Vullaganti, M.; Kwan, J. Advances in molecular pathology, diagnosis, and treatment of amyotrophic lateral sclerosis. BMJ 2023, 383, e075037. [Google Scholar] [CrossRef]
- Marin, B.; Boumédiene, F.; Logroscino, G.; Couratier, P.; Babron, M.C.; Leutenegger, A.L.; Copetti, M.; Preux, P.M.; Beghi, E. Variation in worldwide incidence of amyotrophic lateral sclerosis: A meta-analysis. Int. J. Epidemiol. 2017, 46, 57–74. [Google Scholar] [CrossRef]
- Shoesmith, C.; Abrahao, A.; Benstead, T.; Chum, M.; Dupre, N.; Izenberg, A.; Johnston, W.; Kalra, S.; Leddin, D.; O’Connell, C.; et al. Canadian best practice recommendations for the management of amyotrophic lateral sclerosis. CMAJ 2020, 192, E1453–E1468. [Google Scholar] [CrossRef]
- Wada, Y.; Nagai, A.; Sheikh, A.M.; Onoda, K.; Terashima, M.; Shiota, Y.; Araki, A.; Yamaguchi, S. Co-localization of cystatin C and prosaposin in cultured neurons and in anterior horn neurons with amyotrophic lateral sclerosis. J. Neurol. Sci. 2018, 384, 67–74. [Google Scholar] [CrossRef]
- Huang, J.; Yu, Y.; Pang, D.; Li, C.; Wei, Q.; Cheng, Y.; Cui, Y.; Ou, R.; Shang, H. Lnc-HIBADH-4 regulates autophagy-lysosome pathway in amyotrophic lateral sclerosis by targeting cathepsin D. Mol. Neurobiol. 2024, 61, 4768–4782. [Google Scholar] [CrossRef]
- Kibinge, N.K.; Relton, C.L.; Gaunt, T.R.; Richardson, T.G. Characterizing the causal pathway for genetic variants associated with neurological phenotypes using human brain-derived proteome data. Am. J. Hum. Genet. 2020, 106, 885–892. [Google Scholar] [CrossRef]
- Hook, G.; Jacobsen, J.S.; Kindy, M.; Hook, V. Cathepsin B Is a New Drug Target for Traumatic Brain Injury Therapeutics: Evidence for E64d as a Promising Lead Drug Candidate. Front. Neurol. 2015, 6, 885–892. [Google Scholar] [CrossRef]
- Xu, J.; Wang, H.; Lu, X.; Li, T.; Wang, J.; Wang, C.; Wang, J. Inhibition of Cathepsin S Produces Neuroprotective Effects after Traumatic Brain Injury in Mice. Mediat. Inflamm. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ajani, T.A.; Magwebu, Z.E.; Chauke, C.G.; Obikeze, K. Advances in Cathepsin S Inhibition: Challenges and Breakthroughs in Drug Development. Pathophysiology 2024, 31, 471–487. [Google Scholar] [CrossRef] [PubMed]
- Bentley, D.; Fisher, B.A.; Barone, F.; Kolb, F.A.; Attley, G. A Randomized, Double-Blind, Placebo-Controlled, Parallel Group Study on the Effects of a Cathepsin S Inhibitor in Primary Sjögren’s Syndrome. Rheumatology 2023, 62, 3644–3653. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wu, S.; Gusdon, A.M.; Chen, H.; Hu, H.; Paz, A.S.; Aronowski, J.; Savarraj, J.P.; Kitagawa, R.S.; Choi, H.A.; et al. Cathepsin L and Acute Ischemic Stroke: A Mini-Review. Front. Stroke 2022, 1, 1050536. [Google Scholar] [CrossRef]
- Mehra, S.; Kumar, M.; Singh, R.; Thakur, B.; Rani, N.; Arava, S.; Narang, R.; Arya, D.S.; Chauhan, S.S. Clinical Significance of Cathepsin L and Cathepsin B in Dilated Cardiomyopathy. Mol. Cell. Biochem. 2017, 428, 139–147. [Google Scholar] [CrossRef] [PubMed]

| Cathepsin | Key Characteristics | Role in CNS | Disease Associations |
|---|---|---|---|
| D | Aspartic protease; degrades α-synuclein | Protein clearance; overexpression causes neuronal death | Parkinson’s disease, Alzheimer’s disease |
| H | Dual exo- and endopeptidase; in microglia | Modulates immune response; promotes proinflammatory cytokines and neuronal damage | Neuroinflammation |
| B and L | Cysteine proteases; intracellular proteolysis and ECM remodeling | Maintain brain integrity; involved in cholesterol trafficking | Niemann–Pick type C-like pathology |
| X | Carboxypeptidase; expressed in monocytes/macrophages | Mediates microglial activation and neurotoxicity; inhibition reduces inflammatory markers | Neuroinflammatory conditions |
| C | Activates serine proteases; low brain expression | Induced by inflammation in microglia; role in neuroinflammation unclear | Demyelination |
| S | Active at neutral pH | Involved in aging and neurodegeneration; elevated in ALS models | Aging, ALS |
| K | Known for bone resorption; also in brain parenchyma | Influences behavior, learning, and memory | Neurobehavioral regulation |
| Cystatin C | Endogenous inhibitor of cathepsins | Regulates cathepsin activity; imbalance linked to impaired remyelination | Multiple sclerosis, Alzheimer’s disease |
| Cathepsin | Influence | References |
|---|---|---|
| B |
| [11,19,22,31,35,36] |
| D |
| [8,11,13,23,42] |
| L |
| [11,19,31,43] |
| X |
| [39,40,41] |
| Cathepsin | Pathological Role | Key Mechanism | Significance in Stroke Management |
|---|---|---|---|
| C | Aggravates neuroinflammation and neurotoxic microglial polarization | Activates inflammatory signaling pathways | Potential biomarker; inhibitor may reduce inflammation |
| S | Amplifies stroke related injury | BBB disruption, vascular leakage, neuronal injury | Potential biomarker candidate; inhibition reduces infarct size |
| B | Promotes ferroptosis, exacerbates aneurysm progression | Iron-dependent cell death | Inhibition mimics ferroptosis blockade; potential therapeutic target |
| K | Prevention of hemorrhagic transformation following rtPA | Extracellular matrix degradation | Potential therapeutic target for rtPA complication |
| E | Increased ischemic stroke risk | Chronic neuroinflammation and brain injury, development of atherosclerotic plaques | Emerging biomarker candidate |
| O | Increased ischemic stroke risk | Development of atherosclerosis | Emerging biomarker candidate |
| G | Indirect stroke risk via prothrombotic state found in COVID-19 patients | Neutrophil activation, protease activity | Potential biomarker candidate |
| D | Prevention of neuron cell death following protein accumulation in stroke | Supports lysosomal function, prevents protein accumulation | Neuroprotective therapeutic target |
| A | Genetic small vessel disease with stroke and leukoencephalopathy | Endothelin-1 accumulation impairs myelination | Promising gene therapy in models |
| Cathepsin | Key Findings | Role in Epilepsy |
|---|---|---|
| B | Knockout reduces neuronal apoptosis in EPM1; elevated after seizures | Major contributor to neurodegeneration |
| D | Nuclear translocation within 60 min post-status epilepticus | Early mediator of neuronal injury |
| L | Increased 1 week post-seizure; knockout ineffective in EPM1 | Possibly involved in late inflammation |
| S | Knockout does not reduce EPM1 pathology | Minor or redundant role |
| K | Peaks 1 day after seizures; remains elevated | May support chronic damage progression |
| Cystatin B | Deficiency causes oxidative stress; upregulated after seizures | Key endogenous cathepsin inhibitor |
| Cathepsin | Expression in ALS | Proposed Role | Therapeutic Potential |
|---|---|---|---|
| B | ↑ in spinal anterior horns (postmortem) | Motor neuron degeneration, neuroinflammation | Possible target, but likely secondary to neurodegeneration |
| D | Dysregulated via lncRNA (lnc-HIBADH-4) | Autophagy regulation, TDP-43 clearance | Activators may restore autophagic flux |
| H | ↑ CTSH gene expression in ALS | Amplifies microglial inflammation | Gene suppression may reduce neuroinflammation |
| X/Z | ↑ in ALS models | Microglial activation, cytoskeletal dysfunction | Potential target; needs more validation |
| C | Poorly characterized | Neuroimmune modulation (hypothesized) | Requires further study |
| Cystatin C | ↑ in ALS tissues | Counteracts cathepsin B, modulates autophagy | Protective role; potential biomarker |
| Cathepsin | Protease Type | Neurological Role/Application |
|---|---|---|
| Cathepsin A | Serine carboxypeptidase | Associated with CARASAL, a hereditary stroke and leukoencephalopathy syndrome involving cerebral small vessel disease. |
| Cathepsin B | Cysteine protease | Acts as a biomarker and therapeutic target in Alzheimer’s disease (AD), Parkinson’s disease (PD), and epilepsy; degrades amyloid-beta and α-synuclein; contributes to neuroinflammation. |
| Cathepsin C | Cysteine protease | Enhances M1 polarization and demyelination in multiple sclerosis (MS); observed in stroke and neuroinflammation models; potential therapeutic target. |
| Cathepsin D | Aspartic protease | Major lysosomal enzyme involved in α-synuclein degradation in PD; elevated in AD and dementia; biomarker and potential therapeutic agent in stroke and lysosomal storage disorders. |
| Cathepsin E | Aspartic protease | Involved in neuroinflammation and amyloid-beta production in AD; therapeutic target in aging-related neurodegeneration. |
| Cathepsin F | Cysteine protease | Studied for potential role in neurodegeneration; precise neurological significance remains unclear. |
| Cathepsin G | Serine protease | Promotes platelet aggregation and vascular pathology; may contribute to stroke pathogenesis. |
| Cathepsin H | Cysteine protease | Expressed in perivascular microglia; promotes inflammatory cytokine release; implicated in PD, ALS, and other neuroinflammatory disorders. |
| Cathepsin K | Cysteine protease | Linked to behavioral deficits and anxiety; knockout studies show learning/memory impairment; contributes to stroke pathology. |
| Cathepsin L | Cysteine protease | Crucial for lysosomal function; involved in AD, PD, and Huntington’s disease (HD); regulates autophagy and neuronal survival. |
| Cathepsin O | Cysteine protease | Genetic associations suggest involvement in stroke susceptibility; more studies are needed to clarify function in CNS. |
| Cathepsin S | Cysteine protease | Contributes to chronic inflammation in AD, MS, ALS, and stroke; potential biomarker and drug target. |
| Cathepsin V | Cysteine protease | Limited data in CNS context; included in cathepsin family profiling studies. |
| Cathepsin W | Cysteine protease | Limited data in neurology; role in immune regulation and potential neuroinflammatory processes hypothesized. |
| Cathepsin X/Z | Cysteine protease | Key regulator in microglial activity; implicated in PD, MS, HD, and neurodegenerative inflammation; therapeutic potential shown in EAE and toxin models. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewandowski, D.; Konieczny, M.; Różycka, A.; Chrzanowski, K.; Owecki, W.; Kalinowski, J.; Stepura, M.; Jagodziński, P.; Dorszewska, J. Cathepsins in Neurological Diseases. Int. J. Mol. Sci. 2025, 26, 7886. https://doi.org/10.3390/ijms26167886
Lewandowski D, Konieczny M, Różycka A, Chrzanowski K, Owecki W, Kalinowski J, Stepura M, Jagodziński P, Dorszewska J. Cathepsins in Neurological Diseases. International Journal of Molecular Sciences. 2025; 26(16):7886. https://doi.org/10.3390/ijms26167886
Chicago/Turabian StyleLewandowski, Dominik, Mateusz Konieczny, Agata Różycka, Krzysztof Chrzanowski, Wojciech Owecki, Jan Kalinowski, Mikołaj Stepura, Paweł Jagodziński, and Jolanta Dorszewska. 2025. "Cathepsins in Neurological Diseases" International Journal of Molecular Sciences 26, no. 16: 7886. https://doi.org/10.3390/ijms26167886
APA StyleLewandowski, D., Konieczny, M., Różycka, A., Chrzanowski, K., Owecki, W., Kalinowski, J., Stepura, M., Jagodziński, P., & Dorszewska, J. (2025). Cathepsins in Neurological Diseases. International Journal of Molecular Sciences, 26(16), 7886. https://doi.org/10.3390/ijms26167886







