Do Long COVID and COVID Vaccine Side Effects Share Pathophysiological Picture and Biochemical Pathways?
Abstract
1. Introduction
2. Definition of Long COVID. Pathophysiological Manifestations, Predictors and Causes
2.1. Definition of Long COVID and Pathophysiological Manifestations
2.2. Predictors and Causes of Long COVID
2.2.1. Persistence of the Virus
2.2.2. Altered Endothelial Function and Unrepaired Tissue Damage
2.2.3. Absence of Treatment During COVID Episode
2.2.4. Autoimmunity and Immune Dysregulation
2.2.5. Human Leukocyte Antigens (HLA) Variability and Dormant Virus Activation
2.2.6. Impacts of SARS-CoV-2 on the Microbiota
2.2.7. Integration of Reverse-Transcribed SARS-CoV-2 RNA in the Human Genome
3. Long COVID and Vaccine Side Effects: A Focus on the Common Cardiovascular and Neurological Damages and Associated Pathologies
3.1. Long COVID, Acute and Post-Acute COVID Vaccine Syndromes
3.2. Vascular Damages and Pathologies in Long COVID and After COVID Vaccination
3.2.1. Introduction on Cardiovascular Damages
3.2.2. Myocarditis in Long COVID and After COVID Vaccination
3.2.3. Thrombosis in Long COVID and After COVID Vaccination
3.3. Neurological Damages and Consequences on Cognitive Systems in Long COVID and After COVID Vaccination
3.4. Accelerated Biological Aging and Senescence in Long COVID and After COVID Vaccination
4. Possible Common Immuno-Inflammatory Patterns and Biochemical Aspects of Severe COVID, Long COVID, and COVID Vaccine Side Effects and Therapeutic Targets
4.1. Spike Protein: The Common Toxicant
4.2. Dysregulation of the Renin–Angiotensin–Aldosterone System (ACE/Angiotensin II/AT1R Axis)
4.3. Activation of [Des-Arg9]-Bradykinin (ACE2/Bradykinin B1R/DABK Axis)
4.4. Lectin-Complement Pathway
4.5. Common Cytokines Between COVID-19 Pathology, Long COVID, and COVID Vaccine Side Effects
4.6. The Importance of Oxidative Stress
5. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Al-Aly, Z.; Davis, H.; McCorkell, L.; Soares, L.; Wulf-Hanson, S.; Iwasaki, A.; Topol, E.J. Long COVID Science, Research and Policy. Nat. Med. 2024, 30, 2148–2164. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.M.; Qasmieh, S.A.; Kulkarni, S.G.; Teasdale, C.A.; Jones, H.E.; McNairy, M.; Borrell, L.N.; Nash, D. The Epidemiology of Long Coronavirus Disease in US Adults. Clin. Infect. Dis. 2023, 76, 1636–1645. [Google Scholar] [CrossRef]
- National Center for Health Statistics. Long COVID. Household Pulse Survey. Centers for Disease Control and Prevention. 2023. Available online: https://www.cdc.gov/nchs/covid19/pulse/long-covid.htm (accessed on 3 October 2024).
- Institute for Health Metrics and Evaluation. Long COVID is a Serious Health Concern in Europe. Institute for Health Metrics and Evaluation. 2023. Available online: https://www.healthdata.org/news-events/insights-blog/acting-data/long-covid-serious-health-concern-europe (accessed on 3 October 2024).
- Sivan, M.; Rayner, C.; Delaney, B. Fresh evidence of the scale and scope of long covid. BMJ 2021, 373, n853. [Google Scholar] [CrossRef]
- Scholkmann, F.; May, C.A. COVID-19, post-acute COVID-19 syndrome (PACS, “long COVID”) and post-COVID-19 vaccination syndrome (PCVS, “post-COVIDvac-syndrome”): Similarities and differences. Pathol. Res. Pract. 2023, 246, 154497. [Google Scholar] [CrossRef]
- Lesgards, J.F.; Cerdan, D.; Perronne, C.; Sabatier, J.M.; Azalbert, X.; Rodgers, E.A.; McCullough, P.A. Toxicity of SARS-CoV-2 Spike Protein from the Virus and Produced from COVID-19 mRNA or Adenoviral DNA Vaccines. Arch. Microbiol. Immunol. 2023, 7, 121–138. [Google Scholar] [CrossRef]
- Khazaal, S.; Harb, J.; Rima, M.; Annweiler, C.; Wu, Y.; Cao, Z.; Abi Khattar, Z.; Legros, C.; Kovacic, H.; Fajloun, Z.; et al. The Pathophysiology of Long COVID throughout the Renin-Angiotensin System. Molecules 2022, 27, 2903. [Google Scholar] [CrossRef]
- Dennis, A.; Wamil, M.; Alberts, J.; Oben, J.; Cuthbertson, D.J.; Wootton, D.; Crooks, M.; Gabbay, M.; Brady, M.; Hishmeh, L.; et al. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: A prospective, community-based study. BMJ Open 2021, 11, e048391. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022, 28, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Al-Aly, Z. Risks and burdens of incident diabetes in long COVID: A cohort study. Lancet Diabetes Endocrinol. 2022, 10, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Mancini, D.M.; Brunjes, D.L.; Lala, A.; Trivieri, M.G.; Contreras, J.P.; Natelson, B.H. Use of cardiopulmonary stress testing for patients with unexplained dyspnea post–coronavirus disease. JACC Heart Fail. 2021, 9, 927–937. [Google Scholar] [CrossRef]
- Kedor, C.; Freitag, H.; Meyer-Arndt, L.; Wittke, K.; Hanitsch, L.G.; Zoller, T.; Steinbeis, F.; Haffke, M.; Rudolf, G.; Heidecker, B.; et al. A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat. Commun. 2022, 13, 5104. [Google Scholar] [CrossRef] [PubMed]
- Larsen, N.W.; Stiles, L.E.; Shaik, R.; Schneider, L.; Muppidi, S.; Tsui, C.T.; Geng, L.N.; Bonilla, H.; Miglis, M.G. Characterization of autonomic symptom burden in long COVID: A global survey of 2,314 adults. Front. Neurol. 2022, 13, 1012668. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Alimohamadi, Y.; Sepandi, M.; Taghdir, M.; Hosamirudsari, H. Determine the most common clinical symptoms in COVID-19 patients: A systematic review and meta-analysis. J. Prev. Med. Hyg. 2020, 61, E304–E312. [Google Scholar]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef]
- Amanzio, M.; Mitsikostas, D.D.; Giovannelli, F.; Bartoli, M.; Cipriani, G.E.; Brown, W.A. Adverse events of active and placebo groups in SARS-CoV-2 vaccine randomized trials: A systematic review. Lancet Reg. Health Eur. 2022, 12, 100253. [Google Scholar] [CrossRef]
- Sriwastava, S.; Shrestha, A.K.; Khalid, S.H.; Colantonio, M.A.; Nwafor, D.; Srivastava, S. Spectrum of Neuroimaging Findings in Post-COVID-19 Vaccination: A Case Series and Review of Literature. Neurol. Int. 2021, 13, 622–639. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J. Neurological side effects of SARS-CoV-2 vaccinations. Acta Neurol. Scand. 2022, 145, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Merzon, E.; Weiss, M.; Krone, B.; Cohen, S.; Ilani, G.; Vinker, S.; Cohen-Golan, A.; Green, I.; Israel, A.; Schneider, T.; et al. Clinical and Socio-Demographic Variables Associated with the Diagnosis of Long COVID Syndrome in Youth: A Population-Based Study. Int. J. Environ. Res. Public Health 2022, 19, 5993. [Google Scholar] [CrossRef]
- Fitzpatrick, T.; Yamoah, P.; Summerby-Murray, D.; Cowan, J.; Sadarangani, M.; Wright, A.; Belga, S.; Constantinescu, C.; Carignan, A.; McConnell, A.; et al. Canadian Immunization Research Network Investigators. Neurological adverse events following COVID-19 vaccination among Canadians referred to the special immunization clinic network. Vaccine 2025, 59, 127254. [Google Scholar] [CrossRef]
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav. Immun. 2022, 101, 93–135. [Google Scholar] [CrossRef] [PubMed]
- Shafqat, A.; Masters, M.C.; Tripathi, U.; Tchkonia, T.; Kirkland, J.L.; Hashmi, S.K. Long COVID as a disease of accelerated biological aging: An opportunity to translate geroscience interventions. Ageing Res. Rev. 2024, 99, 102400. [Google Scholar] [CrossRef]
- Al-Aly, Z.; Xie, Y.; Bowe, B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021, 594, 259–264. [Google Scholar] [CrossRef]
- Seighali, N.; Abdollahi, A.; Shafiee, A.; Amini, M.J.; Teymouri Athar, M.M.; Safari, O.; Faghfouri, P.; Eskandari, A.; Rostaii, O.; Salehi, A.H.; et al. The global prevalence of depression, anxiety, and sleep disorder among patients coping with Post COVID-19 syndrome (long COVID): A systematic review and meta-analysis. BMC Psychiatry 2024, 24, 105. [Google Scholar]
- Kim, H.J.; Kim, M.H.; Choi, M.G.; Chun, E.M. Psychiatric adverse events following COVID-19 vaccination: A population-based cohort study in Seoul, South Korea. Mol. Psychiatry 2024, 29, 3635–3643. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, I.; Faheem, A.; Padhy, S.K.; Menon, V. Psychiatric adverse reactions to COVID-19 vaccines: A rapid review of published case reports. Asian J. Psychiatr. 2022, 71, 103129. [Google Scholar]
- Ozturk, M.; Kumova Guler, D.; Oskan, E.E.; Onder, F. Long-Term Effects of COVID-19 on Optic Disc and Retinal Microvasculature Assessed by Optical Coherence Tomography Angiography. Diagnostics 2025, 15, 114. [Google Scholar] [CrossRef]
- Srivastava, A.; Nalroad Sundararaj, S.; Bhatia, J.; Singh Arya, D. Understanding long COVID myocarditis: A comprehensive review. Cytokine 2024, 178, 156584. [Google Scholar] [CrossRef]
- Buoninfante, A.; Andeweg, A.; Genov, G.; Cavaleri, M. Myocarditis associated with COVID-19 vaccination. Npj Vaccines 2024, 9, 122. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Martin, S.; Shchendrygina, A.; Hoffmann, J.; Ka, M.M.; Giokoglu, E.; Vanchin, B.; Holm, N.; Karyou, A.; Laux, G.S.; et al. Long-term cardiac pathology in individuals with mild initial COVID-19 illness. Nat. Med. 2022, 28, 2117–2123. [Google Scholar] [CrossRef]
- Le Vu, S.; Bertrand, M.; Jabagi, M.J.; Botton, J.; Drouin, J.; Baricault, B.; Weill, A.; Dray-Spira, R.; Zureik, M. Age and sex-specific risks of myocarditis and pericarditis following Covid-19 messenger RNA vaccines. Nat. Commun. 2022, 13, 3633. [Google Scholar] [CrossRef]
- Diaz, G.A.; Parsons, G.T.; Gering, S.K.; Meier, A.R.; Hutchinson, I.V.; Robicsek, A. Myocarditis and Pericarditis After Vaccination for COVID-19. JAMA 2021, 326, 1210–1212. [Google Scholar] [CrossRef] [PubMed]
- Paruchuri, S.S.H.; Farwa, U.E.; Jabeen, S.; Pamecha, S.; Shan, Z.; Parekh, R.; Lakkimsetti, M.; Alamin, E.; Sharma, V.; Haider, S.; et al. Myocarditis and Myocardial Injury in Long COVID Syndrome: A Comprehensive Review of the Literature. Cureus 2023, 15, e42444. [Google Scholar] [CrossRef] [PubMed]
- Iwu, C.J.; Jordan, T.; Jaca, A.; George, G.; Hansoti, B.; Wiysonge, C.S. Global, Regional, and National Incidence and Mortality of COVID-19 in 237 Countries and Territories, January 2022: A Systematic Analysis for World Health Organization COVID-19 Dashboard. BMJ Glob. Health 2022, 7, e009531. [Google Scholar]
- Turner, S.; Khan, M.A.; Putrino, D.; Woodcock, A.; Kell, D.B.; Pretorius, E. Long COVID: Pathophysiological factors and abnormalities of coagulation. Trends Endocrinol. Metab. 2023, 34, 321–344. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J. Post-SARS-CoV-2 vaccination thrombosis is frequent and ubiquitous. Indian. J. Ophthalmol. 2022, 70, 1864. [Google Scholar] [CrossRef]
- McCullough, P.A.; Hulscher, N. Risk stratification for future cardiac arrest after COVID-19 vaccination. World J. Cardiol. 2025, 17, 103909. [Google Scholar] [CrossRef]
- Pari, B.; Babbili, A.; Kattubadi, A.; Thakre, A.; Thotamgari, S.; Gopinathannair, R.; Olshansky, B.; Dominic, P. COVID-19 Vaccination and Cardiac Arrhythmias: A Review. Curr. Cardiol. Rep. 2023, 25, 925–940. [Google Scholar] [CrossRef]
- Angeli, F.; Reboldi, G.; Zappa, M.; Verdecchia, P. Hypertension and myocarditis following COVID-19 vaccination. Two Sides Coin? Eur. J. Intern. Med. 2023, 113, 107–109. [Google Scholar] [CrossRef]
- Ota, N.; Itani, M.; Aoki, T.; Sakurai, A.; Fujisawa, T.; Okada, Y.; Noda, K.; Arakawa, Y.; Tokuda, S.; Tanikawa, R. Expression of SARS-CoV-2 spike protein in cerebral Arteries: Implications for hemorrhagic stroke Post-mRNA vaccination. J. Clin. Neurosci. 2025, 136, 111223. [Google Scholar] [CrossRef]
- Afrin, L.B.; Weinstock, L.B.; Molderings, G.J. Covid-19 Hyperinflammation and post-COVID-19 illness may be rooted in mast cell activation syndrome. Int. J. Infect. Dis. 2020, 100, 327–332. [Google Scholar] [CrossRef]
- Weinstock, L.B.; Brook, J.B.; Walters, A.S.; Goris, A.; Afrin, L.B.; Molderings, G.J. Mast cell activation symptoms are prevalent in long-COVID. Int. J. Infect. Dis. 2021, 112, 217–226. [Google Scholar] [CrossRef]
- Nazy, I.; Jevtic, S.D.; Moore, J.C.; Huynh, A.; Smith, J.W.; Kelton, J.G.; Arnold, D.M. Platelet-activating immune complexes identified in critically ill COVID-19 patients suspected of heparin-induced thrombocytopenia. J. Thromb. Haemost. 2021, 19, 1342–1347. [Google Scholar] [CrossRef]
- Siddiqi, H.K.; Mehra, M.R. COVID-19 illness in native and immunosuppressed States: A clinical-therapeutic staging proposal. J. Heart Lung Transplant. 2020, 39, 405–407. [Google Scholar] [CrossRef]
- Bassler, K.; Schulte-Schrepping, J.; Warnat-Herresthal, S.; Aschenbrenner, A.C.; Schultze, J.L. The myeloid cell compartment-cell by cell. Annu. Rev. Immunol. 2019, 37, 269–293. [Google Scholar] [CrossRef] [PubMed]
- Franzblau, L.E.; Mauskar, M.; Wysocki, C.A. Macrophage Activation Syndrome Complicated by Toxic Epidermal Necrolysis Following SARS-CoV-2 mRNA Vaccination. J. Clin. Immunol. 2023, 43, 521–524. [Google Scholar]
- Halpin, S.J.; McIvor, C.; Whyatt, G.; Adams, A.; Harvey, O.; McLean, L.; Walshaw, C.; Kemp, S.; Corrado, J.; Singh, R.; et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: A cross-sectional evaluation. J. Med. Virol. 2021, 93, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Patel, K.; Greenwood, D.C.; Halpin, S.; Lewthwaite, P.; Salawu, A.; Eyre, L.; Breen, A.; Jones, A.; Sivan, M. Long-term clinical outcomes in survivors of severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) coronavirus outbreaks after hospitalisation or ICU admission: A systematic review and meta-analysis. medRxiv 2020. [Google Scholar] [CrossRef]
- Ayoubkhani, D.; Khunti, K.; Nafilyan, V.; Maddox, T.; Humberstone, B.; Diamond, I.; Banerjee, A. Post-covid syndrome in individuals admitted to hospital with covid-19: Retrospective cohort study. BMJ 2021, 372, n693. [Google Scholar] [CrossRef]
- Demko, Z.O.; Yu, T.; Mullapudi, S.K.; Varela Heslin, M.G.; Dorsey, C.A.; Payton, C.B.; Tornheim, J.A.; Blair, P.W.; Mehta, S.H.; Thomas, D.L.; et al. Post-acute sequelae of SARS-CoV-2 (PASC) impact quality of life at 6, 12 and 18 months post-infection. medRxiv 2022. [Google Scholar] [CrossRef]
- Bach, K. Is ‘Long Covid’ Worsening the Labor Shortage? 2022. Available online: https://www.brookings.edu/research/is-long-covid-worsening-the-labor-shortage/ (accessed on 11 January 2022).
- Arjun, M.C.; Singh, A.K.; Pal, D.; Das, K.; G, A.; Venkateshan, M.; Mishra, B.; Patro, B.K.; Mohapatra, P.R.; Subba, S.H. Characteristics and predictors of Long COVID among diagnosed cases of COVID-19. PLoS ONE 2022, 17, e0278825. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Yuan, D.; Chen, D.G.; Ng, R.H.; Wang, K.; Choi, J.; Li, S.; Hong, S.; Zhang, R.; Xie, J.; et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 2022, 185, 881–895.e20. [Google Scholar] [CrossRef]
- Renz-Polster, H.; Tremblay, M.-E.; Bienzle, D.; Fischer, J.E. The pathobiology of myalgic encephalomyelitis/chronic fatigue syndrome: The case for neuroglial failure. Front. Cell. Neurosci. 2022, 16, 888232. [Google Scholar] [CrossRef] [PubMed]
- FAIR Health. Patients Diagnosed with Post-COVID Conditions: An Analysis of Private Healthcare Claims Using the Official ICD-10 Diagnostic Code (FAIR Health, 2022). Available online: https://collections.nlm.nih.gov/catalog/nlm:nlmuid-9918504887106676-pdf (accessed on 18 May 2022).
- Kang, H.; Wang, Y.; Tong, Z.; Liu, X. Retest positive for SARS-CoV-2 RNA of “recovered” patients with COVID-19: Persistence, sampling issues, or reinfection? J. Med. Virol. 2020, 92, 2263–2265. [Google Scholar] [CrossRef]
- Fajloun, Z.; Kovacic, H.; Annweiler, C.; Wu, Y.; Cao, Z.; Sabatier, J.M. SARS-CoV-2-Induced Neurological Disorders in Symptomatic Covid-19 and Long Covid Patients: Key Role of Brain Renin-Angiotensin System. Infect. Disord. Drug Targets 2022, 22, e060422203203. [Google Scholar] [CrossRef] [PubMed]
- Swank, Z.; Senussi, Y.; Manickas-Hill, Z.; Yu, X.G.; Li, J.Z.; Alter, G.; Walt, D.R. Persistent Circulating Severe Acute Respiratory Syndrome Coronavirus 2 Spike Is Associated With Post-acute Coronavirus Disease 2019 Sequelae. Clin. Infect. Dis. 2023, 76, e487–e490. [Google Scholar] [CrossRef]
- Proal, A.D.; VanElzakker, M.B. Long COVID or post-acute sequelae of COVID-19 (PASC): An overview of biological factors that may contribute to persistent symptoms. Front. Microbiol. 2021, 12, 698169. [Google Scholar] [CrossRef]
- Tejerina, F.; Catalan, P.; Rodriguez-Grande, C.; Adan, J.; Rodriguez-Gonzalez, C.; Muñoz, P.; Aldamiz, T.; Diez, C.; Perez, L.; Fanciulli, C.; et al. Gregorio Marañon Microbiology ID COVID 19 Study Group. Post-COVID-19 syndrome. SARS-CoV-2 RNA detection in plasma, stool, and urine in patients with persistent symptoms after COVID-19. BMC Infect. Dis. 2022, 22, 211. [Google Scholar] [CrossRef]
- Cheung, C.C.L.; Goh, D.; Lim, X.; Tien, T.Z.; Lim, J.C.T.; Lee, J.N.; Tan, B.; Tay, Z.E.A.; Wan, W.Y.; Chen, E.X.; et al. Residual SARS-CoV-2 viral antigens detected in GI and hepatic tissues from five recovered patients with COVID-19. Gut 2022, 71, 226–229, Erratum in Gut 2022, 71, e9. [Google Scholar] [CrossRef]
- Swank, Z.; Borberg, E.; Chen, Y.; Senussi, Y.; Chalise, S.; Manickas-Hill, Z.; Yu, X.G.; Li, J.Z.; Alter, G.; Henrich, T.J.; et al. RECOVER consortium authors. Measurement of circulating viral antigens post-SARS-CoV-2 infection in a multicohort study. Clin. Microbiol. Infect. 2024, 30, 1599–1605. [Google Scholar] [CrossRef] [PubMed]
- Goh, D.; Lim, J.C.T.; Fernaíndez, S.B.; Joseph, C.R.; Edwards, S.G.; Neo, Z.W.; Lee, J.N.; Caballero, S.G.; Lau, M.C.; Yeong, J.P.S. Case report: Persistence of residual antigen and RNA of the SARS-CoV-2 virus in tissues of two patients with long COVID. Front. Immunol. 2022, 13, 939989. [Google Scholar]
- Gaebler, C.; Wang, Z.; Lorenzi, J.C.C.; Muecksch, F.; Finkin, S.; Tokuyama, M.; Cho, A.; Jankovic, M.; Schaefer-Babajew, D.; Oliveira, T.Y.; et al. Evolution of antibody immunity to SARS-CoV-2. Nature 2021, 591, 639–644. [Google Scholar] [CrossRef]
- Natarajan, A.; Zlitni, S.; Brooks, E.F.; Vance, S.E.; Dahlen, A.; Hedlin, H.; Park, R.M.; Han, A.; Schmidtke, D.T.; Verma, R.; et al. Gastrointestinal symptoms and fecal shedding of SARS-CoV-2 RNA suggest prolonged gastrointestinal infection. Med 2022, 3, 371–387.e9. [Google Scholar] [CrossRef]
- Griffin, D.E. Why does viral RNA sometimes persist after recovery from acute infections? PLoS Biol. 2022, 20, e3001687. [Google Scholar] [CrossRef]
- The Human Protein Atlas. Tissue Expression of ACE2-Summary. Available online: https://www.proteinatlas.org/ENSG00000130234-ACE2/tissue (accessed on 6 June 2023).
- Martínez-Colón, G.J.; Ratnasiri, K.; Chen, H.; Jiang, S.; Zanley, E.; Rustagi, A.; Verma, R.; Chen, H.; Andrews, J.R.; Mertz, K.D.; et al. SARS-CoV-2 infection drives an inflammatory response in human adipose tissue through infection of adipocytes and macrophages. Sci. Transl. Med. 2022, 14, eabm9151. [Google Scholar] [CrossRef]
- Zollner, A.; Koch, R.; Jukic, A.; Pfister, A.; Meyer, M.; Rössler, A.; Kimpel, J.; Adolph, T.E.; Tilg, H. Postacute COVID-19 is Characterized by Gut Viral Antigen Persistence in Inflammatory Bowel Diseases. Gastroenterology 2022, 163, 495–506.e8. [Google Scholar] [CrossRef]
- Zuo, W.; He, D.; Liang, C.; Du, S.; Hua, Z.; Nie, Q.; Zhou, X.; Yang, M.; Tan, H.; Xu, J.; et al. The persistence of SARS-CoV-2 in tissues and its association with long COVID symptoms: A cross-sectional cohort study in China. Lancet Infect. Dis. 2024, 24, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Zanini, G.; Selleri, V.; Roncati, L.; Coppi, F.; Nasi, M.; Farinetti, A.; Manenti, A.; Pinti, M.; Mattioli, A.V. Vascular “Long COVID”: A New Vessel Disease? Angiology 2024, 75, 8–14. [Google Scholar] [CrossRef]
- Haffke, M.; Freitag, H.; Rudolf, G.; Seifert, M.; Doehner, W.; Scherbakov, N.; Hanitsch, L.; Wittke, K.; Bauer, S.; Konietschke, F.; et al. Endothelial dysfunction and altered endothelial biomarkers in patients with post-COVID-19 syndrome and chronic fatigue syndrome (ME/CFS). J. Transl. Med. 2022, 20, 138. [Google Scholar] [CrossRef] [PubMed]
- Charfeddine, S.; Ibn Hadj Amor, H.; Jdidi, J.; Torjmen, S.; Kraiem, S.; Hammami, R.; Bahloul, A.; Kallel, N.; Moussa, N.; Touil, I.; et al. Long COVID 19 Syndrome: Is It Related to Microcirculation and Endothelial Dysfunction? Insights From TUN-EndCOV Study. Front. Cardiovasc. Med. 2021, 8, 745758. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, E.; Venter, C.; Laubscher, G.J.; Kotze, M.J.; Oladejo, S.O.; Watson, L.R.; Rajaratnam, K.; Watson, B.W.; Kell, D.B. Prevalence of symptoms, comorbidities, fibrin amyloid microclots and platelet pathology in individuals with Long COVID/post-acute sequelae of COVID-19 (PASC). Cardiovasc. Diabetol. 2022, 21, 148. [Google Scholar] [CrossRef]
- Ingul, C.B.; Grimsmo, J.; Mecinaj, A.; Trebinjac, D.; Berger Nossen, M.; Andrup, S.; Grenne, B.; Dalen, H.; Einvik, G.; Stavem, K.; et al. Cardiac Dysfunction and Arrhythmias 3 Months After Hospitalization for COVID-19. J. Am. Heart Assoc. 2022, 11, e023473. [Google Scholar] [CrossRef]
- Faverio, P.; Luppi, F.; Rebora, P.; D’Andrea, G.; Stainer, A.; Busnelli, S.; Catalano, M.; Modafferi, G.; Franco, G.; Monzani, A.; et al. One-year pulmonary impairment after severe COVID-19: A prospective, multicenter follow-up study. Respir Res. 2022, 23, 65. [Google Scholar] [CrossRef]
- Corsi, A.; Caroli, A.; Bonaffini, P.A.; Conti, C.; Arrigoni, A.; Mercanzin, E.; Imeri, G.; Anelli, M.; Balbi, M.; Pace, M.; et al. Structural and Functional Pulmonary Assessment in Severe COVID-19 Survivors at 12 Months after Discharge. Tomography 2022, 8, 2588–2603. [Google Scholar] [CrossRef]
- Aranda, J.; Oriol, I.; Feria, L.; Abelenda, G.; Rombauts, A.; Simonetti, A.F.; Catalano, C.; Pallarès, N.; Martín, M.; Vàzquez, N.; et al. Persistent COVID-19 symptoms 1 year after hospital discharge: A prospective multicenter study. PLoS ONE 2022, 17, e0275615. [Google Scholar] [CrossRef]
- Guedj, E.; Campion, J.Y.; Dudouet, P.; Kaphan, E.; Bregeon, F.; Tissot-Dupont, H.; Guis, S.; Barthelemy, F.; Habert, P.; Ceccaldi, M.; et al. 18F-FDG brain PET hypometabolism in patients with long COVID. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2823–2833. [Google Scholar] [CrossRef] [PubMed]
- Vélez, M.; Falconí Paez, A.; Nicolalde, B.; Esquetini-Vernon, C.; Lara-Taranchenko, Y.; Zambrano, K.; Caicedo, A. Cognitive impairment or dementia in post-acute COVID-19 syndrome. Two suspects and a perfect detective: Positron emission tomography (PET) scan. Eur. Neuropsychopharmacol. 2022, 61, 91–93. [Google Scholar] [CrossRef]
- Sollini, M.; Morbelli, S.; Ciccarelli, M.; Cecconi, M.; Aghemo, A.; Morelli, P.; Chiola, S.; Gelardi, F.; Chiti, A. Long COVID hallmarks on [18F]FDG-PET/CT: A case-control study. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3187–3197. [Google Scholar] [CrossRef]
- Colarusso, C.; Terlizzi, M.; Maglio, A.; Molino, A.; Candia, C.; Vitale, C.; Hansbro, P.M.; Vatrella, A.; Pinto, A.; Sorrentino, R. Activation of the AIM2 receptor in circulating cells of post-COVID-19 patients with signs of lung fibrosis is associated with the release of IL-1α, IFN-α and TGF-β. Front Immunol. 2022, 13, 934264. [Google Scholar] [CrossRef] [PubMed]
- Castanares-Zapatero, D.; Chalon, P.; Kohn, L.; Dauvrin, M.; Detollenaere, J.; Maertens de Noordhout, C.; Primus-de Jong, C.; Cleemput, I.; Van den Heede, K. Pathophysiology and mechanism of long COVID: A comprehensive review. Ann. Med. 2022, 54, 1473–1487. [Google Scholar] [CrossRef] [PubMed]
- Frere, J.J.; Serafini, R.A.; Pryce, K.D.; Zazhytska, M.; Oishi, K.; Golynker, I.; Panis, M.; Zimering, J.; Horiuchi, S.; Hoagland, D.A.; et al. SARS-CoV-2 infection in hamsters and humans results in lasting and unique systemic perturbations after recovery. Sci. Transl. Med. 2022, 14, eabq3059. [Google Scholar] [CrossRef] [PubMed]
- Käufer, C.; Schreiber, C.S.; Hartke, A.S.; Denden, I.; Stanelle-Bertram, S.; Beck, S.; Kouassi, N.M.; Beythien, G.; Becker, K.; Schreiner, T.; et al. Microgliosis and neuronal proteinopathy in brain persist beyond viral clearance in SARS-CoV-2 hamster model. EBioMedicine 2022, 79, 103999. [Google Scholar] [CrossRef]
- Besteher, B.; Machnik, M.; Troll, M.; Toepffer, A.; Zerekidze, A.; Rocktäschel, T.; Heller, C.; Kikinis, Z.; Brodoehl, S.; Finke, K.; et al. Larger gray matter volumes in neuropsychiatric long-COVID syndrome. Psychiatry Res. 2022, 317, 114836. [Google Scholar] [CrossRef]
- Bramante, C.T.; Buse, J.B.; Liebovitz, D.M.; Nicklas, J.M.; Puskarich, M.A.; Cohen, K.; Belani, H.K.; Anderson, B.J.; Huling, J.D.; Tignanelli, C.J.; et al. COVID-OUT Study Team. Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): A multicentre, randomised, quadruple-blind, parallel-group, phase 3 trial. Lancet Infect. Dis. 2023, 23, 1119–1129. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Y.; Ci, X.; An, N.; Ju, Y.; Li, H.; Wang, X.; Han, C.; Cui, J.; Deng, X. Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice. Inflamm. Res. 2008, 57, 524–529. [Google Scholar] [CrossRef]
- Ci, X.; Li, H.; Yu, Q.; Zhang, X.; Yu, L.; Chen, N.; Song, Y.; Deng, X. Ivermectin exerts anti-inflammatory effect by downregulating the nuclear transcription factor kappa-B and mitogen-activated protein kinase activation pathway. Fundam. Clin. Pharmacol. 2009, 23, 449–455. [Google Scholar] [CrossRef]
- Kingeter, L.M.; Lin, X. C-type lectin receptor-induced NF-κB activation in innate immune and inflammatory responses. Cell Mol. Immunol. 2012, 9, 105–112. [Google Scholar] [CrossRef]
- Arthur, J.M.; Forrest, J.C.; Boehme, K.W.; Kennedy, J.L.; Owens, S.; Herzog, C.; Liu, J.; Harville, T.O. Development of ACE2 autoantibodies after SARS-CoV-2 infection. PLoS ONE 2021, 16, e0257016, Erratum in PLoS ONE 2024, 19, e0314426. [Google Scholar] [CrossRef]
- Wallukat, G.; Hohberger, B.; Wenzel, K.; Fürst, J.; Schulze-Rothe, S.; Wallukat, A.; Hönicke, A.S.; Müller, J. Functional autoantibodies against G-protein coupled receptors in patients with persistent Long-COVID-19 symptoms. J. Transl. Autoimmun. 2021, 4, 100100. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.; Wood, J.; Jaycox, J.R.; Dhodapkar, R.M.; Lu, P.; Gehlhausen, J.R.; Tabachnikova, A.; Greene, K.; Tabacof, L.; Malik, A.A.; et al. Distinguishing features of long COVID identified through immune profiling. Nature 2023, 623, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Glynne, P.; Tahmasebi, N.; Gant, V.; Gupta, R. Long COVID following mild SARS-CoV-2 infection: Characteristic T cell alterations and response to antihistamines. J. Investig. Med. 2022, 70, 61–67. [Google Scholar] [CrossRef]
- Phetsouphanh, C.; Darley, D.R.; Wilson, D.B.; Howe, A.; Munier, C.M.L.; Patel, S.K.; Juno, J.A.; Burrell, L.M.; Kent, S.J.; Dore, G.J.; et al. Immunological dysfunction persists for 8 months following initial mild-tomoderate SARS-CoV-2 infection. Nat. Immunol. 2022, 23, 210–216. [Google Scholar] [CrossRef]
- Patterson, B.K.; Francisco, E.B.; Yogendra, R.; Long, E.; Pise, A.; Rodrigues, H.; Hall, E.; Herrera, M.; Parikh, P.; Guevara-Coto, J.; et al. Persistence of SARS CoV-2 S1 protein in Cd16+ monocytes in post-acute sequelae of COVID-19 (PASC) up to 15 months post-infection. Front. Immunol. 2021, 12, 746021. [Google Scholar] [CrossRef]
- Peluso, M.J.; Deeks, S.J. Mechanisms of long COVID and the path toward therapeutics. Cell 2024, 187, 5500–5529. [Google Scholar] [CrossRef]
- Georgopoulos, A.P.; James, L.M.; Peterson, P.K. Human Leukocyte Antigen (HLA) at the Root of Persistent Antigens and Long COVID. J. Immunol. Sci. 2025, 9, 1–3. [Google Scholar] [CrossRef]
- Fakhkhari, M.; Caidi, H.; Sadki, K. HLA alleles associated with COVID-19 susceptibility and severity in different populations: A systematic review. Egypt. J. Med. Hum. Genet. 2023, 24, 10. [Google Scholar] [CrossRef]
- Tavasolian, F.; Rashidi, M.; Hatam, G.R.; Jeddi, M.; Hosseini, A.Z.; Mosawi, S.H.; Abdollahi, E.; Inman, R.D. HLA, Immune Response, and Susceptibility to COVID-19. Front. Immunol. 2021, 11, 601886. [Google Scholar] [CrossRef]
- Rohrhofer, J.; Graninger, M.; Lettenmaier, L.; Schweighardt, J.; Gentile, S.A.; Koidl, L.; Ret, D.; Stingl, M.; Puchhammer-Stöckl, E.; Untersmayr, E. Association between Epstein-Barr-Virus reactivation and development of Long-COVID fatigue. Allergy 2023, 78, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Song, J.; Liu, H.; Zheng, H.; Chen, C. Positive Epstein-Barr virus detection in coronavirus disease 2019 (COVID-19) patients. Sci. Rep. 2021, 11, 10902. [Google Scholar] [CrossRef]
- Gold, J.E.; Okyay, R.A.; Licht, W.E.; Hurley, D.J. Investigation of Long COVID Prevalence and Its Relationship to Epstein-Barr Virus Reactivation. Pathogens 2021, 10, 763. [Google Scholar] [CrossRef] [PubMed]
- Peluso, M.J.; Deveau, T.M.; Munter, S.E.; Ryder, D.; Buck, A.; Beck-Engeser, G.; Chan, F.; Lu, S.; Goldberg, S.A.; Hoh, R.; et al. Chronic viral coinfections differentially affect the likelihood of developing long COVID. J. Clin. Investig. 2023, 133, e163669. [Google Scholar] [CrossRef]
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.; Zhang, F.; Liu, Q.; Li, A.Y.; Chung, A.C.; Cheung, C.P.; Tso, E.Y.; Fung, K.S.; et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 2021, 70, 698–706. [Google Scholar] [CrossRef]
- Liu, Q.; Mak, J.W.Y.; Su, Q.; Yeoh, Y.K.; Lui, G.C.; Ng, S.S.S.; Zhang, F.; Li, A.Y.L.; Lu, W.; Hui, D.S. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut 2022, 71, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Hazan, S.; Stollman, N.; Bozkurt, H.S.; Dave, S.; Papoutsis, A.J.; Daniels, J.; Barrows, B.D.; Quigley, E.M.; Borody, T.J. Lost microbes of COVID-19: Bifidobacterium, Faecalibacterium depletion and decreased microbiome diversity associated with SARS-CoV-2 infection severity. BMJ Open Gastroenterol. 2022, 9, e000871. [Google Scholar] [CrossRef]
- Hilpert, K.; Mikut, R. Is There a Connection Between Gut Microbiome Dysbiosis Occurring in COVID-19 Patients and Post-COVID-19 Symptoms? Front. Microbiol. 2021, 12, 732838. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.T.; Khan, H.; Khalid, A.; Mahmood, S.F.; Nasir, N.; Khanum, I.; de Siqueira, I.; Van Voorhis, W. Chronic inflammation in post-acute sequelae of COVID-19 modulates gut microbiome: A review of literature on COVID-19 sequelae and gut dysbiosis. Mol. Med. 2025, 31, 22. [Google Scholar] [CrossRef]
- Zhang, L.; Richards, A.; Barrasa, M.I.; Hughes, S.H.; Young, R.A.; Jaenisch, R. Reverse-transcribed SARS-CoV-2 RNA can integrate into the genome of cultured human cells and can be expressed in patient-derived tissues. Proc. Natl. Acad. Sci. USA 2021, 118, e2105968118. [Google Scholar] [CrossRef]
- Aldén, M.; Olofsson Falla, F.; Yang, D.; Barghouth, M.; Luan, C.; Rasmussen, M.; De Marinis, Y. Intracellular Reverse Transcription of Pfizer BioNTech COVID-19 mRNA Vaccine BNT162b2 In Vitro in Human Liver Cell Line. Curr. Issues Mol. Biol. 2022, 44, 1115–1126. [Google Scholar] [CrossRef]
- Platschek, B.; Boege, F. The Post-Acute COVID-19-Vaccination Syndrome in the Light of Pharmacovigilance. Vaccines 2024, 12, 1378. [Google Scholar] [CrossRef]
- Yaamika, H.; Muralidas, D.; Elumalai, K. Review of adverse events associated with COVID-19 vaccines, highlighting their frequencies and reported cases. J. Taibah Univ. Med. Sci. 2023, 18, 1646–1661. [Google Scholar] [CrossRef]
- Parry, P.I.; Lefringhausen, A.; Turni, C.; Neil, C.J.; Cosford, R.; Hudson, N.J.; Gillespie, J. ‘Spikeopathy’: COVID-19 Spike Protein Is Pathogenic, from Both Virus and Vaccine mRNA. Biomedicines 2023, 11, 2287. [Google Scholar] [CrossRef]
- Riad, A.; Pokorná, A.; Attia, S.; Klugarová, J.; Koščík, M.; Klugar, M. Prevalence of COVID-19 Vaccine Side Effects among Healthcare Workers in the Czech Republic. J. Clin. Med. 2021, 10, 1428. [Google Scholar] [CrossRef]
- Klugar, M.; Riad, A.; Mekhemar, M.; Conrad, J.; Buchbender, M.; Howaldt, H.P.; Attia, S. Side Effects of mRNA-Based and Viral Vector-Based COVID-19 Vaccines among German Healthcare Workers. Biology 2021, 10, 752. [Google Scholar] [CrossRef]
- Luxi, N.; Giovanazzi, A.; Arcolaci, A.; Bonadonna, P.; Crivellaro, M.A.; Cutroneo, P.M.; Ferrajolo, C.; Furci, F.; Guidolin, L.; Moretti, U.; et al. Allergic Reactions to COVID-19 Vaccines: Risk Factors, Frequency, Mechanisms and Management. BioDrugs 2022, 36, 443–458. [Google Scholar] [CrossRef]
- Sobczak, M.; Pawliczak, R. The risk of anaphylaxis behind authorized COVID-19 vaccines: A meta-analysis. Clin. Mol. Allergy 2022, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Akaishi, T.; Onodera, T.; Takahashi, T.; Harigae, H.; Ishii, T. Reports of acute adverse events in mRNA COVID-19 vaccine recipients after the first and second doses in Japan. Sci. Rep. 2022, 12, 15510. [Google Scholar] [CrossRef]
- Boufidou, F.; Hatziantoniou, S.; Theodoridou, K.; Maltezou, H.C.; Vasileiou, K.; Anastassopoulou, C.; Medić, S.; Tsakris, A. Anaphylactic Reactions to COVID-19 Vaccines: An Updated Assessment Based on Pharmacovigilance Data. Vaccines 2023, 11, 613. [Google Scholar] [CrossRef] [PubMed]
- Katsoularis, I.; Fonseca-Rodríguez, O.; Farrington, P.; Jerndal, H.; Lundevaller, E.H.; Sund, M.; Lindmark, K.; Fors Connolly, A.M. Risks of deep vein thrombosis, pulmonary embolism, and bleeding after covid-19: Nationwide self-controlled cases series and matched cohort study. BMJ 2022, 377, e069590. [Google Scholar] [CrossRef] [PubMed]
- Blagova, O.; Lutokhina, Y.; Kogan, E.; Kukleva, A.; Ainetdinova, D.; Novosadov, V.; Rud’, R.; Savina, P.; Zaitsev, A.; Fomin, V. Chronic biopsy proven post-COVID myoendocarditis with SARS-CoV-2 persistence and high level of antiheart antibodies. Clin. Cardiol. 2022, 45, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Ilonze, O.J.; Guglin, M.E. Myocarditis following COVID-19 vaccination in adolescents and adults: A cumulative experience of 2021. Heart Fail. Rev. 2022, 27, 2033–2043. [Google Scholar] [CrossRef] [PubMed]
- Park, D.Y.; An, S.; Kaur, A.; Malhotra, S.; Vij, A. Myocarditis after COVID-19 mRNA vaccination: A systematic review of case reports and case series. Clin. Cardiol. 2022, 45, 691–700. [Google Scholar] [CrossRef]
- Goyal, M.; Ray, I.; Mascarenhas, D.; Kunal, S.; Sachdeva, R.A.; Ish, P. Myocarditis post-SARS-CoV-2 vaccination: A systematic review. QJM 2023, 116, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Muthukumar, A.; Narasimhan, M.; Li, Q.Z.; Mahimainathan, L.; Hitto, I.; Fuda, F.; Batra, K.; Jiang, X.; Zhu, C.; Schoggins, J.; et al. In-Depth Evaluation of a Case of Presumed Myocarditis After the Second Dose of COVID-19 mRNA Vaccine. Circulation 2021, 144, 487–498. [Google Scholar] [CrossRef]
- Gautam, N.; Saluja, P.; Fudim, M.; Jambhekar, K.; Pandey, T.; Al’Aref, S. A Late Presentation of COVID-19 Vaccine-Induced Myocarditis. Cureus 2021, 13, e17890. [Google Scholar] [CrossRef]
- Mörz, M. A Case Report: Multifocal Necrotizing Encephalitis and Myocarditis after BNT162b2 mRNA Vaccination against COVID-19. Vaccines 2022, 10, 1651. [Google Scholar] [CrossRef]
- Baumeier, C.; Aleshcheva, G.; Harms, D.; Gross, U.; Hamm, C.; Assmus, B.; Westenfeld, R.; Kelm, M.; Rammos, S.; Wenzel, P.; et al. Intramyocardial Inflammation after COVID-19 Vaccination: An Endomyocardial Biopsy-Proven Case Series. Int. J. Mol. Sci. 2022, 23, 6940. [Google Scholar] [CrossRef]
- Buchan, S.A.; Seo, C.Y.; Johnson, C.; Alley, S.; Kwong, J.C.; Nasreen, S.; Calzavara, A.; Lu, D.; Harris, T.M.; Yu, K.; et al. Epidemiology of Myocarditis and Pericarditis Following mRNA Vaccination by Vaccine Product, Schedule, and Interdose Interval Among Adolescents and Adults in Ontario, Canada. JAMA Netw. Open. 2022, 5, e2218505. [Google Scholar] [CrossRef]
- Saleh, A.; Hayek, A.; Azar, L.; Tomasevic, D.; Falque, H.; Prieur, C.; Kochly, F.; Bonnefoy-Cudraz, E.; Mewton, N.; Bochaton, T. Safety of SARS-CoV-2 vaccination in people with a history of acute myocarditis. Eur. Heart J. Acute Cardiovasc. Care 2022, 11 (Suppl. 1), zuac041-124. [Google Scholar]
- Nicolai, L.; Kaiser, R.; Stark, K. Thromboinflammation in long COVID-the elusive key to postinfection sequelae? J. Thromb. Haemost. 2023, 21, 2020–2031. [Google Scholar] [CrossRef]
- Klok, F.A.; Kruip, M.J.H.A.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.A.M.P.J.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V.; et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020, 191, 145–147. [Google Scholar] [CrossRef]
- Levi, M.; Hunt, B.J. Thrombosis and coagulopathy in COVID-19: An illustrated review. Res. Pr. Thromb. Haemost. 2020, 4, 744–751. [Google Scholar] [CrossRef]
- Bilotta, C.; Perrone, G.; Adelfio, V.; Spatola, G.F.; Uzzo, M.L.; Argo, A.; Zerbo, S. COVID-19 Vaccine-Related Thrombosis: A Systematic Review and Exploratory Analysis. Front. Immunol. 2021, 12, 729251. [Google Scholar] [CrossRef]
- Brazete, C.; Aguiar, A.; Furtado, I.; Duarte, R. Thrombotic events and COVID-19 vaccines. Int. J. Tuberc. Lung Dis. 2021, 25, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Tobaiqy, M.; MacLure, K.; Elkout, H.; Stewart, D. Thrombotic Adverse Events Reported for Moderna, Pfizer and Oxford-AstraZeneca COVID-19 Vaccines: Comparison of Occurrence and Clinical Outcomes in the EudraVigilance Database. Vaccines 2021, 9, 1326. [Google Scholar] [CrossRef] [PubMed]
- Magro, C.; Mulvey, J.J.; Berlin, D.; Nuovo, G.; Salvatore, S.; Harp, J.; Baxter-Stoltzfus, A.; Laurence, J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl. Res. 2020, 220, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Llitjos, J.F.; Leclerc, M.; Chochois, C.; Monsallier, J.M.; Ramakers, M.; Auvray, M.; Merouani, K. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J. Thromb. Haemost. 2020, 18, 1743–1746. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Kubánková, M.; Hohberger, B.; Hoffmanns, J.; Fürst, J.; Herrmann, M.; Guck, J.; Kräter, M. Physical phenotype of blood cells is altered in COVID-19. Biophys. J. 2021, 120, 2838–2847. [Google Scholar] [CrossRef]
- Osiaevi, I.; Schulze, A.; Evers, G.; Harmening, K.; Vink, H.; Kümpers, P.; Mohr, M.; Rovas, A. Persistent capillary rarefication in long COVID syndrome. Angiogenesis 2023, 26, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef]
- Dionne, A.; Sperotto, F.; Chamberlain, S.; Baker, A.L.; Powell, A.J.; Prakash, A.; Castellanos, D.A.; Saleeb, S.F.; de Ferranti, S.D.; Newburger, J.W.; et al. Association of Myocarditis With BNT162b2 Messenger RNA COVID-19 Vaccine in a Case Series of Children. JAMA Cardiol. 2021, 6, 1446–1450. [Google Scholar] [CrossRef]
- Pretorius, E.; Vlok, M.; Venter, C.; Bezuidenhout, J.A.; Laubscher, G.J.; Steenkamp, J.; Kell, D.B. Persistent clotting protein pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc. Diabetol. 2021, 20, 172. [Google Scholar] [CrossRef]
- Ashmawy, R.; Hammouda, E.A.; El-Maradny, Y.A.; Aboelsaad, I.; Hussein, M.; Uversky, V.N.; Redwan, E.M. Interplay between Comorbidities and Long COVID: Challenges and Multidisciplinary Approaches. Biomolecules 2024, 14, 835. [Google Scholar] [CrossRef]
- Gonçalves de Andrade, E.; Šimončičová, E.; Carrier, M.; Vecchiarelli, H.A.; Robert, M.È.; Tremblay, M.È. Microglia Fighting for Neurological and Mental Health: On the Central Nervous System Frontline of COVID-19 Pandemic. Front. Cell Neurosci. 2021, 15, 647378. [Google Scholar] [CrossRef]
- Maiese, A.; Manetti, A.C.; Bosetti, C.; Del Duca, F.; La Russa, R.; Frati, P.; Di Paolo, M.; Turillazzi, E.; Fineschi, V. SARS-CoV-2 and the brain: A review of the current knowledge on neuropathology in COVID-19. Brain Pathol. 2021, 31, e13013. [Google Scholar] [CrossRef] [PubMed]
- Matschke, J.; Lütgehetmann, M.; Hagel, C.; Sperhake, J.P.; Schröder, A.S.; Edler, C.; Mushumba, H.; Fitzek, A.; Allweiss, L.; Dandri, M.; et al. Neuropathology of patients with COVID-19 in Germany: A post-mortem case series. Lancet Neurol. 2020, 19, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Baig, A.M.; Khaleeq, A.; Ali, U.; Syeda, H. Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanisms. ACS Chem. Neurosci. 2020, 11, 995–998. [Google Scholar] [CrossRef]
- Desforges, M.; Le Coupanec, A.; Dubeau, P.; Bourgouin, A.; Lajoie, L.; Dubé, M.; Talbot, P.J. Human Coronaviruses and Other Respiratory Viruses: Underestimated Opportunistic Pathogens of the Central Nervous System. Viruses 2019, 12, 14. [Google Scholar] [CrossRef]
- Pellegrini, L.; Albecka, A.; Mallery, D.L.; Kellner, M.J.; Paul, D.; Carter, A.P.; James, L.C.; Lancaster, M.A. SARS-CoV-2 Infects the Brain Choroid Plexus and Disrupts the Blood-CSF Barrier in Human Brain Organoids. Cell Stem Cell 2020, 27, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Paniz-Mondolfi, A.; Bryce, C.; Grimes, Z.; Gordon, R.E.; Reidy, J.; Lednicky, J.; Sordillo, E.M.; Fowkes, M. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J. Med. Virol. 2020, 92, 699–702. [Google Scholar] [CrossRef]
- Spudich, S.; Nath, A. Nervous system consequences of COVID-19. Science 2022, 375, 267–269. [Google Scholar] [CrossRef]
- Chen, R.; Wang, K.; Yu, J.; Howard, D.; French, L.; Chen, Z.; Wen, C.; Xu, Z. The Spatial and Cell-Type Distribution of SARS-CoV-2 Receptor ACE2 in the Human and Mouse Brains. Front. Neurol. 2021, 11, 573095. [Google Scholar] [CrossRef]
- Fernández-Castañeda, A.; Lu, P.; Geraghty, A.C.; Song, E.; Lee, M.H.; Wood, J.; O’Dea, M.R.; Dutton, S.; Shamardani, K.; Nwangwu, K.; et al. Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation. Cell 2022, 185, 2452–2468.e16. [Google Scholar] [CrossRef]
- Vasek, M.J.; Garber, C.; Dorsey, D.; Durrant, D.M.; Bollman, B.; Soung, A.; Yu, J.; Perez-Torres, C.; Frouin, A.; Wilton, D.K.A.; et al. A Complement-microglial axis drives synapse loss during virus-induced memory impairment. Nature 2016, 534, 538–543. [Google Scholar] [CrossRef]
- Low, R.N.; Low, R.J.; Akrami, A. A review of cytokine-based pathophysiology of long COVID symptoms. Front. Med. 2023, 10, 1011936. [Google Scholar] [CrossRef]
- de Melo, L.A.; Almeida-Santos, A.F. Neuropsychiatric Properties of the ACE2/Ang-(1-7)/Mas Pathway: A Brief Review. Protein Pept. Lett. 2020, 27, 476–483. [Google Scholar] [CrossRef]
- Almufarrij, I.; Munro, K.J. One year on: An updated systematic review of SARS-CoV-2, COVID-19 and audio-vestibular symptoms. Int. J. Audiol. 2021, 60, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Kandemirli, S.G.; Altundag, A.; Yildirim, D.; Tekcan Sanli, D.E.; Saatci, O. Olfactory Bulb MRI and Paranasal Sinus CT Findings in Persistent COVID-19 Anosmia. Acad. Radiol. 2021, 28, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Dani, M.; Dirksen, A.; Taraborrelli, P.; Torocastro, M.; Panagopoulos, D.; Sutton, R.; Lim, P.B. Autonomic dysfunction in ‘long COVID’: Rationale, physiology and management strategies. Clin. Med. 2021, 21, e63–e67. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J.; Scorza, C.A.; Scorza, F.A. Long post-COVID-vaccination syndrome manifesting as temperature-sensitive myalgia and hyper-CKemia. Clinics 2023, 78, 100175. [Google Scholar] [CrossRef]
- Yepes, M. Neurological Complications of SARS-CoV-2 Infection and COVID-19 Vaccines: From Molecular Mechanisms to Clinical Manifestations. Curr. Drug Targets 2022, 23, 1620–1638. [Google Scholar] [CrossRef]
- Pal, P.K.; Nalini, A.; Vengalil, S. Co-VAN study: COVID-19 vaccine associated neurological diseases- an experience from an apex neurosciences centre and review of the literature. J. Clin. Neurosci. 2023, 108, 37–75. [Google Scholar]
- Buzhdygan, T.P.; DeOre, B.J.; Baldwin-Leclair, A.; Bullock, T.A.; McGary, H.M.; Khan, J.A.; Razmpour, R.; Hale, J.F.; Galie, P.A.; Potula, R.; et al. The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood-brain barrier. Neurobiol. Dis. 2020, 146, 105131. [Google Scholar] [CrossRef]
- Rhea, E.M.; Logsdon, A.F.; Hansen, K.M.; Williams, L.M.; Reed, M.J.; Baumann, K.K.; Holden, S.J.; Raber, J.; Banks, W.A.; et Erickson, M.A. The S1 protein of SARS-CoV-2 crosses the blood-brain barrier in mice. Nat. Neurosci. 2021, 24, 368–378. [Google Scholar] [CrossRef]
- Bahl, K.; Senn, J.J.; Yuzhakov, O.; Bulychev, A.; Brito, L.A.; Hassett, K.J.; Laska, M.E.; Smith, M.; Almarsson, Ö.; Thompson, J.; et al. Preclinical and Clinical Demonstration of Immunogenicity by mRNA Vaccines against H10N8 and H7N9 Influenza Viruses. Mol. Ther. 2017, 25, 1316–1327. [Google Scholar] [CrossRef] [PubMed]
- EMA. Assessment Report. Comirnaty. 19 February 2021(2021a). Available online: https://www.ema.europa.eu/en/documents/assessment-report/comirnaty-epar-public-assessment-report_en.pdf (accessed on 19 February 2021).
- EMA. Assessment Report. COVID-19 Vaccine Moderna. 11 March (2021b). Available online: https://www.ema.europa.eu/en/documents/assessment-report/spikevax-previously-covid-19-vaccine-moderna-epar-public-assessment-report_en.pdf (accessed on 11 March 2021).
- Maroufi, S.F.; Naderi Behdani, F.; Rezania, F.; Tanhapour Khotbehsara, S.; Mirzaasgari, Z. Longitudinally extensive transverse myelitis after Covid-19 vaccination: Case report and review of literature. Hum. Vaccin. Immunother. 2022, 18, 2040239. [Google Scholar] [CrossRef]
- Ostovan, V.R.; Sahraian, M.A.; Karazhian, N.; Rostamihosseinkhani, M.; Salimi, M.; Marbooti, H. Clinical characteristics, radiological features and prognostic factors of transverse myelitis following COVID-19 vaccination: A systematic review. Mult. Scler. Relat. Disord. 2022, 66, 104032. [Google Scholar] [CrossRef] [PubMed]
- Ismail, I.I.; Salama, S. A systematic review of cases of CNS demyelination following COVID-19 vaccination. J. Neuroimmunol. 2022, 362, 577765. [Google Scholar] [CrossRef]
- Román, G.C.; Gracia, F.; Torres, A.; Palacios, A.; Gracia, K.; Harris, D. Acute Transverse Myelitis (ATM): Clinical Review of 43 Patients With COVID-19-Associated ATM and 3 Post-Vaccination ATM Serious Adverse Events With the ChAdOx1 nCoV-19 Vaccine (AZD1222). Front. Immunol. 2021, 12, 653786. [Google Scholar] [CrossRef]
- Adamec, I.; Brecl Jakob, G.; Drulović, J.; Sellner, J.; Bilić, E.; Sitaš, B.; Bilić, H.; Tamaš, O.; Budimkić, M.; Veselinović, N.; et al. Transverse myelitis following COVID-19: Insights from a multi-center study and systematic literature review. J. Neurol. Sci. 2022, 443, 120463. [Google Scholar] [CrossRef]
- Schulte, E.C.; Hauer, L.; Kunz, A.B.; Sellner, J. Systematic review of cases of acute myelitis in individuals with COVID-19. Eur. J. Neurol. 2021, 28, 3230–3244. [Google Scholar] [CrossRef]
- Ismail, I.I.; Salama, S. Association of CNS demyelination and COVID-19 infection: An updated systematic review. J. Neurol. 2022, 269, 541–576. [Google Scholar] [CrossRef]
- Gudlavalleti, A.; Nath, A. Clinical Profile and Outcomes of COVID-19-Associated Transverse Myelitis: A Case Report and Review of Literature. Neurol. Clin. Pract. 2022, 12, e221–e227. [Google Scholar] [CrossRef]
- Aladdin, Y.; Algahtani, H.; Shirah, B. Vaccine-induced immune thrombotic thrombocytopenia with disseminated intravascular coagulation and death following the ChAdOx1 nCoV-19 vaccine. J. Stroke Cerebrovasc Dis. 2021, 30, 105938. [Google Scholar] [CrossRef] [PubMed]
- Steadman, E.; Fandaros, M.; Yin, W. SARS-CoV-2 and plasma hypercoagulability. Cell Mol Bioeng. 2021, 28, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dodig, D.; Fritzler, M.J.; Naraghi, A.; Tarnopolsky, M.A.; Lu, J.Q. Immune-mediated necrotizing myopathy after BNT162b2 vaccination in a patient with antibodies against receptor-binding domain of SARS-CoV-2 and signal recognition particle. Muscle Nerve 2022, 65, E11–E13. [Google Scholar] [CrossRef]
- Vutipongsatorn, K.; Isaacs, A.; Farah, Z. Inflammatory myopathy occurring shortly after severe acute respiratory syndrome coronavirus 2 vaccination: Two case reports. J. Med. Case Rep. 2022, 16, 57. [Google Scholar] [CrossRef] [PubMed]
- Reiken, S.; Sittenfeld, L.; Dridi, H.; Liu, Y.; Liu, X.; Marks, A.R. Alzheimer’s-like signaling in brains of COVID-19 patients. Alzheimers Dement. 2022, 18, 955–965. [Google Scholar] [CrossRef]
- Charnley, M.; Islam, S.; Bindra, G.K.; Engwirda, J.; Ratcliffe, J.; Zhou, J.; Mezzenga, R.; Hulett, M.D.; Han, K.; Berryman, J.T.; et al. Neurotoxic amyloidogenic peptides in the proteome of SARS-COV2: Potential implications for neurological symptoms in COVID-19. Nat. Commun. 2022, 13, 3387. [Google Scholar] [CrossRef] [PubMed]
- Visser, D.; Golla, S.S.V.; Verfaillie, S.C.J.; Coomans, E.M.; Rikken, R.M.; van de Giessen, E.M.; den Hollander, M.E.; Verveen, A.; Yaqub, M.; Barkhof, F.; et al. Long COVID is associated with extensive in-vivo neuroinflammation on [18F]DPA-714 PET. medRxiv 2022. [Google Scholar] [CrossRef]
- Hugon, J.; Queneau, M.; Sanchez Ortiz, M.; Msika, E.F.; Farid, K.; Paquet, C. Cognitive decline and brainstem hypometabolism in long COVID: A case series. Brain Behav. 2022, 12, e2513. [Google Scholar] [CrossRef]
- Apple, A.C.; Oddi, A.; Peluso, M.J.; Asken, B.M.; Henrich, T.J.; Kelly, J.D.; Pleasure, S.J.; Deeks, S.G.; Allen, I.E.; Martin, J.N.; et al. Risk factors and abnormal cerebrospinal fluid associate with cognitive symptoms after mild COVID-19. Ann. Clin. Transl. Neurol. 2022, 9, 221–226. [Google Scholar] [CrossRef]
- Schwabenland, M.; Salié, H.; Tanevski, J.; Killmer, S.; Lago, M.S.; Schlaak, A.E.; Mayer, L.; Matschke, J.; Püschel, K.; Fitzek, A.; et al. Deep spatial profiling of human COVID-19 brains reveals neuroinflammation with distinct microanatomical microglia-T-cell interactions. Immunity 2021, 54, 1594–1610. [Google Scholar] [CrossRef]
- Sanchez-Vazquez, R.; Guío-Carrión, A.; Zapatero-Gaviria, A.; Martínez, P.; Blasco, M.A. Shorter telomere lengths in patients with severe COVID-19 disease. Aging 2021, 13, 1–15. [Google Scholar] [CrossRef]
- Mongelli, A.; Barbi, V.; Gottardi Zamperla, M.; Atlante, S.; Forleo, L.; Nesta, M.; Massetti, M.; Pontecorvi, A.; Nanni, S.; Farsetti, A.; et al. Evidence for Biological Age Acceleration and Telomere Shortening in COVID-19 Survivors. Int. J. Mol. Sci. 2021, 22, 6151. [Google Scholar] [CrossRef]
- Azalbert, X.; Bouthors, A.T.; Brack, M.; Cerdan, D.; Chesnut, W.; Guillaume, G.; Lesgards, J.F.; Montagnier, L.; Perez, J.C. FranceSoir. 2021. Available online: https://www.francesoir.fr/opinions-tribunes/could-sars-cov2-accelerate-biological-age (accessed on 27 May 2023).
- Post COVID-19 Condition (Long COVID); WHO: Geneva, Switzerland, 2023.
- Cheetham, N.J.; Penfold, R.; Giunchiglia, V.; Bowyer, V.; Sudre, C.H.; Canas, L.S.; Deng, J.; Murray, B.; Kerfoot, E.; Antonelli, M.; et al. The effects of COVID-19 on cognitive performance in a community-based cohort: A COVID symptom study biobank prospective cohort study. EClinicalMedicine 2023, 62, 102086. [Google Scholar] [CrossRef]
- Mahmudpour, M.; Roozbeh, J.; Keshavarz, M.; Farrokhi, S.; Nabipour, I. COVID-19 cytokine storm: The anger of inflammation. Cytokine 2020, 133, 155151. [Google Scholar] [CrossRef]
- Gao, T.; Zhu, L.; Liu, H.; Zhang, X.; Wang, T.; Fu, Y.; Li, H.; Dong, Q.; Hu, Y.; Zhang, Z.; et al. Highly pathogenic coronavirus N protein aggravates inflammation by MASP-2-mediated lectin complement pathway overactivation. Signal Transduct. Target. Ther. 2022, 7, 318. [Google Scholar] [CrossRef] [PubMed]
- Ali, Y.M.; Ferrari, M.; Lynch, N.J.; Yaseen, S.; Dudler, T.; Gragerov, S.; Demopulos, G.; Heeney, J.L.; Schwaeble, W.J. Lectin Pathway Mediates Complement Activation by SARS-CoV-2 Proteins. Front. Immunol. 2021, 12, 714511. [Google Scholar] [CrossRef]
- Trypsteen, W.; Van Cleemput, J.; Snippenberg, W.V.; Gerlo, S.; Vandekerckhove, L. On the whereabouts of SARSCoV-2 in the human body: A systematic review. PLoS Pathog. 2020, 16, e1009037. [Google Scholar] [CrossRef]
- Lin, L.; Jiang, X.; Zhang, Z.; Huang, S.; Zhang, Z.; Fang, Z.; Gu, Z.; Gao, L.; Shi, H.; Mai, L.; et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut 2020, 69, 997–1001. [Google Scholar] [CrossRef]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef]
- Mahalingasivam, V.; Faucon, A.L.; Sjölander, A.; Bosi, A.; González-Ortiz, A.; Lando, S.; Fu, E.L.; Nitsch, D.; Bruchfeld, A.; Evans, M.; et al. Kidney Function Decline After COVID-19 Infection. JAMA Netw. Open. 2024, 7, e2450014. [Google Scholar] [CrossRef]
- Ceulemans, L.J.; Khan, M.; Yoo, S.J.; Zapiec, B.; Van Gerven, L.; Van Slambrouck, J.; Vanstapel, A.; Van Raemdonck, D.; Vos, R.; Wauters, E.; et al. Persistence of SARS-CoV-2 RNA in lung tissue after mild COVID-19. Lancet Respir. Med. 2021, 9, e78–e79. [Google Scholar] [CrossRef]
- Menuchin-Lasowski, Y.; Schreiber, A.; Lecanda, A.; Mecate-Zambrano, A.; Brunotte, L.; Psathaki, O.E.; Ludwig, S.; Rauen, T.; Schöler, H.R. SARS-CoV-2 infects and replicates in photoreceptor and retinal ganglion cells of human retinal organoids. Stem Cell Rep. 2022, 17, 789–803. [Google Scholar] [CrossRef] [PubMed]
- Pfizer/PF-07302048: Structural and Biophysical Characterization of SARS-CoV-2 Spike Glycoprotein (P2 S) as a Vaccine Antigen. 2021. Available online: https://media.ellinikahoaxes.gr/uploads/2023/03/125742_S1_M4_4.2.1-vr-vtr-10741.pdf (accessed on 27 December 2020).
- Vogel, A.B.; Kanevsky, I.; Che, Y.; Swanson, K.A.; Muik, A.; Vormehr, M.; Kranz, L.M.; Walzer, K.C.; Hein, S.; Güler, A.; et al. BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature 2021, 592, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Zhang, J.; Schiavon, C.R.; He, M.; Chen, L.; Shen, H.; Zhang, Y.; Yin, Q.; Cho, Y.; Andrade, L.; et al. SARS-CoV-2 Spike Protein Impairs Endothelial Function via Downregulation of ACE 2. Circ. Res. 2021, 128, 1323–1326. [Google Scholar] [CrossRef]
- Hermans, C.; Goldman, M. Thromboses et vaccins: Un nouveau défi de la pandémie COVID-19. Louvain Med. 2021, 140, 207–215. [Google Scholar]
- Konstantinidis, I.; Tsakiropoulou, E.; Hähner, A.; de With, K.; Poulas, K.; Hummel, T. Olfactory dysfunction after coronavirus disease 2019 (COVID-19) vaccination. Int. Forum Allergy Rhinol. 2021, 11, 1399–1401. [Google Scholar] [CrossRef]
- Olajide, O.A.; Iwuanyanwu, V.U.; Adegbola, O.D.; Al-Hindawi, A.A. SARS-CoV-2 Spike Glycoprotein S1 Induces Neuroinflammation in BV-2 Microglia. Mol. Neurobiol. 2022, 59, 445–458. [Google Scholar] [CrossRef]
- Ogata, A.F.; Cheng, C.A.; Desjardins, M.; Senussi, Y.; Sherman, A.C.; Powell, M.; Novack, L.; Von, S.; Li, X.; Baden, L.R.; et al. Circulating Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccine Antigen Detected in the Plasma of mRNA-1273 Vaccine Recipients. Clin. Infect. Dis. 2022, 74, 715–718. [Google Scholar] [CrossRef]
- Röltgen, K.; Nielsen, S.C.A.; Silva, O.; Younes, S.F.; Zaslavsky, M.; Costales, C.; Yang, F.; Wirz, O.F.; Solis, D.; Hoh, R.A.; et al. Immune imprinting, breadth of variant recognition, and germinal center response in human SARS-CoV-2 infection and vaccination. Cell 2022, 185, 1025–1040. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, M.; Marino, F. The spike hypothesis in vaccine-induced adverse effects: Questions and answers. Trends Mol. Med. 2022, 28, 797–799. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S.L.; Boyle, E.; Jefferson, S.R.; Heslop, C.R.A.; Mohan, P.; Mohanraj, G.G.J.; Sidow, H.A.; Tan, R.C.P.; Hill, S.J.; Woolard, J. Role of the Renin-Angiotensin-Aldosterone and Kinin-Kallikrein Systems in the Cardiovascular Complications of COVID-19 and Long COVID. Int. J. Mol. Sci. 2021, 22, 8255. [Google Scholar] [CrossRef] [PubMed]
- Pacurari, M.; Kafoury, R.; Tchounwou, P.B.; Ndebele, K. The Renin-Angiotensin-aldosterone system in vascular inflammation and remodeling. Int. J. Inflam. 2014, 1, 689360. [Google Scholar] [CrossRef]
- Crowley, S.D.; Rudemiller, N.P. Immunologic Effects of the Renin-Angiotensin System. J. Am. Soc. Nephrol. 2017, 28, 1350–1361. [Google Scholar] [CrossRef]
- Jia, H. Pulmonary Angiotensin-Converting Enzyme 2 (ACE2) and Inflammatory Lung Disease. Shock 2016, 46, 239–248. [Google Scholar] [CrossRef]
- Okamoto, H.; Ichikawa, N. The pivotal role of the angiotensin-II-NF-κB axis in the development of COVID-19 pathophysiology. Hypertens. Res. 2021, 44, 126–128. [Google Scholar] [CrossRef]
- Jamaluddin, M.; Meng, T.; Sun, J.; Boldogh, I.; Han, Y.; Brasier, A.R. Angiotensin II induces nuclear factor (NF)-kappaB1 isoforms to bind the angiotensinogen gene acute-phase response element: A stimulus-specific pathway for NFkappaB activation. Mol. Endocrinol. 2000, 14, 99–113. [Google Scholar]
- Ruiz-Ortega, M.; Lorenzo, O.; Suzuki, Y.; Rupérez, M.; Egido, J. Proinflammatory actions of angiotensins. Curr. Opin. Nephrol. Hypertens. 2001, 10, 321–329. [Google Scholar] [CrossRef]
- Medina-Enríquez, M.M.; Lopez-León, S.; Carlos-Escalante, J.A.; Aponte-Torres, Z.; Cuapio, A.; Wegman-Ostrosky, T. ACE2: The molecular doorway to SARS-CoV-2. Cell Biosci. 2020, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Peiró, C.; Moncada, S. Substituting Angiotensin-(1-7) to Prevent Lung Damage in SARS-CoV-2 Infection. Circulation 2020, 141, 1665–1666. [Google Scholar] [CrossRef] [PubMed]
- Verdecchia, P.; Cavallini, C.; Spanevello, A.; Angeli, F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur. J. Intern. Med. 2020, 76, 14–20, 23. [Google Scholar] [CrossRef] [PubMed]
- Roche, R.A. hypothesized role for dysregulated bradykinin signaling in COVID-19 respiratory complications. FASEB J. 2020, 34, 7265–7269. [Google Scholar] [CrossRef]
- Cojocaru, E.; Cojocaru, C.; Vlad, C.E.; Eva, L. Role of the Renin-Angiotensin System in Long COVID’s Cardiovascular Injuries. Biomedicines 2023, 11, 2004. [Google Scholar] [CrossRef]
- Schieffer, E.; Schieffer, B. The rationale for the treatment of long-Covid symptoms-A cardiologist’s view. Front. Cardiovasc. Med. 2022, 9, 992686, Erratum in Front. Cardiovasc. Med. 2023, 10, 1244340. [Google Scholar] [CrossRef]
- Lazebnik, Y. Cell fusion as a link between the SARS-CoV-2 spike protein, COVID-19 complications, and vaccine side effects. Oncotarget 2021, 12, 2476–2488. [Google Scholar] [CrossRef]
- Lehmann, K.J. SARS-CoV-2-Spike Interactions with the Renin-Angiotensin-Aldosterone System -Consequences of Adverse Reactions of Vaccination. J. Biol. Today’s World 2023, 12, 001–013. [Google Scholar]
- Sodhi, C.P.; Wohlford-Lenane, C.; Yamaguchi, Y.; Prindle, T.; Fulton, W.B.; Wang, S.; McCray, P.B., Jr.; Chappell, M.; Hackam, D.J.; Jia, H. Attenuation of pulmonary ACE2 activity impairs inactivation of des-Arg9 bradykinin/BKB1R axis and facilitates LPS-induced neutrophil infiltration. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 314, L17–L31. [Google Scholar] [CrossRef]
- Patel, M.A.; Knauer, M.J.; Nicholson, M.; Daley, M.; Van Nynatten, L.R.; Cepinskas, G.; Fraser, D.D. Organ and cell-specific biomarkers of Long-COVID identified with targeted proteomics and machine learning. Mol. Med. 2023, 29, 26. [Google Scholar] [CrossRef] [PubMed]
- Abassi, Z.; Skorecki, K.; Hamo-Giladi, D.B.; Kruzel-Davila, E.; Heyman, S.N. Kinins and chymase: The forgotten components of the renin-angiotensin system and their implications in COVID-19 disease. Am. J. Physiol. Lung Cell Mol. Physiol. 2021, 320, L422–L429. [Google Scholar] [CrossRef]
- Sriramula, S.; Theobald, D.; Parekh, R.U.; Akula, S.M.; O’Rourke, D.P.; Eells, J.B. Emerging Role of Kinin B1 Receptor in Persistent Neuroinflammation and Neuropsychiatric Symptoms in Mice Following Recovery from SARS-CoV-2 Infection. Cells 2023, 12, 2107. [Google Scholar] [CrossRef] [PubMed]
- Radonjic-Hoesli, S.; Hofmeier, K.S.; Micaletto, S.; Micaletto, S.; Schmid-Grendelmeier, P.; Bircher, A.; Simon, D. Urticaria and Angioedema: An Update on Classification and Pathogenesis. Clin. Rev. Allerg. Immunol. 2018, 54, 88–101. [Google Scholar] [CrossRef]
- van de Veerdonk, F.L.; Netea, M.G.; van Deuren, M.; van der Meer, J.W.; de Mast, Q.; Brüggemann, R.J.; van der Hoeven, H. Kallikreinkinin blockade in patients with COVID-19 to prevent acute respiratory distress syndrome. eLife 2020, 9, e57555. [Google Scholar] [CrossRef]
- Berra, S.; Parolin, D.; Suffritti, C.; Folcia, A.; Zanichelli, A.; Gusso, L.; Cogliati, C.; Riva, A.; Gidaro, A.; Caccia, S. Patterns of C1-Inhibitor Plasma Levels and Kinin-Kallikrein System Activation in Relation to COVID-19 Severity. Life 2024, 14, 1525. [Google Scholar] [CrossRef]
- Cugno, M.; Consonni, D.; Lombardi, A.; Bono, P.; Oggioni, M.; Uceda Renteria, S.; Pesatori, A.C.; Castaldi, S.; Riboldi, L.; Bordini, L.; et al. Increased Risk of Urticaria/Angioedema after BNT162b2 mRNA COVID-19 Vaccine in Health Care Workers Taking ACE Inhibitors. Vaccines 2021, 9, 1011. [Google Scholar] [CrossRef]
- Mansour, E.; Bueno, F.F.; de Lima-Júnior, J.C.; Palma, A.; Monfort-Pires, M.; Bombassaro, B.; Araujo, E.P.; Bernardes, A.F.; Ulaf, R.G.; Nunes, T.A.; et al. Evaluation of the efficacy and safety of icatibant and C1 esterase/kallikrein inhibitor in severe COVID-19: Study protocol for a three-armed randomized controlled trial. Trials 2021, 22, 71. [Google Scholar] [CrossRef]
- Bulla, R.; Rossi, L.; Furlanis, G.; Agostinis, C.; Toffoli, M.; Balduit, A.; Mangogna, A.; Liccari, M.; Morosini, G.; Kishore, U.; et al. A likely association between low mannan-binding lectin level and brain fog onset in long COVID patients. Front. Immunol. 2023, 14, 1191083. [Google Scholar] [CrossRef] [PubMed]
- Cervia-Hasler, C.; Brüningk, S.C.; Hoch, T.; Fan, B.; Muzio, G.; Thompson, R.C.; Ceglarek, L.; Meledin, R.; Westermann, P.; Emmenegger, M.; et al. Persistent complement dysregulation with signs of thromboinflammation in active Long Covid. Science 2024, 383, eadg7942. [Google Scholar] [CrossRef] [PubMed]
- Defendi, F.; Leroy, C.; Epaulard, O.; Clavarino, G.; Vilotitch, A.; Le Marechal, M.; Jacob, M.C.; Raskovalova, T.; Pernollet, M.; Le Gouellec, A.; et al. Complement Alternative and Mannose-Binding Lectin Pathway Activation Is Associated With COVID-19 Mortality. Front. Immunol. 2021, 12, 742446. [Google Scholar] [CrossRef]
- Yu, J.; Yuan, X.; Chen, H.; Chaturvedi, S.; Braunstein, E.M.; Brodsky, R.A. Direct Activation of the Alternative Complement Pathway by SARS-CoV-2 Spike Proteins is Blocked by Factor D Inhibition. Blood 2020, 136, 2080–2089. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lu, K.; Pfefferle, S.; Bertram, S.; Glowacka, I.; Drosten, C.; Pöhlmann, S.; Simmons, G. A single asparagine-linked glycosylation site of the severe acute respiratory syndrome coronavirus spike glycoprotein facilitates inhibition by mannosebinding lectin through multiple mechanisms. J. Virol. 2010, 84, 8753–8764. [Google Scholar] [CrossRef]
- Baillie, K.; Davies, H.E.; Keat, S.B.K.; Ladell, K.; Miners, K.L.; Jones, S.A.; Mellou, E.; Toonen, E.J.M.; Price, D.A.; Morgan, B.P.; et al. Complement dysregulation is a prevalent and therapeutically amenable feature of long COVID. Med 2024, 5, 239–253.e5. [Google Scholar] [CrossRef]
- Zhao, H.; Lv, Y.; Xu, J.; Song, X.; Wang, Q.; Zhai, X.; Ma, X.; Qiu, J.; Cui, L.; Sun, Y. The activation of microglia by the complement system in neurodegenerative diseases. Ageing Res. Rev. 2025, 104, 102636. [Google Scholar] [CrossRef]
- Theoharides, T.C. Could SARS-CoV-2 Spike Protein Be Responsible for Long-COVID Syndrome. Mol. Neurobiol. 2022, 59, 1850–1861. [Google Scholar] [CrossRef]
- Pekna, M.; Pekny, M. The Complement System: A Powerful Modulator and Effector of Astrocyte Function in the Healthy and Diseased Central Nervous System. Cells 2021, 10, 1812. [Google Scholar] [CrossRef]
- Lian, H.; Litvinchuk, A.; Chiang, A.C.; Aithmitti, N.; Jankowsky, J.L.; Zheng, H. Astrocyte-Microglia Cross Talk through Complement Activation Modulates Amyloid Pathology in Mouse Models of Alzheimer’s Disease. J. Neurosci. 2016, 36, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Beja-Glasser, V.F.; Nfonoyim, B.M.; Frouin, A.; Li, S.; Ramakrishnan, S.; Merry, K.M.; Shi, Q.; Rosenthal, A.; Barres, B.A.; et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 2016, 352, 712–716. [Google Scholar] [CrossRef]
- Schultheiß, C.; Willscher, E.; Paschold, L.; Gottschick, C.; Klee, B.; Henkes, S.S.; Bosurgi, L.; Dutzmann, J.; Sedding, D.; Frese, T.; et al. The IL-1β, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19. Cell Rep. Med. 2022, 3, 100663. [Google Scholar] [CrossRef]
- Peluso, M.J.; Lu, S.; Tang, A.F.; Durstenfeld, M.S.; Ho, H.E.; Goldberg, S.A.; Forman, C.A.; Munter, S.E.; Hoh, R.; Tai, V.; et al. Markers of immune activation and inflammation in individuals with Postacute Sequelae of severe acute respiratory syndrome coronavirus 2 infection. J. Infect. Dis. 2021, 224, 1839–1848. [Google Scholar] [CrossRef]
- VanElzakker, M.B.; Brumfield, S.A.; Lara Mejia, P.S. Neuroinflammation and cytokines in Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): A critical review of research methods. Front. Neurol. 2018, 9, 1033. [Google Scholar]
- Jarrott, B.; Head, R.; Pringle, K.G.; Lumbers, E.R.; Martin, J.H. “LONG COVID”—A hypothesis for understanding the biological basis and pharmacological treatment strategy. Pharmacol. Res. Perspect. 2022, 10, e00911. [Google Scholar] [CrossRef]
- Patterson, B.K.; Yogendra, R.; Francisco, E.B.; Guevara-Coto, J.; Long, E.; Pise, A.; Osgood, E.; Bream, J.; Kreimer, M.; Jeffers, D.; et al. Detection of S1 spike protein in CD16+ monocytes up to 245 days in SARS-CoV-2-negative post-COVID-19 vaccine syndrome (PCVS) individuals. Hum. Vaccin. Immunother. 2025, 21, 2494934. [Google Scholar] [CrossRef]
- Lai, Y.J.; Liu, S.H.; Manachevakul, S.; Lee, T.A.; Kuo, C.T.; Bello, D. Biomarkers in long COVID-19: A systematic review. Front. Med. 2023, 10, 1085988. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, R.; Cecchini, A.L. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med. Hypotheses 2020, 143, 110102. [Google Scholar] [CrossRef]
- Delgado-Roche, L.; Mesta, F. Oxidative Stress as Key Player in Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) Infection. Arch. Med. Res. 2020, 51, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Ducastel, M.; Chenevier-Gobeaux, C.; Ballaa, Y.; Meritet, J.F.; Brack, M.; Chapuis, N.; Pene, F.; Carlier, N.; Szwebel, T.A.; Roche, N.; et al. Oxidative Stress and Inflammatory Biomarkers for the Prediction of Severity and ICU Admission in Unselected Patients Hospitalized with COVID-19. Int. J. Mol. Sci. 2021, 22, 7462. [Google Scholar] [CrossRef]
- Vollbracht, C.; Kraft, K. Oxidative Stress and Hyper-Inflammation as Major Drivers of Severe COVID-19 and Long COVID: Implications for the Benefit of High-Dose Intravenous Vitamin, C. Front. Pharmacol. 2022, 13, 899198. [Google Scholar] [CrossRef] [PubMed]
- Çakırca, G.; Damar Çakırca, T.; Üstünel, M.; Torun, A.; Koyuncu, İ. Thiol level and total oxidant/antioxidant status in patients with COVID-19 infection. Ir. J. Med. Sci. 2022, 191, 1925–1930. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.O.; Banerjee, A. Long COVID and cardiovascular disease: A learning health system approach. Nat. Rev. Cardiol. 2022, 19, 287–288. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, I.; Lambadiari, V.; Mitrakou, A.; Kountouri, A.; Katogiannis, K.; Thymis, J.; Korakas, E.; Pavlidis, G.; Kazakou, P.; Panagopoulos, G.; et al. Myocardial work and vascular dysfunction are partially improved at 12 months after COVID-19 infection. Eur. J. Heart Fail. 2022, 24, 727–729. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Sadarani, B.N.; Majumdar, A.S. Resveratrol potentiates the effect of dexamethasone in rat model of acute lung inflammation. Int. Immunopharmacol. 2015, 28, 773–779. [Google Scholar] [CrossRef]
- Belcaro, G.; Cornelli, U.; Cesarone, M.R.; Scipione, C.; Scipione, V.; Hu, S.; Feragalli, B.; Corsi, M.; Cox, D.; Cotellese, R.; et al. Preventive effects of Pycnogenol® on cardiovascular risk factors (including endothelial function) and microcirculation in subjects recovering from coronavirus disease 2019 (COVID-19). Minerva Med. 2022, 113, 300–308. [Google Scholar] [CrossRef]
- du Preez, H.N.; Lin, J.; Maguire, G.E.M.; Aldous, C.; Kruger, H.G. COVID-19 vaccine adverse events: Evaluating the pathophysiology with an emphasis on sulfur metabolism and endotheliopathy. Eur. J. Clin. Investig. 2024, 54, e14296. [Google Scholar] [CrossRef]
- Hou, Y.; Gu, T.; Ni, Z.; Shi, X.; Ranney, M.L.; Mukherjee, B. Global Prevalence of Long COVID, its Subtypes and Risk factors: An Updated Systematic Review and Meta-Analysis. medRxiv 2025. [Google Scholar] [CrossRef]
- Nyasulu, P.S.; Chikobvu, D.; Mapatano, M.A.; Oni, T.; Chipeta, M.; Ngarina, M.; Chirwa, T.; Chikungwa, P.; Dhillon, R.S.; Chirwa, G.C. Risk Factors and Clinical Presentations of Long COVID in Africa: A Scoping Review. BMJ Glob. Health 2023, 8, e011279. [Google Scholar]
- Frallonardo, L.; Segala, F.V.; Chhaganlal, K.D.; Yelshazly, M.; Novara, R.; Cotugno, S.; Guido, G.; Papagni, R.; Colpani, A.; De Vito, A.; et al. Incidence and burden of long COVID in Africa: A systematic review and meta-analysis. Sci. Rep. 2023, 13, 21482. [Google Scholar] [CrossRef]
- Chen, C.; Haupert, S.R.; Zimmermann, L.; Shi, X.; Fritsche, L.G.; Mukherjee, B. Global Prevalence of Post-Coronavirus Disease 2019 (COVID-19) Condition or Long COVID: A Meta-Analysis and Systematic Review. J. Infect. Dis. 2022, 226, 1593–1607. [Google Scholar] [CrossRef] [PubMed]
- Ogar, C.K.; Quick, J.; Gilbert, H.N.; Vreman, R.A.; Mantel-Teeuwisse, A.K.; Mugunga, J.C. Adverse Events to SARS-CoV-2 (COVID-19) Vaccines and Policy Considerations that Inform the Funding of Safety Surveillance in Low- and Middle-Income Countries: A Mixed Methods Study. Drug Saf. 2023, 46, 357–370. [Google Scholar] [CrossRef]
- Autret-Leca, E.; Bensouda-Grimaldi, L.; Jonville-Béra, A.P.; Beau-Salinas, F. Pharmacovigilance of vaccines. Arch. Pediatr. 2006, 13, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Holm, S.; Kared, H.; Michelsen, A.E.; Kong, X.Y.; Dahl, T.B.; Schultz, N.H.; Nyman, T.A.; Fladeby, C.; Seljeflot, I.; Ueland, T.; et al. Immune complexes, innate immunity, and NETosis in ChAdOx1 vaccine-induced thrombocytopenia. Eur. Heart J. 2021, 42, 4064–4072. [Google Scholar] [CrossRef] [PubMed]
- Fajloun, Z.; Tajer, L.; Khattar, Z.A.; Sabatier, J.M. Unveiling the Role of SARS-CoV-2 or mRNA Vaccine Spike Protein in Macrophage Activation Syndrome (MAS). Infect. Disord. Drug Targets. 2025, 25, e220724232138. [Google Scholar] [CrossRef] [PubMed]
- Weiss-Tessbach, M.; Haider, T.; Gowran, A.; Schubert, L.; Mühlbacher, J.; Brankovic, J.; Wahrmann, M.; Jilma, B.; Boehm, T. COVID-19 mRNA-1273 vaccination induced mast cell activation with strongly elevated Th2 cytokines in a systemic mastocytosis patient. Inflamm. Res. 2025, 74, 71. [Google Scholar] [CrossRef]
- Karim, A.; Shameem, M.; Talwar, A.; Talwar, D. Impact of comorbidities and inflammatory markers on mortality of COVID-19 patients. Lung India 2024, 41, 40–46. [Google Scholar] [CrossRef]
- de Lucena, T.M.C.; da Silva Santos, A.F.; de Lima, B.R.; de Albuquerque Borborema, M.E.; de Azevêdo Silva, J. Mechanism of inflammatory response in associated comorbidities in COVID-19. Diabetes Metab. Syndr. 2020, 14, 597–600. [Google Scholar] [CrossRef]
- Li, X.; Cai, Y.; Zhang, Z.; Zhou, J. Glial and Vascular Cell Regulation of the Blood-Brain Barrier in Diabetes. Diabetes Metab. J. 2022, 46, 222–238. [Google Scholar] [CrossRef]
- Pomara, C.; Sessa, F.; Ciaccio, M.; Dieli, F.; Esposito, M.; Garozzo, S.F.; Giarratano, A.; Prati, D.; Rappa, F.; Salerno, M.; et al. Post-mortem findings in vaccine-induced thrombotic thombocytopenia. Haematologica 2021, 106, 2291–2293. [Google Scholar] [CrossRef]
- Götz, M.P.; Skjoedt, M.O.; Bayarri-Olmos, R.; Hansen, C.B.; Pérez-Alós, L.; Jarlhelt, I.; Benfield, T.; Rosbjerg, A.; Garred, P. Lectin Pathway Enzyme MASP-2 and Downstream Complement Activation in COVID-19. J. Innate Immun. 2023, 15, 122–135. [Google Scholar] [CrossRef]
- Shivshankar, P.; Mueller-Ortiz, S.L.; Domozhirov, A.Y.; Bi, W.; Collum, S.D.; Doursout, M.F.; Patel, M.; LeFebvre, I.N.; Akkanti, B.; Yau, S.; et al. Complement activity and autophagy are dysregulated in the lungs of patients with nonresolvable COVID-19 requiring lung transplantation. Respir. Res. 2025, 26, 68. [Google Scholar] [CrossRef]
- Alwan, N.A. The teachings of long COVID. Commun. Med. 2021, 1, 15. [Google Scholar] [CrossRef] [PubMed]
- Au, L.; Ferreira, T.S.; Kovalchuk, A.; Daly, G.; Wu, M.; Choi, K.; Nam, S.E.; Holt, T.; Davis, H.E.; Zafar, R. Long COVID and medical gaslighting: Dismissal, delayed diagnosis, and deferred treatment. SSM Qual. Res. Health 2022, 2, 100167. [Google Scholar] [CrossRef]
- Perego, E.; Callard, F.; Stras, L.; Melville-Jóhannesson, B.; Pope, C.; Alwan, N.A. Why the patient-made term ‘Long Covid’ is needed. Wellcome Open Res. 2020, 5, 224. [Google Scholar] [CrossRef]
- Zuin, M.; Rigatelli, G.; Bilato, C.; Porcari, A.; Merlo, M.; Roncon, L.; Sinagra, G. One-Year Risk of Myocarditis After COVID-19 Infection: A Systematic Review and Meta-analysis. Can. J. Cardiol. 2023, 39, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Barda, N.; Dagan, N.; Ben-Shlomo, Y.; Kepten, E.; Waxman, J.; Ohana, R.; Hernán, M.A.; Lipsitch, M.; Kohane, I.; Netzer, D.; et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N. Engl. J. Med. 2021, 385, 1078–1090. [Google Scholar] [CrossRef]
- Witberg, G.; Barda, N.; Hoss, S.; Richter, I.; Wiessman, M.; Aviv, Y.; Grinberg, T.; Auster, O.; Dagan, N.; Balicer, R.D.; et al. Myocarditis after Covid-19 Vaccination in a Large Health Care Organization. N. Engl. J. Med. 2021, 385, 2132–2139. [Google Scholar] [CrossRef]
- Mevorach, D.; Anis, E.; Cedar, N.; Bromberg, M.; Haas, E.J.; Nadir, E.; Olsha-Castell, S.; Arad, D.; Hasin, T.; Levi, N.; et al. Myocarditis after BNT162b2 mRNA vaccine against Covid-19 in Israel. N. Engl. J. Med. 2021, 385, 2140–2149. [Google Scholar] [CrossRef]
- Durand, J.; Dogné, J.M.; Cohet, C.; Browne, K.; Gordillo-Marañón, M.; Piccolo, L.; Zaccaria, C.; Genov, G. Safety Monitoring of COVID-19 Vaccines: Perspective from the European Medicines Agency. Clin. Pharmacol. Ther. 2023, 113, 1223–1234. [Google Scholar] [CrossRef]
- Karlstad, Ø.; Hovi, P.; Husby, A.; Härkänen, T.; Selmer, R.M.; Pihlström, N.; Hansen, J.V.; Nohynek, H.; Gunnes, N.; Sundström, A.; et al. SARS-CoV-2 Vaccination and Myocarditis in a Nordic Cohort Study of 23 Million Residents. JAMA Cardiol. 2022, 7, 600–612. [Google Scholar] [CrossRef]
- Chua, G.T.; Kwan, M.Y.W.; Chui, C.S.L.; Smith, R.D.; Cheung, E.C.L.; Ma, T.; Leung, M.T.Y.; Tsao, S.S.L.; Kan, E.; Ng, W.K.C.; et al. Epidemiology of Acute Myocarditis/Pericarditis in Hong Kong Adolescents Following Comirnaty Vaccination. Clin. Infect. Dis. 2022, 75, 673–681. [Google Scholar] [CrossRef]
- Couzin-Frankel, J.; Vogel, G. Vaccines may cause rare, Long Covid-like symptoms. Science 2022, 375, 364–366. [Google Scholar] [CrossRef] [PubMed]
- Murphy, W.J.; Longo, D.L. A Possible Role for Anti-idiotype Antibodies in SARS-CoV-2 Infection and Vaccination. N. Engl. J. Med. 2022, 386, 394–396. [Google Scholar] [CrossRef]
- Kim, A.Y.; Woo, W.; Yon, D.K.; Lee, S.W.; Yang, J.W.; Kim, J.H.; Park, S.; Koyanagi, A.; Kim, M.S.; Lee, S.; et al. Thrombosis patterns and clinical outcome of COVID-19 vaccine-induced immune thrombotic thrombocytopenia: A Systematic Review and Meta-Analysis. Int. J. Infect. Dis. 2022, 119, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, H.; Peluso, M.J.; Rodgers, K.; Aberg, J.A.; Patterson, T.F.; Tamburro, R.; Baizer, L.; Goldman, J.D.; Rouphael, N.; Deitchman, A.; et al. Therapeutic trials for long COVID-19: A call to action from the interventions taskforce of the RECOVER initiative. Front. Immunol. 2023, 14, 1129459. [Google Scholar] [CrossRef] [PubMed]
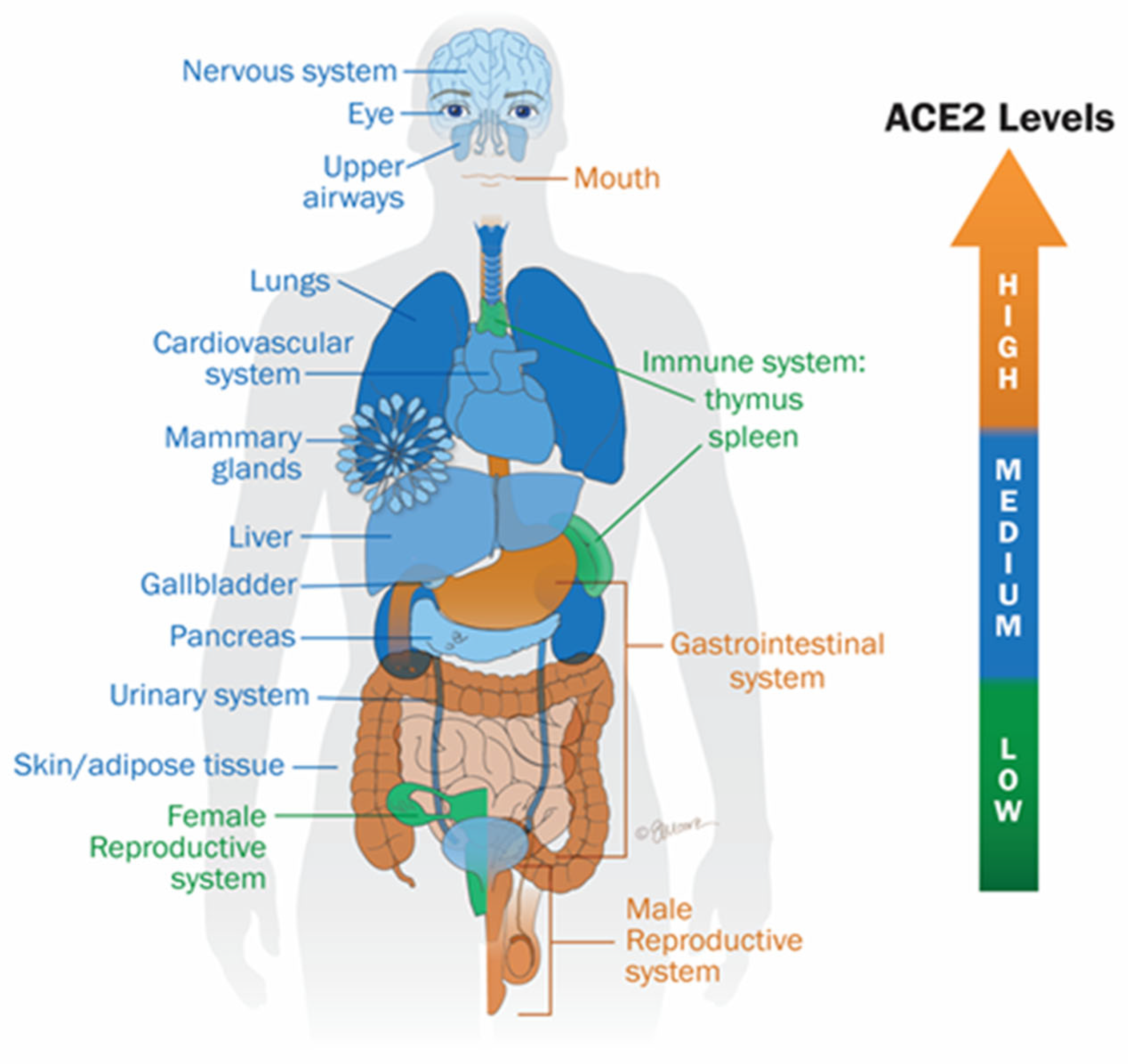
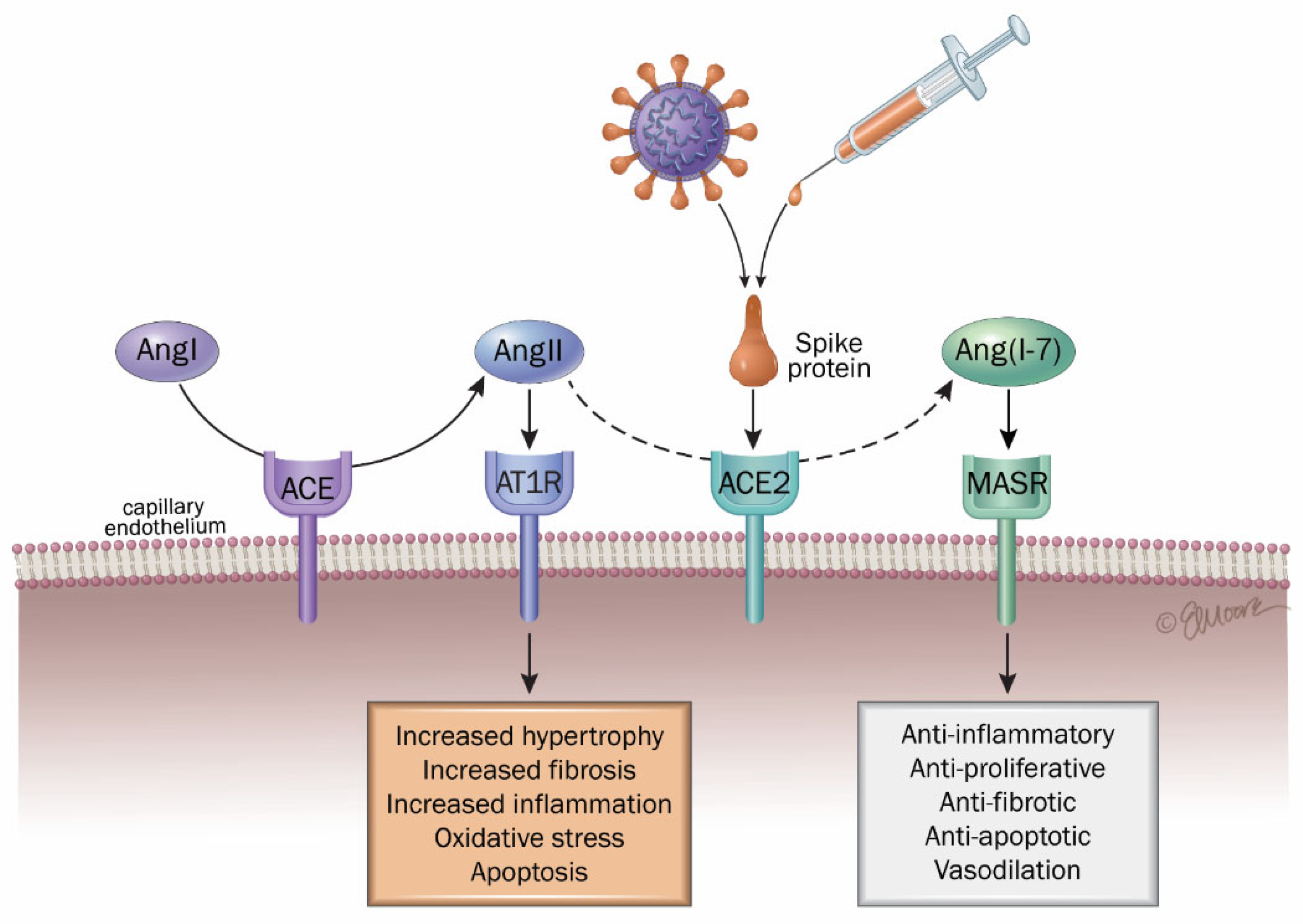
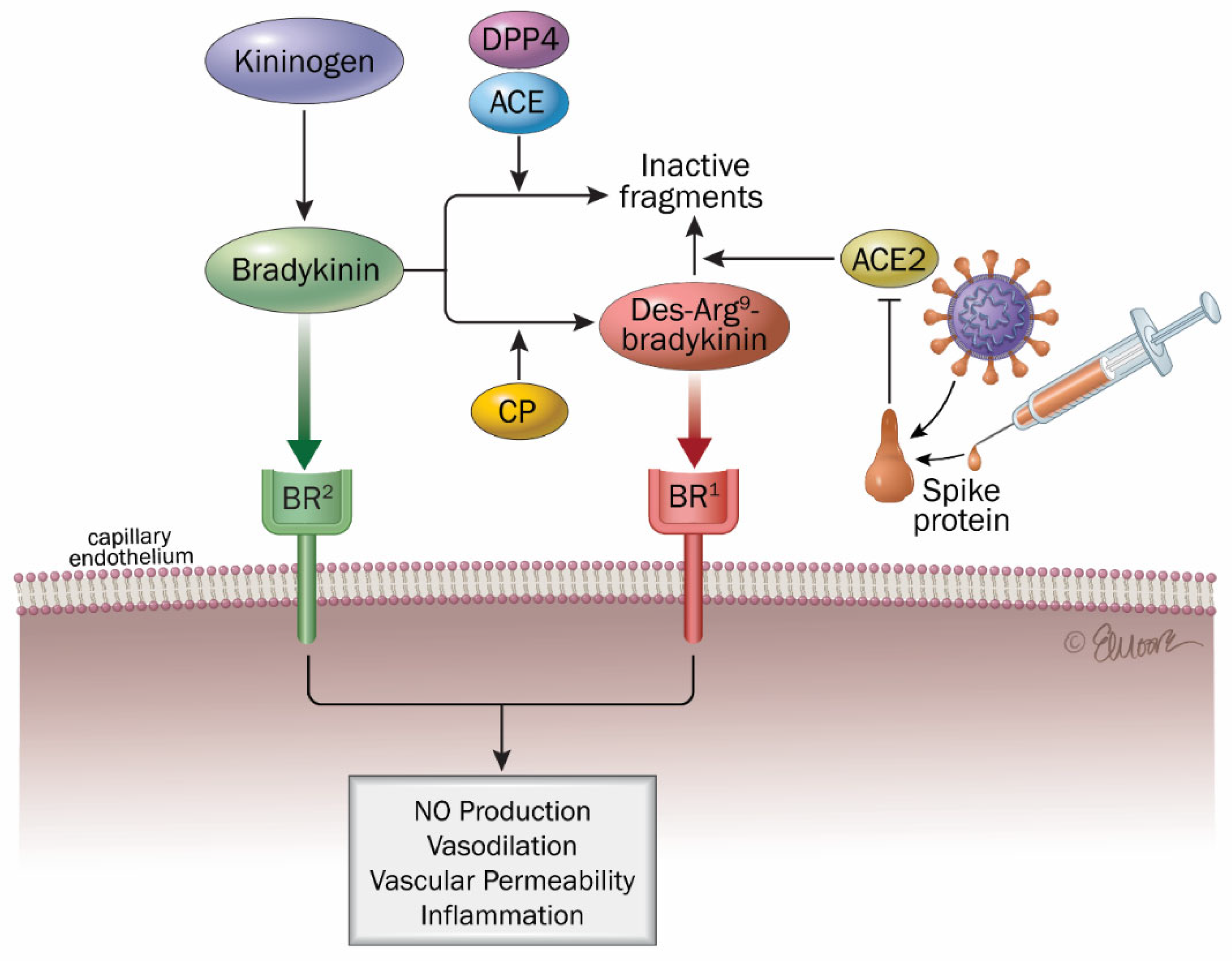
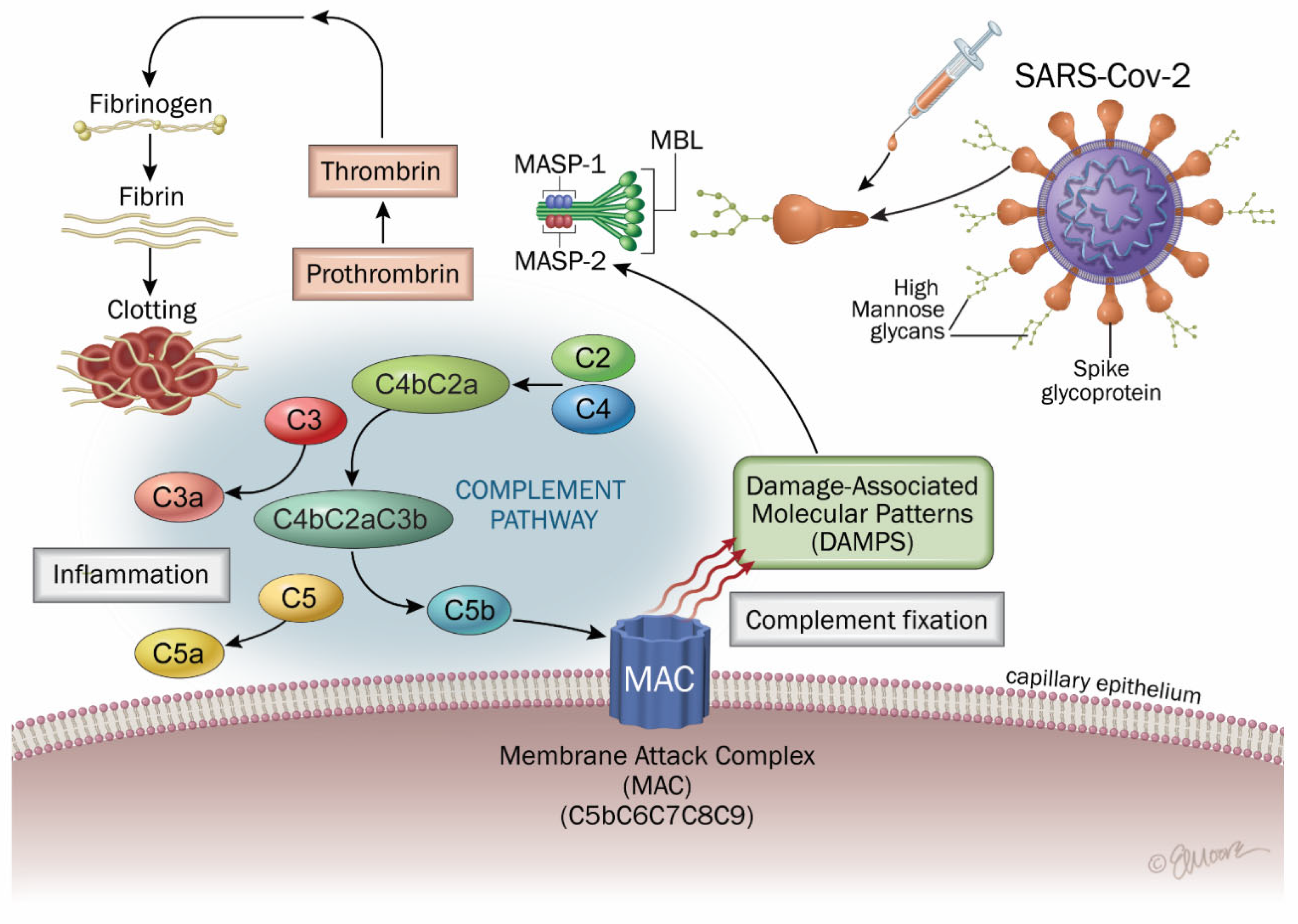
| Symptoms | Systems | Long COVID (References) | Post-COVID-19 Vaccination Syndrome (PCVS) (References) |
|---|---|---|---|
| Fatigue | Nervous system | [6,13,15] | [20,21] |
| Headache | [6] | [20,22] | |
| Attention deficit | [19,23] | [24] | |
| Cognitive impairment | [25,26] | [6] | |
| Muscle pain | [17,27] | [6] | |
| Anxiety | [16,28] | [29] | |
| Depression | [16,28] | [30] | |
| Transverse myelitis | [6] | [21,22] | |
| Guillain–Barré syndrome | [6] | [21,22] | |
| Optic neuritis | [31] | [21,22] | |
| Cerebral venous sinus thrombosis | [6] | [21,22] | |
| Anosmia | [17] | [6] | |
| Ageusia | [17] | [6] | |
| Myocarditis | Cardiovascular system | [17,32] | [7,33] |
| Pericarditis | [17,34] | [35,36] | |
| Heart failure | [27] | [37,38] | |
| Thrombosis | [39] | [7,40] | |
| Ischemic heart disease | [10] | [41] | |
| Dysrhythmias | [16,27] | [42] | |
| Blood pressure disorder | [27] | [43] | |
| Ischemic stroke | [17,27] | [44] | |
| Cardiac arrest | [10] | [41] | |
| Dyspnea | Respiratory system | [8,17,19] | [8] |
| Persistent cough | [27] | [6] | |
| Chest pain | [17] | [41] | |
| Pneumonia | [6] | [7] | |
| Activation of innate immune cells | Immune system | [45,46] | [7,47] |
| Inflammation | [7] | [7] | |
| Mast cell activation syndrome | [45,46] | [43] | |
| Macrophage activation syndrome | [48,49] | [7,50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lesgards, J.-F.; Cerdan, D.; Perronne, C. Do Long COVID and COVID Vaccine Side Effects Share Pathophysiological Picture and Biochemical Pathways? Int. J. Mol. Sci. 2025, 26, 7879. https://doi.org/10.3390/ijms26167879
Lesgards J-F, Cerdan D, Perronne C. Do Long COVID and COVID Vaccine Side Effects Share Pathophysiological Picture and Biochemical Pathways? International Journal of Molecular Sciences. 2025; 26(16):7879. https://doi.org/10.3390/ijms26167879
Chicago/Turabian StyleLesgards, Jean-François, Dominique Cerdan, and Christian Perronne. 2025. "Do Long COVID and COVID Vaccine Side Effects Share Pathophysiological Picture and Biochemical Pathways?" International Journal of Molecular Sciences 26, no. 16: 7879. https://doi.org/10.3390/ijms26167879
APA StyleLesgards, J.-F., Cerdan, D., & Perronne, C. (2025). Do Long COVID and COVID Vaccine Side Effects Share Pathophysiological Picture and Biochemical Pathways? International Journal of Molecular Sciences, 26(16), 7879. https://doi.org/10.3390/ijms26167879





