Benzofuran Derivatives with Antimicrobial and Anti-Inflammatory Activities from Penicillium crustosum SCNU-F0046
Abstract
1. Introduction
2. Results and Discussion
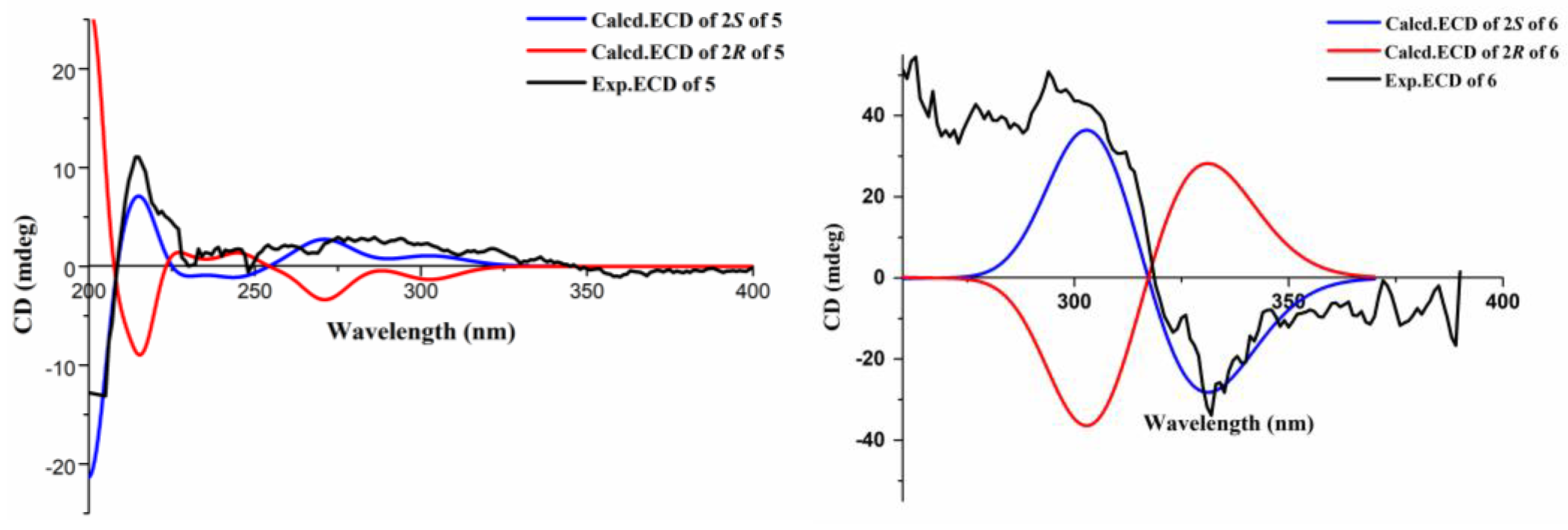
2.1. Structures of Compounds 1, 2, 5 and 6
2.2. Antimicrobial Assay
2.3. Anti-Inflammatory Assay
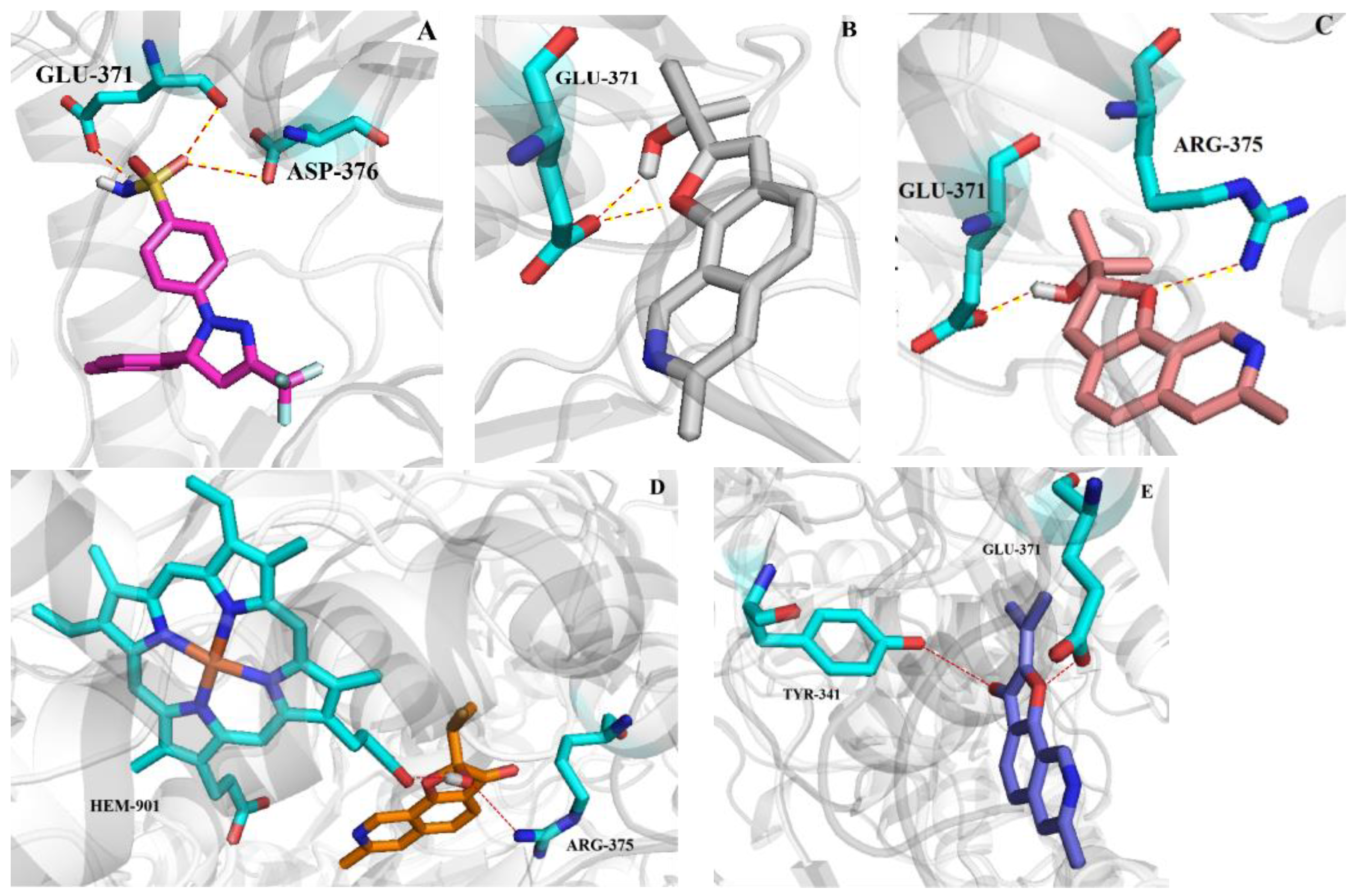
2.4. Molecular Docking Studies
3. Experimental Section
3.1. General Experimental Procedures
3.2. Fungal Material
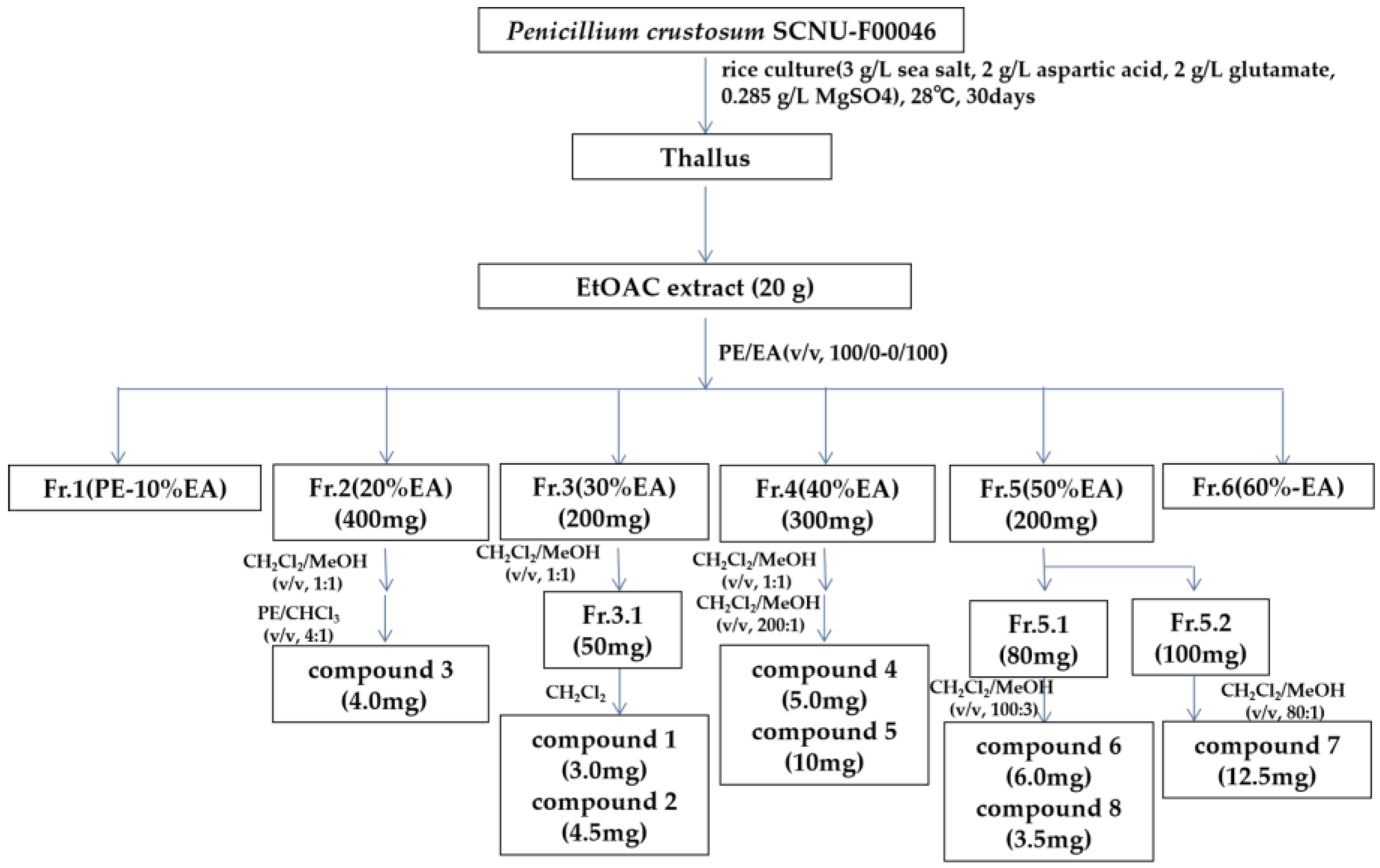
3.3. Fermentation and Isolation
3.4. X-Ray Crystal Data for Compound 1, 2
3.5. ECD Calculation
3.6. In Vitro Antimicrobial Activity
3.7. Anti-Inflammatory and Cytotoxicity Assays
3.8. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Casertano, M.; Menna, M.; Imperatore, C. The Ascidian-Derived Metabolites with Antimicrobial Properties. Antibiotics 2020, 9, 510. [Google Scholar] [CrossRef] [PubMed]
- Millero, F.J. Descriptive Oceanography. In Chemical Oceanography, 4th ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2013. [Google Scholar]
- Jones, M.D.M.; Forn, I.; Gadelha, C.; Egan, M.J.; Bass, D.; Massana, R.; Richards, T.A. Discovery of novel intermediate forms redefines the fungal tree of life. Nature 2011, 474, 200–203. [Google Scholar] [CrossRef]
- Pang, K.L.; Overy, D.P.; Jones, E.B.G.; Calado, M.D.L.; Burgaud, G.; Walker, A.K.; Johnson, J.A.; Kerr, R.G.; Cha, H.J.; Bills, G.F. ‘Marine fungi’ and ‘marine-derived fungi’ in natural product chemistry research: Toward a new consensual definition, fungal. Biol. Rev. 2016, 30, 163–175. [Google Scholar] [CrossRef]
- Gordon, E.M.; Sankhala, K.K.; Chawla, N.; Chawla, S.P. Trabectedin for Soft Tissue Sarcoma: Current Status and Future Perspectives. Adv. Ther. 2016, 33, 1055–1071. [Google Scholar] [CrossRef]
- Olivera, B.M.; Rivier, J.; Clark, C. Diversity of conus neuropeptides. Science 1990, 249, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.Y.; Gao, Y.; Liu, J.Y.; Xu, Y.; Wei, M.Y.; Wang, C.Y.; Gu, Y.C.; Shao, C.L. Design and Characterization of a Natural Arf-GEFs Inhibitor Prodrug CHNQD-01255 with Potent Anti-Hepatocellular Carcinoma Efficacy in Vivo. J. Med. Chem. 2022, 65, 11970–11984. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.X.; Chen, Y.; Zhang, Y.; Li, K.; Wang, S.; Li, L.; Wang, C.; Li, P.L. Isolation of azaphilone derivatives from the marine-derived fungus Talaromyces variabilis. Fitoterapia 2025, 185, 106709. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Grkovic, T.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2024, 41, 162–207. [Google Scholar] [CrossRef]
- Chen, X.H.; Zhou, G.L.; Sun, C.X.; Zhang, X.M.; Zhang, G.J.; Zhu, T.J.; Li, J.; Che, Q.; Li, D.H. Penicacids E−G, three new mycophenolic acid derivatives from the marine-derived fungus Penicillium parvum HDN17-478. Chin. J. Nat. Med. 2020, 18, 850–854. [Google Scholar] [CrossRef]
- Chen, S.H.; Cai, R.L.; Liu, Z.M.; Cui, H.; She, Z.G. Secondary metabolites from mangrove-associated fungi: Source, chemistry and bioactivities. Nat. Prod. Rep. 2022, 39, 560–595. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, Q.; Xu, J.; Li, M.Y.; Pan, J.Y.; Yang, M.H. Natural products from true mangrove flora: Source, chemistry and bioactivities. Nat. Prod. Rep. 2008, 25, 955–981. [Google Scholar] [CrossRef]
- Li, K.L.; Chen, S.Q.; Pang, X.Y.; Cai, J.; Zhang, X.Y.; Liu, Y.H.; Zhu, Y.G.; Zhou, X.F. Natural products from mangrove sediments-derived microbes: Structural diversity, bioactivities, biosynthesis, and total synthesis. Eur. J. Med. Chem. 2022, 230, 114117. [Google Scholar] [CrossRef]
- Wu, Q.; Chang, Y.; Che, Q.; Li, D.; Zhang, G.; Zhu, T. Citreobenzofuran D–F and Phomenone A–B: Five Novel Sesquiterpenoids from the Mangrove-Derived Fungus Penicillium sp. HDN13-494. Mar. Drugs. 2022, 20, 137. [Google Scholar] [CrossRef]
- Xu, J. Bioactive natural products derived from mangrove-associated microbes. RSC Adv. 2015, 5, 841–892. [Google Scholar] [CrossRef]
- Li, H.L.; Li, X.M.; Liu, H.; Meng, L.H.; Wang, B.G. Two new Diphenylketones and a new Xanthone from Talaromyces islandicus EN-501, an endophytic fungus derived from the marine red alga Laurencia okamurai. Mar. Drugs. 2016, 14, 223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Z.; He, X.Q.; Zhang, G.J.; Che, Q.; Zhu, T.J.; Gu, Q.Q.; Li, D.H. Inducing Secondary Metabolite Production by Combined Culture of Talaromyces aculeatus and Penicillium variabile. J. Nat. Prod. 2017, 80, 3167–3171. [Google Scholar] [CrossRef]
- Li, J.L.; Lin, Z.R.; Zeng, H.Q.; Zeng, J.C.; Ye, S.Y.; Chen, C.; Hao, J.; Li, K.; She, Z.G.; Long, Y.H. Talarergosteroids A-C: Three Unusual Steroid-Polyketone Conjugates with Antifungal Activity from a Kandelia Obovata Derived Fungus Talaromyces sp. J. Agric. Food Chem. 2025, 73, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ye, G.T.; Tang, J.; Li, J.L.; Liu, W.B.; Wu, L.; Long, Y.H. New Polyketides from Mangrove Endophytic Fungus Penicillium sp. BJR-P2 and Their Anti-Inflammatory Activity. Mar. Drugs 2022, 20, 583. [Google Scholar] [CrossRef]
- Yan, Z.Y.; Huang, C.Y.; Guo, H.X.; Zheng, S.Y.; He, J.R.; Lin, J.; Long, H.Y. Isobenzofuranone monomer and dimer derivatives from the mangrove endophytic fungus Epicoccum nigrum SCNU-F0002 possess α-glucosidase inhibitory and antioxidant activity. Bioorg. Chem. 2020, 94, 103407. [Google Scholar] [CrossRef]
- Chen, C.; Liu, L.L.; Ye, S.Y.; Li, J.L.; Wu, L.; Li, J.S.; Jia, H.; Long, Y.H. New steroids from mangrove-associated fungus Trichoderma asperellum SCNU-F0048. Steroids 2024, 208, 109449. [Google Scholar] [CrossRef]
- Jia, H.; Wu, L.; Liu, R.R.; Li, J.L.; Liu, L.L.; Chen, C.; Li, J.S.; Zhang, K.; Liao, J.J.; Long, Y.H. Penifuranone A: A Novel Alkaloid from the Mangrove Endophytic Fungus Penicillium crustosum SCNU-F0006. Int. J. Mol. Sci. 2024, 25, 5032. [Google Scholar] [CrossRef]
- Huang, C.Y.; Chen, T.; Yan, Z.Y.; Guo, H.X.; Hou, X.B.; Jiang, L.S.; Long, Y.H. Thiocladospolide E and cladospamide A, novel 12-membered macrolide and macrolide lactam from mangrove endophytic fungus Cladosporium sp. SCNU-F0001. Fitoterapia 2019, 137, 104246. [Google Scholar] [CrossRef] [PubMed]
- Slack, G.J.; Puniani, E.; Frisvad, J.C.; Samson, R.A.; Miller, J.D. Secondary metabolites from Eurotium species, Aspergillus calidoustus and A. insuetus common in Canadian homes with a review of their chemistry and biological activities. Mycol. Res. 2009, 113, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ding, L.; Wang, N.; Xu, J.; Zhang, W.Y.; Zhang, B.; He, S.; Wu, B.; Jin, H. Isolation and Characterization of Two New Metabolites from the Sponge-Derived Fungus Aspergillus sp. LS34 by OSMAC Approach. Mar. Drugs 2019, 17, 283. [Google Scholar] [CrossRef]
- Guo, H.X.; Huang, C.Y.; Yan, Z.Y.; Chen, T.; Hong, K.; Long, Y.H. New furo[3,2-h] isochroman from the mangrove endophytic fungus Aspergillus sp. 085242. Chin. J. Nat. Med. 2020, 18, 855–859. [Google Scholar] [CrossRef]
- Kohno, J.; Hiramastu, H.; Nishio, M.; Sakurai, M.; Okuda, T.; Komatsubara, S. Structures of TMC-120 A, B, C, novel isoquinoline alkaloids from Aspergillus ustus TC 1118. Tetrahedron 1999, 55, 11247–11252. [Google Scholar] [CrossRef]
- Bunbamrung, N.; Intaraudom, C.; Boonyuen, N.; Rachtawee, P.; Laksanacharoen, P.; Pittayakhajonwut, P. Penicisochromans from the endophytic fungus Penicillium sp. BCC18034. Phytochem. Lett. 2014, 10, 13–18. [Google Scholar] [CrossRef]
- Garcin, E.D.; Arvai, A.S.; Rosenfeld, R.J.; Kroeger, M.D.; Crane, B.R.; Andersson, G.; Andrews, G.; Hamley, P.J.; Mallinder, P.R.; Nicholls, D.J.; et al. Anchored plasticity opens doors for selective inhibitor design in nitric oxide synthase. Nat. Chem. Biol. 2008, 4, 700–707. [Google Scholar] [CrossRef]
- Yang, K.L.; Wei, M.Y.; Shao, C.L.; Fu, X.M.; Guo, Z.Y.; Xu, R.F.; Zheng, C.J.; She, Z.G.; Lin, Y.C.; Wang, C.Y. Antibacterial anthraquinone derivatives from a sea anemone-derived fungus Nigrospora sp. J. Nat. Prod. 2012, 75, 935–941. [Google Scholar] [CrossRef]
- Shin, B.; Kim, B.Y.; Cho, E.; Oh, K.B.; Shin, J.; Goodfellow, M.; Oh, D.C. Actinomadurol, an antibacterial norditerpenoid from a rare actinomycete, Actinomadura sp. KC 191. J. Nat. Prod. 2016, 79, 1886–1890. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Lee, J.M.; Jun, S.H.; Lee, S.H.; Kim, N.W.; Lee, J.H.; Ko, N.Y.; Mun, S.H.; Kim, B.K.; Lim, B.O. The anti-inflammatory e ects of Pyrolae herba extract through the inhibition of the expression of inducible nitric oxide synthase (iNOS) and NO production. J. Ethnopharmacol. 2007, 112, 49–54. [Google Scholar] [CrossRef]
- Morikawa, T.; Matsuda, H.; Toguchida, I. Absolute Stereostructures of Three New Sesquiterpenes from the Fruit of Alpinia oxyphylla with Inhibitory Effects on Nitric Oxide Production and Degranulation in RBL-2H3 Cells. J. Nat. Prod. 2002, 65, 1468–1474. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Z.M.; Liu, H.J.; Pan, Y.H.; Li, J.; Liu, L.; She, Z.G. Dichloroisocoumarins with Potential Anti-Inflammatory Activity from the Mangrove Endophytic Fungus Ascomycota sp. CYSK-4. Mar. Drugs 2018, 16, 54. [Google Scholar] [CrossRef] [PubMed]
- Sahaya, A.; Bharat, V.D.; Savita, J.T. Quantitative structure activity relationships studies of non-steroidal anti-inflammatory drugs: A review. Arab. J. Chem. 2019, 12, 3948–3962. [Google Scholar]
- Wang, G.S.; Yuan, Y.L.; Li, Z.K.; Zhu, J.H.; She, Z.G.; Chen, Y. Cytosporones with Anti-Inflammatory Activities from the Mangrove Endophytic Fungus Phomopsis sp. QYM-13. Mar. Drugs 2023, 21, 631. [Google Scholar] [CrossRef] [PubMed]
- Garret, M.M.; Huey, R.; Lingstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar]
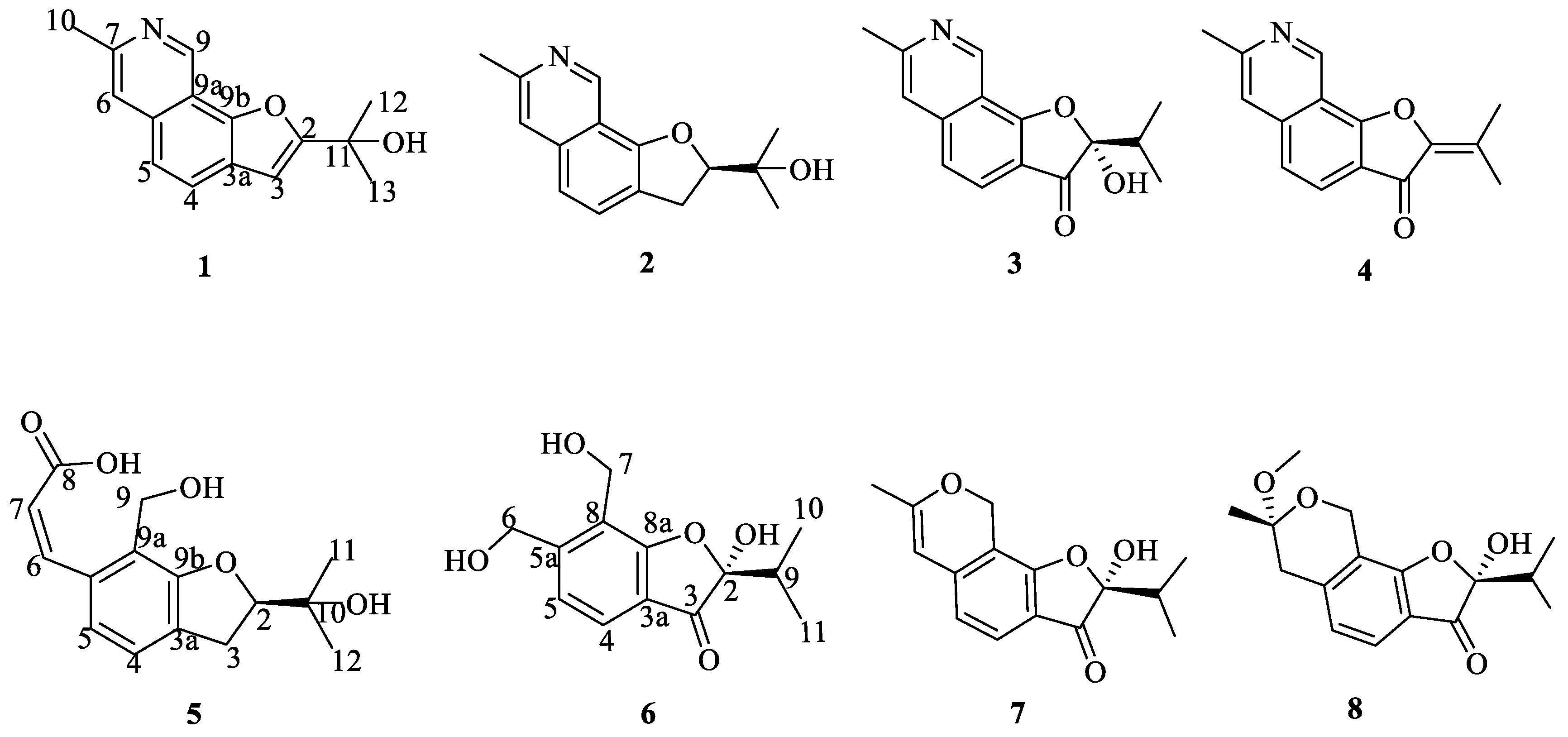
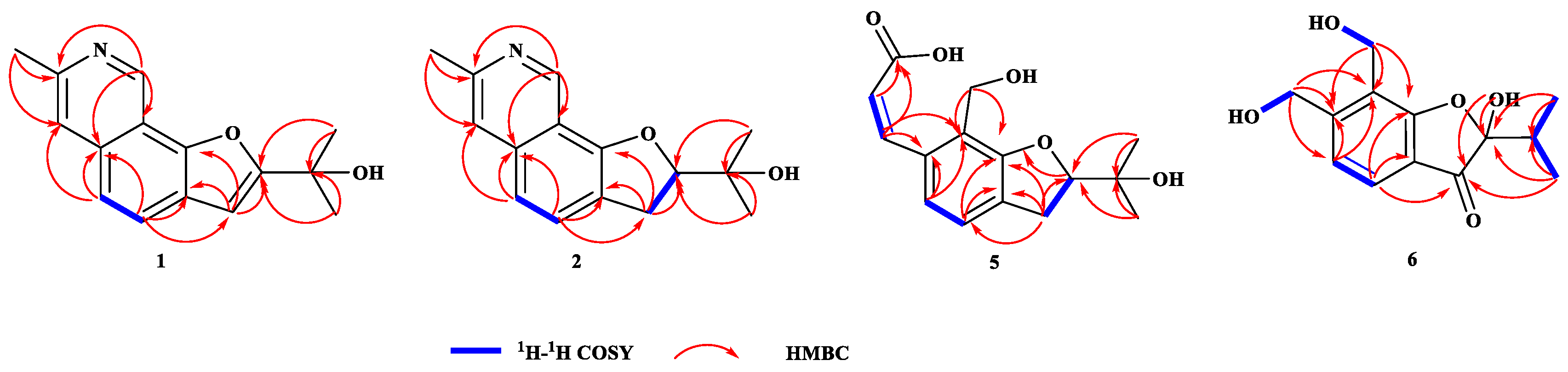
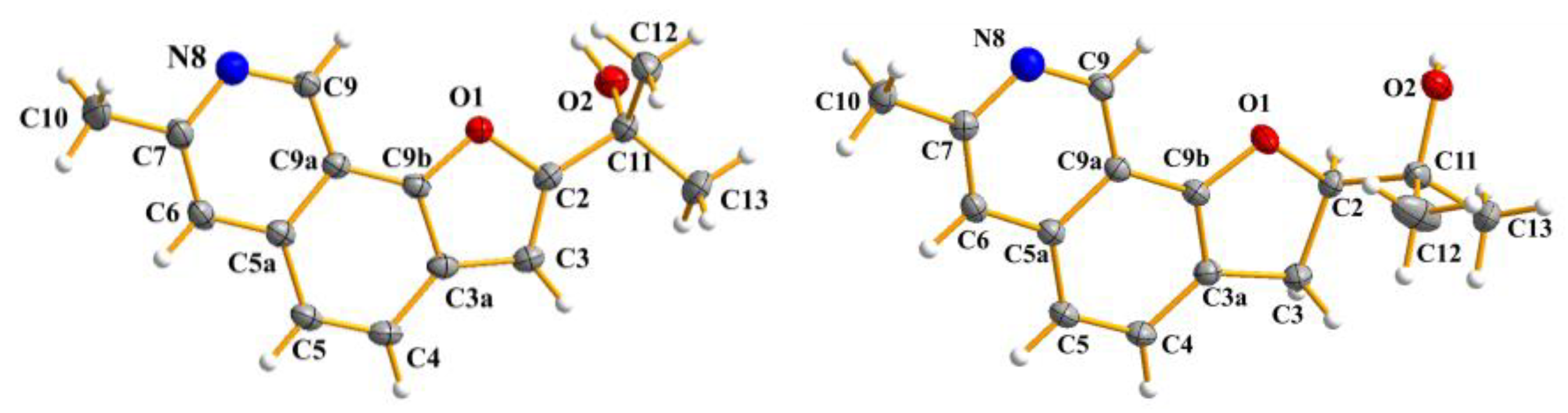

| Position | 1 | |
|---|---|---|
| δH (J in Hz) | δC, Type | |
| 2 | 163.5, C | |
| 3 | 6.75, s | 101.7, CH |
| 3a | 149.5, C | |
| 4 | 7.76, d (8.46) | 124.8, CH |
| 5 | 7.51, d (8.46) | 120.2, CH |
| 5a | 134.8, C | |
| 6 | 7.55, s | 119.2, CH |
| 7 | 151.3, C | |
| 9 | 9.65, s | 144.5, CH |
| 9a | 115.2, C | |
| 9b | 124.7, C | |
| 10 | 2.74, s | 24.2, CH3 |
| 11 | 69.4, C | |
| 12 | 1.78, s | 29.0, CH3 |
| 13 | 1.78, s | 29.0, CH3 |
| Position | 2 | |
|---|---|---|
| δH (J in Hz) | δC, Type | |
| 2 | 3, 28, dd (9.84, 9.84) | 91.3, CH2 |
| 3 | 3.47, m | 30.8, CH |
| 3a | 122.0, C | |
| 4 | 7.42, d (8.16) | 127.8, CH |
| 5 | 7.08, d (8.16) | 120.2, CH |
| 5a | 136.6, C | |
| 6 | 7.19, s | 118.4, CH |
| 7 | 155.7, C | |
| 9 | 9.26, s | 145.8, CH |
| 9a | 113.9, C | |
| 9b | 150.4, C | |
| 10 | 2.48, s | 26.7, CH3 |
| 11 | 71.4, C | |
| 12 | 1.29, s | 24.8, CH3 |
| 13 | 1.54, s | 23.6, CH3 |
| Position | 5 | |
|---|---|---|
| δH (J in Hz) | δC, Type | |
| 2 | 4.64, t (9.1) | 90.5, CH |
| 3 | 3.25, m | 31.6, CH2 |
| 3a | 130.9, C | |
| 4 | 7.17, d (7.8) | 125.5, CH |
| 5 | 7.21, d (7.8) | 120.0, CH |
| 5a | 135.2, C | |
| 6 | 6.41, d (15.9) | 120.6, CH |
| 7 | 8.08, d (15.9) | 143.5, CH |
| 8 | 170.8, C | |
| 9 | 4.75, s | 55.4, CH2 |
| 9a | 121.8, C | |
| 9b | 160.1, C | |
| 10 | 72.4, C | |
| 11 | 1.33, s | 25.7, CH3 |
| 12 | 1.24, s | 24.8, CH3 |
| Position | 6 | |
|---|---|---|
| δH (J in Hz) | δC, Type | |
| 2 | 107.7, C | |
| 3 | 199.7, C | |
| 3a | 118.7, C | |
| 4 | 7.47, d (7.9) | 122.4, CH |
| 5 | 7.25, d (7.9) | 112.0, CH |
| 5a | 153.7, C | |
| 6 | 4.74, m | 60.3, CH2 |
| 7 | 4.56, m | 52.5, CH2 |
| 8 | 122.8, C | |
| 8a | 168.8, C | |
| 9 | 2.07, m | 33.5, CH |
| 10 | 0.80, d (6.8) | 15.9, CH3 |
| 11 | 0.99, d (6.8) | 15.6, CH3 |
| OH-2 | 7.55, s | |
| OH-6 | 5.33, t (5.6) | |
| OH-7 | 5.00, t (5.2) | |
| Compound | Salmonella typhimurium | Escherichia coli | Staphylococcus aureus | Penicillium italicum | Fusarium oxysporum | Colletotrichum musae |
|---|---|---|---|---|---|---|
| 1 | 12.5 | 25 | 12.5 | 50 | 50 | 50 |
| 2 | 50 | 50 | 25 | 50 | 50 | 25 |
| 5 | 50 | 50 | 50 | 12.5 | 50 | 25 |
| 6 | 50 | 100 | 50 | 12.5 | 50 | 12.5 |
| Carbendazim b | - | - | - | 0.78 | 1.56 | 0.78 |
| Ciprofloxacin c | 0.2 | 0.8 | 0.1 | - | - | - |
| Compound | Inhibition of NO Production (IC50/μM) b | Cytotoxicity (CC50/μM) b |
|---|---|---|
| 1 | 17.3 ± 1.6 | >50 |
| 2 | 31.5 ± 2.3 | >50 |
| 3 | 42.8 ± 4.7 | >50 |
| 4 | 16.5 ± 3.4 | >50 |
| Celecoxib a | 32.1 ± 1.7 | >50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Kang, J.; Zhang, R.; Jia, H.; Lin, Z.; Liu, Z.; Liu, R.; Zou, X.; Long, Y. Benzofuran Derivatives with Antimicrobial and Anti-Inflammatory Activities from Penicillium crustosum SCNU-F0046. Int. J. Mol. Sci. 2025, 26, 7861. https://doi.org/10.3390/ijms26167861
Chen C, Kang J, Zhang R, Jia H, Lin Z, Liu Z, Liu R, Zou X, Long Y. Benzofuran Derivatives with Antimicrobial and Anti-Inflammatory Activities from Penicillium crustosum SCNU-F0046. International Journal of Molecular Sciences. 2025; 26(16):7861. https://doi.org/10.3390/ijms26167861
Chicago/Turabian StyleChen, Chen, Jinbi Kang, Ruiqi Zhang, Hao Jia, Zirong Lin, Zhengming Liu, Rongrong Liu, Xinyi Zou, and Yuhua Long. 2025. "Benzofuran Derivatives with Antimicrobial and Anti-Inflammatory Activities from Penicillium crustosum SCNU-F0046" International Journal of Molecular Sciences 26, no. 16: 7861. https://doi.org/10.3390/ijms26167861
APA StyleChen, C., Kang, J., Zhang, R., Jia, H., Lin, Z., Liu, Z., Liu, R., Zou, X., & Long, Y. (2025). Benzofuran Derivatives with Antimicrobial and Anti-Inflammatory Activities from Penicillium crustosum SCNU-F0046. International Journal of Molecular Sciences, 26(16), 7861. https://doi.org/10.3390/ijms26167861






