Elucidating the Complex Structural and Molecular Mechanisms Driving P-Glycoprotein-Mediated Transport of Cardiac Glycosides
Abstract
1. Introduction
2. Results
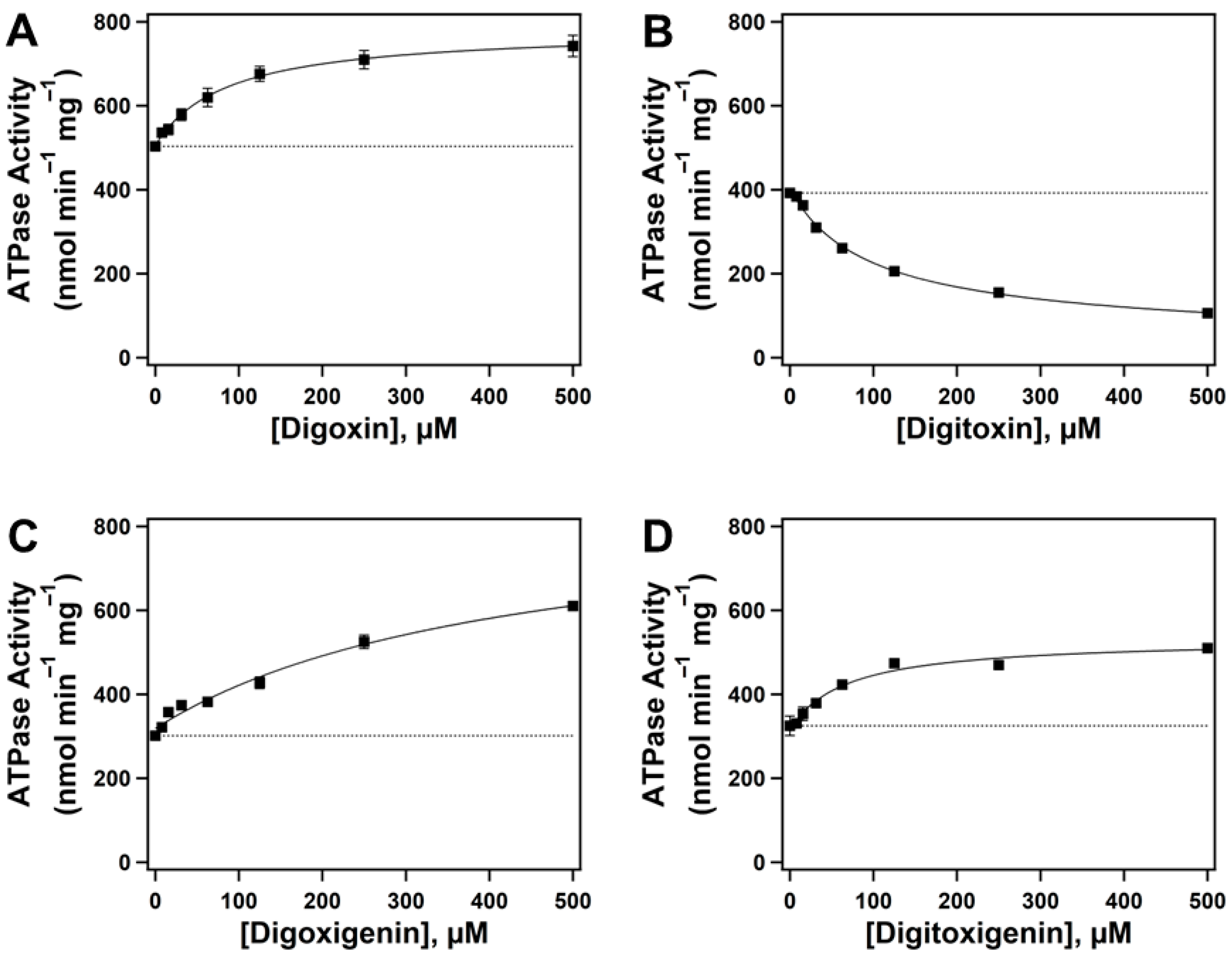
2.1. Effect of Cardiac Glycosides (CGs) on Pgp-Mediated ATP Hydrolysis
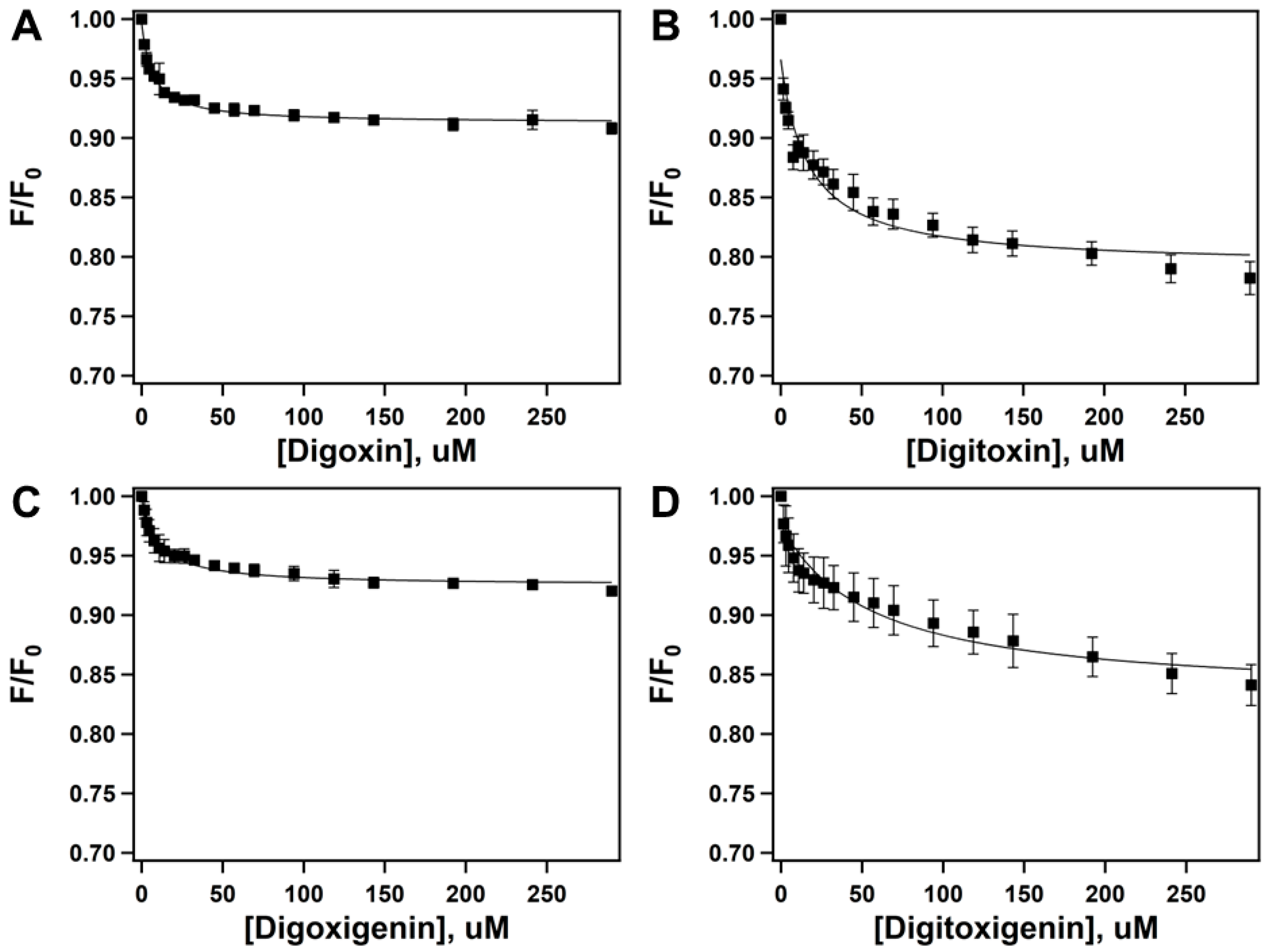
2.2. Quenching of Pgp’s Intrinsic Tryptophan Fluorescence to Determine Drug-Protein Binding Affinity
2.3. Acrylamide Quenching of Protein Fluorescence to Determine CG-Induced Conformational Changes in Pgp
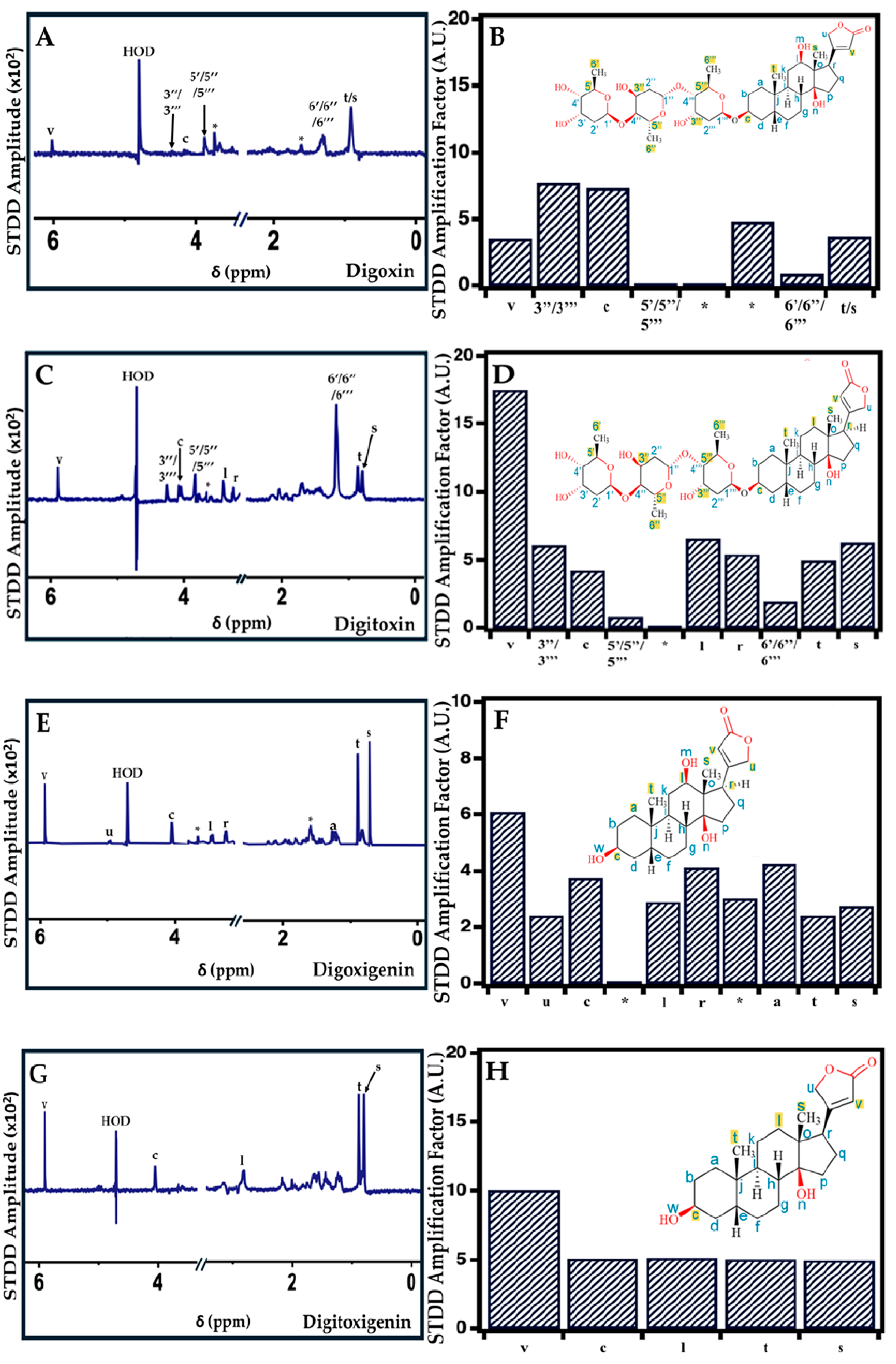
2.4. Interaction of Cardiac Glycosides with Pgp Determined by STDD NMR
3. Discussion
4. Materials and Methods
4.1. Chemical Reagents
4.2. Expression and Purification of P-Glycoprotein (Pgp)
4.3. Preparation of Proteoliposomes
4.4. Measurement of Pgp-Mediated ATPase Activity
4.5. Pgp-Drug Binding Affinity by Intrinsic Tryptophan Quenching
4.6. Drug-Induced Pgp Conformational Changes by Acrylamide Quenching of Protein Fluorescence
4.7. Saturation Transfer Double-Difference (STDD) NMR
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ledwitch, K.V.; Gibbs, M.E.; Barnes, R.W.; Roberts, A.G. Cooperativity between Verapamil and ATP Bound to the Efflux Transporter P-Glycoprotein. Biochem. Pharmacol. 2016, 118, 96–108. [Google Scholar] [CrossRef]
- Mensah, G.A.K.; Schaefer, K.G.; Bartlett, M.G.; Roberts, A.G.; King, G.M. Drug-Induced Conformational Dynamics of P-Glycoprotein Underlies the Transport of Camptothecin Analogs. Int. J. Mol. Sci. 2023, 24, 16058. [Google Scholar] [CrossRef]
- Xu, L.; Schaefer, K.G.; King, G.M.; Xie, Z.-R.; Bartlett, M.G. Insights into Interactions between Taxanes and P-Glycoprotein Using Biophysical and in Silico Methods. J. Pharm. Sci. 2025, 114, 103708. [Google Scholar] [CrossRef] [PubMed]
- Fu, D. Where Is It and How Does It Get There—Intracellular Localization and Traffic of P-Glycoprotein. Front. Oncol. 2013, 3, 321. [Google Scholar] [CrossRef]
- Durmus, S.; Hendrikx, J.J.M.A.; Schinkel, A.H. Chapter One—Apical ABC Transporters and Cancer Chemotherapeutic Drug Disposition. In Advances in Cancer Research; Schuetz, J.D., Ishikawa, T., Eds.; ABC Transporters and Cancer; Academic Press: Cambridge, MA, USA, 2015; Volume 125, pp. 1–41. [Google Scholar] [CrossRef]
- Shchulkin, A.V.; Abalenikhina, Y.V.; Kosmachevskaya, O.V.; Topunov, A.F.; Yakusheva, E.N. Regulation of P-Glycoprotein during Oxidative Stress. Antioxidants 2024, 13, 215. [Google Scholar] [CrossRef] [PubMed]
- Juvale, I.I.A.; Hamid, A.A.A.; Halim, K.B.A.; Has, A.T.C. P-Glycoprotein: New Insights into Structure, Physiological Function, Regulation and Alterations in Disease. Heliyon 2022, 8, e09777. [Google Scholar] [CrossRef] [PubMed]
- Aller, S.G.; Yu, J.; Ward, A.; Weng, Y.; Chittaboina, S.; Zhuo, R.; Harrell, P.M.; Trinh, Y.T.; Zhang, Q.; Urbatsch, I.L.; et al. Structure of P-Glycoprotein Reveals a Molecular Basis for Poly-Specific Drug Binding. Science 2009, 323, 1718–1722. [Google Scholar] [CrossRef]
- Rodriguez, I.; Abernethy, D.R.; Woosley, R.L. P-Glycoprotein in Clinical Cardiology. Circulation 1999, 99, 472–474. [Google Scholar] [CrossRef]
- Wessler, J.D.; Grip, L.T.; Mendell, J.; Giugliano, R.P. The P-Glycoprotein Transport System and Cardiovascular Drugs. JACC 2013, 61, 2495–2502. [Google Scholar] [CrossRef]
- Mowry, J.B.; Spyker, D.A.; Brooks, D.E.; McMillan, N.; Schauben, J.L. 2014 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 32nd Annual Report. Clin. Toxicol. 2015, 53, 962–1147. [Google Scholar] [CrossRef]
- Regina, A.C.; Rehman, R.; Hai, O. Cardiac Glycoside and Digoxin Toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Tomilova, S.V.; Kitashov, A.V.; Nosov, A.M. Cardiac Glycosides: Distribution, Properties and Specificity of Formation in Plant Cell and Organ Cultures In Vitro. Russ. J. Plant Physiol. 2022, 69, 41. [Google Scholar] [CrossRef]
- Fender, J.; Klöcker, J.; Boivin-Jahns, V.; Ravens, U.; Jahns, R.; Lorenz, K. “Cardiac Glycosides”—Quo Vaditis?—Past, Present, and Future? Naunyn. Schmiedebergs Arch. Pharmacol. 2024, 397, 9521–9531. [Google Scholar] [CrossRef]
- Schwinger, R.H.G.; Bundgaard, H.; Müller-Ehmsen, J.; Kjeldsen, K. The Na, K-ATPase in the Failing Human Heart. Cardiovasc. Res. 2003, 57, 913–920. [Google Scholar] [CrossRef]
- Calderón-Montaño, J.M.; Burgos-Morón, E.; López-Lázaro, M. The in Vivo Antitumor Activity of Cardiac Glycosides in Mice Xenografted with Human Cancer Cells Is Probably an Experimental Artifact. Oncogene 2014, 33, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Anderson, A.T.; Meyer, G.; Lauber, K.M.; Gallucci, J.C.; Douglas Kinghorn, A. Digoxin and Its Na+/K+-ATPase-Targeted Actions on Cardiovascular Diseases and Cancer. Bioorg. Med. Chem. 2024, 114, 117939. [Google Scholar] [CrossRef]
- Cornelius, F.; Kanai, R.; Toyoshima, C. A Structural View on the Functional Importance of the Sugar Moiety and Steroid Hydroxyls of Cardiotonic Steroids in Binding to Na,K-ATPase. J. Biol. Chem. 2013, 288, 6602–6616. [Google Scholar] [CrossRef]
- Kowsalya, K.; Vidya, N.; Halka, J.; Preetha, J.S.Y.; Saradhadevi, M.; Sahayarayan, J.J.; Gurusaravanan, P.; Arun, M. Plant Glycosides and Glycosidases: Classification, Sources, and Therapeutic Insights in Current Medicine. Glycoconj. J. 2025, 42, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Akinwumi, I.A.; Ambali, O.A. Cardiac Glycosides from African Medicinal Plants as Promising Therapeutics. Trop. J. Phytochem. Pharm. Sci. 2024, 3, 158–167. [Google Scholar] [CrossRef]
- Akinmoladun, A.C.; Olaleye, M.T.; Farombi, E.O. 13—Cardiotoxicity and Cardioprotective Effects of African Medicinal Plants. In Toxicological Survey of African Medicinal Plants; Kuete, V., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 395–421. [Google Scholar] [CrossRef]
- Prassas, I.; Diamandis, E.P. Novel Therapeutic Applications of Cardiac Glycosides. Nat. Rev. Drug Discov. 2008, 7, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Morsy, N. Cardiac Glycosides in Medicinal Plants. In Aromatic and Medicinal Plants—Back to Nature; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar] [CrossRef]
- López-Lázaro, M.; Pastor, N.; Azrak, S.S.; Ayuso, M.J.; Austin, C.A.; Cortés, F. Digitoxin Inhibits the Growth of Cancer Cell Lines at Concentrations Commonly Found in Cardiac Patients. J. Nat. Prod. 2005, 68, 1642–1645. [Google Scholar] [CrossRef]
- Mijatovic, T.; Mathieu, V.; Gaussin, J.-F.; De Néve, N.; Ribaucour, F.; Van Quaquebeke, E.; Dumont, P.; Darro, F.; Kiss, R. Cardenolide-Induced Lysosomal Membrane Permeabilization Demonstrates Therapeutic Benefits in Experimental Human Non-Small Cell Lung Cancers. Neoplasia 2006, 8, 402–412. [Google Scholar] [CrossRef] [PubMed]
- McConkey, D.J.; Lin, Y.; Nutt, L.K.; Ozel, H.Z.; Newman, R.A. Cardiac Glycosides Stimulate Ca2+ Increases and Apoptosis in Androgen-Independent, Metastatic Human Prostate Adenocarcinoma Cells. Cancer Res. 2000, 60, 3807–3812. [Google Scholar] [PubMed]
- Huang, Y.-T.; Chueh, S.-C.; Teng, C.-M.; Guh, J.-H. Investigation of Ouabain-Induced Anticancer Effect in Human Androgen-Independent Prostate Cancer PC-3 Cells. Biochem. Pharmacol. 2004, 67, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Johansson, S.; Lindholm, P.; Gullbo, J.; Larsson, R.; Bohlin, L.; Claeson, P. Cytotoxicity of Digitoxin and Related Cardiac Glycosides in Human Tumor Cells. Anticancer. Drugs 2001, 12, 475. [Google Scholar] [CrossRef] [PubMed]
- Krishna, A.B.; Manikyam, H.K.; Sharma, V.K. Plant Cardenolides in Therapeutics. Int. J. Indig. Med. Plants 2015, 48, 1871–1896. [Google Scholar]
- Melero, C.P.; Medarde, M.; San Feliciano, A. A Short Review on Cardiotonic Steroids and Their Aminoguanidine Analogues. Molecules 2000, 5, 51–81. [Google Scholar] [CrossRef]
- Pauli-Magnus, C.; Mürdter, T.; Godel, A.; Mettang, T.; Eichelbaum, M.; Klotz, U.; Fromm, M.F. P-Glycoprotein-Mediated Transport of Digitoxin, α-Methyldigoxin and β-Acetyldigoxin. Naunyn. Schmiedebergs Arch. Pharmacol. 2001, 363, 337–343. [Google Scholar] [CrossRef]
- Gozalpour, E.; Wittgen, H.G.M.; van den Heuvel, J.J.M.W.; Greupink, R.; Russel, F.G.M.; Koenderink, J.B. Interaction of Digitalis-Like Compounds with P-Glycoprotein. Toxicol. Sci. 2013, 131, 502–511. [Google Scholar] [CrossRef]
- Khandelwal, R.; Vagha, J.D.; Meshram, R.J.; Patel, A. A Comprehensive Review on Unveiling the Journey of Digoxin: Past, Present, and Future Perspectives. Cureus 2024, 16, e56755. [Google Scholar] [CrossRef]
- Verschraagen, M.; Koks, C.H.W.; Schellens, J.H.M.; Beijnen, J.H. P-GLYCOPROTEIN SYSTEM AS A DETERMINANT OF DRUG INTERACTIONS: THE CASE OF DIGOXIN–VERAPAMIL. Pharmacol. Res. 1999, 40, 301–306. [Google Scholar] [CrossRef]
- Englund, G.; Hallberg, P.; Artursson, P.; Michaëlsson, K.; Melhus, H. Association between the Number of Coadministered P-Glycoprotein Inhibitors and Serum Digoxin Levels in Patients on Therapeutic Drug Monitoring. BMC Med. 2004, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Ledwitch, K.V.; Barnes, R.W.; Roberts, A.G. Unravelling the Complex Drug–Drug Interactions of the Cardiovascular Drugs, Verapamil and Digoxin, with P-Glycoprotein. Biosci. Rep. 2016, 36, e00309. [Google Scholar] [CrossRef]
- Hughes, J.; Crowe, A. Inhibition of P-Glycoprotein–Mediated Efflux of Digoxin and Its Metabolites by Macrolide Antibiotics. J. Pharmacol. Sci. 2010, 113, 315–324. [Google Scholar] [CrossRef]
- Gozalpour, E.; Wilmer, M.J.; Bilos, A.; Masereeuw, R.; Russel, F.G.M.; Koenderink, J.B. Heterogeneous Transport of Digitalis-like Compounds by P-Glycoprotein in Vesicular and Cellular Assays. Toxicol. In Vitro 2016, 32, 138–145. [Google Scholar] [CrossRef]
- Sauna, Z.E.; Ambudkar, S.V. About a Switch: How P-Glycoprotein (ABCB1) Harnesses the Energy of ATP Binding and Hydrolysis to Do Mechanical Work. Mol. Cancer Ther. 2007, 6, 13–23. [Google Scholar] [CrossRef]
- Sauna, Z.E.; Müller, M.; Peng, X.-H.; Ambudkar, S.V. Importance of the Conserved Walker B Glutamate Residues, 556 and 1201, for the Completion of the Catalytic Cycle of ATP Hydrolysis by Human P-Glycoprotein (ABCB1). Biochemistry 2002, 41, 13989–14000. [Google Scholar] [CrossRef]
- Patzlaff, J.S.; van der Heide, T.; Poolman, B. The ATP/Substrate Stoichiometry of the ATP-Binding Cassette (ABC) Transporter OpuA. J. Biol. Chem. 2003, 278, 29546–29551. [Google Scholar] [CrossRef]
- Litman, T.; Zeuthen, T.; Skovsgaard, T.; Stein, W.D. Structure-Activity Relationships of P-Glycoprotein Interacting Drugs: Kinetic Characterization of Their Effects on ATPase Activity. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 1997, 1361, 159–168. [Google Scholar] [CrossRef]
- Seelig, A. P-Glycoprotein: One Mechanism, Many Tasks and the Consequences for Pharmacotherapy of Cancers. Front. Oncol. 2020, 10, 576559. [Google Scholar] [CrossRef] [PubMed]
- Garrigues, A.; Escargueil, A.E.; Orlowski, S. The Multidrug Transporter, P-Glycoprotein, Actively Mediates Cholesterol Redistribution in the Cell Membrane. Proc. Natl. Acad. Sci. USA 2002, 99, 10347–10352. [Google Scholar] [CrossRef] [PubMed]
- von Richter, O.; Glavinas, H.; Krajcsi, P.; Liehner, S.; Siewert, B.; Zech, K. A Novel Screening Strategy to Identify ABCB1 Substrates and Inhibitors. Naunyn. Schmiedebergs Arch. Pharmacol. 2009, 379, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Kerr, K.M.; Sauna, Z.E.; Ambudkar, S.V. Correlation between Steady-State ATP Hydrolysis and Vanadate-Induced ADP Trapping in Human P-Glycoprotein: Evidence for ADP release as the rate-limiting step in the catalytic cycle and its modulation by substrates. J. Biol. Chem. 2001, 276, 8657–8664. [Google Scholar] [CrossRef]
- Nguyen, P.H.; Sigdel, K.P.; Schaefer, K.G.; Mensah, G.A.K.; King, G.M.; Roberts, A.G. The Effects of Anthracycline Drugs on the Conformational Distribution of Mouse P-Glycoprotein Explains Their Transport Rate Differences. Biochem. Pharmacol. 2020, 174, 113813. [Google Scholar] [CrossRef]
- Tran, N.N.B.; Bui, A.T.A.; Jaramillo-Martinez, V.; Weber, J.; Zhang, Q.; Urbatsch, I.L. Lipid Environment Determines the Drug-Stimulated ATPase Activity of P-Glycoprotein. Front. Mol. Biosci. 2023, 10, 1141081. [Google Scholar] [CrossRef]
- Al-Shawi, M.K.; Polar, M.K.; Omote, H.; Figler, R.A. Transition State Analysis of the Coupling of Drug Transport to ATP Hydrolysis by P-Glycoprotein. J. Biol. Chem. 2003, 278, 52629–52640. [Google Scholar] [CrossRef]
- Sharom, F.J. Complex Interplay between the P-Glycoprotein Multidrug Efflux Pump and the Membrane: Its Role in Modulating Protein Function. Front. Oncol. 2014, 4, 41. [Google Scholar] [CrossRef]
- Thangapandian, S.; Kapoor, K.; Tajkhorshid, E. Probing Cholesterol Binding and Translocation in P-Glycoprotein. Biochim. Biophys. Acta BBA-Biomembr. 2020, 1862, 183090. [Google Scholar] [CrossRef] [PubMed]
- Yammine, A.; Gao, J.; Kwan, A. Tryptophan Fluorescence Quenching Assays for Measuring Protein-Ligand Binding Affinities: Principles and a Practical Guide. Bio-Protocol 2019, 9, e3253. [Google Scholar] [CrossRef]
- Gayer, A.V.; Yakimov, B.P.; Budylin, G.S.; Shirshin, E.A. Evaluating the Number of Ligand Binding Sites on Protein from Tryptophan Fluorescence Quenching under Typical Experimental Conditions. J. Biomed. Photonics Eng. 2020, 6, 020303. [Google Scholar] [CrossRef]
- Liu, R.; Siemiarczuk, A.; Sharom, F.J. Intrinsic Fluorescence of the P-Glycoprotein Multidrug Transporter: Sensitivity of Tryptophan Residues to Binding of Drugs and Nucleotides. Biochemistry 2000, 39, 14927–14938. [Google Scholar] [CrossRef] [PubMed]
- Sonveaux, N.; Vigano, C.; Shapiro, A.B.; Ling, V.; Ruysschaert, J.-M. Ligand-Mediated Tertiary Structure Changes of Reconstituted P-Glycoprotein: A tryptophan fluorescence quenching analysis. J. Biol. Chem. 1999, 274, 17649–17654. [Google Scholar] [CrossRef]
- Ward, A.; Reyes, C.L.; Yu, J.; Roth, C.B.; Chang, G. Flexibility in the ABC Transporter MsbA: Alternating Access with a Twist. Proc. Natl. Acad. Sci. USA 2007, 104, 19005–19010. [Google Scholar] [CrossRef]
- Gibbs, M.E.; Wilt, L.A.; Ledwitch, K.V.; Roberts, A.G. A Conformationally Gated Model of Methadone and Loperamide Transport by P-Glycoprotein. J. Pharm. Sci. 2018, 107, 1937–1947. [Google Scholar] [CrossRef]
- Jazaj, D.; Ghadami, S.A.; Bemporad, F.; Chiti, F. Probing Conformational Changes of Monomeric Transthyretin with Second Derivative Fluorescence. Sci. Rep. 2019, 9, 10988. [Google Scholar] [CrossRef] [PubMed]
- Moeller, A.; Lee, S.C.; Tao, H.; Speir, J.A.; Chang, G.; Urbatsch, I.L.; Potter, C.S.; Carragher, B.; Zhang, Q. Distinct Conformational Spectrum of Homologous Multidrug ABC Transporters. Structure 2015, 23, 450–460. [Google Scholar] [CrossRef]
- Szewczyk, P.; Tao, H.; McGrath, A.P.; Villaluz, M.; Rees, S.D.; Lee, S.C.; Doshi, R.; Urbatsch, I.L.; Zhang, Q.; Chang, G. Snapshots of Ligand Entry, Malleable Binding and Induced Helical Movement in P-Glycoprotein. Acta Crystallogr. D Biol. Crystallogr. 2015, 71, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Chen, J. Molecular Structure of Human P-Glycoprotein in the ATP-Bound, Outward-Facing Conformation. Science 2018, 359, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.; Meyer, B. Group Epitope Mapping by Saturation Transfer Difference NMR To Identify Segments of a Ligand in Direct Contact with a Protein Receptor. J. Am. Chem. Soc. 2001, 123, 6108–6117. [Google Scholar] [CrossRef]
- Xu, Y.D.; Lai, R.Y.; Procházková, E.; Stenzel, M.H. Saturation Transfer Difference NMR Spectroscopy for the Elucidation of Supramolecular Albumin–Polymer Interactions. ACS Macro Lett. 2021, 10, 819–824. [Google Scholar] [CrossRef]
- Ozols, R.F.; Cunnion, R.E.; Klecker, R.W.; Hamilton, T.C.; Ostchega, Y.; Parrillo, J.E.; Young, R.C. Verapamil and Adriamycin in the Treatment of Drug-Resistant Ovarian Cancer Patients. J. Clin. Oncol. 1987, 5, 641–647. [Google Scholar] [CrossRef]
- Nanayakkara, A.K.; Follit, C.A.; Chen, G.; Williams, N.S.; Vogel, P.D.; Wise, J.G. Targeted Inhibitors of P-Glycoprotein Increase Chemotherapeutic-Induced Mortality of Multidrug Resistant Tumor Cells. Sci. Rep. 2018, 8, 967. [Google Scholar] [CrossRef]
- PubChem. Digoxin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2724385 (accessed on 10 July 2025).
- Mercer, S.L.; Cunningham, C.W.; Eddington, N.D.; Coop, A. Opioids and Efflux Transporters. Part 3: P-Glycoprotein Substrate Activity of 3-Hydroxyl Addition to Meperidine Analogs. Bioorg. Med. Chem. Lett. 2008, 18, 3638–3640. [Google Scholar] [CrossRef]
- Loo, T.W.; Clarke, D.M. Identification of the Distance between the Homologous Halves of P-Glycoprotein That Triggers the High/Low ATPase Activity Switch. J. Biol. Chem. 2014, 289, 8484–8492. [Google Scholar] [CrossRef][Green Version]
- Cripe, L.D.; Uno, H.; Paietta, E.M.; Litzow, M.R.; Ketterling, R.P.; Bennett, J.M.; Rowe, J.M.; Lazarus, H.M.; Luger, S.; Tallman, M.S. Zosuquidar, a Novel Modulator of P-Glycoprotein, Does Not Improve the Outcome of Older Patients with Newly Diagnosed Acute Myeloid Leukemia: A Randomized, Placebo-Controlled Trial of the Eastern Cooperative Oncology Group 3999. Blood 2010, 116, 4077–4085. [Google Scholar] [CrossRef]
- Baer, M.R.; George, S.L.; Dodge, R.K.; O’Loughlin, K.L.; Minderman, H.; Caligiuri, M.A.; Anastasi, J.; Powell, B.L.; Kolitz, J.E.; Schiffer, C.A.; et al. Phase 3 Study of the Multidrug Resistance Modulator PSC-833 in Previously Untreated Patients 60 Years of Age and Older with Acute Myeloid Leukemia: Cancer and Leukemia Group B Study 9720. Blood 2002, 100, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- List, A.F.; Kopecky, K.J.; Willman, C.L.; Head, D.R.; Persons, D.L.; Slovak, M.L.; Dorr, R.; Karanes, C.; Hynes, H.E.; Doroshow, J.H.; et al. Benefit of Cyclosporine Modulation of Drug Resistance in Patients with Poor-Risk Acute Myeloid Leukemia: A Southwest Oncology Group Study. Blood 2001, 98, 3212–3220. [Google Scholar] [CrossRef] [PubMed]
- Libby, E.; Hromas, R. Dismounting the MDR Horse. Blood 2010, 116, 4037–4038. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wu, S.; Burdette, J.E.; Cheng, X.; Kinghorn, A.D. Structural Insights into the Interactions of Digoxin and Na+/K+-ATPase and Other Targets for the Inhibition of Cancer Cell Proliferation. Molecules 2021, 26, 3672. [Google Scholar] [CrossRef]
- Chen, W.-L.; Ren, Y.; Ren, J.; Erxleben, C.; Johnson, M.E.; Gentile, S.; Kinghorn, A.D.; Swanson, S.M.; Burdette, J.E. (+)-Strebloside-Induced Cytotoxicity in Ovarian Cancer Cells Is Mediated through Cardiac Glycoside Signaling Networks. J. Nat. Prod. 2017, 80, 659–669. [Google Scholar] [CrossRef]
- Ren, Y.; Ribas, H.T.; Heath, K.; Wu, S.; Ren, J.; Shriwas, P.; Chen, X.; Johnson, M.E.; Cheng, X.; Burdette, J.E.; et al. Na+/K+-ATPase-Targeted Cytotoxicity of (+)-Digoxin and Several Semisynthetic Derivatives. J. Nat. Prod. 2020, 83, 638–648. [Google Scholar] [CrossRef]
- Laursen, M.; Gregersen, J.L.; Yatime, L.; Nissen, P.; Fedosova, N.U. Structures and Characterization of Digoxin- and Bufalin-Bound Na+,K+-ATPase Compared with the Ouabain-Bound Complex. Proc. Natl. Acad. Sci. USA 2015, 112, 1755–1760. [Google Scholar] [CrossRef] [PubMed]
- Alevizopoulos, K.; Calogeropoulou, T.; Lang, F.; Stournaras, C. Na+/K+ ATPase Inhibitors in Cancer. Curr. Drug Targets 2014, 15, 988–1000. [Google Scholar] [CrossRef]
- Urbatsch, I.L.; Beaudet, L.; Carrier, I.; Gros, P. Mutations in Either Nucleotide-Binding Site of P-Glycoprotein (Mdr3) Prevent Vanadate Trapping of Nucleotide at Both Sites. Biochemistry 1998, 37, 4592–4602. [Google Scholar] [CrossRef]
- Lerner-Marmarosh, N.; Gimi, K.; Urbatsch, I.L.; Gros, P.; Senior, A.E. Large Scale Purification of Detergent-Soluble P-Glycoprotein fromPichia Pastoris Cells and Characterization of Nucleotide Binding Properties of Wild-Type, Walker A, and Walker B Mutant Proteins. J. Biol. Chem. 1999, 274, 34711–34718. [Google Scholar] [CrossRef] [PubMed]
- Dunn, B.; Wobbe, C.R. Preparation of Protein Extracts from Yeast. Curr. Protoc. Mol. Biol. 1993, 23, 13.13.1–13.13.9. [Google Scholar] [CrossRef]
- Bai, J.; Swartz, D.J.; Protasevich, I.I.; Brouillette, C.G.; Harrell, P.M.; Hildebrandt, E.; Gasser, B.; Mattanovich, D.; Ward, A.; Chang, G.; et al. A Gene Optimization Strategy That Enhances Production of Fully Functional P-Glycoprotein in Pichia Pastoris. PLoS ONE 2011, 6, e22577. [Google Scholar] [CrossRef] [PubMed]
- Chifflet, S.; Torriglia, A.; Chiesa, R.; Tolosa, S. A Method for the Determination of Inorganic Phosphate in the Presence of Labile Organic Phosphate and High Concentrations of Protein: Application to Lens ATPases. Anal. Biochem. 1988, 168, 1–4. [Google Scholar] [CrossRef]
- Segel, I.H. Enzyme Kinetics; Behavior and Analysis of Rapid Equilibrium and Steady-State Enzyme Systems; John Wiley & Sons, Inc.: New York, NY, USA, 1993. [Google Scholar]
- Sharom, F.J.; Russell, P.L.; Qu, Q.; Lu, P. Fluorescence Techniques for Studying Membrane Transport Proteins. In Membrane Transporters: Methods and Protocols; Yan, Q., Ed.; Humana Press: Totowa, NJ, USA, 2003; pp. 109–128. [Google Scholar] [CrossRef]
- Lakowicz, J.R. (Ed.) Instrumentation for Fluorescence Spectroscopy. In Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 2006; pp. 27–61. [Google Scholar] [CrossRef]
- Roberts, A.G.; Sjögren, S.E.A.; Fomina, N.; Vu, K.T.; Almutairi, A.; Halpert, J.R. NMR-Derived Models of Amidopyrine and Its Metabolites in Complexes with Rabbit Cytochrome P450 2B4 Reveal a Structural Mechanism of Sequential N-Dealkylation. Biochemistry 2011, 50, 2123–2134. [Google Scholar] [CrossRef]
- Claasen, B.; Axmann, M.; Meinecke, R.; Meyer, B. Direct Observation of Ligand Binding to Membrane Proteins in Living Cells by a Saturation Transfer Double Difference (STDD) NMR Spectroscopy Method Shows a Significantly Higher Affinity of Integrin αIIbβ3 in Native Platelets than in Liposomes. J. Am. Chem. Soc. 2005, 127, 916–919. [Google Scholar] [CrossRef]
- Haselhorst, T.; Münster-Kühnel, A.K.; Oschlies, M.; Tiralongo, J.; Gerardy-Schahn, R.; Itzstein, M. von. Direct Detection of Ligand Binding to Sepharose-Immobilised Protein Using Saturation Transfer Double Difference (STDD) NMR Spectroscopy. Biochem. Biophys. Res. Commun. 2007, 359, 866–870. [Google Scholar] [CrossRef]
- Venkitakrishnan, R.P.; Benard, O.; Max, M.; Markley, J.L.; Assadi-Porter, F.M. Use of NMR Saturation Transfer Difference Spectroscopy to Study Ligand Binding to Membrane Proteins. In Membrane Protein Structure and Dynamics: Methods and Protocols; Vaidehi, N., Klein-Seetharaman, J., Eds.; Humana Press: Totowa, NJ, USA, 2012; pp. 47–63. [Google Scholar] [CrossRef]
- Viegas, A.; Manso, J.; Nobrega, F.L.; Cabrita, E.J. Saturation-Transfer Difference (STD) NMR: A Simple and Fast Method for Ligand Screening and Characterization of Protein Binding. J. Chem. Educ. 2011, 88, 990–994. [Google Scholar] [CrossRef]
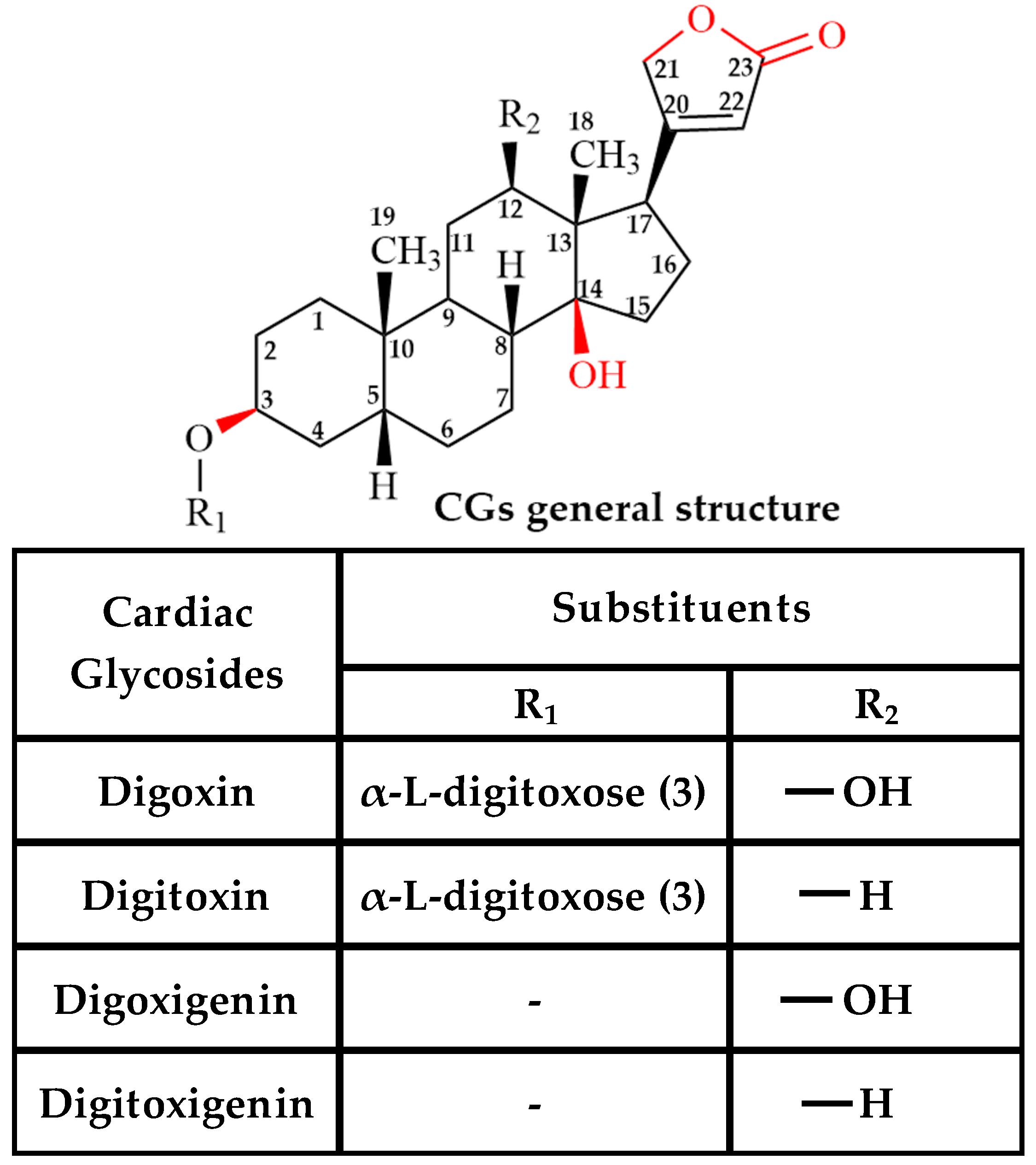

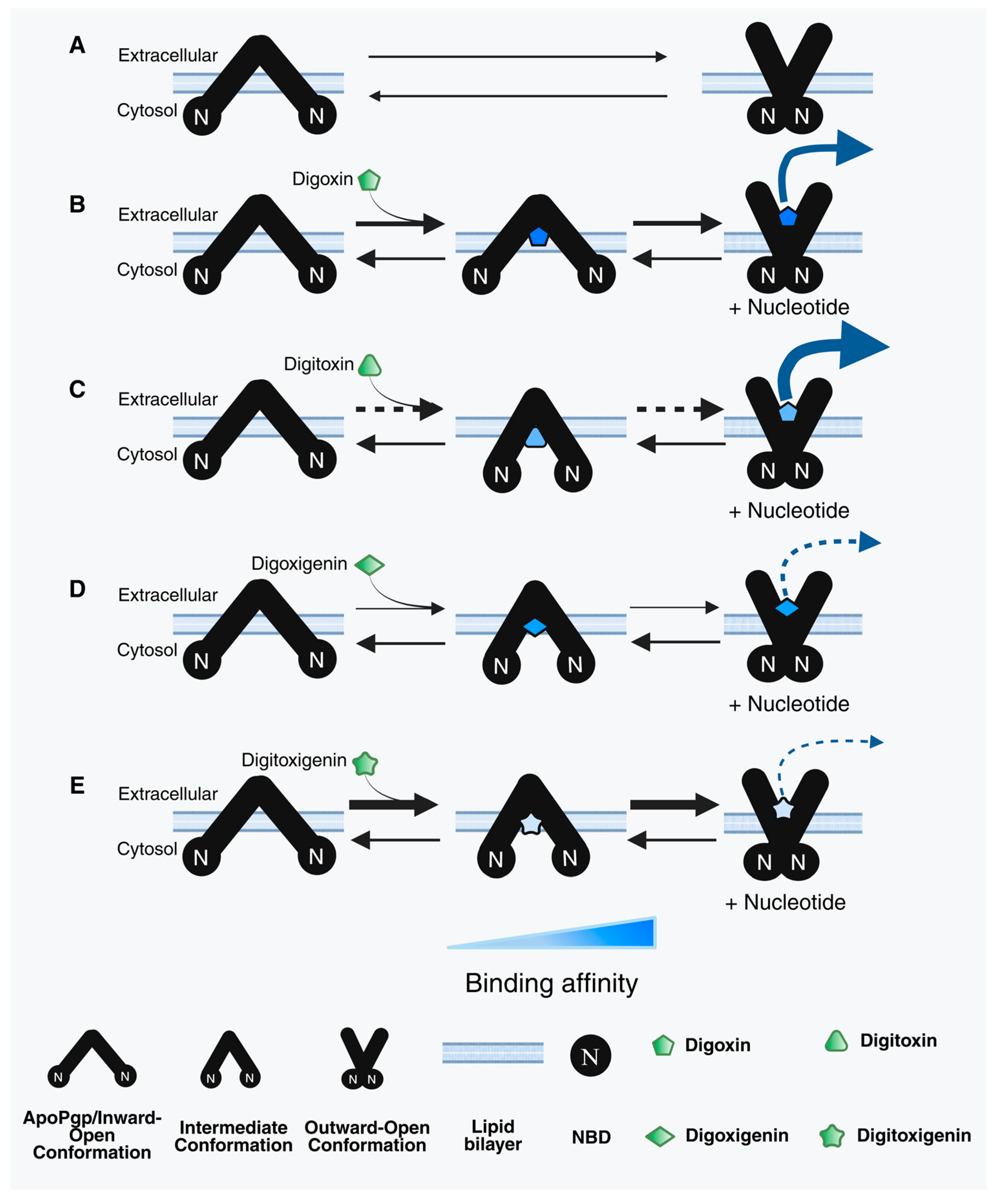
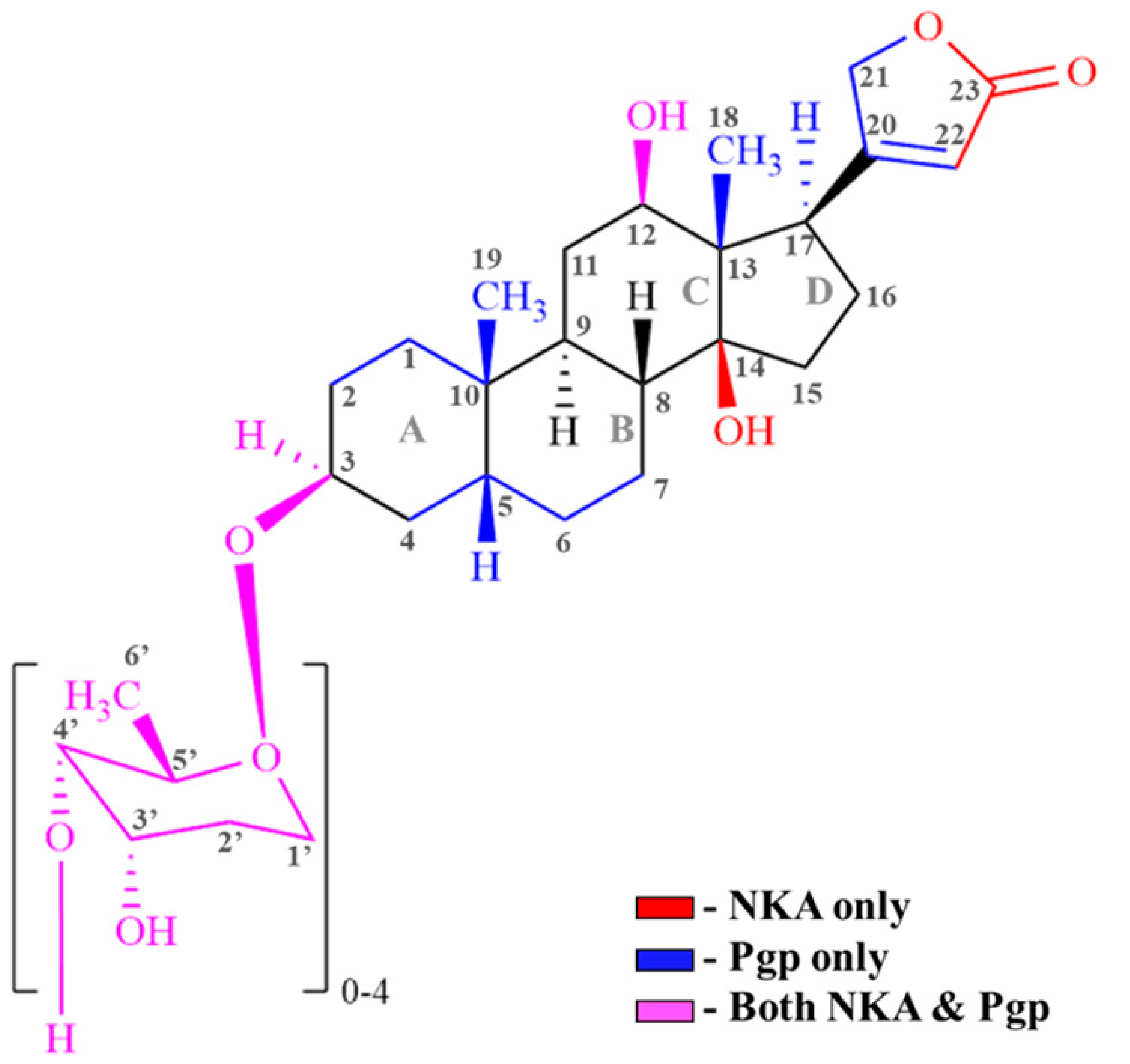
| Cardiac Glycosides (CG) | Interaction (Region/Group) | |
|---|---|---|
| NKA [73] | Pgp (STDD-NMR Data) | |
| Digoxin | C3 (Sugar); C12 (-OH); C14 (-OH); C17 (Carbonyl) [74,75,76] | Protons on C3,C18, C19 of steroid; C22 of lactone; C3,C5,C6 of sugar |
| Digitoxin | C3 (Sugar); C14 (-OH); C17 (Carbonyl) [18,77] | Protons on C3,C17, C18, C19 of steroid; C22 of lactone; C3,C5,C6 of sugar |
| Digoxigenin | C3 (-OH); C12 (-OH); C14 (-OH) [18,73] | Protons on C1, C3,C12, C17, C18, C19 of steroid; C21, C22 of lactone |
| Digitoxigenin | C3 (-OH); C14 (-OH) [18] | Protons on C1, C12, C18, C19 of steroid; C22 of lactone |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katti, R.; Kozarich, A.M.; Mensah, G.A.K.; Bartlett, M.G. Elucidating the Complex Structural and Molecular Mechanisms Driving P-Glycoprotein-Mediated Transport of Cardiac Glycosides. Int. J. Mol. Sci. 2025, 26, 7813. https://doi.org/10.3390/ijms26167813
Katti R, Kozarich AM, Mensah GAK, Bartlett MG. Elucidating the Complex Structural and Molecular Mechanisms Driving P-Glycoprotein-Mediated Transport of Cardiac Glycosides. International Journal of Molecular Sciences. 2025; 26(16):7813. https://doi.org/10.3390/ijms26167813
Chicago/Turabian StyleKatti, Rohit, Amanda M. Kozarich, Gershon A. K. Mensah, and Michael G. Bartlett. 2025. "Elucidating the Complex Structural and Molecular Mechanisms Driving P-Glycoprotein-Mediated Transport of Cardiac Glycosides" International Journal of Molecular Sciences 26, no. 16: 7813. https://doi.org/10.3390/ijms26167813
APA StyleKatti, R., Kozarich, A. M., Mensah, G. A. K., & Bartlett, M. G. (2025). Elucidating the Complex Structural and Molecular Mechanisms Driving P-Glycoprotein-Mediated Transport of Cardiac Glycosides. International Journal of Molecular Sciences, 26(16), 7813. https://doi.org/10.3390/ijms26167813





