Beyond Oxidation: Engineering Functional Anodised Metal Matrices Through Molecular and Surface Modifications
Abstract
1. Introduction
- galvanostatic method (at a constant current density),
- potentiostatic method (at a constant potential),
- combined method (initial oxidation at a constant current.
2. Fundamentals of Anodisation
3. Anodisation of Different Metals: Chemistry and Process Optimisation
3.1. Aluminium
“It is rather remarkable, that a metal, the atomic number of which is so small and the specific gravity of which is so low, should occupy a position in the electromotive scale as to be more negative than zinc in the series.”
“In our early work (16), the regularity of the nanohole array in the replicated structure was unsatisfactory; this resulted from imperfections in the cell arrangement in the mother anodic porous alumina used as a starting material.”
3.2. Titanium
- Generation I—obtained in inorganic aqueous electrolytes (mainly based on HF or HF + H3PO4), 200–500 nm,
- Generation II—obtained in buffered electrolytes (Na2SO4 + NaF or (NH4)2SO4 + NH4F),
- Generation III—obtained in the presence of organic electrolytes containing fluoride ions (NH4F + H2O + glycerol or NH4F + H2O + ethylene glycol),
- Generation IV—obtained in electrolytes that do not contain fluoride ions (mainly HClO4, NaCl and H2O2).
3.3. Other Metals
3.3.1. Niobium
3.3.2. Zinc
3.3.3. Copper
3.3.4. Magnesium
3.3.5. Tantalum
3.3.6. Iron
4. Applications of Functionalised Anodised Matrices
4.1. Energy Applications
4.1.1. Battery Components
4.1.2. Anodes
4.1.3. Cathodes
4.1.4. Interconnectors
4.2. Surface Engineering for Mechanical and Optical Properties
4.3. Biomedical Applications
4.4. Other Applications
5. Limitations and Challenges of Anodisation
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAO | anodic aluminium oxide |
| ATO | anodic tantalum oxide |
| AZIB | aqueous rechargeable zinc-ion battery |
| CuNPA | copper nanopillar array |
| DMSO | dimethyl sulphoxide |
| DSSCs | dye-sensitised solar cells |
| EG | ethylene glycol |
| LIB | lithium-ion batteries |
| NRs | nanorods |
| PAA | polyacrylic acid |
| PECD | pulse electrochemical deposition |
| PEO | plasma electrolytic oxidation |
| SIB | sodium-ion batteries |
| TNA | TiO2 nanotube array |
References
- Hoar, T.P.; Yahalom, J. The Initiation of Pores in Anodic Oxide Films Formed on Aluminum in Acid Solutions. J. Electrochem. Soc. 1963, 10, 614–621. [Google Scholar] [CrossRef]
- Cabrera, N.; Mott, N.F. Theory of the oxidation of metals. Rep. Prog. Phys. 1949, 12, 163–184. [Google Scholar] [CrossRef]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 nanotubes: Synthesis and applications. Angew. Chem.-Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef]
- Vetter, K.J. Electrochemical Kinetics, Theoritical Aspects; Academic Press: Cambridge, MA, USA, 1967. [Google Scholar] [CrossRef]
- Grimes, C.A.; Mor, G.K. Fabrication of TiO2 Nanotube Arrays by Electrochemical Anodization: Four Synthesis Generations. In TiO2 Nanotube Arrays: Synthesis, Properties, and Applications; Springer: Boston, MA, USA, 2009; pp. 1–66. [Google Scholar] [CrossRef]
- Lavos-Valereto, I.C.; Wolynec, S.; Ramires, I.; Carlos, G.A. Electrochemical impedance spectroscopy characterization of passive film formed on implant Ti-6AI-7Nb alloy in Hank’s solution. J. Mater. Sci. Mater. Med. 2004, 15, 55–59. [Google Scholar] [CrossRef]
- Sobieszczyk, S. Self-organized nanotubular oxide layers on Ti and Ti alloys. Adv. Mater. Sci. 2009, 9, 25–41. [Google Scholar] [CrossRef][Green Version]
- Wood, G. Sealing Anodic Oxide Films on Aluminium. In Transactions of the IMF; Taylor and Francis: London, UK, 1959. [Google Scholar]
- Lee, W.; Ji, R.; Gösele, U.; Nielsch, K. Fast fabrication of long-range ordered porous alumina membranes by hard anodization. Nat. Mater. 2006, 5, 741–747. [Google Scholar] [CrossRef]
- Sarkar, J.; Khan, G.; Basumallick, A. Nanowires: Properties, applications and synthesis via porous anodic aluminium oxide template. Bull. Mater. Sci. 2007, 30, 271–290. [Google Scholar] [CrossRef]
- Minagar, S.; Berndt, C.C.; Wang, J.; Ivanova, E.; Wen, C. A review of the application of anodization for the fabrication of nanotubes on metal implant surfaces. Acta Biomater. 2012, 8, 2875–2888. [Google Scholar] [CrossRef] [PubMed]
- Poinern, G.E.J.; Shackleton, R.; Mamun, S.I.; Fawcett, D. Significance of novel bioinorganic anodic aluminum oxide nanoscaffolds for promoting cellular response. Nanotechnol. Sci. Appl. 2011, 4, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Brüggemann, D. Nanoporous aluminium oxide membranes as cell interfaces. J. Nanomater. 2013, 2013, 460870. [Google Scholar] [CrossRef]
- Kumeria, T.; Santos, A.; Losic, D. Nanoporous anodic alumina platforms: Engineered surface chemistry and structure for optical sensing applications. Sensors 2014, 14, 11878–11918. [Google Scholar] [CrossRef] [PubMed]
- Md Jani, A.M.; Habiballah, A.S.; Budiman Abdul Halim, M.Z.; Ahmad Zulkifli, F.W.; Mahmud, A.H.; Yazid, H. Nanoporous Anodic Aluminum Oxide (NAAO) for Catalytic, Biosensing and Template Synthesis Applications (A Review). Curr. Nanosci. 2019, 15, 49–63. [Google Scholar] [CrossRef]
- Diggle, J.; Downie, T.; Goulding, C. Anodic oxide films on aluminum. Chem. Rev. 1969, 69, 365–405. [Google Scholar] [CrossRef]
- Shingubara, S. Fabrication of nanomaterials using porous alumina templates. J. Nanopart. Res. 2003, 5, 17–30. [Google Scholar] [CrossRef]
- Sulka, G.D. Highly ordered anodic porous alumina formation by self-organized anodizing. In Nanostructured Materials in Electrochemistry; Wiley Online Library: Hoboken, NJ, USA, 2008; pp. 1–116. [Google Scholar]
- Kikuchi, T.; Nakajima, D.; Nishinaga, O.; Natsui, S.; Suzuki, R.O. Porous aluminum oxide formed by anodizing in various electrolyte species. Curr. Nanosci. 2015, 11, 560–571. [Google Scholar] [CrossRef]
- Araoyinbo, A.O.; Azmi, A.I.; Ghazali, C.M.R.; Rahmat, A.; Hussin, K.; Abdullah, M.M.A. Nanoporous alumina fabrication: A short review. Nanosci.-Nanotechnol.-Asia 2017, 7, 183–199. [Google Scholar] [CrossRef]
- Norek, M.; Budner, B. Effect of various electrolyte modifiers on anodic alumina (AAO) growth and morphology. Curr. Nanosci. 2019, 15, 76–83. [Google Scholar] [CrossRef]
- Liu, S.; Tian, J.; Zhang, W. Fabrication and application of nanoporous anodic aluminum oxide: A review. Nanotechnology 2021, 32, 222001. [Google Scholar] [CrossRef]
- Manzoor, S.; Waseem Ashraf, M.; Tayyaba, S.; Imran Tariq, M.; Khalid Hossain, M. Recent Progress of Fabrication, Characterization, and Applications of Anodic Aluminum Oxide (AAO) Membrane: A Review. Comput. Model. Eng. Sci. 2023, 135, 1007–1052. [Google Scholar] [CrossRef]
- Fernández-González, C.; Ruiz-Gómez, S.; Arché-Núñez, A.; Pérez, L.; de Sousa, C.T. Nano-patterning using ultra-thin alumina membranes. Mater. Today Nano 2025, 29, 100553. [Google Scholar] [CrossRef]
- Tajima, S. Anodic Oxidation of Aluminum. In Advances in Corrosion Science and Technology; Fontana, M.G., Staehle, R.W., Eds.; Plenum Press: New York, NY, USA, 1970; Volume 1, pp. 229–362. [Google Scholar]
- Del Olmo, R.; Tynkevych, O.; Łazińska, M.; Syrek, K.; Durejko, T.; Czerwiński, M.; Zaraska, L.; Tiwari, R.; Michalska-Domańska, M. Anodizing of iron-based alloys: Fundamentals, recent progress, and applications. Rep. Prog. Phys. 2025, 88, 026501. [Google Scholar] [CrossRef]
- Feil, A.F.; Weibel, D.E.; Corsetti, R.R.; Pierozan, M.D.; Michels, A.F.; Horowitz, F.; Amaral, L.; Teixeira, S.R. Micro and nano-texturization of intermetallic oxide alloys by a single anodization step: Preparation of artificial self-cleaning surfaces. ACS Appl. Mater. Interfaces 2011, 3, 3981–3987. [Google Scholar] [CrossRef]
- Wang, J.; Hu, Y.; Cao, T.; Duan, Z.; Zhao, Z.; Sun, Y.; Gu, J.; Wang, X. Selection and applications of electrocatalysts for electrochemical anodizing oxidation of emerging contaminants in water: A review. Chem. Eng. J. 2025, 505, 159620. [Google Scholar] [CrossRef]
- Kulkarni, M.; Mazare, A.; Gongadz, E.; Perutkova, Š.; Kralj-iglič, V.; Milošev, I.; Schmuki, P.; Iglič, A.; Mozetič, M. Titanium nanostructures for biomedical applications. Nanotechnology 2015, 26, 062002. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Tang, Y.; Wang, K.; Zhoua, X.; Xiang, L. Nanostructured titanium implant surface facilitating osseointegration from protein adsorption to osteogenesis: The example of TiO2 NTAs. Int. J. Nanomed. 2022, 17, 1865–1879. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Mazare, A.; Schmuki, P. Nanostructured One-dimensional titanium dioxide nanomaterials. Nanotubes. Chem. Rev. 2014, 114, 9385–9454. [Google Scholar] [CrossRef]
- Huo, K.; Gao, B.; Fu, J.; Zhao, L.; Chu, P. Fabrication, modification, and biomedical applications of anodized TiO2 nanotube arrays. RSC Adv. 2014, 4, 1730. [Google Scholar] [CrossRef]
- Yan, X.; Chen, X. Titanium Dioxide Nanomaterials. In Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 1–38. [Google Scholar] [CrossRef]
- Cipriano, A.; Miller, C.; Liu, H. Anodic growth and biomedical applications of TiO2 nanotubes. J. Biomed. Nanotechnol. 2014, 10, 2977–3003. [Google Scholar] [CrossRef]
- Crawford, G.; Chawla, N.; Das, K.; Bose, S.; Bandyopadhyay, A. Microstructure and deformation behavior of biocompatible TiO2 nanotubes on titanium substrate. Acta Biomater. 2007, 3, 359–367. [Google Scholar] [CrossRef]
- Macak, J.; Tsuchiya, H.; Ghicov, A.; Yasuda, K.; Hahn, R.; Bauer, S.; Schmuki, P. TiO2 nanotubes: Self-organized electrochemical formation, properties and applications. Curr. Opin. Solid State Mater. Sci. 2007, 11, 3–18. [Google Scholar] [CrossRef]
- Awad, N.; Edwards, S.; Morsii, Y. A review of TiO2 NTs on Ti metal: Electrochemical synthesis, functionalization and potential use as bone implants. Mater. Sci. Eng. C 2017, 76, 1401–1412. [Google Scholar] [CrossRef]
- Aïnouche, L.; Hamadou, L.; Kadri, A.; Benbrahim, N.; Bradai, D. Interfacial barrier layer properties of three generations of TiO2 nanotube arrays. Electrochim. Acta 2014, 133, 597–609. [Google Scholar] [CrossRef]
- Zwilling, V.; Aucouturier, M.; Darque-Ceretti, E. Anodic oxidation of titanium and TA6V alloy in chromic media. An electrochemical approach. Electrochim. Acta 1999, 45, 921–929. [Google Scholar] [CrossRef]
- Gong, D.; Grimes, C.; Varghese, O.; Hu, W.; Singh, R.; Chen, Z.; Dickey, E. Titanium oxide nanotube arrays prepared by anodic oxidation. J. Mater. Sci. 2001, 16, 3331–3334. [Google Scholar] [CrossRef]
- Jakubowicz, J.; Jurczyk, K.; Jurczyk, M. Electrochemical formation and corrosion properties of porous TiOx biomaterials. Materials Science Forum 2010, 636, 15–21. [Google Scholar] [CrossRef]
- Macak, J.; Sirotna, K.; Schmuki, P. Self-organized porous titanium oxide prepared in Na2SO4/NaF electrolytes. Electrochim. Acta 2005, 50, 3679–3684. [Google Scholar] [CrossRef]
- Balakrishnan, M.; Narayanan, R. Synthesis of anodic titania nanotubes in Na2SO4/NaF electrolyte: A comparison between anodization time and specimens with biomaterial based approaches. Thin Solid Film. 2013, 540, 23–30. [Google Scholar] [CrossRef]
- Kulkarni, M.; Mazare, A.; Schmuki, P.; Iglič, A. Biomaterial surface modification of titanium and titanium alloys for medical applications. Nanomedicine 2014, 111, 111–136. [Google Scholar]
- Regonini, D.; Bowen, C.; Jaroenworaluck, A.; Stevens, R. A review of growth mechanism, structure and crystallinity of anodized TiO2 nanotubes. Mater. Sci. Eng. R Rep. 2013, 74, 377–406. [Google Scholar] [CrossRef]
- Rani, S.; Roy, S.; Paulose, M. Synthesis and applications of electrochemically self assembled titania nanotube arrays. Phys. Chem. Chem. Phys. 2010, 12, 2780–2800. [Google Scholar] [CrossRef]
- Hahn, R.; Stark, M.; Killian, M.; Schmuki, P. Photocatalytic properties of in situ doped TiO2-nanotubes grown by rapid breakdown anodization. Catal. Sci. Technol. 2013, 3, 1765–1770. [Google Scholar] [CrossRef]
- Prakasam, H.; Shankar, K.; Paulose, O.; Grimes, A. A new benchmark for TiO2 nanotube array growth by anodization. J. Phys. Chem. C 2007, 111, 7235–7241. [Google Scholar] [CrossRef]
- Oliveira, N.; Ferreira, E.; Duarte, L.; Biaggio, S.; Rocha-Filho, R.; Bocchi, N. Corrosion resistance of anodic oxides on the Ti-50Zr and Ti-13Nb-13Zr alloys. Electrochim. Acta 2006, 51, 2068–2075. [Google Scholar] [CrossRef]
- Wheatstone, C. Note on the position of Aluminum in the Voltaic series. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1855, 4, 143. [Google Scholar]
- Buff, H. Über das elektrische Verhalten des Aluminiums. Justus Liebigs Ann. Chem. 1857, 102, 265–284. [Google Scholar] [CrossRef]
- Bengough, G.D.; Stuart, J.M. Improved Process of Protecting Surfaces of Aluminium or Aluminium Alloys. GB223994A, 3 November 1924. [Google Scholar]
- Keller, F.; Hunter, M.; Robinson, D. Structural features of oxide coatings on aluminum. J. Electrochem. Soc. 1953, 100, 411. [Google Scholar] [CrossRef]
- Masuda, H.; Fukuda, K. Ordered Metal Nanohole Arrays Made by a Two-Step Replication of Honeycomb Structures of Anodic Alumina. Science 1995, 268, 1466–1468. [Google Scholar] [CrossRef]
- Masuda, H.; Tanaka, H.; Baba, N. Preparation of porous material by replacing microstructure of anodic alumina film with metal. Chem. Lett. 1990, 19, 621–622. [Google Scholar] [CrossRef]
- Masuda, H.; Satoh, M. Fabrication of gold nanodot array using anodic porous alumina as an evaporation mask. Jpn. J. Appl. Phys. 1996, 35, L126. [Google Scholar] [CrossRef]
- Limmer, S.; Seraji, S.; Wu, Y.; Chou, T.; Nguyen, C.; Cao, G. Template-Based Growth of Various Oxide Nanorods by Sol–Gel Electrophoresis. Adv. Funct. Mater. 2002, 12, 59–64. [Google Scholar] [CrossRef]
- Caubert, F.; Taberna, P.L.; Arurault, L. Innovating pulsed electrophoretic deposition of boehmite nanoparticles dispersed in an aqueous solution, into a model porous anodic film, prepared on aluminium alloy 1050. Surf. Coat. Technol. 2016, 302, 293–301. [Google Scholar] [CrossRef]
- Gonçalves, I.L.; Vinhosa, R.A.; de Freitas, D.S.; Brasil, S.L. Pulsed electrophoretic deposition of hybrid coatings from aqueous suspensions as surface functionalization and sealing technique of anodized AA2024. Part I: Morphological characterisation, analysis of the interfacial interactions, and evaluation of pore impgregnation of the anodic layer. Prog. Org. Coatings 2023, 178, 107474. [Google Scholar]
- Gonçalves, I.L.; Vinhosa, R.A.; de Freitas, D.S.; Brasil, S.L. Pulsed electrophoretic deposition for surface silanization of anodized aluminum from a silane-ethanol-water suspension. Surf. Coat. Technol. 2024, 478, 130456. [Google Scholar] [CrossRef]
- Miller, C.J.; Majda, M. Microporous Aluminium Oxide Films at Electrodes. J. Am. Chem. Soc. 1985, 107, 1419–1420. [Google Scholar] [CrossRef]
- Miller, C.J.; Majda, M. Microporous Aluminum Oxide Films at Electrodes. 3. Lateral Electron Transport in Self-Assembled Monolayers of N-methyl-N’-octadecyl-4,4’-bipyridinium Chloride. J. Am. Chem. Soc. 1986, 108, 3118–3120. [Google Scholar] [CrossRef]
- Brumlik, C.J.; Martin, C.R. Template Synthesis of Metal Microtubules. J. Am. Chem. Soc. 1991, 113, 3174–3175. [Google Scholar] [CrossRef]
- Brumlik, C.J.; Menon, V.P.; Martin, C.R. Template synthesis of metal microtubule ensembles utilizing chemical, electrochemical, and vacuum deposition techniques. J. Mater. Res. 1994, 9, 1174–1183. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, M.; Zhang, S.; Wu, Q.; Xiong, Z.; Wang, G.; Wu, R.; Wang, X.; Ma, F.; Krit, B. Study on the corrosion resistance and self-cleaning of the superhydrophobic NiCoAl-LDH film on anodic aluminum surface. Surf. Coat. Technol. 2025, 501, 131956. [Google Scholar] [CrossRef]
- Poznyak, A.; Knörnschild, G.; Hoha, A.; Pligovka, A. Porous and Ag-, Cu-, Zn-Doped Al2O3 Fabricated via Barrier Anodizing of Pure Al and Alloys. Coatings 2024, 14, 576. [Google Scholar] [CrossRef]
- Bacakova, L.; Filova, E.; Parizek, M.; Ruml, T.; Svorcik, V. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol. Adv. 2011, 29, 739–767. [Google Scholar] [CrossRef]
- Michalska-Domańska, M.; Norek, M.; Stępniowski, W.J.; Budner, B. Fabrication of high quality anodic aluminum oxide (AAO) on low purity aluminum—A comparative study with the AAO produced on high purity aluminum. Electrochim. Acta 2013, 105, 424–432. [Google Scholar] [CrossRef]
- Wong-ek, K.; Eiamchai, P.; Horprathum, M.; Patthanasettakul, V.; Limnonthakul, P.; Chindaudom, P.; Nuntawong, N. Silver nanoparticles deposited on anodic aluminum oxide template using magnetron sputtering for surface-enhanced Raman scattering substrate. Thin Solid Film. 2010, 518, 7128–7132. [Google Scholar] [CrossRef]
- Zaraska, L.; Sulka, G.D.; Szeremeta, J.; Jaskuła, M. Porous anodic alumina formed by anodization of aluminum alloy (AA1050) and high purity aluminum. Electrochim. Acta 2010, 55, 4377–4386. [Google Scholar] [CrossRef]
- Wu, M.; Wen, L.; Lei, Y.; Ostendorp, S.; Chen, K.; Wilde, G. Ultrathin Alumina Membranes for Surface Nanopatterning in Fabricating Quantum-Sized Nanodots. Small 2010, 6, 695–699. [Google Scholar] [CrossRef]
- Yu, C.U.; Hu, C.C.; Bai, A.; Yang, Y.F. Pore-size dependence of AAO films on surface roughness of Al-1050 sheets controlled by electropolishing coupled with fractional factorial design. Surf. Coat. Technol. 2007, 201, 7259–7265. [Google Scholar] [CrossRef]
- Fernández-Romero, L.; Montero-Moreno, J.; Pellicer, E.; Peiró, F.; Cornet, A.; Morante, J.; Sarret, M.; Müller, C. Assessment of the thermal stability of anodic alumina membranes at high temperatures. Mater. Chem. Phys. 2008, 111, 542–547. [Google Scholar] [CrossRef]
- Masuda, T.; Asoh, H.; Haraguchi, S.; Ono, S. Fabrication and Characterization of Single Phase α-Alumina Membranes with Tunable Pore Diameters. Materials 2015, 8, 1350–1368. [Google Scholar] [CrossRef]
- Domagalski, J.T.; Xifre-Perez, E.; Marsal, L.F. Recent Advances in Nanoporous Anodic Alumina: Principles, Engineering, and Applications. Nanomaterials 2021, 11, 430. [Google Scholar] [CrossRef]
- Bruera, F.A.; Kramer, G.R.; Vera, M.L.; Ares, A.E. Synthesis and Morphological Characterization of Nanoporous Aluminum Oxide Films by Using a Single Anodization Step. Coatings 2019, 9, 115. [Google Scholar] [CrossRef]
- Stępniowski, W.J.; Michalska-Domańska, M.; Norek, M.; Twardosz, E.; Florkiewicz, W.; Polkowski, W.; Zasada, D.; Bojar, Z. Anodization of cold deformed technical purity aluminum (AA1050) in oxalic acid. Surf. Coat. Technol. 2014, 258, 268–274. [Google Scholar] [CrossRef]
- Shih, T.; Wei, P.; Huang, Y. Optical properties of anodic aluminum oxide films on Al1050 alloys. Surf. Coat. Technol. 2008, 202, 3298–3305. [Google Scholar] [CrossRef]
- Schneider, M.; Kremmer, K. The effect of bath aging on the microstructure of anodic oxide layers on AA1050. Surf. Coat. Technol. 2014, 246, 64–70. [Google Scholar] [CrossRef]
- Zaraska, L.; Kurowska, E.; Sulka, G.D.; Senyk, I.; Jaskula, M. The effect of anode surface area on nanoporous oxide formation during anodizing of low purity aluminum (AA1050 alloy). J. Solid State Electrochem. 2014, 18, 361–368. [Google Scholar] [CrossRef]
- Habazaki, H.; Oikawa, Y.; Fushimi, K.; Aoki, Y.; Shimizu, K.; Skeldon, P.; Thompson, G. Importance of water content in formation of porous anodic niobium oxide films in hot phosphate–glycerol electrolyte. Electrochim. Acta 2009, 54, 946–951. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, J.; Choi, J. Understanding of anodization of zinc in an electrolyte containing fluoride ions. Electrochim. Acta 2008, 53, 7941–7945. [Google Scholar] [CrossRef]
- Prakasam, H.E.; Varghese, O.K.; Paulose, M.; Mor, G.K.; Grimes, C.A. Synthesis and photoelectrochemical properties of nanoporous iron (III) oxide by potentiostatic anodization. Nanotechnology 2006, 17, 4285. [Google Scholar] [CrossRef]
- Habazaki, H.; Konno, Y.; Aoki, Y.; Skeldon, P.; Thompson, G.E. Galvanostatic Growth of Nanoporous Anodic Films on Iron in Ammonium Fluoride–Ethylene Glycol Electrolytes with Different Water Contents. J. Phys. Chem. C 2010, 114, 18853–18859. [Google Scholar] [CrossRef]
- Sieber, I.V.; Schmuki, P. Porous tantalum oxide prepared by electrochemical anodic oxidation. J. Electrochem. Soc. 2005, 152, C639. [Google Scholar] [CrossRef]
- Yao, S.; Zhang, X.; Qu, F.; Umar, A.; Wu, X. Hierarchical WO3 nanostructures assembled by nanosheets and their applications in wastewater purification. J. Alloys Compd. 2016, 689, 570–574. [Google Scholar] [CrossRef]
- Yao, S.; Qu, F.; Wang, G.; Wu, X. Facile hydrothermal synthesis of WO3 nanorods for photocatalysts and supercapacitors. J. Alloys Compd. 2017, 724, 695–702. [Google Scholar] [CrossRef]
- Salari, H.; Kohantorabi, M. Facile template-free synthesis of new α-MnO2 nanorod/silver iodide p–n junction nanocomposites with high photocatalytic performance. New J. Chem. 2020, 44, 7401–7411. [Google Scholar] [CrossRef]
- Han, Z.; Fu, Q.; Lv, Y.; Wang, N.; Su, X. A two-dimensional iron-doped carbon-based nanoenzyme with catalase-like activity for the detection of alkaline phosphatase and ascorbate oxidase. Talanta 2024, 272, 125704. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, J.; Bi, J.; Sun, Y.; Zhang, J.; Liu, K.; Kong, F.; Xiao, H.; Chen, J. Effect of Cu-doping on the structure and performance of molybdenum carbide catalyst for low-temperature hydrogenation of dimethyl oxalate to ethanol. Appl. Catal. A Gen. 2017, 529, 143–155. [Google Scholar] [CrossRef]
- Chang, Y.C.; Guo, J.Y.; Chen, C.M.; Di, H.W.; Hsu, C.C. Construction of CuO/In2S3/ZnO heterostructure arrays for enhanced photocatalytic efficiency. Nanoscale 2017, 9, 13235–13244. [Google Scholar] [CrossRef] [PubMed]
- Donat, F.; Corbel, S.; Alem, H.; Pontvianne, S.; Balan, L.; Medjahdi, G.; Schneider, R. ZnO nanoparticles sensitized by CuInZnxS2+x quantum dots as highly efficient solar light driven photocatalysts. Beilstein J. Nanotechnol. 2017, 8, 1080–1093. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, X.; Zhu, B.; Wang, J.; Lan, H.; Chen, X. Synthesis of porous ZnS, ZnO and ZnS/ZnO nanosheets and their photocatalytic properties. RSC Adv. 2017, 7, 30956–30962. [Google Scholar] [CrossRef]
- Roslyakov, I.; Shirin, N.; Evdokimov, P.; Berekchiian, M.; Simonenko, N.; Lyskov, N.; Napolskii, K. High-temperature annealing of porous anodic aluminium oxide prepared in selenic acid electrolyte. Surf. Coat. Technol. 2022, 433, 128080. [Google Scholar] [CrossRef]
- Du, X.; Cai, D.; Ou, Q.; Chen, D.; Zhang, Z.; Liang, P. Fabrication and characterization of the hierarchical AAO film and AAO-MnO2 composite as the anode foil of aluminum electrolytic capacitor. Surf. Coat. Technol. 2021, 419, 127286. [Google Scholar] [CrossRef]
- Liu, M.; Liu, J.C.; Zhang, Y.; Han, X.; Li, H.; Huang, Z.H.; Ma, T. Current Collectors for Supercapacitors: Objectives, Modification Methods and Challenges. ChemElectroChem 2025, 12, e202400513. [Google Scholar] [CrossRef]
- Panjan, P.; Gselman, P.; Kek-Merl, D.; Čekada, M.; Panjan, M.; Dražić, G.; Bončina, T.; Zupanič, F. Growth defect density in PVD hard coatings prepared by different deposition techniques. Surf. Coat. Technol. 2013, 237, 349–356. [Google Scholar] [CrossRef]
- Čekada, M.; Radić, N.; Jerčinović, M.; Panjan, M.; Panjan, P.; Drnovšek, A.; Car, T. Growth defects in magnetron sputtered PVD films deposited in UHV environment. Vacuum 2017, 138, 213–217. [Google Scholar] [CrossRef]
- Mwema, F.; Oladijo, O.; Akinlabi, S.; Akinlabi, E. Properties of physically deposited thin aluminium film coatings: A review. J. Alloys Compd. 2018, 747, 306–323. [Google Scholar] [CrossRef]
- Fleischmann, M.; Thirsk, H. An investigation of electrochemical kinetics at constant overvoltage. The behaviour of the lead dioxide electrode. Part 5.—The formation of lead sulphate and the phase change to lead dioxide. Trans. Faraday Soc. 1955, 51, 71–95. [Google Scholar] [CrossRef]
- Fleischmann, M.; Liler, M. The anodic oxidation of solutions of plumbous salts. Part 1.—The kinetics of deposition of α-lead dioxide from acetate solutions. Trans. Faraday Soc. 1958, 54, 1370–1381. [Google Scholar] [CrossRef]
- Burbank, J. Anodization of lead in sulfuric acid. J. Electrochem. Soc. 1956, 103, 87. [Google Scholar] [CrossRef]
- Burbank, J. Anodization of Lead and Lead Alloys in Sulfuric Acid. J. Electrochem. Soc. 1957, 104, 693. [Google Scholar] [CrossRef]
- Rüetschi, P.; Cahan, B.D. Anodic Corrosion and Hydrogen and Oxygen Overvoltage on Lead and Lead Antimony Alloys. J. Electrochem. Soc. 1957, 104, 406. [Google Scholar] [CrossRef]
- Rüetschi, P.; Angstadt, R.T. Anodic Oxidation of Lead at Constant Potential. J. Electrochem. Soc. 1964, 111, 1323. [Google Scholar] [CrossRef]
- Elaissaoui, I.; Benahmed, W.N.; Bousselmi, L.; Akrout, H. Anodic oxidation as promoting technology for treatment and reuse of industrial textile effluent using ruthenium-doped lead dioxide (PbO2) anode. Clean Technol. Environ. Policy 2025, 27, 1551–1570. [Google Scholar] [CrossRef]
- Yang, Y.; He, Y.; Huang, H.; Guo, Z. Construction of conductive oxide layer on the surface of lead alloy grid for the long life of lead acid batteries. J. Energy Storage 2022, 52, 104877. [Google Scholar] [CrossRef]
- Shin, H.; Dong, J.; Liu, M. Porous Tin Oxides Prepared Using an Anodic Oxidation Process. Adv. Mater. 2004, 16, 237–240. [Google Scholar] [CrossRef]
- Zaraska, L.; Syrek, K.; Hnida, K.E.; Bobruk, M.; Krzysik, A.; Łojewski, T.; Jaskuła, M.; Sulka, G.D. Nanoporous tin oxides synthesized via electrochemical anodization in oxalic acid and their photoelectrochemical activity. Electrochim. Acta 2016, 205, 273–280. [Google Scholar] [CrossRef]
- Lv, X.; Rodriguez, I.; Hu, C.; Shang, J.; Sit, P.H.L.; Ye, C.; Oskam, G.; Teoh, W.Y. Modulated anodization synthesis of Sn-doped iron oxide with enhanced solar water splitting performance. Mater. Today Chem. 2019, 12, 7–15. [Google Scholar] [CrossRef]
- Nico, C.; Monteiro, T.; Graça, M. Niobium oxides and niobates physical properties: Review and prospects. Prog. Mater. Sci. 2016, 80, 1–37. [Google Scholar] [CrossRef]
- Yan, C.; Xue, D. Formation of Nb2O5 Nanotube Arrays Through Phase Transformation**. Adv. Mater. 2008, 20, 1055–1058. [Google Scholar] [CrossRef]
- Mohapatra, B.D.; Sulka, G.D. Review of Anodic Tantalum Oxide Nanostructures: From Morphological Design to Emerging Applications. ACS Appl. Nano Mater. 2024, 7, 13865–13892. [Google Scholar] [CrossRef]
- Usha, N.; Sivakumar, R.; Sanjeeviraja, C.; Arivanandhan, M. Niobium pentoxide (Nb2O5) thin films: Rf Power and substrate temperature induced changes in physical properties. Opt.-Int. J. Light Electron Opt. 2015, 126, 1945–1950. [Google Scholar] [CrossRef]
- Zibrov, I.P.; Filonenko, V.P.; Sundberg, M.; Werner, P.E. Structures and phase transitions of B-Ta2O5 and Z-Ta2O5: Two high-pressure forms of Ta2O5. Acta Crystallogr. Sect. B 2000, 56, 659–665. [Google Scholar] [CrossRef]
- Pang, R.; Wang, Z.; Li, J.; Chen, K. Polymorphs of Nb2O5 Compound and Their Electrical Energy Storage Applications. Materials 2023, 16, 6956. [Google Scholar] [CrossRef]
- Andersson, S. The Crystal Structure of N-Nb2O5, prepared in the presence of small amounts of LiF. Z. Anorg. Allg. Chem. 1967, 351, 106–112. [Google Scholar] [CrossRef]
- Alias, N.; Hussain, Z.; Tan, W.K.; Kawamura, G.; Muto, H.; Matsuda, A.; Lockman, Z. Nanoporous anodic Nb2O5 with pore-in-pore structure formation and its application for the photoreduction of Cr(VI). Chemosphere 2021, 283, 131231. [Google Scholar] [CrossRef]
- Guo, W.; Yang, L.; Lu, J.; Gao, P.; Li, W.; Feng, Z. An Accurate Growth Mechanism and Photocatalytic Degradation Rhodamine B of Crystalline Nb2O5 Nanotube Arrays. Catalysts 2020, 10, 1480. [Google Scholar] [CrossRef]
- Altomare, M.; Cha, G.; Schmuki, P. Anodic nanoporous niobium oxide layers grown in pure molten ortho-phosphoric acid. Electrochim. Acta 2020, 344, 136158. [Google Scholar] [CrossRef]
- Rao, B.M.; Torabi, A.; Varghese, O.K. Anodically grown functional oxide nanotubes and applications. MRS Commun. 2016, 6, 375–396. [Google Scholar] [CrossRef]
- Shen, P.; Zhang, B.; Wang, Y.; Liu, X.; Yu, C.; Xu, T.; Mofarah, S.S.; Yu, Y.; Liu, Y.; Sun, H.; et al. Nanoscale niobium oxides anode for electrochemical lithium and sodium storage: A review of recent improvements. J. Nanostruct. Chem. 2021, 11, 33–68. [Google Scholar] [CrossRef]
- Blanquart, T.; Niinistö, J.; Heikkilä, M.; Sajavaara, T.; Kukli, K.; Puukilainen, E.; Xu, C.; Hunks, W.; Ritala, M.; Leskelä, M. Evaluation and Comparison of Novel Precursors for Atomic Layer Deposition of Nb2O5 Thin Films. Chem. Mater. 2012, 24, 975–980. [Google Scholar] [CrossRef]
- Hu, C.; Teoh, W.Y.; Ji, S.; Ye, C.; Iwase, A. In situ metal doping during modified anodization synthesis of Nb2O5 with enhanced photoelectrochemical water splitting. AIChE J. 2016, 62, 352–358. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, Y.; Li, C.; Li, P.; Li, B.; Zhang, Z.; Zhu, X.; Song, Y. High-temperature anodization route to nanoporous niobium oxides with enhanced supercapacitive properties in aqueous electrolytes. J. Energy Storage 2024, 101, 113887. [Google Scholar] [CrossRef]
- Lim, E.; Jo, C.; Kim, H.; Kim, M.H.; Mun, Y.; Chun, J.; Ye, Y.; Hwang, J.; Ha, K.S.; Roh, K.C.; et al. Facile synthesis of Nb2O5@carbon core–shell nanocrystals with controlled crystalline structure for high-power anodes in hybrid supercapacitors. ACS Nano 2015, 9, 7497–7505. [Google Scholar] [CrossRef]
- Borowski, T.; Zielińska, K.; Spychalski, M.; Adamczyk-Cieślak, B.; Żrodowski, Ł. Effect of oxidation temperature on the properties of niobium in view of its biomedical applications. Surf. Coat. Technol. 2023, 473, 129911. [Google Scholar] [CrossRef]
- Engelkemeier, K.; Sun, A.; Voswinkel, D.; Grydin, O.; Schaper, M.; Bremser, W. Zinc Anodizing: Structural Diversity of Anodic Zinc Oxide Controlled by the Type of Electrolyte. ChemElectroChem 2021, 8, 2155–2168. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions; NACE: Singapore, 1966. [Google Scholar]
- Bhatt, N.A.; Quigley, L.; Zhou, S.; Gnanasabai, A.; Choudhury, A.; Zhang, Y.; Shen, J.; Lu, J.; Siddiqui, A.; Sarma, R.; et al. Morphology and property tuning in ZnO–Ni hybrid metamaterials in vertically aligned nanocomposite (VAN) form. Nanoscale Adv. 2025, 7, 3528–3538. [Google Scholar] [CrossRef] [PubMed]
- Nanto, H.; Minami, T.; Takata, S. Intense white photoluminescence in ZnO thin film formed by anodization. J. Mater. Sci. 1983, 18, 2721–2726. [Google Scholar] [CrossRef]
- Dirkse, T.P. Electrolytic oxidation of zinc in alkaline solutions. J. Electrochem. Soc. 1955, 102, 497. [Google Scholar] [CrossRef]
- Abou Zeid, S.; Leprince-Wang, Y. Advancements in ZnO-Based Photocatalysts for Water Treatment: A Comprehensive Review. Crystals 2024, 14, 611. [Google Scholar] [CrossRef]
- Muktaridha, O.; Adlim, M.; Suhendrayatna, S.; Ismail, I. Progress of 3d metal-doped zinc oxide nanoparticles and the photocatalytic properties. Arab. J. Chem. 2021, 14, 103175. [Google Scholar] [CrossRef]
- Jakobson, S.; Crotty, D.; Griffin, R.; Phipps, D.; Rubin, E. Zinc anodizing. Met. Finish. 1998, 96, 114–118. [Google Scholar] [CrossRef]
- Wang, T.; Li, C.; Xie, X.; Lu, B.; He, Z.; Liang, S.; Zhou, J. Anode Materials for Aqueous Zinc Ion Batteries: Mechanisms, Properties, and Perspectives. ACS Nano 2020, 14, 16321–16347. [Google Scholar] [CrossRef]
- Yan, B.; Zheng, J.; Wang, F.; Zhao, L.; Zhang, Q.; Xu, W.; He, S. Review on porous carbon materials engineered by ZnO templates: Design, synthesis and capacitance performance. Mater. Des. 2021, 201, 109518. [Google Scholar] [CrossRef]
- Liu, G.W.; Chen, T.Y.; Chung, C.H.; Lin, H.P.; Hsu, C.H. Hierarchical Micro/Mesoporous Carbons Synthesized with a ZnO Template and Petroleum Pitch via a Solvent-Free Process for a High-Performance Supercapacitor. ACS Omega 2017, 2, 2106–2113. [Google Scholar] [CrossRef]
- Yang, S.; Wang, J.; Liu, C.; Ren, P.; Yang, Q.; Zhao, G. Morphology control and photocatalytic properties of ZnO sheets grown on an AAO template. Ceram. Int. 2020, 47, 8610–8617. [Google Scholar] [CrossRef]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc Oxide—From Synthesis to Application: A Review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, A.; Kaur, N. Efficiency Investigations of Organic/Inorganic Hybrid ZnO Nanoparticles Based Dye-Sensitized Solar Cells. J. Mater. 2016, 2016, 9081346. [Google Scholar] [CrossRef]
- Tao, F.; Liu, Y.; Ren, X.; Wang, J.; Zhou, Y.; Miao, Y.; Ren, F.; Wei, S.; Ma, J. Different surface modification methods and coating materials of zinc metal anode. J. Energy Chem. 2022, 66, 397–412. [Google Scholar] [CrossRef]
- Elam, J.; Routkevitch, D.; George, S. Properties of ZnO/Al2O3 Alloy Films Grown Using Atomic Layer Deposition Techniques. J. Electrochem. Soc. 2003, 150, G339. [Google Scholar] [CrossRef]
- Song, W.; Brugge, R.; Theodorou, I.G.; Lim, A.L.; Yang, Y.; Zhao, T.; Burgess, C.H.; Johnson, I.D.; Aguadero, A.; Shearing, P.R.; et al. Enhancing Distorted Metal–Organic Framework-Derived ZnO as Anode Material for Lithium Storage by the Addition of Ag2S Quantum Dots. ACS Appl. Mater. Interfaces 2017, 9, 37823–37831. [Google Scholar] [CrossRef]
- Gatou, M.A.; Vagena, I.A.; Lagopati, N.; Pippa, N.; Gazouli, M.; Pavlatou, E.A. Functional MOF-Based Materials for Environmental and Biomedical Applications: A Critical Review. Nanomaterials 2023, 13, 2224. [Google Scholar] [CrossRef]
- Miles, D.O.; Cameron, P.J.; Mattia, D. Hierarchical 3D ZnO nanowire structures via fast anodization of zinc. J. Mater. Chem. A 2015, 3, 17569–17577. [Google Scholar] [CrossRef]
- Magdy Abdo, D.; Nasr El-Shazly, A.; Adel Hamza, M. Effect of acidic and basic leachants on the synthesis of ZnO nanoparticles from Egyptian zinc ore, their optical/surface characteristics and photocatalytic performance. Mater. Lett. 2024, 364, 136377. [Google Scholar] [CrossRef]
- Shrestha, N.K.; Lee, K.; Hahn, R.; Schmuki, P. Anodic growth of hierarchically structured nanotubular ZnO architectures on zinc surfaces using a sulfide based electrolyte. Electrochem. Commun. 2013, 34, 9–13. [Google Scholar] [CrossRef]
- Wang, F.; Huang, F.; Yu, F.; Kang, X.; Wang, Q.; Liu, Y. Metal-sulfide photocatalysts for solar-fuel generation across the solar spectrum. Cell Rep. Phys. Sci. 2023, 4, 101450. [Google Scholar] [CrossRef]
- Gopi, C.V.V.M.; Venkata-Haritha, M.; Lee, Y.S.; Kim, H.J. ZnO nanorods decorated with metal sulfides as stable and efficient counter-electrode materials for high-efficiency quantum dot-sensitized solar cells. J. Mater. Chem. A 2016, 4, 8161–8171. [Google Scholar] [CrossRef]
- Hao, J.; Li, X.; Zhang, S.; Yang, F.; Zeng, X.; Zhang, S.; Bo, G.; Wang, C.; Guo, Z. Designing Dendrite-Free Zinc Anodes for Advanced Aqueous Zinc Batteries. Adv. Funct. Mater. 2020, 30, 2001263. [Google Scholar] [CrossRef]
- Yang, H.; Chang, Z.; Qiao, Y.; Deng, H.; Mu, X.; He, P.; Zhou, H. Constructing a Super-Saturated Electrolyte Front Surface for Stable Rechargeable Aqueous Zinc Batteries. Angew. Chem. Int. Ed. 2020, 59, 9377–9381. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wei, H.; Liu, Y.; Ren, X.; Li, Y.; Wang, F.; Han, X.; Xu, E.; Cao, X.; Wang, G.; et al. Application of Co3O4-based materials in electrocatalytic hydrogen evolution reaction: A review. Int. J. Hydrogen Energy 2020, 45, 21205–21220. [Google Scholar] [CrossRef]
- Dong, J.; Liu, Z.; Dong, J.; Ariyanti, D.; Niu, Z.; Huang, S.; Zhang, W.; Gao, W. Self-organized ZnO nanorods prepared by anodization of zinc in NaOH electrolyte. RSC Adv. 2016, 6, 72968–72974. [Google Scholar] [CrossRef]
- Voon, C.H.; Derman, M.N.B.; Hashim, U.; Lim, B.; Ting, S.S.; Foo, K.L.; Ten, S.T. Synthesis of Nanoporous Zinc Oxide by Anodizing of Zinc in Distilled Water. Appl. Mech. Mater. 2015, 754, 1126–1130. [Google Scholar] [CrossRef]
- Shetty, A.; Nanda, K.K. Synthesis of zinc oxide porous structures by anodization with water as an electrolyte. Appl. Phys. A 2012, 109, 151–157. [Google Scholar] [CrossRef]
- Voon, C.; Tukiman, N.T.; Lim, B.; Hashim, U.; Ten, S.; Derman, M.; Foo, K.; Arshad, M.M. Synthesis of zinc oxide thin film by anodizing. In Proceedings of the 2014 IEEE International Conference on Semiconductor Electronics (ICSE2014), Kuala Lumpur, Malaysia, 27–29 August 2014; pp. 420–423. [Google Scholar]
- Masuda, R.; Kowalski, D.; Kitano, S.; Aoki, Y.; Nozawa, T.; Habazaki, H. Characterization of dark-colored nanoporous anodic films on zinc. Coatings 2020, 10, 1014. [Google Scholar] [CrossRef]
- Ravanbakhsh, A.; Rashchi, F.; Heydarzadeh Sohi, M. Synthesis of nanostructured ZnO thin film using anodization process in KOH solution. In Proceedings of the Ultra-Fine Grained and Nano-Structured Materials (UFGNSM2013), Tehran, Iran, 5–6 November 2013. [Google Scholar]
- Dirkse, T.; Hampson, N. The anodic behaviour of zinc in aqueous KOH solution—II. passivation experiments using linear sweep voltammetry. Electrochim. Acta 1972, 17, 387–394. [Google Scholar] [CrossRef]
- Engelkemeier, K.; Mücke, C.; Hoyer, K.P.; Schaper, M. Anodizing of electrolytically galvanized steel surfaces for improved interface properties in fiber metal laminates. Adv. Compos. Hybrid Mater. 2019, 2, 189–199. [Google Scholar] [CrossRef]
- Ono, S.; Kobayashi, Y.; Kobayashi, R.; Asoh, H. Fabrication of Self-Organized Nanoporous Oxide Semiconductors by Anodization. ECS Trans. 2008, 16, 353. [Google Scholar] [CrossRef]
- Sreekantan, S.; Gee, L.R.; Lockman, Z. Room temperature anodic deposition and shape control of one-dimensional nanostructured zinc oxide. J. Alloys Compd. 2009, 476, 513–518. [Google Scholar] [CrossRef]
- Huang, M.C.; Wang, T.; Wu, B.J.; Lin, J.C.; Wu, C.C. Anodized ZnO nanostructures for photoelectrochemical water splitting. Appl. Surf. Sci. 2016, 360, 442–450. [Google Scholar] [CrossRef]
- Shrestha, N.K.; Hahn, R.; Lee, K.; Tighineanu, A.; Schmuki, P. Electrochemically Assisted Self-Assembling of ZnF2-ZnO Nanospheres: Formation of Hierarchical Thin Porous Films. ECS Electrochem. Lett. 2014, 3, E1. [Google Scholar] [CrossRef]
- Shrestha, S.; Shrestha, B.K.; Tettey-Engmann, F.; Auniq, R.B.Z.; Subedi, K.; Ghimire, S.; Desai, S.; Bhattarai, N. Zein-coated Zn metal particles-incorporated nanofibers: A potent fibrous platform for loading and release of Zn ions for wound healing application. ACS Appl. Mater. Interfaces 2024, 16, 49197–49217. [Google Scholar] [CrossRef] [PubMed]
- Sanz Marco, A.; Sánchez-Tovar, R.; Montes Bajo, M.; Fernández-Domene, R.; García-Antón, J. Cathodoluminescence characterization of Zno/Zns nanostructures anodized under hydrodynamic conditions. Electrochim. Acta 2018, 269, 553–559. [Google Scholar] [CrossRef]
- Cai, L.; Ren, F.; Wang, M.; Cai, G.; Chen, Y.; Liu, Y.; Shen, S.; Guo, L. V ions implanted ZnO nanorod arrays for photoelectrochemical water splitting under visible light. Int. J. Hydrogen Energy 2015, 40, 1394–1401. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, X. Nanomaterial ZnO Synthesis and Its Photocatalytic Applications: A Review. Nanomaterials 2025, 15, 682. [Google Scholar] [CrossRef]
- Baig, A.; Siddique, M.; Panchal, S. A Review of Visible-Light-Active Zinc Oxide Photocatalysts for Environmental Application. Catalysts 2025, 15, 100. [Google Scholar] [CrossRef]
- Pujara, A.; Sharma, R.; Samriti; Bechelany, M.; Mishra, Y.K.; Prakash, J. Novel zinc oxide 3D tetrapod nano-microstructures: Recent progress in synthesis, modification and tailoring of optical properties for photocatalytic applications. Mater. Adv. 2025, 6, 2123–2153. [Google Scholar] [CrossRef]
- Ramirez-Canon, A.; Miles, D.O.; Cameron, P.J.; Mattia, D. Zinc oxide nanostructured films produced via anodization: A rational design approach. RSC Adv. 2013, 3, 25323–25330. [Google Scholar] [CrossRef]
- Mika, K.; Syrek, K.; Uchacz, T.; Sulka, G.D.; Zaraska, L. Dark nanostructured ZnO films formed by anodic oxidation as photoanodes in photoelectrochemical water splitting. Electrochim. Acta 2022, 414, 140176. [Google Scholar] [CrossRef]
- Gulab, H.; Fatima, N.; Tariq, U.; Gohar, O.; Irshad, M.; Khan, M.Z.; Saleem, M.; Ghaffar, A.; Hussain, M.; Khaliq Jan, A.; et al. Advancements in zinc oxide nanomaterials: Synthesis, properties, and diverse applications. Nano-Struct. Nano-Objects 2024, 39, 101271. [Google Scholar] [CrossRef]
- Khan, M.I.; Irfan, M.; Fatima, M.; Somaily, H.H.; Elqahtani, Z.M.; Alwadai, N. Increase the current density and reduce the defects of ZnO by modification of the band gap edges with Cu ions implantation for efficient, flexible dye-sensitized solar cells (FDSSCs). Ceram. Int. 2023, 49, 29622–29629. [Google Scholar]
- Sheikh, A.; Soni, K.; Brajpuriya, R.; Lakshmi, N. Investigation of the structural and electrochemical properties of a ZnO-SnO2 composite and its electrical properties for application in dye-sensitized solar cells. New J. Chem. 2023, 47, 7346–7355. [Google Scholar] [CrossRef]
- Kang, J.; Yun, J.; Oh, Y.Y.; Kim, S.J.; Kamiko, M.; Kim, N.H.; Koh, J.H. Defects controlled stress engineering in Al-doped ZnO transparent multilayered thin films. J. Korean Ceram. Soc. 2022, 59, 742–748. [Google Scholar] [CrossRef]
- Guan, J.J.; Wang, H.Q.; Liang, H.; Cheng, N.P.; Lin, H.; Li, Q.; Li, Y.; Qin, L.Z. Photodeposition synthesis of a ZnO nanoporous layer. RSC Adv. 2015, 5, 52998–53002. [Google Scholar] [CrossRef]
- Cuevas, A.L.; Dominguez, A.; Zamudio-García, J.; Vega, V.; González, A.S.; Marrero-López, D.; Prida, V.M.; Benavente, J. Optical and Electrochemical Properties of a Nanostructured ZnO Thin Layer Deposited on a Nanoporous Alumina Structure via Atomic Layer Deposition. Materials 2024, 17, 1412. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, K.; Xu, D.; Yang, G.; Huang, H.; Nie, F.; Liu, C.; Yang, S. CuO nanostructures: Synthesis, characterization, growth mechanisms, fundamental properties, and applications. Prog. Mater. Sci. 2014, 60, 208–337. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, X.; Yang, Q.; Liang, S.; Zhang, X.; Yang, Z. Cuprous oxide (Cu2O) crystals with tailored architectures: A comprehensive review on synthesis, fundamental properties, functional modifications and applications. Prog. Mater. Sci. 2018, 96, 111–173. [Google Scholar] [CrossRef]
- De Chialvo, M.G.; Zerbino, J.; Marchiano, S.; Arvia, A. Correlation of electrochemical and ellipsometric data in relation to the kinetics and mechanism of Cu2O electroformation in alkaline solutions. J. Appl. Electrochem. 1986, 16, 517–526. [Google Scholar] [CrossRef]
- Stepniowski, W.J.; Misiolek, W.Z. Review of Fabrication Methods, Physical Properties, and Applications of Nanostructured Copper Oxides Formed via Electrochemical Oxidation. Nanomaterials 2018, 8, 379. [Google Scholar] [CrossRef] [PubMed]
- Stepniowski, W.J.; Stojadinović, S.; Vasilić, R.; Tadić, N.; Karczewski, K.; Abrahami, S.T.; Buijnsters, J.G.; Mol, J.M. Morphology and photoluminescence of nanostructured oxides grown by copper passivation in aqueous potassium hydroxide solution. Mater. Lett. 2017, 198, 89–92. [Google Scholar] [CrossRef]
- Yang, X.; Han, Z.; Song, J.; Hu, P.; Teng, F. Novel application of electrochemical test for the controllable electrodeposition of Cu2O and metallic Cu film. J. Alloys Compd. 2024, 970, 172706. [Google Scholar] [CrossRef]
- Han, Z.; Yang, X.; Shi, X.; Wu, L.; Hu, P.; Fan, H.; Teng, F. Adjusting the energy band of Cu2O by changing the deposition conditions and its effect on photoelectrochemistry performance of Cu2O/TiO2 heterojunction. Surfaces Interfaces 2024, 54, 105179. [Google Scholar] [CrossRef]
- Vanpaemel, J.; Abd-Elnaiem, A.M.; De Gendt, S.; Vereecken, P.M. The Formation Mechanism of 3D Porous Anodized Aluminum Oxide Templates from an Aluminum Film with Copper Impurities. J. Phys. Chem. C 2015, 119, 2105–2112. [Google Scholar] [CrossRef]
- Kang, J.S.; Kim, J.; Lee, M.J.; Son, Y.J.; Jeong, J.; Chung, D.Y.; Lim, A.; Choe, H.; Park, H.S.; Sung, Y.E. Electrochemical synthesis of nanoporous tungsten carbide and its application as electrocatalysts for photoelectrochemical cells. Nanoscale 2017, 9, 5413–5424. [Google Scholar] [CrossRef]
- Abd-Elnaiem, A.M.; Mebed, A.; Gaber, A.; Abdel-Rahim, M. Tailoring the porous nanostructure of porous anodic alumina membrane with the impurity control. J. Alloys Compd. 2016, 659, 270–278. [Google Scholar] [CrossRef]
- Mebed, A.; Abd-Elnaiem, A.M. A thermodynamic understanding of horizontal pores formation in anodized doped aluminum with alloying elements. J. Electroanal. Chem. 2018, 829, 138–147. [Google Scholar] [CrossRef]
- Abd-Elnaiem, A.M.; Mebed, A.M.; Alamri, H.R.; Assaedi, H.S. Tailoring Controllable Nanowire Morphologies Using a Multi-layer Porous Anodic Alumina Template for Technological Applications. J. Electrochem. Soc. 2020, 167, 103505. [Google Scholar] [CrossRef]
- Ropertz, M.; Ulbricht, M.; Fischer, L. All-in-one fabrication of bimetallic PdIn-decorated porous PES membranes for the catalytic flow-through reduction of to NH3 with formic acid in water. Chem. Eng. J. Adv. 2024, 20, 100683. [Google Scholar] [CrossRef]
- Ma, L.; Lin, Y.; Wang, Y.; Li, J.; Wang, E.; Qiu, M.; Yu, Y. Aligned 2-D Nanosheet Cu2O Film: Oriented Deposition on Cu Foil and Its Photoelectrochemical Property. J. Phys. Chem. C 2008, 112, 18916–18922. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, S.; Peng, F.; Zhang, H.; Liu, H.; Zhao, H. Electrodeposition of polyhedral Cu2O on TiO2 nanotube arrays for enhancing visible light photocatalytic performance. Electrochem. Commun. 2011, 13, 861–864. [Google Scholar] [CrossRef]
- Mao, Y.; He, J.; Sun, X.; Li, W.; Lu, X.; Gan, J.; Liu, Z.; Gong, L.; Chen, J.; Liu, P.; et al. Electrochemical synthesis of hierarchical Cu2O stars with enhanced photoelectrochemical properties. Electrochim. Acta 2012, 62, 1–7. [Google Scholar] [CrossRef]
- Wang, M.; Sun, L.; Lin, Z.; Cai, J.; Xie, K.; Lin, C. p–n Heterojunction photoelectrodes composed of Cu2O-loaded TiO2 nanotube arrays with enhanced photoelectrochemical and photoelectrocatalytic activities. Energy Environ. Sci. 2013, 6, 1211–1220. [Google Scholar] [CrossRef]
- Fabian, J.; Morton, G.; Sharma, S.; Duffy, B.; Warren, S. Effect of electrodeposition time on physical characteristics and antibacterial activity of copper-incorporated TiO2 nanotubes. RSC Adv. 2025, 15, 142–156. [Google Scholar] [CrossRef]
- Wang, S.; Tian, Y.; Wang, C.; Hang, C.; Zhang, H.; Huang, Y.; Zheng, Z. One-Step Fabrication of Copper Nanopillar Array-Filled AAO Films by Pulse Electrodeposition for Anisotropic Thermal Conductive Interconnectors. ACS Omega 2019, 4, 6092–6096. [Google Scholar] [CrossRef]
- Guo, Y.; Gong, L.; Gao, S.; Zhu, Y.; Zhang, F.; Li, J.; Jin, J. Cupric phosphate mineralized polymer membrane with superior cycle stability for oil/water emulsion separation. J. Membr. Sci. 2020, 612, 118427. [Google Scholar] [CrossRef]
- Abu-Zurayk, R.; Alnairat, N.; Waleed, H.; Al-Khaial, M.Q.; Khalaf, A.; Bozeya, A.; Abu-Dalo, D.; Al-Yousef, S.; Afaneh, R. Polyvinylidene Fluoride (PVDF) and Nanoclay Composites’ Mixed-Matrix Membranes: Exploring Structure, Properties, and Performance Relationships. Polymers 2025, 17, 1120. [Google Scholar] [CrossRef]
- Choi, Y.; Nam, C. Fabrication of PVDF Membranes with a PVA Layer for the Effective Removal of Volatile Organic Compounds in Semiconductor Wastewater. Polymers 2025, 17, 1332. [Google Scholar] [CrossRef]
- Shih, H.H.; Huang, Y.C. Study on the black electrolytic coloring of anodized aluminum in cupric sulfate. J. Mater. Process. Technol. 2008, 208, 24–28. [Google Scholar] [CrossRef]
- Pastore, G.; Montes, S.; Paez, M.; Zagal, J. Electrocolouring of anodized aluminium with copper: Effect of porous and barrier oxide film thicknesses. Thin Solid Film. 1989, 173, 299–308. [Google Scholar] [CrossRef]
- Girginov, C.; Kozhukharov, S.; Tsanev, A.; Dishliev, A. Characterization of Anodized Al 1050 with Electrochemically Deposited Cu, Ni and Cu/Ni and Their Behavior in a Model Corrosive Medium. J. Electrochem. Sci. Technol. 2021, 12, 188–203. [Google Scholar] [CrossRef]
- Yamashita, K.; Hotta, Y.; Kurooka, S.; Abe, H.; Tan, S. Copper-filled anodized aluminum oxide a potential material for low temperature bonding for 3D packaging. In Proceedings of the 2015 International Conference on Electronics Packaging and iMAPS All Asia Conference (ICEP-IAAC), Kyoto, Japan, 14–17 April 2015; pp. 571–574. [Google Scholar] [CrossRef]
- Kanazirski, I.; Girginov, C.; Nedyalkova, P. Electrolytic Colouring of Porous Anodic Aluminum Films. Annu. Univ. Minig Geol. “St. Ivan Rilski” 2021, 64/2021, 49–52. [Google Scholar]
- Wei, H.; Hu, H.; Chang, M.; Zhang, Y.; Chen, D.; Wang, M. Improving solar thermal absorption of an anodic aluminum oxide-based photonic crystal with Cu-Ni composite nanoparticles. Ceram. Int. 2017, 43, 12472–12479. [Google Scholar] [CrossRef]
- Dahlgren, S.D. Correlation of yield strength with internal coherency strains for age-hardened Cu–Ni–Fe alloys. Metall. Trans. A 1977, 8, 347–351. [Google Scholar] [CrossRef]
- Chen, J.; Deng, Y.; Cao, R.; Wang, P.; Mao, Y.; Yi, C.; Zhang, S.; Zhang, T.; Liu, X. Phosphorus-containing nickel-based coatings for enhanced corrosion resistance and mechanical performance: A review. FlatChem 2025, 52, 100887. [Google Scholar] [CrossRef]
- Aliofkhazraei, M.; Walsh, F.C.; Zangari, G.; Köçkar, H.; Alper, M.; Rizal, C.; Magagnin, L.; Protsenko, V.; Arunachalam, R.; Rezvanian, A.; et al. Development of electrodeposited multilayer coatings: A review of fabrication, microstructure, properties and applications. Appl. Surf. Sci. Adv. 2021, 6, 100141. [Google Scholar] [CrossRef]
- Vazquez-Arenas, J.; Treeratanaphitak, T.; Pritzker, M. Formation of Co–Ni alloy coatings under direct current, pulse current and pulse-reverse plating conditions. Electrochim. Acta 2012, 62, 63–72. [Google Scholar] [CrossRef]
- Dadras, S.; Zafarrazaghnia, E. Dielectric study of low-cost porous anodic aluminum oxide (AAO) template and AAO template-copper nanowires (Cu/AAO) nanocomposites as a capacitor for energy storage. J. Phys. Chem. Solids 2024, 195, 112264. [Google Scholar] [CrossRef]
- Selvam, M.A.J.; Arunkumar, S.; Balambica, V.; Padmanabhan, S.; Kumar, T.V.; Shai Sundaram, V. Influence of copper anodization on structural and heat transfer characteristic of aluminum piston. Mater. Today Proc. 2022, 59, 1407–1413. [Google Scholar] [CrossRef]
- Kalinin, I.; Davydov, A.; Leontiev, A.; Napolskii, K.; Sobolev, A.; Shatalov, M.; Zinigrad, M.; Bograchev, D. Influence of natural convection on the electrodeposition of copper nanowires in anodic aluminium oxide templates. Electrochim. Acta 2023, 441, 141766. [Google Scholar] [CrossRef]
- Song, G.; Shi, Z. 9-Anodization and corrosion of magnesium (Mg) alloys Corrosion Prevention of Magnesium Alloys. In Corrosion Prevention of Magnesium Alloys; Guang-Ling, S., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 232–281. [Google Scholar]
- Carter, E.; Barton, T.; Wright, G. Anodisation of magneiusm at high voltages. Trans. Eng. Sci. 1999, 25, 169–177. [Google Scholar]
- Shashikala, A.; Umarani, R.; Mayanna, S.; Sharma, A. Chemical Conversion Coatings on Magnesium Alloys—A Comparative Study. Int. J. Electrochem. Sci. 2008, 3, 993–1004. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A.; Wu, X.; Zhang, B. Corrosion behaviour of AZ21, AZ501 and AZ91 in sodium chloride. Corros. Sci. 1998, 40, 1769–1791. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, J.; Hou, P.; Liu, Y.; Li, Z.; Lu, P.; Wen, G.; Liu, L.; Sun, H. Preparation, optical properties, and color formation mechanism of tantalum oxide anode films. Opt. Mater. 2023, 136, 113425. [Google Scholar] [CrossRef]
- Li, H.; Yu, Y.; Fang, B.; Xiao, P.; Wang, S. Study on properties of Hf6Ta2O17/Ta2O5 system: A potential candidate for environmental barrier coating (EBC). J. Eur. Ceram. Soc. 2022, 42, 4651–4662. [Google Scholar] [CrossRef]
- Luo, D.F.; Ning, P.; Zhang, F.; Zhou, Y.; Zhang, H.M.; Fu, T. Hydrothermal calcification surface modification of biomedical tantalum. Rare Met. 2021, 40, 928–933. [Google Scholar] [CrossRef]
- Manukumar, K.N.; Kishore, B.; Viswanatha, R.; Nagaraju, G. Ta2O5 nanoparticles as an anode material for lithium ion battery. J. Solid State Electrochem. 2020, 24, 1067–1074. [Google Scholar] [CrossRef]
- Almeida Alves, C.; Calderon V., S.; Ferreira, P.; Marques, L.; Carvalho, S. Passivation and dissolution mechanisms in ordered anodic tantalum oxide nanostructures. Appl. Surf. Sci. 2020, 513, 145575. [Google Scholar] [CrossRef]
- Löffler, M.; Fabrichnaya, O.; Hutterer, P.; Lepple, M. Phase equilibrium investigations and thermodynamic modelling of the ZrO2-Ta2O5 system. J. Eur. Ceram. Soc. 2023, 43, 7668–7681. [Google Scholar] [CrossRef]
- Gonçalves, R.V.; Wojcieszak, R.; Uberman, P.M.; Teixeira, S.R.; Rossi, L.M. Insights into the active surface species formed on Ta2O5 nanotubes in the catalytic oxidation of CO. Phys. Chem. Chem. Phys. 2014, 16, 5755–5762. [Google Scholar] [CrossRef] [PubMed]
- Maeng, S.; Axe, L.; Tyson, T.; Jiang, A. An Investigation of Structures of Thermal and Anodic Tantalum Oxide Films. J. Electrochem. Soc. 2005, 152, B60. [Google Scholar] [CrossRef]
- Gonçalves, R.V.; Migowski, P.; Wender, H.; Eberhardt, D.; Weibel, D.E.; Sonaglio, F.C.; Zapata, M.J.M.; Dupont, J.; Feil, A.F.; Teixeira, S.R. Ta2O5 Nanotubes Obtained by Anodization: Effect of Thermal Treatment on the Photocatalytic Activity for Hydrogen Production. J. Phys. Chem. C 2012, 116, 14022–14030. [Google Scholar] [CrossRef]
- Verma, N.; Singh, K.; Marí, B.; Mollar, M.; Jindal, J. Anodic oxide films on niobium and tantalum in different aqueous electrolytes and their impedance characteristics. Acta Phys. Pol. A 2016, 129, 297–303. [Google Scholar] [CrossRef]
- Draper, P.H.G.; Jacobs, P.W.M. Kinetics of the formation of anodic oxide films on tantalum. Trans. Faraday Soc. 1963, 59, 2888–2898. [Google Scholar] [CrossRef]
- Sieber, I.; Kannan, B.; Schmuki, P. Self-Assembled Porous Tantalum Oxide Prepared in H2SO4/HF Electrolytes. Electrochem. Solid-State Lett. 2005, 8, J10. [Google Scholar] [CrossRef]
- Wosu, S.N. Anodic oxidation of tantalum in water and biological solutions using current limiting constant voltage method. J. Mater. Sci. 2007, 42, 4087–4097. [Google Scholar] [CrossRef]
- Pohekar, P.; Upadhyay, B.B.; Parvez, B.; Ganguly, S.; Saha, D. Introduction of charge-trapping Al2O3/Ta2O5/Al2O3 dielectric stack in AlGaN/GaN high electron mobility transistors for programmable threshold voltage. Appl. Phys. Lett. 2025, 126, 112103. [Google Scholar] [CrossRef]
- Vermilyea, D. The oxidation of tantalum at 50–300 °C. Acta Metall. 1958, 6, 166–171. [Google Scholar] [CrossRef]
- Klein, G.P. Oxidation State of Anodic Tantalum Oxide after Heat-Treatment: I. Galvanostatic Method as Applied after Heating in Vacuum. J. Electrochem. Soc. 1972, 119, 1551. [Google Scholar] [CrossRef]
- Arifuku, F.; Iwakura, C.; Yoneyama, H.; Tamura, H. Incorporation of Electrolyte into Anodic Oxide Film on Tantalum. Denki Kagaku Oyobi Kogyo Butsuri Kagaku 1978, 46, 19–24. [Google Scholar] [CrossRef]
- Shimizu, K.; Kobayashi, K.; Thompson, G.E.; Skeldon, P.; Wood, G.C. Anodic oxide films on tantalum: Incorporation and mobilities of electrolyte-derived species. Philos. Mag. B 1996, 73, 461–485. [Google Scholar] [CrossRef]
- Albella, J.; Montero, I.; Fernandez, M.; Gomez-Aleixandre, C.; Martínez-Duart, J. Double anodization experiments in tantalum. Electrochim. Acta 1985, 30, 1361–1364. [Google Scholar] [CrossRef]
- Sieber, I.; Hildebrand, H.; Friedrich, A.; Schmuki, P. Initiation of tantalum oxide pores grown on tantalum by potentiodynamic anodic oxidation. J. Electroceramics 2006, 16, 35–39. [Google Scholar] [CrossRef]
- Singh, S.; Barden, W.R.T.; Kruse, P. Nanopatterning of Transition Metal Surfaces via Electrochemical Dimple Array Formation. ACS Nano 2008, 2, 2453–2464. [Google Scholar] [CrossRef]
- El-Sayed, H.A.; Horwood, C.A.; Abhayawardhana, A.D.; Birss, V.I. New insights into the initial stages of Ta oxide nanotube formation on polycrystalline Ta electrodes. Nanoscale 2013, 5, 1494–1498. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.; Cao, L.; Wang, Y.; Guo, W.; Liu, K.; Xue, J. Fabrication of nanoporous silicon dioxide/silicon nitride membranes using etched ion track technique. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2008, 266, 3166–3169. [Google Scholar] [CrossRef]
- Wei, W.; Macak, J.; Schmuki, P. High aspect ratio ordered nanoporous Ta2O5 films by anodization of Ta. Electrochem. Commun. 2008, 10, 428–432. [Google Scholar] [CrossRef]
- Momeni, M.M.; Mirhosseini, M.; Chavoshi, M.; Hakimizade, A. The effect of anodizing voltage on morphology and photocatalytic activity of tantalum oxide nanostructure. J. Mater. Sci. Mater. Electron. 2016, 27, 3941–3947. [Google Scholar] [CrossRef]
- Lee, Y.K.; Sim, J.J.; Byeon, J.S.; Lee, Y.T.; Cho, Y.W.; Kim, H.C.; Heo, S.G.; Lee, K.A.; Seo, S.J.; Park, K.T. Production of High-Purity Tantalum Metal Powder for Capacitors Using Self-Propagating High-Temperature Synthesis. Arch. Metall. Mater. 2021, 66, 935–939. [Google Scholar] [CrossRef]
- Fialho, L.; Almeida Alves, C.; Marques, L.; Carvalho, S. Development of stacked porous tantalum oxide layers by anodization. Appl. Surf. Sci. 2020, 511, 145542. [Google Scholar] [CrossRef]
- Zhu, Y.; Yu, F.; Man, Y.; Tian, Q.; He, Y.; Wu, N. Preparation and performances of nanosized Ta2O5 powder photocatalyst. J. Solid State Chem. 2005, 178, 224–229. [Google Scholar] [CrossRef]
- Mahala, S.; Arumugam, S.M.; Kunchala, R.K.; Devi, B.; Elumalai, S. A mesoporous Ta2O5/Nb2O5 nanocomposite with Lewis/Brønsted acid sites to enhance stepwise glucose conversion to 5-hydroxymethylfurfural. Sustain. Energy Fuels 2024, 8, 2219–2234. [Google Scholar] [CrossRef]
- Allam, N.K.; Feng, X.J.; Grimes, C.A. Self-Assembled Fabrication of Vertically Oriented Ta2O5 Nanotube Arrays, and Membranes Thereof, by One-Step Tantalum Anodization. Chem. Mater. 2008, 20, 6477–6481. [Google Scholar] [CrossRef]
- Yu, H.; Zhu, S.; Yang, X.; Wang, X.; Sun, H.; Huo, M. Synthesis of coral-like tantalum oxide films via anodization in mixed organic-inorganic electrolytes. PLoS ONE 2013, 8, e66447. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Schmuki, P. Self-organized high aspect ratio porous hafnium oxide prepared by electrochemical anodization. Electrochem. Commun. 2005, 7, 49–52. [Google Scholar] [CrossRef]
- El-Sayed, H.A.; Birss, V.I. Controlled Interconversion of Nanoarray of Ta Dimples and High Aspect Ratio Ta Oxide Nanotubes. Nano Lett. 2009, 9, 1350–1355. [Google Scholar] [CrossRef]
- Chen, W.; Tu, Q.; Wu, H.; Zhao, C.; Yao, X.; Fan, W.; Zhang, S.; Ni, J.; Zhang, X. Study on morphology evolution of anodic tantalum oxide films in different using stages of H2SO4/HF electrolyte. Electrochim. Acta 2017, 236, 140–153. [Google Scholar] [CrossRef]
- El-Sayed, H.A.; Molero, H.M.; Birss, V.I. The impact of fabrication conditions on the quality of Au nanoparticle arrays on dimpled Ta templates. Nanotechnology 2012, 23, 435602. [Google Scholar] [CrossRef]
- Hashimoto, K.; Asami, K.; Kawashima, A.; Habazaki, H.; Akiyama, E. The role of corrosion-resistant alloying elements in passivity. Corros. Sci. 2007, 49, 42–52. [Google Scholar] [CrossRef]
- Robin, A. Comparative study of Nb, Nb–10W, and Nb–16Ta–12W corrosion behavior in sodium hydroxide solutions. Electrochim. Acta 2004, 49, 1915–1923. [Google Scholar] [CrossRef]
- Li, C.Y.; Yu, C.; Zeng, R.C.; Zhang, B.C.; Cui, L.Y.; Wan, J.; Xia, Y. In vitro corrosion resistance of a Ta2O5 nanofilm on MAO coated magnesium alloy AZ31 by atomic layer deposition. Bioact. Mater. 2020, 5, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Xu, Q.; Liang, S.; Zhang, H.; Ma, L.; Hai, L.; Zhang, X. Preparation and defect structure analysis of near-stoichiometric lithium tantalate wafers. RSC Adv. 2022, 12, 19091–19100. [Google Scholar] [CrossRef]
- Cai, H.; Li, W.; Zhu, J.; Wang, X.; Wei, Y.; Hu, C.; Wang, X.; Wu, H.; Yuan, Z. Investigation of the evolution and corrosion resistance mechanism of anodized film on Ta surface. Ceram. Int. 2024, 50, 40151–40160. [Google Scholar] [CrossRef]
- Momeni, M.M.; Mirhosseini, M.; Chavoshi, M. Fabrication of Ta2O5 nanostructure films via electrochemical anodisation of tantalum. Surf. Eng. 2017, 33, 83–89. [Google Scholar] [CrossRef]
- Horwood, C.; El-Sayed, H.; Birss, V. Precise electrochemical prediction of short tantalum oxide nanotube length. Electrochim. Acta 2014, 132, 91–97. [Google Scholar] [CrossRef]
- Xia, S.; Ni, J.; Savilov, S.V.; Li, L. Oxygen-deficient Ta2O5 nanoporous films as self-supported electrodes for lithium microbatteries. Nano Energy 2018, 45, 407–412. [Google Scholar] [CrossRef]
- Wen, T.; Tan, H.; Chen, S.; He, P.; Yang, S.; Deng, C.; Liu, S. Growth behavior of tantalum oxide nanotubes during constant current anodization. Electrochem. Commun. 2021, 128, 107073. [Google Scholar] [CrossRef]
- Brezesinski, T.; Groenewolt, M.; Antonietti, M.; Smarsly, B. Crystal-to-Crystal Phase Transition in Self-Assembled Mesoporous Iron Oxide Films. Angew. Chem. Int. Ed. 2006, 45, 781–784. [Google Scholar] [CrossRef]
- Tian, B.; Liu, X.; Yang, H.; Xie, S.; Yu, C.; Tu, B.; Zhao, D. General Synthesis of Ordered Crystallized Metal Oxide Nanoarrays Replicated by Microwave-Digested Mesoporous Silica. Adv. Mater. 2003, 15, 1370–1374. [Google Scholar] [CrossRef]
- Jiao, F.; Jumas, J.C.; Womes, M.; Chadwick, A.V.; Harrison, A.; Bruce, P.G. Synthesis of Ordered Mesoporous Fe3O4 and γ-Fe2O3 with Crystalline Walls Using Post-Template Reduction/Oxidation. J. Am. Chem. Soc. 2006, 128, 12905–12909. [Google Scholar] [CrossRef] [PubMed]
- Yanagishita, T.; Masuda, T.; Kondo, T.; Masuda, H. Highly ordered anodic porous oxides of transition metals fabricated by anodization combined with a pretexturing process. Electrochem. Commun. 2021, 123, 106916. [Google Scholar] [CrossRef]
- Yu, L.; Wang, Z.; Zhang, L.; Wu, H.B.; Lou, X.W.D. TiO2 nanotube arrays grafted with Fe2O3 hollow nanorods as integrated electrodes for lithium-ion batteries. J. Mater. Chem. A 2013, 1, 122–127. [Google Scholar] [CrossRef]
- Pinilla, S.; Campo, T.; Sanz, J.M.; Márquez, F.; Morant, C. Highly ordered metal-coated alumina membranes: Synthesis and RBS characterization. Surf. Coat. Technol. 2019, 377, 124883. [Google Scholar] [CrossRef]
- Márquez, F.; Morant, C.; Pirota, K.; Borrás, A.; Sanz, J.; Elizalde, E. Fabrication of ordered crystalline zirconium nanoporous membranes by an one-step procedure. Nano Today 2009, 4, 21–26. [Google Scholar] [CrossRef]
- Márquez, F.; Morant, C.; López, V.; Zamora, F.; Campo, T.; Elizalde, E. An alternative route for the synthesis of silicon nanowires via porous anodic alumina masks. Nanoscale Res. Lett. 2011, 6, 495. [Google Scholar] [CrossRef]
- Morant, C.; Márquez, F.; Campo, T.; Sanz, J.; Elizalde, E. Niobium and hafnium grown on porous membranes. Thin Solid Film. 2010, 518, 6799–6803. [Google Scholar] [CrossRef]
- Szczerba, M.; Mohapatra, B.D.; Pisarek, M.; Sulka, G.D. Growth characteristics and physicochemical properties of nanoporous hafnium oxide layers prepared by anodic oxidation of Hf. J. Mater. Res. Technol. 2024, 33, 4137–4148. [Google Scholar] [CrossRef]
- Huang, L.; Lau, S.P.; Zhang, Y.B.; Tay, B.K.; Fu, Y.Q. The synthesis of carbon nanotubes and zirconium carbide composite films on a glass substrate. Nanotechnology 2004, 15, 663. [Google Scholar] [CrossRef]
- Cao, J.; Gu, Q.; Gao, N.; Chen, F.; Cheng, C.; Wang, Y.; Ma, H. Designing micro-nano structure of anodized iron oxide films by metallographic adjustment on T8 steel. Ceram. Int. 2021, 47, 32954–32962. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y. Controllable fabrication of micro-nano structure on Al-Li alloy by hydrothermal treatment. Mater. Lett. 2024, 358, 135851. [Google Scholar] [CrossRef]
- Deng, H.; Gou, X.; Chen, Q.; Tan, B.; Cao, X. Formation of smooth anodic nanoporous iron oxide film for enhancing photocathodic protection on plain carbon steel. Surf. Coat. Technol. 2022, 445, 128724. [Google Scholar] [CrossRef]
- Pawlik, A.; Hnida, K.; Socha, R.P.; Wiercigroch, E.; Małek, K.; Sulka, G.D. Effects of anodizing conditions and annealing temperature on the morphology and crystalline structure of anodic oxide layers grown on iron. Appl. Surf. Sci. 2017, 426, 1084–1093. [Google Scholar] [CrossRef]
- Gao, N.; Ding, G.; Wang, C.; Chen, F.; Wang, Y.; Ma, H. A facile strategy to fabricate bionic superhydrophobic armor based on utilization of metallographic structure heredity and reconstruction of anodized oxide film on alloy. Surf. Interfaces 2022, 34, 102351. [Google Scholar] [CrossRef]
- Jolivet, A.; Labbé, C.; Frilay, C.; Debieu, O.; Marie, P.; Horcholle, B.; Lemarié, F.; Portier, X.; Grygiel, C.; Duprey, S.; et al. Structural, optical, and electrical properties of TiO2 thin films deposited by ALD: Impact of the substrate, the deposited thickness and the deposition temperature. Appl. Surf. Sci. 2023, 608, 155214. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Shen, L.; Dong, S.; Zhang, X. Nb2O5 nanoparticles encapsulated in ordered mesoporous carbon matrix as advanced anode materials for Li ion capacitors. RSC Adv. 2016, 6, 71338–71344. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Z.; Lu, Y.; Li, G.; Augustyn, V.; Dunn, B.; Ma, X.; Wang, G. High-performance supercapacitors based on nanocomposites of Nb2O5 nanocrystals and carbon nanotubes. Adv. Energy Mater. 2011, 1, 1089–1093. [Google Scholar] [CrossRef]
- Wang, J.; Chen, L.; Zhao, B.; Liang, C.; Wang, H.; Zhang, Y. Porous Nb2O5 Formed by Anodic Oxidation as the Sulfur Host for Enhanced Performance Lithium-Sulfur Batteries. Nanomaterials 2023, 13, 777. [Google Scholar] [CrossRef]
- Jiang, L.; Li, P.; Wang, S.; Liu, R.; Zhu, X.; Song, Y.; van Ree, T. Anodization fabrication techniques and energy-related applications for nanostructured anodic films on transition metals. Energy Mater. 2022, 2, 200038. [Google Scholar] [CrossRef]
- Sun, X.D.; Guo, X.; Zhang, J.H.; Wu, J.; Shi, Y.; Sun, H.Y.; Pan, C.F.; Pan, L.J. A new study on formation mechanism of ordered porous anodized metal oxides. Rare Met. 2024, 43, 5410–5418. [Google Scholar] [CrossRef]
- Jose, A.; Mathew, T.; Fernández-Navas, N.; Querebillo, C.J. Porous Inorganic Nanomaterials: Their Evolution towards Hierarchical Porous Nanostructures. Micro 2024, 4, 229–280. [Google Scholar] [CrossRef]
- Lorenzetti, M.; Gongadze, E.; Kulkarni, M.; Junkar, I.; Iglič, A. Electrokinetic properties of TiO2 nanotubular surfaces. Nanoscale Res. Lett. 2016, 11, 111–136. [Google Scholar] [CrossRef] [PubMed]
- Fang, H. Comparison of the rate capability of nanostructured amorphous and anatase TiO2 for lithium insertion using anodic TiO2 nanotube arrays. Nanotechnology 2014, 20, 225701. [Google Scholar] [CrossRef]
- Ortiz, G.; Hanzu, I.; Knauth, P.; Lavela, P.; Tirado, J.; Dienizjan, T. TiO2 nanotubes manufactured by anodization of Ti thin films for on-chip Li-ion 2D microbatteries. Electrochim. Acta 2009, 54, 4262–4268. [Google Scholar] [CrossRef]
- Supriyono; Oktavian, R.; Wulaningfitri, L.P.M.I.; Pradana, J.C.; Feliana, I. Influence of anodizing concentration and electric potential on surface morphology and corrosion behavior of anodized magnesium in seawater activated battery. IOP Conf. Ser. Earth Environ. Sci. 2018, 105, 012054. [Google Scholar] [CrossRef]
- Ridwan, F.; Agusta, D.; Dahlan, D. Employment of anodized aluminum oxide as porous cathode for battery applications. Am. Inst. Phys. Conf. Ser. 2025, 3223, 050003. [Google Scholar] [CrossRef]
- Kruk, A.; Brylewski, T. Structure and Electrical Properties of Fe-Mn-Co-O Spinel. Acta Phys. Pol. A 2019, 135, 439–443. [Google Scholar] [CrossRef]
- Kruk, A.; Stygar, M.; Brylewski, T.; Przybylski, K. Mn1.5Co1.5O4 Spinel Conducting Coatings on Al453 Ferritic Steel with Regard to their Application as Interconnects in IT-SOFC. Arch. Metall. Mater. 2013, 17, 993–1003. [Google Scholar] [CrossRef][Green Version]
- Kruk, A.; Schabikowski, M.; Mitura-Nowak, M.; Brylewski, T. Magnetic and electrical properties of Mn2CoO4 spinel. Phys. B Condens. Matter 2020, 596, 412402. [Google Scholar] [CrossRef]
- Durda, E.; Kruk, A. Physicochemical properties of the Crofer 22 APU steel with La0.6Sr0.4Co0.2Fe0.8O3−δ protective-conductive coatings prepared by pulsed laser deposition. Process. Appl. Ceram. 2023, 17, 301–313. [Google Scholar] [CrossRef]
- Surganov, V.; Mozalev, A. Planar aluminum interconnection formed by electrochemical anodizing technique. Microelectron. Eng. 1997, 37–38, 329–334. [Google Scholar] [CrossRef]
- Brylewski, T.; Gil, A.; Rakowska, A.; Chevalier, S.; Adamczyk, A.; Dabek, J.; Kruk, A.; Stygar, M.; Przybylski, K. Improving the Physicochemical Properties of Fe–25Cr Ferritic Steel for SOFC Interconnects via Y-Implantation and Y2O3-Deposition. Oxid. Met. 2013, 80, 83–111. [Google Scholar] [CrossRef]
- Cong, Z.; Wang, M.; Sun, X.; Liu, L.; Sun, H. Optical and dielectric properties of anodic iron oxide films. Appl. Surf. Sci. 2020, 503, 144159. [Google Scholar] [CrossRef]
- Young, L. Anodic Oxide Films on Niobium: Thickness, Dielectric Constant, Dispersion, Reflection Minima, Formation Field Strength, and Surface Area. Can. J. Chem. 1960, 38, 1141–1147. [Google Scholar] [CrossRef]
- Ko, E.; Weissman, J. Structures of niobium pentoxide and their implications on chemical behavior. Catal. Today 1990, 8, 27–36. [Google Scholar] [CrossRef]
- Le Viet, A.; Jose, R.; Reddy, M.; Chowdari, B.; Ramakrishna, S. Nb2O5 photoelectrodes for dye-sensitized solar cells: Choice of the polymorph. J. Phys. Chem. C 2010, 114, 21795–21800. [Google Scholar] [CrossRef]
- Abe, S. Formation of Nb2O5 matrix and Vis-NIR absorption in Nb-Ge-O thin film. Nanoscale Res. Lett. 2012, 7, 341. [Google Scholar] [CrossRef]
- Jalil, Z. Structural and Optical Properties of Zinc Oxide (ZnO) based Thin Films Deposited by Sol-Gel Spin Coating Method. J. Phys. Conf. Ser. 2018, 1116, 032020. [Google Scholar] [CrossRef]
- Witkowski, B. Applications of ZnO Nanorods and Nanowires–A Review. Acta Phys. Pol. A 2018, 134. [Google Scholar] [CrossRef]
- Potera, P.; Virt, I.S.; Cieniek, B. Structure and Optical Properties of Transparent Cobalt-Doped ZnO Thin Layers. Appl. Sci. 2023, 13, 2701. [Google Scholar] [CrossRef]
- Kruk, A. Fabrication of MgO high transparent ceramics by arc plasma synthesis. Opt. Mater. 2018, 84, 360–366. [Google Scholar] [CrossRef]
- Diachenko, O.; Opanasyuk, A.; Kurbatova, D.; Opanasuy, N.; Kononov, O.; Nam, D.; Cheong, H. Surface Morphology, Structural and Optical Properties of MgO Films Obtained by Spray Pyrolysis Technique. Acta Phys. Pol. A 2016, 130, 805–810. [Google Scholar] [CrossRef]
- Kruk, A.; Madej, D. Structural properties and Faraday effect of arc melted magnesia transparent polycrystal. Opt. Mater. 2020, 108, 110245. [Google Scholar] [CrossRef]
- Al-Gaashani, R.; Radiman, S.; Al-Douri, Y.; Tabet, N.; Daud, A. Investigation of the optical properties of Mg(OH)2 and MgO nanostructures obtained by microwave-assisted methods. J. Alloys Compd. 2012, 521, 71–76. [Google Scholar] [CrossRef]
- Chen, X.; Bai, R.; Huang, M. Optical properties of amorphous Ta2O5 thin films deposited by RF magnetron sputtering. Opt. Mater. 2019, 97, 109404. [Google Scholar] [CrossRef]
- Di Franco, F.; Santamaria, M.; Di Quarto, F.; Tsuji, E.; Habazaki, H. The influence of nitrogen incorporation on the optical properties of anodic Ta2O5. Electrochim. Acta 2012, 59, 382–386. [Google Scholar] [CrossRef]
- Dong, Y.; Ai, F.; Sun-Waterhouse, D.; Murai, K.i.; Moriga, T.; Waterhouse, G.I.N. Optical and Photocatalytic Properties of Three-Dimensionally Ordered Macroporous Ta2O5 and Ta3N5 Inverse Opals. Chem. Mater. 2023, 35, 8281–8300. [Google Scholar] [CrossRef]
- Cheng, H.; Zheng, L.; Tsang, C.K.; Zhang, J.; Wang, H.; Dong, Y.; Li, H.; Liang, F.; Zapien, J.; Li, Y.Y. Electrochemical fabrication and optical properties of periodically structured porous Fe2O3 films. Electrochem. Commun. 2012, 20, 178–181. [Google Scholar] [CrossRef]
- Maabong, K.; Hu, Y.; Braun, A.; Machatine, A.G.J.; Diale, M. Influence of anodization time on the surface modifications on α-Fe2O3 photoanode upon anodization. J. Mater. Res. 2016, 31, 1580–1587. [Google Scholar] [CrossRef]
- Kazmierczak, B.; Kutylowska, M.; Piekarska, K.; Trusz-Zdybek, A. (Eds.) Effects of Aluminium Oxide Nanoparticles on Bacterial Growth. E3s Web Conf. 2017, 17, 00019. [Google Scholar]
- Commission Directive 93/67/EEC of 20 July 1993 Laying Down the Principles for Assessment of Risks to Man and the Environment of Substances Notified in Accordance with Council Directive 67/548/EEC. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:31993L0067 (accessed on 4 July 2025).
- Schabikowski, M.; Kowalczyk, P.; Karczmarska, A.; Gawdzik, B.; Wypych, A.; Kramkowski, K.; Wrzosek, K.; Laskowski, Ł. Aluminium(III) Oxide-The Silent Killer of Bacteria. Molecules 2023, 28, 401. [Google Scholar] [CrossRef] [PubMed]
- Schabikowski, M.; Laskowska, M.; Kowalczyk, P.; Fedorchuk, A.; Szori-Dorogházi, E.; Németh, Z.; Kuźma, D.; Gawdzik, B.; Wypych, A.; Kramkowski, K.; et al. Functionalised Anodised Aluminium Oxide as a Biocidal Agent. Int. J. Mol. Sci. 2022, 23, 8327. [Google Scholar] [CrossRef]
- Laskowski, Ł.; Laskowska, M.; Dulski, M.; Zubko, M.; Jelonkiewicz, J.; Perzanowski, M.; Vila, N.; Walcarius, A. Multi-step functionalization procedure for fabrication of vertically aligned mesoporous silica thin films with metal-containing molecules localized at the pores bottom. Microporous Mesoporous Mater. 2019, 274, 356–362. [Google Scholar] [CrossRef]
- Laskowska, M.; Pastukh, O.; Fedorchuk, A.; Schabikowski, M.; Kowalczyk, P.; Zalasiński, M.; Laskowski, Ł. Nanostructured Silica with Anchoring Units: The 2D Solid Solvent for Molecules and Metal Ions. Int. J. Mol. Sci. 2020, 21, 8137. [Google Scholar] [CrossRef]
- Laskowski, L.; Laskowska, M.; Fijalkowski, K.; Piech, H.; Jelonkiewicz, J.; Jaskulak, M.; Gnatowski, A.; Dulski, M. New Class of Antimicrobial Agents: SBA-15 Silica Containing Anchored Copper Ions. J. Nanomater. 2017, 2017, 1287698. [Google Scholar] [CrossRef]
- Laskowski, L.; Kityk, I.; Konieczny, P.; Pastukh, O.; Schabikowski, M.; Laskowska, M. The separation of the Mn12 single-molecule magnets onto spherical silica nanoparticles. Nanomaterials 2019, 9, 764. [Google Scholar] [CrossRef]
- Bałanda, M.; Pełka, R.; Fitta, M.; Laskowski, Ł.; Laskowska, M. Relaxation and magnetocaloric effect in the Mn12 molecular nanomagnet incorporated into mesoporous silica: A comparative study. RSC Adv. 2016, 6, 49179–49186. [Google Scholar] [CrossRef]
- Laskowska, M.; Oyama, M.; Kityk, I.; Marszalek, M.; Dulski, M.; Laskowski, L. Surface functionalization by silver-containing molecules with controlled distribution of functionalities. Appl. Surf. Sci. 2019, 481, 433–436. [Google Scholar] [CrossRef]
- Koszelewski, D.; Ostaszewski, R.; Śmigielski, P.; Hrunyk, A.; Kramkowski, K.; Laskowski, Ł.; Laskowska, M.; Lizut, R.; Szymczak, M.; Michalski, J.; et al. Pyridine derivatives—a new class of compounds that are toxic to E. coli K12, R2-R4 strains. Materials 2021, 14, 5401. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Cheng, Y.; Yang, H.; Yang, Y.; Huang, J.; Chen, Z.; Wang, X.; Lin, C. Progress in TiO2 nanotube coatings for biomedical applications: A review. J. Mater. Chem. B 2018, 6, 1862–1886. [Google Scholar] [CrossRef]
- Shrestha, N.; Macak, J.; Schmidt-Stein, F. Magnetically guided titania nanotubes for site-selective photocatalysis and drug release. Photoinducted Drug Release 2009, 49, 969–972. [Google Scholar] [CrossRef]
- Ali, H.; Kalashnikova, I.; White, M.; E, R. Preparation, characterization, and transport of dexamethasone-loaded polymeric nanoparticles across a human placental in vitro model. Int. J. Pharm. 2013, 454, 149–157. [Google Scholar] [CrossRef]
- Fan, J.; Chang, S.; Xie, Z. ZnO-Based Light-Emitting Diodes. In Optoelectronics; Pyshkin, S.L., Ballato, J.M., Eds.; IntechOpen: Rijeka, Croatia, 2013. [Google Scholar] [CrossRef]
- Ohta, H.; Hosono, H. Transparent oxide optoelectronics. Mater. Today 2004, 7, 42–51. [Google Scholar] [CrossRef]
- Ginley, D.S.; Bright, C. Transparent Conducting Oxides. MRS Bull. 2000, 25, 15–18. [Google Scholar] [CrossRef]
- Wang, H.J.; Wang, L.N.; Cao, Y. Film-form ZnO/AAO photo-catalysts: Facile synthesis and surface feature-dependent photo-catalytic activity. J. Environ. Chem. Eng. 2015, 3, 2263–2272. [Google Scholar] [CrossRef]
- Bappy, N.F.; Subramani, S. A comprehensive review on Mg-doped ZnO thin film and nanostructure: Properties and applications. Mater. Sci. Eng. B 2025, 318, 118251. [Google Scholar] [CrossRef]
- Najma, B.; Kasi, A.K.; Khan Kasi, J.; Akbar, A.; Bokhari, S.M.A.; Stroe, I.R. ZnO/AAO photocatalytic membranes for efficient water disinfection: Synthesis, characterization and antibacterial assay. Appl. Surf. Sci. 2018, 448, 104–114. [Google Scholar] [CrossRef]
- Lotus, A.; Kang, Y.; Ramsier, R.; Chase, G.G. Investigation of the physical and electronic properties of indium doped zinc oxide nanofibers synthesized by electrospinning. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 2009, 27, 2331–2336. [Google Scholar] [CrossRef]
- Fang, B.; She, J.; Liu, J.; Xu, N. Study of vacuum microelectronic device using zinc oxide nanowires by low temperature solution phase method. In Proceedings of the 2009 22nd International Vacuum Nanoelectronics Conference, Hamamatsu, Japan, 20–24 July 2009; pp. 299–300. [Google Scholar]
- Godlewski, M.; Guziewicz, E.; Kopalko, K.; Łuka, G.; Łukasiewicz, M.; Krajewski, T.; Witkowski, B.; Gierałtowska, S. Zinc oxide for electronic, photovoltaic and optoelectronic applications. Low Temp. Phys. 2011, 37, 235–240. [Google Scholar] [CrossRef]
- Martins, R.; Fortunato, E.; Nunes, P.; Ferreira, I.; Marques, A.; Bender, M.; Katsarakis, N.; Cimalla, V.; Kiriakidis, G. Zinc oxide as an ozone sensor. J. Appl. Phys. 2004, 96, 1398–1408. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, K.; Jiang, D.; Zhu, Z.; Geng, H.; Luo, L. Zinc oxide nanorod and nanowire for humidity sensor. Appl. Surf. Sci. 2005, 242, 212–217. [Google Scholar] [CrossRef]
- Jayaseelan, C.; Rahuman, A.A.; Kirthi, A.V.; Marimuthu, S.; Santhoshkumar, T.; Bagavan, A.; Gaurav, K.; Karthik, L.; Rao, K.B. Novel microbial route to synthesize ZnO nanoparticles using Aeromonas hydrophila and their activity against pathogenic bacteria and fungi. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 90, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Mobaraki, F.; Momeni, M.; Jahromi, M.; Kasmaie, F.M.; Barghbani, M.; Yazdi, M.E.T.; Meshkat, Z.; Shandiz, F.H.; Hosseini, S.M. Apoptotic, antioxidant and cytotoxic properties of synthesized AgNPs using green tea against human testicular embryonic cancer stem cells. Process Biochem. 2022, 119, 106–118. [Google Scholar] [CrossRef]
- Alsaad, A.; Al Dairy, A.R.; Ahmad, A.; Al-anbar, A.S.; Al-Bataineh, Q.M. Synthesis and characterization of as-grown doped polymerized (PMMA-PVA)/ZnO NPs hybrid thin films. Polym. Bull. 2022, 79, 2019–2040. [Google Scholar] [CrossRef]
- Gharbani, P.; Mehrizad, A. Study on the Photocatalytic Activity of Graphene Oxide in Removal of Xylene Solution. Int. J. Biophotonics Biomed. Eng. (IJBBE) 2024, 4, 59–64. [Google Scholar]
- Mirzaei, H.; Darroudi, M. Zinc oxide nanoparticles: Biological synthesis and biomedical applications. Ceram. Int. 2017, 43, 907–914. [Google Scholar] [CrossRef]
- Hutasoit, N.; Kennedy, B.; Hamilton, S.; Luttick, A.; Rahman Rashid, R.A.; Palanisamy, S. Sars-CoV-2 (COVID-19) inactivation capability of copper-coated touch surface fabricated by cold-spray technology. Manuf. Lett. 2020, 25, 93–97. [Google Scholar] [CrossRef]
- Hornberger, H.; Virtanen, S.; Boccaccini, A.R. Biomedical coatings on magnesium alloys—A review. Acta Biomater. 2012, 8, 2442–2455. [Google Scholar] [CrossRef]
- Chaneliere, C.; Four, S.; Autran, J.; Devine, R. Comparison between the properties of amorphous and crystalline Ta2O5 thin films deposited on Si. Microelectron. Reliab. 1999, 39, 261–268. [Google Scholar] [CrossRef]
- Klerer, J. Determination of the Density and Dielectric Constant of Thin Ta2O5 Films. J. Electrochem. Soc. 1965, 112, 896. [Google Scholar] [CrossRef]
- Abbas, Y.; Jeon, Y.R.; Sokolov, A.S.; Kim, S.; Ku, B.; Choi, C. Compliance-Free, Digital SET and Analog RESET Synaptic Characteristics of Sub-Tantalum Oxide Based Neuromorphic Device. Sci. Rep. 2018, 8, 1228. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.; Ge, Z.; Zhao, X.; Liu, J.; Zhao, Y. Electrical and optical properties of tantalum oxide thin films prepared by reactive magnetron sputtering. J. Alloys Compd. 2011, 509, 9758–9763. [Google Scholar] [CrossRef]
- Liu, Y.; Bao, C.; Wismeijer, D.; Wu, G. The physicochemical/biological properties of porous tantalum and the potential surface modification techniques to improve its clinical application in dental implantology. Mater. Sci. Eng. C 2015, 49, 323–329. [Google Scholar] [CrossRef]
- Wei, W.; Macak, J.M.; Shrestha, N.K.; Schmuki, P. Thick self-ordered nanoporous Ta2O5 films with long-range lateral order. J. Electrochem. Soc. 2009, 156, K104. [Google Scholar] [CrossRef]
- Hirasawa, K.; Tomioka, Y.; Kawamura, M.; Hyono, A.; Chiba, M. Self-healing Coating by Using Pore of Porous Film Formed on Al Alloy Anodized and Effect of Pore-size on Self-healing Property of Coating. Corros. Eng. 2022, 71, 63–69. [Google Scholar] [CrossRef]
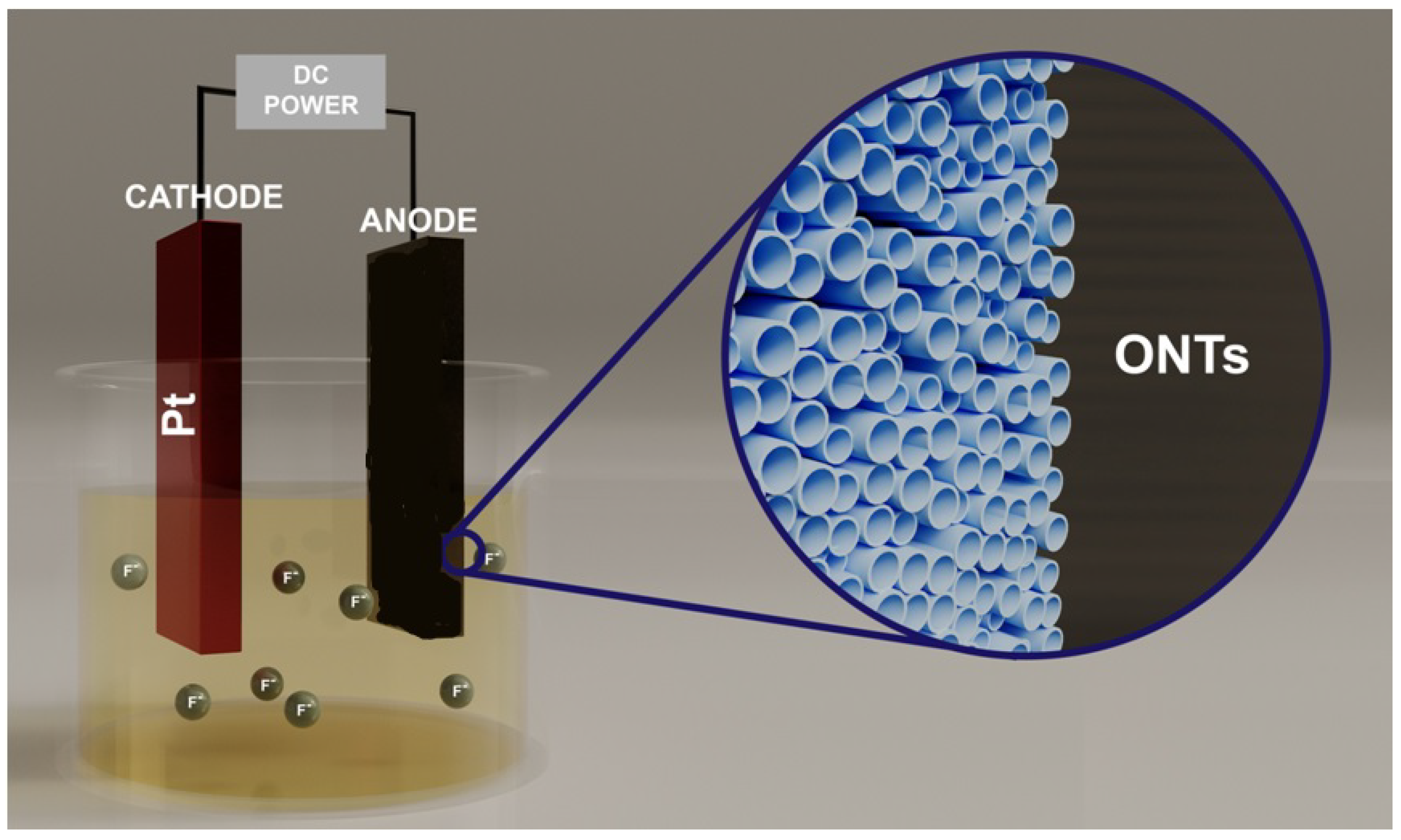
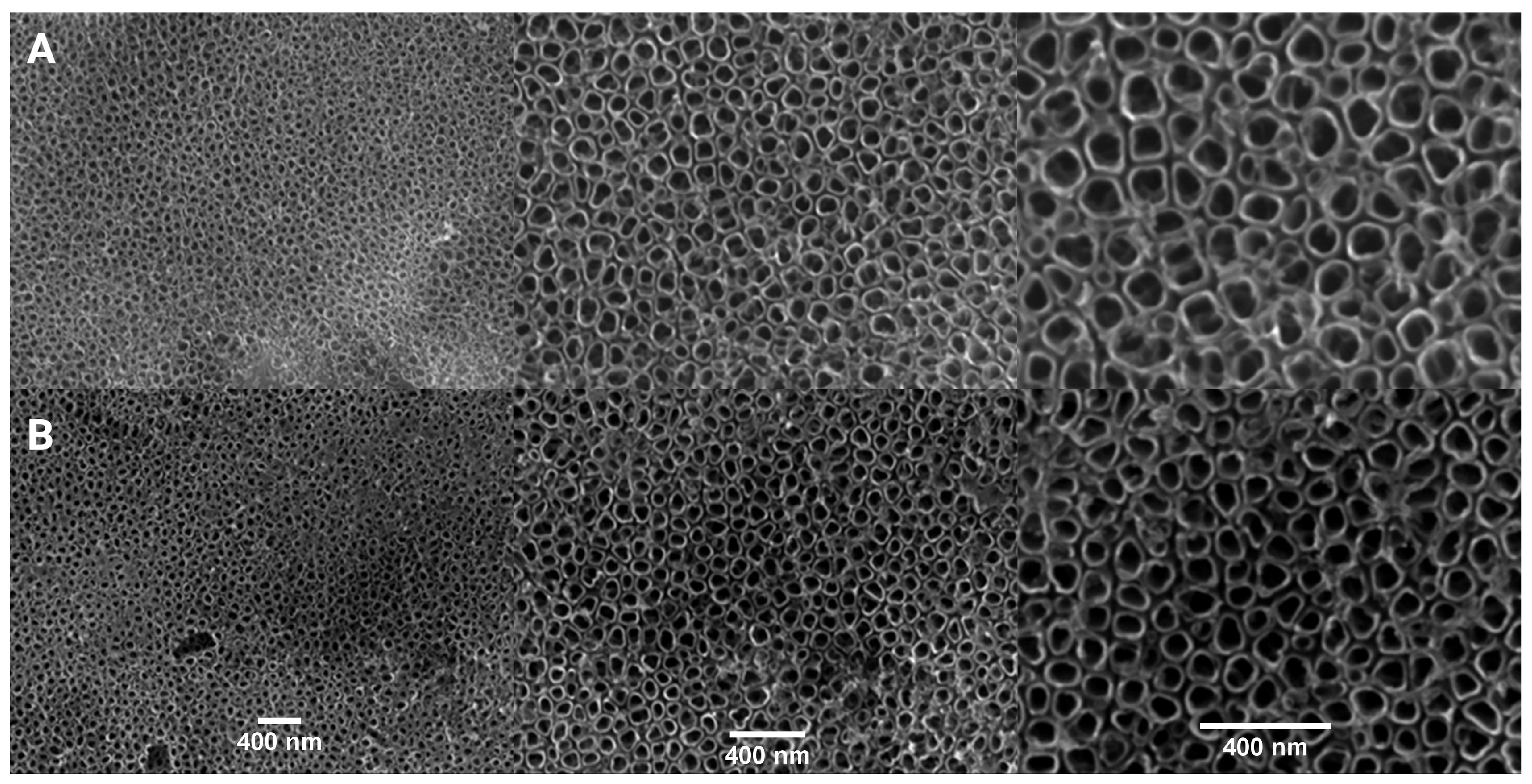

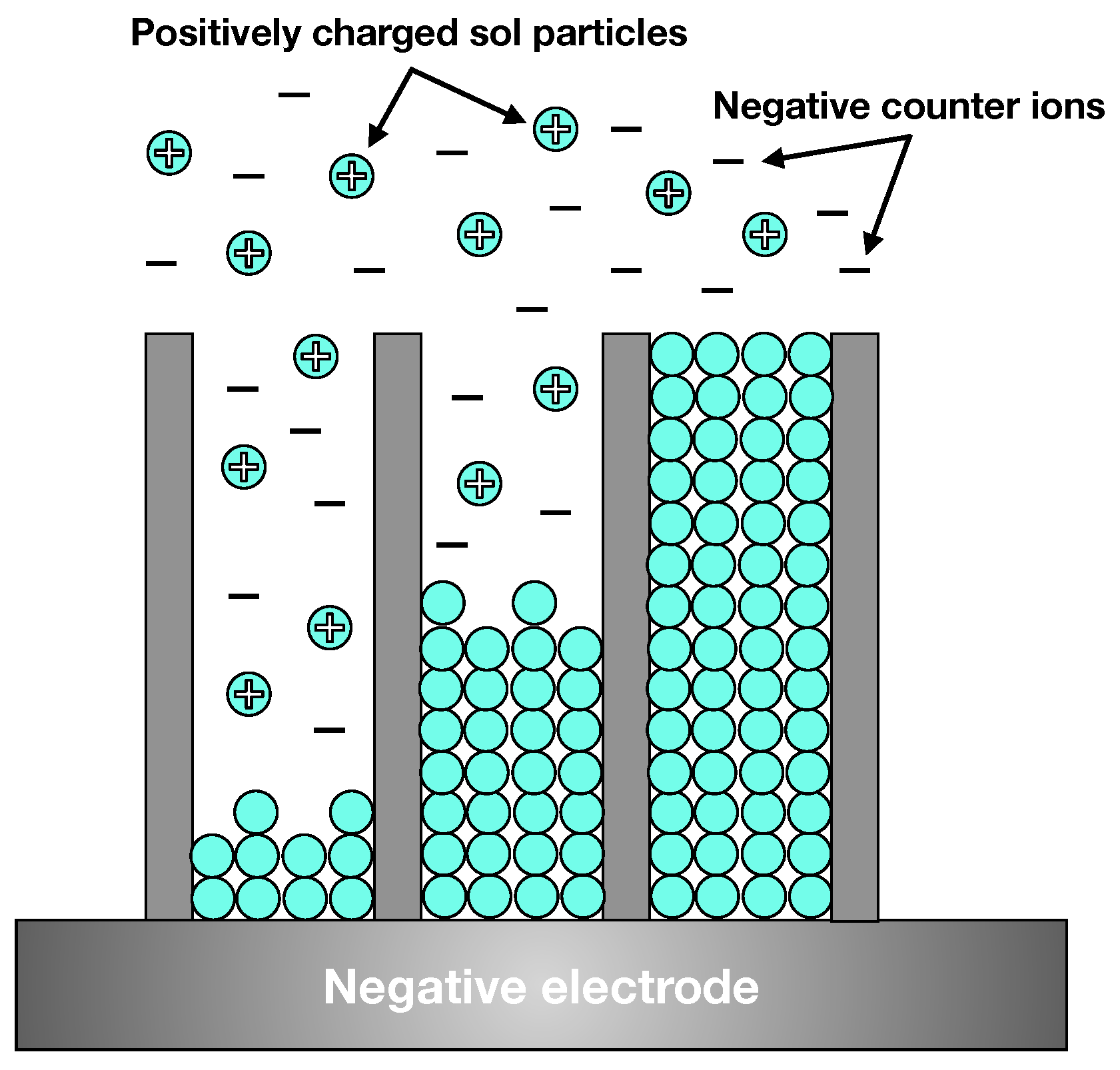
| Biomolecules | Functions |
|---|---|
| Bone Morphogenetic Protein-2 (BMP-2) | Promotes the ability of bone marrow-derived mesenchymal stem cells to differentiate into osteoblasts in vitro |
| Chitosan | Enhances drug elution, osteoblast adhesion and strengthens bone integration |
| Polydopamine | Prolongs drug release duration and maintains constant release kinetics |
| Quercetin | Loads into TNTs and releases in the environment as an alternative for treating postoperative infections, inflammation and promoting faster healing with better integration |
| Trehalose | Has osteogenic potential and anti-inflammatory properties |
| Gelatin | Improves osteoblast adhesion and propagation and serves as a drug release-controlling coating |
| Hemoglobin | Detects hydrogen peroxide |
| Uricase (Urate Oxidase) | Detects uric acid |
| Glucose Oxidase | Detects glucose |
| Polycaprolactone | Improves the solubility and flexibility of nanotubes, enhancing their biocompatibility |
| Antimicrobial Peptides | Exhibit antimicrobial activity |
| Osteogenic Growth Peptide | Enhances osteoblast differentiation |
| Gly-Arg-Gly-Asp-Ser Peptide | Enhances cell adhesion and increases cell spreading and proliferation |
| Arg-Gly-Asp Peptide | Enhances adhesion of bone marrow stem cells (BMSCs) and significantly improves the expression of osteogenic genes in BMSCs |
| Lys-Arg-Ser-Arg Peptide | Enhances preosteoblast adhesion and osteogenic gene expression on TNTs |
| Palmitoyl-oleoyl-phosphatidylcholine | Used as a barrier for controlled and sustained drug release |
| Epidermal Growth Factor | Promotes MSC proliferation and prevents cell apoptosis |
| Small Interfering RNA | Targets tumor necrosis factor-alpha (TNF-α) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schabikowski, M.; Stróż, A.; Kruk, A. Beyond Oxidation: Engineering Functional Anodised Metal Matrices Through Molecular and Surface Modifications. Int. J. Mol. Sci. 2025, 26, 7809. https://doi.org/10.3390/ijms26167809
Schabikowski M, Stróż A, Kruk A. Beyond Oxidation: Engineering Functional Anodised Metal Matrices Through Molecular and Surface Modifications. International Journal of Molecular Sciences. 2025; 26(16):7809. https://doi.org/10.3390/ijms26167809
Chicago/Turabian StyleSchabikowski, Mateusz, Agnieszka Stróż, and Andrzej Kruk. 2025. "Beyond Oxidation: Engineering Functional Anodised Metal Matrices Through Molecular and Surface Modifications" International Journal of Molecular Sciences 26, no. 16: 7809. https://doi.org/10.3390/ijms26167809
APA StyleSchabikowski, M., Stróż, A., & Kruk, A. (2025). Beyond Oxidation: Engineering Functional Anodised Metal Matrices Through Molecular and Surface Modifications. International Journal of Molecular Sciences, 26(16), 7809. https://doi.org/10.3390/ijms26167809






