Discovery of Tricyclic Aromatic Polyketides Reveals Hidden Chain-Length Flexibility in Type II Polyketide Synthases
Abstract
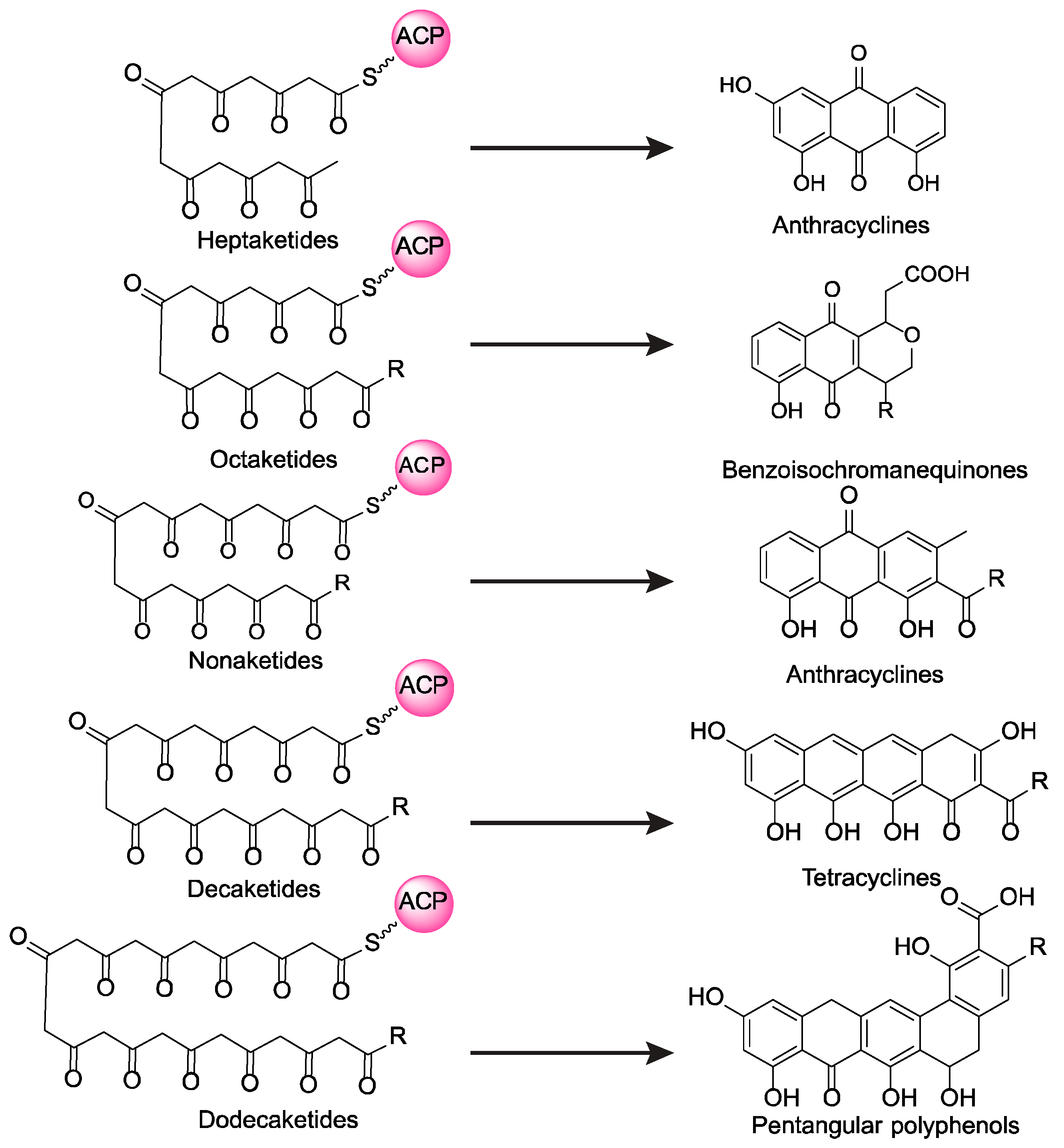
1. Introduction
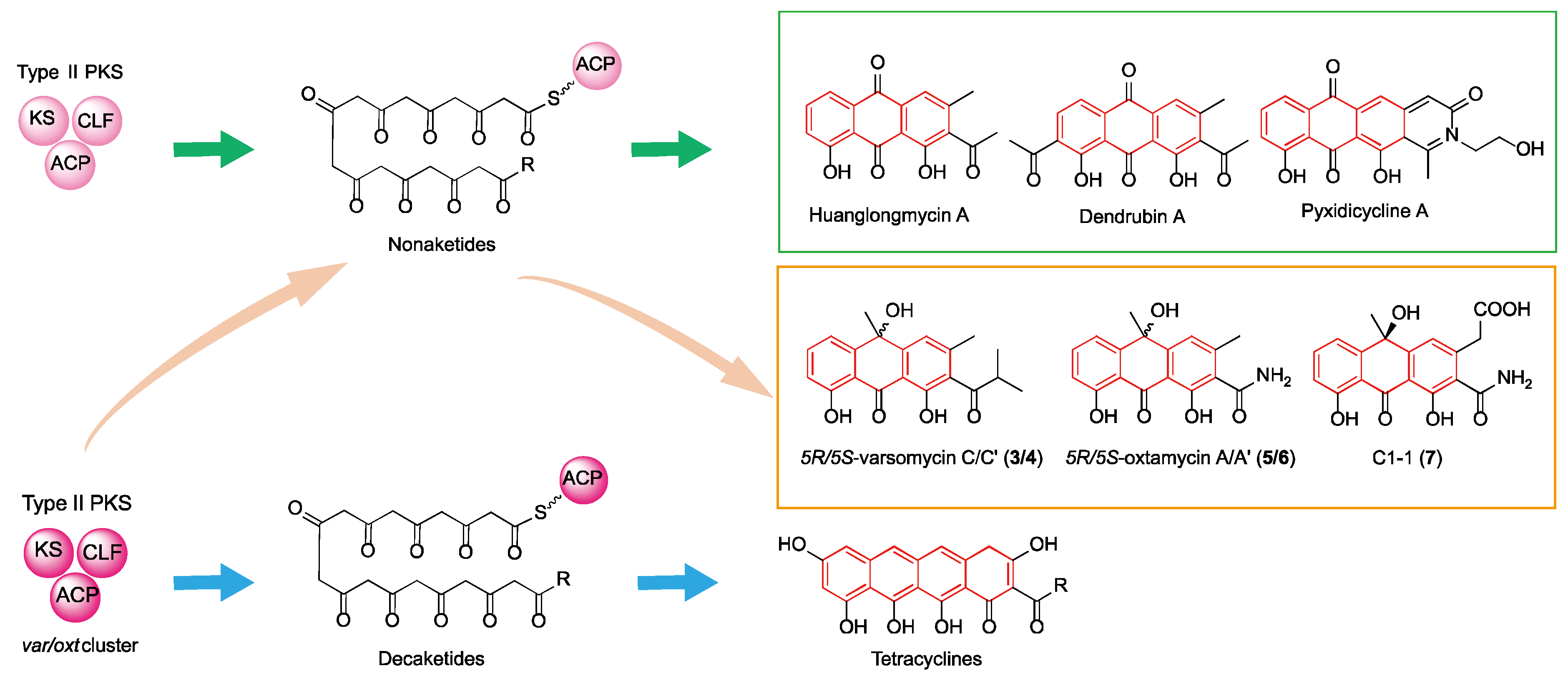
2. Results
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Strains, Growth, and Fermentation Conditions
4.3. Computational ECD Calculation
4.4. Isolation and Purification of Compounds
4.5. Antibacterial Activity and Cytotoxicity Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Rossiter, S.E.; Fletcher, M.H.; Wuest, W.M. Natural Products as Platforms to Overcome Antibiotic Resistance. Chem. Rev. 2017, 117, 12415–12474. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.L.; Levy, S.B. The history of the tetracyclines. Ann. N. Y. Acad. Sci. 2011, 1241, 17–32. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Fan, K.; Pan, G. Tetracycline natural products: Discovery, biosynthesis and engineering. Chin. J. Nat. Med. 2022, 20, 773–794. [Google Scholar] [CrossRef]
- Hulst, M.B.; Grocholski, T.; Neefjes, J.J.C.; van Wezel, G.P.; Metsä-Ketelä, M. Anthracyclines: Biosynthesis, engineering and clinical applications. Nat. Prod. Rep. 2022, 39, 814–841. [Google Scholar] [CrossRef]
- Di Marco, A.; Cassinelli, G.; Arcamone, F. The discovery of daunorubicin. Cancer Treat. Rep. 1981, 65 (Suppl. S4), 3–8. [Google Scholar]
- Damiani, R.M.; Moura, D.J.; Viau, C.M.; Caceres, R.A.; Henriques, J.A.P.; Saffi, J. Pathways of cardiac toxicity: Comparison between chemotherapeutic drugs doxorubicin and mitoxantrone. Arch. Toxicol. 2016, 90, 2063–2076. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Chen, X.; Yan, X.; Li, G.; Lin, Z.; Deng, Z.; Luo, S.; Qu, X. An unusual aromatase/cyclase programs the formation of the phenyldimethylanthrone framework in anthrabenzoxocinones and fasamycin. Proc. Natl. Acad. Sci. USA 2024, 121, e2321722121. [Google Scholar] [CrossRef]
- Chan, Y.A.; Podevels, A.M.; Kevany, B.M.; Thomas, M.G. Biosynthesis of polyketide synthase extender units. Nat. Prod. Rep. 2008, 26, 90–114. [Google Scholar] [CrossRef]
- Hertweck, C.; Luzhetskyy, A.; Rebets, Y.; Bechthold, A. Type II polyketide synthases: Gaining a deeper insight into enzymatic teamwork. Nat. Prod. Rep. 2007, 24, 162–190. [Google Scholar] [CrossRef] [PubMed]
- Fäseke, V.C.; Raps, F.C.; Sparr, C. Polyketide Cyclizations for the Synthesis of Polyaromatics. Angew. Chem. Int. Ed. 2019, 59, 6975–6983. [Google Scholar] [CrossRef]
- Shen, B. Biosynthesis of Aromatic Polyketides. In Biosynthesis: Aromatic Polyketides, Isoprenoids, Alkaloids; Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2000; Volume 209, pp. 1–51. [Google Scholar]
- Hertweck, C. The biosynthetic logic of polyketide diversity. Angew. Chem. Int. Ed. 2009, 48, 4688–4716. [Google Scholar] [CrossRef]
- Olano, C.; Méndez, C.; Salas, J. Post-PKS Tailoring Steps in Natural Product-Producing Actinomycetes from the Perspective of Combinatorial Biosynthesis. Nat. Prod. Rep. 2010, 27, 571–616. [Google Scholar] [CrossRef]
- Lombó, F.; Menéndez, N.; Salas, J.A.; Méndez, C. The aureolic acid family of antitumor compounds: Structure, mode of action, biosynthesis, and novel derivatives. Appl. Microbiol. Biotechnol. 2006, 73, 1–14. [Google Scholar] [CrossRef]
- Xiang, L.; Shi, J.; Zhu, A.; Xu, Z.F.; Liu, S.H.; Wang, Y.S.; Guo, Z.K.; Jiao, R.H.; Tan, R.X.; Ge, H.M. Total Biosynthesis of Mutaxanthene Unveils a Flavoprotein Monooxygenase Catalyzing Xanthene Ring Formation. Angew. Chem. Int. Ed. 2023, 62, e202218660. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Chi, C.; Fan, K.; Zhang, Q.; Xu, Y.; Gao, J.; Hu, H.; Wang, J.; Yang, D.; Ma, M.; et al. Functional Conservation and Divergence of AlpJ-Family Oxygenases Catalyzing C–C Bond Cleavage in Atypical Angucycline Biosynthesis. ACS Chem. Biol. 2025, 20, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, C.; Zhang, L. Investigation of the Molecular Landscape of Bacterial Aromatic Polyketides by Global Analysis of Type II Polyketide Synthases. Angew. Chem. Int. Ed. 2022, 61, e202202286. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Zhang, L. Type II Polyketide Synthases: A Bioinformatics-Driven Approach. ChemBioChem 2023, 24, e202200775. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, B.J. Biosynthesis of polyketides (other than actinomycete macrolides). Nat. Prod. Rep. 1999, 16, 425–484. [Google Scholar] [CrossRef]
- Tang, Y.; Tsai, S.C.; Khosla, C. Polyketide Chain Length Control by Chain Length Factor. J. Am. Chem. Soc. 2003, 125, 12708–12709. [Google Scholar] [CrossRef]
- Chen, A.; Re, R.N.; Burkart, M.D. Type II fatty acid and polyketide synthases: Deciphering protein–protein and protein– substrate interactions. Nat. Prod. Rep. 2018, 10, 1029–1045. [Google Scholar] [CrossRef]
- Nicholson, T.P.; Winfield, C.; Westcott, J.; Crosby, J.; Simpson, T.J.; Cox, R.J. First in vitro directed biosynthesis of new compounds by a minimal type II polyketide synthase: Evidence for the mechanism of chain length determination. Chem. Commun. 2003, 21, 686–687. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Yoon, P.; YU, T.W.; Floss, H.G.; Hopwood, D.S.; Moore, B.S. Ectopic expression of the minimal whiE polyketide synthase generates a library of aromatic polyketides of diverse sizes and shapes. Proc. Natl. Acad. Sci. USA 1999, 96, 3622–3627. [Google Scholar] [CrossRef]
- Peric-Concha, N.; Borovicka, B.; Long, P.F.; Hranueli, D.; Waterman, P.G.; Hunter, I.S. Ablation of the otcC gene encoding a post-polyketide hydroxylase from the oxytetracyline biosynthetic pathway in Streptomyces rimosus results in novel polyketides with altered chain length. J. Biol. Chem. 2005, 280, 37455–37460. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Li, D.; Fan, K.; Yang, Y.; Cao, H.; Sun, J.; Ren, J.; Liu, Y.; Xiang, L.; et al. Uncovering the Molecular Landscape of Tetracycline Family Natural Products through Bacterial Genome Mining. J. Am. Chem. Soc. 2025, 147, 15100–15114. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, W.; Zhan, J.; Tang, Y. Identification of OxyE as an Ancillary Oxygenase during Tetracycline Biosynthesis. ChemBioChem 2009, 10, 1544–1550. [Google Scholar] [CrossRef]
- Jiang, L.; Pu, H.; Xiang, J.; Su, M.; Yan, X.; Yang, D.; Zhu, X.; Shen, B.; Duan, Y.; Huang, Y. Huanglongmycin A-C, Cytotoxic Polyketides Biosynthesized by a Putative Type II Polyketide Synthase from Streptomyces sp. CB09001. Front. Chem. 2018, 6, 254. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Shabuer, G.; Schieferdecker, S.; Pidot, S.J.; Stinear, T.P.; Knuepfer, U.; Cyrulies, M.; Hertweck, C. Oak-Associated Negativicute Equipped with Ancestral Aromatic Polyketide Synthase Produces Antimycobacterial Dendrubins. Chem. A Eur. J. 2020, 26, 13147–13151. [Google Scholar] [CrossRef]
- Panter, F.; Krug, D.; Baumann, S.; Muller, R. Self-resistance guided genome mining uncovers new topoisomerase inhibitors from myxobacteria. Chem. Sci. 2018, 9, 4898–4908. [Google Scholar] [CrossRef]
- Hyun, K.A.; Liang, X.; Xu, Y.; Kim, S.Y.; Boo, K.H.; Park, J.S.; Chi, W.J.; Hyun, C.G. Analysis of the Setomimycin Biosynthetic Gene Cluster from Streptomyces nojiriensis JCM3382 and Evaluation of Its alpha-Glucosidase Inhibitory Activity Using Molecular Docking and Molecular Dynamics Simulations. Int. J. Mol. Sci. 2024, 25, 10758. [Google Scholar] [CrossRef]
- Dong, Y.; Ding, W.; Sun, C.; Ji, X.; Ling, C.; Zhou, Z.; Chen, Z.; Chen, X.; Ju, J. Julichrome Monomers from Marine Gastropod Mollusk-Associated Streptomyces and Stereochemical Revision of Julichromes Q3·5 and Q3·3. Chem. Biodivers. 2020, 17, e2000057. [Google Scholar] [CrossRef] [PubMed]
- Pescitelli, G.; Bruhn, T. Good Computational Practice in the Assignment of Absolute Configurations by TDDFT Calculations of ECD Spectra. Chirality 2016, 28, 749. [Google Scholar] [CrossRef] [PubMed]



| Compound 3/4 | Compound 5/6 | |||
|---|---|---|---|---|
| Position | δH (J in Hz) | δC | δH (J in Hz) | δC |
| 1 | 160.6 | 167.4 | ||
| 2 | 130.0 | 126.6 | ||
| 3 | 146.8 | 144.8 | ||
| 4 | CH, 7.34, s | 120.3 | CH, 7.28, s | 118.5 |
| 4a | 153.6 | 151.3 | ||
| 5 | 71.2 | 69.4 | ||
| 5a | 152.8 | 152.0 | ||
| 6 | CH, 7.42, dd (7.7, 1.0) | 118.0 | CH, 7.42, dd (7.7, 0.8) | 117.2 |
| 7 | CH, 7.63, dd (7.7, 8.3) | 138.5 | CH, 7.68, dd (7.7, 8.3) | 137.4 |
| 8 | CH, 6.91, dd (8.3, 1.0) | 117.5 | CH, 6.94, dd (8.3, 0.8) | 116.0 |
| 9 | 163.8 | 161.5 | ||
| 9a | 114.5 | 113.0 | ||
| 10 | 193.4 | 191.5 | ||
| 10a | 121.6 | 110.8 | ||
| 11 | 212.3 | 167.4 | ||
| 12 | CH, 3.31, m | 42.8 | CH3, 2.36, s | 20.0 |
| 13 | CH3, 1.17, d (7.0) | 18.1 | CH3, 1.50, s | 39.5 |
| 14 | CH3, 1.17, d (7.0) | 18.3 | ||
| 15 | CH3, 2.31, s | 20.7 | ||
| 16 | CH3, 1.58, s | 38.8 | ||
| 1-OH | 7.80, s | |||
| 5-OH | 6.24, s | |||
| 9-OH | 7.55, s | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wang, L.; Wang, H.; Zhu, Y.; Sun, J.; Ma, B.; Liu, L.; Bao, X.; Ren, J.; Fan, K.; et al. Discovery of Tricyclic Aromatic Polyketides Reveals Hidden Chain-Length Flexibility in Type II Polyketide Synthases. Int. J. Mol. Sci. 2025, 26, 7801. https://doi.org/10.3390/ijms26167801
Liu Y, Wang L, Wang H, Zhu Y, Sun J, Ma B, Liu L, Bao X, Ren J, Fan K, et al. Discovery of Tricyclic Aromatic Polyketides Reveals Hidden Chain-Length Flexibility in Type II Polyketide Synthases. International Journal of Molecular Sciences. 2025; 26(16):7801. https://doi.org/10.3390/ijms26167801
Chicago/Turabian StyleLiu, Yao, Lijun Wang, Haiyan Wang, Yuchen Zhu, Jianing Sun, Boyang Ma, Lin Liu, Xunrui Bao, Jinwei Ren, Keqiang Fan, and et al. 2025. "Discovery of Tricyclic Aromatic Polyketides Reveals Hidden Chain-Length Flexibility in Type II Polyketide Synthases" International Journal of Molecular Sciences 26, no. 16: 7801. https://doi.org/10.3390/ijms26167801
APA StyleLiu, Y., Wang, L., Wang, H., Zhu, Y., Sun, J., Ma, B., Liu, L., Bao, X., Ren, J., Fan, K., Wang, L., Li, X., & Pan, G. (2025). Discovery of Tricyclic Aromatic Polyketides Reveals Hidden Chain-Length Flexibility in Type II Polyketide Synthases. International Journal of Molecular Sciences, 26(16), 7801. https://doi.org/10.3390/ijms26167801






