Exploring the Potential Roles of SLC39A8 and POC5 Missense Variants in the Association Between Body Composition, Beverage Consumption, and Chronic Lung Diseases: A Two-Sample Mendelian Randomization Study
Abstract
1. Introduction
2. Results
2.1. Genetic Variants (IVs) Selection
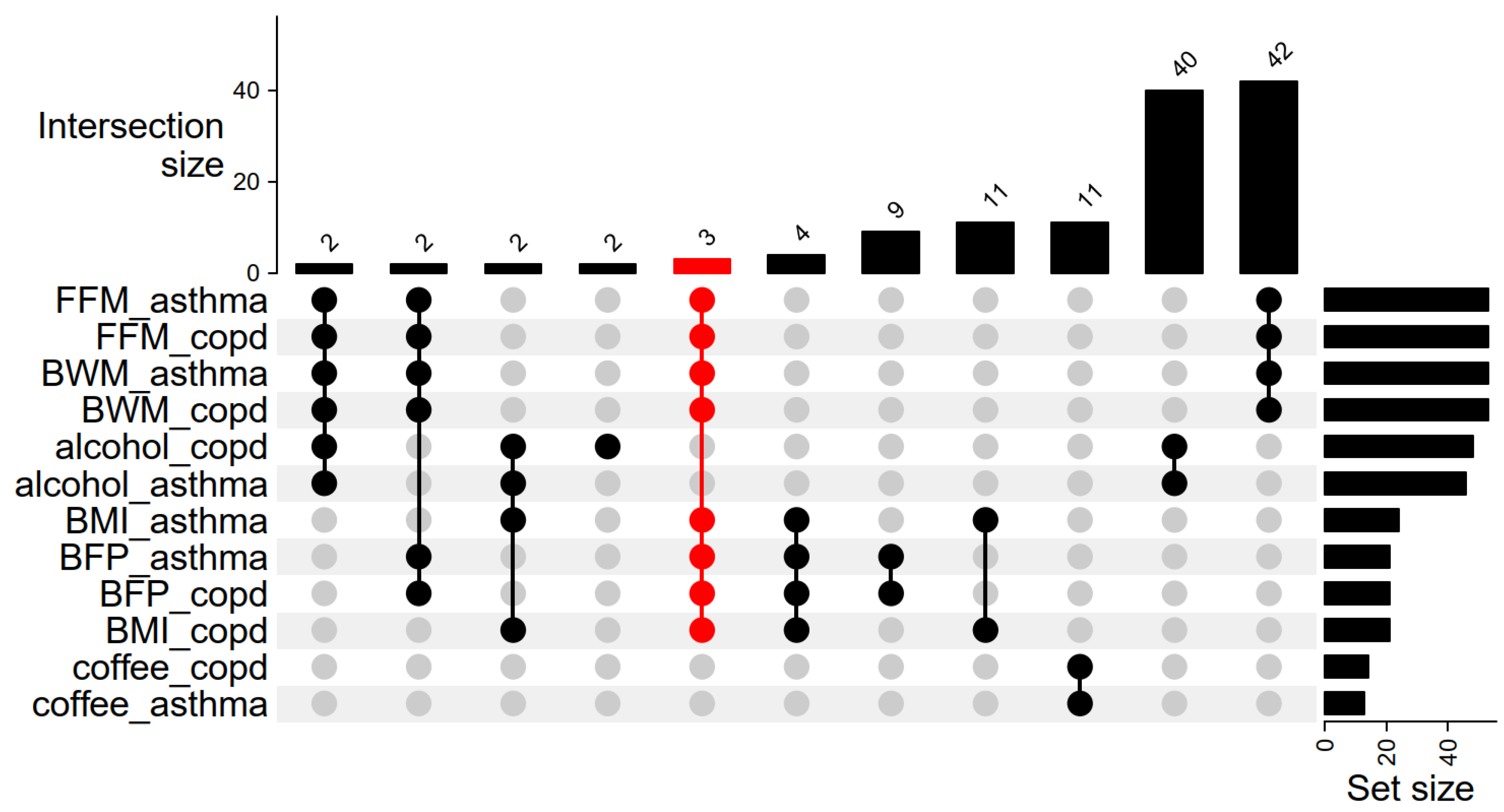
2.2. Association Between Body Fat Percentage and COPD and Asthma
2.3. Association Between BMI and COPD and Asthma
2.4. Association Between Fat-Free Mass and COPD and Asthma
2.5. Association Between Total Body Water Mass and COPD and Asthma
2.6. Association Between Alcohol Intake Frequency and COPD and Asthma
2.7. Association Between Coffee Intake and COPD and Asthma
3. Discussion
4. Methods
4.1. Data Sources
4.2. Selection of Instrumental Variables
4.3. Statistical Analysis
4.4. Enrichment Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Li, X.; Cao, X.; Guo, M.; Xie, M.; Liu, X. Trends and Risk Factors of Mortality and Disability Adjusted Life Years for Chronic Respiratory Diseases from 1990 to 2017: Systematic Analysis for the Global Burden of Disease Study 2017. BMJ 2020, 368, m3150. [Google Scholar] [CrossRef]
- Labaki, W.W.; Han, M.K. Chronic Respiratory Diseases: A Global View. Lancet Respir. Med. 2020, 8, 531–533. [Google Scholar] [CrossRef]
- Bianco, A.; Nigro, E.; Monaco, M.L.; Matera, M.G.; Scudiero, O.; Mazzarella, G.; Daniele, A. The Burden of Obesity in Asthma and COPD: Role of Adiponectin. Pulm. Pharmacol. Ther. 2017, 43, 20–25. [Google Scholar] [CrossRef]
- Sun, T.; Wang, J.; Zheng, M.; Cai, C.; Yu, J.; Fu, L.; Duan, L. Assessment of the Relationship Between Genetic Determinants of Obesity, Unhealthy Eating Habits and Chronic Obstructive Pulmonary Disease: A Mendelian Randomisation Study. COPD J. Chronic Obstr. Pulm. Dis. 2024, 21, 2309236. [Google Scholar] [CrossRef]
- Reitsma, M.B.; Fullman, N.; Ng, M.; Salama, J.S.; Abajobir, A.; Abate, K.H.; Abbafati, C.; Abera, S.F.; Abraham, B.; Abyu, G.Y.; et al. Smoking Prevalence and Attributable Disease Burden in 195 Countries and Territories, 1990–2015: A Systematic Analysis from the Global Burden of Disease Study 2015. Lancet 2017, 390, 1644. [Google Scholar] [CrossRef] [PubMed]
- Nigro, E.; Daniele, A.; Scudiero, O.; Monaco, M.; Roviezzo, F.; D’Agostino, B.; Mazzarella, G.; Bianco, A. Adiponectin in Asthma: Implications for Phenotyping. CPPS 2015, 16, 182–187. [Google Scholar] [CrossRef]
- Harju, M.; Keski-Nisula, L.; Georgiadis, L.; Heinonen, S. Parental Smoking and Cessation during Pregnancy and the Risk of Childhood Asthma. BMC Public Health 2016, 16, 428. [Google Scholar] [CrossRef]
- Dharmage, S.C.; Perret, J.L.; Custovic, A. Epidemiology of Asthma in Children and Adults. Front. Pediatr. 2019, 7, 246. [Google Scholar] [CrossRef] [PubMed]
- Coogan, P.F.; Castro-Webb, N.; Yu, J.; O’Connor, G.T.; Palmer, J.R.; Rosenberg, L. Active and Passive Smoking and the Incidence of Asthma in the Black Women’s Health Study. Am. J. Respir. Crit. Care Med. 2015, 191, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Partridge, L.; Deelen, J.; Slagboom, P.E. Facing up to the Global Challenges of Ageing. Nature 2018, 561, 45–56. [Google Scholar] [CrossRef]
- Ford, E. The Epidemiology of Obesity and Asthma. J. Allergy Clin. Immunol. 2005, 115, 897–909. [Google Scholar] [CrossRef]
- Bermúdez Barón, N.; Kankaanranta, H.; Hedman, L.; Andersson, M.; Stridsman, C.; Lindberg, A.; Rönmark, E.; Backman, H. Body Mass Index Increase: A Risk Factor for Forced Expiratory Volume in 1 s Decline for Overweight and Obese Adults with Asthma. ERJ Open Res. 2022, 8, 00110–02022. [Google Scholar] [CrossRef] [PubMed]
- Kisiel, M.A.; Arnfelt, O.; Lindberg, E.; Jogi, O.; Malinovschi, A.; Johannessen, A.; Benediktsdottir, B.; Franklin, K.; Holm, M.; Real, F.G.; et al. Association between Abdominal and General Obesity and Respiratory Symptoms, Asthma and COPD. Results from the RHINE Study. Respir. Med. 2023, 211, 107213. [Google Scholar] [CrossRef]
- Rönmark, E.; Andersson, C.; Nyström, L.; Forsberg, B.; Järvholm, B.; Lundbäck, B. Obesity Increases the Risk of Incident Asthma among Adults. Eur. Respir. J. 2005, 25, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Schachter, L.M. Obesity Is a Risk for Asthma and Wheeze but Not Airway Hyperresponsiveness. Thorax 2001, 56, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Eisner, M.D.; Blanc, P.D.; Sidney, S.; Yelin, E.H.; Lathon, P.V.; Katz, P.P.; Tolstykh, I.; Ackerson, L.; Iribarren, C. Body Composition and Functional Limitation in COPD. Respir. Res. 2007, 8, 7. [Google Scholar] [CrossRef]
- Vozoris, N.T.; O’Donnell, D.E. Prevalence, Risk Factors, Activity Limitation and Health Care Utilization of an Obese Population-Based Sample with Chronic Obstructive Pulmonary Disease. Can. Respir. J. 2012, 19, 732618. [Google Scholar] [CrossRef]
- Lieberoth, S.; Backer, V.; Kyvik, K.O.; Skadhauge, L.R.; Tolstrup, J.S.; Grønbæk, M.; Linneberg, A.; Thomsen, S.F. Intake of Alcohol and Risk of Adult-Onset Asthma. Respir. Med. 2012, 106, 184–188. [Google Scholar] [CrossRef]
- Yang, W.; Yang, Y.; He, L.; Zhang, M.; Sun, S.; Wang, F.; Han, B. Dietary Factors and Risk for Asthma: A Mendelian Randomization Analysis. Front. Immunol. 2023, 14, 1126457. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and Overweight 2025; World Health Organization: Geneva, Switzerland, 2025. [Google Scholar]
- Gallagher, D.; Heymsfield, S.B.; Heo, M.; Jebb, S.A.; Murgatroyd, P.R.; Sakamoto, Y. Healthy Percentage Body Fat Ranges: An Approach for Developing Guidelines Based on Body Mass Index. Am. J. Clin. Nutr. 2000, 72, 694–701. [Google Scholar] [CrossRef]
- Rubino, F.; Cummings, D.E.; Eckel, R.H.; Cohen, R.V.; Wilding, J.P.H.; Brown, W.A.; Stanford, F.C.; Batterham, R.L.; Farooqi, I.S.; Farpour-Lambert, N.J.; et al. Definition and Diagnostic Criteria of Clinical Obesity. Lancet Diabetes Endocrinol. 2025, 13, e6. [Google Scholar] [CrossRef]
- Davey Smith, G.; Ebrahim, S. ‘Mendelian Randomization’: Can Genetic Epidemiology Contribute to Understanding Environmental Determinants of Disease? Int. J. Epidemiol. 2003, 32, 1–22. [Google Scholar] [CrossRef]
- Burgess, S.; Daniel, R.M.; Butterworth, A.S.; Thompson, S.G.; The EPIC-InterAct Consortium. Network Mendelian Randomization: Using Genetic Variants as Instrumental Variables to Investigate Mediation in Causal Pathways. Int. J. Epidemiol. 2015, 44, 484–495. [Google Scholar] [CrossRef]
- Sekula, P.; Del Greco M, F.; Pattaro, C.; Köttgen, A. Mendelian Randomization as an Approach to Assess Causality Using Observational Data. JASN 2016, 27, 3253–3265. [Google Scholar] [CrossRef]
- Wang, L.; Chen, S.; Wan, S.; Li, K.; Li, X.; Yang, L. Vasectomy and Prostate Cancer Risk: A Pooled of Cohort Studies and Mendelian Randomization Analysis. BMC Cancer 2025, 25, 332. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; An, H.; Gan, H. Unraveling the Causal Association between Inflammatory Bowel Diseases and Uveitis through Mendelian Randomization Analysis. Sci. Rep. 2025, 15, 5686. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, E.; Glymour, M.M.; Holmes, M.V.; Kang, H.; Morrison, J.; Munafò, M.R.; Palmer, T.; Schooling, C.M.; Wallace, C.; Zhao, Q.; et al. Mendelian Randomization. Nat. Rev. Methods Primers 2022, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Timpson, N.J.; Higgins, J.P.T.; Dimou, N.; Langenberg, C.; et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomisation (STROBE-MR): Explanation and Elaboration. BMJ 2021, 375, n2233. [Google Scholar] [CrossRef]
- Sun, B.B.; Kurki, M.I.; Foley, C.N.; Mechakra, A.; Chen, C.-Y.; Marshall, E.; Wilk, J.B.; Biogen Biobank Team; Chahine, M.; Chevalier, P.; et al. Genetic Associations of Protein-Coding Variants in Human Disease. Nature 2022, 603, 95–102. [Google Scholar] [CrossRef]
- Van Der Plaat, D.A. Mendelian Randomisation Supports Causal Link between Obesity and Asthma. Thorax 2020, 75, 194–195. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.; Li, H.; Zhang, Z.; Zhang, Y.; Xi, L.; Zhang, D.; Deng, C.; Wang, D.; Chen, R.; Chen, G.; et al. Association between Uric Acid and Risk of Venous Thromboembolism in East Asian Populations: A Cohort and Mendelian Randomization Study. Lancet Reg. Health-West. Pac. 2023, 39, 100848. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Yang, W. Exploring the Mechanisms and Relationship between Body Mass Index and Asthma: Findings from Mendelian Randomization. J. Asthma 2025, 62, 248–260. [Google Scholar] [CrossRef]
- Dey, K.K.; Gazal, S.; Van De Geijn, B.; Kim, S.S.; Nasser, J.; Engreitz, J.M.; Price, A.L. SNP-to-Gene Linking Strategies Reveal Contributions of Enhancer-Related and Candidate Master-Regulator Genes to Autoimmune Disease. Cell Genom. 2022, 2, 100145. [Google Scholar] [CrossRef]
- Shastry, B.S. SNPs in Disease Gene Mapping, Medicinal Drug Development and Evolution. J. Hum. Genet. 2007, 52, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Fadason, T.; Farrow, S.; Gokuladhas, S.; Golovina, E.; Nyaga, D.; O’Sullivan, J.M.; Schierding, W. Assigning Function to SNPs: Considerations When Interpreting Genetic Variation. Semin. Cell Dev. Biol. 2022, 121, 135–142. [Google Scholar] [CrossRef]
- Cheng, J.; Novati, G.; Pan, J.; Bycroft, C.; Žemgulytė, A.; Applebaum, T.; Pritzel, A.; Wong, L.H.; Zielinski, M.; Sargeant, T.; et al. Accurate Proteome-Wide Missense Variant Effect Prediction with AlphaMissense. Science 2023, 381, eadg7492. [Google Scholar] [CrossRef] [PubMed]
- Samuelson, D.R.; Haq, S.; Knoell, D.L. Divalent Metal Uptake and the Role of ZIP8 in Host Defense Against Pathogens. Front. Cell Dev. Biol. 2022, 10, 924820. [Google Scholar] [CrossRef]
- Nebert, D.W.; Liu, Z. SLC39A8 Gene Encoding a Metal Ion Transporter: Discovery and Bench to Bedside. Hum. Genom. 2019, 13, 51. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, M.; Karlsson, M.J.; Hober, A.; Svensson, A.-S.; Scheffel, J.; Kotol, D.; Zhong, W.; Tebani, A.; Strandberg, L.; Edfors, F.; et al. The Human Secretome. Sci. Signal. 2019, 12, eaaz0274. [Google Scholar] [CrossRef]
- Besecker, B.; Bao, S.; Bohacova, B.; Papp, A.; Sadee, W.; Knoell, D.L. The Human Zinc Transporter SLC39A8 (Zip8) Is Critical in Zinc-Mediated Cytoprotection in Lung Epithelia. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2008, 294, L1127–L1136. [Google Scholar] [CrossRef]
- Thompson, A.; Cook, J.; Choquet, H.; Jorgenson, E.; Yin, J.; Kinnunen, T.; Barclay, J.; Morris, A.P.; Pirmohamed, M. Functional Validity, Role, and Implications of Heavy Alcohol Consumption Genetic Loci. Sci. Adv. 2020, 6, eaay5034. [Google Scholar] [CrossRef]
- Park, J.H.; Hogrebe, M.; Fobker, M.; Brackmann, R.; Fiedler, B.; Reunert, J.; Rust, S.; Tsiakas, K.; Santer, R.; Grüneberg, M.; et al. SLC39A8 Deficiency: Biochemical Correction and Major Clinical Improvement by Manganese Therapy. Genet. Med. 2018, 20, 259–268. [Google Scholar] [CrossRef]
- Boycott, K.M.; Beaulieu, C.L.; Kernohan, K.D.; Gebril, O.H.; Mhanni, A.; Chudley, A.E.; Redl, D.; Qin, W.; Hampson, S.; Küry, S.; et al. Autosomal-Recessive Intellectual Disability with Cerebellar Atrophy Syndrome Caused by Mutation of the Manganese and Zinc Transporter Gene SLC39A8. Am. J. Hum. Genet. 2015, 97, 886–893. [Google Scholar] [CrossRef]
- Park, J.H.; Hogrebe, M.; Grüneberg, M.; DuChesne, I.; von der Heiden, A.L.; Reunert, J.; Schlingmann, K.P.; Boycott, K.M.; Beaulieu, C.L.; Mhanni, A.A.; et al. SLC39A8 Deficiency: A Disorder of Manganese Transport and Glycosylation. Am. J. Hum. Genet. 2015, 97, 894–903. [Google Scholar] [CrossRef]
- Azimzadeh, J.; Hergert, P.; Delouvée, A.; Euteneuer, U.; Formstecher, E.; Khodjakov, A.; Bornens, M. hPOC5 Is a Centrin-Binding Protein Required for Assembly of Full-Length Centrioles. J. Cell Biol. 2009, 185, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Dantas, T.J.; Daly, O.M.; Conroy, P.C.; Tomas, M.; Wang, Y.; Lalor, P.; Dockery, P.; Ferrando-May, E.; Morrison, C.G. Calcium-Binding Capacity of Centrin2 Is Required for Linear POC5 Assembly but Not for Nucleotide Excision Repair. PLoS ONE 2013, 8, e68487. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-W.; Hsu, W.-B.; Tsai, J.-J.; Tang, C.-J.C.; Tang, T.K. CEP295 Interacts with Microtubules and Is Required for Centriole Elongation. J. Cell Sci. 2016, 129, 2501–2513. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Parent, S.; Mathieu, H.; Zaouter, C.; Molidperee, S.; Bagu, E.T.; Barchi, S.; Villemure, I.; Patten, S.A.; Moldovan, F. Adolescent Idiopathic Scoliosis Associated POC5 Mutation Impairs Cell Cycle, Cilia Length and Centrosome Protein Interactions. PLoS ONE 2019, 14, e0213269. [Google Scholar] [CrossRef] [PubMed]
- Patten, S.A.; Margaritte-Jeannin, P.; Bernard, J.-C.; Alix, E.; Labalme, A.; Besson, A.; Girard, S.L.; Fendri, K.; Fraisse, N.; Biot, B.; et al. Functional Variants of POC5 Identified in Patients with Idiopathic Scoliosis. J. Clin. Investig. 2015, 125, 1124–1128. [Google Scholar] [CrossRef]
- Miranda-Lora, A.L.; Molina-Díaz, M.; Cruz, M.; Sánchez-Urbina, R.; Martínez-Rodríguez, N.L.; López-Martínez, B.; Klünder-Klünder, M. Genetic Polymorphisms Associated with Pediatric-onset Type 2 Diabetes: A Family-based Transmission Disequilibrium Test and Case-control Study. Pediatr. Diabetes 2019, 20, 239–245. [Google Scholar] [CrossRef]
- Young, K.L.; Graff, M.; North, K.E.; Richardson, A.S.; Mohlke, K.L.; Lange, L.A.; Lange, E.M.; Harris, K.M.; Gordon-Larsen, P. Interaction of Smoking and Obesity Susceptibility Loci on Adolescent BMI: The National Longitudinal Study of Adolescent to Adult Health. BMC Genet. 2015, 16, 131. [Google Scholar] [CrossRef]
- Granell, R.; Henderson, A.J.; Evans, D.M.; Smith, G.D.; Ness, A.R.; Lewis, S.; Palmer, T.M.; Sterne, J.A.C. Effects of BMI, Fat Mass, and Lean Mass on Asthma in Childhood: A Mendelian Randomization Study. PLoS Med. 2014, 11, e1001669. [Google Scholar] [CrossRef]
- Palma, G.; Sorice, G.P.; Genchi, V.A.; Giordano, F.; Caccioppoli, C.; D’Oria, R.; Marrano, N.; Biondi, G.; Giorgino, F.; Perrini, S. Adipose Tissue Inflammation and Pulmonary Dysfunction in Obesity. Int. J. Mol. Sci. 2022, 23, 7349. [Google Scholar] [CrossRef] [PubMed]
- Pestel, J.; Blangero, F.; Watson, J.; Pirola, L.; Eljaafari, A. Adipokines in Obesity and Metabolic-Related-Diseases. Biochimie 2023, 212, 48–59. [Google Scholar] [CrossRef]
- Wang, K.; Chen, Z.; Wei, Z.; He, L.; Gong, L. Association between Body Fat Distribution and Asthma in Adults: Results from the Cross-Sectional and Bidirectional Mendelian Randomization Study. Front. Nutr. 2024, 11, 1432973. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.-S.; Kim, S.-S.; Lee, S.-H.; Kim, M.-H.; Cho, Y.-J.; Park, H.-W. Fat Mass Index and Airway Hyperresponsiveness in Korean Adults. Postgrad. Med. 2023, 135, 480–485. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity Is Associated with Macrophage Accumulation in Adipose Tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Ying, Y.; Zhang, C.; Wu, S.; Wang, S.; Lian, J.; Lin, Y.; Guan, H.; Cai, D. Health Implications Associated with Fat-Free Mass: A Phenome-Wide Mendelian Randomization Study. Cardiorenal Med. 2025, 15, 295–308. [Google Scholar] [CrossRef]
- Yan, P.; Yao, J.; Ke, B.; Fang, X. Mendelian Randomization Analysis Reveals Higher Whole Body Water Mass May Increase Risk of Bacterial Infections. BMC Med. Genom. 2024, 17, 183. [Google Scholar] [CrossRef]
- Lu, T.; Nakanishi, T.; Yoshiji, S.; Butler-Laporte, G.; Greenwood, C.M.T.; Richards, J.B. Dose-Dependent Association of Alcohol Consumption With Obesity and Type 2 Diabetes: Mendelian Randomization Analyses. J. Clin. Endocrinol. Metab. 2023, 108, 3320–3329. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, E.L.; DiNicolantonio, J.J.; O’Keefe, J.H.; Lavie, C.J. Alcohol and CV Health: Jekyll and Hyde J-Curves. Prog. Cardiovasc. Dis. 2018, 61, 68–75. [Google Scholar] [CrossRef]
- O’Keefe, J.H.; Bybee, K.A.; Lavie, C.J. Alcohol and Cardiovascular Health. J. Am. Coll. Cardiol. 2007, 50, 1009–1014. [Google Scholar] [CrossRef]
- Poli, A.; Marangoni, F.; Avogaro, A.; Barba, G.; Bellentani, S.; Bucci, M.; Cambieri, R.; Catapano, A.L.; Costanzo, S.; Cricelli, C.; et al. Moderate Alcohol Use and Health: A Consensus Document. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 487–504. [Google Scholar] [CrossRef] [PubMed]
- Lomholt, F.K.; Nielsen, S.F.; Nordestgaard, B.G. High Alcohol Consumption Causes High IgE Levels but Not High Risk of Allergic Disease. J. Allergy Clin. Immunol. 2016, 138, 1404–1413.e13. [Google Scholar] [CrossRef] [PubMed]
- Skaaby, T.; Kilpeläinen, T.O.; Taylor, A.E.; Mahendran, Y.; Wong, A.; Ahluwalia, T.S.; Paternoster, L.; Trompet, S.; Stott, D.J.; Flexeder, C.; et al. Association of Alcohol Consumption with Allergic Disease and Asthma: A Multi-centre Mendelian Randomization Analysis. Addiction 2019, 114, 216–225. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zheng, L.; Chen, Z.; Wen, J.; Qin, F.; Mo, H. The Causal Association of Smoking, Alcohol Intake, and Coffee Intake with the Risk of Bacterial Pneumonia: A Mendelian Randomization Study. Medicine 2024, 103, e40702. [Google Scholar] [CrossRef]
- Larsson, S.C.; Woolf, B.; Gill, D. Appraisal of the Causal Effect of Plasma Caffeine on Adiposity, Type 2 Diabetes, and Cardiovascular Disease: Two Sample Mendelian Randomisation Study. BMJ Med. 2023, 2, e000335. [Google Scholar] [CrossRef]
- Lin, F.; Zhu, Y.; Liang, H.; Li, D.; Jing, D.; Liu, H.; Pan, P.; Zhang, Y. Association of Coffee and Tea Consumption with the Risk of Asthma: A Prospective Cohort Study from the UK Biobank. Nutrients 2022, 14, 4039. [Google Scholar] [CrossRef]
- Wee, J.H.; Yoo, D.M.; Byun, S.H.; Song, C.M.; Lee, H.-J.; Park, B.; Park, M.W.; Choi, H.G. Analysis of the Relationship between Asthma and Coffee/Green Tea/Soda Intake. Int. J. Environ. Res. Public Health 2020, 17, 7471. [Google Scholar] [CrossRef]
- Richmond, R.C.; Davey Smith, G. Mendelian Randomization: Concepts and Scope. Cold Spring Harb. Perspect. Med. 2022, 12, a040501. [Google Scholar] [CrossRef]
- Durinck, S.; Spellman, P.T.; Birney, E.; Huber, W. Mapping Identifiers for the Integration of Genomic Datasets with the R/Bioconductor Package biomaRt. Nat. Protoc. 2009, 4, 1184–1191. [Google Scholar] [CrossRef]
- Oscanoa, J.; Sivapalan, L.; Gadaleta, E.; Dayem Ullah, A.Z.; Lemoine, N.R.; Chelala, C. SNPnexus: A Web Server for Functional Annotation of Human Genome Sequence Variation (2020 Update). Nucleic Acids Res. 2020, 48, W185–W192. [Google Scholar] [CrossRef]
- Rainer, J.; Gatto, L.; Weichenberger, C.X. Ensembldb: An R Package to Create and Use Ensembl-Based Annotation Resources. Bioinformatics 2019, 35, 3151–3153. [Google Scholar] [CrossRef] [PubMed]
- Adzhubei, I.; Jordan, D.M.; Sunyaev, S.R. Predicting Functional Effect of Human Missense Mutations Using PolyPhen-2. Curr. Protoc. Hum. Genet. 2013, 76, 7.20.1–7.20.41. [Google Scholar] [CrossRef]
- Hartwig, F.P.; Davey Smith, G.; Bowden, J. Robust Inference in Summary Data Mendelian Randomization via the Zero Modal Pleiotropy Assumption. Int. J. Epidemiol. 2017, 46, 1985–1998. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R Package for Comparing Biological Themes among Gene Clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Eils, R.; Schlesner, M. Complex Heatmaps Reveal Patterns and Correlations in Multidimensional Genomic Data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef] [PubMed]

| GWAS ID | Year | Trait | Consortium | Sample Size | SNPs |
|---|---|---|---|---|---|
| ukb-b-19953 | 2018 | Body mass index | MRC-IEU | 461,460 | 9,851,867 |
| ukb-b-8909 | 2018 | Body fat percentage | MRC-IEU | 454,633 | 9,851,867 |
| ukb-b-13354 | 2018 | Whole-body fat-free mass | MRC-IEU | 454,850 | 9,851,867 |
| ukb-b-14540 | 2018 | Whole-body water mass | MRC-IEU | 454,888 | 9,851,867 |
| ukb-b-5237 | 2018 | Coffee intake | MRC-IEU | 428,860 | 9,851,867 |
| ukb-b-5779 | 2018 | Alcohol intake frequency | MRC-IEU | 462,346 | 9,851,867 |
| finn-b-J10_ASTHMA_MAIN_EXMORE | 2021 | Asthma (only as main-diagnosis), excluding more control | Cases = 17,438 Controls = 131,051 | 16,380,048 | |
| finn-b-K11_CD_NOUC | 2021 | Crohn disease (strict definition, all UC cases excluded) | Cases = 657 Controls = 210,300 | 16,380,454 |
| Exposures | ID | Outcomes | SNPs | MR Analysis | ||||
|---|---|---|---|---|---|---|---|---|
| Method | b | se | p Value | OR (95% CI) | ||||
| BFP | ukb-b-8909 | COPD | 36 | MR Egger | 0.37 | 0.71 | 0.609 | 1.44 (0.36, 5.76) |
| weighted median | 0.40 | 0.24 | 0.094 | 1.49 (0.94, 2.37) | ||||
| Inverse variance weighted | 0.54 | 0.17 | 0.002 | 1.72 (1.23, 2.41) | ||||
| simple mode | 0.80 | 0.51 | 0.125 | 2.22 (0.82, 6.0) | ||||
| weighted mode | 0.29 | 0.40 | 0.478 | 1.34 (0.61, 2.95) | ||||
| BFP | ukb-b-8909 | Asthma | 36 | MR Egger | 0.66 | 0.56 | 0.248 | 1.93 (0.65, 5.78) |
| weighted median | 0.54 | 0.17 | 0.002 | 1.72 (1.23, 2.41) | ||||
| Inverse variance weighted | 0.47 | 0.14 | 0.001 | 1.60 (1.23, 2.09) | ||||
| simple mode | 0.11 | 0.43 | 0.807 | 1.11 (0.48, 2.56) | ||||
| weighted mode | 0.84 | 0.31 | 0.011 | 2.32 (1.25, 4.29) | ||||
| BMI | ukb-b-19953 | COPD | 38 | MR Egger | 0.17 | 0.31 | 0.585 | 1.18 (0.65, 2.16) |
| weighted median | 0.28 | 0.17 | 0.109 | 1.32 (0.94, 1.85) | ||||
| Inverse variance weighted | 0.44 | 0.12 | 0.000 | 1.56 (1.23, 1.98) | ||||
| simple mode | 0.19 | 0.37 | 0.621 | 1.20 (0.58, 2.49) | ||||
| weighted mode | 0.10 | 0.22 | 0.643 | 1.11 (0.72, 1.70) | ||||
| BMI | ukb-b-19953 | Asthma | 42 | MR Egger | 0.41 | 0.24 | 0.095 | 1.50 (0.94, 2.40) |
| weighted median | 0.52 | 0.11 | 0.000 | 1.68 (1.35, 2.10) | ||||
| Inverse variance weighted | 0.43 | 0.09 | 0.000 | 1.54 (1.29, 1.84) | ||||
| simple mode | 0.60 | 0.27 | 0.033 | 1.83 (1.07, 3.11) | ||||
| weighted mode | 0.50 | 0.15 | 0.002 | 1.65 (1.23, 2.19) | ||||
| FFM | ukb-b-13354 | COPD | 98 | MR Egger | 0.33 | 0.39 | 0.398 | 1.39 (0.65, 2.96) |
| weighted median | 0.25 | 0.17 | 0.129 | 1.29 (0.93, 1.78) | ||||
| Inverse variance weighted | 0.24 | 0.13 | 0.073 | 1.27 (0.98, 1.64) | ||||
| simple mode | 0.48 | 0.43 | 0.269 | 1.62 (0.69, 3.80) | ||||
| weighted mode | 0.41 | 0.36 | 0.255 | 1.51 (0.75, 3.04) | ||||
| FFM | ukb-b-13354 | Asthma | 99 | MR Egger | 0.59 | 0.26 | 0.023 | 1.81 (1.10, 2.98) |
| weighted median | 0.12 | 0.11 | 0.273 | 1.13 (0.91, 1.40) | ||||
| Inverse variance weighted | 0.19 | 0.09 | 0.032 | 1.21 (1.02, 1.44) | ||||
| simple mode | −0.32 | 0.35 | 0.365 | 0.73 (0.37, 1.44) | ||||
| weighted mode | 0.61 | 0.29 | 0.037 | 1.84 (1.05, 3.23) | ||||
| BWM | ukb-b-14540 | COPD | 97 | MR Egger | 0.33 | 0.39 | 0.393 | 1.40 (0.65, 2.99) |
| weighted median | 0.22 | 0.17 | 0.195 | 1.25 (0.89, 1.75) | ||||
| Inverse variance weighted | 0.24 | 0.13 | 0.071 | 1.27 (0.98, 1.65) | ||||
| simple mode | 0.44 | 0.43 | 0.311 | 1.55 (0.67, 3.58) | ||||
| weighted mode | 0.36 | 0.37 | 0.328 | 1.44 (0.70, 2.97) | ||||
| BWM | ukb-b-14540 | Asthma | 98 | MR Egger | 0.71 | 0.25 | 0.006 | 2.04 (1.24, 3.34) |
| weighted median | 0.11 | 0.12 | 0.370 | 1.11 (0.88, 1.40) | ||||
| Inverse variance weighted | 0.16 | 0.09 | 0.068 | 1.18 (0.99, 1.40) | ||||
| simple mode | −0.32 | 0.31 | 0.317 | 0.73 (0.40, 1.35) | ||||
| weighted mode | 0.57 | 0.28 | 0.043 | 1.77 (1.03, 3.04) | ||||
| Alcohol | ukb-b-5779 | COPD | 87 | MR Egger | 0.47 | 0.35 | 0.178 | 1.60 (0.81, 3.14) |
| weighted median | 0.18 | 0.16 | 0.260 | 1.20 (0.88, 1.64) | ||||
| Inverse variance weighted | 0.30 | 0.11 | 0.009 | 1.34 (1.08, 1.68) | ||||
| simple mode | −0.30 | 0.42 | 0.478 | 0.74 (0.33, 1.69) | ||||
| weighted mode | −0.27 | 0.36 | 0.467 | 0.77 (0.38, 1.56) | ||||
| Alcohol | ukb-b-5779 | Asthma | 86 | MR Egger | 0.47 | 0.26 | 0.073 | 1.60 (0.96, 2.65) |
| weighted median | 0.22 | 0.11 | 0.040 | 1.25 (1.01, 1.54) | ||||
| Inverse variance weighted | 0.17 | 0.08 | 0.039 | 1.19 (1.01, 1.40) | ||||
| simple mode | 0.22 | 0.27 | 0.422 | 1.24 (0.74, 2.09) | ||||
| weighted mode | 0.29 | 0.18 | 0.113 | 1.33 (0.94, 1.90) | ||||
| Coffee | ukb-b-5237 | COPD | 36 | MR Egger | −0.14 | 0.48 | 0.778 | 0.87 (0.34, 2.25) |
| weighted median | 0.00 | 0.34 | 0.996 | 1.00 (0.51, 1.97) | ||||
| Inverse variance weighted | 0.16 | 0.25 | 0.506 | 1.18 (0.73, 1.91) | ||||
| simple mode | −0.24 | 0.68 | 0.724 | 0.79 (0.21, 2.95) | ||||
| weighted mode | −0.03 | 0.35 | 0.941 | 0.98 (0.50, 1.92) | ||||
| Coffee | ukb-b-5237 | Asthma | 36 | MR Egger | −0.23 | 0.35 | 0.525 | 0.80 (0.40, 1.59) |
| weighted median | −0.19 | 0.23 | 0.411 | 0.83 (0.53, 1.30) | ||||
| Inverse variance weighted | −0.25 | 0.18 | 0.160 | 0.78 (0.55, 1.11) | ||||
| simple mode | −0.34 | 0.47 | 0.473 | 0.71 (0.28, 1.80) | ||||
| weighted mode | −0.33 | 0.23 | 0.164 | 0.72 (0.46, 1.13) | ||||
| Exposures | ID | Outcomes | SNPs | Sensitivity Analysis | ||||
|---|---|---|---|---|---|---|---|---|
| Heterogeneity Test | MR Egger Pleiotropy Test | |||||||
| Cochrane Q | p-Value | b | se | p-Value | ||||
| BFP | ukb-b-8909 | COPD | 36 | 38.96 | 0.296 | 0.00 | 0.02 | 0.799 |
| Asthma | 36 | 55.04 | 0.017 | −0.00 | 0.01 | 0.732 | ||
| BMI | ukb-b-19953 | COPD | 38 | 44.76 | 0.178 | 0.01 | 0.01 | 0.334 |
| Asthma | 42 | 67.97 | 0.005 | 0.00 | 0.01 | 0.919 | ||
| FFM | ukb-b-13354 | COPD | 98 | 152.95 | 0.000 | −0.00 | 0.01 | 0.804 |
| Asthma | 99 | 156.01 | 0.000 | −0.01 | 0.01 | 0.097 | ||
| BWM | ukb-b-14540 | COPD | 97 | 153.20 | 0.000 | −0.00 | 0.01 | 0.799 |
| Asthma | 98 | 154.31 | 0.000 | −0.01 | 0.01 | 0.022 | ||
| Alcohol | ukb-b-5779 | COPD | 87 | 116.93 | 0.015 | −0.00 | 0.01 | 0.597 |
| Asthma | 86 | 131.24 | 0.001 | −0.01 | 0.01 | 0.228 | ||
| Coffee | ukb-b-5237 | COPD | 36 | 40.04 | 0.256 | 0.01 | 0.01 | 0.471 |
| Asthma | 36 | 46.90 | 0.086 | 0.00 | 0.01 | 0.933 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apalowo, O.E.; Walt, H.K.; Alaba, T.E.; Komakech, J.J.; Schilling, M.W. Exploring the Potential Roles of SLC39A8 and POC5 Missense Variants in the Association Between Body Composition, Beverage Consumption, and Chronic Lung Diseases: A Two-Sample Mendelian Randomization Study. Int. J. Mol. Sci. 2025, 26, 7799. https://doi.org/10.3390/ijms26167799
Apalowo OE, Walt HK, Alaba TE, Komakech JJ, Schilling MW. Exploring the Potential Roles of SLC39A8 and POC5 Missense Variants in the Association Between Body Composition, Beverage Consumption, and Chronic Lung Diseases: A Two-Sample Mendelian Randomization Study. International Journal of Molecular Sciences. 2025; 26(16):7799. https://doi.org/10.3390/ijms26167799
Chicago/Turabian StyleApalowo, Oladayo E., Hunter K. Walt, Tolu E. Alaba, Joel J. Komakech, and Mark W. Schilling. 2025. "Exploring the Potential Roles of SLC39A8 and POC5 Missense Variants in the Association Between Body Composition, Beverage Consumption, and Chronic Lung Diseases: A Two-Sample Mendelian Randomization Study" International Journal of Molecular Sciences 26, no. 16: 7799. https://doi.org/10.3390/ijms26167799
APA StyleApalowo, O. E., Walt, H. K., Alaba, T. E., Komakech, J. J., & Schilling, M. W. (2025). Exploring the Potential Roles of SLC39A8 and POC5 Missense Variants in the Association Between Body Composition, Beverage Consumption, and Chronic Lung Diseases: A Two-Sample Mendelian Randomization Study. International Journal of Molecular Sciences, 26(16), 7799. https://doi.org/10.3390/ijms26167799






