External Glands of Nepenthes Traps: Structure and Potential Function
Abstract
1. Introduction
2. Results
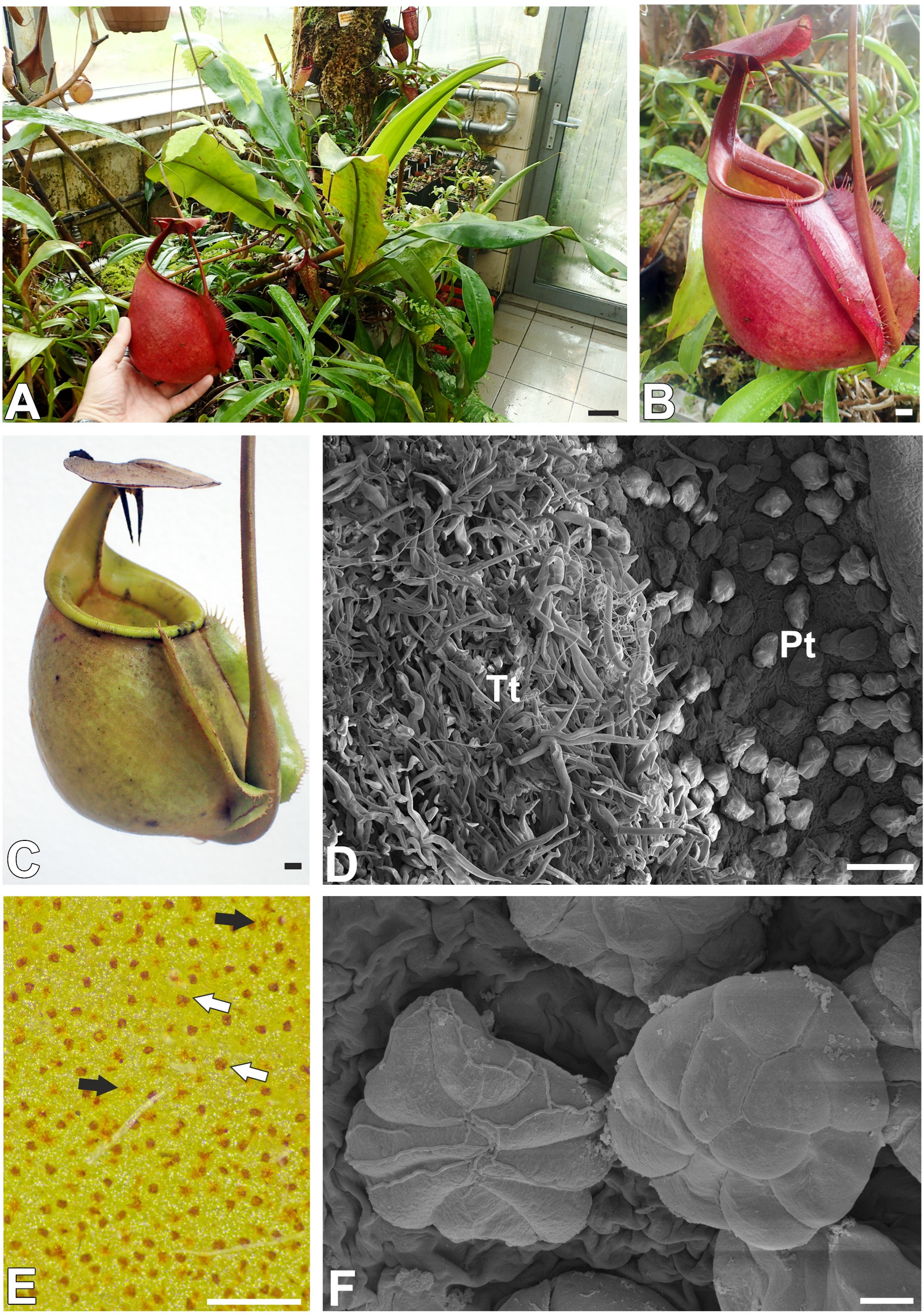
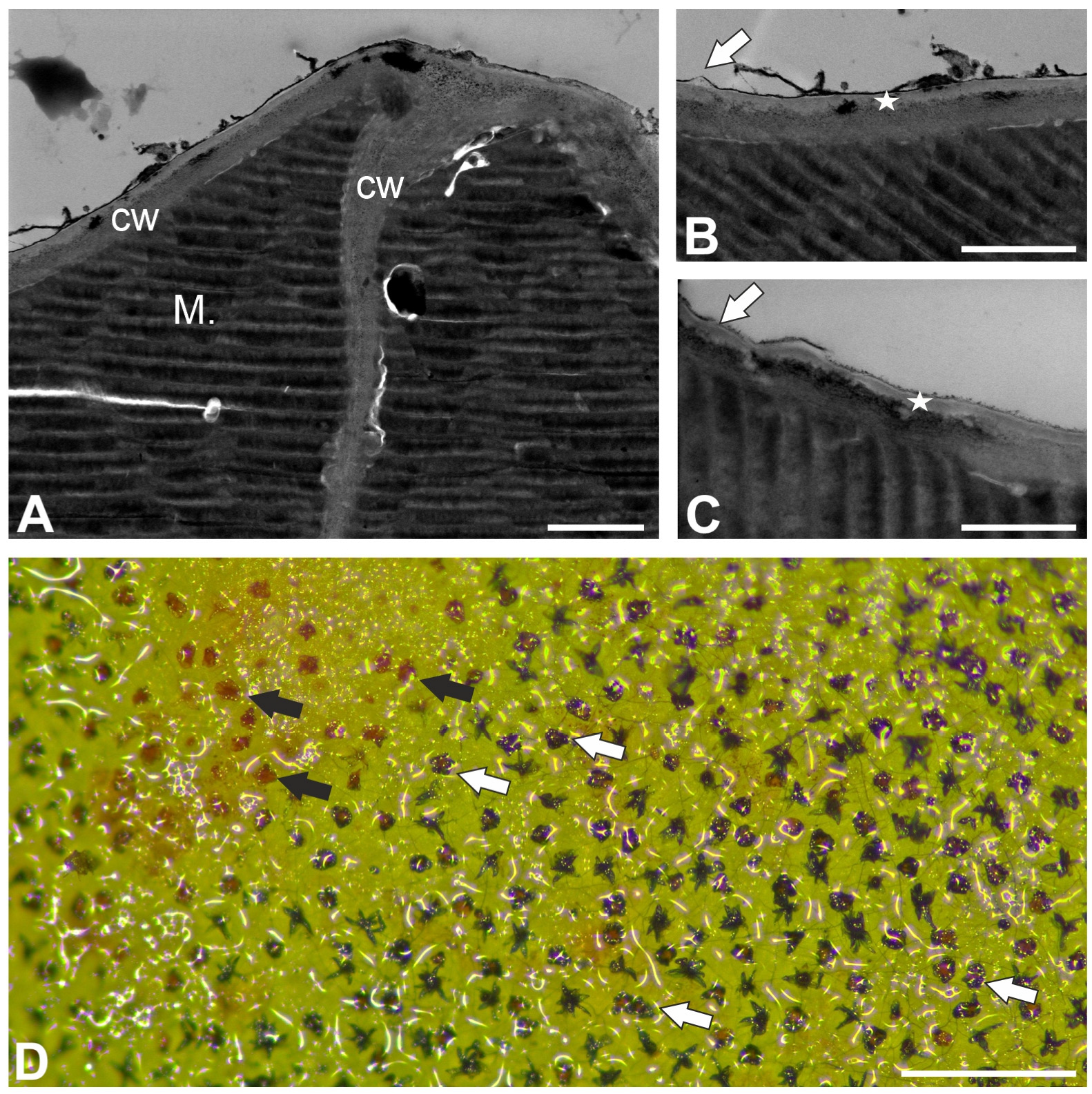
2.1. Trichome Structure (Nepenthes bicalcarata)
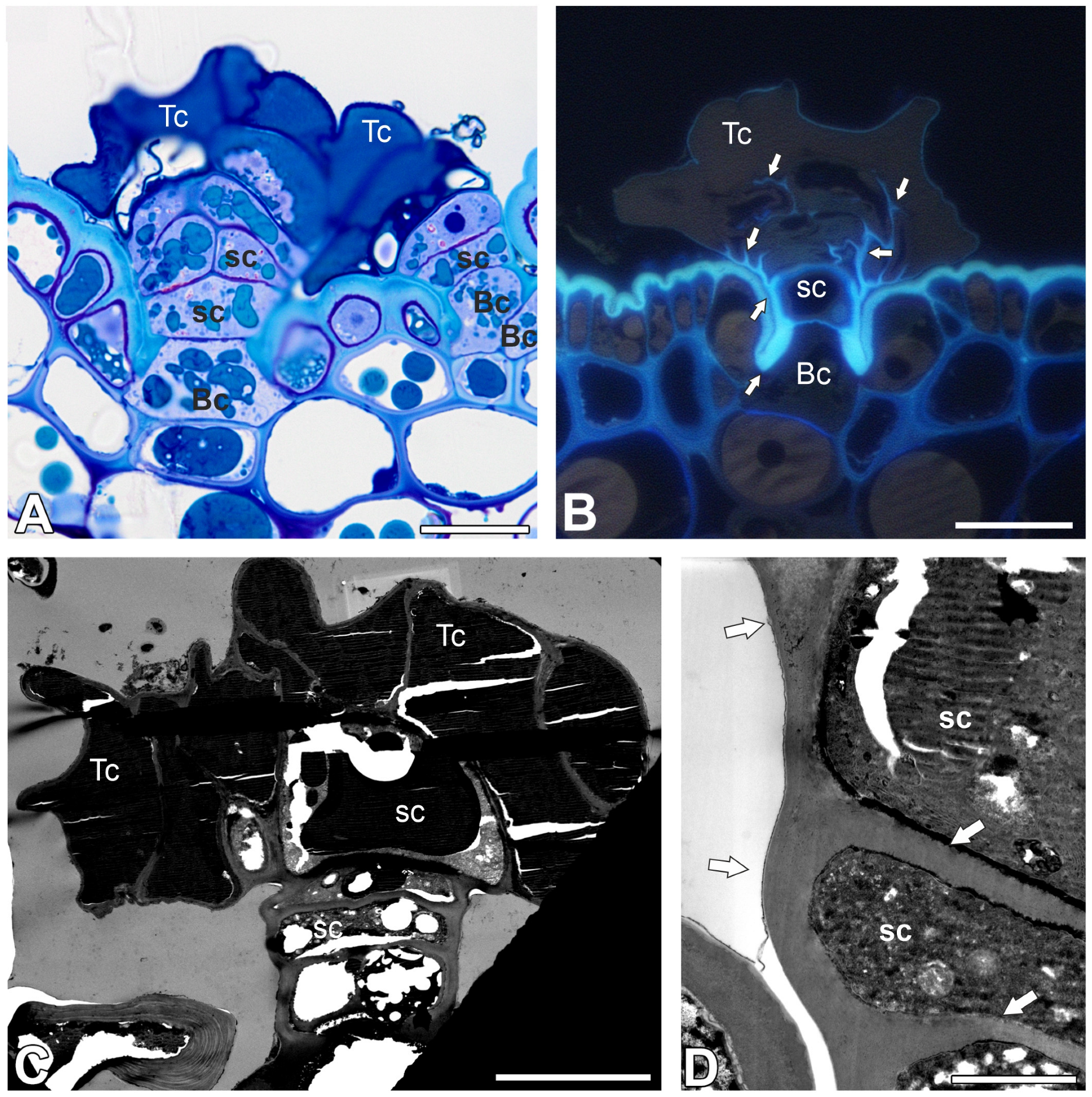
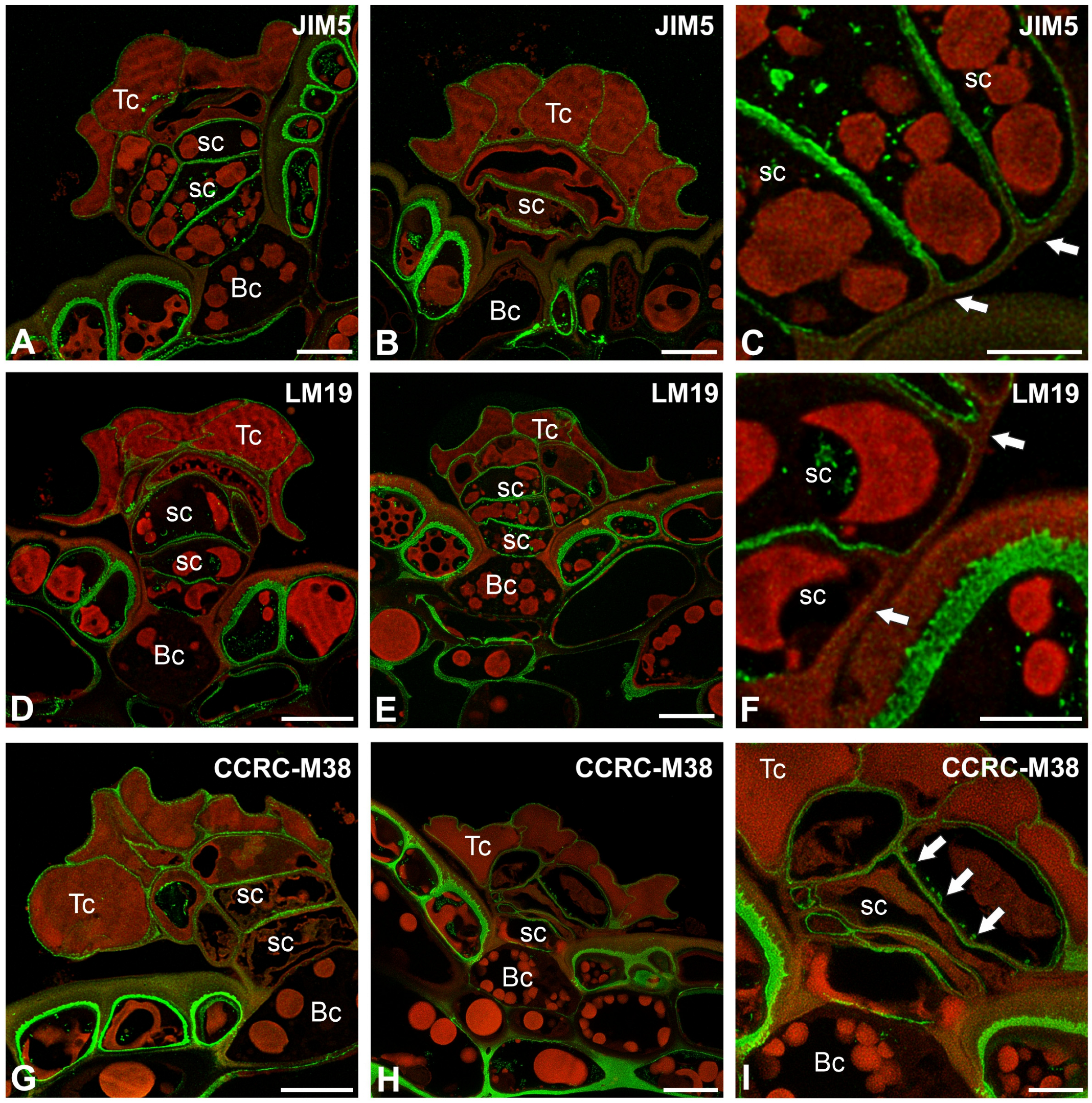
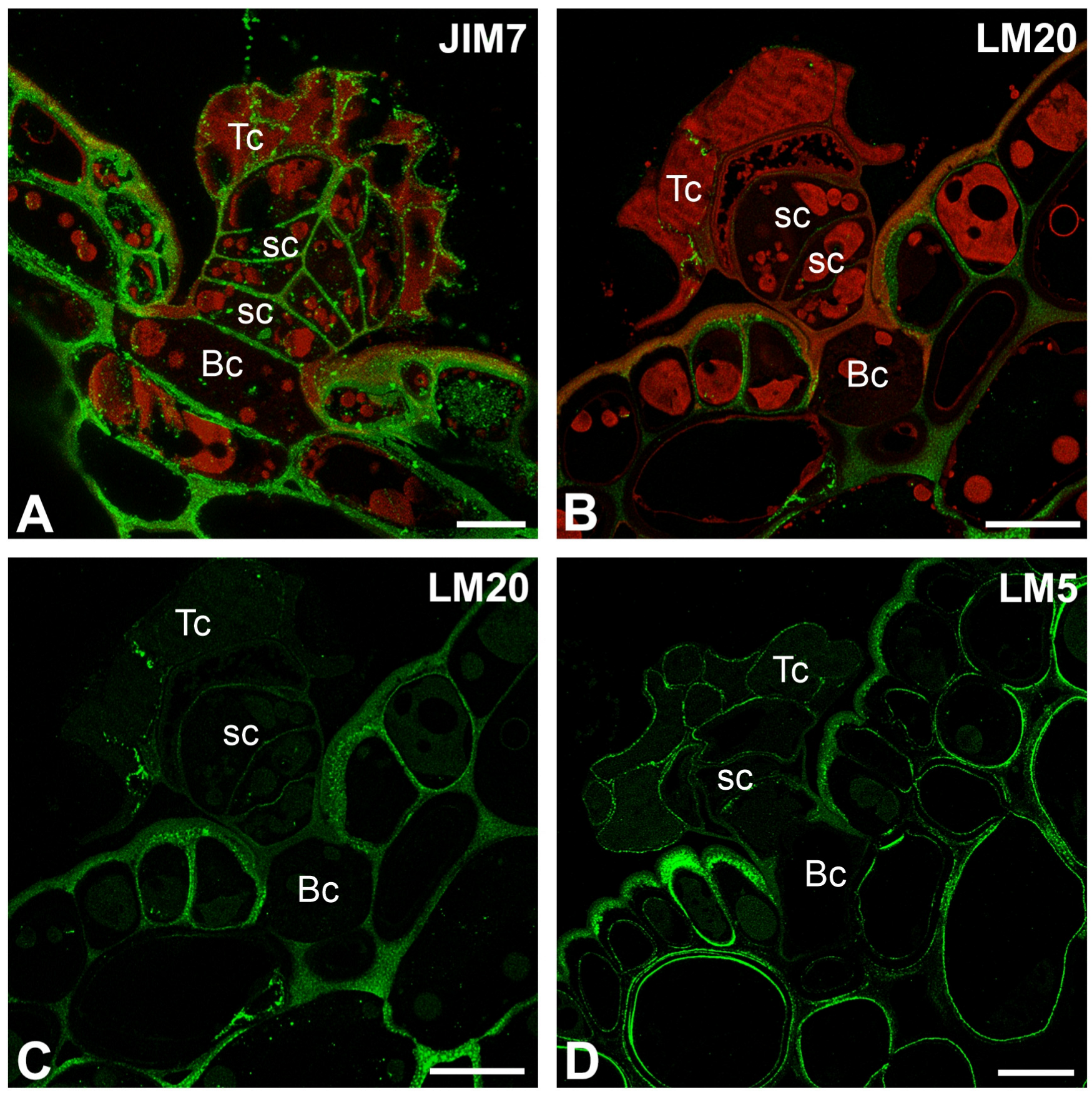
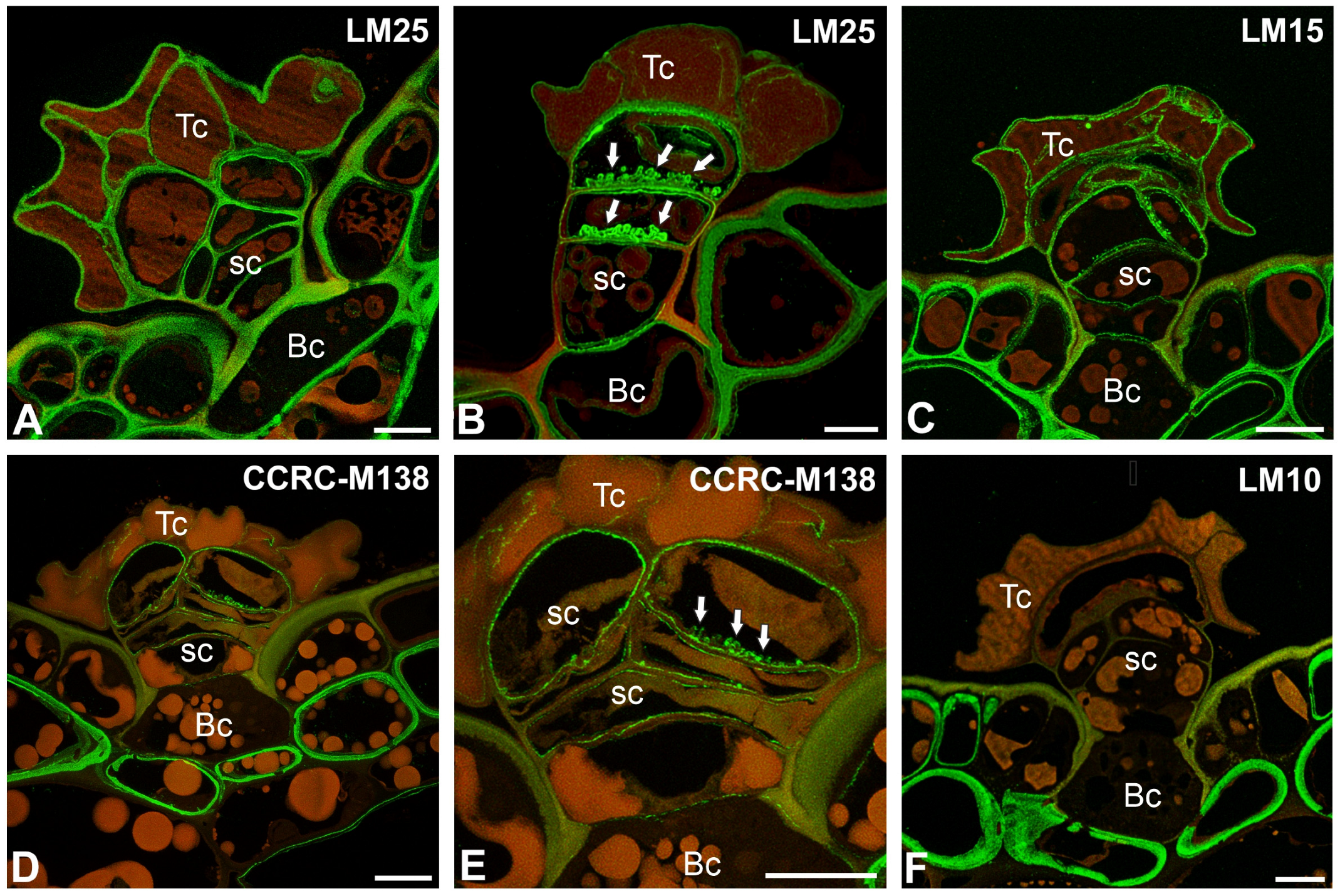
2.2. Pectic Homogalacturonan and Galactan Distribution
2.3. Hemicellulose Distribution
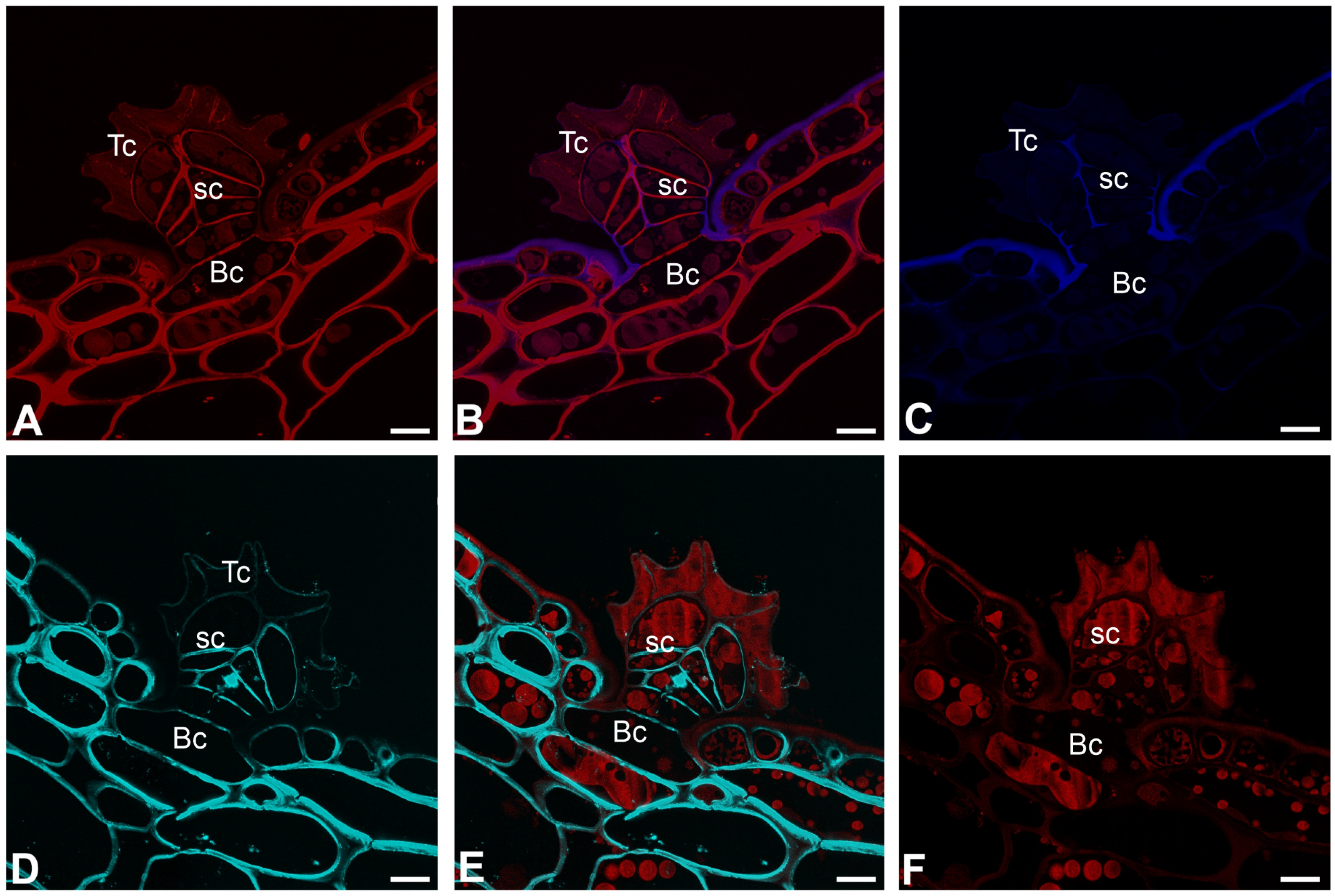
2.4. Histochemistry Staining (Dye Staining)
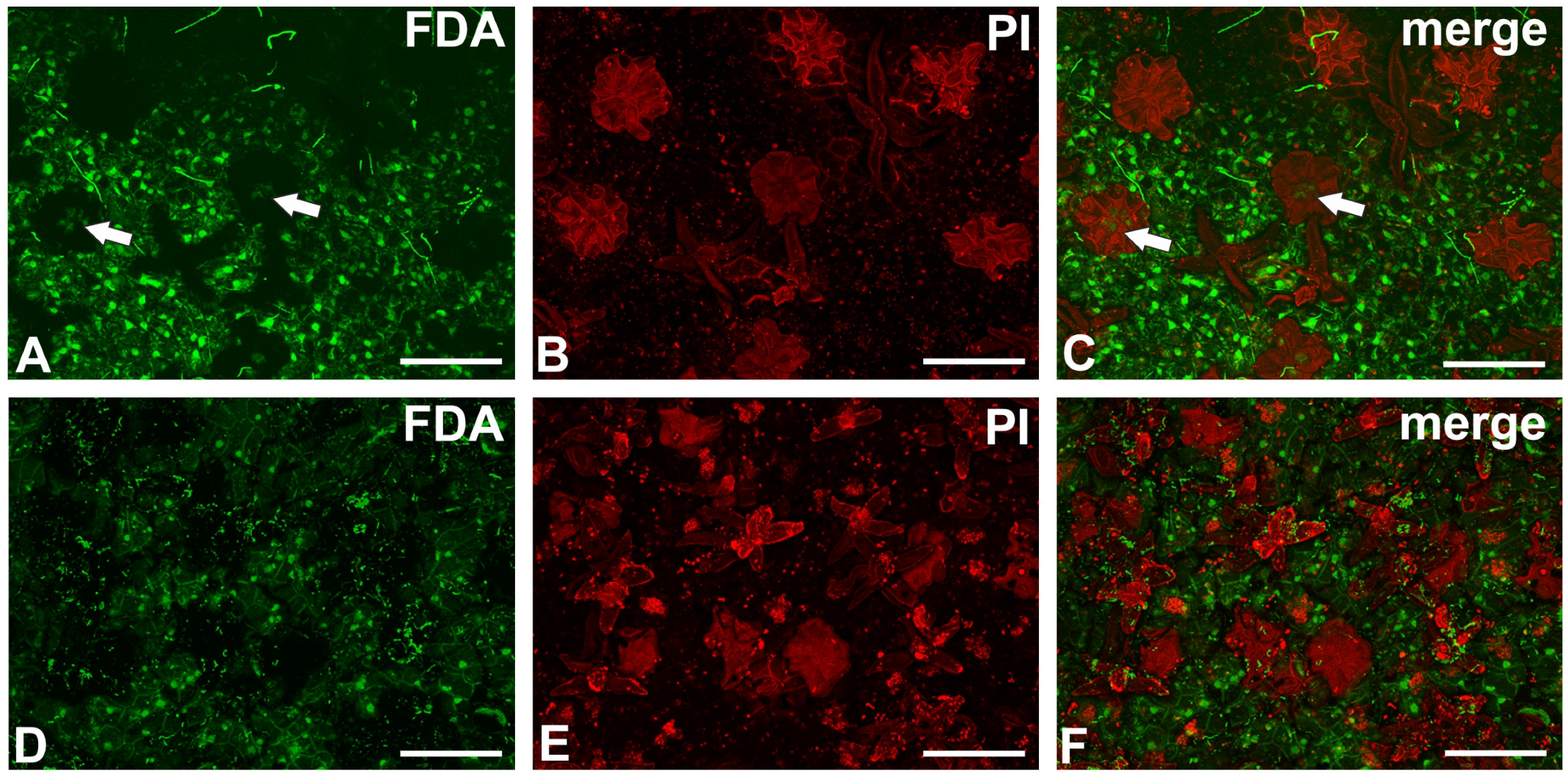
2.5. The Cell Viability Test
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Histological and Immunochemical Analysis
4.3. Scanning Transmission Electron Microscopy (STEM)
4.4. Scanning Electron Microscopy
4.5. Head Cell Viability Test
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Juniper, B.E.; Robbins, R.J.; Joel, D.M. The Carnivorous Plants; Academic Press: London, UK, 1989. [Google Scholar]
- Adamec, L. Mineral nutrition of carnivorous plants: A review. Bot. Rev. 1997, 63, 273–299. [Google Scholar] [CrossRef]
- Król, E.; Płachno, B.J.; Adamec, L.; Stolarz, M.; Dziubińska, H.; Trębacz, K. Quite a few reasons for calling carnivores ‘the most wonderful plants in the world’. Ann. Bot. 2012, 109, 47–64. [Google Scholar]
- Adamec, L. Foliar mineral nutrient uptake in carnivorous plants: What do we know and what should we know? Front. Plant Sci. 2013, 4, 10. [Google Scholar] [CrossRef]
- Ellison, A.M.; Adamec, L. Carnivorous Plants: Physiology, Ecology, and Evolution; Oxford University Press: Oxford, UK, 2018; 510p. [Google Scholar]
- Lloyd, F.E. The Carnivorous Plants; Ronald Press: New York, NY, USA, 1942. [Google Scholar]
- Fleischmann, A.A.; Schlauer, J.; Smith, S.A.; Givnish, T.J. Evolution of carnivory in angiosperms. In Carnivorous Plants: Physiology, Ecology, and Evolution; Ellison, A., Adamec, L., Eds.; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Fleck, S.J.; Jobson, R.W. Molecular Phylogenomics Reveals the Deep Evolutionary History of Carnivory across Land Plants. Plants 2023, 12, 3356. [Google Scholar] [CrossRef]
- McPherson, S. Pitcher Plants of the Old World; Redfern Natural History Productions: Dorset, UK, 2009; Volume 1. [Google Scholar]
- McPherson, S. Nepenthes: The Tropical Pitcher Plants; Redfern Natural History Productions: Dorset, UK, 2023; Volume 1–3. [Google Scholar]
- Miranda, V.F.O.; Silva, S.R.; Reut, M.S.; Dolsan, H.; Stolarczyk, P.; Rutishauser, R.; Płachno, B.J. A Historical Perspective of Bladderworts (Utricularia): Traps, Carnivory and Body Architecture. Plants 2021, 10, 2656. [Google Scholar] [CrossRef]
- Dančák, M.; Majeský, Ľ.; Čermák, V.; Golos, M.R.; Płachno, B.J.; Tjiasmanto, W. First record of functional underground traps in a pitcher plant: Nepenthes pudica (Nepenthaceae), a new species from North Kalimantan, Borneo. PhytoKeys 2022, 201, 77. [Google Scholar] [CrossRef]
- Golos, M.R.; François Sockhom, F.M.; Wistuba, A.; Lim, G.; McPherson, S.R.; Robinson, A.S. Nepenthes limiana (Nepenthaceae), a new pitcher plant from the northern Titiwangsa Range of Peninsular Malaysia. Carniv. Plant Newsl. 2023, 52, 128–153. [Google Scholar] [CrossRef]
- Damit, A.; Yusof, N.A.M.; Jumian, J.; Clarke, C.; Robinson, A.S. Sabah’s hidden giant: Nepenthes pongoides (Nepenthaceae), a micro-endemic tropical pitcher plant from northern Borneo. Aust. J. Bot. 2024, 72, BT24050. [Google Scholar] [CrossRef]
- Lagunday, N.E.; Melbert, J.G.; Baul, R.P.; Ansihagan Amoroso, V.B. Nepenthes higaonon (Nepenthaceae), A New Species of Pitcher Plant from the northern Mindanao, Philippines. Phytotaxa 2025, 689, 285–290. [Google Scholar] [CrossRef]
- Mey, F.S.; Golos, M.; Lim, G.; Wistuba, A.; Hagger, B.; Robinson, A. Hiding in plain sight: Nepenthes batik (Nepenthaceae), an overlooked tropical pitcher plant from Fraser’s Hill, Peninsular Malaysia. Telopea 2025, 29, 15–36. [Google Scholar]
- Moran, J.A.; Clarke, C.M. The carnivorous syndrome in Nepenthes pitcher plants: Current state of knowledge and potential future directions. Plant Signal. Behav. 2010, 5, 644–648. [Google Scholar] [CrossRef]
- Darnowski, D.; Bauer, U.; Méndez, M.; Horner, J.; Płachno, B. Prey selection and specialization by carnivorous plants. In Carnivorous Plants: Physiology, Ecology, and Evolution; Ellison, A., Adamec, L., Eds.; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Moran, J.A. Pitcher dimorphism, prey composition and the mechanisms of prey attraction in the pitcher plant Nepenthes rafflesiana in Borneo. J. Ecol. 1996, 84, 515–525. [Google Scholar] [CrossRef]
- Benz, M.J.; Gorb, E.V.; Gorb, S.N. Diversity of the slippery zone microstructure in pitchers of nine carnivorous Nepenthes taxa. Arthropod-Plant Interact. 2012, 6, 147–158. [Google Scholar] [CrossRef]
- Gaume, L.; Gorb, S.; Rowe, N. Function of epidermal surfaces in the trapping efficiency of Nepenthes alata pitchers. New Phytol. 2002, 156, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Bohn, H.F.; Federle, W. Insect aquaplaning: Nepenthes pitcher plants capture prey with the peristome, a fully wettable water-lubricated anisotropic surface. Proc. Natl. Acad. Sci. USA 2004, 101, 14138–14143. [Google Scholar] [CrossRef]
- Bauer, U.; Bohn, H.F.; Federle, W. Harmless nectar source or deadly trap: Nepenthes pitchers are activated by rain, condensation and nectar. Proc. R. Soc. B Biol. Sci. 2008, 275, 259–265. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, P.; Zhang, L.; Liu, H.; Jiang, Y.; Zhang, D.; Jiang, L. Continuous directional water transport on the peristome surface of Nepenthes alata. Nature 2016, 532, 85–89. [Google Scholar] [CrossRef]
- Moulton, D.E.; Oliveri, H.; Goriely, A.; Thorogood, C.J. Mechanics reveals the role of peristome geometry in prey capture in carnivorous pitcher plants (Nepenthes). Proc. Natl. Acad. Sci. USA 2023, 120, e2306268120. [Google Scholar] [CrossRef]
- Lessware, O.; Mantell, J.; Bauer, U. Carnivorous Nepenthes pitcher plants combine common developmental processes to make a complex epidermal trapping surface. Ann. Bot. 2025, 135, 643–654. [Google Scholar] [CrossRef]
- Gorb, E.; Haas, K.; Henrich, A.; Enders, S.; Barbakadze, N.; Gorb, S. Composite structure of the crystalline epicuticular wax layer of the slippery zone in the pitchers of the carnivorous plant Nepenthes alata and its effect on insect attachment. J. Exp. Bol. 2005, 208, 4651–4662. [Google Scholar] [CrossRef] [PubMed]
- Scholz, I.; Bückins, M.; Dolge, L.; Erlinghagen, T.; Weth, A.; Hischen, F.; Mayer, J.; Hoffmann, S.; Riederer, M.; Riedel, M.; et al. Slippery surfaces of pitcher plants: Nepenthes wax crystals minimize insect attachment via microscopic surface roughness. J. Exp. Biol. 2010, 213, 1115–1125. [Google Scholar] [CrossRef]
- Bonhomme, V.; Pelloux-Prayer, H.; Jousselin, E.; Forterre, Y.; Labat, J.; Gaume, L. Slippery or sticky? Functional diversity in the trapping strategy of Nepenthes carnivorous plants. New Phytol. 2011, 191, 545–554. [Google Scholar] [CrossRef]
- Gorb, E.V.; Baum, M.J.; Gorb, S.N. Development and regeneration ability of the wax coverage in Nepenthes alata pitchers: A cryo-SEM approach. Sci. Rep. 2013, 3, 3078. [Google Scholar] [CrossRef][Green Version]
- Gorb, E.V.; Purtov, J.; Gorb, S.N. Adhesion force measurements on the two wax layers of the waxy zone in Nepenthes alata pitchers. Sci. Rep. 2014, 4, 5154. [Google Scholar] [CrossRef]
- Gorb, E.V.; Gorb, S.N. Chapter 8 Functional Surfaces in the Pitcher of the Carnivorous Plant Nepenthes alata: A Cryo-Sem Approach. In Functional Surfaces in Biology; Gorb, S.N., Ed.; Springer: Dordrecht, The Netherlands, 2009; Volume 2. [Google Scholar]
- Wang, L.; Zhang, S.; Li, S.; Yan, S.; Dong, S. Inner surface of Nepenthes slippery zone: Ratchet effect of lunate cells causes anisotropic superhydrophobicity. R. Soc. Open Sci. 2020, 7, 200066. [Google Scholar] [CrossRef]
- Owen, T.P., Jr.; Lennon, K.A.; Santo, M.J.; Anderson, A.N. Pathways for nutrient transport in the pitchers of the carnivorous plant Nepenthes alata. Ann. Bot. 1999, 84, 459–466. [Google Scholar] [CrossRef]
- Gorb, E.; Kastner, V.; Peressadko, A.; Arzt, E.; Gaume, L.; Rowe, N.; Gorb, S. Structure and properties of the glandular surface in the digestive zone of the pitcher in the carnivorous plant Nepenthes ventrata and its role in insect trapping and retention. J. Exp. Biol. 2004, 207, 2947–2963. [Google Scholar] [CrossRef] [PubMed]
- Gaume, L.; Forterre, Y. A viscoelastic deadly fluid in carnivorous pitcher plants. PLoS ONE 2007, 2, e1185. [Google Scholar] [CrossRef] [PubMed]
- Thornhill, A.H.; Harper, I.S.; Hallam, N.D. The development of the digestive glands and enzymes in the pitchers of three Nepenthes species: N. alata, N. tobaica, and N. ventricosa (Nepenthaceae). Int. J. Mol. Sci. 2008, 169, 615–624. [Google Scholar]
- Rottloff, S.; Müller, U.; Kilper, R.; Mithöfer, A. Micropreparation of single secretory glands from the carnivorous plant Nepenthes. Anal. Biochem. 2009, 394, 135–137. [Google Scholar] [CrossRef]
- Moran, J.A.; Hawkins, B.J.; Gowen, B.E.; Robbins, S.L. Ion fluxes across the pitcher walls of three Bornean Nepenthes pitcher plant species: Flux rates and gland distribution patterns reflect nitrogen sequestration strategies. J. Exp. Bot. 2010, 61, 1365–1374. [Google Scholar] [CrossRef]
- Adlassnig, W.; Koller-Peroutka, M.; Bauer, S.; Koshkin, E.; Lendl, T.; Lichtscheidl, I.K. Endocytotic uptake of nutrients in carnivorous plants. Plant J. 2012, 71, 303–313. [Google Scholar] [CrossRef]
- Freund, M.; Graus, D.; Fleischmann, A.; Gilbert, K.J.; Lin, Q.; Renner, T.; Stigloher, C.; Albert, V.A.; Hedrich, R.; Fukushima, K. The digestive systems of carnivorous plants. Plant Physiol. 2022, 190, 44–59. [Google Scholar] [CrossRef]
- Koch, K.; Barthlott, W. Superhydrophobic and superhydrophilic plant surfaces: An inspiration for biomimetic materials. Philos. Trans A. Math. Phys. Eng. Sci. 2009, 367, 1487–1509. [Google Scholar] [CrossRef] [PubMed]
- Whitney, H.M.; Federle, W. Biomechanics of plant—Insect interactions. Curr. Opin. Plant Biol. 2013, 16, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Poppinga, S.; Körner, A.; Sachse, R.; Born, L.; Westermeier, A.S.; Hesse, L.; Knippers, J.; Bischoff, M.; Gresser, G.T.; Speck, T. Compliant mechanisms in plants and architecture. In Biomimetic Research for Architecture and Building Construction: Biological Design and Integrative Structures; Knippers, J., Speck, T., Nickel, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 169–193. [Google Scholar]
- Zhang, P.; Zhang, L.; Chen, H.; Dong, Z.; Zhang, D. Surfaces inspired by the Nepenthes peristome for unidirectional liquid transport. Adv. Mater. 2017, 29, 1702995. [Google Scholar] [CrossRef] [PubMed]
- Bauer, U.; Poppinga, S. New insights and opportunities from taking a biomechanical perspective on plant ecology. J. Exp. Bot. 2022, 73, 1063–1066. [Google Scholar] [CrossRef]
- Yong, J.; Chen, F.; Yang, Q.; Fang, Y.; Huo, J.; Zhang, J.; Hou, X. Nepenthes inspired design of self-repairing omniphobic slippery liquid infused porous surface (SLIPS) by femtosecond laser direct writing. Adv. Mater. Interfaces 2017, 4, 1700552. [Google Scholar] [CrossRef]
- Villegas, M.; Zhang, Y.; Abu Jarad, N.; Soleymani, L.; Didar, T.F. Liquid-infused surfaces: A review of theory, design, and applications. Acs Nano 2019, 13, 8517–8536. [Google Scholar] [CrossRef]
- Fenner, C.A. Beiträge zur Kenntnis der Anatomie, Entwicklungsgeschichte und Biologie der Laubblätter und Drüsen einiger Insektivoren; Universität Zürich: Zürich, Switzerland, 1904; Volume 23. [Google Scholar]
- Macfarlane, J.M. Nepenthaceae. In: A. Engler. Das Pflanzenreich IV, III, Heft 36: Stern, K., Beitrage zur Kenntnis der Nepenthaceen. Diss., Jena 1916. Flora 1908, 109, 213–283. [Google Scholar]
- Stern, K. Beitrage zur Kenntnis der Nepenthaceen. Diss. Flora 1916, 109, 213–283. [Google Scholar] [CrossRef]
- Metcalfe, C.R.; Chalk, L. Anatomy of the Dicotyledons; Clarendon Press: Oxford, UK, 1957; Volume 2, 557p. [Google Scholar]
- Płachno, B.J.; Kapusta, M.; Stolarczyk, P.; Świątek, P. Stellate Trichomes in Dionaea muscipula Ellis (Venus Flytrap) Traps, Structure and Functions. Int. J. Mol. Sci. 2023, 24, 553. [Google Scholar] [CrossRef]
- Płachno, B.J.; Kapusta, M.; Stolarczyk, P.; Wójciak, M.; Świątek, P. Immunocytochemical Analysis of Bifid Trichomes in Aldrovanda vesiculosa L. Traps. Int. J. Mol. Sci. 2023, 24, 3358. [Google Scholar] [CrossRef]
- Werker, E. Trichome diversity and development. Adv. Bot. Res. 2000, 31, 1–35. [Google Scholar]
- Wagner, G.J.; Wang, E.; Shepherd, R.W. New approaches for studying and exploiting an old protuberance, the plant trichome. Ann Bot. 2004, 93, 3–11. [Google Scholar] [CrossRef]
- Valverde, P.L.; Fornoni, J.; Núñez-Farfán, J. Defensive role of leaf trichomes in resistance to herbivorous insects in Datura stramonium. J. Evol. Biol. 2001, 14, 424–432. [Google Scholar] [CrossRef]
- Voigt, D.; Gorb, E.; Gorb, S. Plant surface–bug interactions: Dicyphus errans stalking along trichomes. Arthropod Plant Interact. 2007, 1, 221–243. [Google Scholar] [CrossRef]
- Hanley, M.E.; Lamont, B.B.; Fairbanks, M.M.; Rafferty, C.M. Plant structural traits and their role in anti-herbivore defence. Perspect. Plant Ecol. Evol. Syst. 2007, 8, 57–78. [Google Scholar] [CrossRef]
- Wati, R.K.; Gravendeel, B.; Langelaan, R.; van Heuven, B.J.; Claessens, J.; Kleynen, J.; Smets, E.F.; de Winter, A.J.; van der Meijden, A. Orchids reduce attachment of herbivorous snails with leaf trichomes. PLoS ONE 2023, 18, e0285731. [Google Scholar] [CrossRef]
- Owen, T.P., Jr.; Lennon, K.A. Structure and development of the pitchers from the carnivorous plant Nepenthes alata (Nepenthaceae). Am. J. Bot. 1999, 86, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Merbach, M.A.; Zizka, G.; Fiala, B.; Merbach, D.; Maschwitz, U. Giant nectaries in the peristome thorns of the pitcher plant Nepenthes bicalcarata Hooker f. Ecotropica 1999, 5, 45–50. [Google Scholar]
- Fahn, A. Secretory tissues in vascular plants. New Phytol. 1988, 108, 229–257. [Google Scholar] [CrossRef]
- Seago, J.L., Jr. Revisiting the occurrence and evidence of endodermis in angiosperm shoots. Flora 2020, 273, 151709. [Google Scholar] [CrossRef]
- Fineran, B.A.; Gilbertson, J.M. Application of lanthanum and uranyl salts as tracers to demonstrate apoplastic pathways for transport in glands of the carnivorous plant Utricularia monanthos. Eur. J. Cell Biol. 1980, 23, 66–72. [Google Scholar]
- Fineran, B.A.; Lee, M.S.L. Organization of mature external glands on the trap and other organs of the bladderwort Utricularia monanthos. Protoplasma 1980, 103, 17–34. [Google Scholar] [CrossRef]
- Płachno, B.J.; Świątek, P.; Adamec, L.; Carvalho, S.; Miranda, V.F.O. The trap architecture of Utricularia multifida and Utricularia westonii (subg. Polypompholyx). Front. Plant Sci. 2019, 10, 336. [Google Scholar] [CrossRef]
- Płachno, B.J.; Kapusta, M.; Feldo, M.; Świątek, P. Homogalacturonans and hemicelluloses in the external glands of Utricularia dichotoma traps. Int. J. Mol. Sci. 2024, 25, 13124. [Google Scholar] [CrossRef] [PubMed]
- Płachno, B.J.; Stpiczyńska, M.; Adamec, L.; Miranda, V.F.O.; Świątek, P. Nectar trichome structure of aquatic bladderworts from the section Utricularia (Lentibulariaceae) with observation of flower visitors and pollinators. Protoplasma 2018, 255, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Płachno, B.J.; Stpiczyńska, M.; Świątek, P.; Lambers, H.; Cawthray, G.R.; Nge, F.J.; Silva, S.R.; Miranda, V.F.O. Floral micromorphology and nectar composition of the early evolutionary lineage Utricularia (subgenus Polypompholyx, Lentibulariaceae). Protoplasma 2019, 256, 1531–1543. [Google Scholar] [CrossRef] [PubMed]
- Lustofin, K.; Świątek, P.; Miranda, V.F.O.; Płachno, B.J. Flower nectar trichome structure of carnivorous plants from the genus butterworts Pinguicula L. (Lentibulariaceae). Protoplasma 2020, 257, 245–259. [Google Scholar] [CrossRef]
- Płachno, B.J.; Lancelle, S.; Świątek, P.; Hepler, P.K.; Weidinger, M.; Lichtscheidl, I. Cyto-architecture of Byblis glands and leaf cells based on freeze-substitution and conventional TEM. Ann. Bot. 2025, 135, 463–482. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.E.; Pickard, B.G. Connections and barriers between cells of Drosera tentacles in relation to their electrophysiology. Planta 1974, 116, 1–16. [Google Scholar] [CrossRef]
- Lichtscheidl, I.; Lancelle, S.; Weidinger, M.; Adlassnig, W.; Koller-Peroutka, M.; Bauer, S.; Krammer, S.; Hepler, P.K. Gland cell responses to feeding in Drosera capensis, a carnivorous plant. Protoplasma 2021, 258, 1291–1306. [Google Scholar] [CrossRef]
- Płachno, B.J.; Kapusta, M.; Stolarczyk, P.; Świątek, P.; Lichtscheidl, I. Differences in the Occurrence of Cell Wall Components between Distinct Cell Types in Glands of Drosophyllum lusitanicum. Int. J. Mol. Sci. 2023, 24, 15045. [Google Scholar] [CrossRef] [PubMed]
- Marburger, J.E. Glandular leaf structure of Triphyophyllum peltatum (Dioncophyllaceae): A “fly-paper” insect trapper. Am. J. Bot. 1979, 66, 404–411. [Google Scholar] [CrossRef]
- Gunning, B.E.S.; Pate, J.S. Transfer cells. In Dynamic Aspects of Plant Ultrastructure; Robardsm, A.W., Ed.; McGraw-Hill: London, UK, 1974; pp. 441–476. [Google Scholar]
- Offler, C.E.; McCurdy, D.W.; Patrick, J.W.; Talbot, M.J. Transfer Cells: Cells Specialized for a Special Purpose. Annu. Rev. Plant Biol. 2003, 54, 431–454. [Google Scholar] [CrossRef]
- Offler, C.E.; Patrick, J.W. Transfer cells: What regulates the development of their intricate wall labyrinths? New Phytol. 2020, 228, 427–444. [Google Scholar] [CrossRef]
- Żytkowicz, M. Budowa Anatomiczna Dwóch Skrajnie Wyspecjalizowanych Gatunków z Rodzaju Nepenthes: Nepenthes bicalcarata Hook. f i Nepenthes albomarginata T.Lobb ex Lindl. Master’s Thesis, Faculty of Biology and Earth Sciences, Jagiellonian University, Kraków, Poland, 2011; 56p. [Google Scholar]
- Fineran, B.A. Ontogeny of external glands in the bladderwort Utricularia monanthos. Protoplasma 1980, 105, 9–25. [Google Scholar] [CrossRef]
- Naidoo, Y.; Heneidak, S. Morphological investigation of glandular hairs on Drosera capensis leaves with an ultrastructural study of the sessile glands. Botany 2013, 91, 234–241. [Google Scholar] [CrossRef]
- Muravnik, L.E. The Structural Peculiarities of the Leaf Glandular Trichomes: A Review. In Plant Cell and Tissue Differentiation and Secondary Metabolites; Reference Series in Phytochemistry; Ramawat, K.G., Ekiert, H.M., Goyal, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Raj, G.; Kurup, R.; Hussain, A.A.; Baby, S. Distribution of Naphthoquinones, Plumbagin, Droserone, and 5-O-Methyl Droserone in Chitin-Induced and Uninduced Nepenthes khasiana: Molecular Events in Prey Capture. J. Exp. Bot. 2011, 62, 5429–5436. [Google Scholar] [CrossRef] [PubMed]
- Rahman-Soad, A.; Dávila-Lara, A.; Paetz, C.; Mithöfer, A. Plumbagin, a potent naphthoquinone from Nepenthes plants with growth inhibiting and larvicidal activities. Molecules 2021, 26, 825. [Google Scholar] [CrossRef] [PubMed]
- Dávila-Lara, A.; Rahman-Soad, A.; Reichelt, M.; Mithöfer, A. Carnivorous Nepenthes x ventrata plants use a naphthoquinone as phytoanticipin against herbivory. PLoS ONE 2021, 16, e0258235. [Google Scholar] [CrossRef]
- Wójciak, M.; Feldo, M.; Stolarczyk, P.; Płachno, B.J. Carnivorous Plants from Nepenthaceae and Droseraceae as a Source of Secondary Metabolites. Molecules 2023, 28, 2155. [Google Scholar] [CrossRef] [PubMed]
- Eilenberg, H.; Pnini-Cohen, S.; Rahamim, Y.; Sionov, E.; Segal, E.; Carmeli, S.; Zilberstein, A. Induced Production of Antifungal Naphthoquinones in the Pitchers of the Carnivorous Plant Nepenthes khasiana. J. Exp. Bot. 2010, 61, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Płachno, B.J.; Kapusta, M.; Stolarczyk, P.; Świątek, P.; Strzemski, M.; Miranda, V.F.O. Immunocytochemical Analysis of the Wall Ingrowths in the Digestive Gland Transfer Cells in Aldrovanda vesiculosa L. (Droseraceae). Cells 2022, 11, 2218. [Google Scholar] [CrossRef]
- Płachno, B.J.; Kapusta, M.; Feldo, M.; Świątek, P. Cell Wall Microdomains Analysis in the Quadrifids of Utricularia dichotoma. Int. J. Mol. Sci. 2025, 26, 832. [Google Scholar] [CrossRef]
- Peaucelle, A.; Braybrook, S.; Höfte, H. Cell wall mechanics and growth control in plants: The role of pectins revisited. Front. Plant Sci. 2012, 3, 121. [Google Scholar] [CrossRef]
- Ridley, M.A.; O’Neill, D.; Mohnen, D. Pectins: Structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 2001, 57, 929–967. [Google Scholar] [CrossRef] [PubMed]
- Choong, F.X.; Lantz, L.; Shirani, H.; Schulz, A.; Nilsson, K.P.R.; Edlund, U.; Richter-Dahlfors, A. Stereochemical identification of glucans by a donor-acceptor-donor conjugated pentamer enables multi-carbohydrate anatomical mapping in plant tissues. Cellulose 2019, 26, 4253–4264. [Google Scholar] [CrossRef]
- Petrova, A.; Gorshkova, T.; Kozlova, L. Gradients of cell wall nano-mechanical properties along and across elongating primary roots of maize. J. Exp. Bot. 2020, 74, 1157. [Google Scholar] [CrossRef]
- Zitzmann, F.L.; Ward, E.; Matharu, A.S. Use of Carbotrace 480 as a Probe for Cellulose and Hydrogel Formation from Defibrillated Microalgae. Gels 2022, 8, 383. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.ebbabiotech.com/collections/carbotrace (accessed on 1 August 2024).
- Bonhomme, V.; Gounand, I.; Alaux, C.; Jousselin, E.; Barthélémy, D.; Gaume, L. The plant-ant Camponotus schmitzi helps its carnivorous host-plant Nepenthes bicalcarata to catch its prey. J. Trop. Ecol. 2011, 27, 15–24. [Google Scholar] [CrossRef]
- Clarke, C.M.; Kitching, R.L. Swimming ants and pitcher plants: A unique ant-plant interaction from Borneo. J. Trop. Ecol. 1995, 11, 589–602. [Google Scholar] [CrossRef]
- Thornham, D.G.; Smith, J.M.; Ulmar Grafe, T.; Federle, W. Setting the trap: Cleaning behaviour of Camponotus schmitzi ants increases long-term capture efficiency of their pitcher plant host, Nepenthes bicalcarata. Funct. Ecol. 2012, 26, 11–19. [Google Scholar] [CrossRef]
- Scharmann, M.; Thornham, D.G.; Grafe, T.U.; Federle, W. A Novel Type of Nutritional Ant–Plant Interaction: Ant Partners of Carnivorous Pitcher Plants Prevent Nutrient Export by Dipteran Pitcher Infauna. PLoS ONE 2013, 8, e63556. [Google Scholar] [CrossRef]
- Moran, J.A.; Merbach, M.A.; Livingston, N.J.; Clarke, C.M.; Booth, W.E. Termite prey specialization in the pitcher plant Nepenthes albomarginata—Evidence from stable isotope analysis. Ann. Bot. 2001, 88, 307–311. [Google Scholar] [CrossRef]
- Bauer, U.; Clemente, C.J.; Renner, T.; Federle, W. Form follows function: Morphological diversification and alternative trapping strategies in carnivorous Nepenthes pitcher plants. J. Evol. Biol. 2012, 25, 90–102. [Google Scholar] [CrossRef]
- Bauer, U.; Di Giusto, B.; Skepper, J.; Grafe, T.U.; Federle, W. With a flick of the lid: A novel trapping mechanism in Nepenthes gracilis pitcher plants. PLoS ONE 2012, 7, e38951. [Google Scholar] [CrossRef]
- Lenz, A.K.; Bauer, U. Pitcher geometry facilitates extrinsically powered ‘springboard trapping’ in carnivorous Nepenthes gracilis pitcher plants. Biol. Lett. 2022, 18, 20220106. [Google Scholar] [CrossRef] [PubMed]
- Liners, F.; Letesson, J.J.; Didembourg, C.; Van Cutsem, P. Monoclonal Antibodies against Pectin: Recognition of a Conformation Induced by Calcium. Plant Physiol. 1989, 91, 1419–1424. [Google Scholar] [CrossRef]
- Paul Knox, PhD, University of Leeds. Available online: https://www.kerafast.com/cat/799/paul-knox-phd (accessed on 13 November 2023).
- Knox, J.P.; Day, S.; Roberts, K. A set of cell surface glycoproteins forms an early marker of cell position, but not cell type, in the root apical meristem of Daucus carota L. Development 1989, 106, 47–56. [Google Scholar] [CrossRef]
- Verhertbruggen, Y.; Marcus, S.E.; Haeger, A.; Ordaz-Ortiz, J.J.; Knox, J.P. An extended set of monoclonal antibodies to pectic homogalacturonan. Carbohydr. Res. 2009, 28, 1858–1862. [Google Scholar] [CrossRef]
- Pattathil, S.; Avci, U.; Baldwin, D.; Swennes, A.G.; McGill, J.A.; Popper, Z.; Bootten, T.; Albert, A.; Davis, R.H.; Chennareddy, C.; et al. A comprehensive toolkit of plant cell wall glycan-directed monoclonal antibodies. Plant Physiol. 2010, 153, 514–525. [Google Scholar] [CrossRef] [PubMed]
- McCartney, L.; Marcus, S.E.; Knox, J.P. Monoclonal antibodies to plant cell wall xylans and arabinoxylans. J. Histochem. Cytochem. 2005, 53, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Marcus, S.E.; Verhertbruggen, Y.; Hervé, C.; Ordaz-Ortiz, J.J.; Farkas, V.; Pedersen, H.L.; Willats, W.G.; Knox, J.P. Pectic homogalacturonan masks abundant sets of xyloglucan epitopes in plant cell walls. BMC Plant Biol. 2008, 22, 60. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.kerafast.com/item/1603/anti-heteroxylan-lm11-antibody (accessed on 26 November 2024).
- Marcus, S.E.; Blake, A.W.; Benians, T.A.S.; Lee, K.J.D.; Poyser, C.; Donaldson, L.; Leroux, O.; Rogowski, A.; Petersen, H.L.; Boraston, A.; et al. Restricted access of proteins to mannan polysaccharides in intact plant cell walls. Plant J. 2010, 64, 191–203. [Google Scholar] [CrossRef]
- Płachno, B.J.; Świątek, P.; Jobson, R.W.; Małota, K.; Brutkowski, W. Serial block face SEM visualization of unusual plant nuclear tubular extensions in a carnivorous plant (Utricularia, Lentibulariaceae). Ann. Bot. 2017, 120, 673–680. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Płachno, B.J.; Kapusta, M.; Feldo, M.; Stolarczyk, P.; Małota, K.; Banaś, K. External Glands of Nepenthes Traps: Structure and Potential Function. Int. J. Mol. Sci. 2025, 26, 7788. https://doi.org/10.3390/ijms26167788
Płachno BJ, Kapusta M, Feldo M, Stolarczyk P, Małota K, Banaś K. External Glands of Nepenthes Traps: Structure and Potential Function. International Journal of Molecular Sciences. 2025; 26(16):7788. https://doi.org/10.3390/ijms26167788
Chicago/Turabian StylePłachno, Bartosz J., Małgorzata Kapusta, Marcin Feldo, Piotr Stolarczyk, Karol Małota, and Krzysztof Banaś. 2025. "External Glands of Nepenthes Traps: Structure and Potential Function" International Journal of Molecular Sciences 26, no. 16: 7788. https://doi.org/10.3390/ijms26167788
APA StylePłachno, B. J., Kapusta, M., Feldo, M., Stolarczyk, P., Małota, K., & Banaś, K. (2025). External Glands of Nepenthes Traps: Structure and Potential Function. International Journal of Molecular Sciences, 26(16), 7788. https://doi.org/10.3390/ijms26167788






