A Reassessment of Sarcopenia from a Redox Perspective as a Basis for Preventive and Therapeutic Interventions
Abstract
1. Introduction
1.1. The Evolving Clinical Definition of Sarcopenia
1.2. Sarcopenia in the Context of Ageing and Frailty
2. Sarcopenia: A Redox Mechanistic Perspective
2.1. The Continuum of Reactive Oxygen/Nitrogen Species (ROS/RNS)-Incited Responses in Skeletal Muscle
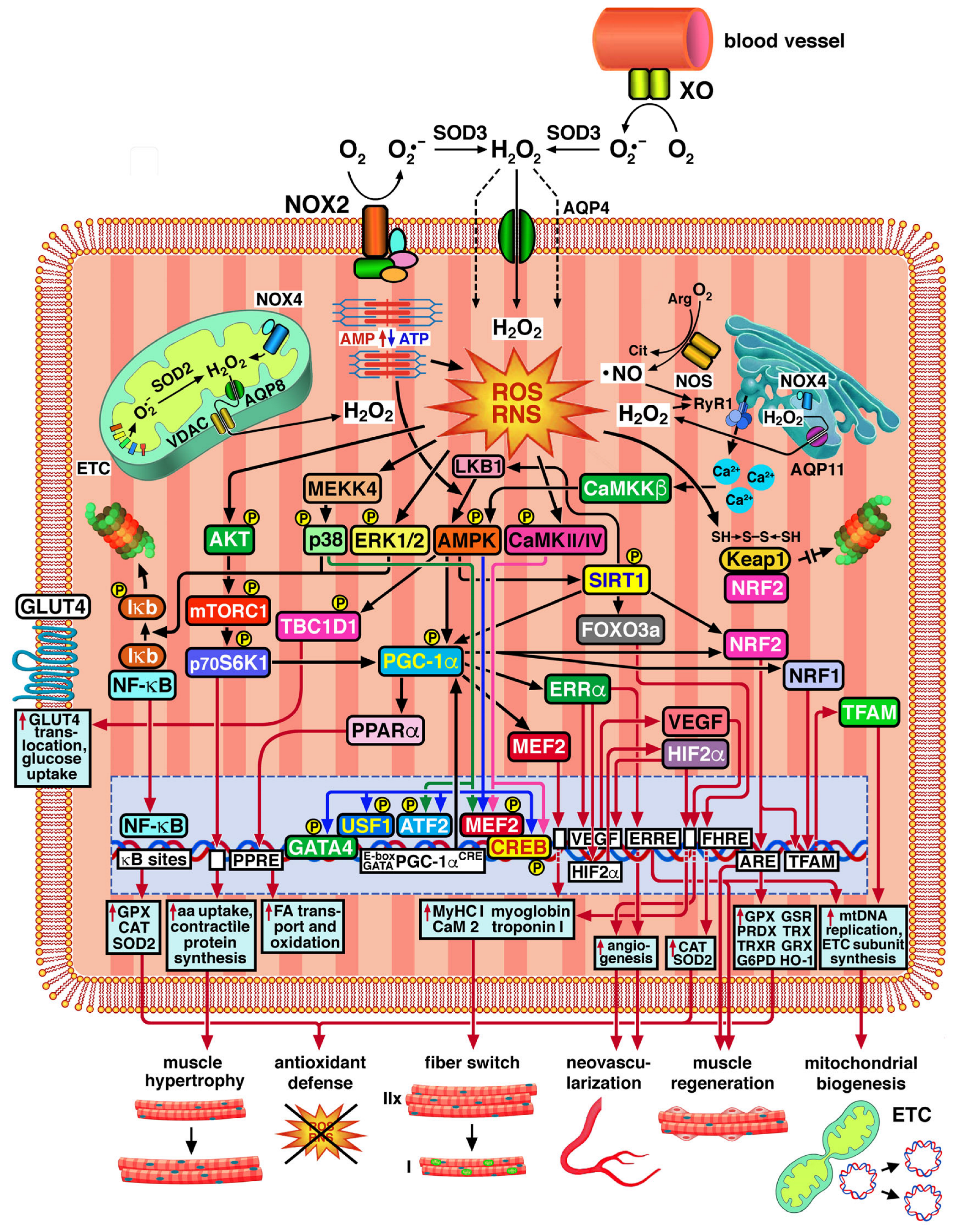
2.2. ROS/RNS-Mediated Adaptation of Skeletal Muscle to Exercise
2.3. Oxidative Stress as a Pathophysiological Response of Muscle Cells in Ageing and Disease
3. Molecular Mechanisms and Targets of Oxidative Damage in Sarcopenia
3.1. Molecular Pathogenesis of Sarcopenia: An Overview
3.2. ROS/RNS and Their Sources
3.3. Direct Modifications of Biological Macromolecules by ROS/RNS
3.4. Lipid Peroxidation and Advanced Lipid Peroxidation End Products (ALEs)
3.5. Advanced Glycation/Glycoxidation End Products (AGEs)
3.6. Interactions of Oxidized Macromolecules with Scavenger Receptors
4. Biochemical Mechanisms of Containment and Disposal of Oxidants in Muscle Cells
- (1)
- Detoxification by enzymatic antioxidants.
- (2)
- Detoxification by non-enzymatic antioxidants.
- (3)
- Expression of protein chaperones, including heat-shock proteins (HSP) and glucose-regulated proteins (GRP).
- (4)
- Removal of damaged molecules by lysosomal or proteasomal digestion and autophagy.
- (5)
- Expression of DNA repair enzymes.
- (6)
- Expression of anti-apoptotic factors and mitochondrial membrane stabilizing factors.
- (7)
- Expression of growth factors, such as brain-derived neurotrophic factor (BDNF), glial-derived neurotrophic factor (GDNF), IGFs, soluble amyloid β precursor protein (AβPP) in brain, and cytokines, such as TNF-α. These activate the expression of genes encoding antioxidant enzymes, anti-apoptotic proteins, and regulators of ion transport (e.g., calbindin and glutamate receptors).
4.1. Endogenous Enzymatic Antioxidants
4.2. Endogenous Non-Enzymatic Antioxidants
4.3. NRF2, the Master Regulator of Antioxidant Responses

5. A Critical Reassessment of Possibly Useful Exogenous Antioxidants in Sarcopenia
5.1. A Chemical Mechanistic and Physiological Classification of Antioxidants
- The reaction mechanisms at the base of the electron/proton- or H-atom-donating abilities of antioxidants;
- Their dependence upon solvent characteristics (pH, polarity, etc.):
- Their water and/or lipid solubility;
- Their abilities to quench different kinds of radicals (O2•−, HO2•, •OH, •NO, •NO2);
- Their abilities to participate in the enzymatic decomposition of ROS/RNS and hydroperoxides;
- Their abilities to restore major antioxidants of biological significance in human metabolism (α-tocopherol, glutathion) to their reduced forms;
- Their prevalent intracytoplasmic or intranuclear activity.
5.2. A Discussion of the Possible Usefulness of Major Antioxidants in Sarcopenia
5.2.1. Phenolic Radical-Trapping Antioxidants
5.2.2. Non-Phenolic Radical-Trapping Antioxidants
5.2.3. Antioxidants That Act Through Different Mechanisms
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, G.; Ostovar, A.; Heshmat, R.; Darabi, H.; Sharifi, F.; Raeisi, A.; Mehrdad, N.; Shadman, Z.; Razi, F.; Amini, M.R.; et al. Bushehr Elderly Health (BEH) programme: Study protocol and design of musculoskeletal system and cognitive function (stage II). BMJ Open 2017, 7, e013606. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rolland, Y.; Czerwinski, S.; Abellan Van Kan, G.; Morley, J.E.; Cesari, M.; Onder, G.; Woo, J.; Baumgartner, R.; Pillard, F.; Boirie, Y.; et al. Sarcopenia: Its assessment, etiology, pathogenesis, consequences and future perspectives. J. Nutr. Health Ageing 2008, 12, 433–450. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jung, H.N.; Jung, C.H.; Hwang, Y.C. Sarcopenia in youth. Metabolism 2023, 144, 155557. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Voulgaridou, G.; Tyrovolas, S.; Detopoulou, P.; Tsoumana, D.; Drakaki, M.; Apostolou, T.; Chatziprodromidou, I.P.; Papandreou, D.; Giaginis, C.; Papadopoulou, S.K. Diagnostic criteria and measurement techniques ofs: A critical evaluation of the up-to-date evidence. Nutrients 2024, 16, 436. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ruan, Z.; Tian, H.; Li, R.; Zhang, J.; Cao, S.; Yang, Z.; Chen, X.; Li, D.; Miao, Q. Joint association of frailty index and biological age with chronic obstructive pulmonary disease: A cohort study from CHARLS. Sci. Rep. 2025, 15, 17616. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yabuuchi, J.; Ueda, S.; Yamagishi, S.I.; Nohara, N.; Nagasawa, H.; Wakabayashi, K.; Matsui, T.; Yuichiro, H.; Kadoguchi, T.; Otsuka, T.; et al. Association of advanced glycation end products with sarcopenia and frailty in chronic kidney disease. Sci. Rep. 2020, 10, 17647. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Freire-Filho, W.A.; Dalboni, M.A.; Elias, R.M. Effects of aging on chronic kidney disease mineral and bone disorder. Curr. Opin. Nephrol. Hypertens. 2025, 34, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Bernabei, R.; Martone, A.M.; Vetrano, D.L.; Calvani, R.; Landi, F.; Marzetti, E. Frailty, physical frailty, sarcopenia: A new conceptual model. Stud. Health Technol. Inform. 2014, 203, 78–84. [Google Scholar] [PubMed]
- Boccardi, V. Sarcopenia: A dive into metabolism to promote a multimodal, preventive, and regenerative approach. Mech. Ageing Dev. 2024, 219, 111941. [Google Scholar] [CrossRef] [PubMed]
- Bolt, J.; Carvalho, V.; Lin, K.; Lee, S.J.; Inglis, C. Systematic review of guideline recommendations for older and frail adults with type 2 diabetes mellitus. Age Ageing 2024, 53, afae259. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuffel, R.L.; Morin, R.T.; Covinsky, K.E.; Boscardin, W.J.; Lohman, M.C.; Li, Y.; Byers, A.L. Association of frailty with risk of suicide attempt in a national cohort of US veterans aged 65 years or older. JAMA Psychiatry 2023, 80, 287–295. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hanna, K.; Ditillo, M.; Joseph, B. The role of frailty and prehabilitation in surgery. Curr. Opin. Crit. Care 2019, 25, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Abi Chebl, J.; Somasundar, P.; Vognar, L.; Kwon, S. Review of frailty in geriatric surgical oncology. Scand. J. Surg. 2024, 114, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Bohn, L.; Drouin, S.M.; McFall, G.P.; Rolfson, D.B.; Andrew, M.K.; Dixon, R.A. Machine learning analyses identify multi-modal frailty factors that selectively discriminate four cohorts in the Alzheimer’s disease spectrum: A COMPASS-ND study. BMC Geriatr. 2023, 23, 837. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Rorke, S.; Tipping, C.J.; Lodge, M.; Mathew, J.; Kimmel, L. Frailty across the adult age spectrum and its effects on outcomes: Experience from a level 1 trauma centre. Injury 2025, 56, 112037. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Shao, Y.; Weir, C.R.; Shah, R.U.; Bray, B.E.; Garvin, J.H.; Zeng-Treitler, Q. Predicting adverse outcomes in heart failure patients using different frailty status measures. Stud. Health Technol. Inform. 2017, 245, 327–331. [Google Scholar] [PubMed] [PubMed Central]
- Xu, W.; Cai, J.; Liu, Y.; Li, L.; Ye, X.; Wang, P.; Lu, J.; Akkaif, M.A.; Zhang, M. Sarcopenia and frailty among older Chinese adults: Findings from the CHARLS study. PLoS ONE 2024, 19, e0312879. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bouviere, J.; Fortunato, R.S.; Dupuy, C.; Werneck-de-Castro, J.P.; Carvalho, D.P.; Louzada, R.A. Exercise-stimulated ROS sensitive signaling pathways in skeletal muscle. Antioxidants 2021, 10, 537. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jackson, M.J. Exercise-induced adaptations to homeostasis of reactive oxygen species in skeletal muscle. Free Radic. Biol. Med. 2024, 225, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrera, M.C.; Close, G.L.; Kayani, A.; McArdle, A.; Viña, J.; Jackson, M.J. Effect of xanthine oxidase-generated extracellular superoxide on skeletal muscle force generation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R2–R8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barbieri, E.; Sestili, P. Reactive oxygen species in skeletal muscle signaling. J. Signal Transduct. 2012, 2012, 982794. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Andrés Juan, C.; Plou, F.J.; Pérez-Lebeña, E. Superoxide Anion Chemistry-Its Role at the Core of the Innate Immunity. Int. J. Mol. Sci. 2023, 24, 1841. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wong, H.S.; Dighe, P.A.; Mezera, V.; Monternier, P.A.; Brand, M.D. Production of superoxide and hydrogen peroxide from specific mitochondrial sites under different bioenergetic conditions. J. Biol. Chem. 2017, 292, 16804–16809. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Molnar, A.M.; Servais, S.; Guichardant, M.; Lagarde, M.; Macedo, D.V.; Pereira-Da-Silva, L.; Sibille, B.; Favier, R. Mitochondrial H2O2 production is reduced with acute and chronic eccentric exercise in rat skeletal muscle. Antioxid. Redox Signal. 2006, 8, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Goldstein, E.; Schrager, M.; Ji, L.L. Exercise training and skeletal muscle antioxidant enzymes: An update. Antioxidants 2022, 12, 39. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Henríquez-Olguín, C.; Boronat, S.; Cabello-Verrugio, C.; Jaimovich, E.; Hidalgo, E.; Jensen, T.E. The emerging roles of nicotinamide adenine dinucleotide phosphate oxidase 2 in skeletal muscle redox signaling and metabolism. Antioxid. Redox Signal. 2019, 31, 1371–1410. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Witherspoon, J.W.; Meilleur, K.G. Review of RyR1 pathway and associated pathomechanisms. Acta Neuropathol. Commun. 2016, 4, 121. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alves, R.; Suehiro, C.L.; Oliveira, F.G.; Frantz, E.D.C.; Medeiros, R.F.; Vieira, R.P.; Martins, M.A.; Lin, C.J.; Nobrega, A.C.L.D.; Toledo-Arruda, A.C. Aerobic exercise modulates cardiac NAD(P)H oxidase and the NRF2/KEAP1 pathway in a mouse model of chronic fructose consumption. J. Appl. Physiol. (1985) 2020, 128, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.J.; Jackson, J.R.; Hao, Y.; Leonard, S.S.; Alway, S.E. Inhibition of xanthine oxidase reduces oxidative stress and improves skeletal muscle function in response to electrically stimulated isometric contractions in aged mice. Free Radic. Biol. Med. 2011, 51, 38–52. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Supruniuk, E.; Górski, J.; Chabowski, A. Endogenous and exogenous antioxidants in skeletal muscle fatigue development during exercise. Antioxidants 2023, 12, 501. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, M.; Liu, Y.; Zhang, G.; Yang, Z.; Xu, W.; Chen, Q. The applications and mechanisms of superoxide dismutase in medicine, food, and cosmetics. Antioxidants 2023, 12, 1675. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aslesh, T.; Al-Aghbari, A.; Yokota, T. Assessing the role of aquaporin 4 in skeletal muscle function. Int. J. Mol. Sci. 2023, 24, 1489. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bienert, G.P.; Chaumont, F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta 2014, 1840, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Eaglesfield, R.; Fernandez-Vizarra, E.; Lacko, E.; Caldwell, S.T.; Sloan, N.L.; Siciarz, D.; Hartley, R.C.; Tokatlidis, K. Sub-organellar mitochondrial hydrogen peroxide observed using a SNAP tag targeted coumarin-based fluorescent reporter. Redox Biol. 2025, 80, 103502. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bestetti, S.; Galli, M.; Sorrentino, I.; Pinton, P.; Rimessi, A.; Sitia, R.; Medraño-Fernandez, I. Human aquaporin-11 guarantees efficient transport of H2O2 across the endoplasmic reticulum membrane. Redox Biol. 2020, 28, 101326. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hirschfield, W.; Moody, M.R.; O’Brien, W.E.; Gregg, A.R.; Bryan, R.M., Jr.; Reid, M.B. Nitric oxide release and contractile properties of skeletal muscles from mice deficient in type III NOS. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 278, R95–R100. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Pannell, B.K. Redox characterization of functioning skeletal muscle. Front. Physiol. 2015, 6, 338. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fleming, I. Molecular mechanisms underlying the activation of eNOS. Pflugers Arch. 2010, 459, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Giordani, L.; He, G.J.; Negroni, E.; Sakai, H.; Law, J.Y.C.; Siu, M.M.; Wan, R.; Corneau, A.; Tajbakhsh, S.; Cheung, T.H.; et al. High-dimensional single-cell cartography reveals novel skeletal muscle-resident cell populations. Mol. Cell 2019, 74, 609–621.e6. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Radak, Z.; Ji, L.L.; Jackson, M. Reactive oxygen species promote endurance exercise-induced adaptations in skeletal muscles. J. Sport Health Sci. 2024, 13, 780–792. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jordan, A.C.; Perry, C.G.R.; Cheng, A.J. Promoting a pro-oxidant state in skeletal muscle: Potential dietary, environmental, and exercise interventions for enhancing endurance-training adaptations. Free Radic. Biol. Med. 2021, 176, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Thirupathi, A.; Pinho, R.A.; Chang, Y.Z. Physical exercise: An inducer of positive oxidative stress in skeletal muscle aging. Life Sci. 2020, 252, 117630. [Google Scholar] [CrossRef] [PubMed]
- Lian, D.; Chen, M.M.; Wu, H.; Deng, S.; Hu, X. The role of oxidative stress in skeletal muscle myogenesis and muscle disease. Antioxidants 2022, 11, 755. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Radak, Z.; Zhao, Z.; Koltai, E.; Ohno, H.; Atalay, M. Oxygen consumption and usage during physical exercise: The balance between oxidative stress and ROS-dependent adaptive signaling. Antioxid. Redox Signal. 2013, 18, 1208–1246. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grgic, J.; Homolak, J.; Mikulic, P.; Botella, J.; Schoenfeld, B.J. Inducing hypertrophic effects of type I skeletal muscle fibers: A hypothetical role of time under load in resistance training aimed at muscular hypertrophy. Med. Hypotheses 2018, 112, 40–42. [Google Scholar] [CrossRef] [PubMed]
- Kerschan-Schindl, K.; Hasenoehrl, T. Exercise in the prevention of age-related fragility fractures (narrative review). Gerontology 2025, 71, 173–184. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Voronina, M.V.; Frolova, A.S.; Kolesova, E.P.; Kuldyushev, N.A.; Parodi, A.; Zamyatnin, A.A., Jr. The intricate balance between life and death: ROS, cathepsins, and their interplay in cell death and autophagy. Int. J. Mol. Sci. 2024, 25, 4087. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Ristow, M. Unraveling the truth about antioxidants: Mitohormesis explains ROS-induced health benefits. Nat. Med. 2014, 20, 709–711. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Ishihara, K.; Tekus, E.; Varga, C.; Posa, A.; Balogh, L.; Boldogh, I.; Koltai, E. Exercise, oxidants, and antioxidants change the shape of the bell-shaped hormesis curve. Redox Biol. 2017, 12, 285–290. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ji, L.L.; Kang, C.; Zhang, Y. Exercise-induced hormesis and skeletal muscle health. Free Radic. Biol. Med. 2016, 98, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Koltai, E.; Taylor, A.W.; Higuchi, M.; Kumagai, S.; Ohno, H.; Goto, S.; Boldogh, I. Redox-regulating sirtuins in aging, caloric restriction, and exercise. Free Radic. Biol. Med. 2013, 58, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Supruniuk, E.; Maciejczyk, M.; Zalewska, A.; Górski, J.; Chabowski, A. Blood profile of cytokines, chemokines, growth factors, and redox biomarkers in response to different protocols of treadmill running in rats. Int. J. Mol. Sci. 2020, 21, 8071. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fittipaldi, S.; Dimauro, I.; Mercatelli, N.; Caporossi, D. Role of exercise-induced reactive oxygen species in the modulation of heat shock protein response. Free Radic. Res. 2014, 48, 52–70. [Google Scholar] [CrossRef] [PubMed]
- VanderVeen, B.N.; Fix, D.K.; Montalvo, R.N.; Counts, B.R.; Smuder, A.J.; Murphy, E.A.; Koh, H.J.; Carson, J.A. The regulation of skeletal muscle fatigability and mitochondrial function by chronically elevated interleukin-6. Exp. Physiol. 2019, 104, 385–397. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, Y.; Zhang, X.; Baker, J.S.; Davison, G.W.; Yan, X. Redox signaling and skeletal muscle adaptation during aerobic exercise. iScience 2024, 27, 109643. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Azzi, A. Oxidative stress: What is it? Can it be measured? Where is it located? Can it be good or bad? Can it be prevented? Can it be cured? Antioxidants 2022, 11, 1431. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sies, H.; Ursini, F. Homeostatic control of redox status and health. IUBMB Life 2022, 74, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Oxidative stress and antioxidants: Distress or eustress? Arch. Biochem. Biophys. 2016, 595, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.J.; Stretton, C.; McArdle, A. Hydrogen peroxide as a signal for skeletal muscle adaptations to exercise: What do concentrations tell us about potential mechanisms? Redox Biol. 2020, 35, 101484. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jackson, M.J.; Pollock, N.; Staunton, C.; Jones, S.; McArdle, A. Redox control of signalling responses to contractile activity and ageing in skeletal muscle. Cells 2022, 11, 1698. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Palomero, J.; Pye, D.; Kabayo, T.; Spiller, D.G.; Jackson, M.J. In situ detection and measurement of intracellular reactive oxygen species in single isolated mature skeletal muscle fibers by real time fluorescence microscopy. Antioxid. Redox Signal. 2008, 10, 1463–1474. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumar, P.; Umakanth, S.; Girish, N. A review of the components of exercise prescription for sarcopenic older adults. Eur. Geriatr. Med. 2022, 13, 1245–1280. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gomez-Cabrera, M.C.; Domenech, E.; Viña, J. Moderate exercise is an antioxidant: Upregulation of antioxidant genes by training. Free Radic. Biol. Med. 2008, 44, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Selles, A.; Galvan-Alvarez, V.; Martinez-Canton, M.; Garcia-Gonzalez, E.; Morales-Alamo, D.; Santana, A.; Gonzalez-Henriquez, J.J.; Dorado, C.; Calbet, J.A.L.; Martin-Rincon, M. Fast regulation of the NF-κB signalling pathway in human skeletal muscle revealed by high-intensity exercise and ischaemia at exhaustion: Role of oxygenation and metabolite accumulation. Redox Biol. 2022, 55, 102398. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ji, L.L. Nuclear factor κB signaling revisited: Its role in skeletal muscle and exercise. Free Radic. Biol. Med. 2025, 232, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; O’Moore, K.M.; Dickman, J.R.; Ji, L.L. Exercise activation of muscle peroxisome proliferator-activated receptor-gamma coactivator-1alpha signaling is redox sensitive. Free Radic. Biol. Med. 2009, 47, 1394–1400. [Google Scholar] [CrossRef] [PubMed]
- Barata, A.G.; Dick, T.P. A role for peroxiredoxins in H2O2- and MEKK-dependent activation of the p38 signaling pathway. Redox Biol. 2020, 28, 101340. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Irrcher, I.; Ljubicic, V.; Hood, D.A. Interactions between ROS and AMP kinase activity in the regulation of PGC-1alpha transcription in skeletal muscle cells. Am. J. Physiol. Cell Physiol. 2009, 296, C116–C123. [Google Scholar] [CrossRef] [PubMed]
- Dent, J.R.; Stocks, B.; Campelj, D.G.; Philp, A. Transient changes to metabolic homeostasis initiate mitochondrial adaptation to endurance exercise. Semin. Cell Dev. Biol. 2023, 143, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Zmijewski, J.W.; Banerjee, S.; Bae, H.; Friggeri, A.; Lazarowski, E.R.; Abraham, E. Exposure to hydrogen peroxide induces oxidation and activation of AMP-activated protein kinase. J. Biol. Chem. 2010, 285, 33154–33164. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kjøbsted, R.; Hingst, J.R.; Fentz, J.; Foretz, M.; Sanz, M.N.; Pehmøller, C.; Shum, M.; Marette, A.; Mounier, R.; Treebak, J.T.; et al. AMPK in skeletal muscle function and metabolism. FASEB J. 2018, 32, 1741–1777. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abu Shelbayeh, O.; Arroum, T.; Morris, S.; Busch, K.B. PGC-1α is a master regulator of mitochondrial lifecycle and ROS stress response. Antioxidants 2023, 12, 1075. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arany, Z. PGC-1 coactivators and skeletal muscle adaptations in health and disease. Curr. Opin. Genet. Dev. 2008, 18, 426–434. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kong, S.; Cai, B.; Nie, Q. PGC-1α affects skeletal muscle and adipose tissue development by regulating mitochondrial biogenesis. Mol. Genet. Genom. 2022, 297, 621–633. [Google Scholar] [CrossRef]
- Quan, Y.; Xin, Y.; Tian, G.; Zhou, J.; Liu, X. Mitochondrial ROS-modulated mtDNA: A potential target for cardiac aging. Oxidative Med. Cell. Longev. 2020, 2020, 9423593. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mesquita, P.H.C.; Vann, C.G.; Phillips, S.M.; McKendry, J.; Young, K.C.; Kavazis, A.N.; Roberts, M.D. Skeletal muscle ribosome and mitochondrial biogenesis in response to different exercise training modalities. Front. Physiol. 2021, 12, 725866. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Esteras, N.; Abramov, A.Y. Nrf2 as a regulator of mitochondrial function: Energy metabolism and beyond. Free Radic. Biol. Med. 2022, 189, 136–153. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.; Yamamoto, M. The molecular mechanisms regulating the KEAP1-NRF2 pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 system: A thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morales-Alamo, D.; Calbet, J.A.L. AMPK signaling in skeletal muscle during exercise: Role of reactive oxygen and nitrogen species. Free Radic. Biol. Med. 2016, 98, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Ubaid, S. Role of Silent Information Regulator 1 (SIRT1) in regulating oxidative stress and inflammation. Inflammation 2020, 43, 1589–1598. [Google Scholar] [CrossRef] [PubMed]
- Vichaiwong, K.; Purohit, S.; An, D.; Toyoda, T.; Jessen, N.; Hirshman, M.F.; Goodyear, L.J. Contraction regulates site-specific phosphorylation of TBC1D1 in skeletal muscle. Biochem. J. 2010, 431, 311–320. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Frøsig, C.; Pehmøller, C.; Birk, J.B.; Richter, E.A.; Wojtaszewski, J.F. Exercise-induced TBC1D1 Ser237 phosphorylation and 14-3-3 protein binding capacity in human skeletal muscle. J. Physiol. 2010, 588, 4539–4548. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Henríquez-Olguin, C.; Knudsen, J.R.; Raun, S.H.; Li, Z.; Dalbram, E.; Treebak, J.T.; Sylow, L.; Holmdahl, R.; Richter, E.A.; Jaimovich, E.; et al. Cytosolic ROS production by NADPH oxidase 2 regulates muscle glucose uptake during exercise. Nat. Commun. 2019, 10, 4623. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Uruno, A.; Yagishita, Y.; Katsuoka, F.; Kitajima, Y.; Nunomiya, A.; Nagatomi, R.; Pi, J.; Biswal, S.S.; Yamamoto, M. Nrf2-mediated regulation of skeletal muscle glycogen metabolism. Mol. Cell. Biol. 2016, 36, 1655–1672. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Specht, K.S.; Kant, S.; Addington, A.K.; McMillan, R.P.; Hulver, M.W.; Learnard, H.; Campbell, M.; Donnelly, S.R.; Caliz, A.D.; Pei, Y.; et al. Nox4 mediates skeletal muscle metabolic responses to exercise. Mol. Metab. 2021, 45, 101160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Korkmaz, E.; Dönmez, G.; Uzuner, K.; Babayeva, N.; Torgutalp, Ş.Ş.; Özçakar, L. Effects of blood flow restriction training on muscle strength and architecture. J. Strength Cond. Res. 2022, 36, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Rasbach, K.A.; Gupta, R.K.; Ruas, J.L.; Wu, J.; Naseri, E.; Estall, J.L.; Spiegelman, B.M. PGC-1alpha regulates a HIF2alpha-dependent switch in skeletal muscle fiber types. Proc. Natl. Acad. Sci. USA 2010, 107, 21866–21871. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yoon, M.S. mTOR as a key regulator in maintaining skeletal muscle mass. Front. Physiol. 2017, 8, 788. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arany, Z.; Foo, S.Y.; Ma, Y.; Ruas, J.L.; Bommi-Reddy, A.; Girnun, G.; Cooper, M.; Laznik, D.; Chinsomboon, J.; Rangwala, S.M.; et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature 2008, 451, 1008–1012. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.; Kruse, C.; Zhang, M.; Schröder, K. Nox4 supports proper capillary growth in exercise and retina neo-vascularization. J. Physiol. 2015, 593, 2145–2154. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al-Sawaf, O.; Fragoulis, A.; Rosen, C.; Keimes, N.; Liehn, E.A.; Hölzle, F.; Kan, Y.W.; Pufe, T.; Sönmez, T.T.; Wruck, C.J. Nrf2 augments skeletal muscle regeneration after ischaemia-reperfusion injury. J. Pathol. 2014, 234, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, Y.; Tang, Y.; Lin, B.; Kong, X.; Oladele, O.A.; Yin, Y. Protective effect of myokine IL-15 against H2O2-mediated oxidative stress in skeletal muscle cells. Mol. Biol. Rep. 2014, 41, 7715–7722. [Google Scholar] [CrossRef] [PubMed]
- Louzada, R.A.; Bouviere, J.; Matta, L.P.; Werneck-de-Castro, J.P.; Dupuy, C.; Carvalho, D.P.; Fortunato, R.S. Redox signaling in widespread health benefits of exercise. Antioxid. Redox Signal. 2020, 33, 745–760. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.S.; Kim, J.H.; Kim, M.J.; Min, B.; Lee, S.M.; Go, G.Y.; Kim, J.W.; Kim, S.; Kwak, J.Y.; Chun, S.W.; et al. Exercise-induced CLCF1 attenuates age-related muscle and bone decline in mice. Nat. Commun. 2025, 16, 4743. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aracena, P.; Tang, W.; Hamilton, S.L.; Hidalgo, C. Effects of S-glutathionylation and S-nitrosylation on calmodulin binding to triads and FKBP12 binding to type 1 calcium release channels. Antioxid. Redox Signal. 2005, 7, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, C.; Sánchez, G.; Barrientos, G.; Aracena-Parks, P. A transverse tubule NADPH oxidase activity stimulates calcium release from isolated triads via ryanodine receptor type 1 S-glutathionylation. J. Biol. Chem. 2006, 281, 26473–26482. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.; Cao, Y.; Zhu, J.; Xu, Y.; Balkan, W.; Buys, E.S.; Diaz, F.; Kerrick, W.G.; Hare, J.M.; Percival, J.M. GSNOR deficiency enhances in situ skeletal muscle strength, fatigue resistance, and RyR1 S-nitrosylation without impacting mitochondrial content and activity. Antioxid. Redox Signal. 2017, 26, 165–181. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kobayashi, T.; Kurebayashi, N.; Murayama, T. The ryanodine receptor as a sensor for intracellular environments in muscles. Int. J. Mol. Sci. 2021, 22, 10795. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mollica, J.P.; Dutka, T.L.; Merry, T.L.; Lamboley, C.R.; McConell, G.K.; McKenna, M.J.; Murphy, R.M.; Lamb, G.D. S-glutathionylation of troponin I (fast) increases contractile apparatus Ca2+ sensitivity in fast-twitch muscle fibres of rats and humans. J. Physiol. 2012, 590, 1443–1463. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dutka, T.L.; Mollica, J.P.; Lamboley, C.R.; Weerakkody, V.C.; Greening, D.W.; Posterino, G.S.; Murphy, R.M.; Lamb, G.D. S-nitrosylation and S-glutathionylation of Cys134 on troponin I have opposing competitive actions on Ca2+ sensitivity in rat fast-twitch muscle fibers. Am. J. Physiol. Cell Physiol. 2017, 312, C316–C327. [Google Scholar] [CrossRef] [PubMed]
- Lamboley, C.R.; Rouffet, D.M.; Dutka, T.L.; McKenna, M.J.; Lamb, G.D. Effects of high-intensity intermittent exercise on the contractile properties of human type I and type II skeletal muscle fibers. J. Appl. Physiol. (1985) 2020, 128, 1207–1216. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Mel’nikova, T.I.; Zavadskiy, S.P.; Kuz’menko, A.N.; Terentiev, A.A. Dual Character of Reactive Oxygen, Nitrogen, and Halogen Species: Endogenous Sources, Interconversions and Neutralization. Biochemistry 2020, 85 (Suppl. S1), S56–S78. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Victor, P.; García-Prat, L.; Muñoz-Cánoves, P. Control of satellite cell function in muscle regeneration and its disruption in ageing. Nat. Rev. Mol. Cell Biol. 2022, 23, 204–226. [Google Scholar] [CrossRef] [PubMed]
- Dalle, S.; Rossmeislova, L.; Koppo, K. The role of inflammation in age-related sarcopenia. Front. Physiol. 2017, 8, 1045. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Santoro, A.; Bientinesi, E.; Monti, D. Immunosenescence and inflammaging in the aging process: Age-related diseases or longevity? Ageing Res. Rev. 2021, 71, 101422. [Google Scholar] [CrossRef] [PubMed]
- Coperchini, F.; Greco, A.; Teliti, M.; Croce, L.; Chytiris, S.; Magri, F.; Gaetano, C.; Rotondi, M. Inflamm-ageing: How cytokines and nutrition shape the trajectory of ageing. Cytokine Growth Factor Rev. 2025, 82, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Mitchell, B.A.; Zampino, M.; Fishbein, K.W.; Spencer, R.G.; Ferrucci, L. Muscle mitochondrial energetics predicts mobility decline in well-functioning older adults: The Baltimore longitudinal study of ageing. Ageing Cell 2022, 21, e13552. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Buonocore, D.; Rucci, S.; Vandoni, M.; Negro, M.; Marzatico, F. Oxidative system in aged skeletal muscle. Muscles Ligaments Tendons J. 2012, 1, 85–90. [Google Scholar] [PubMed] [PubMed Central]
- Shi, Y.; Pulliam, D.A.; Liu, Y.; Hamilton, R.T.; Jernigan, A.L.; Bhattacharya, A.; Sloane, L.B.; Qi, W.; Chaudhuri, A.; Buffenstein, R.; et al. Reduced mitochondrial ROS, enhanced antioxidant defense, and distinct age-related changes in oxidative damage in muscles of long-lived Peromyscus leucopus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R343–R355. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grima-Terrén, M.; Campanario, S.; Ramírez-Pardo, I.; Cisneros, A.; Hong, X.; Perdiguero, E.; Serrano, A.L.; Isern, J.; Muñoz-Cánoves, P. Muscle ageing and sarcopenia: The pathology, etiology, and most promising therapeutic targets. Mol. Asp. Med. 2024, 100, 101319. [Google Scholar] [CrossRef] [PubMed]
- Marzetti, E.; Calvani, R.; Coelho-Júnior, H.J.; Landi, F.; Picca, A. Mitochondrial quantity and quality in age-related sarcopenia. Int. J. Mol. Sci. 2024, 25, 2052. [Google Scholar] [CrossRef] [PubMed]
- Tranah, G.J.; Barnes, H.N.; Cawthon, P.M.; Coen, P.M.; Esser, K.A.; Hepple, R.T.; Huo, Z.; Kramer, P.A.; Toledo, F.G.S.; Zhang, X.; et al. Expression of mitochondrial oxidative stress response genes in muscle is associated with mitochondrial respiration, physical performance, and muscle mass in the Study of Muscle, Mobility, and Ageing. Ageing Cell 2024, 23, e14114. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jang, Y.C.; Lustgarten, M.S.; Liu, Y.; Muller, F.L.; Bhattacharya, A.; Liang, H.; Salmon, A.B.; Brooks, S.V.; Larkin, L.; Hayworth, C.R.; et al. Increased superoxide in vivo accelerates age-associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration. FASEB J. 2010, 24, 1376–1390. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Charles, A.L.; Meyer, A.; Dal-Ros, S.; Auger, C.; Keller, N.; Ramamoorthy, T.G.; Zoll, J.; Metzger, D.; Schini-Kerth, V.; Geny, B. Polyphenols prevent ageing-related impairment in skeletal muscle mitochondrial function through decreased reactive oxygen species production. Exp. Physiol. 2013, 98, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Deepa, S.S.; Van Remmen, H.; Brooks, S.V.; Faulkner, J.A.; Larkin, L.; McArdle, A.; Jackson, M.J.; Vasilaki, A.; Richardson, A. Accelerated sarcopenia in Cu/Zn superoxide dismutase knockout mice. Free Radic. Biol. Med. 2019, 132, 19–23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Musci, R.V.; Fuqua, J.D.; Peelor, F.F., 3rd; Nguyen, H.V.M.; Richardson, A.; Choi, S.; Miller, B.F.; Wanagat, J. Age-induced changes in skeletal muscle mitochondrial DNA synthesis, quantity, and quality in genetically unique rats. Geroscience 2025, 47, 851–862. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Herbst, A.; Lee, C.C.; Vandiver, A.R.; Aiken, J.M.; McKenzie, D.; Hoang, A.; Allison, D.; Liu, N.; Wanagat, J. Mitochondrial DNA deletion mutations increase exponentially with age in human skeletal muscle. Aging Clin. Exp. Res. 2021, 33, 1811–1820. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Herbst, A.; Wanagat, J.; Cheema, N.; Widjaja, K.; McKenzie, D.; Aiken, J.M. Latent mitochondrial DNA deletion mutations drive muscle fiber loss at old age. Aging Cell 2016, 15, 1132–1139. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zanini, G.; Selleri, V.; Malerba, M.; Solodka, K.; Sinigaglia, G.; Nasi, M.; Mattioli, A.V.; Pinti, M. The role of Lonp1 on mitochondrial functions during cardiovascular and muscular diseases. Antioxidants 2023, 12, 598. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Migliavacca, E.; Tay, S.K.H.; Patel, H.P.; Sonntag, T.; Civiletto, G.; McFarlane, C.; Forrester, T.; Barton, S.J.; Leow, M.K.; Antoun, E.; et al. Mitochondrial oxidative capacity and NAD+ biosynthesis are reduced in human sarcopenia across ethnicities. Nat. Commun. 2019, 10, 5808. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hiona, A.; Sanz, A.; Kujoth, G.C.; Pamplona, R.; Seo, A.Y.; Hofer, T.; Someya, S.; Miyakawa, T.; Nakayama, C.; Samhan-Arias, A.K.; et al. Mitochondrial DNA mutations induce mitochondrial dysfunction, apoptosis and sarcopenia in skeletal muscle of mitochondrial DNA mutator mice. PLoS ONE 2010, 5, e11468. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kimoloi, S.; Sen, A.; Guenther, S.; Braun, T.; Brügmann, T.; Sasse, P.; Wiesner, R.J.; Pla-Martín, D.; Baris, O.R. Combined fibre atrophy and decreased muscle regeneration capacity driven by mitochondrial DNA alterations underlie the development of sarcopenia. J. Cachexia Sarcopenia Muscle 2022, 13, 2132–2145. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The chemistry of reactive oxygen species (ROS) revisited: Outlining their role in biological macromolecules (DNA, Lipids and Proteins) and induced pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaludercic, N.; Giorgio, V. The dual function of reactive oxygen/nitrogen species in bioenergetics and cell death: The role of ATP synthase. Oxidative Med. Cell. Longev. 2016, 2016, 3869610. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bonora, M.; Giorgi, C.; Pinton, P. Molecular mechanisms and consequences of mitochondrial permeability transition. Nat. Rev. Mol. Cell Biol. 2022, 23, 266–285. [Google Scholar] [CrossRef] [PubMed]
- Endlicher, R.; Drahota, Z.; Štefková, K.; Červinková, Z.; Kučera, O. The mitochondrial permeability transition pore—Current knowledge of its structure, function, and regulation, and optimized methods for evaluating its functional state. Cells 2023, 12, 1273. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Van Houten, B.; Hunter, S.E.; Meyer, J.N. Mitochondrial DNA damage induced autophagy, cell death, and disease. Front. Biosci. (Landmark Ed.) 2016, 21, 42–54. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Picard, M.; Juster, R.P.; McEwen, B.S. Mitochondrial allostatic load puts the ‘gluc’ back in glucocorticoids. Nat. Rev. Endocrinol. 2014, 10, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Coen, P.M.; Huo, Z.; Tranah, G.J.; Barnes, H.N.; Zhang, X.; Wolff, C.A.; Wu, K.; Cawthon, P.M.; Hepple, R.T.; Toledo, F.G.S.; et al. Autophagy gene expression in skeletal muscle of older individuals is associated with physical performance, muscle volume and mitochondrial function in the study of muscle, mobility and ageing (SOMMA). Ageing Cell 2024, 23, e14118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Picca, A.; Faitg, J.; Auwerx, J.; Ferrucci, L.; D’Amico, D. Mitophagy in human health, ageing and disease. Nat. Metab. 2023, 5, 2047–2061. [Google Scholar] [CrossRef] [PubMed]
- Rodney, G.G.; Pal, R.; Abo-Zahrah, R. Redox regulation of autophagy in skeletal muscle. Free Radic. Biol. Med. 2016, 98, 103–112. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Foreman, N.A.; Hesse, A.S.; Ji, L.L. Redox signaling and sarcopenia: Searching for the primary suspect. Int. J. Mol. Sci. 2021, 22, 9045. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zeng, Z.; Liang, J.; Wu, L.; Zhang, H.; Lv, J.; Chen, N. Exercise-induced autophagy suppresses sarcopenia through Akt/mTOR and Akt/FoxO3a signal pathways and AMPK-mediated mitochondrial quality control. Front. Physiol. 2020, 11, 583478. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rabinovitch, R.C.; Samborska, B.; Faubert, B.; Ma, E.H.; Gravel, S.P.; Andrzejewski, S.; Raissi, T.C.; Pause, A.; St-Pierre, J.; Jones, R.G. AMPK maintains cellular metabolic homeostasis through regulation of mitochondrial reactive oxygen species. Cell Rep. 2017, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrera, M.C.; Arc-Chagnaud, C.; Salvador-Pascual, A.; Brioche, T.; Chopard, A.; Olaso-Gonzalez, G.; Viña, J. Redox modulation of muscle mass and function. Redox Biol. 2020, 35, 101531. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hong, X.; Isern, J.; Campanario, S.; Perdiguero, E.; Ramírez-Pardo, I.; Segalés, J.; Hernansanz-Agustín, P.; Curtabbi, A.; Deryagin, O.; Pollán, A.; et al. Mitochondrial dynamics maintain muscle stem cell regenerative competence throughout adult life by regulating metabolism and mitophagy. Cell Stem Cell 2022, 29, 1298–1314.e10, Erratum in Cell Stem Cell 2022, 29, 1506–1508. [Google Scholar] [CrossRef] [PubMed]
- García-Prat, L.; Martínez-Vicente, M.; Perdiguero, E.; Ortet, L.; Rodríguez-Ubreva, J.; Rebollo, E.; Ruiz-Bonilla, V.; Gutarra, S.; Ballestar, E.; Serrano, A.L.; et al. Autophagy maintains stemness by preventing senescence. Nature 2016, 529, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Szentesi, P.; Csernoch, L.; Dux, L.; Keller-Pintér, A. Changes in redox signaling in the skeletal muscle with ageing. Oxidative Med. Cell. Longev. 2019, 2019, 4617801. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sahu, A.; Mamiya, H.; Shinde, S.N.; Cheikhi, A.; Winter, L.L.; Vo, N.V.; Stolz, D.; Roginskaya, V.; Tang, W.Y.; St Croix, C.; et al. Age-related declines in α-Klotho drive progenitor cell mitochondrial dysfunction and impaired muscle regeneration. Nat. Commun. 2018, 9, 4859. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- White, J.P.; Billin, A.N.; Campbell, M.E.; Russell, A.J.; Huffman, K.M.; Kraus, W.E. The AMPK/p27Kip1 Axis regulates autophagy/apoptosis decisions in aged skeletal muscle stem cells. Stem Cell Rep. 2018, 11, 425–439. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bernet, J.D.; Doles, J.D.; Hall, J.K.; Kelly Tanaka, K.; Carter, T.A.; Olwin, B.B. p38 MAPK signaling underlies a cell-autonomous loss of stem cell self-renewal in skeletal muscle of aged mice. Nat. Med. 2014, 20, 265–271. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tierney, M.T.; Aydogdu, T.; Sala, D.; Malecova, B.; Gatto, S.; Puri, P.L.; Latella, L.; Sacco, A. STAT3 signaling controls satellite cell expansion and skeletal muscle repair. Nat. Med. 2014, 20, 1182–1186. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, C.W.; Yu, K.; Shyh-Chang, N.; Jiang, Z.; Liu, T.; Ma, S.; Luo, L.; Guang, L.; Liang, K.; Ma, W.; et al. Pathogenesis of sarcopenia and the relationship with fat mass: Descriptive review. J. Cachexia Sarcopenia Muscle 2022, 13, 781–794. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lukjanenko, L.; Jung, M.J.; Hegde, N.; Perruisseau-Carrier, C.; Migliavacca, E.; Rozo, M.; Karaz, S.; Jacot, G.; Schmidt, M.; Li, L.; et al. Loss of fibronectin from the aged stem cell niche affects the regenerative capacity of skeletal muscle in mice. Nat. Med. 2016, 22, 897–905. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sugihara, H.; Teramoto, N.; Yamanouchi, K.; Matsuwaki, T.; Nishihara, M. Oxidative stress-mediated senescence in mesenchymal progenitor cells causes the loss of their fibro/adipogenic potential and abrogates myoblast fusion. Ageing 2018, 10, 747–763. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reid, M.B.; Moylan, J.S. Beyond atrophy: Redox mechanisms of muscle dysfunction in chronic inflammatory disease. J. Physiol. 2011, 589, 2171–2179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zuo, L.; Hallman, A.H.; Yousif, M.K.; Chien, M.T. Oxidative stress, respiratory muscle dysfunction, and potential therapeutics in chronic obstructive pulmonary disease. Front. Biol. 2012, 7, 506–513. [Google Scholar] [CrossRef]
- Zampino, M.; Brennan, N.A.; Kuo, P.L.; Spencer, R.G.; Fishbein, K.W.; Simonsick, E.M.; Ferrucci, L. Poor mitochondrial health and systemic inflammation? Test of a classic hypothesis in the Baltimore Longitudinal Study of Ageing. Geroscience 2020, 42, 1175–1182. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sivandzade, F.; Prasad, S.; Bhalerao, A.; Cucullo, L. NRF2 and NF-κB interplay in cerebrovascular and neurodegenerative disorders: Molecular mechanisms and possible therapeutic approaches. Redox Biol. 2019, 21, 101059. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Malik, V.; Rodino-Klapac, L.R.; Mendell, J.R. Emerging drugs for Duchenne muscular dystrophy. Expert Opin. Emerg. Drugs 2012, 17, 261–277. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zuo, L.; Hallman, A.H.; Roberts, W.J.; Wagner, P.D.; Hogan, M.C. Superoxide release from contracting skeletal muscle in pulmonary TNF-α overexpression mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 306, R75–R81. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, M.; Wang, Y.; Deng, S.; Lian, Z.; Yu, K. Skeletal muscle oxidative stress and inflammation in ageing: Focus on antioxidant and anti-inflammatory therapy. Front. Cell Dev. Biol. 2022, 10, 964130. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharma, B.; Dabur, R. Role of pro-inflammatory cytokines in regulation of skeletal muscle metabolism: A systematic review. Curr. Med. Chem. 2020, 27, 2161–2188. [Google Scholar] [CrossRef] [PubMed]
- Ochala, J.; Gustafson, A.M.; Diez, M.L.; Renaud, G.; Li, M.; Aare, S.; Qaisar, R.; Banduseela, V.C.; Hedström, Y.; Tang, X.; et al. Preferential skeletal muscle myosin loss in response to mechanical silencing in a novel rat intensive care unit model: Underlying mechanisms. J. Physiol. 2011, 589, 2007–2026. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative stress: The mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol. 2013, 87, 1157–1180. [Google Scholar] [CrossRef] [PubMed]
- Casati, S.R.; Cervia, D.; Roux-Biejat, P.; Moscheni, C.; Perrotta, C.; De Palma, C. Mitochondria and reactive oxygen species: The therapeutic balance of powers for Duchenne muscular dystrophy. Cells 2024, 13, 574. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Castejon-Vega, B.; Cordero, M.D.; Sanz, A. How the Disruption of Mitochondrial Redox Signalling Contributes to Ageing. Antioxidants 2023, 12, 831. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brioche, T.; Lemoine-Morel, S. Oxidative stress, sarcopenia, antioxidant strategies and exercise: Molecular aspects. Curr. Pharm. Des. 2016, 22, 2664–2678. [Google Scholar] [CrossRef] [PubMed]
- Berlett, B.S.; Stadtman, E.R. Protein oxidation in ageing, disease, and oxidative stress. J. Biol. Chem. 1997, 272, 20313–20316. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R.; Levine, R.L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 2003, 25, 207–218. [Google Scholar] [CrossRef]
- Abello, N.; Kerstjens, H.A.; Postma, D.S.; Bischoff, R. Protein tyrosine nitration: Selectivity, physicochemical and biological consequences, denitration, and proteomics methods for the identification of tyrosine-nitrated proteins. J. Proteome Res. 2009, 8, 3222–3238. [Google Scholar] [CrossRef] [PubMed]
- Zielonka, J.; Sikora, A.; Joseph, J.; Kalyanaraman, B. Peroxynitrite is the major species formed from different flux ratios of co-generated nitric oxide and superoxide: Direct reaction with boronate-based fluorescent probe. J. Biol. Chem. 2010, 285, 14210–14216. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Augusto, O.; Bonini, M.G.; Amanso, A.M.; Linares, E.; Santos, C.C.; De Menezes, S.L. Nitrogen dioxide and carbonate radical anion: Two emerging radicals in biology. Free Radic. Biol. Med. 2002, 32, 841–859. [Google Scholar] [CrossRef] [PubMed]
- Islam, B.U.; Habib, S.; Ahmad, P.; Allarakha, S.; Moinuddin, A.A. Pathophysiological role of peroxynitrite induced DNA damage in human diseases: A special focus on poly(ADP-ribose) polymerase (PARP). Indian J. Clin. Biochem. 2015, 30, 368–385. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jung, T.; Höhn, A.; Grune, T. The proteasome and the degradation of oxidized proteins: Part II—Protein oxidation and proteasomal degradation. Redox Biol. 2014, 2, 99–104. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barreiro, E.; Coronell, C.; Laviña, B.; Ramírez-Sarmiento, A.; Orozco-Levi, M.; Gea, J.; PENAM Project. Ageing, sex differences, and oxidative stress in human respiratory and limb muscles. Free Radic. Biol. Med. 2006, 41, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Day, N.J.; Kelly, S.S.; Lui, L.Y.; Mansfield, T.A.; Gaffrey, M.J.; Trejo, J.B.; Sagendorf, T.J.; Attah, I.K.; Moore, R.J.; Douglas, C.M.; et al. Signatures of cysteine oxidation on muscle structural and contractile proteins are associated with physical performance and muscle function in older adults: Study of Muscle, Mobility and Ageing (SOMMA). Ageing Cell 2024, 23, e14094. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Andersson, D.C.; Betzenhauser, M.J.; Reiken, S.; Meli, A.C.; Umanskaya, A.; Xie, W.; Shiomi, T.; Zalk, R.; Lacampagne, A.; Marks, A.R. Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in ageing. Cell Metab. 2011, 14, 196–207. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xie, T.; Qiao, X.; Sun, C.; Chu, B.; Meng, J.; Chen, C. GAPDH S-nitrosation contributes to age-related sarcopenia through mediating apoptosis. Nitric Oxide 2022, 120, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Baldelli, S.; Ciriolo, M.R. Altered S-nitrosylation of p53 is responsible for impaired antioxidant response in skeletal muscle during ageing. Ageing 2016, 8, 3450–3467. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Samengo, G.; Avik, A.; Fedor, B.; Whittaker, D.; Myung, K.H.; Wehling-Henricks, M.; Tidball, J.G. Age-related loss of nitric oxide synthase in skeletal muscle causes reductions in calpain S-nitrosylation that increase myofibril degradation and sarcopenia. Ageing Cell 2012, 11, 1036–1045. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, B.P. Cellular defenses against damage from reactive oxygen species. Physiol. Rev. 1994, 74, 139–162. [Google Scholar] [CrossRef] [PubMed]
- Jaganjac, M.; Tirosh, O.; Cohen, G.; Sasson, S.; Zarkovic, N. Reactive aldehydes—Second messengers of free radicals in diabetes mellitus. Free Radic. Res. 2013, 47 (Suppl. S1), 39–48. [Google Scholar] [CrossRef] [PubMed]
- Schaur, R.J.; Siems, W.; Bresgen, N.; Eckl, P.M. 4-Hydroxy-nonenal—A bioactive lipid peroxidation product. Biomolecules 2015, 5, 2247–2337. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pizzimenti, S.; Ciamporcero, E.; Daga, M.; Pettazzoni, P.; Arcaro, A.; Cetrangolo, G.; Minelli, R.; Dianzani, C.; Lepore, A.; Gentile, F.; et al. Interaction of aldehydes derived from lipid peroxidation and membrane proteins. Front. Physiol. 2013, 4, 242. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gentile, F.; Arcaro, A.; Pizzimenti, S.; Daga, M.; Cetrangolo, G.P.; Dianzani, C.; Lepore, A.; Graf, M.; Ames, P.R.J.; Barrera, G. DNA damage by lipid peroxidation products: Implications in cancer, inflammation and autoimmunity. AIMS Genet. 2017, 4, 103–137. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, X.; Zhang, Z.; Liu, X.; Wu, D.; Ding, Y.; Li, G.; Wu, Y. Typical reactive carbonyl compounds in food products: Formation, influence on food quality, and detection methods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 503–529. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Liu, Z.; Zhang, W.; Xia, G.; Li, C.; Rakariyatham, K.; Zhou, D. Advance in aldehydes derived from lipid oxidation: A review of the formation mechanism, attributable food thermal processing technology, analytical method and toxicological effect. Food Res. Int. 2025, 203, 115811. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.J.; Siraki, A.G.; Shangari, N. Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit. Rev. Toxicol. 2005, 35, 609–662. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Huang, R.J.; Yang, L.; Dai, W.; Ni, H.; Gong, Y.; Guo, J.; Zhong, H.; Lin, C.; Xu, W. Direct emissions of particulate glyoxal and methylglyoxal from biomass burning and coal combustion. Sci. Total Environ. 2023, 862, 160757. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Feng, Y.; Peng, Y.; Cai, J.; Li, C.; Li, Q.; Zheng, M.; Chen, Y. Emission characteristics and formation mechanism of carbonyl compounds from residential solid fuel combustion based on real-world measurements and tube-furnace experiments. Environ. Sci. Technol. 2022, 56, 15417–15426. [Google Scholar] [CrossRef] [PubMed]
- Colombo, G.; Garavaglia, M.L.; Astori, E.; Giustarini, D.; Rossi, R.; Milzani, A.; Dalle-Donne, I. Protein carbonylation in human bronchial epithelial cells exposed to cigarette smoke extract. Cell Biol. Toxicol. 2019, 35, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Lawal, A.; Ricks, J.; Fox, J.R.; Larson, T.; Navab, M.; Fogelman, A.M.; Rosenfeld, M.E.; Araujo, J.A. Diesel exhaust induces systemic lipid peroxidation and development of dysfunctional pro-oxidant and pro-inflammatory high-density lipoprotein. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Bin, P.; Shen, M.; Li, H.; Sun, X.; Niu, Y.; Meng, T.; Yu, T.; Zhang, X.; Dai, Y.; Gao, W.; et al. Increased levels of urinary biomarkers of lipid peroxidation products among workers occupationally exposed to diesel engine exhaust. Free Radic. Res. 2016, 50, 820–830. [Google Scholar] [CrossRef] [PubMed]
- Barrera, G.; Pizzimenti, S.; Ciamporcero, E.S.; Daga, M.; Ullio, C.; Arcaro, A.; Cetrangolo, G.P.; Ferretti, C.; Dianzani, C.; Lepore, A.; et al. Role of 4-hydroxynonenal-protein adducts in human diseases. Antioxid. Redox Signal. 2015, 22, 1681–1702. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.; Portero-Otin, M.; Pamplona, R.; Ferrer, I. Protein targets of oxidative damage in human neurodegenerative diseases with abnormal protein aggregates. Brain Pathol. 2010, 20, 281–297. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Markesbery, W.R.; Kryscio, R.J.; Lovell, M.A.; Morrow, J.D. Lipid peroxidation is an early event in the brain in amnestic mild cognitive impairment. Ann. Neurol. 2005, 58, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C. Mass Spectrometry Analysis of DNA and Protein Adducts as Biomarkers in Human Exposure to Cigarette Smoking: Acrolein as an Example. Chem. Res. Toxicol. 2023, 36, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Barrera, G.; Pizzimenti, S.; Daga, M.; Dianzani, C.; Arcaro, A.; Cetrangolo, G.P.; Giordano, G.; Cucci, M.A.; Graf, M.; Gentile, F. Lipid peroxidation-derived aldehydes, 4-hydroxynonenal and malondialdehyde in ageing-related disorders. Antioxidants 2018, 7, 102. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feng, J.; Xie, H.; Meany, D.L.; Thompson, L.V.; Arriaga, E.A.; Griffin, T.J. Quantitative proteomic profiling of muscle type-dependent and age-dependent protein carbonylation in rat skeletal muscle mitochondria. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 1137–1152. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eshima, H.; Shahtout, J.L.; Siripoksup, P.; Pearson, M.J.; Mahmassani, Z.S.; Ferrara, P.J.; Lyons, A.W.; Maschek, J.A.; Peterlin, A.D.; Verkerke, A.R.P.; et al. Lipid hydroperoxides promote sarcopenia through carbonyl stress. Elife 2023, 12, e85289. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kovacheva, E.L.; Hikim, A.P.; Shen, R.; Sinha, I.; Sinha-Hikim, I. Testosterone supplementation reverses sarcopenia in ageing through regulation of myostatin, c-Jun NH2-terminal kinase, Notch, and Akt signaling pathways. Endocrinology 2010, 151, 628–638. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brown, J.L.; Peelor, F.F., 3rd; Georgescu, C.; Wren, J.D.; Kinter, M.; Tyrrell, V.J.; O’Donnell, V.B.; Miller, B.F.; Van Remmen, H. Lipid hydroperoxides and oxylipins are mediators of denervation induced muscle atrophy. Redox Biol. 2022, 57, 102518. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pharaoh, G.; Brown, J.L.; Sataranatarajan, K.; Kneis, P.; Bian, J.; Ranjit, R.; Hadad, N.; Georgescu, C.; Rabinovitch, P.; Ran, Q.; et al. Targeting cPLA2 derived lipid hydroperoxides as a potential intervention for sarcopenia. Sci. Rep. 2020, 10, 13968. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Safdar, A.; deBeer, J.; Tarnopolsky, M.A. Dysfunctional Nrf2-Keap1 redox signaling in skeletal muscle of the sedentary old. Free Radic. Biol. Med. 2010, 49, 1487–1493. [Google Scholar] [CrossRef] [PubMed]
- Baraibar, M.A.; Hyzewicz, J.; Rogowska-Wrzesinska, A.; Bulteau, A.L.; Prip-Buus, C.; Butler-Browne, G.; Friguet, B. Impaired energy metabolism of senescent muscle satellite cells is associated with oxidative modifications of glycolytic enzymes. Ageing 2016, 8, 3375–3389. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lourenço Dos Santos, S.; Baraibar, M.A.; Lundberg, S.; Eeg-Olofsson, O.; Larsson, L.; Friguet, B. Oxidative proteome alterations during skeletal muscle ageing. Redox Biol. 2015, 5, 267–274. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Twarda-Clapa, A.; Olczak, A.; Białkowska, A.M.; Koziołkiewicz, M. Advanced glycation end-products (AGEs): Formation, chemistry, classification, receptors, and diseases related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lange, J.N.; Wood, K.D.; Knight, J.; Assimos, D.G.; Holmes, R.P. Glyoxal formation and its role in endogenous oxalate synthesis. Adv. Urol. 2012, 2012, 819202. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pamplona, R.; Dalfó, E.; Ayala, V.; Bellmunt, M.J.; Prat, J.; Ferrer, I.; Portero-Otín, M. Proteins in human brain cortex are modified by oxidation, glycoxidation, and lipoxidation. Effects of Alzheimer disease and identification of lipoxidation targets. J. Biol. Chem. 2005, 280, 21522–21530. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.W.; Hedegaard, R.V.; Andersen, J.M.; de Courten, B.; Bügel, S.; Nielsen, J.; Skibsted, L.H.; Dragsted, L.O. Advanced glycation endproducts in food and their effects on health. Food Chem. Toxicol. 2013, 60, 10–37. [Google Scholar] [CrossRef] [PubMed]
- Sroga, G.E.; Vashishth, D. In vivo glycation-interplay between oxidant and carbonyl stress in bone. JBMR Plus 2024, 8, ziae110. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cepas, V.; Collino, M.; Mayo, J.C.; Sainz, R.M. Redox signaling and advanced glycation endproducts (AGEs) in diet-related diseases. Antioxidants 2020, 9, 142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Perrone, A.; Giovino, A.; Benny, J.; Martinelli, F. Advanced glycation end products (AGEs): Biochemistry, signaling, analytical methods, and epigenetic effects. Oxidative Med. Cell. Longev. 2020, 2020, 3818196. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eguchi, Y.; Toyoguchi, T.; Inage, K.; Fujimoto, K.; Orita, S.; Suzuki, M.; Kanamoto, H.; Abe, K.; Norimoto, M.; Umimura, T.; et al. Advanced glycation end products are associated with sarcopenia in older women: Ageing marker dynamics. J. Women Ageing 2021, 33, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, J.; Zhang, Q.; Lu, A.; Du, Y.; Ye, X. Elevated serum pentosidine is independently associated with the high prevalence of sarcopenia in Chinese middle-aged and elderly men with type 2 diabetes mellitus. J. Diabetes Investig. 2021, 12, 2054–2061. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Waqas, K.; Chen, J.; Lu, T.; van der Eerden, B.C.J.; Rivadeneira, F.; Uitterlinden, A.G.; Voortman, T.; Zillikens, M.C. Dietary advanced glycation end-products (dAGEs) intake and its relation to sarcopenia and frailty—The Rotterdam Study. Bone 2022, 165, 116564. [Google Scholar] [CrossRef] [PubMed]
- Waqas, K.; Chen, J.; Trajanoska, K.; Ikram, M.A.; Uitterlinden, A.G.; Rivadeneira, F.; Zillikens, M.C. Skin autofluorescence, a noninvasive biomarker for advanced glycation end-products, is associated with sarcopenia. J. Clin. Endocrinol. Metab. 2022, 107, e793–e803. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, X.; Chen, X.; Li, S.; Gao, M.; Han, P.; Cao, L.; Gao, J.; Tao, Q.; Zhai, J.; Liang, D.; et al. Association between advanced glycation end products and sarcopenia: The mediating role of osteoporosis. J. Clin. Endocrinol. Metab. 2024, 109, e1105–e1116. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Granic, A.; Hurst, C.; Dismore, L.; Dodds, R.M.; Witham, M.D.; Robinson, S.M.; Sayer, A.A. Advanced glycation end products in skeletal muscle health and sarcopenia: A systematic review of observational studies. Mech. Ageing Dev. 2023, 209, 111744. [Google Scholar] [CrossRef] [PubMed]
- Egawa, T.; Ohno, Y.; Yokoyama, S.; Goto, A.; Ito, R.; Hayashi, T.; Goto, K. The effect of advanced glycation end products on cellular signaling molecules in skeletal muscle. J. Phys. Fit. Sports Med. 2018, 7, 229–238. [Google Scholar] [CrossRef]
- Takata, T.; Sakasai-Sakai, A.; Takeuchi, M. Impact of intracellular toxic advanced glycation end-products (TAGE) on murine myoblast cell death. Diabetol. Metab. Syndr. 2020, 12, 54. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Adachi, N.; Kanazawa, I.; Tanaka, K.I.; Takeno, A.; Notsu, M.; Tanaka, S.; Sugimoto, T. Insulin-like growth factor-I protects against the detrimental effects of advanced glycation end products and high glucose in myoblastic C2C12 cells. Calcif. Tissue Int. 2019, 105, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Olson, L.C.; Redden, J.T.; Schwartz, Z.; Cohen, D.J.; McClure, M.J. Advanced glycation end-products in skeletal muscle ageing. Bioengineering 2021, 8, 168. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Egawa, T.; Hayashi, T. Association of glycative stress with motor and muscle function. Front. Physiol. 2022, 13, 855358. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dozio, E.; Vettoretti, S.; Lungarella, G.; Messa, P.; Corsi Romanelli, M.M. Sarcopenia in chronic kidney disease: Focus on advanced glycation end products as mediators and markers of oxidative stress. Biomedicines 2021, 9, 405. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, Z.; Li, H.; Jiang, S.; Rahmati, M.; Su, J.; Yang, S.; Wu, Y.; Li, Y.; Deng, Z. The role of AGEs in muscle ageing and sarcopenia. Bone Jt. Res. 2025, 14, 185–198. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tian, K.; Xu, Y.; Sahebkar, A.; Xu, S. CD36 in Atherosclerosis: Pathophysiological Mechanisms and Therapeutic Implications. Curr. Atheroscler. Rep. 2020, 22, 59. [Google Scholar] [CrossRef] [PubMed]
- Afonso, C.B.; Spickett, C.M. Lipoproteins as targets and markers of lipoxidation. Redox Biol. 2019, 23, 101066. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, Y.M. CD36, a scavenger receptor implicated in atherosclerosis. Exp. Mol. Med. 2014, 46, e99. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Frijhoff, J.; Winyard, P.G.; Zarkovic, N.; Davies, S.S.; Stocker, R.; Cheng, D.; Knight, A.R.; Taylor, E.L.; Oettrich, J.; Ruskovska, T.; et al. Clinical relevance of biomarkers of oxidative stress. Antioxid. Redox Signal. 2015, 23, 1144–1170. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, H.J.; Jeong, M.S.; Jang, S.B. Molecular characteristics of RAGE and advances in small-molecule inhibitors. Int. J. Mol. Sci. 2021, 22, 6904. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dong, H.; Zhang, Y.; Huang, Y.; Deng, H. Pathophysiology of RAGE in inflammatory diseases. Front. Immunol. 2022, 13, 931473. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Volpe, C.M.O.; Villar-Delfino, P.H.; Dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ighodaro, O.M. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharmacother. 2018, 108, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Riuzzi, F.; Sorci, G.; Sagheddu, R.; Chiappalupi, S.; Salvadori, L.; Donato, R. RAGE in the pathophysiology of skeletal muscle. J. Cachexia Sarcopenia Muscle 2018, 9, 1213–1234. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garay-Sevilla, M.E.; Beeri, M.S.; de la Maza, M.P.; Rojas, A.; Salazar-Villanea, S.; Uribarri, J. The potential role of dietary advanced glycation endproducts in the development of chronic non-infectious diseases: A narrative review. Nutr. Res. Rev. 2020, 33, 298–311. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.Y.; Tseng, Y.T.; Lee, T.Y.; Fu, Y.C.; Chang, W.H.; Lo, W.W.; Lin, C.L.; Lo, Y.C. Triptolide prevents LPS-induced skeletal muscle atrophy via inhibiting NF-κB/TNF-α and regulating protein synthesis/degradation pathway. Br. J. Pharmacol. 2021, 178, 2998–3016. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Li, N.; Jia, W.; Wang, N.; Liang, M.; Yang, X.; Du, G. Skeletal muscle atrophy: From mechanisms to treatments. Pharmacol. Res. 2021, 172, 105807. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.-Y.; Yang, R.-S.; Sheu, M.-L.; Chan, D.-C.; Yang, T.-H.; Tsai, K.-S.; Chiang, C.-K.; Liu, S.-H. Advanced glycation end-products induce skeletal muscle atrophy and dysfunction in diabetic mice via a RAGE-mediated, AMPK-down-regulated, Akt pathway. J. Pathol. 2016, 238, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Ott, C.; Jacobs, K.; Haucke, E.; Navarrete Santos, A.; Grune, T.; Simm, A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014, 2, 411–429. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cai, W.; He, J.C.; Zhu, L.; Chen, X.; Striker, G.E.; Vlassara, H. AGE-receptor-1 counteracts cellular oxidant stress induced by AGEs via negative regulation of p66shc-dependent FKHRL1 phosphorylation. Am. J. Physiol. Cell Physiol. 2008, 294, C145–C152. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Chang, C.H.; Chen, C.Y.; Shih, C.Y.; Peng, J.K.; Huang, H.L.; Chen, P.Y.; Huang, T.L.; Chen, C.Y.; Tsai, J.S. Upregulation of cluster of differentiation 36 mRNA expression in peripheral blood mononuclear cells correlates with frailty severity in older adults. J. Cachexia Sarcopenia Muscle 2022, 13, 1948–1955. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eleutherio, E.C.A.; Silva Magalhães, R.S.; de Araújo Brasil, A.; Monteiro Neto, J.R.; de Holanda Paranhos, L. SOD1, more than just an antioxidant. Arch. Biochem. Biophys. 2021, 697, 108701. [Google Scholar] [CrossRef] [PubMed]
- Fridovich, I. Biological effects of the superoxide radical. Arch. Biochem. Biophys. 2022, 726, 109228. [Google Scholar] [CrossRef] [PubMed]
- Vasileiadou, O.; Nastos, G.G.; Chatzinikolaou, P.N.; Papoutsis, D.; Vrampa, D.I.; Methenitis, S.; Margaritelis, N.V. Redox profile of skeletal muscles: Implications for research design and interpretation. Antioxidants 2023, 12, 1738. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Powers, S.K.; Ji, L.L.; Kavazis, A.N.; Jackson, M.J. Reactive oxygen species: Impact on skeletal muscle. Compr. Physiol. 2011, 1, 941–969. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Halliwell, B. Understanding mechanisms of antioxidant action in health and disease. Nat. Rev. Mol. Cell Biol. 2024, 25, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Flohé, L.; Toppo, S.; Orian, L. The glutathione peroxidase family: Discoveries and mechanism. Free Radic. Biol. Med. 2022, 187, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Lapenna, D. Glutathione and glutathione-dependent enzymes: From biochemistry to gerontology and successful ageing. Ageing Res. Rev. 2023, 92, 102066. [Google Scholar] [CrossRef] [PubMed]
- Perkins, A.; Nelson, K.J.; Parsonage, D.; Poole, L.B.; Karplus, P.A. Peroxiredoxins: Guardians against oxidative stress and modulators of peroxide signaling. Trends Biochem. Sci. 2015, 40, 435–445. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karplus, P.A. A primer on peroxiredoxin biochemistry. Free Radic. Biol. Med. 2015, 80, 183–190. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, M.; Deng, C.; Lo, T.H.; Chan, K.Y.; Li, X.; Wong, C.M. Peroxiredoxin, senescence, and cancer. Cells 2022, 11, 1772. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Villar, S.F.; Ferrer-Sueta, G.; Denicola, A. The multifaceted nature of peroxiredoxins in chemical biology. Curr. Opin. Chem. Biol. 2023, 76, 102355. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Lin, Y.; Huang, Y.; Shen, Y.Q.; Chen, Q. Thioredoxin (Trx): A redox target and modulator of cellular senescence and aging-related diseases. Redox Biol. 2024, 70, 103032. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, J.; Duan, D.; Osama, A.; Fang, J. Natural molecules targeting thioredoxin system and their therapeutic potential. Antioxid. Redox Signal. 2021, 34, 1083–1107. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Peng, Y.; Li, J.; Holmgren, A.; Lu, J. Modulation of thiol-dependent redox system by metal ions via thioredoxin and glutaredoxin systems. Metallomics 2018, 10, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Manabe, Y.; Takagi, M.; Nakamura-Yamada, M.; Goto-Inoue, N.; Taoka, M.; Isobe, T.; Fujii, N.L. Redox proteins are constitutively secreted by skeletal muscle. J. Physiol. Sci. 2014, 64, 401–409. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rohrbach, S.; Gruenler, S.; Teschner, M.; Holtz, J. The thioredoxin system in ageing muscle: Key role of mitochondrial thioredoxin reductase in the protective effects of caloric restriction? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R927–R935. [Google Scholar] [CrossRef] [PubMed]
- Bao, F.; Zhao, X.; You, J.; Liu, Y.; Xu, Z.; Wu, Y.; Wu, Y.; Xu, Z.; Yu, L.; Li, J.; et al. Aerobic exercise alleviates skeletal muscle ageing in male rats by inhibiting apoptosis via regulation of the Trx system. Exp. Gerontol. 2024, 194, 112523. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Kim, M.; Lee, M.; Bamann, K.; Olguin, F.; He, H.; Wang, Y.; Jiang, B.; Fang, S.; Zhu, J.; et al. Overexpression of thioredoxin-2 attenuates age-related muscle loss by suppressing mitochondrial oxidative stress and apoptosis. JCSM Rapid Commun. 2022, 5, 130–145. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vašková, J.; Kočan, L.; Vaško, L.; Perjési, P. Glutathione-related enzymes and proteins: A review. Molecules 2023, 28, 1447. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, Y.; Liao, Z.; Xiao, Q. Metformin ameliorates skeletal muscle atrophy in Grx1 KO mice by regulating intramuscular lipid accumulation and glucose utilization. Biochem. Biophys. Res. Commun. 2020, 533, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Chalker, J.; Gardiner, D.; Kuksal, N.; Mailloux, R.J. Characterization of the impact of glutaredoxin-2 (GRX2) deficiency on superoxide/hydrogen peroxide release from cardiac and liver mitochondria. Redox Biol. 2018, 15, 216–227. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Brien, M.; Chalker, J.; Slade, L.; Gardiner, D.; Mailloux, R.J. Protein S-glutathionylation alters superoxide/hydrogen peroxide emission from pyruvate dehydrogenase complex. Free Radic. Biol. Med. 2017, 106, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yu, Y.; David, L.; Ho, Y.S.; Lou, M.F. Glutaredoxin 2 (Grx2) gene deletion induces early onset of age-dependent cataracts in mice. J. Biol. Chem. 2014, 289, 36125–36139. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Young, A.; Gardiner, D.; Kuksal, N.; Gill, R.; O’Brien, M.; Mailloux, R.J. Deletion of the glutaredoxin-2 gene protects mice from diet-induced weight gain, which correlates with increased mitochondrial respiration and proton leaks in skeletal muscle. Antioxid. Redox Signal. 2019, 31, 1272–1288. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Pain, J.; Singh, P.; Dancis, A.; Pain, D. Mitochondrial glutaredoxin Grx5 functions as a central hub for cellular iron-sulfur cluster assembly. J. Biol. Chem. 2025, 301, 108391. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mancuso, C. Biliverdin as a disease-modifying agent: An integrated viewpoint. Free Radic. Biol. Med. 2023, 207, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Sun, C.C.; Tang, C.F. Heme oxygenase-1: A potential therapeutic target for improving skeletal muscle atrophy. Exp. Gerontol. 2023, 184, 112335. [Google Scholar] [CrossRef] [PubMed]
- Xanthis, V.; Mantso, T.; Dimtsi, A.; Pappa, A.; Fadouloglou, V.E. Human aldehyde dehydrogenases: A superfamily of similar yet different proteins highly related to cancer. Cancers 2023, 15, 4419. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mol, M.; Regazzoni, L.; Altomare, A.; Degani, G.; Carini, M.; Vistoli, G.; Aldini, G. Enzymatic and non-enzymatic detoxification of 4-hydroxynonenal: Methodological aspects and biological consequences. Free Radic. Biol. Med. 2017, 111, 328–344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, B.; Fan, X.; Wang, W.; Xu, T.; Wei, S.; Zheng, W.; Yuan, Q.; Gao, L.; Yin, X.; et al. Aldehyde dehydrogenase 2 protects against post-cardiac arrest myocardial dysfunction through a novel mechanism of suppressing mitochondrial reactive oxygen species production. Front. Pharmacol. 2020, 11, 373. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stockwell, B.R. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell 2022, 185, 2401–2421. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bolt, J.; Sandhu, S.; Mohammadi, A. Effect of coenzyme Q10 supplementation on sarcopenia, frailty, and falls: A scoping review. J. Nutr. Health Ageing 2023, 27, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Kurajoh, M.; Fukumoto, S.; Yoshida, S.; Akari, S.; Murase, T.; Nakamura, T.; Ishii, H.; Yoshida, H.; Nagata, Y.; Morioka, T.; et al. Uric acid shown to contribute to increased oxidative stress level independent of xanthine oxidoreductase activity in MedCity21 health examination registry. Sci. Rep. 2021, 11, 7378. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, X.; Li, H.; Chen, L.; Wu, Y.; Li, Y. NRF2 in age-related musculoskeletal diseases: Role and treatment prospects. Genes Dis. 2023, 11, 101180. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fourquet, S.; Guerois, R.; Biard, D.; Toledano, M.B. Activation of NRF2 by nitrosative agents and H2O2 involves KEAP1 disulfide formation. J. Biol. Chem. 2010, 285, 8463–8471. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McMahon, M.; Lamont, D.J.; Beattie, K.A.; Hayes, J.D. Keap1 perceives stress via three sensors for the endogenous signaling molecules nitric oxide, zinc, and alkenals. Proc. Natl. Acad. Sci. USA 2010, 107, 18838–18843. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kansanen, E.; Bonacci, G.; Schopfer, F.J.; Kuosmanen, S.M.; Tong, K.I.; Leinonen, H.; Woodcock, S.R.; Yamamoto, M.; Carlberg, C.; Ylä-Herttuala, S.; et al. Electrophilic nitro-fatty acids activate NRF2 by a KEAP1 cysteine 151-independent mechanism. J. Biol. Chem. 2011, 286, 14019–14027. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.J.; Gounder, S.S.; Kannan, S.; Goutam, K.; Muthusamy, V.R.; Firpo, M.A.; Symons, J.D.; Paine, R., 3rd; Hoidal, J.R.; Rajasekaran, N.S. Disruption of Nrf2/ARE signaling impairs antioxidant mechanisms and promotes cell degradation pathways in aged skeletal muscle. Biochim. Biophys. Acta 2012, 1822, 1038–1050. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Park, J.S.; Lee, Y.S.; Han, J.; Lee, D.K.; Kwon, S.W.; Han, D.H.; Lee, Y.H.; Bae, S.H. SQSTM1/p62 activates NFE2L2/NRF2 via ULK1-mediated autophagic KEAP1 degradation and protects mouse liver from lipotoxicity. Autophagy 2020, 16, 1949–1973. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Crilly, M.J.; Tryon, L.D.; Erlich, A.T.; Hood, D.A. The role of Nrf2 in skeletal muscle contractile and mitochondrial function. J. Appl. Physiol. (1985) 2016, 121, 730–740. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahn, B.; Pharaoh, G.; Premkumar, P.; Huseman, K.; Ranjit, R.; Kinter, M.; Szweda, L.; Kiss, T.; Fulop, G.; Tarantini, S.; et al. Nrf2 deficiency exacerbates age-related contractile dysfunction and loss of skeletal muscle mass. Redox Biol. 2018, 17, 47–58. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kitaoka, Y.; Tamura, Y.; Takahashi, K.; Takeda, K.; Takemasa, T.; Hatta, H. Effects of Nrf2 deficiency on mitochondrial oxidative stress in aged skeletal muscle. Physiol. Rep. 2019, 7, e13998. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Du, J.; Kang, Z.; Huang, L.; Zhou, F.; Feng, X.; Huang, J. Protective effects of Hirudin against compartment syndrome in rabbits through the activation of Nrf2/HO-1. Injury 2022, 53, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Martyn, J.A.J.; Kaneki, M. Muscle atrophy and the sestrins. N. Engl. J. Med. 2020, 383, 1279–1282. [Google Scholar] [CrossRef] [PubMed]
- Machado, I.F.; Palmeira, C.M.; Rolo, A.P. Sestrin2 is a central regulator of mitochondrial stress responses in disease and aging. Ageing Res. Rev. 2025, 109, 102762. [Google Scholar] [CrossRef] [PubMed]
- Zeng, N.; D’Souza, R.F.; Mitchell, C.J.; Cameron-Smith, D. Sestrins are differentially expressed with age in the skeletal muscle of men: A cross-sectional analysis. Exp. Gerontol. 2018, 110, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Shin, B.Y.; Jin, S.H.; Cho, I.J.; Ki, S.H. Nrf2-ARE pathway regulates induction of Sestrin-2 expression. Free Radic. Biol. Med. 2012, 53, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.H.; Sung, S.H.; Oh, S.Y.; Lim, J.M.; Lee, S.K.; Park, Y.N.; Lee, H.E.; Kang, D.; Rhee, S.G. Sestrins activate Nrf2 by promoting p62-dependent autophagic degradation of Keap1 and prevent oxidative liver damage. Cell Metab. 2013, 17, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Segalés, J.; Perdiguero, E.; Serrano, A.L.; Sousa-Victor, P.; Ortet, L.; Jardí, M.; Budanov, A.V.; Garcia-Prat, L.; Sandri, M.; Thomson, D.M.; et al. Sestrin prevents atrophy of disused and ageing muscles by integrating anabolic and catabolic signals. Nat. Commun. 2020, 11, 189. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, S.; Li, H.; Zhang, Y.; Song, H.; Fu, L. Exercise ameliorates chronic inflammatory response induced by high-fat diet via Sestrin2 in an Nrf2-dependent manner. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166792. [Google Scholar] [CrossRef] [PubMed]
- Taherkhani, S.; Valaei, K.; Arazi, H.; Suzuki, K. An overview of physical exercise and antioxidant supplementation influences on skeletal muscle oxidative stress. Antioxidants 2021, 10, 1528. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Millan-Domingo, F.; Garcia-Dominguez, E.; Gambini, J.; Olaso-Gonzalez, G.; Viña, J.; Gomez-Cabrera, M.C. Diet and exercise in frailty and sarcopenia. Molecular aspects. Mol. Asp. Med. 2024, 100, 101322. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, A.V.; Peruca, G.F.; Braga, R.R.; Brícola, R.S.; Lenhare, L.; Silva, V.R.R.; Anaruma, C.P.; Katashima, C.K.; Crisol, B.M.; Barbosa, L.T.; et al. High-intensity exercise training induces mitonuclear imbalance and activates the mitochondrial unfolded protein response in the skeletal muscle of aged mice. Geroscience 2021, 43, 1513–1518. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goh, J.; Wong, E.; Soh, J.; Maier, A.B.; Kennedy, B.K. Targeting the molecular & cellular pillars of human ageing with exercise. FEBS J. 2023, 290, 649–668. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Reactive oxygen species (ROS), oxygen radicals and antioxidants: Where are we now, where is the field going and where should we go? Biochem. Biophys. Res. Commun. 2022, 633, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Foti, M.C. Antioxidant properties of phenols. J. Pharm. Pharmacol. 2007, 59, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- Ingold, K.U.; Pratt, D.A. Advances in radical-trapping antioxidant chemistry in the 21st century: A kinetics and mechanisms perspective. Chem. Rev. 2014, 114, 9022–9046. [Google Scholar] [CrossRef] [PubMed]
- Foti, M.C.; Amorati, R. Non-phenolic radical-trapping antioxidants. J. Pharm. Pharmacol. 2009, 61, 1435–1448. [Google Scholar] [CrossRef] [PubMed]
- Gironés-Vilaplana, A.; Villaño, D.; Marhuenda, J.; Moreno, D.A.; García-Viguera, C. Vitamins. In Nutraceutical and Functional Food Components. Effects of Innovative Processing Techniques; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; Chapter 6; pp. 159–201. ISBN 9780128052570. [Google Scholar] [CrossRef]
- Yao, Y.; Yao, J.; Tang, D.; Wang, H.; Zhang, H.; Qiu, J.; Shu, X. Dietary antioxidant capacity and sarcopenia: A study from US population. Nutrition 2025, 130, 112613. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ding, X.; Zhang, Y.; Li, T.; Xu, P.; Ma, Y.; Xing, H.; Niu, Q.; Keerman, M. Serum concentrations of different or multiple vitamins and Sarcopenia risk among US adults: Insights from NHANES. BMC Public Health 2024, 24, 3372. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bhagavan, H.N.; Chopra, R.K. Coenzyme Q10: Absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic. Res. 2006, 40, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhou, J.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Health benefits and molecular mechanisms of Resveratrol: A narrative review. Foods 2020, 9, 340. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Masuoka, N. Stilbene compounds are specific inhibitors of the superoxide anion generation catalyzed by xanthine oxidase. Food Chem. X 2021, 12, 100146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Toniolo, L.; Formoso, L.; Torelli, L.; Crea, E.; Bergamo, A.; Sava, G.; Giacomello, E. Long-term resveratrol treatment improves the capillarization in the skeletal muscles of ageing C57BL/6J mice. Int. J. Food Sci. Nutr. 2021, 72, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Sirago, G.; Toniolo, L.; Crea, E.; Giacomello, E. A short-term treatment with resveratrol improves the inflammatory conditions of Middle-aged mice skeletal muscles. Int. J. Food Sci. Nutr. 2022, 73, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhu, X.; Chen, K.; Lang, H.; Zhang, Y.; Hou, P.; Ran, L.; Zhou, M.; Zheng, J.; Yi, L.; et al. Resveratrol prevents sarcopenic obesity by reversing mitochondrial dysfunction and oxidative stress via the PKA/LKB1/AMPK pathway. Aging 2019, 11, 2217–2240. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mohar, D.S.; Malik, S. The sirtuin system: The holy grail of resveratrol? J. Clin. Exp. Cardiolog. 2012, 3, 216. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, J.; Liao, Z.; Jia, J.; Chen, J.L.; Xiao, Q. The effects of resveratrol feeding and exercise training on the skeletal muscle function and transcriptome of aged rats. PeerJ 2019, 7, e7199. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tuntevski, K.; Hajira, A.; Nichols, A.; Alway, S.E.; Mohamed, J.S. Muscle-specific sirtuin1 gain-of-function ameliorates skeletal muscle atrophy in a pre-clinical mouse model of cerebral ischemic stroke. FASEB BioAdv. 2020, 2, 387–397. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hosoda, R.; Nakashima, R.; Yano, M.; Iwahara, N.; Asakura, S.; Nojima, I.; Saga, Y.; Kunimoto, R.; Horio, Y.; Kuno, A. Resveratrol, a SIRT1 activator, attenuates aging-associated alterations in skeletal muscle and heart in mice. J. Pharmacol. Sci. 2023, 152, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Alway, S.E.; McCrory, J.L.; Kearcher, K.; Vickers, A.; Frear, B.; Gilleland, D.L.; Bonner, D.E.; Thomas, J.M.; Donley, D.A.; Lively, M.W.; et al. Resveratrol enhances exercise-induced cellular and functional adaptations of skeletal muscle in older men and women. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1595–1606. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jitoe-Masuda, A.; Fujimoto, A.; Masuda, T. Curcumin: From chemistry to chemistry-based functions. Curr. Pharm. Des. 2013, 19, 2084–2092. [Google Scholar] [PubMed]
- Wang, M.Y.; Yang, J.M.; Wu, Y.; Li, H.; Zhong, Y.B.; Luo, Y.; Xie, R.L. Curcumin-activated Wnt5a pathway mediates Ca2+ channel opening to affect myoblast differentiation and skeletal muscle regeneration. J. Cachexia Sarcopenia Muscle 2024, 15, 1834–1849. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gorza, L.; Germinario, E.; Tibaudo, L.; Vitadello, M.; Tusa, C.; Guerra, I.; Bondì, M.; Salmaso, S.; Caliceti, P.; Vitiello, L.; et al. Chronic systemic curcumin administration antagonizes murine sarcopenia and presarcopenia. Int. J. Mol. Sci. 2021, 22, 11789. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, D.Y.; Chun, Y.S.; Kim, J.K.; Lee, J.O.; Ku, S.K.; Shim, S.M. Curcumin attenuates sarcopenia in chronic forced exercise executed aged mice by regulating muscle degradation and protein synthesis with antioxidant and anti-inflammatory effects. J. Agric. Food Chem. 2021, 69, 6214–6228. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.A.; Steward, W.P.; Gescher, A.J. Pharmacokinetics and pharmacodynamics of curcumin. Adv. Exp. Med. Biol. 2007, 595, 453–470. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.J.; Yang, I.H.; Lin, Y.W.; Lin, J.N.; Wu, C.C.; Chiang, C.Y.; Lai, K.H.; Lin, F.H. Curcumin-loaded hydrophobic surface-modified hydroxyapatite as an antioxidant for sarcopenia prevention. Antioxidants 2021, 10, 616. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Varma, K.; Amalraj, A.; Divya, C.; Gopi, S. The efficacy of the novel bioavailable curcumin (Cureit) in the management of sarcopenia in healthy elderly subjects: A randomized, placebo-controlled, double-blind clinical study. J. Med. Food 2021, 24, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Lykkesfeldt, J.; Tveden-Nyborg, P. The pharmacokinetics of vitamin C. Nutrients 2019, 11, 2412. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gęgotek, A.; Skrzydlewska, E. Ascorbic acid as antioxidant. Vitam. Horm. 2023, 121, 247–270. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 4th ed.; Oxford University Press: New York, NY, USA, 2007. [Google Scholar]
- Fujii, J.; Tsukasa, O.; Bo, T. Ascorbate is a primary antioxidant in mammals. Molecules 2022, 27, 6187. [Google Scholar] [CrossRef]
- Lewis, L.N.; Hayhoe, R.P.G.; Mulligan, A.A.; Luben, R.N.; Khaw, K.T.; Welch, A.A. Lower dietary and circulating vitamin C in middle- and older-aged men and women are associated with lower estimated skeletal muscle mass. J. Nutr. 2020, 150, 2789–2798. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, C.Y.; Shin, S. Low dietary vitamin C intake is associated with low muscle strength among elderly Korean women. Nutr. Res. 2024, 127, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Berkay Yılmaz, Y.; Antika, G.; Boyunegmez Tumer, T.; Fawzi Mahomoodally, M.; Lobine, D.; Akram, M.; Riaz, M.; Capanoglu, E.; Sharopov, F.; et al. Insights on the use of α-lipoic acid for therapeutic purposes. Biomolecules 2019, 9, 356. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Superti, F.; Russo, R. Alpha-lipoic acid: Biological mechanisms and health benefits. Antioxidants 2024, 13, 1228. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petersen Shay, K.; Moreau, R.F.; Smith, E.J.; Hagen, T.M. Is alpha-lipoic acid a scavenger of reactive oxygen species in vivo? Evidence for its initiation of stress signaling pathways that promote endogenous antioxidant capacity. IUBMB Life 2008, 60, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Ghibu, S.; Muresan, A.; Vergely, C. Alpha-lipoic acid: Molecular mechanisms and therapeutic potential in diabetes. Can. J. Physiol. Pharmacol. 2015, 93, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, Y.; Wang, D.; Ren, Q.; Wang, Y.; Zang, Z.; Guo, A.; Guo, J.; Wang, L.; Wang, R.; et al. Nanoparticle-driven skeletal muscle repair and regeneration through macrophage-muscle stem cell interaction. Small 2025, 21, e2412611. [Google Scholar] [CrossRef] [PubMed]
- Carazo, A.; Macáková, K.; Matoušová, K.; Krčmová, L.K.; Protti, M.; Mladěnka, P. Vitamin A update: Forms, sources, kinetics, detection, function, deficiency, therapeutic use and toxicity. Nutrients 2021, 13, 1703. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, W.; Peng, Q.; Zhang, X.; Guo, J.; Tong, H.; Li, S. Vitamin A promotes the repair of mice skeletal muscle injury through RARα. Nutrients 2023, 15, 3674. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fang, M.; Ma, J.; Bai, Y.; Ying, Y.; Shen, X.; Feng, Z. Systematic exploration of the association between vitamin A intakes and sarcopenia prevalence in American adults. Food Sci. Nutr. 2024, 12, 10786–10799. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Borodina, I.; Kenny, L.C.; McCarthy, C.M.; Paramasivan, K.; Pretorius, E.; Roberts, T.J.; van der Hoek, S.A.; Kell, D.B. The biology of ergothioneine, an antioxidant nutraceutical. Nutr. Res. Rev. 2020, 33, 190–217. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheah, I.K.; Tang, R.M.; Yew, T.S.; Lim, K.H.; Halliwell, B. Administration of pure ergothioneine to healthy human subjects: Uptake, metabolism, and effects on biomarkers of oxidative damage and inflammation. Antioxid. Redox Signal. 2017, 26, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Cheah, I. Ergothioneine, where are we now? FEBS Lett. 2022, 596, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N. Prolonging healthy aging: Longevity vitamins and proteins. Proc. Natl. Acad. Sci. USA 2018, 115, 10836–10844. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ceglia, L.; Harris, S.S. Vitamin D and its role in skeletal muscle. Calcif. Tissue Int. 2013, 92, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Achari, A.E.; Jain, S.K. 1,25(OH)2-vitamin D3 upregulates glucose uptake mediated by SIRT1/IRS1/GLUT4 signaling cascade in C2C12 myotubes. Mol. Cell. Biochem. 2018, 444, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, K.R.; Cruzat, V.; Carlessi, R.; Newsholme, P. Mechanisms of vitamin D action in skeletal muscle. Nutr. Res. Rev. 2019, 32, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Girgis, C.M.; Clifton-Bligh, R.J.; Hamrick, M.W.; Holick, M.F.; Gunton, J.E. The roles of vitamin D in skeletal muscle: Form, function, and metabolism. Endocr. Rev. 2013, 34, 33–83. [Google Scholar] [CrossRef] [PubMed]
- Mi, W.; Zhang, H.; Zhang, L.; Li, X.; Wang, Z.; Sun, Y.; Shen, T.; Fan, K.; Liu, C.; Xu, S. Age but not vitamin D is related to sarcopenia in vitamin D sufficient male elderly in rural China. Sci. Rep. 2025, 15, 765. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Welford, A.E.; Lanham-New, S.; Lord, J.; Doyle, A.; Robinson, J.; Nightingale, P.; Gittoes, N.; Greig, C.A. Influence of combined vitamin D3 supplementation and resistance exercise training on musculoskeletal health in older men and women (EXVITD): Protocol for a randomised controlled trial. BMJ Open 2020, 10, e033824. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
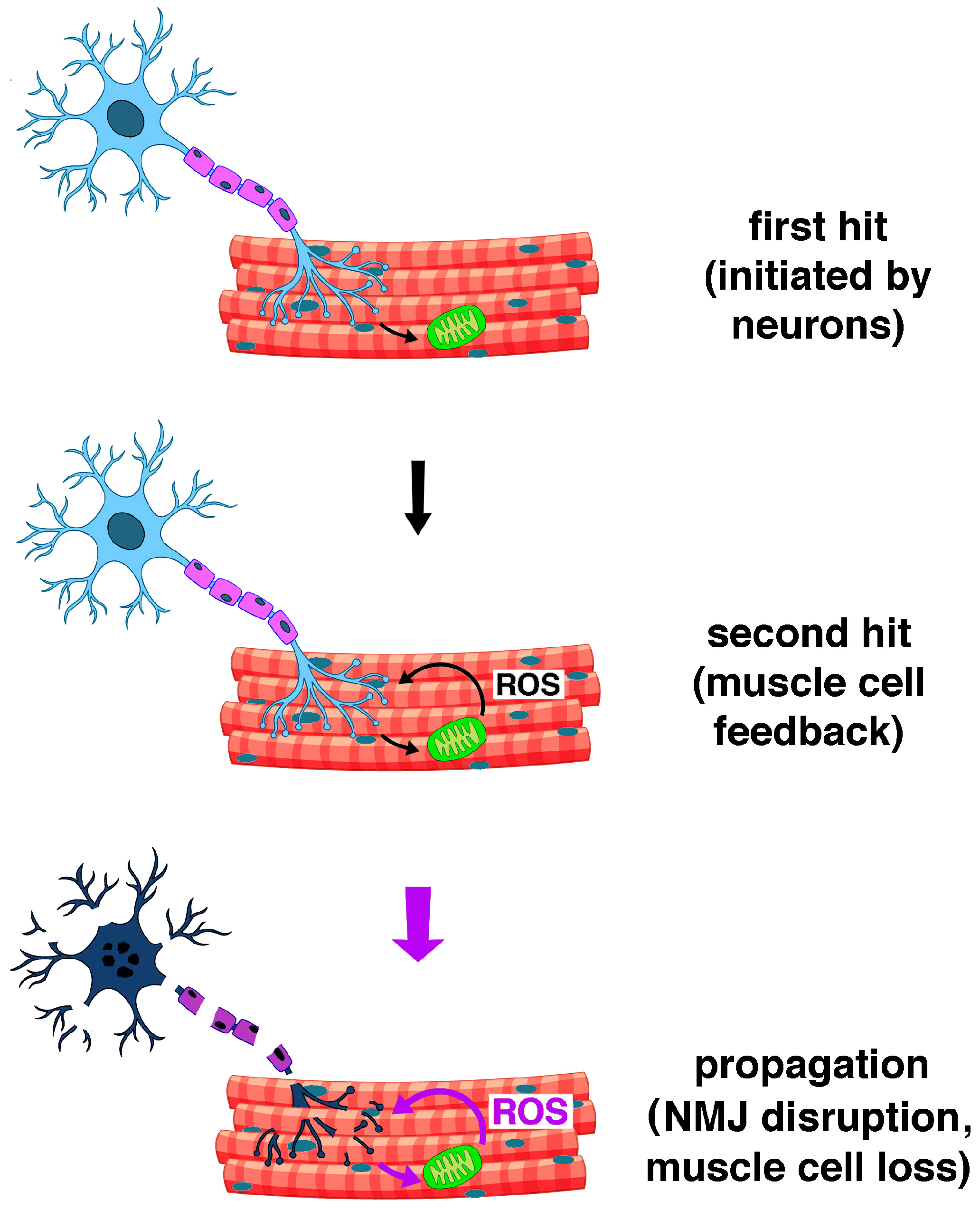
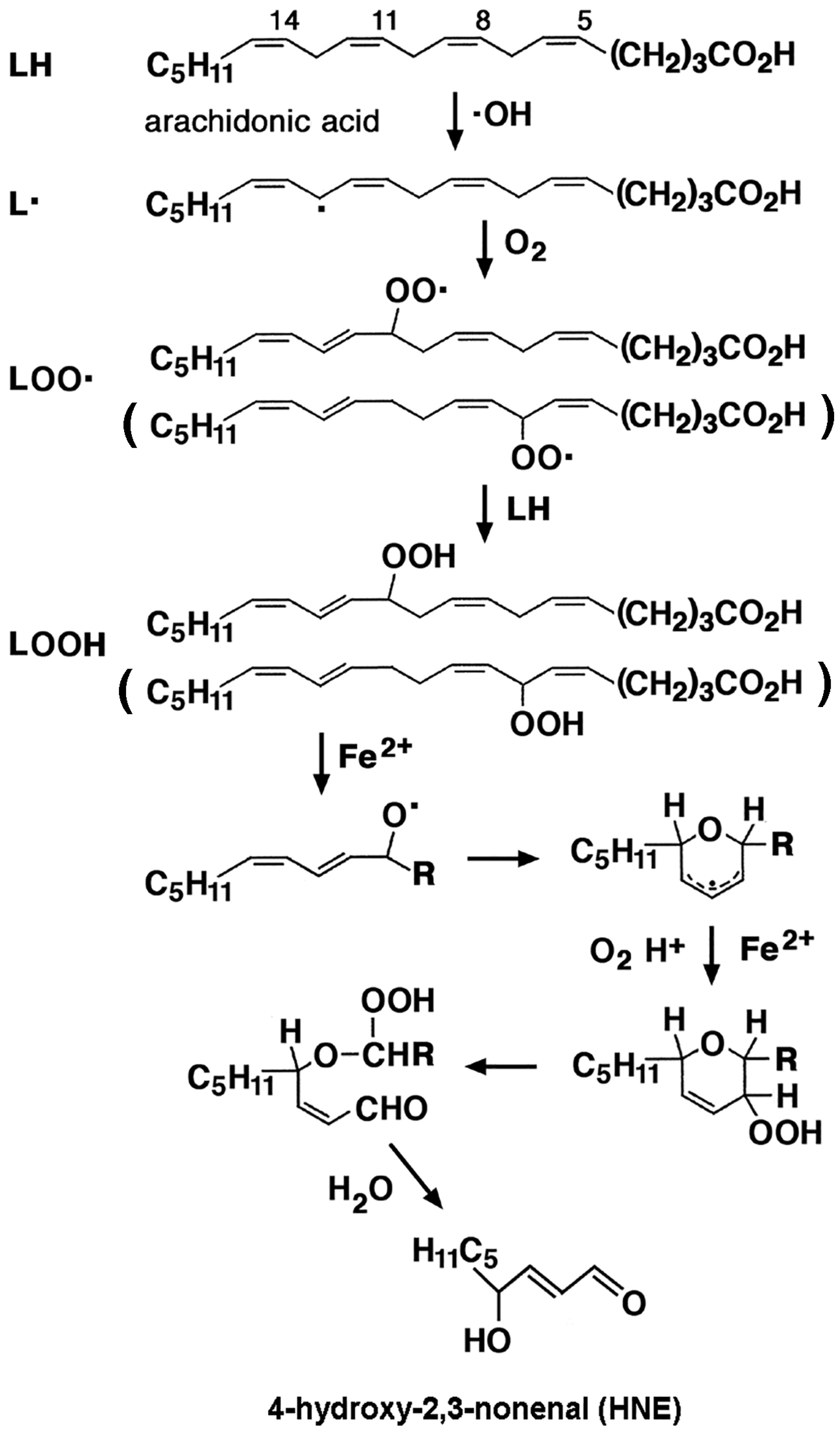
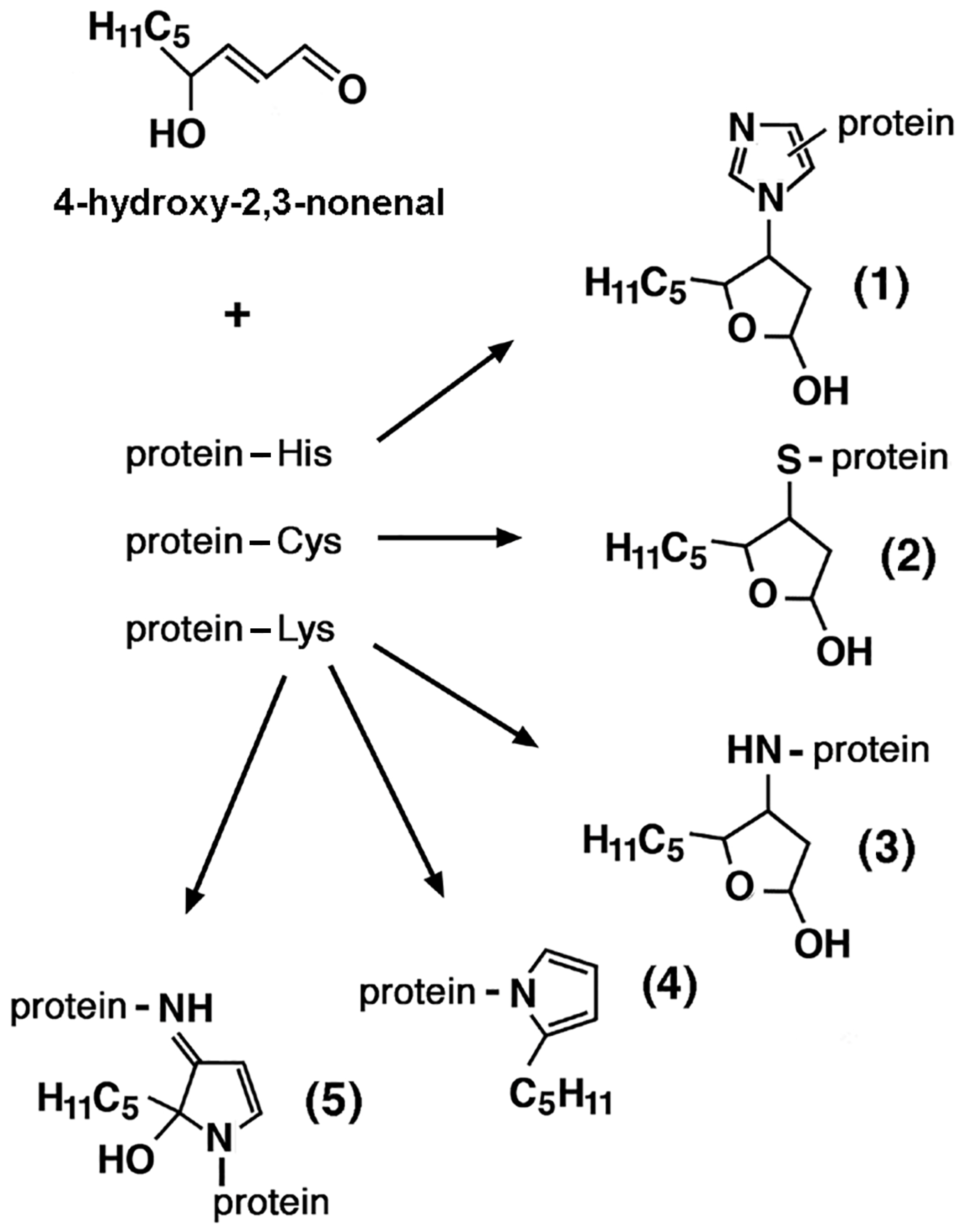
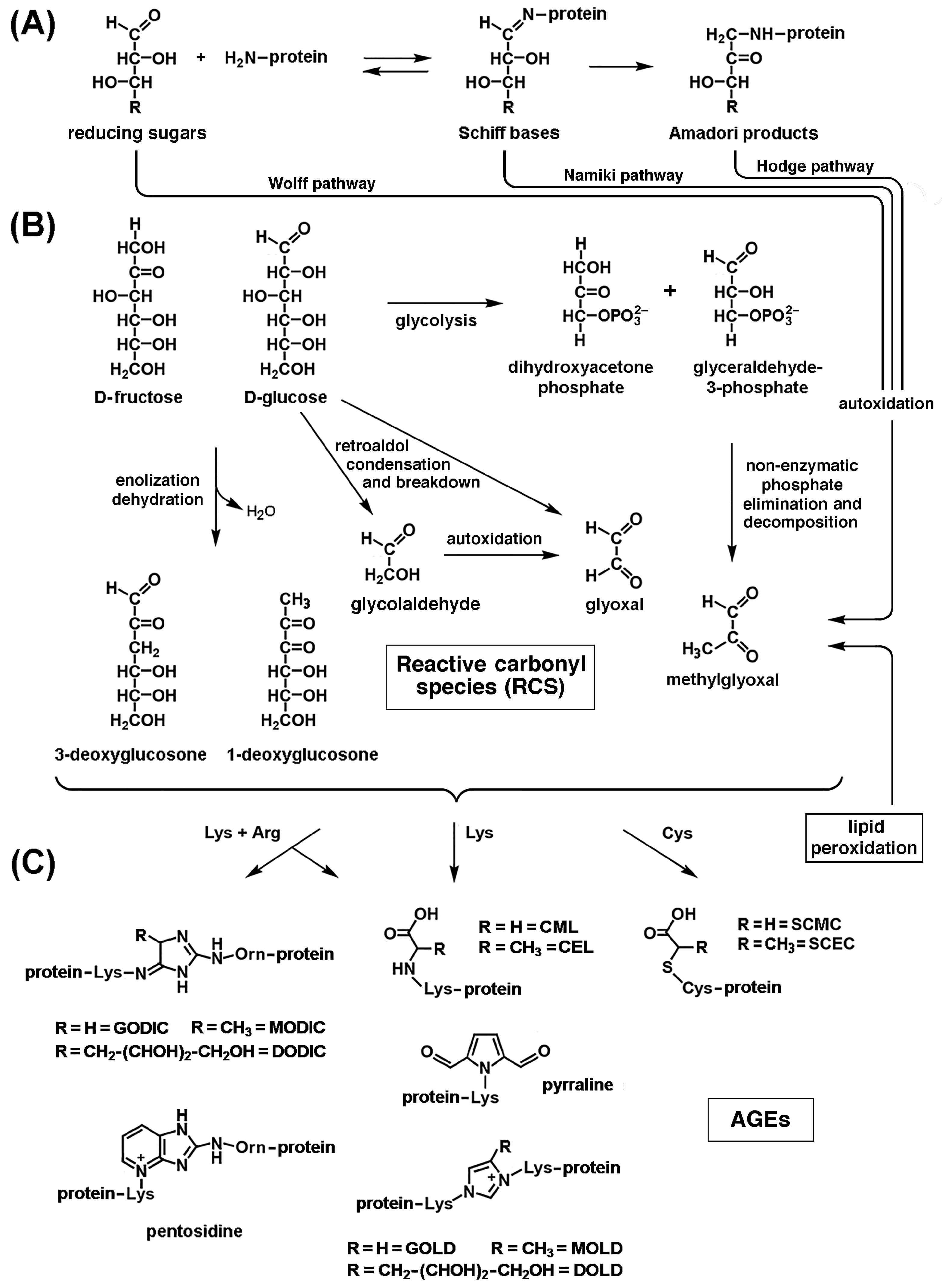
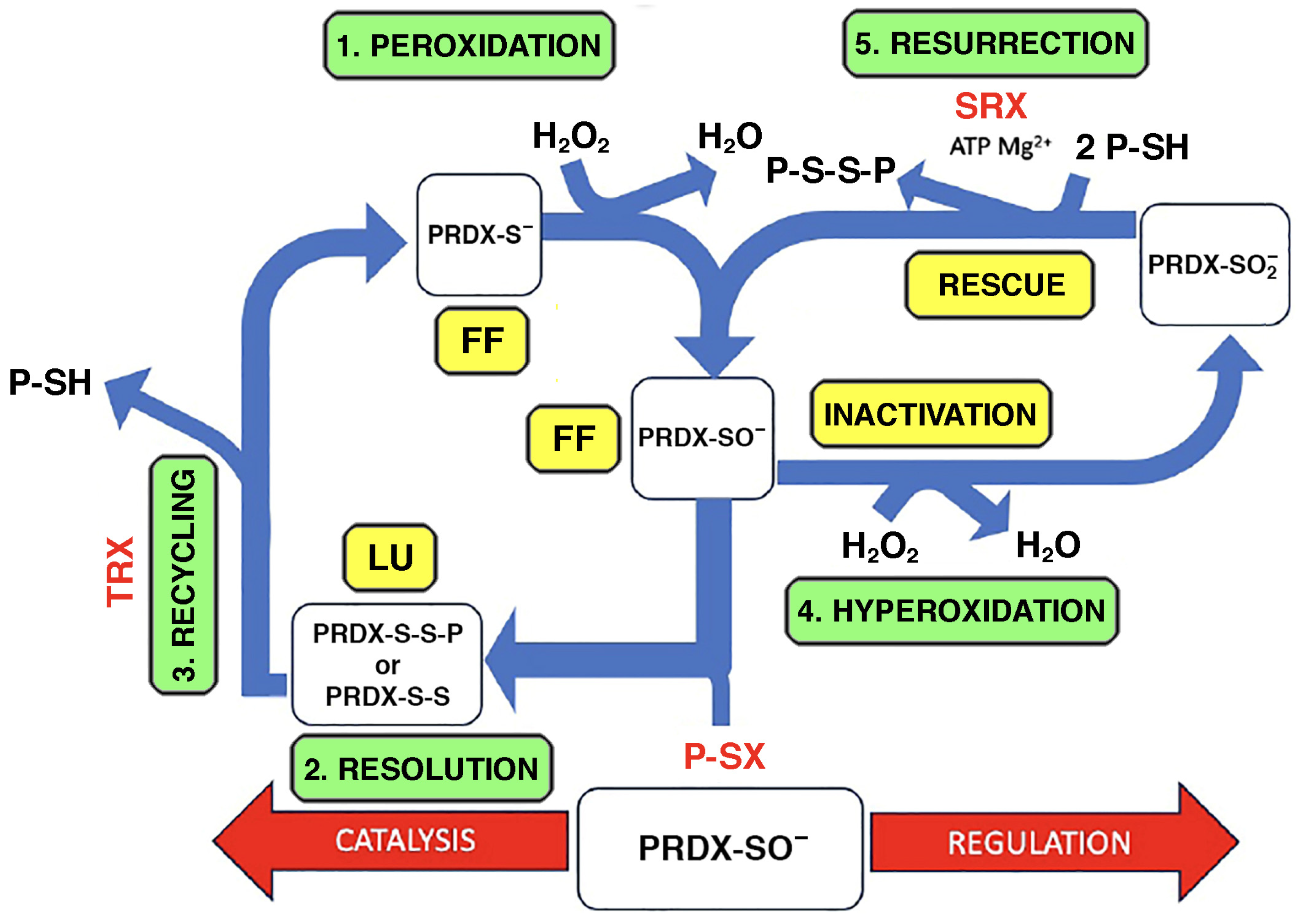
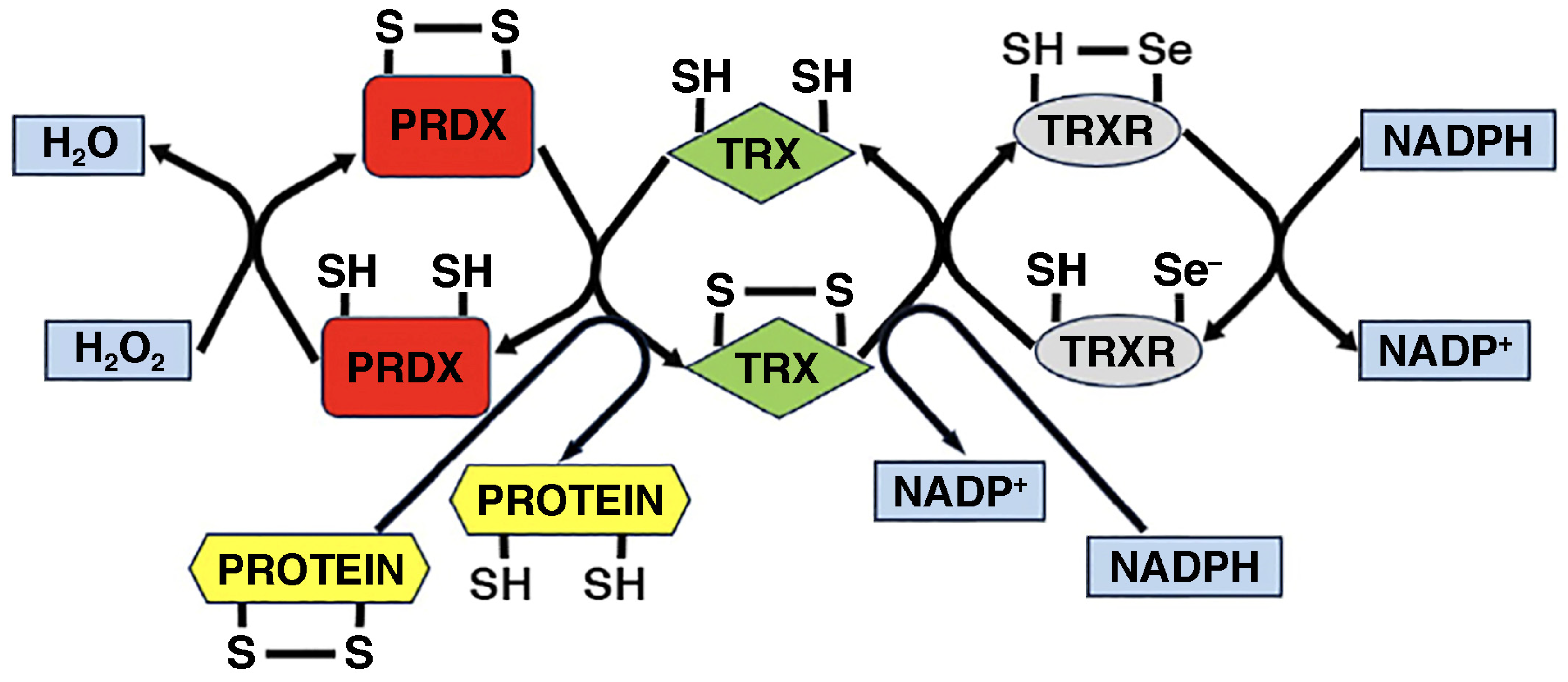
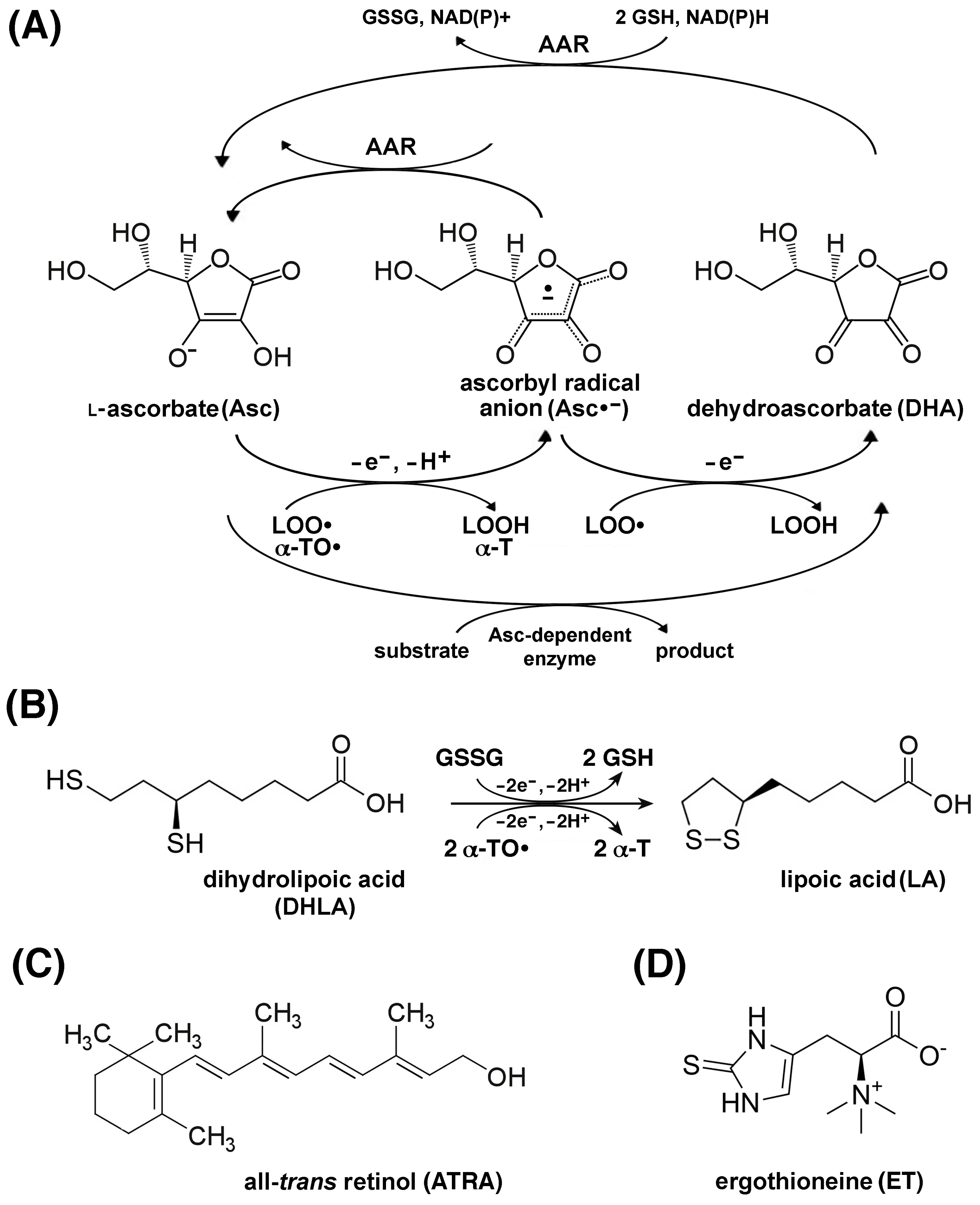
| Test | Cut-Off Points (Men) | Cut-Off Points (Women) |
|---|---|---|
| EWGSOP2 sarcopenia cut-off points for low strength by chair and grip strength | ||
| Grip strength | <27 kg | <16 kg |
| Chair strength | >15 s for 5 rises | |
| EWGSOP2 sarcopenia cut-off points for low muscle quantity | ||
| ASM | <20 kg | <15 kg |
| ASM/height2 | <7.0 kg/m2 | <5.5 kg/m2 |
| EWGSOP2 sarcopenia cut-off points for low performance | ||
| Gait speed | ≤0.8 m/s | |
| SPPB | ≤0.8 point score | |
| TUG | ≥20 s | |
| 400 m walk test | Non-completion or ≥6 min for completion | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arcaro, A.; Lepore, A.; Cetrangolo, G.P.; Paventi, G.; Ames, P.R.J.; Gentile, F. A Reassessment of Sarcopenia from a Redox Perspective as a Basis for Preventive and Therapeutic Interventions. Int. J. Mol. Sci. 2025, 26, 7787. https://doi.org/10.3390/ijms26167787
Arcaro A, Lepore A, Cetrangolo GP, Paventi G, Ames PRJ, Gentile F. A Reassessment of Sarcopenia from a Redox Perspective as a Basis for Preventive and Therapeutic Interventions. International Journal of Molecular Sciences. 2025; 26(16):7787. https://doi.org/10.3390/ijms26167787
Chicago/Turabian StyleArcaro, Alessia, Alessio Lepore, Giovanni Paolo Cetrangolo, Gianluca Paventi, Paul Richard Julian Ames, and Fabrizio Gentile. 2025. "A Reassessment of Sarcopenia from a Redox Perspective as a Basis for Preventive and Therapeutic Interventions" International Journal of Molecular Sciences 26, no. 16: 7787. https://doi.org/10.3390/ijms26167787
APA StyleArcaro, A., Lepore, A., Cetrangolo, G. P., Paventi, G., Ames, P. R. J., & Gentile, F. (2025). A Reassessment of Sarcopenia from a Redox Perspective as a Basis for Preventive and Therapeutic Interventions. International Journal of Molecular Sciences, 26(16), 7787. https://doi.org/10.3390/ijms26167787






