Short-term effects of DAPAgliflozin on Lung fUNction, sleep apneas, and circulatinG surfactant protein B in Heart Failure with reduced ejection fraction (DAPA-LUNG-HF)
Abstract
1. Introduction
2. Results
3. Discussion
Limitations
4. Materials and Methods
4.1. Lung Function Tests
4.2. Venous Blood Sample Collection, Specimen Handling and Assay
4.3. Transthoracic Echocardiography
4.4. Nocturnal Cardiorespiratory Monitoring
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Kober, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Belohlavek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, P.W.; Pieske, B.; Anstrom, K.J.; Ezekowitz, J.; Hernandez, A.F.; Butler, J.; Lam, C.S.P.; Ponikowski, P.; Voors, A.A.; Jia, G.; et al. Vericiguat in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2020, 382, 1883–1893. [Google Scholar] [CrossRef]
- McDonald, M.; Virani, S.; Chan, M.; Ducharme, A.; Ezekowitz, J.A.; Giannetti, N.; Heckman, G.A.; Howlett, J.G.; Koshman, S.L.; Lepage, S.; et al. CCS/CHFS Heart Failure Guidelines Update: Defining a New Pharmacologic Standard of Care for Heart Failure With Reduced Ejection Fraction. Can. J. Cardiol. 2021, 37, 531–546. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
- Mapelli, M.; Mattavelli, I.; Salvioni, E.; Capra, N.; Mantegazza, V.; Garlaschè, A.; Campodonico, J.; Rubbo, F.M.; Paganin, C.; Capovilla, T.M.; et al. Dapagliflozin effects on exercise, cardiac remodeling, biomarkers, and renal and pulmonary function in heart failure patients: Not as good as expected? Front. Cardiovasc. Med. 2025, 12, 1542870. [Google Scholar] [CrossRef]
- Omar, M.; Jensen, J.; Frederiksen, P.H.; Kistorp, C.; Videbaek, L.; Poulsen, M.K.; Moller, S.; Ali, M.; Gustafsson, F.; Kober, L.; et al. Effect of Empagliflozin on Hemodynamics in Patients With Heart Failure and Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2020, 76, 2740–2751. [Google Scholar] [CrossRef]
- Lytvyn, Y.; Bjornstad, P.; Udell, J.A.; Lovshin, J.A.; Cherney, D.Z.I. Sodium Glucose Cotransporter-2 Inhibition in Heart Failure: Potential Mechanisms, Clinical Applications, and Summary of Clinical Trials. Circulation 2017, 136, 1643–1658. [Google Scholar] [CrossRef]
- Brata, R.; Pascalau, A.V.; Fratila, O.; Paul, I.; Muresan, M.M.; Camarasan, A.; Ilias, T. Hemodynamic Effects of SGLT2 Inhibitors in Patients with and Without Diabetes Mellitus-A Narrative Review. Healthcare 2024, 12, 2464. [Google Scholar] [CrossRef]
- Banfi, C.; Agostoni, P. Surfactant protein B: From biochemistry to its potential role as diagnostic and prognostic marker in heart failure. Int. J. Cardiol. 2016, 221, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, P.; Banfi, C.; Ghilardi, S.; Magri, D.; Giovannardi, M.; Bonomi, A.; Salvioni, E.; Battaia, E.; Filardi, P.P.; Tremoli, E.; et al. Surfactant-derived proteins as markers of alveolar membrane damage in heart failure. PLoS ONE 2014, 9, e115030. [Google Scholar] [CrossRef] [PubMed]
- Puri, S.; Baker, B.L.; Dutka, D.P.; Oakley, C.M.; Hughes, J.M.; Cleland, J.G. Reduced alveolar-capillary membrane diffusing capacity in chronic heart failure. Its pathophysiological relevance and relationship to exercise performance. Circulation 1995, 91, 2769–2774. [Google Scholar] [CrossRef] [PubMed]
- Magri, D.; Banfi, C.; Maruotti, A.; Farina, S.; Vignati, C.; Salvioni, E.; Morosin, M.; Brioschi, M.; Ghilardi, S.; Tremoli, E.; et al. Plasma immature form of surfactant protein type B correlates with prognosis in patients with chronic heart failure. A pilot single-center prospective study. Int. J. Cardiol. 2015, 201, 394–399. [Google Scholar] [CrossRef]
- Guazzi, M.; Pontone, G.; Brambilla, R.; Agostoni, P.; Reina, G. Alveolar--capillary membrane gas conductance: A novel prognostic indicator in chronic heart failure. Eur. Heart J. 2002, 23, 467–476. [Google Scholar] [CrossRef]
- Campodonico, J.; Mapelli, M.; Spadafora, E.; Ghilardi, S.; Agostoni, P.; Banfi, C.; Sciomer, S. Surfactant proteins changes after acute hemodynamic improvement in patients with advanced chronic heart failure treated with Levosimendan. Respir. Physiol. Neurobiol. 2018, 252–253, 47–51. [Google Scholar] [CrossRef]
- Mapelli, M.; Mattavelli, I.; Salvioni, E.; Banfi, C.; Ghilardi, S.; De Martino, F.; Gugliandolo, P.; Mantegazza, V.; Volpato, V.; Basile, C.; et al. Impact of Sacubitril/Valsartan on surfactant binding proteins, central sleep apneas, lung function tests and heart failure biomarkers: Hemodynamic or pleiotropism? Front. Cardiovasc. Med. 2022, 9, 971108. [Google Scholar] [CrossRef]
- Shah, R.V.; Januzzi, J.L., Jr. ST2: A novel remodeling biomarker in acute and chronic heart failure. Curr. Heart Fail. Rep. 2010, 7, 9–14. [Google Scholar] [CrossRef]
- Weinberg, E.O.; Shimpo, M.; De Keulenaer, G.W.; MacGillivray, C.; Tominaga, S.; Solomon, S.D.; Rouleau, J.L.; Lee, R.T. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation 2002, 106, 2961–2966. [Google Scholar] [CrossRef]
- Parati, G.; Lombardi, C.; Castagna, F.; Mattaliano, P.; Filardi, P.P.; Agostoni, P.; Italian Society of Cardiology (SIC) Working Group on Heart Failure Members. Heart failure and sleep disorders. Nat. Rev. Cardiol. 2016, 13, 389–403. [Google Scholar] [CrossRef]
- Oldenburg, O.; Wellmann, B.; Buchholz, A.; Bitter, T.; Fox, H.; Thiem, U.; Horstkotte, D.; Wegscheider, K. Nocturnal hypoxaemia is associated with increased mortality in stable heart failure patients. Eur. Heart J. 2016, 37, 1695–1703. [Google Scholar] [CrossRef]
- Emdin, M.; Mirizzi, G.; Giannoni, A.; Poletti, R.; Iudice, G.; Bramanti, F.; Passino, C. Prognostic Significance of Central Apneas Throughout a 24-Hour Period in Patients With Heart Failure. J. Am. Coll. Cardiol. 2017, 70, 1351–1364. [Google Scholar] [CrossRef]
- Brack, T.; Thuer, I.; Clarenbach, C.F.; Senn, O.; Noll, G.; Russi, E.W.; Bloch, K.E. Daytime Cheyne-Stokes respiration in ambulatory patients with severe congestive heart failure is associated with increased mortality. Chest 2007, 132, 1463–1471. [Google Scholar] [CrossRef]
- Wang, H.; Parker, J.D.; Newton, G.E.; Floras, J.S.; Mak, S.; Chiu, K.L.; Ruttanaumpawan, P.; Tomlinson, G.; Bradley, T.D. Influence of obstructive sleep apnea on mortality in patients with heart failure. J. Am. Coll. Cardiol. 2007, 49, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Lanfranchi, P.A.; Braghiroli, A.; Bosimini, E.; Mazzuero, G.; Colombo, R.; Donner, C.F.; Giannuzzi, P. Prognostic value of nocturnal Cheyne-Stokes respiration in chronic heart failure. Circulation 1999, 99, 1435–1440. [Google Scholar] [CrossRef] [PubMed]
- Apostolo, A.; Paolillo, S.; Contini, M.; Vignati, C.; Tarzia, V.; Campodonico, J.; Mapelli, M.; Massetti, M.; Bejko, J.; Righini, F.; et al. Comprehensive effects of left ventricular assist device speed changes on alveolar gas exchange, sleep ventilatory pattern, and exercise performance. J. Heart Lung Transplant. 2018, 37, 1361–1371. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, P.G.; Guazzi, M.; Bussotti, M.; Grazi, M.; Palermo, P.; Marenzi, G. Lack of improvement of lung diffusing capacity following fluid withdrawal by ultrafiltration in chronic heart failure. J. Am. Coll. Cardiol. 2000, 36, 1600–1604. [Google Scholar] [CrossRef]
- Serenelli, M.; Bohm, M.; Inzucchi, S.E.; Kober, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Solomon, S.D.; DeMets, D.L.; et al. Effect of dapagliflozin according to baseline systolic blood pressure in the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure trial (DAPA-HF). Eur. Heart J. 2020, 41, 3402–3418. [Google Scholar] [CrossRef]
- Jaffuel, D.; Bouchaut, Y.; Mallet, J.P.; Vidal, C.; Molinari, N.; Bourdin, A.; Roubille, F. Dapagliflozin initiation in chronic heart failure patients improves central sleep apnoea. ERJ Open Res. 2023, 9, 00123–2023. [Google Scholar] [CrossRef]
- Xie, L.; Li, S.; Yu, X.; Wei, Q.; Yu, F.; Tong, J. DAHOS Study: Efficacy of dapagliflozin in treating heart failure with reduced ejection fraction and obstructive sleep apnea syndrome—A 3-month, multicenter, randomized controlled clinical trial. Eur. J. Clin. Pharmacol. 2024, 80, 771–780. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Coresh, J.; Greene, T.; Marsh, J.; Stevens, L.A.; Kusek, J.W.; Van Lente, F.; Chronic Kidney Disease Epidemiology Collaboration. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin. Chem. 2007, 53, 766–772. [Google Scholar] [CrossRef]
- Graham, B.L.; Brusasco, V.; Burgos, F.; Cooper, B.G.; Jensen, R.; Kendrick, A.; MacIntyre, N.R.; Thompson, B.R.; Wanger, J. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur. Respir. J. 2017, 49, 1600016. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef]
- Macintyre, N.; Crapo, R.O.; Viegi, G.; Johnson, D.C.; van der Grinten, C.P.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Enright, P.; et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur. Respir. J. 2005, 26, 720–735. [Google Scholar] [CrossRef] [PubMed]
- Roughton, F.J.; Forster, R.E. Relative importance of diffusion and chemical reaction rates in determining rate of exchange of gases in the human lung, with special reference to true diffusing capacity of pulmonary membrane and volume of blood in the lung capillaries. J. Appl. Physiol. 1957, 11, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Ramage, J.E., Jr.; Coleman, R.E.; MacIntyre, N.R. Rest and exercise cardiac output and diffusing capacity assessed by a single slow exhalation of methane, acetylene, and carbon monoxide. Chest 1987, 92, 44–50. [Google Scholar] [CrossRef]
- Magri, D.; Brioschi, M.; Banfi, C.; Schmid, J.P.; Palermo, P.; Contini, M.; Apostolo, A.; Bussotti, M.; Tremoli, E.; Sciomer, S.; et al. Circulating plasma surfactant protein type B as biological marker of alveolar-capillary barrier damage in chronic heart failure. Circ. Heart Fail. 2009, 2, 175–180. [Google Scholar] [CrossRef]
- Schagger, H.; von Jagow, G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 1987, 166, 368–379. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 16, 233–271. [Google Scholar]
- Berry, R.B.; Brooks, R.; Gamaldo, C.; Harding, S.M.; Lloyd, R.M.; Quan, S.F.; Troester, M.T.; Vaughn, B.V. AASM Scoring Manual Updates for 2017 (Version 2.4). J. Clin. Sleep Med. 2017, 13, 665–666. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
| Mean ± SD Median [IQR] | |
|---|---|
| Males (n, %) | 56 (85%) |
| Age (years) | 66.5 [59.0–72.8] |
| BMI (kg/m2) | 26.57 ± 3.51 |
| Ischemic etiology (n, %) | 33 (50%) |
| Hypertension (n, %) | 33 (50%) |
| Type II diabetes (n, %) | 7 (11%) |
| Dyslipidemia (n, %) | 40 (61%) |
| Active smoker (n, %) | 12 (18%) |
| Former smoker (n, %) | 26 (39%) |
| COPD (n, %) | 1 (2%) |
| Atrial fibrillation (n, %) | 5 (8%) |
| ICD (n, %) | 35 (53%) |
| CRT (n, %) | 11 (17%) |
| ACEi (n, %) | 6 (9%) |
| ARBs (n, %) | 7 (11%) |
| Sacubitril/valsartan (n, %) | 53 (80%) |
| β-blocker (n, %) | 63 (95%) |
| MRA (n, %) | 55 (83%) |
| Loop diuretic (n, %) | 35 (53%) |
| Loop diuretic dose (mg/die) (n = 35) | 25.0 [12.5–37.5] |
| N | T0 | T1 | T2 | p TREND ANOVA | p Value Bonferroni Adjusted | |||
|---|---|---|---|---|---|---|---|---|
| T0 vs. T1 | T0 vs. T2 | T1 vs. T2 | ||||||
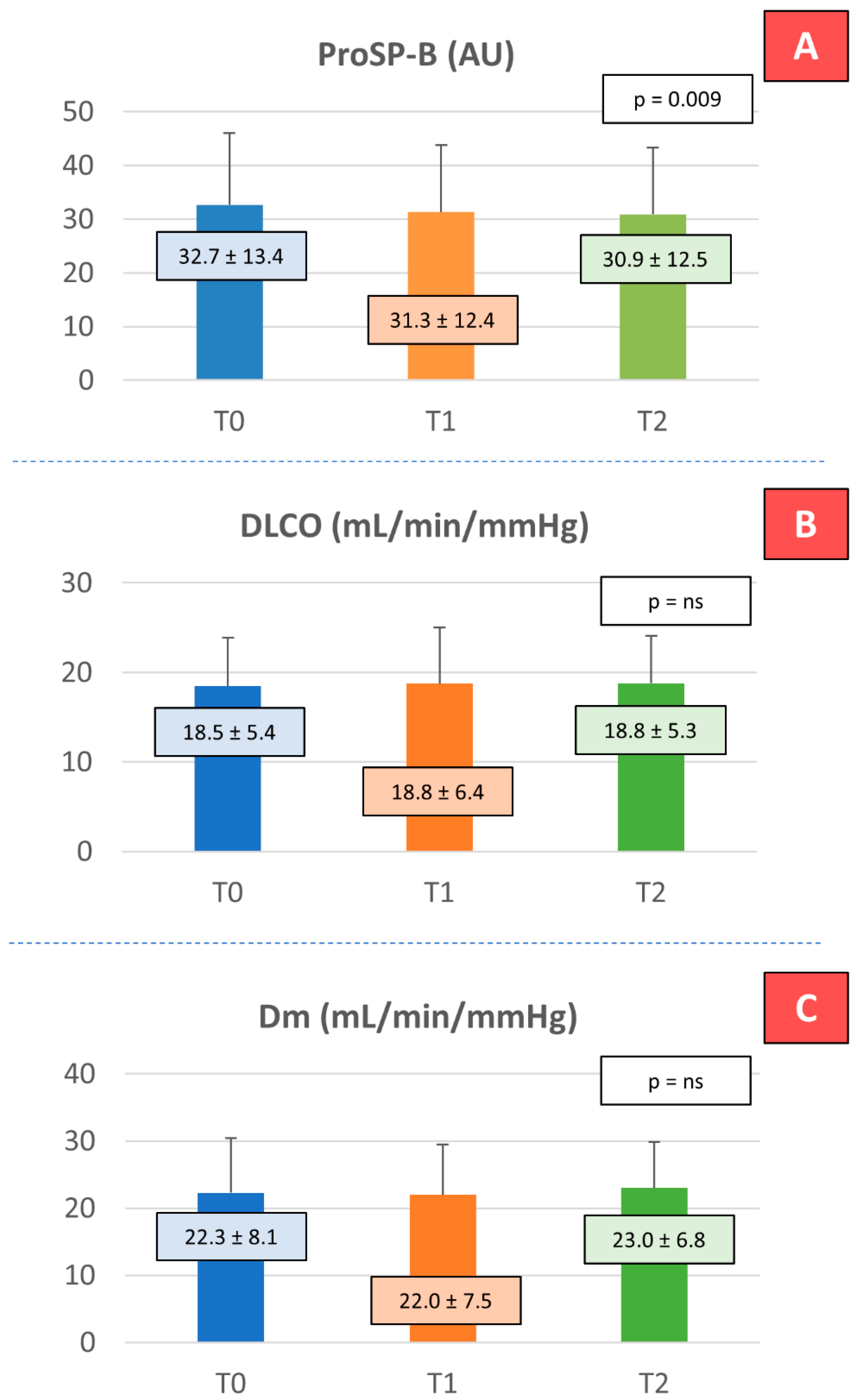
| ProSP-B (AU) | 64 | 32.65 ± 13.36 | 31.29 ± 12.43 | 30.86 ± 12.45 | 0.0092 | 0.0277 | 0.0209 | 1.0000 |
| DLCO (mL/min/mmHg) | 51 | 18.46 ± 5.4 | 18.75 ± 6.26 | 18.76 ± 5.3 | 0.4175 | 1.0000 | 1.0000 | 1.0000 |
| DLCOHb (mL/min/mmHg) | 51 | 18.65 ± 5.44 | 18.55 ± 5.58 | 18.85 ± 5.27 | 0.6241 | 1.0000 | 1.0000 | 1.0000 |
| DLCO% | 51 | 70 ± 16 | 69 ± 15 | 71 ± 15 | 0.6241 | 1.0000 | 1.0000 | 0.7748 |
| Dm (mL/min/mmHg) | 47 | 22.27 ± 8.11 | 21.97 ± 7.53 | 23.0 ± 6.82 | 0.1783 | 1.0000 | 1.0000 | 0.3640 |
| FEV1 (L) | 61 | 2.68 ± 0.69 | 2.7 ± 0.67 | 2.66 ± 0.64 | 0.5193 | 0.7952 | 1.0000 | 0.2881 |
| FEV1% | 61 | 87.02 ± 16.31 | 87.83 ± 15.99 | 86.9 ± 15.14 | 0.8521 | 0.6004 | 1.0000 | 0.7355 |
| FCV (L) | 61 | 3.36 ± 0.86 | 3.4 ± 0.84 | 3.39 ± 0.84 | 0.4112 | 0.5215 | 1.0000 | 1.0000 |
| FVC% | 61 | 84.3 ± 15.71 | 85.18 ± 14.72 | 85.22 ± 14.76 | 0.2642 | 0.8538 | 1.0000 | 1.0000 |
| Hb (g/dL) | 66 | 13.74 ± 1.55 | 13.93 ± 1.44 | 14.59 ± 1.7 | <0.0001 | 0.1141 | <0.0001 | <0.0001 |
| Creatinine (mg/dL) * | 66 | 1.07 [0.91–1.33] | 1.12 [0.94–1.39] | 1.08 [0.89–1.36] | 0.0123 | 0.0017 | 0.1352 | 0.9555 |
| Potassium (mmol/L) | 66 | 4.44 ± 0.39 | 4.52 ± 0.47 | 4.4 ± 0.37 | 0.7714 | 0.2833 | 1.0000 | 0.1098 |
| NT-proBNP (ng/mL) * | 64 | 852 [466–1858] | 793 [298–1771] | 944 [320–1854] | 0.0717 | 0.0453 | 0.4037 | 1.0000 |
| ST-2 (ng/mL) | 61 | 27.28 ± 6.81 | 27.02 ± 6.48 | 28.08 ± 6.8 | 0.2628 | 1.0000 | 0.6841 | 0.5015 |
| VA (L) | 51 | 5.57 ± 1.32 | 5.57 ± 1.34 | 5.59 ± 1.32 | 0.9264 | 1.0000 | 1.0000 | 1.0000 |
| Vcap (mL) * | 43 | 98 [79–115] | 101 [80–126] | 94 [75–117] | 0.7040 | 1.0000 | 1.0000 | 1.0000 |
| EDV (mL) | 66 | 186 [145–232] | 177 [129–225] | <0.0001 | ||||
| ESV (mL) | 66 | 113 [87–163] | 110 [76–145] | <0.0001 | ||||
| LVEF (%) | 66 | 34.60 ± 7.82 | 37.54 ± 9.20 | <0.0001 | ||||
| E/E′ | 60 | 8.5 [7.0–13.0] | 8.0 [6.0–9.8] | 0.0050 | ||||
| PAPs (mmHg) | 56 | 27 [24–29] | 25 [23–28] | 0.0460 | ||||
| TAPSE (mm) | 65 | 20.13 ± 5.72 | 19.34 ± 4.29 | 0.2010 | ||||
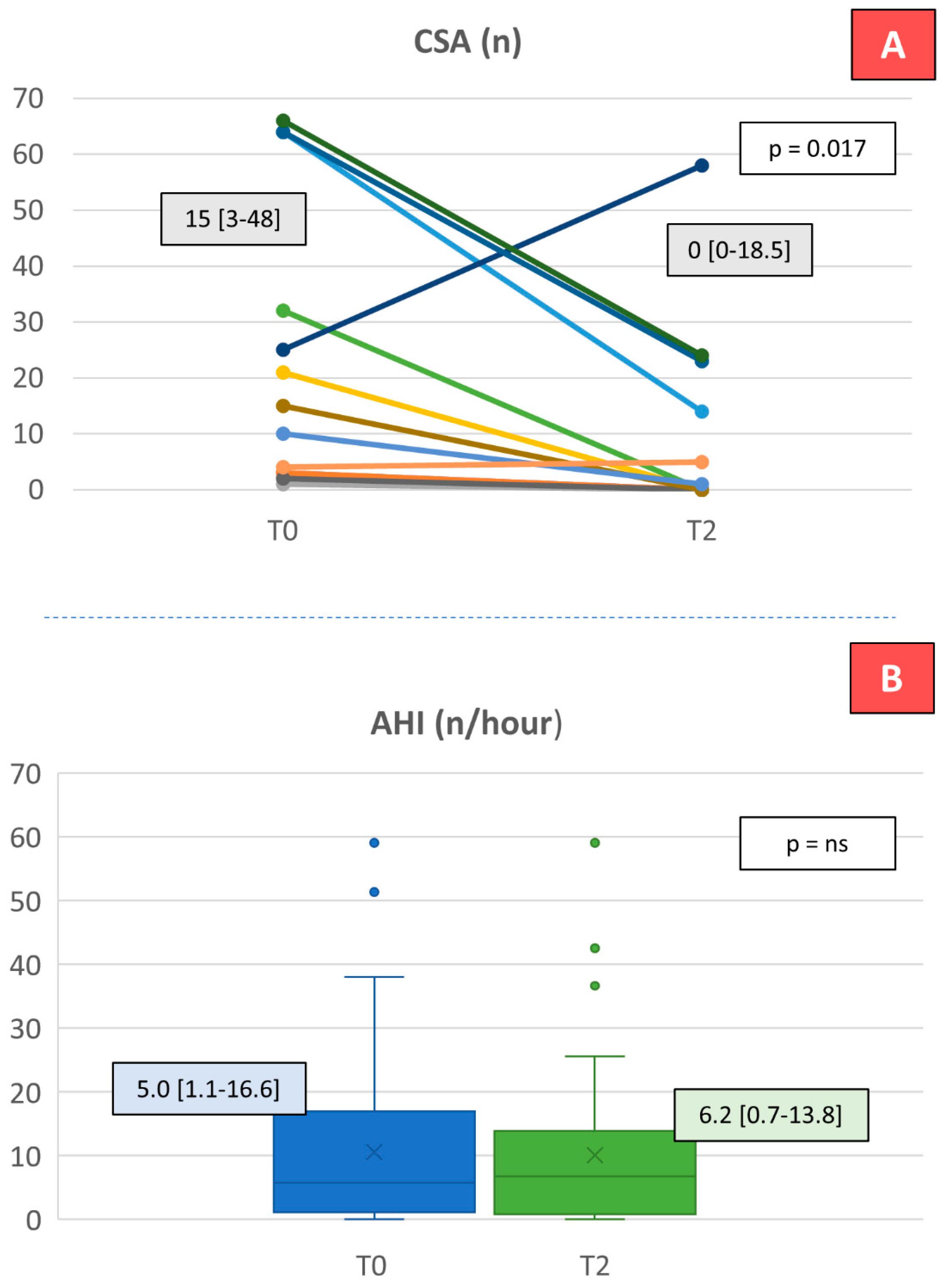
| AHI (n) | 59 | 5.0 [1.1–16.6] | 6.2 [0.7–13.8] | 0.3660 | ||||
| Hypopneas (n) | 49 | 8.0 [3.5–23.0] | 10.5 [2.0–23.0] | 0.8110 | ||||
| CSA (n) | 50 | 0.0 [0.0–2.3] | 0.0 [0.0–1.0] | 0.8090 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mapelli, M.; Mattavelli, I.; Salvioni, E.; Banfi, C.; Mallia, A.; Galotta, A.; Mantegazza, V.; Garlaschè, A.; Campodonico, J.; Rubbo, F.M.; et al. Short-term effects of DAPAgliflozin on Lung fUNction, sleep apneas, and circulatinG surfactant protein B in Heart Failure with reduced ejection fraction (DAPA-LUNG-HF). Int. J. Mol. Sci. 2025, 26, 7696. https://doi.org/10.3390/ijms26167696
Mapelli M, Mattavelli I, Salvioni E, Banfi C, Mallia A, Galotta A, Mantegazza V, Garlaschè A, Campodonico J, Rubbo FM, et al. Short-term effects of DAPAgliflozin on Lung fUNction, sleep apneas, and circulatinG surfactant protein B in Heart Failure with reduced ejection fraction (DAPA-LUNG-HF). International Journal of Molecular Sciences. 2025; 26(16):7696. https://doi.org/10.3390/ijms26167696
Chicago/Turabian StyleMapelli, Massimo, Irene Mattavelli, Elisabetta Salvioni, Cristina Banfi, Alice Mallia, Arianna Galotta, Valentina Mantegazza, Anna Garlaschè, Jeness Campodonico, Filippo Maria Rubbo, and et al. 2025. "Short-term effects of DAPAgliflozin on Lung fUNction, sleep apneas, and circulatinG surfactant protein B in Heart Failure with reduced ejection fraction (DAPA-LUNG-HF)" International Journal of Molecular Sciences 26, no. 16: 7696. https://doi.org/10.3390/ijms26167696
APA StyleMapelli, M., Mattavelli, I., Salvioni, E., Banfi, C., Mallia, A., Galotta, A., Mantegazza, V., Garlaschè, A., Campodonico, J., Rubbo, F. M., Paganin, C., Capovilla, T. M., Caputo, R., Contini, M., Gugliandolo, P., Vignati, C., Pezzuto, B., Grilli, G., Scatigna, M., ... Agostoni, P. (2025). Short-term effects of DAPAgliflozin on Lung fUNction, sleep apneas, and circulatinG surfactant protein B in Heart Failure with reduced ejection fraction (DAPA-LUNG-HF). International Journal of Molecular Sciences, 26(16), 7696. https://doi.org/10.3390/ijms26167696








