1. Introduction
Age-related macular degeneration (AMD) is the major cause of irreversible blindness in developed countries among individuals aged 50 and older [
1], affecting 8.7% of the world population, with estimates reaching over 288 million people by 2040 [
2]. AMD manifests in two forms, dry (atrophic) and wet (neovascular or exudative), with the milestone of the latter being choroidal neovascularization, the formation of new blood vessels in the choroid, a layer of tissue beneath the retina. These new vessels can leak fluid and blood, leading to scarring and central vision loss.
Choroidal neovascularization in AMD is driven by basal lamina and basal linear deposits of cellular debris between the retina pigment epithelium (RPE) and Bruch’s membrane [
3,
4]. In wet AMD, inflammatory factors are overexpressed, as hypoxia inducible factor 1 (HIF-1) triggers the release of several pro-angiogenic factors, notably vascular endothelial growth factor A (VEGF-A), alongside angiopoietin 2, platelet-derived growth factor (PDGF), and fibroblast growth factor 2 (FGF2) [
5]. When up-regulated, these molecules start complex processes of extracellular matrix remodeling through interactions with local molecules, such as glycosaminoglycans (GAGs), facilitating vascular sprouting, cellular migration, and tube formation through processes known as angiogenesis and vasculogenesis [
6]. Without intervention, these processes can lead to fibrosis, scarring, and ultimately, irreversible vision loss [
5,
7].
Among the variety of sulfated glycosaminoglycans (GAGs), heparin and heparan sulfate (HS) are particularly recognized for their pivotal roles in extracellular matrix remodeling during angiogenesis and inflammation [
8,
9]. The biological roles of these GAGs extend beyond anticoagulation to critically regulate angiogenesis, cell proliferation, migration, and inflammatory responses. While heparin is predominantly stored in mast cell granules, HS proteoglycans are ubiquitously expressed at the cell surface and in the extracellular matrix (ECM), where they modulate key signaling pathways [
9,
10]. These effects are mediated by the ability of their structurally diverse polysaccharide chains to interact selectively with a range of signaling proteins. Specific sulfation motifs and conformational domains within HS chains enable binding to angiogenic regulators such as VEGF, FGF-2, HGF, PDGF-B, TGF-β, thrombospondin-1 (TSP-1), platelet factor 4 (PF4), and endostatin, as well as to inflammatory cytokines, chemokines, morphogens, and enzymes [
10,
11,
12]. Notably, VEGF165, the most clinically relevant isoform, contains a defined heparin/HS-binding domain that is critical for its bioavailability, receptor engagement, and angiogenic activity [
8,
11]. A key example is FGF-2, whose mitogenic and pro-angiogenic signaling depend on HS acting as a co-receptor: HS stabilizes the FGF-FGFR complex, promoting receptor dimerization and activation of the MAPK and PI3K/Akt pathways, with 6-O- and N-sulfation playing essential roles in binding specificity and signaling efficacy [
10,
11,
13,
14]. These cascades drive endothelial cell proliferation, migration, and cytoskeletal remodeling. In inflammatory conditions, HS modulates chemokine gradients and leukocyte adhesion, further contributing to vascular remodeling. Altogether, the multifaceted regulatory functions of heparin and HS underscore their importance in the molecular control of pathological neovascularization and inflammation. These multifaceted roles highlight the importance of heparin and HS in coordinating the molecular events underlying neovascularization and inflammation and support the rationale for targeting their structure–function relationships in the development of novel antiangiogenic therapies.
The advent of anti-VEGF therapies has represented a major breakthrough in the management of neovascular age-related macular degeneration (AMD), diabetic retinopathy, and retinal vein occlusion by targeting vascular endothelial growth factor (VEGF), one of the most potent endogenous inducers of angiogenesis [
15,
16,
17]. These therapies have led to significant improvements in visual outcomes [
18]. However, their long-term efficacy tends to plateau in many patients, and the need for frequent intravitreal injections imposes a substantial treatment burden, negatively impacting patient adherence and quality of life [
19]. In addition, anti-VEGF agents are expensive and inaccessible to the underserved populations residing mainly in developing countries, limiting their global impact. Moreover, the systemic administration of anti-VEGF agents—particularly in oncology—has been associated with serious adverse effects, including hypertension and renal dysfunction, and emerging evidence suggests that systemic effects may also arise with chronic local administration [
20]. Given the critical role of the VEGF system in vascular homeostasis and the clinical outcome of anti-VEGF therapies, current strategies remain a cornerstone in the treatment of neovascular eye diseases. Nevertheless, the limitations related to this treatment have stimulated growing interest in identifying alternative or complementary target pathways involved in pathological angiogenesis.
Heparin and its structural mimetics have emerged as promising agents for the modulation of pathological angiogenesis through mechanisms that are not limited to direct VEGF inhibition. These compounds influence several signaling pathways involved in endothelial proliferation, migration, and neovessel formation, thus acting at multiple levels of the angiogenic cascade [
21,
22]. Although the clinical use of native heparin is limited by its strong anticoagulant and hemorrhagic potential, chemically modified derivatives and mimetics have been shown to retain antiangiogenic activity while minimizing coagulation-related risks [
22]. Recent studies have demonstrated that such compounds can inhibit choroidal neovascularization and modulate fibroblast growth factor (FGF) and VEGF signaling in ocular models, supporting their use as complementary or alternative strategies to current anti-VEGF therapies [
21,
22]. These findings highlight a valuable therapeutic avenue for improving efficacy, safety, and accessibility in the treatment of age-related macular degeneration and other vascular proliferative retinopathies.
This study aims to explore the therapeutic potential of 6-O-desulfated heparin, designed to retain antiangiogenic activity with reduced anticoagulant effects. Heparin was selected due to its well-documented ability to modulate angiogenesis and inflammation through specific interactions with key molecular mediators. We assessed the cytocompatibility of 6-O-desfulated heparin (Hep-6Od) in retinal pigment epithelial cells via in vitro cytotoxicity assays. Moreover, the effects of Hep-6Od on endothelial cell proliferation, migration, and capillary tube formation were then evaluated to characterize its anti-angiogenic profile. Additionally, biochemical interaction assays were conducted to examine its binding with FGF-2, a central mediator of neovascularization. Finally, its efficacy was tested in vivo using an animal model of laser-induced choroidal neovascularization, where Hep-6Od was administered via intravitreal injection. By targeting complementary pathways in angiogenesis, this study aims to contribute to the development of better, safer and more accessible therapeutic options for retinal vascular diseases such as neovascular AMD.
3. Discussion
Age-related macular degeneration (AMD) remains a leading cause of irreversible vision loss among older adults in developed countries, with choroidal neovascularization (CNV) constituting the principal pathological hallmark of the neovascular (wet) form of the disease [
2]. Although anti-vascular endothelial growth factor (anti-VEGF) therapies have improved visual outcomes and are currently the standard of care, significant limitations persist, including the need for repeated intravitreal injections, the potential development of tachyphylaxis, partial or non-responsiveness in a subset of patients, and the associated treatment burden [
18,
19]. These challenges highlight the critical need for adjunct or alternative therapeutic strategies that can enhance or replace the current regimens.
Heparin, a highly sulfated glycosaminoglycan, is traditionally recognized for its potent anticoagulant and anti-inflammatory activities [
9,
10]. Recent research has expanded interest in heparin’s role as a modulator of angiogenesis, particularly in ocular neovascular disorders. However, the systemic use of native heparin is constrained by a significant risk of hemorrhagic complications. To circumvent these limitations, chemically modified derivatives of heparin have been developed, aiming to retain or enhance the antiangiogenic efficacy while minimizing anticoagulant activity [
21].
In this context, 6-O-desulfated heparin (Hep-6Od), a selectively modified form of heparin, emerges as a promising candidate. Prior studies have demonstrated that selective desulfation can significantly attenuate anticoagulant properties without abolishing the antiangiogenic potential. Based on this rationale, we investigated the therapeutic efficacy of 6-O-desulfated heparin in a preclinical model of CNV, seeking to explore its ability to suppress pathological neovascularization—a central feature in the pathogenesis of neovascular AMD.
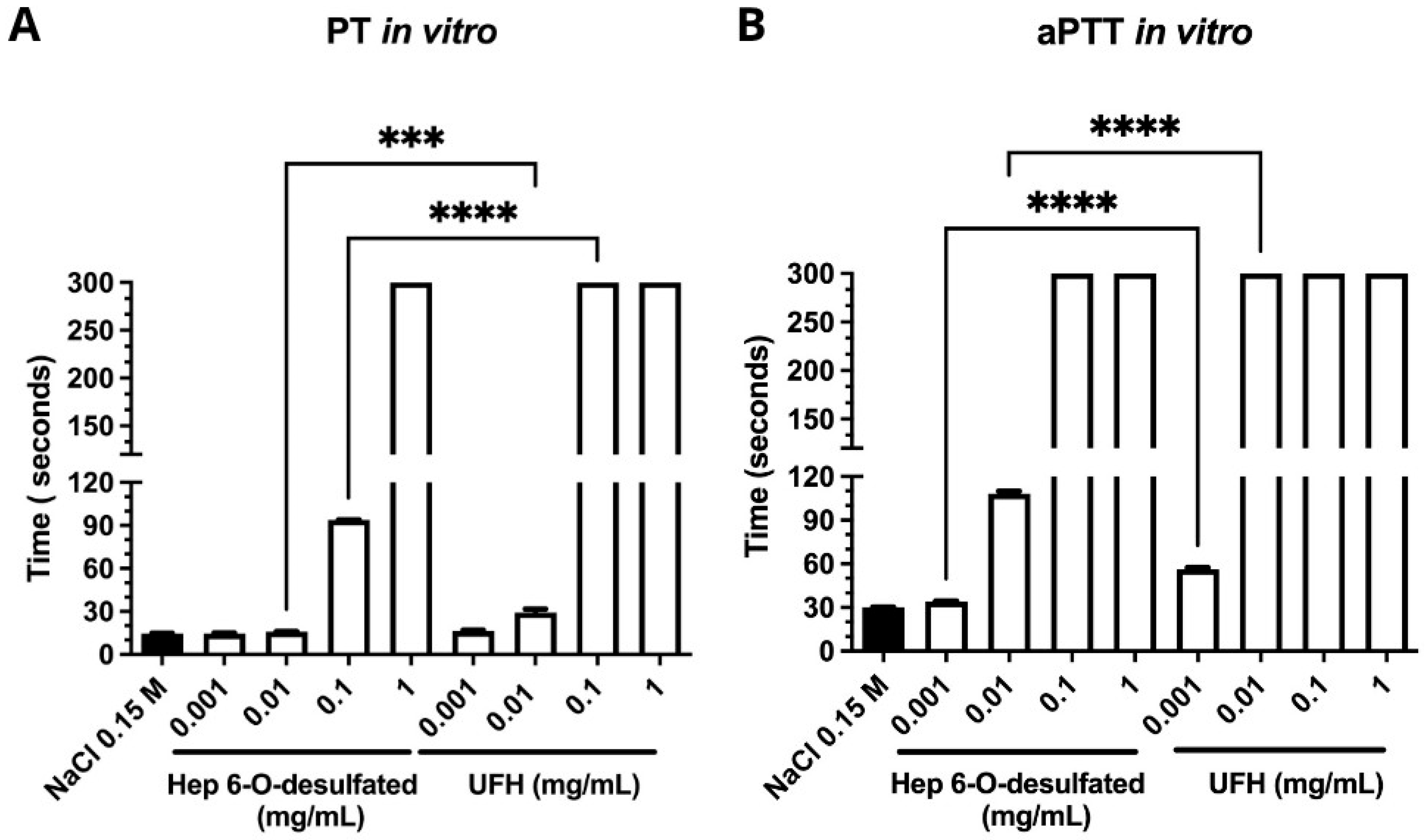
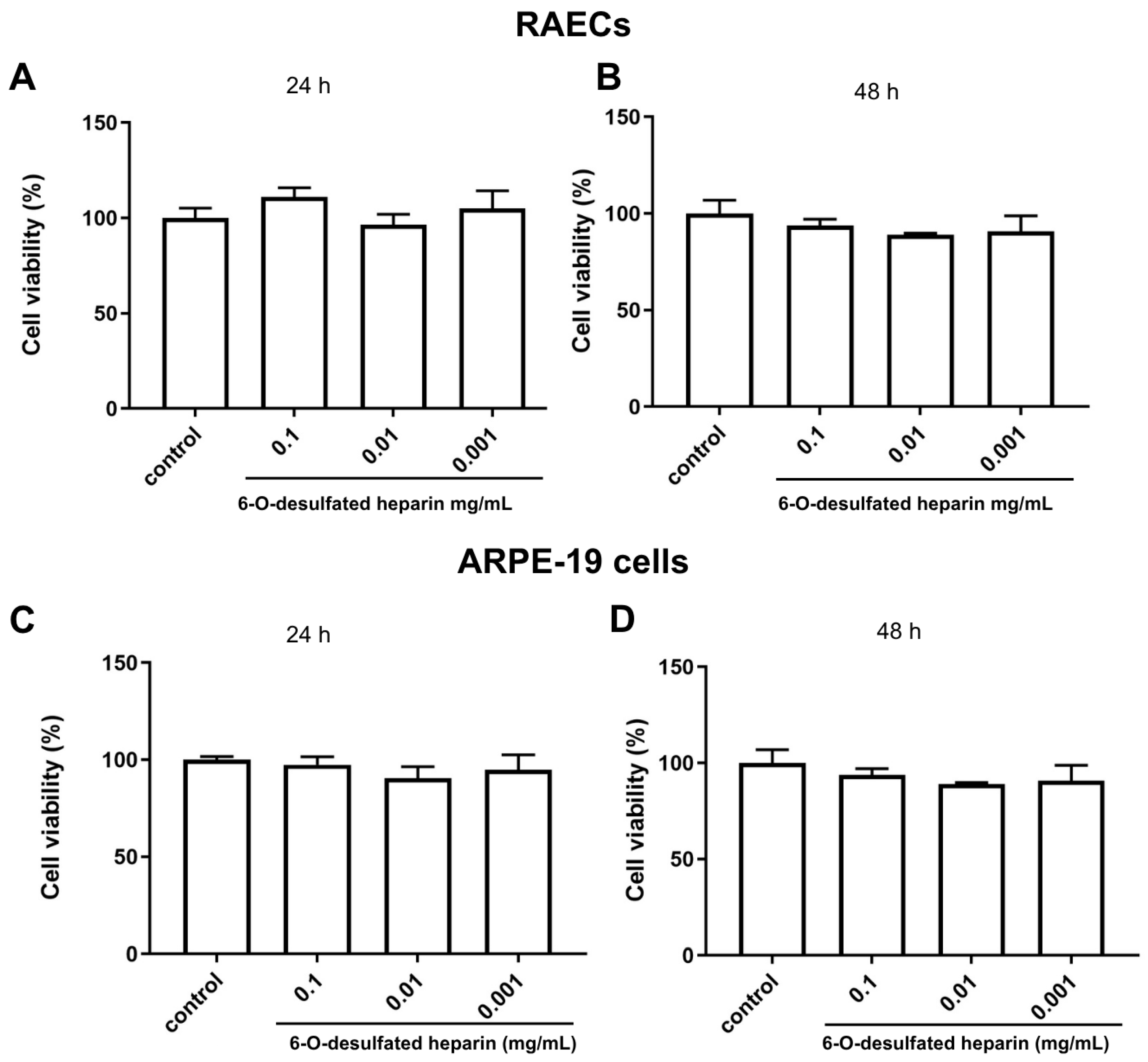
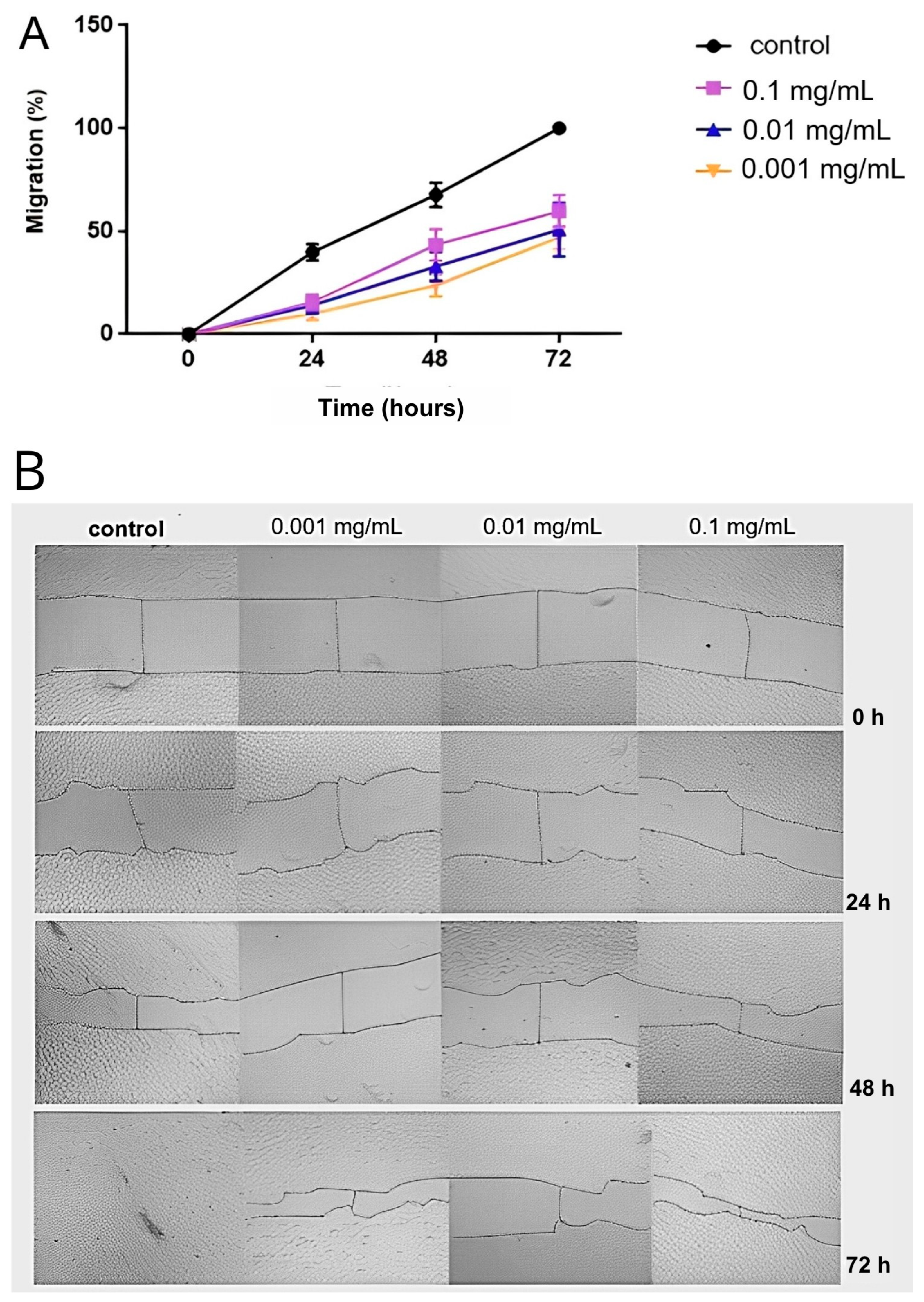
In vitro assays provided robust evidence supporting the antiangiogenic potential of 6-O-desulfated heparin (Hep-6Od). The compound significantly inhibited key cellular processes involved in angiogenesis, including endothelial cell proliferation and migration. DAPI-based nuclear counts revealed a marked reduction in cell proliferation following treatment with Hep-6Od at all tested concentrations, while wound healing assays demonstrated a potent and dose-dependent inhibition of RAEC migration over 72 h. Importantly, MTT viability assay indicated no cytotoxicity, similar result of retinal cells. Together, these results suggest that Hep-6Od effectively impairs essential angiogenic mechanisms. Moreover, coagulation assays (PT and aPTT) demonstrated that Hep-6Od exhibited significantly reduced anticoagulant activity compared to unfractionated heparin (UFH), further supporting its safety profile for potential therapeutic applications targeting pathological angiogenesis, such as in neovascular eye diseases.
For instance, Kniggendorf et al. [
22] demonstrated that a chemically modified heparin, N-desulfated re-N-acetylated heparin (mHep), exerted antiproliferative and antimigratory effects on endothelial cells in vitro. Similarly, Dreyfuss et al. [
21] reported that a heparin mimetic isolated from marine shrimp suppressed neovascularization in vitro and in vivo.
Interestingly, the dose–response behavior of Hep-6Od in the in vitro assays did not follow a linear pattern. In both cell proliferation and tube formation assays, the lower concentration (10 µg/mL) appeared to exert a more potent antiangiogenic effect than higher doses. This phenomenon is consistent with the concept that angiogenesis is regulated by highly dynamic and concentration-sensitive interactions between glycosaminoglycans and angiogenic mediators. Heparins modulate this process by influencing the spatial availability and receptor engagement of key growth factors such as FGF2 and VEGF, as well as by regulating inflammatory cytokines and matrix-bound signaling reservoirs. At lower doses, Hep-6Od may more effectively sequester growth factors or prevent their binding to receptors, whereas at higher doses, saturation effects, altered electrostatic interactions, or compensatory cellular responses may diminish efficacy.
This nonlinear response has been previously observed in our group’s work with other chemically modified heparins, where lower doses frequently produced more pronounced biological effects. Moreover, similar patterns have been reported in the literature. Dreyfuss et al. [
21] described a marine-derived heparin mimetic with reduced anticoagulant activity that more effectively inhibited neovascularization at intermediate concentrations. Likewise, Kniegendorf et al. [
22] demonstrated that structural changes in modified heparins significantly influence their antiangiogenic activity in a dose-dependent yet nonlinear manner. Together, these findings reinforce the importance of fine structural tuning and concentration optimization in the development of heparin-based antiangiogenic therapies.
Beyond the cellular assays, our protein thermal stability studies by DSF provided critical insights into molecular interactions. Specifically, we demonstrated a direct binding between Hep-6Od and FGF-2, a key proangiogenic growth factor. This interaction appears to interfere with the FGF-2 signaling cascade, a pathway intricately involved in neovascularization, and is consistent with the established concept that native heparin and its derivatives can modulate the bioavailability and receptor binding of various growth factors [
9]. These findings suggest that the antiangiogenic effects of Hep-6Od are mediated, at least in part, through the disruption of growth factor signaling networks.
Heparan sulfate proteoglycans (HSPGs) are key regulators of cellular signaling, broadly influencing cell proliferation, migration, adhesion, and apoptosis through their interactions with a wide array of proteins, including growth factors, morphogens, extracellular matrix components, cytokines, and enzymes. Their structurally diverse GAG chains act as critical modulators of the extracellular environment, facilitating or restraining the signaling events essential for tissue homeostasis and pathological processes like angiogenesis [
10].
In the context of angiogenesis, heparan sulfate chains function predominantly as co-receptors, presenting, facilitating and stabilizing the binding of growth factors such as VEGF, FGF-2, HGF, and PDGF to their high-affinity receptors. In addition Heparan sulfate provides a reservoir of readily available growth factors within the ECM. This organization facilitates receptor dimerization and activation, triggering downstream pathways such as MAPK, PI3K/Akt, and PLCγ, which collectively drive endothelial cell proliferation, migration, survival, and cytoskeletal reorganization. The sulfation patterns of heparan sulfate, particularly at the N-, 2-O-, and 6-O- positions, are critical for these interactions, dictating the selectivity and strength of protein binding [
24,
25].
The chemically modified heparin derivative Hep-6Od, characterized by selective 6-O-desulfation, acts as a heparan sulfate mimetic that may strategically disrupt these protein–GAG interactions without abolishing the overall binding affinity. By maintaining the ability to bind growth factors like FGF-2 but impairing proper signaling complex formation, Hep-6Od effectively attenuates endothelial cell responses essential for angiogenesis, as shown in this work. This disruption inhibits endothelial cell proliferation, migration, and capillary-like tube formation, as demonstrated in both in vitro assays and in vivo models of choroidal neovascularization.
Moreover, heparan sulfate not only serves as a platform for growth factor signaling but also regulates endocytic uptake pathways and the spatial distribution of morphogens, chemokines, and cytokines [
25]. Alterations in the heparan sulfate structure, such as those introduced by selective desulfation, can modulate these pathways, impacting the cellular dynamics crucial for angiogenic progression [
25,
26]. By mimicking an under-sulfated heparan sulfate environment, Hep-6Od decreases growth factor bioavailability and signal transduction, thus shifting the cellular balance toward decreased proliferation, impaired migration, and potentially enhanced apoptosis.
In vivo, a dose-dependent reduction in CNV lesion areas was observed following the intravitreal injection of Hep-6Od in a laser-induced CNV model in lean Zucker rats. The concentrations of Hep-6Od used in vitro were selected to reflect the estimated intraocular levels achieved after an intravitreal injection in Zucker rats. Based on a vitreous volume of 50 μL [
27,
28], we calculated that doses of 0.5 to 5.0 μg/eye correspond to final concentrations of approximately 10 to 100 μg/mL, which were matched in the in vitro assays to support translational relevance.
Although UFH was used as a reference compound in the anticoagulant assays, it was not included in the in vivo experiments. This decision was based on prior observations in our laboratory showing that an intravitreal injection of UFH in Zucker rats induces severe intraocular hemorrhage, making it incompatible with subsequent imaging, lesion quantification, and animal welfare standards. Given its high anticoagulant potency, UFH proved to be excessively hemorrhagic when administered intraocularly. For these reasons, and in accordance with the ethical principles of animal experimentation, we opted not to include UFH in the in vivo arm of this study. The reduced hemorrhagic potential of Hep-6Od reinforces its safety profile and translational relevance for ocular applications.
This finding provides further evidence for the antiangiogenic activity of Hep-6Od and suggests that it may be effective at suppressing neovascularization in vivo. The reduction in the CNV area observed in our study is consistent with the results reported by Dreyfuss et al. [
21] and Kniggendorf et al. [
22], who found that a heparin mimetic isolated from marine shrimp and a chemical modification of UFH heparin, respectively, reduced the CNV area in a similar animal model.
Collectively, these findings validate the antiangiogenic activity of Hep-6Od, rooted not merely in its capacity to interfere with specific sulfation-dependent growth factor interactions but in a broader capacity to modulate the heparan sulfate-dependent regulation of cellular behavior. This highlights Hep-6Od as a promising therapeutic agent to target pathological neovascularization through mechanisms extending beyond VEGF inhibition.
This study provides compelling evidence for the antiangiogenic potential of Hep-6Od, and may have important clinical implications in the future studies. The fact that Hep-6Od exhibited antiangiogenic activity without significant cytotoxicity to ARPE-19 cells suggests that it may be a safe therapy for the treatment of wet AMD. Furthermore, the ability of 6-OHep to interact with FGF-2 suggests that it may be effective in patients who are non-responsive to anti-VEGF therapies, as it targets a different signaling pathway involved in angiogenesis.
Future studies should focus on validating the efficacy and safety of 6-OHep in other animal models and, ultimately, in human clinical trials. In addition, further research is needed to elucidate the precise mechanisms by which Hep-6Od exerts its antiangiogenic effects, including its interactions with other growth factors and signaling pathways.
In conclusion, our study provides compelling evidence for the antiangiogenic potential of 6-OHep and suggests that it may be a promising new therapeutic agent for the treatment of wet AMD. Further research is warranted to fully explore its clinical potential and to optimize its use in combination with existing therapies.
4. Materials and Methods
4.1. Synthesis and Characterization of Modified Heparin
Unfractionated heparin (UFH) from porcine intestinal mucosa (Extrasul, Jaguapita, Brazil) was used to generate the 6-O-desulfated heparin according to the procedures outlined in the protocol developed by Yates et al. in 1996 [
23] (
Figure 9).
Initially, a Dowex 50WX8 ion-exchange column was prepared with 1 M hydrochloric acid, then washed to remove free protons. UFH was passed through the column, washed again, eluted and its pH adjusted to 7.0 using pyridine. The resulting pyridine salt-form heparin solution was frozen and lyophilized for storage.
Selective O-desulfation at the C6 position and N-desulfation at the C2 position of glucosamine residues were performed by solvolytic desulfation of the heparin–pyridine salt in a DMSO/methanol (9:1) mixture at 60 °C for 4 h. The pH was adjusted to 7.0 with NaOH. The reaction product was precipitated with cold ethanol saturated with sodium acetate at 4 °C for 16 h. Selective O-desulfation occurs predominantly at the C6 position of the glucosamine units, along with partial N-desulfation at the C2 position, due to the lability of the N-sulfate group. Consequently, the next step involved N-resulfation in this position. N-resulfation at C2 of glucosamine was carried out by reacting the partially desulfated heparin with a trimethylamine–sulfur trioxide complex in the presence of sodium bicarbonate, adjusting the pH to 9.0. Following the reaction, the modified heparin was precipitated again with cold ethanol saturated with sodium acetate and incubated at 4 °C for 16 h. Afterwards the precipitate was desalted by gel filtration chromatography using an FPLC system (GE Healthcare, Uppsala, Sweden).
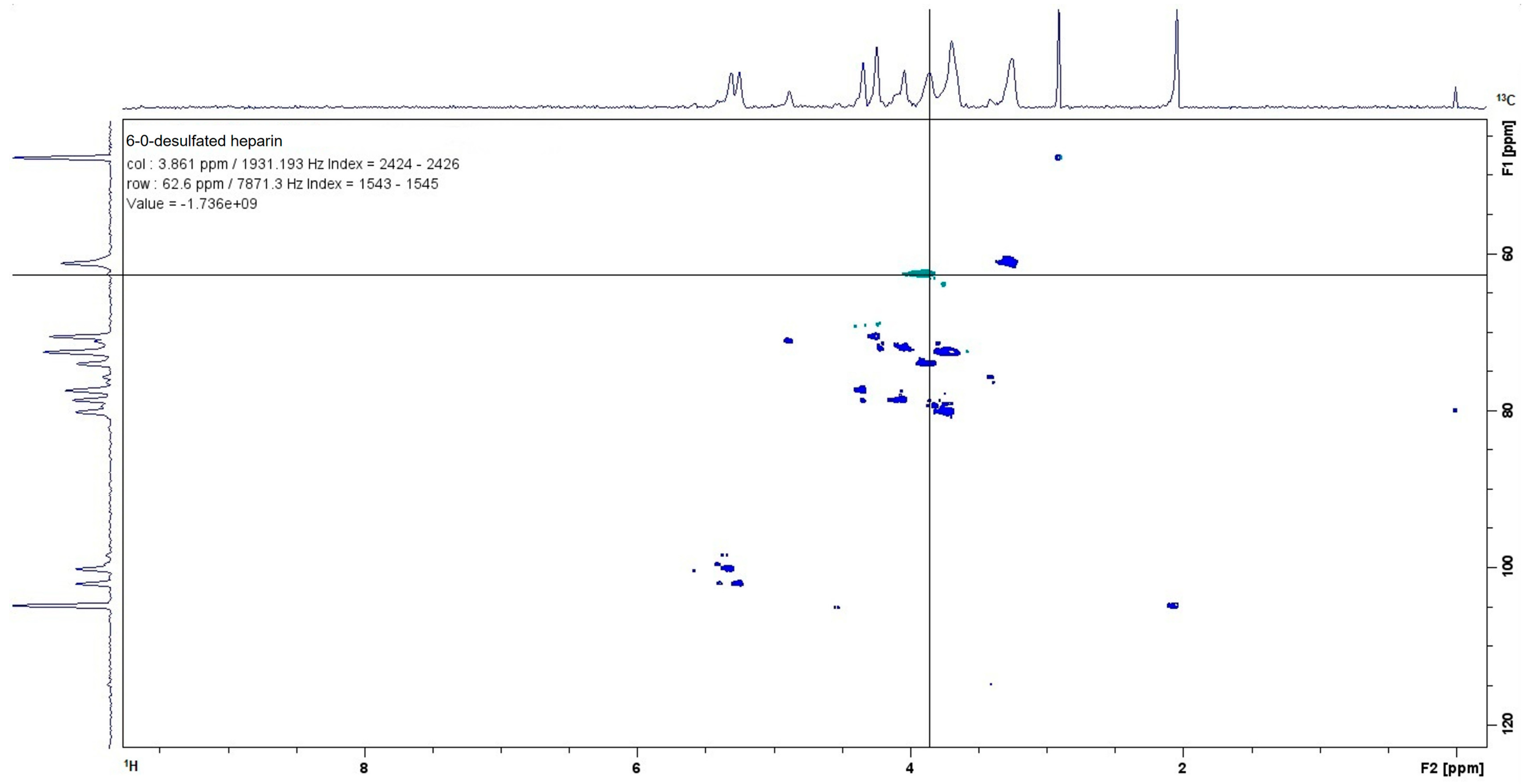
4.2. Characterization of 6-O-Desulfated Heparin by 13C–1H NMR
The chemically modified 6-O-desulfated heparin was dissolved in deuterium oxide (D
2O, 99.9% atom D; Cambridge Isotope Laboratories Inc., Andover, MA, USA) at a final concentration of 10 mg/mL. The NMR spectra were acquired at 22 °C using a Bruker Ascend 500 MHz spectrometer (Billerica, MA, USA) [
30].
4.3. Coagulation Assay
4.3.1. Prothrombin Time (PT) Determination
The prothrombin time assessment utilized a semi-automatic coagulometer (BFT II—Dade Behring) with reconstituted lyophilized rabbit brain thromboplastin from Dade Behring. The procedure involved combining 50 μL of human pooled plasma with 50 μL of either a saline solution (as control) or various concentrations of 6-O-desulfated heparin (6-OHep) (ranging from 1 mg/mL to 0.001 mg/mL) in tubes maintained at 37 °C. Experiments were performed in biological triplicates (n = 3) and analyzed using unpaired two-tailed t-tests. Following a 60 s incubation, 100 μL of thromboplastin was added. The experiments, conducted in duplicate, measured clotting times up to 300 s, providing quantitative data on heparin’s anticoagulant properties.
4.3.2. Activated Partial Thromboplastin Time (aPTT) Determination
The activated partial thromboplastin time (aPTT) was determined using a semi-automatic coagulometer (BFT II—Dade Behring). Activated cephalin derived from rabbit brain, containing celite as the activator, was used by following the procedure described by Silva et al. [
31]. In a standardized tube preheated to 37 °C, 50 µL of plasma and 50 µL of cephalin (with either 50 µL of saline as a control or one of various concentrations of 6-O-desulfated heparin [1 mg/mL, 0.1 mg/mL, 0.01 mg/mL, or 0.001 mg/mL]) were added. Experiments were performed in biological triplicates (
n = 3) and analyzed using unpaired two-tailed
t-tests After an incubation at 37 °C for 120 s, 50 µL of 0.025 M CaCl
2, preheated to 37 °C, was added while simultaneously starting the timer. Measurements were performed in duplicate, and the results were expressed as the clotting time for the different plasma tests. Clotting times are reported in seconds, with the maximum assay duration set at 300 s.
4.4. Cell Culture
In vitro experiments employed two cell lineages: rabbit aortic endothelial cells (RAECs) and retinal pigment epithelial cells (ARPE-19 cells). These cell lines were cryogenically preserved in 1 mL tubes containing culture medium supplemented with 20% fetal bovine serum (FBS) (Cultilab, Campinas, São Paulo, Brazil) and 10% dimethyl sulfoxide (DMSO) (v/v) from Aldrich Chemical Company, Inc. (Milwaukee, WI, USA) in liquid nitrogen.
Rabbit aortic endothelial cells (RAECs) were cultured in F-12 culture medium (Gibco
®, Life Technologies, Rockville, MD, USA) enriched with 10% fetal bovine serum (FBS), 20 mM sodium bicarbonate, and 1% penicillin/streptomycin (Sigma Chemical Co., St. Louis, MO, USA) in adhesive culture dishes (100 mm × 20 mm) (BD Falcon
TM, San Jose, CA, USA). They were incubated at 37 °C in a 2.5% CO
2 atmosphere [
9].
Adult retinal pigment epithelial cells (ARPE-19 cells) were maintained in DMEM/F12 medium (Life Technologies/Gibco®, Rockville, MD, USA) supplemented with 10% fetal bovine serum (FBS; Cultilab, Campinas, SP, Brazil), 15 mM HEPES, 2.0 mM L-glutamine, 0.5 mM sodium pyruvate, 20 mM sodium bicarbonate, and 1% penicillin/streptomycin (Sigma Chemical Co., St. Louis, MO, USA). Cultures were plated in culture dishes (BD FalconTM, San Jose, CA, USA) and incubated at 37 °C in a humidified atmosphere containing 5.0% CO2, ensuring a proper pH balance.
4.5. Cell Viability (Cytotoxicity) Assay
To assess cytotoxicity, the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay was employed. RAECs and ARPE-19 cells were initially seeded in 96-well plates at densities of 2.5 × 104 and 5.0 × 104 cells per well, respectively. After five days, the cultures were treated with 6-O-desulfated heparin at concentrations of 0.001 mg/dL, 0.01 mg/dL, or 0.1 mg/dL prepared in a culture medium supplemented with 10% FBS. After the treatment periods, the culture medium was removed, and the wells were washed with a phosphate-buffered saline (PBS) solution. Subsequently, the MTT solution (0.5 mg/mL), which was diluted in culture medium without fetal bovine serum (FBS), was added to the wells, and the plates were incubated for 2 h. After the incubation, the MTT solution was removed, and 100 µL of DMSO was added to each well. Finally, the absorbance was measured at 540 nm using an Elisa ELx800 microplate reader (BioTek Instruments, Inc., Winooski, VT, USA) with the SoftMax Pro software version 5.4.1.
4.6. Cell Proliferation Assay
Cell proliferation was assessed by the direct counting of nuclei using DAPI (4′,6-diamidino-2-phenylindole) for nuclear staining (Molecular Probes, Eugene, OR, USA). RAECs (2.5 × 103) and ARPE-19 cells (1 × 104) were seeded in 96-well plates and cultured for 18 h in 10% FBS. They were then treated with different concentrations of 6-O-desulfated heparin (0.001 mg/mL, 0.01 mg/mL, or 0.1 mg/dL) in complete culture medium. At 24 and 48 h post-treatment, the cells were washed with PBS and fixed with methanol for 20 min. Subsequently, cells were washed with PBS and stained with DAPI diluted 1:10,000 for 1 h in the dark. The cells were then washed with PBS and analyzed using the Incell Analyzer 2200 (GE Healthcare Bio-Sciences Corp., Uppsala, Sweden).
4.7. Capillary Tube Formation Assay
For this assay, 70 µL of Matrigel
® (Corning, New York, NY, USA) was plated per well in a 96-well plate [
21]. After a polymerization period of 4 h of incubation (37 °C, 2.5% CO
2), 4 × 10
4 RAECs were plated on top of the Matrigel and treated with different concentrations of Hep-6Od (0.001 mg/mL, 0.01 mg/mL, or 0.1 mg/mL). Subsequently, the cultures were maintained at 37 °C in a 2.5% CO
2 atmosphere for 16 h. Tube formation was examined under an inverted bright-field microscope (Zeiss Primovert, Carl Zeiss Microscopy, LLC., Jena, Germany) at 40× magnification. The total length of tubular structures in Matrigel was measured and determined using ImageJ analysis software (version 1.54p, angiogenesis plugin) (NIH, Bethesda, MD, USA) and expressed as tube length in millimeters (mm) [
21].
4.8. Cell Migration Assay (Wound Healing Assay)
The wound healing assay was conducted to evaluate cell migration, simulating in vitro healing processes [
32]. RAECs were seeded in a 24-well plate at a density of 5 × 10
4 cells/well in F12 culture medium and cultured in a 2.5% CO
2 atmosphere at 37 °C. Upon reaching confluence, wounds were created using a 200 µL micropipette tip in each well, followed by the addition of different concentrations of Hep-6Od (0.1, 0.01, or 0.001 mg/mL) or a control (PBS). Sequential images were taken at 0, 24, 48, and 72 h post-injury using a bright-field microscope (Zeiss Primovert, Carl Zeiss Microscopy, LLC., Jena, Germany) at 100× magnification. The area of wound closure (μm
2) was quantified using ImageJ software (NIH, Bethesda, MD, USA).
4.9. Molecular Interaction Assay: 6-O-Desulfated Heparin and Growth Factors (FGF-2)
To assess the possible interaction between FGF-2 and both unfractionated heparin (UFH) and Hep-6Od, a protein thermal stability assay was performed using differential scanning fluorimetry (DSF). FGF-2 (0.2 mg/mL) (Thermo Fisher Scientific Inc., Waltham, MA, USA(, UFH (1 mg/mL), or 6-O-desulfated heparin (10 mg/mL) were mixed with SYPRO® Orange protein gel stain (Invitrogen, Carlsbad, CA, USA) that was previously diluted in PBS (1:50) to achieve a final volume of 35 µL. These solutions were then dispensed into a 96-well optical plate, with 10 µL per well across six wells for each solution. FGF-2 without ligands served as a control. The plate was sealed with optical adhesive film from Applied Biosystems, Waltham, MA, USA, with a 2 min preheating phase at 32 °C, followed by a temperature gradient ramping from 32 °C to 99.9 °C. During this phase, the temperature increased by 0.7 °C per cycle, with each cycle lasting 5 s. Data collection was performed every 5 s with the Applied Biosystems 7500 Real-Time PCR system. The fluorescence versus temperature curves were processed by calculating their first derivatives and processed using GraphPad Prism 7.0 (GraphPad Software, La Jolla, CA, USA).
4.10. In Vivo Experiment
The in vivo study received ethical approval from the Federal University of São Paulo’s Ethics Committee on Animal Use (CEUA/UNIFESP, protocol no. 990022081) and was conducted at the Center for the Development of Experimental Models for Biology and Medicine (CEDEME) at UNIFESP, in accordance with the guidelines of the Association for Research in Vision and Ophthalmology (ARVO).
Sixteen male isogenic lean Zucker rats (aged 8–12 weeks, weighing 180–250 g) were used and randomly allocated into four groups, with four animals per group. The rats were housed under a 12:12 h light–dark cycle at a constant room temperature, with ad libitum access to water and standard chow.
4.10.1. Induction of Choroidal Neovascularization
Anesthesia was induced by an intraperitoneal injection of a mixture of ketamine (10%) and xylazine (2%) (Syntec do Brasil Ltda, Santana do Parnaíba, São Paulo, Brazil) in an 8:2 ratio (80 μL of ketamine to 20 μL of xylazine). A volume of 0.1 mL of this anesthetic solution was administered per 100 g of body weight. Pupil dilation and topical anesthesia were achieved using tropicamide (10 mg/mL) and phenylephrine (2.5%) (Allergan, Guarulhos, São Paulo, Brazil).
For the laser procedure, each animal was positioned in front of a slit lamp, with a microscope slide coated with 2% methylcellulose serving as a contact lens substitute. Only one eye per animal was subjected to the experimental procedure.
Choroidal neovascularization (CNV) was induced using a green argon laser (Quantel Medical, Cournon-d’Auvergne, France; 532 nm wavelength) set at a power of 100–120 mW with a spot size of approximately 100 microns and a duration of 100 milliseconds per shot. Four laser burns were applied around the optic disc in each treated eye, with the rupture of Bruch’s membrane confirmed by the appearance of a subretinal whitish air bubble [
21].
4.10.2. Intravitreal Injection of 6-O-Desulfated Heparin (6-OHep) and Control Treatment
Following Bruch’s membrane rupture, 6-O-desulfated heparin (3 μL) or BSS (for control) was intravitreally injected using a micro syringe (Hamilton Co, Reno, NV, USA). The animals received 0.05 µg, 0.5 µg, or 5 µg of Hep-6Od (in distilled water) or 5 µL of the BSS control solution (Alcon, São Paulo, Brazil).
To calculate the intravitreal concentrations of Hep-6Od in vivo, we assumed an average vitreous volume of 50 μL in Zucker rats, as previously reported [
27,
28] The same concentration range (10–100 μg/mL) was then applied to endothelial cell cultures in vitro to ensure consistency and physiological relevance between the experimental models.
Injections were precisely administered using a Stemi 508 stereo microscope (Carl Zeiss, Oberkochen, Germany) to ensure correct drug delivery into the vitreous cavity.
The following day, animals underwent fundoscopic examinations to identify and exclude any with traumatic lens damage or vitreous hemorrhage [
21].
4.10.3. Euthanasia, Enucleation, and Immunofluorescence Analysis
Fourteen days after the intravitreal injection, the animals were euthanized by the intraperitoneal administration of an anesthetic cocktail consisting of 250 µL of 10% ketamine hydrochloride and 250 µL of 2% xylazine hydrochloride (both from Syntec do Brasil Ltd.a, Santana do Parnaíba, São Paulo, Brazil), followed by an additional 100 µL of the same mixture to induce cardiopulmonary arrest. One eye per rat was immediately enucleated and fixed with 200 µL of 4% paraformaldehyde for 2 h at room temperature.
Subsequently, the eyes were rinsed with PBS and stored overnight at 4 °C in 20% sucrose (Sigma-Aldrich, St. Louis, MO, USA). After an additional PBS rinse, the eyecups were dissected, flat-mounted, and permeabilized using a blocking solution containing 2% bovine serum albumin (BSA) and 0.1% saponin in PBS.
The following day, the eyecups were incubated with an anti-von Willebrand factor primary antibody (sc-8068, Santa Cruz Biotechnology, Dallas, TX, USA) diluted 1:50 in PBS with 1% BSA, and maintained overnight at 4 °C. Subsequently, they were incubated with an Alexa Fluor 488-conjugated secondary antibody (Thermo Fisher Scientific Inc., Waltham, MA, USA) at a 1:300 dilution for 30 min at room temperature, protected from light.
For the choroidal neovascularization analysis, choroidal flat mounts were immunostained for von Willebrand factor (vWF), a marker constitutively expressed throughout the native choroidal vasculature. The eyecups were mounted onto microscope slides using Fluoromount-G mounting medium (Electron Microscopy Sciences, Hatfield, PA, USA) for the lesion analysis. To distinguish neovascular lesions from physiological vWF expression, images were taken using an Axio Observer Z1 inverted fluorescence microscope (Zeiss, Oberkochen, Germany) and the laser intensity was normalized using choroidal areas that had not received laser shots, allowing the visualization exclusively of regions with pathologically increased vWF fluorescence. These areas of focal hyperfluorescence, corresponding to neovascularization, were located in the quadrants where the laser spots had been applied.
Measurements of lesion areas (in µm
2) were performed using ImageJ software (National Institutes of Health, Bethesda, MD, USA). The hyperfluorescent circular lesions were manually delineated and analyzed with this software and the neovascularized area was calculated based on the defined perimeter/area. The immunofluorescence analysis focused on areas indicative of laser-induced disruption of Bruch’s membrane, while hemorrhagic regions were excluded from quantification [
21].
4.11. Statistical Analysis
The evaluation of significant differences was performed using one-way ANOVA complemented by the Bonferroni correction for multiple comparisons utilizing the software GraphPad Prism 7.0 (La Jolla, CA, USA). The results are expressed as the mean values ± the standard errors. The analysis was framed within a 95% confidence level, setting the alpha for significance at 0.05. Therefore, outcomes yielding p values below 0.05 were acknowledged as statistically significant.