Combining Time-Restricted Wheel Running and Feeding During the Light Phase Increases Running Intensity Under High-Fat Diet Conditions Without Altering the Total Amount of Daily Running
Abstract
1. Introduction
2. Results
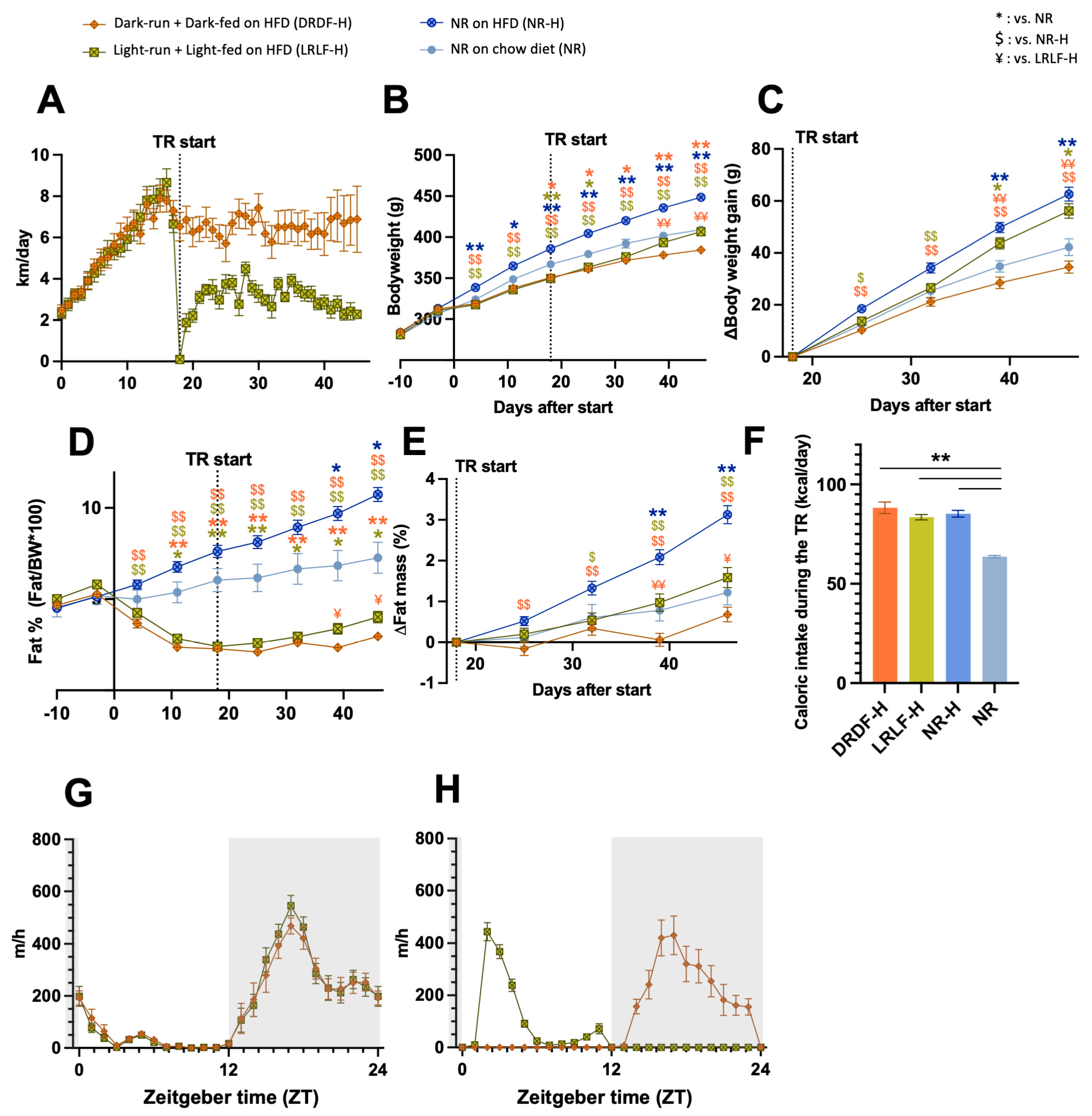
2.1. Effects of Simultaneous Voluntary Running and HFD Consumption Restricted to Dark or Light Phase on Locomotor Activity and Physiology
2.2. Comparing an HFD Versus Chow Diet During Time-Restricted Running and Feeding
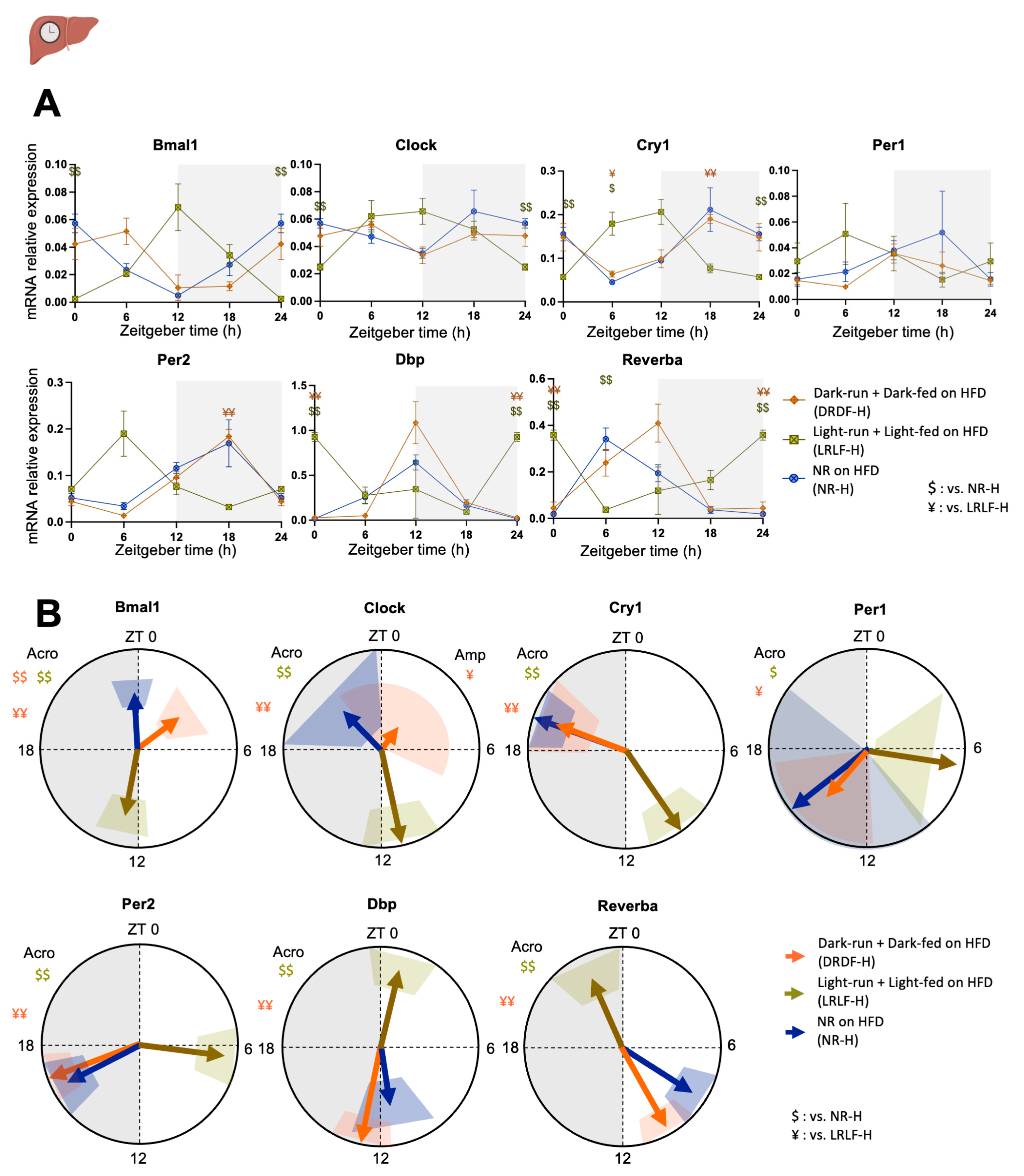
2.3. Effect of Time-Restricted Running and Eating HFD on the Liver Clock
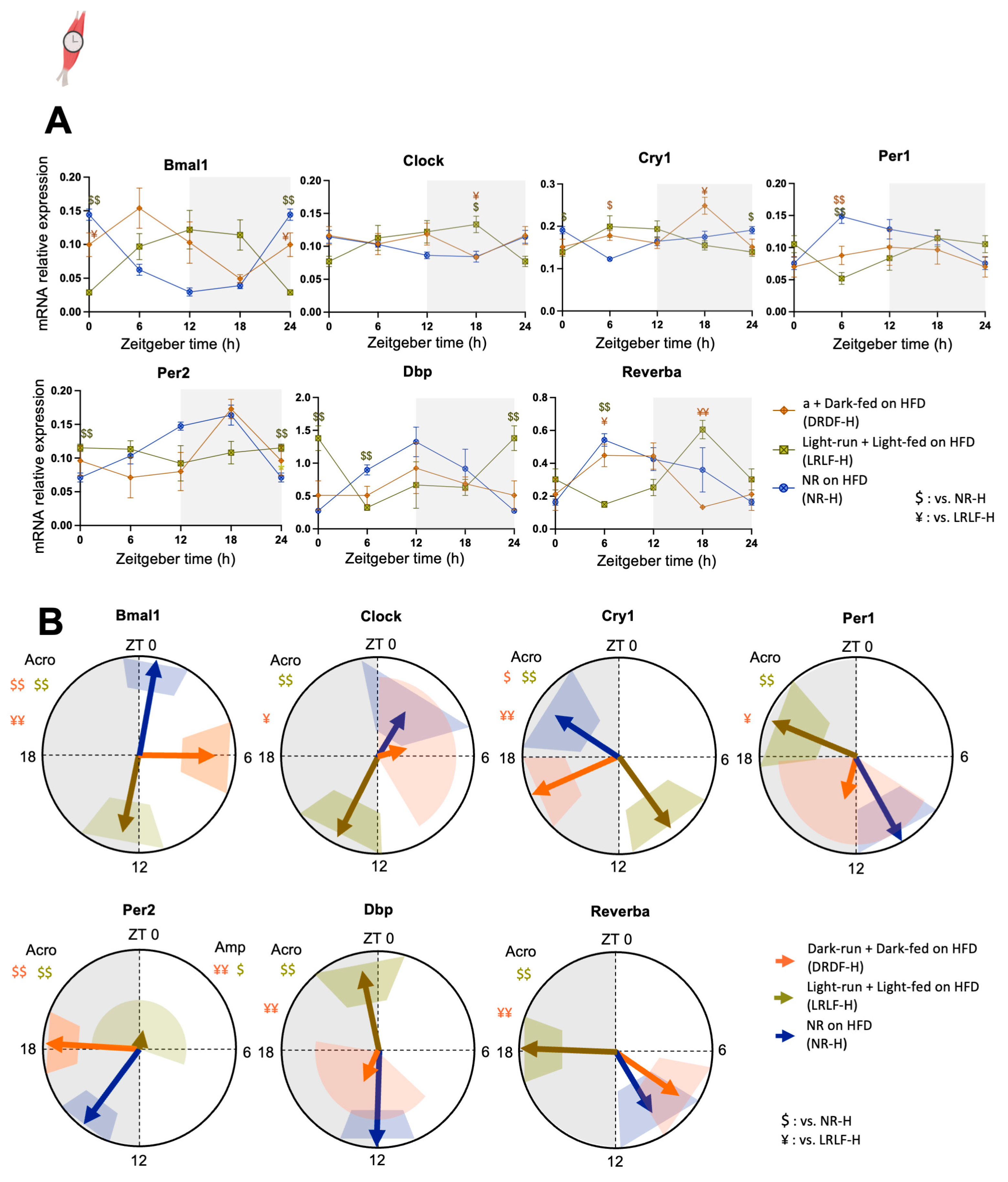
2.4. Effect of Time-Restricted Running and Eating HFD on the Soleus Clock
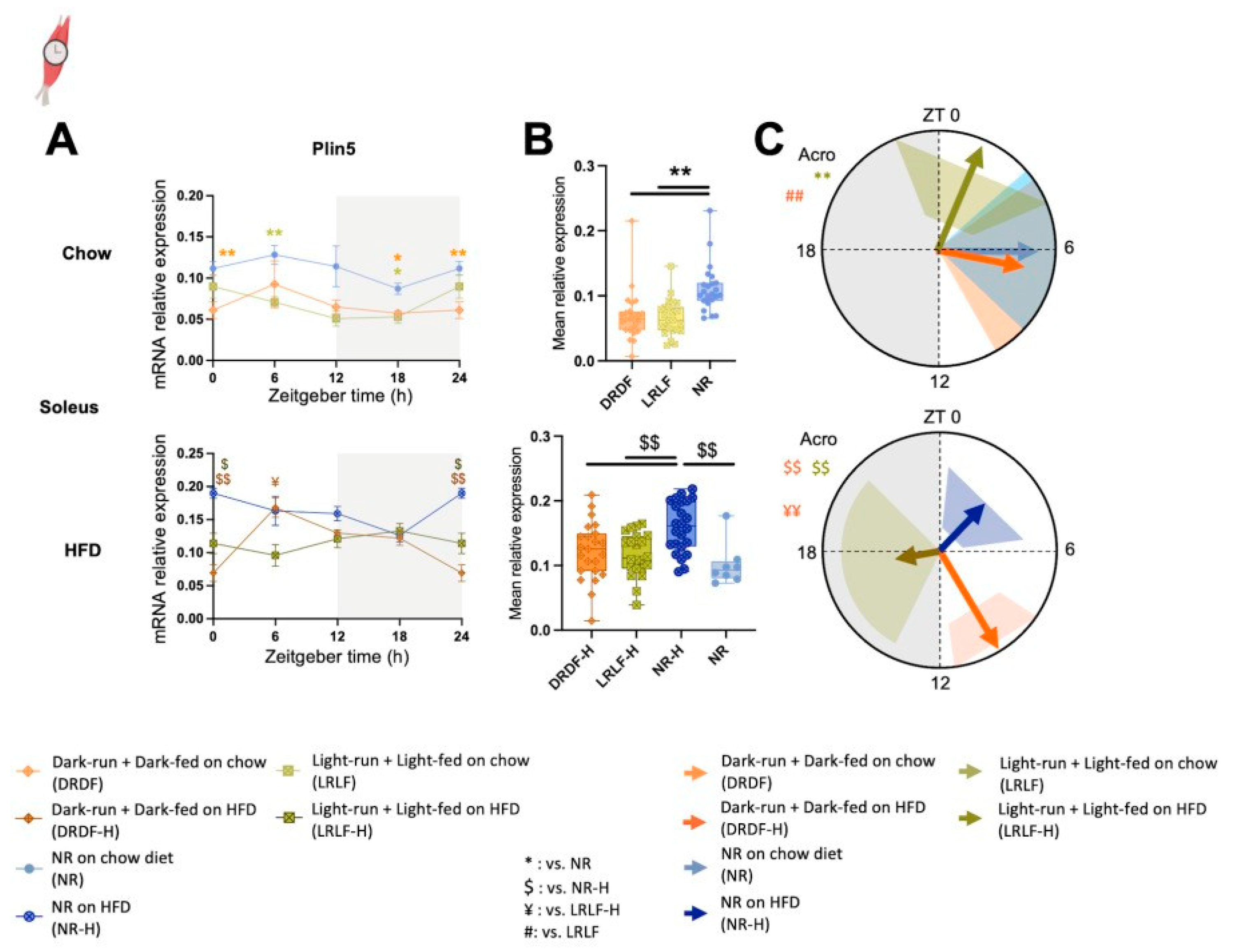
2.5. Effect of Time-Restricted HFD and Running on the Lipid Droplet Associated Protein Gene
3. Discussion
4. Materials and Methods
4.1. Animals and Housing
4.2. Experimental Design
4.2.1. Experimental Cohorts for RT-qPCR Analysis
4.2.2. RNA Isolation, cDNA Synthesis, and RT-qPCR
4.3. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morris, C.J.; Purvis, T.E.; Mistretta, J.; Scheer, F.A.J.L. Effects of the Internal Circadian System and Circadian Misalignment on Glucose Tolerance in Chronic Shift Workers. J. Clin. Endocrinol. Metab. 2016, 101, 1066–1074. [Google Scholar] [CrossRef]
- de Goede, P.; Foppen, E.; Ritsema, W.I.G.R.; Korpel, N.L.; Yi, C.-X.; Kalsbeek, A. Time-Restricted Feeding Improves Glucose Tolerance in Rats, but Only When in Line with the Circadian Timing System. Front. Endocrinol. 2019, 10, 554. [Google Scholar] [CrossRef]
- Joo, J.; Cox, C.C.; Kindred, E.D.; Lashinger, L.M.; Young, M.E.; Bray, M.S. The acute effects of time-of-day-dependent high fat feeding on whole body metabolic flexibility in mice. Int. J. Obes. 2016, 40, 1444–1451. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Clemmensen, C.; Müller, T.D.; Woods, S.C.; Berthoud, H.-R.; Seeley, R.J.; Tschöp, M.H. Gut-Brain Cross-Talk in Metabolic Control. Cell 2017, 168, 758–774. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, J.; Pévet, P.; Challet, E. High-fat feeding alters the clock synchronization to light. J. Physiol. 2008, 586, 5901–5910. [Google Scholar] [CrossRef]
- Oosterman, J.E.; Koekkoek, L.L.; Foppen, E.; Unmehopa, U.A.; Eggels, L.; Verheij, J.; Fliers, E.; La Fleur, S.E.; Kalsbeek, A. Synergistic Effect of Feeding Time and Diet on Hepatic Steatosis and Gene Expression in Male Wistar Rats. Obesity 2020, 28 (Suppl. S1), S81–S92. [Google Scholar] [CrossRef]
- de Goede, P.; Sen, S.; Oosterman, J.E.; Foppen, E.; Jansen, R.; la Fleur, S.E.; Challet, E.; Kalsbeek, A. Differential effects of diet composition and timing of feeding behavior on rat brown adipose tissue and skeletal muscle peripheral clocks. Neurobiol. Sleep Circadian Rhythm. 2018, 4, 24–33. [Google Scholar] [CrossRef]
- Dalbram, E.; Basse, A.L.; Zierath, J.R.; Treebak, J.T. Voluntary wheel running in the late dark phase ameliorates diet-induced obesity in mice without altering insulin action. J. Appl. Physiol. 2019, 126, 993–1005. [Google Scholar] [CrossRef]
- Pendergrast, L.A.; Lundell, L.S.; Ehrlich, A.M.; Ashcroft, S.P.; Schönke, M.; Basse, A.L.; Krook, A.; Treebak, J.T.; Dollet, L.; Zierath, J.R. Time of day determines postexercise metabolism in mouse adipose tissue. Proc. Natl. Acad. Sci. USA 2023, 120, e2218510120. [Google Scholar] [CrossRef]
- Sato, S.; Basse, A.L.; Schönke, M.; Chen, S.; Samad, M.; Altıntaş, A.; Laker, R.C.; Dalbram, E.; Barrès, R.; Baldi, P.; et al. Time of Exercise Specifies the Impact on Muscle Metabolic Pathways and Systemic Energy Homeostasis. Cell Metab. 2019, 30, 92–110.e4. [Google Scholar] [CrossRef]
- Schönke, M.; Ying, Z.; Kovynev, A.; In Het Panhuis, W.; Binnendijk, A.; van der Poel, S.; Pronk, A.C.M.; Streefland, T.C.M.; Hoekstra, M.; Kooijman, S.; et al. Time to run: Late rather than early exercise training in mice remodels the gut microbiome and reduces atherosclerosis development. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2023, 37, e22719. [Google Scholar] [CrossRef]
- Pendergast, J.S.; Branecky, K.L.; Huang, R.; Niswender, K.D.; Yamazaki, S. Wheel-running activity modulates circadian organization and the daily rhythm of eating behavior. Front. Psychol. 2014, 5, 177. [Google Scholar] [CrossRef]
- Shiba, A.; de Goede, P.; Tandari, R.; Foppen, E.; Korpel, N.L.; Coopmans, T.V.; Hellings, T.P.; Jansen, M.W.; Ruitenberg, A.; Ritsema, W.I.G.R.; et al. Synergy between time-restricted feeding and time-restricted running is necessary to shift the muscle clock in male wistar rats. Neurobiol. Sleep Circadian Rhythm. 2024, 17, 100106. [Google Scholar] [CrossRef]
- Xin, H.; Huang, R.; Zhou, M.; Chen, J.; Zhang, J.; Zhou, T.; Ji, S.; Liu, X.; Tian, H.; Lam, S.M.; et al. Daytime-restricted feeding enhances running endurance without prior exercise in mice. Nat. Metab. 2023, 5, 1236–1251. [Google Scholar] [CrossRef]
- Moholdt, T.; Parr, E.B.; Devlin, B.L.; Debik, J.; Giskeødegård, G.; Hawley, J.A. The effect of morning vs evening exercise training on glycaemic control and serum metabolites in overweight/obese men: A randomised trial. Diabetologia 2021, 64, 2061–2076. [Google Scholar] [CrossRef]
- Damasceno de Lima, R.; Fudoli Lins Vieira, R.; Rosetto Muñoz, V.; Chaix, A.; Azevedo Macedo, A.P.; Calheiros Antunes, G.; Felonato, M.; Rosseto Braga, R.; Castelo Branco Ramos Nakandakari, S.; Calais Gaspar, R.; et al. Time-restricted feeding combined with resistance exercise prevents obesity and improves lipid metabolism in the liver of mice fed a high-fat diet. Am. J. Physiol.-Endocrinol. Metab. 2023, 325, E513–E528. [Google Scholar] [CrossRef] [PubMed]
- Vieira, R.F.L.; Muñoz, V.R.; Junqueira, R.L.; Oliveira, F.d.; Gaspar, R.C.; Nakandakari, S.C.B.R.; Costa, S.d.O.; Torsoni, M.A.; da Silva, A.S.R.; Cintra, D.E.; et al. Time-restricted feeding combined with aerobic exercise training can prevent weight gain and improve metabolic disorders in mice fed a high-fat diet. J. Physiol. 2022, 600, 797–813. [Google Scholar] [CrossRef] [PubMed]
- Reijne, A.C.; Talarovicova, A.; Ciapaite, J.; Bruggink, J.E.; Bleeker, A.; Groen, A.K.; Reijngoud, D.-J.; Bakker, B.M.; Dijk, G.V. Running wheel access fails to resolve impaired sustainable health in mice feeding a high fat sucrose diet. Aging 2019, 11, 1564–1579. [Google Scholar] [CrossRef]
- Deng, G.; Jiang, Z.; Lu, H.; Lu, N.; Zhu, R.; Zhu, C.; Zhou, P.; Tang, X. A Study on the Amelioration of Circadian Rhythm Disorders in Fat Mice Using High-Protein Diets. Nutrients 2023, 15, 3459. [Google Scholar] [CrossRef]
- Grimaldi, B.; Bellet, M.M.; Katada, S.; Astarita, G.; Hirayama, J.; Amin, R.H.; Granneman, J.G.; Piomelli, D.; Leff, T.; Sassone-Corsi, P. PER2 Controls Lipid Metabolism by Direct Regulation of PPARγ. Cell Metab. 2010, 12, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Kentish, S.; Hatzinikolas, G.; Li, H.; Frisby, C.; Wittert, G.; Page, A. Time-Restricted Feeding Prevents Ablation of Diurnal Rhythms in Gastric Vagal Afferent Mechanosensitivity Observed in High-Fat Diet-Induced Obese Mice. J. Neurosci. 2018, 38, 5088–5095. [Google Scholar] [CrossRef]
- Begemann, K.; Oster, H. Snack timing affects tissue clock and metabolic responses in male mice. Front. Nutr. 2022, 9, 956641. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, Z.; Ma, J.; Liu, B.; Huang, Z.; Hu, G.; Huang, J.; Xu, Y.; Wang, G.-Z. Circadian-driven tissue specificity is constrained under caloric restricted feeding conditions. Commun. Biol. 2024, 7, 752. [Google Scholar] [CrossRef]
- Kinouchi, K.; Mikami, Y.; Kanai, T.; Itoh, H. Circadian rhythms in the tissue-specificity from metabolism to immunity: Insights from omics studies. Mol. Aspects Med. 2021, 80, 100984. [Google Scholar] [CrossRef]
- Deota, S.; Lin, T.; Chaix, A.; Williams, A.; Le, H.; Calligaro, H.; Ramasamy, R.; Huang, L.; Panda, S. Diurnal transcriptome landscape of a multi-tissue response to time-restricted feeding in mammals. Cell Metab. 2023, 35, 150–165.e4. [Google Scholar] [CrossRef] [PubMed]
- Marcaletti, S.; Thomas, C.; Feige, J.N. Exercise Performance Tests in Mice. Curr. Protoc. Mouse Biol. 2011, 1, 141–154. [Google Scholar] [CrossRef]
- Allen, D.L.; Harrison, B.C.; Maass, A.; Bell, M.L.; Byrnes, W.C.; Leinwand, L.A. Cardiac and skeletal muscle adaptations to voluntary wheel running in the mouse. J. Appl. Physiol. 2001, 90, 1900–1908. [Google Scholar] [CrossRef]
- Claghorn, G.C.; Fonseca, I.A.T.; Thompson, Z.; Barber, C.; Garland, T. Serotonin-mediated central fatigue underlies increased endurance capacity in mice from lines selectively bred for high voluntary wheel running. Physiol. Behav. 2016, 161, 145–154. [Google Scholar] [CrossRef]
- Manzanares, G.; Brito-da-Silva, G.; Gandra, P.G. Voluntary wheel running: Patterns and physiological effects in mice. Braz. J. Med. Biol. Res. 2018, 52, e7830. [Google Scholar] [CrossRef] [PubMed]
- Meek, T.H.; Lonquich, B.P.; Hannon, R.M.; Garland, T. Endurance capacity of mice selectively bred for high voluntary wheel running. J. Exp. Biol. 2009, 212, 2908–2917. [Google Scholar] [CrossRef] [PubMed]
- Bedford, T.G.; Tipton, C.M.; Wilson, N.C.; Oppliger, R.A.; Gisolfi, C.V. Maximum oxygen consumption of rats and its changes with various experimental procedures. J. Appl. Physiol. 1979, 47, 1278–1283. [Google Scholar] [CrossRef]
- Herrera, J.J.; Fedynska, S.; Ghasem, P.R.; Wieman, T.; Clark, P.J.; Gray, N.; Loetz, E.; Campeau, S.; Fleshner, M.; Greenwood, B.N. Neurochemical and behavioral indices of exercise reward are independent of exercise controllability. Eur. J. Neurosci. 2016, 43, 1190–1202. [Google Scholar] [CrossRef]
- Huang, T.-H.; Chang, F.-L.; Lin, S.-C.; Liu, S.-H.; Hsieh, S.S.; Yang, R.-S. Endurance treadmill running training benefits the biomaterial quality of bone in growing male Wistar rats. J. Bone Miner. Metab. 2008, 26, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Patch, L.D.; Brooks, G.A. Effects of training on VO2 max and VO2 during two running intensities in rats. Pflugers Arch. 1980, 386, 215–219. [Google Scholar] [CrossRef]
- Wang, R.; Tian, H.; Guo, D.; Tian, Q.; Yao, T.; Kong, X. Impacts of exercise intervention on various diseases in rats. J. Sport Health Sci. 2020, 9, 211–227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, W.; Xu, R.; Wang, Z.; Zhang, X.; Wang, P.; Peng, K.; Li, M.; Li, J.; Tan, Y.; et al. Plin5 Bidirectionally Regulates Lipid Metabolism in Oxidative Tissues. Oxid. Med. Cell. Longev. 2022, 2022, 4594956. [Google Scholar] [CrossRef]
- Rinnankoski-Tuikka, R.; Hulmi, J.J.; Torvinen, S.; Silvennoinen, M.; Lehti, M.; Kivelä, R.; Reunanen, H.; Kujala, U.M.; Kainulainen, H. Lipid droplet-associated proteins in high-fat fed mice with the effects of voluntary running and diet change. Metabolism 2014, 63, 1031–1040. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, Y.; Gao, X.; Li, L.; Yuan, Y.; Liu, F.; Zhang, L.; Wu, J.; Hu, P.; Zhang, X.; et al. Perilipin 5 improves hepatic lipotoxicity by inhibiting lipolysis. Hepatology 2015, 61, 870–882. [Google Scholar] [CrossRef]
- Baumgarten, P.; Jung, T.; Ott, C.; Grune, T. Differential Plin5 response to high-fat diet in cardiomyocytes isolated from young and aged mice. Mech. Ageing Dev. 2024, 222, 112004. [Google Scholar] [CrossRef] [PubMed]
- Bindesbøll, C.; Berg, O.; Arntsen, B.; Nebb, H.I.; Dalen, K.T. Fatty acids regulate perilipin5 in muscle by activating PPARδ. J. Lipid Res. 2013, 54, 1949–1963. [Google Scholar] [CrossRef]
- Mehdi, F.; Keihan, G.S.; Asadollah, A.S.; Effat, F. The Effects of Resveratrol, Metformin, Cold and Strength Training on the Level of Perilipin 5 in the Heart, Skeletal Muscle and Brown Adipose Tissues in Mouse. Cell Biochem. Biophys. 2018, 76, 471–476. [Google Scholar] [CrossRef]
- Harris, L.-A.L.S.; Skinner, J.R.; Shew, T.M.; Pietka, T.A.; Abumrad, N.A.; Wolins, N.E. Perilipin 5–Driven Lipid Droplet Accumulation in Skeletal Muscle Stimulates the Expression of Fibroblast Growth Factor 21. Diabetes 2015, 64, 2757–2768. [Google Scholar] [CrossRef] [PubMed]
- Porflitt-Rodríguez, M.; Guzmán-Arriagada, V.; Sandoval-Valderrama, R.; Tam, C.S.; Pavicic, F.; Ehrenfeld, P.; Martínez-Huenchullán, S. Effects of aerobic exercise on fibroblast growth factor 21 in overweight and obesity. A systematic review. Metabolism 2022, 129, 155137. [Google Scholar] [CrossRef] [PubMed]
- van Eenige, R.; Verhave, P.S.; Koemans, P.J.; Tiebosch, I.A.C.W.; Rensen, P.C.N.; Kooijman, S. RandoMice, a novel, user-friendly randomization tool in animal research. PLoS ONE 2020, 15, e0237096. [Google Scholar] [CrossRef] [PubMed]
- Moškon, M. CosinorPy: A python package for cosinor-based rhythmometry. BMC Bioinform. 2020, 21, 485. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiba, A.; Tandari, R.; Foppen, E.; Yi, C.-X.; Mul, J.D.; Stenvers, D.J.; Kalsbeek, A. Combining Time-Restricted Wheel Running and Feeding During the Light Phase Increases Running Intensity Under High-Fat Diet Conditions Without Altering the Total Amount of Daily Running. Int. J. Mol. Sci. 2025, 26, 7658. https://doi.org/10.3390/ijms26157658
Shiba A, Tandari R, Foppen E, Yi C-X, Mul JD, Stenvers DJ, Kalsbeek A. Combining Time-Restricted Wheel Running and Feeding During the Light Phase Increases Running Intensity Under High-Fat Diet Conditions Without Altering the Total Amount of Daily Running. International Journal of Molecular Sciences. 2025; 26(15):7658. https://doi.org/10.3390/ijms26157658
Chicago/Turabian StyleShiba, Ayano, Roberta Tandari, Ewout Foppen, Chun-Xia Yi, Joram D. Mul, Dirk Jan Stenvers, and Andries Kalsbeek. 2025. "Combining Time-Restricted Wheel Running and Feeding During the Light Phase Increases Running Intensity Under High-Fat Diet Conditions Without Altering the Total Amount of Daily Running" International Journal of Molecular Sciences 26, no. 15: 7658. https://doi.org/10.3390/ijms26157658
APA StyleShiba, A., Tandari, R., Foppen, E., Yi, C.-X., Mul, J. D., Stenvers, D. J., & Kalsbeek, A. (2025). Combining Time-Restricted Wheel Running and Feeding During the Light Phase Increases Running Intensity Under High-Fat Diet Conditions Without Altering the Total Amount of Daily Running. International Journal of Molecular Sciences, 26(15), 7658. https://doi.org/10.3390/ijms26157658






