Mephedrone and Its Metabolites: A Narrative Review
Abstract
1. Introduction
1.1. Clinical Background and Relevance of Mephedrone
1.2. Importance of Metabolite Research
1.3. Aim of Review and Methodology
2. Structure and Pharmacokinetic Properties of Mephedrone
3. Preclinical Studies
3.1. Metabolism and Pharmacokinetics
3.2. Neurochemical and Behavioral Effects
3.3. Thermoregulatory Response
4. Detection of Mephedrone in Biological Samples
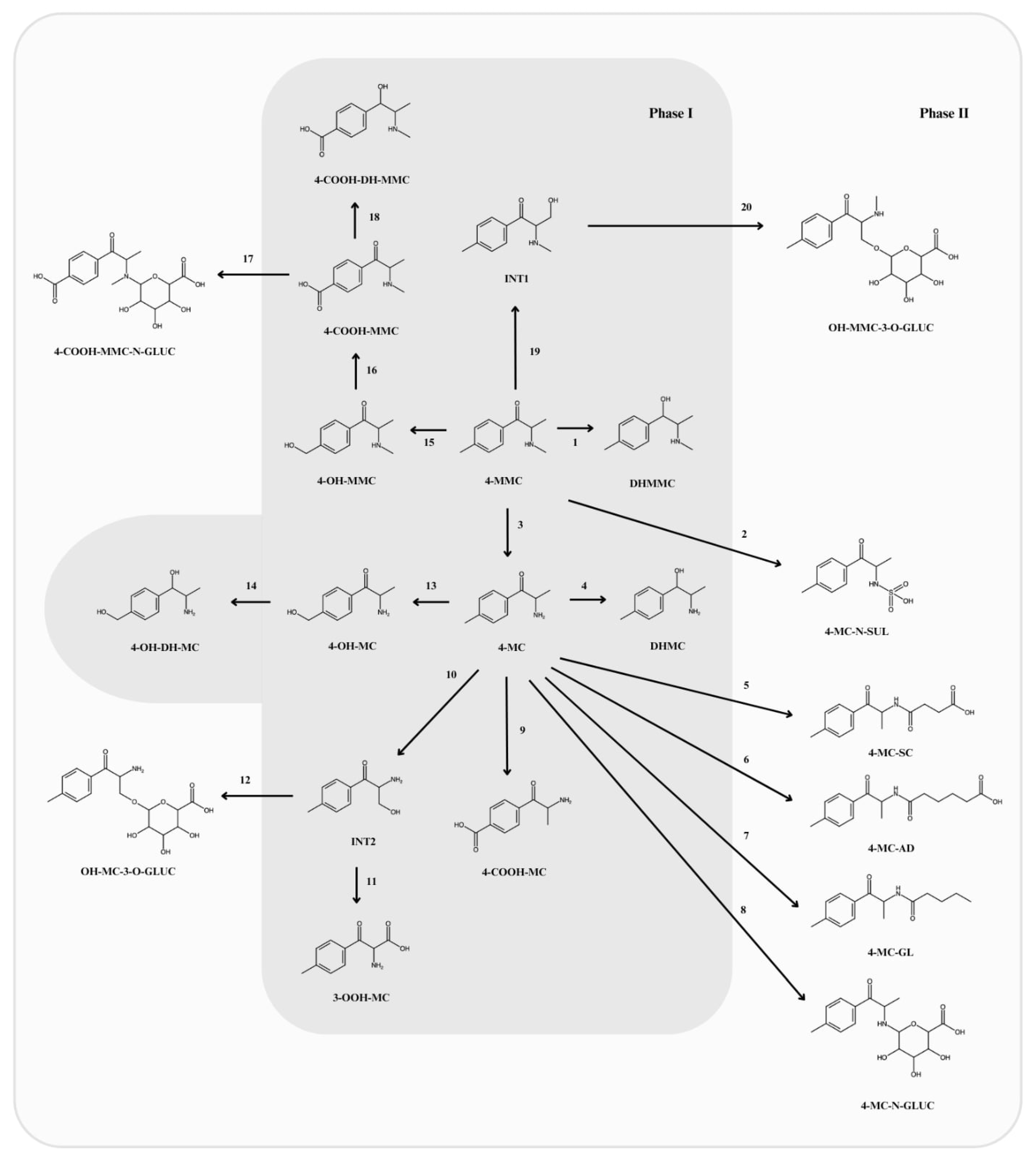
5. Phase I Metabolites
5.1. Normephedrone (4-MC)
5.2. Dihydromephedrone (DHMMC)
5.3. Dihydronormephedrone (DHMC)
5.4. 4-Carboxymephedrone (4-COOH-MMC)
5.5. 4-Hydroxymephedrone (4-OH-MMC)
5.6. Other Phase I Metabolites
6. Phase II Metabolites
6.1. N-Succinyl-Normephedrone (4-MC-SC)
6.2. N-Glutaryl-Normephedrone (4-MC-GL)
6.3. N-Adipoyl-Normephedrone (4-MC-AD)
6.4. Glucuronide Conjugates
6.5. Normephedrone-N-Sulfate (4-MC-N-SUL)
7. Discussion
7.1. Metabolic Complexity and Pharmacological Implications
7.2. Integration of Metabolism with Analytical and Forensic Frameworks
7.3. Environmental and Public Health Relevance of Metabolic Pathways
7.4. Comparison with Previous Reviews and Added Value of Present Study
7.5. Limitations and Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3-OOH-MC | normephedrone-ω-carboxylic acid |
| 4-COOH-DH-MMC | 4-carboxydihydromephedrone |
| 4-COOH-MC | 4-carboxynormephedrone |
| 4-COOH-MMC | 4-carboxymephedrone |
| 4-COOH-MMC-N-GLUC | 4-carboxymephedrone-N-glucuronide |
| 4-MC | normephedrone, 4-methylcathinone |
| 4-MC-AD | N-adipoyl-normephedrone |
| 4-MC-GL | N-glutaryl-normephedrone |
| 4-MC-N-GLUC | normephedrone-N-glucuronide |
| 4-MC-N-SUL | normephedrone-N-sulphate |
| 4-MC-SC | N-succinyl-normephedrone |
| 4-MMC | mephedrone, 4-methylmethcathinone |
| 4-OH-DH-MC | hydroxytolyldihydronormephedrone |
| 4-OH-MC | 4-hydroxynormephedrone |
| 4-OH-MMC | 4-hydroxymephedrone |
| 5-HT | 5-hydroxytryptamine |
| DA | dopamine |
| DAT | dopamine transporter |
| DHMC | dihydronormephedrone |
| DHMMC | dihydromephedrone |
| DLLME | dispersive liquid–-liquid microextraction |
| GC | gas chromatography |
| GC-MS | gas chromatography–mass spectrometry |
| GC-MS/MS | gas chromatography–-tandem mass spectrometry |
| HPLC/HRMS | high-performance liquid chromatography–-high-resolution mass spectrometry |
| LC-QE | liquid chromatography coupled with Q-Exactive system |
| LC-HRMS | liquid chromatography–high resolution mass spectrometry |
| LC-MS | liquid chromatography–-mass spectrometry |
| LC-MS/MS | liquid chromatography–tandem mass spectrometry |
| LC-QTOF | liquid chromatography–quadrupole time-of-flight mass spectrometry |
| LLE | liquid–--liquid extraction |
| MDMA | 3,4-methylenedioxy-methamphetamine |
| mi-SPE | molecularly imprinted solid-phase extraction |
| MMP-9 | matrix metallopeptidase 9 |
| NA | noradrenaline |
| NAT | noradrenaline transporter |
| NMDA | N-methyl-D-aspartate receptor |
| OH-MC-3-O-GLUC | hydroxylnormephedrone-3-O-glucuronide |
| OH-MMC-3-O-GLUC | hydroxylmephedrone-3-O-glucuronide |
| pHLM | human pooled urine |
| SPE | solid-phase extraction |
| SERT | serotonin transporter |
| SPME | solid-phase microextraction |
| TAAR1 | trace amine-associated receptor 1 |
| UHPLC-QTOF/MS | ultra-high-performance liquid chromatography–-hybrid quadrupole time-of-flight mass spectrometry |
| UPLC-TOF/MS | ultra-performance liquid chromatography–-time-of-flight mass spectrometry |
References
- Hasan, M.; Sarker, S.A. New Psychoactive Substances: A Potential Threat to Developing Countries. Addict. Health 2023, 15, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Shafi, A.; Berry, A.J.; Sumnall, H.; Wood, D.M.; Tracy, D.K. New psychoactive substances: A review and updates. Ther. Adv. Psychopharmacol. 2020, 10, 2045125320967197. [Google Scholar] [CrossRef] [PubMed]
- Hockenhull, J.; Murphy, K.G.; Paterson, S. Mephedrone use is increasing in London. Lancet 2016, 387, 1719–1720. [Google Scholar] [CrossRef][Green Version]
- Ordak, M.; Nasierowski, T.; Muszynska, E. The growing problem of mephedrone use in Warsaw, Poland, 2010–18. Lancet Psychiat. 2018, 5, 787. [Google Scholar] [CrossRef]
- Ordak, M.; Nasierowski, T.; Muszynska, E.; Bujalska-Zadrozny, M. The Psychiatric Characteristics of People on a Mephedrone (“bath salts”) Binge. Subst. Use Misuse 2020, 55, 1610–1617. [Google Scholar] [CrossRef]
- Matey, J.M.; Zapata, F.; Menéndez-Quintanal, L.M.; Montalvo, G.; García-Ruiz, C. Identification of new psychoactive substances and their metabolites using non-targeted detection with high-resolution mass spectrometry through diagnosing fragment ions/neutral loss analysis. Talanta 2023, 265, 124816. [Google Scholar] [CrossRef]
- Wagmann, L.; Frankenfeld, F.; Park, Y.M.; Herrmann, J.; Fischmann, S.; Westphal, F.; Müller, R.; Flockerzi, V.; Meyer, M.R. How to study the metabolism of new psychoactive substances for the purpose of toxicological screenings-a follow-up study comparing pooled human liver S9, HepaRG cells, and zebrafish larvae. Front. Chem. 2020, 8, 539. [Google Scholar] [CrossRef]
- Meyer, M.R. New psychoactive substances: An overview on recent publications on their toxicodynamics and toxicokinetics. Arch. Toxicol. 2016, 90, 2421–2444. [Google Scholar] [CrossRef]
- Peacock, A.; Bruno, R.; Gisev, N.; Degenhardt, L.; Hall, W.; Sedefov, R.; White, J.; Thomas, K.V.; Farrell, M.; Griffiths, P. New psychoactive substances: Challenges for drug surveillance, control, and public health responses. Lancet 2019, 394, 1668–1684. [Google Scholar] [CrossRef]
- Řezanka, P.; Macková, D.; Jurok, R.; Himl, M.; Kuchař, M. Enantioseparation and determination of mephedrone and its metabolites by capillary electrophoresis using cyclodextrins as chiral selectors. Molecules 2020, 25, 2879. [Google Scholar] [CrossRef]
- Šíchová, K.; Pinterová, N.; Židková, M.; Horsley, R.R.; Lhotková, E.; Štefková, K.; Vejmola, Č.; Uttl, L.; Balíková, M.; Kuchař, M.; et al. Mephedrone (4-Methylmethcathinone): Acute behavioral effects, hyperthermic, and pharmacokinetic profile in rats. Front. Psychiatry 2018, 8, 306. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Clemente, J.; López-Arnau, R.; Carbó, M.; Pubill, D.; Camarasa, J.; Escubedo, E. Mephedrone pharmacokinetics after intravenous and oral administration in rats: Relation to pharmacodynamics. Psychopharmacology 2013, 229, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.J.; Reitzel, L.A.; Johansen, S.S.; Linnet, K. In vitro metabolism studies on mephedrone and analysis of forensic cases. Drug Test. Anal. 2012, 5, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Niello, M.; Cintulová, D.; Raithmayr, P.; Holy, M.; Jäntsch, K.; Colas, C.; Ecker, G.F.; Sitte, H.H.; Mihovilovic, M.D. Effects of hydroxylated mephedrone metabolites on monoamine transporter activity in vitro. Front. Pharmacol. 2021, 12, 654061. [Google Scholar] [CrossRef]
- Castrignanò, E.; Mardal, M.; Rydevik, A.; Miserez, B.; Ramsey, J.; Shine, T.; Pantoș, G.D.; Meyer, M.R.; Kasprzyk-Hordern, B. Publisher correction: A new approach towards biomarker selection in estimation of human exposure to chiral chemicals: A case study of mephedrone. Sci. Rep. 2018, 8, 4295. [Google Scholar] [CrossRef]
- Docherty, J.R.; Alsufyani, H.A. Pharmacology of drugs used as stimulants. J. Clin. Pharmacol. 2021, 61, S53–S69. [Google Scholar] [CrossRef]
- Martínez-Clemente, J.; Escubedo, E.; Pubill, D.; Camarasa, J. Interaction of mephedrone with dopamine and serotonin targets in rats. Eur. Neuropsychopharmacol. 2012, 22, 231–236. [Google Scholar] [CrossRef]
- Cozzi, N.V.; Sievert, M.K.; Shulgin, A.T.; Jacob, P., 3rd; Ruoho, A.E. Inhibition of plasma membrane monoamine transporters by beta-ketoamphetamines. Eur. J. Pharmacol. 1999, 381, 63–69. [Google Scholar] [CrossRef]
- Wronikowska, O.; Zykubek, M.; Michalak, A.; Pankowska, A.; Kozioł, P.; Boguszewska-Czubara, A.; Kurach, Ł.; Łazorczyk, A.; Kochalska, K.; Talarek, S.; et al. Insight into glutamatergic involvement in rewarding effects of mephedrone in rats: In vivo and ex vivo study. Mol. Neurobiol. 2021, 58, 4413–4424. [Google Scholar] [CrossRef]
- Grochecki, P.; Smaga, I.; Lopatynska-Mazurek, M.; Gibula-Tarlowska, E.; Kedzierska, E.; Listos, J.; Talarek, S.; Marszalek-Grabska, M.; Hubalewska-Mazgaj, M.; Korga-Plewko, A.; et al. Effects of mephedrone and amphetamine exposure during adolescence on spatial memory in adulthood: Behavioral and neurochemical analysis. Int. J. Mol. Sci. 2021, 22, 589. [Google Scholar] [CrossRef]
- Alexandridou, A.; Mouskeftara, T.; Raikos, N.; Gika, H.G. GC-MS analysis of underivatised new psychoactive substances in whole blood and urine. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2020, 1156, 122308. [Google Scholar] [CrossRef]
- Linhart, I.; Himl, M.; Židková, M.; Balíková, M.; Lhotková, E.; Páleníček, T. Metabolic profile of mephedrone: Identification of nor-mephedrone conjugates with dicarboxylic acids as a new type of xenobiotic phase II metabolites. Toxicol. Lett. 2016, 240, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.P.; Wimmer, L.; Dillon-Carter, O.; Partilla, J.S.; Burchardt, N.V.; Mihovilovic, M.D.; Baumann, M.H.; Sitte, H.H. Phase I metabolites of mephedrone display biological activity as substrates at monoamine transporters. Br. J. Pharmacol. 2016, 173, 2657–2668. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.P.; Cintulova, D.; Pittrich, D.A.; Wimmer, L.; Luethi, D.; Holy, M.; Jaentsch, K.; Tischberger, S.; Gmeiner, G.; Hoener, M.C.; et al. Stereochemistry of phase-1 metabolites of mephedrone determines their effectiveness as releasers at the serotonin transporter. Neuropharmacology 2019, 148, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Pozo, Ó.J.; Ibáñez, M.; Sancho, J.V.; Lahoz-Beneytez, J.; Farré, M.; Papaseit, E.; de la Torre, R.; Hernández, F. Mass spectrometric evaluation of mephedrone in vivo human metabolism: Identification of phase I and phase II metabolites, including a novel succinyl conjugate. Drug Metab. Dispos. 2015, 43, 248–257. [Google Scholar] [CrossRef]
- Czerwinska, J.; Parkin, M.C.; George, C.; Kicman, A.T.; Dargan, P.I.; Abbate, V. Excretion of mephedrone and its phase I metabolites in urine after a controlled intranasal administration to healthy human volunteers. Drug Test. Anal. 2022, 14, 741–746. [Google Scholar] [CrossRef]
- Czerwinska, J.; Parkin, M.C.; George, C.; Kicman, A.T.; Dargan, P.I.; Abbate, V. Pharmacokinetics of mephedrone and its metabolites in whole blood and plasma after controlled intranasal administration to healthy human volunteers. J. Anal. Toxicol. 2021, 45, 730–738. [Google Scholar] [CrossRef]
- Steuer, A.E.; Kaelin, D.; Boxler, M.I.; Eisenbeiss, L.; Holze, F.; Vizeli, P.; Czerwinska, J.; Dargan, P.I.; Abbate, V.; Liechti, M.E.; et al. Comparative untargeted metabolomics analysis of the psychostimulants 3,4-methylenedioxy-methamphetamine (MDMA), amphetamine, and the novel psychoactive substance mephedrone after controlled drug administration to humans. Metabolites 2020, 10, 306. [Google Scholar] [CrossRef]
- Czerwinska, J.; Parkin, M.C.; Cilibrizzi, A.; George, C.; Kicman, A.T.; Dargan, P.I.; Abbate, V. Pharmacokinetics of mephedrone enantiomers in whole blood after a controlled intranasal administration to healthy human volunteers. Pharmaceuticals 2020, 14, 5. [Google Scholar] [CrossRef]
- Olesti, E.; Farré, M.; Papaseit, E.; Krotonoulas, A.; Pujadas, M.; de la Torre, R.; Pozo, Ó.J. Pharmacokinetics of mephedrone and its metabolites in human by LC-MS/MS. AAPS J. 2017, 19, 1767–1778. [Google Scholar] [CrossRef]
- Olesti, E.; Pujadas, M.; Papaseit, E.; Pérez-Mañá, C.; Pozo, Ó.J.; Farré, M.; de la Torre, R. GC-MS quantification method for mephedrone in plasma and urine: Application to human pharmacokinetics. J. Anal. Toxicol. 2017, 41, 100–106. [Google Scholar] [CrossRef]
- Schifano, F.; Albanese, A.; Fergus, S.; Stair, J.L.; Deluca, P.; Corazza, O.; Davey, Z.; Corkery, J.; Siemann, H.; Scherbaum, N.; et al. Mephedrone (4-methylmethcathinone; ‘meow meow’): Chemical, pharmacological and clinical issues. Psychopharmacology 2011, 214, 593–602. [Google Scholar] [CrossRef]
- Angoa-Pérez, M.; Kuhn, D.M. Chapter 3—Mephedrone: An Overview of Its Neurotoxic Potential, Neuropathology of Drug Addictions and Substance Misuse. In Neuropathology of Drug Addictions and Substance Misuse, 1st ed.; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2016; Volume 2, pp. 25–35. [Google Scholar] [CrossRef]
- Mead, J.; Parrott, A. Mephedrone and MDMA: A comparative review. Brain Res. 2020, 1735, 146740. [Google Scholar] [CrossRef]
- Chen, Y.K.; Shih, C.P.; Wang, C.H.; Lin, C.C. Mephedrone concentrations in clinical intoxications and fatal cases: A systematic review. Forensic Toxicol. 2025, 43, 46–61. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michal, O.; Daria, T.; Izabela, J.; Gorecka, W.; Tadeusz, N.; Elzbieta, M.; Magdanena, B.-Z. Mephedrone and Its Metabolites: A Narrative Review. Int. J. Mol. Sci. 2025, 26, 7656. https://doi.org/10.3390/ijms26157656
Michal O, Daria T, Izabela J, Gorecka W, Tadeusz N, Elzbieta M, Magdanena B-Z. Mephedrone and Its Metabolites: A Narrative Review. International Journal of Molecular Sciences. 2025; 26(15):7656. https://doi.org/10.3390/ijms26157656
Chicago/Turabian StyleMichal, Ordak, Tkacz Daria, Juzwiuk Izabela, Wiktoria Gorecka, Nasierowski Tadeusz, Muszynska Elzbieta, and Bujalska-Zadrozny Magdanena. 2025. "Mephedrone and Its Metabolites: A Narrative Review" International Journal of Molecular Sciences 26, no. 15: 7656. https://doi.org/10.3390/ijms26157656
APA StyleMichal, O., Daria, T., Izabela, J., Gorecka, W., Tadeusz, N., Elzbieta, M., & Magdanena, B.-Z. (2025). Mephedrone and Its Metabolites: A Narrative Review. International Journal of Molecular Sciences, 26(15), 7656. https://doi.org/10.3390/ijms26157656





