Evaluation of Ultrasonic Spray Method for Application of Sirolimus-Eluting Coating on Bioresorbable Vascular Scaffolds
Abstract
1. Introduction
2. Results
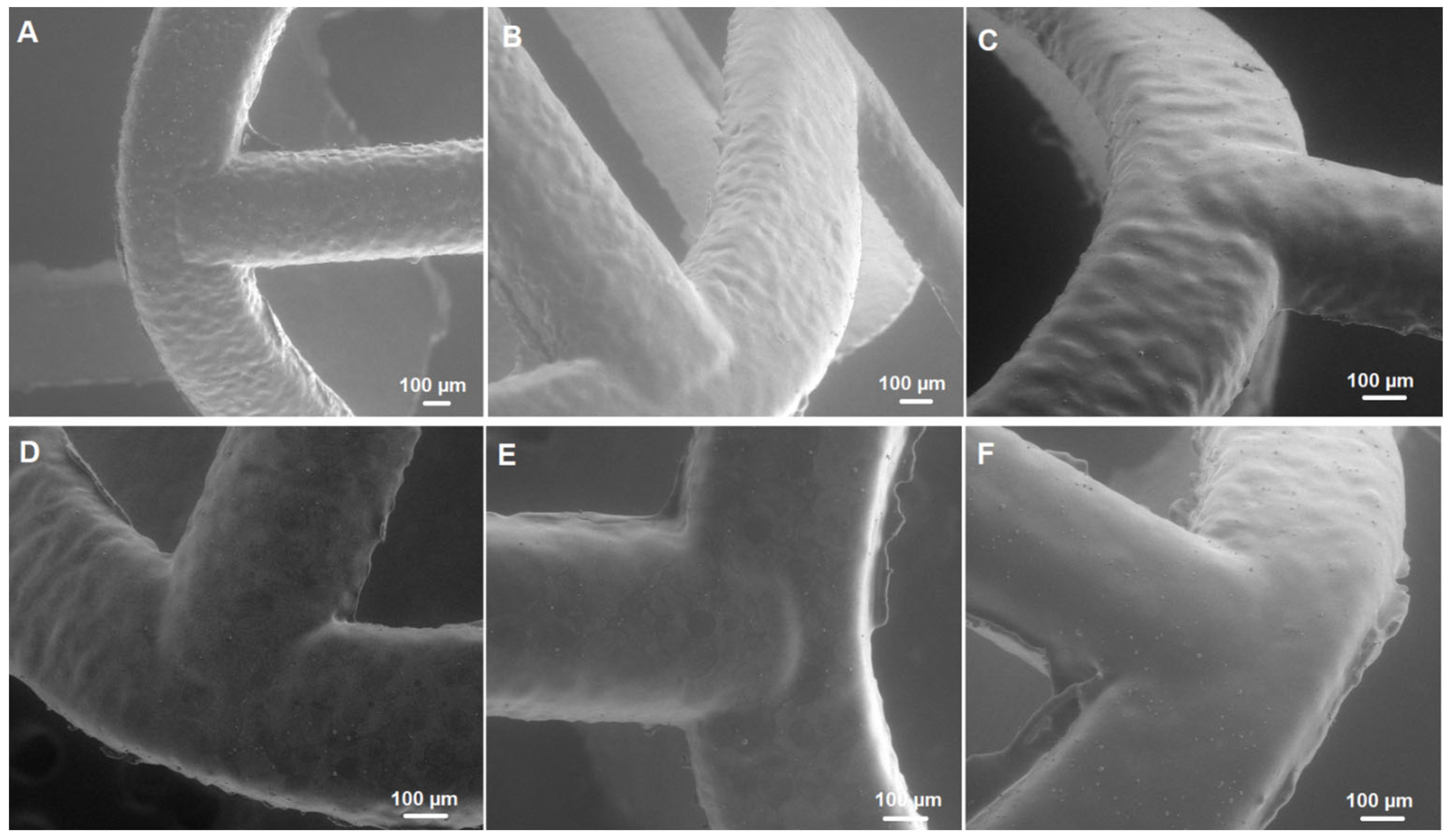
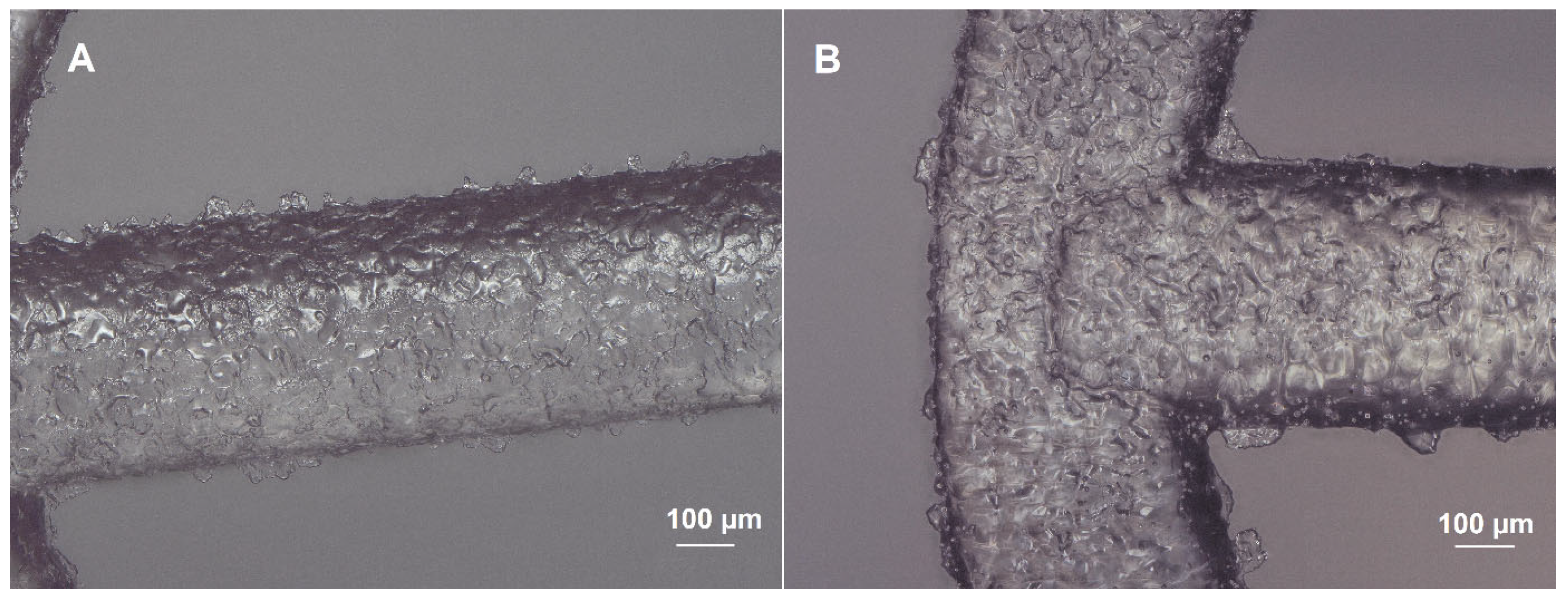
2.1. Morphology of Scaffolds
2.2. Drug Dose
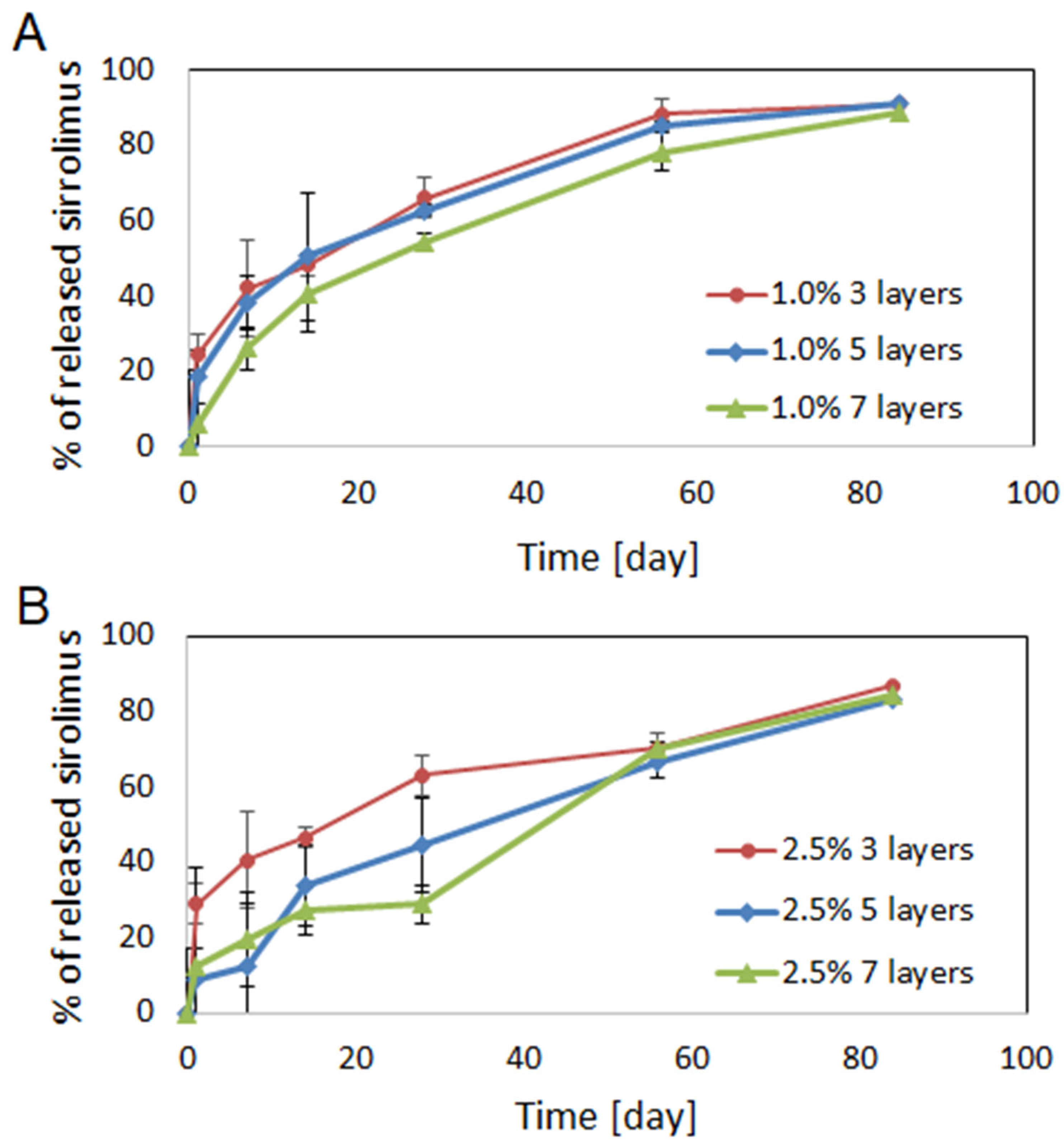
2.3. Drug Release
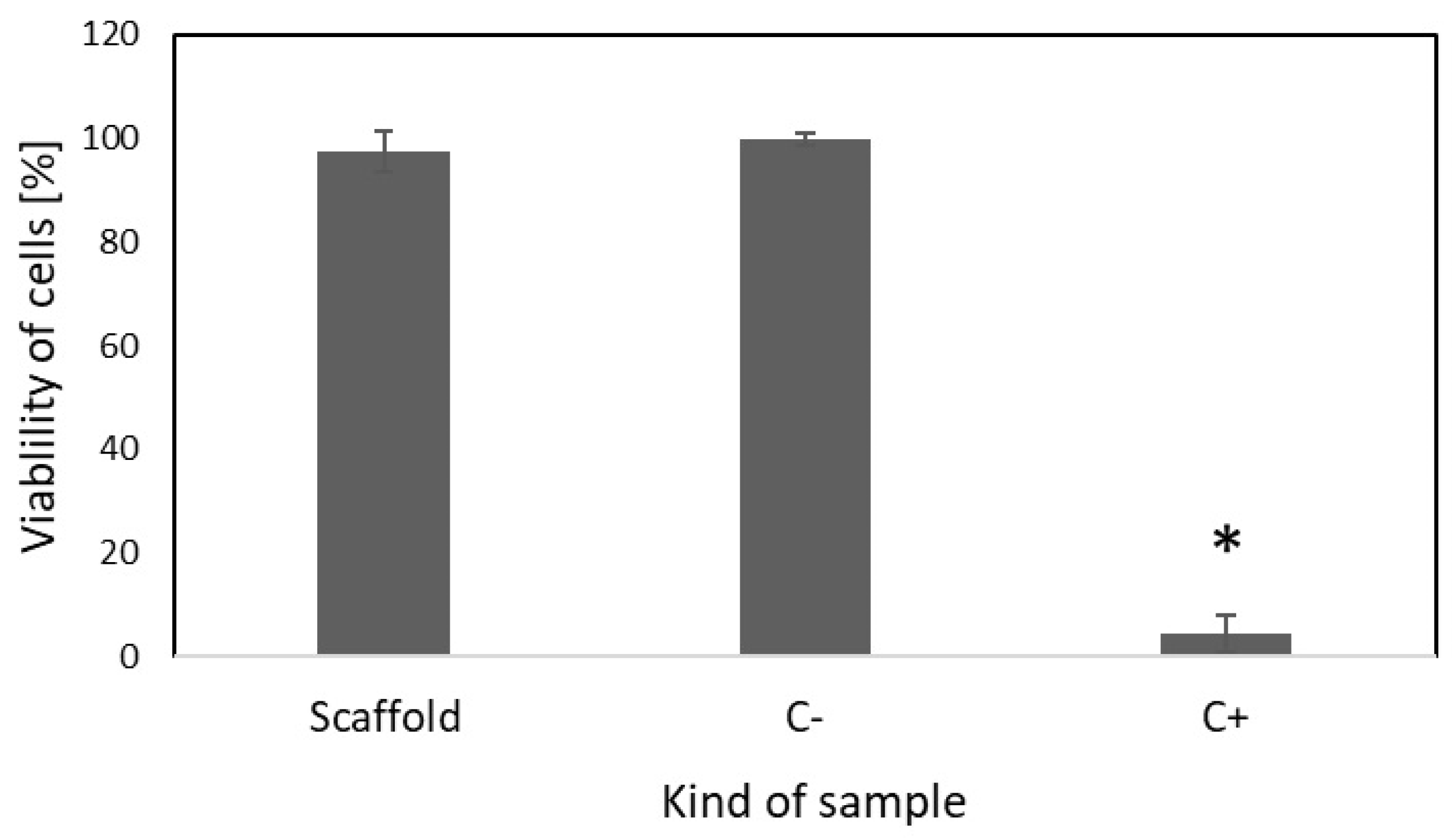
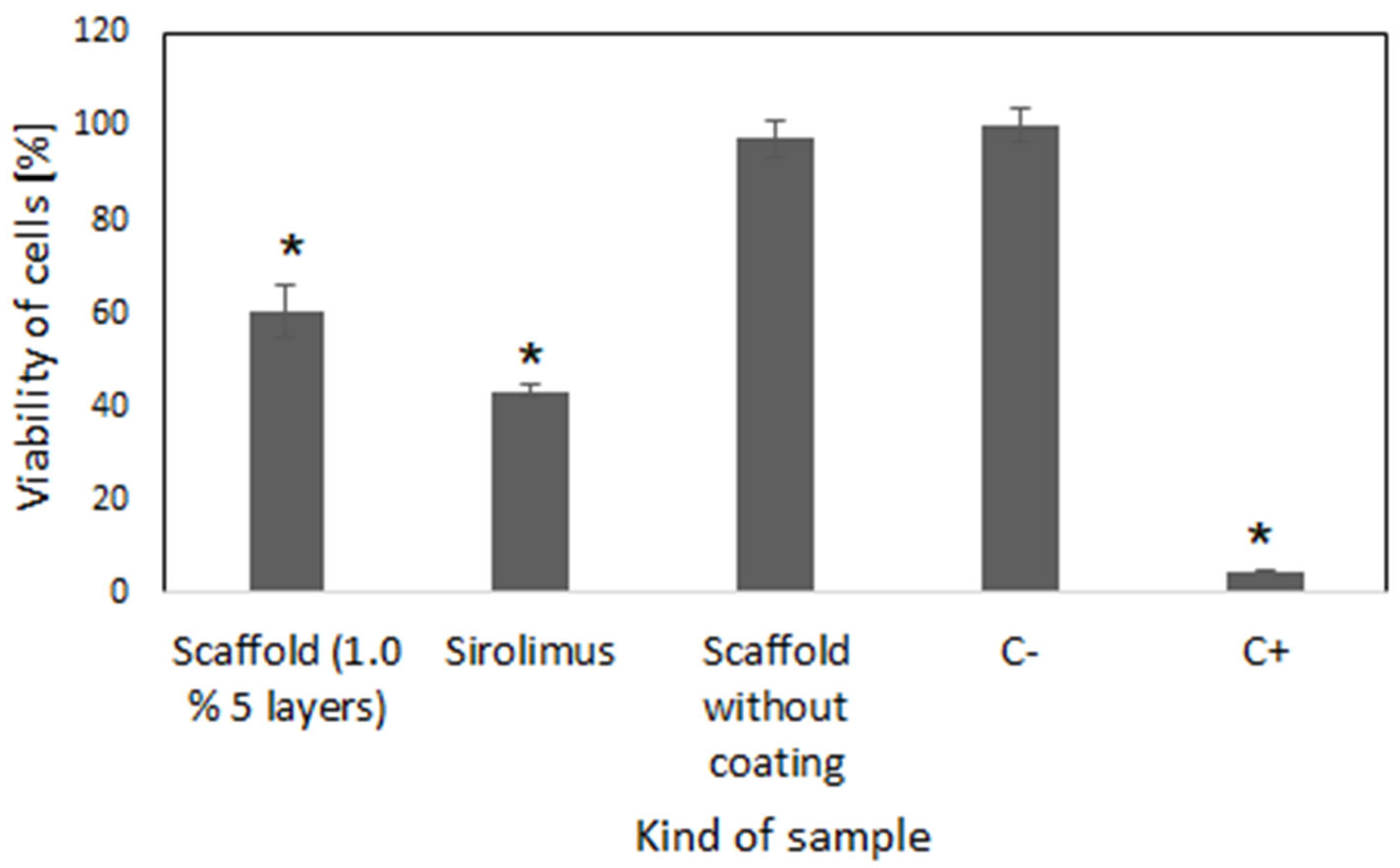
2.4. Cell Culture Study
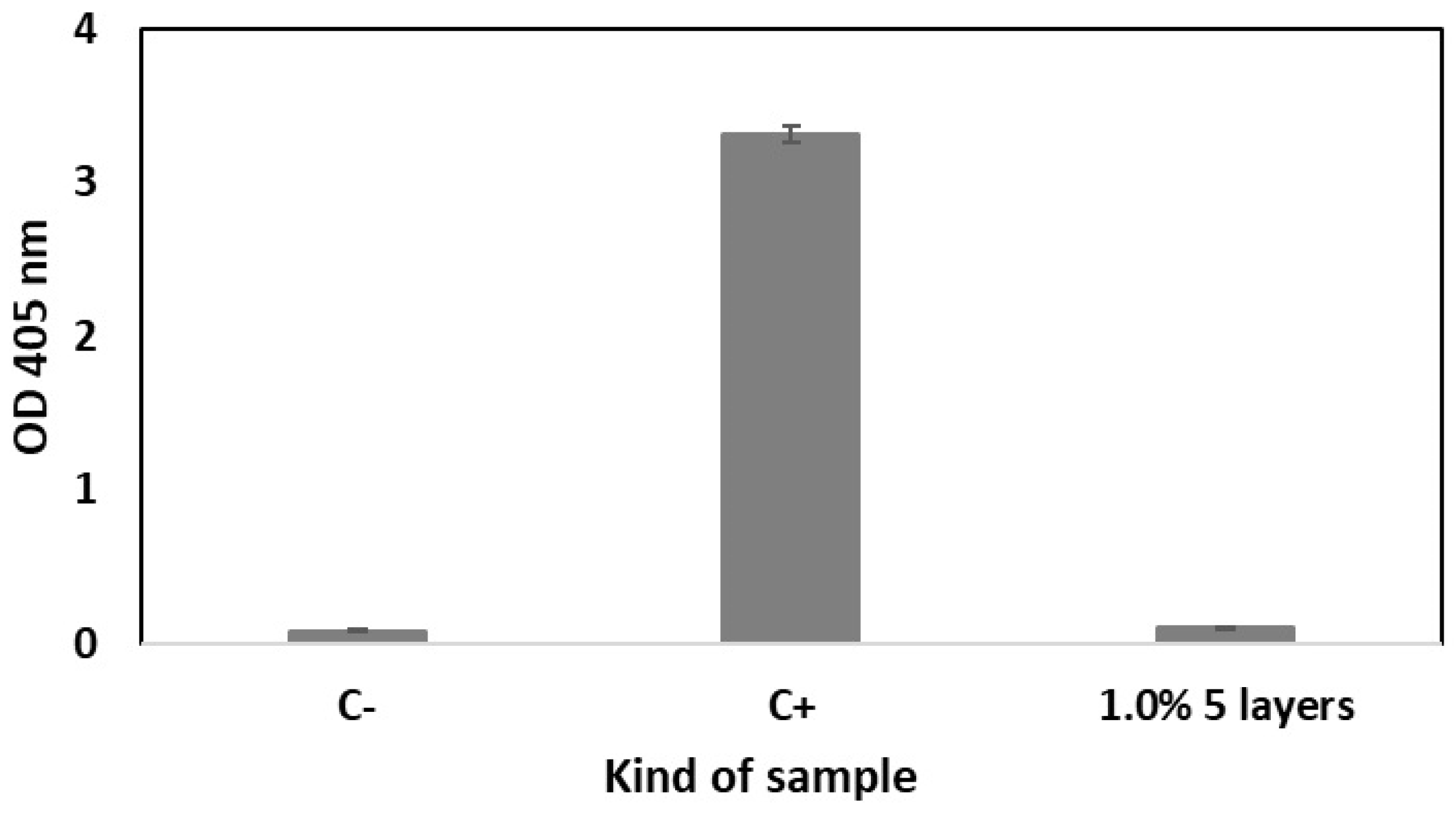
2.5. Hemolytic and Thrombogenic Effect
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Coating Procedure
4.3. Characterization of Scaffold Morphology
4.4. Evaluation of Drug Dose and In Vitro Release
4.5. Cytotoxicity Study
4.6. Compatibility with Blood
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Diffusion of eHealth: Making Universal Health Coverage Achievable: Report of The Third Global Survey on eHealth; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Sane, M.; Dighe, V.; Patil, R.; Hassan, P.A.; Gawali, S.; Patravale, V. Bivalirudin and sirolimus co-eluting coronary stent: Potential strategy for the prevention of stent thrombosis and restenosis. Int. J. Pharm. 2021, 600, 120403. [Google Scholar] [CrossRef] [PubMed]
- Strohbach, A.; Busch, R. Polymers for Cardiovascular Stent Coatings. Int. J. Polym. Sci. 2015, 2015, 782653. [Google Scholar] [CrossRef]
- Ono, M.; Takahashi, K.; Gao, C.; Kawashima, H.; Wu, X.; Hara, H.; Wang, R.; Wykrzykowska, J.J.; Piek, J.J.; Sharif, F.; et al. The state-of-the-art coronary stent with crystallized sirolimus: The MiStent technology and its clinical program. Future Cardiol. 2021, 17, 593–607. [Google Scholar] [CrossRef]
- Mattke, S.; Hanson, M.; Dallmann, A.C.; Bentele, M. Health Economic Evaluation of an Ultrathin, Bioresorbable-Polymer Sirolimus-Eluting Coronary Stent Compared to a Thin, Durable-Polymer Everolimus-Eluting Stent. Cardiovasc. Revascularization Med. 2019, 20, 752–757. [Google Scholar] [CrossRef]
- Ploumen, E.H.; Wolcherink, M.J.O.; Buiten, R.A.; Pinxterhuis, T.H.; Doggen, C.J.M.; Schotborgh, C.E.; Danse, P.W.; Scholte, M.; van Houwelingen, K.G.; Zocca, P.; et al. Cost-Effectiveness of Three Different New-Generation Drug-Eluting Stents in the Randomised BIO-RESORT Trial at 3 Years. PharmacoEconomics—Open 2025, 9, 137–145. [Google Scholar] [CrossRef]
- Mehryab, F.; Rabbani, S.; Shekari, F.; Nazari, A.; Goshtasbi, N.; Haeri, A. Sirolimus-loaded exosomes as a promising vascular delivery system for the prevention of post-angioplasty restenosis. Drug Deliv. Transl. Res. 2024, 14, 158–176. [Google Scholar] [CrossRef]
- Guo, T.; Fang, M.; Zhang, D.; Li, X. Combination treatment with asiaticoside and rapamycin: A new hope for in-stent restenosis. Exp. Ther. Med. 2013, 6, 557–561. [Google Scholar] [CrossRef]
- Kommineni, N.; Saka, R.; Khan, W.; Domb, A.J. Non-polymer drug-eluting coronary stents. Drug Deliv. Transl. Res. 2018, 8, 903–917. [Google Scholar] [CrossRef]
- Marx, S.O.; Totary-Jain, H.; Marks, A.R. Vascular smooth muscle cell proliferation in restenosis. Circulation Cardiovasc. Interv. 2011, 4, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Conway, C. Coronary Stent Fracture: Clinical Evidence Vs. the Testing Paradigm. Cardiovasc. Eng. Technol. 2018, 9, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Carretero, E.; Mayoral-González, I.; Jesús Morón, F.; Fernández-Quero, M.; Domínguez-Rodríguez, A.; Ordóñez, A.; Smani, T. miR-30b-5p Downregulation as a Predictive Biomarker of Coronary In-Stent Restenosis. Biomedicines 2021, 9, 354. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Brott, B.C.; Anderson, P.G.; Hwang, P.T.J.; Sherwood, J.; Huskin, G.; Yoon, Y.-S.; Virmani, R.; Jun, H.-W. Pro-Healing Nanomatrix-Coated Stent Analysis in an In Vitro Vascular Double-Layer System and in a Rabbit Model. ACS Appl. Mater. Interfaces 2022, 14, 51728–51743. [Google Scholar] [CrossRef]
- Park, K. Dual drug-eluting stent. J. Control. Release 2012, 159, 1. [Google Scholar] [CrossRef]
- Calori, I.R.; Braga, G.; de Jesus, P.d.C.C.; Bi, H.; Tedesco, A.C. Polymer scaffolds as drug delivery systems. Eur. Polym. J. 2020, 129, 109621. [Google Scholar] [CrossRef]
- Su, L.-C.; Chen, Y.-H.; Chen, M.-C. Dual Drug-Eluting Stents Coated with Multilayers of Hydrophobic Heparin and Sirolimus. ACS Appl. Mater. Inter. 2013, 5, 12944–12953. [Google Scholar] [CrossRef]
- Kersani, D.; Mougin, J.; Lopez, M.; Degoutin, S.; Tabary, N.; Cazaux, F.; Janus, L.; Maton, M.; Chai, F.; Sobocinski, J.; et al. Stent coating by electrospinning with chitosan/poly-cyclodextrin based nanofibers loaded with simvastatin for restenosis prevention. Eur. J. Pharm. Biopharm. 2020, 150, 156–167. [Google Scholar] [CrossRef]
- Gu, X.; Huang, J.; Ni, Z. Optimization of Coatings for Vascular Stent with Ultrasonic Spray Technology. In Proceedings of the Advances in Control and Communication, Shenzhen, China, 24–25 December 2011; Springer: Berlin/Heidelberg, Germany, 2012; pp. 547–556. [Google Scholar]
- Livingston, M.; Tan, A. Coating Techniques and Release Kinetics of Drug-Eluting Stents. J. Med. Devices 2019, 10, MED-15-1164. [Google Scholar] [CrossRef]
- Yuk, S.H.; Oh, K.S.; Park, J.; Kim, S.-J.; Kim, J.H.; Kwon, I.K. Paclitaxel-loaded poly(lactide-co-glycolide)/poly(ethylene vinyl acetate) composite for stent coating by ultrasonic atomizing spray. Sci. Technol. Adv. Mater. 2012, 13, 025005. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zou, H.; Shi, W.; Huang, J.; Yang, M.; Xiang, X.; Huang, Y.; Li, Y.; Wang, G.; Wang, Y.; et al. Innovative double-layer coated stent for enhanced Sca-1+ stem cell recruitment and vascular repair. Chem. Eng. J. 2024, 499, 156004. [Google Scholar] [CrossRef]
- Varma, M.V.S.; Kaushal, A.M.; Garg, A.; Garg, S. Factors affecting mechanism and kinetics of drug release from matrix-based oral controlled drug delivery systems. Am. J. Drug Deliv. 2004, 2, 43–57. [Google Scholar] [CrossRef]
- Le Devedec, F.; Boucher, H.; Dubins, D.; Allen, C. Factors Controlling Drug Release in Cross-linked Poly(valerolactone) Based Matrices. Mol. Pharm. 2018, 15, 1565–1577. [Google Scholar] [CrossRef]
- Wan, L.S.C.; Lai, W.F. Factors affecting drug release from drug-coated granules prepared by fluidized-bed coating. Int. J. Pharm. 1991, 72, 163–174. [Google Scholar] [CrossRef]
- Maderuelo, C.; Zarzuelo, A.; Lanao, J.M. Critical factors in the release of drugs from sustained release hydrophilic matrices. J. Control Release 2011, 154, 2–19. [Google Scholar] [CrossRef]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—A review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef]
- Jelonek, K.; Jaworska, J.; Pastusiak, M.; Sobota, M.; Włodarczyk, J.; Karpeta-Jarzabek, P.; Kaczmarczyk, B.; Kasperczyk, J.; Dobrzyński, P. Effect of vascular scaffold composition on release of sirolimus. Eur. J. Pharm. Biopharm. 2018, 132, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Pego, A.P.; Poot, A.A.; Grijpma, D.W.; Feijen, J. Physical properties of high molecular weight 1,3-trimethylene carbonate and D,L-lactide copolymers. J. Mater. Sci.-Mater. Med. 2003, 14, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Jaworska, J.; Jelonek, K.; Wąsik, T.J.; Miklasińska-Majdanik, M.; Kępa, M.; Bratosiewicz-Wąsik, J.; Kaczmarczyk, B.; Marcinkowski, A.; Janeczek, H.; Szewczenko, J.; et al. Poly(lactide-co-trimethylene carbonate) coatings with ciprofloxacin, fusidic acid and azithromycin. The effect of the drug on the degradation and biological activity against different Staphylococcus reference strains. Eur. Polym. J. 2021, 155, 110579. [Google Scholar] [CrossRef]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.G.; Idrovo, J.P.; Nicastro, J.; McMullen, H.F.; Molmenti, E.P.; Coppa, G. A retrospective analysis of the incidence of hemolysis in type and screen specimens from trauma patients. Int. J. Angiol. 2009, 18, 182–183. [Google Scholar] [CrossRef][Green Version]
- Gegenschatz-Schmid, K.; Buzzi, S.; Grossmann, J.; Roschitzki, B.; Urbanet, R.; Heuberger, R.; Glück, D.; Zucker, A.; Ehrbar, M. Reduced thrombogenicity of surface-treated Nitinol implants steered by altered protein adsorption. Acta Biomater. 2022, 137, 331–345. [Google Scholar] [CrossRef]
- Milleret, V.; Lienemann, P.S.; Bauer, S.; Ehrbar, M. Quantitative in vitro comparison of the thrombogenicity of commercial dental implants. Clin. Implant. Dent. Relat. Res. 2019, 21, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.F.; Girdhar, G.; Anderson, A.A.; Ubl, S.R.; Thinamany, S.; Jeffers, H.N.; DeRusha, C.E.; Rodriguez-Fernandez, J.; Hoffmann, S.; Strief, C.A. In vitro methodology for medical device material thrombogenicity assessments: A use condition and bioanalytical proof-of-concept approach. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 358–376. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, F.; Byrne, R.A.; Rivero, F.; Kastrati, A. Current Treatment of In-Stent Restenosis. J. Am. Coll. Cardiol. 2014, 63, 2659–2673. [Google Scholar] [CrossRef] [PubMed]
- Tesfamariam, B. Bioresorbable vascular scaffolds: Biodegradation, drug delivery and vascular remodeling. Pharmacol. Res. 2016, 107, 163–171. [Google Scholar] [CrossRef]
- Hu, T.Z.; Yang, J.L.; Cui, K.; Rao, Q.; Yin, T.Y.; Tan, L.L.; Zhang, Y.; Li, Z.G.; Wang, G.X. Controlled Slow-Release Drug-Eluting Stents for the Prevention of Coronary Restenosis: Recent Progress and Future Prospects. ACS Appl. Mater. Inter. 2015, 7, 11695–11712. [Google Scholar] [CrossRef]
- Hou, L.-D.; Li, Z.; Pan, Y.; Sabir, M.; Zheng, Y.-F.; Li, L. A review on biodegradable materials for cardiovascular stent application. Front. Mater. Sci. 2016, 10, 238–259. [Google Scholar] [CrossRef]
- Forrestal, B.J.; Case, B.C.; Yerasi, C.; Garcia-Garcia, H.M.; Waksman, R. The Orsiro Ultrathin, Bioresorbable-Polymer Sirolimus-Eluting Stent: A Review of Current Evidence. Cardiovasc. Revascularization Med. Incl. Mol. Interv. 2020, 21, 540–548. [Google Scholar] [CrossRef]
- Wang, J.; Song, C.; Xiao, Y.; Liu, B. In vivo and in vitro analyses of the effects of a novel high-nitrogen low-nickel coronary stent on reducing in-stent restenosis. J. Biomater. Appl. 2018, 33, 64–71. [Google Scholar] [CrossRef]
- Hertault, A.; Chai, F.; Maton, M.; Sobocinski, J.; Woisel, P.; Maurel, B.; Lyskawa, J.; Blanchemain, N. In vivo evaluation of a pro-healing polydopamine coated stent through an in-stent restenosis rat model. Biomater. Sci. 2021, 9, 212–220. [Google Scholar] [CrossRef]
- Dobrzynski, P. Synthesis of biodegradable copolymers with low-toxicity zirconium compounds. III. Synthesis and chain-microstructure analysis of terpolymer obtained from L-lactide, glycolide, and ϵ-caprolactone initiated by zirconium(IV) acetylacetonate. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 3129–3143. [Google Scholar] [CrossRef]
- Dobrzynski, P.; Kasperczyk, J. Synthesis of biodegradable copolymers with low-toxicity zirconium compounds. V. Multiblock and random copolymers of L-lactide with trimethylene carbonate obtained in copolymerizations initiated with zirconium(IV) acetylacetonate. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 3184–3201. [Google Scholar] [CrossRef]
- Jelonek, K.; Karpeta, P.; Jaworska, J.; Pastusiak, M.; Włodarczyk, J.; Kasperczyk, J.; Dobrzyński, P. Comparison of extraction methods of sirolimus from polymeric coatings of bioresorbable vascular scaffolds. Mater. Lett. 2018, 214, 220–223. [Google Scholar] [CrossRef]
- Jelonek, K.; Zajdel, A.; Wilczok, A.; Kaczmarczyk, B.; Musiał-Kulik, M.; Hercog, A.; Foryś, A.; Pastusiak, M.; Kasperczyk, J. Comparison of PLA-Based Micelles and Microspheres as Carriers of Epothilone B and Rapamycin. The Effect of Delivery System and Polymer Composition on Drug Release and Cytotoxicity against MDA-MB-231 Breast Cancer Cells. Pharmaceutics 2021, 13, 1881. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An Add-In Program for Modeling and Comparison of Drug Dissolution Profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2001, 48, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Sæbø, I.P.; Bjørås, M.; Franzyk, H.; Helgesen, E.; Booth, J.A. Optimization of the Hemolysis Assay for the Assessment of Cytotoxicity. Int. J. Mol. Sci. 2023, 24, 2914. [Google Scholar] [CrossRef]
- Sarode, D.N.; Roy, S. In Vitro models for thrombogenicity testing of blood-recirculating medical devices. Expert Rev. Med. Devices 2019, 16, 603–616. [Google Scholar] [CrossRef] [PubMed]


| Concentration of Coating Solution | Sirolimus Content in Coating of Scaffold (µg) | ||
|---|---|---|---|
| 3 Layers | 5 Layers | 7 Layers | |
| 1.0% | 51.0 ± 0.7 | 100.3 ± 10.3 | 143.1 ± 5.6 |
| 2.5% | 193.7 ± 15.8 | 300.1 ± 0.7 | 415.3 ± 17.9 |
| Model | Kind of Sample | |||||
|---|---|---|---|---|---|---|
| 1.0% 3 Layers | 1.0% 5 Layers | 1.0% 7 Layers | 2.5% 3 Layers | 2.5% 5 Layers | 2.5% 7 Layers | |
| Higuchi R2 | 0.9158 | 0.9499 | 0.9936 | 0.8481 | 0.9781 | 0.9315 |
| Korsmeyer–Peppas R2 n | 0.9897 0.326 | 0.9963 0.357 | 0.9938 0.490 | 0.9851 0.278 | 0.9876 0.590 | 0.9575 0.676 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jelonek, K.; Jaworska, J.; Musiał-Kulik, M.; Stojko, M.; Włodarczyk, J.; Sobota, M.; Pastusiak, M.; Smola-Dmochowska, A.; Szewczenko, J.; Goldsztajn, K.; et al. Evaluation of Ultrasonic Spray Method for Application of Sirolimus-Eluting Coating on Bioresorbable Vascular Scaffolds. Int. J. Mol. Sci. 2025, 26, 7649. https://doi.org/10.3390/ijms26157649
Jelonek K, Jaworska J, Musiał-Kulik M, Stojko M, Włodarczyk J, Sobota M, Pastusiak M, Smola-Dmochowska A, Szewczenko J, Goldsztajn K, et al. Evaluation of Ultrasonic Spray Method for Application of Sirolimus-Eluting Coating on Bioresorbable Vascular Scaffolds. International Journal of Molecular Sciences. 2025; 26(15):7649. https://doi.org/10.3390/ijms26157649
Chicago/Turabian StyleJelonek, Katarzyna, Joanna Jaworska, Monika Musiał-Kulik, Mateusz Stojko, Jakub Włodarczyk, Michał Sobota, Małgorzata Pastusiak, Anna Smola-Dmochowska, Janusz Szewczenko, Karolina Goldsztajn, and et al. 2025. "Evaluation of Ultrasonic Spray Method for Application of Sirolimus-Eluting Coating on Bioresorbable Vascular Scaffolds" International Journal of Molecular Sciences 26, no. 15: 7649. https://doi.org/10.3390/ijms26157649
APA StyleJelonek, K., Jaworska, J., Musiał-Kulik, M., Stojko, M., Włodarczyk, J., Sobota, M., Pastusiak, M., Smola-Dmochowska, A., Szewczenko, J., Goldsztajn, K., Dobrzyński, P., & Kasperczyk, J. (2025). Evaluation of Ultrasonic Spray Method for Application of Sirolimus-Eluting Coating on Bioresorbable Vascular Scaffolds. International Journal of Molecular Sciences, 26(15), 7649. https://doi.org/10.3390/ijms26157649










