Cysteine Surface Engineering of Green-Synthesized Gold Nanoparticles for Enhanced Antimicrobial and Antifungal Activity
Abstract
1. Introduction
2. Results
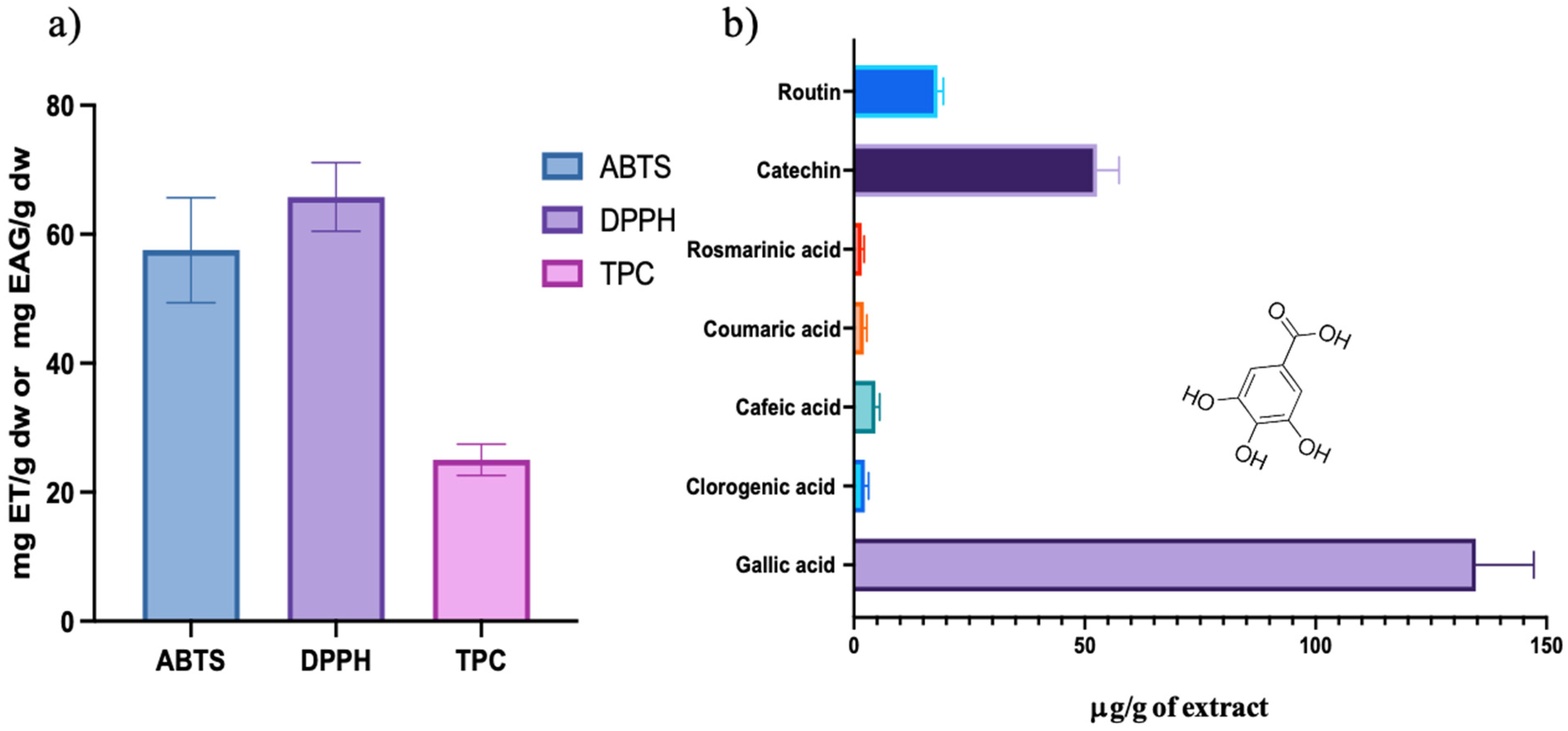
2.1. Antioxidant Activity and Total Phenolic Content of Schinus Molle Extract
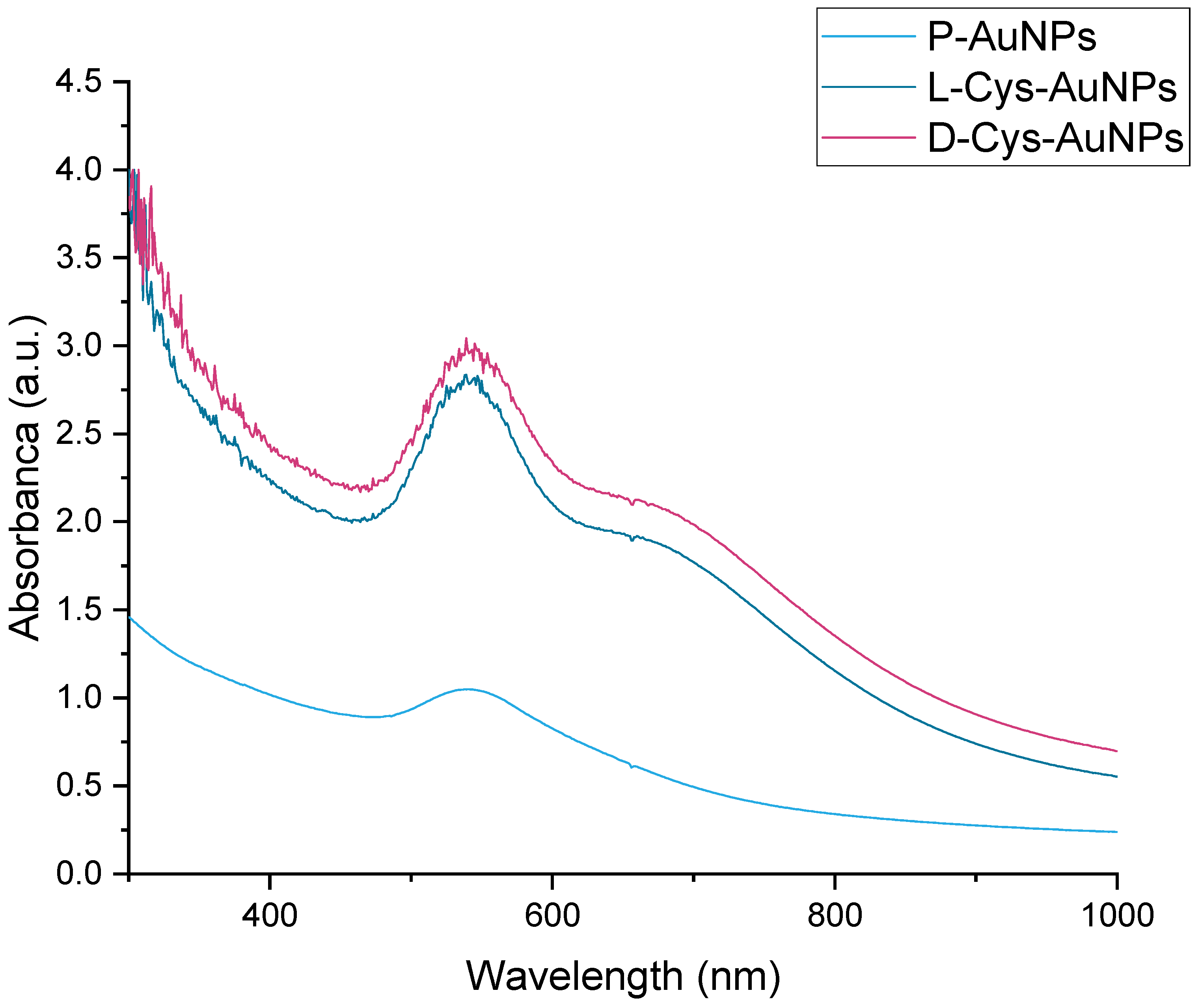
2.2. UV-Vis Spectra
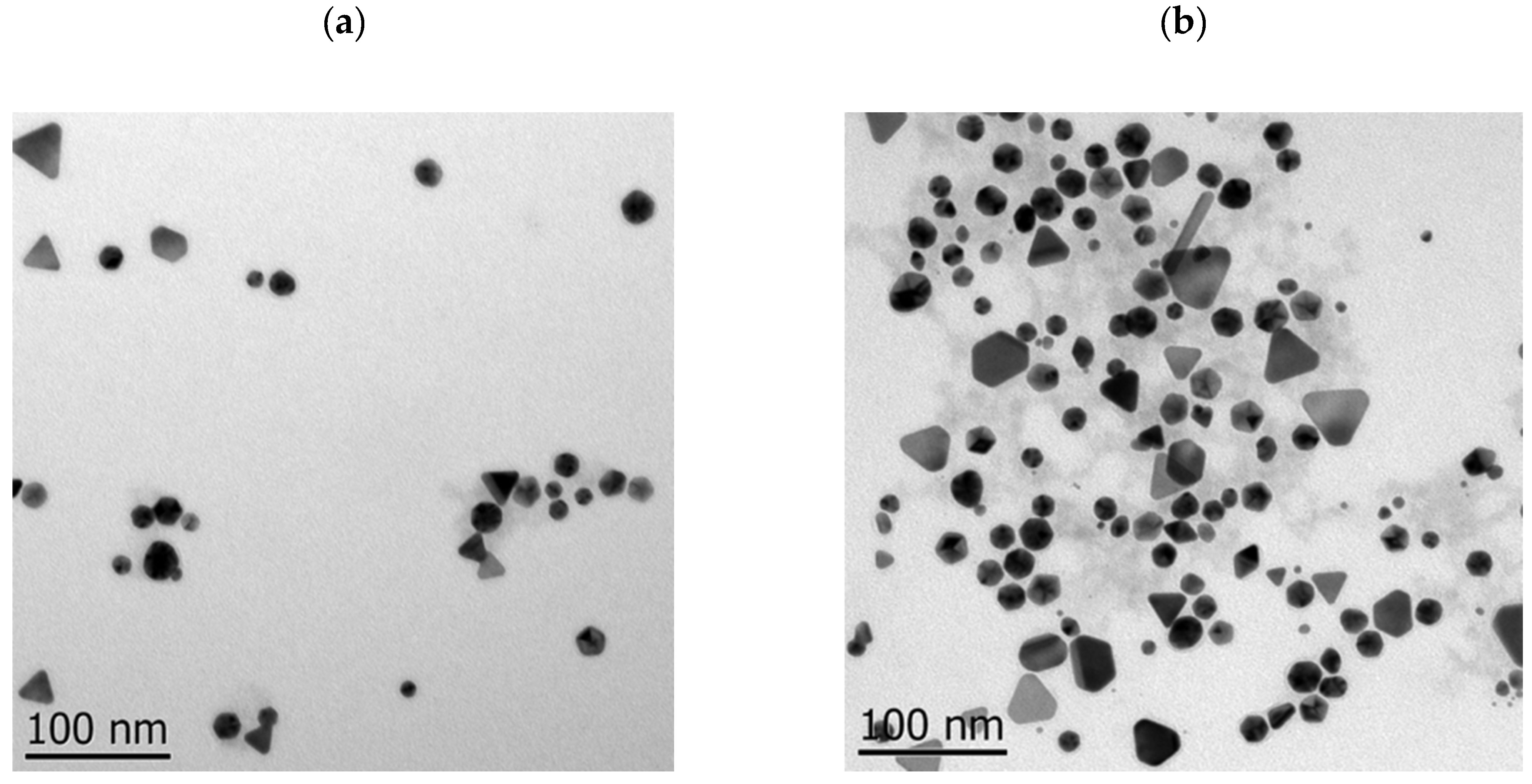
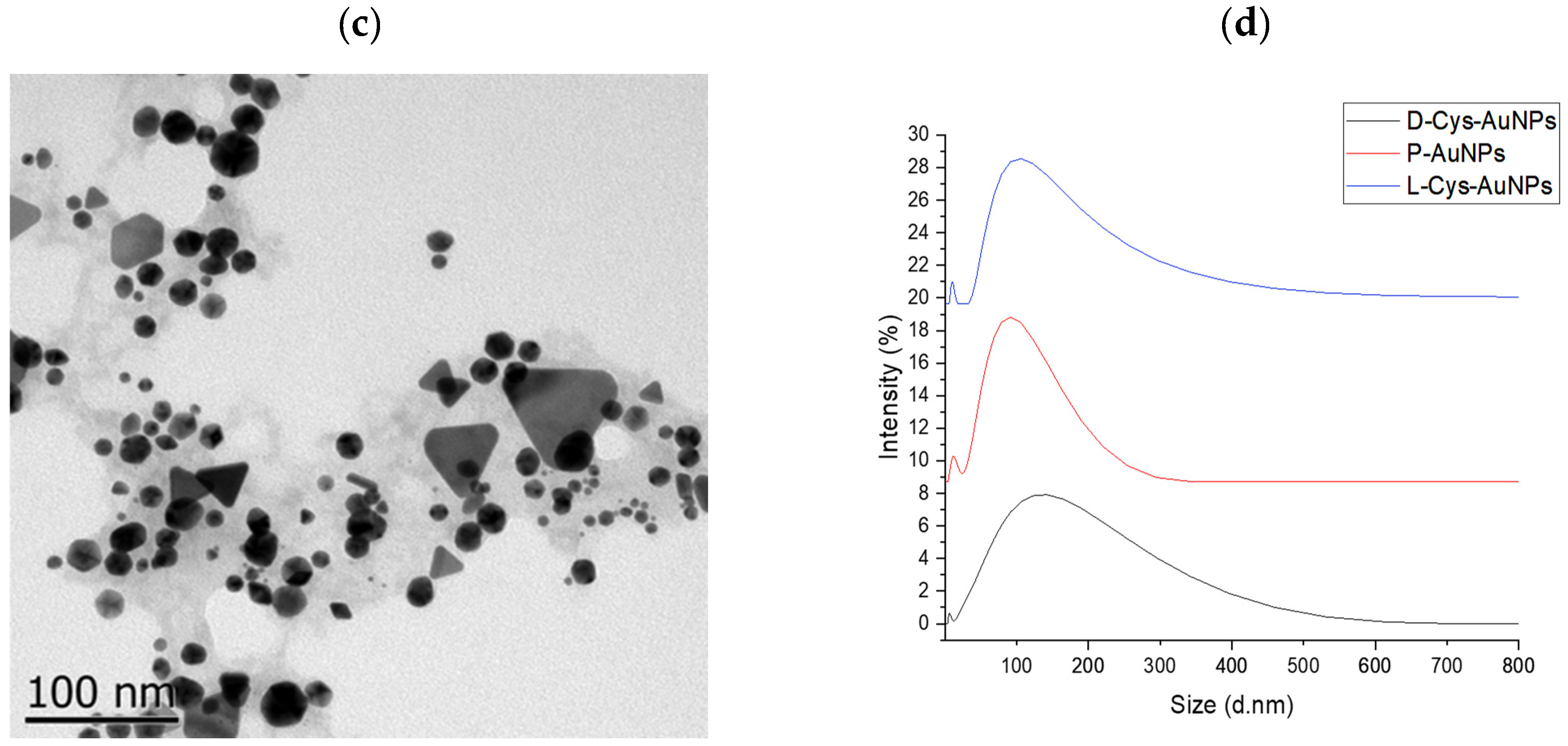
2.3. TEM and DLS Analysis
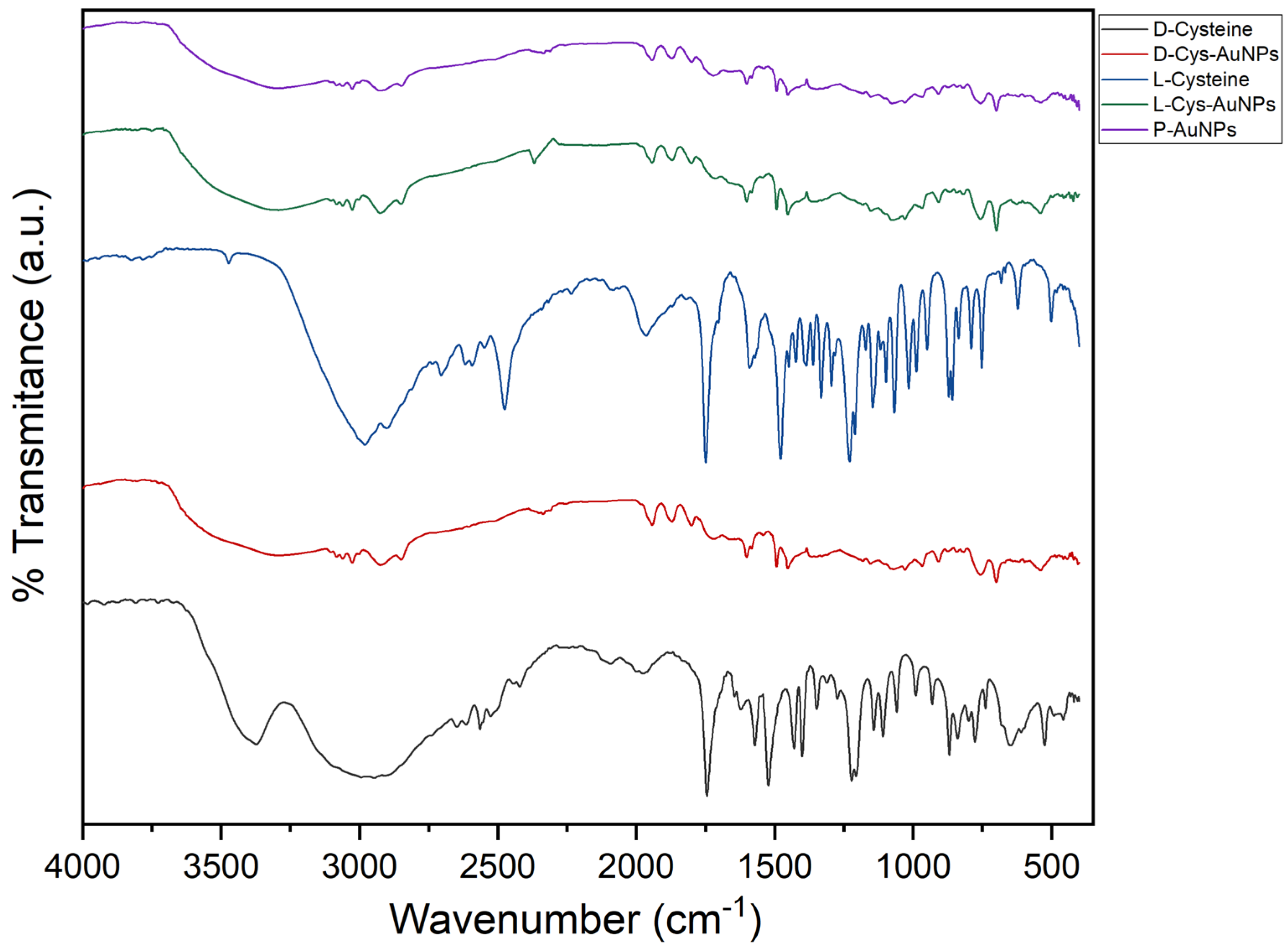
2.4. Fourier Transform Infrared Spectroscopy (FTIR)
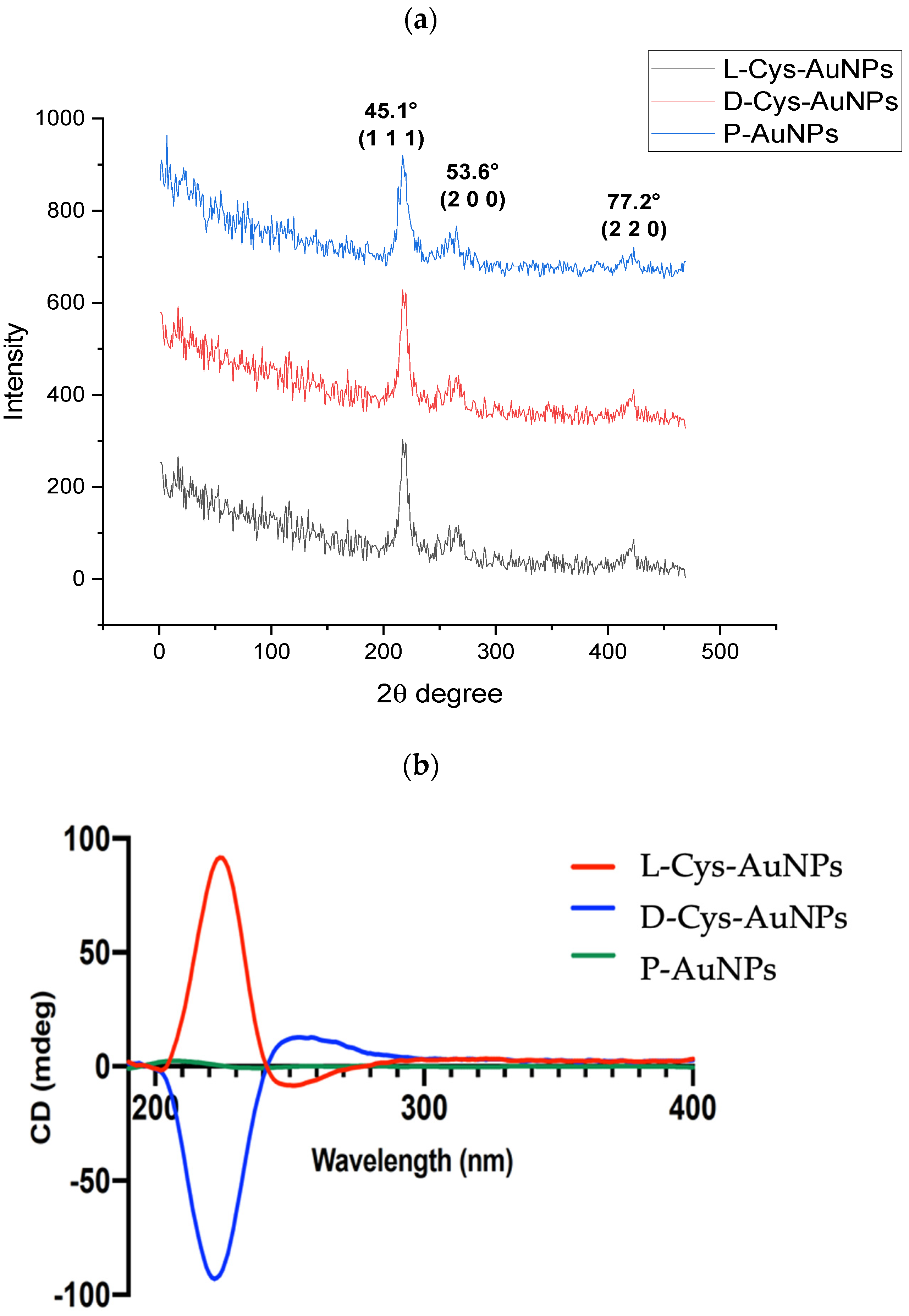
2.5. X-Ray Diffraction Analysis (XDR)
2.6. Circular Dichroism (CD) Analysis
2.7. Antimicrobial Activity
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Extraction and Characterization
3.3. Green Synthesis of Gold Nanoparticles with Pirul SEED Extract
3.4. Synthesis of L/D-Cys-AuNPs
3.5. AuNPs Characterization
3.6. Antimicrobial Activity
3.6.1. Pathogens Strains
3.6.2. Antimicrobial Susceptibility Testing
3.6.3. Well-Diffusion Antimicrobial Analysis
3.6.4. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of AuNPs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soto, K.M.; Hernández-Iturriaga, M.; Cárdenas, A.; Mendoza, S. Biosynthesis of Silver Nanoparticles Mediated by Lippia Graveolens Aqueous Extract. Regul. Issue J. Mex. Chem. Soc 2024, 68, 402–411. [Google Scholar] [CrossRef]
- Soto, K.M.; Luzardo-Ocampo, I.; López-Romero, J.M.; Mendoza, S.; Loarca-Piña, G.; Rivera-Muñoz, E.M.; Manzano-Ramírez, A. Gold Nanoparticles Synthesized with Common Mullein (Verbascum thapsus) and Castor Bean (Ricinus communis) Ethanolic Extracts Displayed Antiproliferative Effects and Induced Caspase 3 Activity in Human HT29 and SW480 Cancer Cells. Pharmaceutics 2022, 14, 2069. [Google Scholar] [CrossRef] [PubMed]
- Bvenura, C.; Kambizi, L. Composition of Phenolic Compounds in South African Schinus molle L. Berries. Foods 2022, 11, 1376. [Google Scholar] [CrossRef] [PubMed]
- Tlili, N.; Yahia, Y.; Feriani, A.; Labidi, A.; Ghazouani, L.; Nasri, N.; Saadaoui, E.; Khaldi, A. Schinus terebinthifolius vs Schinus molle: A Comparative Study of the Effect of Species and Location on the Phytochemical Content of Fruits. Ind. Crops Prod. 2018, 122, 559–565. [Google Scholar] [CrossRef]
- Martins, M.D.R.; Arantes, S.; Candeias, F.; Tinoco, M.T.; Cruz-Morais, J. Antioxidant, Antimicrobial and Toxicological Properties of Schinus molle L. Essential Oils. J. Ethnopharmacol. 2014, 151, 485–492. [Google Scholar] [CrossRef]
- Mares-Briones, F.; Rosas, G. Structure and Stability of Gold Nanoparticles Synthesized Using Schinus molle L. Extract. J. Clust. Sci. 2017, 28, 1995–2003. [Google Scholar] [CrossRef]
- Erenler, R.; Chaoui, R.; Yildiz, I.; Genc, N.; Gecer, E.N.; Temiz, C.; Akkal, S. Biosynthesis, Characterisation, and Antioxidant Activity of Silver Nanoparticles Using Schinus molle L. Trends Sci. 2023, 20, 6105. [Google Scholar] [CrossRef]
- Zhou, C.; Hao, G.; Thomas, P.; Liu, J.; Yu, M.; Sun, S.; Öz, O.K.; Sun, X.; Zheng, J. Near-Infrared Emitting Radioactive Gold Nanoparticles with Molecular Pharmacokinetics. Angew. Chem. Int. Ed. 2012, 51, 10118–10122. [Google Scholar] [CrossRef]
- Ma, W.; Xu, L.; De Moura, A.F.; Wu, X.; Kuang, H.; Xu, C.; Kotov, N.A. Chiral Inorganic Nanostructures. Chem. Rev. 2017, 117, 8041–8093. [Google Scholar] [CrossRef]
- Cho, N.H.; Kim, H.; Kim, J.W.; Lim, Y.C.; Kim, R.M.; Lee, Y.H.; Nam, K.T. Chiral Inorganic Nanomaterials for Biomedical Applications. Chem 2024, 10, 1052–1070. [Google Scholar] [CrossRef]
- Wang, G.; Qu, A.; Sun, M.; Xu, J.; Kuang, H. Chemical Mechanisms and Biological Effects of Chiral Nanomaterials. Acc. Mater. Res. 2024, 5, 1221–1236. [Google Scholar] [CrossRef]
- Dolamic, I.; Knoppe, S.; Dass, A.; Bürgi, T. First Enantioseparation and Circular Dichroism Spectra of Au38 Clusters Protected by Achiral Ligands. Nat. Commun. 2012, 3, 798. [Google Scholar] [CrossRef] [PubMed]
- Parvekar, P.; Palaskar, J.; Metgud, S.; Maria, R.; Dutta, S. The Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of Silver Nanoparticles against Staphylococcus aureus. Biomater. Investig. Dent. 2020, 7, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Pal, I.; Bhattacharyya, D.; Kar, R.K.; Zarena, D.; Bhunia, A.; Atreya, H.S. A Peptide-Nanoparticle System with Improved Efficacy against Multidrug Resistant Bacteria. Sci. Rep. 2019, 9, 4485. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wei, P.H.; Zhu, X.; Wirth, M.J.; Bhunia, A.; Narsimhan, G. Effect of Immobilization on the Antimicrobial Activity of a Cysteine-Terminated Antimicrobial Peptide Cecropin P1 Tethered to Silica Nanoparticle against E. coli O157:H7 EDL933. Colloids Surf. B Biointerfaces 2017, 156, 305–312. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, H.; Xing, C.; Zhang, J.; Yan, W. Antibacterial Mechanism of D-Cysteine/Polyethylene Glycol-Functionalized Gold Nanoparticles and Their Potential for the Treatment of Bacterial Infections. ACS Appl. Mater. Interfaces 2024, 16, 37722–37733. [Google Scholar] [CrossRef]
- Shehata, M.E.; El-Sherbiny, G.M.; Sharaf, M.H.; Kalaba, M.H.; Shaban, A.S. Phytochemical Analysis, Antimicrobial, Antioxidant, and Cytotoxicity Activities of Schinus molle L. Extracts. Biomass Convers. Biorefinery 2024, 15, 3753–3770. [Google Scholar] [CrossRef]
- Rebolledo, V.; Otero, M.C.; Delgado, J.M.; Torres, F.; Herrera, M.; Ríos, M.; Cabañas, M.; Martinez, J.L.; Rodríguez-Díaz, M. Phytochemical Profile and Antioxidant Activity of Extracts of the Peruvian Peppertree Schinus areira L. from Chile. Saudi J. Biol. Sci. 2021, 28, 1052–1062. [Google Scholar] [CrossRef]
- Mariani, E.; Enemark, G.K.G.; Juncos, N.S.; Cravero, C.F.; Olmedo, R.H. The Pinene-to-Five Principal Components Ratio in Schinus molle L. Essential Oil as an Antioxidant Activity Indicator. Food Chem. 2025, 486, 144653. [Google Scholar] [CrossRef]
- Azhar, B.; Angkawijaya, A.E.; Santoso, S.P.; Gunarto, C.; Ayucitra, A.; Go, A.W.; Tran-Nguyen, P.L.; Ismadji, S.; Ju, Y.H. Aqueous Synthesis of Highly Adsorptive Copper–Gallic Acid Metal–Organic Framework. Sci. Rep. 2020, 10, 19212. [Google Scholar] [CrossRef]
- Shen, F.; Lin, Y.; Höhn, M.; Luo, X.; Döblinger, M.; Wagner, E.; Lächelt, U. Iron-Gallic Acid Peptide Nanoparticles as a Versatile Platform for Cellular Delivery with Synergistic ROS Enhancement Effect. Pharmaceutics 2023, 15, 1789. [Google Scholar] [CrossRef]
- Lunkov, A.; Shagdarova, B.; Konovalova, M.; Zhuikova, Y.; Drozd, N.; Il’ina, A.; Varlamov, V. Synthesis of Silver Nanoparticles Using Gallic Acid-Conjugated Chitosan Derivatives. Carbohydr. Polym. 2020, 234, 115916. [Google Scholar] [CrossRef]
- Farzaneh, N.; Arab Chamjangali, M.; Goudarzi, N.; Rezakazemi, M. Green Synthesis of Gallic Acid–Capped Gold Nanoparticles as Novel Electro-Catalyst for Electro-Oxidation of Ethylene Glycol in Alkaline Media. Int. J. Hydrogen Energy 2024, 51, 245–258. [Google Scholar] [CrossRef]
- Soto, K.M.; López-Romero, J.M.; Mendoza, S.; Peza-Ledesma, C.; Rivera-Muñoz, E.M.; Velazquez-Castillo, R.R.; Pineda-Piñón, J.; Méndez-Lozano, N.; Manzano-Ramírez, A. Rapid and Facile Synthesis of Gold Nanoparticles with Two Mexican Medicinal Plants and a Comparison with Traditional Chemical Synthesis. Mater. Chem. Phys. 2023, 295, 127109. [Google Scholar] [CrossRef]
- Acres, R.G.; Feyer, V.; Tsud, N.; Carlino, E.; Prince, K.C. Mechanisms of Aggregation of Cysteine Functionalized Gold Nanoparticles. J. Phys. Chem. C 2014, 118, 10481–10487. [Google Scholar] [CrossRef]
- Falamas, A.; Tosa, N.; Tosa, V. Dynamics of Laser Excited Colloidal Gold Nanoparticles Functionalized with Cysteine Derivatives. J. Quant. Spectrosc. Radiat. Transf. 2014, 162, 207–212. [Google Scholar] [CrossRef]
- Carone, A.; Emilsson, S.; Mariani, P.; Désert, A.; Parola, S. Gold Nanoparticle Shape Dependence of Colloidal Stability Domains. Nanoscale Adv. 2023, 5, 2017–2026. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, H.; Kong, N.; Wang, Z.; Fu, H.; Zhang, Y.; Xiao, Y.; Yang, W.; Yan, F. L-Cysteine-Modified Chiral Gold Nanoparticles Promote Periodontal Tissue Regeneration. Bioact. Mater. 2021, 6, 3288–3299. [Google Scholar] [CrossRef]
- Morales-Vidal, J.; López, N.; Ortuño, M.A. Chirality Transfer in Gold Nanoparticles by l-Cysteine Amino Acid: A First-Principles Study. J. Phys. Chem. C 2019, 123, 13758–13764. [Google Scholar] [CrossRef]
- Devi, S.; Singh, B.; Paul, A.K.; Tyagi, S. Highly Sensitive and Selective Detection of Trinitrotoluene Using Cysteine-Capped Gold Nanoparticles. Anal. Methods 2016, 8, 4398–4405. [Google Scholar] [CrossRef]
- Aabdin, Z.; Lu, J.; Zhu, X.; Anand, U.; Loh, N.D.; Su, H.; Mirsaidov, U. Bonding Pathways of Gold Nanocrystals in Solution. Nano Lett. 2014, 14, 6639–6643. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L.; Mohamed, M.B.; Link, S.; El-Sayed, M.A. Crystallographic facets and shapes of gold nanorods of different aspect ratios. Surf. Sci. 1999, 440, L809–L814. [Google Scholar] [CrossRef]
- Chai, F.; Wang, C.; Wang, T.; Ma, Z.; Su, Z. L-Cysteine Functionalized Gold Nanoparticles for the Colorimetric Detection of Hg2+ Induced by Ultraviolet Light. Nanotechnology 2010, 21, 025501. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.E.; Kim, R.M.; Ahn, H.Y.; Lee, Y.Y.; Byun, G.H.; Im, S.W.; Mun, J.; Rho, J.; Nam, K.T. Cysteine-Encoded Chirality Evolution in Plasmonic Rhombic Dodecahedral Gold Nanoparticles. Nat. Commun. 2020, 11, 263. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yue, X.; Ding, Q.; Lin, H.; Xu, C.; Li, S. Chiral Inorganic Nanomaterials for Biological Applications. Nanoscale 2023, 15, 2541–2552. [Google Scholar] [CrossRef]
- Govorov, A.O.; Fan, Z. Theory of Chiral Plasmonic Nanostructures Comprising Metal Nanocrystals and Chiral Molecular Media. ChemPhysChem 2012, 13, 2551–2560. [Google Scholar] [CrossRef]
- Xiao, L.; An, T.; Wang, L.; Xu, X.; Sun, H. Novel Properties and Applications of Chiral Inorganic Nanostructures. Nano Today 2020, 30, 100824. [Google Scholar] [CrossRef]
- Shukla, N.; Bartel, M.A.; Gellman, A.J. Enantioselective Separation on Chiral Au Nanoparticles. J. Am. Chem. Soc. 2010, 132, 8575–8580. [Google Scholar] [CrossRef]
- Bainova, P.; Joly, J.P.; Urbanova, M.; Votkina, D.; Erzina, M.; Vokata, B.; Trelin, A.; Fitl, P.; Audran, G.; Vanthuyne, N.; et al. Plasmon-Assisted Chemistry Using Chiral Gold Helicoids: Toward Asymmetric Organic Catalysis. ACS Catal. 2023, 13, 12859–12867. [Google Scholar] [CrossRef]
- Stoian, I.-A.; Iacob, B.C.; Prates Ramalho, J.P.; Marian, I.O.; Chiș, V.; Bodoki, E.; Oprean, R. A Chiral Electrochemical System Based on L-Cysteine Modified Gold Nanoparticles for Propranolol Enantiodiscrimination: Electroanalysis and Computational Modelling. Electrochim. Acta 2019, 326, 134961. [Google Scholar] [CrossRef]
- Vaseashta, A.; Achour, M.E.; Mabrouki, M.; Tachafine, A.; Aitali, M. (Eds.) Proceedings of the Eighth International Symposium on Dielectric Materials and Applications (ISyDMA’8); Springer Nature: Cham, Switzerland, 2025. [Google Scholar]
- Ernst, E.J.; Klepser, M.E.; Pfaller, M.A. In Vitro Interaction of Fluconazole and Amphotericin B Administered Sequentially Against Candida albicans: Effect of Concentration and Exposure Time. Diagn. Microbiol. Infect. Dis. 1998, 32, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Arakha, M.; Saleem, M.; Mallick, B.C.; Jha, S. The Effects of Interfacial Potential on Antimicrobial Propensity of ZnO Nanoparticle. Sci. Rep. 2015, 5, 9578. [Google Scholar] [CrossRef] [PubMed]
- López-Lorente, Á.I.; Cárdenas, S.; González-Sánchez, Z.I. Effect of Synthesis, Purification and Growth Determination Methods on the Antibacterial and Antifungal Activity of Gold Nanoparticles. Mater. Sci. Eng. C 2019, 103, 109805. [Google Scholar] [CrossRef] [PubMed]
- González-Silva, N.; Nolasco-González, Y.; Aguilar-Hernández, G.; Sáyago-Ayerdi, S.G.; Villagrán, Z.; Acosta, J.L.; Montalvo-González, E.; Anaya-Esparza, L.M. Ultrasound-Assisted Extraction of Phenolic Compounds from Psidium cattleianum Leaves: Optimization Using the Response Surface Methodology. Molecules 2022, 27, 3557. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Original Contribution Antioxidant Activity Applying an Improved Abts Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Russier-Antoine, I.; Bertorelle, F.; Kulesza, A.; Soleilhac, A.; Bensalah-Ledoux, A.; Guy, S.; Dugourd, P.; Brevet, P.F.; Antoine, R. Chiral Supramolecular Gold-Cysteine Nanoparticles: Chiroptical and Nonlinear Optical Properties. Prog. Nat. Sci. Mater. Int. 2016, 26, 455–460. [Google Scholar] [CrossRef]
- Patel, J.B.; Cockerill, F.R.; Bradford, P.A. Performance Standards for Antimicrobial Susceptibility, Twenty-Fifth Informational Supplement, 27th ed.; Clinical and Laboratory Standards Institute, Ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Wikler, M.A.; Clinical and Laboratory Standards Institute. CLSI Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2009; ISBN 1562386891. [Google Scholar]
| Antimicrobial Suspension | Microorganisms | ||||||
|---|---|---|---|---|---|---|---|
| E. coli | S. enterica | L. monocytogenes | S. aureus | S. epidermidis | C. albicans | ||
| Schinus molle extract | 10 mg/mL | 11.7 ± 0.6 | 14.3 ± 0.6 | 17.0 ± 1.7 | 17.7 ± 1.5 | 17.7 ± 0.6 | PI (18 ± 0.0) |
| 5 mg/mL | NI | NI | 13.3 ± 1.5 | 13.7 ± 1.5 | 14.0 ± 1.0 | PI (10.7 ± 0.6) | |
| 2.5 mg/mL | NI | NI | 12.3 ± 0.6 | 13.0 ± 1.0 | 12.0 ± 0.0 | NI | |
| 1.25 mg/mL | NI | NI | NI | 9.7 ± 0.6 | NI | NI | |
| 0.1 mg/mL | NI | NI | NI | 9.0 ± 0.0 | NI | NI | |
| P-AuNPs | 10 mg/mL | 21 ± 1.0 | 22.7 ± 1.5 | 19.7 ± 0.6 | 25.7 ± 1.5 | 26.7 ± 0.6 | NI |
| 5 mg/mL | 12.3 ± 1.5 | 16.7 ± 1.2 | NI | 13.0 ± 1.0 | 9.3 ± 0.6 | NI | |
| 2.5 mg/mL | NI | NI | NI | 10 ± 0.0 | NI | NI | |
| 1.25 mg/mL | NI | NI | NI | NI | NI | NI | |
| 0.1 mg/mL | NI | NI | NI | NI | NI | NI | |
| D-Cys-AuNPs | 10 mg/mL | 15.7 ± 1.2 | 17.0 ± 1.7 | 14.7 ± 1.5 | 21.3 ± 1.7 | 21.0 ± 1.7 | NI |
| 5 mg/mL | 9.7 ± 0.6 | 10.7 ± 1.2 | NI | 15.7 ± 1.2 | 9.3 ± 0.6 | NI | |
| 2.5 mg/mL | NI | NI | NI | 12.7 ± 0.6 | NI | NI | |
| 1.25 mg/mL | NI | NI | NI | NI | NI | NI | |
| 0.1 mg/mL | NI | NI | NI | NI | NI | NI | |
| L-Cys-AuNPs | 10 mg/mL | 24.0 ± 1.0 | 25.3 ± 0.6 | 26.3 ± 0.6 | 28.0 ± 1.0 | 32.0 ± 1.0 | PI (23.0 ± 0.0) |
| 5 mg/mL | 18.7 ± 1.5 | 22.0 ± 1.0 | 16.3 ± 0.6 | 20.3 ± 0.6 | 18.0 ± 0.0 | PI (10.7 ± 0.6) | |
| 2.5 mg/mL | 13 ± 1.0 | 15.0 ± 1.0 | NI | 15.7 ± 0.6 | 13.3 ± 0.6 | NI | |
| 1.25 mg/mL | NI | 11.0 ± 1.0 | NI | 13.7 ± 0.6 | 11.3 ± 0.6 | NI | |
| 0.1 mg/mL | NI | 10.0 ± 1.0 | NI | 11.3 ± 0.6 | NI | NI | |
| Microorganisms | P-AuNPs | D-Cys-AuNPs | L-Cys-AuNPs | |||
|---|---|---|---|---|---|---|
| MIC (mg/mL) | MBC (mg/mL) | MIC (mg/mL) | MBC (mg/mL) | MIC (mg/mL) | MBC (mg/mL) | |
| E. coli | 0.42 | 5.0 | 0.36 | 5.0 | 0.25 | 1.4 |
| S. enterica | 0.42 | 5.0 | 0.36 | 2.5 | 0.25 | 1.4 |
| L. monocytogenes | 0.50 | 5.0 | 0.42 | 5.0 | 0.25 | 1.8 |
| S. aureus | 0.50 | -- | 0.42 | 5.0 | 0.25 | 5.0 |
| S. epidermidis | 0.50 | 5.0 | 0.36 | 5.0 | 0.25 | 2.5 |
| C. albicans | 2.5 | -- | 2.5 | -- | 1.25 | 2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto, K.M.; Gódinez-Oviedo, A.; Romo-Pérez, A.; Mendoza, S.; López-Romero, J.M.; Torres-Delgado, G.; Pineda-Piñón, J.; Apátiga-Castro, L.M.; de Jesús Pérez Bueno, J.; Manzano-Ramírez, A. Cysteine Surface Engineering of Green-Synthesized Gold Nanoparticles for Enhanced Antimicrobial and Antifungal Activity. Int. J. Mol. Sci. 2025, 26, 7645. https://doi.org/10.3390/ijms26157645
Soto KM, Gódinez-Oviedo A, Romo-Pérez A, Mendoza S, López-Romero JM, Torres-Delgado G, Pineda-Piñón J, Apátiga-Castro LM, de Jesús Pérez Bueno J, Manzano-Ramírez A. Cysteine Surface Engineering of Green-Synthesized Gold Nanoparticles for Enhanced Antimicrobial and Antifungal Activity. International Journal of Molecular Sciences. 2025; 26(15):7645. https://doi.org/10.3390/ijms26157645
Chicago/Turabian StyleSoto, Karen M., Angelica Gódinez-Oviedo, Adriana Romo-Pérez, Sandra Mendoza, José Mauricio López-Romero, Gerardo Torres-Delgado, Jorge Pineda-Piñón, Luis M. Apátiga-Castro, José de Jesús Pérez Bueno, and Alejandro Manzano-Ramírez. 2025. "Cysteine Surface Engineering of Green-Synthesized Gold Nanoparticles for Enhanced Antimicrobial and Antifungal Activity" International Journal of Molecular Sciences 26, no. 15: 7645. https://doi.org/10.3390/ijms26157645
APA StyleSoto, K. M., Gódinez-Oviedo, A., Romo-Pérez, A., Mendoza, S., López-Romero, J. M., Torres-Delgado, G., Pineda-Piñón, J., Apátiga-Castro, L. M., de Jesús Pérez Bueno, J., & Manzano-Ramírez, A. (2025). Cysteine Surface Engineering of Green-Synthesized Gold Nanoparticles for Enhanced Antimicrobial and Antifungal Activity. International Journal of Molecular Sciences, 26(15), 7645. https://doi.org/10.3390/ijms26157645







