Exploring Adverse Event Associations of Predicted PXR Agonists Using the FAERS Database
Abstract
1. Introduction
2. Results
2.1. Results of the PXR Agonist Prediction Model
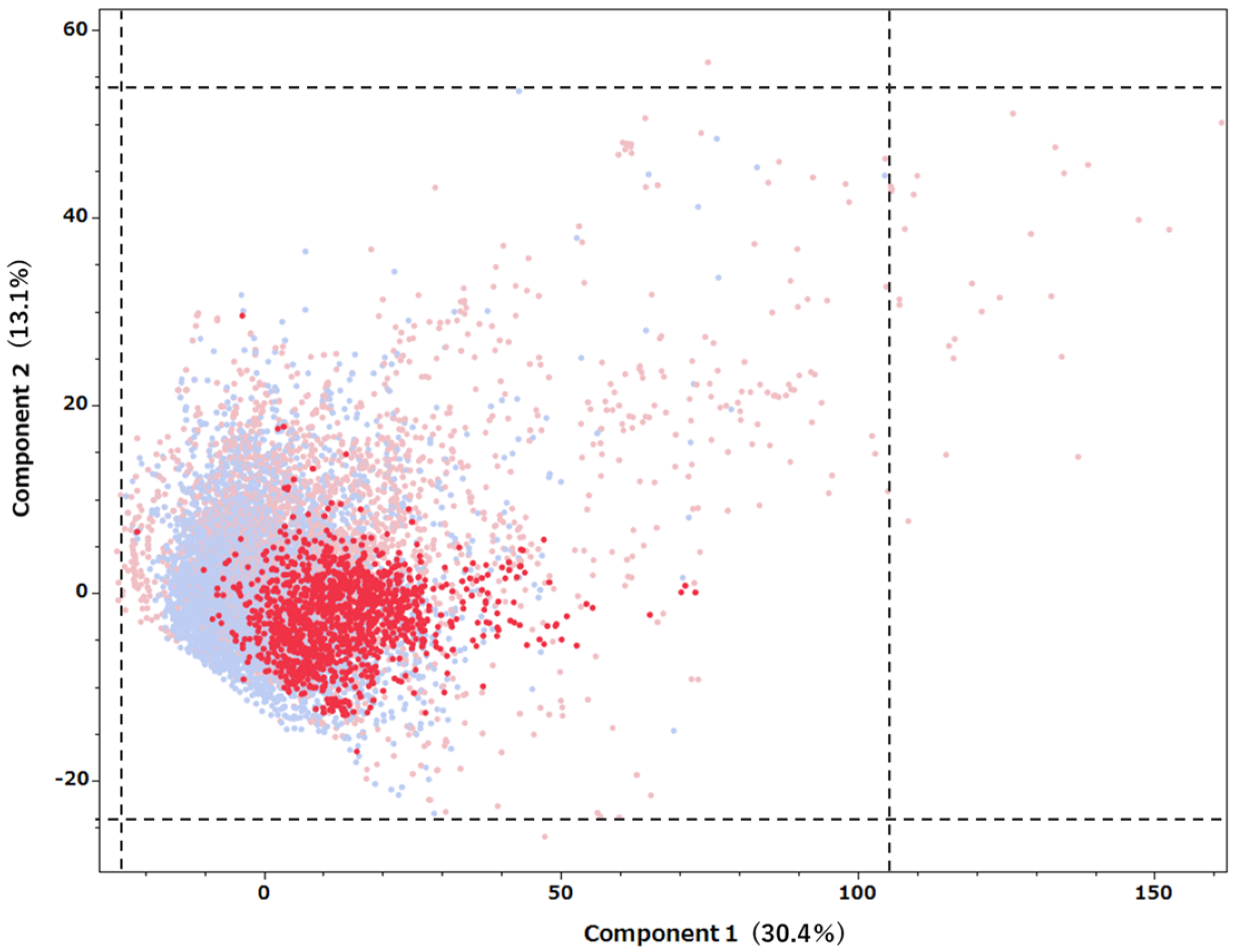
2.2. Predictive Model Application to Drugs Listed in the FAERS Database
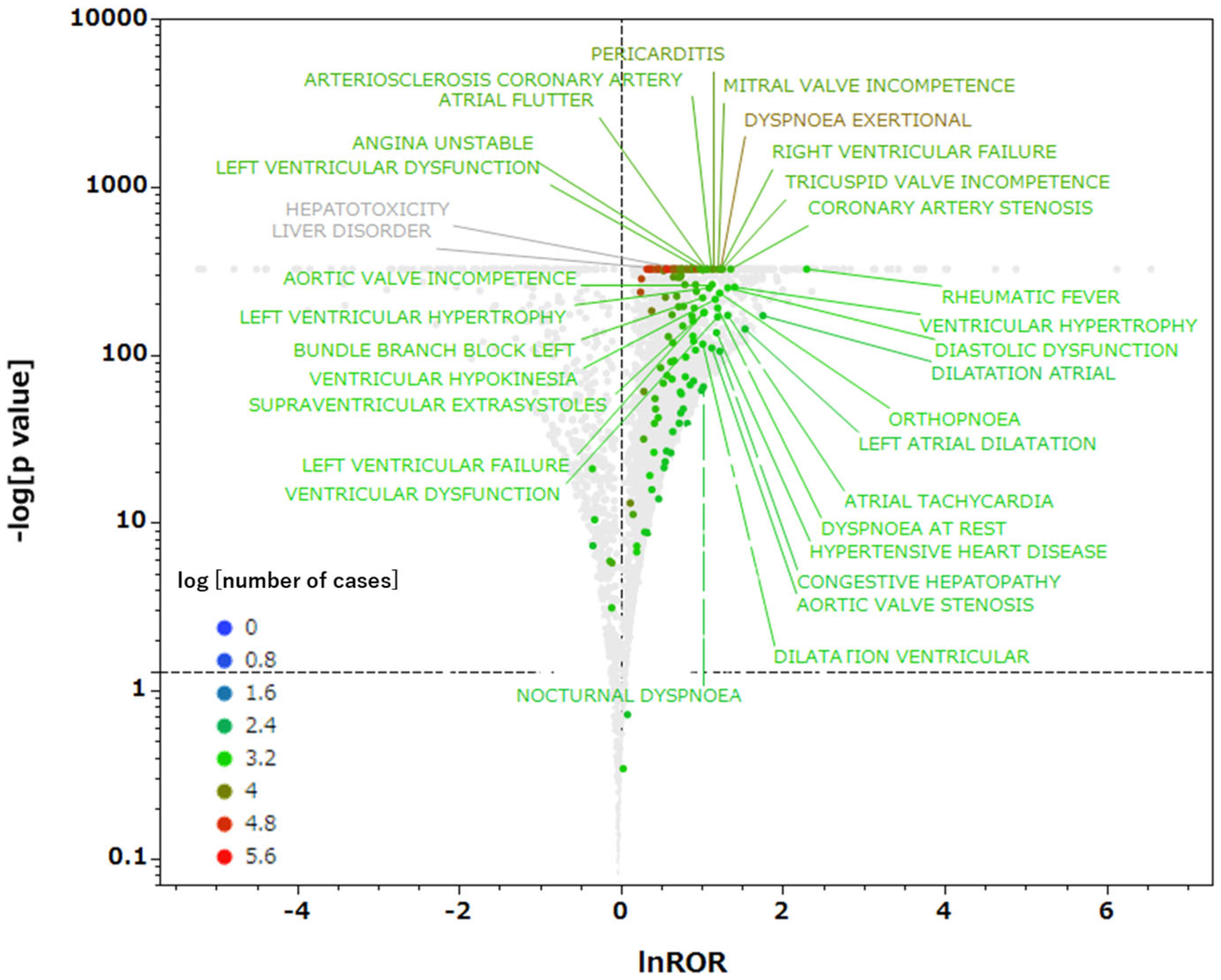
2.3. Relationships Between Drugs with Predicted PXR Agonist Activity and Adverse Events
3. Discussion
3.1. Applicability of the PXR Agonist Prediction Model to Drugs Listed in FAERS
3.2. Relationships Between Drugs with Predicted PXR Agonist Activity and Adverse Events
3.3. Limitations
4. Materials and Methods
4.1. PXR Agonist Database
4.2. Creation of the PXR Agonist Prediction Model
4.3. Evaluation Metrics
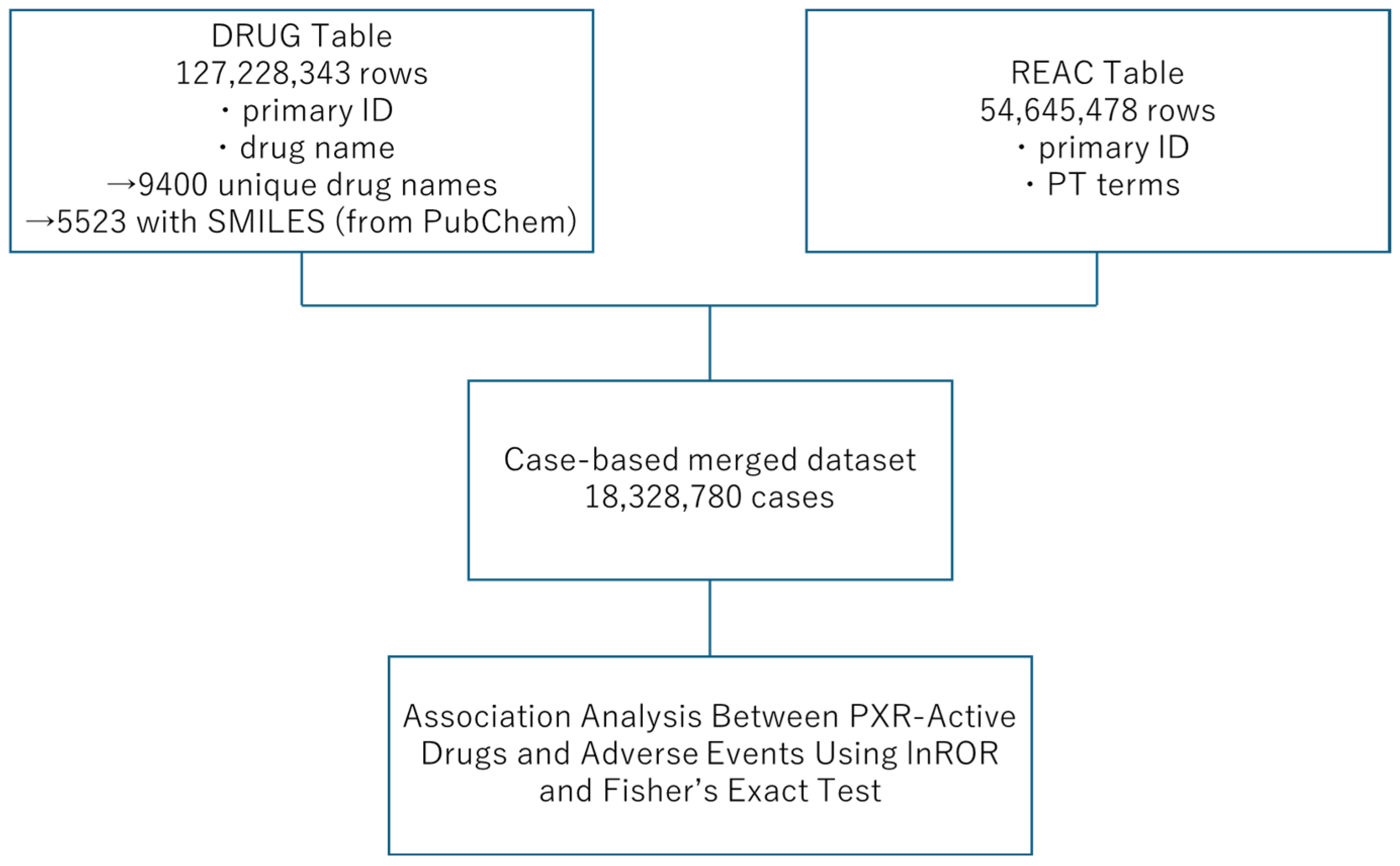
4.4. FAERS Database
4.5. Application of the Predictive Model to Drugs Listed in the FAERS Database
4.6. Relationship Between Drugs with Predicted PXR Agonist Activity and Adverse Events
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kliewer, S.A.; Moore, J.T.; Wade, L.; Staudinger, J.L.; Watson, M.A.; Jones, S.A.; McKee, D.D.; Oliver, B.B.; Willson, T.M.; Zetterström, R.H.; et al. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell 1998, 92, 73–82. [Google Scholar] [CrossRef]
- Zhang, B.; Xie, W.; Krasowski, M.D. PXR: A xenobiotic receptor of diverse function implicated in pharmacogenetics. Pharmacogenomics 2008, 9, 1695–1709. [Google Scholar] [CrossRef]
- Kliewer, S.A.; Goodwin, B.; Willson, T.M. The nuclear pregnane X receptor: A key regulator of xenobiotic metabolism. Endocr. Rev. 2002, 23, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Timsit, Y.E.; Negishi, M. CAR and PXR: The xenobiotic-sensing receptors. Steroids 2007, 72, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Kakehashi, A.; Wei, M.; Fukushima, S.; Wanibuchi, H. Oxidative stress in the carcinogenicity of chemical carcinogens. Cancers 2013, 5, 1332–1354. [Google Scholar] [CrossRef]
- Shah, Y.M.; Ma, X.; Morimura, K.; Kim, I.; Gonzalez, F.J. Pregnane X receptor activation ameliorates DSS-induced inflammatory bowel disease via inhibition of NF-κB target gene expression. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G1114–G1122. [Google Scholar] [CrossRef]
- Moreau, A.; Vilarem, M.J.; Maurel, P.; Pascussi, J.M. Xenoreceptors CAR and PXR activation and consequences on lipid metabolism, glucose homeostasis, and inflammatory response. Mol. Pharmacol. 2008, 5, 35–41. [Google Scholar] [CrossRef]
- Wagner, M.; Halilbasic, E.; Marschall, H.-U.; Zollner, G.; Fickert, P.; Langner, C.; Zatloukal, K.; Denk, H.; Trauner, M. CAR and PXR agonists stimulate hepatic bile acid and bilirubin detoxification and elimination pathways in mice. Hepatology 2005, 42, 420–430. [Google Scholar] [CrossRef]
- Kodama, S.; Negishi, M. PXR Cross-talks with internal and external signals in physiological and pathophysiological responses. Drug Metab. Rev. 2013, 45, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Kodama, S.; Koike, C.; Negishi, M.; Yamamoto, Y. Nuclear receptors CAR and PXR cross talk with FOXO1 to regulate genes that encode drug-metabolizing and gluconeogenic enzymes. Mol. Cell. Biol. 2004, 24, 7931–7940. [Google Scholar] [CrossRef] [PubMed]
- Harmsen, S.; Meijerman, I.; Beijnen, J.H.; Schellens, J.H.M. The role of nuclear receptors in pharmacokinetic drug-drug interactions in oncology. Cancer Treat. Rev. 2007, 33, 369–380. [Google Scholar] [CrossRef]
- Pinne, M.; Raucy, J.L. Advantages of cell-based high-volume screening assays to assess nuclear receptor activation during drug discovery. Expert Opin. Drug Discov. 2014, 9, 669–686. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Raymond, K. Roles of rifampicin in drug-drug interactions: Underlying molecular mechanisms involving the nuclear pregnane X receptor. Ann. Clin. Microbiol. Antimicrob. 2006, 5, 3. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, M.; Deng, M.; Li, Z.; Wang, P.; Ren, S.; Guo, Y.; Ma, X.; Fan, J.; Billiar, T.R.; et al. Activation of pregnane X receptor sensitizes mice to hemorrhagic shock induced liver injury. Hepatology 2019, 70, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- Shehu, A.I.; Lu, J.; Wang, P.; Zhu, J.; Wang, Y.; Yang, D.; McMahon, D.; Xie, W.; Gonzalez, F.J.; Ma, X. Pregnane X receptor activation potentiates ritonavir hepatotoxicity. J. Clin. Investig. 2019, 129, 2898–2903. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.L.; Moffit, J.S.; Nicol, C.J.; Ward, J.M.; Aleksunes, L.A.; Slitt, A.L.; Kliewer, S.A.; Manautou, J.E.; Gonzalez, F.J. Enhanced acetaminophen toxicity by activation of the pregnane X receptor. Toxicol. Sci. 2004, 82, 374–380. [Google Scholar] [CrossRef]
- Liu, P.; Jiang, L.; Kong, W.; Xie, Q.; Li, P.; Liu, X.; Zhang, J.; Liu, M.; Wang, Z.; Zhu, L.; et al. PXR activation impairs hepatic glucose metabolism partly via inhibiting the HNF4α–GLUT2 pathway. Acta Pharm. Sin. B 2022, 12, 2391–2405. [Google Scholar] [CrossRef]
- Hassani-Nezhad-Gashti, F.; Rysä, J.; Kummu, O.; Näpänkangas, J.; Buler, M.; Karpale, M.; Hukkanen, J.; Hakkola, J. Activation of nuclear receptor PXR impairs glucose tolerance and dysregulates GLUT2 expression and subcellular localization in liver. Biochem. Pharmacol. 2018, 148, 253–264. [Google Scholar] [CrossRef]
- Attema, B.; Kummu, O.; Krutáková, M.; Pavek, P.; Hakkola, J.; Hooiveld, G.J.E.J.; Kersten, S. The fungicide propiconazole induces hepatic steatosis and activates PXR in a mouse model of diet-induced obesity. Arch. Toxicol. 2025, 99, 1203–1221. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. FDA Adverse Event Reporting System (FAERS) Database. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-adverse-event-reporting-system-faers-database (accessed on 13 June 2025).
- Ngan, C.-H.; Beglov, D.; Rudnitskaya, A.N.; Kozakov, D.; Waxman, D.J.; Vajda, S. The structural basis of pregnane X receptor binding promiscuity. Biochemistry 2009, 48, 11572–11581. [Google Scholar] [CrossRef]
- Khandelwal, A.; Krasowski, M.D.; Reschly, E.J.; Sinz, M.W.; Swaan, P.W.; Ekins, S. Machine learning methods and docking for predicting human pregnane X receptor activation. Chem. Res. Toxicol. 2008, 21, 1457–1467. [Google Scholar] [CrossRef]
- Dong, H.; Lin, W.; Wu, J.; Chen, T. Flavonoids activate pregnane X receptor-mediated CYP3A4 gene expression by inhibiting cyclin-dependent kinases in HepG2 liver carcinoma cells. BMC Biochem. 2010, 11, 23. [Google Scholar] [CrossRef]
- Li, Y.; Ross-Viola, J.S.; Shay, N.F.; Moore, D.D.; Ricketts, M.-L. Human CYP3A4 and murine Cyp3A11 are regulated by equol and genistein via the pregnane x receptor in a species-specific manner. J. Nutr. 2009, 139, 898–904. [Google Scholar] [CrossRef]
- Svecova, L.; Vrzal, R.; Burysek, L.; Anzenbacherova, E.; Cerveny, L.; Grim, J.; Trejtnar, F.; Kunes, J.; Pour, M.; Staud, F.; et al. Azole antimycotics differentially affect rifampicin-induced pregnane X receptor-mediated CYP3A4 gene expression. Drug Metab. Dispos. 2008, 36, 339–348. [Google Scholar] [CrossRef]
- Deng, R.; Xu, C.; Chen, X.; Chen, P.; Wang, Y.; Zhou, X.; Jin, J.; Niu, L.; Ying, M.; Huang, M.; et al. Resveratrol suppresses the inducible expression of CYP3A4 through the pregnane X receptor. J. Pharmacol. Sci. 2014, 126, 326–334. [Google Scholar] [CrossRef]
- Dolezelova, E.; Prasnicka, A.; Cermanova, J.; Carazo, A.; Hyrsova, L.; Hroch, M.; Mokry, J.; Adamcova, M.; Mrkvicova, A.; Pavek, P.; et al. Resveratrol modifies biliary secretion of cholephilic compounds in sham-operated and cholestatic rats. World J. Gastroenterol. 2017, 23, 7678–7688. [Google Scholar] [CrossRef] [PubMed]
- Helsley, R.N. The Role of PXR and IKKβ Signaling in Cardiometabolic Disease. Doctoral Dissertation, University of Kentucky, Lexington, KY, USA, 2016. [Google Scholar]
- Sui, Y.; Park, S.-H.; Helsley, R.N.; Sunkara, M.; Gonzalez, F.J.; Morris, A.J.; Zhou, C. Bisphenol a increases atherosclerosis in pregnane X receptor-humanized ApoE deficient mice. J. Am. Heart Assoc. 2014, 3, e000492. [Google Scholar] [CrossRef]
- Hukkanen, J.; Hakkola, J. PXR and 4β-Hydroxycholesterol axis and the components of metabolic syndrome. Cells 2020, 9, 2445. [Google Scholar] [CrossRef] [PubMed]
- Staudinger, J.L.; Ding, X.; Lichti, K. Pregnane X receptor and natural products: Beyond drug–drug interactions. Expert Opin. Drug Metab. Toxicol. 2006, 2, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Rácz, A.; Bajusz, D.; Héberger, K. Intercorrelation limits in molecular descriptor preselection for QSAR/QSPR. Mol. Inform. 2019, 38, 1800154. [Google Scholar] [CrossRef]
- Sakai, T. Role and applicability of spontaneous reporting databases in medical big data. Yakugaku Zasshi 2021, 141, 165–168. (In Japanese) [Google Scholar] [CrossRef]
- Alemayehu, D. Evaluation of reporting bias in postmarketing risk assessment based on spontaneous reporting systems. Pharm. Med. 2009, 23, 195–200. [Google Scholar] [CrossRef]
- Pariente, A.; Avillach, P.; Salvo, F.; Thiessard, F.; Miremont-Salamé, G.; Fourrier-Reglat, A.; Haramburu, F.; Bégaud, B.; Moore, N. Association Française des Centres Régionaux de Pharmacovigilance (CRPV). Effect of competition bias in safety signal generation: Analysis of a research database of spontaneous reports in France. Pharm. Med. 2012, 26, 115–123. [Google Scholar]
- Tox21. About Tox21. Toxicology in the 21st Century (Tox21). Available online: https://tox21.gov/overview/about-tox21/ (accessed on 13 June 2025).
- MOLSIS Inc. MOE—Molecular Operating Environment. Available online: https://www.molsis.co.jp/lifescience/moe/?taxonomy=product_summary (accessed on 13 June 2025).
- Ke, G.; Meng, Q.; Finley, T.; Wang, T.; Chen, W.; Ma, W.; Ye, Q.; Liu, T.-Y. LightGBM: A Highly Efficient Gradient Boosting Decision Tree. In Proceedings of the 31st International Conference on Neural Information Processing Systems (NeurIPS 2017), Long Beach, CA, USA, 4–9 December 2017; Curran Associates, Inc.: Red Hook, NY, USA, 2017; pp. 3149–3157. [Google Scholar]
- Hajihosseinlou, M.; Maghsoudi, A.; Ghezelbash, R. A novel scheme for mapping of MVT-Type Pb–Zn prospectivity: LightGBM, a highly efficient gradient boosting decision tree machine learning algorithm. Nat. Resour. Res. 2023, 32, 2417–2438. [Google Scholar] [CrossRef]
- Wilimitis, D.; Walsh, C.G. practical considerations and applied examples of cross-validation for model development and evaluation in health care: Tutorial. JMIR AI 2023, 2, e49023. [Google Scholar] [CrossRef] [PubMed]
- Kurosaki, K.; Wu, R.; Uesawa, Y. A Toxicity prediction tool for potential agonist/antagonist activities in molecular initiating events based on chemical structures. Int. J. Mol. Sci. 2020, 21, 7853. [Google Scholar] [CrossRef]
- Matthews, B.W. Comparison of the predicted and observed secondary structure of T4 phage lysozyme. Biochim. Biophys. Acta 1975, 405, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, T. An Introduction to ROC analysis. Pattern Recognit. Lett. 2006, 27, 861–874. [Google Scholar] [CrossRef]
- Fluss, R.; Faraggi, D.; Reiser, B. Estimation of the youden index and its associated cutoff point. Biom. J. 2005, 47, 458–472. [Google Scholar] [CrossRef] [PubMed]
- Swain, M. PubChemPy: A Way to Interact with PubChem in Python. Available online: https://github.com/mcs07/pubchempy (accessed on 13 June 2025).
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem substance and compound databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef]
- Weber, F.; Knapp, G.; Ickstadt, K.; Kundt, G.; Glass, Ä. Zero-cell corrections in random-effects meta-analyses. Res. Synth. Methods 2022, 13, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Gravel, C.A.; Douros, A. Considerations on the use of different comparators in pharmacovigilance: A methodological review. Br. J. Clin. Pharmacol. 2023, 89, 3466–3475. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Wang, S.-J.; Tsai, C.-A.; Lin, C.-J. Selection of differentially expressed genes in microarray data analysis. Pharmacogenomics J. 2007, 7, 212–220. [Google Scholar] [CrossRef] [PubMed]

| SOC Category | Significant Terms | Total Terms in SOC | Significant Term Rate (%) |
|---|---|---|---|
| Cardiac disorders | 30 | 719 | 4.17 |
| Product issues | 5 | 203 | 2.46 |
| Endocrine disorders | 14 | 639 | 2.19 |
| Blood and lymphatic system disorders | 22 | 1165 | 1.89 |
| Reproductive system and breast disorders | 24 | 1350 | 1.78 |
| Metabolism and nutrition disorders | 16 | 918 | 1.74 |
| Respiratory, thoracic and mediastinal disorders | 22 | 1329 | 1.66 |
| General disorders and administration site conditions | 22 | 1426 | 1.54 |
| Hepatobiliary disorders | 6 | 490 | 1.22 |
| Psychiatric disorders | 11 | 908 | 1.21 |
| Gastrointestinal disorders | 22 | 1986 | 1.11 |
| Immune system disorders | 9 | 936 | 0.96 |
| Renal and urinary disorders | 6 | 826 | 0.73 |
| Pregnancy, puerperium and perinatal conditions | 5 | 700 | 0.71 |
| Neoplasms benign, malignant and unspecified (incl. cysts and polyps) | 15 | 2510 | 0.60 |
| Musculoskeletal and connective tissue disorders | 9 | 1562 | 0.58 |
| Vascular disorders | 11 | 1916 | 0.57 |
| Nervous system disorders | 13 | 2361 | 0.55 |
| Injury, poisoning and procedural complications | 14 | 2817 | 0.50 |
| Infections and infestations | 10 | 2353 | 0.42 |
| Investigations | 27 | 6407 | 0.42 |
| Surgical and medical procedures | 7 | 2607 | 0.27 |
| Congenital, familial and genetic disorders | 2 | 1859 | 0.11 |
| Eye disorders | 1 | 1196 | 0.08 |
| Adverse Event Present | Adverse Event Absent | |
|---|---|---|
| PXR-Active Drug Exposure | a | b |
| Other Drug Exposure | c | d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, S.; Uesawa, Y. Exploring Adverse Event Associations of Predicted PXR Agonists Using the FAERS Database. Int. J. Mol. Sci. 2025, 26, 7630. https://doi.org/10.3390/ijms26157630
Yamada S, Uesawa Y. Exploring Adverse Event Associations of Predicted PXR Agonists Using the FAERS Database. International Journal of Molecular Sciences. 2025; 26(15):7630. https://doi.org/10.3390/ijms26157630
Chicago/Turabian StyleYamada, Saki, and Yoshihiro Uesawa. 2025. "Exploring Adverse Event Associations of Predicted PXR Agonists Using the FAERS Database" International Journal of Molecular Sciences 26, no. 15: 7630. https://doi.org/10.3390/ijms26157630
APA StyleYamada, S., & Uesawa, Y. (2025). Exploring Adverse Event Associations of Predicted PXR Agonists Using the FAERS Database. International Journal of Molecular Sciences, 26(15), 7630. https://doi.org/10.3390/ijms26157630







