Unmasking Pediatric Asthma: Epigenetic Fingerprints and Markers of Respiratory Infections
Abstract
1. Introduction
2. Asthma Endotypes
2.1. T2-High Asthma Endotype
2.2. T2-Low Asthma Endotype
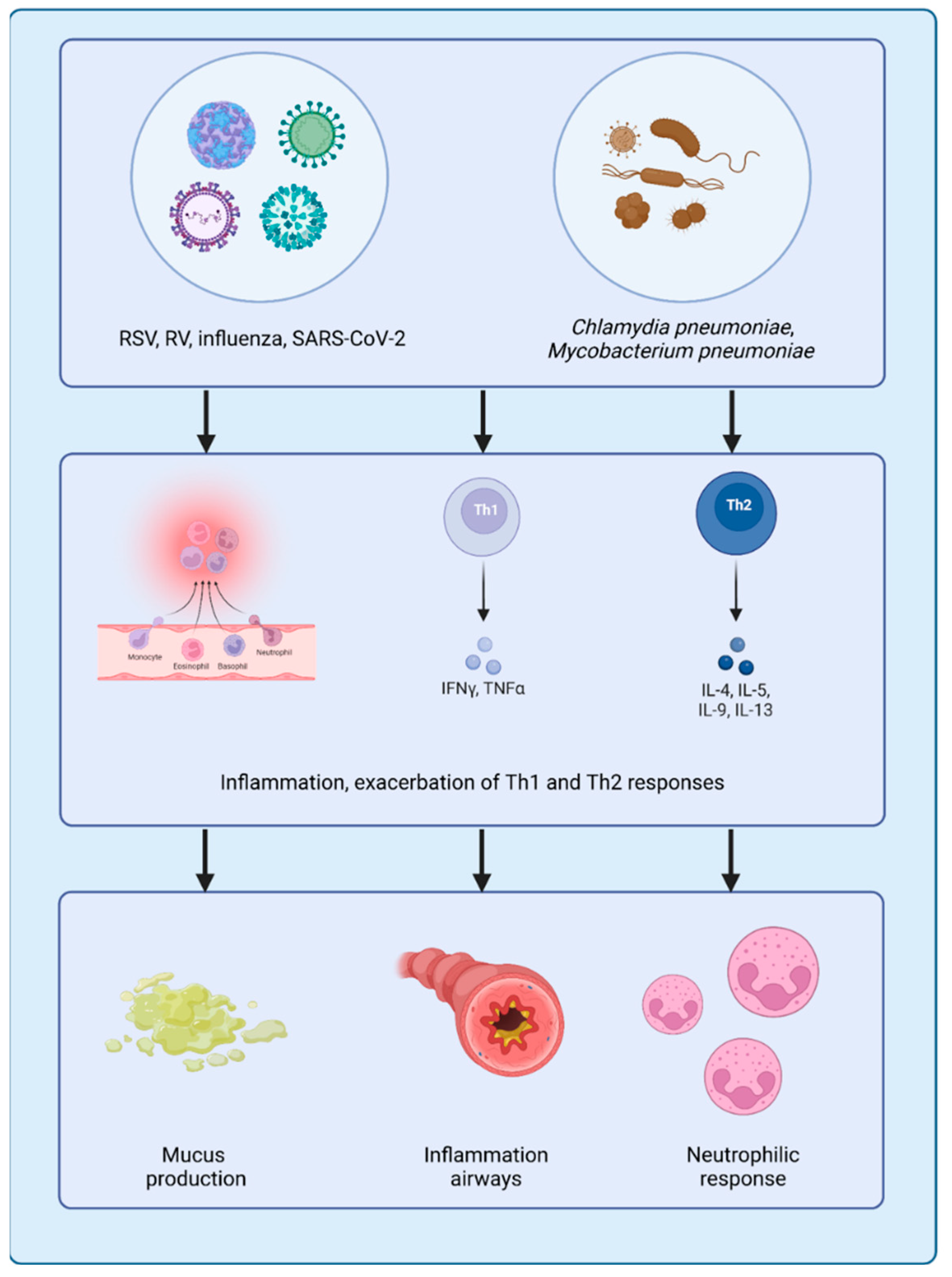
3. Role of Pathogens in Asthma Exacerbation
3.1. Respiratory Viral Pathogens
3.2. Bacterial Pathogens
3.3. Fungal Pathogens
3.4. Coinfections
3.4.1. Coinfections Involving Viruses and Pathogenic Bacteria
3.4.2. Coinfections Involving Different Respiratory Viruses
4. Epigenetic Mechanisms in Asthma
4.1. Histones
4.2. Non-Coding RNAs
5. Novel Therapeutic Approaches
5.1. Epigenetic Modifications
5.2. DNA Methylation
5.3. miRNA-Based Therapies
5.4. Probiotics
5.5. Personalized Medicine
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yuan, L.; Tao, J.; Wang, J.; She, W.; Zou, Y.; Li, R.; Ma, Y.; Sun, C.; Bi, S.; Wei, S.; et al. regional, national burden of asthma from 1990 to 2021, with projections of incidence to 2050: A systematic analysis of the global burden of disease study 2021. eClinicalMedicine 2025, 80, 103051. [Google Scholar] [CrossRef]
- Global Initiative for Asthma (GINA), Global Strategy for Asthma Management and Prevention (2024 Update). 2024. Available online: http://www.ginasthma.org/ (accessed on 1 March 2025).
- Cheng, F.; He, L.; Deng, D.; Zhang, J.; Liu, C. Analysis of asthma incidence and mortality rates among children aged 0–14 in 204 countries from 1990 to 2019. J. Asthma 2025, 62, 45–55. [Google Scholar] [CrossRef]
- Pijnenburg, M.W.; Frey, U.; De Jongste, J.C.; Saglani, S. Childhood asthma: Pathogenesis; phenotypes. Eur. Respir. J. 2022, 59, 2100731. [Google Scholar] [CrossRef]
- Roberto, G.; Barberi, S.; Marseglia, G.L.; Licari, A. What’s new in pediatric asthma and rhinitis phenotypes and endotypes? Curr. Opin. Allergy Clin. Immunol. 2024, 24, 73–78. [Google Scholar] [CrossRef]
- Albano, G.D.; Zhao, J.; Etling, E.B.; Park, S.Y.; Hu, H.; Trudeau, J.B.; Profita, M.; Wenzel, S.E. IL-13 desensitizes β2-adrenergic receptors in human airway epithelial cells through a 15-lipoxygenase/G protein receptor kinase 2 mechanism. J. Allergy Clin. Immunol. 2015, 135, 1144–1153. [Google Scholar] [CrossRef]
- Ricciardolo, F.L.M.; Sprio, A.E.; Baroso, A.; Gallo, F.; Riccardi, E.; Bertolini, F.; Carriero, V.; Arrigo, E.; Ciprandi, G. Characterization of T2-Low and T2-High Asthma Phenotypes in Real-Life. Biomedicines 2021, 9, 1684. [Google Scholar] [CrossRef] [PubMed]
- Tamari, M.; Trier, A.M.; Kim, B.S. Emerging targeted therapeutics underscore immunologic heterogeneity of asthma. J. Allergy Clin. Immunol. 2021, 148, 719–721. [Google Scholar] [CrossRef]
- Hillson, K.; Saglani, S.; Custovic, A. Preschool wheeze and asthma endotypes- implications for future therapy. Expert Rev. Respir. Med. 2024, 18, 1025–1039. [Google Scholar] [CrossRef] [PubMed]
- Skov, F.R.; Sultan, T.; Fischer-Rasmussen, K.; Chawes, B.L.; Stokholm, J.; Vahman, N.; Bønnelykke, K.; Schoos, A.M. Type 2-high airway inflammation in childhood asthma distinguishes a more severe phenotype. Pediatr. Allergy Immunol. 2025, 36, e70032. [Google Scholar] [CrossRef]
- Hinks, T.S.C.; Levine, S.J.; Brusselle, G.G. Treatment options in type-2 low asthma. Eur. Respir. J. 2021, 57, 2000528. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Yang, I.V.; Schwartz, D.A. Epigenetic regulation of immune function in asthma. J. Allergy Clin. Immunol. 2022, 150, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Jartti, T.; Liimatainen, U.; Xepapadaki, P.; Vahlberg, T.; Bachert, C.; Finotto, S.; Kowalski, M.L.; Sobanska, A.; Lukkarinen, H.; Pasioti, M.; et al. Clinical correlates of rhinovirus infection in preschool asthma. Allergy 2021, 76, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Pech, M.; Weckmann, M.; König, I.R.; Franke, A.; Heinsen, F.-A.; Oliver, B.; Ricklefs, I.; Fuchs, O.; Rabe, K.; Hansen, G.; et al. ALLIANCE-study group, Rhinovirus infections change DNA methylation and mRNA expression in children with asthma. PLoS ONE 2018, 13, e0205275. [Google Scholar] [CrossRef]
- Spalluto, C.M.; Singhania, A.; Cellura, D.; Woelk, C.H.; Sanchez-Elsner, T.; Staples, K.J.; Wilkinson, T.M.A. IFN-γ Influences Epithelial Antiviral Responses via Histone Methylation of the RIG-I Promoter. Am. J. Respir. Cell Mol. Biol. 2017, 57, 428–438. [Google Scholar] [CrossRef]
- Nicodemus-Johnson, J.; Myers, R.A.; Sakabe, N.J.; Sobreira, D.R.; Hogarth, D.K.; Naureckas, E.T.; Sperling, A.I.; Solway, J.; White, S.R.; Nobrega, M.A.; et al. DNA methylation in lung cells is associated with asthma endotypes and genetic risk. JCI Insight 2016, 1, e90151. [Google Scholar] [CrossRef]
- Potaczek, D.P.; Miethe, S.; Schindler, V.; Alhamdan, F.; Garn, H. Role of airway epithelial cells in the development of different asthma phenotypes. Cell. Signal. 2020, 69, 109523. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Hammad, H.; Fahy, J.V. The Cytokines of Asthma. Immunity 2019, 50, 975–991. [Google Scholar] [CrossRef]
- Wang, W.; Li, Y.; Lv, Z.; Chen, Y.; Li, Y.; Huang, K.; Corrigan, C.J.; Ying, S. Bronchial Allergen Challenge of Patients with Atopic Asthma Triggers an Alarmin (IL-33, TSLP, and IL-25) Response in the Airways Epithelium and Submucosa. Immunity 2018, 201, 2221–2231. [Google Scholar] [CrossRef]
- Hammad, H.; Lambrecht, B.N. The basic immunology of asthma. Cell 2021, 184, 1469–1485. [Google Scholar] [CrossRef]
- Liu, Y.-J. Thymic stromal lymphopoietin and OX40 ligand pathway in the initiation of dendritic cell-mediated allergic inflammation. J. Allergy Clin. Immunol. 2007, 120, 238–244; quiz 245–246. [Google Scholar] [CrossRef] [PubMed]
- Rochman, Y.; Dienger-Stambaugh, K.; Richgels, P.K.; Lewkowich, I.P.; Kartashov, A.V.; Barski, A.; Hershey, G.K.K.; Leonard, W.J.; Singh, H. TSLP signaling in CD4+ T cells programs a pathogenic T helper 2 cell state. Sci. Signal. 2018, 11, eaam8858. [Google Scholar] [CrossRef]
- Soumelis, V.; Reche, P.A.; Kanzler, H.; Yuan, W.; Edward, G.; Homey, B.; Gilliet, M.; Ho, S.; Antonenko, S.; Lauerma, A.; et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002, 3, 673–680. [Google Scholar] [CrossRef]
- West, E.E.; Kashyap, M.; Leonard, W.J. TSLP: A Key Regulator of Asthma Pathogenesis. Drug Discov. Today Dis. Mech. 2012, 9, e83–e88. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Wang, R.; Zhang, W.; Wen, C.; Chen, C.; Liu, S.; Lei, Q.; Zhang, P.; Zeng, S. The production; function, and clinical applications of IL-33 in type 2 inflammation-related respiratory diseases. Front. Immunol. 2024, 15, 1436437. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Sun, L.; Zhang, M.; Yan, H.; Shi, G.; Xia, Z.; Dai, R.; Tang, W. Role of IL-25 on Eosinophils in the Initiation of Th2 Responses in Allergic Asthma. Front. Immunol. 2022, 13, 842500. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Hammad, H. The immunology of asthma. Nat. Immunol. 2015, 16, 45–56. [Google Scholar] [CrossRef]
- Galli, S.J.; Tsai, M. IgE and mast cells in allergic disease. Nat. Med. 2012, 18, 693–704. [Google Scholar] [CrossRef]
- Truong-Thanh, T.; Vo-Thi-Kim, A.; Vu-Minh, T.; Truong-Viet, D.; Tran-Van, H.; Duong-Quy, S. The beneficial role of FeNO in association with GINA guidelines for titration of inhaled corticosteroids in adult asthma: A randomized study. Adv. Med. Sci. 2020, 65, 244–251. [Google Scholar] [CrossRef]
- Smith, S.G.; Chen, R.; Kjarsgaard, M.; Huang, C.; Oliveria, J.-P.; O’Byrne, P.M.; Gauvreau, G.M.; Boulet, L.-P.; Lemiere, C.; Martin, J.; et al. Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J. Allergy Clin. Immunol. 2016, 137, 75–86.e8. [Google Scholar] [CrossRef] [PubMed]
- Aritomi, M.; Ohta, S. Structural biology of apoptosis proteins: Recent advances in structural analysis of TNF-related, Fas-related, Bcl-2 family and caspase family proteins. Tanpakushitsu Kakusan Koso 1999, 44, 395–403. [Google Scholar]
- Shrine, N.; Portelli, M.A.; John, C.; Artigas, M.S.; Bennett, N.; Hall, R.; Lewis, J.; Henry, A.P.; Billington, C.K.; Ahmad, A.; et al. Moderate-to-severe asthma in individuals of European ancestry: A genome-wide association study. Lancet Respir. Med. 2019, 7, 20–34. [Google Scholar] [CrossRef]
- Wang, C.-M.; Chang, C.-B.; Wu, S.-F. Differential DNA methylation in allergen-specific immunotherapy of asthma. Cell Mol. Immunol. 2020, 17, 1017–1018. [Google Scholar] [CrossRef]
- Cardenas, A.; Sordillo, J.E.; Rifas-Shiman, S.L.; Chung, W.; Liang, L.; Coull, B.A.; Hivert, M.-F.; Lai, P.S.; Forno, E.; Celedón, J.C.; et al. The nasal methylome as a biomarker of asthma and airway inflammation in children. Nat. Commun. 2019, 10, 3095. [Google Scholar] [CrossRef] [PubMed]
- Breton, C.V.; Byun, H.-M.; Wang, X.; Salam, M.T.; Siegmund, K.; Gilliland, F.D. DNA methylation in the arginase-nitric oxide synthase pathway is associated with exhaled nitric oxide in children with asthma. Am. J. Respir. Crit. Care Med. 2011, 184, 191–197. [Google Scholar] [CrossRef]
- Kuriakose, J.; Rosa, M.J.; Perzanowski, M.; Miller, R. Bronchial nitric oxide flux may be better associated with inducible nitric oxide synthase promoter methylation. Am. J. Respir. Crit. Care Med. 2012, 185, 460–461. [Google Scholar] [CrossRef][Green Version]
- Stikker, B.S.; Hendriks, R.W.; Stadhouders, R. Decoding the genetic and epigenetic basis of asthma. Allergy 2023, 78, 940–956. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Huang, P.; Qiu, C. Progresses in epigenetic studies of asthma from the perspective of high-throughput analysis technologies: A narrative review. Ann. Transl. Med. 2022, 10, 493. [Google Scholar] [CrossRef] [PubMed]
- Ricciardolo, F.L.M.; Carriero, V.; Bertolini, F. Which Therapy for Non-Type(T)2/T2-Low Asthma. J. Pers. Med. 2021, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Kuruvilla, M.E.; Lee, F.E.-H.; Lee, G.B. Understanding Asthma Phenotypes, Endotypes, and Mechanisms of Disease. Clin. Rev. Allergy Immunol. 2019, 56, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Yang, J.; Gul, A.; Li, Y.; Zhang, R.; Yalikun, M.; Lv, X.; Lin, Y.; Luo, Q.; Gao, H. Immunologic aspects of asthma: From molecular mechanisms to disease pathophysiology and clinical translation. Front. Immunol. 2024, 15, 1478624. [Google Scholar] [CrossRef]
- Peri, F.; Amaddeo, A.; Badina, L.; Maschio, M.; Barbi, E.; Ghirardo, S. T2-Low Asthma: A Discussed but Still Orphan Disease. Biomedicines 2023, 11, 1226. [Google Scholar] [CrossRef] [PubMed]
- Galeone, C.; Scelfo, C.; Bertolini, F.; Caminati, M.; Ruggiero, P.; Facciolongo, N.; Menzella, F. Precision Medicine in Targeted Therapies for Severe Asthma: Is There Any Place for “Omics” Technology? Biomed. Res. Int. 2018, 2018, 4617565. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lloyd, C.M.; Noble, A. Th17 responses in chronic allergic airway inflammation abrogate regulatory T-cell-mediated tolerance and contribute to airway remodeling. Mucosal Immunol. 2013, 6, 335–346. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Pan, Y.-Q.; He, B.-S.; Zhong, T.-Y. Regulatory T cells and Th1/Th2 in peripheral blood and their roles in asthmatic children. Transl. Pediatr. 2013, 2, 27–33. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Voo, K.S.; Liu, B.; Chen, C.-Y.; Uygungil, B.; Spoede, W.; Bernstein, J.A.; Huston, D.P.; Liu, Y.-J. A novel subset of CD4(+) T(H)2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J. Exp. Med. 2010, 207, 2479–2491. [Google Scholar] [CrossRef]
- Gagliardo, R.; Chanez, P.; Mathieu, M.; Bruno, A.; Costanzo, G.; Gougat, C.; Vachier, I.; Bousquet, J.; Bonsignore, G.; Vignola, A.M. Persistent activation of nuclear factor-kappaB signaling pathway in severe uncontrolled asthma. Am. J. Respir. Crit. Care Med. 2003, 168, 1190–1198. [Google Scholar] [CrossRef]
- Gavino, A.C.; Nahmod, K.; Bharadwaj, U.; Makedonas, G.; Tweardy, D.J. STAT3 inhibition prevents lung inflammation, remodeling, and accumulation of Th2 and Th17 cells in a murine asthma model. Allergy 2016, 71, 1684–1692. [Google Scholar] [CrossRef]
- Nikolskii, A.A.; Shilovskiy, I.P.; Barvinskaia, E.D.; Korneev, A.V.; Sundukova, M.S.; Khaitov, M.R. Role of STAT3 Transcription Factor in Pathogenesis of Bronchial Asthma. Biochemistry 2021, 86, 1489–1501. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhou, Y.; Bao, W.; Fu, Q.; Hao, H.; Han, L.; Zhang, X.; Tian, X.; Zhang, M. STAT3 and IL-6 Contribute to Corticosteroid Resistance in an OVA and Ozone-induced Asthma Model with Neutrophil Infiltration. Front. Mol. Biosci. 2021, 8, 717962. [Google Scholar] [CrossRef]
- Yang, B.-H.; Floess, S.; Hagemann, S.; Deyneko, I.V.; Groebe, L.; Pezoldt, J.; Sparwasser, T.; Lochner, M.; Huehn, J. Development of a unique epigenetic signature during in vivo Th17 differentiation. Nucleic Acids Res. 2015, 43, 1537–1548. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, C.; Thevin, V.; Fesneau, O.; Matias, M.I.; Perrault, J.; Abid, A.H.; Taylor, N.; Dardalhon, V.; Marie, J.C.; Hernandez-Vargas, H. Single-Molecule DNA Methylation Reveals Unique Epigenetic Identity Profiles of T Helper Cells. Immunology 2024, 212, 1029–1039. [Google Scholar] [CrossRef]
- Clifford, R.L.; Patel, J.K.; John, A.E.; Tatler, A.L.; Mazengarb, L.; Brightling, C.E.; Knox, A.J. CXCL8 histone H3 acetylation is dysfunctional in airway smooth muscle in asthma: Regulation by BET. Am. J. Physiol.-Lung Cell Mol. Physiol. 2015, 308, L962–L972. [Google Scholar] [CrossRef]
- Fitzpatrick, A.M.; Chipps, B.E.; Holguin, F.; Woodruff, P.G. T2-“Low” Asthma: Overview and Management Strategies. J. Allergy Clin. Immunol. Pract. 2020, 8, 452–463. [Google Scholar] [CrossRef]
- Kyriakopoulos, C.; Gogali, A.; Bartziokas, K.; Kostikas, K. Identification and treatment of T2-low asthma in the era of biologics. ERJ Open Res. 2021, 7, 00309–02020. [Google Scholar] [CrossRef]
- Lindsley, A.; Lugogo, N.; Reeh, K.; Spahn, J.; Parnes, J. Asthma Biologics Across the T2 Spectrum of Inflammation in Severe Asthma: Biomarkers and Mechanism of Action. JAA Vol. 2025, 18, 33–57. [Google Scholar] [CrossRef]
- Kardas, G.; Panek, M.; Kuna, P.; Damiański, P.; Kupczyk, M. Monoclonal antibodies in the management of asthma: Dead ends, current status and future perspectives. Front. Immunol. 2022, 13, 983852. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.G.; Yang, I.A.; Upham, J.W.; Reynolds, P.N.; Hodge, S.; James, A.L.; Jenkins, C.; Peters, M.J.; Marks, G.B.; Baraket, M.; et al. Efficacy of azithromycin in severe asthma from the AMAZES randomised trial. ERJ Open Res. 2019, 5, 00056–02019. [Google Scholar] [CrossRef]
- Morris, S.B.; Ocadiz-Ruiz, R.; Asai, N.; Malinczak, C.-A.; Rasky, A.J.; Lombardo, G.K.; Velarde, E.M.; Ptaschinski, C.; Zemans, R.L.; Lukacs, N.W.; et al. Long-term alterations in lung epithelial cells after EL-RSV infection exacerbate allergic responses through IL-1β-induced pathways. Mucosal Immunol. 2024, 17, 1072–1088. [Google Scholar] [CrossRef] [PubMed]
- Mthembu, N.; Ikwegbue, P.; Brombacher, F.; Hadebe, S. Respiratory Viral and Bacterial Factors That Influence Early Childhood Asthma. Front. Allergy 2021, 2, 692841. [Google Scholar] [CrossRef] [PubMed]
- Hansbro, N.G.; Horvat, J.C.; Wark, P.A.; Hansbro, P.M. Understanding the mechanisms of viral induced asthma: New therapeutic directions. Pharmacol. Ther. 2008, 117, 313–353. [Google Scholar] [CrossRef]
- Romero-Tapia, S.; Priego, C.G.; Del-Río-Navarro, B.; Sánchez-Solis, M. Advances in the Relationship between Respiratory Viruses and Asthma. J. Clin. Med. 2023, 12, 5501. [Google Scholar] [CrossRef]
- Kim, R.Y.; Horvat, J.C.; Pinkerton, J.W.; Starkey, M.R.; Essilfie, A.T.; Mayall, J.R.; Nair, P.M.; Hansbro, N.G.; Jones, B.; Haw, T.J.; et al. MicroRNA-21 drives severe, steroid-insensitive experimental asthma by amplifying phosphoinositide 3-kinase-mediated suppression of histone deacetylase 2. J. Allergy Clin. Immunol. 2017, 139, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Jakwerth, C.A.; Kitzberger, H.; Pogorelov, D.; Müller, A.; Blank, S.; Schmidt-Weber, C.B.; Zissler, U.M. Role of microRNAs in type 2 diseases and allergen-specific immunotherapy. Front. Allergy 2022, 3, 993937. [Google Scholar] [CrossRef]
- Jackson, D.J.; Gangnon, R.E.; Evans, M.D.; Roberg, K.A.; Anderson, E.L.; Pappas, T.E.; Printz, M.C.; Lee, W.-M.; Shult, P.A.; Reisdorf, E.; et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am. J. Respir. Crit. Care Med. 2008, 178, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Calışkan, M.; Bochkov, Y.A.; Kreiner-Møller, E.; Bønnelykke, K.; Stein, M.M.; Du, G.; Bisgaard, H.; Jackson, D.J.; Gern, J.E.; Lemanske, R.F.; et al. Rhinovirus wheezing illness and genetic risk of childhood-onset asthma. N. Engl. J. Med. 2013, 368, 1398–1407. [Google Scholar] [CrossRef]
- Acevedo, N.; Reinius, L.E.; Greco, D.; Gref, A.; Orsmark-Pietras, C.; Persson, H.; Pershagen, G.; Hedlin, G.; Melén, E.; Scheynius, A.; et al. Risk of childhood asthma is associated with CpG-site polymorphisms, regional DNA methylation and mRNA levels at the GSDMB/ORMDL3 locus. Hum. Mol. Genet. 2015, 24, 875–890. [Google Scholar] [CrossRef]
- Lund, R.J.; Osmala, M.; Malonzo, M.; Lukkarinen, M.; Leino, A.; Salmi, J.; Vuorikoski, S.; Turunen, R.; Vuorinen, T.; Akdis, C.; et al. Atopic asthma after rhinovirus-induced wheezing is associated with DNA methylation change in the SMAD3 gene promoter. Allergy 2018, 73, 1735–1740. [Google Scholar] [CrossRef]
- Ptaschinski, C.; Mukherjee, S.; Moore, M.L.; Albert, M.; Helin, K.; Kunkel, S.L.; Lukacs, N.W. RSV-Induced H3K4 Demethylase KDM5B Leads to Regulation of Dendritic Cell-Derived Innate Cytokines and Exacerbates Pathogenesis In Vivo. PLoS Pathog. 2015, 11, e1004978. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.-R.; Peng, D.; Chen, C.-M.; Qin, X.-Q. Nonstructural protein-1 of respiratory syncytial virus regulates HOX gene expression through interacting with histone. Mol. Biol. Rep. 2013, 40, 675–679. [Google Scholar] [CrossRef]
- Teo, S.M.; Mok, D.; Pham, K.; Kusel, M.; Serralha, M.; Troy, N.; Holt, B.J.; Hales, B.J.; Walker, M.L.; Hollams, E.; et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe 2015, 17, 704–715. [Google Scholar] [CrossRef]
- Busse, W.W.; Lemanske, R.F. Asthma. N. Engl. J. Med. 2001, 344, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Vasileiou, E.; Sheikh, A.; Butler, C.; El Ferkh, K.; von Wissmann, B.; McMenamin, J.; Ritchie, L.; Schwarze, J.; Papadopoulos, N.G.; Johnston, S.L.; et al. Effectiveness of Influenza Vaccines in Asthma: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2017, 65, 1388–1395. [Google Scholar] [CrossRef]
- Hamelin, M.-E.; Boivin, G. Human metapneumovirus: A ubiquitous and long-standing respiratory pathogen. Pediatr. Infect Dis. J. 2005, 24, S203–S207. [Google Scholar] [CrossRef]
- Yousafzai, M.T.; Ibrahim, R.; Thobani, R.; Aziz, F.; Ali, A. Human metapneumovirus in hospitalized children less than 5 years of age in Pakistan. J. Med. Virol. 2018, 90, 1027–1032. [Google Scholar] [CrossRef]
- Weinberg, G.A.; Hall, C.B.; Iwane, M.K.; Poehling, K.A.; Edwards, K.M.; Griffin, M.R.; Staat, M.A.; Curns, A.T.; Erdman, D.D.; Szilagyi, P.G. New Vaccine Surveillance Network, Parainfluenza virus infection of young children: Estimates of the population-based burden of hospitalization. J. Pediatr. 2009, 154, 694–699. [Google Scholar] [CrossRef]
- Wrede, D.; Bordak, M.; Abraham, Y.; Mehedi, M. Pulmonary Pathogen-Induced Epigenetic Modifications. Epigenomes 2023, 7, 13. [Google Scholar] [CrossRef]
- Ferrari, R.; Pellegrini, M.; Horwitz, G.A.; Xie, W.; Berk, A.J.; Kurdistani, S.K. Epigenetic reprogramming by adenovirus e1a. Science 2008, 321, 1086–1088. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.; Su, T.; Li, B.; Bonora, G.; Oberai, A.; Chan, Y.; Sasidharan, R.; Berk, A.J.; Pellegrini, M.; Kurdistani, S.K. Reorganization of the host epigenome by a viral oncogene. Genome Res. 2012, 22, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Lynch, K.L.; Gooding, L.R.; Garnett-Benson, C.; Ornelles, D.A.; Avgousti, D.C. Epigenetics and the dynamics of chromatin during adenovirus infections. FEBS Lett. 2019, 593, 3551–3570. [Google Scholar] [CrossRef] [PubMed]
- Avgousti, D.C.; Herrmann, C.; Kulej, K.; Pancholi, N.J.; Sekulic, N.; Petrescu, J.; Molden, R.C.; Blumenthal, D.; Paris, A.J.; Reyes, E.D.; et al. A core viral protein binds host nucleosomes to sequester immune danger signals. Nature 2016, 535, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Hogg, J.C. Adenovirus infections and lung disease. Curr. Opin. Pharmacol. 2007, 7, 237–243. [Google Scholar] [CrossRef]
- Chlamydas, S.; Papavassiliou, A.G.; Piperi, C. Epigenetic mechanisms regulating COVID-19 infection. Epigenetics 2021, 16, 263–270. [Google Scholar] [CrossRef]
- Skevaki, C.; Karsonova, A.; Karaulov, A.; Fomina, D.; Xie, M.; Chinthrajah, S.; Nadeau, K.C.; Renz, H. SARS-CoV-2 infection COVID-19 in asthmatics: A complex relationship. Nat. Rev. Immunol. 2021, 21, 202–203. [Google Scholar] [CrossRef]
- Konwar, C.; Asiimwe, R.; Inkster, A.M.; Merrill, S.M.; Negri, G.L.; Aristizabal, M.J.; Rider, C.F.; MacIsaac, J.L.; Carlsten, C.; Kobor, M.S. Risk-focused differences in molecular processes implicated in SARS-CoV-2 infection: Corollaries in DNA methylation and gene expression. Epigenet. Chromatin 2021, 14, 54. [Google Scholar] [CrossRef]
- Rathod, R.; Rathod, A.; Rahimabad, P.K.; Duan, J.; Zhang, H.; Arshad, S.H.; Karmaus, W. Methylation of Host Genes Associated with Coronavirus Infection from Birth to 26 Years. Genes 2021, 12, 1198. [Google Scholar] [CrossRef]
- Scorpo, M.L.; Ferrante, G.; La Grutta, S. An Overview of Asthma and COVID-19: Protective Factors Against SARS-COV-2 in Pediatric Patients. Front. Pediatr. 2021, 9, 661206. [Google Scholar] [CrossRef]
- Calmes, D.; Huynen, P.; Paulus, V.; Henket, M.; Guissard, F.; Moermans, C.; Louis, R.; Schleich, F. Chronic infection with Chlamydia pneumoniae in asthma: A type-2 low infection related phenotype. Respir. Res. 2021, 22, 72. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Jiang, T.; Wang, T.; Wang, D.; Tang, H.; Chu, Y.; Bi, J. Clinical value of monitoring cytokine levels for assessing the severity of mycoplasma pneumoniae pneumonia in children. Am. J. Transl. Res. 2024, 16, 3964–3977. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Principi, N. Asthma in children: Are chlamydia or mycoplasma involved? Paediatr. Drugs 2001, 3, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Douradinha, B. Should multidrug resistant Klebsiella pneumoniae strains displaying hypervirulent traits be reclassified as either ultravirulent or supervirulent? Microbiol. Res. 2023, 275, 127446. [Google Scholar] [CrossRef]
- Paczosa, M.K.; Mecsas, J. Klebsiella pneumoniae: Going on the Offense with a Strong Defense. Microbiol. Mol. Biol. Rev. 2016, 80, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Cuscino, N.; Fatima, A.; Di Pilato, V.; Bulati, M.; Alfano, C.; Monaca, E.; Di Mento, G.; Di Carlo, D.; Cardinale, F.; Monaco, F.; et al. Computational design and characterization of a multiepitope vaccine against carbapenemase-producing Klebsiella pneumoniae strains, derived from antigens identified through reverse vaccinology. Comput. Struct. Biotechnol. J. 2022, 20, 4446–4463. [Google Scholar] [CrossRef] [PubMed]
- Bulati, M.; Busà, R.; Carcione, C.; Iannolo, G.; Di Mento, G.; Cuscino, N.; Di Gesù, R.; Piccionello, A.P.; Buscemi, S.; Carreca, A.P.; et al. Klebsiella pneumoniae Lipopolysaccharides Serotype O2afg Induce Poor Inflammatory Immune Responses Ex Vivo. Microorganisms 2021, 9, 1317. [Google Scholar] [CrossRef]
- Douradinha, B. Exploring the journey: A comprehensive review of vaccine development against Klebsiella pneumoniae. Microbiol. Res. 2024, 287, 127837. [Google Scholar] [CrossRef]
- Grubwieser, P.; Hilbe, R.; Gehrer, C.M.; Grander, M.; Brigo, N.; Hoffmann, A.; Seifert, M.; Berger, S.; Theurl, I.; Nairz, M.; et al. Klebsiella pneumoniae manipulates human macrophages to acquire iron. Front. Microbiol. 2023, 14, 1223113. [Google Scholar] [CrossRef] [PubMed]
- Dulek, D.E.; Newcomb, D.C.; Goleniewska, K.; Cephus, J.; Zhou, W.; Reiss, S.; Toki, S.; Ye, F.; Zaynagetdinov, R.; Sherrill, T.P.; et al. Allergic airway inflammation decreases lung bacterial burden following acute Klebsiella pneumoniae infection in a neutrophil- and CCL8-dependent manner. Infect. Immun. 2014, 82, 3723–3739. [Google Scholar] [CrossRef]
- Russell, S.L.; Gold, M.J.; Willing, B.P.; Thorson, L.; McNagny, K.M.; Finlay, B.B. Perinatal antibiotic treatment affects murine microbiota, immune responses and allergic asthma. Gut Microbes 2013, 4, 158–164. [Google Scholar] [CrossRef]
- Atarashi, K.; Tanoue, T.; Shima, T.; Imaoka, A.; Kuwahara, T.; Momose, Y.; Cheng, G.; Yamasaki, S.; Saito, T.; Ohba, Y.; et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 2011, 331, 337–341. [Google Scholar] [CrossRef]
- Greenberger, P.A. Allergic bronchopulmonary aspergillosis. J. Allergy Clin. Immunol. 2002, 110, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; O’Driscoll, B.R.; Hogaboam, C.M.; Bowyer, P.; Niven, R.M. The link between fungi and severe asthma: A summary of the evidence. Eur. Respir. J. 2006, 27, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Niedzwiecki, M.; Zhu, H.; Corson, L.; Grunig, G.; Factor, P.H.; Chu, S.; Jiang, H.; Miller, R.L. Prenatal exposure to allergen, DNA methylation, and allergy in grandoffspring mice. Allergy 2012, 67, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, K.E.; Sitarik, A.R.; Havstad, S.; Lin, D.L.; Levan, S.; Fadrosh, D.; Panzer, A.R.; LaMere, B.; Rackaityte, E.; Lukacs, N.W.; et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat. Med. 2016, 22, 1187–1191. [Google Scholar] [CrossRef]
- Choo, S.; Lee, Y.Y.; Lee, E. Clinical significance of respiratory virus coinfection in children with Mycoplasma pneumoniae pneumonia. BMC Pulm. Med. 2022, 22, 212. [Google Scholar] [CrossRef]
- Kouni, S.; Karakitsos, P.; Chranioti, A.; Theodoridou, M.; Chrousos, G.; Michos, A. Evaluation of viral co-infections in hospitalized and non-hospitalized children with respiratory infections using microarrays. Clin. Microbiol. Infect. 2013, 19, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Busslinger, M.; Tarakhovsky, A. Epigenetic Control of Immunity. Cold Spring Harb. Perspect. Biol. 2014, 6, a019307. [Google Scholar] [CrossRef]
- Buchwald, A.G.; Tamboura, B.; Tennant, S.M.; Haidara, F.C.; Coulibaly, F.; Doumbia, M.; Diallo, F.; Keita, A.M.; Sow, S.O.; Kotloff, K.L.; et al. Epidemiology, Risk Factors, and Outcomes of Respiratory Syncytial Virus Infections in Newborns in Bamako, Mali. Clin. Infect. Dis. 2020, 70, 59–66. [Google Scholar] [CrossRef]
- Zhong, Q.; Feng, H.; Lu, Q.; Liu, X.; Zhao, Q.; Du, Y.; Zhang, X.-H.; Wang, J.-R. Recurrent wheezing in neonatal pneumonia is associated with combined infection with Respiratory Syncytial Virus and Staphylococcus aureus or Klebsiella pneumoniae. Sci. Rep. 2018, 8, 995. [Google Scholar] [CrossRef]
- Huang, Y.J.; Nariya, S.; Harris, J.M.; Lynch, S.V.; Choy, D.F.; Arron, J.R.; Boushey, H. The airway microbiome in patients with severe asthma: Associations with disease features and severity. J. Allergy Clin. Immunol. 2015, 136, 874–884. [Google Scholar] [CrossRef]
- Russell, C.D.; Unger, S.A.; Walton, M.; Schwarze, J. The Human Immune Response to Respiratory Syncytial Virus Infection. Clin. Microbiol. Rev. 2017, 30, 481–502. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Garvey, P.B.; Zhang, P.; Nelson, S.; Bagby, G.; Summer, W.R.; Schwarzenberger, P.; Shellito, J.E.; Kolls, J.K. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am. J. Respir. Cell Mol. Biol. 2001, 25, 335–340. [Google Scholar] [CrossRef]
- Roberts, S.; Salmon, S.L.; Steiner, D.J.; Williams, C.M.; Metzger, D.W.; Furuya, Y. Allergic Airway Disease Prevents Lethal Synergy of Influenza A Virus-Streptococcus pneumoniae Coinfection. mBio 2019, 10, e01335-19. [Google Scholar] [CrossRef] [PubMed]
- Halwani, R.; Al-Muhsen, S.; Al-Jahdali, H.; Hamid, Q. Role of transforming growth factor-β in airway remodeling in asthma. Am. J. Respir. Cell Mol. Biol. 2011, 44, 127–133. [Google Scholar] [CrossRef]
- LeMessurier, K.S.; Iverson, A.R.; Chang, T.-C.; Palipane, M.; Vogel, P.; Rosch, J.W.; Samarasinghe, A.E. Allergic inflammation alters the lung microbiome and hinders synergistic co-infection with H1N1 influenza virus and Streptococcus pneumoniae in C57BL/6 mice. Sci. Rep. 2019, 9, 19360. [Google Scholar] [CrossRef]
- Martínez-Roig, A.; Salvadó, M.; Caballero-Rabasco, M.A.; Sánchez-Buenavida, A.; López-Segura, N.; Bonet-Alcaina, M. Viral coinfection in childhood respiratory tract infections. Arch. Bronconeumol. 2015, 51, 5–9. [Google Scholar] [CrossRef]
- Antalis, E.; Oikonomopoulou, Z.; Kottaridi, C.; Kossyvakis, A.; Spathis, A.; Magkana, M.; Katsouli, A.; Tsagris, V.; Papaevangelou, V.; Mentis, A.; et al. Mixed viral infections of the respiratory tract; an epidemiological study during consecutive winter seasons. J. Med. Virol. 2018, 90, 663–670. [Google Scholar] [CrossRef]
- Soudani, N.; Caniza, M.A.; Assaf-Casals, A.; Shaker, R.; Lteif, M.; Su, Y.; Tang, L.; Akel, I.; Muwakkit, S.; Chmaisse, A.; et al. Prevalence and characteristics of acute respiratory virus infections in pediatric cancer patients. J. Med. Virol. 2019, 91, 1191–1201. [Google Scholar] [CrossRef]
- Jacobs, S.E.; Lamson, D.M.; George, K.S.; Walsh, T.J. Human rhinoviruses. Clin. Microbiol. Rev. 2013, 26, 135–162. [Google Scholar] [CrossRef]
- Amat, F.; Plantard, C.; Mulliez, A.; Petit, I.; Rochette, E.; Verdan, M.; Henquell, C.; Labbé, G.; Heraud, M.C.; Evrard, B.; et al. RSV-hRV co-infection is a risk factor for recurrent bronchial obstruction and early sensitization 3 years after bronchiolitis. J. Med. Virol. 2018, 90, 867–872. [Google Scholar] [CrossRef]
- Garcia-Garcia, M.L.; Calvo, C.; Ruiz, S.; Pozo, F.; Del Pozo, V.; Remedios, L.; Exposito, N.; Tellez, A.; Casas, I. Role of viral coinfections in asthma development. PLoS ONE 2017, 12, e0189083. [Google Scholar] [CrossRef] [PubMed]
- Míguez, A.; Iftimi, A.; Montes, F. Temporal association between the influenza virus and respiratory syncytial virus (RSV): RSV as a predictor of seasonal influenza. Epidemiol. Infect. 2016, 144, 2621–2632. [Google Scholar] [CrossRef] [PubMed]
- Appak, Ö.; Duman, M.; Belet, N.; Sayiner, A.A. Viral respiratory infections diagnosed by multiplex polymerase chain reaction in pediatric patients. J. Med. Virol. 2019, 91, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, J.; Zou, X.; Fan, Y.; Xiong, Z.; Li, B.; Wang, C.; Li, H.; Han, J.; Liu, X.; et al. CAP-China network, Severity of influenza virus and respiratory syncytial virus coinfections in hospitalized adult patients. J. Clin. Virol. 2020, 133, 104685. [Google Scholar] [CrossRef]
- Aberle, J.H.; Aberle, S.W.; Pracher, E.; Hutter, H.-P.; Kundi, M.; Popow-Kraupp, T. Single versus dual respiratory virus infections in hospitalized infants: Impact on clinical course of disease and interferon-gamma response. Pediatr. Infect. Dis. J. 2005, 24, 605–610. [Google Scholar] [CrossRef]
- Meskill, S.D.; Revell, P.A.; Chandramohan, L.; Cruz, A.T. Prevalence of co-infection between respiratory syncytial virus and influenza in children. Am. J. Emerg. Med. 2017, 35, 495–498. [Google Scholar] [CrossRef]
- Drori, Y.; Jacob-Hirsch, J.; Pando, R.; Glatman-Freedman, A.; Friedman, N.; Mendelson, E.; Mandelboim, M. Influenza A Virus Inhibits RSV Infection via a Two-Wave Expression of IFIT Proteins. Viruses 2020, 12, 1171. [Google Scholar] [CrossRef] [PubMed]
- van den Hoogen, B.G.; de Jong, J.C.; Groen, J.; Kuiken, T.; de Groot, R.; Fouchier, R.A.; Osterhaus, A.D. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat. Med. 2001, 7, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pillai, P.; Miyake, F.; Nair, H. The role of viral co-infections in the severity of acute respiratory infections among children infected with respiratory syncytial virus (RSV): A systematic review and meta-analysis. J. Glob. Health 2020, 10, 010426. [Google Scholar] [CrossRef]
- McNamara, P.S.; Flanagan, B.F.; Smyth, R.L.; Hart, C.A. Impact of human metapneumovirus and respiratory syncytial virus co-infection in severe bronchiolitis. Pediatr. Pulmonol. 2007, 42, 740–743. [Google Scholar] [CrossRef] [PubMed]
- Greensill, J.; McNamara, P.S.; Dove, W.; Flanagan, B.; Smyth, R.L.; Hart, C.A. Human metapneumovirus in severe respiratory syncytial virus bronchiolitis. Emerg. Infect. Dis. 2003, 9, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Geiser, J.; Boivin, G.; Huang, S.; Constant, S.; Kaiser, L.; Tapparel, C.; Essaidi-Laziosi, M. RSV and HMPV Infections in 3D Tissue Cultures: Mechanisms Involved in Virus-Host and Virus-Virus Interactions. Viruses 2021, 13, 139. [Google Scholar] [CrossRef]
- Magnaye, K.M.; Clay, S.M.; Nicodemus-Johnson, J.; Naughton, K.A.; Huffman, J.; Altman, M.C.; Jackson, D.J.; Gern, J.E.; Hogarth, D.K.; Naureckas, E.T.; et al. DNA methylation signatures in airway cells from adult children of asthmatic mothers reflect subtypes of severe asthma. Proc. Natl. Acad. Sci. USA 2022, 119, e2116467119. [Google Scholar] [CrossRef]
- Bae, D.J.; Jun, J.A.; Chang, H.S.; Park, J.S.; Park, C.S. Epigenetic Changes in Asthma: Role of DNA CpG Methylation. Tuberc. Respir. Dis. 2020, 83, 1–13. [Google Scholar] [CrossRef]
- Lin, P.-I.; Shu, H.; Mersha, T.B. Comparing DNA methylation profiles across different tissues associated with the diagnosis of pediatric asthma. Sci. Rep. 2020, 10, 151. [Google Scholar] [CrossRef]
- Sheikhpour, M.; Maleki, M.; Vargoorani, M.E.; Amiri, V. A review of epigenetic changes in asthma: Methylation and acetylation. Clin. Epigenet. 2021, 13, 65. [Google Scholar] [CrossRef]
- Miller, R.L.; Ho, S.-M. Environmental epigenetics and asthma: Current concepts and call for studies. Am. J. Respir. Crit. Care Med. 2008, 177, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Stefanowicz, D.; Hackett, T.-L.; Garmaroudi, F.S.; Günther, O.P.; Neumann, S.; Sutanto, E.N.; Ling, K.-M.; Kobor, M.S.; Kicic, A.; Stick, S.M.; et al. DNA methylation profiles of airway epithelial cells and PBMCs from healthy, atopic and asthmatic children. PLoS ONE 2012, 7, e44213. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Jiang, Y.; Yang, I.V.; Forno, E.; Wang, T.; Vonk, J.M.; Gehring, U.; Smit, H.A.; Milanzi, E.B.; Carpaij, O.A.; et al. Nasal DNA methylation profiling of asthma and rhinitis. J. Allergy Clin. Immunol. 2020, 145, 1655–1663. [Google Scholar] [CrossRef]
- Rastogi, D.; Suzuki, M.; Greally, J.M. Differential epigenome-wide DNA methylation patterns in childhood obesity-associated asthma. Sci. Rep. 2013, 3, 2164. [Google Scholar] [CrossRef]
- Chen, L.; Collado, K.; Rastogi, D. Contribution of systemic and airway immune responses to pediatric obesity-related asthma. Paediatr. Respir. Rev. 2021, 37, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Potaczek, D.P.; Harb, H.; Michel, S.; Alhamwe, B.A.; Renz, H.; Tost, J. Epigenetics and allergy: From basic mechanisms to clinical applications. Epigenomics 2017, 9, 539–571. [Google Scholar] [CrossRef] [PubMed]
- Alhamwe, B.A.; Alhamdan, F.; Ruhl, A.; Potaczek, D.P.; Renz, H. The role of epigenetics in allergy and asthma development. Curr. Opin. Allergy Clin. Immunol. 2020, 20, 48–55. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Zhang, L.-Y.; Wen, R.; Yang, N.; Zhang, T.-N. Histone deacetylases and their inhibitors in inflammatory diseases. Biomed. Pharmacother. 2024, 179, 117295. [Google Scholar] [CrossRef]
- Zwinderman, M.R.H.; de Weerd, S.; Dekker, F.J. Targeting HDAC Complexes in Asthma and COPD. Epigenomes 2019, 3, 19. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H. The emerging role of histone deacetylase 1 in allergic diseases. Front. Immunol. 2022, 13, 1027403. [Google Scholar] [CrossRef]
- Barnes, P.J. Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2013, 131, 636–645. [Google Scholar] [CrossRef]
- Koh, K.D.; Bonser, L.R.; Eckalbar, W.L.; Yizhar-Barnea, O.; Shen, J.; Zeng, X.; Hargett, K.L.; Sun, D.I.; Zlock, L.T.; Finkbeiner, W.E.; et al. Genomic characterization and therapeutic utilization of IL-13-responsive sequences in asthma. Cell Genom. 2023, 3, 100229. [Google Scholar] [CrossRef]
- Wasti, B.; Liu, S.-K.; Xiang, X.-D. Role of Epigenetics in the Pathogenesis, Treatment, Prediction, and Cellular Transformation of Asthma. Mediat. Inflamm. 2021, 2021, 9412929. [Google Scholar] [CrossRef]
- Xiao, C.; Fan, T.; Zheng, Y.; Tian, H.; Deng, Z.; Liu, J.; Li, C.; He, J. H3K4 trimethylation regulates cancer immunity: A promising therapeutic target in combination with immunotherapy. J. Immunother. Cancer 2023, 11, e005693. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, Q.; Liu, Z.; Luo, D.; Li, L.; Zhong, Y. Low expression and hypermethylation of FOXP3 in regulatory T cells are associated with asthma in children. Exp. Ther. Med. 2020, 19, 2045–2052. [Google Scholar] [CrossRef]
- Harb, H.; Raedler, D.; Ballenberger, N.; Böck, A.; Kesper, D.A.; Renz, H.; Schaub, B. Childhood allergic asthma is associated with increased IL-13 and FOXP3 histone acetylation. J. Allergy Clin. Immunol. 2015, 136, 200–202. [Google Scholar] [CrossRef]
- Yu, F.; Sun, Y.; Yu, J.; Ding, Z.; Wang, J.; Zhang, L.; Zhang, T.; Bai, Y.; Wang, Y. ORMDL3 is associated with airway remodeling in asthma via the ERK/MMP-9 pathway. Mol. Med. Rep. 2017, 15, 2969–2976. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Zhang, H.; Hu, L.; Liu, J.; Wang, L.; Wang, T.; Zhang, H.; Cong, L.; Wang, Q. Pathogenesis of allergic diseases and implications for therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 138. [Google Scholar] [CrossRef]
- Gil-Martínez, M.; Lorente-Sorolla, C.; Naharro, S.; Rodrigo-Muñoz, J.M.; Del Pozo, V. Advances and Highlights of miRNAs in Asthma: Biomarkers for Diagnosis and Treatment. Int. J. Mol. Sci. 2023, 24, 1628. [Google Scholar] [CrossRef] [PubMed]
- Albano, G.D.; Gagliardo, R.; Montalbano, A.M.; Profita, M. Non-Coding RNAs in Airway Diseases: A Brief Overview of Recent Data. Cancers 2022, 15, 54. [Google Scholar] [CrossRef]
- Klein, M.; Gagnon, P.-A.; Salem, M.; Rouabhia, M.; Chakir, J. MicroRNA-155-5p Differentially Regulates IL-13Rα1 and IL-13Rα2 Expression and Signaling Driving Abnormal Lung Epithelial Cell Phenotype in Severe Asthma. Am. J. Respir. Cell Mol. Biol. 2024, 71, 603–616. [Google Scholar] [CrossRef]
- Zhou, H.; Li, J.; Gao, P.; Wang, Q.; Zhang, J. miR-155: A Novel Target in Allergic Asthma. Int. J. Mol. Sci. 2016, 17, 1773. [Google Scholar] [CrossRef]
- Svitich, O.A.; Sobolev, V.V.; Gankovskaya, L.V.; Zhigalkina, P.V.; Zverev, V.V. The role of regulatory RNAs (miRNAs) in asthma. Allergol. Immunopathol. 2018, 46, 201–205. [Google Scholar] [CrossRef]
- Gutierrez, M.J.; Gomez, J.L.; Perez, G.F.; Pancham, K.; Val, S.; Pillai, D.K.; Giri, M.; Ferrante, S.; Freishtat, R.; Rose, M.C.; et al. Airway Secretory microRNAome Changes during Rhinovirus Infection in Early Childhood. PLoS ONE 2016, 11, e0162244. [Google Scholar] [CrossRef]
- ElKashef, S.M.M.A.E.; Ahmad, S.E.-A.; Soliman, Y.M.A.; Mostafa, M.S. Role of microRNA-21 and microRNA-155 as biomarkers for bronchial asthma. Innate Immun. 2021, 27, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, K.; Shi, H.; Xu, J.; Zhang, D.; Wu, Y.; Zhou, S.; Sun, X. MiR-21 modulates human airway smooth muscle cell proliferation and migration in asthma through regulation of PTEN expression. Exp. Lung Res. 2015, 41, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Wang, X.; Sun, Q.; Papakonstantinou, E.; S’ng, C.; Tamm, M.; Stolz, D.; Roth, M. IgE Downregulates PTEN through MicroRNA-21-5p and Stimulates Airway Smooth Muscle Cell Remodeling. Int. J. Mol. Sci. 2019, 20, 875. [Google Scholar] [CrossRef]
- Xu, X.; Hong, P.; Wang, Z.; Tang, Z.; Li, K. MicroRNAs in Transforming Growth Factor-Beta Signaling Pathway Associated With Fibrosis Involving Different Systems of the Human Body. Front. Mol. Biosci. 2021, 8, 707461. [Google Scholar] [CrossRef]
- Wadhwa, R.; Dua, K.; Adcock, I.M.; Horvat, J.C.; Kim, R.Y.; Hansbro, P.M. Cellular mechanisms underlying steroid-resistant asthma. Eur. Respir. Rev. 2019, 28, 190096. [Google Scholar] [CrossRef]
- Yan, F.; Wufuer, D.; Ding, J.; Wang, J. MicroRNA miR-146a-5p inhibits the inflammatory response and injury of airway epithelial cells via targeting TNF receptor-associated factor 6. Bioengineered 2021, 12, 1916–1926. [Google Scholar] [CrossRef]
- Gilyazova, I.; Asadullina, D.; Kagirova, E.; Sikka, R.; Mustafin, A.; Ivanova, E.; Bakhtiyarova, K.; Gilyazova, G.; Gupta, S.; Khusnutdinova, E.; et al. MiRNA-146a-A Key Player in Immunity and Diseases. Int. J. Mol. Sci. 2023, 24, 12767. [Google Scholar] [CrossRef]
- Cañas, J.A.; Rodrigo-Muñoz, J.M.; Sastre, B.; Gil-Martinez, M.; Redondo, N.; Del Pozo, V. MicroRNAs as Potential Regulators of Immune Response Networks in Asthma and Chronic Obstructive Pulmonary Disease. Front. Immunol. 2020, 11, 608666. [Google Scholar] [CrossRef]
- Sharma, R.; Tiwari, A.; McGeachie, M.J. Recent miRNA Research in Asthma. Curr. Allergy Asthma Rep. 2022, 22, 231–258. [Google Scholar] [CrossRef]
- Rodrigo-Muñoz, J.M.; Cañas, J.A.; Sastre, B.; Rego, N.; Greif, G.; Rial, M.; Mínguez, P.; Mahíllo-Fernández, I.; Fernández-Nieto, M.; Mora, I.; et al. Asthma diagnosis using integrated analysis of eosinophil microRNAs. Allergy 2019, 74, 507–517. [Google Scholar] [CrossRef]
- Alhamwe, B.A.; Potaczek, D.P.; Miethe, S.; Alhamdan, F.; Hintz, L.; Magomedov, A.; Garn, H. Extracellular Vesicles and Asthma—More Than Just a Co-Existence. Int. J. Mol. Sci. 2021, 22, 4984. [Google Scholar] [CrossRef] [PubMed]
- Kothalawala, D.M.; Kadalayil, L.; Curtin, J.A.; Murray, C.S.; Simpson, A.; Custovic, A.; Tapper, W.J.; Arshad, S.H.; Rezwan, F.I.; Holloway, J.W. null On Behalf Of Stelar/Unicorn Investigators, Integration of Genomic Risk Scores to Improve the Prediction of Childhood Asthma Diagnosis. J. Pers. Med. 2022, 12, 75. [Google Scholar] [CrossRef] [PubMed]
- Ntontsi, P.; Photiades, A.; Zervas, E.; Xanthou, G.; Samitas, K. Genetics and Epigenetics in Asthma. Int. J. Mol. Sci. 2021, 22, 2412. [Google Scholar] [CrossRef]
- Gonzalez-Uribe, V.; Romero-Tapia, S.J.; Castro-Rodriguez, J.A. Asthma Phenotypes in the Era of Personalized Medicine. J. Clin. Med. 2023, 12, 6207. [Google Scholar] [CrossRef]
- Uddin, M.G.; Fandy, T.E. DNA methylation inhibitors: Retrospective and perspective view. Adv. Cancer Res. 2021, 152, 205–223. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-J.; Yang, C.-Y.; Chen, Y.-H.; Chen, C.-M.; Chen, L.-C.; Kuo, M.-L. The DNA methylation inhibitor 5-azacytidine increases regulatory T cells and alleviates airway inflammation in ovalbumin-sensitized mice. Int. Arch. Allergy Immunol. 2013, 160, 356–364. [Google Scholar] [CrossRef]
- Kuehl, P.J.; Tellez, C.S.; Grimes, M.J.; March, T.H.; Tessema, M.; Revelli, D.A.; Mallis, L.M.; Dye, W.W.; Sniegowski, T.; Badenoch, A.; et al. 5-Azacytidine inhaled dry powder formulation profoundly improves pharmacokinetics and efficacy for lung cancer therapy through genome reprogramming. Br. J. Cancer 2020, 122, 1194–1204. [Google Scholar] [CrossRef] [PubMed]
- Zwinderman, M.R.H.; Cao, F.; Dekker, F.J. Acetylation and Methylation in Asthma, COPD, and Lung Cancer. In Chemical Epigenetics; Mai, A., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 429–453. [Google Scholar] [CrossRef]
- Comer, B.S.; Ba, M.; Singer, C.A.; Gerthoffer, W.T. Epigenetic targets for novel therapies of lung diseases. Pharmacol. Ther. 2015, 147, 91–110. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Tsai, Y.-H.; Wang, C.-C.; Liu, S.-F.; Chen, T.-W.; Fang, W.-F.; Lee, C.-P.; Hsu, P.-Y.; Chao, T.-Y.; Wu, C.-C.; et al. Taiwan Clinical Trial Consortium of Respiratory Disease (TCORE) group, Epigenome-wide association study on asthma and chronic obstructive pulmonary disease overlap reveals aberrant DNA methylations related to clinical phenotypes. Sci. Rep. 2021, 11, 5022. [Google Scholar] [CrossRef]
- Brightling, C.E.; Gupta, S.; Gonem, S.; Siddiqui, S. Lung damage and airway remodelling in severe asthma. Clin. Exp. Allergy 2012, 42, 638–649. [Google Scholar] [CrossRef] [PubMed]
- Ruzic, D.; Djoković, N.; Srdić-Rajić, T.; Echeverria, C.; Nikolic, K.; Santibanez, J.F. Targeting Histone Deacetylases: Opportunities for Cancer Treatment and Chemoprevention. Pharmaceutics 2022, 14, 209. [Google Scholar] [CrossRef]
- Weidner, J.; Bartel, S.; Kılıç, A.; Zissler, U.M.; Renz, H.; Schwarze, J.; Schmidt-Weber, C.B.; Maes, T.; Rebane, A.; Krauss-Etschmann, S.; et al. Spotlight on microRNAs in allergy and asthma. Allergy 2021, 76, 1661–1678. [Google Scholar] [CrossRef]
- Gagliardo, R.; Ferrante, G.; Fasola, S.; Di Vincenzo, S.; Pace, E.; La Grutta, S. Resolvin D1 and miR-146a are independent distinctive parameters in children with moderate and severe asthma. Clin. Exp. Allergy 2021, 51, 350–353. [Google Scholar] [CrossRef]
- Segal, M.; Slack, F.J. Challenges identifying efficacious miRNA therapeutics for cancer. Expert Opin. Drug Discov. 2020, 15, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Ding, Z.; Liu, Z.; Shi, Y.; Zhang, Q. miR-21-5p Modulates Airway Inflammation and Epithelial-Mesenchymal Transition Processes in a Mouse Model of Combined Allergic Rhinitis and Asthma Syndrome. Int. Arch. Allergy Immunol. 2024, 185, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Weidner, J.; Malmhäll, C.; Rådinger, M. microRNAs in Asthma Pathogenesis—From Mouse to Man; JTGG: Vienna, Austria, 2019. [Google Scholar] [CrossRef]
- Hernández-Díazcouder, A.; Romero-Nava, R.; Del-Río-Navarro, B.E.; Sánchez-Muñoz, F.; Guzmán-Martín, C.A.; Reyes-Noriega, N.; Rodríguez-Cortés, O.; Leija-Martínez, J.J.; Vélez-Reséndiz, J.M.; Villafaña, S.; et al. The Roles of MicroRNAs in Asthma and Emerging Insights into the Effects of Vitamin D3 Supplementation. Nutrients 2024, 16, 341. [Google Scholar] [CrossRef]
- Duan, W.; Huang, J.; Wasti, B.; Chen, Z.; Yuan, Y.; He, Y.; Li, D.; Jia, J.; Liu, S.; Liu, Y.; et al. miR-146a-3p as a potential novel therapeutic by targeting MBD2 to mediate Th17 differentiation in Th17 predominant neutrophilic severe asthma. Clin. Exp. Med. 2023, 23, 2839–2854. [Google Scholar] [CrossRef]
- Calazans, A.P.C.T.; Milani, T.M.S.; Prata, A.S.; Clerici, M.T.P.S.; Nicoli, J.R.; Martins, F.S.; Borges, M.C. A Functional Bread Fermented with Saccharomyces cerevisiae UFMG A-905 Prevents Allergic Asthma in Mice. Curr. Dev. Nutr. 2024, 8, 102142. [Google Scholar] [CrossRef]
- Chen, P.-C.; Hsu, H.-Y.; Liao, Y.-C.; Lee, C.-C.; Hsieh, M.-H.; Kuo, W.-S.; Wu, L.S.-H.; Wang, J.-Y. Oral administration of Lactobacillus delbrueckii subsp. lactis LDL557 attenuates airway inflammation and changes the gut microbiota in a Der p-sensitized mouse model of allergic asthma. Asian Pac. J. Allergy Immunol. 2024, 46, 8969–8980. [Google Scholar] [CrossRef]
- Wang, H.; He, Y.; Dang, D.; Feng, L.; Huang, L.; Zhao, J.; Lu, S.; Lu, W. Bifidobacterium animalis subsp. lactis CCFM1274 relieved allergic asthma symptoms by modifying intestinal tryptophan metabolism in mice. Food Funct. 2024, 15, 8810–8822. [Google Scholar] [CrossRef]
- Dotterud, C.K.; Storrø, O.; Johnsen, R.; Oien, T. Probiotics in pregnant women to prevent allergic disease: A randomized, double-blind trial. Br. J. Dermatol. 2010, 163, 616–623. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Ge, T.; Xiao, Y.; Liao, Y.; Cui, Y.; Zhang, Y.; Ho, W.; Yu, G.; Zhang, T. Probiotics for prevention and treatment of respiratory tract infections in children: A systematic review and meta-analysis of randomized controlled trials. Medicine 2016, 95, e4509. [Google Scholar] [CrossRef]
- West, C.E.; Renz, H.; Jenmalm, M.C.; Kozyrskyj, A.L.; Allen, K.J.; Vuillermin, P.; Prescott, S.L. in-FLAME Microbiome Interest Group, The gut microbiota and inflammatory noncommunicable diseases: Associations and potentials for gut microbiota therapies. J. Allergy Clin. Immunol. 2015, 135, 3–13; quiz 14. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, A.; Huoman, J.; Söderholm, S.; Mehta, R.B.; Nilsson, L.; Abrahamsson, T.R.; Ernerudh, J.; Gustafsson, M.; Jenmalm, M.C. Pre- and postnatal Lactobacillus reuteri treatment alters DNA methylation of infant T helper cells. Pediatr. Allergy Immunol. 2020, 31, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Vähämiko, S.; Laiho, A.; Lund, R.; Isolauri, E.; Salminen, S.; Laitinen, K. The impact of probiotic supplementation during pregnancy on DNA methylation of obesity-related genes in mothers and their children. Eur. J. Nutr. 2019, 58, 367–377. [Google Scholar] [CrossRef]
- Valverde-Molina, J.; García-Marcos, L. Microbiome and Asthma: Microbial Dysbiosis and the Origins, Phenotypes, Persistence, and Severity of Asthma. Nutrients 2023, 15, 486. [Google Scholar] [CrossRef]
- Potaczek, D.P.; Alhamwe, B.A.; Miethe, S.; Garn, H. Epigenetic Mechanisms in Allergy Development and Prevention. Handb. Exp. Pharmacol. 2022, 268, 331–357. [Google Scholar] [CrossRef] [PubMed]
- Ruokolainen, L.; Paalanen, L.; Karkman, A.; Laatikainen, T.; von Hertzen, L.; Vlasoff, T.; Markelova, O.; Masyuk, V.; Auvinen, P.; Paulin, L.; et al. Significant disparities in allergy prevalence and microbiota between the young people in Finnish and Russian Karelia. Clin. Exp. Allergy 2017, 47, 665–674. [Google Scholar] [CrossRef]
- Conrad, M.L.; Ferstl, R.; Teich, R.; Brand, S.; Blümer, N.; Yildirim, A.O.; Patrascan, C.C.; Hanuszkiewicz, A.; Akira, S.; Wagner, H.; et al. Maternal TLR signaling is required for prenatal asthma protection by the nonpathogenic microbe Acinetobacter lwoffii F78. J. Exp. Med. 2009, 206, 2869–2877. [Google Scholar] [CrossRef]
- Brand, S.; Teich, R.; Dicke, T.; Harb, H.; Yildirim, A.Ö.; Tost, J.; Schneider-Stock, R.; Waterland, R.A.; Bauer, U.-M.; von Mutius, E.; et al. Epigenetic regulation in murine offspring as a novel mechanism for transmaternal asthma protection induced by microbes. J. Allergy Clin. Immunol. 2011, 128, 618–625.e1–7. [Google Scholar] [CrossRef]
- Alhamwe, B.A.; Gao, Z.; Alhamdan, F.; Harb, H.; Pichene, M.; Garnier, A.; El Andari, J.; Kaufmann, A.; Graumann, P.L.; Kesper, D.; et al. Intranasal administration of Acinetobacter lwoffii in a murine model of asthma induces IL-6-mediated protection associated with cecal microbiota changes. Allergy 2023, 78, 1245–1257. [Google Scholar] [CrossRef]
- Palma, M.L.; Zamith-Miranda, D.; Martins, F.S.; Bozza, F.A.; Nimrichter, L.; Montero-Lomeli, M.; Marques, E.T.A.; Douradinha, B. Probiotic Saccharomyces cerevisiae strains as biotherapeutic tools: Is there room for improvement? Appl. Microbiol. Biotechnol. 2015, 99, 6563–6570. [Google Scholar] [CrossRef]
- Palma, M.L.; Garcia-Bates, T.M.; Martins, F.S.; Douradinha, B. Genetically engineered probiotic Saccharomyces cerevisiae strains mature human dendritic cells and stimulate Gag-specific memory CD8+ T cells ex vivo. Appl. Microbiol. Biotechnol. 2019, 103, 5183–5192. [Google Scholar] [CrossRef] [PubMed]
- Batista, T.M.; Marques, E.T.A.; Franco, G.R.; Douradinha, B. Draft Genome Sequence of the Probiotic Yeast Saccharomyces cerevisiae var. boulardii Strain ATCC MYA-796. Genome Announc. 2014, 2, e01345-14. [Google Scholar] [CrossRef] [PubMed]
- Hussen, B.M.; Najmadden, Z.B.; Abdullah, S.R.; Rasul, M.F.; Mustafa, S.A.; Ghafouri-Fard, S.; Taheri, M. CRISPR/Cas9 gene editing: A novel strategy for fighting drug resistance in respiratory disorders. Cell Commun. Signal. 2024, 22, 329. [Google Scholar] [CrossRef] [PubMed]
- Solazzo, G.; Ferrante, G.; La Grutta, S. DNA Methylation in Nasal Epithelium: Strengths and Limitations of an Emergent Biomarker for Childhood Asthma. Front. Pediatr. 2020, 8, 256. [Google Scholar] [CrossRef] [PubMed]


| FEATURE | T2-HIGH ASTHMA | T2-LOW ASTHMA |
|---|---|---|
| DOMINANT IMMUNE CELLS | Th2, ILC2, eosinophils, mast cells, B cells | Th1, Th17, neutrophils, antigen-presenting cells (APCs) |
| KEY CYTOKINES | IL-4, IL-5, IL-13, IL-9 | IFN-γ, IL-17, TNF-α |
| ALARMINS INVOLVED | TSLP, IL-33, IL-25 (epithelial-derived) | Minimal role |
| INFLAMMATORY PATTERN | Eosinophilia, elevated IgE and FeNO | Neutrophilia, normal/low FeNO |
| RESPONSE TO CORTICOSTEROIDS | Responsive | Poor responsive or absent |
| STRUCTURAL ALTERATIONS | Airway remodeling, epithelial hyperplasia, mucus overproduction | Persistent epithelial damage, remodeling, impaired repair |
| Virus | Epigenetic Modification | Genes Affected | Function/Effect |
|---|---|---|---|
| RSV | DNA methylation, histone modification, miRNA | DDX58, KDM5B, HOX, miR-21 | ↑ DDX58 → antiviral defense; ↓ KDM5B → shift to Th1; HOX activation → lung remodeling; miR-21 ↑ inflammation/fibrosis |
| Rhinovirus | DNA methylation, miRNA | ORMDL3, GSDMB, SMAD3, DDO, METTL24, miR-155 | Hypomethylation → ↑ ORMDL3/GSDMB → inflammation; ↑ miR-155 → ↑ Th1/Th2; ↑ asthma severity |
| Influenza | Histone modification, ISG activation (indirect) | IFIT1–3, IFI44 (indirect); general chromatin structure | ↑ cytokines → ↑ mucus/bronchial reactivity; ISG expression may inhibit RSV replication |
| Adenovirus | Histone acetylation, chromatin remodeling | Histone H3 (K9, K18), chromatin targets (via E1A, protein VII) | H3K9/K18 acetylation → transcriptional reprogramming; ↑ proinflammatory gene expression |
| SARS-CoV-2 | DNA hypomethylation, histone modification | ACE2, H3K4me1, H3K4me3, H3K27Ac marks | Hypomethylation → ↑ ACE2; histone marks → ↑ ACE2 transcription → ↑ viral entry/inflammation |
| hMPV | Not specified | - | ↑ mucus, bronchial reactivity, hospitalization risk |
| Parainfluenza | Not specified | - | ↑ airway inflammation and obstruction |
| Modification Type | Endotype | Involved Gene(s) | Etiological Agent | Implications |
|---|---|---|---|---|
| DNA methylation | T2-high | ORMDL3, GSDMB | Rhinovirus (implicated) | ↑ Inflammation via CD8+ T cells; ↑ Expression linked to asthma susceptibility (17q21 locus) |
| Anti-inflammatory genes (unspecified) | - | Hypermethylation leads to gene silencing and poor disease control | ||
| Th2-associated CpG islands (nasal, buccal) | - | Methylation markers in nasal epithelium and buccal cells linked to Th2 activation | ||
| IL6R, IL5, CD38, STAT3 | Probiotics (L. rhamnosus GG, B. lactis) (implicated) | Global hypomethylation in immune genes after prenatal/postnatal supplementation | ||
| GATA3, IL4 | - | Hypo/hypermethylation regulates Th2 immune response | ||
| SMAD3, DDO, METTL24 | Rhinovirus (implicated) | Asthma susceptibility, early wheezing | ||
| - | Rhodotorula species (implicated) | Associated with higher risk of atopy and asthma in children | ||
| - | Candida species (implicated) | Early gut colonization linked to increased atopy and asthma risk | ||
| ACE2 | SARS-CoV-2 (implicated) | Hypomethylation → ↑ ACE2 expression → ↑ viral entry and inflammation | ||
| T2-low | IL17A, IFNG | - | Epigenetic regulation of Th1 and Th17 response in neutrophilic asthma | |
| CXCL8, CXCL1 | - | Genes involved in neutrophil recruitment and steroid resistance | ||
| IFN-γ, IL-4 promoters | Aspergillus (prenatal) (implicated) | CpG methylation linked to protective effect in offspring | ||
| Histone modification | T2-high | H3K4, CCR4, CCL5 | - | Trimethylation and dimethylation promote Th2 inflammation |
| Foxp3, IL13 | - | Acetylation → immune regulation, ↑ cytokine expression | ||
| H3K4me3, H3K9ac | - | Linked to expression of IL-4 and IFN-γ | ||
| H3K4me1, H3K4me3, H3K27Ac | SARS-CoV-2 (implicated) | Active chromatin marks → ↑ ACE2 transcription | ||
| Histone H3 (K9, K18) | Adenoviruses (implicated) | E1A protein alters H3 acetylation → transcriptional reprogramming ↑ inflammation | ||
| HDAC2 | - | Loss linked to steroid resistance | ||
| IL10 | Acinetobacter lwoffii (implicated) | IL-6-dependent epigenetic activation reduces asthma susceptibility | ||
| IFNG promoter (H4 acetylation) | Acinetobacter lwoffii (implicated) | Stabilized acetylation via TLR signaling in mouse offspring | ||
| Chromatin (via protein VII) | Adenoviruses (implicated) | Alters chromatin structure, enhances proinflammatory state | ||
| ORMDL3 | - | Hyperacetylation → airway remodeling | ||
| KDM5B (H3K4 demethylation) | RSV (implicated) | ↓ KDM5B → ↑ Th1, ↓ Th2 → improved balance | ||
| HOX genes | RSV (implicated) | NS1 interaction with H2BD → HOX activation → lung remodeling | ||
| T2-low | STAT3 | - | Altered histone signaling via STAT3 in neutrophilic inflammation | |
| miRNA | T2-high | miR-21 | RSV (implicated) | ↓ PTEN, ↑ PI3K/Akt → inflammation, fibrosis, steroid resistance; increased in asthma; promotes inflammation via PTEN and TGF-β pathways |
| miR-126 | - | Suppresses proinflammatory genes; reduced in asthma | ||
| miR-146a | - | ↓ NF-κB (TRAF6, IRAK1) → ↓ inflammation, ↑ Treg phenotype | ||
| miR-155 | Rhinovirus (implicated) | ↑ Th1/Th2 responses → ↑ inflammation, asthma severity |
| Full Name | Role | Therapeutic Target | References |
|---|---|---|---|
| DNA Methyltransferases (DNMTs) | Enzymes responsible for adding methyl groups to DNA, contributing to hypermethylation and gene silencing. | Inhibition by DNMT inhibitors (DMNTi) like 5-azacytidine to reverse silencing of anti-inflammatory genes. | [12,15,24,28,37,38,41,83,131,135,136,141,148,153,155,163,174,175,176,179,198,206] |
| Histone Deacetylases (HDACs) | Enzymes that remove acetyl groups from histones, leading to tighter chromatin and gene silencing. | Inhibition by HDAC inhibitors like vorinostat (SAHA) to promote gene expression and reduce inflammation. | [38,51,53,63,83,135,137,141,142,143,144,145,146,147,148,149,151,152,153,154,164,201,202] |
| MicroRNA-21 (miR-21) | miRNA upregulated in asthma, associated with inflammation and exacerbations, particularly after viral infections. | miR-21 inhibitors to reduce inflammation and improve lung function. | [12,13,16,27,28,37,38,49,50,63,64,106,136,141,142,143,148,154,158,160,161,164,206,207] |
| MicroRNA-146a (miR-146a) | Regulates Toll-like receptor (TLR) signaling pathways and inflammatory cytokine production. | Inhibitors to modulate immune responses, particularly in T2-high asthma. | [64,158,165,166,167,168,172,174,182,183,198] |
| Probiotic strains (e.g., Lactobacillus, Bifidobacterium, and Saccharomyces species) | Probiotic strains that modulate DNA methylation and histone modifications to balance proinflammatory and anti-inflammatory immune responses. | Normalizing hypermethylation of anti-inflammatory genes and promoting histone acetylation for immune tolerance. | [95,153,189,190,191,192,193,194,195,196,197,198,199,203,204,205] |
| CRISPR-Cas9 | Gene-editing technology that targets DNA methylation sites or miRNA binding regions contributing to asthma. | Editing specific genetic and epigenetic alterations in asthma-related endotypes. | [52,68,147,206] |
| T2-high asthma (Epigenetic Endotype) | DNA methylation of anti-inflammatory genes; miR-21 and miR-146a dysregulation. | DNMT inhibitors, miRNA inhibitors, and immune-modulating probiotics. | [2,18,20,22,27,37,40,41,43,84,87,109,112,113,114,118,119,120,132,133,134,145,147,148,150,151,153,154,155,156,157,158,172,173,182] |
| T2-low asthma (Epigenetic Endotype) | HDAC-mediated histone modifications. | HDAC inhibitors to alleviate corticosteroid-resistant inflammation. | [2,11,18,20,27,37,39,40,41,42,43,50,84,87,88,109,118,146,148,153,154,155,158,173,182] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandolfo, A.; Gagliardo, R.P.; Lazzara, V.; Perri, A.; Malizia, V.; Ferrante, G.; Licari, A.; La Grutta, S.; Albano, G.D. Unmasking Pediatric Asthma: Epigenetic Fingerprints and Markers of Respiratory Infections. Int. J. Mol. Sci. 2025, 26, 7629. https://doi.org/10.3390/ijms26157629
Pandolfo A, Gagliardo RP, Lazzara V, Perri A, Malizia V, Ferrante G, Licari A, La Grutta S, Albano GD. Unmasking Pediatric Asthma: Epigenetic Fingerprints and Markers of Respiratory Infections. International Journal of Molecular Sciences. 2025; 26(15):7629. https://doi.org/10.3390/ijms26157629
Chicago/Turabian StylePandolfo, Alessandra, Rosalia Paola Gagliardo, Valentina Lazzara, Andrea Perri, Velia Malizia, Giuliana Ferrante, Amelia Licari, Stefania La Grutta, and Giusy Daniela Albano. 2025. "Unmasking Pediatric Asthma: Epigenetic Fingerprints and Markers of Respiratory Infections" International Journal of Molecular Sciences 26, no. 15: 7629. https://doi.org/10.3390/ijms26157629
APA StylePandolfo, A., Gagliardo, R. P., Lazzara, V., Perri, A., Malizia, V., Ferrante, G., Licari, A., La Grutta, S., & Albano, G. D. (2025). Unmasking Pediatric Asthma: Epigenetic Fingerprints and Markers of Respiratory Infections. International Journal of Molecular Sciences, 26(15), 7629. https://doi.org/10.3390/ijms26157629







