A Retrospective Study of Clinical and Genetic Features in a Long-Term Cohort of Mexican Children with Alagille Syndrome
Abstract
1. Introduction
2. Results
2.1. Study Population
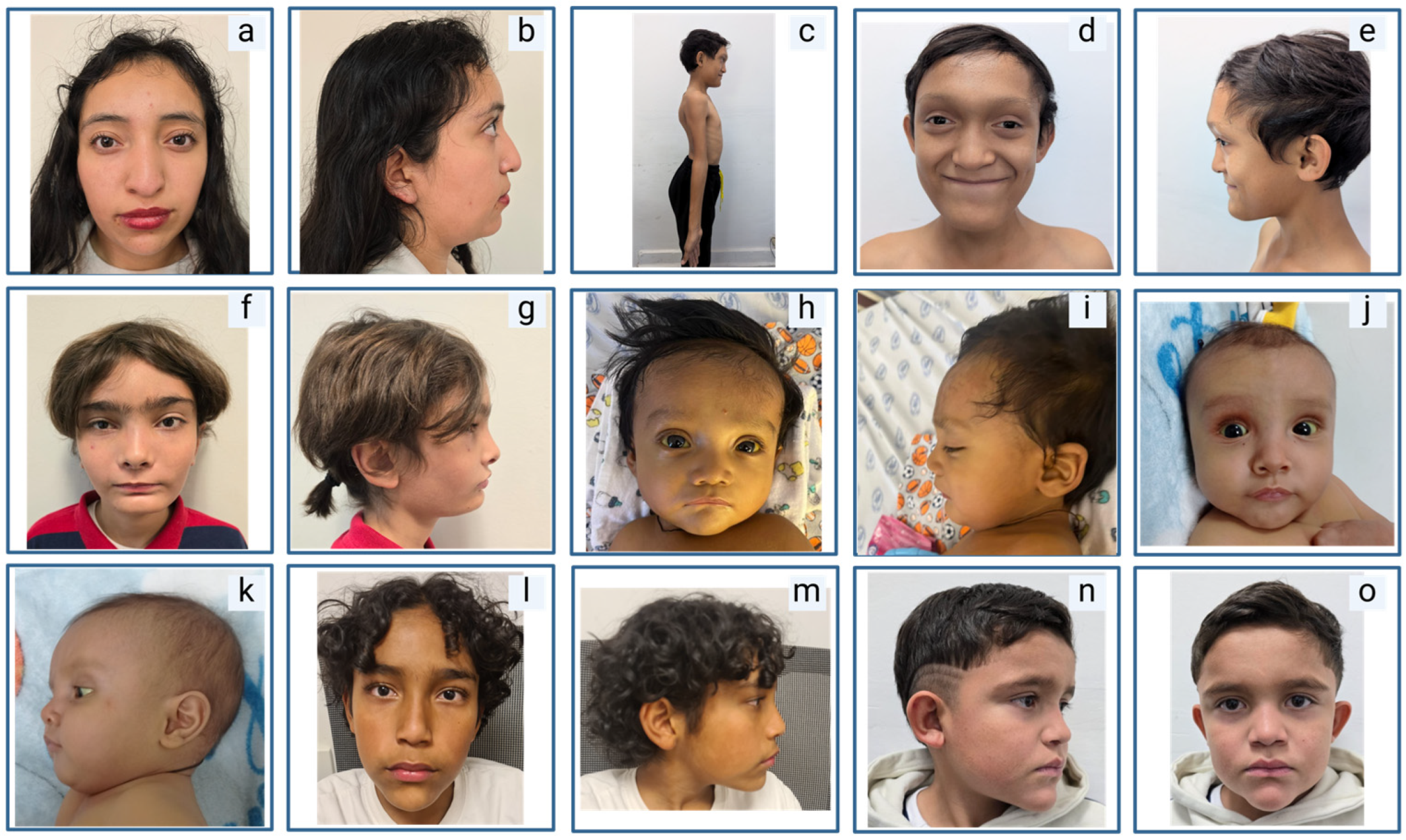
2.2. Clinical Manifestations
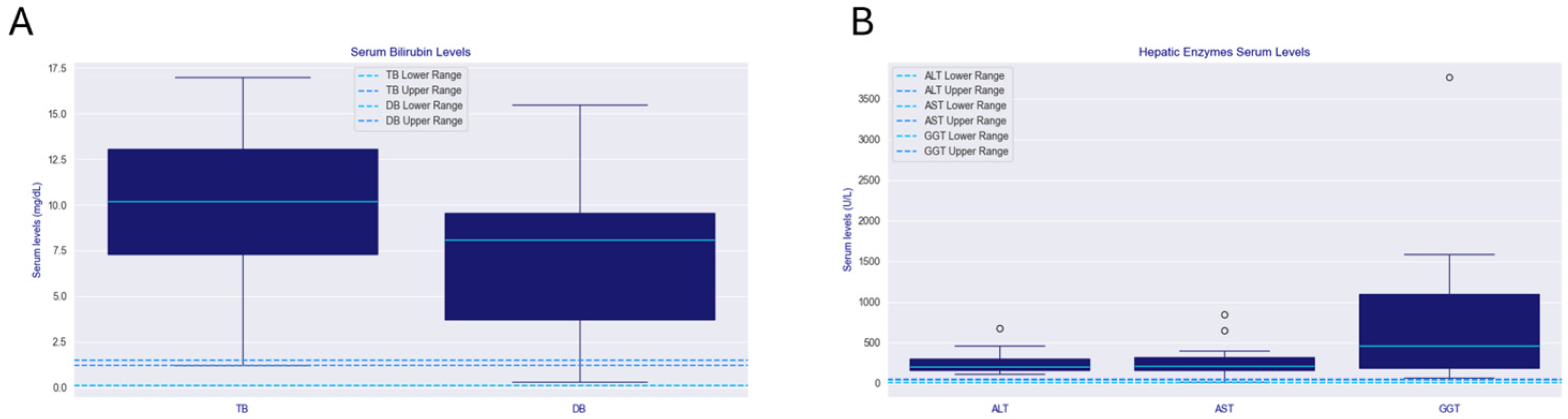
2.3. Histopathology
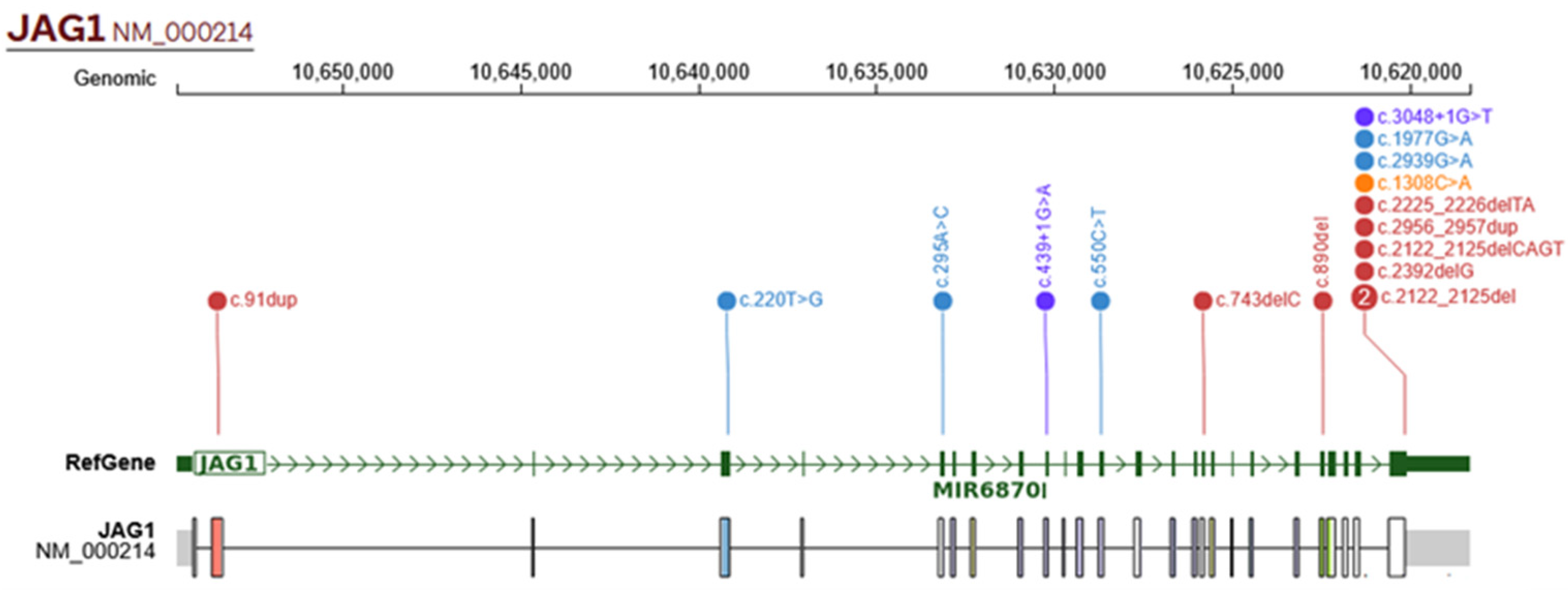
2.4. Genetic Findings
2.5. Treatment and Outcomes
3. Discussion
3.1. Hepatic Manifestations
3.2. Extrahepatic Manifestations
3.3. Notable Phenotypes
3.4. Genetic Findings
3.5. Perspectives
3.6. Strengths and Limitations
4. Materials and Methods
4.1. Study Design, Population, and Data Collection
4.2. Genetic Analysis
4.3. Liver Histopathology
4.4. Other Assessments
4.5. Statistical Analysis
4.6. Ethical Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACMG | American College of Medical Genetics and Genomics |
| AI | Auricles with adequate implantation |
| ALGS | Alagille syndrome |
| ALT | Alanine aminotransferase |
| ASD | Atrial septal defect |
| AST | Aspartate aminotransferase |
| BFH | Broad forehead |
| BNT | Bulbous nasal tip |
| CNV | Copy number variant |
| DB | Direct bilirubin |
| Dol | Dolichocephaly |
| DSEs | Deep-set eyes |
| EF | Epicanthus |
| EVs | Esophageal varices |
| GGT | Gamma-glutamyl transferase |
| H&E | Hematoxylin and eosin |
| HypT | Hypertelorism |
| ICHU | Inferior crus of helix underdevelopment |
| IBAT | Ileal bile acid transporter |
| MRI | Magnetic resonance imaging |
| NGS | Next-generation sequencing |
| PAS | Pulmonary artery stenosis |
| PDA | Patent ductus arteriosus |
| PE | Prominent ears |
| PC | Pointed chin |
| PFO | Patent foramen ovale |
| PM | Pathogenic moderate |
| PP | Pathogenic supporting |
| PS | Pathogenic strong (ACMG classification evidence code) |
| PVS | Pathogenic very strong (ACMG classification evidence code) |
| RTA | Renal tubular acidosis |
| SVPS | Supravalvular pulmonary stenosis |
| TB | Total bilirubin |
| Tc | Telecanthus |
| TUL | Thin upper lip vermilion |
| TOF | Tetralogy of Fallot |
| UPF | Up-slanting palpebral fissures |
| UPJ | Ureteropelvic junction |
| VSD | Ventricular septal defect |
| VUR | Vesicoureteral reflux |
References
- Chitayat, D.; Kamath, B.; Saleh, M. Alagille Syndrome: Clinical Perspectives. Appl. Clin. Genet. 2016, 9, 75–82. [Google Scholar] [CrossRef]
- Gilbert, M.A.; Bauer, R.C.; Rajagopalan, R.; Grochowski, C.M.; Chao, G.; McEldrew, D.; Nassur, J.A.; Rand, E.B.; Krock, B.L.; Kamath, B.M.; et al. Alagille Syndrome Mutation Update: Comprehensive Overview of JAG1 and NOTCH2 Mutation Frequencies and Insight into Missense Variant Classification. Hum. Mutat. 2019, 40, 2197–2220. [Google Scholar] [CrossRef]
- Kohut, T.J.; Gilbert, M.A.; Loomes, K.M. Alagille Syndrome: A Focused Review on Clinical Features, Genetics, and Treatment. Semin. Liver Dis. 2021, 41, 525–537. [Google Scholar] [CrossRef]
- Spinner, N.B.; Colliton, R.P.; Crosnier, C.; Krantz, I.D.; Hadchouel, M.; Meunier-Rotival, M. Jagged1 Mutations in Alagille Syndrome. Hum. Mutat. 2001, 17, 18–33. [Google Scholar] [CrossRef]
- Vandriel, S.M.; Li, L.; She, H.; Wang, J.; Gilbert, M.A.; Jankowska, I.; Czubkowski, P.; Gliwicz-Miedzińska, D.; Gonzales, E.M.; Jacquemin, E.; et al. Natural History of Liver Disease in a Large International Cohort of Children with Alagille Syndrome: Results from the GALA Study. Hepatology 2023, 77, 512–529. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, M.D.; Kamath, B.M. Alagille Syndrome: Diagnostic Challenges and Advances in Management. Diagnostics 2020, 10, 907. [Google Scholar] [CrossRef]
- Yan, J.; Huang, Y.; Cao, L.; Dong, Y.; Xu, Z.; Wang, F.; Gao, Y.; Feng, D.; Zhang, M. Clinical, Pathological and Genetic Characteristics of 17 Unrelated Children with Alagille Syndrome. BMC Pediatr. 2024, 24, 532. [Google Scholar] [CrossRef] [PubMed]
- Leonard, L.D.; Chao, G.; Baker, A.; Loomes, K.; Spinner, N.B. Clinical Utility Gene Card for: Alagille Syndrome (ALGS). Eur. J. Hum. Genet. 2014, 22, 435. [Google Scholar] [CrossRef]
- Le Gloan, L.; Pichon, O.; Isidor, B.; Boceno, M.; Rival, J.-M.; David, A.; Le Caignec, C. A 8.26Mb Deletion in 6q16 and a 4.95Mb Deletion in 20p12 Including JAG1 and BMP2 in a Patient with Alagille Syndrome and Wolff-Parkinson-White Syndrome. Eur. J. Med. Genet. 2008, 51, 651–657. [Google Scholar] [CrossRef]
- Roy, C.C.; Alvarez, F.; Grand, R.J. Obituary for Daniel Alagille. J. Pediatr. Gastroenterol. Nutr. 2006, 42, 127–128. [Google Scholar] [CrossRef]
- MacMahon, H.E. Biliary Xanthomatosis (Xanthomatous Biliary Cirrhosis). Am. J. Pathol. 1948, 24, 527–543. [Google Scholar]
- Kamath, B.M.; Baker, A.; Houwen, R.; Todorova, L.; Kerkar, N. Systematic Review. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 148–156. [Google Scholar] [CrossRef]
- Ayoub, M.D.; Bakhsh, A.A.; Vandriel, S.M.; Keitel, V.; Kamath, B.M. Management of Adults with Alagille Syndrome. Hepatol. Int. 2023, 17, 1098–1112. [Google Scholar] [CrossRef]
- Kremer, A.E.; Bolier, R.; van Dijk, R.; Oude Elferink, R.P.J.; Beuers, U. Advances in Pathogenesis and Management of Pruritus in Cholestasis. Dig. Dis. 2014, 32, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, E.; Hardikar, W.; Stormon, M.; Baker, A.; Hierro, L.; Gliwicz, D.; Lacaille, F.; Lachaux, A.; Sturm, E.; Setchell, K.D.R.; et al. Efficacy and Safety of Maralixibat Treatment in Patients with Alagille Syndrome and Cholestatic Pruritus (ICONIC): A Randomised Phase 2 Study. Lancet 2021, 398, 1581–1592. [Google Scholar] [CrossRef]
- Semenova, N.; Kamenets, E.; Annenkova, E.; Marakhonov, A.; Gusarova, E.; Demina, N.; Guseva, D.; Anisimova, I.; Degtyareva, A.; Taran, N.; et al. Clinical Characterization of Alagille Syndrome in Patients with Cholestatic Liver Disease. Int. J. Mol. Sci. 2023, 24, 11758. [Google Scholar] [CrossRef]
- Micaglio, E.; Andronache, A.A.; Carrera, P.; Monasky, M.M.; Locati, E.T.; Pirola, B.; Presi, S.; Carminati, M.; Ferrari, M.; Giamberti, A.; et al. Novel JAG1 Deletion Variant in Patient with Atypical Alagille Syndrome. Int. J. Mol. Sci. 2019, 20, 6247. [Google Scholar] [CrossRef]
- Vázquez-Martínez, E.R.; Varela-Fascinetto, G.; García-Delgado, C.; Rodríguez-Espino, B.A.; Sánchez-Boiso, A.; Valencia-Mayoral, P.; Heller-Rosseau, S.; Pelcastre-Luna, E.L.; Zenteno, J.C.; Cerbón, M.; et al. Polymorphism Analysis and New JAG1 Gene Mutations of Alagille Syndrome in Mexican Population. Meta Gene 2014, 2, 32–40. [Google Scholar] [CrossRef]
- Ruiz-Castillo, M.; Michel-Penichet, F.; Cervantes-Bustamante, R.; Zarate-Mondragón, F.; Mata-Rivera, N.; Montijo-Barrios, E.; García-Campos, M.; Ramírez-Mayans, J.A. Síndrome de Alagille: Informe de 12 casos en el Instituto Nacional de Pediatría. Rev. Enf. Inf. Ped. 2007, 21, 13–17. [Google Scholar]
- Zhou, X.; Edmonson, M.N.; Wilkinson, M.R.; Patel, A.; Wu, G.; Liu, Y.; Li, Y.; Zhang, Z.; Rusch, M.C.; Parker, M.; et al. Exploring Genomic Alteration in Pediatric Cancer Using ProteinPaint. Nat. Genet. 2016, 48, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, H.; Dong, C.; Feng, J.; Huang, Z. Clinical Features and Genetic Analysis of Pediatric Patients with Alagille Syndrome Presenting Initially with Liver Function Abnormalities. Curr. Med. Sci. 2018, 38, 304–309. [Google Scholar] [CrossRef]
- Cho, J.M.; Oh, S.H.; Kim, H.J.; Kim, J.S.; Kim, K.M.; Kim, G.; Yu, E.; Lee, B.H.; Yoo, H. Clinical Features, Outcomes, and Genetic Analysis in Korean Children with Alagille Syndrome. Pediatr. Int. 2015, 57, 552–557. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, M.; Teng, X. Clinical and Genetic Analysis in Chinese Children with Alagille Syndrome. BMC Pediatr. 2022, 22, 688. [Google Scholar] [CrossRef]
- Moldovan, G.E.; Miele, L.; Fazleabas, A.T. Notch Signaling in Reproduction. Trends Endocrinol. Metab. 2021, 32, 1044–1057. [Google Scholar] [CrossRef]
- Xie, Q.; Cheng, Z.; Chen, X.; Lobe, C.G.; Liu, J. The Role of Notch Signalling in Ovarian Angiogenesis. J. Ovarian Res. 2017, 10, 13. [Google Scholar] [CrossRef]
- Brooks, J.K. A Review of Syndromes Associated with Blue Sclera, with Inclusion of Malformations of the Head and Neck. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 126, 252–263. [Google Scholar] [CrossRef]
- Hingorani, M.; Nischal, K.K.; Davies, A.; Bentley, C.; Vivian, A.; Baker, A.J.; Mieli-Vergani, G.; Bird, A.C.; Aclimandos, W.A. Ocular Abnormalities in Alagille Syndrome11None of the Authors Has Any Commercial Interest Arising from the Findings Presented in This Article. Ophthalmology 1999, 106, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Kamath, B.M.; Bauer, R.C.; Loomes, K.M.; Chao, G.; Gerfen, J.; Hutchinson, A.; Hardikar, W.; Hirschfield, G.; Jara, P.; Krantz, I.D.; et al. NOTCH2 Mutations in Alagille Syndrome. J. Med. Genet. 2012, 49, 138–144. [Google Scholar] [CrossRef]
- Flück, C.E.; Audí, L.; Fernández-Cancio, M.; Sauter, K.-S.; Martinez de LaPiscina, I.; Castaño, L.; Esteva, I.; Camats, N. Broad Phenotypes of Disorders/Differences of Sex Development in MAMLD1 Patients Through Oligogenic Disease. Front. Genet. 2019, 10, 746. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.S.; Yen, H.-Y.; Barske, L.; Smith, B.; Llamas, J.; Segil, N.; Go, J.; Sanchez-Lara, P.A.; Maxson, R.E.; Crump, J.G. Requirement for Jagged1-Notch2 Signaling in Patterning the Bones of the Mouse and Human Middle Ear. Sci. Rep. 2017, 7, 2497. [Google Scholar] [CrossRef] [PubMed]
- Lalani, S.R.; Thakuria, J.V.; Cox, G.F.; Wang, X.; Bi, W.; Bray, M.S.; Shaw, C.; Cheung, S.W.; Chinault, A.C.; Boggs, B.A.; et al. 20p12. 3 Microdeletion Predisposes to Wolff-Parkinson-White Syndrome with Variable Neurocognitive Deficits. J. Med. Genet. 2008, 46, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Halma, J.; Lin, H.C. Alagille Syndrome: Understanding the Genotype-Phenotype Relationship and Its Potential Therapeutic Impact. Expert Rev. Gastroenterol. Hepatol. 2023, 17, 883–892. [Google Scholar] [CrossRef]
- Giugliani, R.; Castillo Taucher, S.; Hafez, S.; Oliveira, J.B.; Rico-Restrepo, M.; Rozenfeld, P.; Zarante, I.; Gonzaga-Jauregui, C. Opportunities and Challenges for Newborn Screening and Early Diagnosis of Rare Diseases in Latin America. Front. Genet. 2022, 13, 1053559. [Google Scholar] [CrossRef]
- Calvo Aspiros, C.E.; Gonzaga-Jauregui, C. First Year Results and Insights from the Mexican Rare Disease Patient Registry. Rare 2024, 2, 100046. [Google Scholar] [CrossRef]
- Huang, L.; Li, S.; Chen, J.; Zhu, Y.; Lan, K.; Zeng, L.; Jiang, X.; Zhang, L. Efficacy and Safety of Ursodeoxycholic Acid in Children with Cholestasis: A Systematic Review and Meta-Analysis. PLoS ONE 2023, 18, e0280691. [Google Scholar] [CrossRef]
- Arnon, R.; Annunziato, R.; Miloh, T.; Suchy, F.; Sakworawich, A.; Hiroshi, S.; Kishore, I.; Kerkar, N. Orthotopic Liver Transplantation for Children with Alagille Syndrome. Pediatr. Transplant. 2010, 14, 622–628. [Google Scholar] [CrossRef]
- Hori, T.; Egawa, H.; Takada, Y.; Oike, F.; Kasahara, M.; Ogura, Y.; Sakamoto, S.; Ogawa, K.; Yonekawa, Y.; Nguyen, J.H.; et al. Long-Term Outcomes After Living-Donor Liver Transplantation for Alagille Syndrome: A Single Center 20-Year Experience in Japan. Am. J. Transplant. 2010, 10, 1951–1952. [Google Scholar] [CrossRef] [PubMed]
- Gesprasert, G.; Chongsrisawat, V.; Tantemsapya, N.; Thirapattaraphan, C.; Nonthasoot, B.; Tovikkai, C.; Pugkhem, A.; Limsrichamrern, S.; Lumpaopong, A.; Supaporn, T.; et al. The First Report Of Pediatric Liver Transplantation In Thailand From The Thai Liver Transplant Registry. Transplantation 2020, 104, S536. [Google Scholar] [CrossRef]
- Centro Nacional de Trasplantes. Estado Actual de Receptores, Donación y Trasplantes En México; Anual 2024; Centro Nacional de Trasplantes: Ciudad de México, México, 2025.
- Varela-Fascinetto, G.; Dávila-Pérez, R.; Hernández-Plata, A.; Castañeda-Martínez, P.; Fuentes-García, V.; Nieto-Zermeño, J. Pediatric Liver Transplantation. Rev. Invest. Clin. 2005, 57, 273–282. [Google Scholar] [PubMed]
- Hansen, B.E.; Vandriel, S.M.; Vig, P.; Garner, W.; Mogul, D.B.; Loomes, K.M.; Piccoli, D.A.; Rand, E.B.; Jankowska, I.; Czubkowski, P.; et al. Event-Free Survival of Maralixibat-Treated Patients with Alagille Syndrome Compared to a Real-World Cohort from GALA. Hepatology 2024, 79, 1279–1292. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and Guidelines for the Interpretation of Sequence Variants: A Joint Consensus Recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]

| Patient | Sex | Age at Onset | Age at Diagnosis | Age at Last Follow-Up | Family History | Neonatal Cholestasis (n = 17) | Cardiovascular Findings (n = 15) | Renal Findings (n = 13) | Vascular Findings (n = 11) | Skeletal Finding (n = 14) | Ophthalmic Findings (n = 17) | Facial Features (n = 10) | Pruritus (n = 5) | Age at Transplant (If Performed) (n = 15) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | Male | - | 1 mo | 11 y | No | Yes | PAS | No | No | Butterfly vertebrae | Retinal pigmentary changes, right exotropia | BFH, HypT, PC | - | 6 y |
| P2 | Female | 3 mo | 3 y | 19 y | No | Yes | PAS | Double right collecting system | No | Scoliosis | No | - | No | 9 y |
| P3 | Male | 2 mo | 2 mo | 9 y | No | Yes | Crossed pulmonary arteries | Left renal atrophy, right solitary kidney | No | Hemivertebra | Posterior embryotoxon | - | - | Unspecified |
| P4 | Male | - | 3 mo | 2 y | No | Yes, with intrahepatic portal hypertension | VSD | - | No | No | No | - | - | Waiting list |
| P5 | Male | - | 3 mo | 10 y | Mother with suspected but unconfirmed ALGS | Yes | PAS | No | No | Scoliosis | Posterior embryotoxon | BFH, DSE, PC | No | Waiting list |
| P6 | Male | 15 d | NA | 2 y | No | Yes | - | - | - | - | - | - | - | Waiting list |
| P7 | Female | - | - | 17 y | No | Yes | PA, tricuspid insufficiency | No | - | Butterfly vertebrae | Posterior embryotoxon | Tc, PC | - | Waiting list |
| P8 | Female | 1 mo | - | 6 y | No | Yes | PAS | No | EV | Butterfly vertebrae | Posterior embryotoxon | BFH, DSE, PC | - | Waiting list |
| P9 | Male | - | - | - | - | - | - | - | - | - | - | - | - | - |
| P10 | Male | - | - | - | - | - | - | - | - | - | - | - | - | - |
| P11 | Male | - | - | Deceased | - | - | - | - | - | - | - | - | - | - |
| P12 | Male | - | - | - | - | - | - | - | - | - | - | - | - | - |
| P13 | Female | - | - | 9 y | No | No | ASD | No | - | Butterfly vertebrae | Posterior embryotoxon, blue sclerae, deep-set eyes | - | - | - |
| P14 | Male | 1 w | 1 w | - | No | Yes | PAS | No | - | Butterfly vertebrae | No | No | - | 0 y, deceased 2 weeks after transplant |
| P15 | Male | 2 mo | 2 mo | 6 y | Yes: one parent and a parent’s cousin | Yes | SVPS, PAS, PFO, PDA | VUR, post-operative left UPJ stenosis, RTA | No | Butterfly vertebrae | No | BFH, DSE, PE, BNT, PC | - | Waiting list |
| P16 | Male | 2 mo | 2 mo | 1 y | No | Yes | VSD | No | No | No | No | Dol, BFH, SE, UPF, EF, DSE, BNT | - | Waiting list |
| P17 | Male | 15 d | 3 y | - | Yes, one of the parents | Yes | PDA | No | No | No | Posterior embryotoxon | SE, UPF, HypT, BNT, TUL, PC, AI, ICHU | - | Waiting list |
| P18 | Female | - | - | 1 y | Yes: two paternal cousins deceased at age 2 with unspecified hepatopathy | No | TOF with pulmonary valve agenesis | No | No | Butterfly vertebrae | Posterior embryotoxon | DSE, PC | Yes, on treatment with cholestyramine | Waiting list |
| P19 | Male | - | - | - | - | - | - | - | - | - | Lisch Nodules | - | - | No |
| P20 | Male | 4 y | - | No | No | PAS | - | - | - | Posterior embryotoxon | - | Yes, on treatment with ursodeoxycholic acid | - | |
| P21 | Male | - | - | Deceased | No | No | - | No | No | No | Posterior embryotoxon | - | - | - |
| P22 | Male | 2 mo | 5 mo | 5 mo | No | Yes | PAS | - | - | - | Visual immaturity due to delayed conduction | BFH, HypT, PC | No | Waiting list |
| Patient | Liver Biopsy |
|---|---|
| P1 | Reduced portal spaces and bile ducts; no cholangial proliferation |
| P2 | Decreased intrahepatic bile ducts, bile retention in hepatocytes, dilated sinusoids |
| P3 | Intrahepatic bile duct hypoplasia |
| P4 | Partial biliary flow obstruction, stage 3 fibrosis |
| P7 | Decreased interlobular bile ducts |
| P13 | Minimal changes suggestive of portal hypertension secondary to efferent flow obstruction |
| P14 | No significant changes |
| P16 | Giant cell hepatitis, lobular disarray, cholestasis, mild portal fibrosis, no ductular proliferation, no microorganisms detected |
| P17 | Expanded portal spaces with lymphoplasmacytic infiltrate, fibrosis with septal formation, biliary pigment in hepatocytes |
| P18 | Bile duct paucity, giant cell hepatitis |
| P21 | Fibrosis |
| P22 | Bile duct hypoplasia, cholangial proliferation, intracanalicular and intracytoplasmic cholestasis, scattered apoptotic cells |
| Patient | Gene | NM ID | cDNA | Amino Acid Change | rsID | Mutation Type | Classification | Previous Reports in the Literature (PMID) |
|---|---|---|---|---|---|---|---|---|
| P1 | JAG1 | NM_000214.3 (JAG1) | c.550C>T | p.Arg184Cys | rs121918350 | Missense | Likely Pathogenic (PM1, PM2, PM5, PP2, PP3, PP4, PP5) | 24748328 |
| P3 | JAG1 | NM_000214.3 (JAG1) | c.295A>C | p.Thr99Pro | - | Missense | Likely Pathogenic (PM1, PM2, PM5, PP2, PP3, PP4) | Novel |
| P4 | JAG1 | NM_000214.3 (JAG1) | c.743delC | p.Pro248Glnfs*164 | - | Frameshift | Pathogenic (PVS1, PS3, PM2, PP4) | Novel |
| P5 | JAG1 | NM_000214.3 (JAG1) | c.2392delG | p.Val798Trpfs*22 | - | Frameshift | Pathogenic (PVS1, PS3, PM2, PP4) | Novel |
| P7 | JAG1 | NM_000214.3 (JAG1) | c.1308C>A | p.Cys436* | rs764485729 | Nonsense | Pathogenic (PVS1, PM2, PP2, PP4) | 25676721 |
| P8 | JAG1 | NM_000214.3 (JAG1) | c.220T>G | p.Tyr74Asp | - | Missense | Likely pathogenic (PM1, PM2, PM5, PP3, PP4) | - |
| P10 | JAG1 | NM_000214.3 (JAG1) | c.2122_2125delCAGT | p.Gln708Valfs*34 | rs727504412 | Frameshift | Pathogenic (PVS1, PS3, PM2, PP3, PP4, PP5) | 25676721 |
| P11 | JAG1 | NM_000214.3 (JAG1) | c.439+1G>A | - | rs863223648 | Splice donor variant | Pathogenic (PVS1, PM1, PM2, PP3, PP4, PP5) | 24748328 |
| P12 | JAG1 | NM_000214.3 (JAG1) | c.2939G>A | p.Cys980Tyr | - | Missense | Likely pathogenic (PM1, PM2, PM5, PP2, PP3, PP4) | Novel |
| P13 | JAG1 | NM_000214.3 (JAG1) | c.890del | p.Gly270AspfsTer145 | - | Frameshift | Pathogenic (PVS1, PS3, PM1, PM2, PP4) | Novel |
| P14 | JAG1 | NM_000214.3 (JAG1) | c.91dup | p.Ala31Glyfs*42 | - | Frameshift | Pathogenic (PVS1, PS3, PM2, PP4). | Novel |
| P15 | JAG1 | NM_000214.3 (JAG1) | c.2956_2957dup | p.Leu986fs | rs2122595849 | Frameshift | Pathogenic (PVS1, PS3 PM2, PP4, PP5). | - |
| P16 | JAG1 | NM_000214.3 (JAG1) | c.2122_2125del | p.Gln708Valfs*34 | rs727504412 | Frameshift | Pathogenic (PVS1, PS3, PM2, PP4, PP5) | 15712272 |
| P17 | JAG1 | NM_000214.3 (JAG1) | c.2122_2125del | p.Gln708Valfs*34 | rs727504412 | Frameshift | Pathogenic (PVS1, PS3, PM2, PP4, PP5) | 22488849 |
| P18 | JAG1 | NM_000214.3 (JAG1) | c.2225_2226delTA | p.Ile742SerfsTer5 | rs1555828209 | Frameshift | Pathogenic (PVS1, PS3, PM2, PP4, PP5). | 21752016 |
| P19 | JAG1 | NM_000214.3 (JAG1) | c.1977G>A | p.Trp659* | rs1600182107 | Nonsense | Pathogenic (PVS1, PS3, PM1, PM2, PP4, PP5) | 12497640 |
| P20 | JAG1 | NM_000214.3 (JAG1) | c.3048+1G>T | - | rs876661121 | Splice donor variant | Pathogenic (PVS1, PM2, PP3, PP4, PP5) | 31343788 |
| P21 | NOTCH2 | NM_024408.4 (NOTCH2) | c.3878G>A | p.Arg1293His | rs201968231 | Missense | Variant of Uncertain Significance (PM1, PM2, PP4) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-Frias, R.; Varela-Fascinetto, G.; Acosta-Rodríguez-Bueno, C.P.; Consuelo, A.; Carrillo, A.; Reyes-Apodaca, M.; Moreno-Salgado, R.; López-Valdez, J.; Hernández-Chávez, E.; González-Ortiz, B.; et al. A Retrospective Study of Clinical and Genetic Features in a Long-Term Cohort of Mexican Children with Alagille Syndrome. Int. J. Mol. Sci. 2025, 26, 7626. https://doi.org/10.3390/ijms26157626
Vázquez-Frias R, Varela-Fascinetto G, Acosta-Rodríguez-Bueno CP, Consuelo A, Carrillo A, Reyes-Apodaca M, Moreno-Salgado R, López-Valdez J, Hernández-Chávez E, González-Ortiz B, et al. A Retrospective Study of Clinical and Genetic Features in a Long-Term Cohort of Mexican Children with Alagille Syndrome. International Journal of Molecular Sciences. 2025; 26(15):7626. https://doi.org/10.3390/ijms26157626
Chicago/Turabian StyleVázquez-Frias, Rodrigo, Gustavo Varela-Fascinetto, Carlos Patricio Acosta-Rodríguez-Bueno, Alejandra Consuelo, Ariel Carrillo, Magali Reyes-Apodaca, Rodrigo Moreno-Salgado, Jaime López-Valdez, Elizabeth Hernández-Chávez, Beatriz González-Ortiz, and et al. 2025. "A Retrospective Study of Clinical and Genetic Features in a Long-Term Cohort of Mexican Children with Alagille Syndrome" International Journal of Molecular Sciences 26, no. 15: 7626. https://doi.org/10.3390/ijms26157626
APA StyleVázquez-Frias, R., Varela-Fascinetto, G., Acosta-Rodríguez-Bueno, C. P., Consuelo, A., Carrillo, A., Reyes-Apodaca, M., Moreno-Salgado, R., López-Valdez, J., Hernández-Chávez, E., González-Ortiz, B., Cadena-León, J. F., Villalpando-Carrión, S., Worona-Dibner, L., Martínez-Montoya, V., Cerón-Muñiz, A., Ramírez-Ramírez, E., & Barragán-Arévalo, T. (2025). A Retrospective Study of Clinical and Genetic Features in a Long-Term Cohort of Mexican Children with Alagille Syndrome. International Journal of Molecular Sciences, 26(15), 7626. https://doi.org/10.3390/ijms26157626








