Evaluation of Collagenic Porcine Bone Blended with a Collagen Gel for Bone Regeneration: An In Vitro Study
Abstract
1. Introduction
2. Results
2.1. Viability
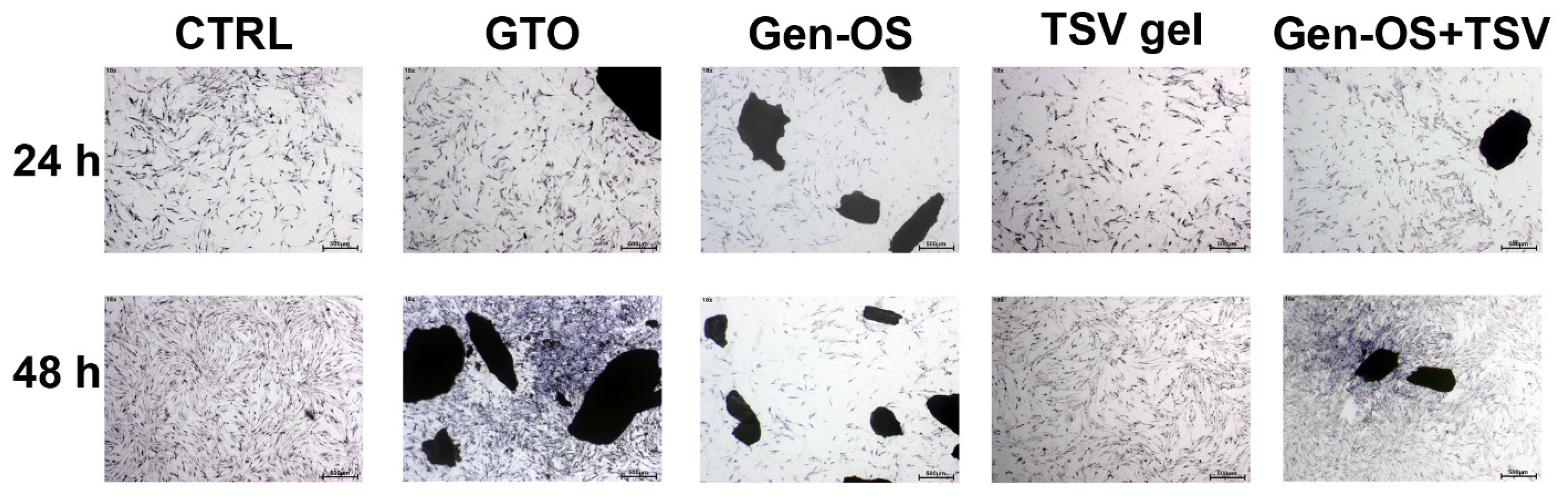
2.2. Morphology and Adhesion
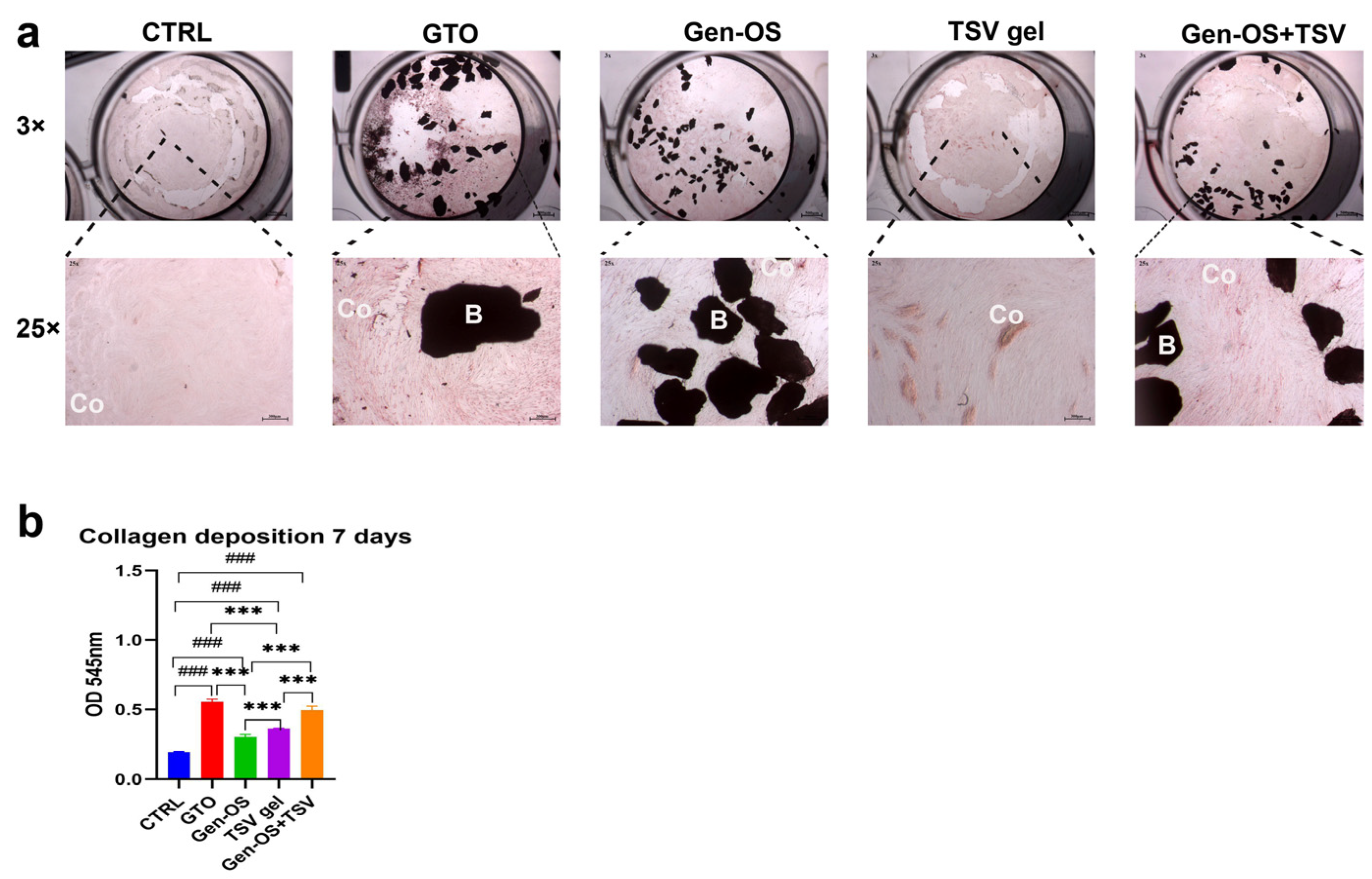
2.3. Collagen I Deposition
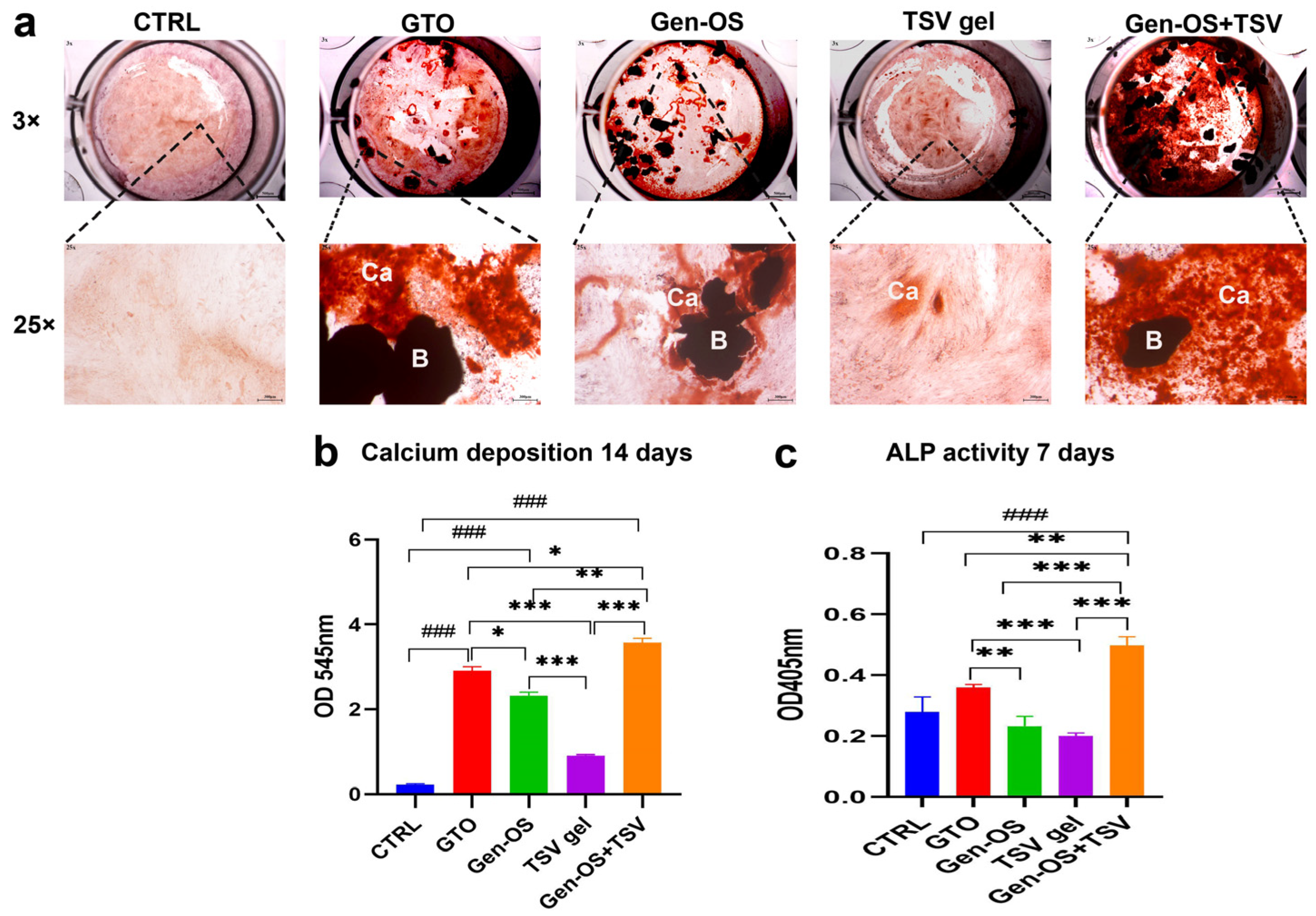
2.4. Mineralization
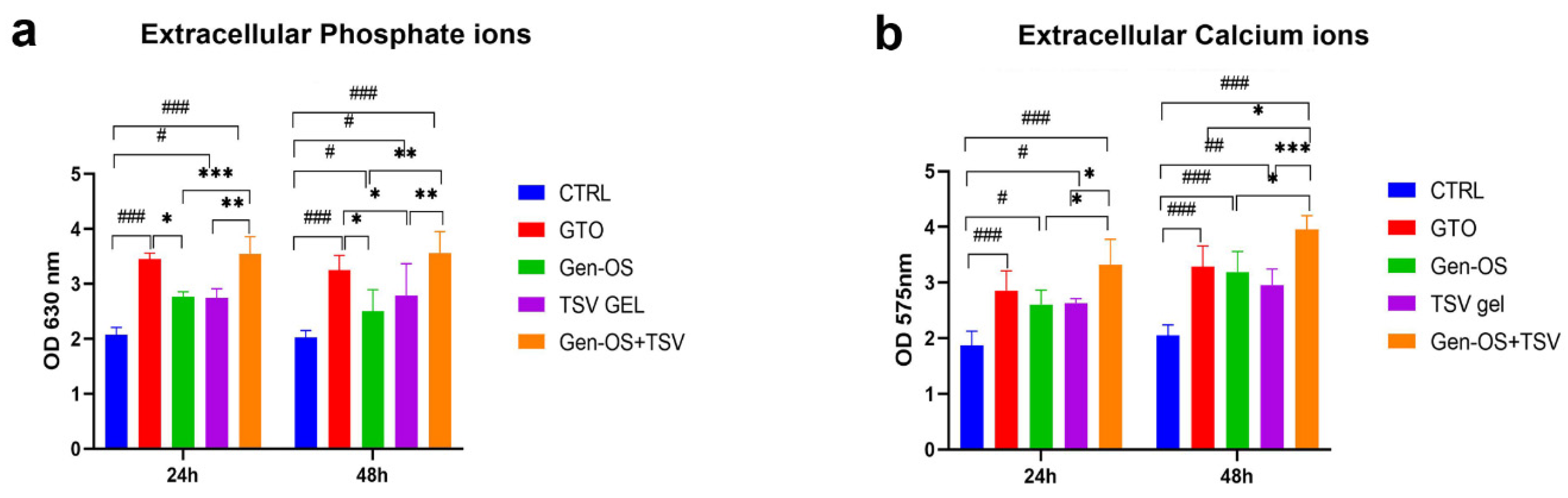
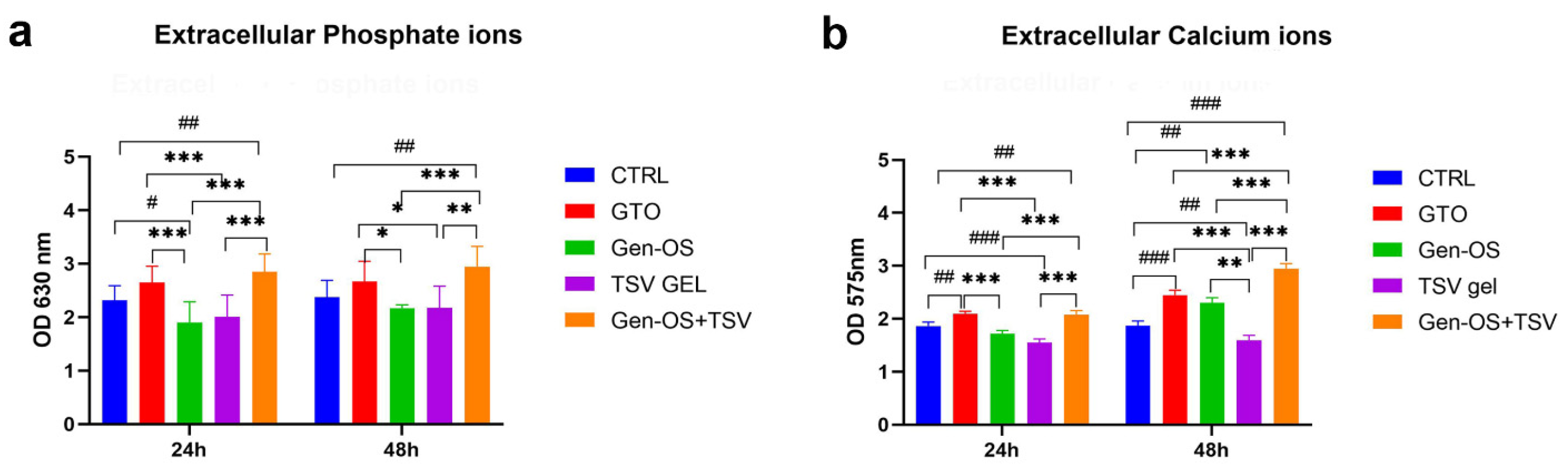
2.5. Ions in the Extracellular Environment
2.6. Changes in Medium pH During Cell Culture
2.7. Gene Expression
3. Discussion
4. Materials and Methods
4.1. Study Design and Bone Substitutes
- The GTO group included osteoblasts cultured with OsteoBiol® GTO®, composed of 80% xenogeneic collagenic cortico-cancellous granules, ranging in size from 600 to 1000 μm, and 20% TSV gel. OsteoBiol® GTO® was used as the manufacturer with an applicator syringe.
- The Gen-OS group included osteoblasts cultured with OsteoBiol® Gen-OS®, composed of xenogeneic collagenic cortico-cancellous granules, ranging in size from 250 to 1000 μm.
- The TSV gel group included osteoblasts cultured with TSV gel, a synthetic copolymer containing type I and III collagen along with polyunsaturated fatty acids. TSV gel was used as the manufacturer with an applicator syringe.
- The Gen-OS+TSV group included osteoblasts cultured with OsteoBiol® Gen-OS® granules mixed with TSV gel for this study at a ratio of 50:50 (%).
- The CTRL group included untreated osteoblasts.
- i.
- Cell viability assessment at 24 and 48 h with the 3-(4,5-dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide (MTT) assay.
- ii.
- Assessment of hOBs adhesion and morphology at 24 and 48 h using Toluidine Blue staining.
- iii.
- Assessment of collagen secretion by hOBs at 7 days using Picro-Sirius Red staining and spectrophotometric analysis.
- iv.
- Assessment of mineralization by hOBs at 14 days with Alizarin Red staining and spectrophotometric quantification and ALP activity at 7 days.
- v.
- Measurement of extracellular phosphatase and calcium ions in culture media at 24 and 48 h by a colorimetric assay.
- vi.
- Measurement of pH in culture media at 24 and 48 h by pH meter.
- vii.
- Assessment of osteogenic markers and integrins gene expression in hOBs at 48 h using real-time quantitative polymerase chain reactions (RT-qPCRs).
4.2. Cell Culture
4.3. Viability 1 mg/mL, 5 mg/mL, 10 mg/mL, 24 h, 48 h
4.4. Toluidine Blue Staining 1 mg/mL, 24 h, 48 h
4.5. Picro-Sirius 1 mg/mL, 7 Days
4.6. Alizarin Red Staining, 1 mg/mL, 14 Days
4.7. ALP Activity 1 mg/mL, 7 Days
4.8. Phosphate Ions (Extracellular) 1 mg/mL, 24 h, 48 h
4.9. Calcium Ions (Extracellular) 1 mg/mL, 24 h, 48 h
4.10. pH Measurements 1 mg/mL, 24 h, 48 h
4.11. Gene Expression, 1 mg/mL, 48 h
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jamjoom, A.; Cohen, R. Grafts for Ridge Preservation. J. Funct. Biomater. 2015, 6, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Chavda, S.; Levin, L. Human Studies of Vertical and Horizontal Alveolar Ridge Augmentation Comparing Different Types of Bone Graft Materials: A Systematic Review. J. Oral Implantol. 2018, 44, 74–84. [Google Scholar] [CrossRef]
- Covani, U.; Marconcini, S.; Crespi, R.; Barone, A. Immediate Implant Placement After Removal of a Failed Implant: A Clinical and Histological Case Report. J. Oral Implantol. 2009, 35, 189–195. [Google Scholar] [CrossRef]
- Barone, A.; Toti, P.; Menchini-Fabris, G.; Derchi, G.; Marconcini, S.; Covani, U. Extra Oral Digital Scanning and Imaging Superimposition for Volume Analysis of Bone Remodeling after Tooth Extraction with and without 2 Types of Particulate Porcine Mineral Insertion: A Randomized Controlled Trial. Clin. Implant. Dent. Relat. Res. 2017, 19, 750–759. [Google Scholar] [CrossRef]
- Correia, F.; Pozza, D.H.; Gouveia, S.; Felino, A.C.; Faria-Almeida, R. Advantages of Porcine Xenograft over Autograft in Sinus Lift: A Randomised Clinical Trial. Materials 2021, 14, 3439. [Google Scholar] [CrossRef]
- Yamada, R.; Xavier, S.P.; Nakajima, Y.; Silva, E.R.; Botticelli, D.; Teranishi, Y.; Baba, S. Impact of Collagenated and Non-Collagenated Deproteinized Bovine Bone Mineral on Schneiderian Membrane Integrity in Rabbits. Dent. J. 2025, 13, 19. [Google Scholar] [CrossRef]
- Miyauchi, Y.; Izutani, T.; Teranishi, Y.; Iida, T.; Nakajima, Y.; Xavier, S.P.; Baba, S. Healing Patterns of Non-Collagenated Bovine and Collagenated Porcine Xenografts Used for Sinus Floor Elevation: A Histological Study in Rabbits. J. Funct. Biomater. 2022, 13, 276. [Google Scholar] [CrossRef]
- Giuliani, A.; Iezzi, G.; Mazzoni, S.; Piattelli, A.; Perrotti, V.; Barone, A. Regenerative Properties of Collagenated Porcine Bone Grafts in Human Maxilla: Demonstrative Study of the Kinetics by Synchrotron Radiation Microtomography and Light Microscopy. Clin. Oral Investig. 2018, 22, 505–513. [Google Scholar] [CrossRef]
- Akita, K.; Fukuda, N.; Takamaru, N.; Kudoh, K.; Ishikawa, K.; Miyamoto, Y. Fabrication of a Carbonate Apatite Granules Sponge as a New Bone Substitute and Its Histological Evaluation at Rat Calvarial Bone Defects. J. Oral Maxillofac. Surg. Med. Pathol. 2025, 37, 42–49. [Google Scholar] [CrossRef]
- Allori, A.C.; Sailon, A.M.; Warren, S.M. Biological Basis of Bone Formation, Remodeling, and Repair—Part II: Extracellular Matrix. Tissue Eng. Part B Rev. 2008, 14, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Abdelgawad, M.E.; Søe, K.; Andersen, T.L.; Merrild, D.M.H.; Christiansen, P.; Kjærsgaard-Andersen, P.; Delaisse, J.-M. Does Collagen Trigger the Recruitment of Osteoblasts into Vacated Bone Resorption Lacunae during Bone Remodeling? Bone 2014, 67, 181–188. [Google Scholar] [CrossRef]
- Barone, A.; Ricci, M.; Covani, U.; Nannmark, U.; Azarmehr, I.; Calvo-Guirado, J.L. Maxillary Sinus Augmentation Using Prehydrated Corticocancellous Porcine Bone: Hystomorphometric Evaluation after 6 Months. Clin. Implant. Dent. Relat. Res. 2012, 14, 373–379. [Google Scholar] [CrossRef]
- Kim, D.; Lee, B.; Thomopoulos, S.; Jun, Y.-S. The Role of Confined Collagen Geometry in Decreasing Nucleation Energy Barriers to Intrafibrillar Mineralization. Nat. Commun. 2018, 9, 962. [Google Scholar] [CrossRef]
- Dirckx, N.; Van Hul, M.; Maes, C. Osteoblast Recruitment to Sites of Bone Formation in Skeletal Development, Homeostasis, and Regeneration. Birth Defects Res. C Embryo Today 2013, 99, 170–191. [Google Scholar] [CrossRef]
- Romasco, T.; Tumedei, M.; Inchingolo, F.; Pignatelli, P.; Montesani, L.; Iezzi, G.; Petrini, M.; Piattelli, A.; Di Pietro, N. A Narrative Review on the Effectiveness of Bone Regeneration Procedures with OsteoBiol® Collagenated Porcine Grafts: The Translational Research Experience over 20 Years. J. Funct. Biomater. 2022, 13, 121. [Google Scholar] [CrossRef]
- Anselme, K. Osteoblast Adhesion on Biomaterials. Biomaterials 2000, 21, 667–681. [Google Scholar] [CrossRef]
- Kübler, A.; Neugebauer, J.; Oh, J.-H.; Scheer, M.; Zöller, J.E. Growth and Proliferation of Human Osteoblasts on Different Bone Graft Substitutes An In Vitro Study. Implant. Dent. 2004, 13, 171–179. [Google Scholar] [CrossRef]
- Yamada, M.; Egusa, H. Current Bone Substitutes for Implant Dentistry. J. Prosthodont. Res. 2018, 62, 152–161. [Google Scholar] [CrossRef]
- Siebers, M.C.; ter Brugge, P.J.; Walboomers, X.F.; Jansen, J.A. Integrins as Linker Proteins between Osteoblasts and Bone Replacing Materials. A Critical Review. Biomaterials 2005, 26, 137–146. [Google Scholar] [CrossRef]
- Mao, L.; Wang, L.; Xu, J.; Zou, J. The Role of Integrin Family in Bone Metabolism and Tumor Bone Metastasis. Cell Death Discov. 2023, 9, 119. [Google Scholar] [CrossRef]
- Mizuno, M.; Fujisawa, R.; Kuboki, Y. Type I Collagen-Induced Osteoblastic Differentiation of Bone-Marrow Cells Mediated by Collagen-?2?1 Integrin Interaction. J. Cell. Physiol. 2000, 184, 207–213. [Google Scholar] [CrossRef]
- Lin, X.; Patil, S.; Gao, Y.G.; Qian, A. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front. Pharmacol. 2020, 11, 757. [Google Scholar] [CrossRef]
- Glimcher, M.J. Bone: Nature of the Calcium Phosphate Crystals and Cellular, Structural, and Physical Chemical Mechanisms in Their Formation. Rev. Miner. Geochem. 2006, 64, 223–282. [Google Scholar] [CrossRef]
- Millán, J.L. The Role of Phosphatases in the Initiation of Skeletal Mineralization. Calcif. Tissue Int. 2013, 93, 299–306. [Google Scholar] [CrossRef]
- Salamanca, E.; Hsu, C.C.; Yao, W.L.; Choy, C.S.; Pan, Y.H.; Teng, N.-C.; Chang, W.-J. Porcine Collagen–Bone Composite Induced Osteoblast Differentiation and Bone Regeneration In Vitro and In Vivo. Polymers 2020, 12, 93. [Google Scholar] [CrossRef]
- Rodrigues, W.C.; da Silva Fabris, A.L.; Hassumi, J.S.; Gonçalves, A.; Sonoda, C.K.; Okamoto, R. Kinetics of Gene Expression of Alkaline Phosphatase during Healing of Alveolar Bone in Rats. Br. J. Oral Maxillofac. Surg. 2016, 54, 531–535. [Google Scholar] [CrossRef]
- Blair, H.C.; Robinson, L.J.; Huang, C.L.-H.; Sun, L.; Friedman, P.A.; Schlesinger, P.H.; Zaidi, M. Calcium and Bone Disease. BioFactors 2011, 37, 159–167. [Google Scholar] [CrossRef]
- Wang, B.; Yang, Y.; Liu, L.; Blair, H.C.; Friedman, P.A. NHERF1 Regulation of PTH-Dependent Bimodal Pi Transport in Osteoblasts. Bone 2013, 52, 268–277. [Google Scholar] [CrossRef]
- Anderson, H.C. Matrix Vesicles and Calcification. Curr. Rheumatol. Rep. 2003, 5, 222–226. [Google Scholar] [CrossRef]
- Gadaleta, S.J.; Paschalis, E.P.; Betts, F.; Mendelsohn, R.; Boskey, A.L. Fourier Transform Infrared Spectroscopy of the Solution-Mediated Conversion of Amorphous Calcium Phosphate to Hydroxyapatite: New Correlations between X-Ray Diffraction and Infrared Data. Calcif. Tissue Int. 1996, 58, 9–16. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Sakamoto, K.; Minamizato, T.; Katsube, K.; Nakanishi, S. Regulation of Osteoblast Differentiation Mediated by BMP, Notch, and CCN3/NOV. Jpn. Dent. Sci. Rev. 2008, 44, 48–56. [Google Scholar] [CrossRef]
- Celil, A.B.; Campbell, P.G. BMP-2 and Insulin-like Growth Factor-I Mediate Osterix (Osx) Expression in Human Mesenchymal Stem Cells via the MAPK and Protein Kinase D Signaling Pathways. J. Biol. Chem. 2005, 280, 31353–31359. [Google Scholar] [CrossRef]
- Zoch, M.L.; Clemens, T.L.; Riddle, R.C. New Insights into the Biology of Osteocalcin. Bone 2016, 82, 42–49. [Google Scholar] [CrossRef]
- Pierfelice, T.V.; D’Amico, E.; Iezzi, G.; Piattelli, A.; Di Pietro, N.; D’Arcangelo, C.; Comuzzi, L.; Petrini, M. Nanoporous Titanium Enriched with Calcium and Phosphorus Promotes Human Oral Osteoblast Bioactivity. Int. J. Environ. Res. Public Health 2022, 19, 6212. [Google Scholar] [CrossRef]



| 1 mg/mL | CTRL | +GTO | +Gen-OS | +TSV gel | +Gen-OS+TSV |
|---|---|---|---|---|---|
| 24 h | 7.74 | 8.87 | 7.72 | 8.14 | 9.11 |
| 48 h | 8.01 | 8.62 | 8.07 | 8.60 | 8.62 |
| 1 mg/mL | DMEM | +GTO | +Gen-OS | +TSV gel | +Gen-OS+TSV |
|---|---|---|---|---|---|
| 24 h | 7.51 | 8.84 | 7.74 | 8.65 | 9.06 |
| 48 h | 7.46 | 8.63 | 8.07 | 8.60 | 8.62 |
| Gene | Primer Sequences (5’-3’) |
|---|---|
| OCN | F TCAGCCAACTCGTCACAGTC R GGCGCTACCTGTATCAATGG |
| RUNX2 | F ACAAGGTGGTCGAAGACTGG R TCTGGTCCTAGTGCCTCACAT |
| BMP2 | F GGAAGCAGCAACGCTAGAAG R GACTGCGGTCTCCTAAAGGTC |
| ALP | F AATGAGTGAGTGACCATCCTGG R GCACCCCAAGACCTGCTTTAT |
| INTEGRIN α2 | F TCAGGCACACCAAAAGAATTG R CGTCTTTCAACCAGCAGGTAA |
| INTEGRIN α5 | F ACATCTGTGTGCCTGACCTG R CCAGGTACACATGGTTCTGC |
| INTEGRIN β3 | F GTGTGCCTGCTCTGAT R AGCAGATTCTCCTTCAGGTCA |
| β-ACTIN | F CCAGAGGCGTACAGGGATAG R GAGAAGATGACCCAGGACTCTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pierfelice, T.V.; Cinquini, C.; Petrini, M.; D’Amico, E.; D’Arcangelo, C.; Barone, A.; Iezzi, G. Evaluation of Collagenic Porcine Bone Blended with a Collagen Gel for Bone Regeneration: An In Vitro Study. Int. J. Mol. Sci. 2025, 26, 7621. https://doi.org/10.3390/ijms26157621
Pierfelice TV, Cinquini C, Petrini M, D’Amico E, D’Arcangelo C, Barone A, Iezzi G. Evaluation of Collagenic Porcine Bone Blended with a Collagen Gel for Bone Regeneration: An In Vitro Study. International Journal of Molecular Sciences. 2025; 26(15):7621. https://doi.org/10.3390/ijms26157621
Chicago/Turabian StylePierfelice, Tania Vanessa, Chiara Cinquini, Morena Petrini, Emira D’Amico, Camillo D’Arcangelo, Antonio Barone, and Giovanna Iezzi. 2025. "Evaluation of Collagenic Porcine Bone Blended with a Collagen Gel for Bone Regeneration: An In Vitro Study" International Journal of Molecular Sciences 26, no. 15: 7621. https://doi.org/10.3390/ijms26157621
APA StylePierfelice, T. V., Cinquini, C., Petrini, M., D’Amico, E., D’Arcangelo, C., Barone, A., & Iezzi, G. (2025). Evaluation of Collagenic Porcine Bone Blended with a Collagen Gel for Bone Regeneration: An In Vitro Study. International Journal of Molecular Sciences, 26(15), 7621. https://doi.org/10.3390/ijms26157621










