Genome-Wide Identification and Characterisation of the 4-Coumarate–CoA Ligase (4CL) Gene Family in Gastrodia elata and Their Transcriptional Response to Fungal Infection
Abstract
1. Introduction
2. Results
2.1. Identification and Chromosomal Location Analysis of G. elata Ge4CL Gene Family Members
2.2. Collinearity Analysis
2.3. Phylogenetic Tree Analysis
2.4. Analysis of Conserved Motifs and Gene Structures
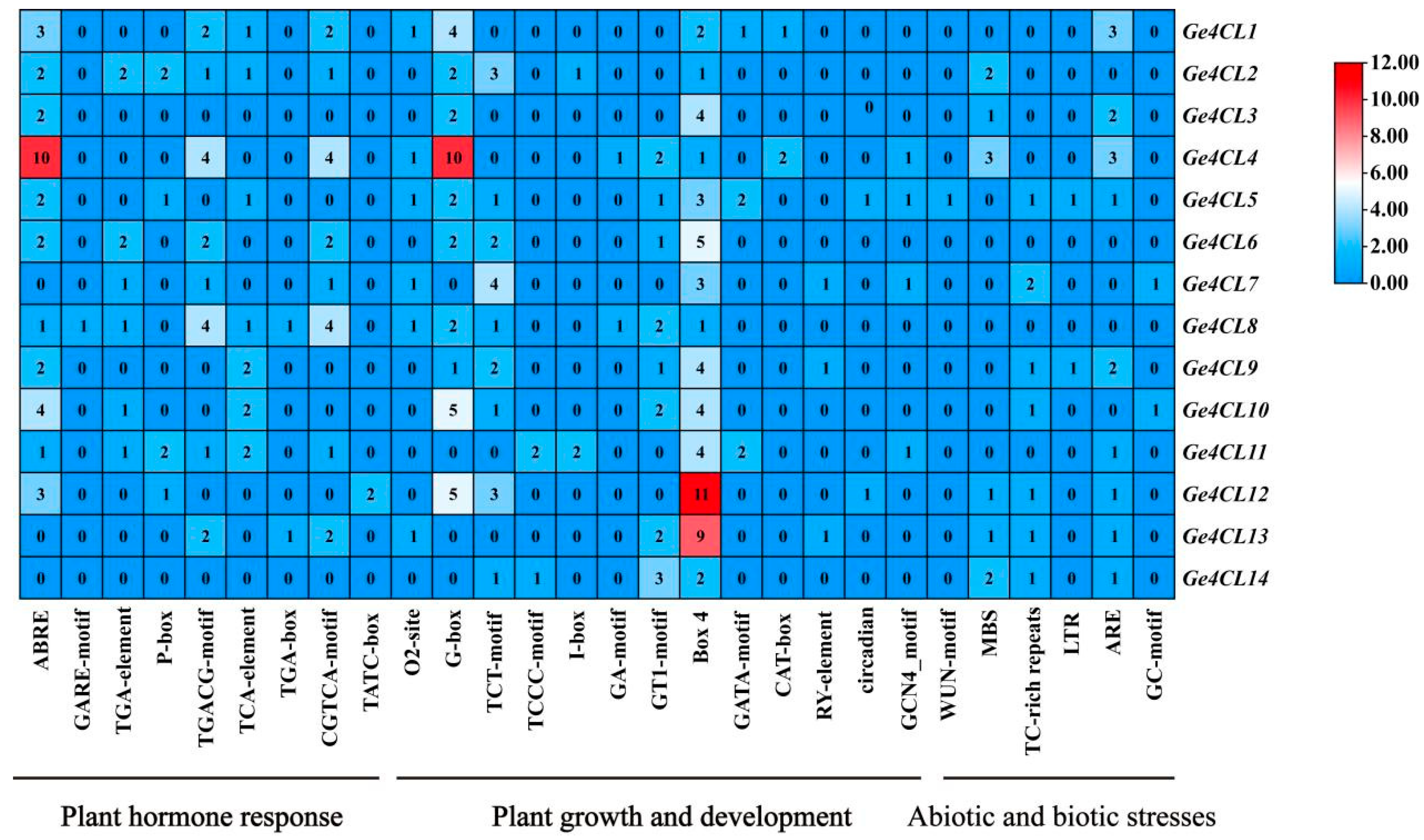
2.5. Analysis of Promoter Cis-Acting Elements
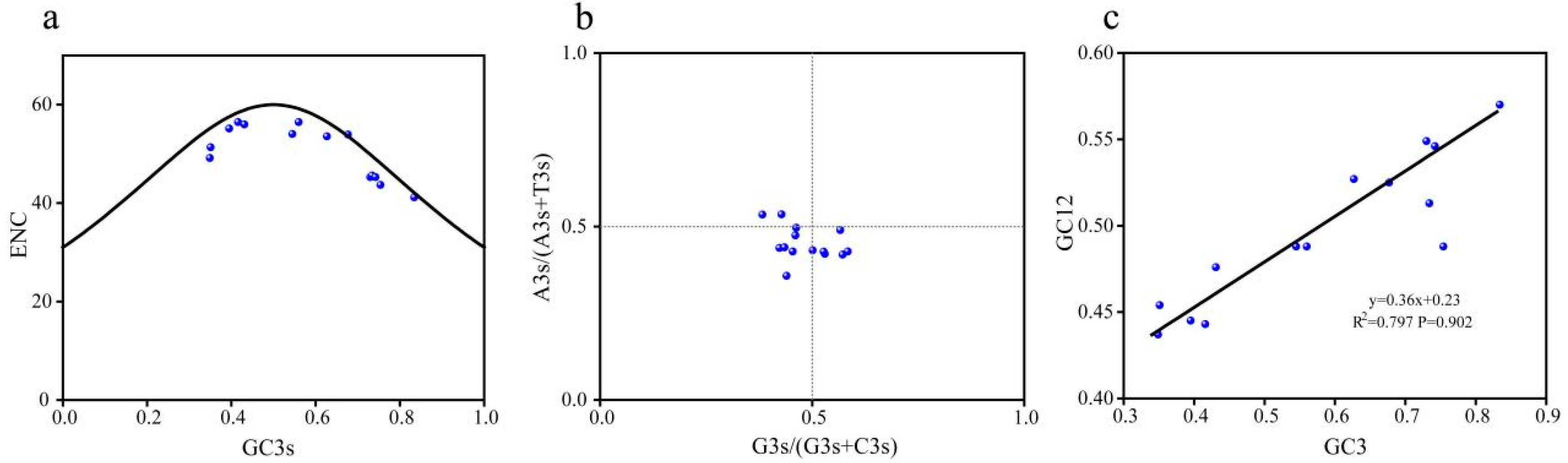
2.6. Analysis of Codon-Related Parameters of the Gene Family
2.7. Analysis of Influencing Factors on Codon Usage Bias of the Gene Family
2.8. Selection of the Recipient System of the Ge4CLs
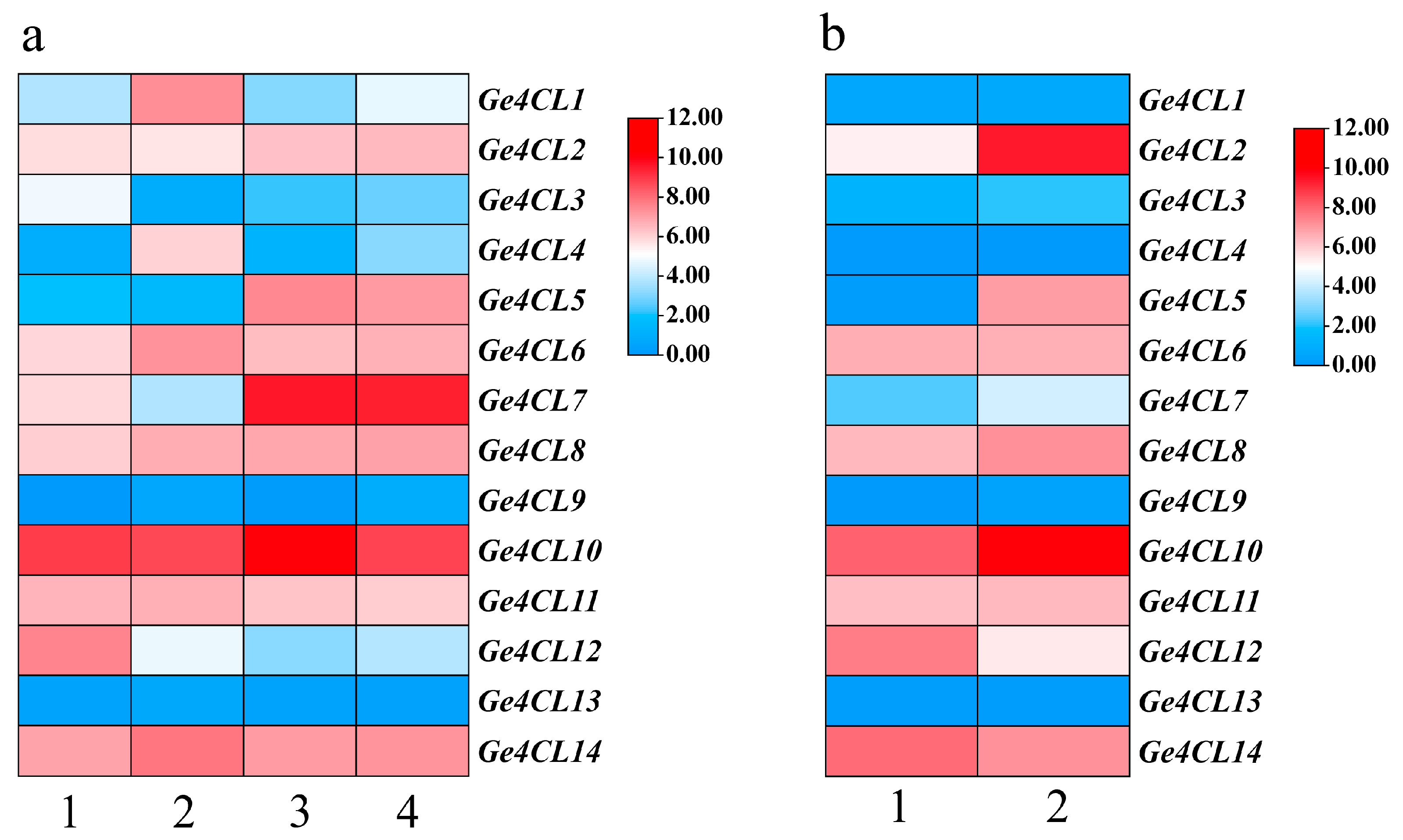
2.9. Expression Patterns of the Ge4CLs in G. elata
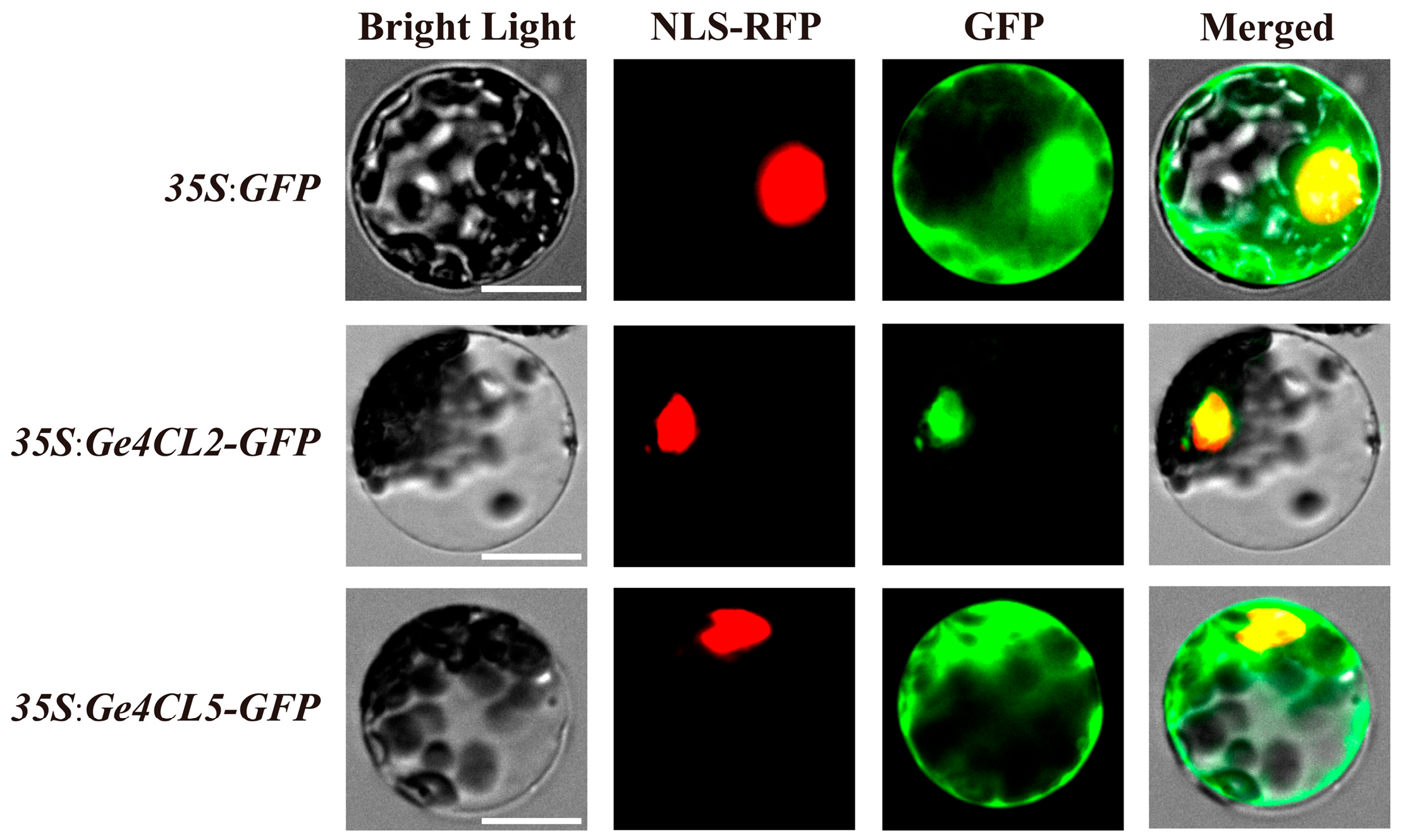
2.10. Subcellular Localisation Analysis
3. Discussion
3.1. Evolutionary Expansion and Diversification of the Ge4CL Gene Family in G. elata
3.2. Role of Ge4CL Genes in Fungal Infection Response and Plant Defence Mechanisms
3.3. Codon Usage Bias and Its Implications for Gene Expression in G. elata
3.4. Evolutionary Implications of the Reduced 4CL Repertoire in G. elata
3.5. Future Directions: Functional Validation and Applications in Plant Breeding
4. Materials and Methods
4.1. Identification and Chromosomal Localisation of Ge4CL Genes
4.2. Collinearity Analysis of the Ge4CL Gene Family
4.3. Phylogenetic Analysis of the Ge4CLs
4.4. Conserved Motifs and Gene Structures of Ge4CLs
4.5. Analysis of Cis-Acting Elements in the Promoters of Ge4CLs
4.6. Analysis of Codon Usage Bias in the Ge4CLs
4.7. Data Sources for Codon Usage Bias
4.8. Analysis of Gene Family Expression Patterns
4.9. Cloning and Subcellular Localisation of Ge4CL2 and Ge4CL5
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 4CL | 4-Coumarate–CoA Ligase |
| Ge4CL | Gastrodia elata 4CL Gene |
| G. elata | Gastrodia elata |
| TPM | Transcripts Per Million |
| NGDC | National Genomics Data Center |
| CDD | Conserved Domain Database |
| MEME | Multiple EM for Motif Elicitation |
| ENC | Effective Number of Codons |
| CAI | Codon Adaptation Index |
| CBI | Codon Bias Index |
| FOP | Frequency of Optimal Codons |
| GC | Guanine–Cytosine Content |
| GFF | General Feature Format |
| PSORT | Protein Subcellular Localisation Prediction Tool |
| TBtools | Toolbox for Biologists |
| GFP | Green Fluorescent Protein |
| RFP | Red Fluorescent Protein |
| RNAi | RNA Interference |
References
- Hu, J.; Feng, Y.; Zhong, H.; Liu, W.; Tian, X.; Wang, Y.; Tan, T.; Hu, Z.; Liu, Y. Impact of climate change on the geographical distribution and niche dynamics of Gastrodia elata. PeerJ 2023, 11, e15741. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Yin, C.; Lai, Y.; Lin, H.; Jian, Z.; Tao, A. Extraction, purification, characteristics, bioactivities, application, and toxicity of Gastrodia R. Br. polysaccharides: A review. Int. J. Biol. Macromol. 2025, 301, 140084. [Google Scholar] [CrossRef]
- Su, Z.; Yang, Y.; Chen, S.; Tang, Z.; Xu, H. The processing methods, phytochemistry and pharmacology of Gastrodia elata Bl.: A comprehensive review. J. Ethnopharmacol. 2023, 314, 116467. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, J.; Wang, Y.Z. A Review of Gastrodia elata Bl.: Extraction, Analysis and Application of Functional Food. Crit. Rev. Anal. Chem. 2024, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Tang, R.; Yang, N.; Chen, Y. Review on pharmacological effects of gastrodin. Arch. Pharm. Res. 2023, 46, 744–770. [Google Scholar] [CrossRef]
- Zhan, H.D.; Zhou, H.Y.; Sui, Y.P.; Du, X.L.; Wang, W.H.; Dai, L.; Sui, F.; Huo, H.R.; Jiang, T.L. The rhizome of Gastrodia elata Blume—An ethnopharmacological review. J. Ethnopharmacol. 2016, 189, 361–385. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, M.; Wang, X.; Peng, Z.; Cai, C.; Xi, J.; Yan, C.; Luo, J.; Li, X. Gastrodin: A comprehensive pharmacological review. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 3781–3802. [Google Scholar] [CrossRef]
- Yu, C.J.; Wang, S.B. Research on Pollution-free Comprehensive Prevention and Control Technology for Sclerotium Rot of G. elata. Hubei Agric. Sci. 2010, 49, 92–94. [Google Scholar]
- Tian, W.G. Research on Fungal Diseases of G. elata in Zhaotong, Yunnan. Master’s Thesis, Yunnan University, Kunming, China, 2020. [Google Scholar]
- Zhang, J.Q.; Tang, X.; Xiao, C.H.; Xu, J.; Yuan, Q.S.; Wang, X.; Liu, D.H.; Zhang, G.W.; Liu, F.M.; Jiang, W.K.; et al. [Investigation, analysis and identification of disease of Gastrodia elata f. glauca]. Zhongguo Zhong Yao Za Zhi 2020, 45, 478–484. [Google Scholar]
- Kang, L.; Wu, Y.; Jia, Y.; Chen, Z.; Kang, D.; Zhang, L.; Pan, C. Nano-selenium enhances melon resistance to Podosphaera xanthii by enhancing the antioxidant capacity and promoting alterations in the polyamine, phenylpropanoid and hormone signaling pathways. J. Nanobiotechnol. 2023, 21, 377. [Google Scholar] [CrossRef]
- Sun, J.; Fan, Z.; Chen, Y.; Jiang, Y.; Lin, M.; Wang, H.; Lin, Y.; Chen, Y.; Lin, H. The effect of ε-poly-l-lysine treatment on molecular, physiological and biochemical indicators related to resistance in longan fruit infected by Phomopsis longanae Chi. Food Chem. 2023, 416, 135784. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Fang, X.; Zhang, Y.; Wang, X.; Zhang, C.; Yan, X.; Zhao, Y.; Wu, J.; Xu, P.; Zhang, S. Overexpression of a soybean 4-coumaric acid: Coenzyme A ligase (GmPI4L) enhances resistance to Phytophthora sojae in soybean. Funct. Plant Biol. 2019, 46, 304–313. [Google Scholar] [CrossRef]
- Lavhale, S.G.; Kalunke, R.M.; Giri, A.P. Structural, functional and evolutionary diversity of 4-coumarate-CoA ligase in plants. Planta 2018, 248, 1063–1078. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Zhou, R.; Liang, L.; Zang, X.; Lin, J.; Wang, Q.; Wang, L.; Wang, W.; Li, Z.; Ren, P. 4-Coumarate-CoA ligase (4-CL) enhances flavonoid accumulation, lignin synthesis, and fruiting body formation in Ganoderma lucidum. Gene 2024, 899, 148147. [Google Scholar] [CrossRef]
- Wei, C.; Wang, C.; Zhang, X.; Huang, W.; Xing, M.; Han, C.; Lei, C.; Zhang, Y.; Zhang, X.; Cheng, K.; et al. Histone deacetylase GhHDA5 negatively regulates Verticillium wilt resistance in cotton. Plant Physiol. 2024, 196, 2918–2935. [Google Scholar] [CrossRef]
- Ehlting, J.; Büttner, D.; Wang, Q.; Douglas, C.J.; Somssich, I.E.; Kombrink, E. Three 4-coumarate:coenzyme A ligases in Arabidopsis thaliana represent two evolutionarily divergent classes in angiosperms. Plant J. 1999, 19, 9–20. [Google Scholar] [CrossRef]
- Alariqi, M.; Ramadan, M.; Wang, Q.; Yang, Z.; Hui, X.; Nie, X.; Ahmed, A.; Chen, Q.; Wang, Y.; Zhu, L.; et al. Cotton 4-coumarate-CoA ligase 3 enhanced plant resistance to Verticillium dahliae by promoting jasmonic acid signaling-mediated vascular lignification and metabolic flux. Plant J. 2023, 115, 190–204. [Google Scholar] [CrossRef]
- Li, W.; Wang, K.; Chern, M.; Liu, Y.; Zhu, Z.; Liu, J.; Zhu, X.; Yin, J.; Ran, L.; Xiong, J.; et al. Sclerenchyma cell thickening through enhanced lignification induced by OsMYB30 prevents fungal penetration of rice leaves. New Phytol. 2020, 226, 1850–1863. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, L.; Zhao, Y.; Zhao, X.; Yuan, Z. Systematic Analysis and Expression Profiles of the 4-Coumarate: CoA Ligase (4CL) Gene Family in Pomegranate (Punica granatum L.). Int. J. Mol. Sci. 2022, 23, 3509. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.H.; Nan, X.T.; Li, W.F.; Mao, J.; Chen, B.H. Comprehensive genomic identification and expression analysis 4CL gene family in apple. Gene 2023, 858, 147197. [Google Scholar] [CrossRef]
- Ran, F.; Xiang, C.; Wang, C.; Zang, Y.; Liu, L.; Wu, S.; Wang, C.; Cai, J.; Wang, D.; Min, Y. Identification of the 4CL family in cassava (Manihot esculenta Crantz) and expression pattern analysis of the Me4CL32 gene. Biochem. Biophys. Res. Commun. 2024, 735, 150731. [Google Scholar] [CrossRef]
- Zhang, C.; Zang, Y.; Liu, P.; Zheng, Z.; Ouyang, J. Characterization, functional analysis and application of 4-Coumarate: CoA ligase genes from Populus trichocarpa. J. Biotechnol. 2019, 302, 92–100. [Google Scholar] [CrossRef]
- Li, Y.; Kim, J.I.; Pysh, L.; Chapple, C. Four Isoforms of Arabidopsis 4-Coumarate:CoA Ligase Have Overlapping yet Distinct Roles in Phenylpropanoid Metabolism. Plant Physiol. 2015, 169, 2409–2421. [Google Scholar]
- Gong, M.Q.; Lai, F.F.; Chen, J.Z.; Li, X.H.; Chen, Y.J.; He, Y. Traditional uses, phytochemistry, pharmacology, applications, and quality control of Gastrodia elata Blume: A comprehensive review. J. Ethnopharmacol. 2024, 319 Pt 1, 117128. [Google Scholar] [CrossRef]
- Sun, H.; Li, Y.; Feng, S.; Zou, W.; Guo, K.; Fan, C.; Si, S.; Peng, L. Analysis of five rice 4-coumarate:coenzyme A ligase enzyme activity and stress response for potential roles in lignin and flavonoid biosynthesis in rice. Biochem. Biophys. Res. Commun. 2013, 430, 1151–1156. [Google Scholar] [CrossRef]
- Sun, S.C.; Xiong, X.P.; Zhang, X.L.; Feng, H.J.; Zhu, Q.H.; Sun, J.; Li, Y.J. Characterization of the Gh4CL gene family reveals a role of Gh4CL7 in drought tolerance. BMC Plant Biol. 2020, 20, 125. [Google Scholar] [CrossRef]
- Feng, X.; Wang, Y.; Zhang, N.; Gao, S.; Wu, J.; Liu, R.; Huang, Y.; Zhang, J.; Qi, Y. Comparative phylogenetic analysis of CBL reveals the gene family evolution and functional divergence in Saccharum spontaneum. BMC Plant Biol. 2021, 21, 395. [Google Scholar] [CrossRef]
- Nie, T.; Sun, X.; Wang, S.; Wang, D.; Ren, Y.; Chen, Q. Genome-Wide Identification and Expression Analysis of the 4-Coumarate: CoA Ligase Gene Family in Solanum tuberosum. Int. J. Mol. Sci. 2023, 24, 1642. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef]
- Baker, D.D.; Chu, M.; Oza, U.; Rajgarhia, V. The value of natural products to future pharmaceutical discovery. Nat. Prod. Rep. 2007, 24, 1225–1244. [Google Scholar] [CrossRef]
- Gill, U.S.; Uppalapati, S.R.; Gallego-Giraldo, L.; Ishiga, Y.; Dixon, R.A.; Mysore, K.S. Metabolic flux towards the (iso)flavonoid pathway in lignin modified alfalfa lines induces resistance against Fusarium oxysporum f. sp. medicaginis. Plant Cell Environ. 2018, 41, 1997–2007. [Google Scholar]
- Shinde, B.A.; Dholakia, B.B.; Hussain, K.; Panda, S.; Meir, S.; Rogachev, I.; Aharoni, A.; Giri, A.P.; Kamble, A.C. Dynamic metabolic reprogramming of steroidal glycol-alkaloid and phenylpropanoid biosynthesis may impart early blight resistance in wild tomato (Solanum arcanum Peralta). Plant Mol. Biol. 2017, 95, 411–423. [Google Scholar] [CrossRef]
- Wang, S. Cloning of Phenylpropanoid Metabolism and Key Gene Cl4CL in the Resistance Response of Watermelon to Fusarium Wilt. Master’s Thesis, Hebei Agricultural University, Baoding, China, 2021. [Google Scholar]
- Zhang, M.; Tian, M.; Weng, Z.; Yang, Y.; Pan, N.; Shen, S.; Zhao, H.; Du, H.; Qu, C.; Yin, N. Genome-Wide Identification Analysis of the 4-Coumarate: Coa Ligase (4CL) Gene Family in Brassica U’s Triangle Species and Its Potential Role in the Accumulation of Flavonoids in Brassica napus L. Plants 2025, 14, 211. Plants 2025, 14, 211. [Google Scholar]
- Chen, X.; Wang, H.; Li, X.; Ma, K.; Zhan, Y.; Zeng, F. Molecular cloning and functional analysis of 4-Coumarate:CoA ligase 4(4CL-like 1) from Fraxinus mandshurica and its role in abiotic stress tolerance and cell wall synthesis. BMC Plant Biol. 2019, 19, 231. [Google Scholar] [CrossRef]
- Zhou, X.; Cao, J.; Liu, X.M.; Wang, L.N.; Zhang, W.W.; Ye, J.B.; Xu, F.; Cheng, S. Cloning and functional analysis of Gb4CL1 and Gb4CL2 from Ginkgo biloba. Plant Genome 2024, 17, e20440. [Google Scholar] [CrossRef]
- Chtioui, W.; Balmas, V.; Delogu, G.; Migheli, Q.; Oufensou, S. Bioprospecting Phenols as Inhibitors of Trichothecene-Producing Fusarium: Sustainable Approaches to the Management of Wheat Pathogens. Toxins 2022, 14, 72. [Google Scholar] [CrossRef]
- Irais, C.M.; María-de-la-Luz, S.G.; Dealmy, D.G.; Agustina, R.M.; Nidia, C.H.; Mario-Alberto, R.G.; Luis-Benjamín, S.G.; María-Del-Carmen, V.M.; David, P.E. Plant Phenolics as Pathogen-Carrier Immunogenicity Modulator Haptens. Curr. Pharm. Biotechnol. 2020, 21, 897–905. [Google Scholar] [CrossRef]
- Angellotti, M.C.; Bhuiyan, S.B.; Chen, G.; Wan, X.F. CodonO: Codon usage bias analysis within and across genomes. Nucleic Acids Res. 2007, 35 (Suppl. S2), W132–W136. [Google Scholar] [CrossRef]
- Gao, Y.; Lu, Y.; Song, Y.; Jing, L. Analysis of codon usage bias of WRKY transcription factors in Helianthus annuus. BMC Genom. Data 2022, 23, 46. [Google Scholar] [CrossRef]
- Cao, Y.; Han, Y.; Li, D.; Lin, Y.; Cai, Y. Systematic analysis of the 4-Coumarate: Coenzyme a ligase (4CL) related genes and expression profiling during fruit development in the Chinese pear. Genes 2016, 7, 89. [Google Scholar] [CrossRef]
- Kaur, A.; Sharma, K.; Pawar, S.V.; Sembi, J.K. Genome-wide characterization of PAL, C4H, and 4CL genes regulating the phenylpropanoid pathway in Vanilla planifolia. Sci. Rep. 2025, 15, 10714. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chen, T.; Qiu, X.; Liu, J.; Guo, S. Evolutionary differences in gene loss and pseudogenization among mycoheterotrophic orchids in the tribe Vanilleae (subfamily Vanilloideae). Front. Plant Sci. 2023, 14, 1160446. [Google Scholar] [CrossRef] [PubMed]
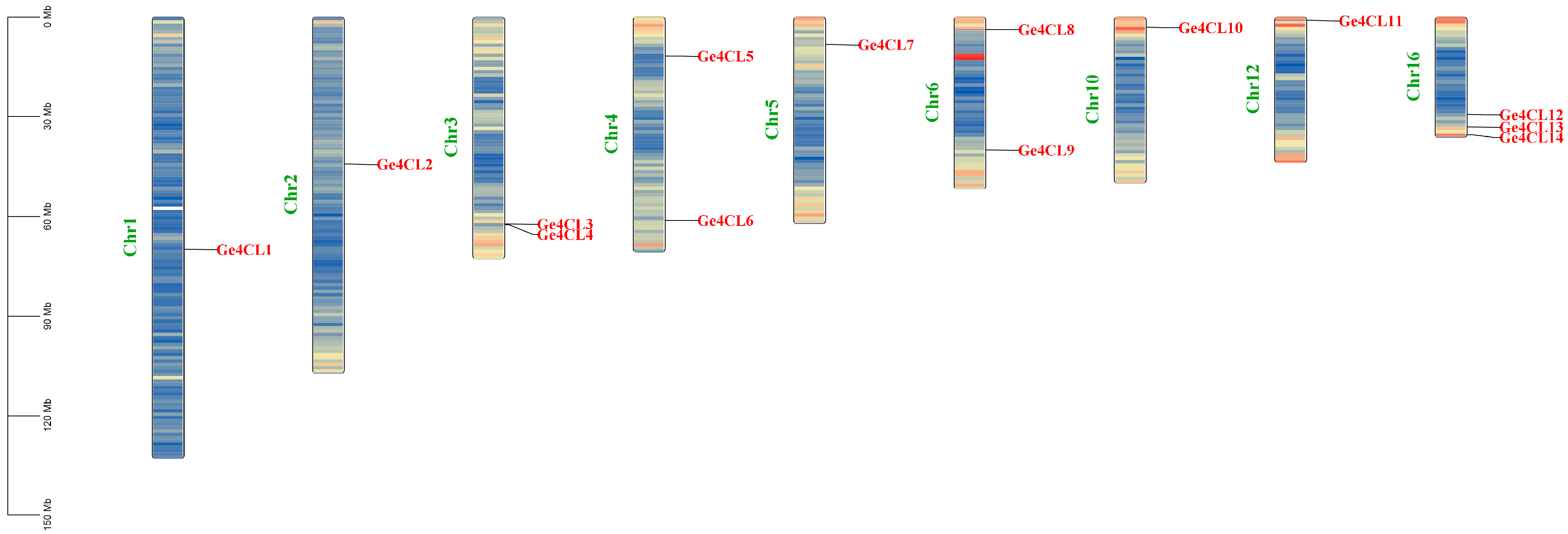
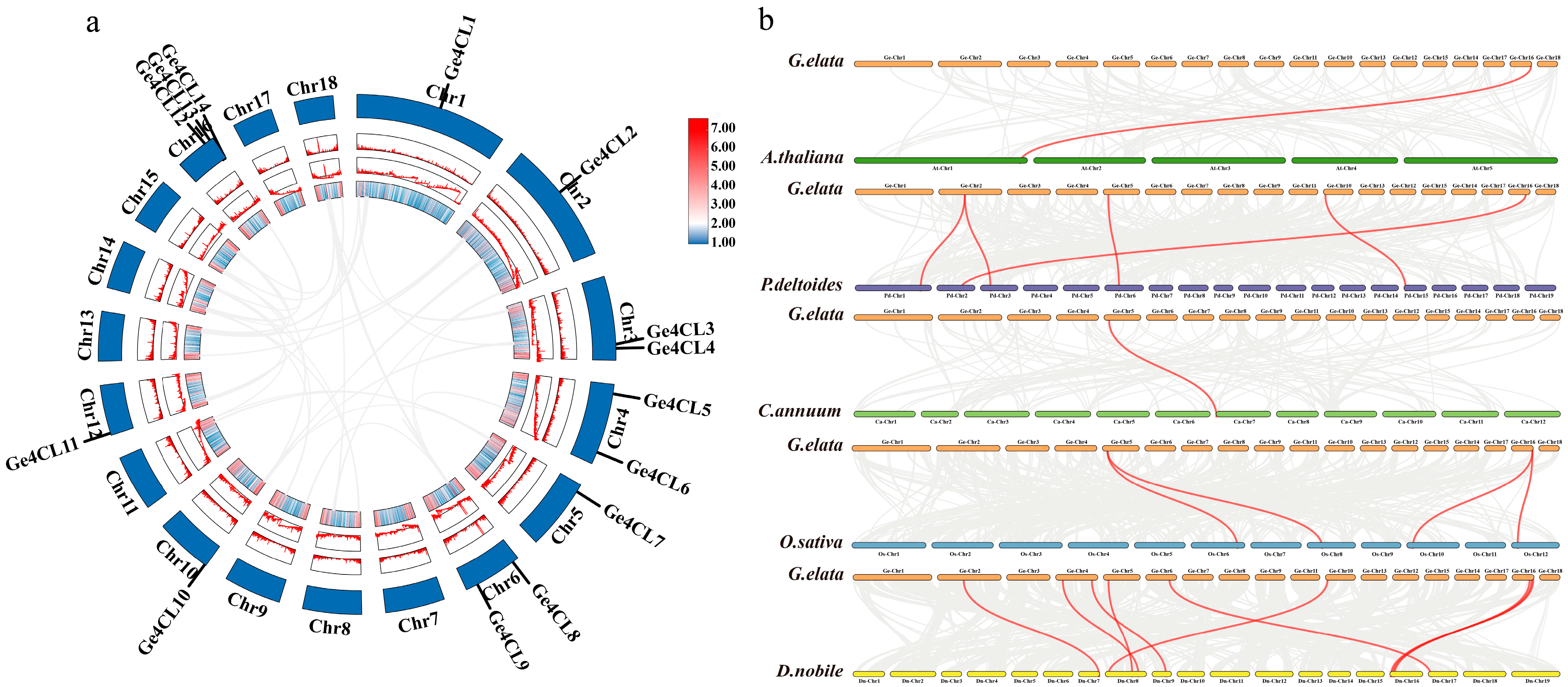
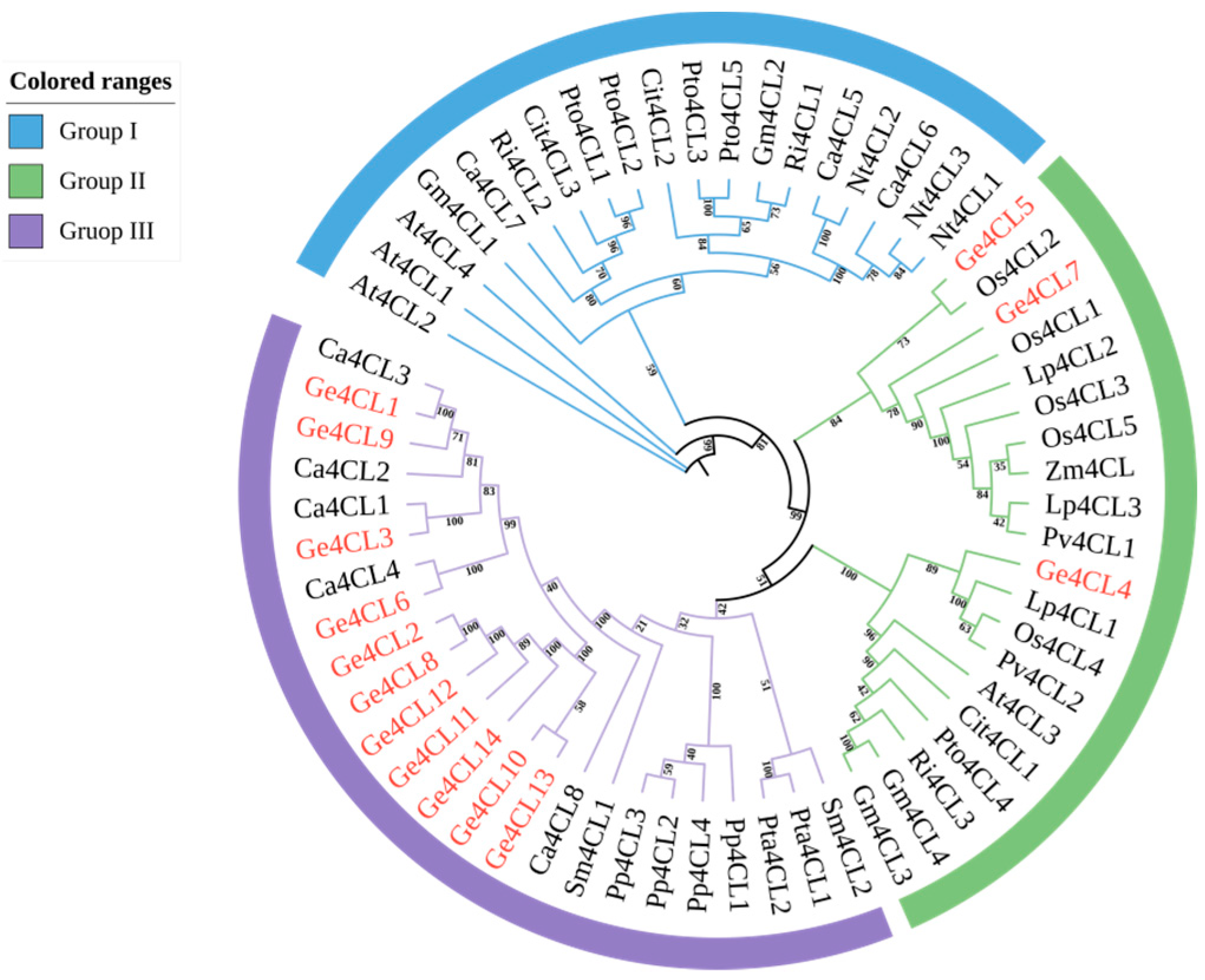
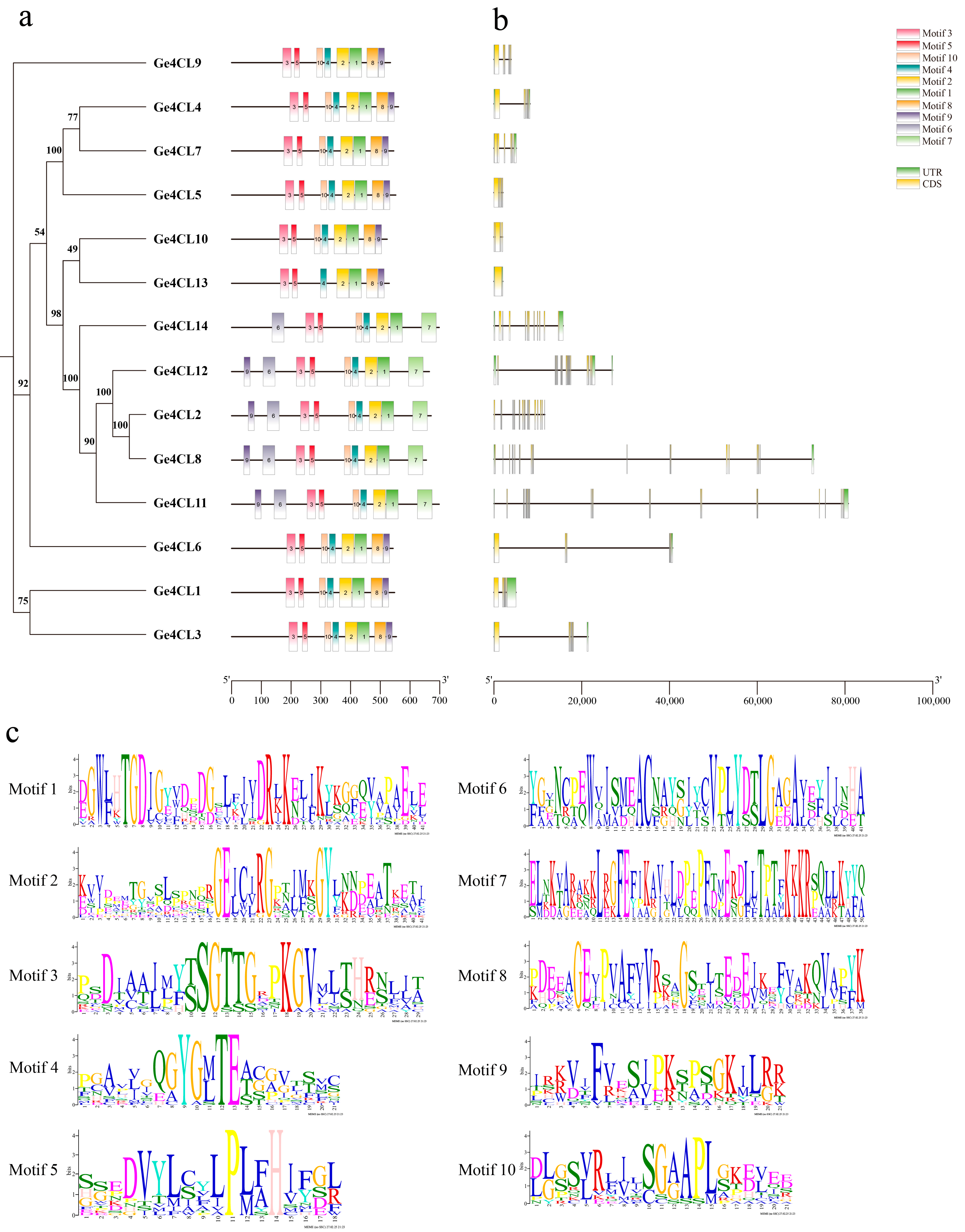
| Gene Name | Gene ID | Number of aa | Molecular Weight/kD | pI Value | Instability Index | GRAVY | Subcellular Localisation |
|---|---|---|---|---|---|---|---|
| Ge4CL1 | GelC01G00992.1 | 547 | 58.15 | 8.82 | 49.47 | 0.163 | Plasma membrane |
| Ge4CL2 | GelC02G00823.1 | 670 | 74.27 | 7.15 | 33.04 | −0.064 | Cytoplasm |
| Ge4CL3 | GelC03G01279.2 | 553 | 60.16 | 8.51 | 41.85 | 0.095 | Plasma membrane |
| Ge4CL4 | GelC03G01284.1 | 560 | 60.16 | 6.29 | 35.89 | 0.179 | Plasma membrane |
| Ge4CL5 | GelC04G00359.1 | 551 | 60.10 | 5.42 | 43.37 | 0.06 | Chloroplast |
| Ge4CL6 | GelC04G01345.1 | 542 | 58.78 | 6.19 | 40.94 | 0.1 | Plasma membrane |
| Ge4CL7 | GelC05G00275.1 | 544 | 59.27 | 5.59 | 38.71 | 0.077 | Plasma membrane |
| Ge4CL8 | GelC06G00150.1 | 654 | 73.00 | 6.23 | 27.81 | −0.081 | Cytoplasm |
| Ge4CL9 | GelC06G00789.1 | 533 | 57.28 | 6.47 | 44.49 | 0.166 | Plasma membrane |
| Ge4CL10 | GelC10G00141.1 | 522 | 55.70 | 5.88 | 42.5 | 0.022 | Endoplasmic reticulum |
| Ge4CL11 | GelC12G00053.2 | 697 | 77.41 | 6.53 | 34.69 | −0.113 | Cytoplasm |
| Ge4CL12 | GelC16G00539.1 | 664 | 75.08 | 6.49 | 35.78 | −0.191 | Cytoplasm |
| Ge4CL13 | GelC16G00628.1 | 529 | 57.48 | 6.34 | 36.82 | 0.043 | Chloroplast |
| Ge4CL14 | GelC16G00735.1 | 697 | 76.24 | 6.47 | 36.1 | 0.005 | Plasma membrane |
| Gene Name | T3s | C3s | A3s | G3s | GC3s | GC | GC1 | GC2 | GC3 | CAI | CBI | Fop | ENC | L_sym | Aromo |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ge4CL1 | 0.15 | 0.53 | 0.17 | 0.33 | 0.73 | 0.61 | 0.61 | 0.49 | 0.73 | 0.21 | 0.14 | 0.49 | 45.27 | 531 | 0.07 |
| Ge4CL2 | 0.43 | 0.24 | 0.32 | 0.27 | 0.42 | 0.43 | 0.51 | 0.37 | 0.42 | 0.20 | −0.07 | 0.38 | 56.44 | 646 | 0.10 |
| Ge4CL3 | 0.32 | 0.35 | 0.24 | 0.29 | 0.55 | 0.51 | 0.54 | 0.44 | 0.55 | 0.21 | 0.03 | 0.43 | 54.02 | 533 | 0.09 |
| Ge4CL4 | 0.22 | 0.41 | 0.17 | 0.41 | 0.68 | 0.58 | 0.61 | 0.44 | 0.68 | 0.22 | 0.07 | 0.45 | 53.90 | 546 | 0.07 |
| Ge4CL5 | 0.17 | 0.53 | 0.13 | 0.39 | 0.75 | 0.58 | 0.56 | 0.41 | 0.75 | 0.22 | 0.10 | 0.47 | 43.68 | 530 | 0.06 |
| Ge4CL6 | 0.30 | 0.38 | 0.23 | 0.29 | 0.56 | 0.51 | 0.54 | 0.43 | 0.56 | 0.20 | 0.02 | 0.42 | 56.44 | 531 | 0.08 |
| Ge4CL7 | 0.21 | 0.50 | 0.12 | 0.39 | 0.73 | 0.59 | 0.61 | 0.41 | 0.73 | 0.20 | 0.06 | 0.43 | 45.60 | 524 | 0.07 |
| Ge4CL8 | 0.45 | 0.21 | 0.33 | 0.28 | 0.40 | 0.43 | 0.52 | 0.37 | 0.40 | 0.20 | −0.06 | 0.38 | 55.16 | 634 | 0.11 |
| Ge4CL9 | 0.22 | 0.40 | 0.22 | 0.35 | 0.63 | 0.56 | 0.58 | 0.47 | 0.63 | 0.21 | 0.13 | 0.48 | 53.56 | 518 | 0.06 |
| Ge4CL10 | 0.16 | 0.48 | 0.15 | 0.41 | 0.74 | 0.61 | 0.61 | 0.48 | 0.74 | 0.23 | 0.13 | 0.49 | 45.28 | 513 | 0.07 |
| Ge4CL11 | 0.47 | 0.18 | 0.35 | 0.25 | 0.35 | 0.42 | 0.48 | 0.42 | 0.35 | 0.20 | −0.13 | 0.35 | 51.35 | 677 | 0.10 |
| Ge4CL12 | 0.43 | 0.19 | 0.42 | 0.24 | 0.35 | 0.41 | 0.50 | 0.38 | 0.35 | 0.18 | −0.12 | 0.35 | 49.18 | 639 | 0.11 |
| Ge4CL13 | 0.09 | 0.56 | 0.11 | 0.42 | 0.83 | 0.66 | 0.65 | 0.49 | 0.83 | 0.20 | 0.10 | 0.46 | 41.15 | 506 | 0.07 |
| Ge4CL14 | 0.41 | 0.25 | 0.30 | 0.28 | 0.43 | 0.46 | 0.53 | 0.42 | 0.43 | 0.21 | −0.02 | 0.41 | 55.96 | 675 | 0.09 |
| Codon | AA | Ge/Ec | Ge/Nt | Ge/At | Ge/Sc | Ge/Os | Codon | AA | Ge/Ec | Ge/Nt | Ge/At | Ge/Sc | Ge/Os |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UUU | Phe | 0.86 | 0.84 | 0.97 | 0.81 | 1.61 | UAU | Tyr | 0.6 | 0.73 | 0.89 | 0.69 | 1.3 |
| UUC | 1.51 | 1.17 | 1.01 | 1.14 | 0.94 | UAC | 1.58 | 1.37 | 1.35 | 1.25 | 1.23 | ||
| UUA | Leu | 0.47 | 0.6 | 0.64 | 0.31 | 1.33 | UAA | TER | 0.1 | 0.18 | 0.22 | 0.18 | 0.29 |
| UUG | 1.21 | 0.7 | 0.75 | 0.57 | 1.06 | UAG | 2 | 1.2 | 1.2 | 1.2 | 0.75 | ||
| CUU | 1.21 | 0.73 | 0.73 | 1.42 | 1.15 | CAU | His | 0.85 | 0.78 | 0.76 | 0.77 | 0.93 | |
| CUC | 3.07 | 2.37 | 1.81 | 5.4 | 1.13 | CAC | 1.34 | 1.13 | 1.13 | 1.26 | 0.71 | ||
| CUA | 1.34 | 0.8 | 0.76 | 0.56 | 0.97 | CAA | Gln | 0.68 | 0.47 | 0.99 | 0.36 | 0.73 | |
| CUG | 0.57 | 2.09 | 2.17 | 2.03 | 1.01 | CAG | 0.56 | 1 | 0.99 | 1.24 | 0.72 | ||
| AUU | Ile | 0.85 | 0.91 | 1.18 | 0.84 | 1.78 | AAU | Asn | 0.58 | 0.6 | 0.76 | 0.47 | 1.12 |
| AUC | 1.3 | 1.82 | 1.37 | 1.47 | 1.3 | AAC | 0.67 | 0.77 | 0.66 | 0.55 | 1.74 | ||
| AUA | 1 | 0.95 | 1.06 | 0.75 | 1.51 | AAA | Lys | 0.56 | 0.63 | 0.67 | 0.49 | 1.29 | |
| AUG | Met | 0.92 | 0.87 | 0.89 | 1.04 | 0.91 | AAG | 1.99 | 0.91 | 0.93 | 0.99 | 0.94 | |
| GUU | Val | 1.06 | 0.86 | 0.85 | 1.04 | 1 | GAU | Asp | 0.82 | 0.75 | 0.75 | 0.73 | 1.09 |
| GUC | 1.79 | 2.1 | 1.83 | 1.98 | 1.16 | GAC | 1.14 | 1.21 | 1.19 | 1.01 | 0.73 | ||
| GUA | 0.71 | 0.82 | 0.94 | 0.79 | 1.37 | GAA | Glu | 0.77 | 0.75 | 0.79 | 0.59 | 1.25 | |
| GUG | 1.16 | 1.38 | 1.33 | 2.14 | 0.95 | GAG | 1.67 | 0.8 | 1.01 | 1.69 | 0.84 | ||
| UCU | Ser | 1.20 | 0.79 | 0.62 | 0.67 | 1.24 | UGU | Cys | 1 | 0.6 | 1.56 | 0.73 | 0.95 |
| UCC | 2.11 | 2.01 | 1.83 | 1.44 | 1.26 | UGC | 2.29 | 1.75 | 1.75 | 2.63 | 1.02 | ||
| UCA | 0.89 | 0.66 | 0.64 | 0.63 | 0.94 | UGA | TER | 0.73 | 0.8 | 0.67 | 1.42 | 0.67 | |
| UCG | 1.5 | 1.32 | 1.32 | 1.43 | 1 | UGG | Trp | 0.72 | 0.8 | 0.78 | 0.93 | 0.7 | |
| CCU | Pro | 1.85 | 0.94 | 0.94 | 1.3 | 1.29 | CGU | Arg | 0.32 | 0.68 | 0.57 | 0.8 | 0.71 |
| CCC | 2.43 | 2.29 | 2.85 | 2.22 | 1.25 | CGC | 0.74 | 2.64 | 2.71 | 3.96 | 0.64 | ||
| CCA | 1.16 | 0.54 | 0.66 | 0.58 | 0.75 | CGA | 0.96 | 0.87 | 0.73 | 1.53 | 0.72 | ||
| CCG | 1.03 | 3 | 1.74 | 2.83 | 0.83 | CGG | 1.05 | 2.24 | 1.69 | 4.88 | 0.62 | ||
| ACU | Thr | 0.89 | 0.57 | 0.66 | 0.57 | 1.09 | AGU | Ser | 0.39 | 0.39 | 0.37 | 0.37 | 0.59 |
| ACC | 0.77 | 1.5 | 1.41 | 1.14 | 0.97 | AGC | 0.92 | 1.32 | 1.17 | 1.35 | 0.83 | ||
| ACA | 0.8 | 0.7 | 0.77 | 0.68 | 1.04 | AGA | Arg | 1.39 | 0.62 | 0.52 | 0.46 | 0.94 | |
| ACG | 1.99 | 2.29 | 1.34 | 1.29 | 0.9 | AGG | 0.93 | 0.96 | 1.06 | 1.27 | 0.73 | ||
| GCU | Ala | 1.08 | 0.65 | 0.72 | 0.96 | 1.04 | GGU | Gly | 0.5 | 0.53 | 0.53 | 0.49 | 0.8 |
| GCC | 1.32 | 2.29 | 2.78 | 0.27 | 0.93 | GGC | 1.23 | 2.26 | 2.75 | 2.58 | 0.86 | ||
| GCA | 0.78 | 1.78 | 1.03 | 1.11 | 1.04 | GGA | 1.63 | 0.96 | 0.92 | 2.04 | 1.4 | ||
| GCG | 0.85 | 3.09 | 1.99 | 2.89 | 0.67 | GGG | 1.56 | 2.78 | 1.88 | 3.2 | 1.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sha, S.; Mu, K.; Luo, Q.; Yao, S.; Tang, T.; Sun, W.; Ju, Z.; Pang, Y. Genome-Wide Identification and Characterisation of the 4-Coumarate–CoA Ligase (4CL) Gene Family in Gastrodia elata and Their Transcriptional Response to Fungal Infection. Int. J. Mol. Sci. 2025, 26, 7610. https://doi.org/10.3390/ijms26157610
Sha S, Mu K, Luo Q, Yao S, Tang T, Sun W, Ju Z, Pang Y. Genome-Wide Identification and Characterisation of the 4-Coumarate–CoA Ligase (4CL) Gene Family in Gastrodia elata and Their Transcriptional Response to Fungal Infection. International Journal of Molecular Sciences. 2025; 26(15):7610. https://doi.org/10.3390/ijms26157610
Chicago/Turabian StyleSha, Shan, Kailang Mu, Qiumei Luo, Shi Yao, Tianyu Tang, Wei Sun, Zhigang Ju, and Yuxin Pang. 2025. "Genome-Wide Identification and Characterisation of the 4-Coumarate–CoA Ligase (4CL) Gene Family in Gastrodia elata and Their Transcriptional Response to Fungal Infection" International Journal of Molecular Sciences 26, no. 15: 7610. https://doi.org/10.3390/ijms26157610
APA StyleSha, S., Mu, K., Luo, Q., Yao, S., Tang, T., Sun, W., Ju, Z., & Pang, Y. (2025). Genome-Wide Identification and Characterisation of the 4-Coumarate–CoA Ligase (4CL) Gene Family in Gastrodia elata and Their Transcriptional Response to Fungal Infection. International Journal of Molecular Sciences, 26(15), 7610. https://doi.org/10.3390/ijms26157610







