PSMA-Targeted Radiolabeled Peptide for Imaging and Therapy in Prostate Cancer: Preclinical Evaluation of Biodistribution and Therapeutic Efficacy
Abstract
1. Introduction
2. Results
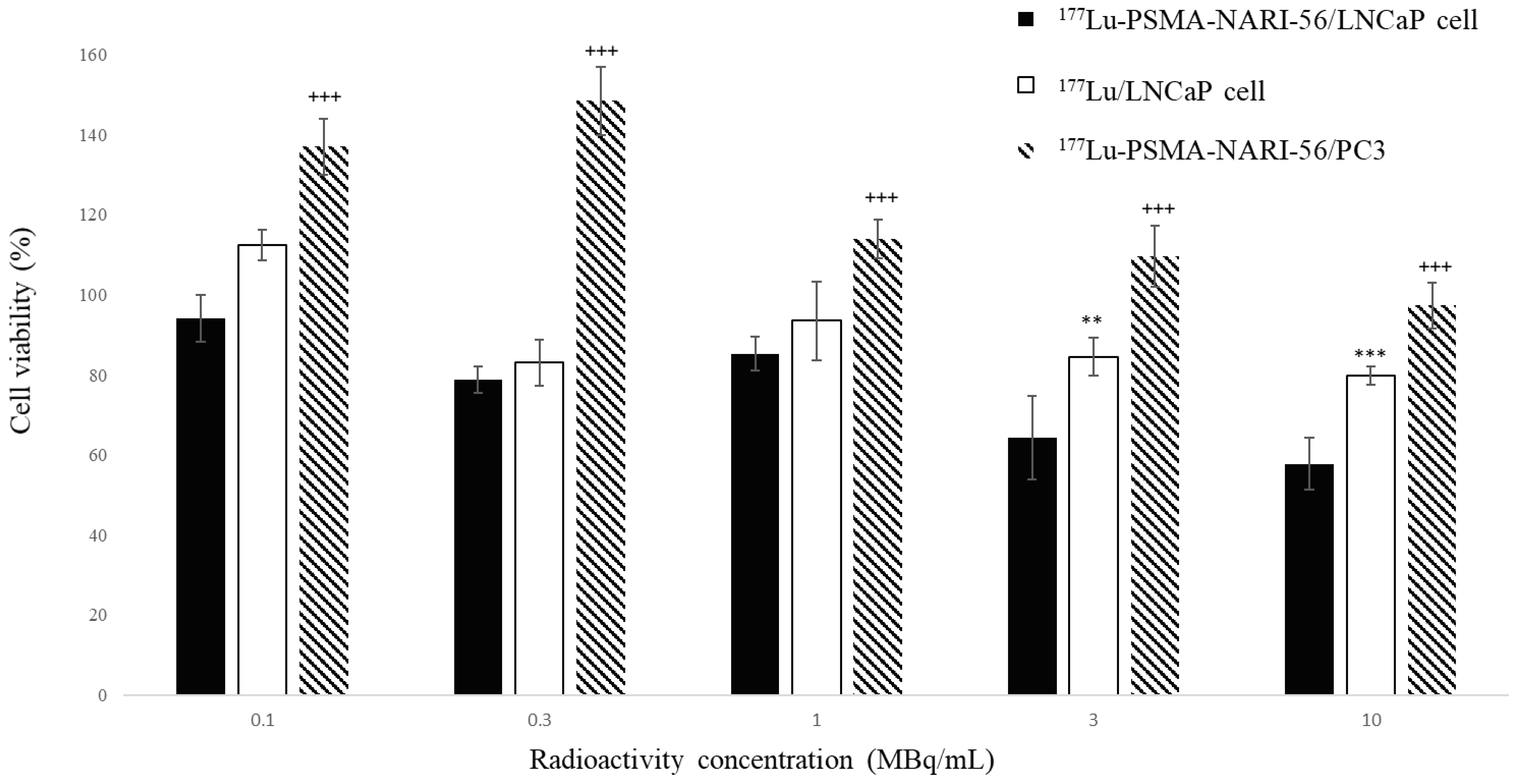
2.1. LNCaP Cell Viability Assay with 177Lu-PSMA-NARI-56
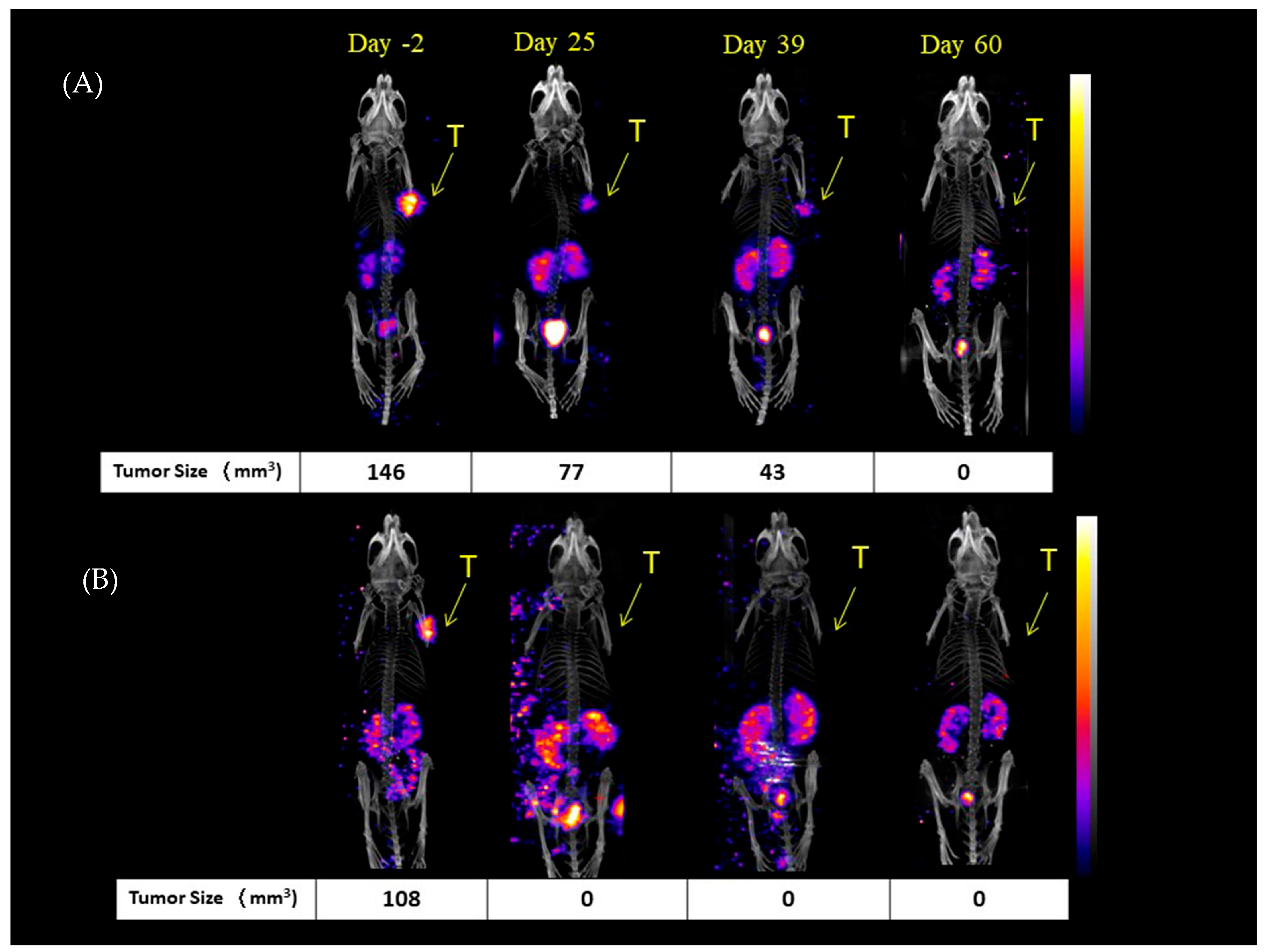
2.2. Mice NanoSPECT/CT Images
2.3. Biodistribution of 177Lu-PSMA-NARI-56 in LNCaP Xenografts Tumor-Bearing Mice
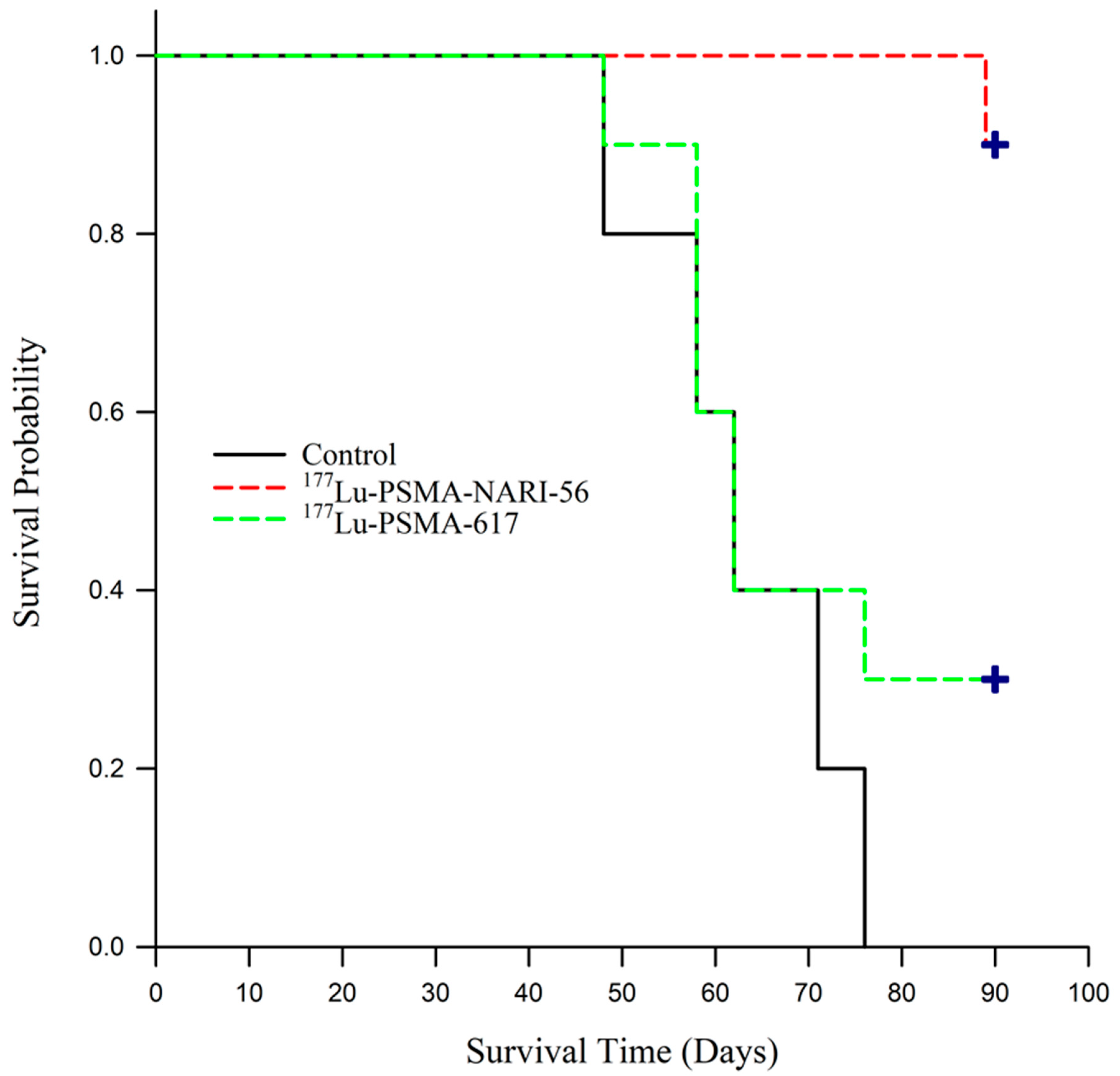
2.4. Efficacy of 177Lu-PSMA-NARI-56 vs. 177Lu-PSMA-617 Radionuclide Therapy
3. Discussion
4. Materials and Methods
4.1. Radio-Synthesis of Peptides with Indium-111 and Lutetium-177
4.2. Cell Culture and Tumor-Bearing Mice
4.3. Cell Viability Assay
4.4. NanoSPECT/CT Imaging of 111In-PSMA-NARI-56 on Tumor-Bearing Mice
4.5. Biodistribution of 177Lu-PSMA-NARI-56 in LNCaP Tumor-Bearing Mice
4.6. Radionuclide Therapy Study of 177Lu-PSMA-NARI-56 and 177Lu-PSMA-617
4.7. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Halabi, S.; Vogelzang, N.J.; Kornblith, A.B.; Ou, S.S.; Kantoff, P.W.; Dawson, N.A.; Small, E.J. Pain predicts overall survival in men with metastatic castration-refractory prostate cancer. J. Clin. Oncol. 2008, 26, 2544–2549. [Google Scholar] [CrossRef]
- Cornford, P.; Bellmunt, J.; Bolla, M.; Briers, E.; De Santis, M.; Gross, T.; Henry, A.M.; Joniau, S.; Lam, T.B.; Mason, M.D.; et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur. Urol. 2017, 71, 630–642. [Google Scholar] [CrossRef]
- de Bono, J.S.; Oudard, S.; Ozguroglu, M.; Hansen, S.; Machiels, J.P.; Kocak, I.; Gravis, G.; Bodrogi, I.; Mackenzie, M.J.; Shen, L.; et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 2010, 376, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Elgqvist, J.; Frost, S.; Pouget, J.P.; Albertsson, P. The potential and hurdles of targeted alpha therapy—Clinical trials and beyond. Front. Oncol. 2014, 3, 324. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef]
- Scher, H.I.; Fizazi, K.; Saad, F.; Taplin, M.E.; Sternberg, C.N.; Miller, K.; de Wit, R.; Mulders, P.; Chi, K.N.; Shore, N.D.; et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 2012, 367, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.L.; Marks, G.; Levine, A. The influence of symptoms of disease and side effects of treatment on compliance with cancer therapy. J. Clin. Oncol. 1988, 6, 1746–1752. [Google Scholar] [CrossRef]
- Mhawech-Fauceglia, P.; Zhang, S.; Terracciano, L.; Sauter, G.; Chadhuri, A.; Herrmann, F.R.; Penetrante, R. Prostate-specific membrane antigen (PSMA) protein expression in normal and neoplastic tissues and its sensitivity and specificity in prostate adenocarcinoma: An immunohistochemical study using mutiple tumour tissue microarray technique. Histopathology 2007, 50, 472–483. [Google Scholar] [CrossRef]
- Sweat, S.D.; Pacelli, A.; Murphy, G.P.; Bostwick, D.G. Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology 1998, 52, 637–640. [Google Scholar] [CrossRef]
- Yao, V.; Berkman, C.E.; Choi, J.K.; O’Keefe, D.S.; Bacich, D.J. Expression of prostate-specific membrane antigen (PSMA), increases cell folate uptake and proliferation and suggests a novel role for PSMA in the uptake of the non-polyglutamated folate, folic acid. Prostate 2010, 70, 305–316. [Google Scholar] [CrossRef]
- Nishida, H.; Kondo, Y.; Kusaba, T.; Kadowaki, H.; Daa, T. Immunohistochemical Reactivity of Prostate-Specific Membrane Antigen in Salivary Gland Tumors. Head Neck Pathol. 2022, 16, 427–433. [Google Scholar] [CrossRef]
- Bostwick, D.G.; Pacelli, A.; Blute, M.; Roche, P.; Murphy, G.P. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: A study of 184 cases. Cancer 1998, 82, 2256–2261. [Google Scholar] [CrossRef]
- Alam, M.R.; Singh, S.B.; Thapaliya, S.; Shrestha, S.; Deo, S.; Khanal, K. A Review of 177Lutetium-PSMA and 225Actinium-PSMA as Emerging Theranostic Agents in Prostate Cancer. Cureus 2022, 14, e29369. [Google Scholar] [CrossRef] [PubMed]
- Virgolini, I.; Decristoforo, C.; Haug, A.; Fanti, S.; Uprimny, C. Current status of theranostics in prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 471–495. [Google Scholar] [CrossRef] [PubMed]
- Benešová, M.; Umbricht, C.A.; Schibli, R.; Müller, C. Albumin-Binding PSMA Ligands: Optimization of the Tissue Distribution Profile. Mol. Pharm. 2018, 15, 934–946. [Google Scholar] [CrossRef]
- Choy, C.J.; Ling, X.; Geruntho, J.J.; Beyer, S.K.; Latoche, J.D.; Langton-Webster, B.; Anderson, C.J.; Berkman, C.E. (177)Lu-Labeled Phosphoramidate-Based PSMA Inhibitors: The Effect of an Albumin Binder on Biodistribution and Therapeutic Efficacy in Prostate Tumor-Bearing Mice. Theranostics 2017, 7, 1928–1939. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.M.; Amor-Coarasa, A.; Nikolopoulou, A.; Wüstemann, T.; Barelli, P.; Kim, D.; Williams, C., Jr.; Zheng, X.; Bi, C.; Hu, B.; et al. Dual-Target Binding Ligands with Modulated Pharmacokinetics for Endoradiotherapy of Prostate Cancer. J. Nucl. Med. 2017, 58, 1442–1449. [Google Scholar] [CrossRef]
- Kuo, H.T.; Merkens, H.; Zhang, Z.; Uribe, C.F.; Lau, J.; Zhang, C.; Colpo, N.; Lin, K.S.; Bénard, F. Enhancing Treatment Efficacy of (177)Lu-PSMA-617 with the Conjugation of an Albumin-Binding Motif: Preclinical Dosimetry and Endoradiotherapy Studies. Mol. Pharm. 2018, 15, 5183–5191. [Google Scholar] [CrossRef]
- Lo, W.L.; Lo, S.W.; Chen, S.J.; Chen, M.W.; Huang, Y.R.; Chen, L.C.; Chang, C.H.; Li, M.H. Molecular Imaging and Preclinical Studies of Radiolabeled Long-Term RGD Peptides in U-87 MG Tumor-Bearing Mice. Int. J. Mol. Sci. 2021, 22, 5459. [Google Scholar] [CrossRef]
- Wang, Z.; Jacobson, O.; Tian, R.; Mease, R.C.; Kiesewetter, D.O.; Niu, G.; Pomper, M.G.; Chen, X. Radioligand Therapy of Prostate Cancer with a Long-Lasting Prostate-Specific Membrane Antigen Targeting Agent (90)Y-DOTA-EB-MCG. Bioconjug. Chem. 2018, 29, 2309–2315. [Google Scholar] [CrossRef] [PubMed]
- Eichten, A.; Adler, A.P.; Cooper, B.; Griffith, J.; Wei, Y.; Yancopoulos, G.D.; Lin, H.C.; Thurston, G. Rapid decrease in tumor perfusion following VEGF blockade predicts long-term tumor growth inhibition in preclinical tumor models. Angiogenesis 2013, 16, 429–441. [Google Scholar] [CrossRef][Green Version]
- Yadav, M.P.; Ballal, S.; Bal, C.; Sahoo, R.K.; Damle, N.A.; Tripathi, M.; Seth, A. Efficacy and Safety of 177Lu-PSMA-617 Radioligand Therapy in Metastatic Castration-Resistant Prostate Cancer Patients. Clin. Nucl. Med. 2020, 45, 19–31. [Google Scholar] [CrossRef]
- Kuo, H.T.; Lin, K.S.; Zhang, Z.; Uribe, C.F.; Merkens, H.; Zhang, C.; Bénard, F. (177)Lu-Labeled Albumin-Binder-Conjugated PSMA-Targeting Agents with Extremely High Tumor Uptake and Enhanced Tumor-to-Kidney Absorbed Dose Ratio. J. Nucl. Med. 2021, 62, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Umbricht, C.A.; Gracheva, N.; Tschan, V.J.; Pellegrini, G.; Bernhardt, P.; Zeevaart, J.R.; Köster, U.; Schibli, R.; van der Meulen, N.P. Terbium-161 for PSMA-targeted radionuclide therapy of prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1919–1930. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.J.; Chang, C.H.; Chang, T.J.; Yu, C.Y.; Chen, L.C.; Jan, M.L.; Luo, T.Y.; Lee, T.W.; Ting, G. Biodistribution, pharmacokinetics and microSPECT/CT imaging of 188Re-bMEDA-liposome in a C26 murine colon carcinoma solid tumor animal model. Anticancer Res. 2007, 27, 2217–2225. [Google Scholar] [PubMed]
- Umbricht, C.A.; Benešová, M.; Schibli, R.; Müller, C. Preclinical Development of Novel PSMA-Targeting Radioligands: Modulation of Albumin-Binding Properties To Improve Prostate Cancer Therapy. Mol. Pharm. 2018, 15, 2297–2306. [Google Scholar] [CrossRef]
- Bacich, D.J.; Pinto, J.T.; Tong, W.P.; Heston, W.D. Cloning, expression, genomic localization, and enzymatic activities of the mouse homolog of prostate-specific membrane antigen/NAALADase/folate hydrolase. Mamm. Genome 2001, 12, 117–123. [Google Scholar] [CrossRef]
- Wang, Z.; Tian, R.; Niu, G.; Ma, Y.; Lang, L.; Szajek, L.P.; Kiesewetter, D.O.; Jacobson, O.; Chen, X. Single Low-Dose Injection of Evans Blue Modified PSMA-617 Radioligand Therapy Eliminates Prostate-Specific Membrane Antigen Positive Tumors. Bioconjug. Chem. 2018, 29, 3213–3221. [Google Scholar] [CrossRef]
- Rathke, H.; Giesel, F.L.; Flechsig, P.; Kopka, K.; Mier, W.; Hohenfellner, M.; Haberkorn, U.; Kratochwil, C. Repeated (177)Lu-Labeled PSMA-617 Radioligand Therapy Using Treatment Activities of Up to 9.3 GBq. J. Nucl. Med. 2018, 59, 459–465. [Google Scholar] [CrossRef]
- Vegt, E.; de Jong, M.; Wetzels, J.F.; Masereeuw, R.; Melis, M.; Oyen, W.J.; Gotthardt, M.; Boerman, O.C. Renal toxicity of radiolabeled peptides and antibody fragments: Mechanisms, impact on radionuclide therapy, and strategies for prevention. J. Nucl. Med. 2010, 51, 1049–1058. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.L.; Leotta, K.; Eder, M.; Hoppe-Tich, T.; Youssoufian, H.; Kopka, K.; Babich, J.W.; Haberkorn, U. PMPA for nephroprotection in PSMA-targeted radionuclide therapy of prostate cancer. J. Nucl. Med. 2015, 56, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Fendler, W.P.; Stuparu, A.D.; Evans-Axelsson, S.; Lückerath, K.; Wei, L.; Kim, W.; Poddar, S.; Said, J.; Radu, C.G.; Eiber, M.; et al. Establishing (177)Lu-PSMA-617 Radioligand Therapy in a Syngeneic Model of Murine Prostate Cancer. J. Nucl. Med. 2017, 58, 1786–1792. [Google Scholar] [CrossRef] [PubMed]
- Matteucci, F.; Mezzenga, E.; Caroli, P.; Di Iorio, V.; Sarnelli, A.; Celli, M.; Fantini, L.; Moretti, A.; Galassi, R.; De Giorgi, U.; et al. Reduction of (68)Ga-PSMA renal uptake with mannitol infusion: Preliminary results. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 2189–2194. [Google Scholar] [CrossRef] [PubMed]

| Mean ± SD (%ID/g) | Biodistribution of 177Lu-PSMA-NARI-56 in LNCaP Tumor-Bearing Mice (n = 4) | |||||
|---|---|---|---|---|---|---|
| 1 h | 4 h | 24 h | 48 h | 72 h | 96 h | |
| Blood | 27.69 ± 8.21 | 12.55 ± 1.45 | 2.13 ± 0.66 | 0.67 ± 0.30 | 0.26 ± 0.15 | 0.13 ± 0.02 |
| Skin | 8.85 ± 1.96 | 3.55 ± 0.54 | 1.01 ± 0.45 | 0.35 ± 0.16 | 0.19 ± 0.08 | 0.13 ± 0.03 |
| Muscle | 3.38 ± 1.15 | 1.48 ± 0.11 | 0.28 ± 0.18 | 0.10 ± 0.04 | 0.05 ± 0.03 | 0.07 ± 0.07 |
| Bone | 1.98 ± 0.49 | 0.84 ± 0.12 | 0.44 ± 0.05 | 0.28 ± 0.08 | 0.17 ± 0.09 | 0.20 ± 0.10 |
| Brain | 0.52 ± 0.14 | 0.30 ± 0.06 | 0.10 ± 0.05 | 0.05 ± 0.02 | 0.03 ± 0.01 | 0.02 ± 0.00 |
| Bladder | 7.74 ± 1.24 | 7.50 ± 6.70 | 1.14 ± 0.58 | 0.26 ± 0.11 | 0.13 ± 0.06 | 0.04 ± 0.03 |
| Pancreas | 3.48 ± 0.83 | 1.67 ± 0.41 | 0.35 ± 0.15 | 0.12 ± 0.06 | 0.06 ± 0.02 | 0.03 ± 0.01 |
| Spleen | 9.66 ± 4.22 | 3.57 ± 1.15 | 1.14 ± 0.57 | 0.44 ± 0.17 | 0.29 ± 0.13 | 0.16 ± 0.06 |
| Stomach | 3.96 ± 1.35 | 1.75 ± 0.50 | 0.51 ± 0.30 | 0.15 ± 0.06 | 0.06 ± 0.02 | 0.04 ± 0.01 |
| Small intestine | 5.68 ± 0.70 | 3.21 ± 0.94 | 0.56 ± 0.27 | 0.19 ± 0.05 | 0.08 ± 0.03 | 0.05 ± 0.02 |
| Large intestine | 4.34 ± 1.19 | 2.27 ± 0.53 | 0.46 ± 0.20 | 0.24 ± 0.09 | 0.10 ± 0.02 | 0.08 ± 0.02 |
| Bile | 6.63 ± 8.40 | 2.46 ± 2.13 | 0.53 ± 0.52 | 0.46 ± 0.29 | 0.24 ± 0.20 | 0.06 ± 0.04 |
| Liver | 7.15 ± 1.73 | 3.01 ± 1.04 | 0.66 ± 0.16 | 0.32 ± 0.11 | 0.16 ± 0.03 | 0.10 ± 0.02 |
| Adrenal | 14.72 ± 4.25 | 8.50 ± 3.38 | 2.42 ± 1.35 | 1.35 ± 0.82 | 0.86 ± 0.30 | 0.50 ± 0.34 |
| Kidney | 107.65 ± 37.19 | 92.05 ± 19.62 | 37.27 ± 20.02 | 8.13 ± 4.46 | 3.29 ± 2.47 | 1.55 ± 0.26 |
| Heart | 7.43 ± 2.11 | 2.75 ± 0.41 | 0.60 ± 0.31 | 0.18 ± 0.08 | 0.08 ± 0.05 | 0.05 ± 0.01 |
| Lung | 28.70 ± 7.1 | 9.90 ± 2.69 | 2.47 ± 1.15 | 0.77 ± 0.33 | 0.35 ± 0.15 | 0.19 ± 0.04 |
| Tumor | 26.52 ± 12.11 | 28.91 ± 6.05 | 40.56 ± 10.01 | 31.32 ± 10.37 | 18.57 ± 6.02 | 13.41 ± 2.89 |
| Mean ± SD (%) | Tumor Volume Ratio Post-Treatment/Pre-Treatment | ||
|---|---|---|---|
| Day of Injection | Control | Lu-177-PSMA-NARI-56 | Lu-177-PSMA-617 |
| 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| 7 | 152 ± 44 | 73 ± 23 | 72 ± 27 |
| 10 | 196 ± 54 | 46 ± 19 | 75 ± 34 |
| 13 | 215 ± 66 | 38 ± 14 | 71 ± 34 |
| 16 | 248 ± 64 | 34 ± 11 | 72 ± 36 |
| 20 | 282 ± 63 | 36 ± 13 | 87 ± 66 |
| 23 | 337 ± 82 | 32 ± 15 | 93 ± 68 |
| 27 | 358 ± 100 | 23 ± 12 | 86 ± 66 |
| 30 | 368 ± 133 | 22 ± 13 | 106 ± 102 |
| 34 | 408 ± 150 | 20 ± 13 | 145 ± 129 |
| 37 | 437 ± 137 | 20 ± 15 | 153 ± 129 |
| 41 | 483 ± 157 | 17 ± 15 | 199 ± 185 |
| 44 | 480 ± 175 | 19 ± 17 | 217 ± 121 |
| 48 | 594 ± 232 | 18 ± 14 | 266 ± 229 |
| 51 | 763 ± 314 | 17 ± 16 | 288 ± 281 |
| 55 | 758 ± 313 | 21 ± 25 | 299 ± 319 |
| 58 | 906 ± 400 | 31 ± 39 | 380 ± 446 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.-W.; Huang, Y.-R.; Lo, W.-L.; Lee, S.-Y.; Lo, S.-N.; Wang, S.-M.; Chang, K.-W. PSMA-Targeted Radiolabeled Peptide for Imaging and Therapy in Prostate Cancer: Preclinical Evaluation of Biodistribution and Therapeutic Efficacy. Int. J. Mol. Sci. 2025, 26, 7580. https://doi.org/10.3390/ijms26157580
Chen M-W, Huang Y-R, Lo W-L, Lee S-Y, Lo S-N, Wang S-M, Chang K-W. PSMA-Targeted Radiolabeled Peptide for Imaging and Therapy in Prostate Cancer: Preclinical Evaluation of Biodistribution and Therapeutic Efficacy. International Journal of Molecular Sciences. 2025; 26(15):7580. https://doi.org/10.3390/ijms26157580
Chicago/Turabian StyleChen, Ming-Wei, Yuan-Ruei Huang, Wei-Lin Lo, Shih-Ying Lee, Sheng-Nan Lo, Shih-Ming Wang, and Kang-Wei Chang. 2025. "PSMA-Targeted Radiolabeled Peptide for Imaging and Therapy in Prostate Cancer: Preclinical Evaluation of Biodistribution and Therapeutic Efficacy" International Journal of Molecular Sciences 26, no. 15: 7580. https://doi.org/10.3390/ijms26157580
APA StyleChen, M.-W., Huang, Y.-R., Lo, W.-L., Lee, S.-Y., Lo, S.-N., Wang, S.-M., & Chang, K.-W. (2025). PSMA-Targeted Radiolabeled Peptide for Imaging and Therapy in Prostate Cancer: Preclinical Evaluation of Biodistribution and Therapeutic Efficacy. International Journal of Molecular Sciences, 26(15), 7580. https://doi.org/10.3390/ijms26157580






