Meconium and Amniotic Fluid IgG Fc Binding Protein (FcGBP) Concentrations in Neonates Delivered by Cesarean Section and by Vaginal Birth in the Third Trimester of Pregnancy
Abstract
1. Introduction
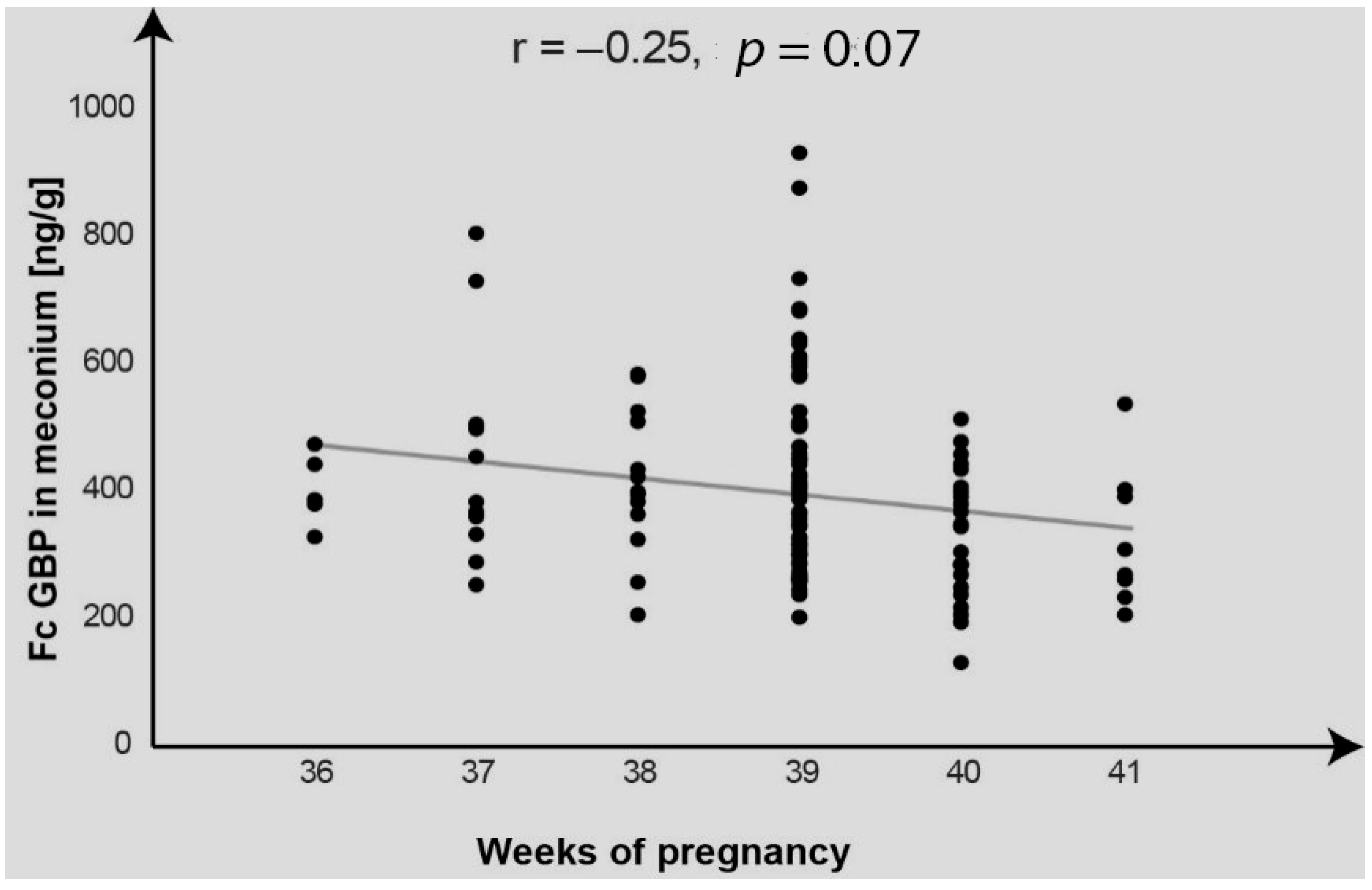
2. Results
3. Discussion
4. Materials and Methods
4.1. Neonates
- -
- Birth weight (mean± SD; range) [g]: 3481 ± 485; (1940–4960)
- -
- Birth length (mean± SD; range) [cm]: 55.1 ± 2.4; (47–60)
- -
- Apgar score: 10/10/10/10 (n = 97), 9/10/10/10 (n = 3), 8/9/10/10 (n = 3), 9/9/10/10 (n = 5), 9/9/9/10 (n = 2), 8/9/9/10 (n = 1), 8/8/9/10 (n = 1), 9/9/9/9 (n = 3), 9/8/9/9 (n = 2), 8/8/8/9 (n = 1), 7/8/8/9 (n = 1), 7/7/8/9 (n = 1).
4.2. Materials
4.2.1. Meconium
4.2.2. Amniotic Fluid
4.3. Methods
4.4. Statistical Analysis
5. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kobayashi, K.; Ogata, H.; Morikawa, M.; Iijima, S.; Harada, N.; Yoshida, T.; Brown, W.R.; Inoue, N.; Hamada, Y.; Ishii, H.; et al. Distribution and partial characterisation of IgG Fc binding protein in various mucin producing cells and body fluids. Gut 2002, 51, 169–176. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, N.; Fischer, A.; Abdo, E.M.; Simon, F.; Peter, H.J.; Gerber, H.; Buergi, U.; Marti, U. Differential expression of IgG Fc binding protein (FcgammaBP) in human normal thyroid tissue, thyroid adenomas and thyroid carcinomas. J. Endocrinol. 2002, 174, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Ehrencrona, E.; van der Post, S.; Gallego, P.; Recktenwald, C.V.; Rodriguez-Pineiro, A.M.; Garcia-Bonete, M.J.; Trillo-Muyo, S.; Bäckström, M.; Hansson, G.C.; Johansson, M.E.V. The IgGFc-binding protein FCGBP is secreted with all GDPH sequences cleaved but maintained by interfragment disulfide bonds. J. Biol. Chem. 2021, 297, 100871. [Google Scholar] [CrossRef]
- Mimoun, A.; Delignat, S.; Peyron, I.; Daventure, V.; Lecerf, M.; Dimitrov, J.D.; Kaveri, S.V.; Bayry, J.; Lacroix-Desmazes, S. Relevance of the materno-fetal interface for the induction of antigen-specific immune tolerance. Front. Immunol. 2020, 11, 810. [Google Scholar] [CrossRef]
- Frazer, L.C.; Good, M. Intestinal epithelium in early life. Mucosal Immunol. 2022, 15, 1181–1187. [Google Scholar] [CrossRef]
- Hossain, Z.; Reza, A.H.M.M.; Qasem, W.A.; Friel, J.K.; Omri, A. Development of the immune system in the human embryo. Pediatr. Res. 2022, 92, 951–955. [Google Scholar] [CrossRef]
- Pirker, A.-L.; Vogl, T. Development of systemic and mucosal immune responses against gut microbiota in early life and implications for the onset of allergies. Front. Allergy 2024, 5, 1439303. [Google Scholar] [CrossRef]
- Fouda, G.G.; Martinez, D.R.; Swamy, G.K.; Permar, S.R. The Impact of IgG transplacental transfer on early life immunity. Immunohorizons 2018, 2, 14–25. [Google Scholar] [CrossRef]
- Jain, N. The early life education of the immune system: Moms, microbes and (missed) opportunities. Gut Microbes 2020, 12, 1824564. [Google Scholar] [CrossRef]
- Ohsaki, A.; Venturelli, N.; Buccigrosso, T.M.; Osganian, S.K.; Lee, J.; Blumberg, R.S.; Oyoshi, M.K. Maternal IgG immune complexes induce food allergen-specific tolerance in offspring. J. Exp. Med. 2018, 215, 91–113. [Google Scholar] [CrossRef] [PubMed]
- Husso, A.; Pessa-Morikawa, T.; Koistinen, V.M.; Kärkkäinen, O.; Kwon, H.N.; Lahti, L.; Iivanainen, A.; Hanhineva, K.; Niku, M. Impacts of maternal microbiota and microbial metabolites on fetal intestine, brain, and placenta. BMC Biol. 2023, 21, 207. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, K.; Henriksen, L. Cesarean section and disease associated with immune function. J. Allergy Clin. Immunol. 2016, 137, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.L. Caesarean delivery, immune function and inflammation in early life among Ecuadorian infants and young children. J. Dev. Orig. Health Dis. 2019, 10, 555–562. [Google Scholar] [CrossRef]
- Turunen, J.; Tejesvi, M.V.; Paalanne, N.; Hekkala, J.; Lindgren, O.; Kaakinen, M.; Pokka, T.; Kaisanlahti, A.; Reunanen, J.; Tapiainen, T. Presence of distinctive microbiome in the first-pass meconium of newborn infants. Sci. Rep. 2021, 11, 19449. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Arya, S.; Choudhary, S.; Jain, S.K. Amniotic fluid: Source of trophic factors for the developing intestine. World J. Gastrointest. Pathophysiol. 2016, 7, 38–47. [Google Scholar] [CrossRef]
- Patel, D.D.; Bussel, J.B. Neonatal Fc receptor in human immunity: Function and role in therapeutic intervention. J. Allergy Clin. Immunol. 2020, 146, 467–478. [Google Scholar] [CrossRef]
- Shah, U.; Dickinson, B.L.; Blumberg, R.S.; Simister, N.E.; Lencer, W.I.; Walker, W.A. Distribution of the IgG Fc receptor, FcRn, in the human fetal intestine. Pediatr. Res. 2003, 53, 295–301. [Google Scholar] [CrossRef]
- Pyzik, M.; Sand, K.M.K.; Hubbard, J.J.; Andersen, J.T.; Sandlie, I.; Blumberg, R.S. The Neonatal Fc Receptor (FcRn): A Misnomer? Front. Immunol. 2019, 10, 1540. [Google Scholar] [CrossRef]
- Peter, H.H.; Ochs, H.D.; Cunningham-Rundles, C.; Vinh, D.C.; Kiessling, P.; Greve, B.; Jolles, S. Targeting FcRn for immunomodulation: Benefits, risks, and practical considerations. J. Allergy Clin. Immunol. 2020, 146, 479–491.e5. [Google Scholar] [CrossRef]
- Aaen, K.H.; Anthi, A.K.; Sandlie, I.; Nilsen, J.; Mester, S.; Andersen, J.T. The neonatal Fc receptor in mucosal immune regulation. Scand. J. Immunol. 2021, 93, e13017. [Google Scholar] [CrossRef]
- Apostol, A.C.; Jensen, K.D.C.; Beaudin, A.E. Training the Fetal Immune System Through Maternal Inflammation-A Layered Hygiene Hypothesis. Front. Immunol. 2020, 11, 123. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Claypool, S.M.; Wagner, J.S.; Mizoguchi, E.; Mizoguchi, A.; Roopenian, D.C.; Lencer, W.I.; Blumberg, R.S. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity 2004, 20, 769–783. [Google Scholar] [CrossRef]
- Zheng, W.; Zhao, W.; Wu, M.; Song, X.; Caro, F.; Sun, X.; Gazzaniga, F.; Stefanetti, G.; Oh, S.; Mekalanos, J.J.; et al. Microbiota-targeted maternal antibodies protect neonates from enteric infection. Nature 2020, 577, 543–548. [Google Scholar] [CrossRef]
- Hussain, T.; Murtaza, G.; Kalhoro, D.H.; Kalhoro, M.S.; Yin, Y.; Chughtai, M.I.; Tan, B.; Yaseen, A.; Rehman, Z.U. Understanding the immune system in fetal protection and maternal infections during pregnancy. J. Immunol. Res. 2022, 24, 7567708. [Google Scholar] [CrossRef]
- Rackaityte, E.; Halkias, J.; Fukui, E.M.; Mendoza, V.F.; Hayzelden, C.; Crawford, E.D.; Fujimura, K.E.; Burt, T.D.; Lynch, S.V. Viable bacterial colonization is highly limited in the human intestine in utero. Nat. Med. 2020, 26, 599–607. [Google Scholar] [CrossRef]
- Abu Raya, B.; Bamberger, E.; Almog, M.; Peri, R.; Srugo, I.; Kessel, A. Immunization of pregnant women against pertussis: The effect of timing on antibody avidity. Vaccine 2015, 33, 1948–1952. [Google Scholar] [CrossRef] [PubMed]
- Lisowska-Myjak, B.; Skarżyńska, E.; Wojdan, K.; Nasierowska-Guttmejer, A. Protein and peptide profiles in neonatal meconium. J. Obs. Gynaecol. Res. 2019, 45, 556–564. [Google Scholar] [CrossRef] [PubMed]

| Biological Material | FcGBP Concentration | ||
|---|---|---|---|
| Mean ± SD | Median | Range | |
| Meconium [ng/g] | 405.78 ± 145.27 | 388.94 | 134.96–933.68 |
| Amniotic fluid [ng/mL] | 135.70 ± 35.83 | 138.24 | 39.36–193.67 |
| concentration FcGBP Mean ± SD; median (range) | Delivery method | |
| Vaginal birth (n = 35) | Cesarean section (n = 85) | |
| Meconium [ng/g] | 352.73 ± 142.16; 327.66 (134.96–880.13) | 427.63 ± 141.62; 410.62 (202.84–933.68) |
| Amniotic fluid [ng/mL] | 121.56 ± 38.09; 126.70 (39.36–182.51) | 141.54 ± 33.42; 148.57 (61.99–196.43) |
| Parameter | Correlation Coefficient |
|---|---|
| Birth weight vs. meconium FcGBP | r = 0.01, p = 0.914 |
| Birth weight vs. amniotic fluid FcGBP | r = 0.02, p = 0.871 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisowska-Myjak, B.; Szczepanik, K.; Skarżyńska, E.; Jakimiuk, A. Meconium and Amniotic Fluid IgG Fc Binding Protein (FcGBP) Concentrations in Neonates Delivered by Cesarean Section and by Vaginal Birth in the Third Trimester of Pregnancy. Int. J. Mol. Sci. 2025, 26, 7579. https://doi.org/10.3390/ijms26157579
Lisowska-Myjak B, Szczepanik K, Skarżyńska E, Jakimiuk A. Meconium and Amniotic Fluid IgG Fc Binding Protein (FcGBP) Concentrations in Neonates Delivered by Cesarean Section and by Vaginal Birth in the Third Trimester of Pregnancy. International Journal of Molecular Sciences. 2025; 26(15):7579. https://doi.org/10.3390/ijms26157579
Chicago/Turabian StyleLisowska-Myjak, Barbara, Kamil Szczepanik, Ewa Skarżyńska, and Artur Jakimiuk. 2025. "Meconium and Amniotic Fluid IgG Fc Binding Protein (FcGBP) Concentrations in Neonates Delivered by Cesarean Section and by Vaginal Birth in the Third Trimester of Pregnancy" International Journal of Molecular Sciences 26, no. 15: 7579. https://doi.org/10.3390/ijms26157579
APA StyleLisowska-Myjak, B., Szczepanik, K., Skarżyńska, E., & Jakimiuk, A. (2025). Meconium and Amniotic Fluid IgG Fc Binding Protein (FcGBP) Concentrations in Neonates Delivered by Cesarean Section and by Vaginal Birth in the Third Trimester of Pregnancy. International Journal of Molecular Sciences, 26(15), 7579. https://doi.org/10.3390/ijms26157579





