Exploring Human Sperm Metabolism and Male Infertility: A Systematic Review of Genomics, Proteomics, Metabolomics, and Imaging Techniques
Abstract
1. Introduction
2. Methods
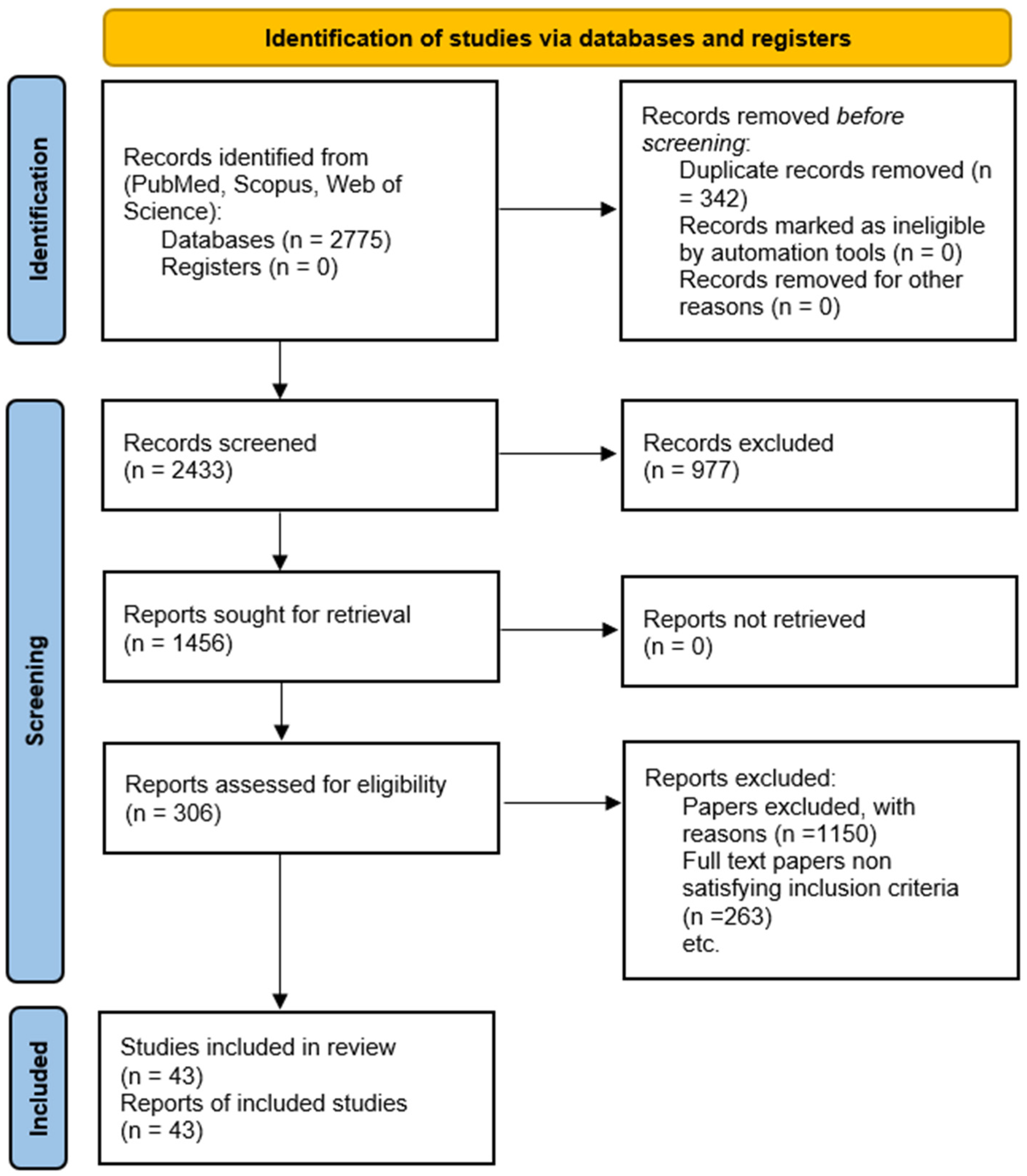
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Synthesis
3. Results
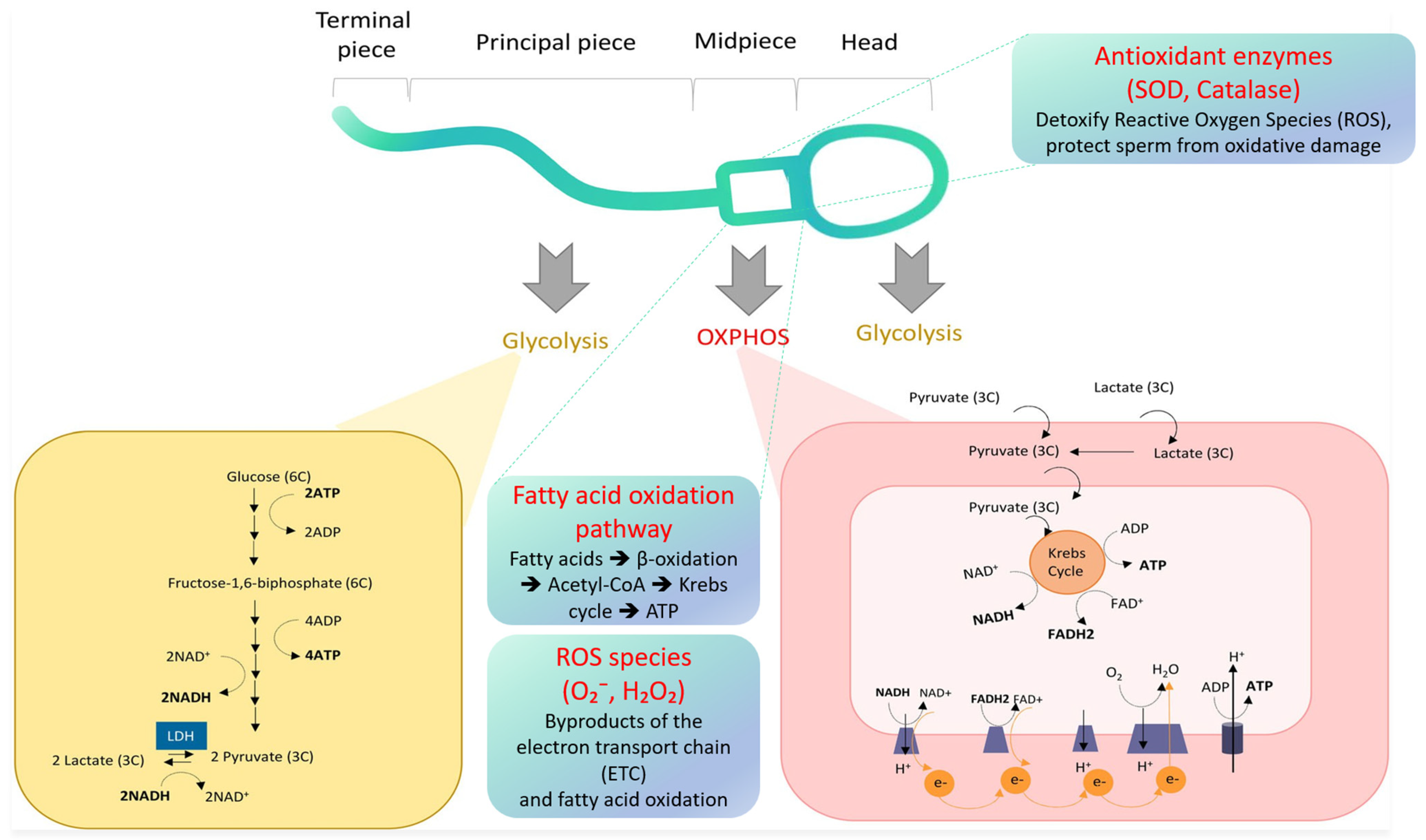
4. Energy Metabolism Pathways in Spermatozoa
5. Metabolic Substrates and Adaptations
5.1. Metabolic Substrates and Transport
5.2. Metabolic Adaptations During Maturation and Transit
6. Molecular Biomarkers and Oxidative Stress
7. Influence of the Seminal Microbiome
8. Advanced Techniques and Tools for Evaluating Sperm Metabolism
9. Clinical Implications: Metabolic Dysfunction in Male Infertility
9.1. Diagnostic Implications
9.2. Prognostic Potential
9.3. Therapeutic Strategies
9.4. Integration with Clinical Workflows
9.5. Disadvantages of Current Methods and Tools to Evaluate Sperm Metabolism
9.6. Complementary Strengths of Metabolic Assessment Techniques
10. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bohnensack, R.; Halangk, W. Control of respiration and of motility in ejaculated bull spermatozoa. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 1986, 850, 72–79. [Google Scholar] [CrossRef]
- Du Plessis, S.S.; Agarwal, A.; Halabi, J.; Tvrda, E. Contemporary evidence on the physiological role of reactive oxygen species in human sperm function. J. Assist. Reprod. Genet. 2015, 32, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Visconti, P. Sperm Bioenergetics in a Nutshell1. Biol. Reprod. 2012, 87, 72. [Google Scholar] [CrossRef]
- Sciorio, R.; Fleming, S.D. Intracytoplasmic sperm injection vs. in-vitro fertilization in couples in whom the male partners had a semen analysis within normal reference ranges: An open debate. Andrology 2023, 12, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Ombelet, W.; Van Blerkom, J.; Boshoff, G.; Huyser, C.; Lopes, F.; Nargund, G.; Sallam, H.; Vanmechelen, K.; Campo, R. Now is the time to introduce new innovative assisted reproduction methods to implement accessible, affordable, and demonstrably successful advanced infertility services in resource-poor countries. Hum. Reprod. Open 2025, 2025, hoaf001. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Infertility. Available online: https://www.who.int/news-room/fact-sheets/detail/infertility (accessed on 17 April 2025).
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef]
- Amaral, A.; Castillo, J.; Estanyol, J.M.; Ballescà, J.L.; Ramalho-Santos, J.; Oliva, R. Human Sperm Tail Proteome Suggests New Endogenous Metabolic Pathways. Mol. Cell Proteom. 2013, 12, 330–342. [Google Scholar] [CrossRef]
- He, J.; Ma, M.; Xu, Z.; Guo, J.; Chen, H.; Yang, X.; Chen, P.; Liu, G. Association between semen microbiome disorder and sperm DNA damage. Microbiol. Spectr. 2024, 12, e00759-24. [Google Scholar] [CrossRef]
- Milardi, D.; Grande, G.; Vincenzoni, F.; Giampietro, A.; Messana, I.; Castagnola, M.; Marana, R.; De Marinis, L.; Pontecorvi, A. Novel Biomarkers of Androgen Deficiency From Seminal Plasma Profiling Using High-Resolution Mass Spectrometry. J. Clin. Endocrinol. Metab. 2014, 99, 2813–2820. [Google Scholar] [CrossRef][Green Version]
- Sharma, R.; Agarwal, A.; Mohanty, G.; Jesudasan, R.; Gopalan, B.; Willard, B.; Yadav, S.P.; Sabanegh, E. Functional proteomic analysis of seminal plasma proteins in men with various semen parameters. Reprod. Biol. Endocrinol. 2013, 11, 38. [Google Scholar] [CrossRef]
- Sharma, R.; Agarwal, A.; Mohanty, G.; Hamada, A.J.; Gopalan, B.; Willard, B.; Yadav, S.; du Plessis, S. Proteomic analysis of human spermatozoa proteins with oxidative stress. Reprod. Biol. Endocrinol. 2013, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Abruzzese, G.A.; Sanchez-Rodriguez, A.; Roldan, E.R. Sperm Metabolism. Mol. Reprod. Dev. 2024, 91, e23772. [Google Scholar] [CrossRef] [PubMed]
- Barceló, M.; Mata, A.; Bassas, L.; Larriba, S. Exosomal microRNAs in seminal plasma are markers of the origin of azoospermia and can predict the presence of sperm in testicular tissue. Hum. Reprod. 2018, 33, 1087–1098. [Google Scholar] [CrossRef] [PubMed]
- Kyrgiafini, M.-A.; Giannoulis, T.; Chatziparasidou, A.; Christoforidis, N.; Mamuris, Z. Unveiling the Genetic Complexity of Teratozoospermia: Integrated Genomic Analysis Reveals Novel Insights into lncRNAs’ Role in Male Infertility. Int. J. Mol. Sci. 2023, 24, 15002. [Google Scholar] [CrossRef]
- Larriba, S.; Sánchez-Herrero, J.F.; Pluvinet, R.; López-Rodrigo, O.; Bassas, L.; Sumoy, L. Seminal extracellular vesicle sncRNA sequencing reveals altered miRNA/isomiR profiles as sperm retrieval biomarkers for azoospermia. Andrology 2023, 12, 137–156. [Google Scholar] [CrossRef]
- Li, J.; Xu, J.; Yang, T.; Chen, J.; Larriba Li, F.; Shen, B.; Fan, C. Genome-Wide Methylation Analyses of Human Sperm Unravel Novel Differentially Methylated Regions in Asthenozoospermia. Epigenomics 2022, 14, 951–964. [Google Scholar] [CrossRef]
- Wainstein, A.; Hassan, S.; Barda, S.; Lehavi, O.; Azem, F.; Ben-Dov, I.Z.; Hauser, R.; Kleiman, S.E. MicroRNAs expression in semen and testis of azoospermic men. Andrology 2023, 11, 687–697. [Google Scholar] [CrossRef]
- Wang, C.; Yang, C.; Chen, X.; Yao, B.; Yang, C.; Zhu, C.; Li, L.; Wang, J.; Li, X.; Shao, Y.; et al. Altered Profile of Seminal Plasma MicroRNAs in the Molecular Diagnosis of Male Infertility. Clin. Chem. 2011, 57, 1722–1731. [Google Scholar] [CrossRef]
- Agarwal, A.; Ayaz, A.; Samanta, L.; Sharma, R.; Assidi, M.; Abuzenadah, A.M.; Sabanegh, E. Comparative proteomic network signatures in seminal plasma of infertile men as a function of reactive oxygen species. Clin. Proteom. 2015, 12, 23. [Google Scholar] [CrossRef]
- Bragado, M.; Garcia-Marin, L.; Calle-Guisado, V.; de Llera, A.; Martin-Hidalgo, D.; Mijares, J.; Gil, M.; Alvarez, I. AMP-activated kinase in human spermatozoa: Identification, intracellular localization, and key function in the regulation of sperm motility. Asian J. Androl. 2017, 19, 707–714. [Google Scholar] [CrossRef]
- Deng, T.; Wang, W.; Fu, Z.; Xie, Y.; Zhou, Y.; Pu, J.; Chen, K.; Yao, B.; Li, X.; Yao, J. Lipidomics random forest algorithm of seminal plasma is a promising method for enhancing the diagnosis of necrozoospermia. Metabolomics 2024, 20, 57. [Google Scholar] [CrossRef]
- Fietz, D.; Sgaier, R.; O’donnell, L.; Stanton, P.G.; Dagley, L.F.; Webb, A.I.; Schuppe, H.-C.; Diemer, T.; Pilatz, A. Proteomic biomarkers in seminal plasma as predictors of reproductive potential in azoospermic men. Front. Endocrinol. 2024, 15, 1327800. [Google Scholar] [CrossRef] [PubMed]
- Hashemitabar, M.; Sabbagh, S.; Orazizadeh, M.; Ghadiri, A.; Bahmanzadeh, M. A proteomic analysis on human sperm tail: Comparison between normozoospermia and asthenozoospermia. J. Assist. Reprod. Genet. 2015, 32, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zheng, Y.; Zeng, W.; Chen, L.; Yang, S.; Du, P.; Wang, Y.; Yu, X.; Zhang, X. Proteomic Profile of Sperm in Infertile Males Reveals Changes in Metabolic Pathways. Protein J. 2021, 40, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Torra-Massana, M.; Jodar, M.; Barragán, M.; Soler-Ventura, A.; Delgado-Dueñas, D.; Rodríguez, A.; Oliva, R.; Vassena, R. Altered mitochondrial function in spermatozoa from patients with repetitive fertilization failure after ICSI revealed by proteomics. Andrology 2021, 9, 1192–1204. [Google Scholar] [CrossRef]
- Martins, A.D.; Selvam, M.K.P.; Agarwal, A.; Alves, M.G.; Baskaran, S. Alterations in seminal plasma proteomic profile in men with primary and secondary infertility. Sci. Rep. 2020, 10, 7539. [Google Scholar] [CrossRef]
- Soler, L.; Labas, V.; Thélie, A.; Grasseau, I.; Teixeira-Gomes, A.-P.; Blesbois, E. Intact Cell MALDI-TOF MS on Sperm: A Molecular Test For Male Fertility Diagnosis. Mol. Cell Proteom. 2016, 15, 1998–2010. [Google Scholar] [CrossRef]
- Sulc, A.; Czétány, P.; Máté, G.; Balló, A.; Semjén, D.; Szántó, Á.; Márk, L. MALDI Imaging Mass Spectrometry Reveals Lipid Alterations in Physiological and Sertoli Cell-Only Syndrome Human Testicular Tissue Sections. Int. J. Mol. Sci. 2024, 25, 8358. [Google Scholar] [CrossRef]
- Xu, Y.; Fan, Y.; Fan, W.; Jing, J.; Xue, K.; Zhang, X.; Ye, B.; Ji, Y.; Liu, Y.; Ding, Z. RNASET2 impairs the sperm motility via PKA/PI3K/calcium signal pathways. Reproduction 2018, 155, 383–392. [Google Scholar] [CrossRef]
- Boguenet, M.; Bocca, C.; Bouet, P.; Serri, O.; Chupin, S.; Tessier, L.; Blanchet, O.; El Hachem, H.; de la Barca, J.M.C.; Reynier, P.; et al. Metabolomic signature of the seminal plasma in men with severe oligoasthenospermia. Andrology 2020, 8, 1859–1866. [Google Scholar] [CrossRef]
- Calvert, S.J.; Reynolds, S.; Paley, M.N.; Walters, S.J.; Pacey, A.A. Probing human Kyrgiafini using 13C-magnetic resonance spectroscopy. Mol. Hum. Reprod. 2018, 25, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, V.; Ghosh, S.; Sengupta, A.; Srivastava, S.; Sonawat, H.M.; Narayan, P.K. Identification of biochemical differences between different forms of male infertility by nuclear magnetic resonance (NMR) spectroscopy. J. Assist. Reprod. Genet. 2014, 31, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Olesti, E.; Boccard, J.; Rahban, R.; Girel, S.; Moskaleva, N.E.; Zufferey, F.; Rossier, M.F.; Nef, S.; Rudaz, S.; González-Ruiz, V. Low-polarity untargeted metabolomic profiling as a tool to gain insight into seminal fluid. Metabolomics 2023, 19, 53. [Google Scholar] [CrossRef] [PubMed]
- Olesti, E.; Garcia, A.; Rahban, R.; Rossier, M.F.; Boccard, J.; Nef, S.; González-Ruiz, V.; Rudaz, S. Steroid profile analysis by LC-HRMS in human seminal fluid. J. Chromatogr. B 2020, 1136, 121929. [Google Scholar] [CrossRef]
- Paiva, C.; Amaral, A.; Rodriguez, M.; Canyellas, N.; Correig, X.; Ballescà, J.L.; Ramalho-Santos, J.; Oliva, R. Identification of endogenous metabolites in human sperm cells using proton nuclear magnetic resonance (1H-NMR) spectroscopy and gas chromatography-mass spectrometry (GC-MS). Andrology 2015, 3, 496–505. [Google Scholar] [CrossRef]
- Qiao, S.; Wu, W.; Chen, M.; Tang, Q.; Xia, Y.; Jia, W.; Wang, X. Seminal plasma metabolomics approach for the diagnosis of unexplained male infertility. PLoS ONE 2017, 12, e0181115. [Google Scholar] [CrossRef]
- Reynolds, S.; Calvert, S.J.; Paley, M.N.; Pacey, A.A. 1H Magnetic Resonance Spectroscopy of live human sperm. Mol. Hum. Reprod. 2017, 23, 441–451. [Google Scholar] [CrossRef]
- Zhao, K.; Zhang, J.; Xu, Z.; Xu, Y.; Xu, A.; Chen, W.; Miao, C.; Liu, S.; Wang, Z.; Jia, R. Metabolomic Profiling of Human Spermatozoa in Idiopathic Asthenozoospermia Patients Using Gas Chromatography-Mass Spectrometry. BioMed Res. Int. 2018, 2018, 8327506. [Google Scholar] [CrossRef]
- Zhao, K.; Zhang, Q.; Cong, R.; Xu, Z.; Xu, Y.; Han, J. Metabolomic profiling of human semen in patients with oligospermia using high performance liquid chromatography-tandem mass spectrometry. Sci. Rep. 2024, 14, 23739. [Google Scholar] [CrossRef]
- Almeida, C.; Cunha, M.; Ferraz, L.; Silva, J.; Barros, A.; Sousa, M. Caspase-3 detection in human testicular spermatozoa from azoospermic and non-azoospermic patients. Int. J. Androl. 2011, 34, e407–e414. [Google Scholar] [CrossRef]
- Cassina, A.; Silveira, P.; Cantu, L.; Montes, J.M.; Radi, R.; Sapiro, R. Defective Human Sperm Cells Are Associated with Mitochondrial Dysfunction and Oxidant Production1. Biol. Reprod. 2015, 93, 119. [Google Scholar] [CrossRef]
- Irigoyen, P.; Pintos-Polasky, P.; Rosa-Villagran, L.; Skowronek, M.F.; Cassina, A.; Sapiro, R. Mitochondrial metabolism determines the functional status of human sperm and correlates with semen parameters. Front. Cell Dev. Biol. 2022, 10, 926684. [Google Scholar] [CrossRef]
- Fukuda, T.; Miyake, H.; Enatsu, N.; Matsushita, K.; Fujisawa, M. Seminal level of clusterin in infertile men as a significant biomarker reflecting spermatogenesis. Andrologia 2016, 48, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Paoli, D.; Gallo, M.; Rizzo, F.; Baldi, E.; Francavilla, S.; Lenzi, A.; Lombardo, F.; Gandini, L. Mitochondrial membrane potential profile and its correlation with increasing sperm motility. Fertil. Steril. 2011, 95, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, R.; Amaral, S.; Redmann, K.; Kliesch, S.; Schlatt, S. Spectral features of nuclear DNA in human sperm assessed by Raman Microspectroscopy: Effects of UV-irradiation and hydration. PLoS ONE 2018, 13, e0207786. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, Y.; Di, L.; Osterberg, E.C.; Liu, F.; He, L.; Hu, H.; Huang, Y.; Li, P.S.; Li, Z. Raman spectroscopy as an ex vivo noninvasive approach to distinguish complete and incomplete spermatogenesis within human seminiferous tubules. Fertil. Steril. 2014, 102, 54–60.e2. [Google Scholar] [CrossRef]
- Aziz, N.; Novotny, J.; Oborna, I.; Fingerova, H.; Brezinova, J.; Svobodova, M. Comparison of chemiluminescence and flow cytometry in the estimation of reactive oxygen and nitrogen species in human semen. Fertil. Steril. 2010, 94, 2604–2608. [Google Scholar] [CrossRef]
- Torrezan-Nitao, E.; Brown, S.G.; Mata-Martínez, E.; Treviño, C.L.; Barratt, C.; Publicover, S. [Ca2+]i oscillations in human sperm are triggered in the flagellum by membrane potential-sensitive activity of CatSper. Hum. Reprod. 2020, 36, 293–304. [Google Scholar] [CrossRef]
- Vashisht, A.; Ahluwalia, P.K.; Gahlay, G.K. A Comparative Analysis of the Altered Levels of Human Seminal Plasma Constituents as Contributing Factors in Different Types of Male Infertility. Curr. Issues Mol. Biol. 2021, 43, 1307–1324. [Google Scholar] [CrossRef]
- Quijano, C.; Trujillo, M.; Castro, L.; Trostchansky, A. Interplay between oxidant species and energy metabolism. Redox Biol. 2016, 8, 28–42. [Google Scholar] [CrossRef]
- Tombes, R.M.; Shapiro, B.M. Metabolite channeling: A phosphorylcreatine shuttle to mediate high energy phosphate transport between sperm mitochondrion and tail. Cell 1985, 41, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Ford, W. Glycolysis and sperm motility: Does a spoonful of sugar help the flagellum go round? Hum. Reprod. Update 2006, 12, 269–274. [Google Scholar] [CrossRef]
- Mann, T. Fructose, a Constituent of Semen. Nature 1946, 157, 79. [Google Scholar] [CrossRef] [PubMed]
- Frenette, G.; Lessard, C.; Madore, E.; Fortier, M.A.; Sullivan, R. Aldose Reductase and Macrophage Migration Inhibitory Factor Are Associated with Epididymosomes and Spermatozoa in the Bovine Epididymis1. Biol. Reprod. 2003, 69, 1586–1592. [Google Scholar] [CrossRef]
- Rigau, T.; Rivera, M.; Palomo, M.; Fernandez-Novell, J.; Mogas, T.; Ballester, J.; Pena, A.; Otaegui, P.; Guinovart, J.; Rodriguez-Gil, J. Differential effects of glucose and fructose on hexose metabolism in dog spermatozoa. Reproduction 2002, 123, 579–591. [Google Scholar] [CrossRef]
- Burant, C.; Takeda, J.; Brot-Laroche, E.; Bell, G.; Davidson, N. Fructose transporter in human spermatozoa and small intestine is GLUT5. J. Biol. Chem. 1992, 267, 14523–14526. [Google Scholar] [CrossRef] [PubMed]
- Bucci, D.; Rodriguez-Gil, J.E.; Vallorani, C.; Spinaci, M.; Galeati, G.; Tamanini, C. GLUTs and Mammalian Sperm Metabolism. J. Androl. 2010, 32, 348–355. [Google Scholar] [CrossRef]
- King, T.E.; Mann, T.R.R. Sorbitol metabolism in spermatozoa. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1959, 151, 226–243. [Google Scholar] [CrossRef]
- Cao, W.; Aghajanian, H.K.; Haig-Ladewig, L.A.; Gerton, G.L. Sorbitol Can Fuel Mouse Sperm Motility and Protein Tyrosine Phosphorylation via Sorbitol Dehydrogenase1. Biol. Reprod. 2009, 80, 124–133. [Google Scholar] [CrossRef]
- Darr, C.R.; Varner, D.D.; Teague, S.; Cortopassi, G.A.; Datta, S.; Meyers, S.A. Lactate and Pyruvate Are Major Sources of Energy for Stallion Sperm with Dose Effects on Mitochondrial Function, Motility, and ROS Production. Biol. Reprod. 2016, 95, 34. [Google Scholar] [CrossRef]
- Halestrap, A.P.; Price, N.T. The proton-linked monocarboxylate transporter (MCT) family: Structure, function and regulation. Biochem. J. 1999, 343, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, J.; Lee, D.R. The genetic variation in Monocarboxylic acid transporter 2 (MCT2) has functional and clinical relevance with male infertility. Asian J. Androl. 2014, 16, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Blanco, A.; Zinkham, W.H. Lactate Dehydrogenases in Human Testes. Science 1963, 139, 601–602. [Google Scholar] [CrossRef]
- Swegen, A.; Curry, B.J.; Gibb, Z.; Lambourne, S.R.; Smith, N.D.; Aitken, R.J. Investigation of the stallion sperm proteome by mass spectrometry. Reproduction 2015, 149, 235–244. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, R.; Feng, C.; Liu, R.; Zheng, Y.; Hoque, S.A.M.; Wu, D.; Lu, H.; Zhang, T.; Zeng, W. Exogenous Oleic Acid and Palmitic Acid Improve Boar Sperm Motility via Enhancing Mitochondrial Β-Oxidation for ATP Generation. Animals 2020, 10, 591. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Umehara, T.; Tsujita, N.; Shimada, M. Saturated fatty acids accelerate linear motility through mitochondrial ATP production in bull sperm. Reprod. Med. Biol. 2021, 20, 289–298. [Google Scholar] [CrossRef]
- Amaral, A.; Lourenço, B.; Marques, M.; Ramalho-Santos, J. Mitochondria functionality and sperm quality. Reproduction 2013, 146, R163–R174. [Google Scholar] [CrossRef]
- Ballester, J.; Rutllant, J.; Mogas, T.; Rigau, T.; Guinovart, J.J.; Fernández-Novell, J.M.; García-Rocha, M.; Palomo, M.J.; Peña, A.; Rodríguez-Gil, J.E. Evidence for a functional glycogen metabolism in mature mammalian spermatozoa. Mol. Reprod. Dev. 2000, 56, 207–219. [Google Scholar] [CrossRef]
- Palomo, M.J.; Fernández-Novell, J.M.; Peña, A.; Guinovart, J.J.; Rigau, T.; Rodríguez-Gil, J.E. Glucose- and fructose-induced dog-sperm glycogen synthesis shows specific changes in the location of the sperm glycogen deposition. Mol. Reprod. Dev. 2003, 64, 349–359. [Google Scholar] [CrossRef]
- Losano, J.; Angrimani, D.; Dalmazzo, A.; Rui, B.; Brito, M.; Mendes, C.; Kawai, G.; Vannucchi, C.; Assumpção, M.; Barnabe, V.; et al. Effect of mitochondrial uncoupling and glycolysis inhibition on ram sperm functionality. Reprod. Domest. Anim. 2017, 52, 289–297. [Google Scholar] [CrossRef]
- Moraes, C.R.; Moraes, L.E.; Blawut, B.; Benej, M.; Papandreou, I.; Denko, N.C.; da Silva, M.C. Effect of glucose concentration and cryopreservation on mitochondrial functions of bull spermatozoa and relationship with sire conception rate. Anim. Reprod. Sci. 2021, 230, 106779. [Google Scholar] [CrossRef] [PubMed]
- Bulkeley, E.A.; Foutouhi, A.; Wigney, K.; Santistevan, A.C.; Collins, C.; McNabb, B.; Meyers, S. Effects from disruption of mitochondrial electron transport chain function on bull sperm motility. Theriogenology 2021, 176, 63–72. [Google Scholar] [CrossRef]
- Balbach, M.; Ghanem, L.; Violante, S.; Kyaw, A.; Romarowski, A.; Cross, J.R.; Visconti, P.E.; Levin, L.R.; Buck, J. Capacitation induces changes in metabolic pathways supporting motility of epididymal and ejaculated sperm. Front. Cell Dev. Biol. 2023, 11, 1160154. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.-K.; Yang, W.-X. Factors and pathways involved in capacitation: How are they regulated? Oncotarget 2016, 8, 3600–3627. [Google Scholar] [CrossRef] [PubMed]
- Tourmente, M.; Sansegundo, E.; Roldan, E.R.S. Capacitation promotes a shift in energy metabolism in murine sperm. Front. Cell Dev. Biol. 2022, 10, 950979. [Google Scholar] [CrossRef]
- Mateo-Otero, Y.; Madrid-Gambin, F.; Llavanera, M.; Gomez-Gomez, A.; Haro, N.; Pozo, O.J.; Yeste, M. Sperm physiology and in vitro fertilising ability rely on basal metabolic activity: Insights from the pig model. Commun. Biol. 2023, 6, 344. [Google Scholar] [CrossRef]
- Li, L.; Hao, X.; Chen, H.; Wang, L.; Chen, A.; Song, X.; Hu, Z.; Su, Y.; Lin, H.; Fan, P. Metabolomic characterization of semen from asthenozoospermic patients using ultra-high-performance liquid chromatography–tandem quadrupole time-of-flight mass spectrometry. Biomed. Chromatogr. 2020, 34, e4897. [Google Scholar] [CrossRef]
| (A) Genomics Studies Based on Techniques Used in Sperm Metabolism and Male Infertility | |||||
| Methods/Tools | Study Aim | Results | Sample Size | Study Type | References |
| Evaluate seminal plasma exosomal MiRNAs as markers for Azoospermia origin and sperm presence | Mir-31-5p as biomarker for azoospermia origin combined with FSH improves prediction | 39 | Case-control prospective study | Barceló et al., 2018 [14] |
| Identify lncRNA mutations and expression in teratozoospermia | 1166 unique mutations in Differentially Expressed lncRNAs Variants affect structure/function and MiRNA interactions | N/A * | Observational study | Kyrgiafini et al., 2023 [15] |
| Small RNA profiling in seminal extracellular vesicles for azoospermia classification | Canonical and isomiR microRNAs discriminate azoospermia origin tRNA and piRNAs less discriminatory | 17 | Observational case-control study | Larriba et al., 2024 [16] |
| Investigate DNA methylation patterns in asthenozoospermia | 238 differentially methylated regions annotated to 114 genes related to spermatogenesis and motility | 12 | Comparative observational study | Li et al., 2022 [17] |
| Study microRNA profiles in semen and testicular tissue of azoospermic men | Mir-202-3p reduced in azoospermic semen mir-370-3p elevated in azoospermia without sperm in testis | 54 | Observational comparative study | Wainstein et al., 2023 [18] |
| Seminal plasma miRNAs in infertile men and diagnostic value | Seven miRNAs altered miRNAs better diagnostic markers than routine parameters | 457 | Observational case-control study | Wang et al., 2011 [19] |
| (B) Proteomics studies based on techniques used in sperm metabolism and male infertility | |||||
| Methods/Tools | Study Aim | Results | Sample size | Study Type | References |
| Identify seminal plasma proteins involved in ROS-mediated male infertility | Membrane Metallo-Endopeptidase (MME) overexpressed; Proteins modulated in infertile groups 35-protein pathway linked to sperm dysfunction | 59 | Comparative observational study | Agarwal et al., 2015 [20] |
| Characterize human sperm tail proteome, focusing on metabolism-related proteins | 1049 proteins identified; lipid metabolism enzymes prominent Mitochondrial and peroxisomal pathways active | N/A * | Experimental proteomic study | Amaral et al., 2013 [8] |
| Identify and localize AMP- activated protein kinase (AMPK) in human sperm and evaluate its role in sperm motility | AMPK localized in acrosome, midpiece, tail Active AMPK higher in high motility sperm; inhibition reduces motility | N/A * | Experimental study | Calle-Guisado et al., 2017 [21] |
| Profile seminal plasma lipid composition in necrozoospermia and evaluate lipid biomarkers | Identified 1267 lipids; 20 lipids predictive for necrozoospermia Lipidomics improves diagnosis | 56 | Comparative observational study | Deng et al., 2024 [22] |
| Discover seminal plasma biomarkers for non-invasive differential diagnosis of Obstructive Azoospermia (OA) vs. Non-Obstructive Azoospermia (NOA) | 42 proteins downregulated in NOA SCO patients Testis-specific proteins as biomarkers | 30 | Prospective observational proteomic study | Fietz et al., 2024 [23] |
| Identify novel biomarkers for asthenozoospermia via sperm tail proteomic analysis | 14 proteins altered in asthenozoospermia including tubulin beta 2B, glutathione S-transferase Mu 3, clusterin | N/A * | Experimental proteomic study | Hashemitabar et al., 2015 [24] |
| Proteomic profiling of sperm in severe oligoasthenoteratozoospermia | 938 proteins differentially expressed Metabolic pathways enriched YBX1 upregulated AK1 and ACO2 downregulated | N/A * | Experimental proteomic study | Liang et al., 2021 [25] |
| Proteomic profile changes in spermatozoa with elevated ROS | 15 proteins differentially expressed Energy metabolism and oxidative stress pathways affected | 52 | Prospective observational proteomic study | Sharma et al., 2013 [12] |
| Proteomic analysis of sperm in fertilization failure after ICSI | Altered mitochondrial and proteasomal proteins in fertilization failure | 17 | Observational proteomic study | Massana et al., 2021 [26] |
| Identify seminal biomarkers for secondary male hypogonadism (HH) | 33 proteins absent in hypogonadic patients 14 recovered after testosterone therapy | 30 | Observational proteomic study | Milardi et al., 2014 [10] |
| Identify seminal plasma proteins as biomarkers of sperm quality | 20 proteins differentially expressed Biological regulation affected | N/A * | Observational proteomic study | Sharma et al., 2013 [11] |
| Characterize seminal plasma proteome in primary and secondary infertility | Dysregulated proteins linked to secretion and immune response | 59 | Pilot observational proteomic study | Martins et al., 2020 [27] |
| Fertility-predictive model profiles of spermatozoa | High diagnostic accuracy Proteins involved in energy metabolism and sperm structure | N/A * | Experimental study | Soler et al., 2016 [28] |
| Phospholipid expression in Sertoli cell-only syndrome (SCOS) testis | Phospholipid levels correlated with spermatogenesis Potential microTESE diagnostic tool | N/A * | Experimental study | Sulc et al., 2024 [29] |
| Ribonuclease (RNASET2) levels in sperm and relation to motility | RNASET2 elevated in asthenozoospermia Inversely correlated with motility Interacts with AKAP4 | 205 | Experimental observational study | Xu et al., 2018 [30] |
| (C) Metabolomics studies based on techniques used in sperm metabolism and male infertility | |||||
| Methods/Tools | Study Aim | Results | Sample size | Study Type | References |
| Assess metabolomic signatures of seminal plasma in severe Oligoasthenospermia | Decreased metabolites linked to sperm membrane deterioration and energy defects | 40 | Comparative observational study | Boguenet et al., 2020 [31] |
| Investigate sperm metabolism | Sperm metabolize glucose, fructose, pyruvate to lactate Motile sperm show higher glycolytic activity | 97 | Experimental observational study | Calvert et al., 2019 [32] |
| Investigate seminal microbiome and metabolome role in high sperm DNA fragmentation index (HDFI) | Lactobacillus iners enriched in HDFI Microbial profiles linked to sperm DNA fragmentation Butanoate fermentation implicated | 102 | Observational case-control study | He et al., 2024 [9] |
| Analyze seminal plasma metabolic profiles in idiopathic/male factor infertility | Distinct metabolic profiles in idiopathic infertility: Altered lysine, arginine, tyrosine, citrate, fructose | 103 | Observational comparative study | Jayaraman et al., 2014 [33] |
| Correlate metabolomic profiles with semen quality in young men | Metabolites including acyl-carnitines and steroids distinguish low vs. high sperm count | 2700 | Observational cohort study | Olesti et al., 2023 [34] |
| Develop steroidomics strategy for human seminal fluid | Detected 41 steroids including androgens; steroid profile stable | 7 | Method development and validation study | Olesti et al., 2020 [35] |
| Comprehensive metabolomic characterization of human sperm cell | Identified 69 metabolites Pathways linked to sperm physiology and dysfunction | N/A * | Methodological study | Paiva et al., 2015 [36] |
| Metabolic profiling of unexplained male infertility (UMI) | Identified 44 differential metabolites Metabolic pathways altered | N/A * | Two-stage population observational study | Qiao et al., 2017 [37] |
| Examine sperm molecules | Density gradient concentration (DGC) with two washes minimized contamination Metabolite peaks differed between sperm populations | 20 | Experimental observational study | Reynolds et al., 2017 [38] |
| Metabolic profiling of idiopathic asthenozoospermia sperm cells | 33 metabolites identified Disturbed energy and amino acid metabolism | 213 | Experimental observational study | Zhao et al., 2018 [39] |
| Semen metabolic profiling in oligospermia patients | 72–89 metabolites as potential markers Altered amino acid and ketone body metabolism | 40 | Comparative observational study | Zhao et al., 2024 [40] |
| (D) Imaging, functional assays, and other techniques used in sperm metabolism and male infertility | |||||
| Methods/Tools | Study Aim | Results | Sample size | Study Type | References |
| Quantify testicular sperm apoptosis via active caspase-3 in normal and impaired spermatogenesis | Higher active caspase-3 rates in obstructive azoospermia (OA) and hypospermatogenesis Distinct localization patterns | 24 | Observational comparative study | Almeida et al., 2011 [41] |
| Analyze mitochondrial function and oxidative stress in human sperm affecting fertility | Mitochondrial dysfunction correlated with reduced motility with nitro-oxidative modifications in midpiece and head | N/A * | Experimental observational study | Cassina et al., 2015 [42] |
| Assess sperm mitochondrial metabolism and ROS production as tools to complement semen analysis | Mitochondrial respiratory control ratio correlates with motility; H2O2 inversely correlated | N/A * | Experimental observational study | Irigoyen et al., 2022 [43] |
| Assess impact of seminal clusterin level on spermatogenesis and sperm retrieval in infertile men | Seminal clusterin lower in NOA and oligozoospermia Correlation with testicular expression FSH and clusterin predict retrieval | 89 | Observational clinical study | Fukuda et al., 2016 [44] |
| Correlate sperm mitochondrial integrity with motility | Positive correlation between mitochondrial membrane potential and sperm motility | 213 | Experimental study | Paoli et al., 2011 [45] |
| Assessement of sperm DNA damage and biochemical features | DNA damage detected only in dry conditions Technique applicable to live sperm analysis | N/A * | Experimental methodological study | Costa et al., 2018 [46] |
| Identify spermatogenesis in testicular tissue | Raman spectra distinguished NOA from OA with ~90% sensitivity and 86% specificity | 52 | Observational diagnostic study | Liu et al., 2014 [47] |
| Determine cell type contributions to intracellular H2O2 and peroxynitrite production in sperm | Cell-type specific ROS production H2O2 and peroxynitrite correlated | 197 | Prospective observational study | Aziz et al., 2010 [48] |
| Progesterone-induced Ca2+ oscillations in human sperm | Ca2+ oscillations generated by CatSper channels Membrane potential modulates oscillations | 20 | Laboratory experimental study | Nitao et al., 2021 [49] |
| Evaluate seminal plasma biochemical and immunological markers in male infertility | 15 markers significantly altered Monocytes/lymphocytes in NOA Platelets in asthenozoospermia | 100 | Observational case-control study | Vashisht et al., 2021 [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakaria, A.; Diawara, I.; Bouziyane, A.; Louanjli, N. Exploring Human Sperm Metabolism and Male Infertility: A Systematic Review of Genomics, Proteomics, Metabolomics, and Imaging Techniques. Int. J. Mol. Sci. 2025, 26, 7544. https://doi.org/10.3390/ijms26157544
Zakaria A, Diawara I, Bouziyane A, Louanjli N. Exploring Human Sperm Metabolism and Male Infertility: A Systematic Review of Genomics, Proteomics, Metabolomics, and Imaging Techniques. International Journal of Molecular Sciences. 2025; 26(15):7544. https://doi.org/10.3390/ijms26157544
Chicago/Turabian StyleZakaria, Achraf, Idrissa Diawara, Amal Bouziyane, and Noureddine Louanjli. 2025. "Exploring Human Sperm Metabolism and Male Infertility: A Systematic Review of Genomics, Proteomics, Metabolomics, and Imaging Techniques" International Journal of Molecular Sciences 26, no. 15: 7544. https://doi.org/10.3390/ijms26157544
APA StyleZakaria, A., Diawara, I., Bouziyane, A., & Louanjli, N. (2025). Exploring Human Sperm Metabolism and Male Infertility: A Systematic Review of Genomics, Proteomics, Metabolomics, and Imaging Techniques. International Journal of Molecular Sciences, 26(15), 7544. https://doi.org/10.3390/ijms26157544






