Carbon Dot-Enhanced Doxorubicin Liposomes: A Dual-Functional Nanoplatform for Cancer Therapy
Abstract
1. Introduction
2. Results and Discussion
2.1. Preparation and Characterization of LPs Formulations
2.1.1. LPs Formulations Preparation
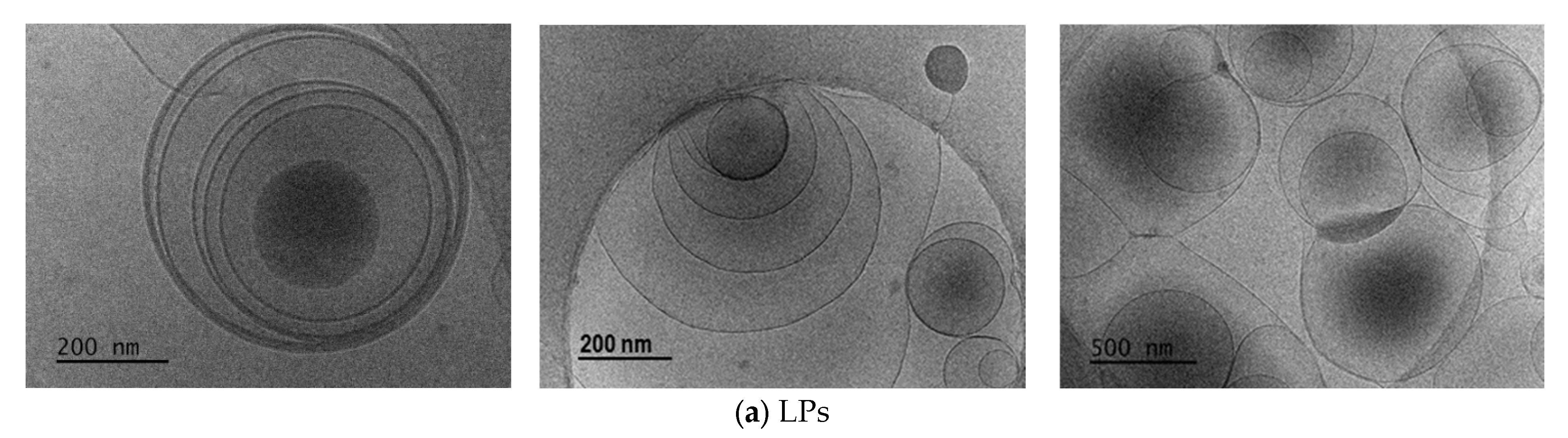
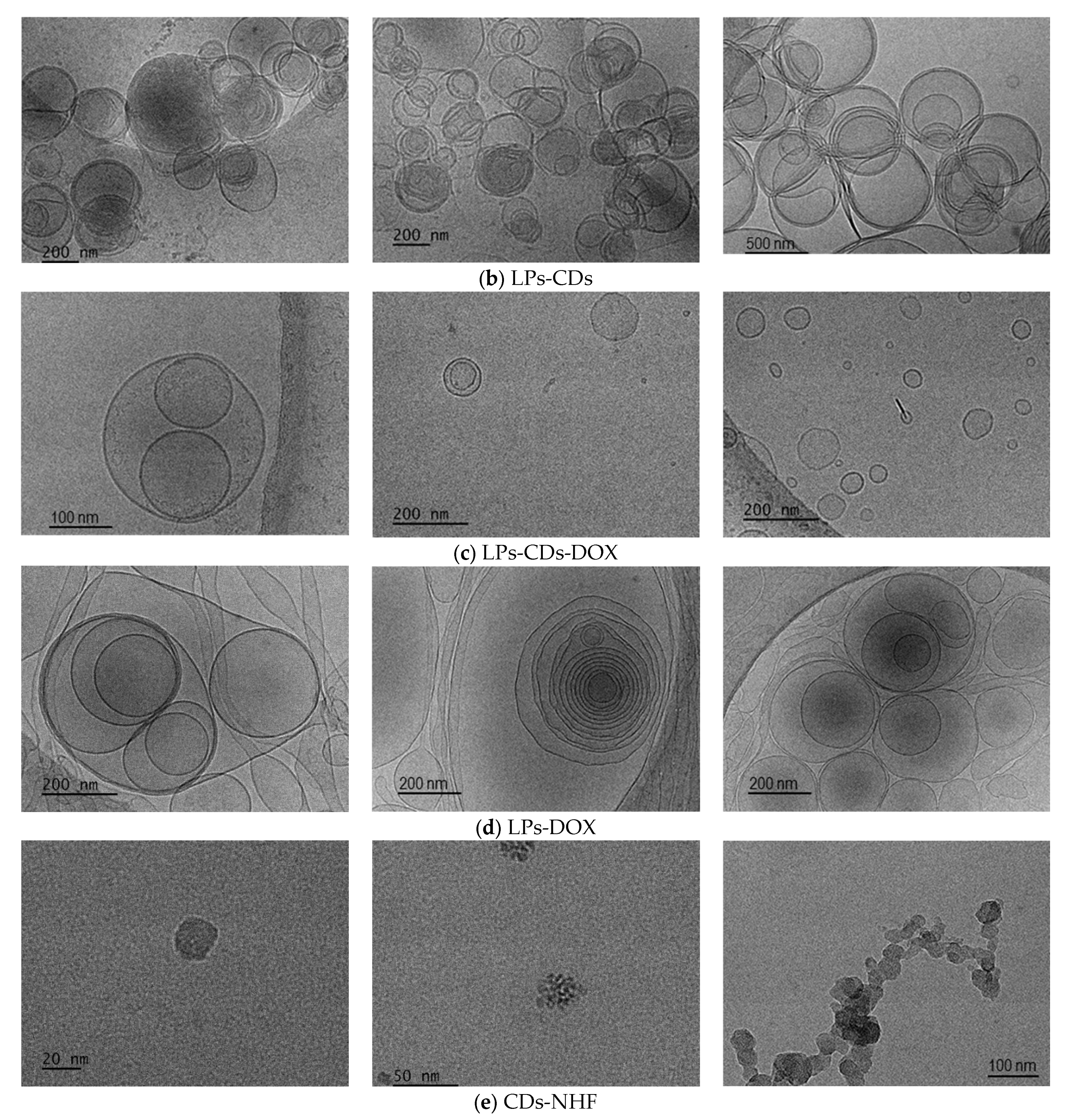
2.1.2. LPs Formulations Morphology
2.1.3. LPs Formulations Size and Zeta Potential
- η—viscosity;
- ε—dielectric constant;
- k and α—Debye-Hűckel parameter, and LPs radius.
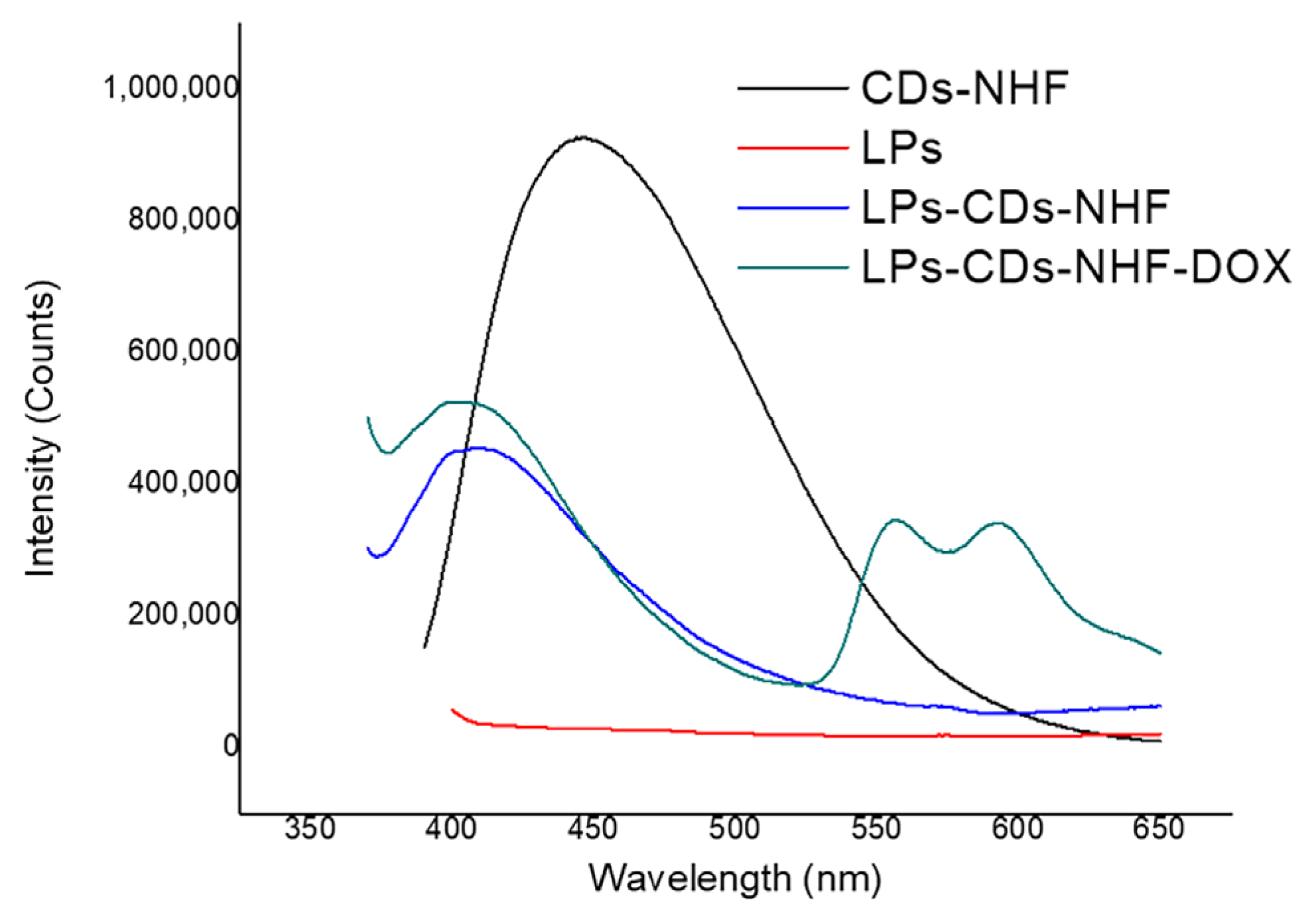
2.1.4. LPs Formulations Fluorescence Abilities
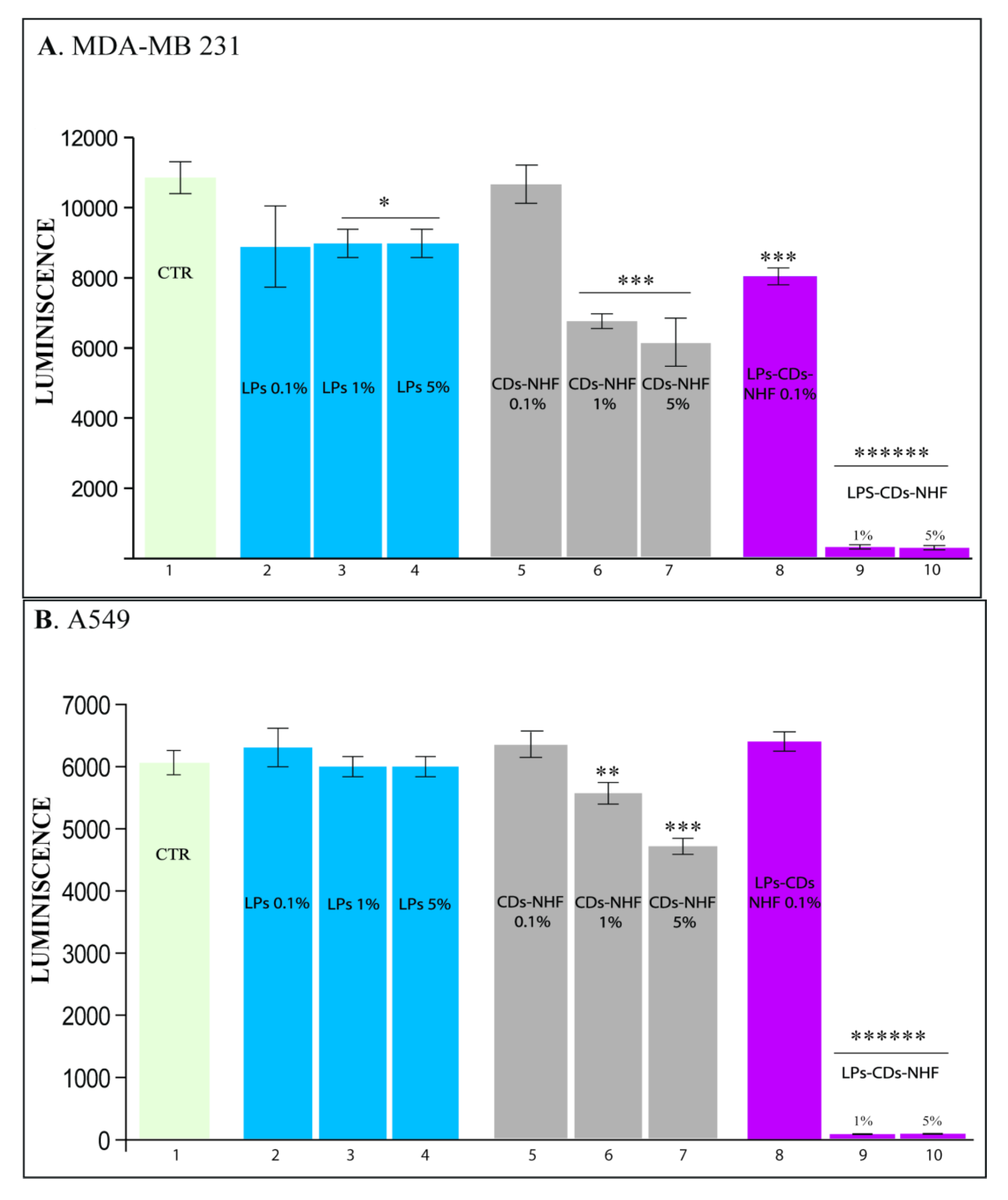
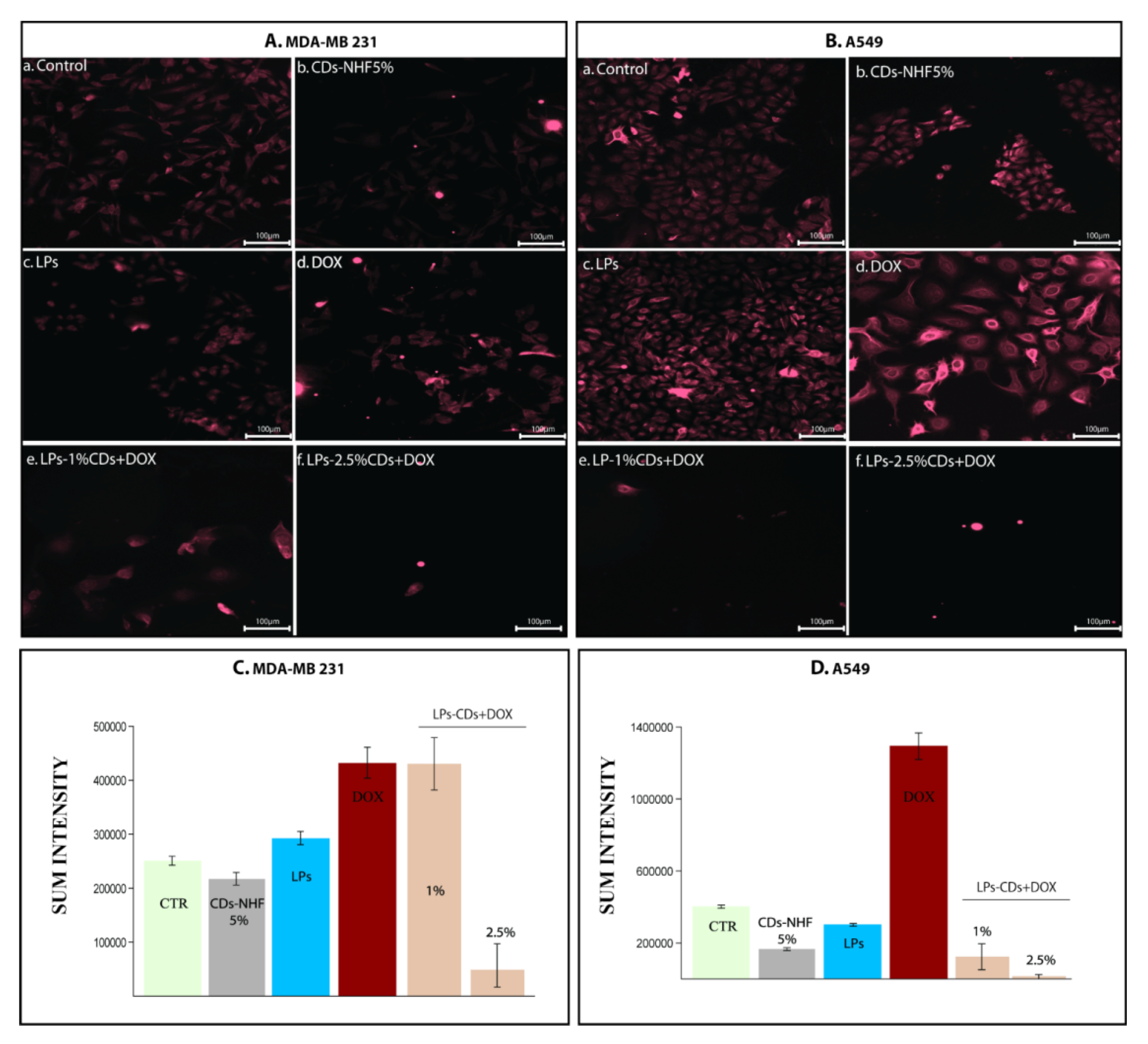
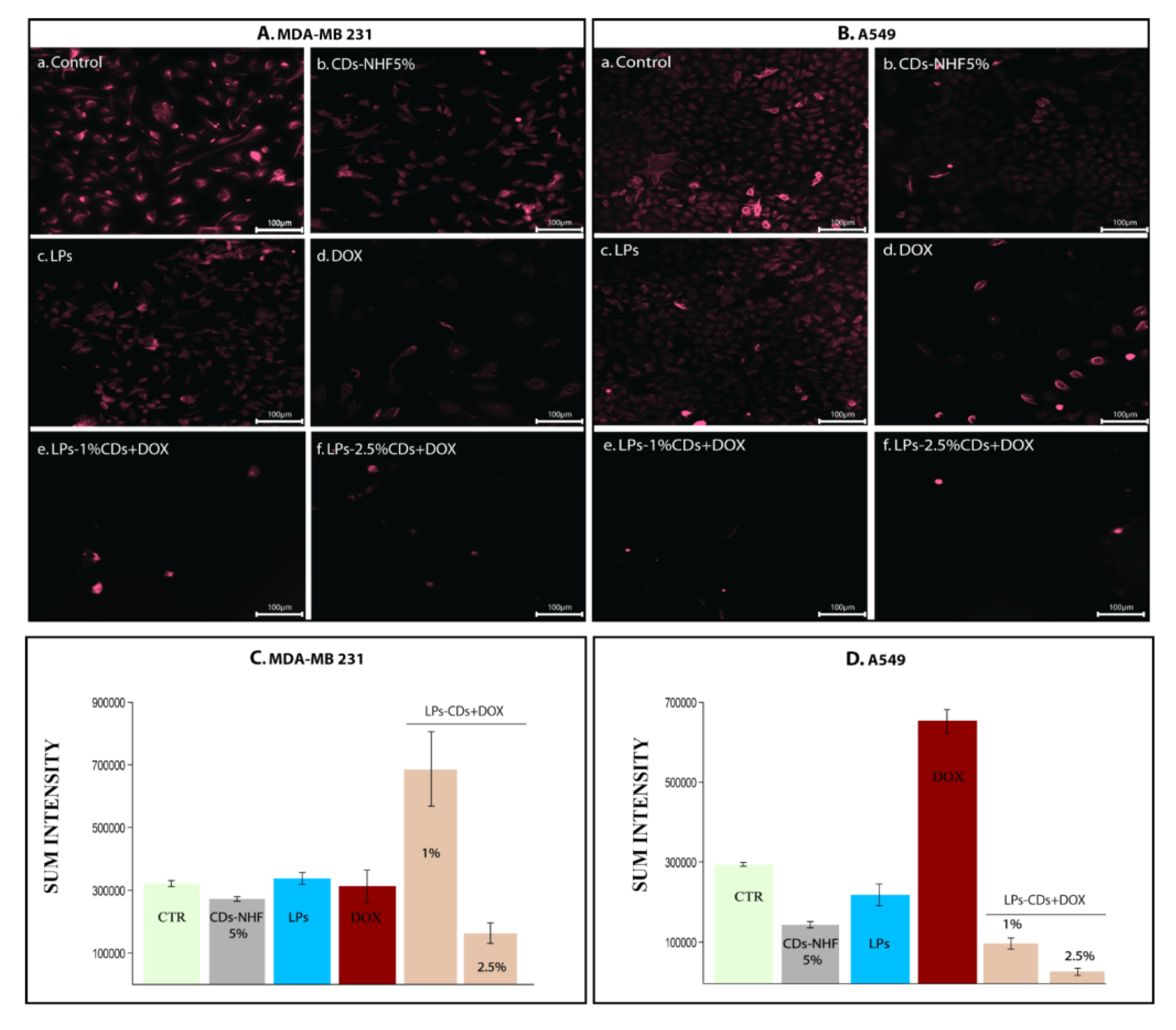
2.2. In Vitro Studies
3. Materials and Methods
3.1. Materials
3.2. Instrumentation
- DH is the hydrodynamic diameter;
- D is the diffusion coefficient;
- kB is Boltzmann’s constant;
- T is the temperature;
- η is the viscosity of the medium.
3.3. Methods
3.3.1. Preparation of LPs Formulations
3.3.2. Determination of Phospholipids Concentration by Stewart Assay
3.3.3. In Vitro Studies
Cell Viability
Immunofluorescence (IF)
Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kasouni, A.; Chatzimitakos, T.; Stalikas, C. Bioimaging Applications of Carbon Nanodots: A Review. J. Carbon Res. 2019, 5, 19. [Google Scholar] [CrossRef]
- Ghosal, K.; Ghosh, A. Carbon dots: The next generation platform for biomedical applications. Mater. Sci. Eng. C 2019, 96, 887–903. [Google Scholar] [CrossRef] [PubMed]
- Maiti, D.; Tong, X.; Mou, X.; Yang, K. Carbon-Based Nanomaterials for Biomedical Applications: A Recent Study. Front. Pharmacol. 2019, 9, 1401. [Google Scholar] [CrossRef]
- Malliappan, S.P.; Kandasamy, P.; Chidambaram, S.; Venkatasubbu, D.; Perumal, S.K.; Sugumaran, A. Breast Cancer Targeted Treatment Strategies: Promising Nanocarrier Approaches. Anti-Cancer Agents Med. Chem. 2020, 20, 1300–1310. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Kang, Y.; Zhang, X.F. Nanotechnology for the theranostic opportunity of breast cancer lung metastasis: Recent advancements and future challenges. Front. Bioeng. Biotechnol. 2024, 12, 1410017. [Google Scholar] [CrossRef]
- Jin, K.-T.; Lu, Z.-B.; Chen, J.-Y.; Liu, Y.-Y.; Lan, H.-R.; Dong, H.-Y.; Yang, F.; Zhao, Y.-Y.; Chen, X.-Y. Recent Trends in Nanocarrier Based Targeted Chemotherapy: Selective Delivery of Anticancer Drugs for Effective Lung, Colon, Cervical, and Breast Cancer Treatment. J. Nanomater. 2020, 2020, 9184284. [Google Scholar] [CrossRef]
- Baskaran, S.; Siew, Q.Y.; Tan, M.T.T.; Loh, H.S. Theranostic tools against lung and breast cancers: Through the lens of mature gold nanoparticles and emerging graphene. RPS Pharm. Pharmacol. Rep. 2024, 3, rqae003. [Google Scholar] [CrossRef]
- Brijendra, K.K.; Virendra, V.S.; Manoj, K.S.; Anil, K.; Janne, R.; Kavindra, K.K. Smart Nanomaterials in Cancer Theranostics: Challenges and Opportunities. ACS Omega 2023, 8, 14290–14320. [Google Scholar] [CrossRef]
- Faghihi, H.; Mozafari, M.R.; Bumrungpert, A.; Parsaei, H.; Taheri, S.V.; Mardani, P.; Dehkharghani, F.M.; Pudza, M.Y.; Alavi, M. Prospects and challenges of synergistic effect of fluorescent carbon dots, liposomes and nanoliposomes for theragnostic applications. Photodiagnosis Photodyn Ther. 2023, 42, 103614. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Saraf, S.; Jain, A.; Tiwari, A.; Verma, A.; Panda, P.K.; Jain, S.K. Advances in liposomal drug delivery to cancer: An overview. J. Drug Deliv. Sci. Technol. 2020, 56, 101549. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Ji, P.; Zhang, Y.; Wang, S.-J.; Ge, H.-L.; Zhao, G.-P.; Xu, Y.-C.; Wang, Y. CD44hiCD24lo mammosphere-forming cells from primary breast cancer display resistance to multiple chemotherapeutic drugs. Oncol. Rep. 2016, 35, 3293–3302. [Google Scholar] [CrossRef][Green Version]
- Maio, M.D.; Basch, E.; Bryce, J.; Perrone, F. Patient-reported outcomes in the evaluation of toxicity of anticancer treatments. Nat. Rev. Clin. Oncol. 2016, 13, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Naghizadeh, S.; Mohammadi, A.; Baradaran, B.; Mansoori, B. Overcoming multiple drug resistance in lung cancer using siRNA targeted therapy. Gene 2019, 714, 143972. [Google Scholar] [CrossRef] [PubMed]
- Cantley, L.C. The phosphoinositide 3-kinase pathway. Science 2002, 296, 1655–1657. [Google Scholar] [CrossRef] [PubMed]
- LoRusso, P.M. Inhibition of the PI3K/AKT/mTOR pathway in solid tumors. J. Clin. Oncol. 2016, 34, 3803–3815. [Google Scholar] [CrossRef]
- Sarris, E.G.; Saif, M.W.; Syrigos, K.N. The biological role of PI3K pathway in lung cancer. Pharmaceuticals 2012, 5, 1236–1264. [Google Scholar] [CrossRef]
- Fruman, D.A.; Rommel, C. PI3K and cancer: Lessons, challenges and opportunities. Nat. Rev. Drug Discov. 2014, 13, 140–156. [Google Scholar] [CrossRef]
- Dienstmann, R.; Rodon, J.; Serra, V.; Tabernero, J. Picking the point of inhibition: A comparative review of PI3K/AKT/mTOR pathway inhibitors. Mol. Cancer Ther. 2014, 13, 1021–1031. [Google Scholar] [CrossRef]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharm. Pharmacol. 2013, 65, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Mignania, S.; Bryszewska, M.; Klajnert-Maculewicz, B.; Zablocka, M.; Majoral, J.-P. Advances in Combination Therapies Based on Nanoparticles for Efficacious Cancer Treatment: An Analytical Report. Biomacromolecules 2014, 16, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kang, M.-H.; Qasimand, M.; Kim, J.-H. Nanoparticle-Mediated Combination Therapy: Two-in-One Approach for Cancer. Int. J. Mol. Sci. 2018, 19, 3264. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.; Caster, J.M.; Eblan, M.J.; Wang, A.Z. Clinical Translation of Nanomedicine. Chem. Rev. 2015, 115, 11147–11190. [Google Scholar] [CrossRef]
- Caster, J.M.; Wang, A.Z. Applying nanotherapeutics to improve chemoradiotherapy treatment for cancer. Ther. Deliv. 2017, 8, 791–803. [Google Scholar] [CrossRef]
- Fang, J.; Nakamura, H.; Maeda, H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef]
- Maeda, H. Macromolecular therapeutics in cancer treatment: The EPR effect and beyond. J. Control Release 2012, 164, 138–144. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Peer, D.; Margalit, R. Tumor-targeted hyaluronan nanoliposomes increase the antitumor activity of liposomal doxorubicin in syngeneic and human xenograft mouse tumor models. Neoplasia 2004, 6, 343–353. [Google Scholar] [CrossRef]
- Risi, G.; Bloise, N.; Merli, D.; Cornaglia, A.I.; Profumo, A.; Fagnoni, M.; Quartarone, E.; Imbriani, M.; Visai, L. In vitro study of multiwall carbon nanotubes (MWCNTs) with adsorbed mitoxantrone (MTO) as a drug delivery system to treat breast cancer. RSC Adv. 2014, 4, 18683–18693. [Google Scholar] [CrossRef]
- Li, D.; Fan, Y.; Shen, M.; Bányai, I.; Shi, X. Design of dual drug-loaded dendrimer/carbon dot nanohybrids for fluorescence imaging and enhanced chemotherapy of cancer cells. J. Mater. Chem. B 2019, 7, 5306. [Google Scholar] [CrossRef]
- Lee, W.; Im, H.J. Theranostics Based on Liposome: Looking Back and Forward. Nucl. Med. Mol. Imaging 2019, 53, 242–246. [Google Scholar] [CrossRef]
- Demir, B.; Moulahoum, H.; Ghorbanizamani, F.; Barlas, F.B.; Yesiltepe, O.; Gumus, Z.P.; Meral, K.; Demirkol, D.O.; Timur, S. Carbon dots and curcumin-loaded CD44-Targeted liposomes for imaging and tracking cancer chemotherapy: A multi-purpose tool for theranostics. J. Drug Deliv. Sci. Technol. 2021, 62, 102363. [Google Scholar] [CrossRef]
- Guan, C.; Zhao, Y.; Hou, Y.; Shan, G.; Yan, D.; Liu, Y. Glycosylated liposomes loading carbon dots for targeted recognition to HepG2 cells. Talanta 2018, 182, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Chen, S.; Liao, Y.; Li, S.; Ge, J.; Tao, F.; Huo, Q.; Zhang, Y.; Zhao, Z. Near-infrared fluorescent carbon dots encapsulated liposomes as multifunctional nano-carrier and tracer of the anticancer agent cinobufagin in vivo and in vitro. Colloids Surf. B Biointerfaces 2019, 174, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Savin, C.-L.; Tiron, C.; Carasevici, E.; Stan, C.S.; Ibanescu, S.A.; Simionescu, B.C.; Peptu, C.A. Entrapment of N-Hydroxyphthalimide Carbon Dots in Different Topical Gel Formulations: New Composites with Anticancer Activity. Pharmaceutics 2019, 11, 303. [Google Scholar] [CrossRef]
- Tiron, C.E.; Zugun-Eloae, F.; Peptu, C.A.; Tiron, A.; Stan, C.S. Imide Derived Carbon Dots Exhibit Promising Antitumoral Properties on Multiple In vitro Experimental Designs. Nanosci. Nanotechnol. Indian J. 2019, 13, 131. [Google Scholar]
- Xue, X.; Fang, T.; Yin, L.; Jiang, J.; He, Y.; Dai, Y.; Wang, D. Multistage delivery of CDs-DOX/ICG-loaded liposome for highly penetration and effective chemo-photothermal combination therapy. Drug Deliv. 2018, 25, 1826–1839. [Google Scholar] [CrossRef]
- Abizaid, A.; Costa, J.R., Jr. New drug-eluting stents: An overview on biodegradable and polymer-free next-generation stent systems. Circ. Cardiovasc. Interv. 2010, 3, 384–393. [Google Scholar] [CrossRef]
- Mozafari, M.R. Liposomes: An overview of manufacturing techniques. Cell Mol. Biol. Lett. 2005, 10, 711–719. [Google Scholar] [PubMed]
- Lombardo, D.; Kiselev, M.A. Methods of Liposomes Preparation: Formation and Control Factors of Versatile Nanocarriers for Biomedical and Nanomedicine Application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Irie, H.Y.; Pearline, R.V.; Grueneberg, D.; Hsia, M.; Ravichandran, P.; Kothari, N.; Natesan, S.; Brugge, J.S. Distinct roles of Akt1 and Akt2 in regulating cell migration and epithelial–mesenchymal transition. JCB 2005, 171, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, X.-M.; Ding, L.; Zhang, X.-J.; Ma, Z.-L. mTOR signaling-related MicroRNAs and Cancer involvement. J. Cancer 2018, 9, 667–673. [Google Scholar] [CrossRef]
- Santarpia, L.; Lippman, S.M.; El-Naggar, A.K. Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert Opin. Ther. Targets 2012, 16, 103–119. [Google Scholar] [CrossRef]
- Luta, G.; Butura, M.; Tiron, A.; Tiron, C.E. Enhancing Anti-Tumoral Potential of CD-NHF by Modulating PI3K/Akt Axis in U87 Ex Vivo Glioma Model. Int. J. Mol. Sci. 2021, 22, 3873. [Google Scholar] [CrossRef]
- Tiron, C.E.; Luta, G.; Butura, M.; Zugun-Eloae, F.; Stan, C.S.; Coroaba, A.; Ursu, E.L.; Stanciu, G.D.; Tiron, A. NHF-derived carbon dots: Prevalidation approach in breast cancer treatment. Sci. Rep. 2020, 10, 12662. [Google Scholar] [CrossRef]
- Carragher, N.O.; Frame, M.C. Focal adhesion and actin dynamics: A place where kinases and proteases meet to promote invasion. Trends Cell Biol. 2004, 14, 241–249. [Google Scholar] [CrossRef]
- Bartholomeusz, C.; Oishi, T.; Saso, H.; Akar, U.; Liu, P.; Kondo, K.; Kazansky, A.; Krishnamurthy, S.; Lee, J.; Esteva, F.J.; et al. MEK1/2 inhibitor selumetinib (AZD6244) inhibits growth of ovarian clear cell carcinoma in a PEA-15-dependent manner in a mouse xenograft model. Mol. Cancer Ther. 2012, 11, 360–369. [Google Scholar] [CrossRef]
- Kim, B.Y.; Rutka, J.T.; Chan, W.C. Nanomedicine. N. Engl. J. Med. 2010, 363, 2434–2443. [Google Scholar] [CrossRef]
- Hu, Q.; Sun, W.; Wang, C.; Gu, Z. Recent advances of cocktail chemotherapy by combination drug delivery systems. Adv. Drug Deliv. Rev. 2016, 98, 19–34. [Google Scholar] [CrossRef]
- Cabeza, L.; Ortiz, R.; Arias, J.L.; Entrena, J.M.; Luque, R.; Melguizo, C. Enhanced antitumor activity of doxorubicin in breast cancer through the use of poly(butylcyanoacrylate) nanoparticles. Int. J. Nanomed. 2015, 10, 1291–1306. [Google Scholar] [CrossRef]
- Gupta, A.A.; Yao, S.; Mackay, H.; Hopkins, L. Chemotherapy (gemcitabine, docetaxel plus gemcitabine, doxorubicin, or trabectedin) for inoperable, locally advanced, recurrent, or metastatic uterine leiomyosarcoma, in a quality initiative of the program in Evidence-Based Care (PEBC). Cancer Care Ont. 2013, 20, 448–454. [Google Scholar] [CrossRef]
- Xu, Y.; Li, N.; Xiang, R.; Sun, P. Emerging roles of hep38MAPK and PI3K/AKT/mTOR pathways in oncogene-induced senescence. Trends Biochem. Sci. 2014, 39, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Stan, C.S.; Albu, C.; Coroaba, A.; Popa, M.; Sutiman, D. One-step synthesis of fluorescent carbon dots through pyrolysis of N-hydroxysuccinimide. J. Mater. Chem. C 2015, 3, 789–795. [Google Scholar] [CrossRef]
- New, R.R.C. Liposomes a practical approach. In Practical Approach Series; Rickwood, D., Hames, B.D., Eds.; Oxford University Press: Oxford, UK, 2003; p. 108. ISBN 0199636540/978-0199636549. [Google Scholar]



| Sample ID | Pre-Purification | Post-Purification | |||
|---|---|---|---|---|---|
| PC-G, mg/mL | CHOL, mg/mL | Diethyl Ether, mL | PC-G, mg/mL | CDs-NHF or DOX/Lipid, mg/mL | |
| Control cells | - | - | - | - | - |
| Cells-loaded LPs | - | - | - | - | - |
| Cells-loaded LPs-CDs-NHF | - | - | - | - | - |
| CDs-NHF | - | - | - | - | - |
| Control LPs | 60 | 40 | 10 | 23 | - |
| LPs-CDs-NHF | 60 | 40 | 10 | 17 | 0.13 |
| LPs-DOX | 60 | 40 | 10 | 13 | 0.35 |
| LPs-CDs-NHF-DOX | 60 | 40 | 10 | 12 | 0.219 (CDs) |
| 0.373 (DOX) | |||||
| Sample ID | Diameter (DLS ± SD), nm | Zeta Potential (mV) |
|---|---|---|
| Control cells | - | −8.11 |
| Cells-loaded LPs | - | −6.10 |
| Cells-loaded LPs-CDs-NHF | - | −11.98 |
| CDs-NHF | 44 | −10.47 |
| LPs | 384 | −33.12 |
| LPs-CDs-NHF | 1016 | −24.86 |
| LPs-DOX | 917 | −23.67 |
| LPs-CDs-NHF-DOX | 801 | −26.57 |
| Sample ID | Excitation (nm) | Emission Peaks (nm) | Intensity (Counts) |
|---|---|---|---|
| CDs-NHF | 370 | 447 | 9.27641 × 105 |
| Control LPs | 370 | 0 | 0 |
| LPs-CDs-NHF | 370 | 408 | 4.55403 × 105 |
| LPs-CDs-NHF-DOX | 370 | 405 | 5.26137 × 105(CDs-NHF) |
| LPs-CDs-NHF-DOX | 370 | 557 | 3.46898 × 105(DOX) |
| LPs-CDs-NHF-DOX | 370 | 593 | 3.42876 × 105(DOX) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Logigan, C.-L.; Peptu, C.; Stan, C.S.; Luta, G.; Tiron, C.E.; Pinteala, M.; Foryś, A.; Simionescu, B.; Ibanescu, C.; Tiron, A.; et al. Carbon Dot-Enhanced Doxorubicin Liposomes: A Dual-Functional Nanoplatform for Cancer Therapy. Int. J. Mol. Sci. 2025, 26, 7535. https://doi.org/10.3390/ijms26157535
Logigan C-L, Peptu C, Stan CS, Luta G, Tiron CE, Pinteala M, Foryś A, Simionescu B, Ibanescu C, Tiron A, et al. Carbon Dot-Enhanced Doxorubicin Liposomes: A Dual-Functional Nanoplatform for Cancer Therapy. International Journal of Molecular Sciences. 2025; 26(15):7535. https://doi.org/10.3390/ijms26157535
Chicago/Turabian StyleLogigan, Corina-Lenuta, Cristian Peptu, Corneliu S. Stan, Gabriel Luta, Crina Elena Tiron, Mariana Pinteala, Aleksander Foryś, Bogdan Simionescu, Constanta Ibanescu, Adrian Tiron, and et al. 2025. "Carbon Dot-Enhanced Doxorubicin Liposomes: A Dual-Functional Nanoplatform for Cancer Therapy" International Journal of Molecular Sciences 26, no. 15: 7535. https://doi.org/10.3390/ijms26157535
APA StyleLogigan, C.-L., Peptu, C., Stan, C. S., Luta, G., Tiron, C. E., Pinteala, M., Foryś, A., Simionescu, B., Ibanescu, C., Tiron, A., & Peptu, C. A. (2025). Carbon Dot-Enhanced Doxorubicin Liposomes: A Dual-Functional Nanoplatform for Cancer Therapy. International Journal of Molecular Sciences, 26(15), 7535. https://doi.org/10.3390/ijms26157535








