Upregulating ANKHD1 in PS19 Mice Reduces Tau Phosphorylation and Mitigates Tau Toxicity-Induced Cognitive Deficits
Abstract
1. Introduction
2. Results
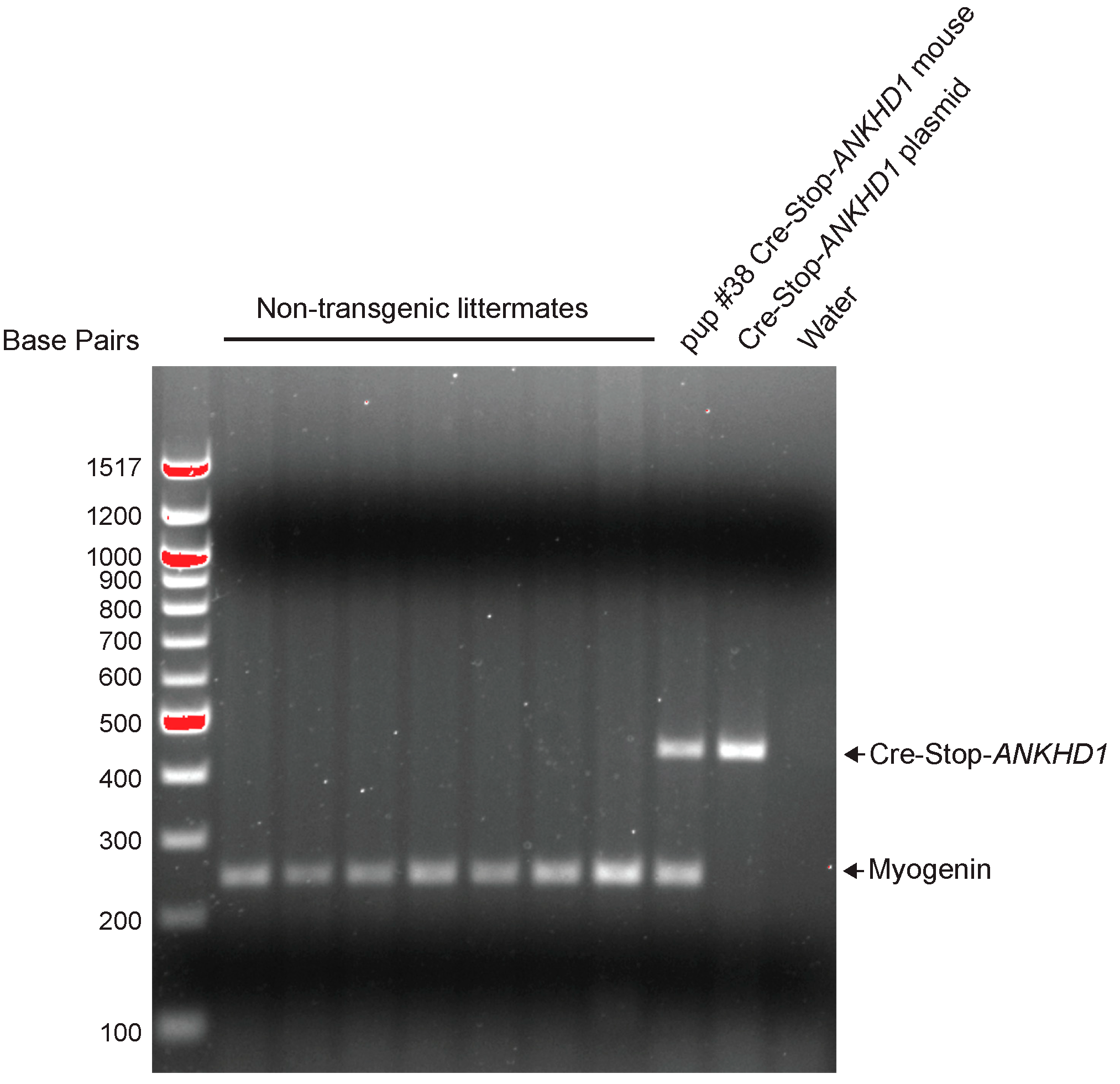
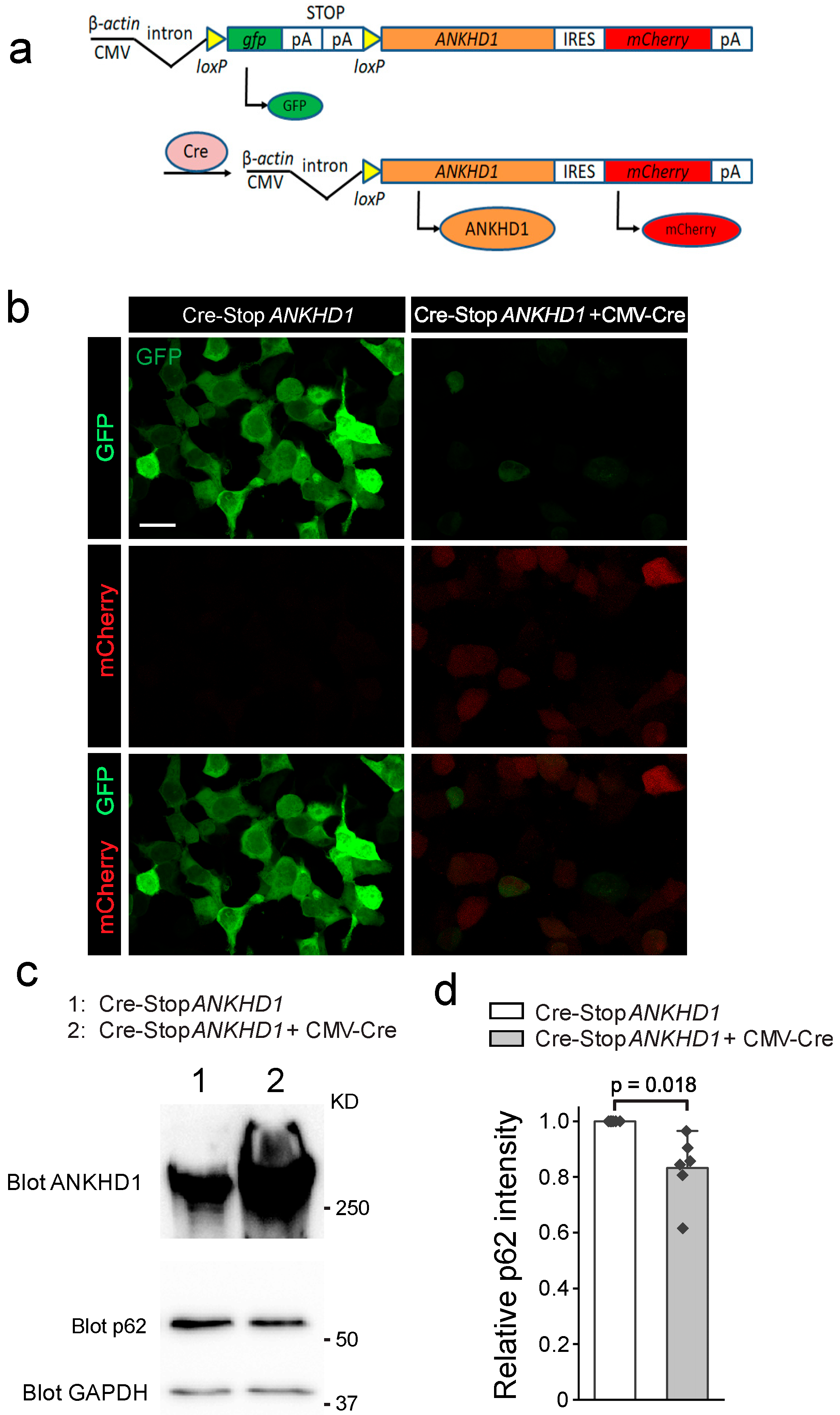
2.1. Generation and Validation of Cre-Inducible ANKHD1 Expression Mouse Lines
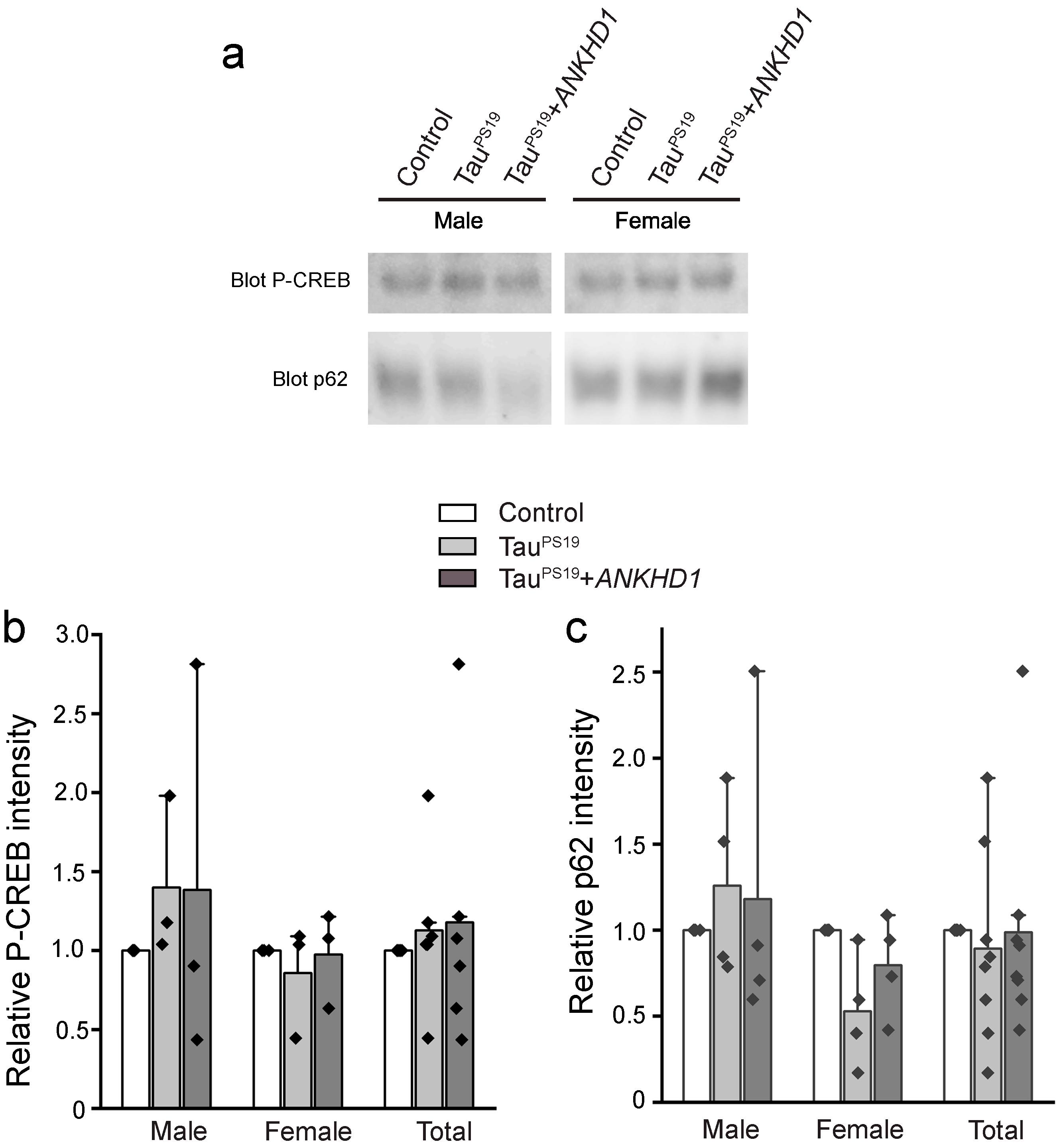
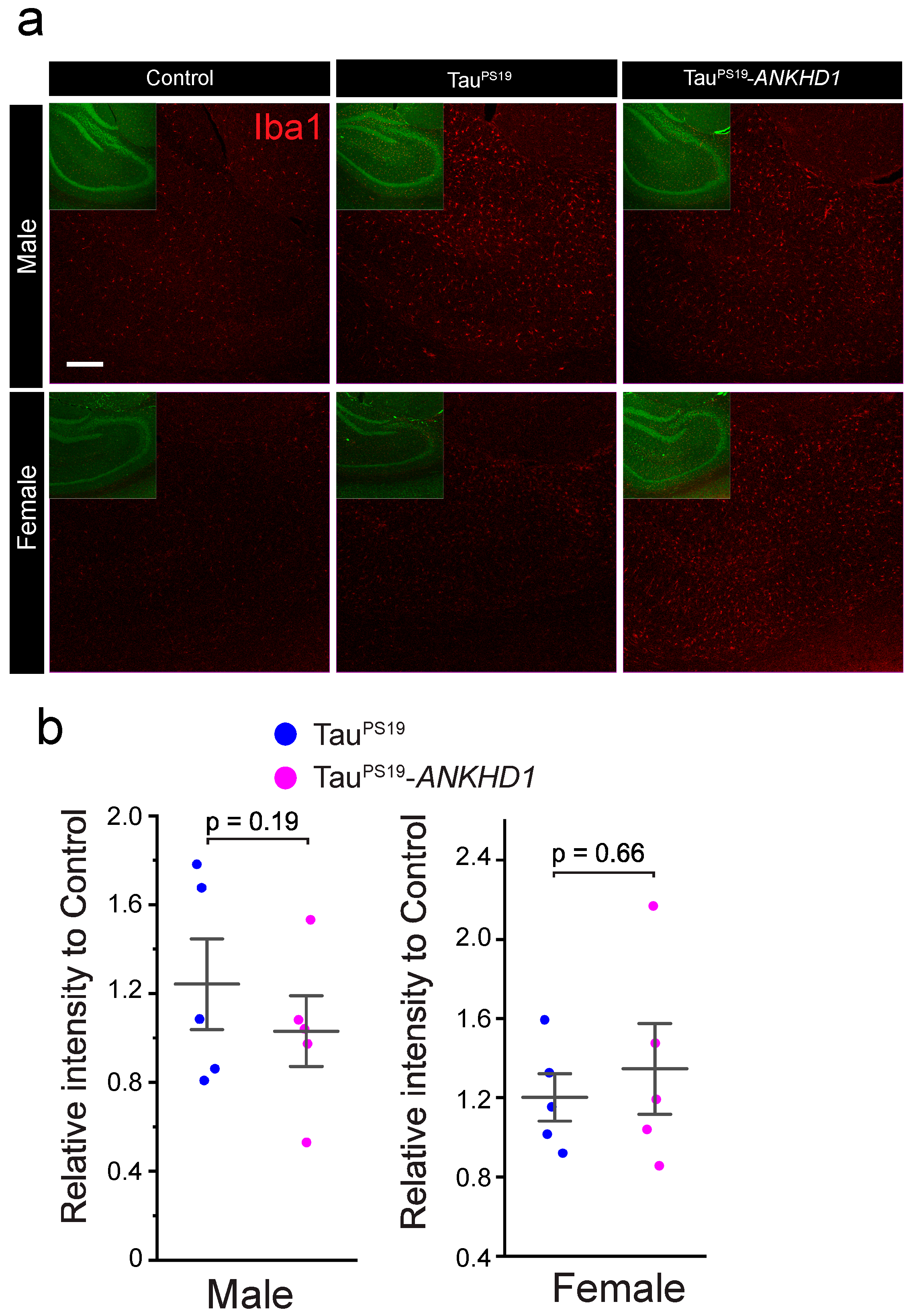
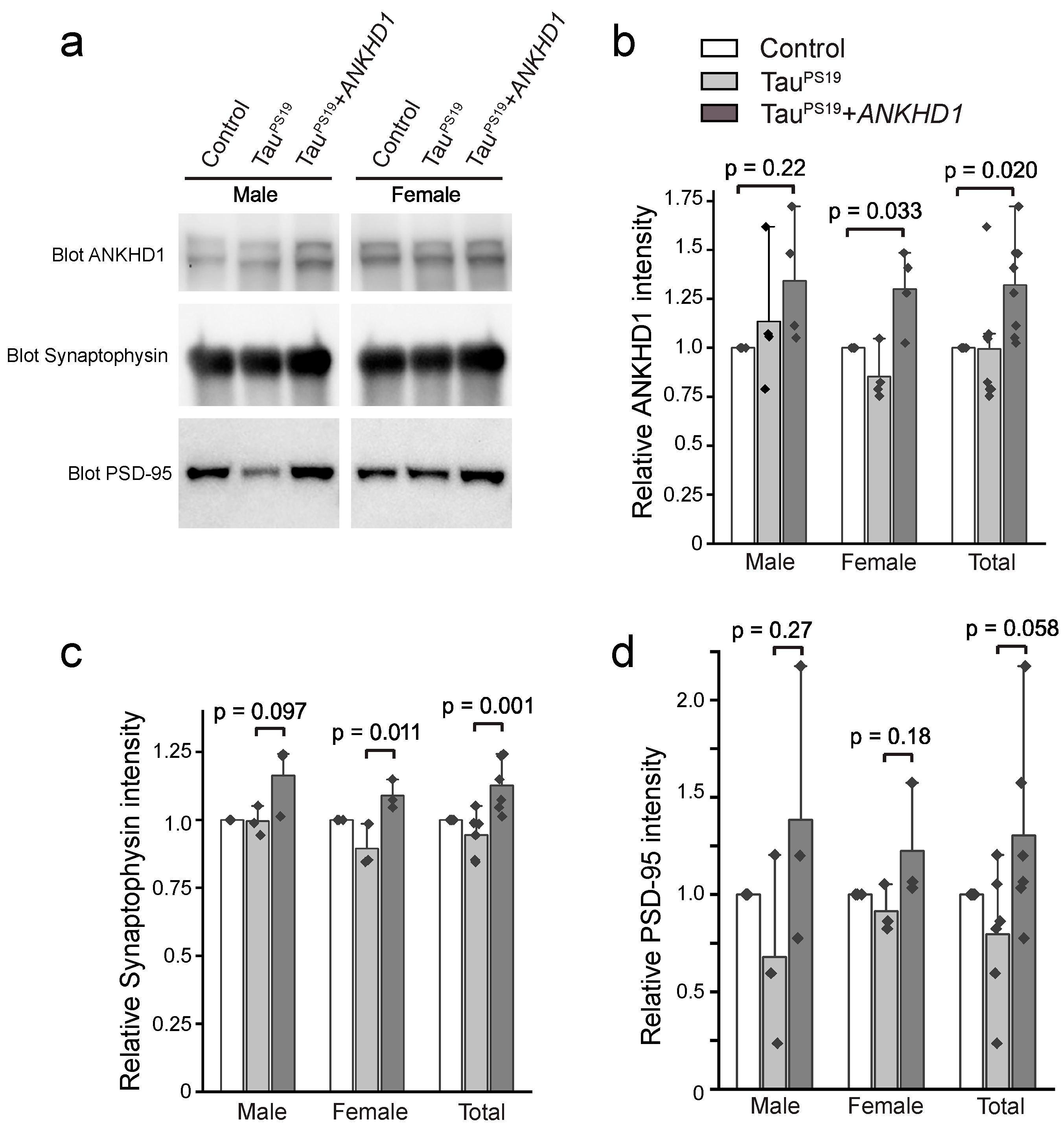
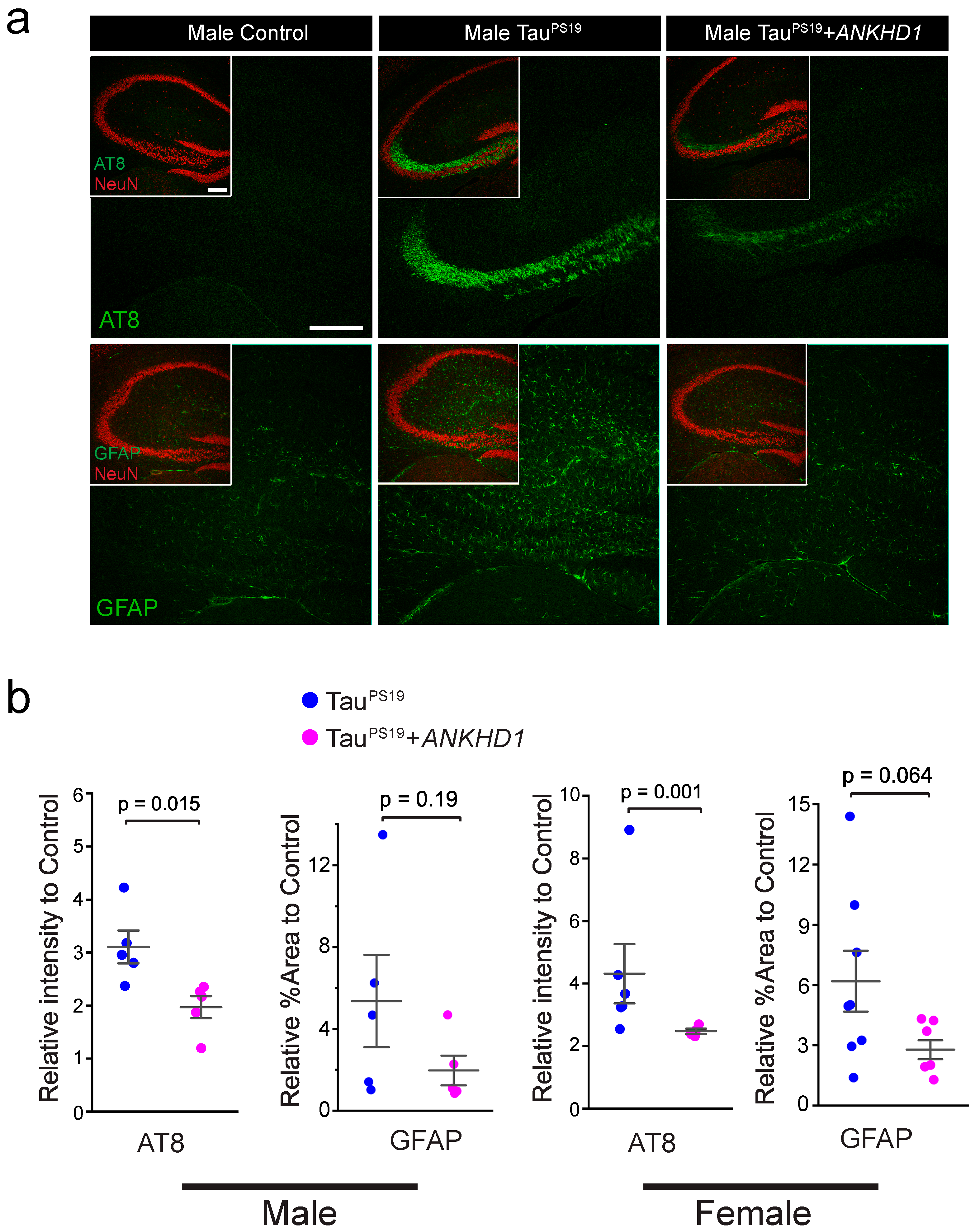
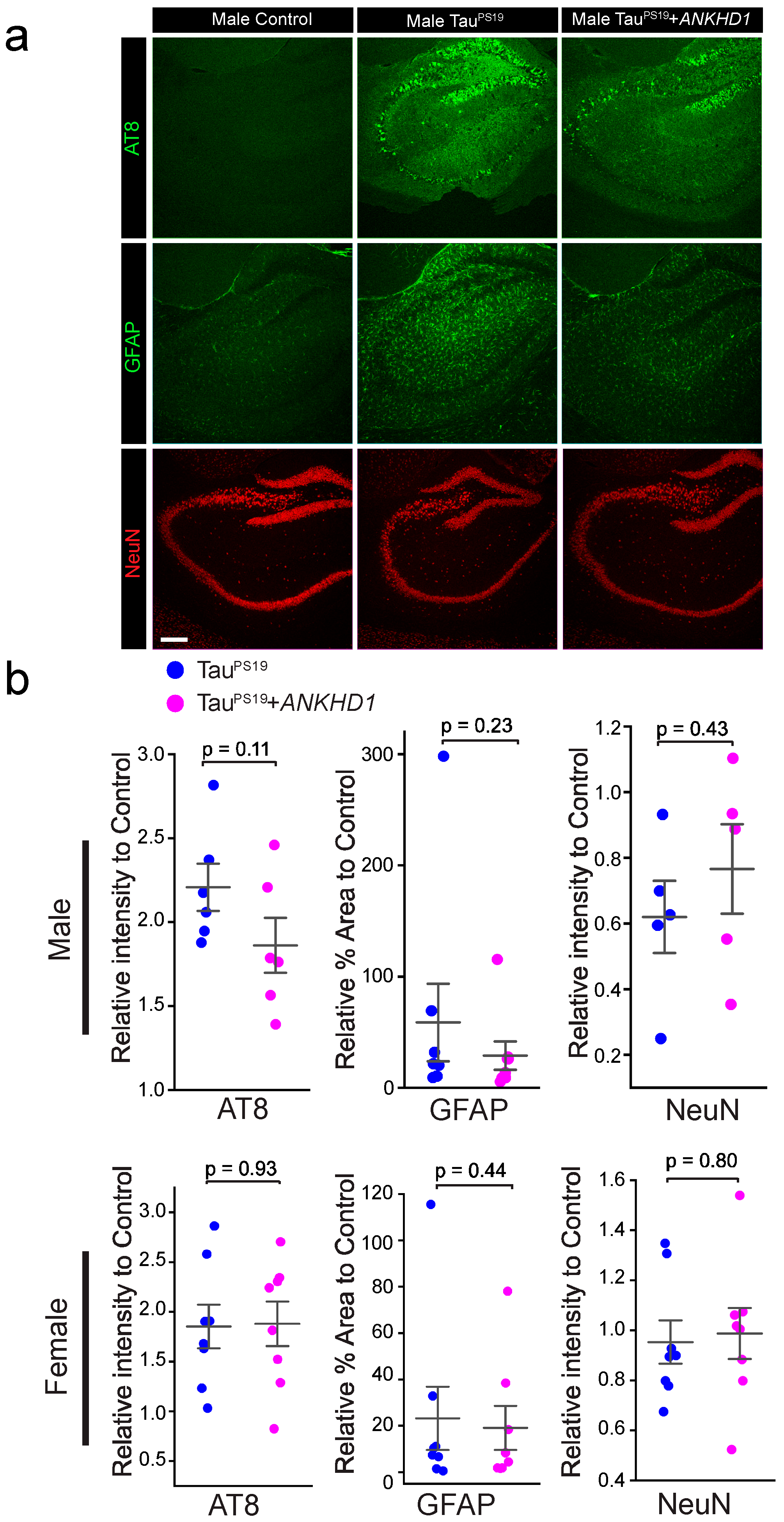
2.2. ANKHD1 Suppresses Neuron-Specific Histopathological Lesions Induced by Mutant Human Tau Proteins in the Mouse Brain
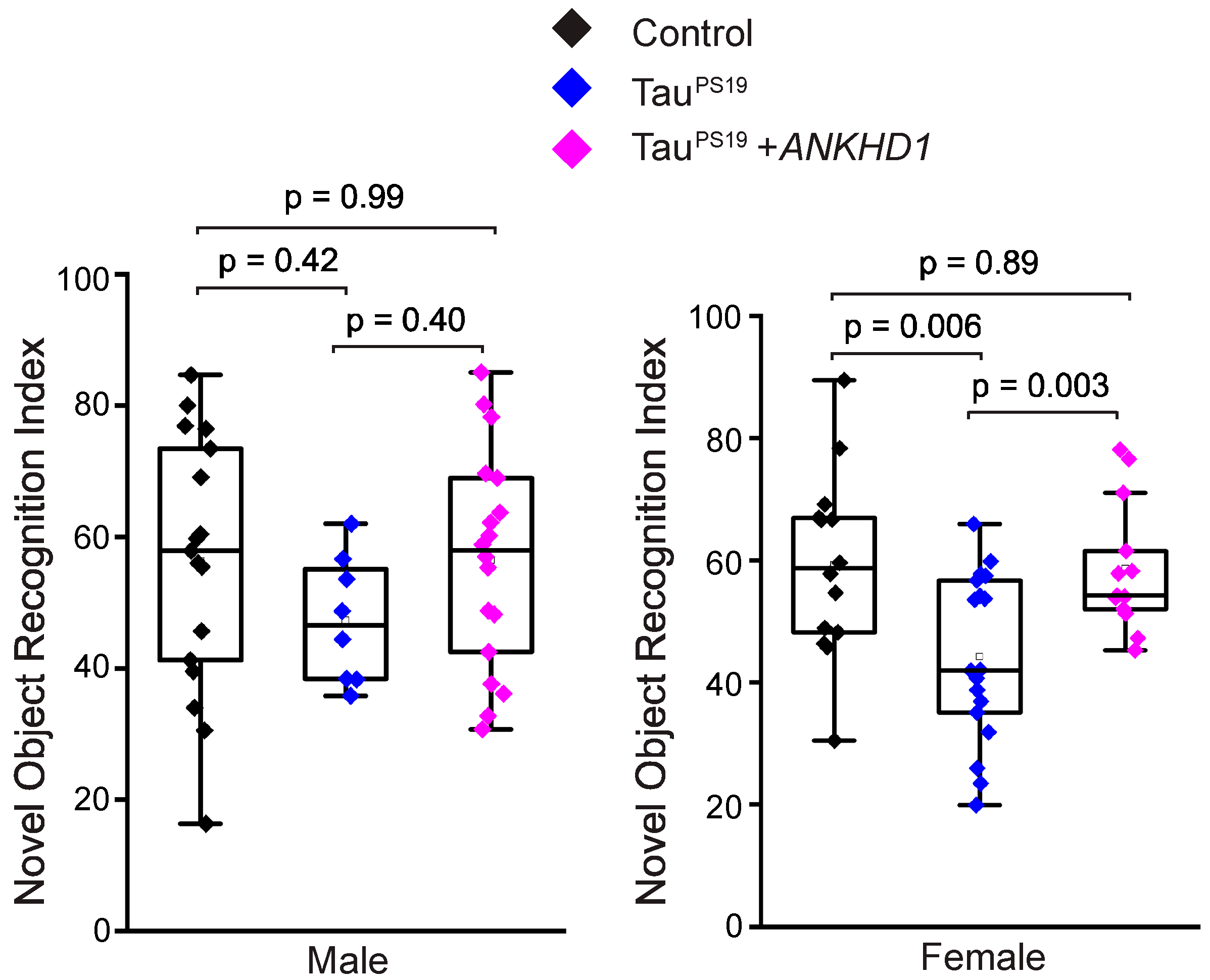
2.3. ANKHD1 Partially Restores Cognitive Functions in the PS19 Mice
3. Discussion
4. Materials and Methods
4.1. Generation of Cre-Inducible ANKHD1 Transgenic Mouse
4.2. Cell Culture and Transfection
4.3. Western Blot Analysis
4.4. Immunohistochemistry
4.5. Confocal Microscopy and Quantification
4.6. Y-Maze
4.7. Novel Object Recognition
4.8. Data and Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MT | microtubule |
| PHF | paired helical filaments |
| PS19 | TauPS19, or pnp-Tau-P301S |
| NOR | Novel Object Recognition |
| GFAP | glial fibrillary acidic protein (GFAP) |
| AT8 | phosphorylated Tau |
Appendix A


References
- Arendt, T.; Stieler, J.T.; Holzer, M. Tau and Tauopathies. Brain Res. Bull. 2016, 126, 238–292. [Google Scholar] [CrossRef]
- Götz, J.; Halliday, G.; Nisbet, R.M. Molecular Pathogenesis of the Tauopathies. Annu. Rev. Pathol. 2019, 14, 239–261. [Google Scholar] [CrossRef] [PubMed]
- Igaev, M.; Janning, D.; Sündermann, F.; Niewidok, B.; Brandt, R.; Junge, W. A Refined Reaction-Diffusion Model of Tau-Microtubule Dynamics and Its Application in FDAP Analysis. Biophys. J. 2014, 107, 2567–2578. [Google Scholar] [CrossRef] [PubMed]
- Dixit, R.; Ross, J.L.; Goldman, Y.E.; Holzbaur, E.L.F. Differential Regulation of Dynein and Kinesin Motor Proteins by Tau. Science 2008, 319, 1086–1089. [Google Scholar] [CrossRef]
- Crowther, R.A. Straight and Paired Helical Filaments in Alzheimer Disease Have a Common Structural Unit. Proc. Natl. Acad. Sci. USA 1991, 88, 2288–2292. [Google Scholar] [CrossRef]
- Guo, J.L.; Lee, V.M.-Y. Seeding of Normal Tau by Pathological Tau Conformers Drives Pathogenesis of Alzheimer-like Tangles. J. Biol. Chem. 2011, 286, 15317–15331. [Google Scholar] [CrossRef]
- Nonaka, T.; Watanabe, S.T.; Iwatsubo, T.; Hasegawa, M. Seeded Aggregation and Toxicity of {alpha}-Synuclein and Tau: Cellular Models of Neurodegenerative Diseases. J. Biol. Chem. 2010, 285, 34885–34898. [Google Scholar] [CrossRef]
- Clavaguera, F.; Bolmont, T.; Crowther, R.A.; Abramowski, D.; Frank, S.; Probst, A.; Fraser, G.; Stalder, A.K.; Beibel, M.; Staufenbiel, M.; et al. Transmission and Spreading of Tauopathy in Transgenic Mouse Brain. Nat. Cell Biol. 2009, 11, 909–913. [Google Scholar] [CrossRef]
- Colom-Cadena, M.; Davies, C.; Sirisi, S.; Lee, J.-E.; Simzer, E.M.; Tzioras, M.; Querol-Vilaseca, M.; Sánchez-Aced, É.; Chang, Y.Y.; Holt, K.; et al. Synaptic Oligomeric Tau in Alzheimer’s Disease—A Potential Culprit in the Spread of Tau Pathology through the Brain. Neuron 2023, 111, 2170–2183.e6. [Google Scholar] [CrossRef]
- Kopeikina, K.J.; Hyman, B.T.; Spires-Jones, T.L. Soluble Forms of Tau Are Toxic in Alzheimer’s Disease. Transl. Neurosci. 2012, 3, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Spires, T.L.; Orne, J.D.; SantaCruz, K.; Pitstick, R.; Carlson, G.A.; Ashe, K.H.; Hyman, B.T. Region-Specific Dissociation of Neuronal Loss and Neurofibrillary Pathology in a Mouse Model of Tauopathy. Am. J. Pathol. 2006, 168, 1598–1607. [Google Scholar] [CrossRef] [PubMed]
- Bain, H.D.C.; Davidson, Y.S.; Robinson, A.C.; Ryan, S.; Rollinson, S.; Richardson, A.; Jones, M.; Snowden, J.S.; Pickering-Brown, S.; Mann, D.M.A. The Role of Lysosomes and Autophagosomes in Frontotemporal Lobar Degeneration. Neuropathol. Appl. Neurobiol. 2019, 45, 244–261. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Akiyama, H.; Arai, T.; Kondo, H.; Haga, C.; Iritani, S.; Tsuchiya, K. Alz-50/Gallyas-Positive Lysosome-like Intraneuronal Granules in Alzheimer’s Disease and Control Brains. Neurosci. Lett. 1998, 258, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Nixon, R.A. Amyloid Precursor Protein and Endosomal-Lysosomal Dysfunction in Alzheimer’s Disease: Inseparable Partners in a Multifactorial Disease. FASEB J. 2017, 31, 2729–2743. [Google Scholar] [CrossRef]
- Schaeffer, V.; Lavenir, I.; Ozcelik, S.; Tolnay, M.; Winkler, D.T.; Goedert, M. Stimulation of Autophagy Reduces Neurodegeneration in a Mouse Model of Human Tauopathy. Brain 2012, 135, 2169–2177. [Google Scholar] [CrossRef]
- Berger, Z.; Ravikumar, B.; Menzies, F.M.; Oroz, L.G.; Underwood, B.R.; Pangalos, M.N.; Schmitt, I.; Wullner, U.; Evert, B.O.; O’Kane, C.J.; et al. Rapamycin Alleviates Toxicity of Different Aggregate-Prone Proteins. Hum. Mol. Genet. 2006, 15, 433–442. [Google Scholar] [CrossRef]
- Silva, M.C.; Nandi, G.A.; Tentarelli, S.; Gurrell, I.K.; Jamier, T.; Lucente, D.; Dickerson, B.C.; Brown, D.G.; Brandon, N.J.; Haggarty, S.J. Prolonged Tau Clearance and Stress Vulnerability Rescue by Pharmacological Activation of Autophagy in Tauopathy Neurons. Nat. Commun. 2020, 11, 3258. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, S.; Tian, X.; Wu, C. Mask Mitigates MAPT- and FUS-Induced Degeneration by Enhancing Autophagy through Lysosomal Acidification. Autophagy 2017, 13, 1924–1938. [Google Scholar] [CrossRef]
- Chopra, M.; McEntagart, M.; Clayton-Smith, J.; Platzer, K.; Shukla, A.; Girisha, K.M.; Kaur, A.; Kaur, P.; Pfundt, R.; Veenstra-Knol, H.; et al. Heterozygous ANKRD17 Loss-of-Function Variants Cause a Syndrome with Intellectual Disability, Speech Delay, and Dysmorphism. Am. J. Hum. Genet. 2021, 108, 1138–1150. [Google Scholar] [CrossRef]
- Silverstein, R.; Kuwabara, M.; Appavu, B. Neonatal Aneurysm Rupture in a Child with a De Novo Variant to ANKRD17. Child Neurol. Open 2022, 9, 2329048X221134600. [Google Scholar] [CrossRef]
- Sveden, A.; Gordon, C.T.; Amiel, J.; Chopra, M. ANKRD17-Related Neurodevelopmental Syndrome. In GeneReviews(®); Adam, M.P., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Farghaian, H.; Turnley, A.M.; Sutherland, C.; Cole, A.R. Bioinformatic Prediction and Confirmation of Beta-Adducin as a Novel Substrate of Glycogen Synthase Kinase 3. J. Biol. Chem. 2011, 286, 25274–25283. [Google Scholar] [CrossRef]
- Mullenger, J.L.; Zeidler, M.P.; Fragiadaki, M. Evaluating the Molecular Properties and Function of ANKHD1, and Its Role in Cancer. Int. J. Mol. Sci. 2023, 24, 12834. [Google Scholar] [CrossRef]
- Sansores-Garcia, L.; Atkins, M.; Moya, I.M.; Shahmoradgoli, M.; Tao, C.; Mills, G.B.; Halder, G. Mask Is Required for the Activity of the Hippo Pathway Effector Yki/YAP. Curr. Biol. 2013, 23, 229–235. [Google Scholar] [CrossRef]
- Sun, W.; Huang, R.; Li, Z.; Zhu, Y.; Bai, Y.; Wu, S.; Wang, J.; Xiao, Y.; Xian, S.; Tong, X.; et al. Alternative ANKHD1 Transcript Promotes Proliferation and Inhibits Migration in Uterine Corpus Endometrial Carcinoma. NPJ Genom. Med. 2022, 7, 56. [Google Scholar] [CrossRef]
- Traina, F.; Favaro, P.M.; Medina Sde, S.; Duarte Ada, S.; Winnischofer, S.M.; Costa, F.F.; Saad, S.T. ANKHD1, Ankyrin Repeat and KH Domain Containing 1, Is Overexpressed in Acute Leukemias and Is Associated with SHP2 in K562 Cells. Biochim. Biophys. Acta 2006, 1762, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zheng, J.; Liu, X.; Xue, Y.; He, Q.; Dong, Y.; Wang, D.; Li, Z.; Liu, L.; Ma, J.; et al. Role of ANKHD1/LINC00346/ZNF655 Feedback Loop in Regulating the Glioma Angiogenesis via Staufen1-Mediated mRNA Decay. Mol. Ther. Nucleic Acids 2020, 20, 866–878. [Google Scholar] [CrossRef] [PubMed]
- Almeida, B.O.; Machado-Neto, J.A. Emerging Functions for ANKHD1 in Cancer-Related Signaling Pathways and Cellular Processes. BMB Rep. 2020, 53, 413–418. [Google Scholar] [CrossRef]
- Dhyani, A.; Duarte, A.S.; Machado-Neto, J.A.; Favaro, P.; Ortega, M.M.; Olalla Saad, S.T. ANKHD1 Regulates Cell Cycle Progression and Proliferation in Multiple Myeloma Cells. FEBS Lett. 2012, 586, 4311–4318. [Google Scholar] [CrossRef]
- Dhyani, A.; Machado-Neto, J.A.; Favaro, P.; Saad, S.T. ANKHD1 Represses P21 (WAF1/CIP1) Promoter and Promotes Multiple Myeloma Cell Growth. Eur. J. Cancer 2015, 51, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Fragiadaki, M.; Zeidler, M.P. Ankyrin Repeat and Single KH Domain 1 (ANKHD1) Drives Renal Cancer Cell Proliferation via Binding to and Altering a Subset of miRNAs. J. Biol. Chem. 2018, 293, 9570–9579. [Google Scholar] [CrossRef] [PubMed]
- Yoshiyama, Y.; Higuchi, M.; Zhang, B.; Huang, S.M.; Iwata, N.; Saido, T.C.; Maeda, J.; Suhara, T.; Trojanowski, J.Q.; Lee, V.M. Synapse Loss and Microglial Activation Precede Tangles in a P301S Tauopathy Mouse Model. Neuron 2007, 53, 337–351. [Google Scholar] [CrossRef]
- Dutta, D.; Jana, M.; Paidi, R.K.; Majumder, M.; Raha, S.; Dasarathy, S.; Pahan, K. Tau Fibrils Induce Glial Inflammation and Neuropathology via TLR2 in Alzheimer’s Disease–Related Mouse Models. J. Clin. Invest. 2023, 133, e161987. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-Y.; Dong, Q.-X.; Zhu, J.; Sun, X.; Zhang, L.-F.; Qiu, M.; Yu, X.-L.; Liu, R.-T. Resveratrol Rescues Tau-Induced Cognitive Deficits and Neuropathology in a Mouse Model of Tauopathy. Curr. Alzheimer Res. 2019, 16, 710–722. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Gao, D.; Wang, Y.; Wang, Z.-H.; Wang, X.; Ye, J.; Wu, D.; Fang, L.; Pi, G.; Yang, Y.; et al. Tau Accumulation Induces Synaptic Impairment and Memory Deficit by Calcineurin-Mediated Inactivation of Nuclear CaMKIV/CREB Signaling. Proc. Natl. Acad. Sci. 2016, 113, E3773–E3781. [Google Scholar] [CrossRef]
- Togo, T.; Dickson, D.W. Tau Accumulation in Astrocytes in Progressive Supranuclear Palsy Is a Degenerative Rather than a Reactive Process. Acta Neuropathol. 2002, 104, 398–402. [Google Scholar] [CrossRef]
- Hovakimyan, A.; Antonyan, T.; Shabestari, S.K.; Svystun, O.; Chailyan, G.; Coburn, M.A.; Carlen-Jones, W.; Petrushina, I.; Chadarevian, J.P.; Zagorski, K.; et al. A MultiTEP Platform-Based Epitope Vaccine Targeting the Phosphatase Activating Domain (PAD) of Tau: Therapeutic Efficacy in PS19 Mice. Sci. Rep. 2019, 9, 15455. [Google Scholar] [CrossRef]
- Takeuchi, H.; Iba, M.; Inoue, H.; Higuchi, M.; Takao, K.; Tsukita, K.; Karatsu, Y.; Iwamoto, Y.; Miyakawa, T.; Suhara, T.; et al. P301S Mutant Human Tau Transgenic Mice Manifest Early Symptoms of Human Tauopathies with Dementia and Altered Sensorimotor Gating. PLoS ONE 2011, 6, e21050. [Google Scholar] [CrossRef]
- Lopez-Lee, C.; Torres, E.R.S.; Carling, G.; Gan, L. Mechanisms of Sex Differences in Alzheimer’s Disease. Neuron 2024, 112, 1208–1221. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, J.P.; Leung, L.W.; Cai, D.; Kaasik, K.; Gross, R.S.; Rodriguez-Boulan, E.; Greengard, P.; Xu, H. Estrogen Lowers Alzheimer Beta-Amyloid Generation by Stimulating Trans-Golgi Network Vesicle Biogenesis. J. Biol. Chem. 2002, 277, 12128–12136. [Google Scholar] [CrossRef]
- Pinto-Almazán, R.; Calzada-Mendoza, C.C.; Campos-Lara, M.G.; Guerra-Araiza, C. Effect of Chronic Administration of Estradiol, Progesterone, and Tibolone on the Expression and Phosphorylation of Glycogen Synthase Kinase-3β and the Microtubule-Associated Protein Tau in the Hippocampus and Cerebellum of Female Rat. J. Neurosci. Res. 2012, 90, 878–886. [Google Scholar] [CrossRef]
- Xu, H.; Gouras, G.K.; Greenfield, J.P.; Vincent, B.; Naslund, J.; Mazzarelli, L.; Fried, G.; Jovanovic, J.N.; Seeger, M.; Relkin, N.R.; et al. Estrogen Reduces Neuronal Generation of Alzheimer Beta-Amyloid Peptides. Nat. Med. 1998, 4, 447–451. [Google Scholar] [CrossRef]
- Congdon, E.E. Sex Differences in Autophagy Contribute to Female Vulnerability in Alzheimer’s Disease. Front. Neurosci. 2018, 12, 372. [Google Scholar] [CrossRef] [PubMed]
- Gegenhuber, B.; Wu, M.V.; Bronstein, R.; Tollkuhn, J. Gene Regulation by Gonadal Hormone Receptors Underlies Brain Sex Differences. Nature 2022, 606, 153–159. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, Y.; Feng, X.; Jia, M.; Ai, N.; Dong, Y.; Zheng, Y.; Fu, L.; Yu, B.; Zhang, H.; et al. The Behavioural and Neuropathologic Sexual Dimorphism and Absence of MIP-3α in Tau P301S Mouse Model of Alzheimer’s Disease. J. Neuroinflamm. 2020, 17, 72. [Google Scholar] [CrossRef]
- Zampar, S.; Wirths, O. Characterization of a Mouse Model of Alzheimer’s Disease Expressing Aβ4-42 and Human Mutant Tau. Int. J. Mol. Sci. 2021, 22, 5191. [Google Scholar] [CrossRef]
- Martinez, D.; Zhu, M.; Guidry, J.J.; Majeste, N.; Mao, H.; Yanofsky, S.T.; Tian, X.; Wu, C. Mask, the Drosophila Ankyrin Repeat and KH Domain-Containing Protein, Affects Microtubule Stability. J. Cell Sci. 2021, 134, jcs258512. [Google Scholar] [CrossRef]
- Tian, X. Enhancing Mask Activity in Dopaminergic Neurons Extends Lifespan in Flies. Aging Cell 2021, 20, e13493. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gibb, S.L.; Zhao, J.; Moore, A.N.; Hylin, M.J.; Menge, T.; Xue, H.; Baimukanova, G.; Potter, D.; Johnson, E.M.; et al. Wnt3a, a Protein Secreted by Mesenchymal Stem Cells Is Neuroprotective and Promotes Neurocognitive Recovery Following Traumatic Brain Injury. Stem Cells 2016, 34, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Kraeuter, A.-K.; Guest, P.C.; Sarnyai, Z. The Y-Maze for Assessment of Spatial Working and Reference Memory in Mice. Methods Mol. Biol. 2019, 1916, 105–111. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, X.; Le, N.; Zhao, Y.; Alawamleh, D.; Schwartz, A.; Meyer, L.; Helm, E.; Wu, C. Upregulating ANKHD1 in PS19 Mice Reduces Tau Phosphorylation and Mitigates Tau Toxicity-Induced Cognitive Deficits. Int. J. Mol. Sci. 2025, 26, 7524. https://doi.org/10.3390/ijms26157524
Tian X, Le N, Zhao Y, Alawamleh D, Schwartz A, Meyer L, Helm E, Wu C. Upregulating ANKHD1 in PS19 Mice Reduces Tau Phosphorylation and Mitigates Tau Toxicity-Induced Cognitive Deficits. International Journal of Molecular Sciences. 2025; 26(15):7524. https://doi.org/10.3390/ijms26157524
Chicago/Turabian StyleTian, Xiaolin, Nathan Le, Yuhai Zhao, Dina Alawamleh, Andrew Schwartz, Lauren Meyer, Elizabeth Helm, and Chunlai Wu. 2025. "Upregulating ANKHD1 in PS19 Mice Reduces Tau Phosphorylation and Mitigates Tau Toxicity-Induced Cognitive Deficits" International Journal of Molecular Sciences 26, no. 15: 7524. https://doi.org/10.3390/ijms26157524
APA StyleTian, X., Le, N., Zhao, Y., Alawamleh, D., Schwartz, A., Meyer, L., Helm, E., & Wu, C. (2025). Upregulating ANKHD1 in PS19 Mice Reduces Tau Phosphorylation and Mitigates Tau Toxicity-Induced Cognitive Deficits. International Journal of Molecular Sciences, 26(15), 7524. https://doi.org/10.3390/ijms26157524





