Perfluoroalkyl Substance (PFAS) Mixtures Drive Rheumatoid Arthritis Risk Through Immunosuppression: Integrating Epidemiology and Mechanistic Evidence
Abstract
1. Introduction
2. Results
2.1. Population Characteristics and PFAS Exposure
2.2. Logistic Regression Analysis of PFAS Metabolites Exposure and RA Risk
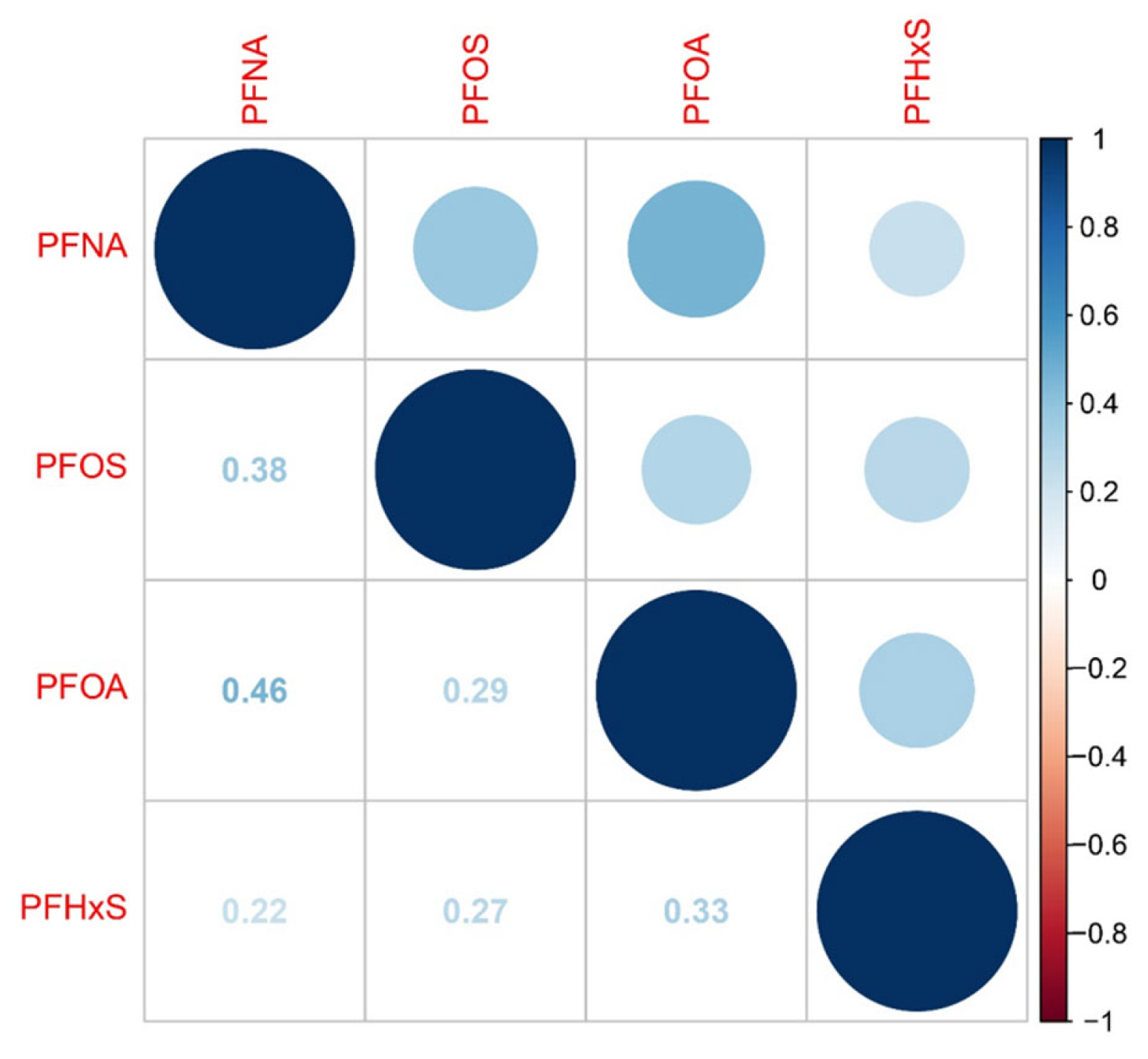
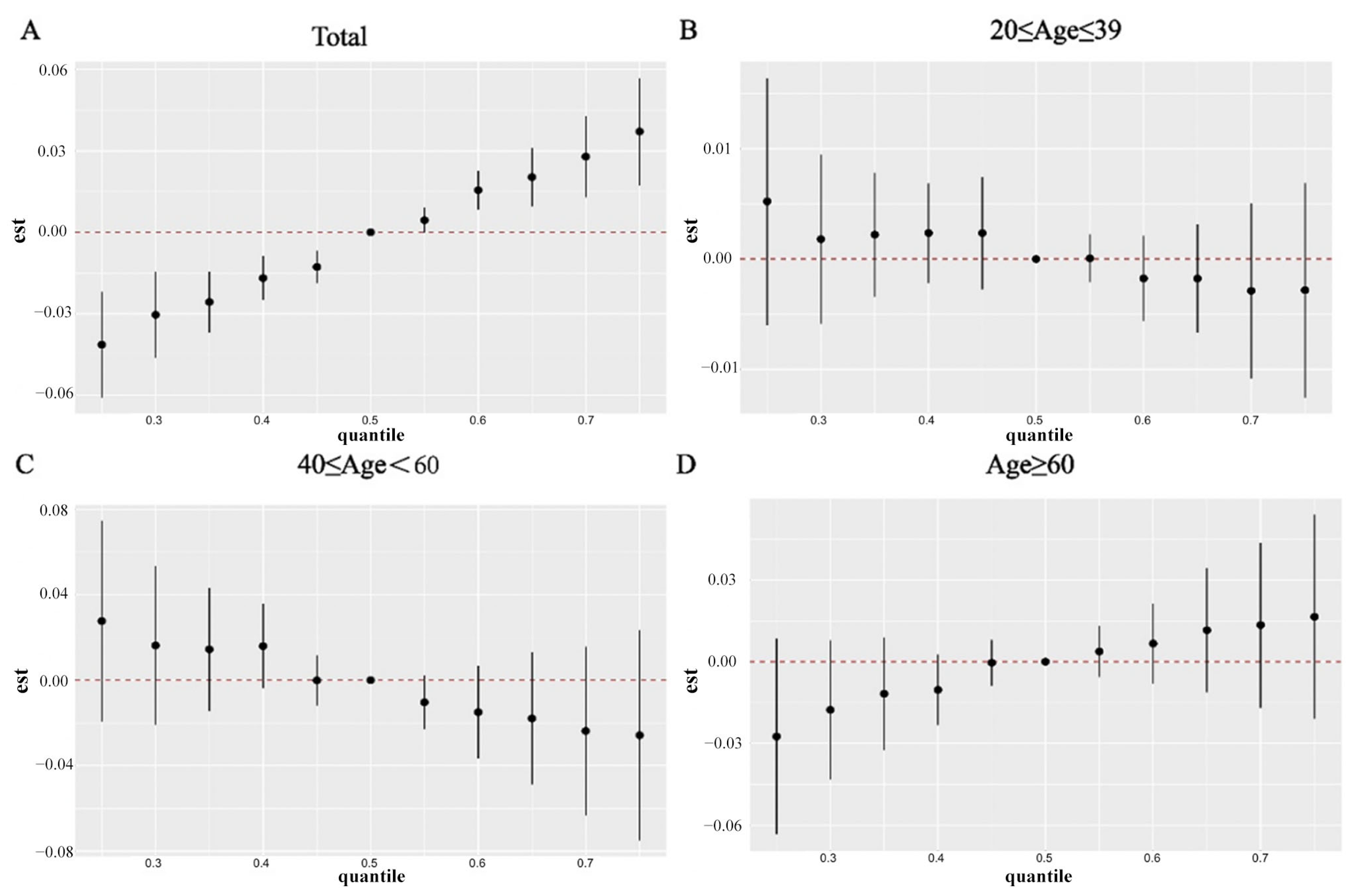
2.3. WQS, Qgcomp, and BKMR Modeling for Joint Effect of PFAS Co-Exposure on RA Incidence
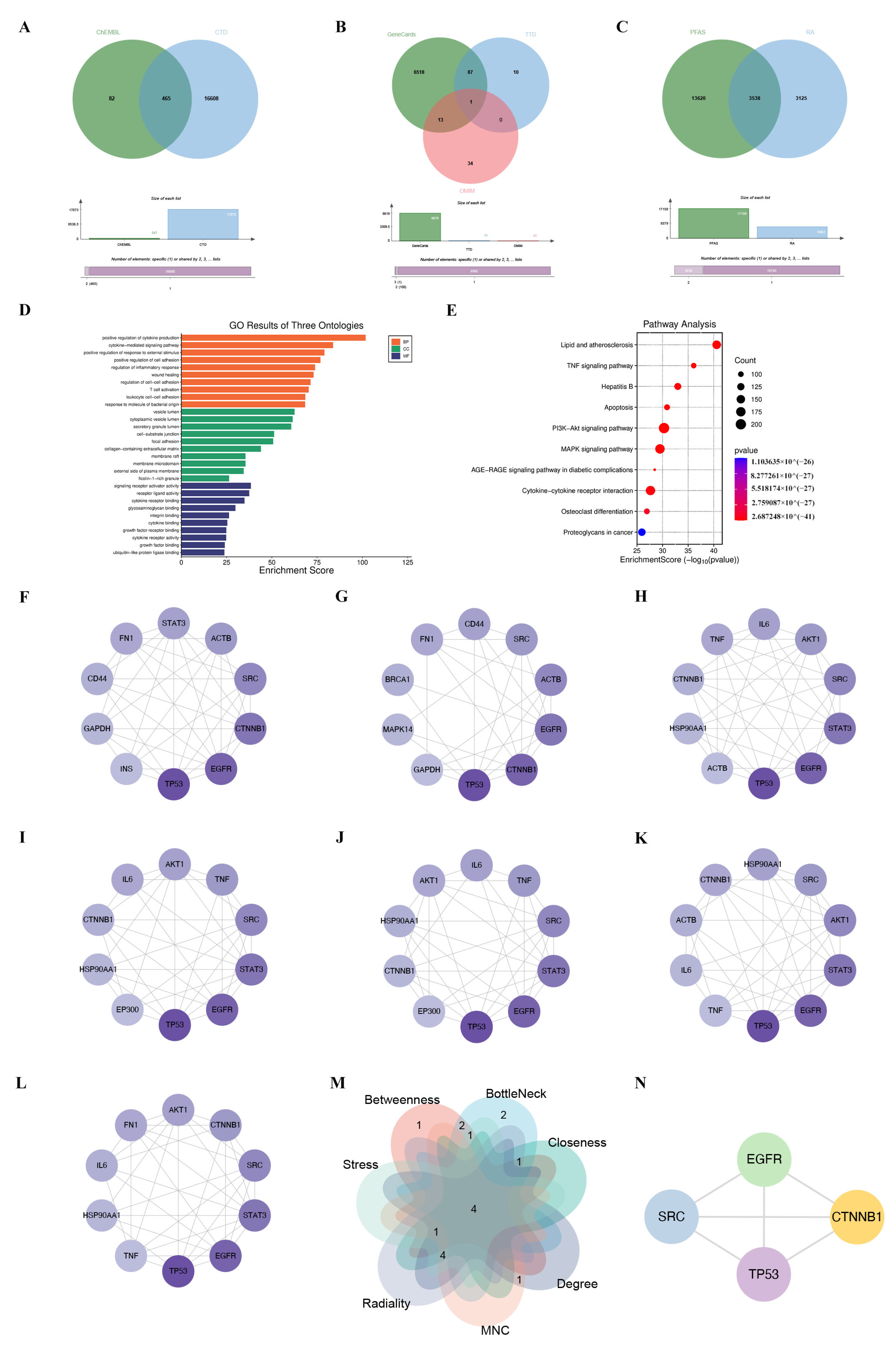
2.4. Screening of PFAS- and RA-Related Target Genes
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Serum PFAS Measurement
4.3. Definition of RA
4.4. Collection of Covariates
4.5. Screening of Potential Molecular Targets
4.6. Molecular Docking
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, J.H.; Barbagallo, B.; Annunziato, K.; Farias-Pereira, R.; Doherty, J.J.; Lee, J.; Zina, J.; Tindal, C.; McVey, C.; Aresco, R.; et al. Maternal Preconception PFOS Exposure of Drosophila Melanogaster Alters Reproductive Capacity, Development, Morphology and Nutrient Regulation. Food Chem. Toxicol. 2021, 151, 112153. [Google Scholar] [CrossRef]
- Huang, R.; Chen, Q.; Zhang, L.; Luo, K.; Chen, L.; Zhao, S.; Feng, L.; Zhang, J. Prenatal Exposure to Perfluoroalkyl and Polyfluoroalkyl Substances and the Risk of Hypertensive Disorders of Pregnancy. Environ. Health 2019, 18, 5. [Google Scholar] [CrossRef]
- Ding, N.; Harlow, S.D.; Randolph, J.F.; Loch-Caruso, R.; Park, S.K. Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) and Their Effects on the Ovary. Hum. Reprod. Update 2020, 26, 724–752. [Google Scholar] [CrossRef]
- Wan, H.; Mills, R.; Wang, Y.; Wang, K.; Xu, S.; Bhattacharyya, D.; Xu, Z. Gravity-Driven Electrospun Membranes for Effective Removal of Perfluoro-Organics from Synthetic Groundwater. J. Membr. Sci. 2022, 644, 120180. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Jung, H.W.; Kim, H.Y.; Choi, Y.-J.; Lee, Y.A. Early-Life Exposure to Per- and Poly-Fluorinated Alkyl Substances and Growth, Adiposity, and Puberty in Children: A Systematic Review. Front. Endocrinol. 2021, 12, 683297. [Google Scholar] [CrossRef] [PubMed]
- Berg, V.; Sandanger, T.M.; Hanssen, L.; Rylander, C.; Nøst, T.H. Time Trends of Perfluoroalkyl Substances in Blood in 30-Year Old Norwegian Men and Women in the Period 1986-2007. Environ. Sci. Pollut. Res. Int. 2021, 28, 43897–43907. [Google Scholar] [CrossRef] [PubMed]
- Lauria, M.Z.; Naim, A.; Plassmann, M.; Fäldt, J.; Sühring, R.; Benskin, J.P. Widespread Occurrence of Non-Extractable Fluorine in Artificial Turfs from Stockholm, Sweden. Environ. Sci. Technol. Lett. 2022, 9, 666–672. [Google Scholar] [CrossRef]
- Wu, W.; Patel, K.B.; Booth, J.L.; Zhang, W.; Metcalf, J.P. Cigarette Smoke Extract Suppresses the RIG-I-Initiated Innate Immune Response to Influenza Virus in the Human Lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 300, L821–L830. [Google Scholar] [CrossRef]
- Wrangel, O.; Graff, P.; Bryngelsson, I.-L.; Fornander, L.; Wiebert, P.; Vihlborg, P. Silica Dust Exposure Increases Risk for Rheumatoid Arthritis: A Swedish National Registry Case-Control Study. J. Occup. Environ. Med. 2021, 63, 951–955. [Google Scholar] [CrossRef]
- Irfan, S.; Rani, A.; Riaz, N.; Arshad, M.; Kashif Nawaz, S. Comparative Evaluation of Heavy Metals in Patients with Rheumatoid Arthritis and Healthy Control in Pakistani Population. Iran. J. Public Health 2017, 46, 626–633. [Google Scholar]
- Gargaro, M.; Pirro, M.; Romani, R.; Zelante, T.; Fallarino, F. Aryl Hydrocarbon Receptor-Dependent Pathways in Immune Regulation. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2016, 16, 2270–2276. [Google Scholar] [CrossRef]
- Thambirajah, A.A.; Wade, M.G.; Verreault, J.; Buisine, N.; Alves, V.A.; Langlois, V.S.; Helbing, C.C. Disruption by Stealth—Interference of Endocrine Disrupting Chemicals on Hormonal Crosstalk with Thyroid Axis Function in Humans and Other Animals. Environ. Res. 2022, 203, 111906. [Google Scholar] [CrossRef]
- Wu, L.; Wang, R.; Zhou, Y.; Zhao, D.; Chen, F.; Wu, X.; Chen, X.; Chen, S.; Li, J.; Zhu, J. Natural Killer Cells Infiltration in the Joints Exacerbates Collagen-Induced Arthritis. Front. Immunol. 2022, 13, 860761. [Google Scholar] [CrossRef]
- Song, J.J.; Song, Y.W.; Bae, S.C.; Cha, H.-S.; Choe, J.-Y.; Choi, S.J.; Kim, H.A.; Kim, J.; Kim, S.-S.; Lee, C.-K.; et al. Treat-to-Target Strategy for Asian Patients with Early Rheumatoid Arthritis: Result of a Multicenter Trial in Korea. J. Korean Med. Sci. 2018, 33, e346. [Google Scholar] [CrossRef]
- Ba, X.; Huang, Y.; Shen, P.; Huang, Y.; Wang, H.; Han, L.; Lin, W.J.; Yan, H.J.; Xu, L.J.; Qin, K.; et al. WTD Attenuating Rheumatoid Arthritis via Suppressing Angiogenesis and Modulating the PI3K/AKT/mTOR/HIF-1α Pathway. Front. Pharmacol. 2021, 12, 696802. [Google Scholar] [CrossRef] [PubMed]
- Ha, E.; Bae, S.-C.; Kim, K. Large-Scale Meta-Analysis across East Asian and European Populations Updated Genetic Architecture and Variant-Driven Biology of Rheumatoid Arthritis, Identifying 11 Novel Susceptibility Loci. Ann. Rheum. Dis. 2021, 80, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Pamphlett, R.; Kum Jew, S. Mercury Is Taken Up Selectively by Cells Involved in Joint, Bone, and Connective Tissue Disorders. Front. Med. 2019, 6, 168. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Parra, G.; Gilbert, E.L.; Valenzuela-Almada, M.O.; Vallejo, S.; Neville, M.R.; Patel, N.J.; Cook, C.; Fu, X.; Hagi, R.; McDermott, G.C.; et al. Risk of Severe COVID-19 Outcomes Associated with Rheumatoid Arthritis and Phenotypic Subgroups: A Retrospective, Comparative, Multicentre Cohort Study. Lancet Rheumatol. 2022, 4, e765–e774. [Google Scholar] [CrossRef]
- Bulka, C.M.; Avula, V.; Fry, R.C. Associations of Exposure to Perfluoroalkyl Substances Individually and in Mixtures with Persistent Infections: Recent Findings from NHANES 1999-2016. Environ. Pollut. 2021, 275, 116619. [Google Scholar] [CrossRef]
- DeWitt, J.C.; Peden-Adams, M.M.; Keller, J.M.; Germolec, D.R. Immunotoxicity of Perfluorinated Compounds: Recent Developments. Toxicol. Pathol. 2012, 40, 300–311. [Google Scholar] [CrossRef]
- Khalil, N.; Ducatman, A.M.; Sinari, S.; Billheimer, D.; Hu, C.; Littau, S.; Burgess, J.L. Per- and Polyfluoroalkyl Substance and Cardio Metabolic Markers in Firefighters. J. Occup. Environ. Med. 2020, 62, 1076–1081. [Google Scholar] [CrossRef]
- Rockwell, C.E.; Turley, A.E.; Cheng, X.; Fields, P.E.; Klaassen, C.D. Persistent Alterations in Immune Cell Populations and Function from a Single Dose of Perfluorononanoic Acid (PFNA) in C57Bl/6 Mice. Food Chem. Toxicol. 2017, 100, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Barton, K.E.; Zell-Baran, L.M.; DeWitt, J.C.; Brindley, S.; McDonough, C.A.; Higgins, C.P.; Adgate, J.L.; Starling, A.P. Cross-Sectional Associations between Serum PFASs and Inflammatory Biomarkers in a Population Exposed to AFFF-Contaminated Drinking Water. Int. J. Hyg. Environ. Health 2022, 240, 113905. [Google Scholar] [CrossRef] [PubMed]
- Steenland, K.; Zhao, L.; Winquist, A. A Cohort Incidence Study of Workers Exposed to Perfluorooctanoic Acid (PFOA). Occup. Environ. Med. 2015, 72, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.-C.; Li, Z.-H.; Ma, Y.-B.; Ma, H.-Y.; Zhang, M.-Y.; Zhang, X.-J.; Hu, C.-Y. Associations of Per- and Polyfluoroalkyl Substances (PFAS) and Their Mixture with Risk of Rheumatoid Arthritis in the U.S. Adult Population. Environ. Health 2024, 23, 38. [Google Scholar] [CrossRef]
- Jefferis, B.J.; Whincup, P.H.; Lennon, L.T.; Papacosta, O.; Goya Wannamethee, S. Physical Activity in Older Men: Longitudinal Associations with Inflammatory and Hemostatic Biomarkers, N-Terminal pro-Brain Natriuretic Peptide, and Onset of Coronary Heart Disease and Mortality. J. Am. Geriatr. Soc. 2014, 62, 599–606. [Google Scholar] [CrossRef]
- Lin, P.-I.D.; Cardenas, A.; Hauser, R.; Gold, D.R.; Kleinman, K.P.; Hivert, M.-F.; Fleisch, A.F.; Calafat, A.M.; Sanchez-Guerra, M.; Osorio-Yáñez, C.; et al. Dietary Characteristics Associated with Plasma Concentrations of Per- and Polyfluoroalkyl Substances among Adults with Pre-Diabetes: Cross-Sectional Results from the Diabetes Prevention Program Trial. Environ. Int. 2020, 137, 105217. [Google Scholar] [CrossRef]
- Barton, K.E.; Starling, A.P.; Higgins, C.P.; McDonough, C.A.; Calafat, A.M.; Adgate, J.L. Sociodemographic and Behavioral Determinants of Serum Concentrations of Per- and Polyfluoroalkyl Substances in a Community Highly Exposed to Aqueous Film-Forming Foam Contaminants in Drinking Water. Int. J. Hyg. Environ. Health 2020, 223, 256–266. [Google Scholar] [CrossRef]
- Tian, J.; Hong, Y.; Li, Z.; Yang, Z.; Lei, B.; Liu, J.; Cai, Z. Immunometabolism-Modulation and Immunotoxicity Evaluation of Perfluorooctanoic Acid in Macrophage. Ecotoxicol. Environ. Saf. 2021, 215, 112128. [Google Scholar] [CrossRef]
- Träger, U.; Andre, R.; Lahiri, N.; Magnusson-Lind, A.; Weiss, A.; Grueninger, S.; McKinnon, C.; Sirinathsinghji, E.; Kahlon, S.; Pfister, E.L.; et al. HTT-Lowering Reverses Huntington’s Disease Immune Dysfunction Caused by NFκB Pathway Dysregulation. Brain J. Neurol. 2014, 137, 819–833. [Google Scholar] [CrossRef]
- Yuan, F.-L.; Li, X.; Lu, W.-G.; Sun, J.-M.; Jiang, D.-L.; Xu, R.-S. Epidermal Growth Factor Receptor (EGFR) as a Therapeutic Target in Rheumatoid Arthritis. Clin. Rheumatol. 2013, 32, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Yasuda, K.; Mizuta, K.; Kawaue, H.; Kokabu, S. Tyrosine Kinase Src Is a Regulatory Factor of Bone Homeostasis. Int. J. Mol. Sci. 2022, 23, 5508. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-A.; Joo, N.-R.; Park, J.-H.; Oh, S.-M. Role of the SIRT1/P53 Regulatory Axis in Oxidative Stress-mediated Granulosa Cell Apoptosis. Mol. Med. Rep. 2021, 23, 20. [Google Scholar] [CrossRef]
- Huang, G.; Chubinskaya, S.; Liao, W.; Loeser, R.F. Wnt5a Induces Catabolic Signaling and Matrix Metalloproteinase Production in Human Articular Chondrocytes. Osteoarthr. Cartil. 2017, 25, 1505–1515. [Google Scholar] [CrossRef]
- Moroney, M.R.; Davies, K.D.; Wilberger, A.C.; Sheeder, J.; Post, M.D.; Berning, A.A.; Fisher, C.; Lefkowits, C.; Guntupalli, S.R.; Behbakht, K.; et al. Molecular Markers in Recurrent Stage I, Grade 1 Endometrioid Endometrial Cancers. Gynecol. Oncol. 2019, 153, 517–520. [Google Scholar] [CrossRef]
- Full, F.; Hahn, A.S.; Großkopf, A.K.; Ensser, A. Gammaherpesviral Tegument Proteins, PML-Nuclear Bodies and the Ubiquitin-Proteasome System. Viruses 2017, 9, 308. [Google Scholar] [CrossRef]
- Alijagic, A.; Sinisalu, L.; Duberg, D.; Kotlyar, O.; Scherbak, N.; Engwall, M.; Orešič, M.; Hyötyläinen, T. Metabolic and Phenotypic Changes Induced by PFAS Exposure in Two Human Hepatocyte Cell Models. Environ. Int. 2024, 190, 108820. [Google Scholar] [CrossRef]
- Johnson, C.L.; Paulose-Ram, R.; Ogden, C.L.; Carroll, M.D.; Kruszon-Moran, D.; Dohrmann, S.M.; Curtin, L.R. National Health and Nutrition Examination Survey: Analytic Guidelines, 1999–2010. Vital Health Stat. 2013, 1–24. [Google Scholar]
- Bernat, J.L. Informed Consent. Muscle Nerve 2001, 24, 614–621. [Google Scholar] [CrossRef]
- Jackson-Browne, M.S.; Eliot, M.; Patti, M.; Spanier, A.J.; Braun, J.M. PFAS (Per- and Polyfluoroalkyl Substances) and Asthma in Young Children: NHANES 2013-2014. Int. J. Hyg. Environ. Health 2020, 229, 113565. [Google Scholar] [CrossRef]
- Gui, J.; Ding, R.; Huang, D.; Wang, L.; Han, Z.; Yang, X.; Yang, J.; Luo, H.; Jiang, L. Associations between Urinary Heavy Metals and Anxiety among Adults in the National Health and Nutrition Examination Survey (NHANES), 2007-2012. Chemosphere 2023, 341, 140085. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Pan, C.; Su, W.; Vinturache, A.; Hu, Y.; Dong, X.; Ding, G. Associations between Exposure to a Mixture of Phenols, Parabens, and Phthalates and Sex Steroid Hormones in Children 6-19 Years from NHANES, 2013–2016. Sci. Total Environ. 2022, 822, 153548. [Google Scholar] [CrossRef]
- Zhu, X.; Yin, T.; Yue, X.; Liao, S.; Cheang, I.; Zhu, Q.; Yao, W.; Lu, X.; Shi, S.; Tang, Y.; et al. Association of Urinary Phthalate Metabolites with Cardiovascular Disease among the General Adult Population. Environ. Res. 2021, 202, 111764. [Google Scholar] [CrossRef] [PubMed]



| Characteristic | Overall, N = 8743 (100%) | Non-RA, N = 7832 (89%) | RA, N = 911 (11%) | p-Value |
|---|---|---|---|---|
| Age, N (%) | <0.001 | |||
| Young (20–39) | 3315 (39.89%) | 3257 (43.77%) | 58 (7.21%) | |
| Middle (40–59) | 2648 (35.49%) | 2392 (35.82%) | 256 (32.75%) | |
| Old (≥60) | 2780 (24.61%) | 2183 (20.42%) | 597 (60.03%) | |
| Gender, N (%) | <0.001 | |||
| Female | 4316 (49.29%) | 3752 (47.93%) | 564 (60.73%) | |
| Male | 4427 (50.71%) | 4080 (52.07%) | 347 (39.27%) | |
| Race, N (%) | <0.001 | |||
| Mexican American | 1456 (8.55%) | 1368 (9.19%) | 88 (3.15%) | |
| Non-Hispanic Black | 1789 (10.33%) | 1615 (10.58%) | 174 (8.25%) | |
| Non-Hispanic White | 3848 (68.97%) | 3341 (67.64%) | 507 (80.17%) | |
| Other Hispanic | 753 (5.36%) | 684 (5.61%) | 69 (3.30%) | |
| Other Race | 897 (6.79%) | 824 (6.98%) | 73 (5.13%) | |
| Educational level, N (%) | 0.4 | |||
| Greater than high school | 4762 (62.66%) | 4284 (62.82%) | 478 (61.37%) | |
| High school | 1963 (22.92%) | 1751 (22.68%) | 212 (24.99%) | |
| Lower than high school | 2018 (14.41%) | 1797 (14.51%) | 221 (13.63%) | |
| Marital status, N (%) | <0.001 | |||
| Married/living with partner | 5347 (64.22%) | 4825 (64.18%) | 522 (64.56%) | |
| Never married | 1714 (19.42%) | 1642 (21.03%) | 72 (5.88%) | |
| Widowed/divorced/separated | 1682 (16.36%) | 1365 (14.79%) | 317 (29.56%) | |
| Family PIR, N (%) | 0.6 | |||
| <1.30 | 2603 (19.93%) | 2327 (20.06%) | 276 (18.85%) | |
| >3.50 | 2821 (44.49%) | 2545 (44.54%) | 276 (44.04%) | |
| 1.30–3.5 | 3319 (35.58%) | 2960 (35.39%) | 359 (37.11%) | |
| BMI (kg/m2), N (%) | <0.001 | |||
| <25 | 2699 (32.04%) | 2495 (33.21%) | 204 (22.18%) | |
| >30 | 3063 (33.61%) | 2609 (31.89%) | 454 (48.12%) | |
| 25–30 | 2981 (34.35%) | 2728 (34.91%) | 253 (29.70%) | |
| Smoking, N (%) | 3778 (43.41%) | 3299 (42.41%) | 479 (51.89%) | <0.001 |
| Drinking alcohol status, N (%) | 6561 (80.73%) | 5885 (80.64%) | 676 (81.45%) | 0.6 |
| Diabetes, N (%) | 1059 (9.41%) | 813 (7.83%) | 246 (22.77%) | <0.001 |
| Crude Model | Model I | Model II | ||||
|---|---|---|---|---|---|---|
| Crude OR (95%CI) | p-Value | Adjusted OR (95%CI) | p-Value | Adjusted OR (95%CI) | P for Trend | |
| PFOA | ||||||
| Continuous | 1.31 (1.18–1.46) | <0.001 | 1.60 (1.40–1.83) | <0.001 | 1.63 (1.41–1.89) | <0.001 |
| Q1 | Reference | Reference | Reference | |||
| Q2 | 1.10 (0.83–1.44) | 1.38 (1.04–1.82) | 1.51 (1.14–2.00) | |||
| Q3 | 1.04 (0.80–1.35) | <0.001 | 1.48 (1.10–1.98) | <0.001 | 1.50 (1.11–2.02) | <0.001 |
| Q4 | 2.21 (1.68–2.91) | 3.24 (2.23–4.48) | 3.48 (2.46–4.92) | |||
| PFOS | ||||||
| Continuous | 1.17 (1.06–1.28) | <0.001 | 1.43 (1.28–1.60) | <0.001 | 1.41 (1.25–1.58) | <0.001 |
| Q1 | Reference | Reference | Reference | |||
| Q2 | 1.05 (0.81–1.37) | 1.40 (1.04–1.88) | 1.36 (1.00–1.83) | |||
| Q3 | 1.21 (0.91–1.61) | 0.003 | 1.90 (1.41–2.57) | <0.001 | 1.86 (1.37–2.51) | <0.001 |
| Q4 | 1.61 (1.20–2.16) | 2.79 (2.01–3.87) | 2.65 (1.87–3.76) | |||
| PFHxS | ||||||
| Continuous | 0.99 (0.90–1.09) | 0.8 | 1.10 (0.99–1.22) | 0.085 | 1.11 (0.99–1.24) | 0.079 |
| Q1 | Reference | Reference | Reference | |||
| Q2 | 0.71 (0.54–0.93) | 0.84 (0.62–1.15) | 0.89 (0.65–1.23) | |||
| Q3 | 0.80 (0.61–1.06) | 0.020 | 1.13 (0.84–1.52) | 0.066 | 1.17 (0.86–1.59) | 0.10 |
| Q4 | 0.97 (0.72–1.31) | 1.27 (0.92–1.76) | 1.33 (0.94–1.87) | |||
| PFNA | ||||||
| Continuous | 1.21 (1.08–1.37) | <0.001 | 1.47 (1.27–1.70) | <0.001 | 1.40 (1.20–1.63) | <0.001 |
| Q1 | Reference | Reference | Reference | |||
| Q2 | 0.92 (0.68–1.26) | 1.18 (0.85–1.63) | 1.12 (0.80–1.15) | |||
| Q3 | 1.24 (0.89–1.73) | 0.004 | 1.70 (1.18–2.45) | <0.001 | 1.65 (1.15–2.39) | <0.001 |
| Q4 | 1.49 (1.08–2.06) | 2.24 (1.54–3.25) | 2.01 (1.38–2.93) | |||
| Compounds | Targets | PDB ID | Vina Source (kcal/mol) |
|---|---|---|---|
| PFOA | CTNNB1 | 1jpw | −6.6 |
| EGFR | 1ivo | −7.7 | |
| TP53 | 1kzy | −6.3 | |
| SRC | 1a07 | −6.0 | |
| PFOS | CTNNB1 | 1jpw | −7.0 |
| EGFR | 1ivo | −8.6 | |
| TP53 | 1kzy | −6.9 | |
| SRC | 1a07 | −6.1 | |
| PFNA | CTNNB1 | 1jpw | −6.9 |
| EGFR | 1ivo | −7.9 | |
| TP53 | 1kzy | −6.5 | |
| SRC | 1a07 | −6.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, Y.; Zhao, C.; Xiang, Y.; Fu, W.; Li, J.; Wang, F.; Li, X. Perfluoroalkyl Substance (PFAS) Mixtures Drive Rheumatoid Arthritis Risk Through Immunosuppression: Integrating Epidemiology and Mechanistic Evidence. Int. J. Mol. Sci. 2025, 26, 7518. https://doi.org/10.3390/ijms26157518
Lv Y, Zhao C, Xiang Y, Fu W, Li J, Wang F, Li X. Perfluoroalkyl Substance (PFAS) Mixtures Drive Rheumatoid Arthritis Risk Through Immunosuppression: Integrating Epidemiology and Mechanistic Evidence. International Journal of Molecular Sciences. 2025; 26(15):7518. https://doi.org/10.3390/ijms26157518
Chicago/Turabian StyleLv, Yanming, Chunlong Zhao, Yi Xiang, Wenhao Fu, Jiaqi Li, Fan Wang, and Xueting Li. 2025. "Perfluoroalkyl Substance (PFAS) Mixtures Drive Rheumatoid Arthritis Risk Through Immunosuppression: Integrating Epidemiology and Mechanistic Evidence" International Journal of Molecular Sciences 26, no. 15: 7518. https://doi.org/10.3390/ijms26157518
APA StyleLv, Y., Zhao, C., Xiang, Y., Fu, W., Li, J., Wang, F., & Li, X. (2025). Perfluoroalkyl Substance (PFAS) Mixtures Drive Rheumatoid Arthritis Risk Through Immunosuppression: Integrating Epidemiology and Mechanistic Evidence. International Journal of Molecular Sciences, 26(15), 7518. https://doi.org/10.3390/ijms26157518





