When DNA Tells the Tale: High-Resolution Melting as a Forensic Tool for Mediterranean Cetacean Identification
Abstract
1. Introduction
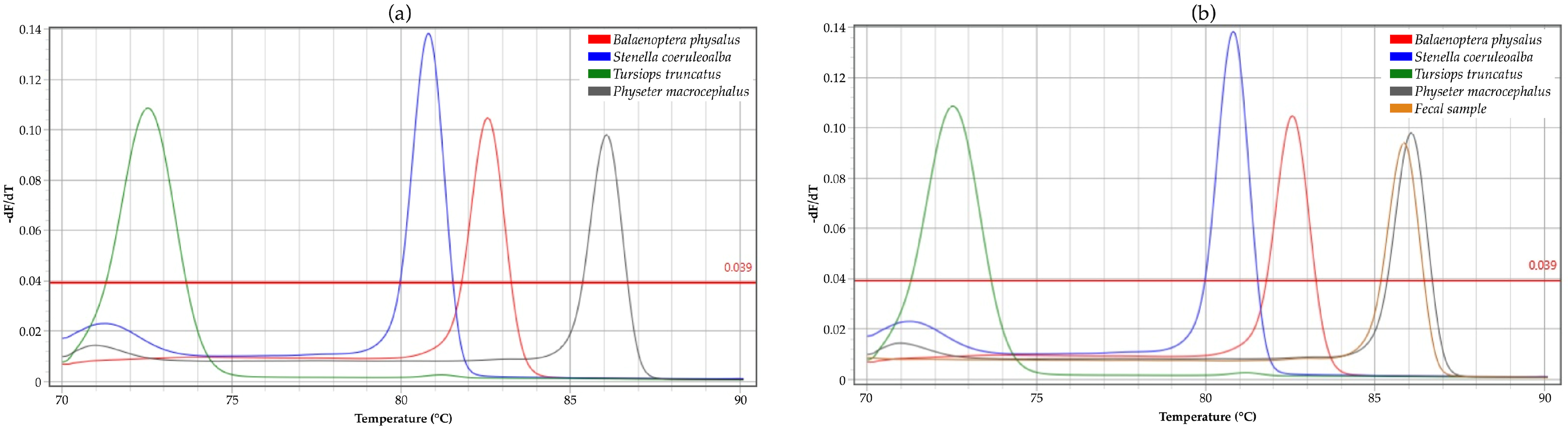
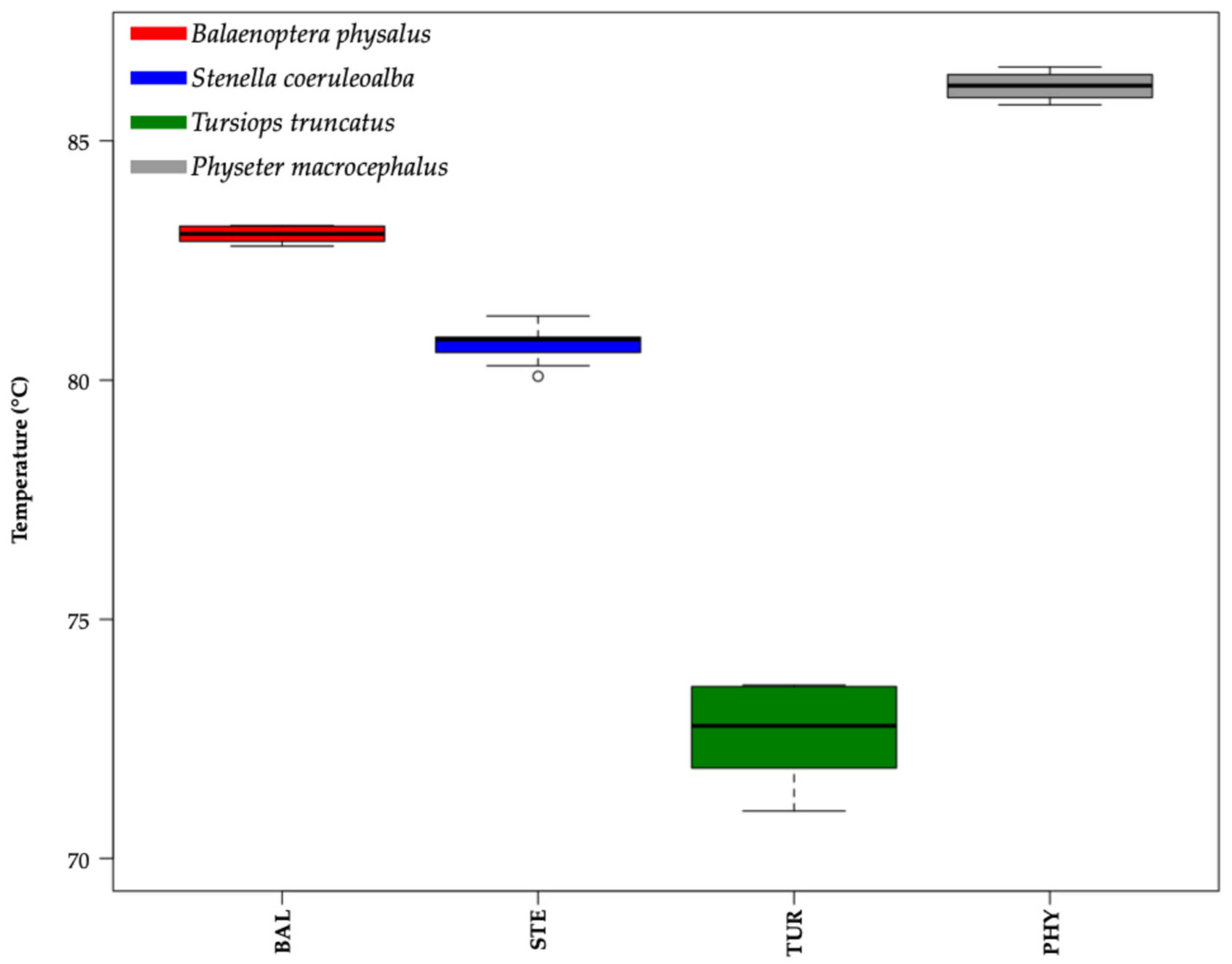
2. Results
3. Discussion
3.1. Applicability of HRM on Cetacean DNA Genotyping
3.2. Application in Wildlife Forensics
4. Materials and Methods
4.1. Sampling and DNA Extraction
4.2. Coprological Sampling and Analyses
4.3. Primer Design
4.4. High-Resolution Melting (HRM)
4.5. Quality Control and Validation Procedures
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gnone, G.; Bellingeri, M.; Airoldi, S.; Gonzalvo, J.; David, L.; Di-Méglio, N.; Cañadas, A.M.; Akkaya, A.; Awbery, T.; Mussi, B.; et al. Cetaceans in the Mediterranean Sea: Encounter Rate, Dominant Species, and Diversity Hotspots. Diversity 2023, 15, 321. [Google Scholar] [CrossRef]
- Torreblanca, E.; Báez, J.-C.; Real, R.; Macías, D.; García-Barcelona, S.; Ferri-Yañez, F.; Camiñas, J.-A. Factors associated with the differential distribution of cetaceans linked with deep habitats in the Western Mediterranean Sea. Sci. Rep. 2022, 12, 12918. [Google Scholar] [CrossRef]
- Notarbartolo di Sciara, G.; Tonay, A.M. Conserving Whales, Dolphins and Porpoises in the Mediterranean Sea, Black Sea and Adjacent Areas: An ACCOBAMS Status Report; ACCOBAMS: Monaco, 2021; 160p, ISBN 978-2-9579273-1-9. [Google Scholar]
- Panigada, S.; Boisseau, O.; Canadas, A.; Lambert, C.; Laran, S.; McLanaghan, R.; Moscrop, A. Estimates of Abundance and Distribution of Cetaceans, Marine Mega-Fauna and Marine Litter in the Mediterranean Sea from 2018–2019 Surveys; ACCOBAMS: Monaco, 2021; 177p. [Google Scholar]
- Cañadas, A.; Aissi, M.; Airoldi, S.; Alemany, X.; Arcangeli, A.; Atzori, F.; Marta, A.; Bellingeri, M.; Benamer, I.; Benmessaoud, R. Spatially-Explicit Cetacean Abundance Estimates from Multiple Data Sources in the Mediterranean. In Proceedings of the 36th Conference of European Cetacean Society, Ponta Delgada, Azores, Portugal, 12–16 May 2025. [Google Scholar]
- Panigada, S.; Lauriano, G.; Donovan, G.; Pierantonio, N.; Cañadas, A.; Vázquez, J.A.; Burt, L. Estimating cetacean density and abundance in the Central and Western Mediterranean Sea through aerial surveys: Implications for management. Deep-Sea Res. II Top. Stud. Oceanogr. 2017, 141, 41–58. [Google Scholar] [CrossRef]
- Mannocci, L.; Roberts, J.J.; Halpin, P.N.; Authier, M.; Boisseau, O.; Bradai, M.N.; Cañadas, A.; Chicote, C.; David, L.; Di-Méglio, N.; et al. Assessing cetacean surveys throughout the Mediterranean Sea: A gap analysis in environmental space. Sci. Rep. 2018, 8, 3126. [Google Scholar] [CrossRef] [PubMed]
- IUCN. The Conservation Status of Cetaceans in the Mediterranean Sea: Trends and Changes After a Decade of Conservation Efforts; IUCN: Gland, Switzerland, 2023.
- Aguilar, A.; Gaspari, S. Stenella coeruleoalba Mediterranean subpopulation, in The IUCN Red List of Threatened Species. 2012. Available online: https://www.iucnredlist.org/species/16674437/210833690 (accessed on 30 July 2025).
- Lauriano, G. Stenella coeruleoalba (Mediterranean subpopulation). The IUCN Red List of Threatened Species 2022: e.T16674437A210833690. 2022. Available online: https://www.iucnredlist.org (accessed on 30 July 2025).
- Bearzi, G.; Fortuna, C.M.; Reeves, R.R. Ecology and conservation of common bottlenose dolphins Tursiops truncatus in the Mediterranean Sea. Mammal Rev. 2009, 39, 92–123. [Google Scholar] [CrossRef]
- Reeves, R.R.; McClellan, K.; Werner, T.B. Marine mammal bycatch in gillnet and other entangling net fisheries, 1990 to 2011. Endanger. Species Res. 2013, 20, 71–97. [Google Scholar] [CrossRef]
- Petitguyot, M.A.C.; Bearzi, G.; van den Hurk, Y.; Fuentes, M.T.; Pierce, G.J. Intentional Killings and Culling of Small Cetaceans due to Perceived Competition with Fisheries in the Mediterranean Sea and Northeast Atlantic between the Eighteenth and Twentieth Centuries. In Oceanography and Marine Biology; CRC Press: Boca Raton, FL, USA, 2024; Volume 62, 43p. [Google Scholar]
- Bearzi, G.; Fortuna, C.; Reeves, R. Tursiops truncatus (Mediterranean subpopulation). 2012. Available online: https://www.iucnredlist.org/species/16369383/16369386 (accessed on 30 July 2025).
- Natoli, A.; Genov, T.; Kerem, D.; Gonzalvo, J.; Lauriano, G.; Holcer, D.; Labach, H.; Marsili, L.; Mazzariol, S.; Moura, A.E.; et al. Tursiops truncatus (Mediterranean subpopulation); IUCN: Gland, Switzerland, 2021.
- Cañadas, A. Ziphius cavirostris (Mediterranean subpopulation). 2012. Available online: https://www.iucnredlist.org/species/16381144/16382769 (accessed on 30 July 2025).
- Cañadas, A.; Notarbartolo di Sciara, G. Ziphius cavirostris (Mediterranean subpopulation). IUCN Red List Threat. Species 2018, e.T16381144A199549199. [Google Scholar] [CrossRef]
- Notarbartolo di Sciara, G.; Frantzis, A.; Bearzi, G.; Reeves, R. Physeter macrocephalus (Mediterranean subpopulation). 2012. Available online: https://www.iucnredlist.org/species/16370739/16370477 (accessed on 30 July 2025).
- Pirotta, E.; Carpinelli, E.; Frantzis, A.; Gauffier, P.; Lanfredi, C.; Pace, D.S.; Rendell, L.E. Physeter macrocephalus (Mediterranean subpopulation). 2012. Available online: https://www.iucnredlist.org/species/16370739/50285671 (accessed on 30 July 2025).
- Panigada, S.; Notarbartolo di Sciara, G. Balaenoptera physalus (Mediterranean subpopulation). 2012. The IUCN Red List of Threatened Species 2012 e.T16208224A17549588. Available online: https://www.iucnredlist.org/species/16208224/17549588 (accessed on 1 August 2025). [CrossRef]
- Taylor, B.L.; Chivers, S.J.; Larese, J.; Perrin, W.F. Generation Length and Percent Mature Estimates for IUCN Assessments of Cetaceans. Southwest Fish. Sci. Cent. Publ. Database 2007, 24. [Google Scholar]
- Panigada, S.; Gauffier, P.; Notarbartolo di Sciara, G. Balaenoptera physalus (Mediterranean subpopulation). IUCN: Gland, Switzerland, 2021. [Google Scholar]
- Siciliano, S.; de Moura, J.F.; Filgueiras, H.R.; Rodrigues, P.P.; de Oliveira Leite, N.J. Sightings of humpback whales on the Vitória-Trindade chain and around Trindade Island, Brazil. Braz. J. Oceanogr. 2012, 60, 455–459. [Google Scholar] [CrossRef][Green Version]
- Silva, V.S.; Skueresky, N.; Lopes, F.; Koch, T.K.; Ott, P.H.; Siciliano, S.; Barreto, A.S.; Secchi, E.R.; de Meirelles, A.C.O.; Carvalho, V.L.; et al. Integrating morphology and DNA barcoding to assess cetacean diversity in Brazil. Mamm. Res. 2021, 66, 349–369. [Google Scholar] [CrossRef]
- Mattioda, V.; Giorda, F.; Consales, G.; Testori, C.; Zoppi, S.; Goria, M.; Crescio, M.I.; Serracca, L.; Varello, K.; Carta, V.; et al. Anthropic Pressure on Cetaceans Stranded Along the Ligurian Coast Within the Pelagos Sanctuary: A Case Series. Animals 2024, 14, 3207. [Google Scholar] [CrossRef]
- Esposito, E.; Oliviero, M.; Iaccarino, D.; Paduano, G.; Serra, F.; Levante, M.; Amoroso, M.G.; Auriemma, C.; Gallo, A.; Lucibelli, M.G.; et al. Post Mortem Findings of Cetaceans Stranded Along the Campania Coast from 2016 to 2022. Animals 2025, 15, 1812. [Google Scholar] [CrossRef]
- Grattarola, C.; Pietroluongo, G.; Belluscio, D.; Berio, E.; Canonico, C.; Centelleghe, C.; Cocumelli, C.; Crotti, S.; Denurra, D.; Di Donato, A.; et al. Pathogen Prevalence in Cetaceans Stranded along the Italian Coastline between 2015 and 2020. Pathogens 2024, 13, 762. [Google Scholar] [CrossRef]
- Cuvertoret-Sanz, M.; López-Figueroa, C.; O’Byrne, A.; Canturri, A.; Martí-Garcia, B.; Pintado, E.; Pérez, L.; Ganges, L.; Cobos, A.; Abarca, M.L.; et al. Causes of cetacean stranding and death on the Catalonian coast (western Mediterranean Sea), 2012–2019. Dis. Aquat. Organ. 2020, 142, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Delgado, J.; Fernández, A.; Sierra, E.; Sacchini, S.; Andrada, M.; Vela, A.I.; Quesada-Canales, Ó.; Paz, Y.; Zucca, D.; Groch, K.; et al. Pathologic findings and causes of death of stranded cetaceans in the Canary Islands (2006–2012). PLoS ONE 2018, 13, e0204444. [Google Scholar] [CrossRef] [PubMed]
- Notarbartolo di Sciara, G. Chapter One—Marine Mammals in the Mediterranean Sea: An Overview. In Advances in Marine Biology; Notarbartolo Di Sciara, G., Podestà, M., Curry, B.E., Eds.; Academic Press: Cambridge, MA, USA, 2016; Volume 75, pp. 1–36. [Google Scholar]
- Laran, S.; Authier, M.; Blanck, A.; Doremus, G.; Falchetto, H.; Monestiez, P.; Pettex, E.; Stephan, E.; Van Canneyt, O.; Ridoux, V. Seasonal distribution and abundance of cetaceans within French waters—Part II: The Bay of Biscay and the English Channel. Deep-Sea Res. II Top. Stud. Oceanogr. 2017, 141, 31–40. [Google Scholar] [CrossRef]
- Laran, S.; Pettex, E.; Authier, M.; Blanck, A.; David, L.; Dorémus, G.; Falchetto, H.; Monestiez, P.; Van Canneyt, O.; Ridoux, V. Seasonal distribution and abundance of cetaceans within French waters–Part I: The North-Western Mediterranean, including the Pelagos sanctuary. Deep-Sea Res. II Top. Stud. Oceanogr. 2017, 141, 20–30. [Google Scholar] [CrossRef]
- Hammond, P.S.; Francis, T.B.; Heinemann, D.; Long, K.J.; Moore, J.E.; Punt, A.E.; Reeves, R.R.; Sepúlveda, M.; Sigurðsson, G.M.; Siple, M.C.; et al. Estimating the Abundance of Marine Mammal Populations. Front. Mar. Sci. 2021, 8, 3–12. [Google Scholar] [CrossRef]
- Panigada, S.; Pierantonio, N.; Araújo, H.; David, L.; Di-Méglio, N.; Dorémus, G.; Gonzalvo, J.; Holcer, D.; Laran, S.; Lauriano, G.; et al. The ACCOBAMS survey initiative: The first synoptic assessment of cetacean abundance in the Mediterranean Sea through aerial surveys. Front. Mar. Sci. 2024, 10, 1270513. [Google Scholar] [CrossRef]
- Dawson, S.; Wade, P.; Slooten, E.; Barlow, J. Design and field methods for sighting surveys of cetaceans in coastal and riverine habitats. Mammal Rev. 2008, 38, 19–49. [Google Scholar] [CrossRef]
- Oedekoven, C.S.; Marques, T.A.; Harris, D.; Thomas, L.; Thode, A.M.; Blackwell, S.B.; Conrad, A.S.; Kim, K.H. A comparison of three methods for estimating call densities of migrating bowhead whales using passive acoustic monitoring. Environ. Ecol. Stat. 2022, 29, 101–125. [Google Scholar] [CrossRef]
- Ballance, L. Contributions of Photographs to Cetacean Science. Aquat. Mamm. 2018, 44, 668–682. [Google Scholar] [CrossRef]
- Frasier, K.E.; Garrison, L.P.; Soldevilla, M.S.; Wiggins, S.M.; Hildebrand, J.A. Cetacean distribution models based on visual and passive acoustic data. Res. Ethics 2021, 11, 8240. [Google Scholar] [CrossRef] [PubMed]
- Caruso, F.; Dong, L.; Lin, M.; Liu, M.; Gong, Z.; Xu, W.; Alonge, G.; Li, S. Monitoring of a Nearshore Small Dolphin Species Using Passive Acoustic Platforms and Supervised Machine Learning Techniques. Front. Mar. Sci. 2020, 7, 2020. [Google Scholar] [CrossRef]
- Caruso, F.; Sciacca, V.; Alonge, G.; Bellia, G.; Buscaino, G.; De Domenico, E.; Grammauta, R.; Larosa, G.; Mazzola, S.; Pavan, G.; et al. Long-term monitoring of cetacean bioacoustics using cabled observatories in deep-sea off East Sicily. J. Acoust. Soc. Am. 2017, 141, 4001. [Google Scholar] [CrossRef]
- Leonard, D.; Øien, N. Estimated Abundances of Cetacean Species in the Northeast Atlantic from Norwegian Shipboard Surveys Conducted in 2014–2018; NAMMCO Scientific Publications: Tromsø, Norway, 2020. [Google Scholar] [CrossRef]
- Marques, T.A.; Thomas, L.; Martin, S.W.; Mellinger, D.K.; Ward, J.A.; Moretti, D.J.; Harris, D.; Tyack, P.L. Estimating animal population density using passive acoustics. Biol. Rev. 2013, 88, 287–309. [Google Scholar] [CrossRef]
- Liu, M.; Lin, M.; Dong, L.; Caruso, F.; Li, S. An integrated strategy for monitoring cetaceans in data-poor regions. Biol. Conserv. 2022, 272, 109648. [Google Scholar] [CrossRef]
- Cabrera, A.A.; Bérubé, M.; Lopes, X.M.; Louis, M.; Oosting, T.; Rey-Iglesia, A.; Rivera-León, V.E.; Székely, D.; Lorenzen, E.D.; Palsbøll, P.J. A Genetic Perspective on Cetacean Evolution. Annu. Rev. Ecol. Evol. Syst. 2021, 52, 131–151. [Google Scholar] [CrossRef]
- Kraft, S.; Rodríguez, F.; Olavarría, C.; Poulin, E.; Pérez-Álvarez, M.J. Genetic Analysis as a Tool to Improve the Monitoring of Stranded Cetaceans in Chile. Biology 2023, 12, 748. [Google Scholar] [CrossRef]
- Valsecchi, E.; Amos, W. Microsatellite markers for the study of cetacean populations. Mol. Ecol. 1996, 5, 151–156. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Maxwell, E.A.; Marshall, A.D.; Christensen, A.B. Evaluating manta ray mucus as an alternative DNA source for population genetics study: Underwater-sampling, dry-storage and PCR success. PeerJ 2015, 3, e1188. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kashiwagi, T.; Marshall, A.D.; Bennett, M.B.; Ovenden, J.R. The genetic signature of recent speciation in manta rays (Manta alfredi and M. birostris). Mol. Phylogenetics Evol. 2012, 64, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Larson, S.; Farrer, D.; Lowry, D.; Ebert, D.A. Preliminary Observations of Population Genetics and Relatedness of the Broadnose Sevengill Shark, Notorynchus cepedianus, in Two Northeast Pacific Estuaries. PLoS ONE 2015, 10, e0129278. [Google Scholar] [CrossRef] [PubMed]
- Marsili, L.; Fossi, M.C.; Neri, G.; Casini, S.; Gardi, C.; Palmeri, S.; Tarquini, E.; Panigada, S. Skin biopsies for cell cultures from Mediterranean free-ranging cetaceans. Mar. Environ. Res. 2000, 50, 523–526. [Google Scholar] [CrossRef]
- Noren, D.P.; Mocklin, J.A. Review of cetacean biopsy techniques: Factors contributing to successful sample collection and physiological and behavioral impacts. Mar. Mamm. Sci. 2012, 28, 154–199. [Google Scholar] [CrossRef]
- Krützen, M.; Barre, L.M.; Möller, L.M.; Heithaus, M.R.; Simms, C.; Sherwin, W.B. A biopsy system for small cetaceans: Darting success and wound healing in Tursiops spp. Mar. Mamm. Sci. 2002, 18, 863–878. [Google Scholar] [CrossRef]
- Bearzi, G. First report of a common dolphin (Delphinus delphis) death following penetration of a biopsy dart. J. Cetacean Res. Manag. 2000, 2, 217–221. [Google Scholar] [CrossRef]
- Garrigue, C.; Derville, S. Behavioral responses of humpback whales to biopsy sampling on a breeding ground: The influence of age-class, reproductive status, social context, and repeated sampling. Mar. Mamm. Sci. 2022, 38, 102–117. [Google Scholar] [CrossRef]
- Adams, C.I.M.; Knapp, M.; Gemmell, N.J.; Jeunen, G.J.; Bunce, M.; Lamare, M.D.; Taylor, H.R. Beyond Biodiversity: Can Environmental DNA (eDNA) Cut It as a Population Genetics Tool? Genes 2019, 10, 192. [Google Scholar] [CrossRef]
- Rendell, L.; Mesnick, S.L.; Dalebout, M.L.; Burtenshaw, J.; Whitehead, H. Can genetic differences explain vocal dialect variation in sperm whales, Physeter macrocephalus? Behav. Genet. 2012, 42, 332–343. [Google Scholar] [CrossRef]
- Whitehead, H.; Gordon, J.; Mathews, E.A.; Richard, K.R. Obtaining skin samples from living sperm whales. Mar. Mammal Sci. 1990, 6, 316–326. [Google Scholar] [CrossRef]
- Valsecchi, E.; Glockner-Ferrari, D.; Ferrari, M.; Amos, W. Molecular analysis of the efficiency of sloughed skin sampling in whale population genetics. Mol. Ecol. 1998, 7, 1419–1422. [Google Scholar] [CrossRef]
- Parsons, K.M.; Dallas, J.F.; Claridge, D.E.; Durban, J.W.; Balcomb, I.K.; Thompson, P.M.; Noble, L.R. Amplifying dolphin mitochondrial DNA from faecal plumes. Mol. Ecol. 1999, 8, 1766–1768. [Google Scholar] [CrossRef]
- Alter, S.E.; King, C.D.; Chou, E.; Chin, S.C.; Rekdahl, M.; Rosenbaum, H.C. Using Environmental DNA to Detect Whales and Dolphins in the New York Bight. Front. Conserv. Sci. 2022, 3, 820377. [Google Scholar] [CrossRef]
- Baker, C.S.; Steel, D.; Calambokidis, J.; Falcone, E.A.; Gozález-Peral, U.; Barlow, J.; Burdin, A.M.; Clapham, P.J.; Ford, J.K.B.; Gabriele, C.M.; et al. Strong Maternal Fidelity and Natal Philopatry Shape Genetic Structure in North Pacific Humpback Whales. Mar. Ecol. Prog. Ser. 2013, 494, 291–306. [Google Scholar] [CrossRef]
- Baker, C.S.; Claridge, D.; Dunn, C.; Fetherston, T.; Baker, D.N.; Klinck, H.; Steel, D. Quantification by droplet digital PCR and species identification by metabarcoding of environmental (e)DNA from Blainville’s beaked whales, with assisted localization from an acoustic array. PLoS ONE 2023, 18, e0291187. [Google Scholar] [CrossRef] [PubMed]
- Pinfield, R.; Dillane, E.; Runge, A.K.W.; Evans, A.; Mirimin, L.; Niemann, J.; Reed, T.E.; Reid, D.G.; Rogan, E.; Samarra, F.I.P.; et al. False-negative detections from environmental DNA collected in the presence of large numbers of killer whales (Orcinus orca). Environ. DNA 2019, 1, 316–328. [Google Scholar] [CrossRef]
- Székely, D.; Corfixen, N.L.; Mørch, L.L.; Knudsen, S.W.; McCarthy, M.L.; Teilmann, J.; Heide-Jørgensen, M.P.; Olsen, M.T. Environmental DNA captures the genetic diversity of bowhead whales (Balaena mysticetus) in West Greenland. Environ. DNA 2021, 3, 248–260. [Google Scholar] [CrossRef]
- Boldrocchi, G.; Conte, L.; Galli, P.; Bettinetti, R.; Valsecchi, E. Cuvier’s beaked whale (Ziphius cavirostris) detection through surface-sourced eDNA: A promising approach for monitoring deep-diving cetaceans. Ecol. Indic. 2024, 161, 111966. [Google Scholar] [CrossRef]
- Valsecchi, E.; Arcangeli, A.; Lombardi, R.; Boyse, E.; Carr, I.M.; Galli, P.; Goodman, S.J. Ferries and Environmental DNA: Underway Sampling From Commercial Vessels Provides New Opportunities for Systematic Genetic Surveys of Marine Biodiversity. Front. Mar. Sci. 2021, 8, 2021. [Google Scholar] [CrossRef]
- Hunt, K.E.; Moore, M.J.; Rolland, R.M.; Kellar, N.M.; Hall, A.J.; Kershaw, J.; Raverty, S.A.; Davis, C.E.; Yeates, L.C.; Fauquier, D.A.; et al. Overcoming the challenges of studying conservation physiology in large whales: A review of available methods. Conserv. Physiol. 2013, 1, cot006. [Google Scholar] [CrossRef] [PubMed]
- Yeates, L.C.; Borras, E.; Cumeras, R.; Davis, C.E. Chapter 28—Breath analysis in marine mammals. In Breathborne Biomarkers and the Human Volatilome, 2nd ed.; Beauchamp, J., Davis, C., Pleil, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 461–472. [Google Scholar]
- Valsecchi, E. The answer, my friend, is blowin’ in the wind: Blow sampling provides a new dimension to whale population monitoring. Mol. Ecol. Resour. 2024, 24, e14012. [Google Scholar] [CrossRef]
- Kriangwanich, W.; Buddhachat, K.; Poommouang, A.; Chomdej, S.; Thitaram, C.; Kaewmong, P.; Kittiwattanawong, K.; Nganvongpanit, K. Feasibility of melting fingerprint obtained from ISSR-HRM curves for marine mammal species identification. PeerJ 2021, 9, e11689. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-T.; Chou, L.-S.; Lo, C.; Yang, W.-c. Rapid Species Identification of Morphologically Similar Cetacean Species Kogia sima and K. breviceps by High-resolution Melt Analysis. Pak. J. Zool. 2013, 45, 273–277. [Google Scholar] [CrossRef]
- Chatzoglou, E.; Tsaousi, N.; Apostolidis, A.P.; Exadactylos, A.; Sandaltzopoulos, R.; Giantsis, I.A.; Gkafas, G.A.; Malandrakis, E.E.; Sarantopoulou, J.; Tokamani, M.; et al. High-Resolution Melting (HRM) Analysis for Rapid Molecular Identification of Sparidae Species in the Greek Fish Market. Genes 2023, 14, 1255. [Google Scholar] [CrossRef]
- Alacs, E.A.; Georges, A.; FitzSimmons, N.N.; Robertson, J. DNA detective: A review of molecular approaches to wildlife forensics. Forensic Sci. Med. Pathol. 2010, 6, 180–194. [Google Scholar] [CrossRef]
- Baker, C.S.; Lukoschek, V.; Lavery, S.; Dalebout, M.L.; Yong-un, M.; Endo, T.; Funahashi, N. Incomplete reporting of whale, dolphin and porpoise ‘bycatch’ revealed by molecular monitoring of Korean markets. Anim. Conserv. 2006, 9, 474–482. [Google Scholar] [CrossRef]
- Baker, C.S.; Cooke, J.G.; Lavery, S.; Dalebout, M.L.; Ma, Y.-U.; Funahashi, N.; Carraher, C.; Brownell, R.L. Estimating the number of whales entering trade using DNA profiling and capture-recapture analysis of market products. Mol. Ecol. 2007, 16, 2617–2626. [Google Scholar] [CrossRef]
- Linacre, A.; Tobe, S.S. An overview to the investigative approach to species testing in wildlife forensic science. Investig. Genet. 2011, 2, 2. [Google Scholar] [CrossRef]
- Lo, C.; Chin, L.-T.; Chu, C.-S.; Wang, Y.-T.; Chan, K.-W.; Yang, W.-C. Rapid Immune Colloidal Gold Strip for Cetacean Meat Restraining Illegal Trade and Consumption: Implications for Conservation and Public Health. PLoS ONE 2013, 8, e60704. [Google Scholar] [CrossRef]
- Lukoschek, V.; Funahashi, N.; Lavery, S.; Dalebout, M.L.; Cipriano, F.; Baker, C.S. High proportion of protected minke whales sold on Japanese markets is due to illegal, unreported or unregulated exploitation. Anim. Conserv. 2009, 12, 385–395. [Google Scholar] [CrossRef]
- Ogden, R.; Dawnay, N.; McEwing, R. Wildlife DNA forensics—Bridging the gap between conservation genetics and law enforcement. Endanger. Species Res. 2009, 9, 179–195. [Google Scholar] [CrossRef]
- Porter, L.; Lai, H.Y. Marine Mammals in Asian Societies; Trends in Consumption, Bait, and Traditional Use. Front. Mar. Sci. 2017, 4, 47. [Google Scholar] [CrossRef]
- Martinez, I.; Daníelsdóttir, A.K. Identification of marine mammal species in food products. J. Sci. Food Agric. 2000, 80, 527–533. [Google Scholar] [CrossRef]
- Sholl, T.G.C.; do Nascimento, F.F.; Leoncini, O.; Bonvicino, C.R.; Siciliano, S. Taxonomic identification of dolphin love charms commercialized in the Amazonian region through the analysis of cytochrome b DNA. J. Mar. Biol. Assoc. UK 2008, 88, 1207–1210. [Google Scholar] [CrossRef]
- Sholl, T.G.C.; de Moura, J.F.; Ott, P.H.; Bonvicino, C.R.; Reis, E.C.; Tavares, D.C.; Siciliano, S. Cytochrome b sequencing for the species identification of whale carcasses washed ashore in Brazil. Mar. Biodivers. Rec. 2013, 6, e30. [Google Scholar] [CrossRef]
- Teramitsu, I.; Yamamoto, Y.; Chiba, I.; Iwata, H.; Tanabe, S.; Fujise, Y.; Kazusaka, A.; Akahori, F.; Fujita, S. Identification of novel cytochrome P450 1A genes from five marine mammal species. Aquat. Toxicol. 2000, 51, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Wittwer, C.T. High-resolution DNA melting analysis: Advancements and limitations. Hum. Mutat. 2009, 30, 857–859. [Google Scholar] [CrossRef]
- Erdem, M.; Kesmen, Z.; Özbekar, E.; Çetin, B.; Yetim, H. Application of high-resolution melting analysis for differentiation of spoilage yeasts. J. Microbiol. 2016, 54, 618–625. [Google Scholar] [CrossRef]
- Gopaul, K.K.; Sells, J.; Lee, R.; Beckstrom- Sternberg, S.M.; Foster, J.T.; Whatmore, A.M. Development and assessment of multiplex high resolution melting assay as a tool for rapid single-tube identification of five Brucella species. BMC Res. Notes 2014, 7, 903. [Google Scholar] [CrossRef]
- Jin, Y.; Li, Q.; Kong, L.; Yu, H.; Zhong, X. High-resolution melting (HRM) analysis: A highly sensitive alternative for the identification of commercially important Crassostrea oysters. J. Molluscan Stud. 2014, 81, 167–170. [Google Scholar] [CrossRef]
- Erali, M.; Wittwer, C.T. High resolution melting analysis for gene scanning. Methods 2010, 50, 250–261. [Google Scholar] [CrossRef]
- Power, E.G.M. RAPD typing in microbiology—A technical review. J. Hosp. Infect. 1996, 34, 247–265. [Google Scholar] [CrossRef]
- Reed, G.H.; Kent, J.O.; Wittwer, C.T. High-Resolution DNA Melting Analysis for Simple and Efficient Molecular Diagnostics. Pharmacogenomics 2007, 8, 597–608. [Google Scholar] [CrossRef]
- Tulsiani, S.M.; Craig, S.B.; Graham, G.C.; Cobbold, R.C.; Dohnt, M.F.; Burns, M.A.; Leung, L.K.; Field, H.E.; Smythe, L.D. High-resolution melt-curve analysis of random-amplified-polymorphic-DNA markers, for the characterisation of pathogenic Leptospira. Ann. Trop. Med. Parasitol. 2010, 104, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Vossen, R.H.A.M.; Aten, E.; Roos, A.; den Dunnen, J.T. High-Resolution Melting Analysis (HRMA)—More than just sequence variant screening. Hum. Mutat. 2009, 30, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Wittwer, C.T.; Reed, G.H.; Gundry, C.N.; Vandersteen, J.G.; Pryor, R.J. High-Resolution Genotyping by Amplicon Melting Analysis Using LCGreen. Clin. Chem. 2003, 49, 853–860. [Google Scholar] [CrossRef] [PubMed]
- McGowen, M.R.; Tsagkogeorga, G.; Álvarez-Carretero, S.; Dos Reis, M.; Struebig, M.; Deaville, R.; Jepson, P.D.; Jarman, S.; Polanowski, A.; Morin, P.A.; et al. Phylogenomic Resolution of the Cetacean Tree of Life Using Target Sequence Capture. Syst. Biol. 2020, 69, 479–501. [Google Scholar] [CrossRef]
- Poommouang, A.; Piboon, P.; Kittiwatanawong, K.; Sucharitakul, P.; Kaewmong, P.; Nganvongpanit, K.; Buddhachat, K. Stranded carcass identification of marine mammal species by high resolution melting analysis using barcodes (Bar-HRM). Mar. Biol. 2025, 172, 77. [Google Scholar] [CrossRef]
- Athamanolap, P.; Parekh, V.; Fraley, S.I.; Agarwal, V.; Shin, D.J.; Jacobs, M.A.; Wang, T.-H.; Yang, S. Trainable High Resolution Melt Curve Machine Learning Classifier for Large-Scale Reliable Genotyping of Sequence Variants. PLoS ONE 2014, 9, e109094. [Google Scholar] [CrossRef]
- Villinger, J.; Mbaya, M.K.; Ouso, D.; Kipanga, P.N.; Lutomiah, J.; Masiga, D.K. Arbovirus and insect-specific virus discovery in Kenya by novel six genera multiplex high-resolution melting analysis. Mol. Ecol. Resour. 2017, 17, 466–480. [Google Scholar] [CrossRef] [PubMed]
- Eaton, M.J.; Meyers, G.L.; Kolokotronis, S.-O.; Leslie, M.S.; Martin, A.P.; Amato, G. Barcoding bushmeat: Molecular identification of Central African and South American harvested vertebrates. Conserv. Genet. 2010, 11, 1389–1404. [Google Scholar] [CrossRef]
- Rezaei, F.; Haeili, M.; Fooladi, A.I.; Feizabadi, M.M. High Resolution Melting Curve Analysis for Rapid Detection of Streptomycin and Ethambutol Resistance in Mycobacterium tuberculosis. Maedica 2017, 12, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Prasetyo, A.P.; Cusa, M.; Murray, J.M.; Agung, F.; Muttaqin, E.; Mariani, S.; McDevitt, A.D. Universal closed-tube barcoding for monitoring the shark and ray trade in megadiverse conservation hotspots. iScience 2023, 26, 107065. [Google Scholar] [CrossRef]
- Battistini, R.; Masotti, C.; Giorda, F.; Grattarola, C.; Peletto, S.; Testori, C.; Zoppi, S.; Berio, E.; Crescio, M.I.; Pussini, N.; et al. Photobacterium damselae subsp. damselae in Stranded Cetaceans: A 6-Year Monitoring of the Ligurian Sea in Italy. Animals 2024, 14, 2825. [Google Scholar] [CrossRef]
- Yagi, G.; Qi, H.; Arai, K.; Kita, Y.F.; Kogi, K.; Morisaka, T.; Yoshioka, M.; Inoue-Murayama, M. Non-invasive age estimation based on faecal DNA using methylation-sensitive high-resolution melting for Indo-Pacific bottlenose dolphins. Mol. Ecol. Resour. 2024, 24, e13906. [Google Scholar] [CrossRef]
- Robinson, C.V.; Dracott, K.; Glover, R.D.; Warner, A.; Migneault, A. DNA from dives: Species detection of humpback whales (Megaptera novaeangliae) from flukeprint eDNA. Environ. DNA 2024, 6, e524. [Google Scholar] [CrossRef]
- Afonso, L.; Costa, J.; Correia, A.M.; Valente, R.; Lopes, E.; Tomasino, M.P.; Gil, Á.; Oliveira-Rodrigues, C.; Sousa Pino, I.; López, A.; et al. Environmental DNA as a complementary tool for biodiversity monitoring: A multi-technique and multi-trophic approach to investigate cetacean distribution and feeding ecology. PLoS ONE 2024, 19, e0300992. [Google Scholar] [CrossRef]
- Ouso, D.O.; Otiende, M.Y.; Jeneby, M.M.; Oundo, J.W.; Bargul, J.L.; Miller, S.E.; Wambua, L.; Villinger, J. Three-gene PCR and high-resolution melting analysis for differentiating vertebrate species mitochondrial DNA for biodiversity research and complementing forensic surveillance. Sci. Rep. 2020, 10, 4741. [Google Scholar] [CrossRef]
- Smith, M.A.; Fisher, B.L.; Hebert, P.D. DNA barcoding for effective biodiversity assessment of a hyperdiverse arthropod group: The ants of Madagascar. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005, 360, 1825–1834. [Google Scholar] [CrossRef]
- Anabalón, L.; Encina-Montoya, F.; Sánchez, P.; Solano, J.; Benavente, F.; Guiñez, B.; Olivares, F.; Oberti, C.; Vega, R. High-resolution melting of the cytochrome B gene in fecal DNA: A powerful approach for fox species identification of the Lycalopex genus in Chile. Ecol. Evol. 2019, 9, 7448–7454. [Google Scholar] [CrossRef]
- Buglione, M.; Petrelli, S.; Notomista, T.; de Filippo, G.; Gregorio, R.; Fulgione, D. Who is who? High Resolution Melting analysis to discern between hare species using non-invasive sampling. Conserv. Genet. Resour. 2020, 12, 727–732. [Google Scholar] [CrossRef]
- Würsig, B.; Thewissen, J.G.M.; Kovacs, K.M. Encyclopedia of Marine Mammals, 3rd ed.; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Traffic (UK charity, Number 1076722). Wildlife Trade in the European Union: An Analysis of EU-TWIX Seizure Data. 2020. Available online: https://www.traffic.org (accessed on 30 July 2025).
- WWF Italia. Successful Wildlife Crime Prosecution in Europe. ITALIA, Report Nazionale. Escalation invisibile dei crimini di natura—Analisi E proposte del wwf. 2022. Available online: https://stopwildlifecrime.eu/wp-content/uploads/2022/11/SWiPE_Italy_National_report_EN-compressed.pdf (accessed on 30 July 2025).
- Endo, T.; Haraguchi, K.; Sakata, M. Mercury and selenium concentrations in the internal organs of toothed whales and dolphins marketed for human consumption in Japan. Sci. Total Environ. 2003, 300, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Smart, U.; Cihlar, J.C.; Budowle, B. International Wildlife Trafficking: A perspective on the challenges and potential forensic genetics solutions. Forensic Sci. Int. Genet. 2021, 54, 102551. [Google Scholar] [CrossRef] [PubMed]
- Stolen, M. Forensic Science in Marine Mammalogy: Applications and Limitations. In Wildlife Biodiversity Conservation: Multidisciplinary and Forensic Approaches; Underkoffler, S.C., Adams, H.R., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 383–399. [Google Scholar]
- Deiner, K.; Renshaw, M.A.; Li, Y.; Olds, B.P.; Lodge, D.M.; Pfrender, M.E. Long-range PCR allows sequencing of mitochondrial genomes from environmental DNA. Methods Ecol. Evol. 2017, 8, 1888–1898. [Google Scholar] [CrossRef]
- Suarez-Bregua, P.; Álvarez-González, M.; Parsons, K.M.; Rotllant, J.; Pierce, G.J.; Saavedra, C. Environmental DNA (eDNA) for monitoring marine mammals: Challenges and opportunities. Front. Mar. Sci. 2022, 9, 987774. [Google Scholar] [CrossRef]
- Mussi, B.; Vivaldi, C.; Zucchini, A.; Miragliuolo, A.; Pace, D.S. The decline of short-beaked common dolphin (Delphinus delphis) in the waters off the island of Ischia (Gulf of Naples, Italy). Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 87–100. [Google Scholar] [CrossRef]
- Mussi, B.; Miragliuolo, A.; Zucchini, A.; Pace, D.S. Occurrence and spatio-temporal distribution of sperm whale (Physeter macrocephalus) in the submarine canyon of Cuma (Tyrrhenian Sea, Italy). Aquat. Conserv. Mar. Freshw. Ecosyst. 2014, 24, 59–70. [Google Scholar] [CrossRef]
- Mussi, B.; Miragliuolo, A.; Monzini, E.; Díaz López, B.; Battaglia, M. Fin whale (Balaenoptera physalus) feeding ground in the coastal waters of Ischia (Archipelago Campano). Eur. Res. Cetaceans 1999, 13, 330–335. [Google Scholar]
- Notarbartolo di Sciara, G.; Birkun, A. Conservation of Cetaceans in the Mediterranean and Black Seas: ACCOBAMS Status Report; ACCOBAMS and Department of External Relations: Monaco, 2010; 212p. [Google Scholar]
- Bianchi, C.N.; Morri, C. Marine Biodiversity of the Mediterranean Sea: Situation, Problems and Prospects for Future Research. Mar. Pollut. Bull. 2000, 40, 367–376. [Google Scholar] [CrossRef]
- Pace, D.; Tizzi, R.; Mussi, B. Cetaceans Value and Conservation in the Mediterranean Sea. J. Biodivers. Endang. Species 2015, S1, 004. [Google Scholar] [CrossRef]
- Papastavrou, V.; Ryan, C. Ethical standards for research on marine mammals. Res. Ethics 2023, 19, 390–408. [Google Scholar] [CrossRef]
- Gales, N.J.; Bowen, W.D.; Johnston, D.W.; Kovacs, K.M.; Littnan, C.L.; Perrin, W.F.; Reynolds, J.E., III; Thompson, P.M. Guidelines for the treatment of marine mammals in field research. Mar. Mamm. Sci. 2009, 25, 725–736. [Google Scholar] [CrossRef]

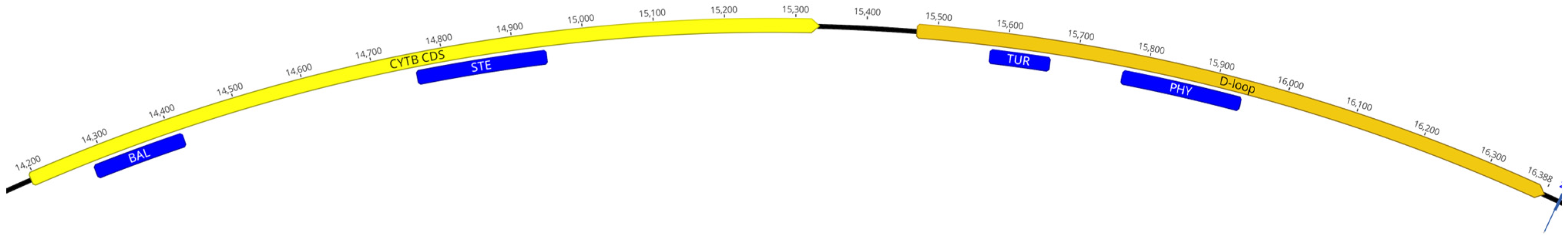
| Species | Primers | Size (bp) | %A | %C | %G | %T | %GC |
|---|---|---|---|---|---|---|---|
| Balaenoptera physalus | B1F-B1R | 133 | 29.3 | 33.9 | 13.5 | 23.2 | 47.7 |
| Stenella coeruleoalba | S1F-S1R | 189 | 32.9 | 32.9 | 10.2 | 24.7 | 43.1 |
| Tursiops truncatus | T1F-T11R | 86 | 26.5 | 17.4 | 11.7 | 44.4 | 29.1 |
| Physeter macrocephalus | P1F-P1R | 175 | 21.7 | 30.9 | 22.7 | 24.8 | 53.6 |
| Species | Primers Name | SNPsIntraspecific | SNPsInterspecific |
|---|---|---|---|
| Balaenoptera physalus | B1F-B1R | 1 | 8 B. musculus (north of the Antarctic Convergence, most abundant in waters off Australia, Madagascar, and New Zealand); 9 B. borealis (MED, CAA); 10 B. acutorostrata (MED, CAA) |
| Stenella coeruleoalba | S1F-S1R | 4 | 11 S. longirostris (Pacific, Atlantic, and Indian Oceans, including the Persian Gulf and the Red Sea; they do not occur in the Mediterranean Sea); 1 S. clymene (northwestern Atlantic, Gulf of Mexico, the Caribbean, and along the coasts of West Africa and southern Brazil); 6 Delphinus delphis (MED, CAA); 12 T. truncatus (MED, CAA) |
| Tursiops truncatus | T1F-T11R | 2 | 3 Tursiops aduncus (Indo-pacific); 3 S. coeruleoalba (MED, CAA) |
| Physeter macrocephalus | P1F-P1R | 0 | 59 Kogia sima (MED); 57 K. breviceps (Atlantic, Pacific, and Indian Oceans) |
| Species | Primer Name | Sequence 5′-3′ | Size (bp) | Region |
|---|---|---|---|---|
| Balaenoptera physalus | B1F | TGGAACTTCGGCTCCCTACT | 133 | Cytochrome b |
| B1R | AATTCACGTCTCGGCAGATG | |||
| Stenella coeruleoalba | S1F | CACAGCATTAGCAGCCGTTC | 189 | Cytochrome b |
| S1R | CCTAGTAGGTCGGGGGTGAA | |||
| Tursiops truncatus | T1F | TGCGCATGCTAATATTTAGTCTCT | 86 | D-loop |
| T11R | TCGTATGGAAAATAAATGAATGCACAA | |||
| Physeter macrocephalus | P1F | TGAGCTCTCGGATCAGACCA | 175 | D-loop |
| P1R | GCAGGTGCCTCGAGTTATGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Norcia, M.; Illiano, A.; Mussi, B.; Di Nocera, F.; Esposito, E.; Di Cosmo, A.; Fulgione, D.; Maselli, V. When DNA Tells the Tale: High-Resolution Melting as a Forensic Tool for Mediterranean Cetacean Identification. Int. J. Mol. Sci. 2025, 26, 7517. https://doi.org/10.3390/ijms26157517
Norcia M, Illiano A, Mussi B, Di Nocera F, Esposito E, Di Cosmo A, Fulgione D, Maselli V. When DNA Tells the Tale: High-Resolution Melting as a Forensic Tool for Mediterranean Cetacean Identification. International Journal of Molecular Sciences. 2025; 26(15):7517. https://doi.org/10.3390/ijms26157517
Chicago/Turabian StyleNorcia, Mariangela, Alessia Illiano, Barbara Mussi, Fabio Di Nocera, Emanuele Esposito, Anna Di Cosmo, Domenico Fulgione, and Valeria Maselli. 2025. "When DNA Tells the Tale: High-Resolution Melting as a Forensic Tool for Mediterranean Cetacean Identification" International Journal of Molecular Sciences 26, no. 15: 7517. https://doi.org/10.3390/ijms26157517
APA StyleNorcia, M., Illiano, A., Mussi, B., Di Nocera, F., Esposito, E., Di Cosmo, A., Fulgione, D., & Maselli, V. (2025). When DNA Tells the Tale: High-Resolution Melting as a Forensic Tool for Mediterranean Cetacean Identification. International Journal of Molecular Sciences, 26(15), 7517. https://doi.org/10.3390/ijms26157517








