Protective Efficacy of Lactobacillus plantarum Postbiotic beLP-K in a Dexamethasone-Induced Sarcopenia Model
Abstract
1. Introduction
2. Results
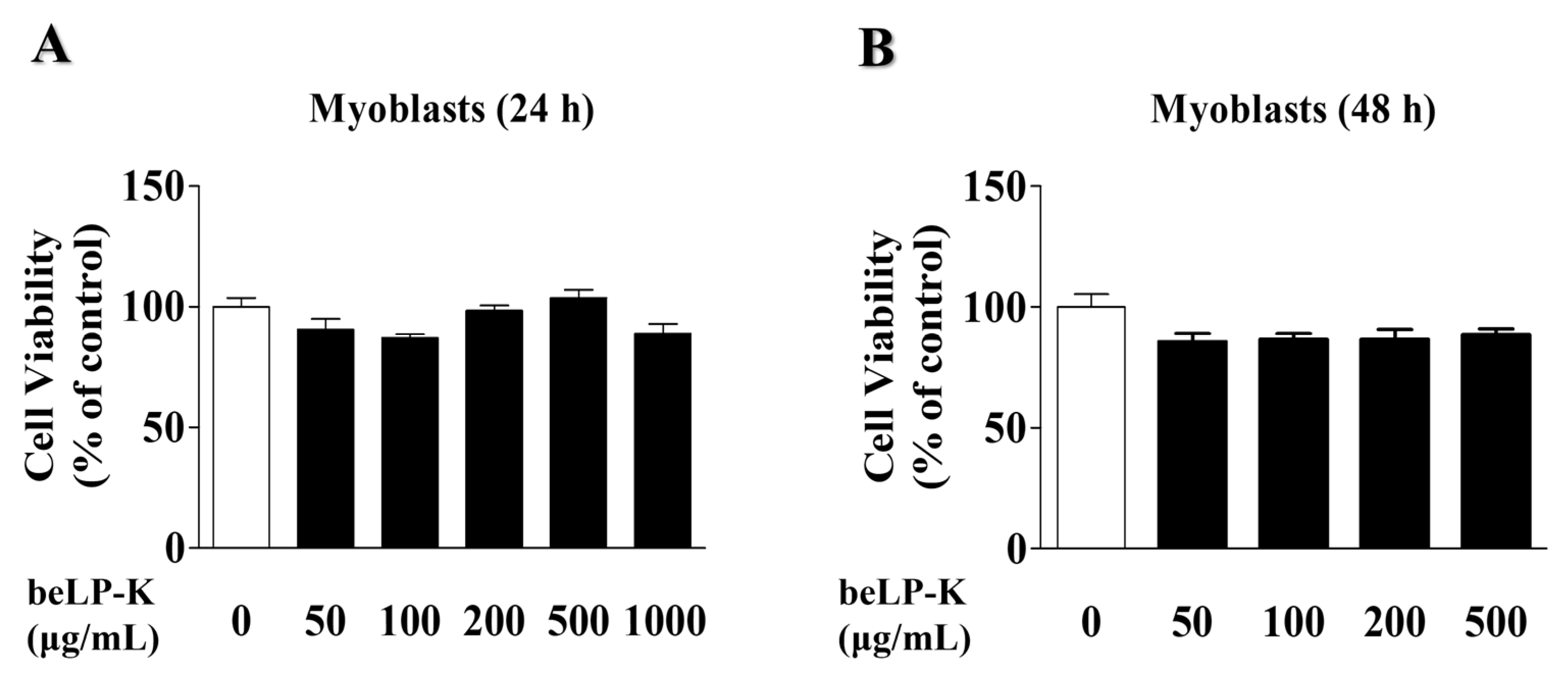
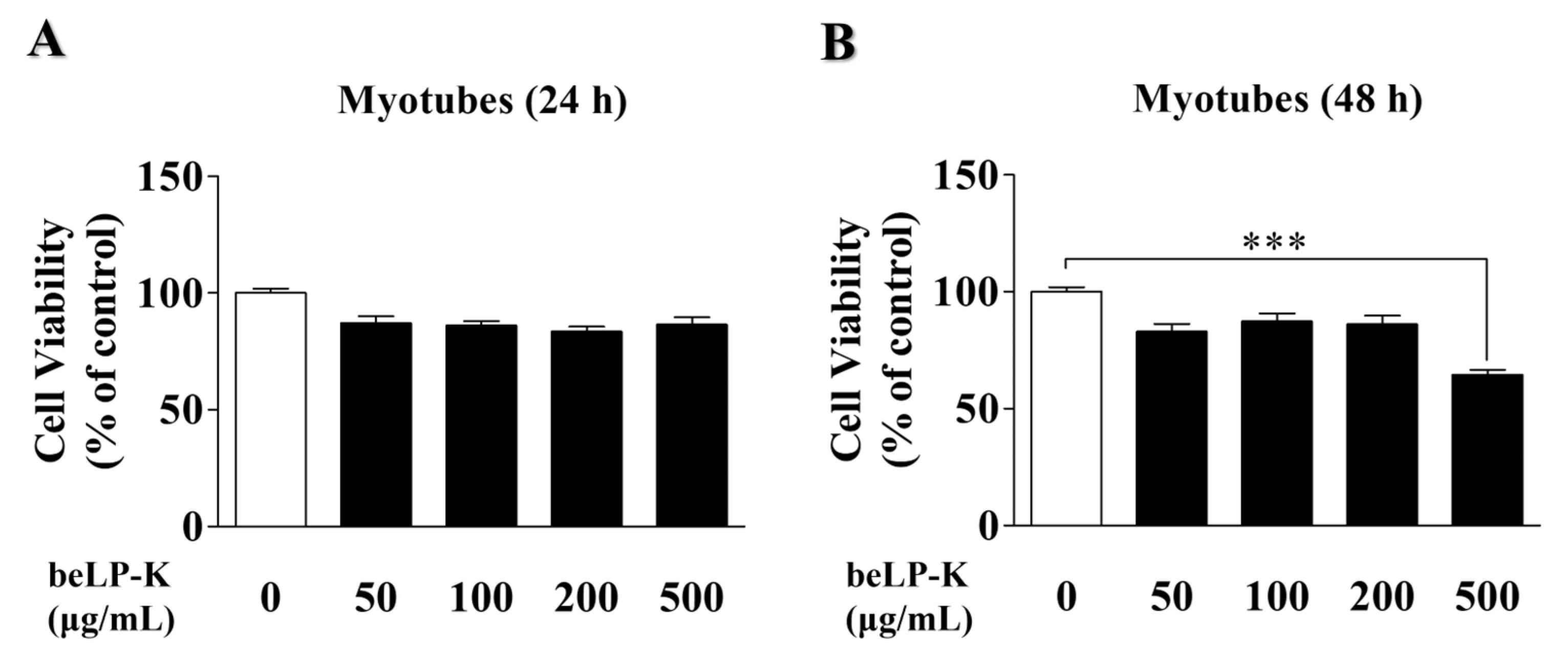
2.1. Effects of beLP-K on the Viability of C2C12 Myoblasts and Myotubes
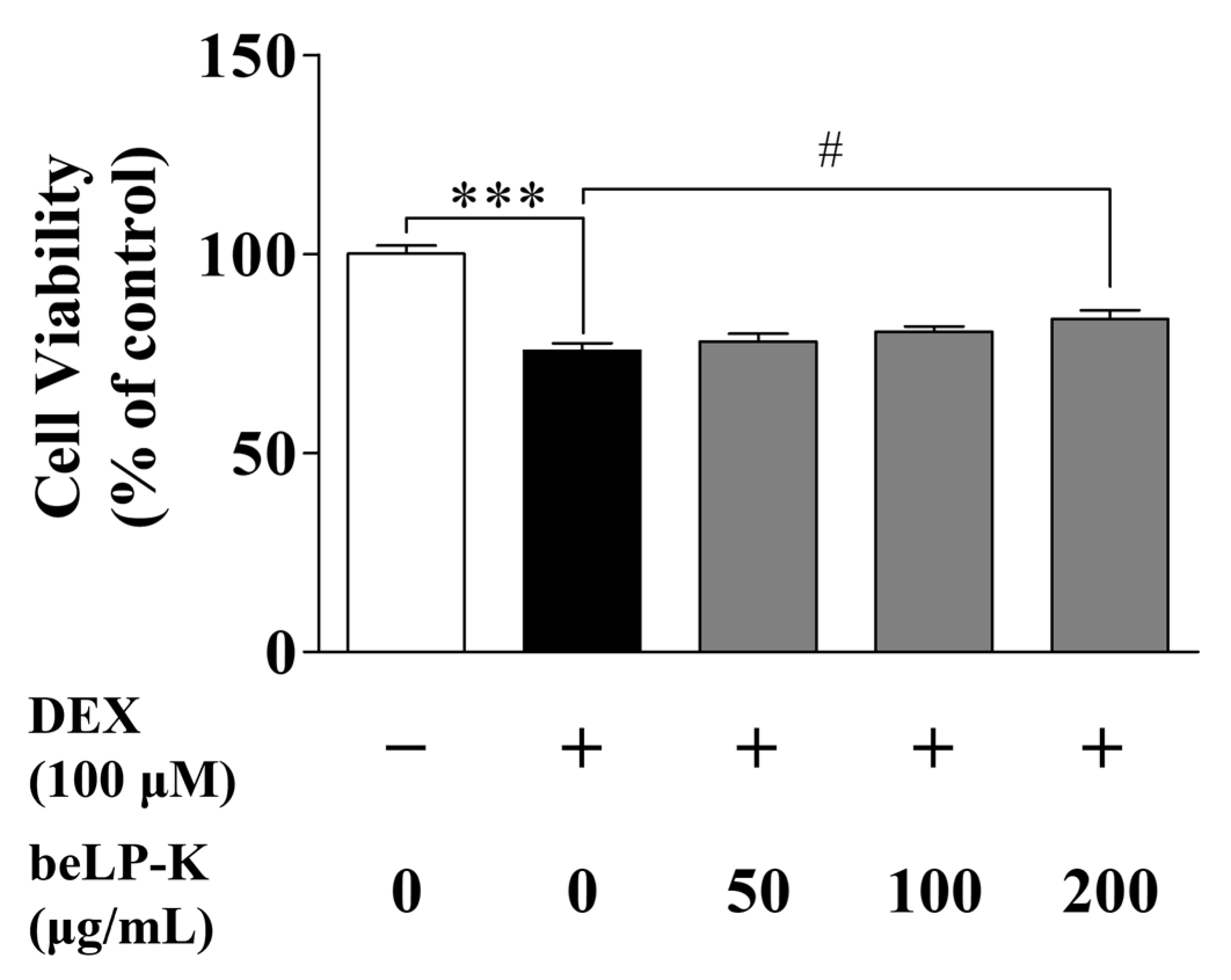
2.2. Evaluation of the Protective Efficacy of beLP-K in DEX-Induced C2C12 Myotubes
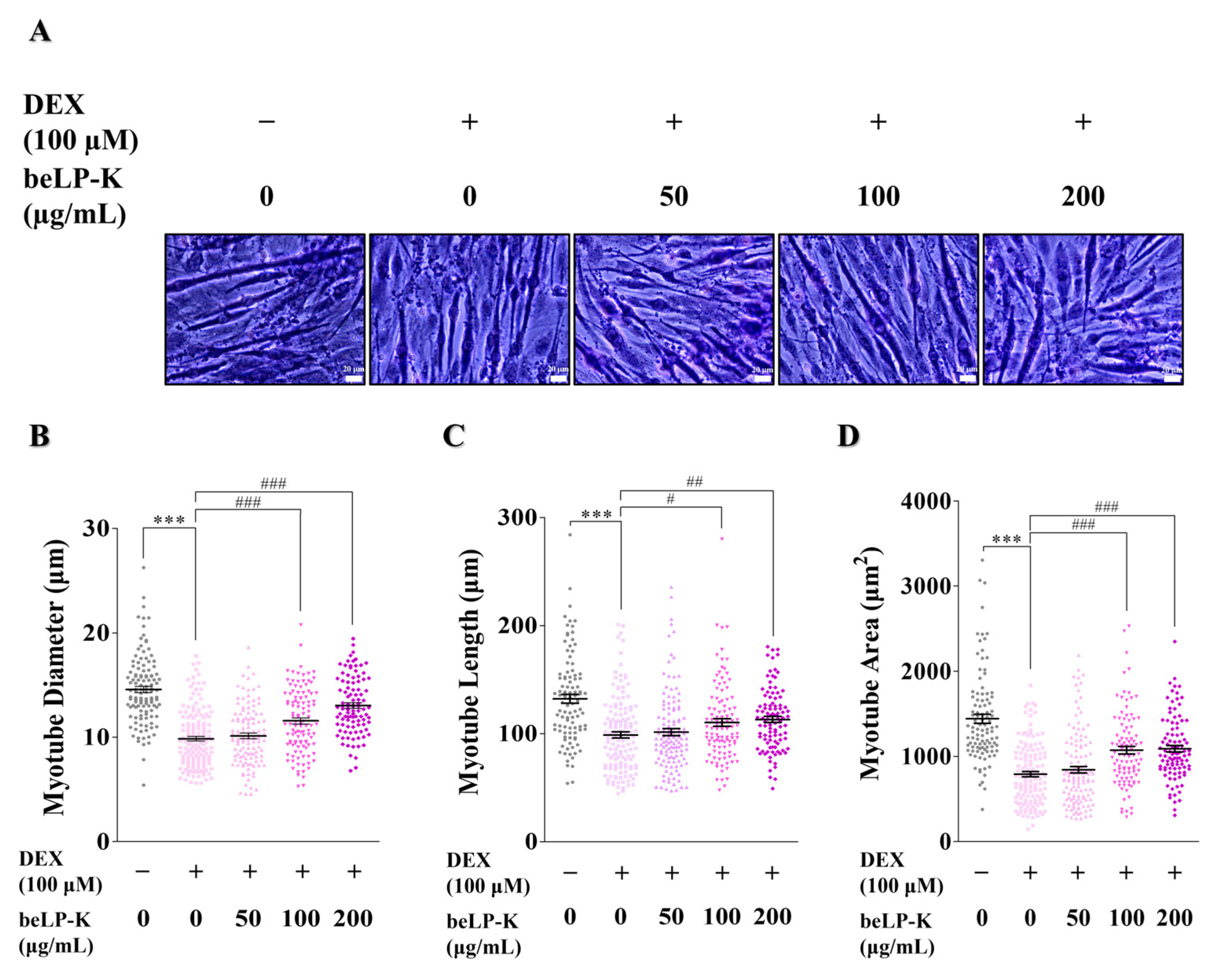
2.3. Morphometric Analysis of the Efficacy of beLP-K Against DEX-Induced Cell Damage in C2C12 Myotubes
2.4. Effects of beLP-K on E3 Ubiquitin Proteasome Pathway Regulation in DEX-Induced C2C12 Myotubes
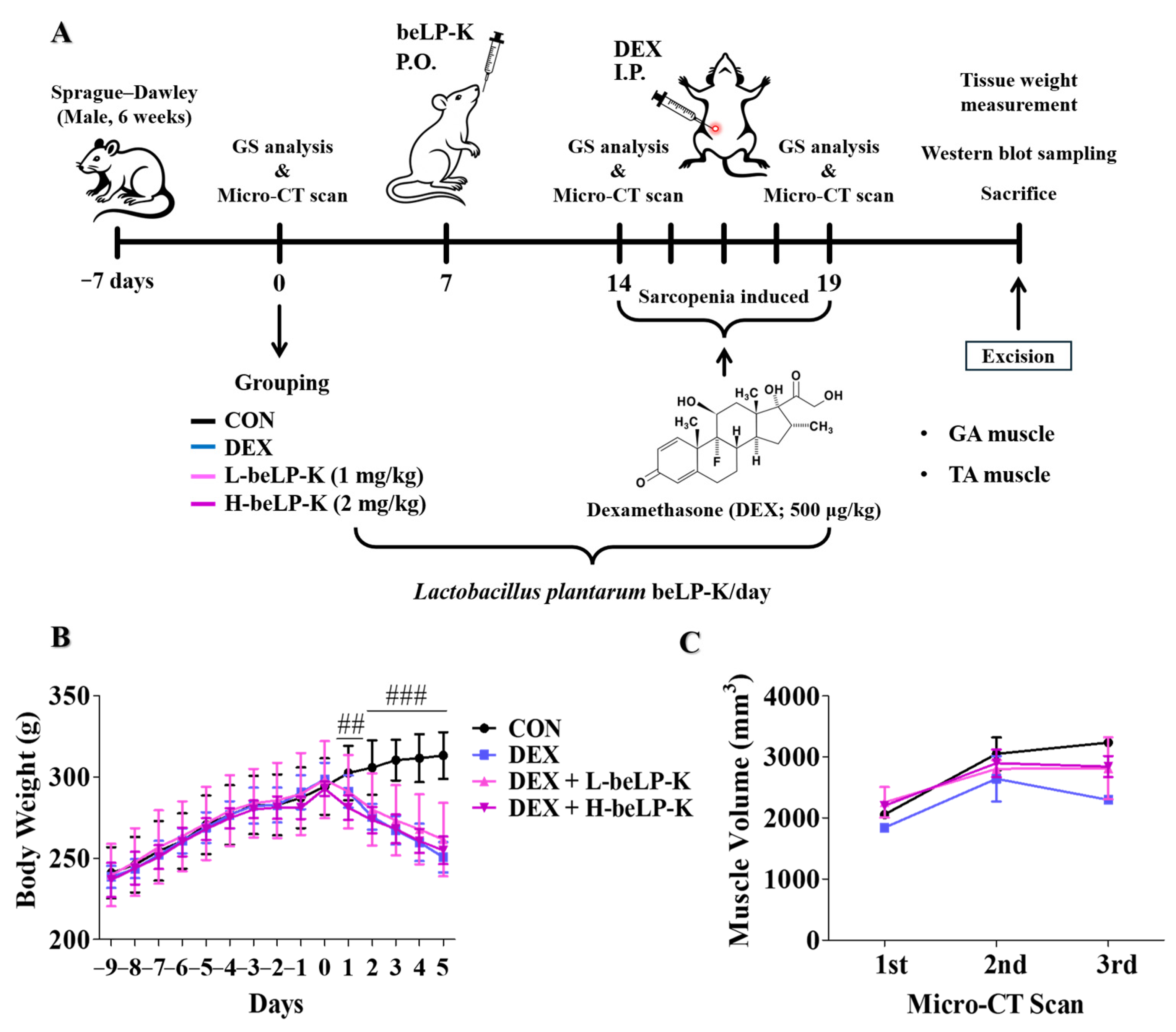
2.5. beLP-K Prevents Body Weight and Muscle Volume in DEX-Induced Sarcopenia Rat Model
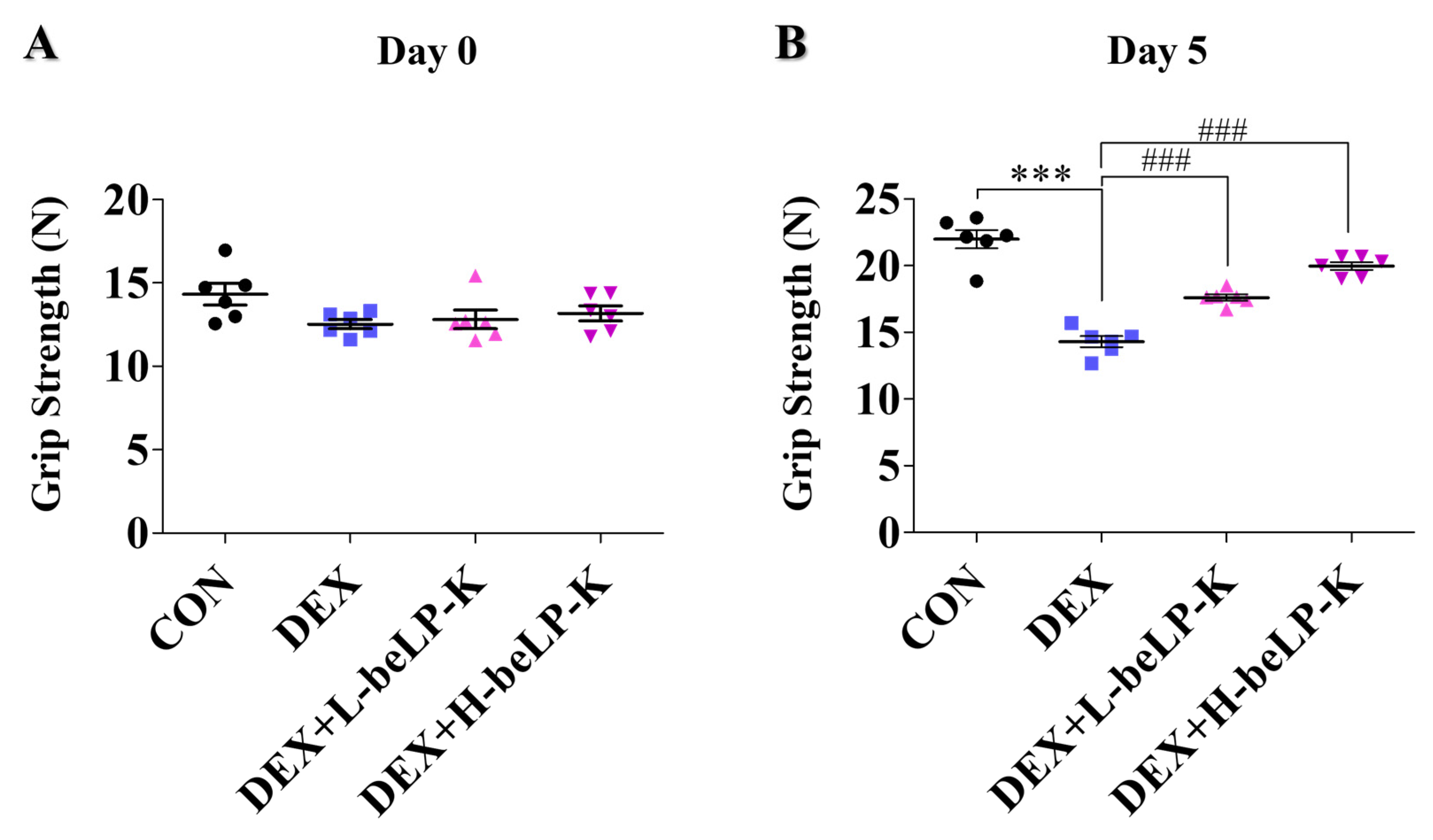
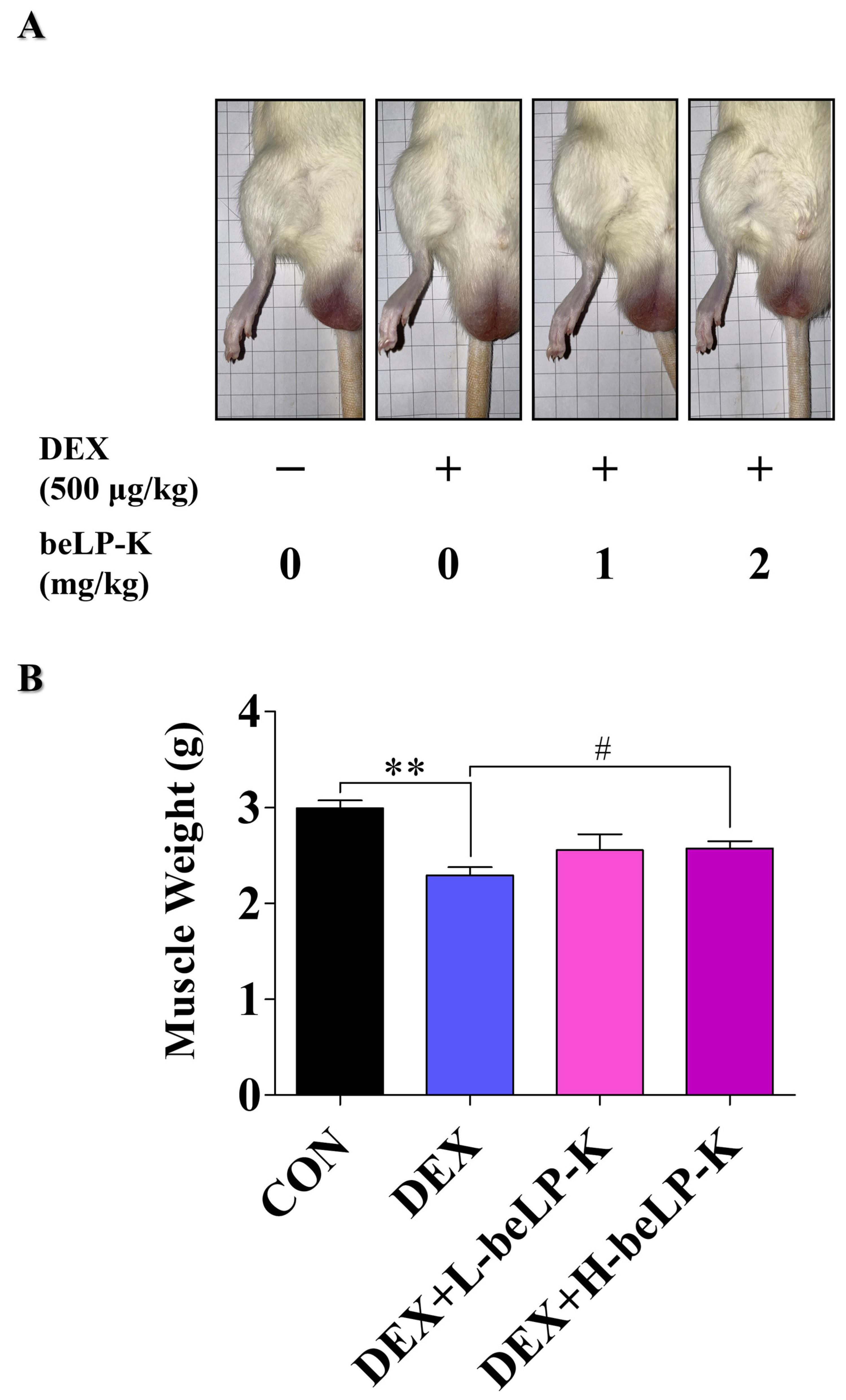
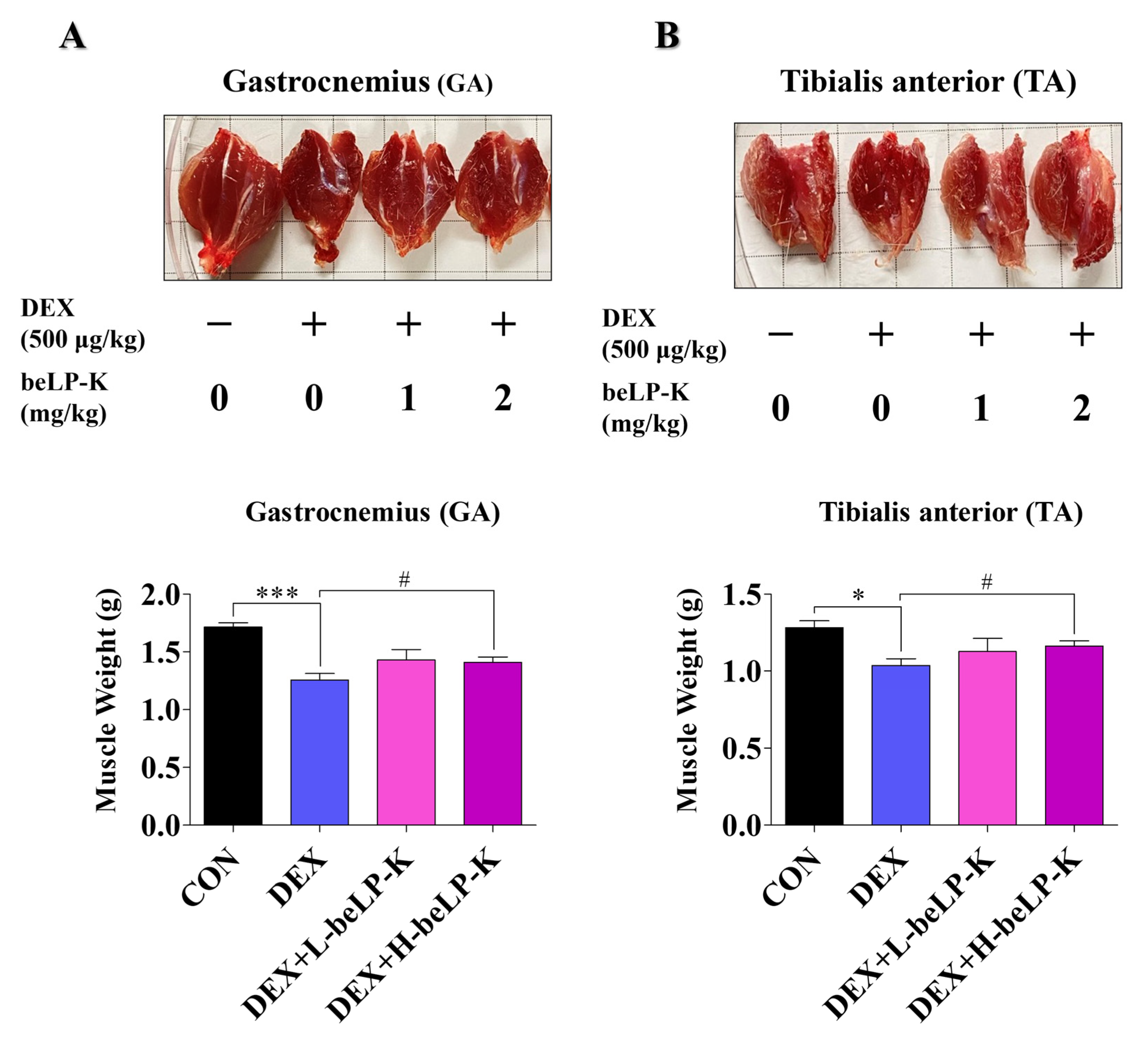
2.6. beLP-K Alleviated DEX-Induced Functional Decline and Muscle Loss in SD Rats
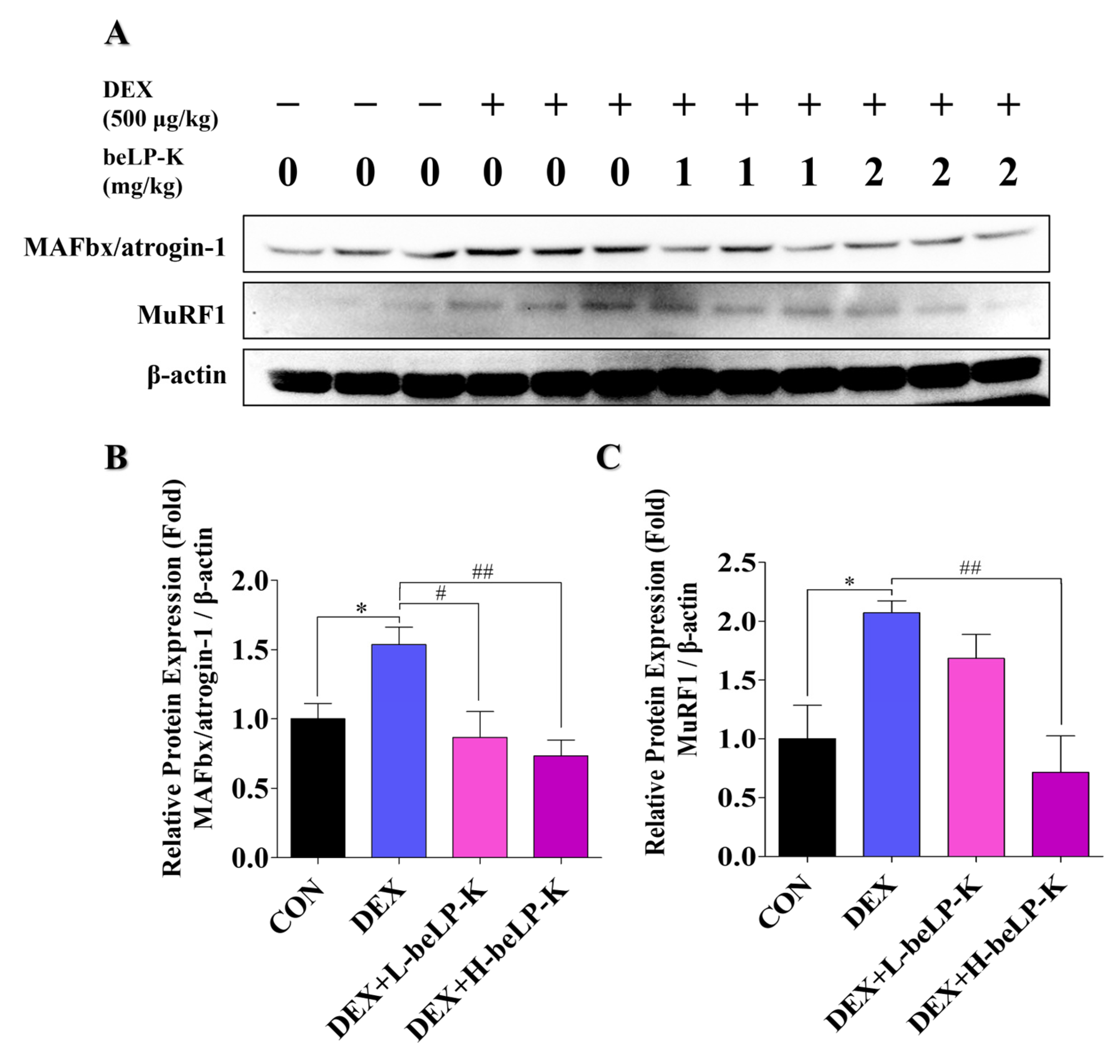
2.7. beLP-K Decreases E3-Ubiquitin Ligase Expression in DEX-Treated SD Rats
3. Discussion
4. Materials and Methods
4.1. Preparation of Heat-Killed L. plantarum Postbiotic beLP-K
4.2. Cell Experiment Design Using C2C12 Myoblasts
4.3. Cell Viability Assay
4.4. Morphological Assessment
4.5. Western Blot Analysis and Antibodies
4.6. Animal Experiment Design
4.7. GS Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| beLP-K | Lactobacillus plantarum beLP-K |
| CON | Control |
| CT | Computed tomography |
| DEX | Dexamethasone |
| DM | Differentiation medium |
| DMEM | Dulbecco’s -Modified Eagle Medium |
| DW | Distilled water |
| FTMFs | Fast-twitch muscle fibers |
| FoxO3α | Forkhead box O3 α |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| GA | Gastrocnemius |
| GC | Glucocorticoid |
| GS | Grip strength |
| GSM | Grip strength meter |
| HRP | Horseradish peroxidase |
| ICD | International classification of disease |
| MAFbx | Muscle atrophy f-box |
| MuRF1 | Muscle RING-finger protein-1 |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| OD | Optical density |
| PBS | Phosphate-buffered saline |
| RT | Room temperature |
| SD | Sprague–Dawley |
| STMFs | Slow-twitch muscle fibers |
| TA | Tibialis anterior |
| UPS | Ubiquitin–proteasome system |
| WHO | World Health Organization |
References
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Jeong, J.-W.; Choi, Y.H. Induction of muscle atrophy by dexamethasone and hydrogen peroxide in differentiated C2C12 myotubes. J. Life Sci. 2017, 27, 1479–1485. [Google Scholar]
- Cohen, S.; Nathan, J.A.; Goldberg, A.L. Muscle wasting in disease: Molecular mechanisms and promising therapies. Nat. Rev. Drug Discov. 2015, 14, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Tournadre, A.; Vial, G.; Capel, F.; Soubrier, M.; Boirie, Y. Sarcopenia. Jt. Bone Spine 2019, 86, 309–314. [Google Scholar] [CrossRef]
- Chang, S.-J.; Lee, M.-H.; Kim, W.-J.; Chae, Y.; Iwasa, M.; Han, K.-I.; Kim, W.-J.; Kim, T.-J. Effect of heat-killed Enterococcus faecalis, EF-2001 on C2C12 myoblast damage induced by oxidative stress and muscle volume decreased by sciatic denervation in C57BL/6 mice. J. Life Sci. 2019, 29, 215–222. [Google Scholar]
- Jeon, S.K.; Kim, O.H.; Park, S.M.; Lee, J.-H.; Park, S.-D. Effects of glucoraphanin in dexamethasone-induced skeletal muscle atrophy in vitro model. Herb. Formula Sci. 2020, 28, 29–39. [Google Scholar]
- Schakman, O.; Kalista, S.; Barbé, C.; Loumaye, A.; Thissen, J.P. Glucocorticoid-induced skeletal muscle atrophy. Int. J. Biochem. Cell Biol. 2013, 45, 2163–2172. [Google Scholar] [CrossRef]
- Lee, M.K.; Jeong, H.H.; Kim, M.J.; Ryu, H.; Baek, J.; Lee, B. Nutrients against glucocorticoid-induced muscle atrophy. Foods 2022, 11, 687. [Google Scholar] [CrossRef]
- Walston, J.D. Sarcopenia in older adults. Curr. Opin. Rheumatol. 2012, 24, 623–627. [Google Scholar] [CrossRef]
- Anker, S.D.; Morley, J.E.; von Haehling, S. Welcome to the ICD-10 code for sarcopenia. J. Cachexia Sarcopenia Muscle 2016, 7, 512–514. [Google Scholar] [CrossRef]
- Baek, J.Y.; Jung, H.W.; Kim, K.M.; Kim, M.; Park, C.Y.; Lee, K.P.; Lee, S.Y.; Jang, I.-Y.; Jeon, O.H.; Lim, J. Y Korean Working Group on Sarcopenia guideline: Expert consensus on sarcopenia screening and diagnosis by the Korean Society of Sarcopenia, the Korean Society for Bone and Mineral Research, and the Korean Geriatrics Society. Ann. Geriatr. Med. Res. 2023, 27, 9–21. [Google Scholar] [CrossRef]
- Gill, T.K. Global, regional, and national burden of other musculoskeletal disorders, 1990–2020, and projections to 2050: A systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e670–e682. [Google Scholar] [CrossRef]
- Jang, J.Y.; Kim, D.; Kim, N.D. Pathogenesis, intervention, and current status of drug development for sarcopenia: A review. Biomedicines 2023, 11, 1635. [Google Scholar] [CrossRef]
- Choo, Y.J.; Chang, M.C. Prevalence of sarcopenia among the elderly in Korea: A meta-analysis. J. Prev. Med. Public Health 2021, 54, 96. [Google Scholar] [CrossRef]
- Kim, S.; Ha, Y.C.; Kim, D.Y.; Yoo, J.I. Recent update on the prevalence of sarcopenia in Koreans: Findings from the Korea National Health and Nutrition Examination Survey. J. Bone Metab. 2024, 31, 150. [Google Scholar] [CrossRef] [PubMed]
- Cegielski, J.; Dawe, R.J.; Paris, M.T.; Landi, F.; Schaap, L.A.; Cesari, M.; Reginster, J.Y.; Cruz-Jentoft, A.J.; Heymsfield, S.B.; Prado, C.M. Exploring the variability of sarcopenia prevalence in a research population using different disease definitions. Aging Clin. Exp. Res. 2023, 35, 2271–2275. [Google Scholar] [CrossRef] [PubMed]
- Jagoe, R.T.; Goldberg, A.L. What do we really know about the ubiquitin-proteasome pathway in muscle atrophy? Curr. Opin. Clin. Nutr. Metab. Care 2001, 4, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Sartori, R.; Romanello, V.; Sandri, M. Mechanisms of muscle atrophy and hypertrophy: Implications in health and disease. Nat. Commun. 2021, 12, 330. [Google Scholar] [CrossRef]
- Schäcke, H.; Döcke, W.D.; Asadullah, K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol. Ther. 2002, 96, 23–43. [Google Scholar] [CrossRef]
- Moghadam-Kia, S.; Werth, V.P. Prevention and treatment of systemic glucocorticoid side effects. Int. J. Dermatol. 2010, 49, 239–248. [Google Scholar] [CrossRef]
- Shimizu, N.; Yoshikawa, N.; Ito, N.; Maruyama, T.; Suzuki, Y.; Takeda, S.; Nakae, J.; Tagata, Y.; Nishitani, S.; Takehana, K.; et al. Crosstalk between glucocorticoid receptor and nutritional sensor mTOR in skeletal muscle. Cell Metab. 2011, 13, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-W.; Chang, Y.B.; Park, C.W.; Han, S.H.; Suh, H.J.; Ahn, Y. Protein hydrolysate from Spirulina platensis prevents dexamethasone-induced muscle atrophy via Akt/Foxo3 signaling in C2C12 myotubes. Mar. Drugs 2022, 20, 365. [Google Scholar] [CrossRef] [PubMed]
- Schakman, O.; Gilson, H.; Kalista, S.; Thissen, J.P. Mechanisms of muscle atrophy induced by glucocorticoids. Horm. Res. 2009, 72 (Suppl. 1), 36–41. [Google Scholar] [CrossRef] [PubMed]
- Barel, M.; Perez, O.A.B.; Giozzet, V.A.; Rafacho, A.; Bosqueiro, J.R.; do Amaral, S.L. Exercise training prevents hyperinsulinemia, muscular glycogen loss and muscle atrophy induced by dexamethasone treatment. Eur. J. Appl. Physiol. 2010, 108, 999–1007. [Google Scholar] [CrossRef]
- Krug, A.L.O.; Macedo, A.G.; Zago, A.S.; Rush, J.W.E.; Santos, C.F.; Amaral, S.L. High-intensity resistance training attenuates dexamethasone-induced muscle atrophy. Muscle Nerve 2016, 53, 779–788. [Google Scholar] [CrossRef]
- Choe, M.A.; Shin, G.S.; An, G.J.; Lee, E.J. Effect of DHEA Administration before, during and after Dexamethasone Treatment on Body Weight and Mass of Type I, II Muscles in Rats. J. Korean Acad. Nurs. 2002, 32, 727–734. [Google Scholar] [CrossRef]
- Khalil, R. Ubiquitin-proteasome pathway and muscle atrophy. Adv. Exp. Med. Biol. 2018, 1088, 235–248. [Google Scholar]
- Dupont-Versteegden, E.E. Apoptosis in skeletal muscle and its relevance to atrophy. World J. Gastroenterol. 2006, 12, 7463. [Google Scholar] [CrossRef]
- Mitch, W.E.; Goldberg, A.L. Mechanisms of muscle wasting.The role of the ubiquitin–proteasome pathway. N. Engl. J. Med. 1996, 335, 1897–1905. [Google Scholar] [CrossRef]
- Foletta, V.C.; White, L.J.; Larsen, A.E.; Léger, B.; Russell, A.P. The role and regulation of MAFbx/atrogin-1 and MuRF1 in skeletal muscle atrophy. Pflug. Arch. 2011, 461, 325–335. [Google Scholar] [CrossRef]
- Chen, L.; Chen, L.; Wan, L.; Huo, Y.; Huang, J.; Li, J.; Lu, J.; Xin, B.; Yang, Q.; Guo, C. Matrine improves skeletal muscle atrophy by inhibiting E3 ubiquitin ligases and activating the Akt/mTOR/FoxO3α signaling pathway in C2C12 myotubes and mice. Oncol. Rep. 2019, 42, 479–494. [Google Scholar] [CrossRef]
- Sakai, H.; Kimura, M.; Tsukimura, Y.; Yabe, S.; Isa, Y.; Kai, Y.; Sato, F.; Kon, R.; Ikarashi, N.; Narita, M.; et al. Dexamethasone exacerbates cisplatin-induced muscle atrophy. Clin. Exp. Pharmacol. Physiol. 2019, 46, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-J.; Lim, J.-M.; Ku, B.-H.; Cheon, D.-M.; Jung, Y.J.; Kim, Y.-S.; Oh, T.W. Effects of polysaccharide (polycan) derived from black yeast in dexamethasone-induced muscle atrophy cell model. Herb. Formula Sci. 2021, 29, 45–55. [Google Scholar]
- Deshaies, R.J.; Joazeiro, C.A.P. RING domain E3 ubiquitin ligases. Annu. Rev. Biochem. 2009, 78, 399–434. [Google Scholar] [CrossRef] [PubMed]
- Castillero, E.; Alamdari, N.; Lecker, S.H.; Hasselgren, P.O. Suppression of atrogin-1 and MuRF1 prevents dexamethasone-induced atrophy of cultured myotubes. Metabolism 2013, 62, 1495–1502. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, J.; Chen, D.; Wang, Y. E3 ubiquitin ligases: Styles, structures and functions. Mol. Biomed. 2021, 2, 23. [Google Scholar] [CrossRef]
- Sandri, M.; Sandri, C.; Gilbert, A.; Skurk, C.; Calabria, E.; Picard, A.; Walsh, K.; Schiaffino, S.; Lecker, S.H.; Goldberg, A.L. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 2004, 117, 399–412. [Google Scholar] [CrossRef]
- Bodine, S.C.; Latres, E.; Baumhueter, S.; Lai, V.K.; Nunez, L.; Clarke, B.A.; Poueymirou, W.T.; Panaro, F.J.; Na, E.; Dharmarajan, K.; et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 2001, 294, 1704–1708. [Google Scholar] [CrossRef]
- Bodine, S.C.; Baehr, L.M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E469–E484. [Google Scholar] [CrossRef]
- Zeng, Z.; Xie, Y.; Jiang, H.; Yang, L.; Yu, G.; Zhou, X.; Li, J. Exercise-induced autophagy suppresses sarcopenia through Akt/mTOR and Akt/FoxO3a signal pathways and AMPK-mediated mitochondrial quality control. Front. Physiol. 2020, 11, 583478. [Google Scholar] [CrossRef]
- Rolland, Y.; Dray, C.; Vellas, B.; Barreto, P.D.S. Current and investigational medications for the treatment of sarcopenia. Metabolism 2023, 149, 155597. [Google Scholar] [CrossRef]
- Saud Gany, S.L.; Chin, K.-Y.; Tan, J.K.; Aminuddin, A.; Makpol, S. Curcumin as a therapeutic agent for sarcopenia. Nutrients 2023, 15, 2526. [Google Scholar] [CrossRef]
- Oh, G.; Men, X.; La, I.-J.; Han, X.; Lee, S.-J.; Im, J.-H.; Fu, X.; Lim, J.-S.; Bae, K.S.; Seong, G.-S.; et al. Fermented Red Ginseng Extract Improves Sarcopenia-Related Muscle Atrophy in Old Mice through Regulation of Muscle Protein Metabolism. Food Sci. Biotechnol. 2024, 34, 793–802. [Google Scholar] [CrossRef]
- Jeong, Y.-J.; Kim, J.-H.; Jung, Y.-J.; Kwak, M.-S.; Sung, M.-H.; Imm, J.-Y. KL-Biome (Postbiotic Formulation of Lactiplantibacillus plantarum KM2) Improves Dexamethasone-Induced Muscle Atrophy in Mice. Int. J. Mol. Sci. 2024, 25, 7499. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Kang, M.; Yoo, J.; Lee, J.; Lee, S.; Yun, B.; Song, M.; Kim, J.M.; Kim, H.W.; Yang, J.; et al. Dietary supplementation with Lacticaseibacillus rhamnosus IDCC3201 alleviates sarcopenia by modulating the gut microbiota and metabolites in dexamethasone-induced models. Food Funct. 2024, 15, 4936–4953. [Google Scholar] [CrossRef] [PubMed]
- Malashree, L.; Angadi, V.; Yadav, K.S.; Prabha, R. “Postbiotics.”—One Step Ahead of Probiotics. Int. J. Curr. Microbiol. App. Sci. 2019, 8, 2049–2053. [Google Scholar] [CrossRef]
- Yeşilyurt, N.; Yılmaz, B.; Ağagündüz, D.; Capasso, R. Involvement of probiotics and postbiotics in the immune system modulation. Biologics 2021, 1, 89–110. [Google Scholar] [CrossRef]
- Kim, B.-Y.; Park, S.S. The concepts and applications of postbiotics for the development of health functional food product. Curr. Top. Lact. Acid Bact. Probiotics 2021, 7, 14–22. [Google Scholar] [CrossRef]
- Podgórski, R.; Wojasiński, M.; Ciach, T. Nanofibrous materials affect the reaction of cytotoxicity assays. Sci. Rep. 2022, 12, 9047. [Google Scholar] [CrossRef]
- Lee, M.K.; Kim, Y.M.; Kim, I.H.; Choi, Y.H.; Nam, T.J. Pyropia yezoensis peptide PYP1-5 protects against dexamethasone-induced muscle atrophy through the downregulation of atrogin1/MAFbx and MuRF1 in mouse C2C12 myotubes. Mol. Med. Rep. 2017, 15, 3507–3514. [Google Scholar] [CrossRef]
- Chang, J.S.; Kong, I.D. Irisin prevents dexamethasone-induced atrophy in C2C12 myotubes. Pflug. Arch. 2020, 472, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Mallidis, C.; Bhasin, S.; Mahabadi, V.; Artaza, J.; Gonzalez-Cadavid, N.; Salehian, B. Glucocorticoid-induced skeletal muscle atrophy is associated with upregulation of myostatin gene expression. Am. J. Physiol. Endocrinol. Metab. 2003, 285, e363–e371. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Park, J.; Shin, H.; Bae, Y. Gastrocnemius medial head stiffness is associated with potential fall risk in community-dwelling older adults. Healthcare 2022, 10, 785. [Google Scholar] [CrossRef]
- Oeppen, J.; Vaupel, J.W. Broken limits to life expectancy. Science 2002, 296, 1029–1031. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, T.; Su, Q.; Luo, H.; Lou, L.; Zhao, L.; Kang, X.; Pan, Y.; Nie, Y. Prevalence of polypharmacy in elderly population worldwide: A systematic review and meta-analysis. Pharmacoepidemiol. Drug Saf. 2024, 33, e5880. [Google Scholar] [CrossRef]
- Getzen, T.E. Measuring national health expenditures. SSRN J. 2020, 1–83. [Google Scholar] [CrossRef]
- Alexanian, R.; Dimopoulos, M.A.; Delasalle, K.; Barlogie, B. Primary dexamethasone treatment of multiple myeloma. Blood 1992, 80, 887–890. [Google Scholar] [CrossRef][Green Version]
- Cheng, Y.; Wong, R.S.M.; Soo, Y.O.Y.; Chui, C.H.; Lau, F.Y.; Chan, N.P.H.; Wong, W.S.; Cheng, G. Initial treatment of immune thrombocytopenic purpura with high-dose dexamethasone. N. Engl. J. Med. 2003, 349, 831–836. [Google Scholar] [CrossRef]
- Gupta, P.; Bhatia, V. Corticosteroid physiology and principles of therapy. Indian J. Pediatr. 2008, 75, 1039–1044. [Google Scholar] [CrossRef]
- Salehian, B.; Mahabadi, V.; Bilas, J.; Taylor, W.E.; Ma, K. The effect of glutamine on prevention of glucocorticoid-induced skeletal muscle atrophy is associated with myostatin suppression. Metabolism 2006, 55, 1239–1247. [Google Scholar] [CrossRef]
- Zhao, W.; Pan, J.; Zhao, Z.; Wu, Y.; Bauman, W.A.; Cardozo, C.P. Testosterone protects against dexamethasone-induced muscle atrophy, protein degradation and MAFbx upregulation. J. Steroid Biochem. Mol. Biol. 2008, 110, 125–129. [Google Scholar] [CrossRef]
- Shang, Y.; Kuang, M.; Wang, Z.; Huang, Y.; Liu, L.; Zhao, X.; Zhang, R.; Zhao, Y.; Peng, R.; Sun, S.; et al. An ultrashort peptide-based supramolecular hydrogel mimicking IGF-1 to alleviate glucocorticoid-induced sarcopenia. ACS Appl. Mater. Interfaces 2020, 12, 34678–34688. [Google Scholar] [CrossRef]
- Lee, K.; Kim, J.; Park, S.D.; Shim, J.J.; Lee, J.L. Lactobacillus plantarum HY7715 ameliorates sarcopenia by improving skeletal muscle mass and function in aged BALB/c mice. Int. J. Mol. Sci. 2021, 22, 10023. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kang, M.; Yoo, J.; Lee, S.; Kang, M.; Yun, B.; Kim, J.N.; Moon, H.; Chung, Y.; Oh, S. Lactobacillus rhamnosus JY02 ameliorates sarcopenia by anti-atrophic effects in a dexamethasone-induced cellular and murine model. J. Microbiol. Biotechnol. 2023, 33, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Noh, K.K.; Chung, K.W.; Choi, Y.J.; Park, M.H.; Jang, E.J.; Park, C.H.; Yoon, C.; Kim, N.D.; Kim, M.K.; Chung, H.Y. β–hydroxy β–methylbutyrate improves dexamethasone-induced muscle atrophy by modulating the muscle degradation pathway in SD rat. PLoS ONE 2014, 9, e102947. [Google Scholar] [CrossRef]
- Noh, K.K.; Chung, K.W.; Sung, B.; Kim, M.J.; Park, C.H.; Yoon, C.; Choi, J.S.; Kim, M.K.; Kim, C.M.; Kim, N.D.; et al. Loquat (Eriobotrya japonica) extract prevents dexamethasone-induced muscle atrophy by inhibiting the muscle degradation pathway in Sprague Dawley rats. Mol. Med. Rep. 2015, 12, 3607–3614. [Google Scholar] [CrossRef] [PubMed]
- Hah, Y.-S.; Lee, W.K.; Lee, S.; Kim, E.J.; Lee, J.H.; Lee, S.-J.; Ji, Y.H.; Kim, S.G.; Lee, H.-H.; Hong, S.Y.; et al. β-sitosterol attenuates dexamethasone-induced muscle atrophy via regulating FoxO1-dependent signaling in C2C12 cell and mice model. Nutrients 2022, 14, 2894. [Google Scholar] [CrossRef]
- Sacheck, J.M.; Ohtsuka, A.; McLary, S.C.; Goldberg, A.L. IGF-I stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E591–E601. [Google Scholar] [CrossRef]
- Yamamoto, D.; Ikeshita, N.; Matsubara, T.; Tasaki, H.; Herningtyas, E.H.; Toda, K.; Iida, K.; Takahashi, Y.; Kaji, H.; Chihara, K.; et al. GHRP-2, a GHS-R agonist, directly acts on myocytes to attenuate the dexamethasone-induced expressions of muscle-specific ubiquitin ligases, Atrogin-1 and MuRF1. Life Sci. 2008, 82, 460–466. [Google Scholar] [CrossRef]
- Gumucio, J.P.; Mendias, C.L. Atrogin-1, MuRF-1, and sarcopenia. Endocrine 2013, 43, 12–21. [Google Scholar] [CrossRef]
- Bollinger, L.M.; Witczak, C.A.; Houmard, J.A.; Brault, J.J. SMAD3 augments FoxO3-induced MuRF-1 promoter activity in a DNA-binding-dependent manner. Am. J. Physiol. Cell Physiol. 2014, 307, C278–C287. [Google Scholar] [CrossRef]
- Moritani, T.; Oddsson, L.; Thorstensson, A. Activation patterns of the soleus and gastrocnemius muscles during different motor tasks. J. Electromyogr. Kinesiol. 1991, 1, 81–88. [Google Scholar] [CrossRef]
- DeNies, M.S.; Johnson, J.; Maliphol, A.B.; Bruno, M.; Kim, A.; Rizvi, A.; Rustici, K.; Medler, S. Diet-induced obesity alters skeletal muscle fiber types of male but not female mice. Physiol. Rep. 2014, 2, e00204. [Google Scholar] [CrossRef] [PubMed]
- Hata, J.; Nakashima, D.; Tsuji, O.; Fujiyoshi, K.; Yasutake, K.; Sera, Y.; Komaki, Y.; Hikishima, K.; Nagura, T.; Matsumoto, M.; et al. Noninvasive technique to evaluate the muscle fiber characteristics using q-space imaging. PLoS ONE 2019, 14, e0214805. [Google Scholar] [CrossRef] [PubMed]
- Dumas, J.F.; Bielicki, G.; Renou, J.P.; Roussel, D.; Ducluzeau, P.H.; Malthièry, Y.; Simard, G.; Ritz, P. Dexamethasone impairs muscle energetics, studied by (31)P NMR, in rats. Diabetologia 2005, 48, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Nilwik, R.; Snijders, T.; Leenders, M.; Groen, B.B.L.; van Kranenburg, J.; Verdijk, L.B.; van Loon, L.J.C. The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp. Gerontol. 2013, 48, 492–498. [Google Scholar] [CrossRef]
- Kim, Y. Dexamethasone-Induced Skeletal Muscle Atrophy in Young Rats and Aged Rats Comparison to Myosin Heavy Chain Transition. Master’s Thesis, Kyung Hee University, Yong in-Si, Republic of Korea, 2018. [Google Scholar]
- Wang, B.Y.-H.; Hsiao, A.W.-T.; Wong, N.; Chen, Y.-F.; Lee, C.-W.; Lee, W.Y.W. Is dexamethasone-induced muscle atrophy an alternative model for naturally aged sarcopenia model? J. Orthop. Transl. 2023, 39, 12–20. [Google Scholar] [CrossRef]
- Tanganelli, F.; Meinke, P.; Hofmeister, F.; Jarmusch, S.; Baber, L.; Mehaffey, S.; Hintze, S.; Ferrari, U.; Neuerburg, C.; Kammerlander, C.; et al. Type-2 muscle fiber atrophy is associated with sarcopenia in elderly men with hip fracture. Exp. Gerontol. 2021, 144, 111171. [Google Scholar] [CrossRef]
- Sandri, M. Protein breakdown in muscle wasting: Role of autophagy-lysosome and ubiquitin-proteasome. Int. J. Biochem. Cell Biol. 2013, 45, 2121–2129. [Google Scholar] [CrossRef]
- Zhao, J.; Zhai, B.; Gygi, S.P.; Goldberg, A.L. mTOR inhibition activates overall protein degradation by the ubiquitin proteasome system as well as by autophagy. Proc. Natl Acad. Sci. USA 2015, 112, 15790–15797. [Google Scholar] [CrossRef]
- Ono, Y.; Sakamoto, K. Lipopolysaccharide inhibits myogenic differentiation of C2C12 myoblasts through the Toll-like receptor 4-nuclear factor-κB signaling pathway and myoblast-derived tumor necrosis factor-α. PLoS ONE 2017, 12, e0182040. [Google Scholar] [CrossRef]
- Mori, K.; Sun, H.; Miura, K.; Simizu, S. Involvement of DPY19L3 in myogenic differentiation of C2C12 myoblasts. Molecules 2021, 26, 5685. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, J.; Lee, J.-H.; Jeong, E.; Park, H.; Song, H.-Y.; Choi, J.; Kim, M.-a.; Han, K.-I.; Kim, D.; Kim, H.S.; et al. Protective Efficacy of Lactobacillus plantarum Postbiotic beLP-K in a Dexamethasone-Induced Sarcopenia Model. Int. J. Mol. Sci. 2025, 26, 7504. https://doi.org/10.3390/ijms26157504
Moon J, Lee J-H, Jeong E, Park H, Song H-Y, Choi J, Kim M-a, Han K-I, Kim D, Kim HS, et al. Protective Efficacy of Lactobacillus plantarum Postbiotic beLP-K in a Dexamethasone-Induced Sarcopenia Model. International Journal of Molecular Sciences. 2025; 26(15):7504. https://doi.org/10.3390/ijms26157504
Chicago/Turabian StyleMoon, Juyeong, Jin-Ho Lee, Eunwoo Jeong, Harang Park, Hye-Yeong Song, Jinsu Choi, Min-ah Kim, Kwon-Il Han, Doyong Kim, Han Sung Kim, and et al. 2025. "Protective Efficacy of Lactobacillus plantarum Postbiotic beLP-K in a Dexamethasone-Induced Sarcopenia Model" International Journal of Molecular Sciences 26, no. 15: 7504. https://doi.org/10.3390/ijms26157504
APA StyleMoon, J., Lee, J.-H., Jeong, E., Park, H., Song, H.-Y., Choi, J., Kim, M.-a., Han, K.-I., Kim, D., Kim, H. S., & Kim, T.-J. (2025). Protective Efficacy of Lactobacillus plantarum Postbiotic beLP-K in a Dexamethasone-Induced Sarcopenia Model. International Journal of Molecular Sciences, 26(15), 7504. https://doi.org/10.3390/ijms26157504





