The Redox Revolution in Brain Medicine: Targeting Oxidative Stress with AI, Multi-Omics and Mitochondrial Therapies for the Precision Eradication of Neurodegeneration
Abstract
1. Introduction
1.1. Defining Oxidative Stress and Its Role in Neurology
1.2. Overview of ROS, RNS, and Redox Homeostasis
1.3. Relevance of Oxidative Stress to Neurological Disorders
1.4. Objectives and Significance of This Review
2. Mechanisms of Oxidative Stress in Neurological Disorders
2.1. Biochemistry of Oxidative Stress
2.1.1. Reactive Oxygen Species and Reactive Nitrogen Species
2.1.2. Cellular Targets and Oxidative Damage
2.1.3. Antioxidant Defense Systems
2.2. Pathophysiological Mechanisms in the Nervous System
2.2.1. Mitochondrial Dysfunction in Oxidative Stress
2.2.2. Impaired Autophagy and Proteostasis
2.2.3. Neuroinflammation and Oxidative Stress Interplay
2.2.4. Blood–Brain Barrier Integrity and Oxidative Stress
2.2.5. Interaction with Other Cellular Processes
3. Oxidative Stress and Major Neurological Disorders
3.1. Neurodegenerative Diseases
3.1.1. AD
3.1.2. PD
3.1.3. ALS
3.2. Cerebrovascular Disorders
3.2.1. Ischemic Stroke and Reperfusion Injury
3.2.2. Oxidative Stress in Blood–Brain Barrier Dysfunction
3.3. Demyelinating Disorders
3.4. Neurodevelopmental Disorders
3.5. Psychiatric Disorders
3.5.1. Depression
3.5.2. Schizophrenia
3.5.3. Bipolar Disorder
4. Diagnostic and Biomarker Advances
4.1. Emerging Biomarkers of Oxidative Stress in Neurology
4.1.1. Biomarkers of Oxidative Damage to Biomolecules
4.1.2. Antioxidant Status Biomarkers
4.1.3. Disease-Specific Oxidative Stress Markers
4.2. Imaging Techniques to Detect Oxidative Damage In Vivo
4.2.1. Magnetic Resonance Imaging (MRI)-Based Techniques
4.2.2. Positron Emission Tomography (PET)
4.2.3. Optical Imaging Techniques
4.2.4. Emerging Multimodal Imaging Approaches
4.2.5. Clinical Implications
4.3. Multi-Omics Integration
4.3.1. Genomics and Epigenomics: The Genetic Basis of Oxidative Vulnerability
4.3.2. Proteomics: Oxidatively Modified Proteins as Dynamic Biomarkers
4.3.3. Metabolomics: Tracking Dynamic Redox Changes
4.3.4. Multi-Omics Integration: Toward Personalized Redox Medicine
5. Therapeutic Strategies Targeting Oxidative Stress
5.1. Advanced Antioxidant Therapies: Beyond Traditional Free Radical Scavenging
5.2. Nrf2 Pathway Activators: Enhancing Endogenous Antioxidant Defenses
5.3. Mitochondria-Targeted Therapies: The Next Frontier in Neuroprotection
5.4. Redox Gene Therapy and Epigenetic Reprogramming
5.5. Future Directions: AI-Driven Personalized Redox Medicine
6. Clinical Trials and Translational Advances
6.1. Clinical Trials in Neurodegenerative Diseases
6.1.1. NAC: Clinical Trials Targeting GSH Depletion
6.1.2. Coenzyme Q10 (CoQ10) in Mitochondrial Protection
6.2. Nrf2 Activator Trials: Translating Preclinical Success to Clinical Practice
6.2.1. Dimethyl Fumarate in MS and Neurodegenerative Diseases
6.2.2. Sulforaphane Trials in AD, ASD, and Stroke
6.3. Mitochondria-Targeted Clinical Trials: Addressing Energy Deficits and ROS Overload
6.3.1. MitoQ in Parkinson’s Disease and AD
6.3.2. SS-31 (Elamipretide) in ALS and Stroke
6.4. Emerging Gene and Epigenetic Therapies
6.4.1. Gene Therapy Trials Targeting Antioxidant Enzymes
6.4.2. Epigenetic Reprogramming to Restore Antioxidant Defenses
6.5. Future Directions: Personalized Redox Medicine and AI-Driven Clinical Trials
7. Future Directions and Emerging Therapeutic Frontiers
7.1. AI-Driven Personalized Redox Medicine: Precision at an Unprecedented Scale
7.2. Combination Therapies: Targeting the Complexity of Oxidative Stress
7.3. Nanomedicine: Precision Delivery of Antioxidants to ROS Hotspots
7.4. Gene Editing and Epigenetic Therapies: Reprogramming Redox Homeostasis
7.5. Multi-Omics Integration and Digital Twin Simulations: The Future of Redox Precision Medicine
8. Conclusions: Redefining the Battle Against Oxidative Stress
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Espinosa-Vellarino, F.L.; Garrido, I.; Ortega, A.; Casimiro, I.; Espinosa, F. Effects of Antimony on Reactive Oxygen and Nitrogen Species (ROS and RNS) and Antioxidant Mechanisms in Tomato Plants. Front. Plant Sci. 2020, 11, 674. [Google Scholar] [CrossRef]
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive Oxygen Species Signaling and Oxidative Stress: Transcriptional Regulation and Evolution. Antioxidants 2024, 13, 312. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Khalil, A.A.; Awadallah, S.; Khan, S.A.; Abu-Izneid, T.; Kamran, M.; Hemeg, H.A.; Mubarak, M.S.; Khalid, A.; Wilairatana, P. Reactive oxygen species in biological systems: Pathways, associated diseases, and potential inhibitors—A review. Food Sci. Nutr. 2023, 12, 675–693. [Google Scholar] [CrossRef]
- Garbarino, V.R.; Orr, M.E.; Rodriguez, K.A.; Buffenstein, R. Mechanisms of Oxidative Stress Resistance in The Brain: Lessons Learned From Hypoxia Tolerant Extremophilic Vertebrates. Arch. Biochem. Biophys. 2015, 576, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Danielli, M.; Perne, L.; Jovičić, E.J.; Petan, T. Lipid droplets and polyunsaturated fatty acid trafficking: Balancing life and death. Front. Cell Dev. Biol. 2023, 11, 1104725. [Google Scholar] [CrossRef]
- Giblin, A.; Cammack, A.J.; Blomberg, N.; Anoar, S.; Mikheenko, A.; Carcolé, M.; Atilano, M.L.; Hull, A.; Shen, D.; Wei, X.; et al. Neuronal polyunsaturated fatty acids are protective in ALS/FTD. Nat. Neurosci. 2025, 28, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Cenini, G.; Lloret, A.; Cascella, R. Oxidative Stress in Neurodegenerative Diseases: From a Mitochondrial Point of View. Oxid. Med. Cell. Longev. 2019, 2019, 2105607. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Lan, Y.-W.; Chen, W.-R.; Chang, G.R.-L.; Chen, Y.-C.; Chong, K.-Y.; Chuang, K.-C.; Kao, Y.-T.; Chen, M.-S.; Chen, C.-M. Aldo-keto reductase family 1 member A1 (AKR1A1) exerts a protective function in alcohol-associated liver disease by reducing 4-HNE accumulation and p53 activation. Cell Biosci. 2024, 14, 1–17. [Google Scholar] [CrossRef]
- Li, S.; Wang, Z.; Liu, G.; Chen, M. Neurodegenerative diseases and catechins: (−)-epigallocatechin-3-gallate is a modulator of chronic neuroinflammation and oxidative stress. Front. Nutr. 2024, 11, 1425839. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, U.J.; Lee, B.H.; Cha, M. Safeguarding the brain from oxidative damage. Free. Radic. Biol. Med. 2024, 226, 143–157. [Google Scholar] [CrossRef]
- Üremiş, N.; Üremiş, M.M. Oxidative/Nitrosative Stress, Apoptosis, and Redox Signaling: Key Players in Neurodegenerative Diseases. J. Biochem. Mol. Toxicol. 2025, 39, e70133. [Google Scholar] [CrossRef]
- Vázquez-Galán, Y.I.; Guzmán-Silahua, S.; Trujillo-Rangel, W.Á.; Rodríguez-Lara, S.Q. Role of Ischemia/Reperfusion and Oxidative Stress in Shock State. Cells 2025, 14, 808. [Google Scholar] [CrossRef]
- Vornic, I.; Buciu, V.; Furau, C.G.; Gaje, P.N.; Ceausu, R.A.; Dumitru, C.-S.; Barb, A.C.; Novacescu, D.; Cumpanas, A.A.; Latcu, S.C.; et al. Oxidative Stress and Placental Pathogenesis: A Contemporary Overview of Potential Biomarkers and Emerging Therapeutics. Int. J. Mol. Sci. 2024, 25, 12195. [Google Scholar] [CrossRef]
- Salvagno, M.; Sterchele, E.D.; Zaccarelli, M.; Mrakic-Sposta, S.; Welsby, I.J.; Balestra, C.; Taccone, F.S. Oxidative Stress and Cerebral Vascular Tone: The Role of Reactive Oxygen and Nitrogen Species. Int. J. Mol. Sci. 2024, 25, 3007. [Google Scholar] [CrossRef]
- de la Lastra, J.M.P.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. The Nitration of Proteins, Lipids and DNA by Peroxynitrite Derivatives-Chemistry Involved and Biological Relevance. Stresses 2022, 2, 53–64. [Google Scholar] [CrossRef]
- Rao, M.J.; Duan, M.; Zhou, C.; Jiao, J.; Cheng, P.; Yang, L.; Wei, W.; Shen, Q.; Ji, P.; Yang, Y.; et al. Antioxidant Defense System in Plants: Reactive Oxygen Species Production, Signaling, and Scavenging During Abiotic Stress-Induced Oxidative Damage. Horticulturae 2025, 11, 477. [Google Scholar] [CrossRef]
- Zheng, M.; Liu, Y.; Zhang, G.; Yang, Z.; Xu, W.; Chen, Q. The Applications and Mechanisms of Superoxide Dismutase in Medicine, Food, and Cosmetics. Antioxidants 2023, 12, 1675. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Fujii, J.; Homma, T.; Osaki, T. Superoxide Radicals in the Execution of Cell Death. Antioxidants 2022, 11, 501. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Shang, J.; Chen, Q. Superoxide Dismutases in Immune Regulation and Infectious Diseases. Antioxidants 2025, 14, 809. [Google Scholar] [CrossRef]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’andrea, L.; Napolitano, P.; D’Alessandro, A.G. Free Radical Properties, Source and Targets, Antioxidant Consumption and Health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Wang, P.; Liu, W.-C.; Han, C.; Wang, S.; Bai, M.-Y.; Song, C.-P. Reactive oxygen species: Multidimensional regulators of plant adaptation to abiotic stress and development. J. Integr. Plant Biol. 2024, 66, 330–367. [Google Scholar] [CrossRef]
- Pei, J.; Pan, X.; Wei, G.; Hua, Y. Research progress of glutathione peroxidase family (GPX) in redoxidation. Front. Pharmacol. 2023, 14, 1147414. [Google Scholar] [CrossRef]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox system in health and disease: The latest update. Biomed. Pharmacother. 2023, 162, 114606. [Google Scholar] [CrossRef]
- Lirussi, L.; Nilsen, H.L. DNA Glycosylases Define the Outcome of Endogenous Base Modifications. Int. J. Mol. Sci. 2023, 24, 10307. [Google Scholar] [CrossRef] [PubMed]
- Jurcau, M.-C.; Jurcau, A.; Diaconu, R.-G. Oxidative Stress in the Pathogenesis of Neurodegenerative Diseases. Stresses 2024, 4, 827–849. [Google Scholar] [CrossRef]
- Toader, C.; Tataru, C.P.; Munteanu, O.; Serban, M.; Covache-Busuioc, R.-A.; Ciurea, A.V.; Enyedi, M. Decoding Neurodegeneration: A Review of Molecular Mechanisms and Therapeutic Advances in Alzheimer’s, Parkinson’s, and ALS. Int. J. Mol. Sci. 2024, 25, 12613. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H.; Li, R.; Sterling, K.; Song, W. Amyloid β-based therapy for Alzheimer’s disease: Challenges, successes and future. Signal Transduct. Target. Ther. 2023, 8, 1–26. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, P.; Bailey, T.; Bhattarai, S.; Subedi, U.; Miller, C.; Ara, H.; Kidambi, S.; Sun, H.; Panchatcharam, M.; et al. Electrophilic Aldehyde 4-Hydroxy-2-Nonenal Mediated Signaling and Mitochondrial Dysfunction. Biomolecules 2022, 12, 1555. [Google Scholar] [CrossRef]
- Kim, S.; Jung, U.J.; Kim, S.R. Role of Oxidative Stress in Blood–Brain Barrier Disruption and Neurodegenerative Diseases. Antioxidants 2024, 13, 1462. [Google Scholar] [CrossRef]
- Liu, X.; Lin, J.; Zhang, H.; Khan, N.U.; Zhang, J.; Tang, X.; Cao, X.; Shen, L. Oxidative Stress in Autism Spectrum Disorder—Current Progress of Mechanisms and Biomarkers. Front. Psychiatry 2022, 13, 813304. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, Y.; Yang, A.; Meng, F.; Zhang, J. The Expanding Burden of Neurodegenerative Diseases: An Unmet Medical and Social Need. Aging Dis. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Li, M.; Ye, X.; Huang, Z.; Ye, L.; Chen, C. Global burden of Parkinson’s disease from 1990 to 2021: A population-based study. BMJ Open 2025, 15, e095610. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Z.; Li, Q. Global trends and projections of Parkinson’s disease incidence: A 30-year analysis using GBD 2021 data. J. Neurol. 2025, 272, 286. [Google Scholar] [CrossRef]
- Selvaraj, N.R.; Nandan, D.; Nair, B.G.; Nair, V.A.; Venugopal, P.; Aradhya, R. Oxidative Stress and Redox Imbalance: Common Mechanisms in Cancer Stem Cells and Neurodegenerative Diseases. Cells 2025, 14, 511. [Google Scholar] [CrossRef] [PubMed]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef] [PubMed]
- Sinenko, S.A.; Starkova, T.Y.; Kuzmin, A.A.; Tomilin, A.N. Physiological Signaling Functions of Reactive Oxygen Species in Stem Cells: From Flies to Man. Front. Cell Dev. Biol. 2021, 9, 714370. [Google Scholar] [CrossRef] [PubMed]
- Peggion, C.; Calì, T.; Brini, M. Mitochondria Dysfunction and Neuroinflammation in Neurodegeneration: Who Comes First? Antioxidants 2024, 13, 240. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; de la Lastra, J.M.P.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Superoxide Anion Chemistry—Its Role at the Core of the Innate Immunity. Int. J. Mol. Sci. 2023, 24, 1841. [Google Scholar] [CrossRef]
- Sánchez-Alcázar, J.; Villalón-García, I.; Povea-Cabello, S.; Álvarez-Córdoba, M.; Talaverón-Rey, M.; Suárez-Rivero, J.; Suárez-Carrillo, A.; Munuera-Cabeza, M.; Reche-López, D.; Cilleros-Holgado, P.; et al. Vicious cycle of lipid peroxidation and iron accumulation in neurodegeneration. Neural Regen. Res. 2023, 18, 1196–1202. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E. Nitric oxide signaling in health and disease. Cell 2022, 185, 2853–2878. [Google Scholar] [CrossRef]
- Eo, H.; Kim, S.; Jung, U.J.; Kim, S.R. Alpha-Synuclein and Microglia in Parkinson’s Disease: From Pathogenesis to Therapeutic Prospects. J. Clin. Med. 2024, 13, 7243. [Google Scholar] [CrossRef]
- Carapeto, A.P.; Marcuello, C.; Faísca, P.F.N.; Rodrigues, M.S. Morphological and Biophysical Study of S100A9 Protein Fibrils by Atomic Force Microscopy Imaging and Nanomechanical Analysis. Biomolecules 2024, 14, 1091. [Google Scholar] [CrossRef]
- Ziaunys, M.; Sakalauskas, A.; Mikalauskaite, K.; Smirnovas, V. Polymorphism of Alpha-Synuclein Amyloid Fibrils Depends on Ionic Strength and Protein Concentration. Int. J. Mol. Sci. 2021, 22, 12382. [Google Scholar] [CrossRef]
- Afzal, S.; Manap, A.S.A.; Attiq, A.; Albokhadaim, I.; Kandeel, M.; Alhojaily, S.M. From imbalance to impairment: The central role of reactive oxygen species in oxidative stress-induced disorders and therapeutic exploration. Front. Pharmacol. 2023, 14, 1269581. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Sun, J.; Luo, Z.; Li, Y.; Huang, Y. Emerging mechanisms of lipid peroxidation in regulated cell death and its physiological implications. Cell Death Dis. 2024, 15, 1–19. [Google Scholar] [CrossRef]
- Anjo, S.I.; He, Z.; Hussain, Z.; Farooq, A.; McIntyre, A.; Laughton, C.A.; Carvalho, A.N.; Finelli, M.J. Protein Oxidative Modifications in Neurodegenerative Diseases: From Advances in Detection and Modelling to Their Use as Disease Biomarkers. Antioxidants 2024, 13, 681. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, X.; Du, X.; Bi, M.; Ma, F.; Xie, J.; Jiang, H. The S-nitrosylation of parkin attenuated the ubiquitination of divalent metal transporter 1 in MPP+-treated SH-SY5Y cells. Sci. Rep. 2020, 10, 15542. [Google Scholar] [CrossRef] [PubMed]
- Palmisano, M.; Kulp, J.; Bender, S.; Stefanovski, D.; Robinson, M.; Johnson, A. Measurement of 8-hydroxy-2′-deoxyguanosine in serum and cerebrospinal fluid of horses with neuroaxonal degeneration and other causes of proprioceptive ataxia. J. Veter-Intern. Med. 2024, 38, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Hosaka, T.; Tsuji, H.; Tamaoka, A. Biomolecular Modifications Linked to Oxidative Stress in Amyotrophic Lateral Sclerosis: Determining Promising Biomarkers Related to Oxidative Stress. Processes 2021, 9, 1667. [Google Scholar] [CrossRef]
- Suzen, S.; Tucci, P.; Profumo, E.; Buttari, B.; Saso, L. A Pivotal Role of Nrf2 in Neurodegenerative Disorders: A New Way for Therapeutic Strategies. Pharmaceuticals 2022, 15, 692. [Google Scholar] [CrossRef]
- Aran, K.R.; Singh, S. Mitochondrial dysfunction and oxidative stress in Alzheimer’s disease–A step towards mitochondria based therapeutic strategies. Aging Health Res. 2023, 3, 100169. [Google Scholar] [CrossRef]
- Ganley, I.G. Strengthening the link between mitophagy and Parkinson’s disease. Brain 2022, 145, 4154–4156. [Google Scholar] [CrossRef]
- Li, J.; Yang, D.; Li, Z.; Zhao, M.; Wang, D.; Sun, Z.; Wen, P.; Dai, Y.; Gou, F.; Ji, Y.; et al. PINK1/Parkin-mediated mitophagy in neurodegenerative diseases. Ageing Res. Rev. 2022, 84, 101817. [Google Scholar] [CrossRef] [PubMed]
- Percy, K.C.M.; Liu, Z.; Qi, X. Mitochondrial dysfunction in Alzheimer’s disease: Guiding the path to targeted therapies. Neurotherapeutics 2025, 22, e00525. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jing, S.; Liu, S.; Shen, X.; Cai, L.; Zhu, C.; Zhao, Y.; Pang, M. Double-activation of mitochondrial permeability transition pore opening via calcium overload and reactive oxygen species for cancer therapy. J. Nanobiotechnol. 2022, 20, 188. [Google Scholar] [CrossRef]
- Panes, J.D.; Wendt, A.; Ramirez-Molina, O.; Castro, P.A.; Fuentealba, J. Deciphering the role of PGC-1α in neurological disorders: From mitochondrial dysfunction to synaptic failure. Neural Regen. Res. 2022, 17, 237–245. [Google Scholar] [CrossRef]
- Piccinin, E.; Sardanelli, A.M.; Seibel, P.; Moschetta, A.; Cocco, T.; Villani, G. PGC-1s in the Spotlight with Parkinson’s Disease. Int. J. Mol. Sci. 2021, 22, 3487. [Google Scholar] [CrossRef]
- Tutas, J.; Tolve, M.; Özer-Yildiz, E.; Ickert, L.; Klein, I.; Silverman, Q.; Liebsch, F.; Dethloff, F.; Giavalisco, P.; Endepols, H.; et al. Autophagy regulator ATG5 preserves cerebellar function by safeguarding its glycolytic activity. Nat. Metab. 2025, 7, 297–320. [Google Scholar] [CrossRef]
- Oh, C.-K.; Sultan, A.; Platzer, J.; Dolatabadi, N.; Soldner, F.; McClatchy, D.B.; Diedrich, J.K.; Yates, J.R., III; Ambasudhan, R.; Nakamura, T.; et al. S-Nitrosylation of PINK1 Attenuates PINK1/Parkin-Dependent Mitophagy in hiPSC-Based Parkinson’s Disease Models. Cell Rep. 2017, 21, 2171–2182. [Google Scholar] [CrossRef]
- George, J.; Shafiq, K.; Kapadia, M.; Kalia, L.V.; Kalia, S.K. High frequency electrical stimulation reduces α-synuclein levels and α-synuclein-mediated autophagy dysfunction. Sci. Rep. 2024, 14, 16091. [Google Scholar] [CrossRef]
- Zheng, Q.; Wang, X. Alzheimer’s disease: Insights into pathology, molecular mechanisms, and therapy. Protein Cell 2024, 16, 83–120. [Google Scholar] [CrossRef]
- Guan, T.; Zhou, T.; Zhang, X.; Guo, Y.; Yang, C.; Lin, J.; Zhang, J.V.; Cheng, Y.; Marzban, H.; Wang, Y.T.; et al. Selective removal of misfolded SOD1 delays disease onset in a mouse model of amyotrophic lateral sclerosis. Cell. Mol. Life Sci. 2023, 80, 304. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.J.; Choi, B.; Kim, E.J.; Ohk, J.; Yang, C.; Choi, Y.-G.; Lee, J.; Kang, C.; Song, H.K.; Kim, Y.K.; et al. UXT chaperone prevents proteotoxicity by acting as an autophagy adaptor for p62-dependent aggrephagy. Nat. Commun. 2021, 12, 1955. [Google Scholar] [CrossRef] [PubMed]
- Granatiero, V.; Sayles, N.M.; Savino, A.M.; Konrad, C.; Kharas, M.G.; Kawamata, H.; Manfredi, G. Modulation of the IGF1R-MTOR pathway attenuates motor neuron toxicity of human ALS SOD1G93A astrocytes. Autophagy 2021, 17, 4029–4042. [Google Scholar] [CrossRef]
- Tu, D.; Velagapudi, R.; Gao, Y.; Hong, J.-S.; Zhou, H.; Gao, H.-M. Activation of neuronal NADPH oxidase NOX2 promotes inflammatory neurodegeneration. Free Radic. Biol. Med. 2023, 200, 47–58. [Google Scholar] [CrossRef]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in neurodegenerative diseases: Mechanism and potential therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 1–37. [Google Scholar] [CrossRef]
- Onciul, R.; Brehar, F.-M.; Toader, C.; Covache-Busuioc, R.-A.; Glavan, L.-A.; Bratu, B.-G.; Costin, H.P.; Dumitrascu, D.-I.; Serban, M.; Ciurea, A.V. Deciphering Glioblastoma: Fundamental and Novel Insights into the Biology and Therapeutic Strategies of Gliomas. Curr. Issues Mol. Biol. 2024, 46, 2402–2443. [Google Scholar] [CrossRef]
- Hu, C.-F.; Wu, S.-P.; Lin, G.-J.; Shieh, C.-C.; Hsu, C.-S.; Chen, J.-W.; Chen, S.-H.; Hong, J.-S.; Chen, S.-J. Microglial Nox2 Plays a Key Role in the Pathogenesis of Experimental Autoimmune Encephalomyelitis. Front. Immunol. 2021, 12, 638381. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Moon, J.-S. Molecular Roles of NADPH Oxidase-Mediated Oxidative Stress in Alzheimer’s Disease: Isoform-Specific Contributions. Int. J. Mol. Sci. 2024, 25, 12299. [Google Scholar] [CrossRef]
- Pereira, J.B.; Janelidze, S.; Strandberg, O.; Whelan, C.D.; Zetterberg, H.; Blennow, K.; Palmqvist, S.; Stomrud, E.; Mattsson-Carlgren, N. Microglial activation protects against accumulation of tau aggregates in nondemented individuals with underlying Alzheimer’s disease pathology. Nat. Aging 2022, 2, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jung, U.J.; Kim, S.R. The Crucial Role of the Blood–Brain Barrier in Neurodegenerative Diseases: Mechanisms of Disruption and Therapeutic Implications. J. Clin. Med. 2025, 14, 386. [Google Scholar] [CrossRef]
- Peña-Bautista, C.; Vigor, C.; Galano, J.-M.; Oger, C.; Durand, T.; Ferrer, I.; Cuevas, A.; López-Cuevas, R.; Baquero, M.; López-Nogueroles, M.; et al. New screening approach for Alzheimer’s disease risk assessment from urine lipid peroxidation compounds. Sci. Rep. 2019, 9, 14244. [Google Scholar] [CrossRef]
- Majerníková, N.; Marmolejo-Garza, A.; Salinas, C.S.; Luu, M.D.A.; Zhang, Y.; Trombetta-Lima, M.; Tomin, T.; Birner-Gruenberger, R.; Lehtonen, Š.; Koistinaho, J.; et al. The link between amyloid β and ferroptosis pathway in Alzheimer’s disease progression. Cell Death Dis. 2024, 15, 782. [Google Scholar] [CrossRef]
- Park, H.-J.; Nam, M.-H.; Park, J.-H.; Lee, J.-M.; Hong, H.-S.; Kim, T.-W.; Lee, I.-H.; Shin, C.-H.; Lee, S.-H.; Seo, Y.-K. Comparison of Malondialdehyde, Acetylcholinesterase, and Apoptosis-Related Markers in the Cortex and Hippocampus of Cognitively Dysfunctional Mice Induced by Scopolamine. Biomedicines 2024, 12, 2475. [Google Scholar] [CrossRef]
- Abeti, R.; Baccaro, A.; Esteras, N.; Giunti, P. Novel Nrf2-Inducer Prevents Mitochondrial Defects and Oxidative Stress in Friedreich’s Ataxia Models. Front. Cell. Neurosci. 2018, 12, 188. [Google Scholar] [CrossRef]
- Gan, W.; Liu, X.-L.; Yu, T.; Zou, Y.-G.; Li, T.-T.; Wang, S.; Deng, J.; Wang, L.-L.; Cai, J.-P. Urinary 8-oxo-7,8-dihydroguanosine as a Potential Biomarker of Aging. Front. Aging Neurosci. 2018, 10, 34. [Google Scholar] [CrossRef]
- Basu, S.; Song, M.; Adams, L.; Jeong, I.; Je, G.; Guhathakurta, S.; Jiang, J.; Boparai, N.; Dai, W.; Cardozo-Pelaez, F.; et al. Transcriptional mutagenesis of α-synuclein caused by DNA oxidation in Parkinson’s disease pathogenesis. Acta Neuropathol. 2023, 146, 685–705. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Parker, K.; Zhan, Y.; Miller, M.; Zhu, M.-Y. Upregulated DNA Damage-Linked Biomarkers in Parkinson’s Disease Model Mice. ASN Neuro 2023, 15, 17590914231152099. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Chen, H.; Li, M.; Fan, X.; Jiang, F.; Xu, C.; Wang, Y.; Wei, W.; Song, J.; Zhong, D.; et al. NRF2 activation ameliorates blood–brain barrier injury after cerebral ischemic stroke by regulating ferroptosis and inflammation. Sci. Rep. 2024, 14, 5300. [Google Scholar] [CrossRef]
- Medeiros, R.; Sousa, B.; Rossi, S.; Afonso, C.; Bonino, L.; Pitt, A.; López, E.; Spickett, C.; Borthagaray, G. Identification and relative quantification of 3-nitrotyrosine residues in fibrinogen nitrated in vitro and fibrinogen from ischemic stroke patient plasma using LC-MS/MS. Free. Radic. Biol. Med. 2021, 165, 334–347. [Google Scholar] [CrossRef]
- Fan, R.; Schrott, L.M.; Snelling, S.; Felty, J.; Graham, D.; McGauly, P.L.; Arnold, T.; Korneeva, N.L. Carbonyl-protein content increases in brain and blood of female rats after chronic oxycodone treatment. BMC Neurosci. 2020, 21, 4. [Google Scholar] [CrossRef]
- Riggs, P.K.; Anderson, A.M.; Tang, B.; Rubin, L.H.; Morgello, S.; Marra, C.M.; Gelman, B.B.; Clifford, D.B.; Franklin, D.; Heaton, R.K.; et al. Elevated Plasma Protein Carbonyl Concentration Is Associated with More Abnormal White Matter in People with HIV. Viruses 2023, 15, 2410. [Google Scholar] [CrossRef]
- Alkazemi, D.; Rahman, A.; Habra, B. Alterations in glutathione redox homeostasis among adolescents with obesity and anemia. Sci. Rep. 2021, 11, 3034. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Matsuba, Y.; Takahashi, M.; Kamano, N.; Watamura, N.; Sasaguri, H.; Takado, Y.; Yoshihara, Y.; Saito, T.; Saido, T.C. Neuronal glutathione loss leads to neurodegeneration involving gasdermin activation. Sci. Rep. 2023, 13, 1109. [Google Scholar] [CrossRef] [PubMed]
- González-García, P.; Hidalgo-Gutiérrez, A.; Mascaraque, C.; Barriocanal-Casado, E.; Bakkali, M.; Ziosi, M.; Abdihankyzy, U.B.; Sánchez-Hernández, S.; Escames, G.; Prokisch, H.; et al. Coenzyme Q10 modulates sulfide metabolism and links the mitochondrial respiratory chain to pathways associated to one carbon metabolism. Hum. Mol. Genet. 2020, 29, 3296–3311. [Google Scholar] [CrossRef]
- Millichap, L.; Turton, N.; Damiani, E.; Marcheggiani, F.; Orlando, P.; Silvestri, S.; Tiano, L.; Hargreaves, I.P. The Effect of Neuronal CoQ10 Deficiency and Mitochondrial Dysfunction on a Rotenone-Induced Neuronal Cell Model of Parkinson’s Disease. Int. J. Mol. Sci. 2024, 25, 6622. [Google Scholar] [CrossRef]
- Daud, D.M.A.; Ahmedy, F.; Baharuddin, D.M.P.; Zakaria, Z.A. Oxidative Stress and Antioxidant Enzymes Activity after Cycling at Different Intensity and Duration. Appl. Sci. 2022, 12, 9161. [Google Scholar] [CrossRef]
- Gusti, A.M.T.; Qusti, S.Y.; Alshammari, E.M.; Toraih, E.A.; Fawzy, M.S. Antioxidants-Related Superoxide Dismutase (SOD), Catalase (CAT), Glutathione Peroxidase (GPX), Glutathione-S-Transferase (GST), and Nitric Oxide Synthase (NOS) Gene Variants Analysis in an Obese Population: A Preliminary Case-Control Study. Antioxidants 2021, 10, 595. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Z.; Xiong, Y.; Zhou, Q.; Zhu, L.-Q.; Liu, D. The emerging role of nitric oxide in the synaptic dysfunction of vascular dementia. Neural Regen. Res. 2024, 20, 402–415. [Google Scholar] [CrossRef]
- Katusic, Z.S.; d’Uscio, L.V.; He, T. Emerging Roles of Endothelial Nitric Oxide in Preservation of Cognitive Health. Stroke 2023, 54, 686–696. [Google Scholar] [CrossRef]
- Ashraf, M.I.; Ebner, M.; Wallner, C.; Haller, M.; Khalid, S.; Schwelberger, H.; Koziel, K.; Enthammer, M.; Hermann, M.; Sickinger, S.; et al. A p38MAPK/MK2 signaling pathway leading to redox stress, cell death and ischemia/reperfusion injury. Cell Commun. Signal. 2014, 12, 6. [Google Scholar] [CrossRef]
- Belenichev, I.; Popazova, O.; Bukhtiyarova, N.; Ryzhenko, V.; Pavlov, S.; Suprun, E.; Oksenych, V.; Kamyshnyi, O. Targeting Mitochondrial Dysfunction in Cerebral Ischemia: Advances in Pharmacological Interventions. Antioxidants 2025, 14, 108. [Google Scholar] [CrossRef] [PubMed]
- Andreadi, A.; Bellia, A.; Di Daniele, N.; Meloni, M.; Lauro, R.; Della-Morte, D.; Lauro, D. The molecular link between oxidative stress, insulin resistance, and type 2 diabetes: A target for new therapies against cardiovascular diseases. Curr. Opin. Pharmacol. 2022, 62, 85–96. [Google Scholar] [CrossRef]
- Shadfar, S.; Parakh, S.; Jamali, M.S.; Atkin, J.D. Redox dysregulation as a driver for DNA damage and its relationship to neurodegenerative diseases. Transl. Neurodegener. 2023, 12, 18. [Google Scholar] [CrossRef]
- Meng, K.; Jia, H.; Hou, X.; Zhu, Z.; Lu, Y.; Feng, Y.; Feng, J.; Xia, Y.; Tan, R.; Cui, F.; et al. Mitochondrial Dysfunction in Neurodegenerative Diseases: Mechanisms and Corresponding Therapeutic Strategies. Biomedicines 2025, 13, 327. [Google Scholar] [CrossRef] [PubMed]
- Aishwarya, R.; Abdullah, C.S.; Remex, N.S.; Bhuiyan, M.A.N.; Lu, X.-H.; Dhanesha, N.; Stokes, K.Y.; Orr, A.W.; Kevil, C.G.; Bhuiyan, S. Diastolic dysfunction in Alzheimer’s disease model mice is associated with Aβ-amyloid aggregate formation and mitochondrial dysfunction. Sci. Rep. 2024, 14, 16715. [Google Scholar] [CrossRef]
- Juan, C.A.; de la Lastra, J.M.P.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Hyeon, S.J.; McQuade, A.; Lim, J.; Baek, S.H.; Diep, Y.N.; Do, K.V.; Jeon, Y.; Jo, D.; Lee, C.J.; et al. Neurotoxic Microglial Activation via IFNγ-Induced Nrf2 Reduction Exacerbating Alzheimer’s Disease. Adv. Sci. 2024, 11, e2304357. [Google Scholar] [CrossRef]
- Foret, M.K.; Orciani, C.; Welikovitch, L.A.; Huang, C.; Cuello, A.C.; Carmo, S.D. Early oxidative stress and DNA damage in Aβ-burdened hippocampal neurons in an Alzheimer’s-like transgenic rat model. Commun. Biol. 2024, 7, 861. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Li, S.; Li, M.; Ke, D.; Wang, Q.; Yang, Y.; Liu, G.-P.; Wang, X.-C.; Liu, E.; Wang, J.-Z. Human tau accumulation promotes glycogen synthase kinase-3β acetylation and thus upregulates the kinase: A vicious cycle in Alzheimer neurodegeneration. EBioMedicine 2022, 78, 103970. [Google Scholar] [CrossRef]
- Zhou, Z.D.; Yi, L.X.; Wang, D.Q.; Lim, T.M.; Tan, E.K. Role of dopamine in the pathophysiology of Parkinson’s disease. Transl. Neurodegener. 2023, 12, 44. [Google Scholar] [CrossRef]
- Mahul-Mellier, A.-L.; Burtscher, J.; Maharjan, N.; Weerens, L.; Croisier, M.; Kuttler, F.; Leleu, M.; Knott, G.W.; Lashuel, H.A. The process of Lewy body formation, rather than simply α-synuclein fibrillization, is one of the major drivers of neurodegeneration. Proc. Natl. Acad. Sci. USA 2020, 117, 4971–4982. [Google Scholar] [CrossRef] [PubMed]
- Teil, M.; Arotcarena, M.-L.; Faggiani, E.; Laferriere, F.; Bezard, E.; Dehay, B. Targeting α-Synuclein for PD Therapeutics: A Pursuit on All Fronts. Biomolecules 2020, 10, 391. [Google Scholar] [CrossRef] [PubMed]
- Tresse, E.; Marturia-Navarro, J.; Sew, W.Q.G.; Cisquella-Serra, M.; Jaberi, E.; Riera-Ponsati, L.; Fauerby, N.; Hu, E.; Kretz, O.; Aznar, S.; et al. Mitochondrial DNA damage triggers spread of Parkinson’s disease-like pathology. Mol. Psychiatry 2023, 28, 4902–4914. [Google Scholar] [CrossRef]
- Lee, S.-Y.; An, H.-J.; Kim, J.M.; Sung, M.-J.; Kim, D.K.; Kim, H.K.; Oh, J.; Jeong, H.Y.; Lee, Y.H.; Yang, T.; et al. PINK1 deficiency impairs osteoblast differentiation through aberrant mitochondrial homeostasis. Stem Cell Res. Ther. 2021, 12, 589. [Google Scholar] [CrossRef]
- Henrich, M.T.; Oertel, W.H.; Surmeier, D.J.; Geibl, F.F. Mitochondrial dysfunction in Parkinson’s disease—A key disease hallmark with therapeutic potential. Mol. Neurodegener. 2023, 18, 83. [Google Scholar] [CrossRef]
- Sahasrabudhe, S.A.; Terluk, M.R.; Kartha, R.V. N-acetylcysteine Pharmacology and Applications in Rare Diseases—Repurposing an Old Antioxidant. Antioxidants 2023, 12, 1316. [Google Scholar] [CrossRef]
- Peggion, C.; Scalcon, V.; Massimino, M.L.; Nies, K.; Lopreiato, R.; Rigobello, M.P.; Bertoli, A. SOD1 in ALS: Taking Stock in Pathogenic Mechanisms and the Role of Glial and Muscle Cells. Antioxidants 2022, 11, 614. [Google Scholar] [CrossRef]
- Kim, B.W.; Jeong, Y.E.; Wong, M.; Martin, L.J. DNA damage accumulates and responses are engaged in human ALS brain and spinal motor neurons and DNA repair is activatable in iPSC-derived motor neurons with SOD1 mutations. Acta Neuropathol. Commun. 2020, 8, 7. [Google Scholar] [CrossRef]
- Nishimura, A.L.; Arias, N. Synaptopathy Mechanisms in ALS Caused by C9orf72 Repeat Expansion. Front. Cell. Neurosci. 2021, 15, 660693. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.-F.; Zhang, T.-Z.; Zhou, Y.-F.; Zhou, Q.-Q.; Gong, H.-B.; Liang, L.; Hai, L.-N.; You, N.-X.; Su, Y.; Chen, Y.-J.; et al. GPX4 deficiency-dependent phospholipid peroxidation drives motor deficits of ALS. J. Adv. Res. 2022, 43, 205–218. [Google Scholar] [CrossRef]
- Huang, M.; Liu, Y.U.; Yao, X.; Qin, D.; Su, H. Variability in SOD1-associated amyotrophic lateral sclerosis: Geographic patterns, clinical heterogeneity, molecular alterations, and therapeutic implications. Transl. Neurodegener. 2024, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, L.; Anakor, E.; Connolly, O.; Vijayakumar, U.G.; Duddy, W.J.; Duguez, S. Molecular and Cellular Mechanisms Affected in ALS. J. Pers. Med. 2020, 10, 101. [Google Scholar] [CrossRef]
- Schmitz, A.; Marques, J.P.; Oertig, I.; Maharjan, N.; Saxena, S. Emerging Perspectives on Dipeptide Repeat Proteins in C9ORF72 ALS/FTD. Front. Cell. Neurosci. 2021, 15, 637548. [Google Scholar] [CrossRef]
- Inigo, J.R.; Chandra, D. The mitochondrial unfolded protein response (UPRmt): Shielding against toxicity to mitochondria in cancer. J. Hematol. Oncol. 2022, 15, 98. [Google Scholar] [CrossRef]
- Balendra, R.; Sreedharan, J.; Hallegger, M.; Luisier, R.; A Lashuel, H.; Gregory, J.M.; Patani, R. Amyotrophic lateral sclerosis caused by TARDBP mutations: From genetics to TDP-43 proteinopathy. Lancet Neurol. 2025, 24, 456–470. [Google Scholar] [CrossRef] [PubMed]
- Verde, E.M.; Secco, V.; Ghezzi, A.; Mandrioli, J.; Carra, S. Molecular Mechanisms of Protein Aggregation in ALS-FTD: Focus on TDP-43 and Cellular Protective Responses. Cells 2025, 14, 680. [Google Scholar] [CrossRef]
- Chen, L.; Shen, Q.; Liu, Y.; Zhang, Y.; Sun, L.; Ma, X.; Song, N.; Xie, J. Homeostasis and metabolism of iron and other metal ions in neurodegenerative diseases. Signal Transduct. Target. Ther. 2025, 10, 31. [Google Scholar] [CrossRef]
- Wang, H.; Kodavati, M.; Britz, G.W.; Hegde, M.L. DNA Damage and Repair Deficiency in ALS/FTD-Associated Neurodegeneration: From Molecular Mechanisms to Therapeutic Implication. Front. Mol. Neurosci. 2021, 14, 784361. [Google Scholar] [CrossRef]
- Bond, S.; Saxena, S.; Sierra-Delgado, J.A. Microglia in ALS: Insights into Mechanisms and Therapeutic Potential. Cells 2025, 14, 421. [Google Scholar] [CrossRef]
- MohanKumar, S.M.J.; Murugan, A.; Palaniyappan, A.; MohanKumar, P.S. Role of Cytokines and Reactive oxygen species in Brain Aging. Mech. Ageing Dev. 2023, 214, 111855. [Google Scholar] [CrossRef]
- Motataianu, A.; Serban, G.; Barcutean, L.; Balasa, R. Oxidative Stress in Amyotrophic Lateral Sclerosis: Synergy of Genetic and Environmental Factors. Int. J. Mol. Sci. 2022, 23, 9339. [Google Scholar] [CrossRef]
- Cha, S.J.; Kim, K. Effects of the Edaravone, a Drug Approved for the Treatment of Amyotrophic Lateral Sclerosis, on Mitochondrial Function and Neuroprotection. Antioxidants 2022, 11, 195. [Google Scholar] [CrossRef]
- Márquez, B.T.; Leung, T.C.S.; Hui, J.; Charron, F.; McKinney, R.A.; Watt, A.J. A mitochondrial-targeted antioxidant (MitoQ) improves motor coordination and reduces Purkinje cell death in a mouse model of ARSACS. Neurobiol. Dis. 2023, 183, 106157. [Google Scholar] [CrossRef] [PubMed]
- Bernoud-Hubac, N.; Van, A.L.; Lazar, A.-N.; Lagarde, M. Ischemic Brain Injury: Involvement of Lipids in the Pathophysiology of Stroke and Therapeutic Strategies. Antioxidants 2024, 13, 634. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Candelario-Jalil, E. Emerging Neuroprotective Strategies for the Treatment of Ischemic Stroke: An Overview of Clinical and Preclinical Studies. Exp. Neurol. 2020, 335, 113518. [Google Scholar] [CrossRef] [PubMed]
- Thapak, P.; Gomez-Pinilla, F. The bioenergetics of traumatic brain injury and its long-term impact for brain plasticity and function. Pharmacol. Res. 2024, 208, 107389. [Google Scholar] [CrossRef]
- Chavda, V.; Chaurasia, B.; Garg, K.; Deora, H.; Umana, G.E.; Palmisciano, P.; Scalia, G.; Lu, B. Molecular mechanisms of oxidative stress in stroke and cancer. Brain Disord. 2022, 5, 100029. [Google Scholar] [CrossRef]
- Zhang, Q.-G.; Laird, M.D.; Han, D.; Nguyen, K.; Scott, E.; Dong, Y.; Dhandapani, K.M.; Brann, D.W.; Combs, C. Critical Role of NADPH Oxidase in Neuronal Oxidative Damage and Microglia Activation following Traumatic Brain Injury. PLoS ONE 2012, 7, e34504. [Google Scholar] [CrossRef]
- Marqués, J.; Zalba, G. Vascular NADPH Oxidases and Atherothrombotic Stroke. Stresses 2024, 4, 558–574. [Google Scholar] [CrossRef]
- Matsushima, S.; Sadoshima, J. Yin and Yang of NADPH Oxidases in Myocardial Ischemia-Reperfusion. Antioxidants 2022, 11, 1069. [Google Scholar] [CrossRef] [PubMed]
- Rahi, V.; Kaundal, R.K. Exploring the intricacies of calcium dysregulation in ischemic stroke: Insights into neuronal cell death and therapeutic strategies. Life Sci. 2024, 347, 122651. [Google Scholar] [CrossRef] [PubMed]
- Gielecińska, A.; Kciuk, M.; Kontek, R. The Impact of Calcium Overload on Cellular Processes: Exploring Calcicoptosis and Its Therapeutic Potential in Cancer. Int. J. Mol. Sci. 2024, 25, 13727. [Google Scholar] [CrossRef]
- Tugasworo, D.; Prasetyo, A.; Kurnianto, A.; Retnaningsih, R.; Andhitara, Y.; Ardhini, R.; Budiman, J. Malondialdehyde (MDA) and 8-hydroxy-2′-deoxyguanosine (8-OHdG) in ischemic stroke: A systematic review. Egypt. J. Neurol. Psychiatry Neurosurg. 2023, 59, 87. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, X.; Geng, Y.; Xiang, L.; Wu, Y.; Li, Y.; Yang, L.; Zhou, K. Exploring the role of parthanatos in CNS injury: Molecular insights and therapeutic approaches. J. Adv. Res. 2024, 70, 271–286. [Google Scholar] [CrossRef]
- Bezerra, F.S.; Lanzetti, M.; Nesi, R.T.; Nagato, A.C.; e Silva, C.P.; Kennedy-Feitosa, E.; Melo, A.C.; Cattani-Cavalieri, I.; Porto, L.C.; Valenca, S.S. Oxidative Stress and Inflammation in Acute and Chronic Lung Injuries. Antioxidants 2023, 12, 548. [Google Scholar] [CrossRef]
- Piacenza, L.; Zeida, A.; Trujillo, M.; Radi, R. The superoxide radical switch in the biology of nitric oxide and peroxynitrite. Physiol. Rev. 2022, 102, 1881–1906. [Google Scholar] [CrossRef]
- Skulachev, V.P.; Vyssokikh, M.Y.; Chernyak, B.V.; Averina, O.A.; Andreev-Andrievskiy, A.A.; Zinovkin, R.A.; Lyamzaev, K.G.; Marey, M.V.; Egorov, M.V.; Frolova, O.J.; et al. Mitochondrion-targeted antioxidant SkQ1 prevents rapid animal death caused by highly diverse shocks. Sci. Rep. 2023, 13, 4326. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Xiong, X.; Zhu, H.; Chen, R.; Zhang, S.; Chen, G.; Jian, Z. Nrf2 Regulates Oxidative Stress and Its Role in Cerebral Ischemic Stroke. Sci. Rep. 2023, 13, 4326. [Google Scholar] [CrossRef]
- Yuan, S.; Liu, K.J.; Qi, Z. Occludin regulation of blood–brain barrier and potential therapeutic target in ischemic stroke. Brain Circ. 2020, 6, 152–162. [Google Scholar] [CrossRef]
- Liu, G.; Liang, Y.; Xu, M.; Sun, M.; Sun, W.; Zhou, Y.; Huang, X.; Song, W.; Liang, Y.; Wang, Z. Protective mechanism of Erigeron breviscapus injection on blood–brain barrier injury induced by cerebral ischemia in rats. Sci. Rep. 2021, 11, 18451. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Song, Z.; Yu, S.; Piazza, A.; Nanda, A.; Penninger, J.M.; Granger, D.N.; Li, G. Phosphatidylinositol-3-Kinase Gamma Plays a Central Role in Blood–Brain Barrier Dysfunction in Acute Experimental Stroke. Stroke 2011, 42, 2033–2044. [Google Scholar] [CrossRef]
- Gao, H.; Chen, H.; Cui, G.-Y.; Hu, J.-X. Damage mechanism and therapy progress of the blood-brain barrier after ischemic stroke. Cell Biosci. 2023, 13, 196. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Chen, Z.; Wen, J. The role of RhoA/ROCK pathway in the ischemic stroke-induced neuroinflammation. Biomed. Pharmacother. 2023, 165, 115141. [Google Scholar] [CrossRef]
- Publik, M.A.; Filipoiu, F.M.; Dumitru, A.V.; Precup, A.; Petrescu, I.-A.; Slavu, I.; Tulin, R.F.; Tulin, A.; Baloiu, A.I.; Cirstoiu, M.M.; et al. An Extensive Study Regarding the Microscopic Anatomy of the Early Fetal Human Optic Nerve. Neurol. Int. 2024, 16, 470–482. [Google Scholar] [CrossRef]
- Kim, Y.; Cho, A.Y.; Kim, H.C.; Ryu, D.; Jo, S.A.; Jung, Y.-S. Effects of Natural Polyphenols on Oxidative Stress-Mediated Blood-Brain Barrier Dysfunction. Antioxidants 2022, 11, 197. [Google Scholar] [CrossRef]
- Robinson, B.D.; Isbell, C.L.; Melge, A.R.; Lomas, A.M.; Shaji, C.A.; Mohan, C.G.; Huang, J.H.; Tharakan, B. Doxycycline prevents blood–brain barrier dysfunction and microvascular hyperpermeability after traumatic brain injury. Sci. Rep. 2022, 12, 5415. [Google Scholar] [CrossRef] [PubMed]
- Kamma, E.; Lasisi, W.; Libner, C.; Ng, H.S.; Plemel, J.R. Central nervous system macrophages in progressive multiple sclerosis: Relationship to neurodegeneration and therapeutics. J. Neuroinflamm. 2022, 19, 45. [Google Scholar] [CrossRef]
- Goidescu, O.-C.; Enyedi, M.; Tulin, A.-D.; Tulin, R.; Vacaroiu, I.A.; Nica, A.E.; Dragos, D.; Ionescu, D.; Georgescu, D.; Miron, A.; et al. Overview of the anatomical basis of the piriformis syndrome-dissection with magnetic resonance correlation. Exp. Ther. Med. 2021, 23, 113. [Google Scholar] [CrossRef]
- Yong, V.W. Microglia in multiple sclerosis: Protectors turn destroyers. Neuron 2022, 110, 3534–3548. [Google Scholar] [CrossRef]
- Barnes-Vélez, J.A.; Yasar, F.B.A.; Hu, J. Myelin lipid metabolism and its role in myelination and myelin maintenance. Innovation 2022, 4, 100360. [Google Scholar] [CrossRef]
- Lei, Z.; Lin, W. Mechanisms Governing Oligodendrocyte Viability in Multiple Sclerosis and Its Animal Models. Cells 2024, 13, 116. [Google Scholar] [CrossRef]
- Blagov, A.V.; Sukhorukov, V.N.; Orekhov, A.N.; Sazonova, M.A.; Melnichenko, A.A. Significance of Mitochondrial Dysfunction in the Progression of Multiple Sclerosis. Int. J. Mol. Sci. 2022, 23, 12725. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, P.P.; Guevara, C.; Olesen, M.A.; Orellana, J.A.; Quintanilla, R.A.; Ortiz, F.C. Neurodegeneration in Multiple Sclerosis: The Role of Nrf2-Dependent Pathways. Antioxidants 2022, 11, 1146. [Google Scholar] [CrossRef] [PubMed]
- García-Revilla, J.; Alonso-Bellido, I.M.; Burguillos, M.A.; Herrera, A.J.; Espinosa-Oliva, A.M.; Ruiz, R.; Cruz-Hernández, L.; García-Domínguez, I.; Roca-Ceballos, M.A.; Santiago, M.; et al. Reformulating Pro-Oxidant Microglia in Neurodegeneration. J. Clin. Med. 2019, 8, 1719. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Silva, E.; Meuth, S.G.; Peixoto, C.A. The role of iron metabolism in the pathogenesis and treatment of multiple sclerosis. Front. Immunol. 2023, 14, 1137635. [Google Scholar] [CrossRef]
- Cheli, V.T.; Correale, J.; Paez, P.M.; Pasquini, J.M. Iron Metabolism in Oligodendrocytes and Astrocytes, Implications for Myelination and Remyelination. ASN Neuro 2020, 12, 1759091420962681. [Google Scholar] [CrossRef]
- Tonev, D.; Momchilova, A. Oxidative Stress and the Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2) Pathway in Multiple Sclerosis: Focus on Certain Exogenous and Endogenous Nrf2 Activators and Therapeutic Plasma Exchange Modulation. Int. J. Mol. Sci. 2023, 24, 17223. [Google Scholar] [CrossRef]
- Moraes, C.A.; Zaverucha-Do-Valle, C.; Fleurance, R.; Sharshar, T.; Bozza, F.A.; D’avila, J.C. Neuroinflammation in Sepsis: Molecular Pathways of Microglia Activation. Pharmaceuticals 2021, 14, 416. [Google Scholar] [CrossRef]
- Mohseni, G.K.; Hosseini, S.A.; Majdinasab, N.; Cheraghian, B. Effects of N-acetylcysteine on oxidative stress biomarkers, depression, and anxiety symptoms in patients with multiple sclerosis. Neuropsychopharmacol. Rep. 2023, 43, 382–390. [Google Scholar] [CrossRef]
- Ashok, A.; Andrabi, S.S.; Mansoor, S.; Kuang, Y.; Kwon, B.K.; Labhasetwar, V. Antioxidant Therapy in Oxidative Stress-Induced Neurodegenerative Diseases: Role of Nanoparticle-Based Drug Delivery Systems in Clinical Translation. Antioxidants 2022, 11, 408. [Google Scholar] [CrossRef]
- Navarro, E.; Esteras, N. Multitarget Effects of Nrf2 Signalling in the Brain: Common and Specific Functions in Different Cell Types. Antioxidants 2024, 13, 1502. [Google Scholar] [CrossRef]
- La Rosa, G.; Lonardo, M.S.; Cacciapuoti, N.; Muscariello, E.; Guida, B.; Faraonio, R.; Santillo, M.; Damiano, S. Dietary Polyphenols, Microbiome, and Multiple Sclerosis: From Molecular Anti-Inflammatory and Neuroprotective Mechanisms to Clinical Evidence. Int. J. Mol. Sci. 2023, 24, 7247. [Google Scholar] [CrossRef]
- Doğan, Ö.E. Investigation of the Effects of Curcumin and Resveratrol Supplements Added to the Mediterranean Diet on Disease Severity and Inflammatory Biomarkers in Patients with Ulcerative Colitis. ClinicalTrials.gov, Clinical Trial Registration NCT05761327. March 2024. Available online: https://clinicaltrials.gov/study/NCT05761327 (accessed on 11 February 2025).
- Kuźniar-Pałka, A. The Role of Oxidative Stress in Autism Spectrum Disorder Pathophysiology, Diagnosis and Treatment. Biomedicines 2025, 13, 388. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Zhao, Y.; Lan, X.; Zhang, Y.; Zhang, R. Prenatal, perinatal and parental risk factors for autism spectrum disorder in China: A case- control study. BMC Psychiatry 2024, 24, 219. [Google Scholar] [CrossRef] [PubMed]
- Toader, C.; Tataru, C.P.; Munteanu, O.; Covache-Busuioc, R.-A.; Serban, M.; Ciurea, A.V.; Enyedi, M. Revolutionizing Neuroimmunology: Unraveling Immune Dynamics and Therapeutic Innovations in CNS Disorders. Int. J. Mol. Sci. 2024, 25, 13614. [Google Scholar] [CrossRef] [PubMed]
- Zaky, E.A.; Elhameed, S.A.A.; Ismail, S.M.; Eldamer, N.M.; Abdelaziz, A.W. Analysis of urinary 8-hydroxy-2-deoxyguanosine as a biomarker of oxidative DNA damage in pediatric children with autism spectrum disorder. Res. Autism Spectr. Disord. 2023, 102, 102129. [Google Scholar] [CrossRef]
- Khaliulin, I.; Hamoudi, W.; Amal, H. The multifaceted role of mitochondria in autism spectrum disorder. Mol. Psychiatry 2024, 30, 629–650. [Google Scholar] [CrossRef]
- Rojas, V.; Carrasco-Gallardo, C.; Tenorio, L.; Olesen, M.A.; Tapia, V.; Carrasco, M.; Araos, P.; Quintanilla, R.A.; Ruiz, L.M. Analysis of mitochondrial DNA replisome in autism spectrum disorder: Exploring the role of replisome genes. Autism Res. 2025, 18, 933–953. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, H.; Rahman, M.S.; der Veen, C.H.J.M.-V.; Covarrubias, A.M.; Rafie, K.; Pendin, D.; Schmidt, M.; Dolga, A.M. Regulation of calcium signaling prevents neuronal death mediated by NIST DEP in xenoferroptotic cell death conditions. J. Hazard. Mater. 2025, 488, 137374. [Google Scholar] [CrossRef]
- Román, P.; Ruiz-González, C.; Rueda-Ruzafa, L.; Cardona, D.; Requena, M.; Alarcón, R. Exposure to Environmental Pesticides and the Risk of Autism Spectrum Disorders: A Population-Based Case-Control Study. Medicina 2024, 60, 479. [Google Scholar] [CrossRef]
- Toledano, J.M.; Puche-Juarez, M.; Moreno-Fernandez, J.; Gonzalez-Palacios, P.; Rivas, A.; Ochoa, J.J.; Diaz-Castro, J. Implications of Prenatal Exposure to Endocrine-Disrupting Chemicals in Offspring Development: A Narrative Review. Nutrients 2024, 16, 1556. [Google Scholar] [CrossRef]
- Parenti, M.; Slupsky, C.M. Disrupted Prenatal Metabolism May Explain the Etiology of Suboptimal Neurodevelopment: A Focus on Phthalates and Micronutrients and their Relationship to Autism Spectrum Disorder. Adv. Nutr. Int. Rev. J. 2024, 15, 100279. [Google Scholar] [CrossRef] [PubMed]
- Binjawhar, D.N.; Alhazmi, A.T.; Jawhar, W.N.B.; MohammedSaeed, W.; Safi, S.Z. Hyperglycemia-induced oxidative stress and epigenetic regulation of ET-1 gene in endothelial cells. Front. Genet. 2023, 14, 1167773. [Google Scholar] [CrossRef]
- Usui, N.; Kobayashi, H.; Shimada, S. Neuroinflammation and Oxidative Stress in the Pathogenesis of Autism Spectrum Disorder. Int. J. Mol. Sci. 2023, 24, 5487. [Google Scholar] [CrossRef]
- Zawadzka, A.; Cieślik, M.; Adamczyk, A. The Role of Maternal Immune Activation in the Pathogenesis of Autism: A Review of the Evidence, Proposed Mechanisms and Implications for Treatment. Int. J. Mol. Sci. 2021, 22, 11516. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-M.; Lee, K.-M.; Lee, C.-Y.; Lee, H.-C.; Tam, K.-W.; Loh, E.-W. Effectiveness of N-acetylcysteine in autism spectrum disorders: A meta-analysis of randomized controlled trials. Aust. N. Z. J. Psychiatry 2020, 55, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.G.; Sears, L.; Watson, W.H.; Gunaratnam, B.; Feygin, Y.; Wright, S.P.; Sullivan, J.E. Glutathione, Vitamin C, and Cysteine Use in Autistic Children With Disruptive Behavior: A Double-Blind, Placebo-Controlled Crossover Pilot Study. J. Dev. Behav. Pediatr. 2025, 46, e17–e24. [Google Scholar] [CrossRef]
- Pérez-Cabral, I.D.; Bernal-Mercado, A.T.; Islas-Rubio, A.R.; Suárez-Jiménez, G.M.; Robles-García, M.Á.; Puebla-Duarte, A.L.; Del-Toro-Sánchez, C.L. Exploring Dietary Interventions in Autism Spectrum Disorder. Foods 2024, 13, 3010. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Albero, M.; Mafla-España, M.A.; Martínez-Raga, J.; Cauli, O. Autistic Children/Adolescents Have Lower Adherence to the Mediterranean Diet and Higher Salivary IL-6 Concentration: Potential Diet–Inflammation Links? Pathophysiology 2024, 31, 376–387. [Google Scholar] [CrossRef]
- Folbergrová, J.; Ješina, P.; Otáhal, J. Protective Effect of Sulforaphane on Oxidative Stress and Mitochondrial Dysfunction Associated with Status Epilepticus in Immature Rats. Mol. Neurobiol. 2023, 60, 2024–2035. [Google Scholar] [CrossRef]
- University of North Carolina. Chapel Hill, A Phase II, Randomized, Double-Blind, Placebo-Controlled Study of Myrosinase-Enriched Glucoraphanin, a Sulforaphane Precursor System, in Autism Spectrum Disorder. ClinicalTrials.gov, Clinical Trial Registration NCT02909959. May 2020. Available online: https://clinicaltrials.gov/study/NCT02909959 (accessed on 11 February 2025).
- Csoka, A.B.; El Kouhen, N.; Bennani, S.; Getachew, B.; Aschner, M.; Tizabi, Y. Roles of Epigenetics and Glial Cells in Drug-Induced Autism Spectrum Disorder. Biomolecules 2024, 14, 437. [Google Scholar] [CrossRef]
- Zhang, S.; Duan, S.; Xie, Z.; Bao, W.; Xu, B.; Yang, W.; Zhou, L. Epigenetic Therapeutics Targeting NRF2/KEAP1 Signaling in Cancer Oxidative Stress. Front. Pharmacol. 2022, 13, 924817. [Google Scholar] [CrossRef]
- Toader, C.; Serban, M.; Munteanu, O.; Covache-Busuioc, R.-A.; Enyedi, M.; Ciurea, A.V.; Tataru, C.P. From Synaptic Plasticity to Neurodegeneration: BDNF as a Transformative Target in Medicine. Int. J. Mol. Sci. 2025, 26, 4271. [Google Scholar] [CrossRef]
- Suprunowicz, M.; Tomaszek, N.; Urbaniak, A.; Zackiewicz, K.; Modzelewski, S.; Waszkiewicz, N. Between Dysbiosis, Maternal Immune Activation and Autism: Is There a Common Pathway? Nutrients 2024, 16, 549. [Google Scholar] [CrossRef]
- Zeng, P.; Zhang, C.-Z.; Fan, Z.-X.; Yang, C.-J.; Cai, W.-Y.; Huang, Y.-F.; Xiang, Z.-J.; Wu, J.-Y.; Zhang, J.; Yang, J. Effect of probiotics on children with autism spectrum disorders: A meta-analysis. Ital. J. Pediatr. 2024, 50, 120. [Google Scholar] [CrossRef]
- Plascencia-Villa, G.; Perry, G. Roles of Oxidative Stress in Synaptic Dysfunction and Neuronal Cell Death in Alzheimer’s Disease. Antioxidants 2023, 12, 1628. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Kim, S.R. Linking Oxidative Stress and Proteinopathy in Alzheimer’s Disease. Antioxidants 2021, 10, 1231. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cai, Y.; He, J. High serum levels of 8-OHdG are an independent predictor of post-stroke depression in Chinese stroke survivors. Neuropsychiatr. Dis. Treat. 2018, 14, 587–596. [Google Scholar] [CrossRef]
- Cubillos, S.; Engmann, O.; Brancato, A. BDNF as a Mediator of Antidepressant Response: Recent Advances and Lifestyle Interactions. Int. J. Mol. Sci. 2022, 23, 14445. [Google Scholar] [CrossRef]
- Wu, A.; Zhang, J. Neuroinflammation, memory, and depression: New approaches to hippocampal neurogenesis. J. Neuroinflamm. 2023, 20, 283. [Google Scholar] [CrossRef]
- Gąsior, Ł.; Pochwat, B.; Zaręba-Kozioł, M.; Włodarczyk, J.; Grabrucker, A.M.; Szewczyk, B. Proteomics analysis in rats reveals convergent mechanisms between major depressive disorder and dietary zinc deficiency. Pharmacol. Rep. 2024, 77, 145–157. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Beltrán-Velasco, A.I.; Ramos-Campo, D.J.; Belinchón-Demiguel, P.; Martinez-Guardado, I.; Dalamitros, A.A.; Yáñez-Sepúlveda, R.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Mitochondria and Brain Disease: A Comprehensive Review of Pathological Mechanisms and Therapeutic Opportunities. Biomedicines 2023, 11, 2488. [Google Scholar] [CrossRef] [PubMed]
- Mikulska, J.; Juszczyk, G.; Gawrońska-Grzywacz, M.; Herbet, M. HPA Axis in the Pathomechanism of Depression and Schizophrenia: New Therapeutic Strategies Based on Its Participation. Brain Sci. 2021, 11, 1298. [Google Scholar] [CrossRef] [PubMed]
- Tenório, M.C.D.S.; Graciliano, N.G.; Moura, F.A.; de Oliveira, A.C.M.; Goulart, M.O.F. N-Acetylcysteine (NAC): Impacts on Human Health. Antioxidants 2021, 10, 967. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Ali, T.; Li, A.; Gao, R.; Yu, X.; Li, S.; Li, T. Ketamine reverses chronic corticosterone-induced behavioral deficits and hippocampal synaptic dysfunction by regulating eIF4E/BDNF signaling. Neuropharmacology 2024, 261, 110156. [Google Scholar] [CrossRef]
- Więdłocha, M.; Zborowska, N.; Marcinowicz, P.; Dębowska, W.; Dębowska, M.; Zalewska, A.; Maciejczyk, M.; Waszkiewicz, N.; Szulc, A. Oxidative Stress Biomarkers among Schizophrenia Inpatients. Brain Sci. 2023, 13, 490. [Google Scholar] [CrossRef]
- Cuenod, M.; Steullet, P.; Cabungcal, J.-H.; Dwir, D.; Khadimallah, I.; Klauser, P.; Conus, P.; Do, K.Q. Caught in vicious circles: A perspective on dynamic feed-forward loops driving oxidative stress in schizophrenia. Mol. Psychiatry 2021, 27, 1886–1897. [Google Scholar] [CrossRef]
- Bassil, K.; Krontira, A.C.; Leroy, T.; Escoto, A.I.; Snijders, C.; Pernia, C.D.; Pasterkamp, R.J.; de Nijs, L.; Hove, D.v.D.; Kenis, G.; et al. In vitro modeling of the neurobiological effects of glucocorticoids: A review. Neurobiol. Stress 2023, 23, 100530. [Google Scholar] [CrossRef]
- Pardo, C.C.; Merino, K.O.; Vera-Montecinos, A. Neuroinflammatory Loop in Schizophrenia, Is There a Relationship with Symptoms or Cognition Decline? Int. J. Mol. Sci. 2025, 26, 310. [Google Scholar] [CrossRef]
- Sedky, A.A.; Raafat, M.H.; Hamam, G.G.; Sedky, K.A.; Magdy, Y. Effects of tamoxifen alone and in combination with risperidone on hyperlocomotion, hippocampal structure and bone in ketamine-induced model of psychosis in rats. Egypt. J. Neurol. Psychiatry Neurosurg. 2022, 58, 44. [Google Scholar] [CrossRef]
- Jang, Y.-J.; Kim, M.-J.; Lee, S.-J.; Lim, S.-W.; Kim, D.-K. Oxidative Stress and Risk of Dementia in Older Patients with Depression: A Longitudinal Cohort Study Using Plasma Biomarkers. Medicina 2025, 61, 108. [Google Scholar] [CrossRef]
- Bodenstein, D.F.; Kim, H.K.; Brown, N.C.; Navaid, B.; Young, L.T.; Andreazza, A.C. Mitochondrial DNA content and oxidation in bipolar disorder and its role across brain regions. Schizophrenia 2019, 5, 21. [Google Scholar] [CrossRef]
- Solmi, M.; Sharma, M.S.; Osimo, E.F.; Fornaro, M.; Bortolato, B.; Croatto, G.; Miola, A.; Vieta, E.; Pariante, C.M.; Smith, L.; et al. Peripheral levels of C-reactive protein, tumor necrosis factor-α, interleukin-6, and interleukin-1β across the mood spectrum in bipolar disorder: A meta-analysis of mean differences and variability. Brain Behav. Immun. 2021, 97, 193–203. [Google Scholar] [CrossRef]
- Hidalgo-Gutiérrez, A.; González-García, P.; Díaz-Casado, M.E.; Barriocanal-Casado, E.; López-Herrador, S.; Quinzii, C.M.; López, L.C. Metabolic Targets of Coenzyme Q10 in Mitochondria. Antioxidants 2021, 10, 520. [Google Scholar] [CrossRef] [PubMed]
- Mas-Bargues, C.; García-Domínguez, E.; Borrás, C. Recent Approaches to Determine Static and Dynamic Redox State-Related Parameters. Antioxidants 2022, 11, 864. [Google Scholar] [CrossRef] [PubMed]
- Bailo, P.S.; Martín, E.L.; Calmarza, P.; Breva, S.M.; Gómez, A.B.; Giráldez, A.P.; Callau, J.J.S.-P.; Santamaría, J.M.V.; Khialani, A.D.; Micó, C.C.; et al. The role of oxidative stress in neurodegenerative diseases and potential antioxidant therapies. Adv. Lab. Med. 2022, 3, 342–360. [Google Scholar] [CrossRef]
- Khan, M.Z.; Khan, A.; Chen, W.; Chai, W.; Wang, C. Advancements in Genetic Biomarkers and Exogenous Antioxidant Supplementation for Safeguarding Mammalian Cells against Heat-Induced Oxidative Stress and Apoptosis. Antioxidants 2024, 13, 258. [Google Scholar] [CrossRef]
- Peña-Bautista, C.; Álvarez-Sánchez, L.; Ferrer, I.; López-Nogueroles, M.; Cañada-Martínez, A.J.; Oger, C.; Galano, J.-M.; Durand, T.; Baquero, M.; Cháfer-Pericás, C. Lipid Peroxidation Assessment in Preclinical Alzheimer Disease Diagnosis. Antioxidants 2021, 10, 1043. [Google Scholar] [CrossRef]
- Milkovic, L.; Zarkovic, N.; Marusic, Z.; Zarkovic, K.; Jaganjac, M. The 4-Hydroxynonenal–Protein Adducts and Their Biological Relevance: Are Some Proteins Preferred Targets? Antioxidants 2023, 12, 856. [Google Scholar] [CrossRef]
- Maier, S.; Barcutean, L.; Andone, S.; Manu, D.; Sarmasan, E.; Bajko, Z.; Balasa, R. Recent Progress in the Identification of Early Transition Biomarkers from Relapsing-Remitting to Progressive Multiple Sclerosis. Int. J. Mol. Sci. 2023, 24, 4375. [Google Scholar] [CrossRef] [PubMed]
- Cadenas-Garrido, P.; Schonvandt-Alarcos, A.; Herrera-Quintana, L.; Vázquez-Lorente, H.; Santamaría-Quiles, A.; de Francisco, J.R.; Moya-Escudero, M.; Martín-Oliva, D.; Martín-Guerrero, S.M.; Rodríguez-Santana, C.; et al. Using Redox Proteomics to Gain New Insights into Neurodegenerative Disease and Protein Modification. Antioxidants 2024, 13, 127. [Google Scholar] [CrossRef]
- Goriuc, A.; Cojocaru, K.-A.; Luchian, I.; Ursu, R.-G.; Butnaru, O.; Foia, L. Using 8-Hydroxy-2′-Deoxiguanosine (8-OHdG) as a Reliable Biomarker for Assessing Periodontal Disease Associated with Diabetes. Int. J. Mol. Sci. 2024, 25, 1425. [Google Scholar] [CrossRef]
- Obrador, E.; Salvador-Palmer, R.; López-Blanch, R.; Jihad-Jebbar, A.; Vallés, S.L.; Estrela, J.M. The Link between Oxidative Stress, Redox Status, Bioenergetics and Mitochondria in the Pathophysiology of ALS. Int. J. Mol. Sci. 2021, 22, 6352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, Q.; Zhao, H.; Shu, M.; Luo, M.; Li, Y.; Ding, Y.; Shi, S.; Cheng, X.; Niu, Q. Neurodegenerative disease and antioxidant biomarkers: A bidirectional Mendelian randomization study. Front. Neurol. 2023, 14, 1158366. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Alrumaihi, F.; Sarwar, T.; Babiker, A.Y.; Khan, A.A.; Prabhu, S.V.; Rahmani, A.H. Exploring Therapeutic Potential of Catalase: Strategies in Disease Prevention and Management. Biomolecules 2024, 14, 697. [Google Scholar] [CrossRef]
- Feng, J.; Zheng, Y.; Guo, M.; Ares, I.; Lopez-Torres, B.; Martínez-Larrañaga, M.-R.; Wang, X.; Anadón, A.; Martínez, M.-A. Oxidative stress, the blood–brain barrier and neurodegenerative diseases: The critical beneficial role of dietary antioxidants. Acta Pharm. Sin. B 2023, 13, 3988–4024. [Google Scholar] [CrossRef]
- Shukla, D.; Goel, A.; Mandal, P.K.; Joon, S.; Punjabi, K.; Arora, Y.; Kumar, R.; Mehta, V.S.; Singh, P.; Maroon, J.C.; et al. Glutathione Depletion and Concomitant Elevation of Susceptibility in Patients with Parkinson’s Disease: State-of-the-Art MR Spectroscopy and Neuropsychological Study. ACS Chem. Neurosci. 2023, 14, 4383–4394. [Google Scholar] [CrossRef]
- Aramouni, K.; Assaf, R.; Shaito, A.; Fardoun, M.; Al-Asmakh, M.; Sahebkar, A.; Eid, A.H. Biochemical and cellular basis of oxidative stress: Implications for disease onset. J. Cell. Physiol. 2023, 238, 1951–1963. [Google Scholar] [CrossRef]
- Fanlo-Ucar, H.; Picón-Pagès, P.; Herrera-Fernández, V.; ILL-Raga, G.; Muñoz, F.J. The Dual Role of Amyloid Beta-Peptide in Oxidative Stress and Inflammation: Unveiling Their Connections in Alzheimer’s Disease Etiopathology. Antioxidants 2024, 13, 1208. [Google Scholar] [CrossRef]
- Sefati, N.; Esmaeilpour, T.; Salari, V.; Zarifkar, A.; Dehghani, F.; Ghaffari, M.K.; Zadeh-Haghighi, H.; Császár, N.; Bókkon, I.; Rodrigues, S.; et al. Monitoring Alzheimer’s disease via ultraweak photon emission. iScience 2023, 27, 108744. [Google Scholar] [CrossRef]
- Hollen, C.; Neilson, L.E.; Barajas, R.F.; Greenhouse, I.; Spain, R.I. Oxidative stress in multiple sclerosis—Emerging imaging techniques. Front. Neurol. 2023, 13, 1025659. [Google Scholar] [CrossRef]
- Prasuhn, J.; Kunert, L.; Brüggemann, N. Neuroimaging Methods to Map In Vivo Changes of OXPHOS and Oxidative Stress in Neurodegenerative Disorders. Int. J. Mol. Sci. 2022, 23, 7263. [Google Scholar] [CrossRef]
- Lowe, A.J.; Rodrigues, F.B.; Arridge, M.; De Vita, E.; Johnson, E.B.; I Scahill, R.; Byrne, L.M.; Tortelli, R.; Heslegrave, A.; Zetterberg, H.; et al. Longitudinal evaluation of proton magnetic resonance spectroscopy metabolites as biomarkers in Huntington’s disease. Brain Commun. 2022, 4, fcac258. [Google Scholar] [CrossRef]
- Carletti, B.; Banaj, N.; Piras, F.; Bossù, P. Schizophrenia and Glutathione: A Challenging Story. J. Pers. Med. 2023, 13, 1526. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Dijkstra, J.M.; Nagatsu, T. Parkinson’s Disease: Cells Succumbing to Lifelong Dopamine-Related Oxidative Stress and Other Bioenergetic Challenges. Int. J. Mol. Sci. 2024, 25, 2009. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, N.; Sousa, A.; Amaral, A.P.; Graça, G.; Verde, I. Searching for Metabolic Markers of Stroke in Human Plasma via NMR Analysis. Int. J. Mol. Sci. 2023, 24, 16173. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Wang, Y.; Qiu, T.; Zhou, Y.; Zhang, Y.; Hu, L.; Zhang, L.; Liang, J.; Ding, M.; Fan, S.; et al. SS-31 alleviated nociceptive responses and restored mitochondrial function in a headache mouse model via Sirt3/Pgc-1α positive feedback loop. J. Headache Pain 2023, 24, 65. [Google Scholar] [CrossRef]
- McCunn, P.; Richardson, J.D.; Jetly, R.; Dunkley, B. Diffusion Tensor Imaging Reveals White Matter Differences in Military Personnel Exposed to Trauma with and without Post-traumatic Stress Disorder. Psychiatry Res. 2021, 298, 113797. [Google Scholar] [CrossRef] [PubMed]
- Steardo, L.; D’Angelo, M.; Monaco, F.; Di Stefano, V.; Steardo, L. Decoding neural circuit dysregulation in bipolar disorder: Toward an advanced paradigm for multidimensional cognitive, emotional, and psychomotor treatment. Neurosci. Biobehav. Rev. 2025, 169, 106030. [Google Scholar] [CrossRef]
- Alshehri, A.; Al-Iedani, O.; Arm, J.; Gholizadeh, N.; Billiet, T.; Lea, R.; Lechner-Scott, J.; Ramadan, S. Neural diffusion tensor imaging metrics correlate with clinical measures in people with relapsing-remitting MS. Neuroradiol. J. 2022, 35, 592–599. [Google Scholar] [CrossRef]
- Arya, R.; Haque, A.K.M.A.; Shakya, H.; Billah, M.; Parvin, A.; Rahman, M.-M.; Sakib, K.M.; Faruquee, H.M.; Kumar, V.; Kim, J.-J. Parkinson’s Disease: Biomarkers for Diagnosis and Disease Progression. Int. J. Mol. Sci. 2024, 25, 12379. [Google Scholar] [CrossRef]
- Menger, K.E.; Logan, A.; Luhmann, U.F.; Smith, A.J.; Wright, A.F.; Ali, R.R.; Murphy, M.P. In vivo measurement of mitochondrial ROS production in mouse models of photoreceptor degeneration. Redox Biochem. Chem. 2023, 5–6, 100007. [Google Scholar] [CrossRef]
- Zhu, Y.; Kohli, N.; Young, A.; Sheldon, M.; Coni, J.; Rajasekaran, M.; Robinson, L.; Chroneos, R.; Riley, S.; Guarnieri, J.W.; et al. PET Imaging with [18F]ROStrace Detects Oxidative Stress and Predicts Parkinson’s Disease Progression in Mice. Antioxidants 2024, 13, 1226. [Google Scholar] [CrossRef]
- Gallagher, E.; Hou, C.; Zhu, Y.; Hsieh, C.-J.; Lee, H.; Li, S.; Xu, K.; Henderson, P.; Chroneos, R.; Sheldon, M.; et al. Positron Emission Tomography with [18F]ROStrace Reveals Progressive Elevations in Oxidative Stress in a Mouse Model of Alpha-Synucleinopathy. Int. J. Mol. Sci. 2024, 25, 4943. [Google Scholar] [CrossRef]
- Tondo, G.; Iaccarino, L.; Cerami, C.; Vanoli, G.E.; Presotto, L.; Masiello, V.; Coliva, A.; Salvi, F.; Bartolomei, I.; Mosca, L.; et al. 11 C-PK11195 PET-based molecular study of microglia activation in SOD1 amyotrophic lateral sclerosis. Ann. Clin. Transl. Neurol. 2020, 7, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Chauveau, F.; Becker, G.; Boutin, H. Have (R)-[11C]PK11195 challengers fulfilled the promise? A scoping review of clinical TSPO PET studies. Eur. J. Nucl. Med. 2021, 49, 201–220. [Google Scholar] [CrossRef] [PubMed]
- Roldán, M.; Kyriacou, P.A. Near-Infrared Spectroscopy (NIRS) in Traumatic Brain Injury (TBI). Oxygen 2023, 3, 222–255. [Google Scholar] [CrossRef]
- Jávega, B.; Herrera, G.; Martínez-Romero, A.; O’Connor, J.-E. Flow Cytometry of Oxygen and Oxygen-Related Cellular Stress. Oxygen 2023, 3, 222–255. [Google Scholar] [CrossRef]
- Rogov, A.G.; Goleva, T.N.; Epremyan, K.K.; Kireev, I.I.; Zvyagilskaya, R.A. Propagation of Mitochondria-Derived Reactive Oxygen Species within the Dipodascus magnusii Cells. Antioxidants 2021, 10, 120. [Google Scholar] [CrossRef]
- Rebelos, E.; Malloggi, E.; Parenti, M.; Dardano, A.; Tura, A.; Daniele, G. Near-Infrared Spectroscopy: A Free-Living Neuroscience Tool to Better Understand Diabetes and Obesity. Metabolites 2023, 13, 814. [Google Scholar] [CrossRef]
- Teniou, A.; Madi, I.A.; Mouhoub, R.; Marty, J.L.; Rhouati, A. One-Step Chemiluminescent Assay for Hydrogen Peroxide Analysis in Water. Chemosensors 2023, 11, 455. [Google Scholar] [CrossRef]
- Kano, R.; Shirakawa, H.; Poole, D.C.; Hoshino, D.; Kano, Y. State of the art in vivo reactive oxygen species measurements in skeletal muscle using fluorescent proteins. Redox Biochem. Chem. 2025, 13, 100056. [Google Scholar] [CrossRef]
- Rajlic, S.; Treede, H.; Münzel, T.; Daiber, A.; Duerr, G.D. Early Detection Is the Best Prevention—Characterization of Oxidative Stress in Diabetes Mellitus and Its Consequences on the Cardiovascular System. Cells 2023, 12, 583. [Google Scholar] [CrossRef]
- Kalinovic, S.; Oelze, M.; Kröller-Schön, S.; Steven, S.; Vujacic-Mirski, K.; Kvandová, M.; Schmal, I.; Al Zuabi, A.; Münzel, T.; Daiber, A. Comparison of Mitochondrial Superoxide Detection Ex Vivo/In Vivo by mitoSOX HPLC Method with Classical Assays in Three Different Animal Models of Oxidative Stress. Antioxidants 2019, 8, 514. [Google Scholar] [CrossRef]
- Perez, R.C.; Kim, D.; Maxwell, A.W.P.; Camacho, J.C. Functional Imaging of Hypoxia: PET and MRI. Cancers 2023, 15, 3336. [Google Scholar] [CrossRef]
- Stefani, A.; Antelmi, E.; Arnaldi, D.; Arnulf, I.; During, E.; Högl, B.; Hu, M.M.T.; Iranzo, A.; Luke, R.; Peever, J.; et al. From mechanisms to future therapy: A synopsis of isolated REM sleep behavior disorder as early synuclein-related disease. Mol. Neurodegener. 2025, 20, 19. [Google Scholar] [CrossRef] [PubMed]
- Patterson, A.P.; Booth, S.A.; Saba, R. The Emerging Use of In Vivo Optical Imaging in the Study of Neurodegenerative Diseases. BioMed Res. Int. 2014, 2014, 401306. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Morgenstern, C.; Lastres-Becker, I.; Demirdöğen, B.C.; Costa, V.M.; Daiber, A.; Foresti, R.; Motterlini, R.; Kalyoncu, S.; Arioz, B.I.; Genc, S.; et al. Biomarkers of NRF2 signalling: Current status and future challenges. Redox Biol. 2024, 72, 103134. [Google Scholar] [CrossRef]
- Muhie, S.; Gautam, A.; Misganaw, B.; Yang, R.; Mellon, S.H.; Hoke, A.; Flory, J.; Daigle, B.; Swift, K.; Hood, L.; et al. Integrated analysis of proteomics, epigenomics and metabolomics data revealed divergent pathway activation patterns in the recent versus chronic post-traumatic stress disorder. Brain Behav. Immun. 2023, 113, 303–316. [Google Scholar] [CrossRef]
- Munteanu, C.; Galaction, A.I.; Turnea, M.; Blendea, C.D.; Rotariu, M.; Poștaru, M. Redox Homeostasis, Gut Microbiota, and Epigenetics in Neurodegenerative Diseases: A Systematic Review. Antioxidants 2024, 13, 1062. [Google Scholar] [CrossRef]
- Tak, Y.J.; Park, J.-H.; Rhim, H.; Kang, S. ALS-Related Mutant SOD1 Aggregates Interfere with Mitophagy by Sequestering the Autophagy Receptor Optineurin. Int. J. Mol. Sci. 2020, 21, 7525. [Google Scholar] [CrossRef]
- Karahalil, B.; Salihoğlu, E.M.; Elkama, A.; Orhan, G.; Saygın, E.; Akaydin, S.Y. Individual susceptibility has a major impact on strong association between oxidative stress, defence systems and Parkinson’s disease. Basic Clin. Pharmacol. Toxicol. 2022, 130, 158–170. [Google Scholar] [CrossRef]
- Klupczyńska, E.A.; Dietz, K.-J.; Małecka, A.; Ratajczak, E. Mitochondrial Peroxiredoxin-IIF (PRXIIF) Activity and Function during Seed Aging. Antioxidants 2022, 11, 1226. [Google Scholar] [CrossRef]
- Santana, D.A.; Bedrat, A.; Puga, R.D.; Turecki, G.; Mechawar, N.; Faria, T.C.; O Gigek, C.; Payão, S.L.; Smith, M.A.; Lemos, B.; et al. The role of H3K9 acetylation and gene expression in different brain regions of Alzheimer’s disease patients. Epigenomics 2022, 14, 651–670. [Google Scholar] [CrossRef]
- Prasanth, M.I.; Sivamaruthi, B.S.; Cheong, C.S.Y.; Verma, K.; Tencomnao, T.; Brimson, J.M.; Prasansuklab, A. Role of Epigenetic Modulation in Neurodegenerative Diseases: Implications of Phytochemical Interventions. Antioxidants 2024, 13, 606. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, E.; Chandrasekhar, G.; Chandrasekar, P.; Anbarasu, K.; Vickram, A.S.; Karunakaran, R.; Rajasekaran, R.; Srikumar, P.S. Alpha-Synuclein Aggregation in Parkinson’s Disease. Front. Med. 2021, 8, 736978. [Google Scholar] [CrossRef] [PubMed]
- Saramowicz, K.; Siwecka, N.; Galita, G.; Kucharska-Lusina, A.; Rozpędek-Kamińska, W.; Majsterek, I. Alpha-Synuclein Contribution to Neuronal and Glial Damage in Parkinson’s Disease. Int. J. Mol. Sci. 2023, 25, 360. [Google Scholar] [CrossRef] [PubMed]
- Bandookwala, M.; Sengupta, P. 3-Nitrotyrosine: A versatile oxidative stress biomarker for major neurodegenerative diseases. Int. J. Neurosci. 2020, 130, 1047–1062. [Google Scholar] [CrossRef] [PubMed]
- Ebanks, B.; Chakrabarti, L. Mitochondrial ATP Synthase is a Target of Oxidative Stress in Neurodegenerative Diseases. Front. Mol. Biosci. 2022, 9, 854321. [Google Scholar] [CrossRef]
- Duarte-Jurado, A.P.; de J, M.; Saucedo-Cardenas, O.; de Oca-Luna, R.M.; Rodriguez-Rocha, H.; Garcia-Garcia, A. Peroxiredoxin 5 overexpression decreases oxidative stress and dopaminergic cell death mediated by paraquat. Cells Dev. 2023, 175, 203860. [Google Scholar] [CrossRef]
- Rovnyagina, N.R.; Budylin, G.S.; Vainer, Y.G.; Tikhonova, T.N.; Vasin, S.L.; Yakovlev, A.A.; Kompanets, V.O.; Chekalin, S.V.; Priezzhev, A.V.; Shirshin, E.A. Fluorescence Lifetime and Intensity of Thioflavin T as Reporters of Different Fibrillation Stages: Insights Obtained from Fluorescence Up-Conversion and Particle Size Distribution Measurements. Int. J. Mol. Sci. 2020, 21, 6169. [Google Scholar] [CrossRef]
- Basha, S.; Mukunda, D.C.; Pai, A.R.; Mahato, K.K. Assessing amyloid fibrils and amorphous aggregates: A review. Int. J. Biol. Macromol. 2025, 311, 143725. [Google Scholar] [CrossRef]
- Šunjić, S.B.; Jaganjac, M.; Vlainić, J.; Halasz, M.; Žarković, N. Lipid Peroxidation-Related Redox Signaling in Osteosarcoma. Int. J. Mol. Sci. 2024, 25, 4559. [Google Scholar] [CrossRef] [PubMed]
- Lorenzano, S.; Rost, N.S.; Khan, M.; Li, H.; Lima, F.O.; Maas, M.B.; Green, R.E.; Thankachan, T.K.; Dipietro, A.J.; Arai, K.; et al. Oxidative Stress Biomarkers of Brain Damage: Hyperacute Plasma F2-Isoprostane Predicts Infarct Growth in Stroke. Stroke 2018, 49, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Yang, W.; Orellana, A.; Frölich, L.; de Rojas, I.; Cano, A.; Boada, M.; Hernández, I.; Hausner, L.; Harms, A.C.; et al. Association of oxidative stress and inflammatory metabolites with Alzheimer’s disease cerebrospinal fluid biomarkers in mild cognitive impairment. Alzheimer’s Res. Ther. 2024, 16, 171. [Google Scholar] [CrossRef]
- El Kodsi, D.N.; Tokarew, J.M.; Sengupta, R.; Lengacher, N.A.; Chatterji, A.; Nguyen, A.P.; Boston, H.; Jiang, Q.; Palmberg, C.; Pileggi, C.; et al. Parkin coregulates glutathione metabolism in adult mammalian brain. Acta Neuropathol. Commun. 2023, 11, 19. [Google Scholar] [CrossRef]
- Pei, X.; Huang, D.; Li, Z. Genetic insights and emerging therapeutics in diabetic retinopathy: From molecular pathways to personalized medicine. Front. Genet. 2024, 15, 1416924. [Google Scholar] [CrossRef]
- Brackhan, M.; Arribas-Blazquez, M.; Lastres-Becker, I. Aging, NRF2, and TAU: A Perfect Match for Neurodegeneration? Antioxidants 2023, 12, 1564. [Google Scholar] [CrossRef] [PubMed]
- Kołodziej-Sobczak, D.; Sobczak, Ł.; Łączkowski, K.Z. Protein Tyrosine Phosphatase 1B (PTP1B): A Comprehensive Review of Its Role in Pathogenesis of Human Diseases. Int. J. Mol. Sci. 2024, 25, 7033. [Google Scholar] [CrossRef]
- Jain, M.; Singh, M.K.; Shyam, H.; Mishra, A.; Kumar, S.; Kumar, A.; Kushwaha, J. Role of JAK/STAT in the Neuroinflammation and its Association with Neurological Disorders. Ann. Neurosci. 2021, 28, 191–200. [Google Scholar] [CrossRef]
- Morén, C.; deSouza, R.M.; Giraldo, D.M.; Uff, C. Antioxidant Therapeutic Strategies in Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 9328. [Google Scholar] [CrossRef]
- University of Minnesota. Repeated-Dose Oral N-Acetylcysteine for the Treatment of Parkinson’s Disease. ClinicalTrials.gov, Clinical Trial Registration NCT02212678. April 2019. Available online: https://clinicaltrials.gov/study/NCT02212678 (accessed on 12 February 2025).
- Khanra, S.; Reddy, P.; Giménez-Palomo, A.; Park, C.H.J.; Panizzutti, B.; McCallum, M.; Arumugham, S.S.; Umesh, S.; Debnath, M.; Das, B.; et al. Metabolic regulation to treat bipolar depression: Mechanisms and targeting by trimetazidine. Mol. Psychiatry 2023, 28, 3231–3242. [Google Scholar] [CrossRef]
- Germano, B.C.d.C.; de Morais, L.C.C.; Neta, F.I.; Fernandes, A.C.L.; Pinheiro, F.I.; Rego, A.C.M.D.; Filho, I.A.; de Azevedo, E.P.; Cavalcanti, J.R.L.d.P.; Guzen, F.P.; et al. Vitamin E and Its Molecular Effects in Experimental Models of Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 11191. [Google Scholar] [CrossRef] [PubMed]
- Tippairote, T.; Bjørklund, G.; Gasmi, A.; Semenova, Y.; Peana, M.; Chirumbolo, S.; Hangan, T. Combined Supplementation of Coenzyme Q10 and Other Nutrients in Specific Medical Conditions. Nutrients 2022, 14, 4383. [Google Scholar] [CrossRef] [PubMed]
- Fišar, Z.; Hroudová, J. CoQ10 and Mitochondrial Dysfunction in Alzheimer’s Disease. Antioxidants 2024, 13, 191. [Google Scholar] [CrossRef]
- Mayer, C.; Riera-Ponsati, L.; Kauppinen, S.; Klitgaard, H.; Erler, J.T.; Hansen, S.N. Targeting the NRF2 pathway for disease modification in neurodegenerative diseases: Mechanisms and therapeutic implications. Front. Pharmacol. 2024, 15, 1437939. [Google Scholar] [CrossRef]
- Sánchez-Sanz, A.; Coronado-Albi, M.J.; Muñoz-Viana, R.; García-Merino, A.; Sánchez-López, A.J. Neuroprotective and Anti-Inflammatory Effects of Dimethyl Fumarate, Monomethyl Fumarate, and Cannabidiol in Neurons and Microglia. Int. J. Mol. Sci. 2024, 25, 13082. [Google Scholar] [CrossRef]
- Biogen. A Multicenter, Postmarketing Study of Dimethyl Fumarate (Tecfidera; BG00012) in Relapsing Forms of Multiple Sclerosis (RMS) Participants in China. ClinicalTrials.gov, Clinical Trial Registration NCT05658484. September 2024. Available online: https://clinicaltrials.gov/study/NCT05658484 (accessed on 12 February 2025).
- Baba, M.Z.; Gomathy, S.; Wahedi, U. Role of Nrf2 Pathway Activation in Neurological Disorder: A Brief Review. J. Pharmacol. Pharmacother. 2022, 13, 229–238. [Google Scholar] [CrossRef]
- Magner, M.; Thorová, K.; Župová, V.; Houška, M.; Švandová, I.; Novotná, P.; Tříska, J.; Vrchotová, N.; Soural, I.; Jílek, L. Sulforaphane Treatment in Children with Autism: A Prospective Randomized Double-Blind Study. Nutrients 2023, 15, 718. [Google Scholar] [CrossRef] [PubMed]
- Bono, S.; Feligioni, M.; Corbo, M. Impaired antioxidant KEAP1-NRF2 system in amyotrophic lateral sclerosis: NRF2 activation as a potential therapeutic strategy. Mol. Neurodegener. 2021, 16, 71. [Google Scholar] [CrossRef]
- Hibino, M.; Maeki, M.; Tokeshi, M.; Ishitsuka, Y.; Harashima, H.; Yamada, Y. A system that delivers an antioxidant to mitochondria for the treatment of drug-induced liver injury. Sci. Rep. 2023, 13, 6961. [Google Scholar] [CrossRef]
- Suárez-Rivero, J.M.; Pastor-Maldonado, C.J.; Povea-Cabello, S.; Álvarez-Córdoba, M.; Villalón-García, I.; Munuera-Cabeza, M.; Suárez-Carrillo, A.; Talaverón-Rey, M.; Sánchez-Alcázar, J.A. Coenzyme Q10 Analogues: Benefits and Challenges for Therapeutics. Antioxidants 2021, 10, 236. [Google Scholar] [CrossRef] [PubMed]
- Medicine. Effects of High-dose Coenzyme Q10 on Biomarkers of Oxidative Damage and Clinical Outcome in Parkinson Disease. ClinicalTrials.gov, Clinical Trial Registration NCT01892176. July 2013. Available online: https://clinicaltrials.gov/study/NCT01892176 (accessed on 12 February 2025).
- Yip, J.M.X.; Chiang, G.S.H.; Lee, I.C.J.; Lehming-Teo, R.; Dai, K.; Dongol, L.; Wang, L.Y.-T.; Teo, D.; Seah, G.T.; Lehming, N. Mitochondria and the Repurposing of Diabetes Drugs for Off-Label Health Benefits. Int. J. Mol. Sci. 2025, 26, 364. [Google Scholar] [CrossRef]
- Lynch, D. A Pilot Investigator Initiated Study to Evaluate the Safety, Tolerability and Efficacy of Elamipretide in the Treatment of Advanced Symptoms of Friedreich Ataxia (FRDA). ClinicalTrials.gov, Clinical Trial Registration NCT05168774. August 2024. Available online: https://clinicaltrials.gov/study/NCT05168774 (accessed on 1 February 2025).
- Liu, R.; Zhao, E.; Yu, H.; Yuan, C.; Abbas, M.N.; Cui, H. Methylation across the central dogma in health and diseases: New therapeutic strategies. Signal Transduct. Target. Ther. 2023, 8, 310. [Google Scholar] [CrossRef]
- Nojadeh, J.N.; Eryilmaz, N.S.B.; Ergüder, B.I. CRISPR/Cas9 genome editing for neurodegenerative diseases. EXCLI J. 2023, 22, 567–582. [Google Scholar] [CrossRef]
- Zhang, L.-Y.; Zhang, S.-Y.; Wen, R.; Zhang, T.-N.; Yang, N. Role of histone deacetylases and their inhibitors in neurological diseases. Pharmacol. Res. 2024, 208, 107410. [Google Scholar] [CrossRef] [PubMed]
- Chushak, Y.; Clewell, R.A. An integrated approach to predict activators of NRF2—The transcription factor for oxidative stress response. Artif. Intell. Life Sci. 2024, 5, 100097. [Google Scholar] [CrossRef]
- Modi, H.R.; Musyaju, S.; Ratcliffe, M.; Shear, D.A.; Scultetus, A.H.; Pandya, J.D. Mitochondria-Targeted Antioxidant Therapeutics for Traumatic Brain Injury. Antioxidants 2024, 13, 303. [Google Scholar] [CrossRef]
- Houldsworth, A. Role of oxidative stress in neurodegenerative disorders: A review of reactive oxygen species and prevention by antioxidants. Brain Commun. 2023, 6, fcad356. [Google Scholar] [CrossRef]
- Pant, T.; Uche, N.; Juric, M.; Zielonka, J.; Bai, X. Regulation of immunomodulatory networks by Nrf2-activation in immune cells: Redox control and therapeutic potential in inflammatory diseases. Redox Biol. 2024, 70, 103077. [Google Scholar] [CrossRef]
- Bastyr University. Phase IIb Study of Intranasal Glutathione in Parkinson’s Disease. ClinicalTrials.gov, Clinical Trial Registration NCT02424708. June 2016. Available online: https://clinicaltrials.gov/study/NCT02424708 (accessed on 12 February 2025).
- El-Habta, R.; Bjerkén, S.A.; Virel, A. N-acetylcysteine increases dopamine release and prevents the deleterious effects of 6-OHDA on the expression of VMAT2, α-synuclein, and tyrosine hydroxylase. Neurol. Res. 2024, 46, 406–415. Available online: https://www.tandfonline.com/doi/abs/10.1080/01616412.2024.2325312 (accessed on 11 November 2024). [CrossRef] [PubMed]
- German Parkinson Study Group (GPS). Mono-Center, Prospective, Double-blind, Placebo-Controlled, Randomized Clinical Phase IIa Trial to Assess the Safety, Tolerability, and Immediate Biological Effects of Coenzyme Q10—NanoQuinon® in Progressive Supranuclear Palsy. ClinicalTrials.gov, Clinical Trial Registration NCT00328874. January 2020. Available online: https://clinicaltrials.gov/study/NCT00328874 (accessed on 12 February 2025).
- Columbia University. Clinical Trial of High Dose CoQ10 in ALS. ClinicalTrials.gov, Clinical Trial Registration NCT00243932. April 2024. Available online: https://clinicaltrials.gov/study/NCT00243932 (accessed on 12 February 2025).
- Mantle, D.; Heaton, R.A.; Hargreaves, I.P. Coenzyme Q10, Ageing and the Nervous System: An Overview. Antioxidants 2021, 11, 2. [Google Scholar] [CrossRef]
- Berger, A.A.; Sottosanti, E.R.; Winnick, A.; Izygon, J.; Berardino, K.; Cornett, E.M.; Kaye, A.D.; Varrassi, G.; Viswanath, O.; Urits, I. Monomethyl Fumarate (MMF, Bafiertam) for the Treatment of Relapsing Forms of Multiple Sclerosis (MS). Neurol. Int. 2021, 13, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Wu, R. A 6-Month Study to Evaluate Sulforaphane Add-On Effects in Treatment of Negative Symptoms and Cognition Impairment of Schizophrenia Patients. ClinicalTrials.gov, Clinical Trial Registration NCT04521868. April 2023. Available online: https://clinicaltrials.gov/study/NCT04521868 (accessed on 11 November 2024).
- Sandouka, S.; Singh, P.K.; Saadi, A.; Taiwo, R.O.; Sheeni, Y.; Zhang, T.; Deeb, L.; Guignet, M.; White, S.H.; Shekh-Ahmad, T. Repurposing dimethyl fumarate as an antiepileptogenic and disease-modifying treatment for drug-resistant epilepsy. J. Transl. Med. 2023, 21, 796. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.K. A Double-Blind, Prospective, Randomized Comparison of 2 Doses of MitoQ and Placebo for the Treatment of Patients with Parkinson’s Disease. ClinicalTrials.gov, Clinical Trial Registration NCT00329056. July 2010. Available online: https://clinicaltrials.gov/study/NCT00329056 (accessed on 1 February 2025).
- Talbot, J.L. Dimethyl Fumarate Treatment of Primary Progressive Multiple Sclerosis. ClinicalTrials.gov, Clinical Trial Registration NCT02959658. December 2020. Available online: https://clinicaltrials.gov/study/NCT02959658 (accessed on 1 February 2025).
- Weill Medical College of Cornell University. N-Acetylcysteine for Neuroprotection in Parkinson’s Disease. ClinicalTrials.gov, Clinical Trial Registration NCT01470027. July 2018. Available online: https://clinicaltrials.gov/study/NCT01470027 (accessed on 1 February 2025).
- Himmelfarb, J. Assessing the Effect of the Dietary Supplement Coenzyme Q10 on Biomarkers of Oxidative Stress, Systemic Inflammation, and Endothelial Function in Hemodialysis Patients. ClinicalTrials.gov, Clinical Trial Registration NCT01408680. December 2014. Available online: https://clinicaltrials.gov/study/NCT01408680 (accessed on 1 February 2025).
- Rodriguez-Leyva, I. Efficacy of Memantine Compared With Sodium Valproate in the Prophylactic Treatment of Episodic Migraine. ClinicalTrials.gov, Clinical Trial Registration NCT04698525. May 2024. Available online: https://clinicaltrials.gov/study/NCT04698525 (accessed on 1 February 2025).
- Lange, D.D.J. Phase I/II Study of SOD1 Inhibition by Pyrimethamine in Familial ALS. ClinicalTrials.gov, Clinical Trial Registration NCT01083667. May 2017. Available online: https://clinicaltrials.gov/study/NCT01083667 (accessed on 1 February 2025).
- Otoo, R.A.; Allen, A.R. Sulforaphane’s Multifaceted Potential: From Neuroprotection to Anticancer Action. Molecules 2023, 28, 6902. [Google Scholar] [CrossRef]
- Jiang, Q.; Yin, J.; Chen, J.; Ma, X.; Wu, M.; Liu, G.; Yao, K.; Tan, B.; Yin, Y.; Hou, D.-X. Mitochondria-Targeted Antioxidants: A Step towards Disease Treatment. Oxidative Med. Cell. Longev. 2020, 2020, 8837893. [Google Scholar] [CrossRef]
- Pal, C. Targeting mitochondria with small molecules: A promising strategy for combating Parkinson’s disease. Mitochondrion 2024, 79, 101971. [Google Scholar] [CrossRef]
- Rondeau, J.D.; Lipari, S.; Mathieu, B.; Beckers, C.; Van de Velde, J.A.; Mignion, L.; Morais, M.D.S.; Kreuzer, M.; Colauzzi, I.; Capeloa, T.; et al. Mitochondria-targeted antioxidant MitoQ radiosensitizes tumors by decreasing mitochondrial oxygen consumption. Cell Death Discov. 2024, 10, 514. [Google Scholar] [CrossRef] [PubMed]
- Scorziello, A.; Sirabella, R.; Sisalli, M.J.; Tufano, M.; Giaccio, L.; D’apolito, E.; Castellano, L.; Annunziato, L. Mitochondrial Dysfunction in Parkinson’s Disease: A Contribution to Cognitive Impairment? Int. J. Mol. Sci. 2024, 25, 11490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wei, H.; Zhao, A.; Yan, X.; Zhang, X.; Gan, J.; Guo, M.; Wang, J.; Zhang, F.; Jiang, Y.; et al. Mitochondrial DNA leakage: Underlying mechanisms and therapeutic implications in neurological disorders. J. Neuroinflamm. 2025, 22, 34. [Google Scholar] [CrossRef]
- Xu, J.; Shi, J.; Zhang, J.; Zhang, Y. Vorinostat: A histone deacetylases (HDAC) inhibitor ameliorates traumatic brain injury by inducing iNOS/Nrf2/ARE pathway. Folia Neuropathol. 2018, 56, 179–186. [Google Scholar] [CrossRef]
- Chopra, H.; Annu; Kil Shin, D.; Munjal, K.; Priyanka; Dhama, K.; Bin Emran, T. Revolutionizing clinical trials: The role of AI in accelerating medical breakthroughs. Int. J. Surg. 2023, 109, 4211–4220. [Google Scholar] [CrossRef]
- Krsek, A.; Baticic, L. Nanotechnology-Driven Therapeutic Innovations in Neurodegenerative Disorders: A Focus on Alzheimer’s and Parkinson’s Disease. Futur. Pharmacol. 2024, 4, 352–379. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Kloska, D.; Grochot-Przęczek, A.; Feelisch, M.; Cuadrado, A.; van Goor, H. Personalized redox medicine in inflammatory bowel diseases: An emerging role for HIF-1α and NRF2 as therapeutic targets. Redox Biol. 2023, 60, 102603. [Google Scholar] [CrossRef]
- Toader, C.; Brehar, F.-M.; Radoi, M.P.; Covache-Busuioc, R.-A.; Glavan, L.-A.; Grama, M.; Corlatescu, A.-D.; Costin, H.P.; Bratu, B.-G.; Popa, A.A.; et al. Machine Learning-Based Prediction of Clinical Outcomes in Microsurgical Clipping Treatments of Cerebral Aneurysms. Diagnostics 2024, 14, 2156. [Google Scholar] [CrossRef]
- Rudroff, T.; Rainio, O.; Klén, R. Leveraging Artificial Intelligence to Optimize Transcranial Direct Current Stimulation for Long COVID Management: A Forward-Looking Perspective. Brain Sci. 2024, 14, 831. [Google Scholar] [CrossRef]
- Trevena, W.; Zhong, X.; Lal, A.; Rovati, L.; Cubro, E.; Dong, Y.; Schulte, P.; Gajic, O. Model-driven engineering for digital twins: A graph model-based patient simulation application. Front. Physiol. 2024, 15, 1424931. [Google Scholar] [CrossRef]
- Hickey, J.P.; Collins, A.E.; Nelson, M.L.; Chen, H.; Kalisch, B.E. Modulation of Oxidative Stress and Neuroinflammation by Cannabidiol (CBD): Promising Targets for the Treatment of Alzheimer’s Disease. Curr. Issues Mol. Biol. 2024, 46, 4379–4402. [Google Scholar] [CrossRef]
- Kuo, C.-L.; Babuharisankar, A.P.; Lin, Y.-C.; Lien, H.-W.; Lo, Y.K.; Chou, H.-Y.; Tangeda, V.; Cheng, L.-C.; Cheng, A.N.; Lee, A.Y.-L. Mitochondrial oxidative stress in the tumor microenvironment and cancer immunoescape: Foe or friend? J. Biomed. Sci. 2022, 29, 74. [Google Scholar] [CrossRef]
- Xi, Y.; Feng, D.; Tao, K.; Wang, R.; Shi, Y.; Qin, H.; Murphy, M.P.; Yang, Q.; Zhao, G. MitoQ protects dopaminergic neurons in a 6-OHDA induced PD model by enhancing Mfn2-dependent mitochondrial fusion via activation of PGC-1α. Biochim. Biophys. Acta 2018, 1864, 2859–2870. [Google Scholar] [CrossRef]
- Delgado-Minjares, K.M.; Martinez-Fong, D.; Martínez-Dávila, I.A.; Bañuelos, C.; Gutierrez-Castillo, M.E.; Blanco-Alvarez, V.M.; Cardenas-Aguayo, M.-D.; Luna-Muñoz, J.; Pacheco-Herrero, M.; Soto-Rojas, L.O. Mechanistic Insight from Preclinical Models of Parkinson’s Disease Could Help Redirect Clinical Trial Efforts in GDNF Therapy. Int. J. Mol. Sci. 2021, 22, 11702. [Google Scholar] [CrossRef]
- Crinelli, R.; Zara, C.; Galluzzi, L.; Buffi, G.; Ceccarini, C.; Smietana, M.; Mari, M.; Magnani, M.; Fraternale, A. Activation of NRF2 and ATF4 Signaling by the Pro-Glutathione Molecule I-152, a Co-Drug of N-Acetyl-Cysteine and Cysteamine. Antioxidants 2021, 10, 175. [Google Scholar] [CrossRef]
- Vedaei, F.; Newberg, A.B.; Alizadeh, M.; Zabrecky, G.; Navarreto, E.; Hriso, C.; Wintering, N.; Mohamed, F.B.; Monti, D. Treatment effects of N-acetyl cysteine on resting-state functional MRI and cognitive performance in patients with chronic mild traumatic brain injury: A longitudinal study. Front. Neurol. 2024, 15, 1282198. [Google Scholar] [CrossRef]
- Atlante, A.; Amadoro, G.; Latina, V.; Valenti, D. Therapeutic Potential of Targeting Mitochondria for Alzheimer’s Disease Treatment. J. Clin. Med. 2022, 11, 6742. [Google Scholar] [CrossRef] [PubMed]
- Kurul, F.; Turkmen, H.; Cetin, A.E.; Topkaya, S.N. Nanomedicine: How nanomaterials are transforming drug delivery, bio-imaging, and diagnosis. Next Nanotechnol. 2025, 7, 100129. [Google Scholar] [CrossRef]
- Ye, D.; Liu, H.; Dai, E.; Fan, J.; Wu, L. Recent advances in nanomedicine design strategies for targeting subcellular structures. iScience 2024, 28, 111597. [Google Scholar] [CrossRef]
- Guo, Z.-H.; Khattak, S.; Rauf, M.A.; Ansari, M.A.; Alomary, M.N.; Razak, S.; Yang, C.-Y.; Wu, D.-D.; Ji, X.-Y. Role of Nanomedicine-Based Therapeutics in the Treatment of CNS Disorders. Molecules 2023, 28, 1283. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Li, W.-J.; Xie, D.-D.; Liu, B.; Gong, L.; Han, H.-H. Stimuli-Responsive Nano Drug Delivery Systems for the Treatment of Neurological Diseases. Small 2025, 21, e2410030. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.A.; Kankel, M.W.; Hana, S.; Lau, S.K.; Zavodszky, M.I.; McKissick, O.; Mastrangelo, N.; Dion, J.; Wang, B.; Ferretti, D.; et al. In vivo genome editing using novel AAV-PHP variants rescues motor function deficits and extends survival in a SOD1-ALS mouse model. Gene Ther. 2023, 30, 443–454. [Google Scholar] [CrossRef]
- Silva-Llanes, I.; Shin, C.H.; Jiménez-Villegas, J.; Gorospe, M.; Lastres-Becker, I. The Transcription Factor NRF2 Has Epigenetic Regulatory Functions Modulating HDACs, DNMTs, and miRNA Biogenesis. Antioxidants 2023, 12, 641. [Google Scholar] [CrossRef]
- Vallée, A. Envisioning the Future of Personalized Medicine: Role and Realities of Digital Twins. J. Med Internet Res. 2024, 26, e50204. [Google Scholar] [CrossRef] [PubMed]
- Puccetti, M.; Pariano, M.; Schoubben, A.; Giovagnoli, S.; Ricci, M. Biologics, theranostics, and personalized medicine in drug delivery systems. Pharmacol. Res. 2024, 201, 107086. [Google Scholar] [CrossRef] [PubMed]
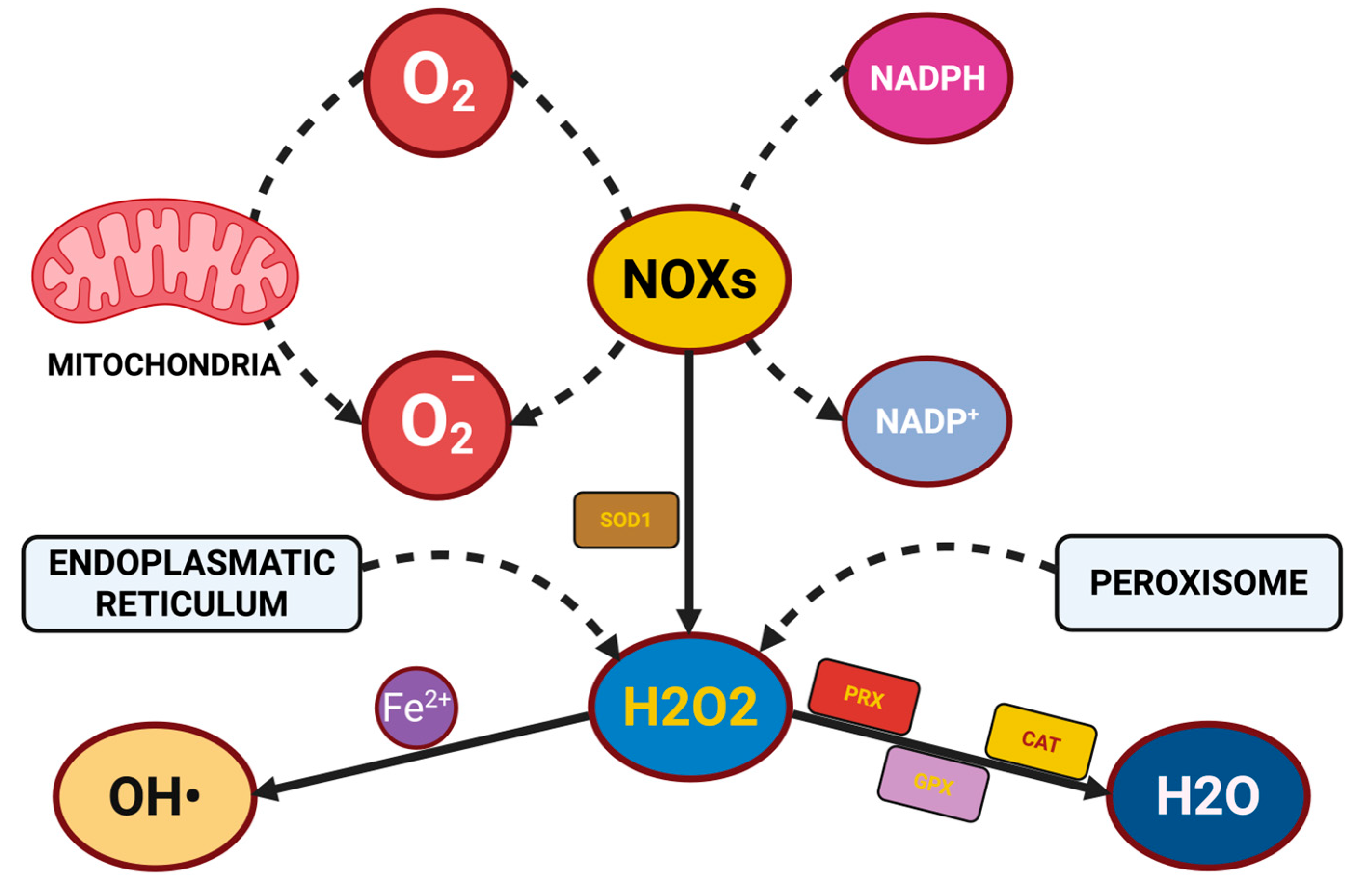
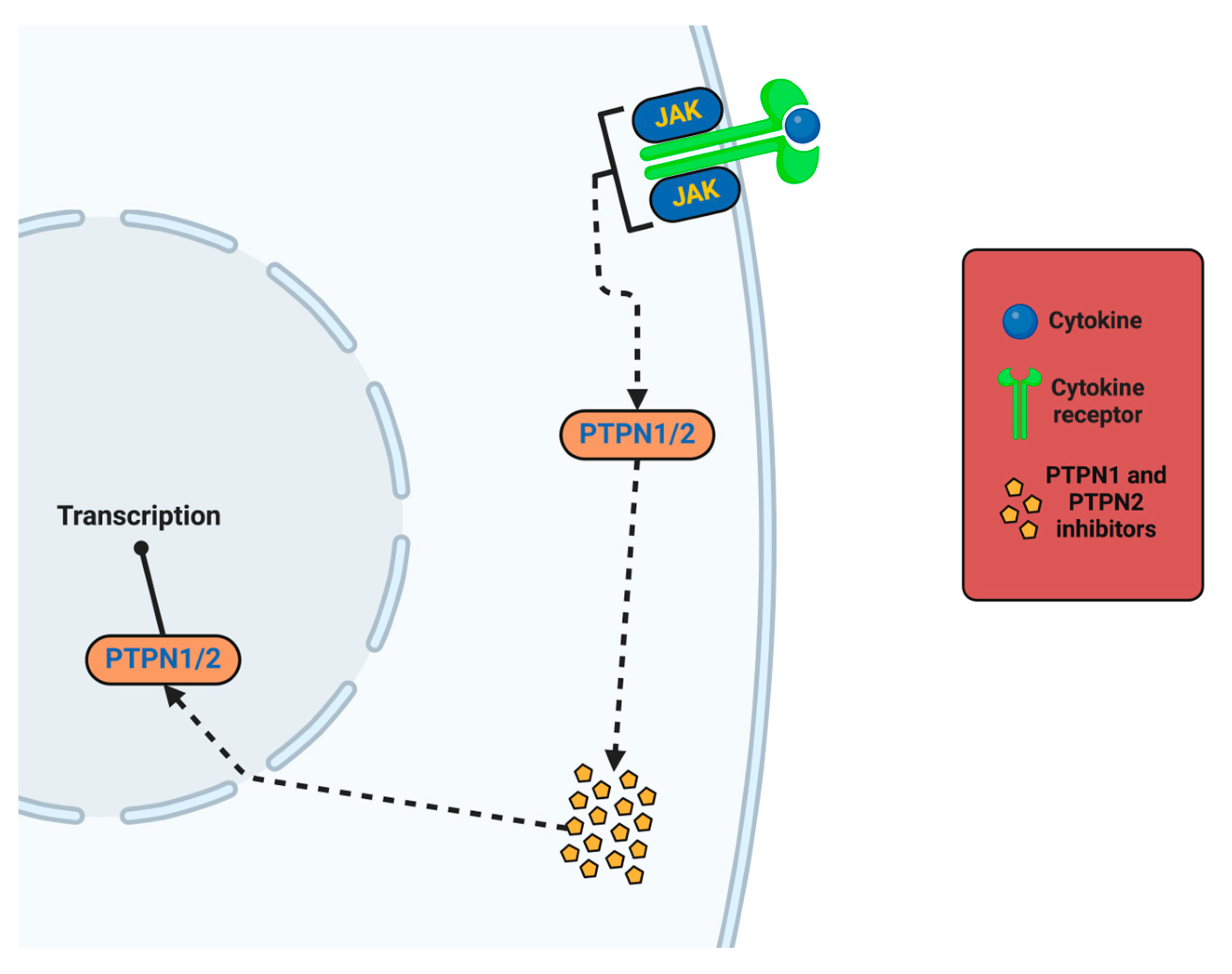
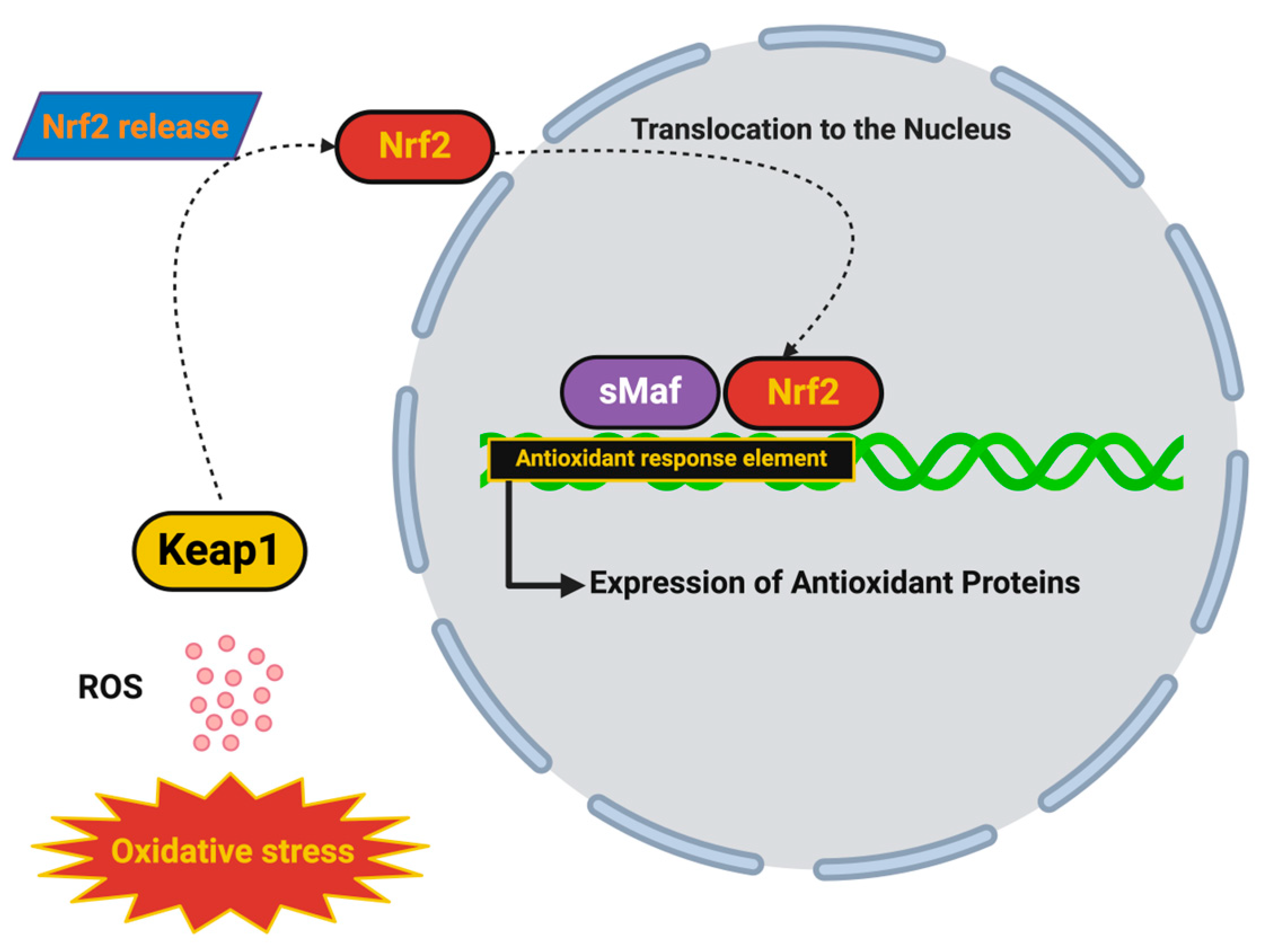
| Biomarker | Type of Damage | Associated Neurological Disorders | Biological Significance | Detection Method | Reference |
|---|---|---|---|---|---|
| F2-isoprostanes | Lipid peroxidation products | AD, PD, Stroke | Are stable and reliable markers of free radical-induced lipid peroxidation; correlate with cognitive decline and neuroinflammation | LC-MS/MS (liquid chromatography–tandem mass spectrometry), GC-MS (gas chromatography-mass spectrometry) | [75] |
| 4-Hydroxynonenal (4-HNE) | Lipid peroxidation byproduct | AD, PD, ALS, Stroke | Is a highly reactive aldehyde that forms covalent adducts with proteins and DNA; promotes protein misfolding and neurotoxicity | ELISA, HPLC, mass spectrometry | [76] |
| Malondialdehyde (MDA) | Lipid peroxidation marker | AD, PD, ALS, Schizophrenia | Indicates oxidative damage to cell membranes; correlates with mitochondrial dysfunction and neurodegeneration | TBARS (thiobarbituric acid reactive substance) assay, HPLC, spectrophotometry | [77,78] |
| 8-Hydroxy-2′-deoxyguanosine (8-OHdG) | DNA oxidation marker | AD, PD, ALS, Stroke, MS | Indicates oxidative damage to nuclear and mitochondrial DNA; correlates with disease severity and progression when elevated levels are present | ELISA, HPLC, electrochemical biosensors | [79,80,81] |
| Nitrotyrosine | Protein nitration marker | PD, AD, ALS, Ischemic Stroke | Is a marker of peroxynitrite-induced oxidative stress; is associated with nitrated α-synuclein in PD and neuroinflammation | ELISA, Western blot, immunohistochemistry | [82,83] |
| Protein Carbonyls | Oxidatively modified proteins | AD, PD, ALS, MS | Indicates irreversible oxidation of proteins, resulting in enzyme inactivation, aggregation, and neurotoxicity | DNPH (2,4-Dinitrophenylhydrazine) derivatization assay, ELISA | [84,85] |
| Glutathione (GSH/GSSG ratio) | Antioxidant capacity marker | AD, PD, Schizophrenia | Reflects intracellular redox balance; indicates oxidative stress and impaired detoxification when the ratio is low | HPLC, Fluorescence-based assays | [86,87] |
| Coenzyme Q10 (oxidized form) | Mitochondrial dysfunction marker | PD, ALS, AD | Reduced CoQ10 levels correlate with impaired electron transport chain function and ATP production | HPLC, LC-MS/MS | [88,89] |
| SOD, Catalase, GPx Activity | Antioxidant enzyme activity | AD, PD, MS, ALS | Decreased activity reflects impaired detoxification of ROS and contributes to sustained oxidative stress | Spectrophotometric enzyme assays, ELISA | [90,91] |
| Therapeutic Strategy | Agent/Intervention | Clinical Trial Name/Phase | Targeted Mechanism | Disease/Condition | Key Outcomes | Advantages | Limitations | Reference |
|---|---|---|---|---|---|---|---|---|
| Mitochondria-Targeted Antioxidant | MitoQ (Mitochondria-targeted CoQ10 derivative) | Phase II PD Trial | Scavenges mitochondrial ROS, protects ETC complexes, and improves ATP production | PD | Reduced oxidative biomarkers (F2-isoprostanes, 4-HNE), improved motor function, and preserved dopaminergic neurons | High mitochondrial selectivity, direct ROS scavenging, and clinical feasibility | Variable efficacy across individuals and limited BBB permeability | NCT00329056 [309] |
| Nrf2 Activator | Dimethyl fumarate (DMF) | DEFINE and CONFIRM Trials (MS); Phase II ALS | Activates Nrf2, induces antioxidant enzyme production (SOD, catalase, GPx), and reduces lipid peroxidation | Multiple Sclerosis (MS), ALS (repurposed) | Reduced relapse rates and slowed disability progression (MS); ongoing ALS trial testing reduced oxidative damage | Oral availability and upregulation of broad antioxidant response | Possible GI side effects and immunomodulatory concerns | NCT02959658 [310] |
| Glutathione Precursor | NAC | PD; Cognitive Decline Study (MCI) | Replenishes intracellular GSH, neutralizes ROS, and prevents protein and lipid peroxidation | PD, Mild Cognitive Impairment (MCI) | Improved motor outcomes in PD, preserved cognitive function in MCI, and reduced oxidative stress biomarkers | Clinically approved, good safety profile, and restores GSH levels | Limited CNS bioavailability and requires long-term administration | NCT01470027 [311] |
| Mitochondrial Membrane Stabilizer | SS-31 (Elamipretide) | Phase II ALS Trial | Binds to cardiolipin, stabilizes mitochondrial membrane potential, and prevents mPTP opening | ALS, Stroke | Improved mitochondrial bioenergetics, reduced muscle weakness, and delayed ALS progression | Targets mitochondrial structure and function directly, and preserves neuromuscular performance | Still in trials, unknown long-term safety, and cost-intensive | NCT05168774 [293] |
| Nanoparticle-Based Delivery | Nanoparticle-encapsulated CoQ10 | Preclinical (PD/AD models) | BBB penetration, targeted release at ROS hotspots, and sustained antioxidant action | Parkinson’s Disease, Alzheimer’s Disease | Enhanced antioxidant efficacy, reduced oxidative stress, and greater preservation of synaptic function | Overcomes BBB limitations, and localized action minimizes systemic toxicity | Still preclinical and requires advanced formulation techniques | NCT01408680 [312] |
| Epigenetic Therapy | Valproic acid (HDAC inhibitor) | Preclinical AD/PD models | Reactivates silenced antioxidant genes and restores Nrf2 expression | AD, PD | Reduced amyloid burden, improved cognitive outcomes, and reactivated endogenous antioxidant defenses | Modifies gene expression and long-lasting effect on redox balance | Epigenetic off-target effects and dose-dependent toxicity | NCT04698525 [313] |
| Gene Therapy | CRISPR-Cas9 for SOD1 mutations | Preclinical ALS models | Corrects inherited mutations driving oxidative damage | Familial ALS | Reduced ROS, delayed motor neuron death, and extended survival | Permanent correction of genetic defects and disease-modifying potential | Ethical and safety concerns and high technical complexity | NCT01083667 [314] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Șerban, M.; Toader, C.; Covache-Busuioc, R.-A. The Redox Revolution in Brain Medicine: Targeting Oxidative Stress with AI, Multi-Omics and Mitochondrial Therapies for the Precision Eradication of Neurodegeneration. Int. J. Mol. Sci. 2025, 26, 7498. https://doi.org/10.3390/ijms26157498
Șerban M, Toader C, Covache-Busuioc R-A. The Redox Revolution in Brain Medicine: Targeting Oxidative Stress with AI, Multi-Omics and Mitochondrial Therapies for the Precision Eradication of Neurodegeneration. International Journal of Molecular Sciences. 2025; 26(15):7498. https://doi.org/10.3390/ijms26157498
Chicago/Turabian StyleȘerban, Matei, Corneliu Toader, and Răzvan-Adrian Covache-Busuioc. 2025. "The Redox Revolution in Brain Medicine: Targeting Oxidative Stress with AI, Multi-Omics and Mitochondrial Therapies for the Precision Eradication of Neurodegeneration" International Journal of Molecular Sciences 26, no. 15: 7498. https://doi.org/10.3390/ijms26157498
APA StyleȘerban, M., Toader, C., & Covache-Busuioc, R.-A. (2025). The Redox Revolution in Brain Medicine: Targeting Oxidative Stress with AI, Multi-Omics and Mitochondrial Therapies for the Precision Eradication of Neurodegeneration. International Journal of Molecular Sciences, 26(15), 7498. https://doi.org/10.3390/ijms26157498





