TMAO Activates the NLRP3 Inflammasome, Disrupts Gut–Kidney Interaction, and Promotes Intestinal Inflammation
Abstract
1. Introduction
2. Results
2.1. General Conditions of Mice
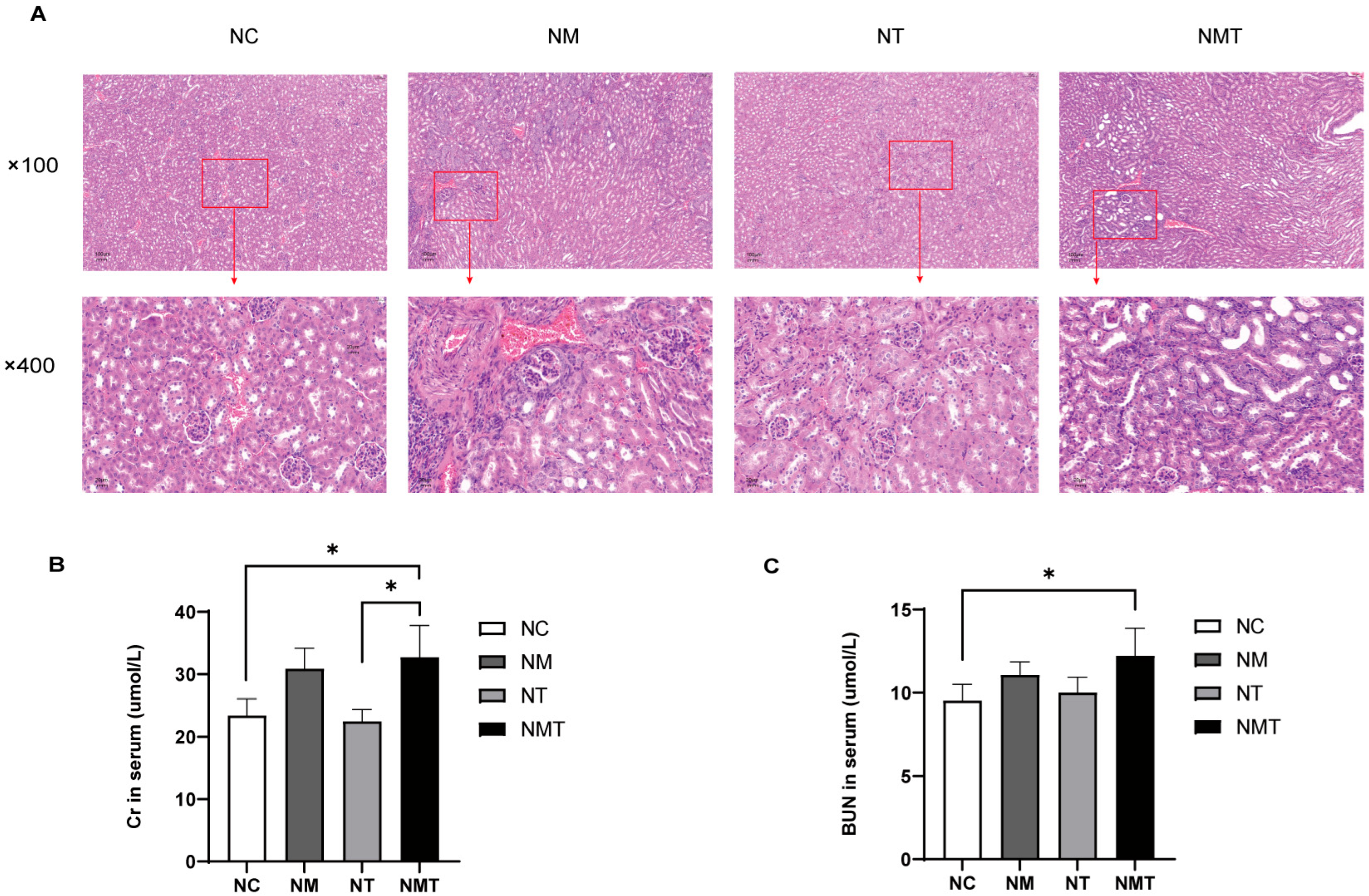
2.2. Effect of TMAO on Renal Histology and Function in Mice
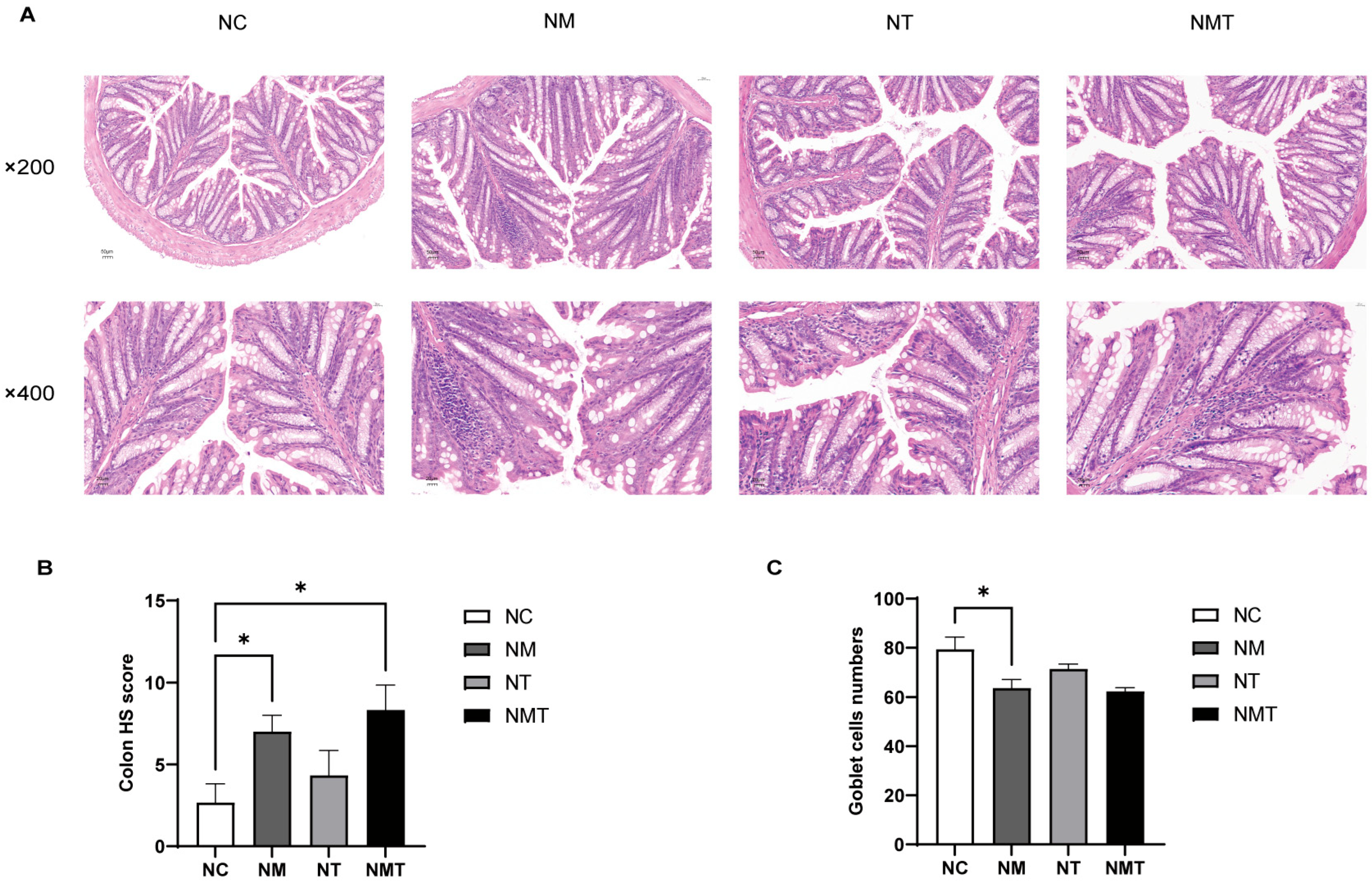
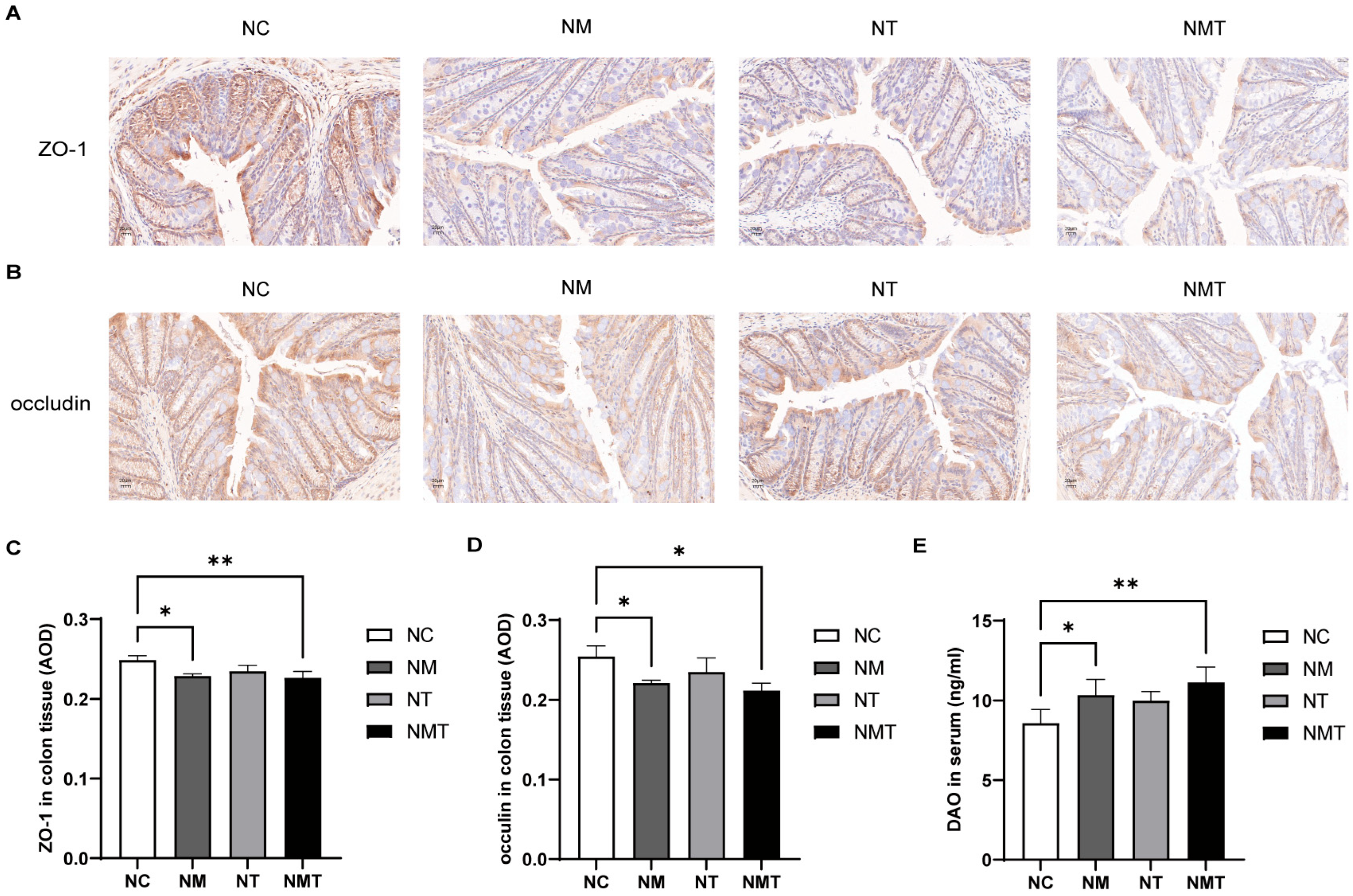
2.3. Effects of TMAO on Colonic Tissue and Intestinal Barrier Function in Mice
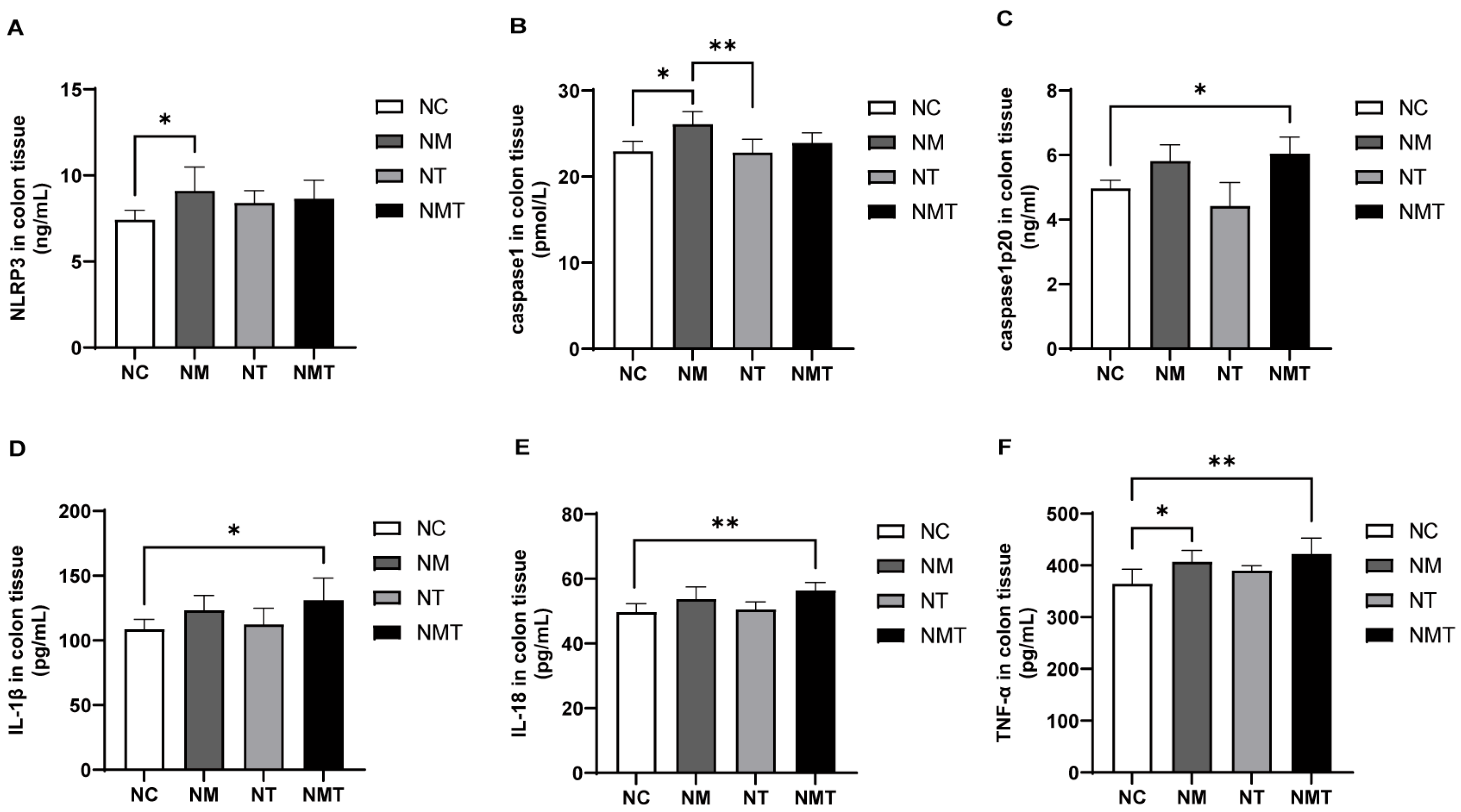
2.4. Colonic Inflammasomes and Inflammatory Cytokines
2.5. Kidney Inflammasomes and Inflammatory Cytokines
2.6. TMAO and TMA Levels Are Altered in Mice
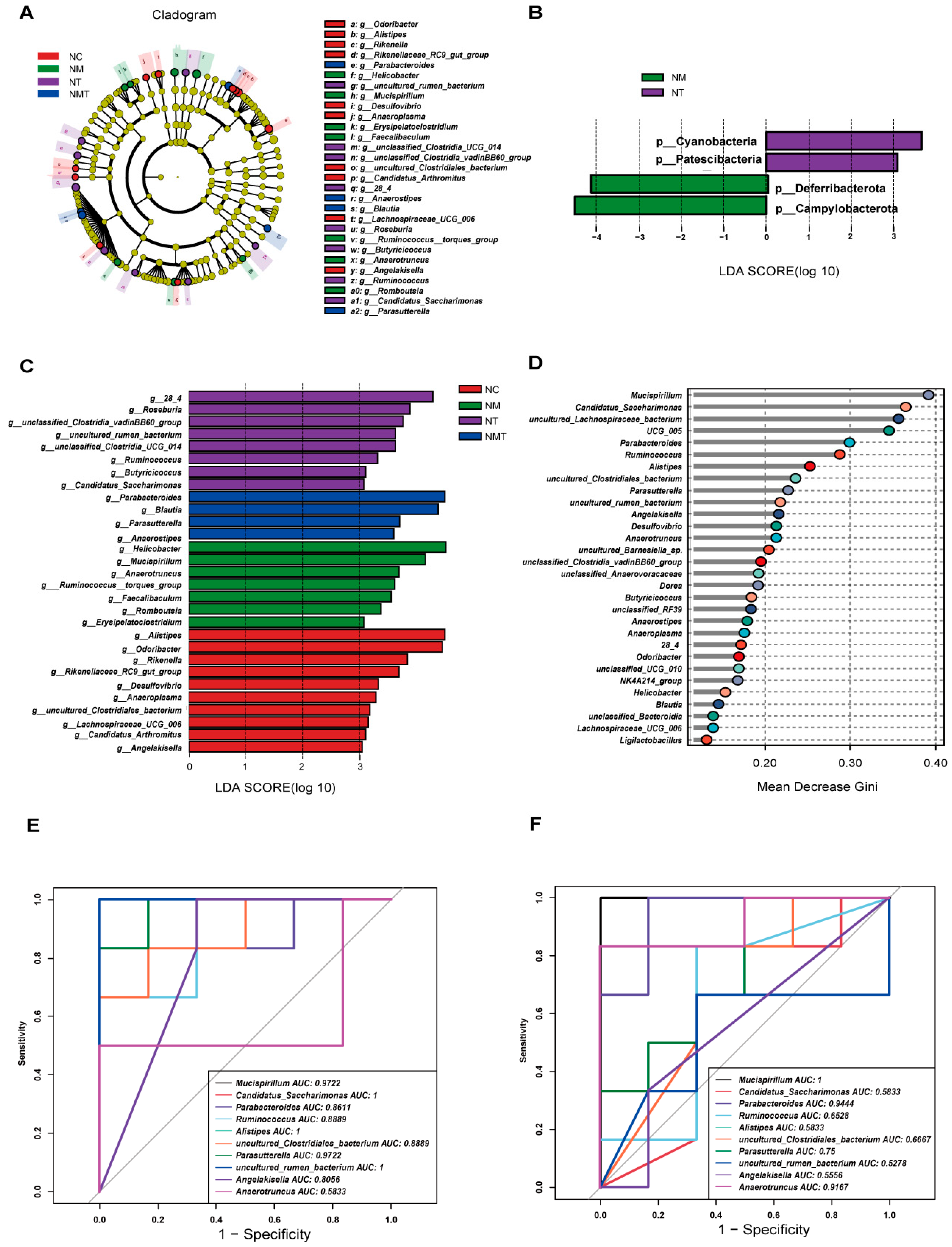
2.7. Effect of TMAO on Characteristic Microbiota in Mice
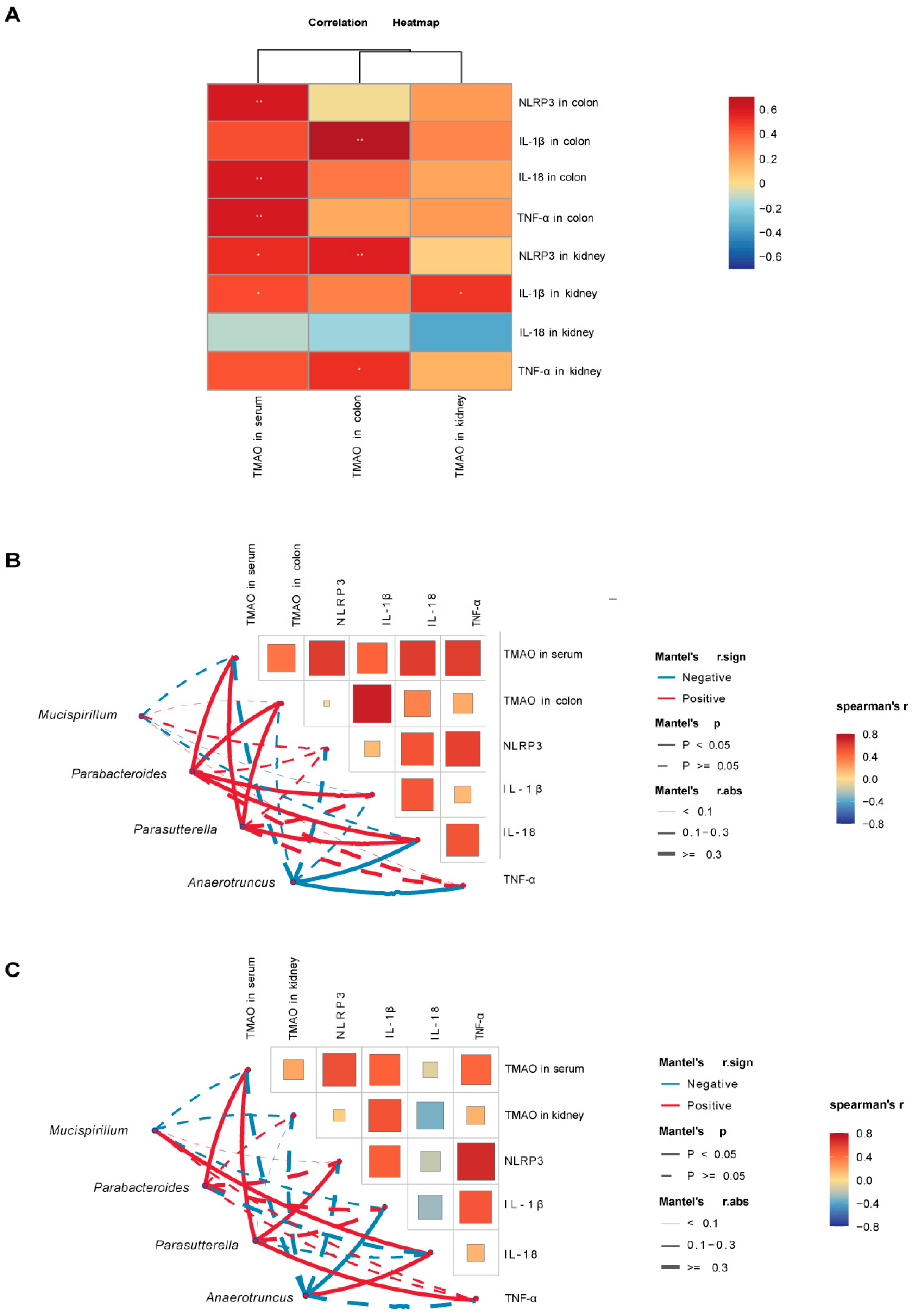
2.8. Correlation Analysis
3. Discussion
3.1. TMAO Elevation Is Linked to Gut Microbiota Dysbiosis in Mice with Diarrhea
3.2. The Aggravation of Diarrhea in Model Mice Is Closely Associated with TMAO Levels
3.3. TMAO May Contribute to Gut–Kidney Interaction During Diarrhea Progression
4. Materials and Methods
4.1. Medication Preparation
4.2. Grouping and Modeling
4.3. General Condition and Fecal Water Content of Mice
4.4. Biochemical Methods
4.5. Enzyme-Linked Immunosorbent Assay
4.6. Histological Examination of Colon and Kidney Tissues
4.7. Immunohistochemical Detection of ZO-1 and Occludin Expression in the Colon
4.8. Collection of Intestinal Contents and 16S rRNA Sequencing
4.9. Characteristic Gut Microbiota Analysis
4.10. Correlation Analysis Method
4.11. Statistical Analysis
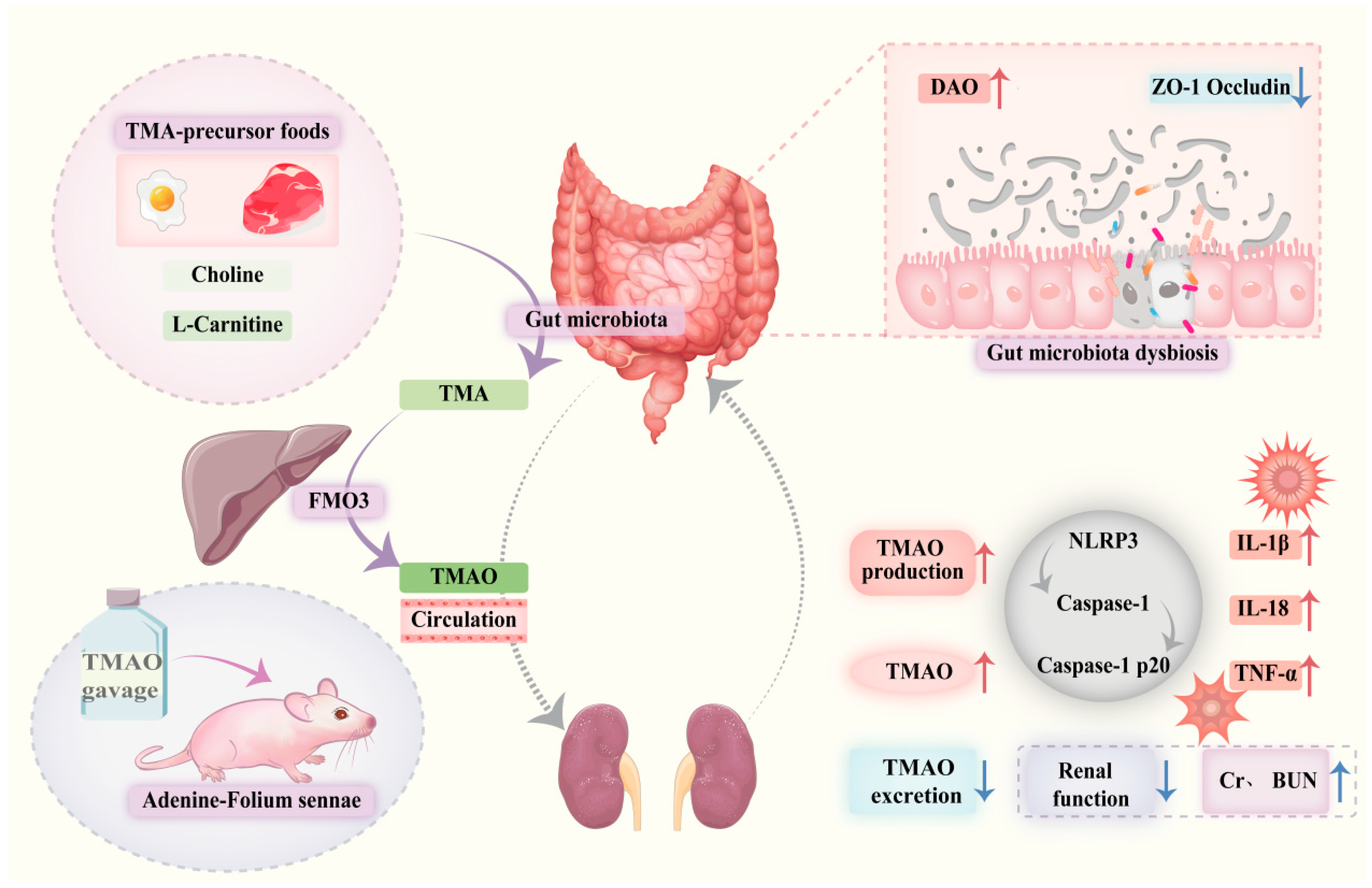
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TMAO | trimethylamine N-oxide |
| CKD | chronic kidney disease |
| TMA | trimethylamine |
| FMO3 | flavin-containing monooxygenase 3 |
| ZO-1 | zonula occludens-1 |
| DAO | diamine oxidase |
| Cr | creatinine |
| BUN | blood urea nitrogen |
| NLRP3 | nucleotide-binding oligomerization domain-like receptor protein |
| IL-1β | interleukin-1 beta |
| IL-18 | interleukin-18 |
| TNF-α | tumor necrosis factor-alpha |
| H&E | hematoxylin and eosin |
| DAB | 3,3′-diaminobenzidine |
| AOD | average optical density |
| LDA | linear discriminant analysis |
| ROC | receiver operating characteristic |
| AUC | area under the curve |
References
- Xiao, L.; Liu, Q.; Luo, M.; Xiong, L. Gut Microbiota-Derived Metabolites in Irritable Bowel Syndrome. Front. Cell. Infect. Microbiol. 2021, 11, 729346. [Google Scholar] [CrossRef]
- Gong, J.; Noel, S.; Pluznick, J.L.; Hamad, A.R.A.; Rabb, H. Gut Microbiota-Kidney Cross-Talk in Acute Kidney Injury. Semin. Nephrol. 2019, 39, 107–116. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Chen, D.Q.; Chen, L.; Liu, J.R.; Vaziri, N.D.; Guo, Y.; Zhao, Y.Y. Microbiome-metabolome reveals the contribution of gut-kidney axis on kidney disease. J. Transl. Med. 2019, 17, 5. [Google Scholar] [CrossRef] [PubMed]
- Rysz, J.; Franczyk, B.; Ławiński, J.; Olszewski, R.; Ciałkowska-Rysz, A.; Gluba-Brzózka, A. The Impact of CKD on Uremic Toxins and Gut Microbiota. Toxins 2021, 13, 252. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Lockwood, M.B.; Rhee, C.M.; Tantisattamo, E.; Andreoli, S.; Balducci, A.; Laffin, P.; Harris, T.; Knight, R.; Kumaraswami, L.; et al. Patient-centred approaches for the management of unpleasant symptoms in kidney disease. Nat. Rev. Nephrol. 2022, 18, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Belo, L.; Carvalho, M. Chronic Kidney Disease: Underlying Molecular Mechanisms-A Special Issue Overview. Int. J. Mol. Sci. 2023, 24, 12363. [Google Scholar] [CrossRef]
- Shin, H.S.; Chandraker, A. Causes and management of postrenal transplant diarrhea: An underappreciated cause of transplant-associated morbidity. Curr. Opin. Nephrol. Hypertens. 2017, 26, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Gioco, R.; Corona, D.; Ekser, B.; Puzzo, L.; Inserra, G.; Pinto, F.; Schipa, C.; Privitera, F.; Veroux, P.; Veroux, M. Gastrointestinal complications after kidney transplantation. World J. Gastroenterol. 2020, 26, 5797–5811. [Google Scholar] [CrossRef] [PubMed]
- Gatarek, P.; Kaluzna-Czaplinska, J. Trimethylamine N-oxide (TMAO) in human health. EXCLI J. 2021, 20, 301–319. [Google Scholar] [CrossRef]
- Banno, Y.; Nomura, M.; Hara, R.; Asami, M.; Tanaka, K.; Mukai, Y.; Tomata, Y. Trimethylamine N-oxide and risk of inflammatory bowel disease: A Mendelian randomization study. Medicine 2023, 102, e34758. [Google Scholar] [CrossRef]
- Rath, S.; Rud, T.; Pieper, D.H.; Vital, M. Potential TMA-Producing Bacteria Are Ubiquitously Found in Mammalia. Front. Microbiol. 2019, 10, 2966. [Google Scholar] [CrossRef]
- Chen, Y.; Weng, Z.; Liu, Q.; Shao, W.; Guo, W.; Chen, C.; Jiao, L.; Wang, Q.; Lu, Q.; Sun, H.; et al. FMO3 and its metabolite TMAO contribute to the formation of gallstones. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2576–2585. [Google Scholar] [CrossRef]
- Xie, J.; Ma, X.; Zheng, Y.; Mao, N.; Ren, S.; Fan, J. Panax notoginseng saponins alleviate damage to the intestinal barrier and regulate levels of intestinal microbes in a rat model of chronic kidney disease. Ren. Fail. 2022, 44, 1948–1960. [Google Scholar] [CrossRef]
- Yu, P.S.; Wu, P.H.; Hung, W.W.; Lin, M.Y.; Zhen, Y.Y.; Hung, W.C.; Chang, J.M.; Tsai, J.R.; Chiu, Y.W.; Hwang, S.J.; et al. Association Between Trimethylamine N-oxide and Adverse Kidney Outcomes and Overall Mortality in Type 2 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2024, 109, 2097–2105. [Google Scholar] [CrossRef]
- Lai, Y.; Tang, H.; Zhang, X.; Zhou, Z.; Zhou, M.; Hu, Z.; Zhu, F.; Zhang, L.; Nie, J. Trimethylamine-N-Oxide Aggravates Kidney Injury via Activation of p38/MAPK Signaling and Upregulation of HuR. Kidney Blood Press. Res. 2022, 47, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Yong, C.; Huang, G.; Ge, H.; Zhu, Y.; Yang, Y.; Yu, Y.; Tian, F.; Gao, K.; Zhou, E. Perilla frutescens L. alleviates trimethylamine N-oxide-induced apoptosis in the renal tubule by regulating ASK1-JNK phosphorylation. Phytother. Res. 2023, 37, 1274–1292. [Google Scholar] [CrossRef]
- Ge, H.; Wei, Y.; Zhang, W.; Yong, C.; Chen, Y.; Zhou, E. Suyin Detoxification Granule alleviates trimethylamine N-oxide-induced tubular ferroptosis and renal fibrosis to prevent chronic kidney disease progression. Phytomedicine 2024, 135, 156195. [Google Scholar] [CrossRef]
- Rodrigues, C.; Ismael, S.; Castela, I.; Barreiros-Mota, I.; Almeida, M.J.; Santos, G.M.; Calhau, C.; Rocha, J.C.; Faria, A.; Araújo, J.R. Trimethylamine increases intestinal fatty acid absorption: In vitro studies in a Caco-2 cell culture system. J. Nutr. Sci. 2023, 12, e108. [Google Scholar] [CrossRef]
- Xie, S.; Fang, L.; Deng, N.; Shen, J.; Tan, Z.; Peng, X. Targeting the Gut-Kidney Axis in Diarrhea with Kidney-Yang Deficiency Syndrome: The Role of Sishen Pills in Regulating TMAO-Mediated Inflammatory Response. Med. Sci. Monit. 2024, 30, e944185. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Peng, X.; Qiao, B.; Peng, M.; Deng, N.; Yu, R.; Tan, Z. Gut-Kidney Impairment Process of Adenine Combined with Folium sennae-Induced Diarrhea: Association with Interactions between Lactobacillus intestinalis, Bacteroides acidifaciens and Acetic Acid, Inflammation, and Kidney Function. Cells 2022, 11, 3261. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ye, Q.; Liu, Y.L.; Kang, J.; Chen, Y.; Dong, W.G. Schistosoma japonicum attenuates dextran sodium sulfate-induced colitis in mice via reduction of endoplasmic reticulum stress. World J. Gastroenterol. 2017, 23, 5700–5712. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Zhang, B.; Lu, M.; Ma, J.; Liu, Z.; Huang, J.; Ma, J.; Yang, X.; Wang, F.; et al. Modified Gegen Qinlian decoction ameliorated ulcerative colitis by attenuating inflammation and oxidative stress and enhancing intestinal barrier function in vivo and in vitro. J. Ethnopharmacol. 2023, 313, 116538. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Bergeron, N.; Levison, B.S.; Li, X.S.; Chiu, S.; Jia, X.; Koeth, R.A.; Li, L.; Wu, Y.; Tang, W.H.W.; et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur. Heart J. 2019, 40, 583–594. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Ivey, K.L.; Wang, D.D.; Wilkinson, J.E.; Franke, A.; Lee, K.H.; Chan, A.; Huttenhower, C.; Hu, F.B.; et al. Interplay between diet and gut microbiome, and circulating concentrations of trimethylamine N-oxide: Findings from a longitudinal cohort of US men. Gut 2022, 71, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.E.; Caudill, M.A. Trimethylamine-N-Oxide: Friend, Foe, or Simply Caught in the Cross-Fire? Trends Endocrinol. Metab. 2017, 28, 121–130. [Google Scholar] [CrossRef]
- Jia, X.; Osborn, L.J.; Wang, Z. Simultaneous Measurement of Urinary Trimethylamine (TMA) and Trimethylamine N-Oxide (TMAO) by Liquid Chromatography-Mass Spectrometry. Molecules 2020, 25, 1862. [Google Scholar] [CrossRef]
- Yu, Y.; Yin, Y.; Deng, J.; Yang, X.; Bai, S.; Yu, R. Unveiling the causal effects of gut microbiome on trimethylamine N-oxide: Evidence from Mendelian randomization. Front. Microbiol. 2024, 15, 1465455. [Google Scholar] [CrossRef]
- Li, X.; Song, J.; Shi, X.; Huang, M.; Liu, L.; Yi, G.; Yang, N.; Xu, G.; Zheng, J. FMO3 deficiency of duck leads to decreased lipid deposition and increased antibacterial activity. J. Anim. Sci. Biotechnol. 2022, 13, 119. [Google Scholar] [CrossRef]
- Qiu, L.; Tao, X.; Xiong, H.; Yu, J.; Wei, H. Lactobacillus plantarum ZDY04 exhibits a strain-specific property of lowering TMAO via the modulation of gut microbiota in mice. Food Funct. 2018, 9, 4299–4309. [Google Scholar] [CrossRef] [PubMed]
- Shirouchi, B.; Fukuda, A.; Akasaka, T. Unlike Glycerophosphocholine or Choline Chloride, Dietary Phosphatidylcholine Does Not Increase Plasma Trimethylamine-N-Oxide Levels in Sprague-Dawley Rats. Metabolites 2022, 12, 64. [Google Scholar] [CrossRef]
- Laryushina, Y.; Samoilova-Bedych, N.; Turgunova, L.; Kozhakhmetov, S.; Alina, A.; Suieubayev, M.; Mukhanbetzhanov, N. Alterations of the Gut Microbiome and TMAO Levels in Patients with Ulcerative Colitis. J. Clin. Med. 2024, 13, 5794. [Google Scholar] [CrossRef]
- Zysset-Burri, D.C.; Keller, I.; Berger, L.E.; Neyer, P.J.; Steuer, C.; Wolf, S.; Zinkernagel, M.S. Retinal artery occlusion is associated with compositional and functional shifts in the gut microbiome and altered trimethylamine-N-oxide levels. Sci. Rep. 2019, 9, 15303. [Google Scholar] [CrossRef]
- Li, X.; Qiao, B.; Wu, Y.; Deng, N.; Yuan, J.; Tan, Z. Sishen Pill inhibits intestinal inflammation in diarrhea mice via regulating kidney-intestinal bacteria-metabolic pathway. Front. Pharmacol. 2024, 15, 1360589. [Google Scholar] [CrossRef]
- Pahl, M.V.; Vaziri, N.D. The Chronic Kidney Disease–Colonic Axis. Semin. Dial. 2015, 28, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xin, W.; Xiong, J.; Yao, M.; Zhang, B.; Zhao, J. The Intestinal Microbiota and Metabolites in the Gut-Kidney-Heart Axis of Chronic Kidney Disease. Front. Pharmacol. 2022, 13, 837500. [Google Scholar] [CrossRef]
- Guo, M.; Wu, Y.; Peng, M.; Xiao, N.; Lei, Z.; Tan, Z. Decreasing of Trimethylamine N-Oxide by Cecal Microbiota and Choline-Trimethylamine Lyase are Associated with Sishen Pill on Diarrhea with Kidney-Yang Deficiency Syndrome. J. Inflamm. Res. 2024, 17, 7275–7294. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J.K.; Johansson, M.E.V. The role of goblet cells and mucus in intestinal homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 785–803. [Google Scholar] [CrossRef]
- Nian, F.; Chen, Y.; Xia, Q.; Zhu, C.; Wu, L.; Lu, X. Gut microbiota metabolite trimethylamine N-oxide promoted NAFLD progression by exacerbating intestinal barrier disruption and intrahepatic cellular imbalance. Int. Immunopharmacol. 2024, 142, 113173. [Google Scholar] [CrossRef]
- Linsalata, M.; Riezzo, G.; Clemente, C.; D’Attoma, B.; Russo, F. Noninvasive Biomarkers of Gut Barrier Function in Patients Suffering from Diarrhea Predominant-IBS: An Update. Dis. Markers 2020, 2020, 2886268. [Google Scholar] [CrossRef]
- Zeng, Y.; Guo, M.; Fang, X.; Teng, F.; Tan, X.; Li, X.; Wang, M.; Long, Y.; Xu, Y. Gut Microbiota-Derived Trimethylamine N-Oxide and Kidney Function: A Systematic Review and Meta-Analysis. Adv. Nutr. 2021, 12, 1286–1304. [Google Scholar] [CrossRef]
- Yue, C.; Yang, X.; Li, J.; Chen, X.; Zhao, X.; Chen, Y.; Wen, Y. Trimethylamine N-oxide prime NLRP3 inflammasome via inhibiting ATG16L1-induced autophagy in colonic epithelial cells. Biochem. Biophys. Res. Commun. 2017, 490, 541–551. [Google Scholar] [CrossRef]
- Chen, X.; Kong, Q.; Zhao, X.; Zhao, C.; Hao, P.; Irshad, I.; Lei, H.; Kulyar, M.F.; Bhutta, Z.A.; Ashfaq, H.; et al. Sodium acetate/sodium butyrate alleviates lipopolysaccharide-induced diarrhea in mice via regulating the gut microbiota, inflammatory cytokines, antioxidant levels, and NLRP3/Caspase-1 signaling. Front. Microbiol. 2022, 13, 1036042. [Google Scholar] [CrossRef]
- Chen, M.L.; Zhu, X.H.; Ran, L.; Lang, H.D.; Yi, L.; Mi, M.T. Trimethylamine-N-Oxide Induces Vascular Inflammation by Activating the NLRP3 Inflammasome Through the SIRT3-SOD2-mtROS Signaling Pathway. J. Am. Heart Assoc. 2017, 6, e006347. [Google Scholar] [CrossRef]
- Wu, Y.; Peng, X.; Li, X.; Li, D.; Tan, Z.; Yu, R. Sex hormones influence the intestinal microbiota composition in mice. Front. Microbiol. 2022, 13, 964847. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Shen, J.; Wu, Y.; Tan, Z. Involvement of intestinal mucosal microbiota in adenine-induced liver function injury. 3 Biotech 2025, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Koeth, R.A.; Levison, B.S.; Culley, M.K.; Buffa, J.A.; Wang, Z.; Gregory, J.C.; Org, E.; Wu, Y.; Li, L.; Smith, J.D.; et al. γ-Butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO. Cell Metab. 2014, 20, 799–812. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, L.; Shen, J.; Xiao, N.; Tan, Z. TMAO Activates the NLRP3 Inflammasome, Disrupts Gut–Kidney Interaction, and Promotes Intestinal Inflammation. Int. J. Mol. Sci. 2025, 26, 7441. https://doi.org/10.3390/ijms26157441
Fang L, Shen J, Xiao N, Tan Z. TMAO Activates the NLRP3 Inflammasome, Disrupts Gut–Kidney Interaction, and Promotes Intestinal Inflammation. International Journal of Molecular Sciences. 2025; 26(15):7441. https://doi.org/10.3390/ijms26157441
Chicago/Turabian StyleFang, Leyao, Junxi Shen, Nenqun Xiao, and Zhoujin Tan. 2025. "TMAO Activates the NLRP3 Inflammasome, Disrupts Gut–Kidney Interaction, and Promotes Intestinal Inflammation" International Journal of Molecular Sciences 26, no. 15: 7441. https://doi.org/10.3390/ijms26157441
APA StyleFang, L., Shen, J., Xiao, N., & Tan, Z. (2025). TMAO Activates the NLRP3 Inflammasome, Disrupts Gut–Kidney Interaction, and Promotes Intestinal Inflammation. International Journal of Molecular Sciences, 26(15), 7441. https://doi.org/10.3390/ijms26157441




