Fused-Linked and Spiro-Linked N-Containing Heterocycles
Abstract
1. Introduction
2. Fused- and Spiro-Linked 3-, 4-, and 5-Membered Nitrogen Heterocycles
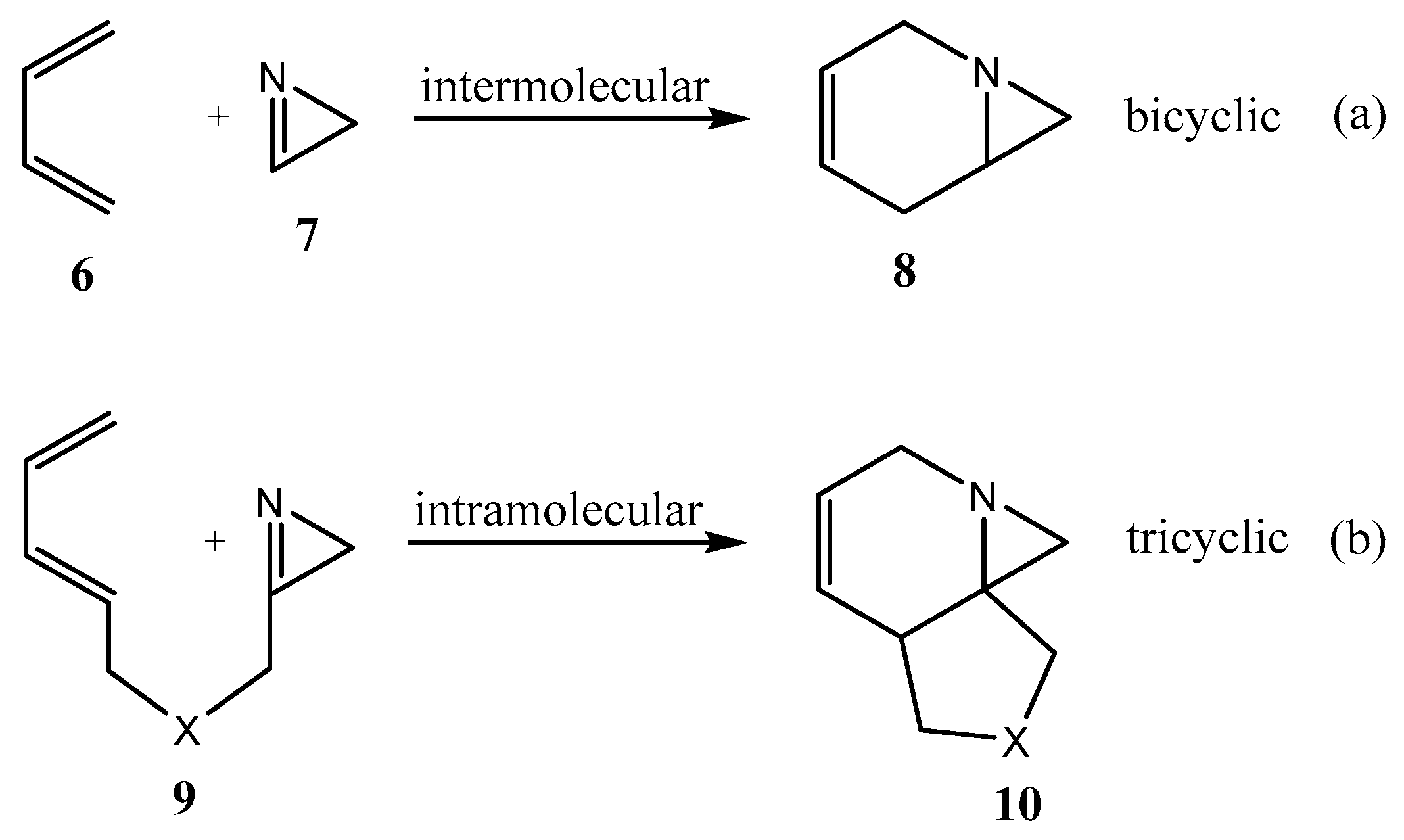
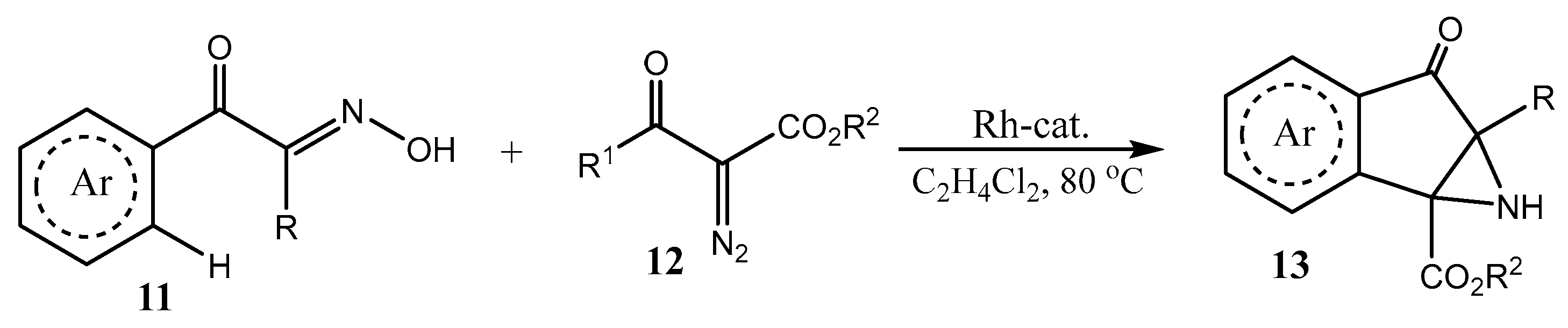
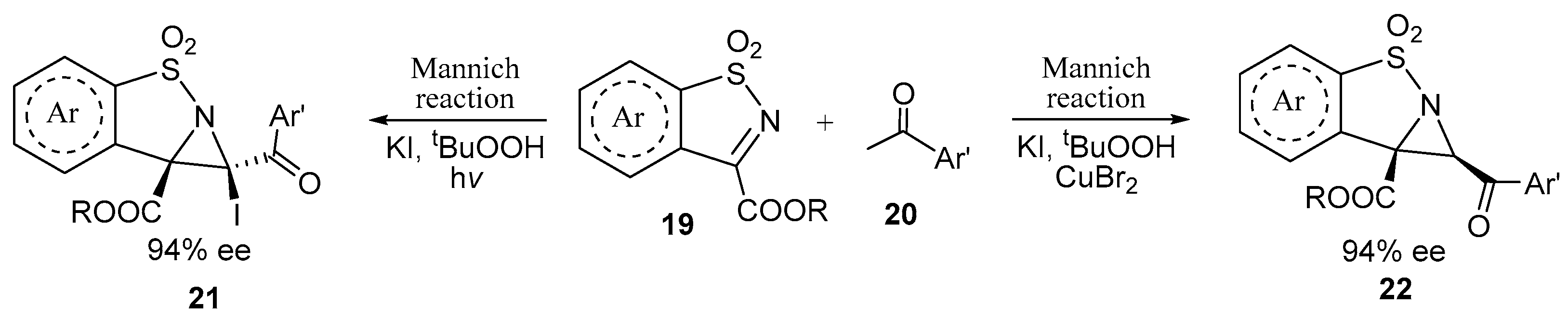
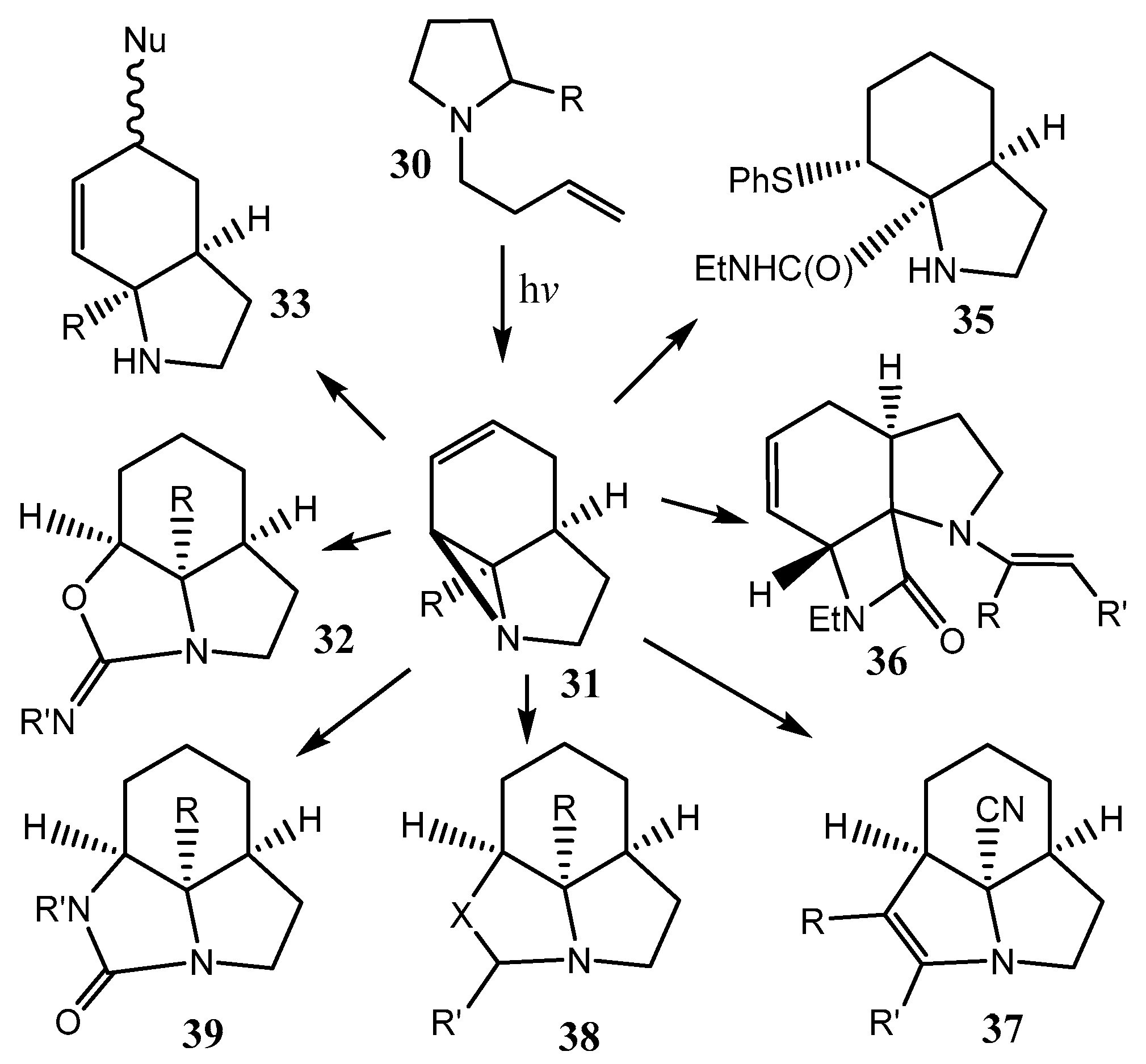
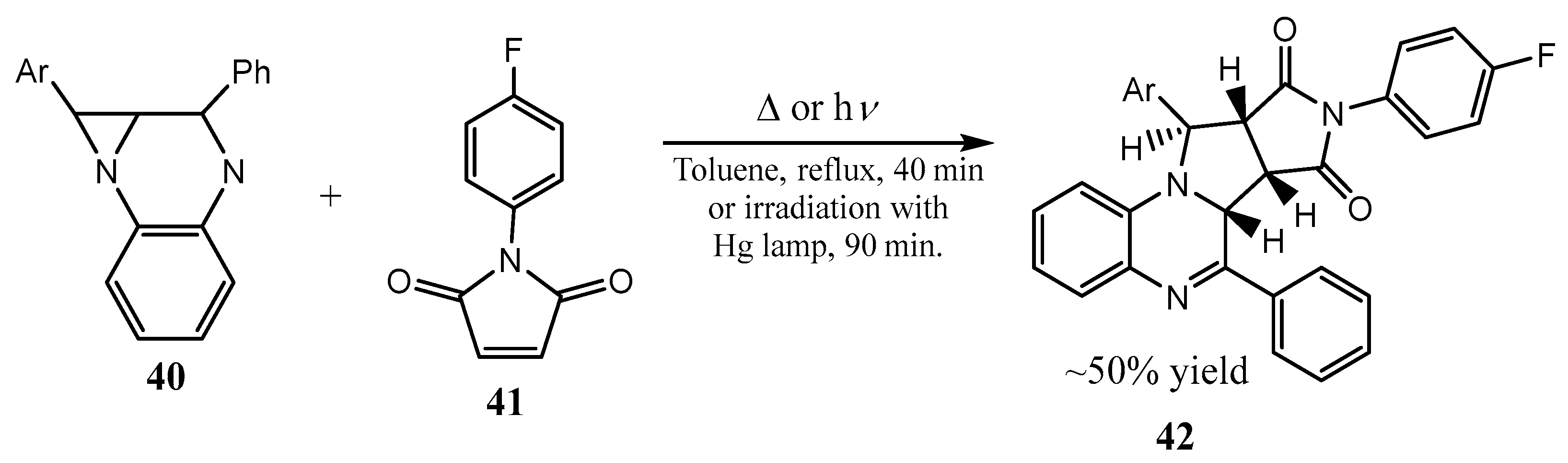
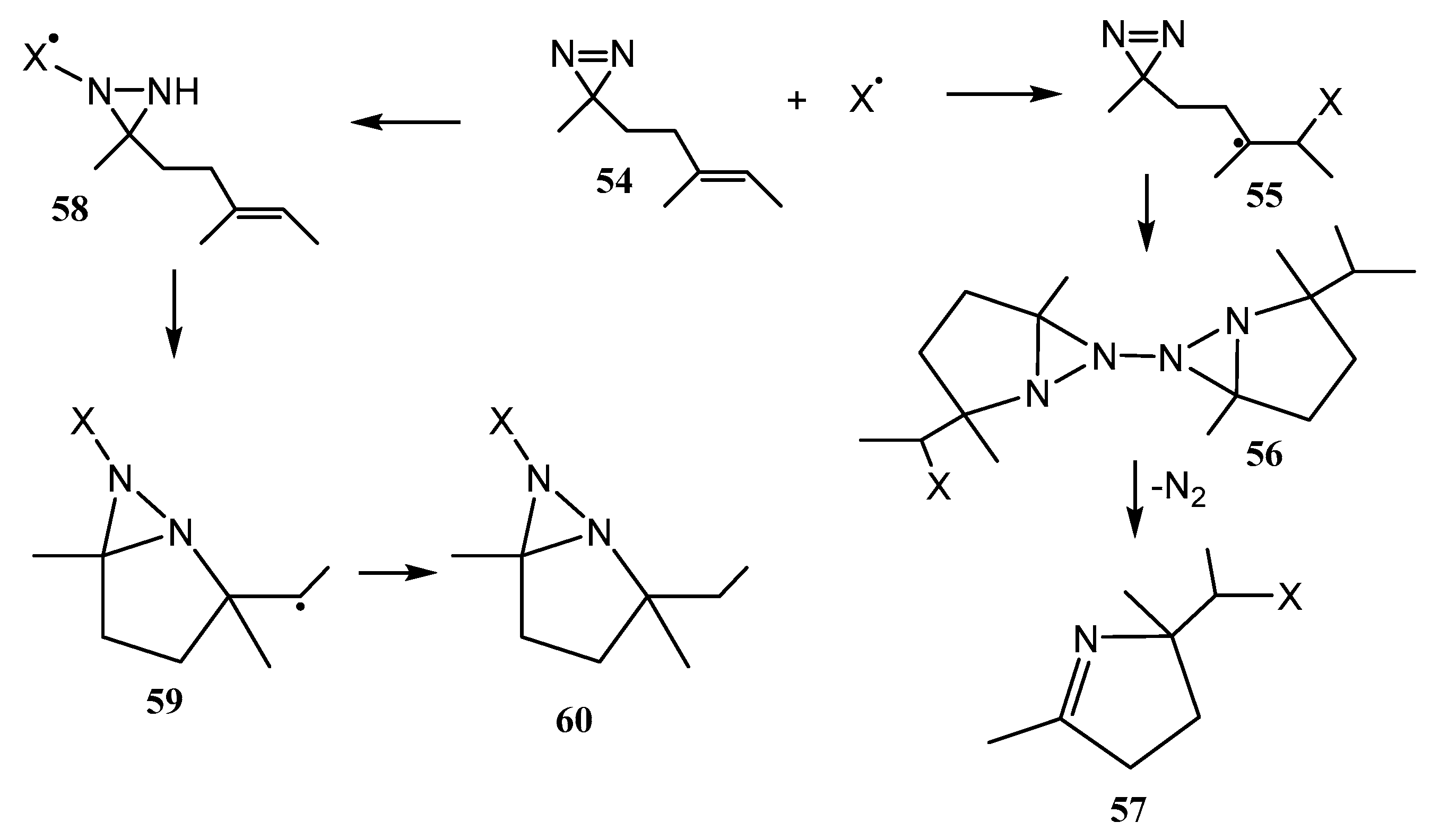
2.1. Fused Aziridines and Diaziridines
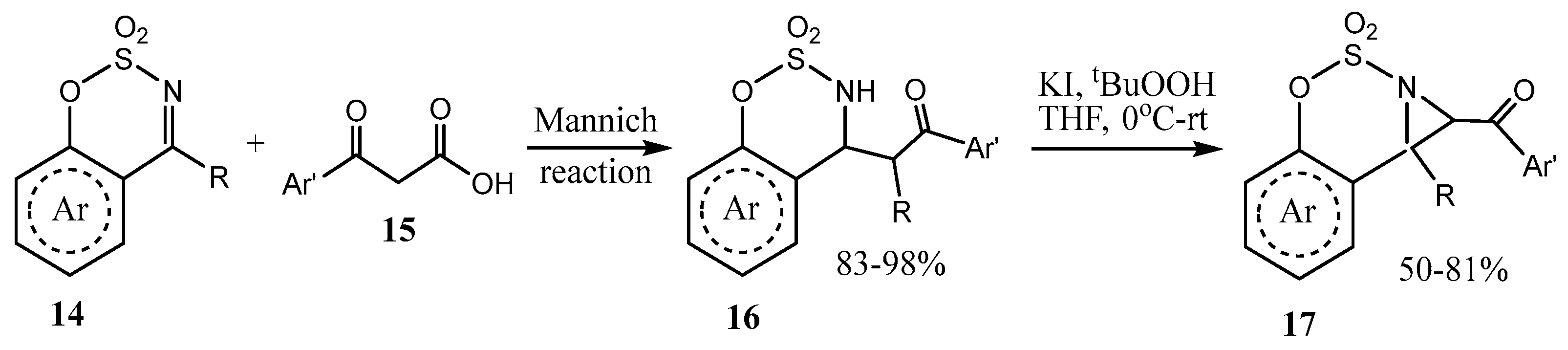
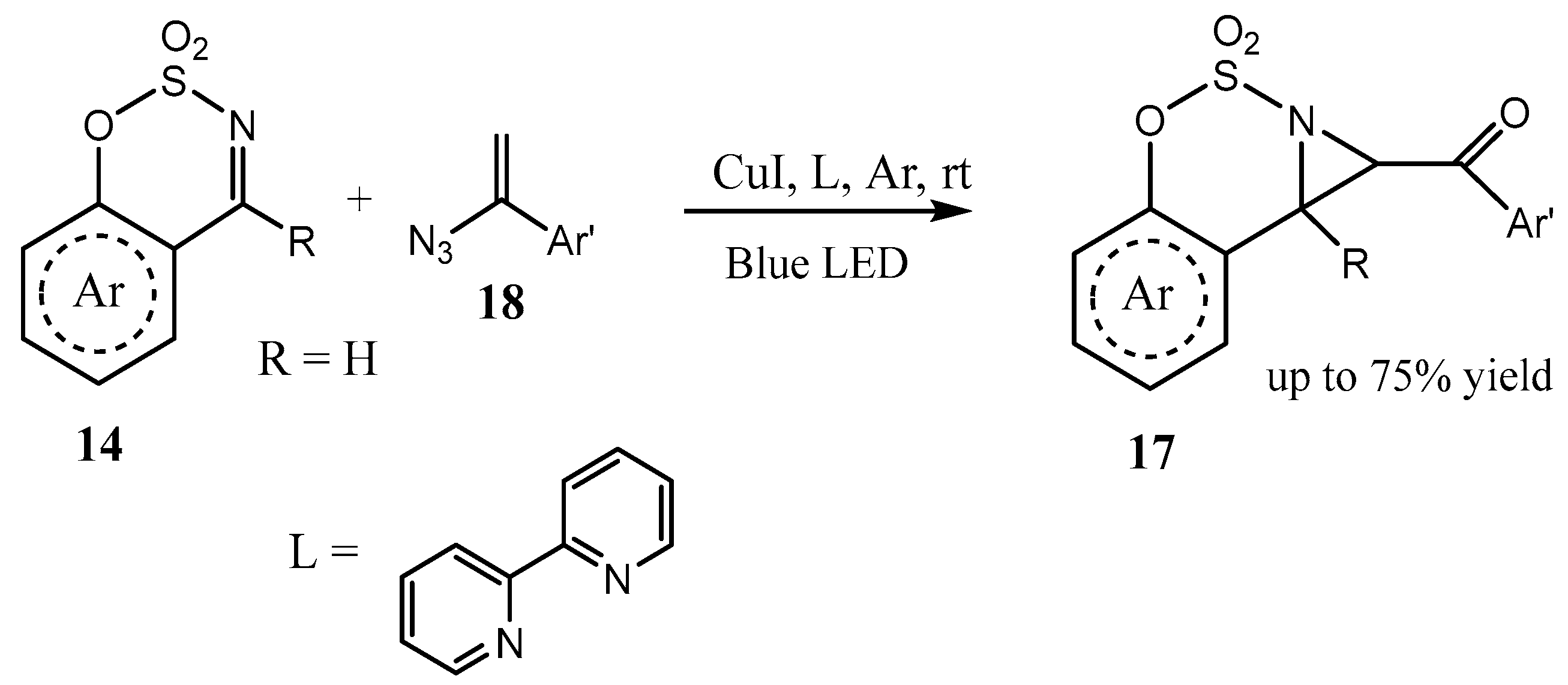
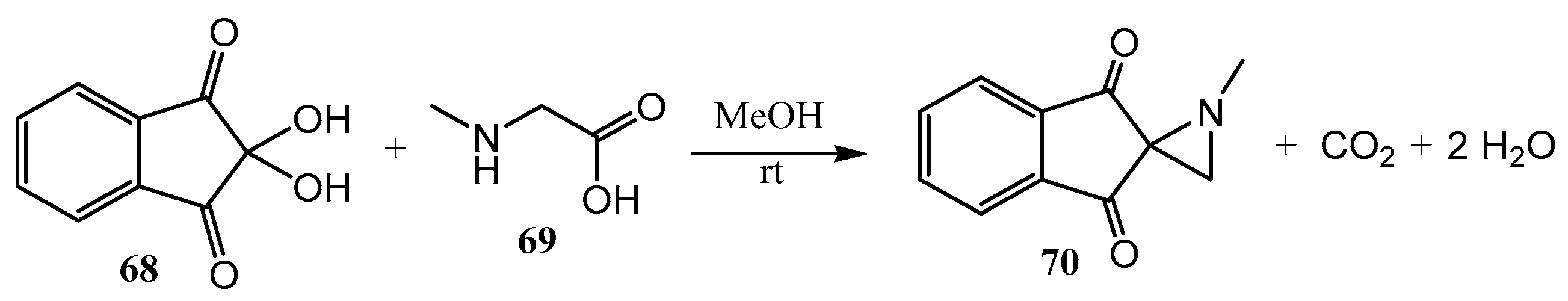
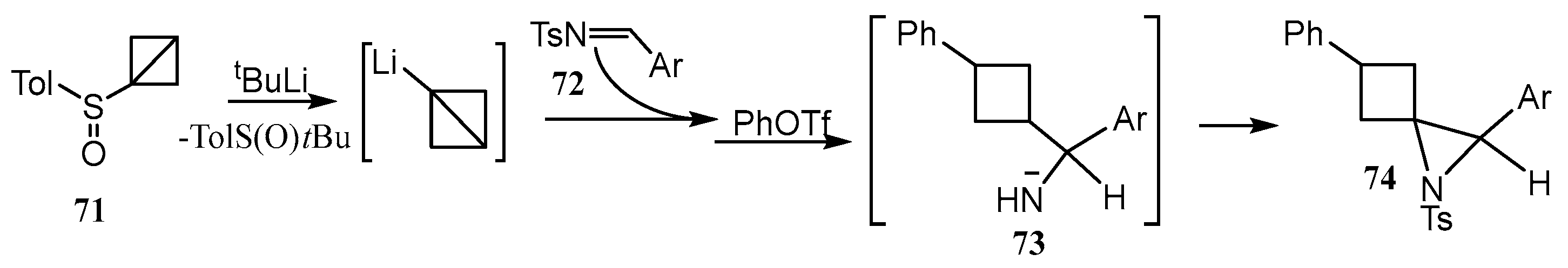
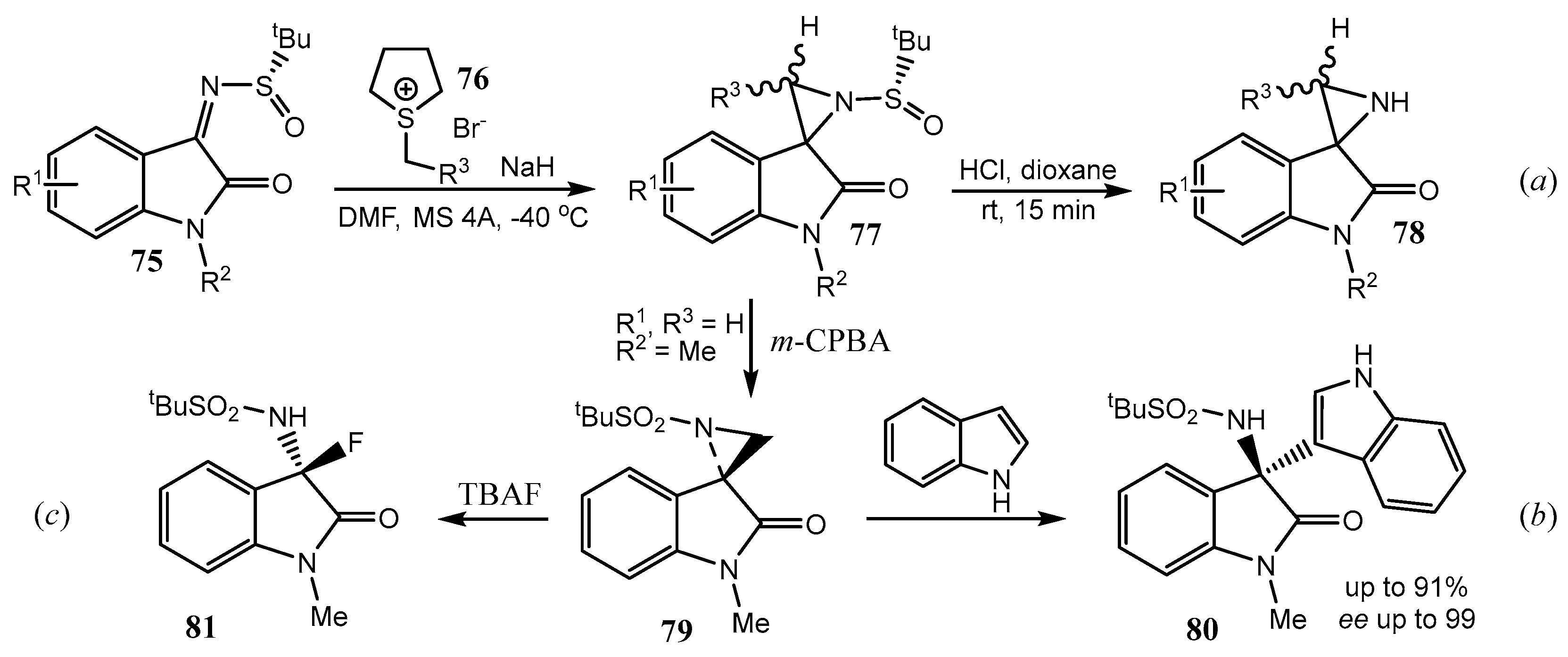
2.2. Spiro Aziridines and Diaziridines
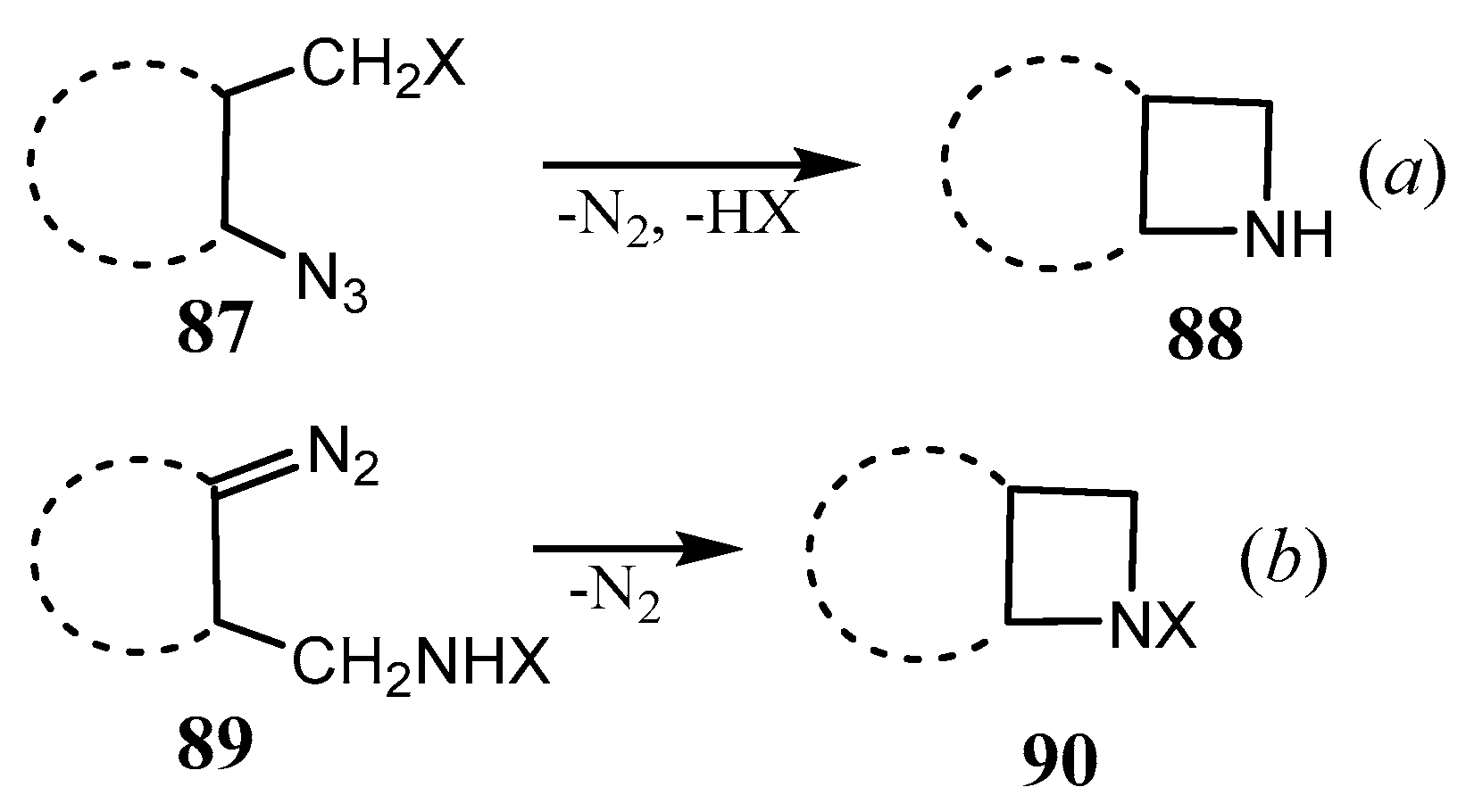
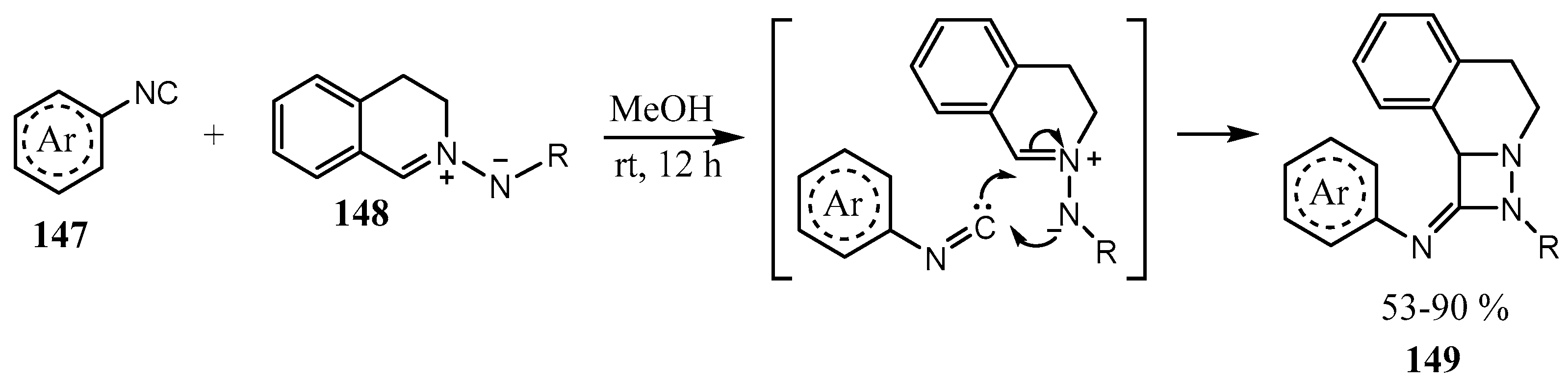
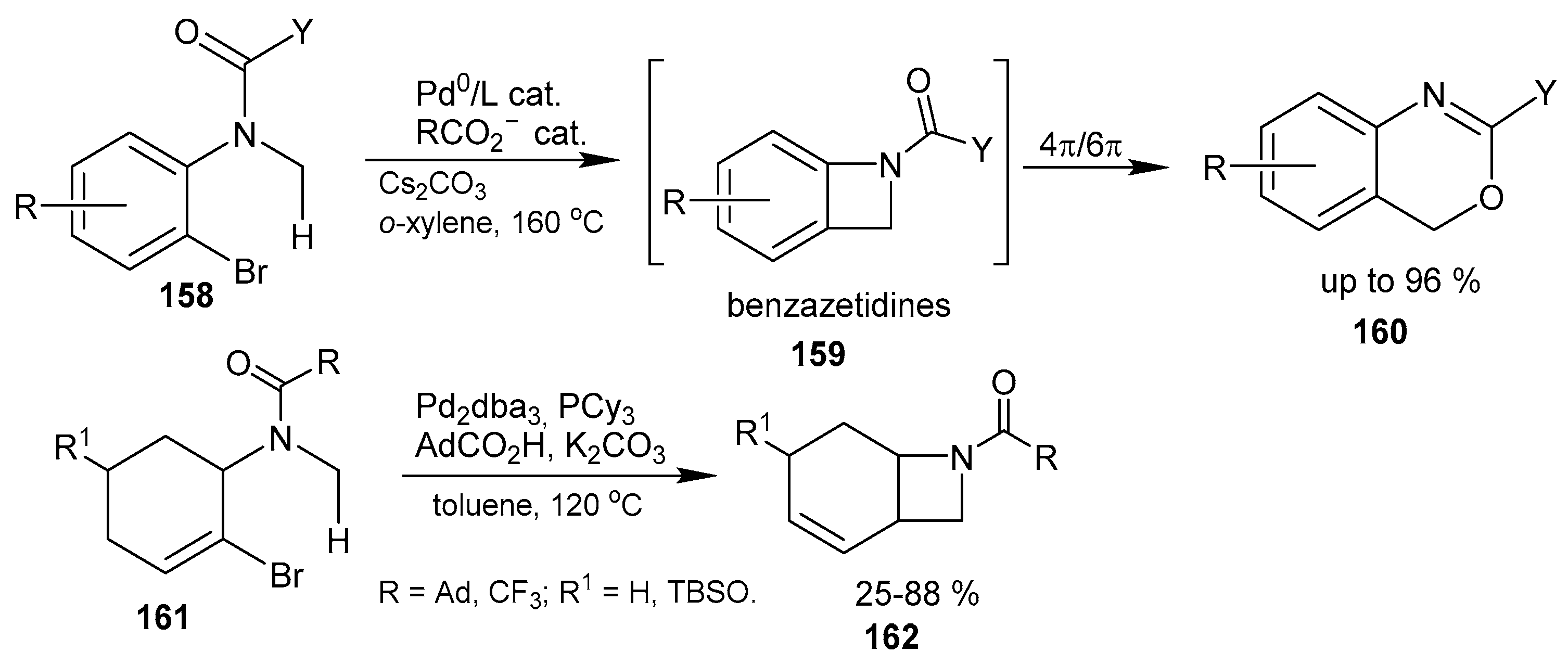
2.3. Fused Azetidines and Diazetidines
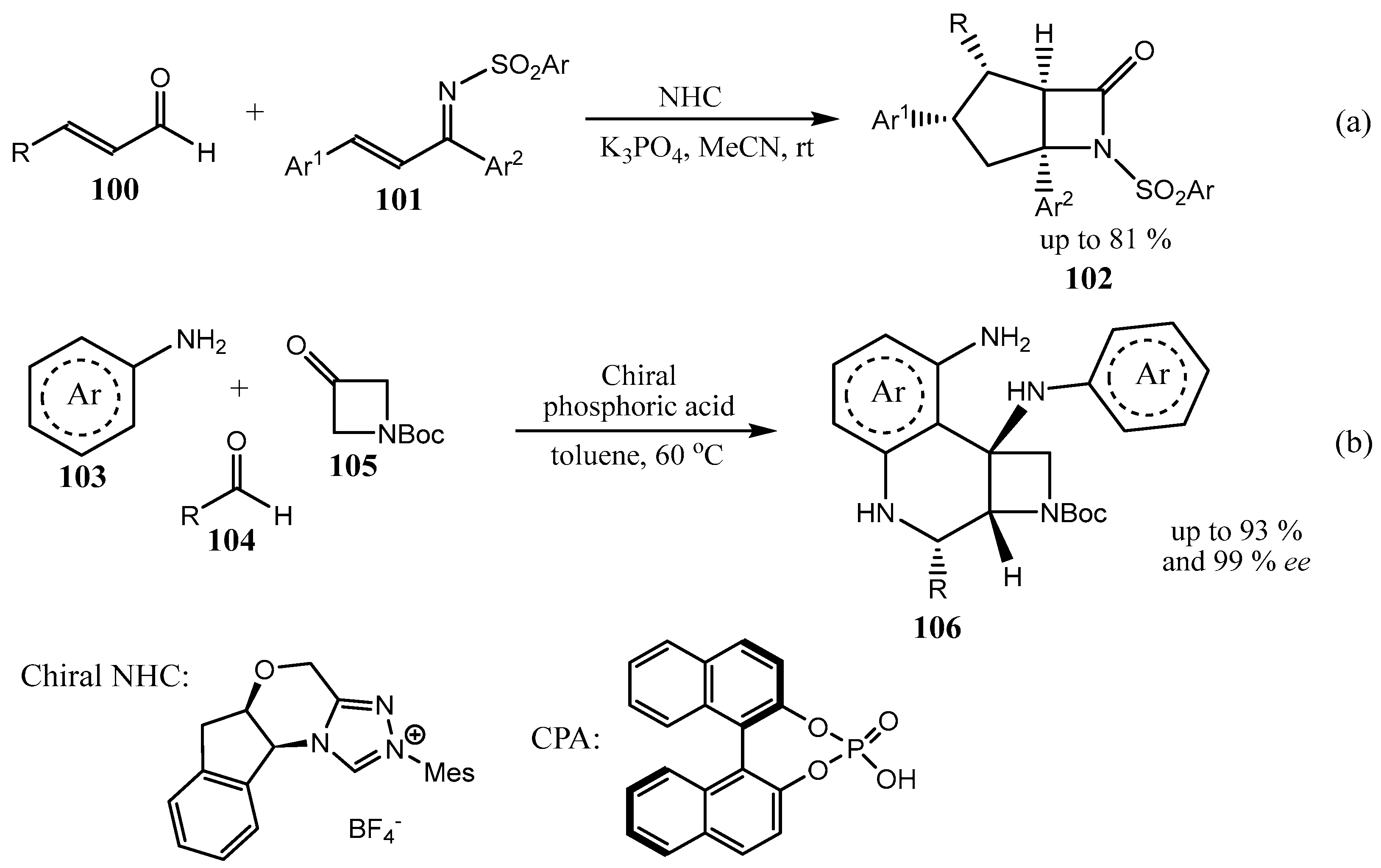
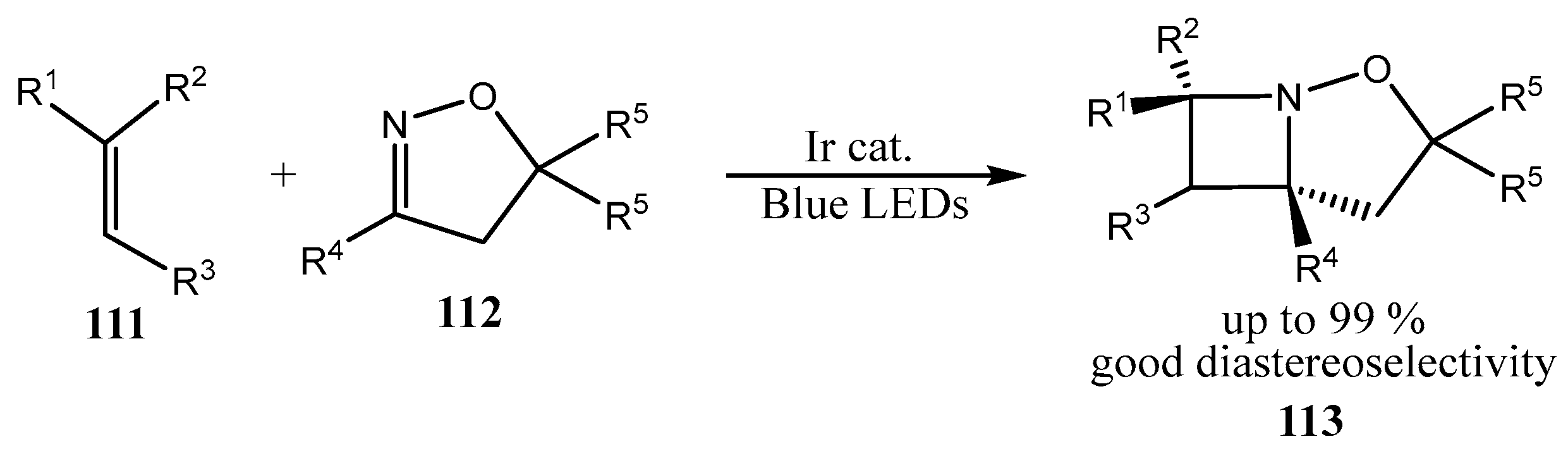
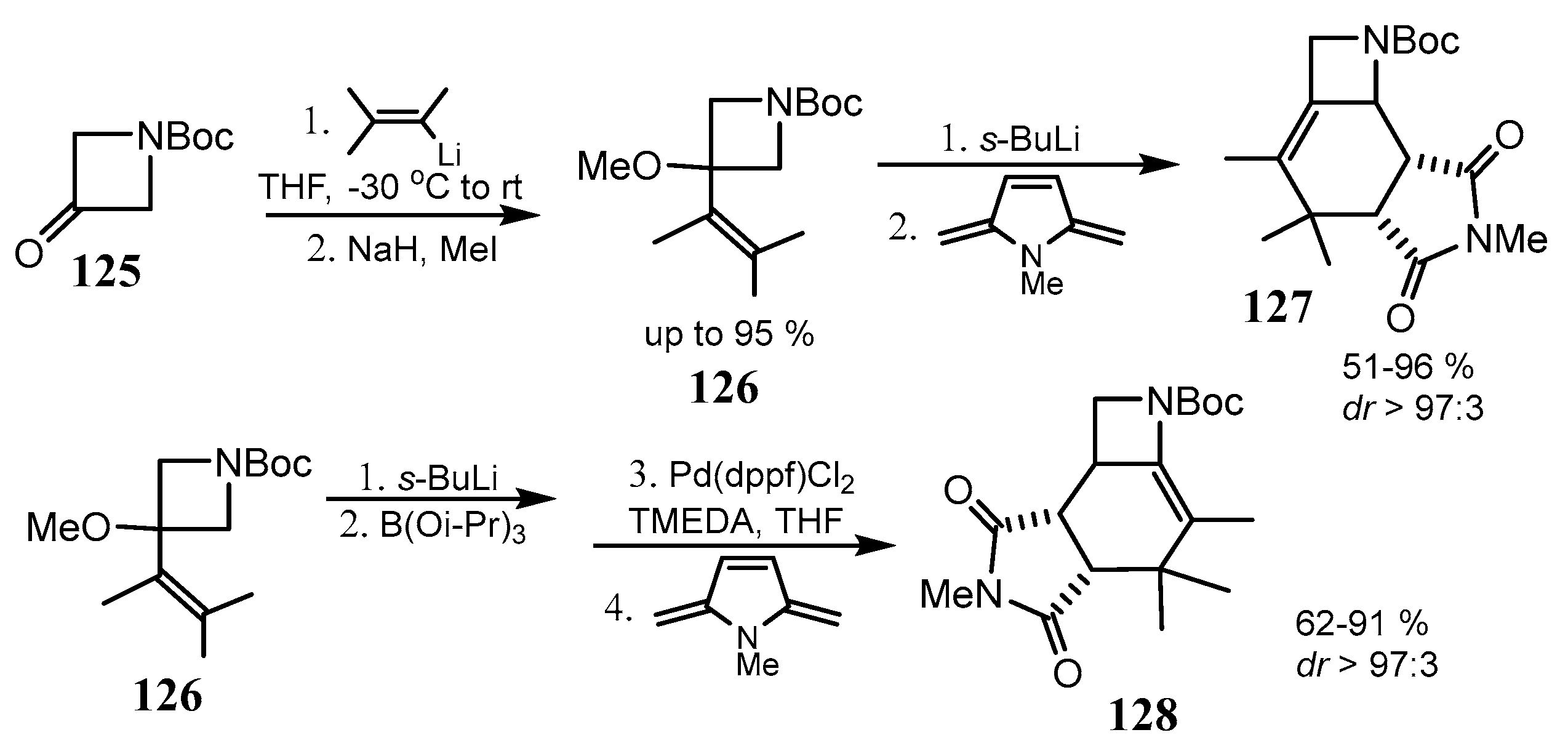
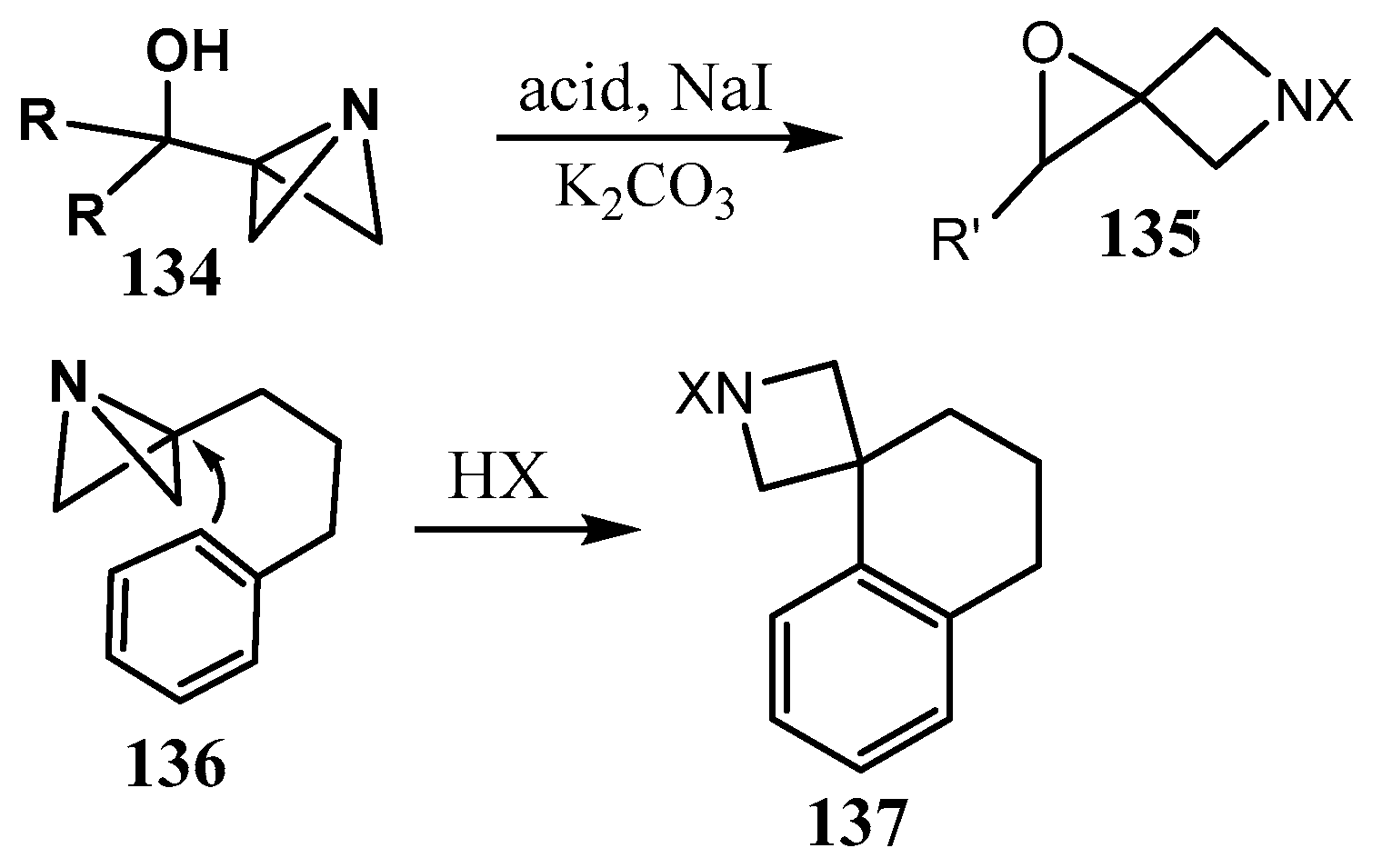
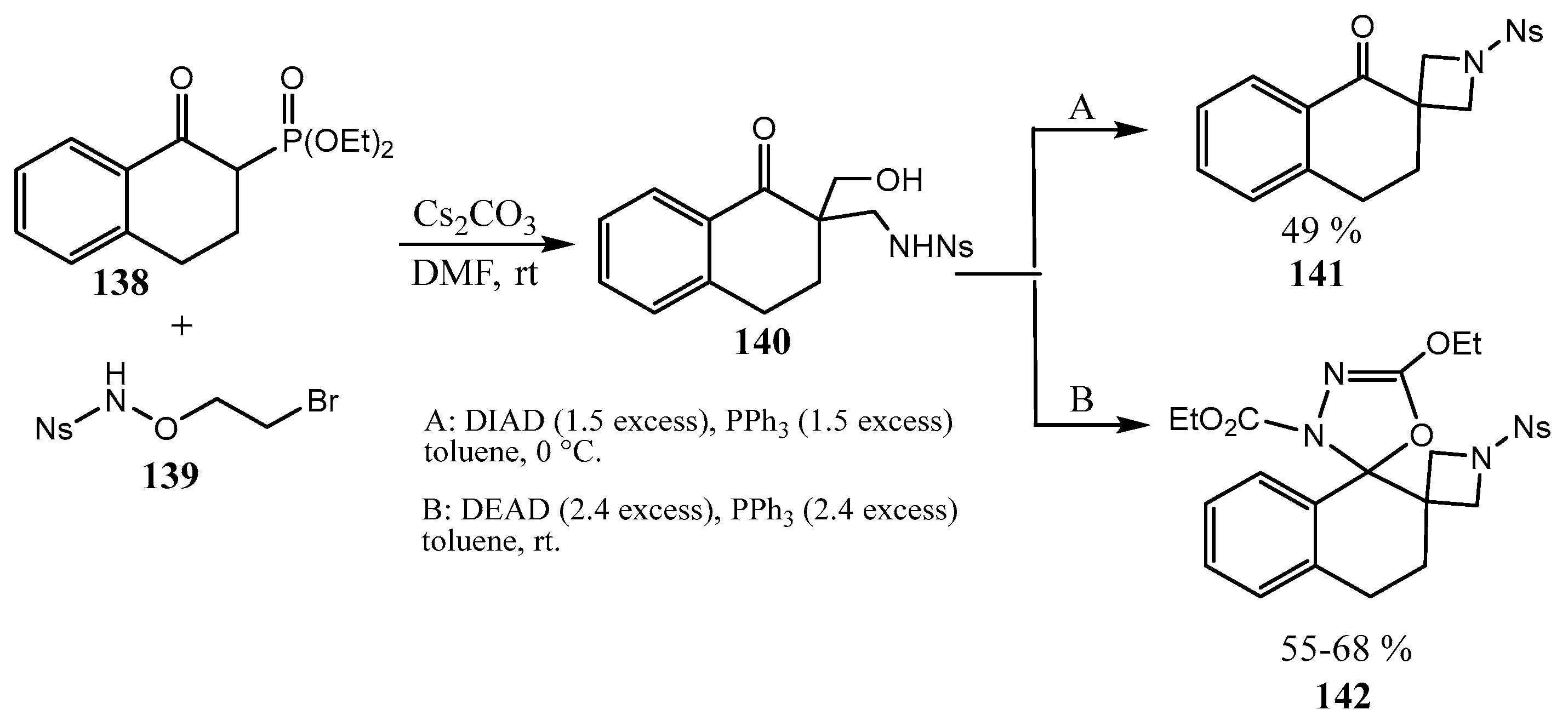
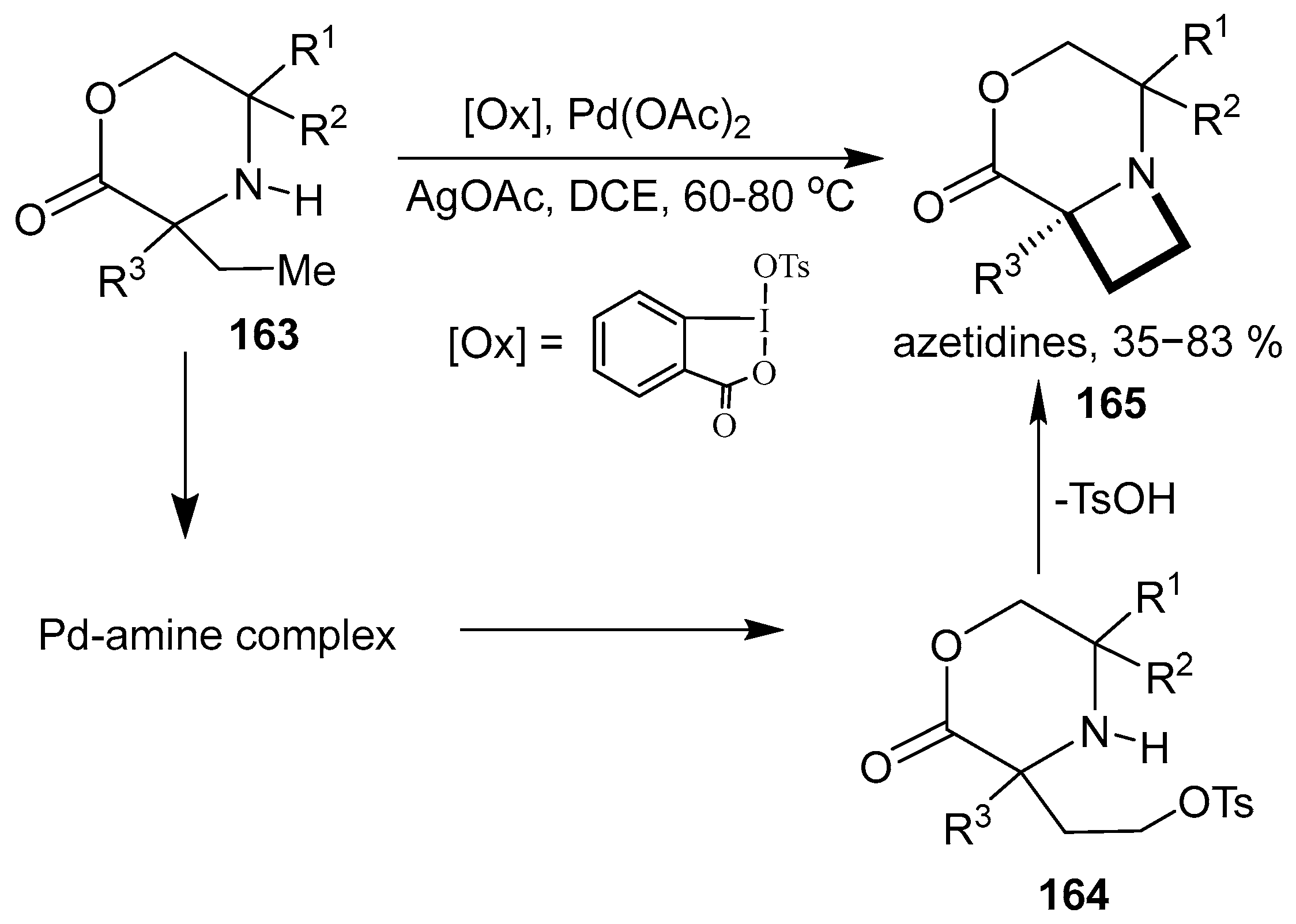
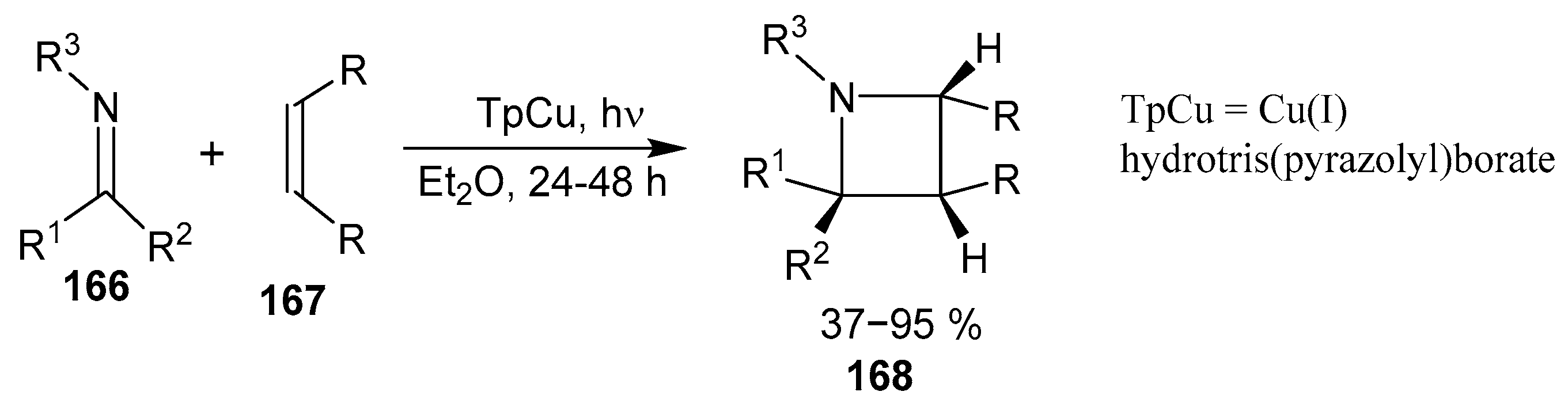
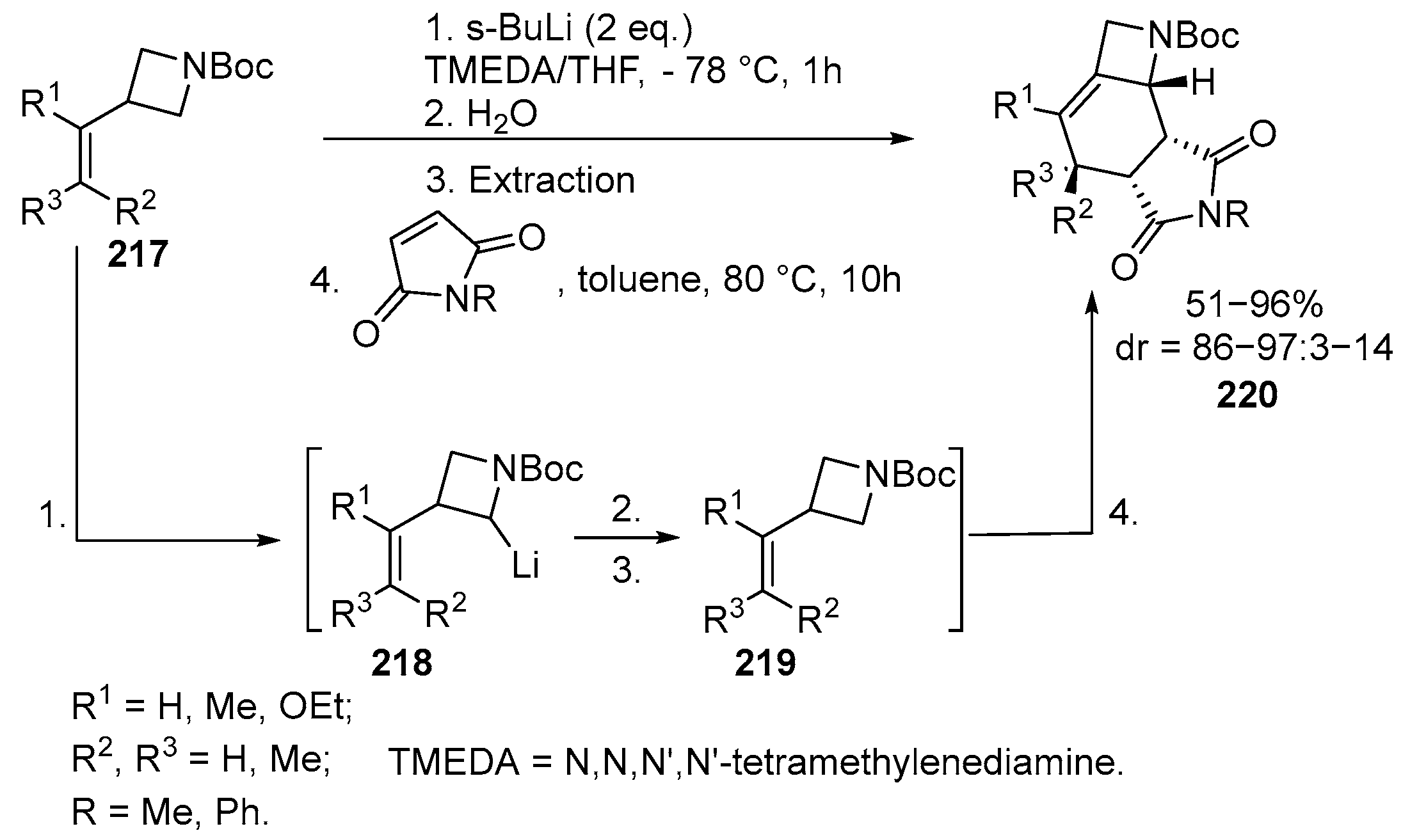
2.4. Spiro-Fused Azetidines
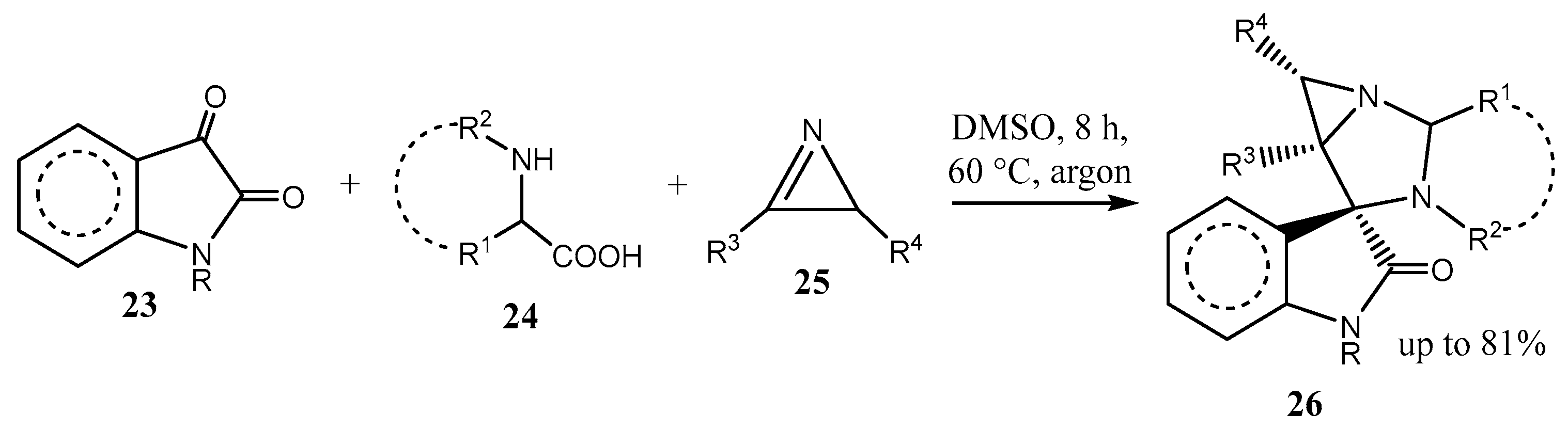
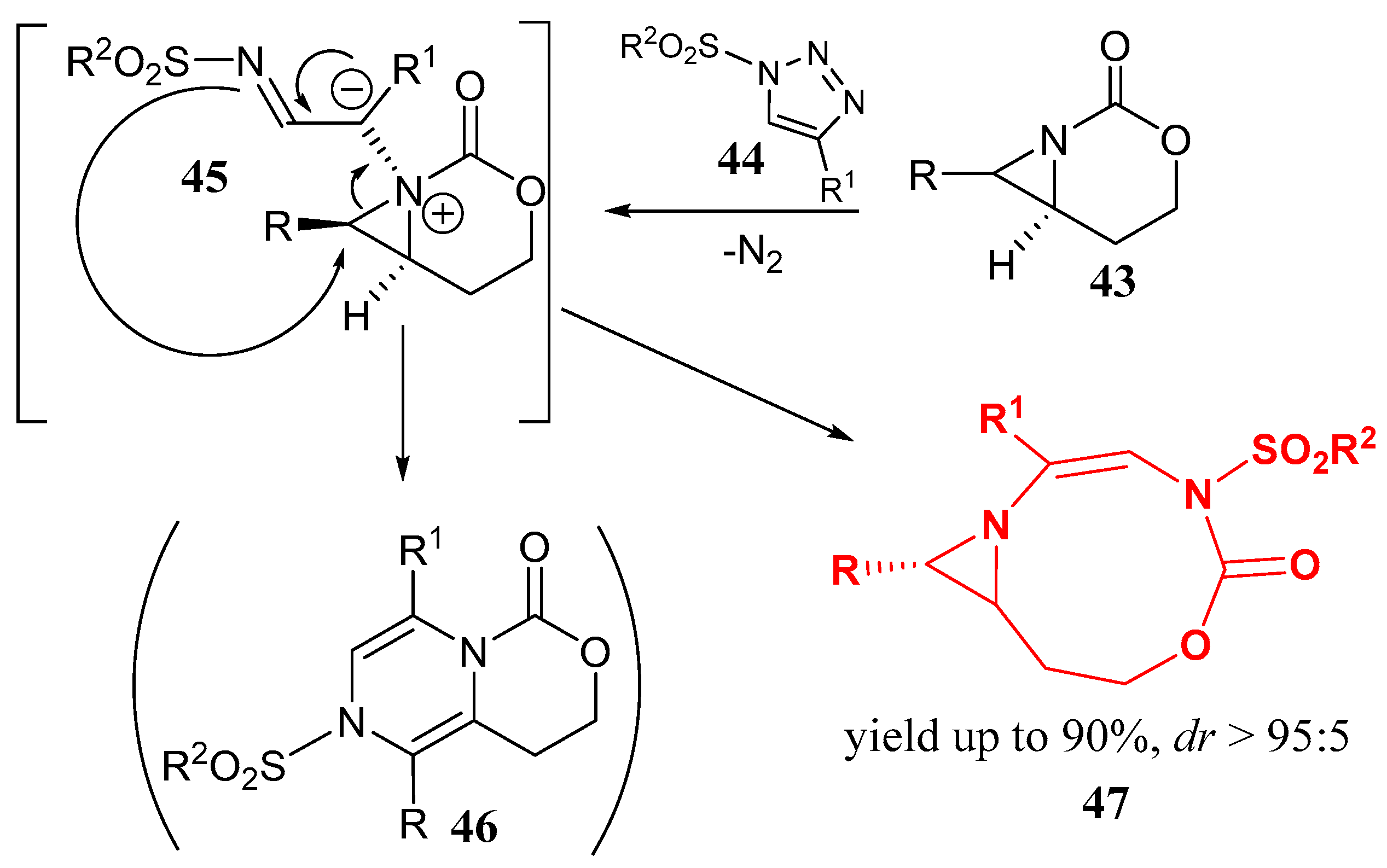
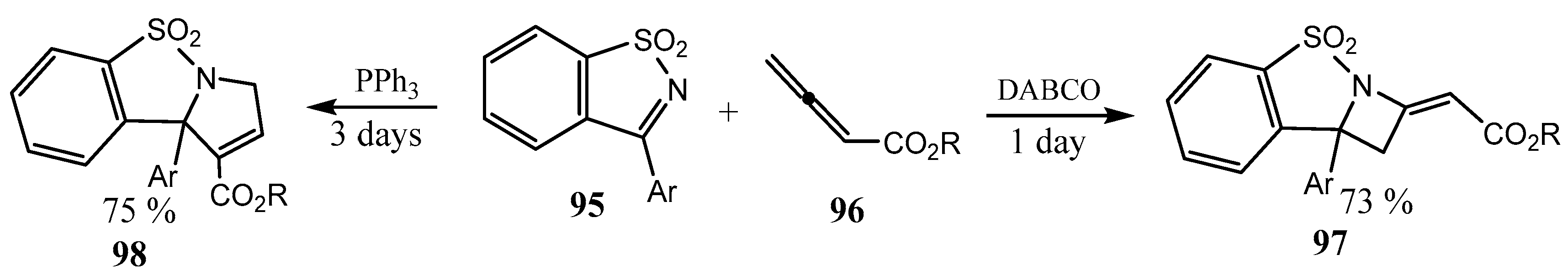
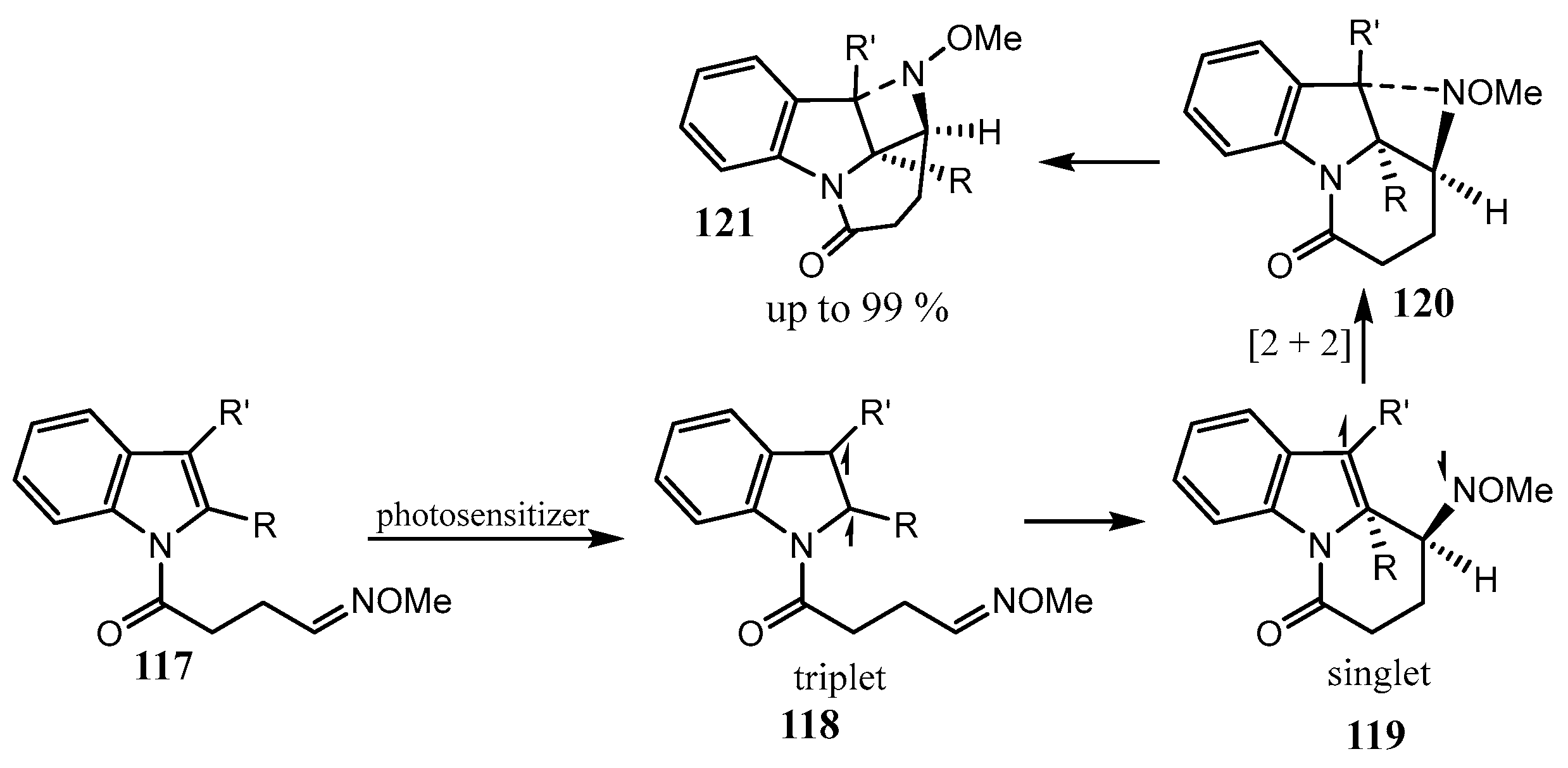
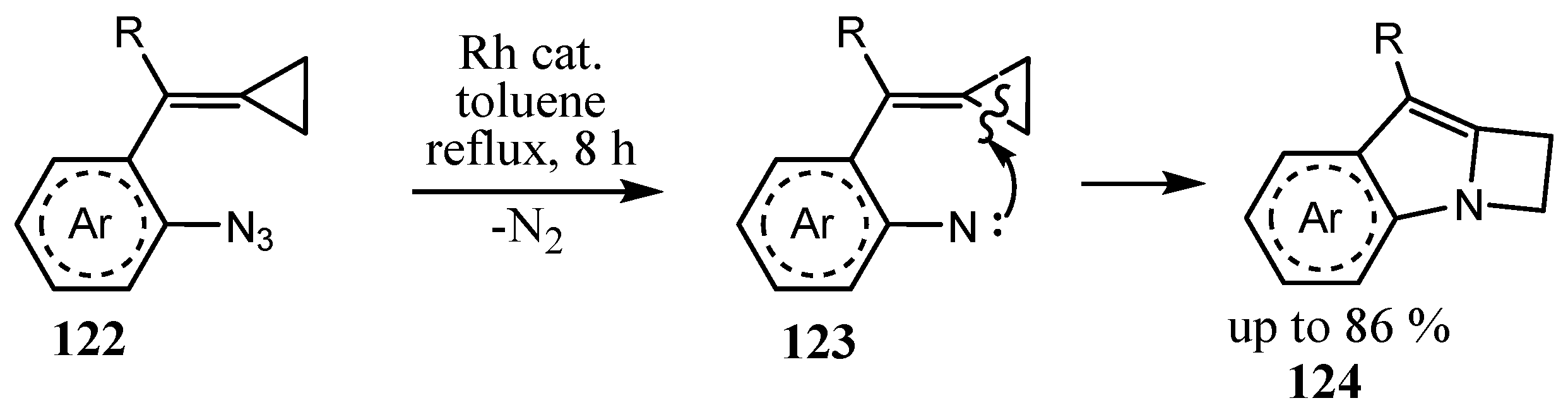
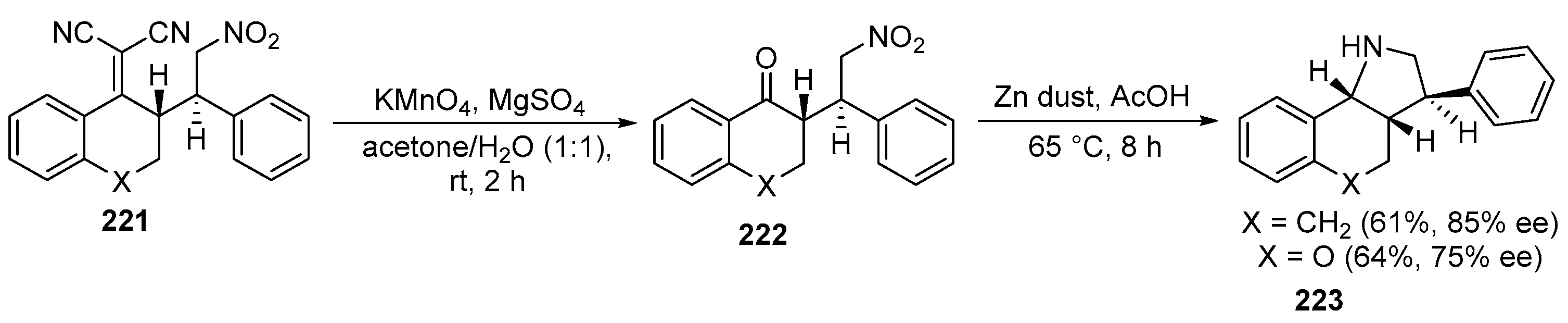
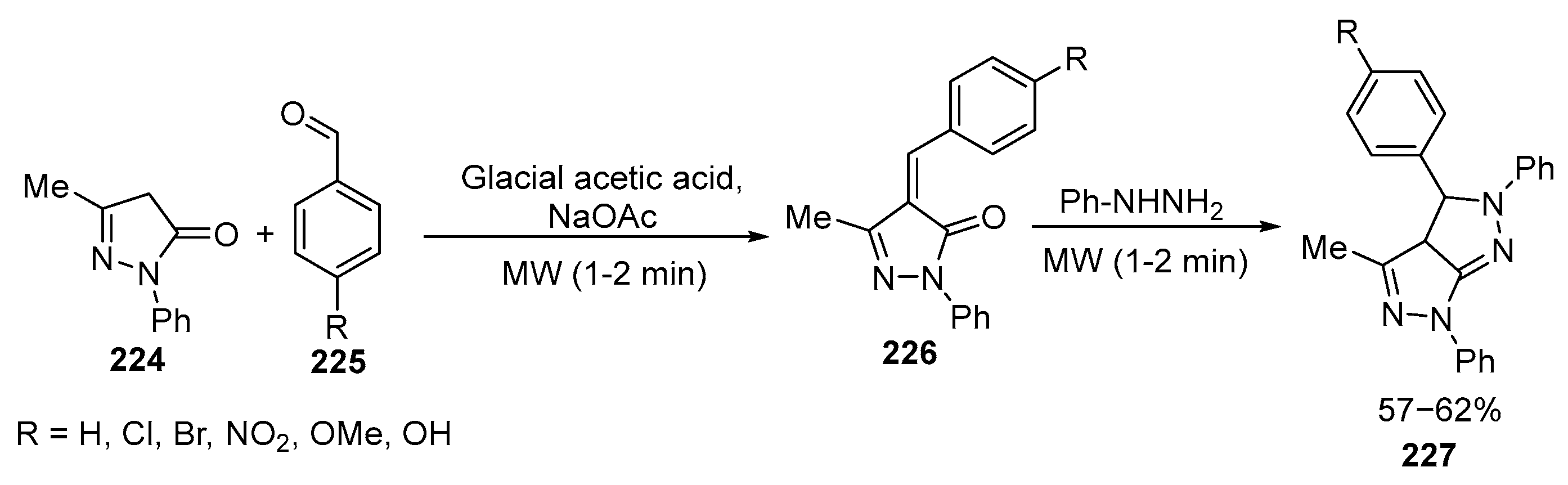
2.5. Fused 5-Membered Heterocycles
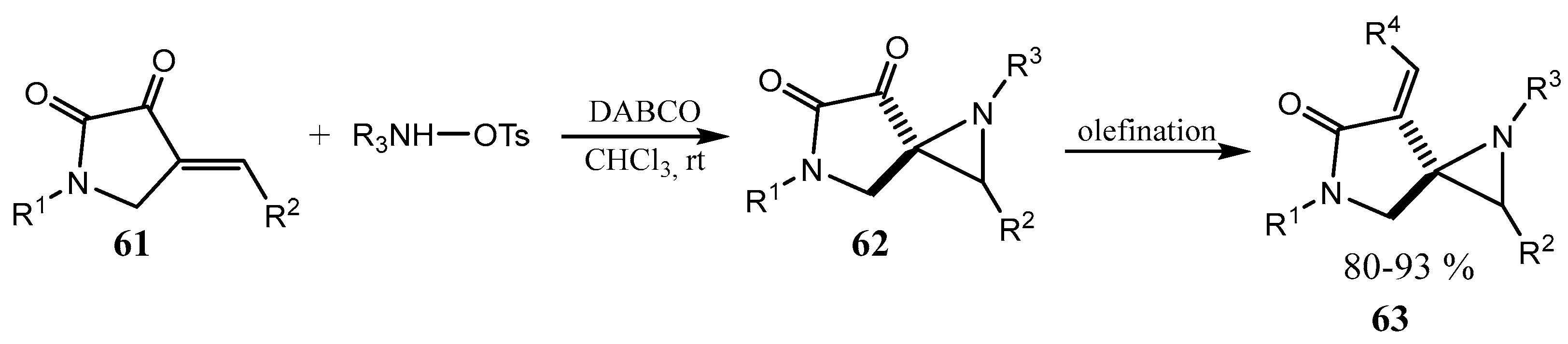
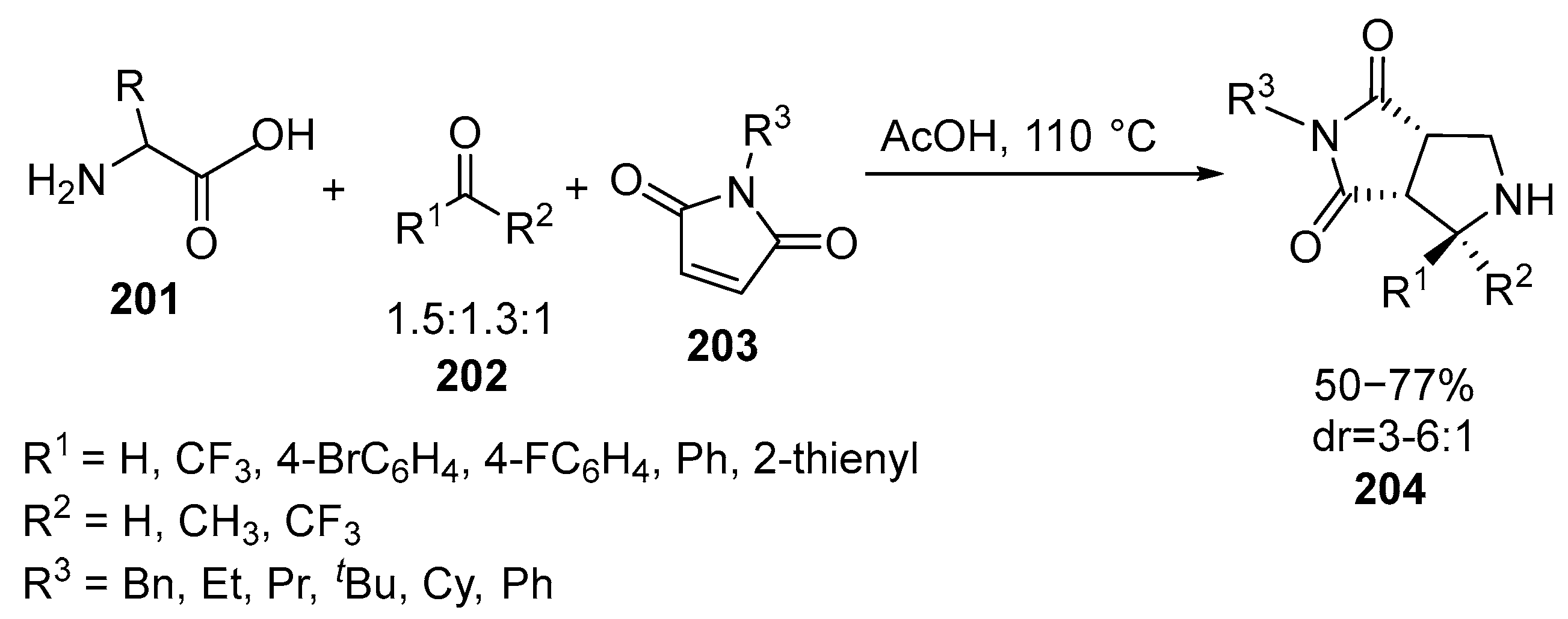
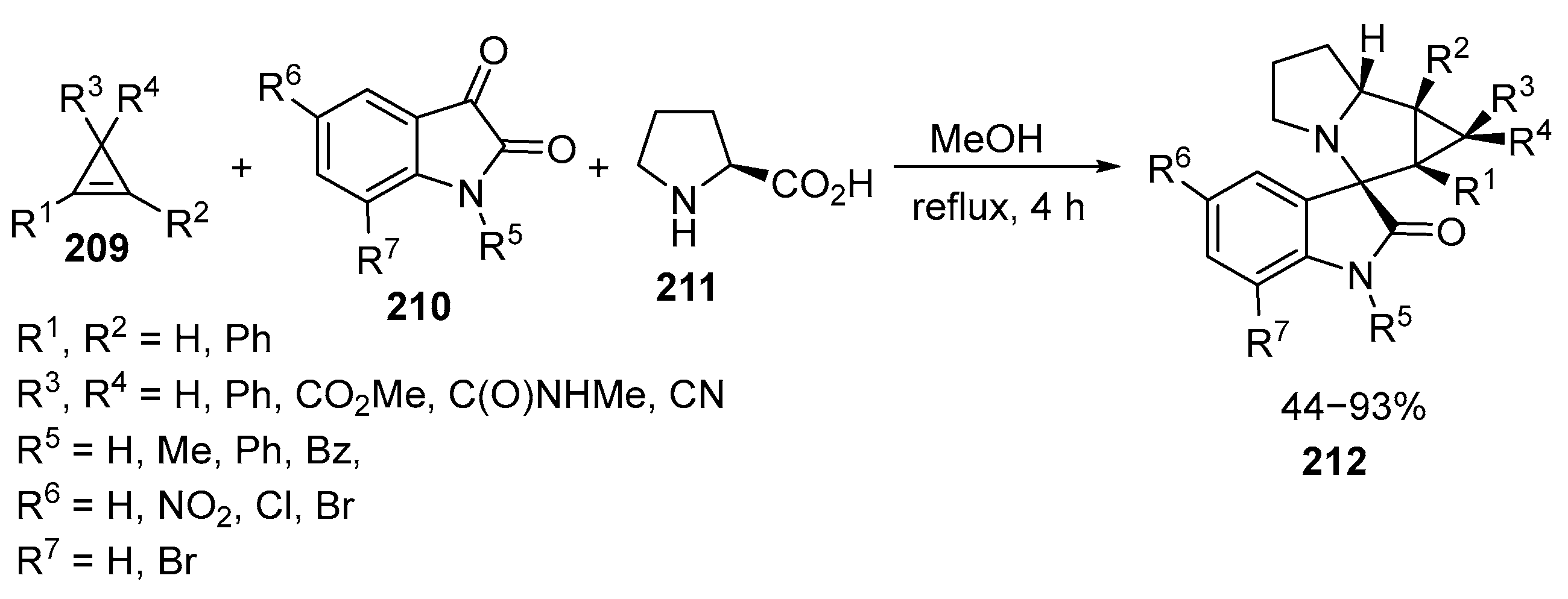
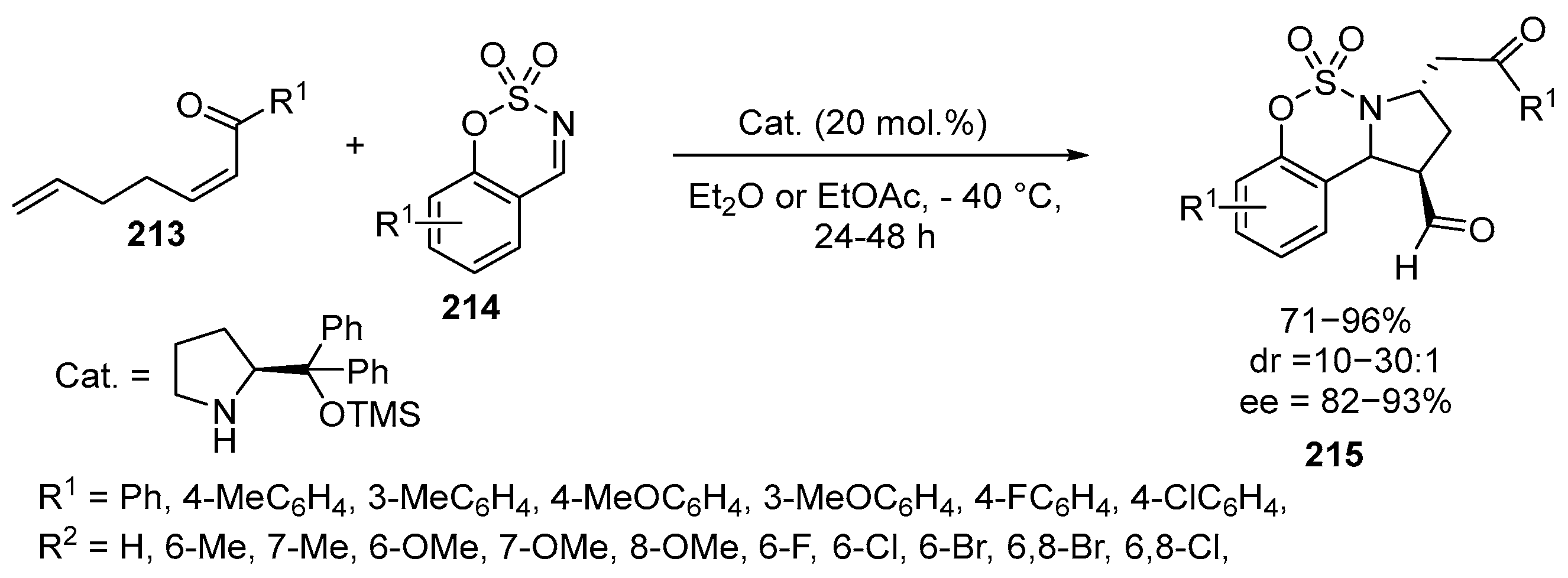
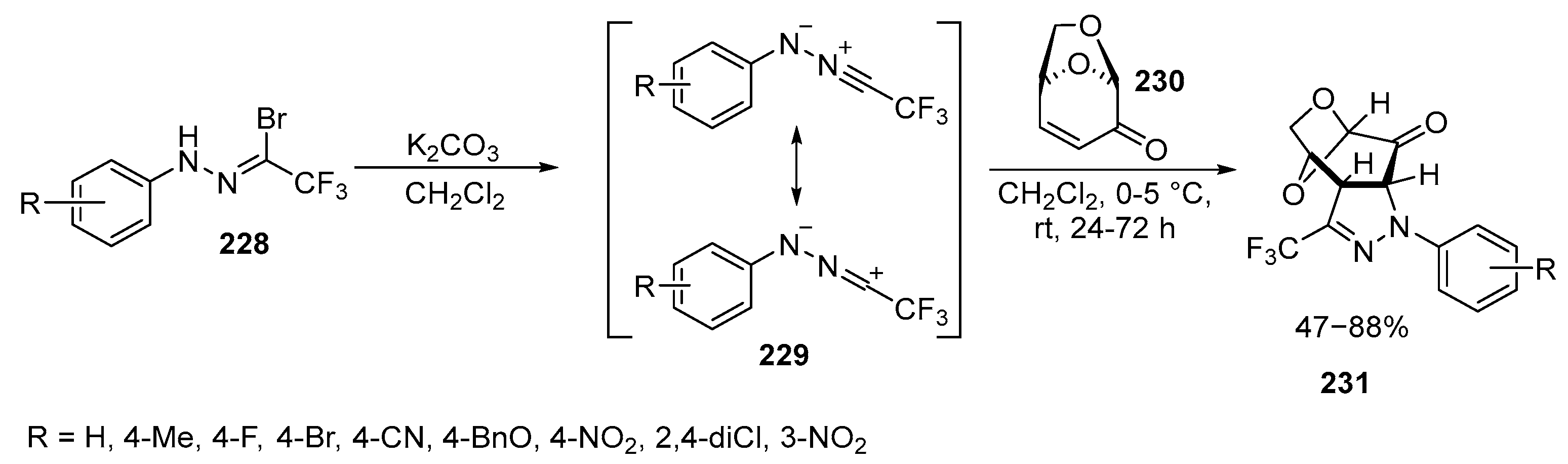
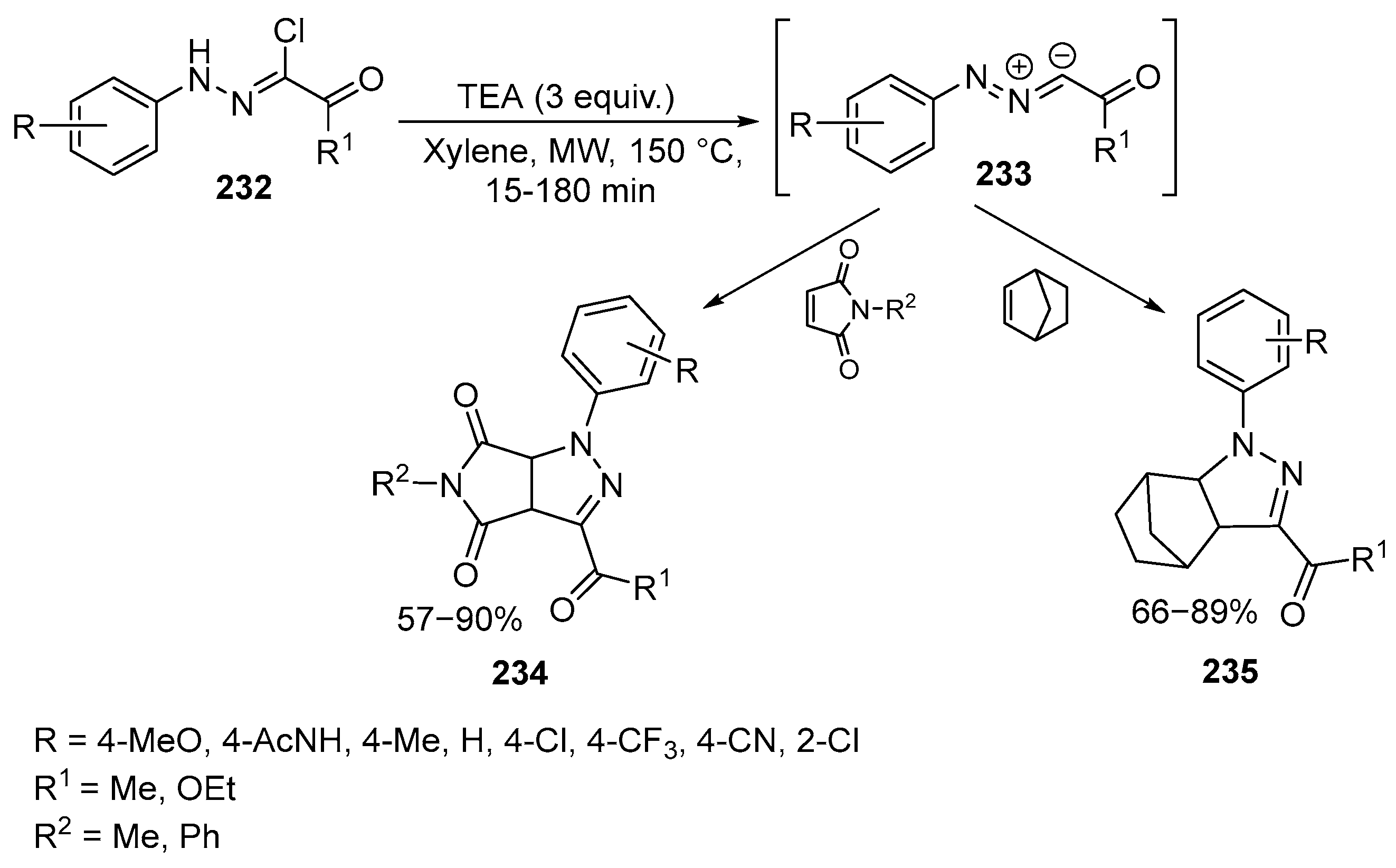
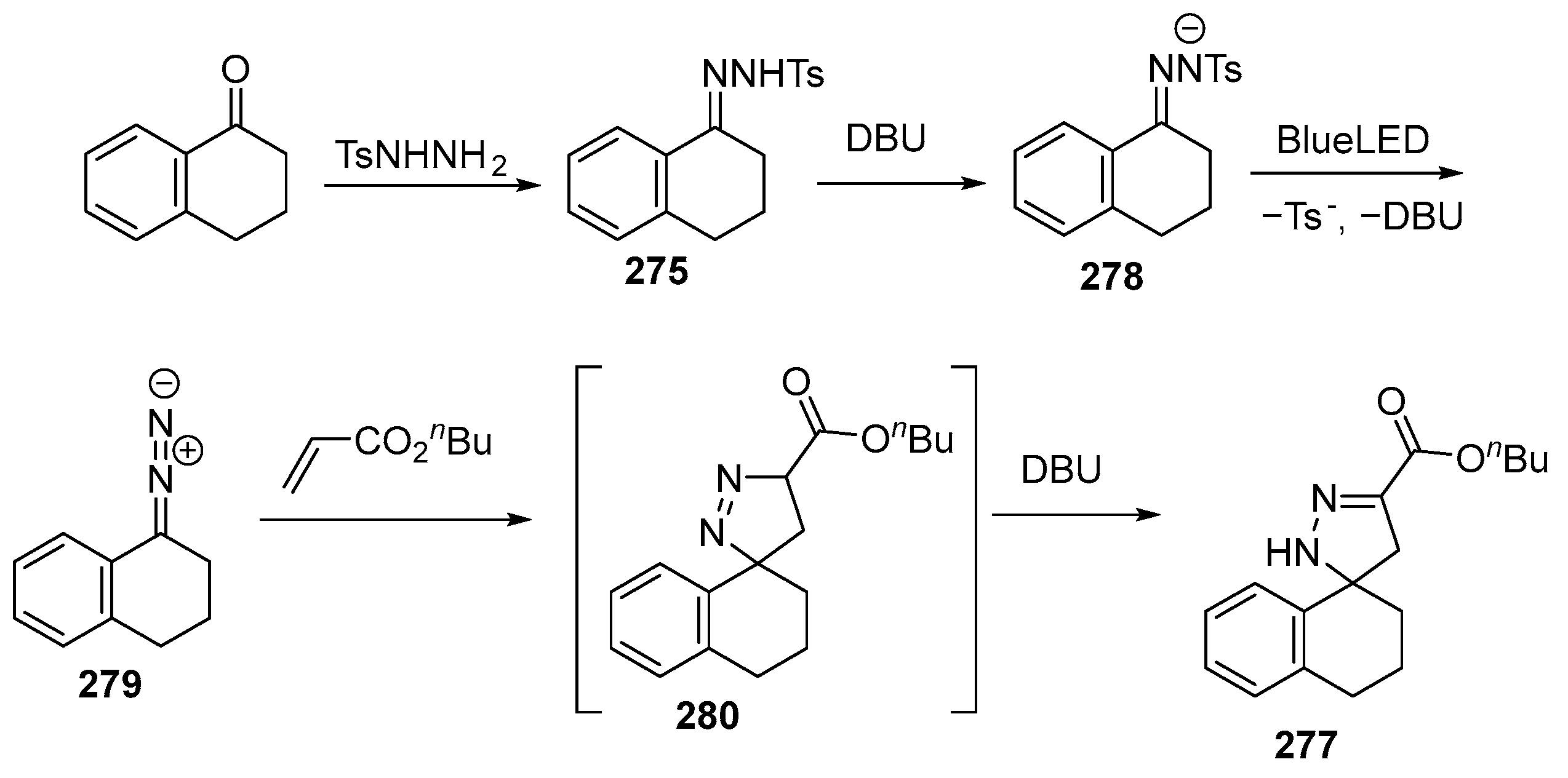
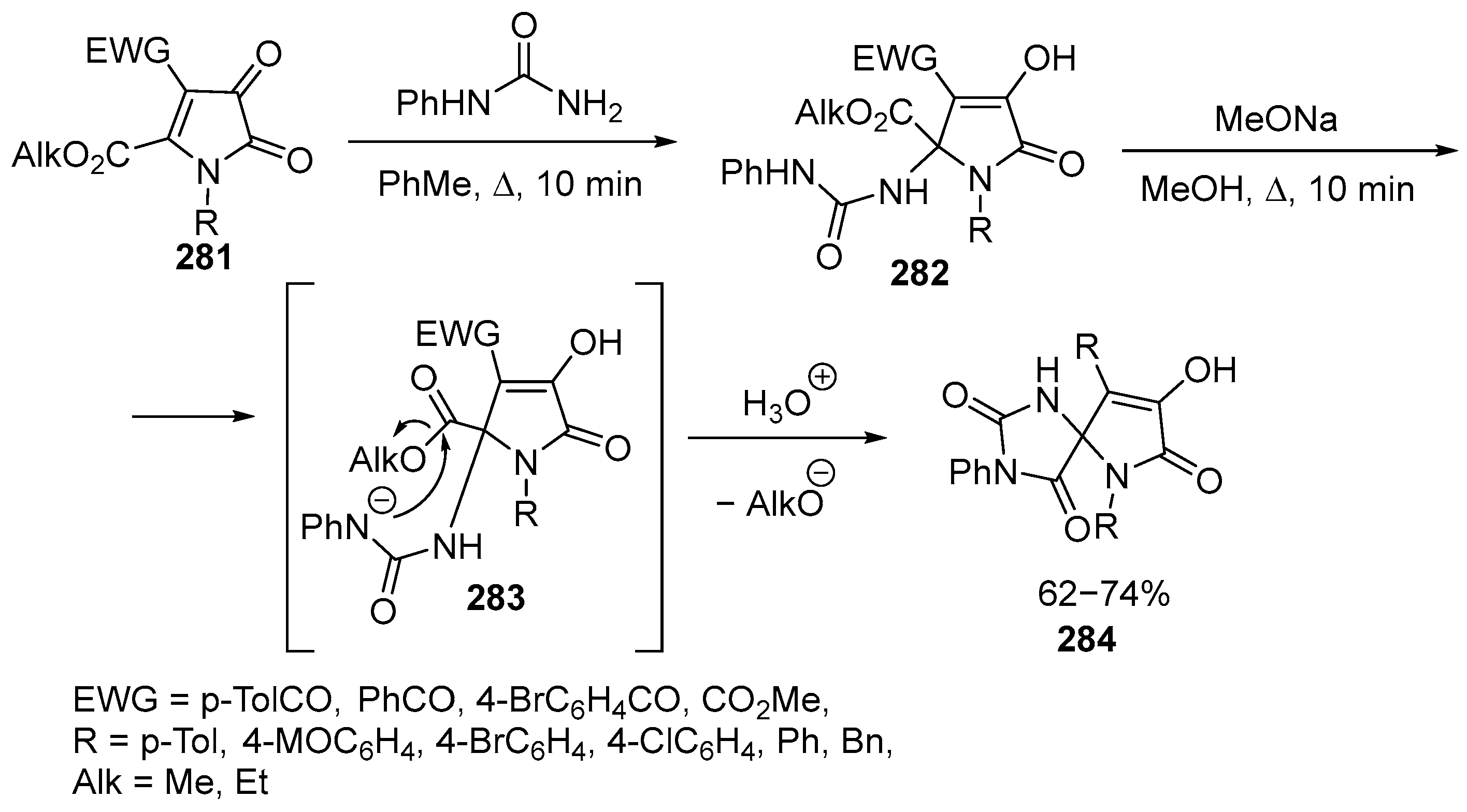
2.6. Spiro-5-Membered Heterocycles
3. Conclusions
Funding
Conflicts of Interest
References
- Heravi, M.M.; Zadsirjan, V. Prescribed drugs containing nitrogen heterocycles: An overview. RSC Adv. 2020, 10, 44247–44311. [Google Scholar] [CrossRef]
- Jampilek, J. Heterocycles in Medicinal Chemistry. Molecules 2019, 24, 3839. [Google Scholar] [CrossRef]
- Lee, B.; Gyu, K.D.; Aram, L.; Mi, K.Y.; Lianji, C.; Sunghoon, K.; Choi, I. Synthesis and discovery of the first potent proteolysis targeting chimaera (PROTAC) degrader of AIMP2-DX2 as a lung cancer drug. J. Enzym. Inhib. Med. Chem. 2023, 38, 51–66. [Google Scholar] [CrossRef]
- Barreca, M.; Spanò, V.; Rocca, R.; Bivacqua, R.; Gualtieri, G.; Raimondi, M.V.; Gaudio, E.; Bortolozzi, R.; Manfreda, L.; Bai, R.; et al. Identification of pyrrolo [3,4:3,4] cyclohepta [1,2-d][1,2] oxazoles as promising new candidates for the treatment of lymphomas. Eur. J. Med. Chem. 2023, 254, 115372. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Liu, Y.; Qin, H.; Zhao, Z.; Wang, S.; He, W.; Tang, S.; Peng, J. Nitrogen-containing heterocyclic drug products approved by the FDA in 2023: Synthesis and biological activity. Eur. J. Med. Chem. 2024, 279, 116838. [Google Scholar] [CrossRef] [PubMed]
- Bivacqua, R.; Barreca, M.; Spanò, V.; Raimondi, M.V.; Romeo, I.; Alcaro, S.; Andrei, G.; Barraja, P.; Montalbano, A. Insight into non-nucleoside triazole-based systems as viral polymerases inhibitors. Eur. J. Med. Chem. 2023, 249, 115136. [Google Scholar] [CrossRef]
- Moskalik, M.Y. Sulfonamides with Heterocyclic Periphery as Antiviral Agents. Molecules 2023, 28, 51. [Google Scholar] [CrossRef]
- Anton, S.G.; Mariya, M.S.; Mikhail Yu, M. Synthesis of N-Heterocycles and Linear Adducts from Allyl-Containing Substrates and Sulfonamides. Cur. Org. Chem. 2025, 29, 1145–1158. [Google Scholar]
- Dank, C.; Ielo, L. Recent advances in the accessibility, synthetic utility, and biological applications of aziridines. Org. Biomol. Chem. 2023, 21, 4553–4573. [Google Scholar] [CrossRef]
- da Silva, A.R.; dos Santos, D.A.; Paixão, M.W.; Corrêa, A.G. Stereoselective Multicomponent Reactions in the Synthesis or Transformations of Epoxides and Aziridines. Molecules 2019, 24, 630. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.S.; Sudheesh, S.; Keroletswe, N. Recent applications of aziridine ring expansion reactions in heterocyclic synthesis. ARKIVOC 2017, 2018, 50–113. [Google Scholar] [CrossRef]
- Liu, Q.-H.; Liu, J.; Han, Y.-P.; Liang, Y.-M.; Peng, L.-Z. Synthetic Approaches for the Construction of Chiral Aziridines. Eur. J. Org. Chem. 2025, 28, e202401048. [Google Scholar] [CrossRef]
- Gatazka, M.R.; McFee, E.C.; Ng, C.H.; Wearing, E.R.; Schindler, C.S. New strategies for the synthesis of 1- and 2-azetines and their applications as value-added building blocks. Org. Biomol. Chem. 2022, 20, 9052–9068. [Google Scholar] [CrossRef] [PubMed]
- Malarney, K.P.; Kc, S.; Schmidt, V.A. Recent strategies used in the synthesis of saturated four-membered heterocycles. Org. Biomol. Chem. 2021, 19, 8425–8441. [Google Scholar] [CrossRef]
- Li Petri, G.; Raimondi, M.V.; Spanò, V.; Holl, R.; Barraja, P.; Montalbano, A. Pyrrolidine in Drug Discovery: A Versatile Scaffold for Novel Biologically Active Compounds. Top. Curr. Chem. 2021, 379, 34. [Google Scholar] [CrossRef]
- Kumar, A.; Khan, W.A.; Ahamad, S.; Mohanan, K. Trifluorodiazoethane: A versatile building block to access trifluoromethylated heterocycles. J. Heterocycl. Chem. 2022, 59, 607–632. [Google Scholar] [CrossRef]
- Mótyán, G.; Molnár, B.; Wölfling, J.; Frank, É. Microwave-Assisted Stereoselective Heterocyclization to Novel Ring d-fused Arylpyrazolines in the Estrone Series. Molecules 2019, 24, 569. [Google Scholar] [CrossRef]
- Sadjadi, S.; Heravi, M.M.; Nazari, N. Isocyanide-based multicomponent reactions in the synthesis of heterocycles. RSC Adv. 2016, 6, 53203–53272. [Google Scholar] [CrossRef]
- Ding, A.; Meazza, M.; Guo, H.; Yang, J.W.; Rios, R. New development in the enantioselective synthesis of spiro compounds. Chem. Soc. Rev. 2018, 47, 5946–5996. [Google Scholar] [CrossRef]
- Gataullin, R.R. Advances in the Synthesis of Benzo-Fused Spiro Nitrogen Heterocycles: New Approaches and Modification of Old Strategies. Helv. Chim. Acta 2020, 103, e2000137. [Google Scholar] [CrossRef]
- Zhou, H.; Shen, M.-H.; Xu, H.-D. Evolution of the Aza-Diels–Alder Reaction of 2H-Azirines. Synlett 2016, 27, 2171–2177. [Google Scholar]
- Haghdadi, M.; Norouzi, K.; Hamzehloueian, M. Evaluation of the mechanism, regio-, and diastereoselectivity of aza-Diels–Alder reactions of 2H-azirine under a Lewis acid catalyst. Struct. Chem. 2022, 33, 445–456. [Google Scholar] [CrossRef]
- Xu, H.D.; Zhou, H.; Pan, Y.P.; Ren, X.T.; Wu, H.; Han, M.; Han, R.Z.; Shen, M.H. Stereoselective Synthesis of Polycycles Containing an Aziridine Group: Intramolecular aza-Diels–Alder Reactions of Unactivated 2H-Azirines with Unactivated Dienes. Angew. Chem. Int. Ed. 2016, 55, 2540–2544. [Google Scholar] [CrossRef]
- Zheng, F.; Zhou, J.; Fang, F.; Li, J.; Wang, J.; Zheng, M.; Liu, H.; Xu, Y.; Zhou, Y. Rh(III)-Catalyzed C–H Activation and [4+1+1] Sequential Cyclization Cascade to Give Highly Fused Indano [1,2-b]azirines. Org. Lett. 2022, 24, 5688–5692. [Google Scholar] [CrossRef]
- Lai, B.-N.; Qiu, J.-F.; Zhang, H.-X.; Nie, J.; Ma, J.-A. Stereoselective Synthesis of Fused Aziridines via One-Pot Sequential Decarboxylative Mannich Reaction and Oxidative C–H Amination of Cyclic Imines with β-Ketoacids. Org. Lett. 2016, 18, 520–523. [Google Scholar] [CrossRef]
- Banuprakash Goud, S.; Lal Dhakar, R.; Samanta, S. Copper(I)-Photocatalyzed Diastereoselective Aziridination of N-Sulfonyl Imines with Vinyl Azides: Application to Benzo-[1,2,3]oxathiazepines Dioxides and Fused Isoxazolines. Chem. Asian J. 2023, 19, e202300904. [Google Scholar] [CrossRef]
- Lei, X.; Sun, Y.; Guo, Q.; Shi, J. Base mediated aza-[2+1] annulation and regioselective aziridine ring-opening cascade: Mild synthesis of functionalized β-amino ketones from cyclic N-sulfonyl aldimines and α-carbonyl sulfonium salts. RSC Adv. 2024, 14, 17178–17183. [Google Scholar] [CrossRef]
- Wu, J.-H.; Pan, J.; Wang, T. Dipeptide-Based Phosphonium Salt Catalysis: Application to Enantioselective Synthesis of Fused Tri- and Tetrasubstituted Aziridines. Synlett 2019, 30, 2101–2106. [Google Scholar] [CrossRef]
- Pan, J.; Wu, J.H.; Zhang, H.; Ren, X.; Tan, J.P.; Zhu, L.; Zhang, H.S.; Jiang, C.; Wang, T. Highly Enantioselective Synthesis of Fused Tri-and Tetrasubstituted Aziridines: Aza-Darzens Reaction of Cyclic Imines with α-Halogenated Ketones Catalyzed by Bifunctional Phosphonium Salt. Angew. Chem. Int. Ed. 2019, 58, 7425–7430. [Google Scholar] [CrossRef]
- Zhang, S.; Li, L.; Xin, L.; Liu, W.; Xu, K. Asymmetric Aziridination of N-Sulphonyl Ketimines with Unfunctionalized Ketones: A One-pot Approach to Multisubstituted Fused Aziridines. J. Org. Chem. 2017, 82, 2399–2406. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Wang, M.C.; Wie, P.; Xing, Q.; Liu, C.H. A One-Pot Approach to Multi-Substituted Fused Aziridines: Formal Intermolecular [2+1] Cycloaddition Reaction between Saccharin-Derived Cyclic Ketimines and Sulfur Ylides. Eur. J. Org. Chem. 2023, 26, e202300300. [Google Scholar] [CrossRef]
- Angyal, A.; Demjén, A.; Harmat, V.; Wölfling, J.; Puskás, L.G.; Kanizsai, I. 1,3-Dipolar Cycloaddition of Isatin-Derived Azomethine Ylides with 2H-Azirines: Stereoselective Synthesis of 1,3-Diazaspiro [bicyclo [3.1.0] hexane]oxindoles. J. Org. Chem. 2019, 84, 4273–4281. [Google Scholar] [CrossRef]
- Nonn, M.; Kiss, L.; Haukka, M.; Fustero, S.; Fülöp, F. A Novel and Selective Fluoride Opening of Aziridines by XtalFluor-E. Synthesis of Fluorinated Diamino Acid Derivatives. Org. Lett. 2015, 17, 1074–1077. [Google Scholar] [CrossRef]
- Nonn, M.; Remete, A.M.; Fülöp, F.; Kiss, L. Recent advances in the transformations of cycloalkane-fused oxiranes and aziridines. Tetrahedron 2017, 73, 5461–5483. [Google Scholar] [CrossRef]
- Blackham, E.E.; Knowles, J.P.; Burgess, J.; Booker-Milburn, K.I. Combining photochemistry and catalysis: Rapid access to sp3-rich polyheterocycles from simple pyrroles. Chem. Sci. 2016, 7, 2302–2307. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-Y.; Angamuthu, V.; Chen, W.-C.; Hou, D.-R. Synthesis of Methyl -Kijanosides by Regio- and Stereoselective Ring Opening of 2-Oxazolidinone-Fused Aziridines. Org. Lett. 2020, 22, 2246–2250. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-C.; Liu, C.-Y.; Angamuthu, V.; Chen, W.-C.; Wen, C.-S.; Hou, D.-R. Synthesis of Optically Active Organofluorides by Ring Opening of Oxazolidinone-Fused Aziridines. Org. Lett. 2023, 25, 190–194. [Google Scholar] [CrossRef]
- Zbruyev, A.I.; Shishkin, O.V.; Doroshenko, A.O.; Desenko, S.M.; Chebanov, V.A. Stepwise photoinduced transformation of fused aziridines via stable biradicals and azomethine ylides. J. Photoch. Photobiol. A Chem. 2018, 353, 469–476. [Google Scholar] [CrossRef]
- Akter, M.; Rupa, K.; Anbarasan, P. 1,2,3-Triazole and Its Analogues: New Surrogates for Diazo Compounds. Chem. Rev. 2022, 122, 13108–13205. [Google Scholar] [CrossRef]
- Dequina, H.J.; Eshon, J.; Schmid, S.C.; Raskopf, W.T.; Sanders, K.M.; Fernández, I.; Schomaker, J.M. Re-Evaluation of Product Outcomes in the Rh-Catalyzed Ring Expansion of Aziridines with N-Sulfonyl-1,2,3-Triazoles. J. Org. Chem. 2022, 87, 10902–10907. [Google Scholar] [CrossRef]
- Makhova, N.N.; Petukhova, V.Y.; Kuznetsov, V.V. Synthesis of monocyclic diaziridines and their fused derivatives. Arkivoc 2008, 2008, 128–152. [Google Scholar] [CrossRef]
- De, A.; Majee, A. Synthesis of various functionalized 2H-azirines: An updated library. J. Het. Chem. 2021, 59, 422–448. [Google Scholar] [CrossRef]
- Stájer, G.; Sohár, P.; Csámpai, A.; Sillanpää, R.; Fülöp, F. Preparation and Structure of Bicycloalkane-Condensed Aryldiaziridines Accompanied by Pyrimidines. HETEROCYCLES 2007, 71, 1315. [Google Scholar] [CrossRef]
- Kiyosu, Y.; Tanaka, S.; Okumura, S.; Kiyokawa, K.; Minakata, S. Synthesis of Fused Diaziridine Derivatives from Cyclic Secondary Amines by Utilizing N-Bromosulfonamides as an Aminating Reagent. Synthesis 2021, 53, 3101–3109. [Google Scholar] [CrossRef]
- Ma, Z.; Wu, X.; Li, H.; Cao, Z.; Zhu, C. Access to pyrrolines and fused diaziridines by selective radical addition to homoallylic diazirines. Chem. Sci. 2023, 15, 1879–1884. [Google Scholar] [CrossRef]
- Yang, K.-C.; Li, J.-L.; Shen, X.-D.; Li, Q.; Leng, H.-J.; Huang, Q.; Zheng, P.-K.; Gou, X.-J.; Zhi, Y.-G. Facile synthesis of novel spiroheterocycles via diastereoselective aziridination of cyclic enones. RSC Adv. 2017, 7, 21175–21179. [Google Scholar] [CrossRef]
- Liu, T.-S.; Zhou, H.; Chen, P.; Huang, X.-R.; Bao, L.-Q.; Zhuang, C.-L.; Xu, Q.-S.; Shen, M.-H.; Xu, H.-D. Intramolecular Imino-ene Reaction of 2H-azirines with Alkenes: Rapid Construction of Spiro NH Aziridines from Vinyl Azides. Org. Lett. 2018, 20, 3156–3160. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.-H.; Qu, H.-Y.; Dai, P.; Zhou, H.; Liu, T.-S.; Bao, X.; Xu, D.; Xu, H.-D. Intramolecular Imino-ene Reaction of Azirines: Regioselectivity, Diastereoselectivity, and Computational Insights. J. Org. Chem. 2019, 84, 4095–4103. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Filatov, A.S.; Lozovskiy, S.V.; Shmakov, S.V.; Khoroshilova, O.V.; Larina, A.G.; Selivanov, S.I.; Boitsov, V.M.; Stepakov, A.V. Construction of Spiro [3-azabicyclo [3.1.0] hexanes] via 1,3-Dipolar Cycloaddition of 1,2-Diphenylcyclopropenes to Ninhydrin-Derived Azomethine Ylides. Synthesis 2021, 53, 2114–2132. [Google Scholar]
- Ashitha, K.T.; Vinaya, P.P.; Krishna, A.; Vincent, D.C.; Jalaja, R.; Varughese, S.; Balappa Somappa, S. I2/TBHP mediated diastereoselective synthesis of spiroaziridines. Org. Biomol. Chem. 2020, 18, 1588–1593. [Google Scholar] [CrossRef] [PubMed]
- Wölfl, B.; Winter, N.; Li, J.; Noble, A.; Aggarwal, V.K. Strain-Release Driven Epoxidation and Aziridination of Bicyclo [1.1.0] butanes via Palladium Catalyzed σ-Bond Nucleopalladation. Angew. Chem. Int. Ed. 2023, 62, e202217064. [Google Scholar] [CrossRef]
- Hajra, S.; Aziz, S.M.; Jana, B.; Mahish, P.; Das, D. Synthesis of Chiral Spiro-Aziridine Oxindoles via Aza-Corey–Chaykovsky Reaction of Isatin Derived N-tert-Butanesulfinyl Ketimines. Org. Lett. 2016, 18, 532–535. [Google Scholar] [CrossRef] [PubMed]
- Hajra, S.; Singha Roy, S.; Aziz, S.M.; Das, D. Catalyst-Free “On-Water” Regio- and Stereospecific Ring-Opening of Spiroaziridine Oxindole: Enantiopure Synthesis of Unsymmetrical 3,3′-Bisindoles. Org. Lett. 2017, 19, 4082–4085. [Google Scholar] [CrossRef]
- Hajra, S.; Hazra, A.; Mandal, P. Stereocontrolled Nucleophilic Fluorination at the Tertiary sp3-Carbon Center for Enantiopure Synthesis of 3-Fluorooxindoles. Org. Lett. 2018, 20, 6471–6475. [Google Scholar] [CrossRef] [PubMed]
- Hajra, S.; Singha Roy, S.; Biswas, A.; Saleh, S.A. Catalyst-Free Ring Opening of Spiroaziridine Oxindoles by Heteronucleophiles: An Approach to the Synthesis of Enantiopure 3-Substituted Oxindoles. J. Org. Chem. 2018, 83, 3633–3644. [Google Scholar] [CrossRef] [PubMed]
- Hajra, S.; Hazra, A.; Abu Saleh, S.K. One-Pot Synthesis of Enantiopure Spiro [3,4-dihydrobenzo [b][1,4] oxazine-2,3′-oxindole] via Regio- and Stereoselective Tandem Ring Opening/Cyclization of Spiroaziridine Oxindoles with Bromophenols. J. Org. Chem. 2019, 84, 10412–10421. [Google Scholar] [CrossRef]
- Hajra, S.; Hazra, A.; Saleh, S.K.A.; Mondal, A.S. Aqueous tert-Butyl Hydroperoxide Mediated Regioselective Ring-Opening Reactions of Spiro-aziridine-epoxy Oxindoles: Synthesis of 3-Peroxy-3-substituted Oxindoles and Their Acid-Mediated Rearrangement. Org. Lett. 2019, 21, 10154–10158. [Google Scholar] [CrossRef]
- Hajra, S.; Abu Saleh, S.K.; Hazra, A.; Singh, M.S. Organocatalytic Domino Reaction of Spiroaziridine Oxindoles and Malononitrile for the Enantiopure Synthesis of Spiro [dihydropyrrole-3,3′-oxindoles]. J. Org. Chem. 2019, 84, 8194–8201. [Google Scholar] [CrossRef]
- Hajra, S.; Biswas, A. Catalyst-Free Stereocontrolled Formal [3+2]-Cycloaddition of CO2 for the Synthesis of Enantiopure Spiro[indoline-3,5′-oxazolidine]-2,2′-diones under Aqueous and Ambient Conditions. Org. Lett. 2020, 22, 4990–4994. [Google Scholar] [CrossRef]
- Sakla, A.P.; Panda, B.; Laxmikeshav, K.; Soni, J.P.; Bhandari, S.; Godugu, C.; Shankaraiah, N. Dithiocarbamation of spiro-aziridine oxindoles: A facile access to C3-functionalised 3-thiooxindoles as apoptosis inducing agents. Org. Biomol. Chem. 2021, 19, 10622–10634. [Google Scholar] [CrossRef]
- Saleh, S.K.A.; Hazra, A.; Singh, M.S.; Hajra, S. Selective C3-Allylation and Formal [3+2]-Annulation of Spiro-Aziridine Oxindoles: Synthesis of 5′-Substituted Spiro[pyrrolidine-3,3′-oxindoles] and Coerulescine. J. Org. Chem. 2022, 87, 8656–8671. [Google Scholar] [CrossRef]
- Bhandari, S.; Sakla, A.P.; Shankaraiah, N. FeCl3 Catalyzed [3+2] Cycloaddition Reaction: A Mild Synthetic Approach to Spirooxindolo-2-iminothiazolidine Scaffolds. ChemistrySelect 2020, 5, 2886–2891. [Google Scholar] [CrossRef]
- Bhandari, S.; Kulkarni, N.; Sakla, A.P.; Shankaraiah, N. Lewis-acid catalyzed dehydrative [3+2] cycloaddition reaction: A facile synthetic approach to spiro-benzoindoline oxindoles. Tetrahedron Lett. 2020, 61, 152007. [Google Scholar] [CrossRef]
- Bhandari, S.; Sana, S.; Lahoti, V.; Tokala, R.; Shankaraiah, N. Ring-opening cyclization of activated spiro-aziridine oxindoles with heteroarenes: A facile synthetic approach to spiro-oxindole-fused pyrroloindolines. RSC Adv. 2020, 10, 16101–16109. [Google Scholar] [CrossRef] [PubMed]
- Sakla, A.P.; Kansal, P.; Shankaraiah, N. Syntheses and reactivity of spiro-epoxy/aziridine oxindole cores: Developments in the past decade. Org. Biomol. Chem. 2020, 18, 8572–8596. [Google Scholar] [CrossRef]
- Biswas, A.; Saleh, S.K.A.; Hazra, A.; Debnath, S.C.; Hajra, S. Sequential one-pot synthesis of N-sulfonyl spiroaziridine oxindoles from spiroepoxy oxindoles. Org. Biomol. Chem. 2021, 19, 5624–5631. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-Q.; Yue, D.-F.; Zhang, X.-M.; Xu, X.-Y.; Yuan, W.-C. The organocatalytic asymmetric Neber reaction for the enantioselective synthesis of spirooxindole 2H-azirines. Org. Biomol. Chem. 2016, 14, 10946–10952. [Google Scholar] [CrossRef]
- Bisht, G.S.; Dunchu, T.D.; Gnanaprakasam, B. Synthesis of Quaternary Spirooxindole 2H-Azirines under Batch and Continuous Flow Condition and Metal Assisted Umpolung Reactivity for the Ring-Opening Reaction. Chem. Asian J. 2021, 16, 656–665. [Google Scholar] [CrossRef]
- Mehra, V.; Lumb, I.; Anand, A.; Kumar, V. Recent advances in synthetic facets of immensely reactive azetidines. RSC Adv. 2017, 7, 45763–45783. [Google Scholar] [CrossRef]
- Brandi, A.; Cicchi, S.; Cordero, F.M. Novel Syntheses of Azetidines and Azetidinones. Chem. Rev. 2008, 108, 3988–4035. [Google Scholar] [CrossRef]
- Gerard, B.; Lee, M.D.t.; Dandapani, S.; Duvall, J.R.; Fitzgerald, M.E.; Kesavan, S.; Lowe, J.T.; Marié, J.-C.; Pandya, B.A.; Suh, B.-C.; et al. Synthesis of stereochemically and skeletally diverse fused ring systems from functionalized C-glycosides. J. Org. Chem. 2013, 78, 5160–5171. [Google Scholar] [CrossRef]
- Alcaide, B.; Almendros, P. Gold-catalyzed heterocyclizations in alkynyl- and allenyl-β-lactams. Beilst. J. Org. Chem. 2011, 7, 622–630. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Lin, R.-C.; Ye, S. Catalytic [2+2] and [3+2] cycloaddition reactions of allenoates with cyclic ketimines. Chem. Commun. 2012, 48, 1317–1319. [Google Scholar] [CrossRef]
- Wang, L.; Li, S.; Blümel, M.; Puttreddy, R.; Peuronen, A.; Rissanen, K.; Enders, D. Switchable Access to Different Spirocyclopentane Oxindoles by N-Heterocyclic Carbene Catalyzed Reactions of Isatin-Derived Enals and N-Sulfonyl Ketimines. Angew. Chem. Int. Ed. 2017, 129, 8636–8641. [Google Scholar] [CrossRef]
- Qian, L.-L.; Hu, Y.-C.; Min, X.-T.; Yang, S.-N.; Shen, B.-X.; Wan, B.; Chen, Q.-A. CPA-catalyzed multicomponent reaction of anilines, aldehydes, and azetidinones: Rapid access to enantiopure-fused azetidines. Chem. Catal. 2022, 2, 2024–2033. [Google Scholar] [CrossRef]
- Kaupang, A.; Bonge-Hansen, T. α-Bromodiazoacetamides—A new class of diazo compounds for catalyst-free, ambient temperature intramolecular C-H insertion reactions. Beilst. J. Org. Chem. 2013, 9, 1407–1413. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.R.; Wearing, E.R.; Schindler, C.S. Synthesis of azetidines via visible-light-mediated intermolecular [2+2] photocycloadditions. Nat. Chem. 2020, 12, 898–905. [Google Scholar] [CrossRef]
- Mughal, H.; Szostak, M. Recent advances in the synthesis and reactivity of azetidines: Strain-driven character of the four-membered heterocycle. Org. Biomol. Chem. 2021, 19, 3274–3286. [Google Scholar] [CrossRef]
- Baumann, A.N.; Reiners, F.; Juli, T.; Didier, D. Chemodivergent and Stereoselective Access to Fused Isoxazoline Azetidines and Thietanes through [3+2]-Cycloadditions. Org. Lett. 2018, 20, 6736–6740. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, X.; Zheng, C.; You, S.-L. Visible-Light-Induced Dearomatization via [2+2] Cycloaddition or 1,5-Hydrogen Atom Transfer: Divergent Reaction Pathways of Transient Diradicals. ACS Catal. 2020, 10, 12618–12626. [Google Scholar] [CrossRef]
- Chen, K.; Zhu, Z.-Z.; Liu, J.-X.; Tang, X.-Y.; Wei, Y.; Shi, M. Substrate-controlled Rh(ii)-catalyzed single-electron-transfer (SET): Divergent synthesis of fused indoles. Chem. Commun. 2016, 52, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Hensienne, R.; Hazelard, D.; Compain, P. Conformationally constrained fused bicyclic iminosugars: Synthetic challenges and opportunities. Arkivoc 2019, 2019, 4–43. [Google Scholar] [CrossRef]
- Music, A.; Baumann, A.N.; Eisold, M.; Didier, D. Regiodivergent Stereoselective Access to Fused Alkylideneazetidines. J. Org. Chem. 2018, 83, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Losa, R.; Lorton, C.; Retailleau, P.; Bignon, J.; Voituriez, A. Fluorinated 2-Azetines: Synthesis, Applications, Biological Tests, and Development of a Catalytic Process. Org. Lett. 2023, 25, 5140–5144. [Google Scholar] [CrossRef]
- Meanwell, N.A.; Loiseleur, O. Applications of Isosteres of Piperazine in the Design of Biologically Active Compounds: Part 2. J. Agric. Food Chem. 2022, 70, 10972–11004. [Google Scholar] [CrossRef]
- Burkhard, J.A.; Guérot, C.; Knust, H.; Rogers-Evans, M.; Carreira, E.M. Synthesis and Structural Analysis of a New Class of Azaspiro [3.3] heptanes as Building Blocks for Medicinal Chemistry. Org. Lett. 2010, 12, 1944–1947. [Google Scholar] [CrossRef]
- Burkhard, J.A.; Wagner, B.; Fischer, H.; Schuler, F.; Müller, K.; Carreira, E.M. Synthesis of Azaspirocycles and their Evaluation in Drug Discovery. Angew. Chem. Int. Ed. 2010, 49, 3524–3527. [Google Scholar] [CrossRef]
- Bauer, M.R.; Di Fruscia, P.; Lucas, S.C.C.; Michaelides, I.N.; Nelson, J.E.; Storer, R.I.; Whitehurst, B.C. Put a ring on it: Application of small aliphatic rings in medicinal chemistry. RSC Med. Chem. 2021, 12, 448–471. [Google Scholar] [CrossRef]
- Pang, L.; Yao, D.; Gao, F.; Bian, X.; Zhang, Y.; Zhong, G. Biosyntheses of azetidine-containing natural products. Org. Biomol. Chem. 2023, 21, 7242–7254. [Google Scholar] [CrossRef]
- Flodén, N.J.; Trowbridge, A.; Willcox, D.; Walton, S.M.; Kim, Y.; Gaunt, M.J. Streamlined Synthesis of C-Rich N-Heterospirocycles Enabled by Visible-Light-Mediated Photocatalysis. J. Am. Chem. Soc. 2019, 141, 8426–8430. [Google Scholar] [CrossRef]
- Tyler, J.L.; Noble, A.; Aggarwal, V.K. Strain-Release-Driven Friedel-Crafts Spirocyclization of Azabicyclo [1.1.0] butanes. Angew. Chem. Int. Ed. 2022, 61, e202114235. [Google Scholar] [CrossRef]
- Reed, M.; Baidilov, D. An Elegant Synthetic Avenue to Azetidine-Containing Spirocycles. Synfacts 2021, 17, 0860. [Google Scholar] [CrossRef]
- Turkowska, J.; Durka, J.; Gryko, D. Strain release—An old tool for new transformations. Chem. Commun. 2020, 56, 5718–5734. [Google Scholar] [CrossRef]
- Shen, H.-C.; Popescu, M.V.; Wang, Z.-S.; de Lescure, L.; Noble, A.; Paton, R.S.; Aggarwal, V.K. Iridium-Catalyzed Asymmetric Difunctionalization of C-C σ-Bonds Enabled by Ring-Strained Boronate Complexes. J. Am. Chem. Soc. 2023, 145, 16508–16516. [Google Scholar] [CrossRef]
- Tyler, J.L.; Noble, A.; Aggarwal, V.K. Strain-Release Driven Spirocyclization of Azabicyclo [1.1.0] butyl Ketones. Angew. Chem. Int. Ed. 2021, 60, 11824–11829. [Google Scholar] [CrossRef] [PubMed]
- Gregson, C.H.U.; Noble, A.; Aggarwal, V.K. Divergent, Strain-Release Reactions of Azabicyclo [1.1.0] butyl Carbinols: Semipinacol or Spiroepoxy Azetidine Formation. Angew. Chem. Int. Ed. 2021, 60, 7360–7365. [Google Scholar] [CrossRef] [PubMed]
- Andresini, M.; Degennaro, L.; Luisi, R. The renaissance of strained 1-azabicyclo [1.1.0] butanes as useful reagents for the synthesis of functionalized azetidines. Org. Biomol. Chem. 2020, 18, 5798–5810. [Google Scholar] [CrossRef]
- Fawcett, A. Recent advances in the chemistry of bicyclo- and 1-azabicyclo [1.1.0] butanes. Pure Appl. Chem. 2019, 92, 751–765. [Google Scholar] [CrossRef]
- Wu, B.; Chen, H.; Gao, M.; Gong, X.; Hu, L. Synthesis of 1,3-Aminoalcohols and Spirocyclic Azetidines via Tandem Hydroxymethylation and Aminomethylation Reaction of β-Keto Phosphonates with N-Nosyl-O-(2-bromoethyl)hydroxylamine. Org. Lett. 2021, 23, 4152–4157. [Google Scholar] [CrossRef] [PubMed]
- Rainoldi, G.; Faltracco, M.; Lo Presti, L.; Silvani, A.; Lesma, G. Highly diastereoselective entry into chiral spirooxindole-based 4-methyleneazetidines via formal [2+2] annulation reaction. Chem. Commun. 2016, 52, 11575–11578. [Google Scholar] [CrossRef]
- Moderhack, D. Dihydro-1,2-diazetes—The preparative chemistry since 1980. Chem. Het. Comp. 2019, 55, 3–24. [Google Scholar] [CrossRef]
- Pancholi, A.K.; Iacobini, G.P.; Clarkson, G.J.; Porter, D.W.; Shipman, M. Synthesis of 4,5-Diazaspiro [2.3] hexanes and 1,2-Diazaspiro [3.3] heptanes as Hexahydropyridazine Analogues. J. Org. Chem. 2017, 83, 491–498. [Google Scholar] [CrossRef]
- Cao, W.-B.; Jiang, S.; Li, H.-Y.; Xu, X.-P.; Ji, S.-J. Synthesis of strained 1,2-diazetidines via [3+1] cycloaddition of C,N-cyclic azomethine imines with isocyanides and synthetic derivation. Org. Chem. Front. 2021, 8, 2494–2503. [Google Scholar] [CrossRef]
- Breton, G.W.; Hughes, J.S.; Pitchko, T.J.; Martin, K.L.; Hardcastle, K. Unexpected σ Bond Rupture during the Reaction of N-Methyl-1,2,4-triazoline-3,5-dione with Acenaphthylene and Indene. J. Org. Chem. 2014, 79, 8212–8220. [Google Scholar] [CrossRef] [PubMed]
- Rocaboy, R.; Dailler, D.; Zellweger, F.; Neuburger, M.; Salomé, C.; Clot, E.; Baudoin, O. Domino Pd0-Catalyzed C(sp3)–H Arylation/Electrocyclic Reactions via Benzazetidine Intermediates. Angew. Chem. Int. Ed. 2018, 57, 12131–12135. [Google Scholar] [CrossRef]
- Tsitopoulou, M.; Clemenceau, A.; Thesmar, P.; Baudoin, O. 1,4-Pd Migration-Enabled Synthesis of Fused 4-Membered Rings. J. Am. Chem. Soc. 2024, 146, 18811–18816. [Google Scholar] [CrossRef] [PubMed]
- Nappi, M.; He, C.; Whitehurst, W.G.; Chappell, B.G.N.; Gaunt, M.J. Selective Reductive Elimination at Alkyl Palladium(IV) by Dissociative Ligand Ionization: Catalytic C(sp3)−H Amination to Azetidines. Angew. Chem. Int. Ed. 2018, 57, 3178–3182. [Google Scholar] [CrossRef]
- Flores, D.M.; Neville, M.L.; Schmidt, V.A. Intermolecular 2+2 imine-olefin photocycloadditions enabled by Cu(I)-alkene MLCT. Nat. Commun. 2022, 13, 2764. [Google Scholar] [CrossRef]
- Ren, X.; Chandgude, A.L.; Fasan, R. Highly Stereoselective Synthesis of Fused Cyclopropane-γ-Lactams via Biocatalytic Iron-Catalyzed Intramolecular Cyclopropanation. ACS Catal. 2020, 10, 2308–2313. [Google Scholar] [CrossRef]
- Lin, H.-Z.; Qi, Z.; Wu, Q.-M.; Jiang, Y.-Y.; Peng, J.-B. Palladium-catalyzed intramolecular asymmetric hydrocyclopropanylation of alkynes: Synthesis of cyclopropane-fused γ-lactams. Chem. Sci. 2023, 14, 7564–7568. [Google Scholar] [CrossRef]
- Wu, X.; Hao, L.; Zhang, Y.; Rakesh, M.; Reddi, R.N.; Yang, S.; Song, B.-A.; Chi, Y.R. Construction of Fused Pyrrolidines and β-Lactones by Carbene-Catalyzed C−N, C−C, and C−O Bond Formations. Angew. Chem. Int. Ed. 2017, 56, 4201–4205. [Google Scholar] [CrossRef]
- Bastrakov, M.A.; Kucherova, A.Y.; Fedorenko, A.K.; Starosotnikov, A.M.; Fedyanin, I.V.; Dalinger, I.L.; Shevelev, S.A. Dearomatization of 3,5-dinitropyridines—An atom-efficient approach to fused 3-nitropyrrolidines. Arkivoc 2017, 2017, 181–190. [Google Scholar] [CrossRef]
- Panda, S.S.; Aziz, M.N.; Stawinski, J.; Girgis, A.S. Azomethine Ylides—Versatile Synthons for Pyrrolidinyl-Heterocyclic Compounds. Molecules 2023, 28, 668. [Google Scholar] [CrossRef]
- Savych, V.I.; Mykhalchuk, V.L.; Melnychuk, P.V.; Isakov, A.O.; Savchuk, T.; Timoshenko, V.M.; Siry, S.A.; Pavlenko, S.O.; Kovalenko, D.V.; Hryshchuk, O.V.; et al. Bicyclic Pyrrolidines for Medicinal Chemistry via [3+2]-Cycloaddition. J. Org. Chem. 2021, 86, 13289–13309. [Google Scholar] [CrossRef]
- Almeida, J.; Silva, A.M.N.; Rebelo, S.L.H.; Cunha-Silva, L.; Rangel, M.; de Castro, B.; Leite, A.; Silva, A.M.G. Synthesis and coordination studies of 5-(4′-carboxyphenyl)-10,15,20-tris(pentafluorophenyl)porphyrin and its pyrrolidine-fused chlorin derivative. New J. Chem. 2018, 42, 8169–8179. [Google Scholar] [CrossRef]
- Almeida, J.; Tomé, A.C.; Rangel, M.; Silva, A.M.G. Microwave-Assisted Synthesis and Spectral Properties of Pyrrolidine-Fused Chlorin Derivatives. Molecules 2023, 28, 3833. [Google Scholar] [CrossRef]
- Ou, C.; Wang, J.; Yin, P.; Chen, B.; Hu, P.; Wang, B.-Q.; Cao, P.; Xu, M. Synthesis of polysubstituted fused pyrrolidines via [2+2]/[2+3] cycloaddition of azomethine ylides. Org. Chem. Front. 2024, 11, 4149–4155. [Google Scholar] [CrossRef]
- Babazadeh, M.; Soleimani-Amiri, S.; Vessally, E.; Hosseinian, A.; Edjlali, L. Transition metal-catalyzed [2+2+2] cycloaddition of nitrogen-linked 1,6-diynes: A straightforward route to fused pyrrolidine systems. RSC Adv. 2017, 7, 43716–43736. [Google Scholar] [CrossRef]
- Blanco-Ania, D.W.M.; Aben, R.; van Berkom, L.W.A.; Scheeren, H.W.; Rutjes, F.P.J.T. Synthesis of Steroidal D-Ring-Fused Pyrrolidines of Dehydroepiandrosterone. Eur. J. Org. Chem. 2017, 2017, 3729–3737. [Google Scholar] [CrossRef]
- Kashyap, S.; Singh, B.; Ghorai, M.K. Magic Blue-Initiated SN2-Type Ring Opening of Activated Aziridines: Friedel–Crafts-Type Alkylation of Electron-Rich Arenes/Heteroarenes. J. Org. Chem. 2024, 89, 11429–11445. [Google Scholar] [CrossRef]
- Feng, J.-J.; Zhang, J. Synthesis of Unsaturated N-Heterocycles by Cycloadditions of Aziridines and Alkynes. ACS Catal. 2016, 6, 6651–6661. [Google Scholar] [CrossRef]
- Sunbal; Alamzeb, M.; Omer, M.; Abid, O.-U.-R.; Ullah, M.; Sohail, M.; Ullah, I. Chemical insights into the synthetic chemistry of five-membered saturated heterocycles—A transition metal–catalyzed approach. Front. Chem. 2023, 11, 2023. [Google Scholar] [CrossRef]
- Wang, F.; Xu, X.; Yan, Y.; Zhang, J.; Bai, W.-J.; Chen, J.; Yang, Y. Diastereoselective Construction of Fused Carbocyclic Pyrrolidines via a Copper-Catalyzed [3+2] Cycloaddition: Total Syntheses of Pancratinines B–C. Org. Lett. 2023, 25, 6853–6857. [Google Scholar] [CrossRef]
- Akhtar, R.; Naqvi, S.A.R.; Zahoor, A.F.; Saleem, S. Nucleophilic ring opening reactions of aziridines. Mol. Divers. 2018, 22, 447–501. [Google Scholar] [CrossRef] [PubMed]
- Loh, J.K.; Asad, N.; Samarakoon, T.B.; Hanson, P.R. Modular, One-Pot, Sequential Aziridine Ring Opening–SNAr Strategy to 7-, 10-, and 11-Membered Benzo-Fused Sultams. J. Org. Chem. 2015, 80, 9926–9941. [Google Scholar] [CrossRef]
- Javahershenas, R.; Makarem, A.; Klika, K.D. Recent advances in microwave-assisted multicomponent synthesis of spiro heterocycles. RSC Adv. 2024, 14, 5547–5565. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, M.; Zhang, W.; Legris, M.; Zhang, W. Synthesis of trifluoromethylated pyrrolidines via decarboxylative [3+2] cycloaddition of non-stabilized N-unsubstituted azomethine ylides. J. Fluor. Chem. 2017, 204, 18–22. [Google Scholar] [CrossRef]
- Deng, H.; He, F.-S.; Li, C.-S.; Yang, W.-L.; Deng, W.-P. Enantioselective construction of tricyclic pyrrolidine-fused benzo[b]thiophene 1,1-dioxide derivatives via copper(i)-catalyzed asymmetric 1,3-dipolar cycloaddition. Org. Chem. Front. 2017, 4, 2343–2347. [Google Scholar] [CrossRef]
- Filatov, A.S.; Knyazev, N.A.; Molchanov, A.P.; Panikorovsky, T.L.; Kostikov, R.R.; Larina, A.G.; Boitsov, V.M.; Stepakov, A.V. Synthesis of Functionalized 3-Spiro[cyclopropa[a]pyrrolizine]- and 3-Spiro [3-azabicyclo [3.1.0] hexane] oxindoles from Cyclopropenes and Azomethine Ylides via [3+2]-Cycloaddition. J. Org. Chem. 2017, 82, 959–975. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, Y.; Kim, S.-G. Organocatalytic Asymmetric Mannich/Aza-Michael Cascade Reaction of δ-Formyl-α,β-unsaturated Ketones with Cyclic N-Sulfimines: Enantioselective Synthesis of Benzosulfamidate-Fused Pyrrolidines. J. Org. Chem. 2017, 82, 8179–8185. [Google Scholar] [CrossRef]
- Łowicki, D.; Przybylski, P. Cascade synthetic strategies opening access to medicinal-relevant aliphatic 3- and 4-membered N-heterocyclic scaffolds. Eur. J. Med. Chem. 2022, 238, 114438. [Google Scholar] [CrossRef]
- Vishwanath, M.; Prakash, M.; Vinayagam, P.; Kesavan, V. Construction of Polycyclic Fused Pyrrolidines with Three Contiguous Stereocenters via Michael Addition of Vinylmalononitriles to Nitrostyrenes Using l-Proline-Derived Thiourea. Synthesis 2016, 48, 2671–2678. [Google Scholar]
- Phirke, J.; Meshram, Y. Mocrowave Assited Synthesis & Microbial Activities of some Pyrazoline Derivatives. Int. J. Sci. Res. 2017, 5, 28–30. [Google Scholar]
- Aparicio, D.A.; Lobo, G.M.; Sánchez, J.L.; Monasterios, M.C.; Acosta, M.E.; De Lima, L.; Llovera, L.J.; Ángel, J.E.; Charris, J.E. Synthesis of Trans- and cis-2-acetyl-3-phenyl-3,3a,4,5-tetrahydro-2H-benzoindazoles: Evaluation as Inhibitors of β-hematin Formation. J. Chem. Res. 2017, 41, 668–672. [Google Scholar] [CrossRef]
- Romero-López, A.; Montiel-Smith, S.; Meza-Reyes, S.; Merino-Montiel, P.; Vega-Baez, J.L. Synthesis of steroidal derivatives containing substituted, fused and spiro pyrazolines. Steroids 2014, 87, 86–92. [Google Scholar] [CrossRef]
- Mlostoń, G.; Urbaniak, K.; Palusiak, M.; Witczak, Z.J.; Würthwein, E.-U. (3+2)-Cycloadditions of Levoglucosenone (LGO) with Fluorinated Nitrile Imines Derived from Trifluoroacetonitrile: An Experimental and Computational Study. Molecules 2023, 28, 7348. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Ma, R.; Li, X.; Wang, K.-H.; Wang, J.; Huang, D.; Lv, X.; Hu, Y. Synthesis of ring fused difluoromethyl pyrazolines and pyrazoles by cycloaddition of difluoroacetohydrazonoyl bromides with cyclic α,β-Unsaturated carbonyl compounds. Tetrahedron 2023, 149, 133711. [Google Scholar] [CrossRef]
- Popova, A.V.; Kanaa, A.; Vavilova, V.S.; Mironova, M.A.; Slepukhin, P.A.; Benassi, E.; Belskaya, N.P. Design, synthesis, and photophysics of bi- and tricyclic fused pyrazolines. New J. Chem. 2021, 45, 6315–6326. [Google Scholar] [CrossRef]
- Belskaya, N.P.; Eliseeva, A.I.; Bakulev, V.A. Hydrazones as substrates for cycloaddition reactions. Rus. Chem. Rev. 2015, 84, 1226. [Google Scholar] [CrossRef]
- Vijay, M.; Kumar, S.V.; Satheesh, V.; Ananthappan, P.; Srivastava, H.K.; Ellairaja, S.; Vasantha, V.S.; Punniyamurthy, T. Stereospecific Assembly of Fused Imidazolidines via Tandem Ring Opening/Oxidative Amination of Aziridines with Cyclic Secondary Amines Using Photoredox Catalysis. Org. Lett. 2019, 21, 7649–7654. [Google Scholar] [CrossRef]
- Das, D.; Kamilya, C.; Ghorai, P. Hydrazine Hydrate in Asymmetric Synthesis: A Bifunctional Squaramide Catalytic Approach toward Fused Pyrazolines. Org. Lett. 2023, 25, 6993–6998. [Google Scholar] [CrossRef]
- Zhao, H.-W.; Yang, Z.; Meng, W.; Tian, T.; Li, B.; Song, X.-Q.; Chen, X.-Q.; Pang, H.-L. Diastereo- and Enantioselective Synthesis of Chiral Pyrrolidine-Fused Spirooxindoles via Organocatalytic [3+2] 1,3-Dipolar Cycloaddition of Azomethine Ylides with Maleimides. Adv. Synth. Catal. 2015, 357, 2492–2502. [Google Scholar] [CrossRef]
- Chen, Z.-J.; Liang, W.; Chen, Z.; Chen, L. Phase-Transfer Catalytic Strategy: Rapid Synthesis of Spiro-Fused Heterocycles, Integrated with Four Pharmacophores-Succinimide, Pyrrolidine, Oxindole, and Trifluoromethyl Group. Eur. J. Org. Chem. 2021, 2021, 788–793. [Google Scholar] [CrossRef]
- Ben Hamadi, N.; Guesmi, A. Synthesis of New Spiro-Cyclopropanes Prepared by Non-Stabilized Diazoalkane Exhibiting an Extremely High Insecticidal Activity. Molecules 2022, 27, 2470. [Google Scholar] [CrossRef]
- Deng, H.; Jia, R.; Yang, W.-L.; Yu, X.; Deng, W.-P. Ligand-controlled switch in diastereoselectivities: Catalytic asymmetric construction of spirocyclic pyrrolidine-azetidine/oxe(thie)tane derivatives. Chem. Commun. 2019, 55, 7346–7349. [Google Scholar] [CrossRef]
- Ren, W.; Zhao, Q.; Zheng, C.; Zhao, Q.; Guo, L.; Huang, W. Efficient Synthesis of Fully Substituted Pyrrolidine-Fused 3-Spirooxindoles via 1,3-Dipolar Cycloaddition of Aziridine and 3-Ylideneoxindole. Molecules 2016, 21, 1113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-H.; Pan, C.-L.; Zhang, H.-H.; Xu, P.-F.; Luo, Y.-C. Sc(OTf)3 catalyzed [3+2]-annulation reaction of donor–acceptor aziridines with methylene exo-glycals: Synthesis of chiral carbohydrate-spiro-heterocycles. Org. Chem. Front. 2021, 8, 2203–2207. [Google Scholar] [CrossRef]
- Li, R.-L.; Fang, Q.-Y.; Li, M.-Y.; Wang, X.-S.; Zhao, L.-M. A rearrangement of saccharin-derived cyclic ketimines with 3-chlorooxindoles leading to spiro-1,3-benzothiazine oxindoles. Chem. Commun. 2021, 57, 11322–11325. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.-J.; Wang, J.-L.; Xu, D.; Burgess, K.S.; Zhu, A.-F.; Rao, Y.-Y.; Chen, X.-B.; Wang, Y.-C. Chemically Sustainable and Green One-Pot Multicomponent Synthesis of Highly Functionalized Polycyclic N-Fused-Pyrrolidine Heterocycles. ChemistrySelect 2018, 3, 662–665. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Wang, J.-L.; Burgess, K.S.; Zhang, J.-W.; Zheng, Q.-M.; Pu, Y.-D.; Yan, L.-J.; Chen, X.-B. Green synthesis of new pyrrolidine-fused spirooxindoles via three-component domino reaction in EtOH/H2O. RSC Adv. 2018, 8, 5702–5713. [Google Scholar] [CrossRef] [PubMed]
- Poomathi, N.; Mayakrishnan, S.; Muralidharan, D.; Perumal, P.T. A facile access to novel spiroxindole fused pyrrolidine and thiazolo pyrrolidine benzimidazole derivatives via 1,3-dipolar cycloaddition reaction. Tetrahedron Lett. 2015, 56, 721–726. [Google Scholar] [CrossRef]
- Molteni, G.; Silvani, A. Spiro-2-oxindoles via 1,3-dipolar cycloadditions. A decade update. Eur. J. Org. Chem. 2021, 2021, 1653–1675. [Google Scholar] [CrossRef]
- Sakla, A.P.; Kansal, P.; Shankaraiah, N. Syntheses and Applications of Spirocyclopropyl Oxindoles: A Decade Review. Eur. J. Org. Chem. 2021, 2021, 757–772. [Google Scholar] [CrossRef]
- Boddy, A.J.; Bull, J.A. Stereoselective synthesis and applications of spirocyclic oxindoles. Org. Chem. Front. 2021, 8, 1026–1084. [Google Scholar] [CrossRef]
- Wang, B.; Liang, M.; Tang, J.; Deng, Y.; Zhao, J.; Sun, H.; Tung, C.-H.; Jia, J.; Xu, Z. Gold/Lewis Acid Catalyzed Cycloisomerization/Diastereoselective [3+2] Cycloaddition Cascade: Synthesis of Diverse Nitrogen-Containing Spiro Heterocycles. Org. Lett. 2016, 18, 4614–4617. [Google Scholar] [CrossRef]
- Cormier, M.; Chardon, A.; Blanchet, J.; Rouden, J.; Maddaluno, J.; De Paolis, M. An Organocatalytic Access to Spiro [4.5] decanes and Spiro [4.6] undecanes Containing Aminolactones and 3-Aminopyrrolidines. Synthesis 2015, 47, 2549–2553. [Google Scholar]
- Kamlar, M.; Urban, M.; Veselý, J. Enantioselective Synthesis of Spiro Heterocyclic Compounds Using a Combination of Organocatalysis and Transition-Metal Catalysis. Chem. Rec. 2023, 23, e202200284. [Google Scholar] [CrossRef]
- Griffiths, O.M.; Ley, S.V. Multicomponent Direct Assembly of N-Heterospirocycles Facilitated by Visible-Light-Driven Photocatalysis. J. Org. Chem. 2022, 87, 13204–13223. [Google Scholar] [CrossRef]
- Zaitseva, E.R.; Smirnov, A.Y.; Baleeva, N.S.; Baranov, M.S. Synthesis of spiro[imidazole-4,3′-quinolin]ones from [2-(dimethylamino)benzylidene]-2-(methylsulfanyl)imidazolones. Chem. Heterocycl. Comp. 2021, 57, 695–699. [Google Scholar] [CrossRef]
- Molchanova, M.V.; Ikonnikova, V.A.; Smirnov, A.Y.; Solyev, P.N.; Baranov, M.S.; Mikhaylov, A.A. Synthesis of Spiro[imidazole-4,3′-pyrrolo [1,2-a] quinolin]-5-ones via 1,3-Dipolar Cycloaddition of Quinolinium Ylides with Arylydeneimidazol-4-ones. ChemistrySelect 2024, 9, e202305123. [Google Scholar] [CrossRef]
- Luo, Y.; Zhou, H.-Y.; Gang, Y.-C.; Dong, L. Formation of Fluorovinyl Spiro-[imidazole-indene] and α-Amino-β-naphthalenones via Rh(III)-Catalyzed Cascade C–H Functionalization. Org. Lett. 2022, 24, 6940–6944. [Google Scholar] [CrossRef]
- Luo, Y.; Pu, W.-Y.; Xu, Y.-J.; Dong, L. Formation of diversified spiro-[imidazole-indene] derivatives from 2H-imidazoles: Based on versatile propargyl alcohols. Org. Chem. Front. 2021, 8, 4549–4553. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Ni, S.-F.; Li, J.-P.; Xia, D.; Han, X.; Lin, J.; Wang, J.; Das, S.; Zhang, W.-D. Visible-light-induced [3+2] cycloadditions of donor/donor diazo intermediates with alkenes to achieve (spiro)-pyrazolines and pyrazoles. Chem. Sci. 2023, 14, 10411–10419. [Google Scholar] [CrossRef]
- Tu, L.; Gao, L.; Wang, Q.; Cao, Z.; Huang, R.; Zheng, Y.; Liu, J. Substrate-Switched Chemodivergent Pyrazole and Pyrazoline Synthesis: [3+2] Cycloaddition/Ring-Opening Rearrangement Reaction of Azadienes with Nitrile Imines. J. Org. Chem. 2022, 87, 3389–3401. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Li, Z.; He, J.; Liu, X.; Feng, X. Asymmetric catalytic 1,3-dipolar cycloaddition of α-diazoesters for synthesis of 1-pyrazoline-based spirochromanones and beyond. Sci. China Chem. 2021, 64, 1355–1360. [Google Scholar] [CrossRef]
- Dadiboyena, S.; Valente, E.J.; Hamme, A.T. Synthesis and tautomerism of spiro-pyrazolines. Tetrahedron Lett. 2014, 55, 2208–2211. [Google Scholar] [CrossRef] [PubMed]
- Dubovtsev, A.Y.; Denislamova, E.S.; Silaichev, P.S.; Dmitriev, M.V.; Maslivets, A.N. Synthesis of Imidazole Spiro Compounds From 5-Alkoxycarbonyl-1H-Pyrrole-2,3-Diones and Phenylurea. Chem. Heterocycl. Comp. 2016, 52, 467–472. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, X.; Luo, X.; de Voogd, N.J.; Li, P.; Li, G. (+)- and (−)-Spiroreticulatine, A Pair of Unusual Spiro Bisheterocyclic Quinoline-imidazole Alkaloids from the South China Sea Sponge Fascaplysinopsis reticulata. Org. Lett. 2015, 17, 3458–3461. [Google Scholar] [CrossRef]
- Yusuf, M.; Jain, P. Synthetic and biological studies of pyrazolines and related heterocyclic compounds. Arab. J. Chem. 2014, 7, 553–596. [Google Scholar] [CrossRef]
- Moskalik, M.Y.; Garagan, I.A.; Borodina, T.N.; Zinchenko, S.V.; Shainyan, B.A. Quinazoline, Imidazoline, and Azetidine Heterocycles via Oxidative Sulfonamidation of Camphene. Asian J. Org. Chem. 2025, 14, e202400671. [Google Scholar] [CrossRef]
- Moskalik, M.Y.; Garagan, I.A.; Shainyan, B.A.; Yarovaya, O.I.; Ganin, A.S.; Astakhova, V.V.; Sterkhova, I.V.; Zinchenko, S.V.; Salakhutdinov, N.F.; Radzhabov, A.D. Halosulfonamidation of camphene: Chemo and stereoselectivity, rearrangement, solvent interception, heterocyclization. New J. Chem. 2024, 48, 13506–13513. [Google Scholar] [CrossRef]



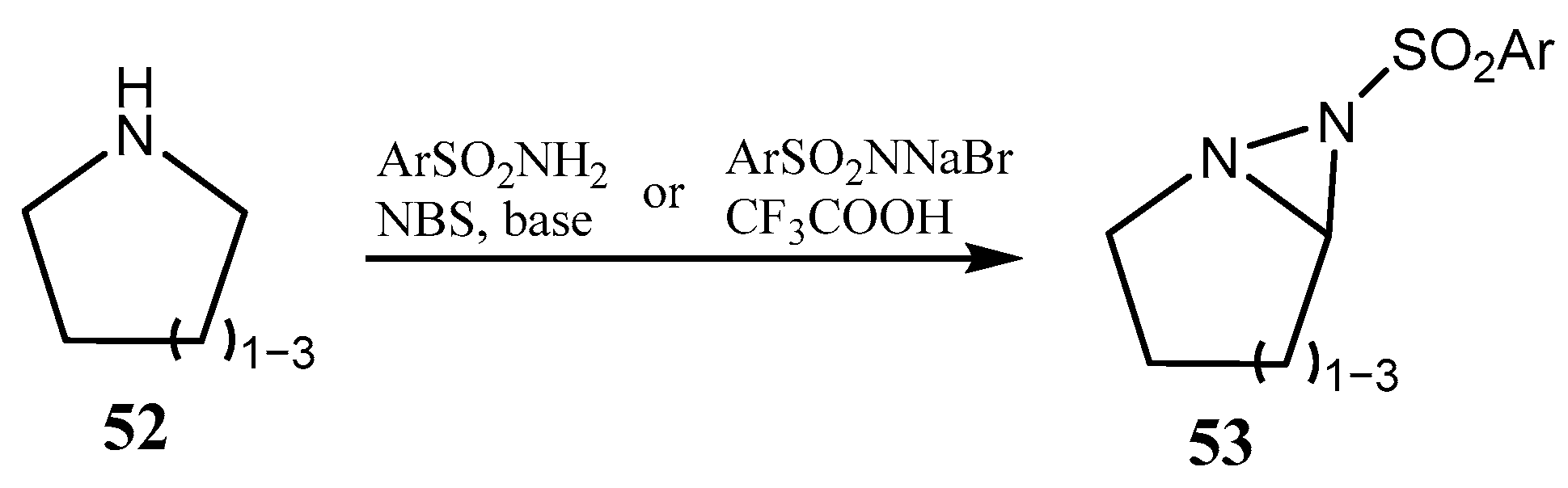

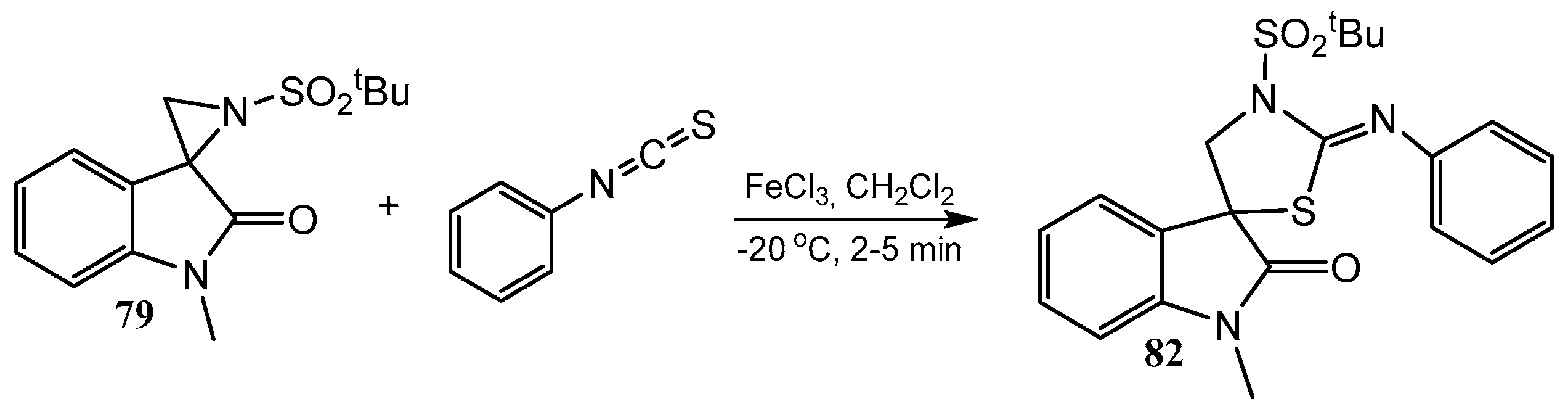

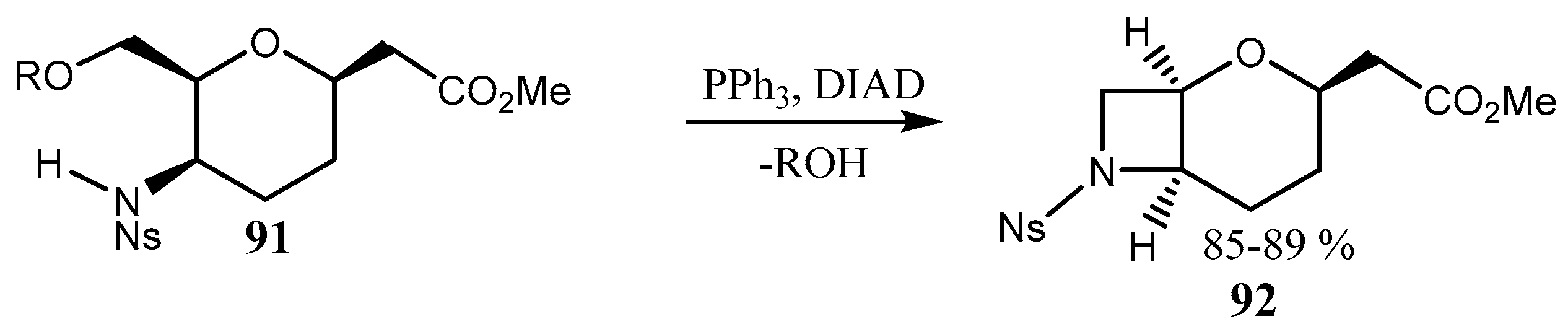

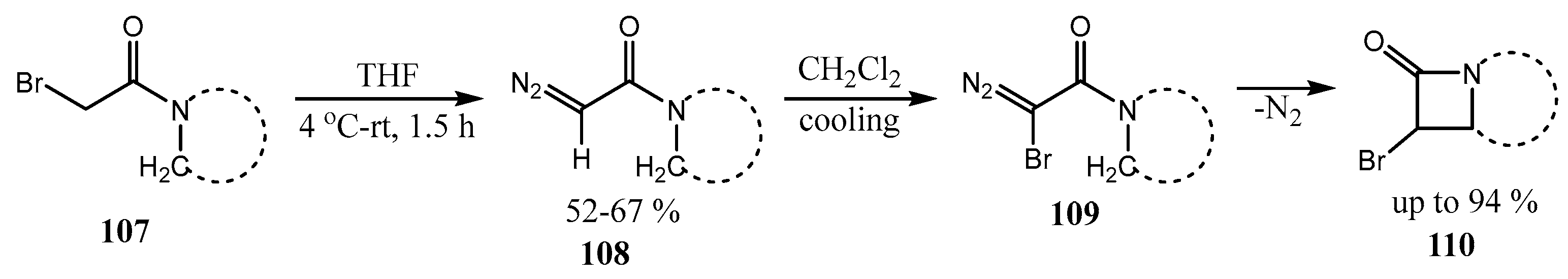
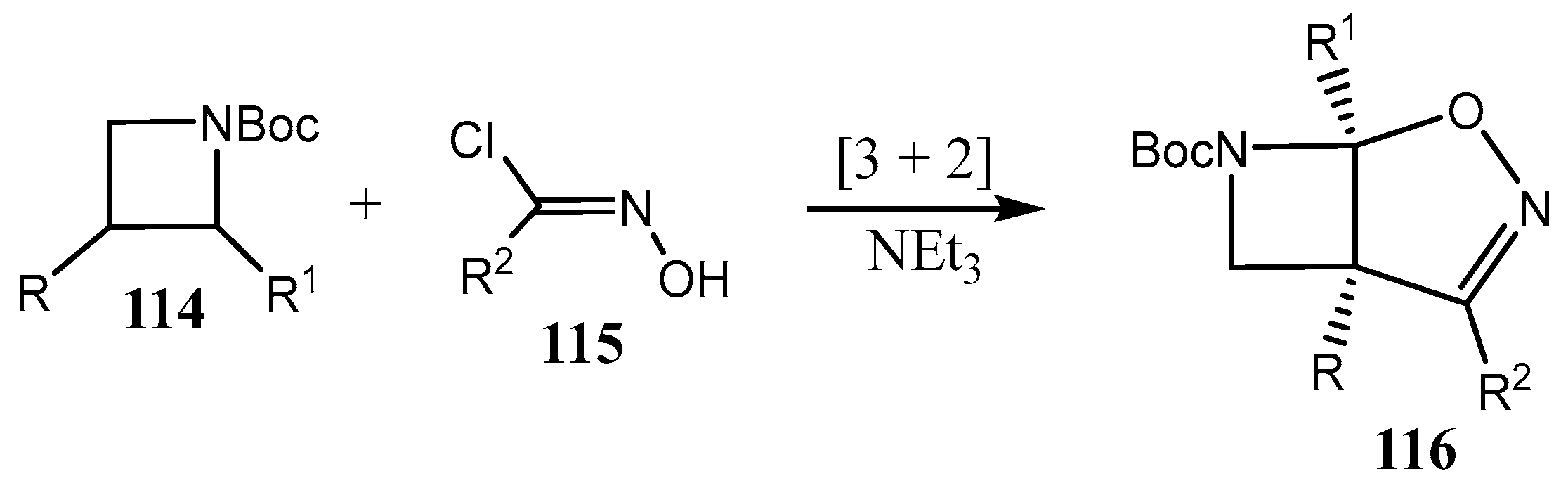



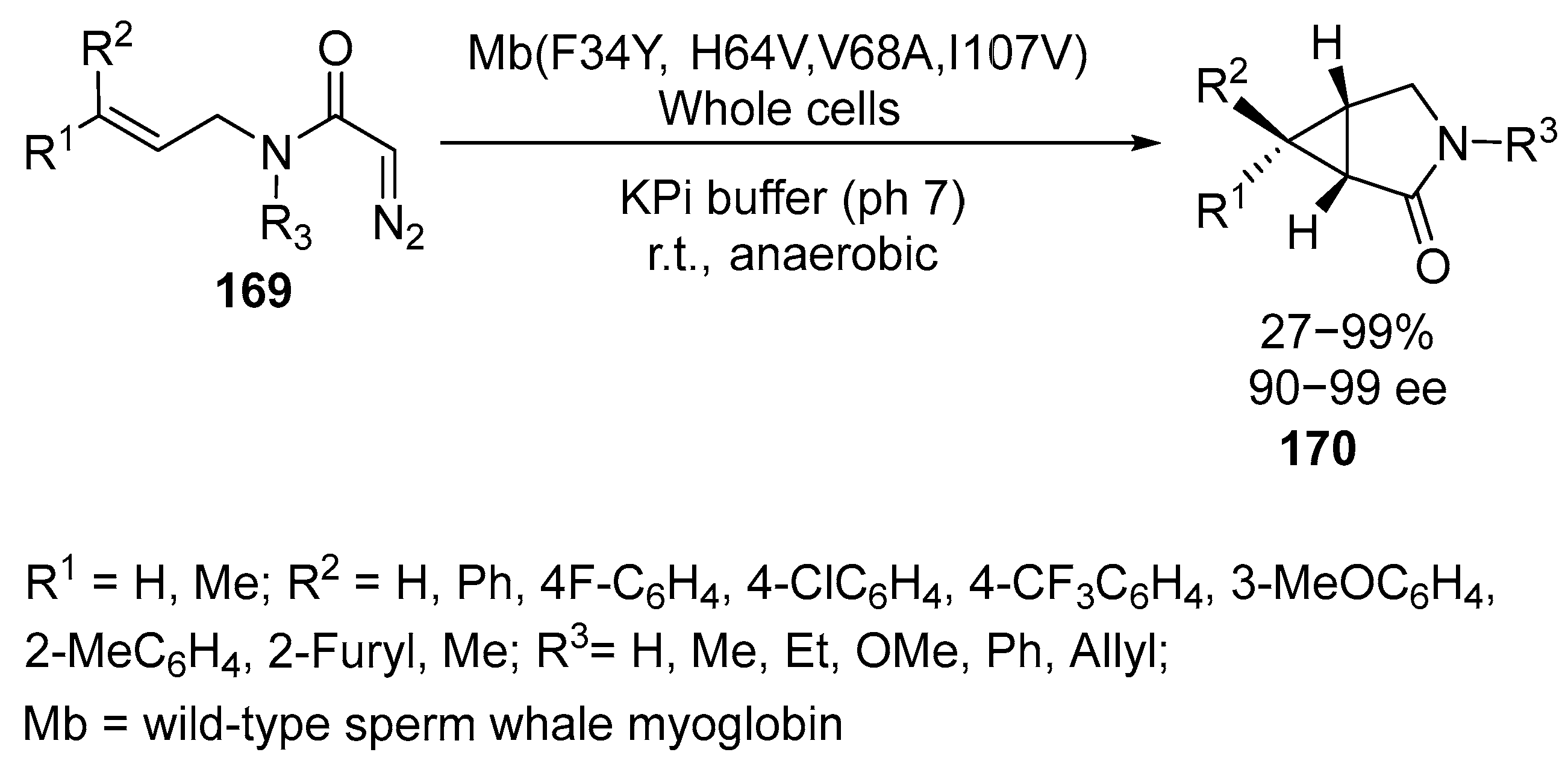
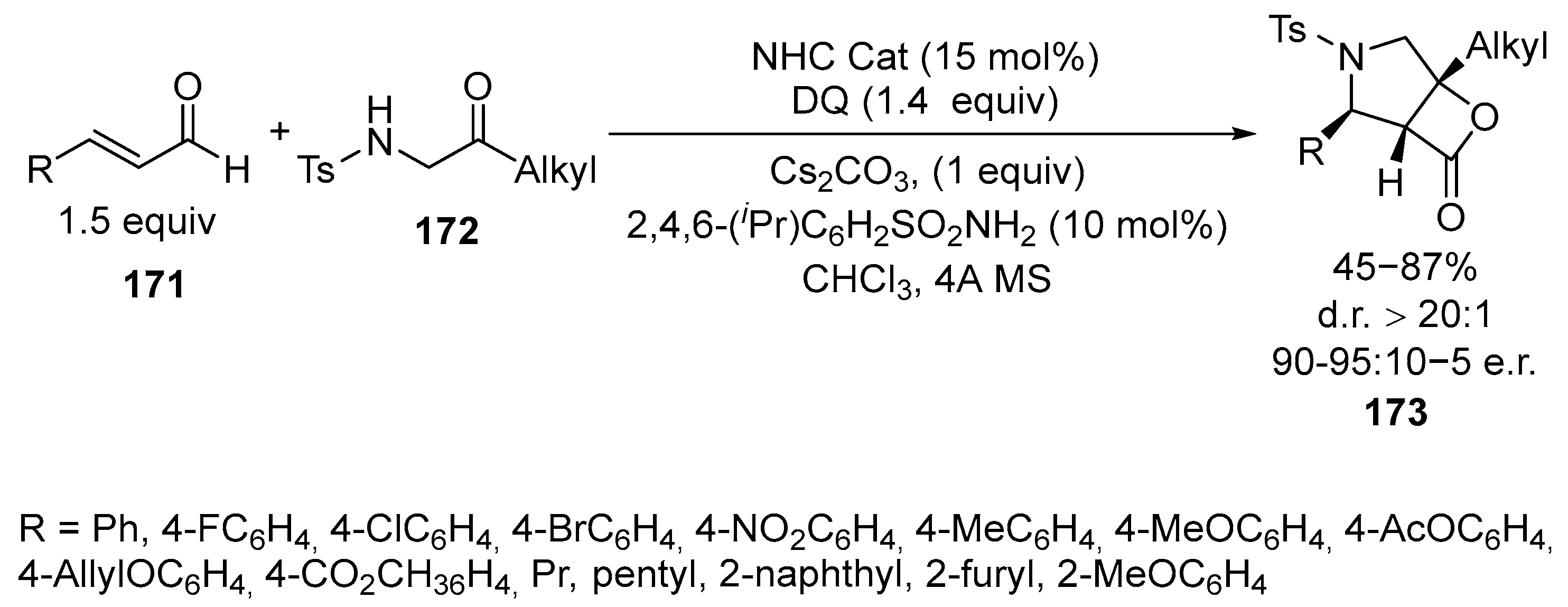


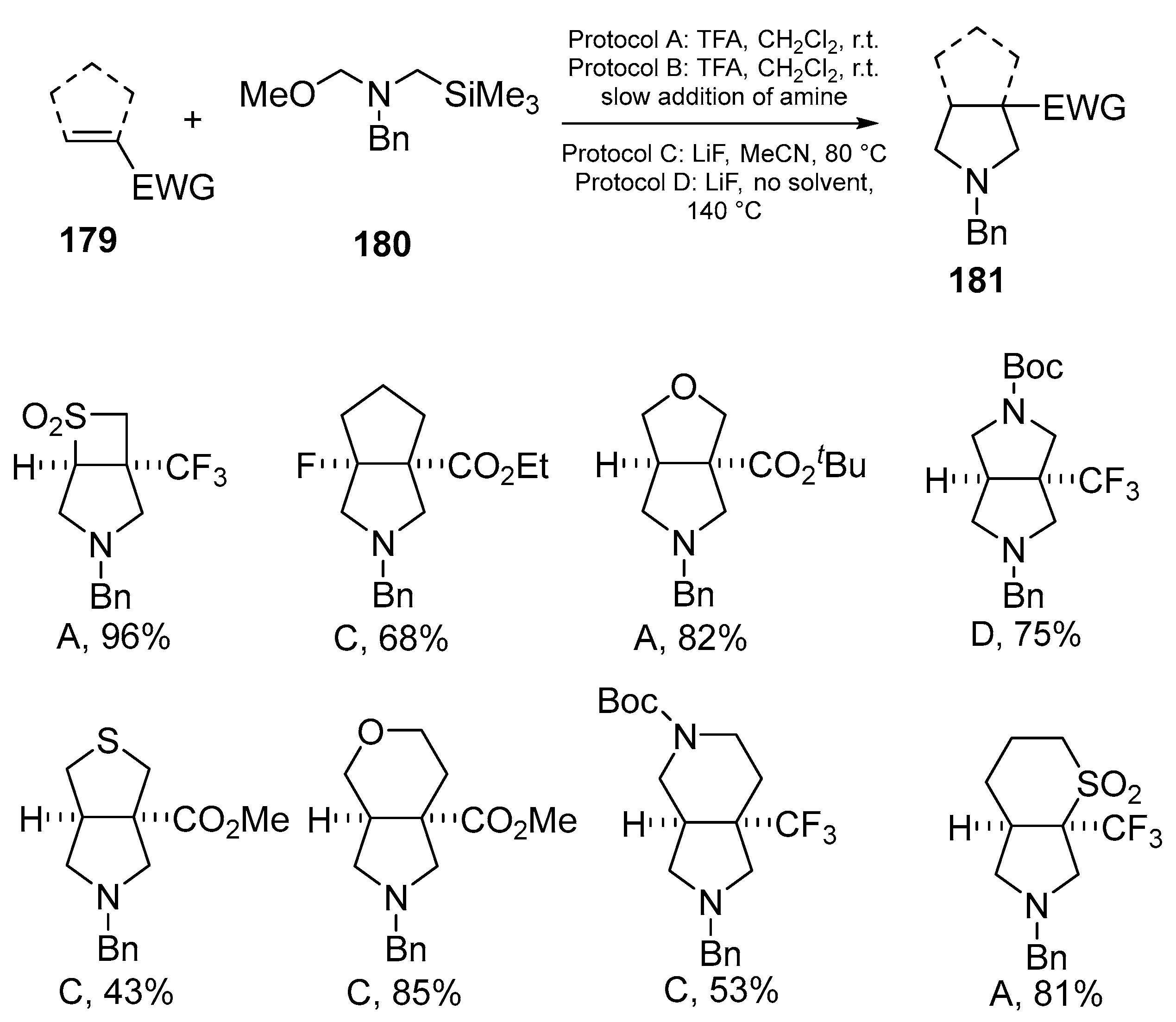
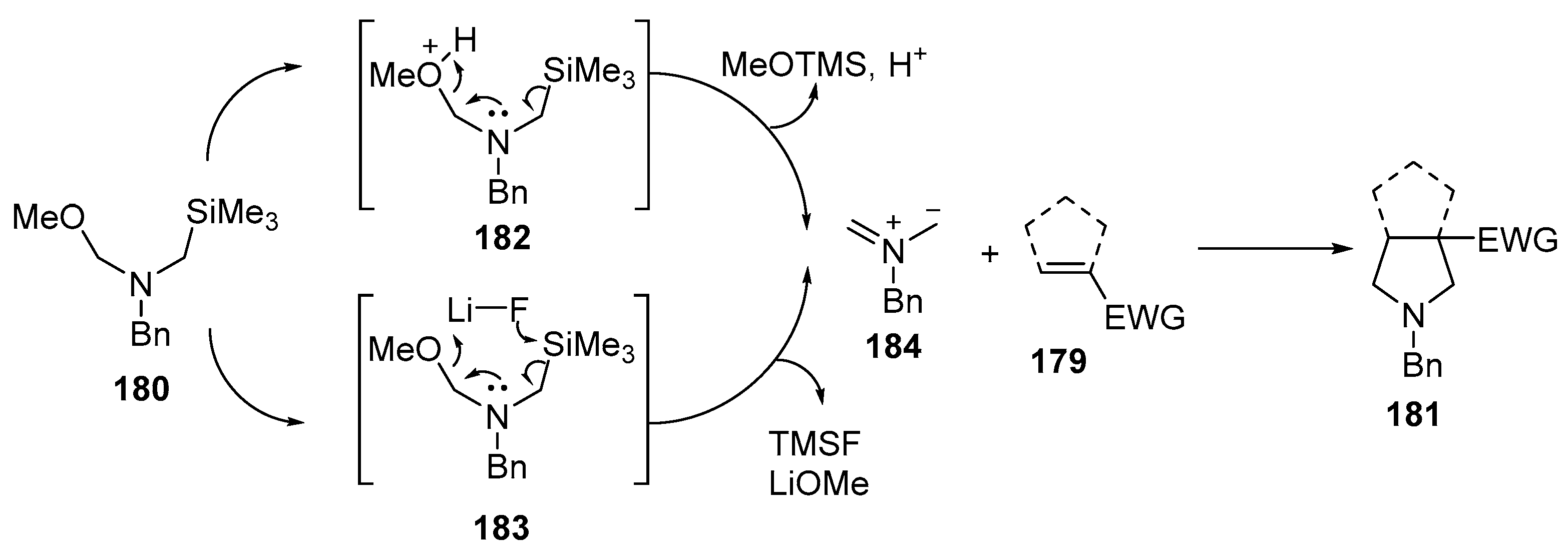
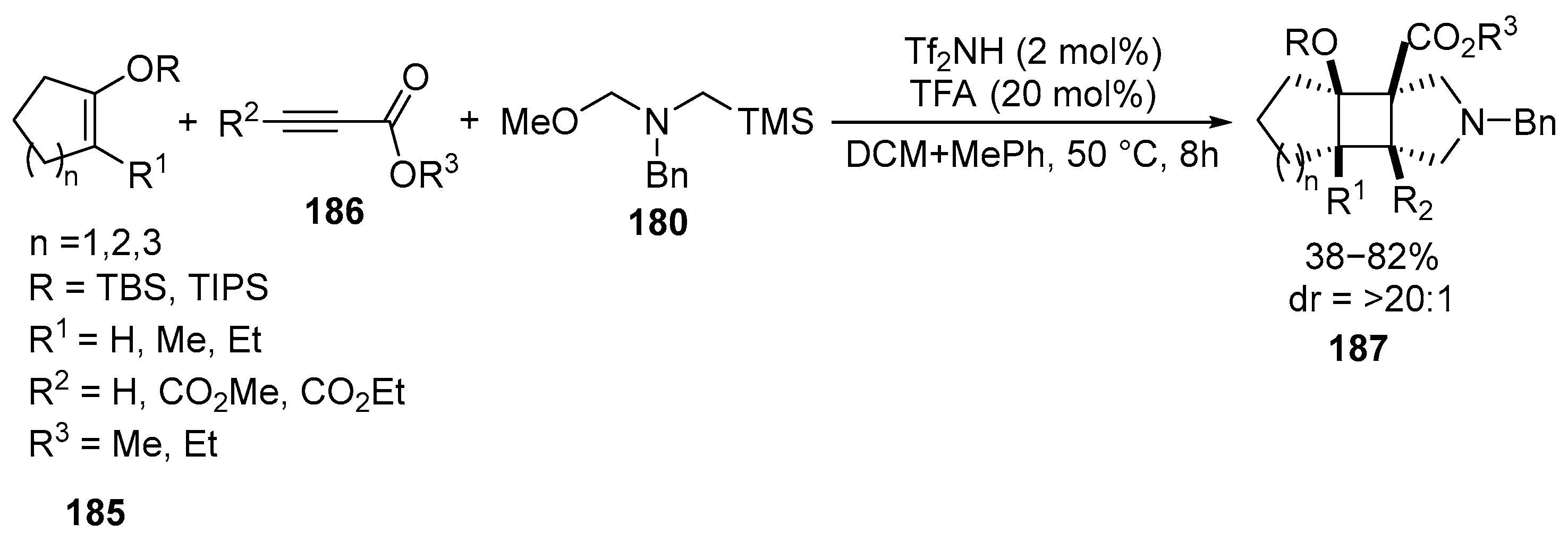
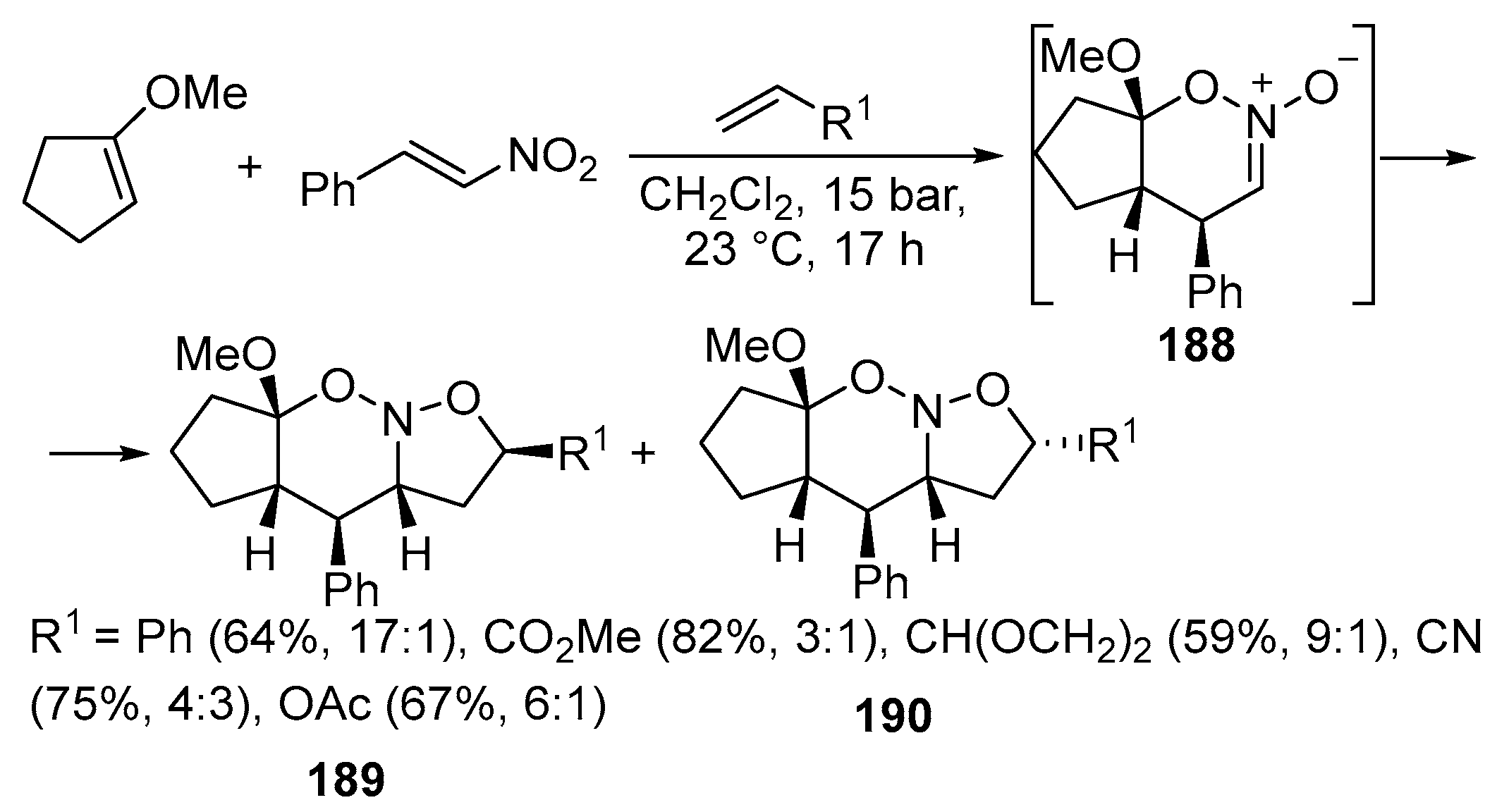

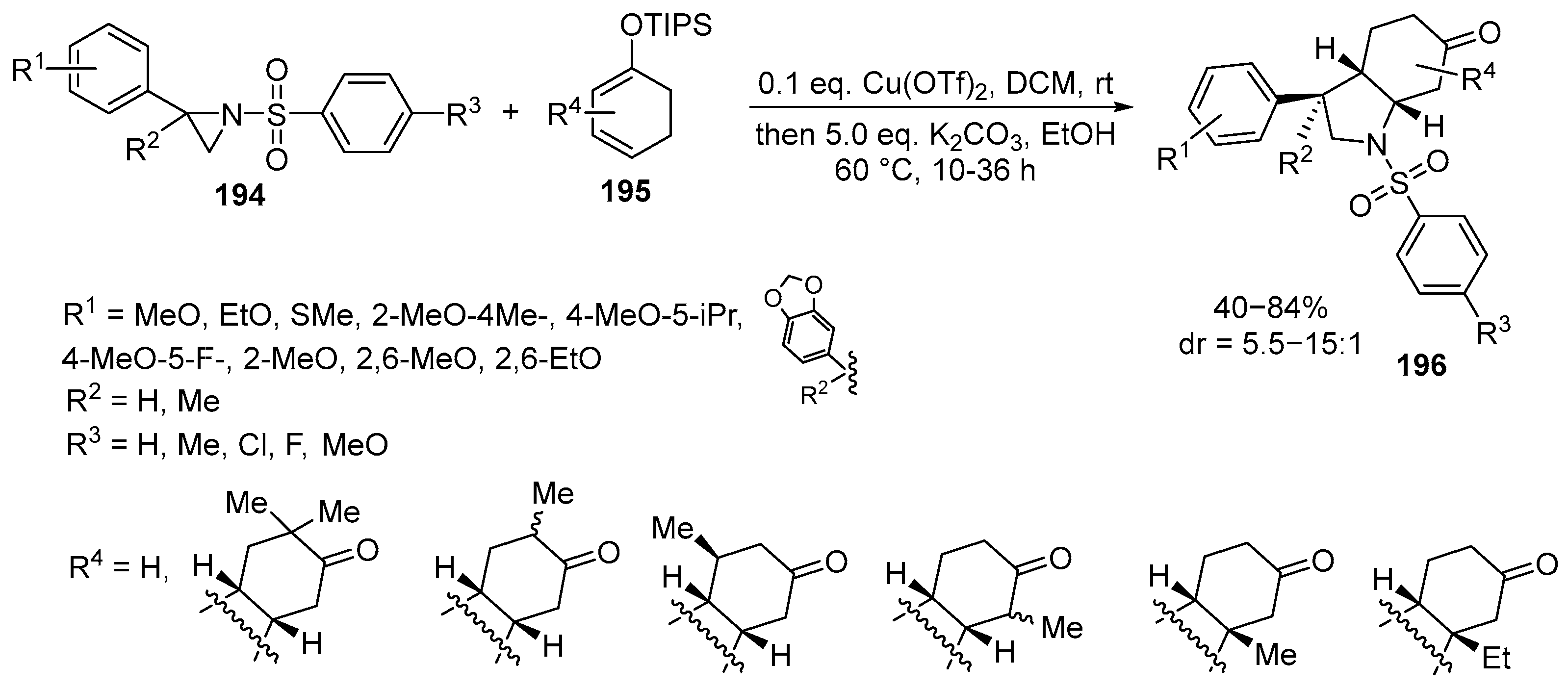
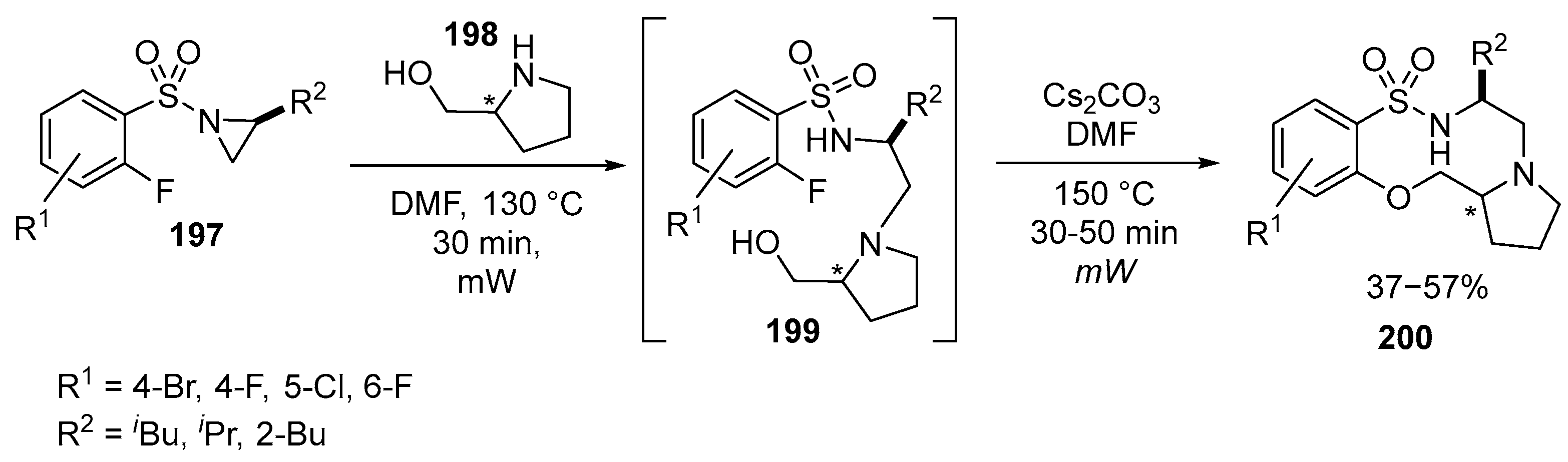



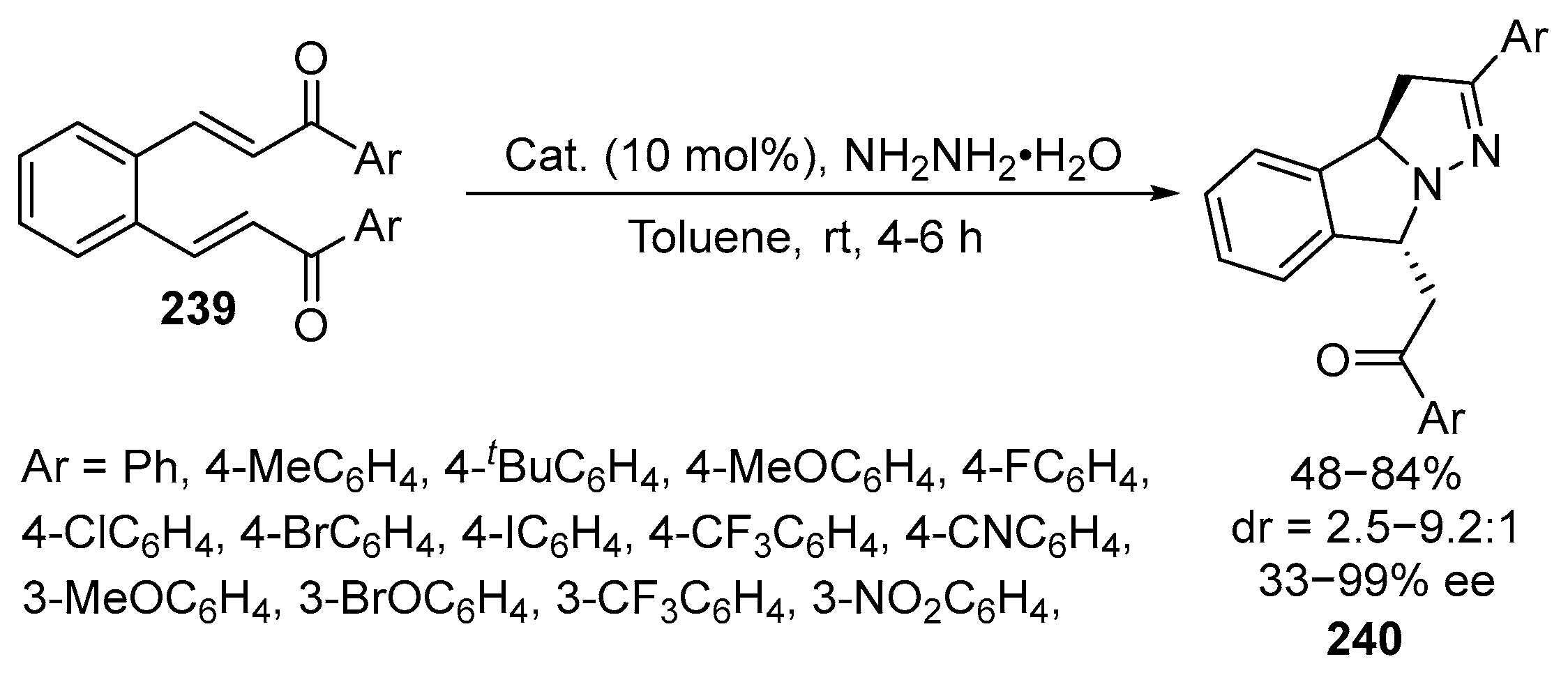

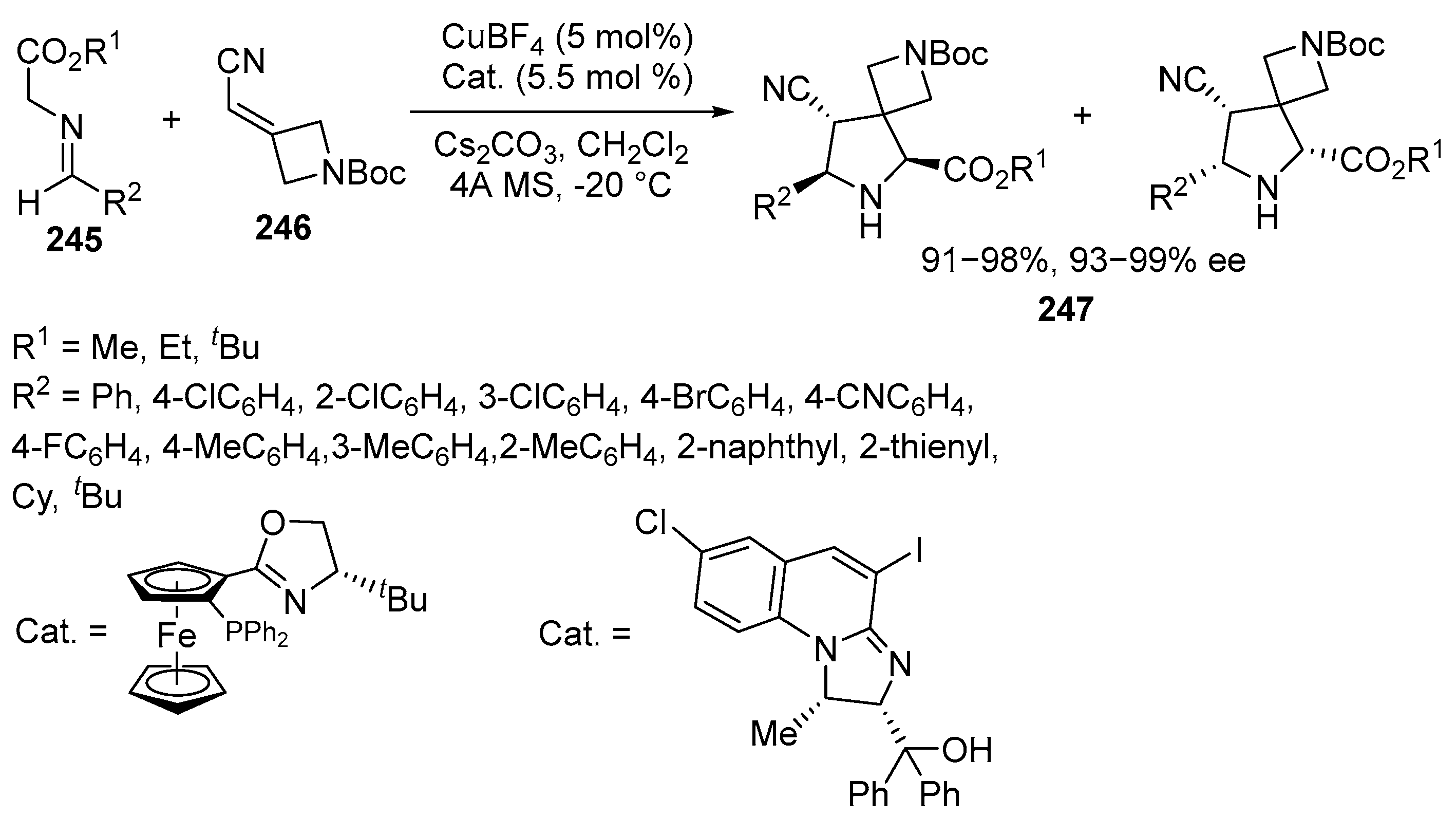

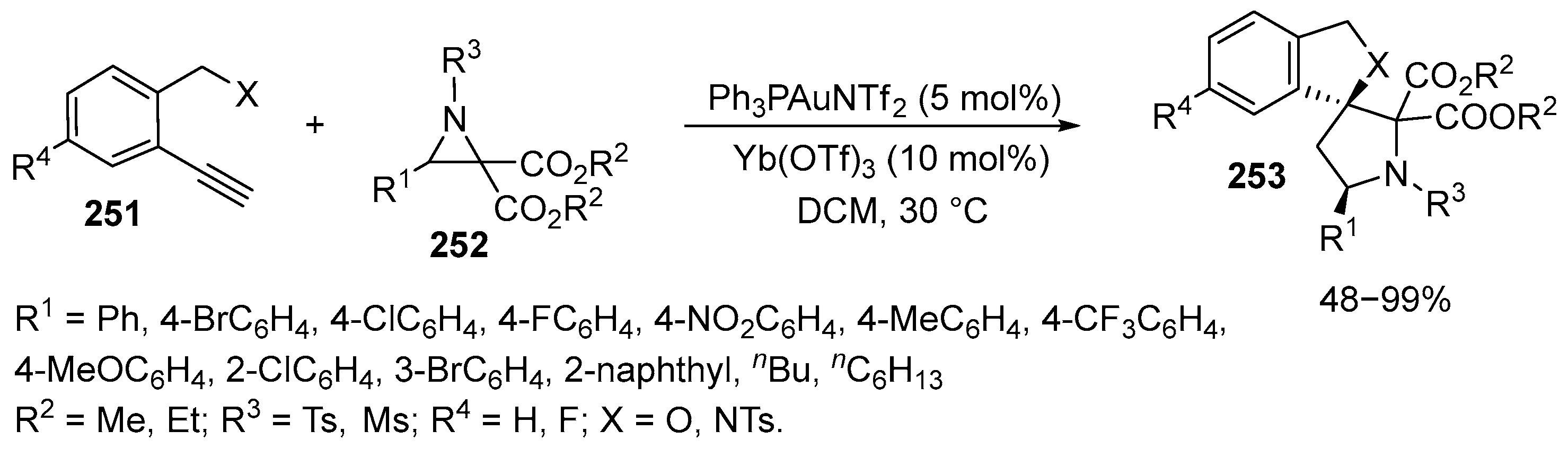
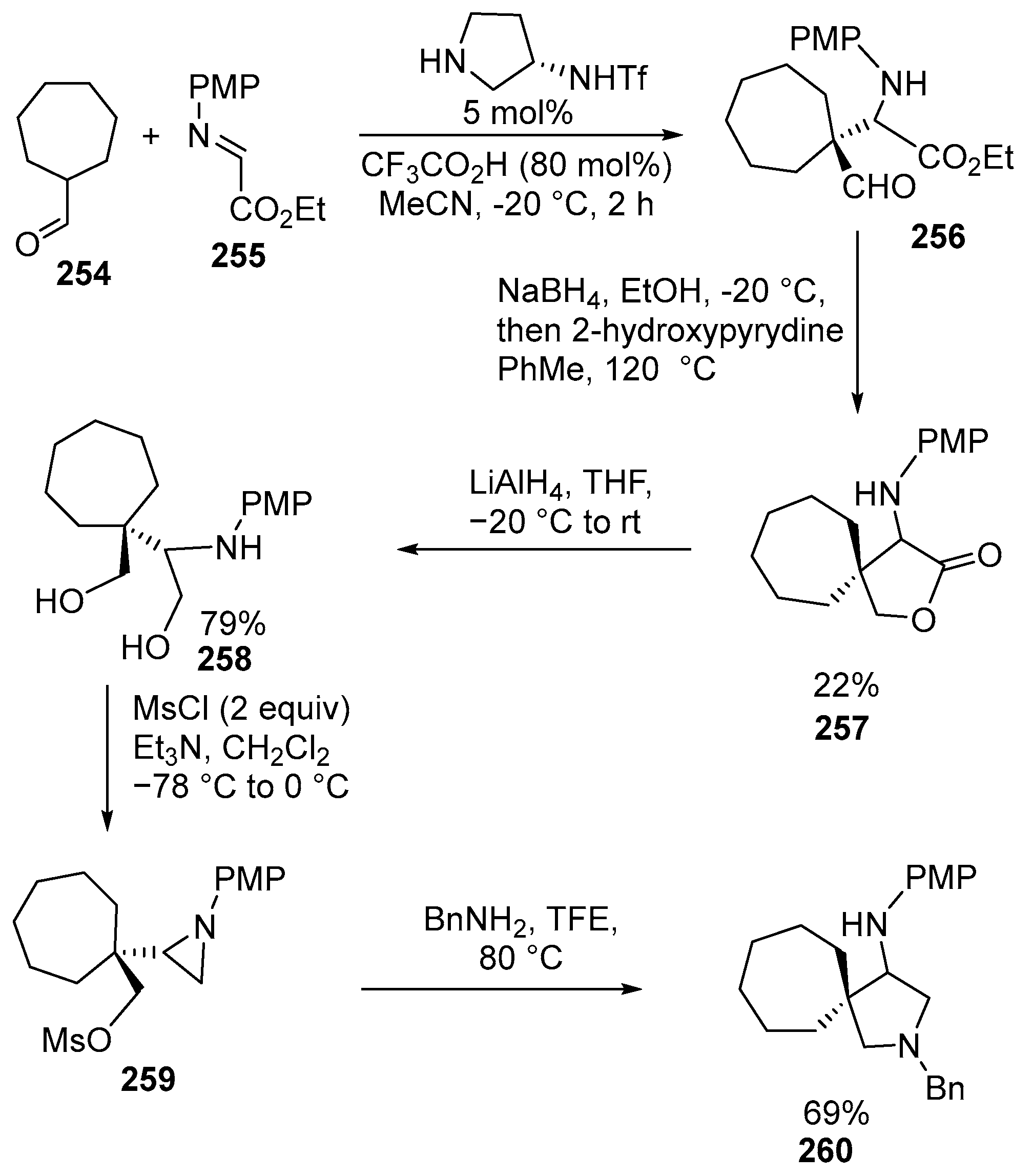
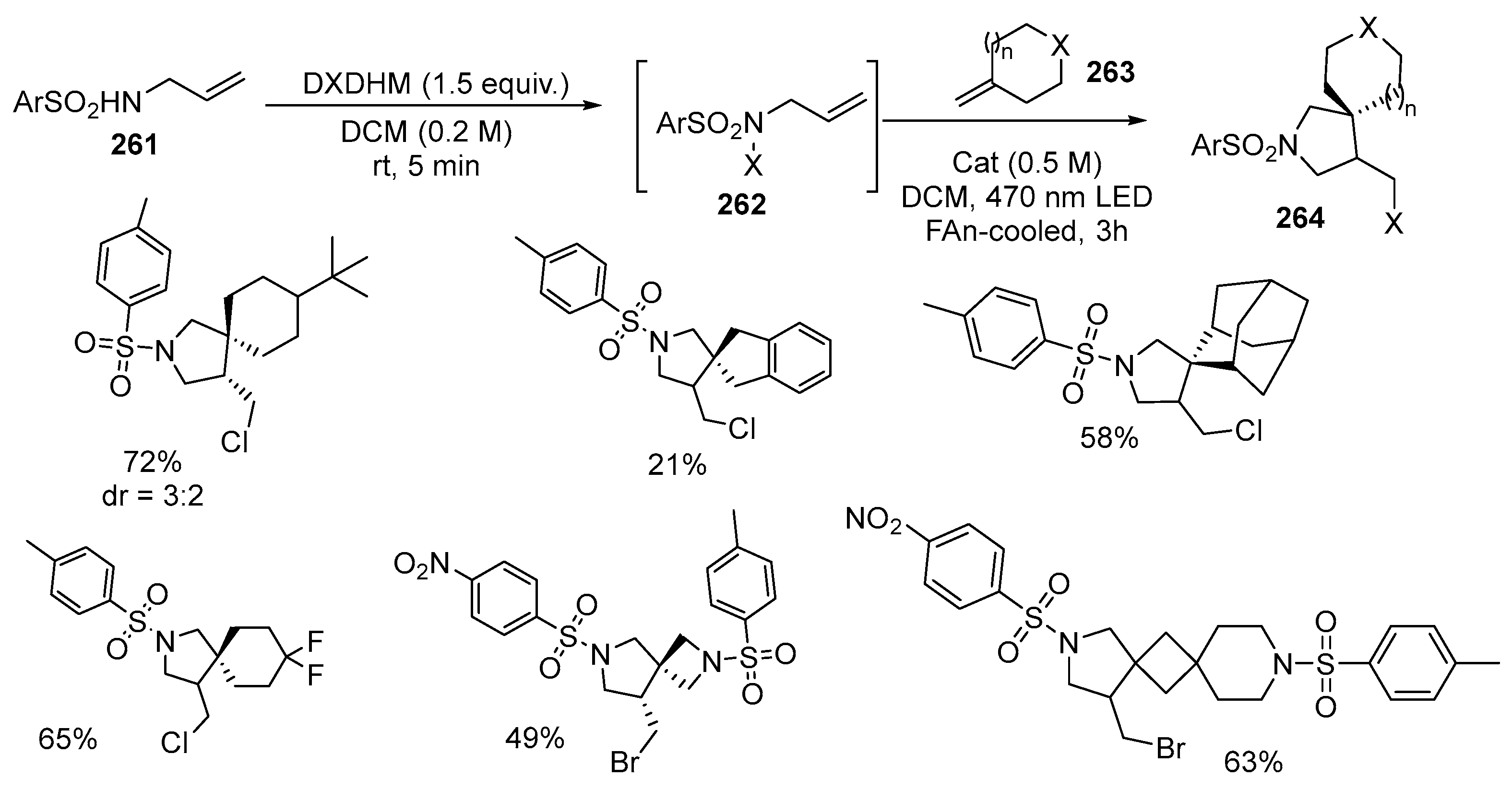
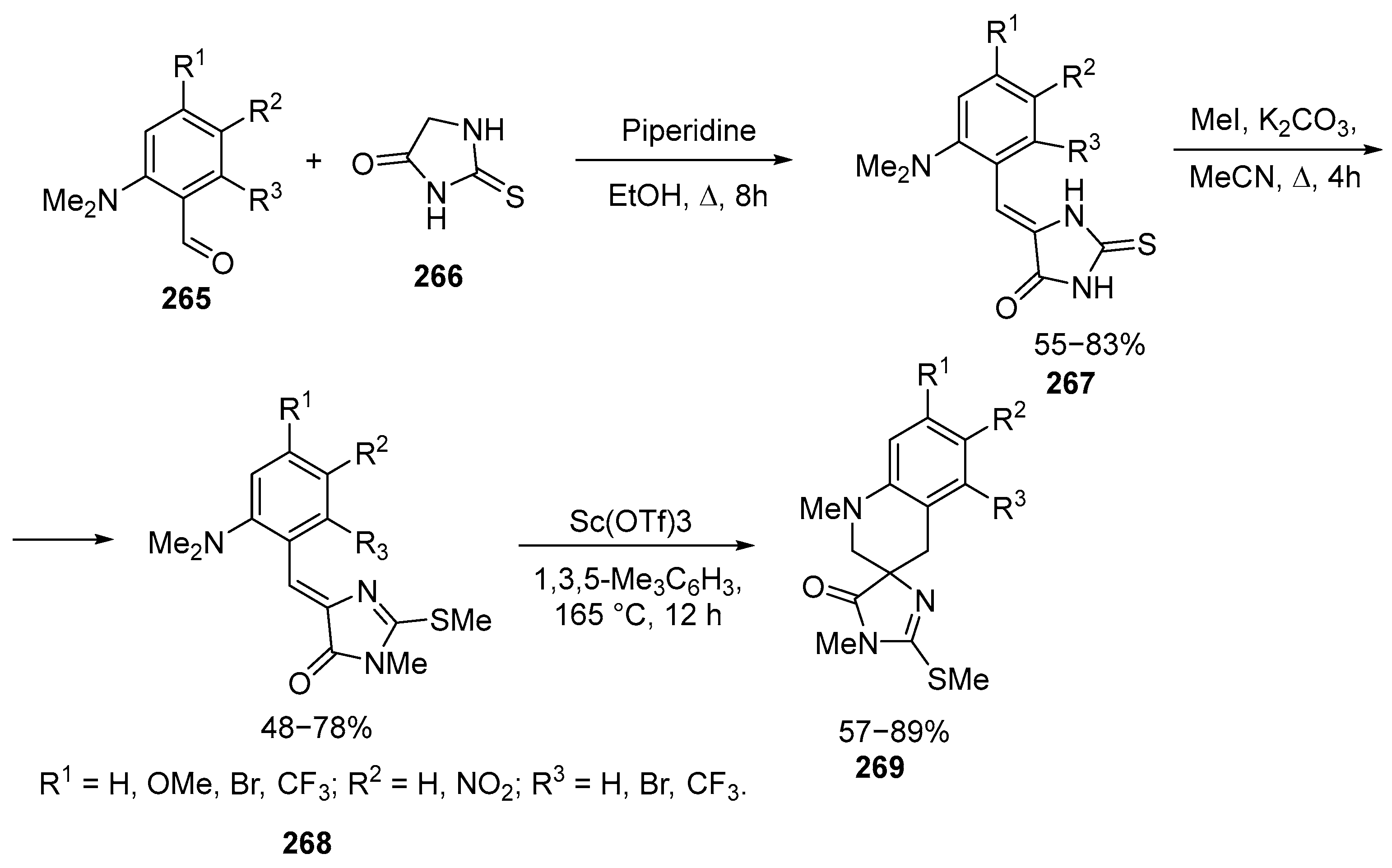
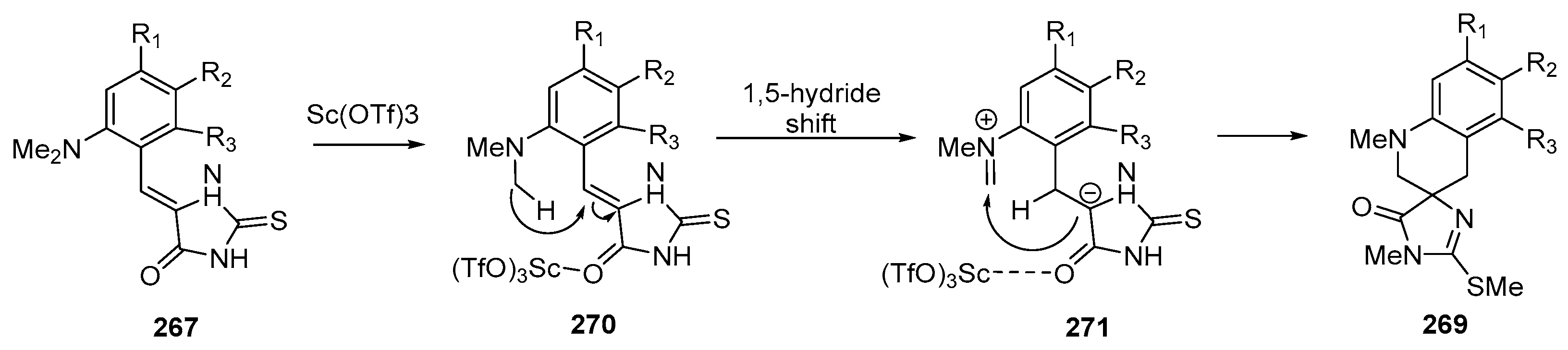

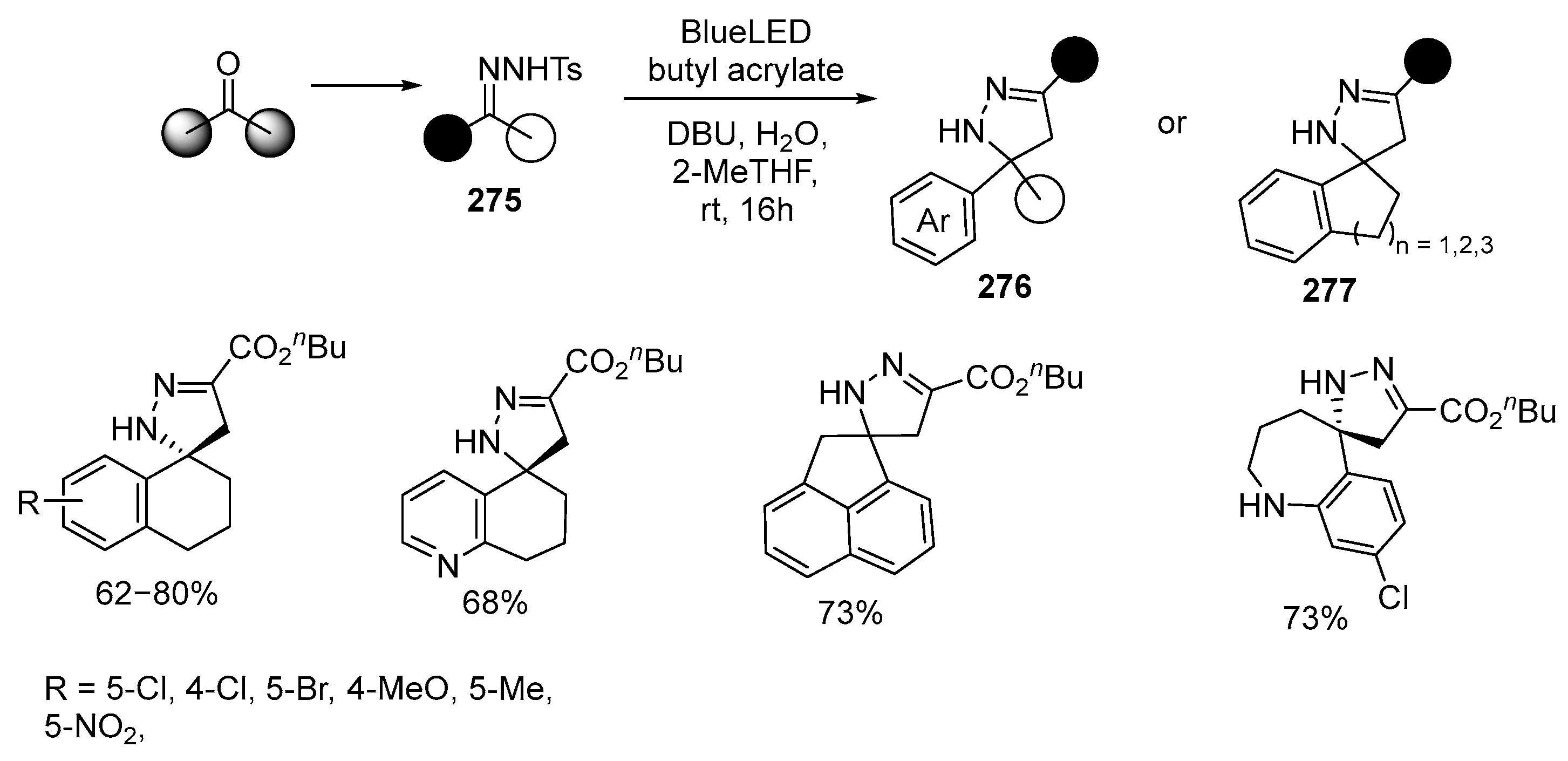
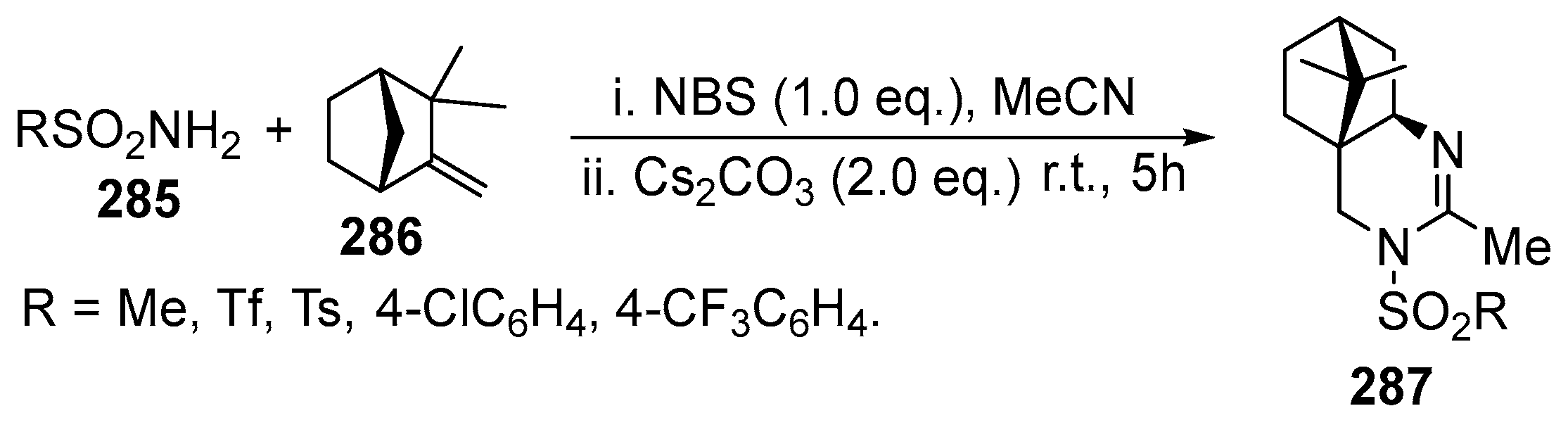

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moskalik, M.Y.; Shainyan, B.A. Fused-Linked and Spiro-Linked N-Containing Heterocycles. Int. J. Mol. Sci. 2025, 26, 7435. https://doi.org/10.3390/ijms26157435
Moskalik MY, Shainyan BA. Fused-Linked and Spiro-Linked N-Containing Heterocycles. International Journal of Molecular Sciences. 2025; 26(15):7435. https://doi.org/10.3390/ijms26157435
Chicago/Turabian StyleMoskalik, Mikhail Yu., and Bagrat A. Shainyan. 2025. "Fused-Linked and Spiro-Linked N-Containing Heterocycles" International Journal of Molecular Sciences 26, no. 15: 7435. https://doi.org/10.3390/ijms26157435
APA StyleMoskalik, M. Y., & Shainyan, B. A. (2025). Fused-Linked and Spiro-Linked N-Containing Heterocycles. International Journal of Molecular Sciences, 26(15), 7435. https://doi.org/10.3390/ijms26157435







