Resveratrol Impairs Insulin Signaling in Hepatic Cells via Activation of PKC and PTP1B Pathways
Abstract
1. Introduction
2. Results
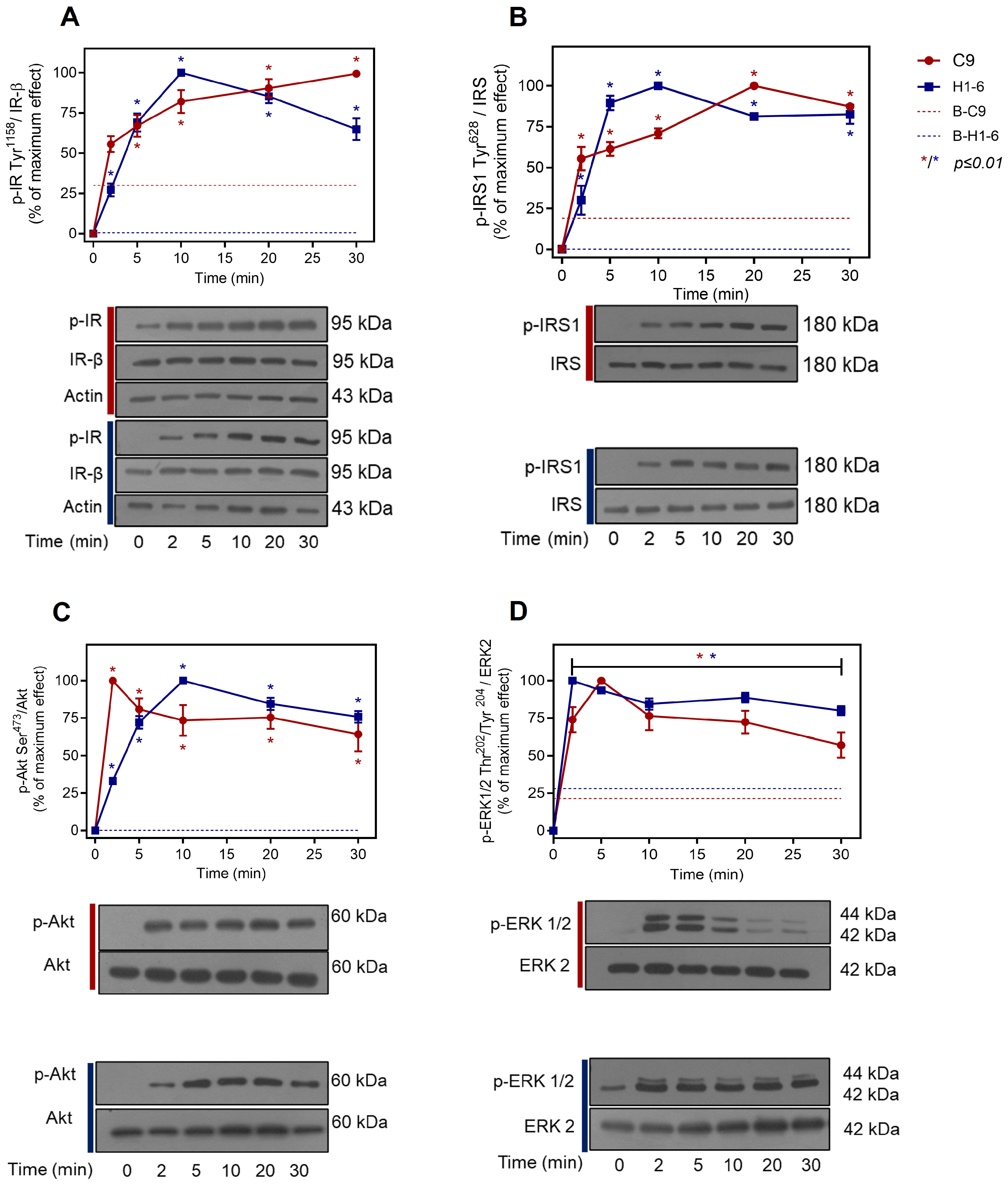
2.1. Insulin Signaling Pathway in Liver Cells
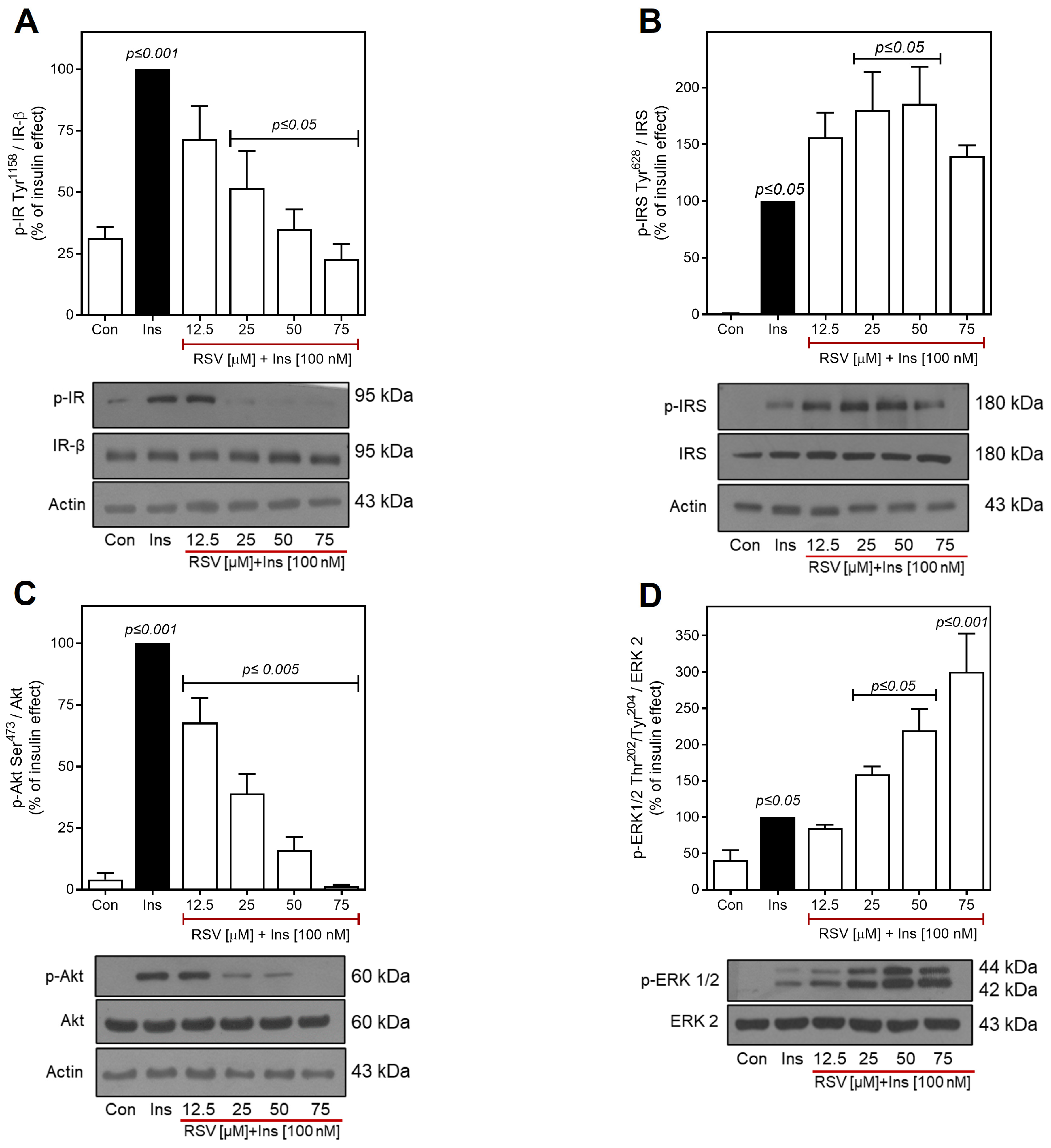
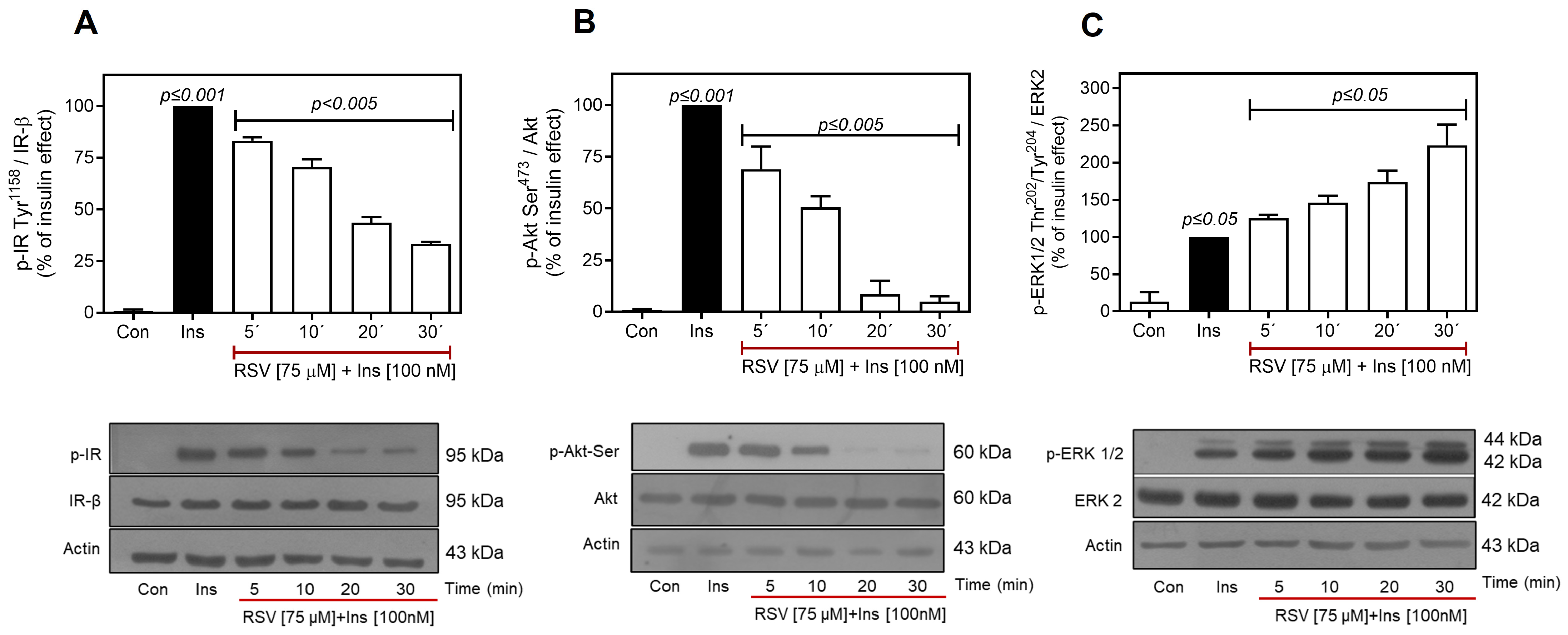
2.2. Resveratrol Desensitizes Insulin Signaling
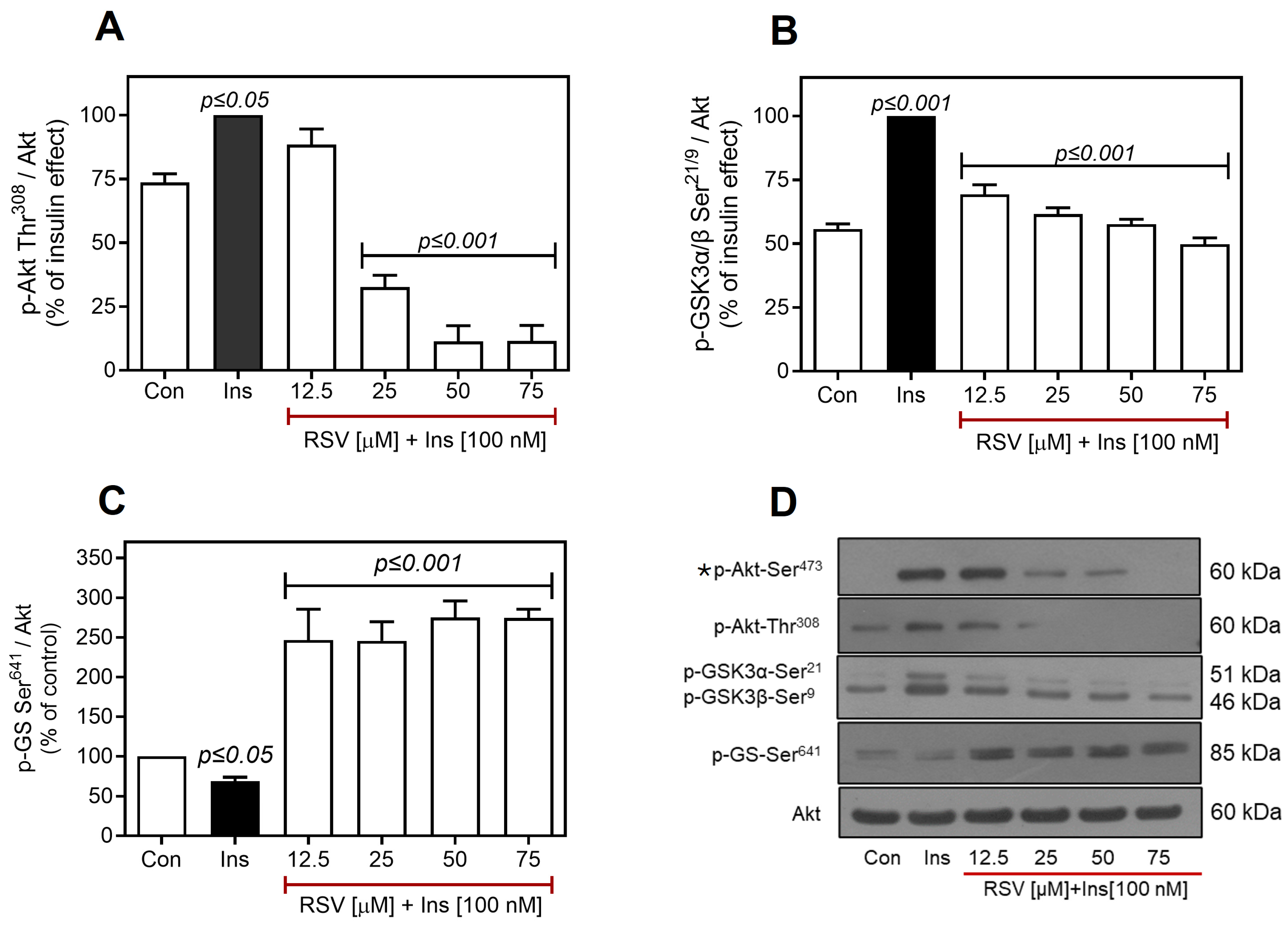
2.3. Resveratrol Negatively Regulates the Insulin Pathway Downstream of Akt
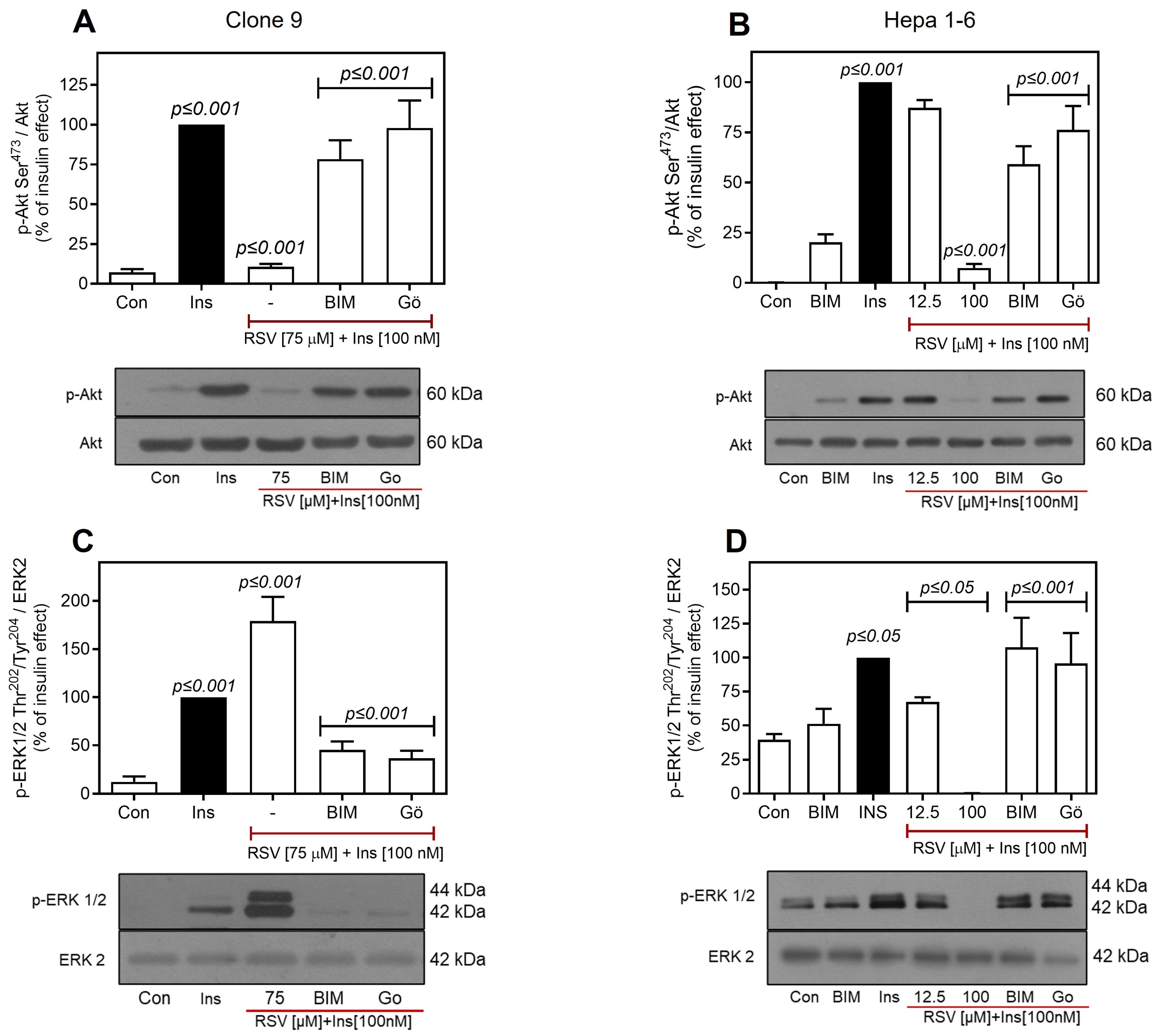
2.4. Resveratrol Downregulates the IR/Akt Pathway via PKC
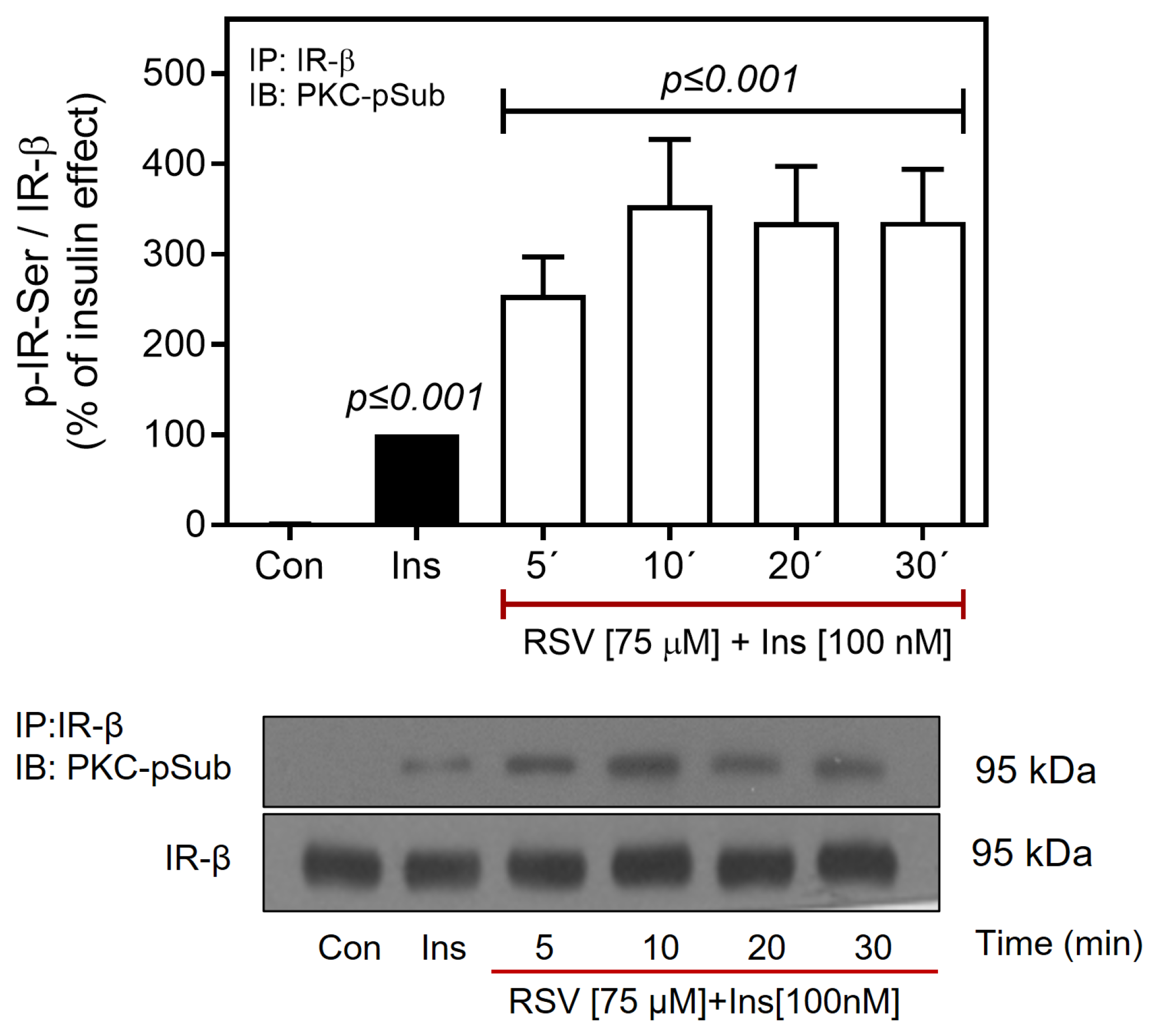
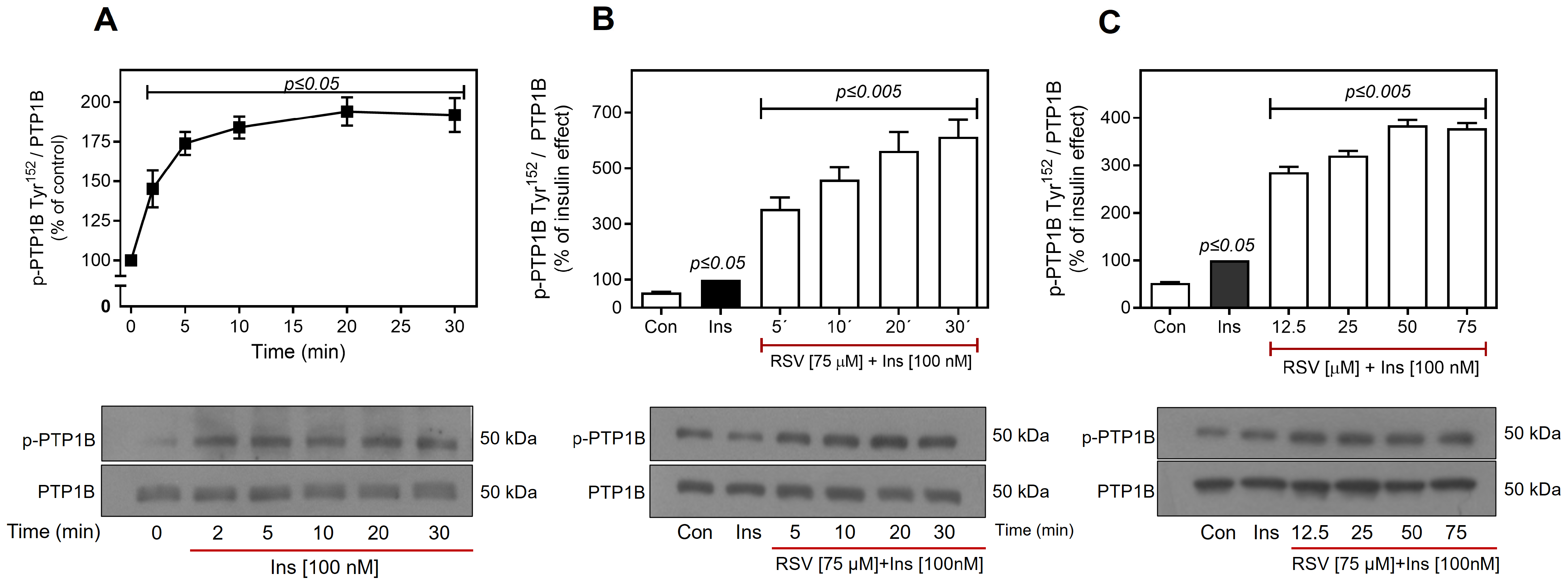
2.5. Resveratrol Promotes the Activation of PTP1B
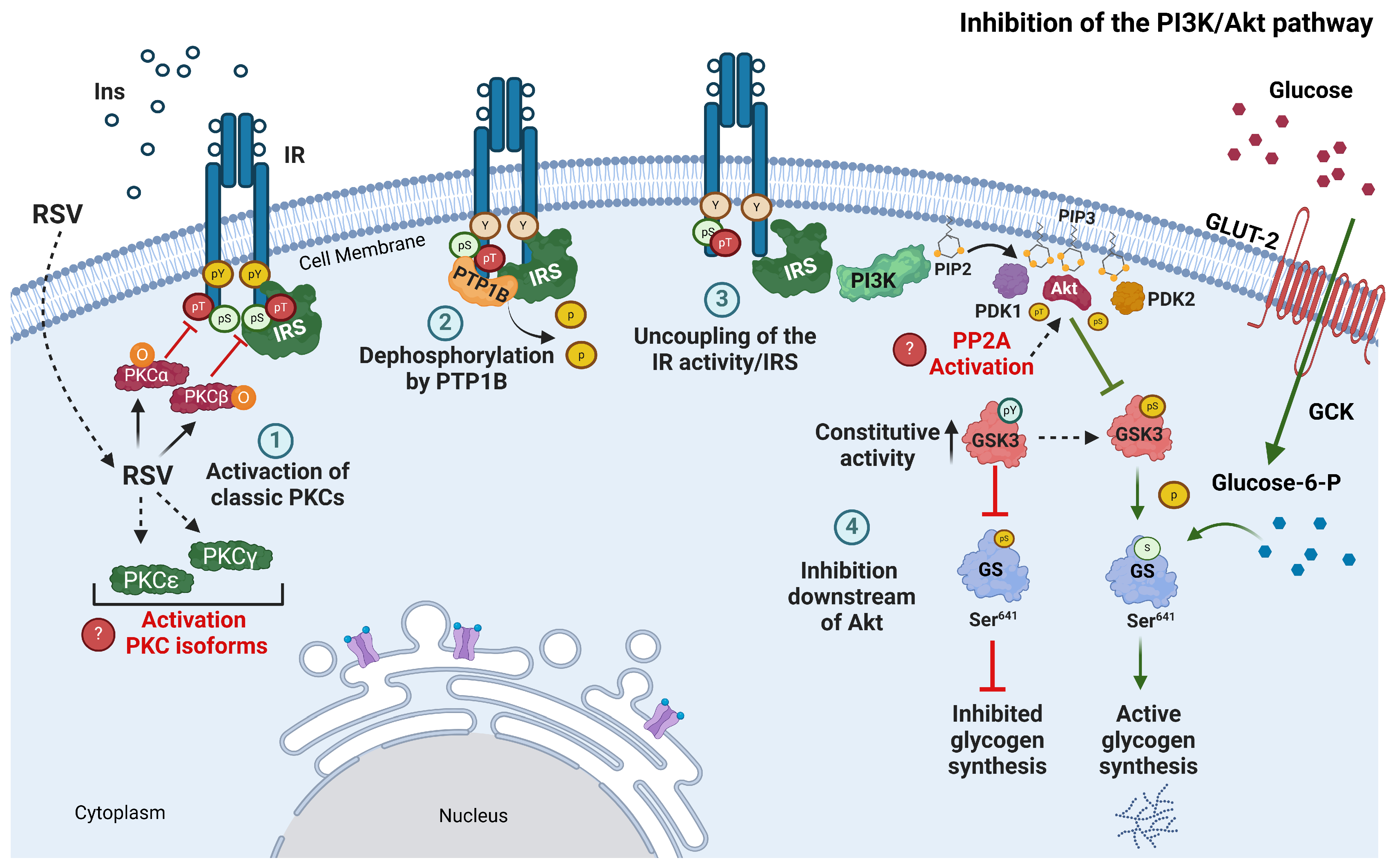
3. Discussion
4. Materials and Methods
4.1. Reagents, Peptides, Inhibitors, and Antibodies
4.2. Cell Culture and Experiments
4.3. Western Blot Methods
4.4. Immunoprecipitation Assay for Ser/Thr Phosphorylated IR
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BIM | Bisindolylmaleimide I |
| C9 cells | Clone 9 cells |
| CAT | Catalases |
| DNL | De novo lipogenesis |
| FFA | Free fatty acid |
| GLUT4 | Glucose transporter 4 |
| GPX | Glutathione peroxidase |
| GS | Glycogen synthase |
| GSK3α/β | Glycogen synthase kinase-3 α/β |
| H1-6 | Hepa 1-6 cells |
| HFSD | High-fat/high-sugar diet |
| InsR | Insulin resistance |
| IR | Insulin receptor |
| IRS | Insulin receptor substrate |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| NIDDM | Non-insulin-dependent diabetes mellitus |
| PI3K | Phosphatidylinositol 3-kinase |
| PKC | Protein kinase C |
| PTP | Protein tyrosine phosphatase |
| PTP1B | Protein tyrosine phosphatase 1B |
| ROS | Reactive oxygen species |
| RSV | Resveratrol |
| Ser | Serine |
| SOD | Superoxide dismutase |
| T2D | Type 2 diabetes |
| Thr | Threonine |
| Tyr | Tyrosine |
References
- Li, Z.; Chen, X.; Liu, G.; Li, J.; Zhang, J.; Cao, Y.; Miao, J. Antioxidant Activity and Mechanism of Resveratrol and Polydatin Isolated from Mulberry (Morus Alba L.). Molecules 2021, 26, 7574. [Google Scholar] [CrossRef]
- Akinwumi, B.; Bordun, K.-A.; Anderson, H. Biological Activities of Stilbenoids. Int. J. Mol. Sci. 2018, 19, 792. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Tong, W.; Chen, X.; Song, X.; Chen, Y.; Jia, R.; Zou, Y.; Li, L.; Yin, L.; He, C.; Liang, X.; et al. Resveratrol Inhibits Lps-Induced Inflammation through Suppressing the Signaling Cascades of Tlr4-Nf-κb/Mapks/Irf3. Exp. Ther. Med. 2020, 19, 1824–1834. [Google Scholar] [CrossRef]
- Kursvietiene, L.; Kopustinskiene, D.M.; Staneviciene, I.; Mongirdiene, A.; Kubová, K.; Masteikova, R.; Bernatoniene, J. Anti-Cancer Properties of Resveratrol: A Focus on Its Impact on Mitochondrial Functions. Antioxidants 2023, 12, 2056. [Google Scholar] [CrossRef] [PubMed]
- Sadi, G.; Şahin, G.; Bostanci, A. Modulation of Renal Insulin Signaling Pathway and Antioxidant Enzymes with Streptozotocin-Induced Diabetes: Effects of Resveratrol. Medicina 2018, 55, 3. [Google Scholar] [CrossRef] [PubMed]
- Reda, D.; Elshopakey, G.E.; Mahgoub, H.A.; Risha, E.F.; Khan, A.A.; Rajab, B.S.; El-Boshy, M.E.; Abdelhamid, F.M. Effects of Resveratrol against Induced Metabolic Syndrome in Rats: Role of Oxidative Stress, Inflammation, and Insulin Resistance. Evid. Based Complement. Alternat. Med. 2022, 2022, 3362005. [Google Scholar] [CrossRef]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between Insulin Resistance and the Development of Cardiovascular Disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef]
- Khattar, S.; Khan, S.A.; Zaidi, S.A.A.; Darvishikolour, M.; Farooq, U.; Naseef, P.P.; Kurunian, M.S.; Khan, M.Z.; Shamim, A.; Khan, M.M.U.; et al. Resveratrol from Dietary Supplement to a Drug Candidate: An Assessment of Potential. Pharmaceuticals 2022, 15, 957. [Google Scholar] [CrossRef]
- Su, M.; Zhao, W.; Xu, S.; Weng, J. Resveratrol in Treating Diabetes and Its Cardiovascular Complications: A Review of Its Mechanisms of Action. Antioxidants 2022, 11, 1085. [Google Scholar] [CrossRef]
- Cottart, C.H.; Nivet-Antoine, V.; Laguillier-Morizot, C.; Beaudeux, J.L. Resveratrol Bioavailability and Toxicity in Humans. Mol. Nutr. Food Res. 2010, 54, 7–16. [Google Scholar] [CrossRef]
- Patel, K.R.; Scott, E.; Brown, V.A.; Gescher, A.J.; Steward, W.P.; Brown, K. Clinical Trials of Resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 161–169. [Google Scholar] [CrossRef]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef]
- Jhanji, M.; Rao, C.N.; Sajish, M. Towards Resolving the Enigma of the Dichotomy of Resveratrol: Cis- and Trans-Resveratrol Have Opposite Effects on Tyrrs-Regulated Parp1 Activation. GeroScience 2021, 43, 1171–1200. [Google Scholar] [CrossRef]
- Jhanji, M.; Rao, C.N.; Massey, J.C.; Hope, M.C.; Zhou, X.; Keene, C.D.; Ma, T.; Wyatt, M.D.; Stewart, J.A.; Sajish, M. Cis- and Trans-Resveratrol Have Opposite Effects on Histone Serine-Adp-Ribosylation and Tyrosine Induced Neurodegeneration. Nat. Commun. 2022, 13, 3244. [Google Scholar] [CrossRef] [PubMed]
- Komorowska, D.; Gajewska, A.; Hikisz, P.; Bartosz, G.; Rodacka, A. Comparison of the Effects of Resveratrol and Its Derivatives on the Radiation Response of Mcf-7 Breast Cancer Cells. Int. J. Mol. Sci. 2021, 22, 9511. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Jia, B.; Tian, X.-T.; Song, X.; Wu, M.-L.; Kong, Q.-Y.; Li, H.; Liu, J. Correlation of Reactive Oxygen Species Levels with Resveratrol Sensitivities of Anaplastic Thyroid Cancer Cells. Oxid. Med. Cell. Longev. 2018, 2018, 6235417. [Google Scholar] [CrossRef] [PubMed]
- Konopko, A.; Litwinienko, G. Unexpected Role of Ph and Microenvironment on the Antioxidant and Synergistic Activity of Resveratrol in Model Micellar and Liposomal Systems. J. Org. Chem. 2022, 87, 1698–1709. [Google Scholar] [CrossRef]
- Pignitter, M.; Schueller, K.; Burkon, A.; Knorr, V.; Esefelder, L.; Doberer, D.; Wolzt, M.; Somoza, V. Concentration-Dependent Effects of Resveratrol and Metabolites on the Redox Status of Human Erythrocytes in Single-Dose Studies. J. Nutr. Biochem. 2016, 27, 164–170. [Google Scholar] [CrossRef]
- Salami, S.A.; Guinguina, A.; Agboola, J.O.; Omede, A.A.; Agbonlahor, E.M.; Tayyab, U. Review: In Vivo and Postmortem Effects of Feed Antioxidants in Livestock: A Review of the Implications on Authorization of Antioxidant Feed Additives. Animal 2016, 10, 1375–1390. [Google Scholar] [CrossRef]
- Desjardins, D.; Cacho-Valadez, B.; Liu, J.-L.; Wang, Y.; Yee, C.; Bernard, K.; Khaki, A.; Breton, L.; Hekimi, S. Antioxidants Reveal an Inverted U-Shaped Dose-Response Relationship between Reactive Oxygen Species Levels and the Rate of Aging in Caenorhabditis Elegans. Aging Cell 2017, 16, 104–112. [Google Scholar] [CrossRef]
- Xia, N.; Daiber, A.; Förstermann, U.; Li, H. Antioxidant Effects of Resveratrol in the Cardiovascular System. Br. J. Pharmacol. 2017, 174, 1633–1646. [Google Scholar] [CrossRef]
- Brioukhanov, A.L.; Netrusov, A.I. Catalase and Superoxide Dismutase: Distribution, Properties, and Physiological Role in Cells of Strict Anaerobes. Biochemistry 2004, 69, 949–962. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraman, M.M.; Al-Yousif, N.S.H.; Singh Mann, A.; Dolinsky, V.W.; Rabbani, R.; Zarychanski, R.; Abou-Setta, A.M. Resveratrol for Adults with Type 2 Diabetes Mellitus. Cochrane Database Syst. Rev. 2020, 2020, CD011919. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.M.; Eckardt, P.; Aleman, J.O.; da Rosa, J.C.; Liang, Y.; Iizumi, T.; Etheve, S.; Blaser, M.J.; Breslow, J.L.; Holt, P.R. The Effects of Trans-Resveratrol on Insulin Resistance, Inflammation, and Microbiota in Men with the Metabolic Syndrome: A Pilot Randomized, Placebo-Controlled Clinical Trial. J. Clin. Transl. Res. 2019, 4, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.H.X.; Howe, P.R.C. Resveratrol Counteracts Insulin Resistance—Potential Role of the Circulation. Nutrients 2018, 10, 1160. [Google Scholar] [CrossRef]
- Szkudelska, K.; Deniziak, M.; Sassek, M.; Szkudelski, I.; Noskowiak, W.; Szkudelski, T. Resveratrol Affects Insulin Signaling in Type 2 Diabetic Goto-Kakizaki Rats. Int. J. Mol. Sci. 2021, 22, 2469. [Google Scholar] [CrossRef]
- Movahed, A.; Raj, P.; Nabipour, I.; Mahmoodi, M.; Ostovar, A.; Kalantarhormozi, M.; Netticadan, T. Efficacy and Safety of Resveratrol in Type 1 Diabetes Patients: A Two-Month Preliminary Exploratory Trial. Nutrients 2020, 12, 161. [Google Scholar] [CrossRef]
- Yang, B.; Wang, Q.; Li, Y.; Zhang, S.; Sun, Y.; Wei, Y.; Jiang, Q.; Huang, Y. Resveratrol Inhibits White Adipose Deposition by the Esr1-Mediated Pi3k/Akt Signaling Pathway. Cell. Signal. 2024, 124, 111448. [Google Scholar] [CrossRef]
- Jimenez-Gomez, Y.; Mattison, J.A.; Pearson, K.J.; Martin-Montalvo, A.; Palacios, H.H.; Sossong, A.M.; Ward, T.M.; Younts, C.M.; Lewis, K.; Allard, J.S.; et al. Resveratrol Improves Adipose Insulin Signaling and Reduces the Inflammatory Response in Adipose Tissue of Rhesus Monkeys on High-Fat, High-Sugar Diet. Cell Metab. 2013, 18, 533–545. [Google Scholar] [CrossRef]
- Gong, L.; Guo, S.; Zou, Z. Resveratrol Ameliorates Metabolic Disorders and Insulin Resistance in High-Fat Diet-Fed Mice. Life Sci. 2020, 242, 117212. [Google Scholar] [CrossRef] [PubMed]
- Quan, Y.; Hua, S.; Li, W.; Zhan, M.; Li, Y.; Lu, L. Resveratrol Bidirectionally Regulates Insulin Effects in Skeletal Muscle through Alternation of Intracellular Redox Homeostasis. Life Sci. 2020, 242, 117188. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, Y.; Shu, L.; Song, G.; Ma, H. Resveratrol Reduces Liver Endoplasmic Reticulum Stress and Improves Insulin Sensitivity in Vivo and in Vitro. Drug Des. Devel. Ther. 2019, 13, 1473–1485. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Huang, R.; Lin, D.; Wang, Y.; Yang, X.; Huang, X.; Zheng, B.; Chen, Z.; Huang, Y.; Wang, X.; et al. Resveratrol Improves Liver Steatosis and Insulin Resistance in Non-Alcoholic Fatty Liver Disease in Association with the Gut Microbiota. Front. Microbiol. 2021, 12, 611323. [Google Scholar] [CrossRef]
- Zhang, J. Resveratrol Inhibits Insulin Responses in a Sirt1-Independent Pathway. Biochem. J. 2006, 397, 519–527. [Google Scholar] [CrossRef]
- Fröjdö, S.; Cozzone, D.; Vidal, H.; Pirola, L. Resveratrol Is a Class Ia Phosphoinositide 3-Kinase Inhibitor. Biochem. J. 2007, 406, 511–518. [Google Scholar] [CrossRef]
- Saltiel, A.R. Insulin Signaling in Health and Disease. J. Clin. Investig. 2021, 131, e142241. [Google Scholar] [CrossRef]
- Esposito, D.L.; Li, Y.; Cama, A.; Quon, M.J. Tyr612 and Tyr632 in Human Insulin Receptor Substrate-1 Are Important for Full Activation of Insulin-Stimulated Phosphatidylinositol 3-Kinase Activity and Translocation of Glut4 in Adipose Cells. Endocrinology 2001, 142, 2833–2840. [Google Scholar] [CrossRef]
- Boucher, J.; Kleinridders, A.; Kahn, C.R. Insulin Receptor Signaling in Normal and Insulin-Resistant States. Cold Spring Harb. Perspect. Biol. 2014, 6, a009191. [Google Scholar] [CrossRef]
- Najjar, S.M.; Perdomo, G. Hepatic Insulin Clearance: Mechanism and Physiology. Physiology 2019, 34, 198–215. [Google Scholar] [CrossRef]
- Roura-Guiberna, A.; Hernandez-Aranda, J.; Ramirez-Flores, C.J.; Mondragon-Flores, R.; Garibay-Nieto, N.; Queipo-Garcia, G.; Laresgoiti-Servitje, E.; Soh, J.-W.; Olivares-Reyes, J.A. Isomers of Conjugated Linoleic Acid Induce Insulin Resistance through a Mechanism Involving Activation of Protein Kinase Cε in Liver Cells. Cell. Signal. 2019, 53, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Whelan, S.A.; Dias, W.B.; Thiruneelakantapillai, L.; Lane, M.D.; Hart, G.W. Regulation of Insulin Receptor Substrate 1 (Irs-1)/Akt Kinase-Mediated Insulin Signaling by O-Linked Beta-N-Acetylglucosamine in 3t3-L1 Adipocytes. J. Biol. Chem. 2010, 285, 5204–5211. [Google Scholar] [CrossRef]
- Fayard, E.; Xue, G.; Parcellier, A.; Bozulic, L.; Hemmings, B.A. Protein Kinase B (Pkb/Akt), a Key Mediator of the Pi3k Signaling Pathway. Curr. Top. Microbiol. Immunol. 2010, 346, 31–56. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, S.Y.; Choi, C.S. Insulin Resistance: From Mechanisms to Therapeutic Strategies. Diabetes Metab. J. 2022, 46, 15–37. [Google Scholar] [CrossRef]
- Rui, L. Energy Metabolism in the Liver. Compr. Physiol. 2014, 4, 177–197. [Google Scholar] [CrossRef] [PubMed]
- Ader, M.; Bergman, R.N. Hyperinsulinemic Compensation for Insulin Resistance Occurs Independent of Elevated Glycemia in Male Dogs. Endocrinology 2021, 162, bqab119. [Google Scholar] [CrossRef]
- Weinstein, I.B.; Orenstein, J.M.; Gebert, R.; Kaighn, M.E.; Stadler, U.C. Growth and Structural Properties of Epithelial Cell Cultures Established from Normal Rat Liver and Chemically Induced Hepatomas1. Cancer Res. 1975, 35, 253–263. [Google Scholar]
- Arellano-Plancarte, A.; Hernandez-Aranda, J.; Catt, K.J.; Olivares-Reyes, J.A. Angiotensin-Induced Egf Receptor Transactivation Inhibits Insulin Signaling in C9 Hepatic Cells. Biochem. Pharmacol. 2010, 79, 733–745. [Google Scholar] [CrossRef][Green Version]
- Defries, D.M.; Taylor, C.G.; Zahradka, P. Glut3 Is Present in Clone 9 Liver Cells and Translocates to the Plasma Membrane in Response to Insulin. Biochem. Biophys. Res. Commun. 2016, 477, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla, R.A.; Porras, O.H.; Castro, J.; Barros, L.F. Cytosolic [Ca(2+)] Modulates Basal Glut1 Activity and Plays a Permissive Role in Its Activation by Metabolic Stress and Insulin in Rat Epithelial Cells. Cell Calcium 2000, 28, 97–106. [Google Scholar] [CrossRef]
- Shetty, M.; Kuruvilla, A.K.; Ismail-Beigi, F.; Loeb, J.N. Stimulation of Glucose Transport in Clone 9 Cells by Insulin and Thyroid Hormone: Role of Glut-1 Activation. Biochim. Biophys. Acta 1996, 1314, 140–146. [Google Scholar] [CrossRef]
- Nellis, M.M.; Doering, C.B.; Kasinski, A.; Danner, D.J. Insulin Increases Branched-Chain Alpha-Ketoacid Dehydrogenase Kinase Expression in Clone 9 Rat Cells. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E853–E860. [Google Scholar] [CrossRef]
- Gao, M.; Liu, D. Resveratrol Suppresses T0901317-Induced Hepatic Fat Accumulation in Mice. AAPS J. 2013, 15, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Butt, M.S.; Nadeem, M.; Peters, D.G.; Mubarak, M.S. Resveratrol as an Anti-Cancer Agent: A Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1428–1447. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, A.; Becattini, B.; Solinas, G. Insulin Signaling and Glucose Metabolism in Different Hepatoma Cell Lines Deviate from Hepatocyte Physiology toward a Convergent Aberrant Phenotype. Sci. Rep. 2020, 10, 12031. [Google Scholar] [CrossRef]
- Salmeen, A.; Andersen, J.N.; Myers, M.P.; Tonks, N.K.; Barford, D. Molecular Basis for the Dephosphorylation of the Activation Segment of the Insulin Receptor by Protein Tyrosine Phosphatase 1b. Mol. Cell 2000, 6, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Hung, M.C. Physiological Regulation of Akt Activity and Stability. Am. J. Transl. Res. 2010, 2, 19–42. [Google Scholar]
- Chen, X.; Hu, X.; Li, Y.; Zhu, C.; Dong, X.; Zhang, R.; Ma, J.; Huang, S.; Chen, L. Resveratrol Inhibits Erk1/2-Mediated Adhesion of Cancer Cells Via Activating Pp2a–Pten Signaling Network. J. Cell. Physiol. 2019, 234, 2822–2836. [Google Scholar] [CrossRef]
- Venkatesan, B.; Ghosh-Choudhury, N.; Das, F.; Mahimainathan, L.; Kamat, A.; Kasinath, B.S.; Abboud, H.E.; Choudhury, G.G. Resveratrol Inhibits Pdgf Receptor Mitogenic Signaling in Mesangial Cells: Role of Ptp1b. FASEB J. 2008, 22, 3469–3482. [Google Scholar] [CrossRef]
- Ye, M.-J.; Meng, N. Resveratrol Acts Via the Mitogen-Activated Protein Kinase (Mapk) Pathway to Protect Retinal Ganglion Cells from Apoptosis Induced by Hydrogen Peroxide. Bioengineered 2021, 12, 4878–4886. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, R.; Sun, C.; Zhang, H.; Xu, C.; Liu, W.; Gao, W.; Huang, S.; Chen, L. Resveratrol Prevents Cadmium Activation of Erk1/2 and Jnk Pathways from Neuronal Cell Death Via Protein Phosphatases 2a and 5. J. Neurochem. 2015, 135, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.-H.; Sethi, G.; Um, J.-Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef]
- Wang, Y.; Romigh, T.; He, X.; Orloff, M.S.; Silverman, R.H.; Heston, W.D.; Eng, C. Resveratrol Regulates the Pten/Akt Pathway through Androgen Receptor-Dependent and -Independent Mechanisms in Prostate Cancer Cell Lines. Hum. Mol. Genet. 2010, 19, 4319–4329. [Google Scholar] [CrossRef]
- Jiang, H.; Shang, X.; Wu, H.; Gautam, S.C.; Al-Holou, S.; Li, C.; Kuo, J.; Zhang, L.; Chopp, M. Resveratrol Downregulates Pi3k/Akt/Mtor Signaling Pathways in Human U251 Glioma Cells. J. Exp. Ther. Oncol. 2009, 8, 25–33. [Google Scholar]
- Lin, H.Y.; Shih, A.; Davis, F.B.; Tang, H.Y.; Martino, L.J.; Bennett, J.A.; Davis, P.J. Resveratrol Induced Serine Phosphorylation of P53 Causes Apoptosis in a Mutant P53 Prostate Cancer Cell Line. J. Urol. 2002, 168, 748–755. [Google Scholar] [CrossRef]
- Lin, C.; Crawford, D.R.; Lin, S.; Hwang, J.; Sebuyira, A.; Meng, R.; Westfall, J.E.; Tang, H.-Y.; Lin, S.; Yu, P.-Y.; et al. Inducible Cox-2-Dependent Apoptosis in Human Ovarian Cancer Cells. Carcinogenesis 2011, 32, 19–26. [Google Scholar] [CrossRef]
- Zhang, S.; Cao, H.J.; Davis, F.B.; Tang, H.-Y.; Davis, P.J.; Lin, H.-Y. Oestrogen Inhibits Resveratrol-Induced Post-Translational Modification of P53 and Apoptosis in Breast Cancer Cells. Br. J. Cancer 2004, 91, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Nakazato, T.; Xian, M.J.; Sagawa, M.; Ikeda, Y.; Kizaki, M. Resveratrol Induces Apoptosis of Human Malignant B Cells by Activation of Caspase-3 and P38 Map Kinase Pathways. Biochem. Pharmacol. 2006, 71, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Miloso, M.; Bertelli, A.A.E.; Nicolini, G.; Tredici, G. Resveratrol-Induced Activation of the Mitogen-Activated Protein Kinases, Erk1 and Erk2, in Human Neuroblastoma Sh-Sy5y Cells. Neurosci. Lett. 1999, 264, 141–144. [Google Scholar] [CrossRef]
- Johnson, H.; Narayan, S.; Sharma, A.K. Altering Phosphorylation in Cancer through Pp2a Modifiers. Cancer Cell Int. 2024, 24, 11. [Google Scholar] [CrossRef]
- Mazhar, S.; Taylor, S.E.; Sangodkar, J.; Narla, G. Targeting Pp2a in Cancer: Combination Therapies. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 51–63. [Google Scholar] [CrossRef]
- Zeng, Y.-H.; Zhou, L.-Y.; Chen, Q.-Z.; Li, Y.; Shao, Y.; Ren, W.-Y.; Liao, Y.-P.; Wang, H.; Zhu, J.-H.; Huang, M.; et al. Resveratrol Inactivates Pi3k/Akt Signaling through Upregulating Bmp7 in Human Colon Cancer Cells. Oncol. Rep. 2017, 38, 456–464. [Google Scholar] [CrossRef]
- Dirimanov, S.; Högger, P. Screening of Inhibitory Effects of Polyphenols on Akt-Phosphorylation in Endothelial Cells and Determination of Structure-Activity Features. Biomolecules 2019, 9, 219. [Google Scholar] [CrossRef]
- Marr, L.; Biswas, D.; Daly, L.A.; Browning, C.; Vial, S.C.M.; Maskell, D.P.; Hudson, C.; Bertrand, J.A.; Pollard, J.; Ranson, N.A.; et al. Mechanism of Glycogen Synthase Inactivation and Interaction with Glycogenin. Nat. Commun. 2022, 13, 3372. [Google Scholar] [CrossRef]
- Rozhkov, S.V.; Sharlo, K.A.; Shenkman, B.S.; Mirzoev, T.M. The Role of Glycogen Synthase Kinase-3 in the Regulation of Ribosome Biogenesis in Rat Soleus Muscle under Disuse Conditions. Int. J. Mol. Sci. 2022, 23, 2751. [Google Scholar] [CrossRef]
- Gutierrez-Rodelo, C.; Roura-Guiberna, A.; Olivares-Reyes, J.A. Molecular Mechanisms of Insulin Resistance: An Update. Gac. Med. Mex. 2017, 153, 197–209. [Google Scholar]
- Gutierrez-Rodelo, C.; Arellano-Plancarte, A.; Hernandez-Aranda, J.; Landa-Galvan, H.V.; Parra-Mercado, G.K.; Moreno-Licona, N.J.; Hernandez-Gonzalez, K.D.; Catt, K.J.; Villalobos-Molina, R.; Olivares-Reyes, J.A. Angiotensin Ii Inhibits Insulin Receptor Signaling in Adipose Cells. Int. J. Mol. Sci. 2022, 23, 6048. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sampat, K.; Hu, N.; Zakari, J.; Yuspa, S.H. Protein Kinase C Negatively Regulates Akt Activity and Modifies Uvc-Induced Apoptosis in Mouse Keratinocytes. J. Biol. Chem. 2006, 281, 3237–3243. [Google Scholar] [CrossRef] [PubMed]
- Samuel, V.T.; Liu, Z.-X.; Wang, A.; Beddow, S.A.; Geisler, J.G.; Kahn, M.; Zhang, X.-M.; Monia, B.P.; Bhanot, S.; Shulman, G.I. Inhibition of Protein Kinase Cε Prevents Hepatic Insulin Resistance in Nonalcoholic Fatty Liver Disease. J. Clin. Investig. 2007, 117, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, V.; Werner, E.D.; Giraud, J.; Lee, Y.H.; Shoelson, S.E.; White, M.F. Phosphorylation of Ser307 in Insulin Receptor Substrate-1 Blocks Interactions with the Insulin Receptor and Inhibits Insulin Action. J. Biol. Chem. 2002, 277, 1531–1537. [Google Scholar] [CrossRef]
- England, K.; Rumsby, M.G. Changes in Protein Kinase C ∊ Phosphorylation Status and Intracellular Localization as 3t3 and 3t6 Fibroblasts Grow to Confluency and Quiescence: A Role for Phosphorylation at Ser-729? Biochem. J. 2000, 352, 19–26. [Google Scholar] [CrossRef]
- Zick, Y. Ser/Thr Phosphorylation of Irs Proteins: A Molecular Basis for Insulin Resistance. Sci. Signal. 2005, 2005, pe4. [Google Scholar] [CrossRef] [PubMed]
- Schmitz-Peiffer, C.; Biden, T.J. Protein Kinase C Function in Muscle, Liver, and Beta-Cells and Its Therapeutic Implications for Type 2 Diabetes. Diabetes 2008, 57, 1774–1783. [Google Scholar] [CrossRef]
- Bossenmaier, B.; Mosthaf, L.; Mischak, H.; Ullrich, A.; Haring, H.U. Protein Kinase C Isoforms β 1 and β 2 Inhibit the Tyrosine Kinase Activity of the Insulin Receptor. Diabetologia 1997, 40, 863–866. [Google Scholar] [CrossRef]
- Kawano, T.; Inokuchi, J.; Eto, M.; Murata, M.; Kang, J.-H. Activators and Inhibitors of Protein Kinase C (Pkc): Their Applications in Clinical Trials. Pharmaceutics 2021, 13, 1748. [Google Scholar] [CrossRef]
- Fang, Z.; Xu, A.; Wu, L.; Hei, T.K.; Hong, M. The Role of Protein Kinase C Alpha Translocation in Radiation-Induced Bystander Effect. Sci. Rep. 2016, 6, 25817. [Google Scholar] [CrossRef]
- Sun, X.J.; Liu, F. Phosphorylation of Irs Proteins Yin-Yang Regulation of Insulin Signaling. Vitam. Horm. 2009, 80, 351–387. [Google Scholar] [CrossRef]
- Boura-Halfon, S.; Zick, Y. Phosphorylation of Irs Proteins, Insulin Action, and Insulin Resistance. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E581–E591. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriou, P.; Geronikaki, A.; Petrou, A. Ptp1b Inhibition, a Promising Approach for the Treatment of Diabetes Type Ii. Curr. Top. Med. Chem. 2019, 19, 246–263. [Google Scholar] [CrossRef] [PubMed]
- Abdelsalam, S.S.; Korashy, H.M.; Zeidan, A.; Agouni, A. The Role of Protein Tyrosine Phosphatase (Ptp)-1b in Cardiovascular Disease and Its Interplay with Insulin Resistance. Biomolecules 2019, 9, 286. [Google Scholar] [CrossRef]
- Ren, L.; Chen, X.; Luechapanichkul, R.; Selner, N.G.; Meyer, T.M.; Wavreille, A.-S.; Chan, R.; Iorio, C.; Zhou, X.; Neel, B.G.; et al. Substrate Specificity of Protein Tyrosine Phosphatases 1b, Rptpα, Shp-1, and Shp-2. Biochemistry 2011, 50, 2339–2356. [Google Scholar] [CrossRef]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol Improves Health and Survival of Mice on a High-Calorie Diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef]
- Zhang, Z.; Lei, B.; Chai, W.; Liu, R.; Li, T. Increased Expression of Insulin-Like Growth Factor-1 Receptor Predicts Poor Prognosis in Patients with Hepatocellular Carcinoma. Medicine 2019, 98, e17680. [Google Scholar] [CrossRef]
- Cai, W.; Ma, Y.; Song, L.; Cao, N.; Gao, J.; Zhou, S.; Tang, X. Igf-1r down Regulates the Sensitivity of Hepatocellular Carcinoma to Sorafenib through the Pi3k/Akt and RasRaf/Erk Signaling Pathways. BMC Cancer 2023, 23, 87. [Google Scholar] [CrossRef]
- Ngo, M.-H.T.; Jeng, H.-Y.; Kuo, Y.-C.; Diony Nanda, J.; Brahmadhi, A.; Ling, T.-Y.; Chang, T.-S.; Huang, Y.-H. The Role of Igf/Igf-1r Signaling in Hepatocellular Carcinomas: Stemness-Related Properties and Drug Resistance. Int. J. Mol. Sci. 2021, 22, 1931. [Google Scholar] [CrossRef]
- Paraiso, K.H.; Van Der Kooi, K.; Messina, J.L.; Smalley, K.S. Measurement of Constitutive Mapk and Pi3k/Akt Signaling Activity in Human Cancer Cell Lines. Methods Enzymol. 2010, 484, 549–567. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Han, G.; Li, X.; Li, B.; Wu, B.; Jin, H.; Wu, L.; Wang, W. Mapk-Rap1a Signaling Enriched in Hepatocellular Carcinoma Is Associated with Favorable Tumor-Infiltrating Immune Cells and Clinical Prognosis. Front. Oncol. 2021, 11, 649980. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C.; Buse, M.; Busuioc, C.; Drula, R.; Gulei, D.; Raduly, L.; Rusu, A.; Irimie, A.; Atanasov, A.G.; Slaby, O.; et al. A Comprehensive Review on Mapk: A Promising Therapeutic Target in Cancer. Cancers 2019, 11, 1618. [Google Scholar] [CrossRef]
- Van Horn, D.J.; Myers, M.G.; Backer, J.M. Direct Activation of the Phosphatidylinositol 3′-Kinase by the Insulin Receptor. J. Biol. Chem. 1994, 269, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Staubs, P.A.; Reichart, D.R.; Saltiel, A.R.; Milarski, K.L.; Maegawa, H.; Berhanu, P.; Olefsky, J.M.; Seely, B.L. Localization of the Insulin Receptor Binding Sites for the Sh2 Domain Proteins P85, Syp, and Gap. J. Biol. Chem. 1994, 269, 27186–27192. [Google Scholar] [CrossRef]
- Yamamoto-Honda, R.; Honda, Z.I.; Ueki, K.; Tobe, K.; Kaburagi, Y.; Takahashi, Y.; Tamemoto, H.; Suzuki, T.; Itoh, K.; Akanuma, Y.; et al. Mutant of Insulin Receptor Substrate-1 Incapable of Activating Phosphatidylinositol 3-Kinase Did Not Mediate Insulin-Stimulated Maturation of Xenopus Laevis Oocytes. J. Biol. Chem. 1996, 271, 28677–28681. [Google Scholar] [CrossRef] [PubMed]
- Tartare-Deckert, S.; Murdaca, J.; Sawka-Verhelle, D.; Holt, K.H.; Pessin, J.E.; Obberghen, E.V. Interaction of the Molecular Weight 85k Regulatory Subunit of the Phosphatidylinositol 3-Kinase with the Insulin Receptor and the Insulin-like Growth Factor-1 (Igf- I) Receptor: Comparative Study Using the Yeast Two-Hybrid System. Endocrinology 1996, 137, 1019–1024. [Google Scholar] [CrossRef]
- Yuan, T.L.; Cantley, L.C. Pi3k Pathway Alterations in Cancer: Variations on a Theme. Oncogene 2008, 27, 5497–5510. [Google Scholar] [CrossRef]
- Liu, R.; Chen, Y.; Liu, G.; Li, C.; Song, Y.; Cao, Z.; Li, W.; Hu, J.; Lu, C.; Liu, Y. Pi3k/Akt Pathway as a Key Link Modulates the Multidrug Resistance of Cancers. Cell Death Dis. 2020, 11, 797. [Google Scholar] [CrossRef]
- Tian, L.-Y.; Smit, D.J.; Jücker, M. The Role of Pi3k/Akt/Mtor Signaling in Hepatocellular Carcinoma Metabolism. Int. J. Mol. Sci. 2023, 24, 2652. [Google Scholar] [CrossRef] [PubMed]
- Sun, E.J.; Wankell, M.; Palamuthusingam, P.; McFarlane, C.; Hebbard, L. Targeting the Pi3k/Akt/Mtor Pathway in Hepatocellular Carcinoma. Biomedicines 2021, 9, 1639. [Google Scholar] [CrossRef]
- Chetram, M.A.; Hinton, C.V. Pten Regulation of Erk1/2 Signaling in Cancer. J. Recept. Signal Transduct. 2012, 32, 190–195. [Google Scholar] [CrossRef]
- Kuo, Y.-C.; Huang, K.-Y.; Yang, C.-H.; Yang, Y.-S.; Lee, W.-Y.; Chiang, C.-W. Regulation of Phosphorylation of Thr-308 of Akt, Cell Proliferation, and Survival by the B55α Regulatory Subunit Targeting of the Protein Phosphatase 2a Holoenzyme to Akt. J. Biol. Chem. 2008, 283, 1882–1892. [Google Scholar] [CrossRef]
- Resjö, S.; Göransson, O.; Härndahl, L.; Zolnierowicz, S.; Manganiello, V.; Degerman, E. Protein Phosphatase 2a Is the Main Phosphatase Involved in the Regulation of Protein Kinase B in Rat Adipocytes. Cell. Signal. 2002, 14, 231–238. [Google Scholar] [CrossRef]
- Schweiger, S.; Matthes, F.; Posey, K.; Kickstein, E.; Weber, S.; Hettich, M.M.; Pfurtscheller, S.; Ehninger, D.; Schneider, R.; Krauß, S. Resveratrol Induces Dephosphorylation of Tau by Interfering with the Mid1-Pp2a Complex. Sci. Rep. 2017, 7, 13753. [Google Scholar] [CrossRef] [PubMed]
- Jian, B.; Yang, S.; Chaudry, I.H.; Raju, R. Resveratrol Restores Sirtuin 1 (Sirt1) Activity and Pyruvate Dehydrogenase Kinase 1 (Pdk1) Expression after Hemorrhagic Injury in a Rat Model. Mol. Med. 2014, 20, 10–16. [Google Scholar] [CrossRef]
- Alayev, A.; Doubleday, P.F.; Berger, S.M.; Ballif, B.A.; Holz, M.K. Phosphoproteomics Reveals Resveratrol-Dependent Inhibition of Akt/Mtorc1/S6k1 Signaling. J. Proteome Res. 2014, 13, 5734–5742. [Google Scholar] [CrossRef]
- Miao, R.; Fang, X.; Wei, J.; Wu, H.; Wang, X.; Tian, J. Akt: A Potential Drug Target for Metabolic Syndrome. Front. Physiol. 2022, 13, 822333. [Google Scholar] [CrossRef]
- Rayasam, G.V.; Tulasi, V.K.; Sodhi, R.; Davis, J.A.; Ray, A. Glycogen Synthase Kinase 3: More Than a Namesake. Br. J. Pharmacol. 2009, 156, 885–898. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Di, L.J. Glycogen Synthesis and Beyond, a Comprehensive Review of Gsk3 as a Key Regulator of Metabolic Pathways and a Therapeutic Target for Treating Metabolic Diseases. Med. Res. Rev. 2022, 42, 946–982. [Google Scholar] [CrossRef]
- Papadopoli, D.; Pollak, M.; Topisirovic, I. The Role of Gsk3 in Metabolic Pathway Perturbations in Cancer. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 119059. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, W.; Fang, H.; Yang, Y.; Li, X.; He, J.; Jiang, X.; Wang, W.; Liu, S.; Hu, J.; et al. Gsk-3β Inhibition Attenuates Clp-Induced Liver Injury by Reducing Inflammation and Hepatic Cell Apoptosis. Mediat. Inflamm. 2014, 2014, 629507. [Google Scholar] [CrossRef]
- Irimia, J.M.; Meyer, C.M.; Segvich, D.M.; Surendran, S.; DePaoli-Roach, A.A.; Morral, N.; Roach, P.J. Lack of Liver Glycogen Causes Hepatic Insulin Resistance and Steatosis in Mice. J. Biol. Chem. 2017, 292, 10455–10464. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Macaulay, K.; Woodgett, J.R. Tissue-Specific Analysis of Glycogen Synthase Kinase-3α (Gsk-3α) in Glucose Metabolism: Effect of Strain Variation. PLoS ONE 2011, 6, e15845. [Google Scholar] [CrossRef] [PubMed]
- Ciaraldi, T.P.; Nikoulina, S.E.; Bandukwala, R.A.; Carter, L.; Henry, R.R. Role of Glycogen Synthase Kinase-3α in Insulin Action in Cultured Human Skeletal Muscle Cells. Endocrinology 2007, 148, 4393–4399. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cortés-Vieyra, R.; Silva-García, O.; Gómez-García, A.; Gutiérrez-Castellanos, S.; Álvarez-Aguilar, C.; Baizabal-Aguirre, V.M. Glycogen Synthase Kinase 3β Modulates the Inflammatory Response Activated by Bacteria, Viruses, and Parasites. Front. Immunol. 2021, 12, 675751. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Shi, C.; Zhang, Q.; Wang, Y.; Guo, J.; Gong, Z. Inhibition of Gsk3β Activity Alleviates Acute Liver Failure Via Suppressing Multiple Programmed Cell Death. J. Inflamm. 2023, 20, 24. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Zhang, L.; Zhang, X.; Shi, H.; Wen, T.; Bai, L.; Zheng, S.; Chen, Y.; Chen, D.; Li, L.; et al. Inhibition of Glycogen Synthase Kinase 3β Promotes Autophagy to Protect Mice from Acute Liver Failure Mediated by Peroxisome Proliferator-Activated Receptor α. Cell Death Dis. 2016, 7, e2151. [Google Scholar] [CrossRef]
- Ludvik, B.; Nolan, J.J.; Roberts, A.; Baloga, J.; Joyce, M.; Bell, J.M.; Olefsky, J.M. A Noninvasive Method to Measure Splanchnic Glucose Uptake after Oral Glucose Administration. J. Clin. Investig. 1995, 95, 2232–2238. [Google Scholar] [CrossRef][Green Version]
- Goldstein, B.J.; Bittner-Kowalczyk, A.; White, M.F.; Harbeck, M. Tyrosine Dephosphorylation and Deactivation of Insulin Receptor Substrate-1 by Protein-Tyrosine Phosphatase 1b. J. Biol. Chem. 2000, 275, 4283–4289. [Google Scholar] [CrossRef]
- Steinberg, S.F. Mechanisms for Redox-Regulation of Protein Kinase C. Front. Pharmacol. 2015, 6, 128. [Google Scholar] [CrossRef]
- Steinberg, S.F. Structural Basis of Protein Kinase C Isoform Function. Physiol. Rev. 2008, 88, 1341–1378. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Hirai, S.-I.; Osada, S.-I.; Suzuki, A.; Mizuno, K.; Ohno, S. Protein Kinase C δ Activates the Mek-Erk Pathway in a Manner Independent of Ras and Dependent on Raf. J. Biol. Chem. 1996, 271, 23512–23519. [Google Scholar] [CrossRef]
- Letiges, M.; Plomann, M.; Standaert, M.L.; Bandyopadhyay, G.; Sajan, M.P.; Kanoh, Y.; Farese, R.V. Knockout of Pkcα Enhances Insulin Signaling through Pi3k. Mol. Endocrinol. 2002, 16, 847–858. [Google Scholar] [CrossRef]
- Maeno, Y.; Li, Q.; Park, K.; Rask-Madsen, C.; Gao, B.; Matsumoto, M.; Liu, Y.; Wu, I.H.; White, M.F.; Feener, E.P.; et al. Inhibition of Insulin Signaling in Endothelial Cells by Protein Kinase C-Induced Phosphorylation of P85 Subunit of Phosphatidylinositol 3-Kinase (Pi3k). J. Biol. Chem. 2012, 287, 4518–4530. [Google Scholar] [CrossRef]
- Turban, S.; Hajduch, E. Protein Kinase C Isoforms: Mediators of Reactive Lipid Metabolites in the Development of Insulin Resistance. FEBS Lett. 2011, 585, 269–274. [Google Scholar] [CrossRef]
- Considine, R.V.; Nyce, M.R.; Allen, L.E.; Morales, L.M.; Triester, S.; Serrano, J.; Colberg, J.; Lanza-Jacoby, S.; Caro, J.F. Protein Kinase C Is Increased in the Liver of Humans and Rats with Non-Insulin-Dependent Diabetes Mellitus: An Alteration Not Due to Hyperglycemia. J. Clin. Investig. 1995, 95, 2938–2944. [Google Scholar] [CrossRef]
- Wen-Sheng, W.; Jun-Ming, H. Activation of Protein Kinase C Alpha Is Required for Tpa-Triggered Erk (Mapk) Signaling and Growth Inhibition of Human Hepatoma Cell Hepg2. J. Biomed. Sci. 2005, 12, 289–296. [Google Scholar] [CrossRef]
- Wu, W. Protein Kinase C α Trigger Ras and Raf-Independent Mek/Erk Activation for Tpa-Induced Growth Inhibition of Human Hepatoma Cell Hepg2. Cancer Lett. 2006, 239, 27–35. [Google Scholar] [CrossRef]
- Rucci, N.; Digiacinto, C.; Orrù, L.; Millimaggi, D.; Baron, R.; Teti, A. A Novel Protein Kinase C α-Dependent Signal to Erk1/2 Activated by αvβ3 Integrin in Osteoclasts and in Chinese Hamster Ovary (Cho) Cells. J. Cell Sci. 2005, 118, 3263–3275. [Google Scholar] [CrossRef]
- Cheng, J.-J.; Wung, B.-S.; Chao, Y.-J.; Wang, D.L. Sequential Activation of Protein Kinase C (Pkc)-α and Pkc-ε Contributes to Sustained Raf/Erk1/2 Activation in Endothelial Cells under Mechanical Strain. J. Biol. Chem. 2001, 276, 31368–31375. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Liu, Y.; Zhou, H.; Dai, Z.; Zhang, J.; Sun, R.; Chen, J.; Sun, Q.; Lu, W.; Kang, X.; et al. Involvement of Protein Kinase C β–Extracellular Signal-Regulating Kinase1/2P38 Mitogen-Activated Protein Kinase–Heat Shock Protein 27 Activation in Hepatocellular Carcinoma Cell Motility and Invasion. Cancer Sci. 2008, 99, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Trollér, U.; Zeidman, R.; Svensson, K.; Larsson, C. A Pkcβ Isoform Mediates Phorbol Ester-Induced Activation of Erk1/2 and Expression of Neuronal Differentiation Genes in Neuroblastoma Cells. FEBS Lett. 2001, 508, 126–130. [Google Scholar] [CrossRef]
- Berti, L.; Mosthaf, L.; Kroder, G.; Kellerer, M.; Tippmer, S.; Mushack, J.; Seffer, E.; Seedorf, K.; Haring, H. Glucose-Induced Translocation of Protein Kinase C Isoforms in Rat-1 Fibroblasts Is Paralleled by Inhibition of the Insulin Receptor Tyrosine Kinase. J. Biol. Chem. 1994, 269, 3381–3386. [Google Scholar] [CrossRef]
- Kellerer, M.; Mushack, J.; Seffer, E.; Mischak, H.; Ullrich, A.; Haring, H.U. Protein Kinase C Isoforms Alpha, Delta and Theta Require Insulin Receptor Substrate-1 to Inhibit the Tyrosine Kinase Activity of the Insulin Receptor in Human Kidney Embryonic Cells (Hek 293 Cells). Diabetologia 1998, 41, 833–838. [Google Scholar] [CrossRef]
- Cipok, M.; Aga-Mizrachi, S.; Bak, A.; Feurstein, T.; Steinhart, R.; Brodie, C.; Sampson, S.R. Protein Kinase Cα Regulates Insulin Receptor Signaling in Skeletal Muscle. Biochem. Biophys. Res. Commun. 2006, 345, 817–824. [Google Scholar] [CrossRef]
- Bakke, J.; Haj, F.G. Protein-Tyrosine Phosphatase 1b Substrates and Metabolic Regulation. Semin. Cell Dev. Biol. 2015, 37, 58–65. [Google Scholar] [CrossRef]
- Haj, F.G.; Zabolotny, J.M.; Kim, Y.-B.; Kahn, B.B.; Neel, B.G. Liver-Specific Protein-Tyrosine Phosphatase 1b (Ptp1b) Re-Expression Alters Glucose Homeostasis of Ptp1b-/-Mice. J. Biol. Chem. 2005, 280, 15038–15046. [Google Scholar] [CrossRef]
- Zinker, B.A.; Rondinone, C.M.; Trevillyan, J.M.; Gum, R.J.; Clampit, J.E.; Waring, J.F.; Xie, N.; Wilcox, D.; Jacobson, P.; Frost, L.; et al. Ptp1b Antisense Oligonucleotide Lowers Ptp1b Protein, Normalizes Blood Glucose, and Improves Insulin Sensitivity in Diabetic Mice. Proc. Natl. Acad. Sci. USA 2002, 99, 11357–11362. [Google Scholar] [CrossRef] [PubMed]
- González-Rodríguez, Á.; Gutierrez, J.A.M.; Sanz-González, S.; Ros, M.; Burks, D.J.; Valverde, Á.M. Inhibition of Ptp1b Restores Irs1-Mediated Hepatic Insulin Signaling in Irs2-Deficient Mice. Diabetes 2010, 59, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Dadke, S.; Kusari, J.; Chernoff, J. Down-Regulation of Insulin Signaling by Protein-Tyrosine Phosphatase 1b Is Mediated by an N-Terminal Binding Region. J. Biol. Chem. 2000, 275, 23642–23647. [Google Scholar] [CrossRef]
- Liu, R.; Mathieu, C.; Berthelet, J.; Zhang, W.; Dupret, J.-M.; Rodrigues Lima, F. Human Protein Tyrosine Phosphatase 1b (Ptp1b): From Structure to Clinical Inhibitor Perspectives. Int. J. Mol. Sci. 2022, 23, 7027. [Google Scholar] [CrossRef]
- Bheri, M.; Pandey, G.K. Protein Phosphatases Meet Reactive Oxygen Species in Plant Signaling Networks. Environ. Exp. Bot. 2019, 161, 26–40. [Google Scholar] [CrossRef]
- Corcoran, A.; Cotter, T.G. Redox Regulation of Protein Kinases. FEBS J. 2013, 280, 1944–1965. [Google Scholar] [CrossRef]
- Lou, Y.W.; Chen, Y.Y.; Hsu, S.F.; Chen, R.K.; Lee, C.L.; Khoo, K.H.; Tonks, N.K.; Meng, T.C. Redox Regulation of the Protein Tyrosine Phosphatase Ptp1b in Cancer Cells. FEBS J. 2008, 275, 69–88. [Google Scholar] [CrossRef]
- Giorgi, C.; Agnoletto, C.; Baldini, C.; Bononi, A.; Bonora, M.; Marchi, S.; Missiroli, S.; Patergnani, S.; Poletti, F.; Rimessi, A.; et al. Redox Control of Protein Kinase C: Cell- and Disease-Specific Aspects. Antioxid. Redox Signal. 2010, 13, 1051–1085. [Google Scholar] [CrossRef]
- Truong, T.H.; Carroll, K.S. Redox Regulation of Protein Kinases. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 332–356. [Google Scholar] [CrossRef]
- Finkel, T. Signal Transduction by Reactive Oxygen Species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.; Andersen, J.N.; Salmeen, A.; Barford, D.; Tonks, N.K. Conformation-Sensing Antibodies Stabilize the Oxidized Form of Ptp1b and Inhibit Its Phosphatase Activity. Cell 2011, 147, 185–198. [Google Scholar] [CrossRef]
- Krishnan, N.; Bonham, C.A.; Rus, I.A.; Shrestha, O.K.; Gauss, C.M.; Haque, A.; Tocilj, A.; Joshua-Tor, L.; Tonks, N.K. Harnessing Insulin- and Leptin-Induced Oxidation of Ptp1b for Therapeutic Development. Nat. Commun. 2018, 9, 283. [Google Scholar] [CrossRef] [PubMed]
- Londhe, A.D.; Bergeron, A.; Curley, S.M.; Zhang, F.; Rivera, K.D.; Kannan, A.; Coulis, G.; Rizvi, S.H.M.; Kim, S.J.; Pappin, D.J.; et al. Regulation of Ptp1b Activation through Disruption of Redox-Complex Formation. Nat. Chem. Biol. 2020, 16, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Senga, T.; Miyazaki, K.; Machida, K.; Iwata, H.; Matsuda, S.; Nakashima, I.; Hamaguchi, M. Clustered Cysteine Residues in the Kinase Domain of V-Src: Critical Role for Protein Stability, Cell Transformation and Sensitivity to Herbimycin A. Oncogene 2000, 19, 273–279. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Giannoni, E.; Buricchi, F.; Raugei, G.; Ramponi, G.; Chiarugi, P. Intracellular Reactive Oxygen Species Activate Src Tyrosine Kinase during Cell Adhesion and Anchorage-Dependent Cell Growth. Mol. Cell. Biol. 2005, 25, 6391–6403. [Google Scholar] [CrossRef] [PubMed]
- Giannoni, E.; Taddei, M.L.; Chiarugi, P. Src Redox Regulation: Again in the Front Line. Free Radic. Biol. Med. 2010, 49, 516–527. [Google Scholar] [CrossRef]
- Wani, R.; Bharathi, N.S.; Field, J.; Tsang, A.W.; Furdui, C.M. Oxidation of Akt2 Kinase Promotes Cell Migration and Regulates G1-S Transition in the Cell Cycle. Cell Cycle 2011, 10, 3263–3268. [Google Scholar] [CrossRef]
- Wani, R.; Qian, J.; Yin, L.; Bechtold, E.; King, S.B.; Poole, L.B.; Paek, E.; Tsang, A.W.; Furdui, C.M. Isoform-Specific Regulation of Akt by Pdgf-Induced Reactive Oxygen Species. Proc. Natl. Acad. Sci. USA 2011, 108, 10550–10555. [Google Scholar] [CrossRef] [PubMed]
- Murata, H.; Ihara, Y.; Nakamura, H.; Yodoi, J.; Sumikawa, K.; Kondo, T. Glutaredoxin Exerts an Antiapoptotic Effect by Regulating the Redox State of Akt. J. Biol. Chem. 2003, 278, 50226–50233. [Google Scholar] [CrossRef]
- Galli, S.; Antico Arciuch, V.G.; Poderoso, C.; Converso, D.P.; Zhou, Q.; De Kier Joffé, E.B.; Cadenas, E.; Boczkowski, J.; Carreras, M.C.; Poderoso, J.J. Tumor Cell Phenotype Is Sustained by Selective Mapk Oxidation in Mitochondria. PLoS ONE 2008, 3, e2379. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, C.E.; Truong, T.H.; Garcia, F.J.; Homann, A.; Gupta, V.; Leonard, S.E.; Carroll, K.S. Peroxide-Dependent Sulfenylation of the Egfr Catalytic Site Enhances Kinase Activity. Nat. Chem. Biol. 2012, 8, 57–64. [Google Scholar] [CrossRef]
- Truong, T.H.; Carroll, K.S. Redox Regulation of Epidermal Growth Factor Receptor Signaling through Cysteine Oxidation. Biochemistry 2012, 51, 9954–9965. [Google Scholar] [CrossRef]
- Cosentino-Gomes, D.; Rocco-Machado, N.; Meyer-Fernandes, J.R. Cell Signaling through Protein Kinase C Oxidation and Activation. Int. J. Mol. Sci. 2012, 13, 10697–10721. [Google Scholar] [CrossRef]
- Martelli, A.M.; Evangelisti, C.; Nyakern, M.; Manzoli, F.A. Nuclear Protein Kinase C. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2006, 1761, 542–551. [Google Scholar] [CrossRef]
- Hsu, A.H.; Lum, M.A.; Shim, K.-S.; Frederick, P.J.; Morrison, C.D.; Chen, B.; Lele, S.M.; Sheinin, Y.M.; Daikoku, T.; Dey, S.K.; et al. Crosstalk between Pkcα and Pi3k/Akt Signaling Is Tumor Suppressive in the Endometrium. Cell Rep. 2018, 24, 655–669. [Google Scholar] [CrossRef]
- Nadel, G.; Yao, Z.; Hacohen-Lev-Ran, A.; Wainstein, E.; Maik-Rachline, G.; Ziv, T.; Naor, Z.; Admon, A.; Seger, R. Phosphorylation of Pp2ac by Pkc Is a Key Regulatory Step in the Pp2a-Switch-Dependent Akt Dephosphorylation That Leads to Apoptosis. Cell Commun. Signal. 2024, 22, 154. [Google Scholar] [CrossRef] [PubMed]
- Hevener, A.L.; Ribas, V.; Moore, T.M.; Zhou, Z. Erα in the Control of Mitochondrial Function and Metabolic Health. Trends Mol. Med. 2021, 27, 31–46. [Google Scholar] [CrossRef]
- González, C.; Alonso, A.; Fernández, R.; Patterson, A.M. Regulation of Insulin Receptor Substrate-1 in the Liver, Skeletal Muscle and Adipose Tissue of Rats Throughout Pregnancy. Gynecol. Endocrinol. 2003, 17, 187–197. [Google Scholar] [CrossRef]
- Tran, H.T.; Liong, S.; Lim, R.; Barker, G.; Lappas, M. Resveratrol Ameliorates the Chemical and Microbial Induction of Inflammation and Insulin Resistance in Human Placenta, Adipose Tissue and Skeletal Muscle. PLoS ONE 2017, 12, e0173373. [Google Scholar] [CrossRef]
- Bo, T.; Gao, L.; Yao, Z.; Shao, S.; Wang, X.; Proud, C.G.; Zhao, J. Hepatic Selective Insulin Resistance at the Intersection of Insulin Signaling and Metabolic Dysfunction-Associated Steatotic Liver Disease. Cell Metab. 2024, 36, 947–968. [Google Scholar] [CrossRef] [PubMed]
- Michael, M.D.; Kulkarni, R.N.; Postic, C.; Previs, S.F.; Shulman, G.I.; Magnuson, M.A.; Kahn, C.R. Loss of Insulin Signaling in Hepatocytes Leads to Severe Insulin Resistance and Progressive Hepatic Dysfunction. Mol. Cell 2000, 6, 87–97. [Google Scholar] [CrossRef]
- Brown, M.S.; Goldstein, J.L. Selective Versus Total Insulin Resistance: A Pathogenic Paradox. Cell Metab. 2008, 7, 95–96. [Google Scholar] [CrossRef]
- Li, S.; Brown, M.S.; Goldstein, J.L. Bifurcation of Insulin Signaling Pathway in Rat Liver: Mtorc1 Required for Stimulation of Lipogenesis, but Not Inhibition of Gluconeogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 3441–3446. [Google Scholar] [CrossRef]
- Titchenell, P.M.; Quinn, W.J.; Lu, M.; Chu, Q.; Lu, W.; Li, C.; Chen, H.; Monks, B.R.; Chen, J.; Rabinowitz, J.D.; et al. Direct Hepatocyte Insulin Signaling Is Required for Lipogenesis but Is Dispensable for the Suppression of Glucose Production. Cell Metab. 2016, 23, 1154–1166. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tian, Z.; Shi, X.; Long, A.; Wang, Y.; Yang, Y.; Wang, Y.; Zhang, J.; Wang, Y. Adipose Tissue-Derived Prxl2a Suppresses Hepatic Lipogenesis in a Study with Male Mice. Nat. Commun. 2025, 16, 6567. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Yang, Y.; Zhao, S.; Bai, Y.; Yao, W.; Gao, X.; Yin, J. Crosstalk in Extrahepatic and Hepatic System in Nafld/Nash. Liver Int. 2024, 44, 1856–1871. [Google Scholar] [CrossRef]
- Song, Z.; Xiaoli, A.M.; Yang, F. Regulation and Metabolic Significance of De Novo Lipogenesis in Adipose Tissues. Nutrients 2018, 10, 1383. [Google Scholar] [CrossRef]
- Pandit, H.; Li, Y.; Li, X.; Zhang, W.; Li, S.; Martin, R.C.G. Enrichment of Cancer Stem Cells Via β-Catenin Contributing to the Tumorigenesis of Hepatocellular Carcinoma. BMC Cancer 2018, 18, 783. [Google Scholar] [CrossRef]
- Landa-Galvan, H.V.; Rios-Castro, E.; Romero-Garcia, T.; Rueda, A.; Olivares-Reyes, J.A. Metabolic Syndrome Diminishes Insulin-Induced Akt Activation and Causes a Redistribution of Akt-Interacting Proteins in Cardiomyocytes. PLoS ONE 2020, 15, e0228115. [Google Scholar] [CrossRef]
- Horinouchi, T.; Hoshi, A.; Harada, T.; Higa, T.; Karki, S.; Terada, K.; Higashi, T.; Mai, Y.; Nepal, P.; Mazaki, Y.; et al. Endothelin-1 Suppresses Insulin-Stimulated Akt Phosphorylation and Glucose Uptake Via Gpcr Kinase 2 in Skeletal Muscle Cells. Br. J. Pharmacol. 2016, 173, 1018–1032. [Google Scholar] [CrossRef]
- Sun, Z.J.; Pan, C.E.; Liu, H.S.; Wang, G.J. Anti-Hepatoma Activity of Resveratrol in Vitro. World J. Gastroenterol. 2002, 8, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak-Drozd, K.; Niziński, P.; Kasprzak, P.; Kondracka, A.; Oniszczuk, T.; Rusinek, A.; Oniszczuk, A. Does Resveratrol Improve Metabolic Dysfunction-Associated Steatotic Liver Disease (Masld)? Int. J. Mol. Sci. 2024, 25, 3746. [Google Scholar] [CrossRef] [PubMed]
- Tomé-Carneiro, J.; Larrosa, M.; González-Sarrías, A.; Tomás-Barberán, F.; García-Conesa, M.; Espín, J. Resveratrol and Clinical Trials: The Crossroad from in Vitro Studies to Human Evidence. Curr. Pharm. Des. 2013, 19, 6064–6093. [Google Scholar] [CrossRef]
- Robinson, K.; Mock, C.; Liang, D. Pre-Formulation Studies of Resveratrol. Drug Dev. Ind. Pharm. 2015, 41, 1464–1469. [Google Scholar] [CrossRef] [PubMed]
- Szkudelska, K.; Szkudelski, T. Resveratrol, Obesity and Diabetes. Eur. J. Pharmacol. 2010, 635, 1–8. [Google Scholar] [CrossRef]
- Parra-Mercado, G.K.; Fuentes-Gonzalez, A.M.; Hernandez-Aranda, J.; Diaz-Coranguez, M.; Dautzenberg, F.M.; Catt, K.J.; Hauger, R.L.; Olivares-Reyes, J.A. Crf1 Receptor Signaling Via the Erk1/2-Map and Akt Kinase Cascades: Roles of Src, Egf Receptor, and Pi3-Kinase Mechanisms. Front. Endocrinol. 2019, 10, 869. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-González, K.D.; Vinchira-Lamprea, M.A.; Hernandez-Aranda, J.; Olivares-Reyes, J.A. Resveratrol Impairs Insulin Signaling in Hepatic Cells via Activation of PKC and PTP1B Pathways. Int. J. Mol. Sci. 2025, 26, 7434. https://doi.org/10.3390/ijms26157434
Hernández-González KD, Vinchira-Lamprea MA, Hernandez-Aranda J, Olivares-Reyes JA. Resveratrol Impairs Insulin Signaling in Hepatic Cells via Activation of PKC and PTP1B Pathways. International Journal of Molecular Sciences. 2025; 26(15):7434. https://doi.org/10.3390/ijms26157434
Chicago/Turabian StyleHernández-González, Karla D., Monica A. Vinchira-Lamprea, Judith Hernandez-Aranda, and J. Alberto Olivares-Reyes. 2025. "Resveratrol Impairs Insulin Signaling in Hepatic Cells via Activation of PKC and PTP1B Pathways" International Journal of Molecular Sciences 26, no. 15: 7434. https://doi.org/10.3390/ijms26157434
APA StyleHernández-González, K. D., Vinchira-Lamprea, M. A., Hernandez-Aranda, J., & Olivares-Reyes, J. A. (2025). Resveratrol Impairs Insulin Signaling in Hepatic Cells via Activation of PKC and PTP1B Pathways. International Journal of Molecular Sciences, 26(15), 7434. https://doi.org/10.3390/ijms26157434







