Development and Evaluation of Graphene Oxide-Enhanced Chitosan Sponges as a Potential Antimicrobial Wound Dressing for Infected Wound Management
Abstract
1. Introduction
2. Results
2.1. Studies of Physico-Mechanical Parameters of Sponges GO-Enhanced Confirm Its Proper Properties to Be Applied as a Dressing
2.1.1. Determination of Mechanical Parameters of Dressing GO-Enhanced Sponges
2.1.2. Studies of the Absorption and Sorption Properties of Biocomposite Dressing Materials in the Form of a Sponge
2.1.3. Assessment of the Internal Structure of Dressing Materials in the Form of a Sponge
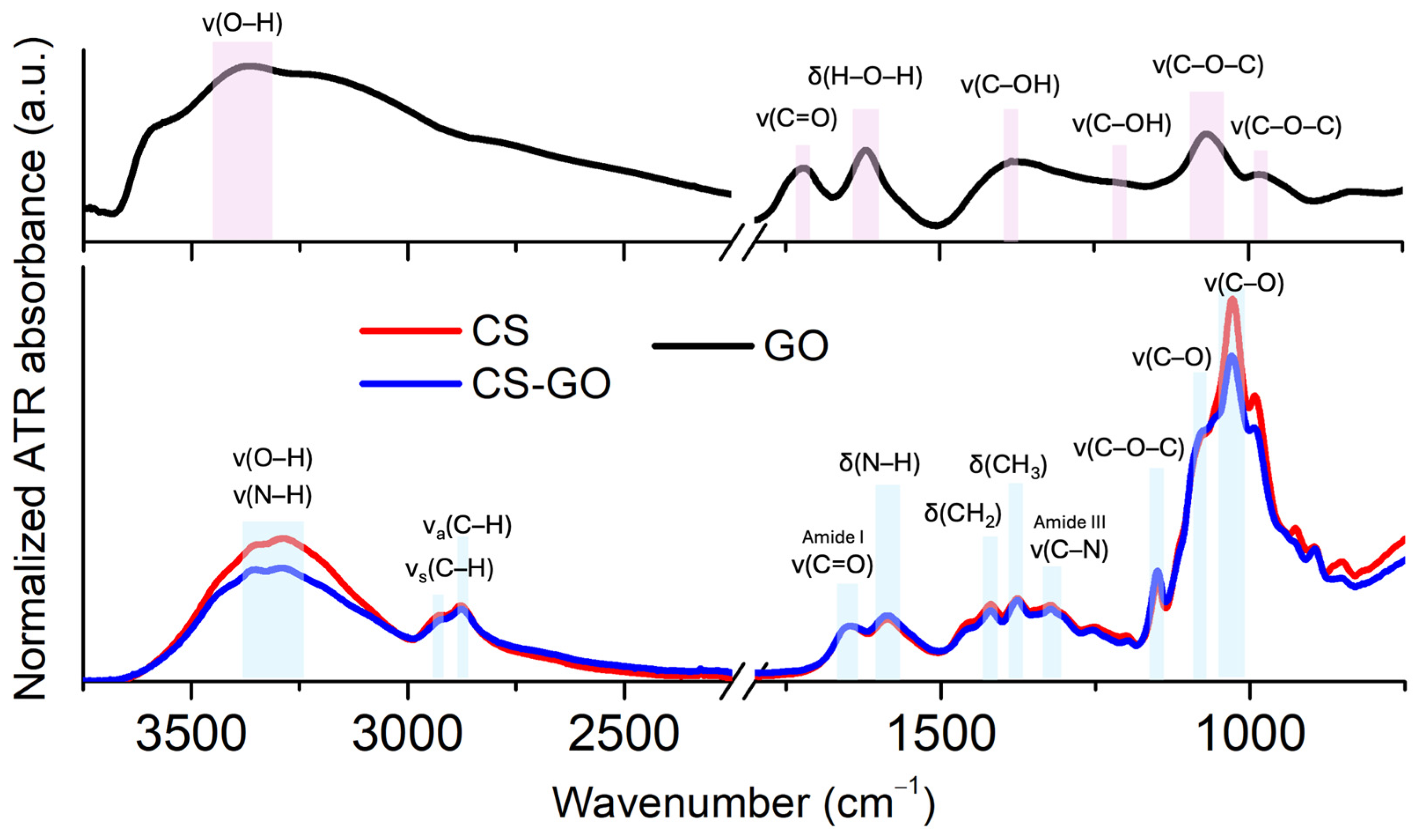
2.1.4. FTIR Spectroscopy Studies of Potential Molecular Interaction Between CS and GO
2.1.5. Raman Spectroscopy in the Characterization of GO in Composite Material
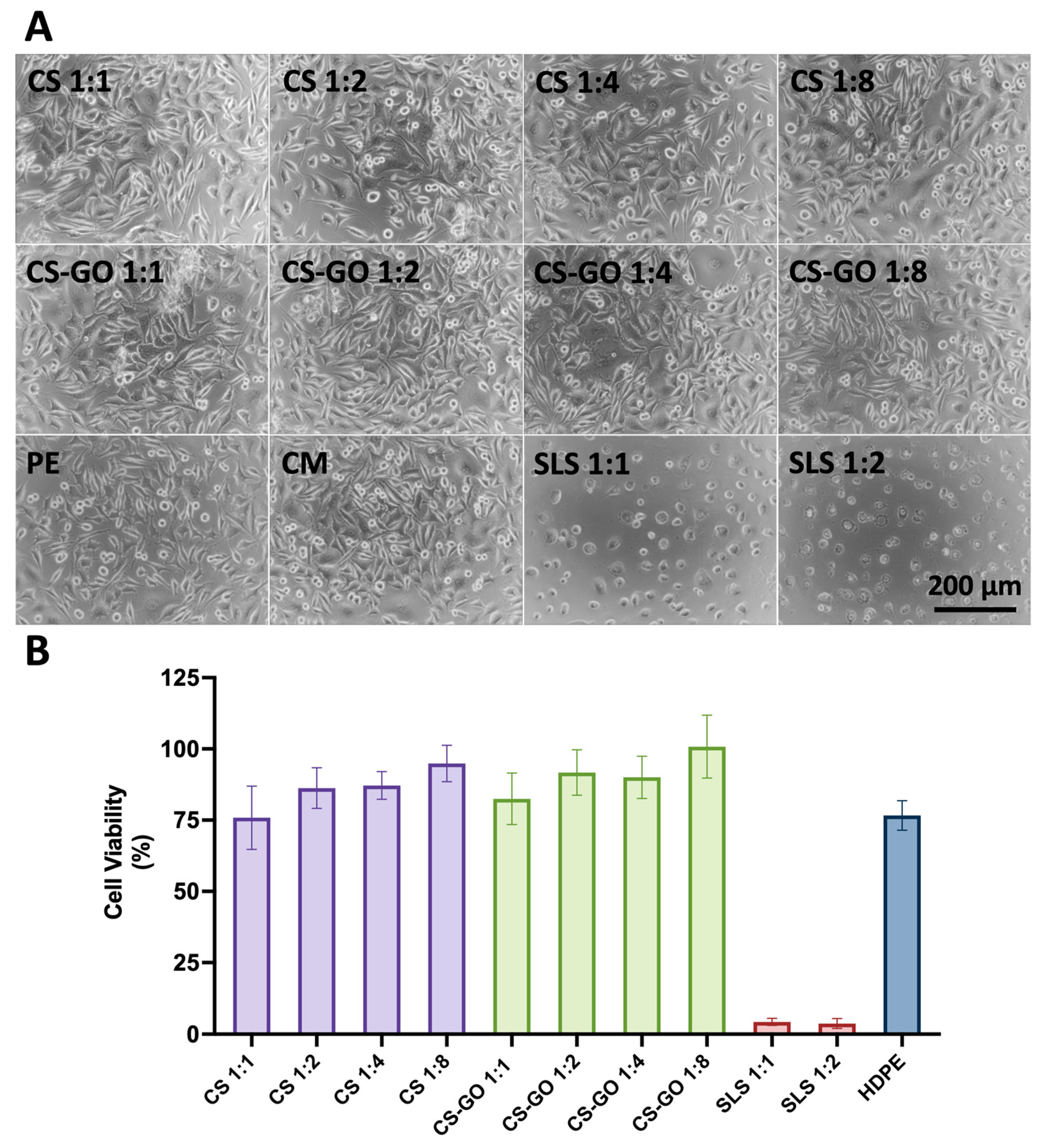
2.2. Cell Morphology and Viability Studies Proved Cytocompatibility Properties of Dressings
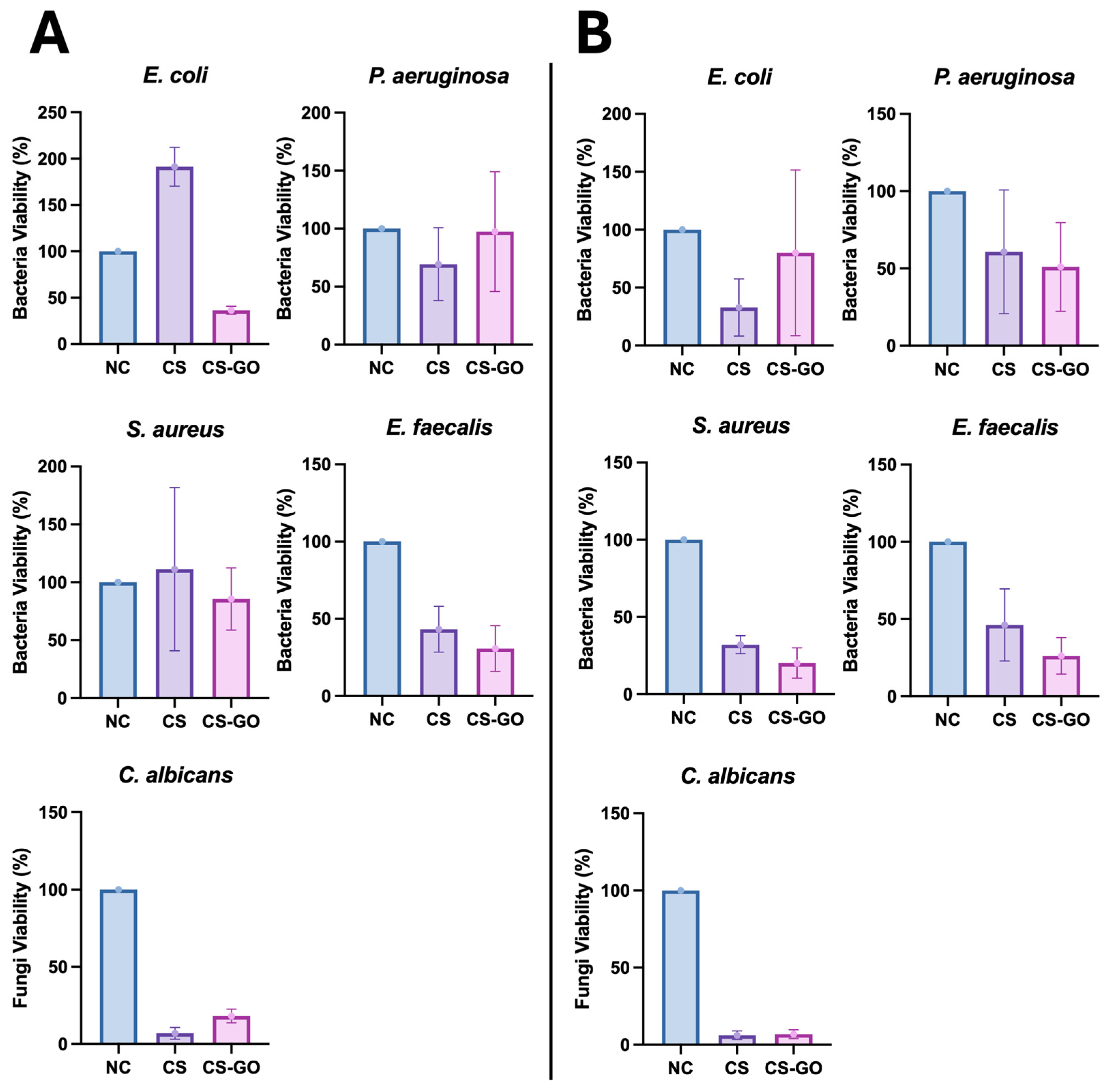
2.3. The Proposed GO-Enhanced CS Dressing Exhibits Potential Anti-Bacterial and Anti-Fungal Activity
3. Discussion
4. Materials and Methods
4.1. GO-Enhanced CS Sponges Preparation
4.1.1. CS
4.1.2. GO
4.1.3. Production of Composite Materials in the Form of a Sponge with the Participation of GO
4.2. Assessment of Physicochemical Parameters of Dressing Sponges Made of MKCh with and Without GO
4.2.1. Determination of Mechanical Parameters of Dressing Sponges
4.2.2. Studies of the Absorption and Sorption Properties of Dressing Materials in the Form of a Sponge
4.2.3. Evaluation of the Internal Structure of Dressing Materials in the Form of a Sponge by Means of SEM
4.2.4. Evaluation of the Composite Employing ATR-FTIR
4.2.5. Evaluation of the Composite Employing Raman Spectroscopy
4.3. Biocompatibility Studies of Biocomposite Dressing Materials in the Form of a Sponge
4.3.1. Cell Line
4.3.2. Indirect Cell Contact Study
4.3.3. Morphology Assessment of the Cells
4.3.4. Assessment of Cell Survival Utilizing Viability and Proliferation Assays
4.4. Antimicrobial Properties Evaluation of Biocomposite Dressing Materials
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CS | Chitosan |
| FBS | Fetal bovine serum |
| FTIR | Fourier transform infrared spectroscopy. |
| GO | Graphene oxide |
| HDPE | High-density polyethylene |
| MKCh | Microcrystalline chitosan |
| Mv | Molecular weight |
| SD | Degree of deacetylation |
| SEM | Scanning electron microscopy |
| SLS | Sodium lauryl sulfate |
| WRV | Secondary swelling index |
| Ws | Sorption index |
References
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef]
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment Strategies for Infected Wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef]
- Ye, S.; Jiang, L.; Wu, J.; Su, C.; Huang, C.; Liu, X.; Shao, W. Flexible Amoxicillin-Grafted Bacterial Cellulose Sponges for Wound Dressing: In Vitro and in Vivo Evaluation. ACS Appl. Mater. Interfaces 2018, 10, 5862–5870. [Google Scholar] [CrossRef]
- Sikder, S.; Toha, M.; Anik, A.H.; Sultan, M.B.; Alam, M.; Parvin, F.; Tareq, S.M. A Comprehensive Review on the Fate and Impact of Antibiotic Residues in the Environment and Public Health: A Special Focus on the Developing Countries. Water Environ. Res. 2024, 96, e10987. [Google Scholar] [CrossRef]
- Kumar, P.; Huo, P.; Zhang, R.; Liu, B. Antibacterial Properties of Graphene-Based Nanomaterials. Nanomaterials 2019, 9, 737. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Hu, X.; He, W.; Zhan, R.; Liu, M.; Zhou, D.; Huang, Y.; Hu, X.; Wang, Z.; Fei, G.; et al. Polydimethylsiloxane Incorporated with Reduced Graphene Oxide (rGO) Sheets for Wound Dressing Application: Preparation and Characterization. Colloids Surf. B Biointerfaces 2018, 166, 61–71. [Google Scholar] [CrossRef]
- Linklater, D.P.; Baulin, V.A.; Juodkazis, S.; Ivanova, E.P. Mechano-Bactericidal Mechanism of Graphene Nanomaterials. Interface Focus 2018, 8, 20170060. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, I.; Bhattacharya, P.; Talukdar, M.; Neogi, S.; Pal, S.K.; Chakraborty, S. Bactericidal Effect of Graphene Oxide and Reduced Graphene Oxide: Influence of Shape of Bacteria. Colloid Interface Sci. Commun. 2019, 28, 60–68. [Google Scholar] [CrossRef]
- Yang, S.; Lei, P.; Shan, Y.; Zhang, D. Preparation and Characterization of Antibacterial Electrospun Chitosan/Poly(Vinyl Alcohol)/Graphene Oxide Composite Nanofibrous Membrane. Appl. Surf. Sci. 2018, 435, 832–840. [Google Scholar] [CrossRef]
- Tu, Q.; Zhang, Q.; Wang, Y.; Jiao, Y.; Xiao, J.; Peng, T.; Wang, J. Antibacterial Properties of Poly(Dimethylsiloxane) Surfaces Modified with Graphene Oxide-Catechol Composite. Prog. Org. Coat. 2019, 129, 247–253. [Google Scholar] [CrossRef]
- Mottaghitalab, F.; Khodadadi Yazdi, M.; Saeb, M.R.; Bączek, T.; Farokhi, M. Green and Sustainable Hydrogels Based on Quaternized Chitosan to Enhance Wound Healing. Chem. Eng. J. 2024, 492, 152288. [Google Scholar] [CrossRef]
- Sikora, M.; Wąsik, S.; Semaniak, J.; Drulis-Kawa, Z.; Wiśniewska-Wrona, M.; Arabski, M. Chitosan-Based Matrix as a Carrier for Bacteriophages. Appl. Microbiol. Biotechnol. 2024, 108, 6. [Google Scholar] [CrossRef]
- Brusko, V.; Khannanov, A.; Rakhmatullin, A.; Dimiev, A.M. Unraveling the Infrared Spectrum of Graphene Oxide. Carbon 2024, 229, 119507. [Google Scholar] [CrossRef]
- Emiru, T.F.; Ayele, D.W. Controlled Synthesis, Characterization and Reduction of Graphene Oxide: A Convenient Method for Large Scale Production. Egypt. J. Basic Appl. Sci. 2017, 4, 74–79. [Google Scholar] [CrossRef]
- Uea-Aree, K.; Thongpool, V.; Sangkhun, W.; Wongyao, N.; Wootthikanokkhan, J. Preparations, Characterizations, and a Comparative Study on Photovoltaic Performance of Two Different Types of Graphene/TiO2 Nanocomposites Photoelectrodes. J. Nanomater. 2017, 2017, 2758294. [Google Scholar] [CrossRef]
- Sudesh, N.; Kumar, N.; Das, S.; Bernhard, C.; Varma, G.D. Effect of Graphene Oxide Doping on Superconducting Properties of Bulk MgB2. Supercond. Sci. Technol. 2013, 26, 095008. [Google Scholar] [CrossRef]
- Fernandes Queiroz, M.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the Use of Chitosan Contribute to Oxalate Kidney Stone Formation? Mar. Drugs 2015, 13, 141–158. [Google Scholar] [CrossRef]
- El Rouby, W.M.A.; Farghali, A.A.; Sadek, M.A.; El-Shahat, M.F.; El-Kemary, M.; El-Sharkawy, R.G. Fast Removal of Sr(II) from Water by Graphene Oxide and Chitosan Modified Graphene Oxide. J. Inorg. Organomet. Polym. 2018, 28, 2336–2349. [Google Scholar] [CrossRef]
- Sabzevari, M.; Cree, D.E.; Wilson, L.D. Graphene Oxide–Chitosan Composite Material for Treatment of a Model Dye Effluent. ACS Omega 2018, 3, 13045–13054. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.; Yang, K.; Anh, N.D.; Park, C.; Lee, S.M.; Lee, T.G.; Jeong, M.S. Raman Study of D* Band in Graphene Oxide and Its Correlation with Reduction. Appl. Surf. Sci. 2021, 536, 147990. [Google Scholar] [CrossRef]
- Kołodziej, A.; Długoń, E.; Świętek, M.; Ziąbka, M.; Dawiec, E.; Gubernat, M.; Michalec, M.; Wesełucha-Birczyńska, A. A Raman Spectroscopic Analysis of Polymer Membranes with Graphene Oxide and Reduced Graphene Oxide. J. Compos. Sci. 2021, 5, 20. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Ryzhkov, S.A.; Kirilenko, D.A.; Baidakova, M.V.; Vul, A.Y. From Graphene Oxide towards Aminated Graphene: Facile Synthesis, Its Structure and Electronic Properties. Sci. Rep. 2020, 10, 6902. [Google Scholar] [CrossRef]
- Nasef, S.M.; Khozemy, E.E.; Kamoun, E.A.; El-Gendi, H. Gamma Radiation-Induced Crosslinked Composite Membranes Based on Polyvinyl Alcohol/Chitosan/AgNO3/Vitamin E for Biomedical Applications. Int. J. Biol. Macromol. 2019, 137, 878–885. [Google Scholar] [CrossRef]
- Barleany, D.R.; Jayanudin; Utama, A.S.; Riyupi, U.; Alwan, H.; Lestari, R.S.D.; Pitaloka, A.B.; Yulvianti, M. Erizal Synthesis and Characterization of Chitosan/Polyvinyl Alcohol Crosslinked Poly(N-isopropylacrylamide) Smart Hydrogels via γ-Radiation. Mater. Today Proc. 2023, 87, 1–7. [Google Scholar] [CrossRef]
- Gefen, A.; Alves, P.; Beeckman, D.; Lázaro-Martínez, J.L.; Lev-Tov, H.; Najafi, B.; Swanson, T.; Woo, K. Mechanical and contact characteristics of foam materials within wound dressings: Theoretical and practical considerations in treatment. Int. Wound J. 2023, 20, 1960–1978. [Google Scholar] [CrossRef] [PubMed]
- Ovington, L.G. Wound care products: How to choose. Adv. Skin Wound Care 2001, 14, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, C.; Tittelbach, J.; Hipler, U.C.; Elsner, P. Clinical Efficacy of Dressings for Treatment of Heavily Exuding Chronic Wounds. Chronic Wound Care Manag. Res. 2015, 2, 101–111. [Google Scholar] [CrossRef]
- Minsart, M.; Van Vlierberghe, S.; Dubruel, P.; Mignon, A. Commercial Wound Dressings for the Treatment of Exuding Wounds: An In-Depth Physico-Chemical Comparative Study. Burns Trauma 2022, 10, tkac024. [Google Scholar] [CrossRef]
- Sinha, A.; Georgoulas, A.; Crua, C.; Saberianpour, S.; Sarker, D.; Forss, R.; Santin, M. Exploring Exudate Absorption via Sessile Droplet Dynamics in Porous Wound Dressings. Exp. Therm. Fluid Sci. 2025, 163, 111408. [Google Scholar] [CrossRef]
- Haider, A.; Khan, S.; Iqbal, D.N.; Khan, S.U.; Haider, S.; Mohammad, K.; Mustfa, G.; Rizwan, M.; Haider, A. Chitosan as a Tool for Tissue Engineering and Rehabilitation: Recent Developments and Future Perspectives—A Review. Int. J. Biol. Macromol. 2024, 278, 134172. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Sudheesh Kumar, P.T.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2015, 144, 51–63. [Google Scholar] [CrossRef]
- Raafat, D.; von Bargen, K.; Haas, A.; Sahl, H.-G. Insights into the mode of action of chitosan as an antibacterial compound. Appl. Environ. Microbiol. 2008, 74, 3764–3773. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Perreault, F.; de Faria, A.F.; Nejati, S.; Elimelech, M. Antimicrobial Properties of Graphene Oxide Nanosheets: Why Size Mat-ters. ACS Nano 2015, 9, 7226–7236. [Google Scholar] [CrossRef]
- Zou, F.; Zhou, H.; Jeong, D.Y.; Kwon, J.; Eom, S.U.; Park, T.J.; Hong, S.W.; Lee, J. Wrinkled surface-mediated antibacterial activity of graphene oxide nanosheets. ACS Appl. Mater. Interfaces 2017, 9, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Unnikrishnan, B.; Wei, S.-C.; Chou, C.P.; Zhang, L.-Z.; Huang, C.-C. Graphene oxide and carbon dots as broad-spectrum antimicrobial agents—A minireview. Nanoscale Horiz. 2019, 4, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Singh, N.B.; Nagpal, G.; Saah, F.K. Antibacterial activity of reduced graphene-silver oxide nanocomposite against gram-negative bacteria. Microbe 2024, 5, 100221. [Google Scholar] [CrossRef]
- Bousiakou, L.G.; Qindeel, R.; Al-Dossary, O.M.; Kalkani, H. Synthesis and characterization of graphene oxide (GO) sheets for pathogen inhibition: Escherichia coli, Staphylococcus aureus and Pseudomonas aeruginosa. Science 2022, 34, 102002. [Google Scholar] [CrossRef]
- Ravikumar, V.; Mijakovic, I.; Pandit, S. Antimicrobial activity of graphene oxide contributes to alteration of key stress-related and membrane-bound proteins. Int. J. Nanomed. 2022, 17, 6707–6721. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxida-tive stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxi-dants. Archiv. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef] [PubMed]
- Aunkor, T.H.; Raihan, T.; Prodhan, S.H.; Metselaar, H.S.C.; Malik, S.U.F.; Azad, A.K. Antibacterial activity of graphene oxide nanosheet against multidrug resistant superbugs isolated from infected patients. R. Soc. Open Sci. 2020, 7, 200640. [Google Scholar] [CrossRef] [PubMed]
- Mokkapati, V.R.; Pandit, S.; Kim, J.; Martensson, A.; Lovmar, M.; Westerlund, F.; Mijakovic, I. Bacterial response to graphene oxide and reduced graphene oxide integrated in agar plates. R. Soc. Open Sci. 2018, 5, 181083. [Google Scholar] [CrossRef] [PubMed]
- Fallatah, H.; Overton, T.; Ali-Boucetta, H.; Gkatzionis, K. Impact of Environmental Stresses on the Antibacterial Activity of Gra-phene Oxide (GO) Nanoparticles against P. putida Biofilms. Microorganisms 2023, 11, 609. [Google Scholar] [CrossRef]
- Marsala, V.; Gerasymchuk, Y.; Saladino, M.L.; Paluch, E.; Wawrzyńska, M.; Boiko, V.; Li, X.; Giordano, C.; Hreniak, D.; Sobieszczańska, B. Structural, Morphological, and Antibacterial Attributes of Graphene Oxide Prepared by Hummers’ and Brodie’s Methods. Molecules 2025, 30, 240. [Google Scholar] [CrossRef]
- Struszczyk, H.; Niekraszewicz, A.; Kucharska, M.; Urbanowski, A.; Wisniewska-Wrona, M.; Wesolowska, E.; Ciechanska, D. Method of Producing Microcrystalline Chitosan Using a Continuous Method. Patent PL 164 247, 27 January 2009. [Google Scholar]
- Szabó, T.; Tombácz, E.; Illés, E.; Dékány, I. Enhanced Acidity and pH-Dependent Surface Charge Characterization of Successively Oxidized Graphite Oxides. Carbon 2006, 44, 537–545. [Google Scholar] [CrossRef]
- Gerasymchuk, Y.; Wędzyńska, A.; Stręk, W. Liquid “Syngas” Based on Supercritical Water and Graphite Oxide/TiO2 Composite as Catalyst for CO2 to Organic Conversion. Catal. Lett. 2022, 152, 2840–2851. [Google Scholar] [CrossRef]
- PN-EN ISO 1923:1999; Porous Plastics and Rubbers. Determination of Linear Dimensions. ISO: Geneva, Switzerland, 1999.
- PN-EN ISO 1798:2009; Flexible Porous Plastics. Determination of Tensile Strength and Elongation at Break. ISO: Geneva, Switzerland, 2009.
- Procedure SPR/BPB/14; Determination of WRV of Starting Chitosan and Microcrystalline Chitosan, According to GLP No. G-016. IBWCh (currently Łukasiewicz Research Network—Łódź Institute of Technology): Lodz, Poland, 2005.
- PN-EN ISO 10993-12:2012; Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials. ISO: Geneva, Switzerland, 2012.
- PN-EN ISO 10993-5:2009; Biological Evaluation of Medical Devices. Part 5: Cytotoxicity Studies. ISO: Geneva, Switzerland, 2009.


| Sample | Thickness (mm) | Max Tensile Force (N) | Tensile Strength (MPa) | Elongation at Max Tension (%) |
|---|---|---|---|---|
| Sponge of MKCh | 3.05 ± 0.06 | 0.374 ± 0.046 | 0.008 ± 0.001 | 14.7 ± 6.4 |
| Sponge of MKCh/25 kGy | 3.34 ± 0.30 | 0.542 ± 0.062 | 0.011 ± 0.002 | 17.3 ± 3.4 |
| Sponge of MKCh + GO | 3.01 ± 0.09 | 0.344 ± 0.009 | 0.008 ± 0.001 | 19.9 ± 2.3 |
| Sponge of MKCh + GO/25 kGy | 3.12 ± 0.12 | 0.320 ± 0.020 | 0.007 ± 0.001 | 13.4 ± 1.8 |
| Time (min) | Sponge of MKCh | Sponge of MKCh + GO | |||
|---|---|---|---|---|---|
| Ws Index (%) | Sorption Capacity (g) | Ws Index (%) | Sorption Capacity (g) | ||
| 15 | Before 25 kGy | 1692.15 | 16.93 | 1661.87 | 16.62 |
| After 25 kGy | 1842.10 | 18.42 | 1840.22 | 18.41 | |
| 30 | Before 25 kGy | 1904.10 | 19.04 | 1818.97 | 18.19 |
| After 25 kGy | 2059.36 | 20.60 | 2012.62 | 20.13 | |
| 180 | Before 25 kGy | 1806.58 | 19.93 | 2011.18 | 20.11 |
| After 25 kGy | 2144.30 | 21.46 | 2105.47 | 21.06 | |
| 300 | Before 25 kGy | 2217.43 | 22.17 | 2078.38 | 20.79 |
| After 25 kGy | 2250.74 | 22.51 | 2217.30 | 22.17 | |
| Sample | WRV (%) |
|---|---|
| Sponge of MKCh | 160.0 |
| Sponge of MKCh/25 kGy | 165.0 |
| Sponge of MKCh + GO | 195.0 |
| Sponge of MKCh + GO/25 kGy | 227.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sareło, P.; Wiśniewska-Wrona, M.; Sikora, M.; Mielan, B.; Gerasymchuk, Y.; Wędzyńska, A.; Boiko, V.; Hreniak, D.; Szymonowicz, M.; Sobieszczańska, B.; et al. Development and Evaluation of Graphene Oxide-Enhanced Chitosan Sponges as a Potential Antimicrobial Wound Dressing for Infected Wound Management. Int. J. Mol. Sci. 2025, 26, 7403. https://doi.org/10.3390/ijms26157403
Sareło P, Wiśniewska-Wrona M, Sikora M, Mielan B, Gerasymchuk Y, Wędzyńska A, Boiko V, Hreniak D, Szymonowicz M, Sobieszczańska B, et al. Development and Evaluation of Graphene Oxide-Enhanced Chitosan Sponges as a Potential Antimicrobial Wound Dressing for Infected Wound Management. International Journal of Molecular Sciences. 2025; 26(15):7403. https://doi.org/10.3390/ijms26157403
Chicago/Turabian StyleSareło, Przemysław, Maria Wiśniewska-Wrona, Monika Sikora, Bartosz Mielan, Yuriy Gerasymchuk, Anna Wędzyńska, Vitalii Boiko, Dariusz Hreniak, Maria Szymonowicz, Beata Sobieszczańska, and et al. 2025. "Development and Evaluation of Graphene Oxide-Enhanced Chitosan Sponges as a Potential Antimicrobial Wound Dressing for Infected Wound Management" International Journal of Molecular Sciences 26, no. 15: 7403. https://doi.org/10.3390/ijms26157403
APA StyleSareło, P., Wiśniewska-Wrona, M., Sikora, M., Mielan, B., Gerasymchuk, Y., Wędzyńska, A., Boiko, V., Hreniak, D., Szymonowicz, M., Sobieszczańska, B., & Wawrzyńska, M. (2025). Development and Evaluation of Graphene Oxide-Enhanced Chitosan Sponges as a Potential Antimicrobial Wound Dressing for Infected Wound Management. International Journal of Molecular Sciences, 26(15), 7403. https://doi.org/10.3390/ijms26157403







