S100A14 as a Potential Biomarker of the Colorectal Serrated Neoplasia Pathway
Abstract
1. Introduction
2. Results
2.1. S100A14 Is Higher in Serrated Lesions Compared to Adenoma
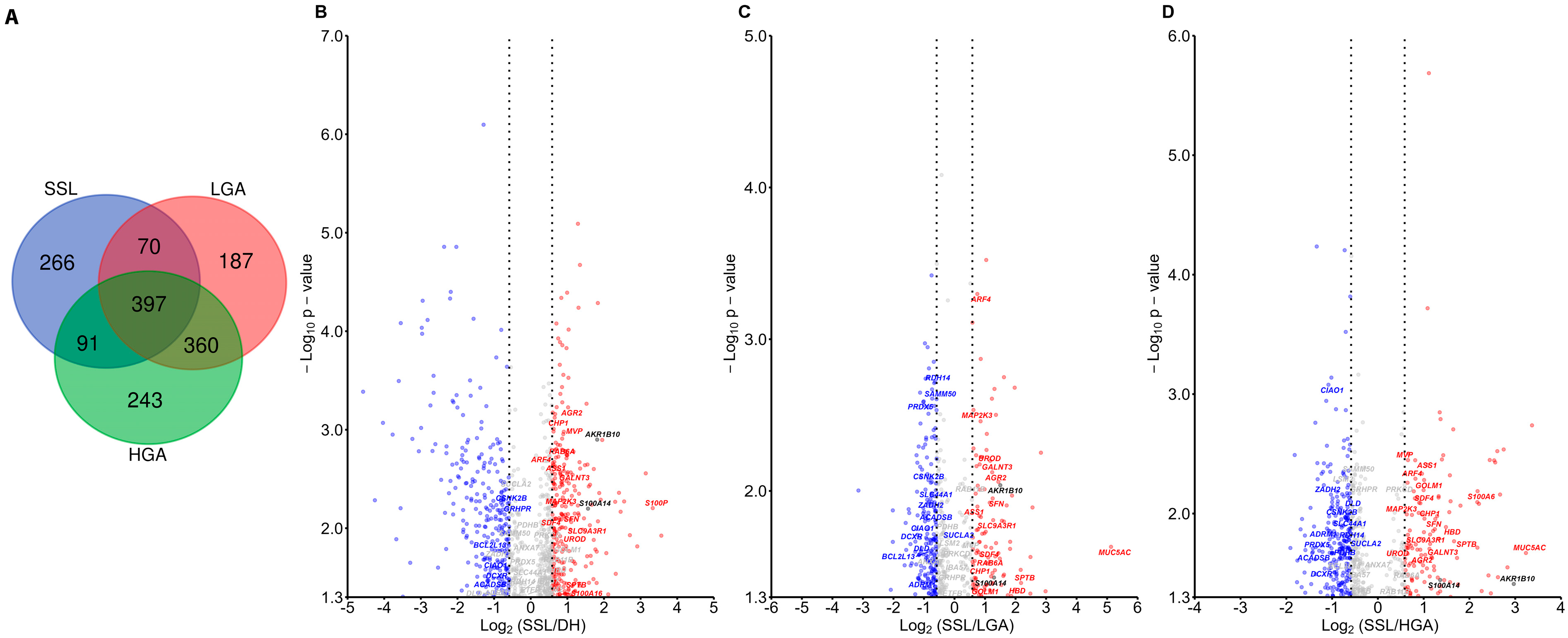
2.1.1. Proteomic Results
2.1.2. Immunohistochemistry Results
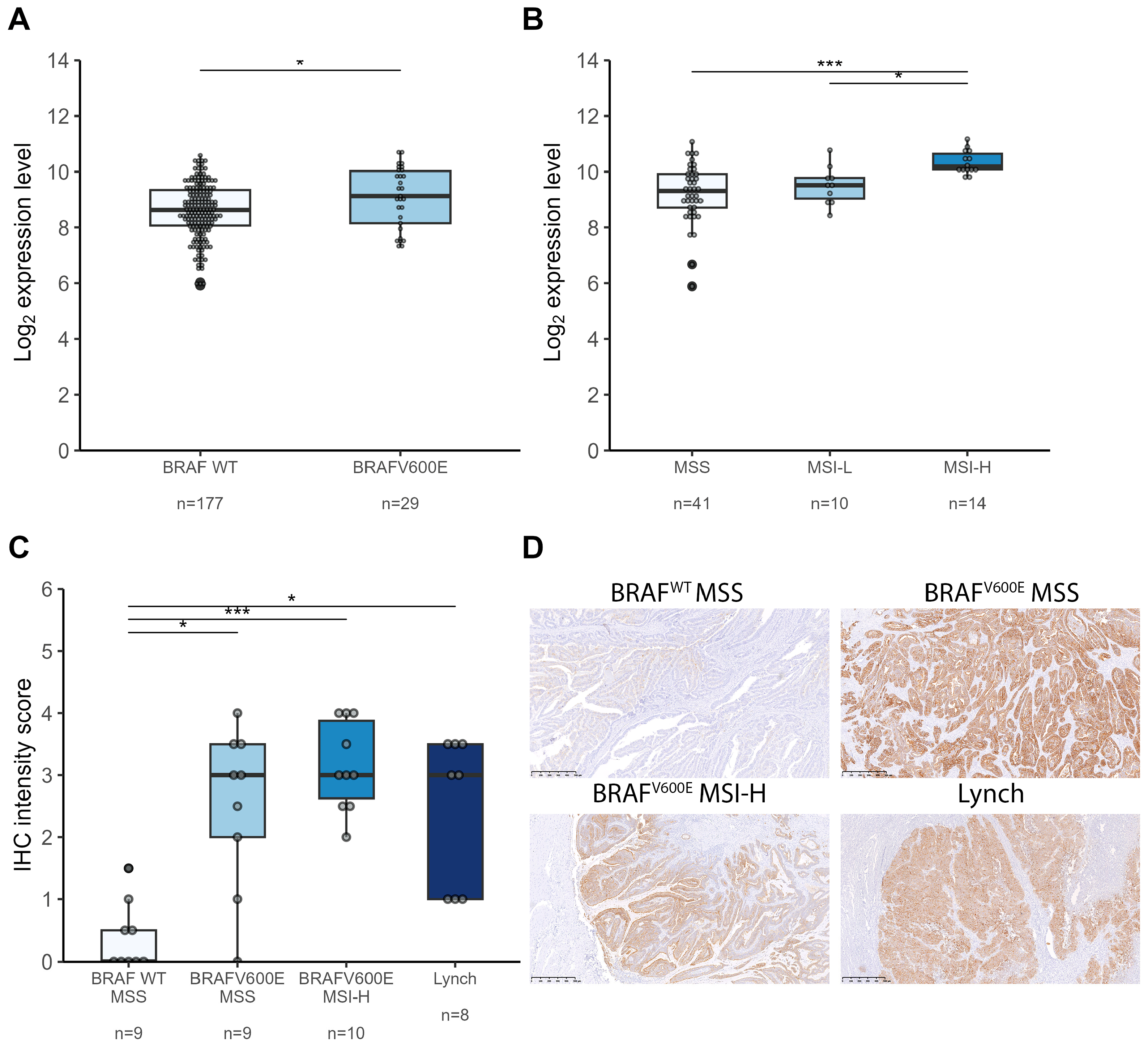
2.2. S100A14 Is Upregulated in Colorectal Cancers Arising from the Serrated Pathway
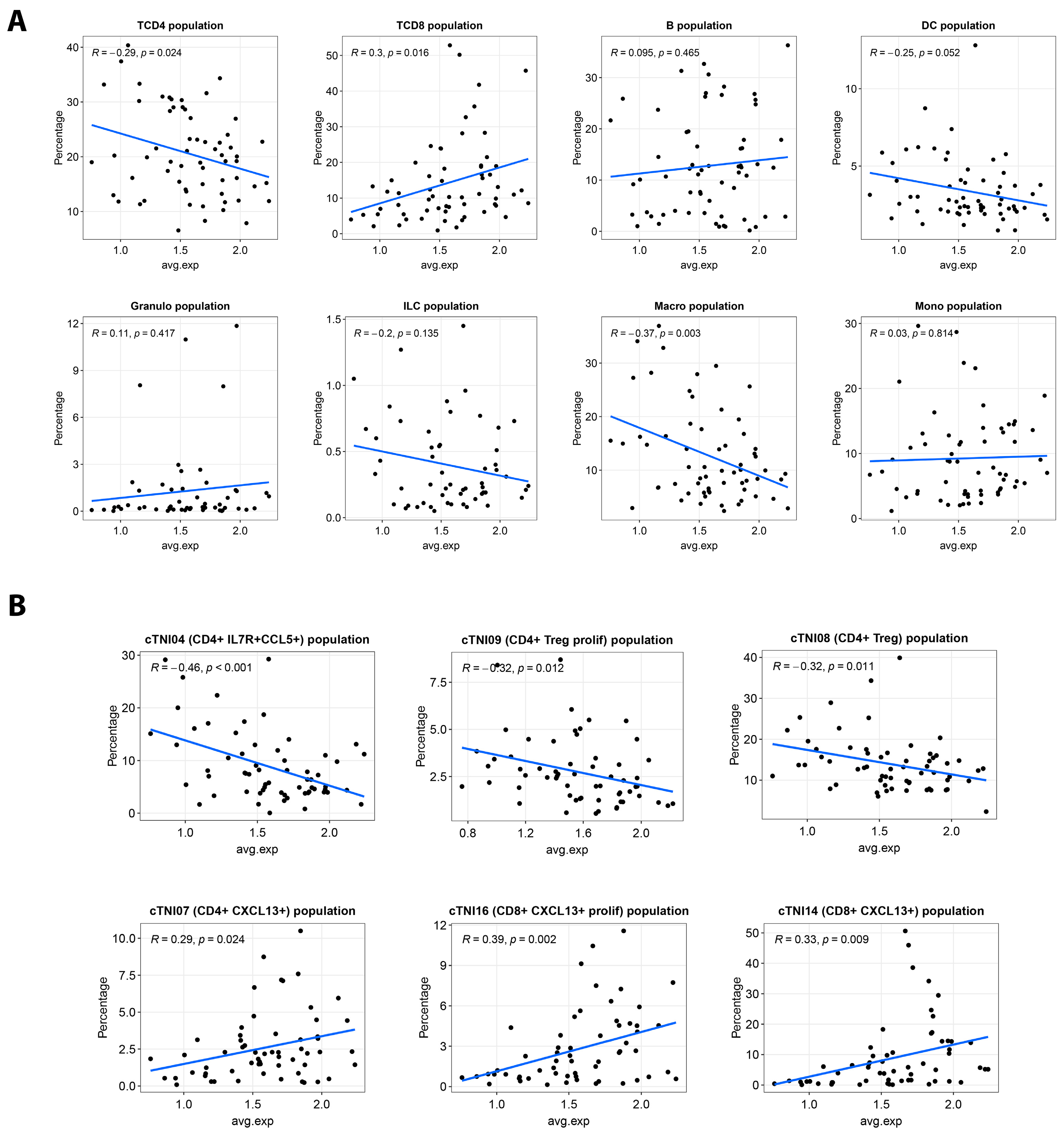
2.3. S100A14 Is Associated with Anti-Tumor Immunity
3. Discussion
4. Materials and Methods
4.1. Patient Inclusions and FFPE Tissue Samples
4.2. Shotgun Proteomic Experiments
4.2.1. Sample Processing
4.2.2. LC-MSMS Analysis
4.2.3. Protein Identifications and Quantifications
4.3. Immunohistochemistry (IHC) Experiments
4.3.1. IHC Sample Preparation
4.3.2. IHC Characterization
4.4. Gene Expression Analysis
4.5. Single-Cell Data Analysis
4.6. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADH | Alcohol Dehydrogenase |
| AGC | Automatic Gain Control |
| AKR1B10 | Aldo-Keto Reductase Family 1 Member B10 |
| BRAF | v-Raf murine sarcoma viral oncogene homolog B |
| CCL5 | Chemokine (C-C motif) Ligand 5 |
| CPI | Checkpoint Inhibitor |
| CRC | Colorectal Cancer |
| CXCL13 | Chemokine (C-X-C motif) Ligand 13 |
| DH | Diverticulitis Healthy |
| ENO1 | Enolase 1 |
| FASP | Filter-Aided Sample Preparation |
| FFPE | Formalin-Fixed Paraffin-Embedded |
| GEO | Gene Expression Omnibus |
| GPB | Glycogen Phosphorylase B |
| H&E | Hematoxylin and Eosin |
| HGA | High-Grade Adenoma |
| HP | Hyperplastic Polyp |
| IHC | Immunohistochemistry |
| IL7R | Interleukin 7 Receptor |
| LC-MS/MS | Liquid Chromatography–Tandem Mass Spectrometry |
| LGA | Low-Grade Adenoma |
| LTF | Lactotransferrin |
| LGR5 | Leucine-Rich Repeat Containing G Protein-Coupled Receptor 5 |
| MMR | Mismatch Repair |
| MMRd | Mismatch Repair Deficient |
| MMRp | Mismatch Repair Proficient |
| MPDS | MassPREP Digestion Standard |
| MSI | Microsatellite Instability |
| MSI-L | Microsatellite Instability Low |
| MSI-H | Microsatellite Instability High |
| MSS | Microsatellite Stable |
| MVHP | Microvesicular Hyperplastic Polyp |
| MUC2 | Mucin 2 |
| MUC5A | Mucin 5AC |
| NGS | Next-Generation Sequencing |
| OLFM4 | Olfactomedin 4 |
| OR | Odds Ratio |
| p53 | Tumor protein p53 |
| PD-L1 | Programmed Death-Ligand 1 |
| PRIDE | PRoteomics IDEntifications Database |
| RCDC | Reducing Agent and Detergent Compatible |
| RRID | Research Resource Identifier |
| S100A14 | S100 Calcium Binding Protein A14 |
| S100A16 | S100 Calcium Binding Protein A16 |
| S100P | S100 Calcium Binding Protein P |
| SCP | Single Cell Portal |
| SSL | Sessile Serrated Lesion |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| TFF3 | Trefoil Factor 3 |
| TRM | Tissue-Resident Memory T cell |
| TSA | Traditional Serrated Adenoma |
| UMAP | Uniform Manifold Approximation and Projection |
| UPLC | Ultra-Performance Liquid Chromatography |
References
- Akimoto, N.; Ugai, T.; Zhong, R.; Hamada, T.; Fujiyoshi, K.; Giannakis, M.; Wu, K.; Cao, Y.; Ng, K.; Ogino, S. Rising incidence of early-onset colorectal cancer: A call for action. Physiol. Behav. 2021, 176, 139–148. [Google Scholar] [CrossRef]
- Müller, M.F.; Ibrahim, A.E.K.; Arends, M.J. Molecular pathological classification of colorectal cancer. Virchows Arch. 2016, 469, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Kitamura, S.; Kimura, T.; Nakagawa, T.; Sogabe, M.; Miyamoto, H.; Muguruma, N.; Takayama, T. Clinicopathological characteristics of serrated polyps as precursors to colorectal cancer: Current status and management. J. Gastroenterol. Hepatol. 2017, 32, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Mezzapesa, M.; Losurdo, G.; Celiberto, F.; Rizzi, S.; D’amati, A.; Piscitelli, D.; Ierardi, E.; Di Leo, A. Serrated Colorectal Lesions: An Up-to-Date Review from Histological Pattern to Molecular Pathogenesis. Int. J. Mol. Sci. 2022, 23, 4461. [Google Scholar] [CrossRef] [PubMed]
- Phipps, A.I.; Limburg, P.J.; Baron, J.A.; Burnett-Hartman, A.N.; Weisenberger, D.J.; Laird, P.W.; Sinicrope, F.A.; Rosty, C.; Buchanan, D.D.; Potter, J.D.; et al. Association between molecular subtypes of colorectal cancer and patient survival. Gastroenterology 2015, 148, 77–87.e2. [Google Scholar] [CrossRef]
- Ahadi, M.; Sokolova, A.; Brown, I.; Chou, A.; Gill, A.J. The 2019 World Health Organization Classification of appendiceal, colorectal and anal canal tumours: An update and critical assessment. Pathology 2021, 53, 454–461. [Google Scholar] [CrossRef]
- Satorres, C.; García-Campos, M.; Bustamante-Balén, M. Molecular Features of the Serrated Pathway to Colorectal Cancer: Current Knowledge and Future Directions. Gut Liver 2021, 15, 31–43. [Google Scholar] [CrossRef]
- Li, D.; Doherty, A.R.; Raju, M.; Liu, L.; Lei, N.Y.; Amsden, L.B.; Lee, J.K.; Levin, T.R.; Corley, D.A.; Herrinton, L.J. Risk stratification for colorectal cancer in individuals with subtypes of serrated polyps. Gut 2022, 71, 2022–2029. [Google Scholar] [CrossRef]
- Yang, J.F.; Tang, S.J.; Lash, R.H.; Wu, R.; Yang, Q. Anatomic distribution of sessile serrated adenoma/polyp with and without cytologic dysplasia. Arch. Pathol. Lab. Med. 2015, 139, 388–393. [Google Scholar] [CrossRef]
- Haque, T.; Greene, K.G.; Crockett, S.D. Serrated neoplasia of the colon: What do we really know? Curr. Gastroenterol. Rep. 2014, 16, 380. [Google Scholar] [CrossRef]
- Crockett, S.D.; Nagtegaal, I.D. Terminology, Molecular Features, Epidemiology, and Management of Serrated Colorectal Neoplasia. Gastroenterology 2019, 157, 949–966.e4. [Google Scholar] [CrossRef]
- Murcia, O.; Juárez, M.; Rodríguez-Soler, M.; Hernández-Illán, E.; Giner-Calabuig, M.; Alustiza, M.; Egoavil, C.; Castillejo, A.; Alenda, C.; Barberá, V.; et al. Colorectal cancer molecular classification using BRAF, KRAS, microsatellite instability and CIMP status: Prognostic implications and response to chemotherapy. PLoS ONE 2018, 13, e0203051. [Google Scholar] [CrossRef] [PubMed]
- De’angelis, G.L.; Bottarelli, L.; Azzoni, C.; De’angelis, N.; Leandro, G.; Di Mario, F.; Gaiani, F.; Negri, F. Microsatellite instability in colorectal cancer. Acta Biomed. 2018, 89, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Li, L.; Jiang, X.; Li, Q. Mismatch repair deficiency/microsatellite instability-high as a predictor for anti-PD-1/PD-L1 immunotherapy efficacy. J. Hematol. Oncol. 2019, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- Sohier, P.; Sanson, R.; Leduc, M.; Audebourg, A.; Broussard, C.; Salnot, V.; Just, P.A.; Pasmant, E.; Mayeux, P.; Guillonneau, F.; et al. Proteome analysis of formalin-fixed paraffin-embedded colorectal adenomas reveals the heterogeneous nature of traditional serrated adenomas compared to other colorectal adenomas. J. Pathol. 2020, 250, 251–261. [Google Scholar] [CrossRef]
- Pelka, K.; Hofree, M.; Chen, J.H.; Sarkizova, S.; Pirl, J.D.; Jorgji, V.; Bejnood, A.; Dionne, D.; Ge, W.H.; Xu, K.H.; et al. Spatially organized multicellular immune hubs in human colorectal cancer. Cell 2021, 184, 4734–4752.e20. [Google Scholar] [CrossRef]
- Rickelt, S.; Condon, C.; Mana, M.; Whittaker, C.; Pfirschke, C.; Roper, J.; Patil, D.T.; Brown, I.; Mattia, A.R.; Zukerberg, L.; et al. Agrin in the Muscularis mucosa serves as a biomarker distinguishing hyperplastic polyps from sessile serrated lesions. Clin. Cancer Res. 2020, 26, 1277–1287. [Google Scholar] [CrossRef]
- Chen, B.; Scurrah, C.R.; McKinley, E.T.; Simmons, A.J.; Ramirez-Solano, M.A.; Zhu, X.; Markham, N.O.; Heiser, C.N.; Vega, P.N.; Rolong, A.; et al. Differential pre-malignant programs and microenvironment chart distinct paths to malignancy in human colorectal polyps. Cell 2021, 184, 6262–6280.e26. [Google Scholar] [CrossRef]
- Basnet, S.; Vallenari, E.M.; Maharjan, U.; Sharma, S.; Schreurs, O.; Sapkota, D. An Update on S100A16 in Human Cancer. Biomolecules 2023, 13, 1070. [Google Scholar] [CrossRef]
- Takahashi, S.; Okamoto, K.; Tanahashi, T.; Fujimoto, S.; Nakagawa, T.; Bando, M.; Ma, B.; Kawaguchi, T.; Fujino, Y.; Mitsui, Y.; et al. S100P Expression via DNA Hypomethylation Promotes Cell Growth in the Sessile Serrated Adenoma/Polyp-Cancer Sequence. Digestion 2021, 102, 789–802. [Google Scholar] [CrossRef]
- Bresnick, A.R.; Weber, D.J.; Zimmer, D.B. S100 proteins in cancer. Nat. Rev. Cancer 2015, 15, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Basnet, S.; Sharma, S.; Costea, D.E.; Sapkota, D. Expression profile and functional role of S100A14 in human cancer. Oncotarget 2019, 10, 2996–3012. [Google Scholar] [CrossRef] [PubMed]
- Hashida, H.; Coffey, R.J. Significance of a calcium-binding protein S100A14 expression in colon cancer progression. J. Gastrointest. Oncol. 2022, 13, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Y.; Zhang, J.-Y.; Cui, J.-T.; Tan, X.-H.; Li, W.-M.; Gu, J.; Lu, Y.-Y. Expression status of S100A14 and S100A4 correlates with metastatic potential and clinical outcome in colorectal cancer after surgery. Oncol. Rep. 2010, 23, 45–52. [Google Scholar] [CrossRef]
- Diamantopoulou, A.; Mantas, D.; Kostakis, I.D.; Agrogiannis, G.; Garoufalia, Z.; Kavantzas, N.; Kouraklis, G. A clinicopathological analysis of S100A14 expression in colorectal cancer. In Vivo 2020, 34, 321–330. [Google Scholar] [CrossRef]
- Sapkota, D.; Costea, D.E.; Blø, M.; Bruland, O.; Lorens, J.B.; Vasstrand, E.N.; Ibrahim, S.O. S100A14 inhibits proliferation of oral carcinoma derived cells through G1-arrest. Oral. Oncol. 2012, 48, 219–225. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, H.; Cui, J.; Li, W.; An, G.; Pan, Y.; Zhang, Q.; Xing, R.; Lu, Y. Calcium-binding protein s100a14 induces differentiation and suppresses metastasis in gastric cancer. Cell Death Dis. 2017, 8, e2938-12. [Google Scholar] [CrossRef]
- Min, H.; Cho, J.; Sim, J.Y.; Boo, H.; Lee, J.; Lee, S.; Lee, Y.; Kim, S.J.; Kim, K.; Park, I.; et al. S100A14: A novel negative regulator of cancer stemness and immune evasion by inhibiting STAT3-mediated programmed death-ligand 1 expression in colorectal cancer. Clin. Transl. Med. 2022, 12, e986. [Google Scholar] [CrossRef]
- Zhou, Y.-J.; Lu, X.-F.; Chen, H.; Wang, X.-Y.; Cheng, W.; Zhang, Q.-W.; Chen, J.-N.; Wang, X.-Y.; Jin, J.-Z.; Yan, F.-R.; et al. Single-cell transcriptomics reveals early molecular and immune alterations underlying the serrated neoplasia pathway toward colorectal cancer. Cell. Mol. Gastroenterol. Hepatol. 2022, 15, 393–424. [Google Scholar] [CrossRef]
- Banchereau, R.; Chitre, A.S.; Scherl, A.; Wu, T.D.; Patil, N.S.; De Almeida, P.; Kadel, E.E.; Madireddi, S.; Au-Yeung, A.; Takahashi, C.; et al. Intratumoral CD103+ CD8+ T cells predict response to PD-L1 blockade. J. Immunother. Cancer 2021, 9, e002231. [Google Scholar] [CrossRef]
- Okla, K.; Farber, D.L.; Zou, W. Tissue-resident memory T cells in tumor immunity and immunotherapy. J. Exp. Med. 2021, 218, e20201605. [Google Scholar] [CrossRef]
- Che, G.; Yin, J.; Wang, W.; Luo, Y.; Chen, Y.; Yu, X.; Wang, H.; Liu, X.; Chen, Z.; Wang, X.; et al. Circumventing drug resistance in gastric cancer: A spatial multi-omics exploration of chemo and immuno-therapeutic response dynamics. Drug Resist. Updat. 2024, 74, 101080, Erratum in: Drug Resist Updat. 2024, 76, 101101. [Google Scholar] [CrossRef] [PubMed]
- Litchfield, K.; Reading, J.L.; Puttick, C.; Thakkar, K.; Abbosh, C.; Bentham, R.; Watkins, T.B.K.; Rosenthal, R.; Biswas, D.; Rowan, A.; et al. Meta-analysis of tumor- and T cell-intrinsic mechanisms of sensitization to checkpoint inhibition. Cell 2021, 184, 596–614.e14. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, D.; Li, Y.; Qi, L.; Si, W.; Bo, Y.; Chen, X.; Ye, Z.; Fan, H.; Liu, B.; et al. Spatiotemporal single-cell analysis decodes cellular dynamics underlying different responses to immunotherapy in colorectal cancer. Cancer Cell 2024, 42, 1268–1285.e7. [Google Scholar] [CrossRef] [PubMed]
- Grasso, C.S.; Giannakis, M.; Wells, D.K.; Hamada, T.; Mu, X.J.; Quist, M.; Nowak, J.A.; Nishihara, R.; Qian, Z.R.; Inamura, K.; et al. Genetic mechanisms of immune evasion in colorectal cancer. Cancer Discov. 2018, 8, 730–749. [Google Scholar] [CrossRef]
- Hébrant, A.; Jouret-Mourin, A.; Froyen, G.; Van Der Meulen, J.; De Man, M.; Salgado, R.; Van Den Eynde, M.; D’haene, N.; Martens, G.; Van Cutsem, E.; et al. Molecular test algorithms for digestive tumours. Belg. J. Med. Oncol. 2019, 13, 4–10. [Google Scholar]
- Quesada-Calvo, F.; Massot, C.; Bertrand, V.; Longuespée, R.; Blétard, N.; Somja, J.; Mazzucchelli, G.; Smargiasso, N.; Baiwir, D.; De Pauw-Gillet, M.-C.; et al. OLFM4, KNG1 and Sec24C identified by proteomics and immunohistochemistry as potential markers of early colorectal cancer stages. Clin. Proteom. 2017, 14, 9. [Google Scholar] [CrossRef]
- Pierre, N.; Salée, C.; Massot, C.; Blétard, N.; Mazzucchelli, G.; Smargiasso, N.; Morsa, D.; Baiwir, D.; De Pauw, E.; Reenaers, C.; et al. Proteomics Highlights Common and Distinct Pathophysiological Processes Associated with Ileal and Colonic Ulcers in Crohn’s Disease. J. Crohn's Colitis 2020, 14, 205–215. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Cox, J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 2016, 11, 2301–2319. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef]
- Deutsch, E.W.; Bandeira, N.; Perez-Riverol, Y.; Sharma, V.; Carver, J.J.; Mendoza, L.; Kundu, D.J.; Wang, S.; Bandla, C.; Kamatchinathan, S.; et al. The ProteomeXchange consortium at 10 years: 2023 update. Nucleic Acids Res. 2023, 51, D1539–D1548. [Google Scholar] [CrossRef] [PubMed]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; García-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE database resources in 2022: A hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022, 50, D543–D552. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adam, P.; Salée, C.; Quesada Calvo, F.; Lavergne, A.; Merli, A.-M.; Massot, C.; Blétard, N.; Somja, J.; Baiwir, D.; Mazzucchelli, G.; et al. S100A14 as a Potential Biomarker of the Colorectal Serrated Neoplasia Pathway. Int. J. Mol. Sci. 2025, 26, 7401. https://doi.org/10.3390/ijms26157401
Adam P, Salée C, Quesada Calvo F, Lavergne A, Merli A-M, Massot C, Blétard N, Somja J, Baiwir D, Mazzucchelli G, et al. S100A14 as a Potential Biomarker of the Colorectal Serrated Neoplasia Pathway. International Journal of Molecular Sciences. 2025; 26(15):7401. https://doi.org/10.3390/ijms26157401
Chicago/Turabian StyleAdam, Pierre, Catherine Salée, Florence Quesada Calvo, Arnaud Lavergne, Angela-Maria Merli, Charlotte Massot, Noëlla Blétard, Joan Somja, Dominique Baiwir, Gabriel Mazzucchelli, and et al. 2025. "S100A14 as a Potential Biomarker of the Colorectal Serrated Neoplasia Pathway" International Journal of Molecular Sciences 26, no. 15: 7401. https://doi.org/10.3390/ijms26157401
APA StyleAdam, P., Salée, C., Quesada Calvo, F., Lavergne, A., Merli, A.-M., Massot, C., Blétard, N., Somja, J., Baiwir, D., Mazzucchelli, G., Coimbra Marques, C., Delvenne, P., Louis, E., & Meuwis, M.-A. (2025). S100A14 as a Potential Biomarker of the Colorectal Serrated Neoplasia Pathway. International Journal of Molecular Sciences, 26(15), 7401. https://doi.org/10.3390/ijms26157401





