Identification of Common Cancer Antigens Useful for Specific Immunotherapies to Colorectal Cancer and Liver Metastases
Abstract
1. Introduction
2. Results
2.1. Expression of Seven Common Cancer Antigens and HLA Class I on Cell Membrane in 25 Cases of Primary CRC
2.2. Expression of Seven Common Cancer Antigens and HLA Class I on Cell Membrane in 60 Cases of CRC Liver Metastases
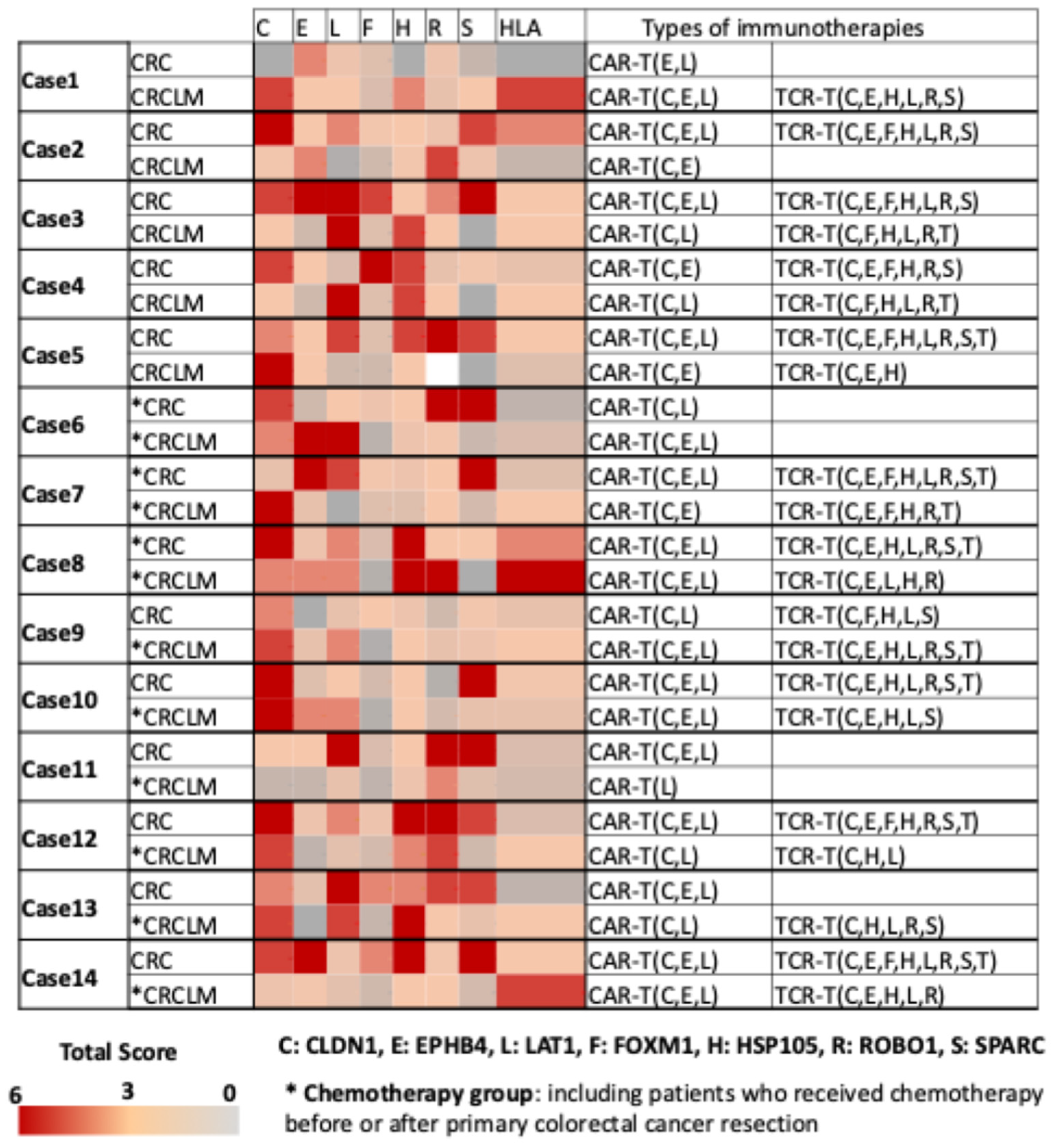
2.3. Expression of Seven Common Cancer Antigens and HLA Class I on Cell Membrane in 14 Cases of Primary CRCLM
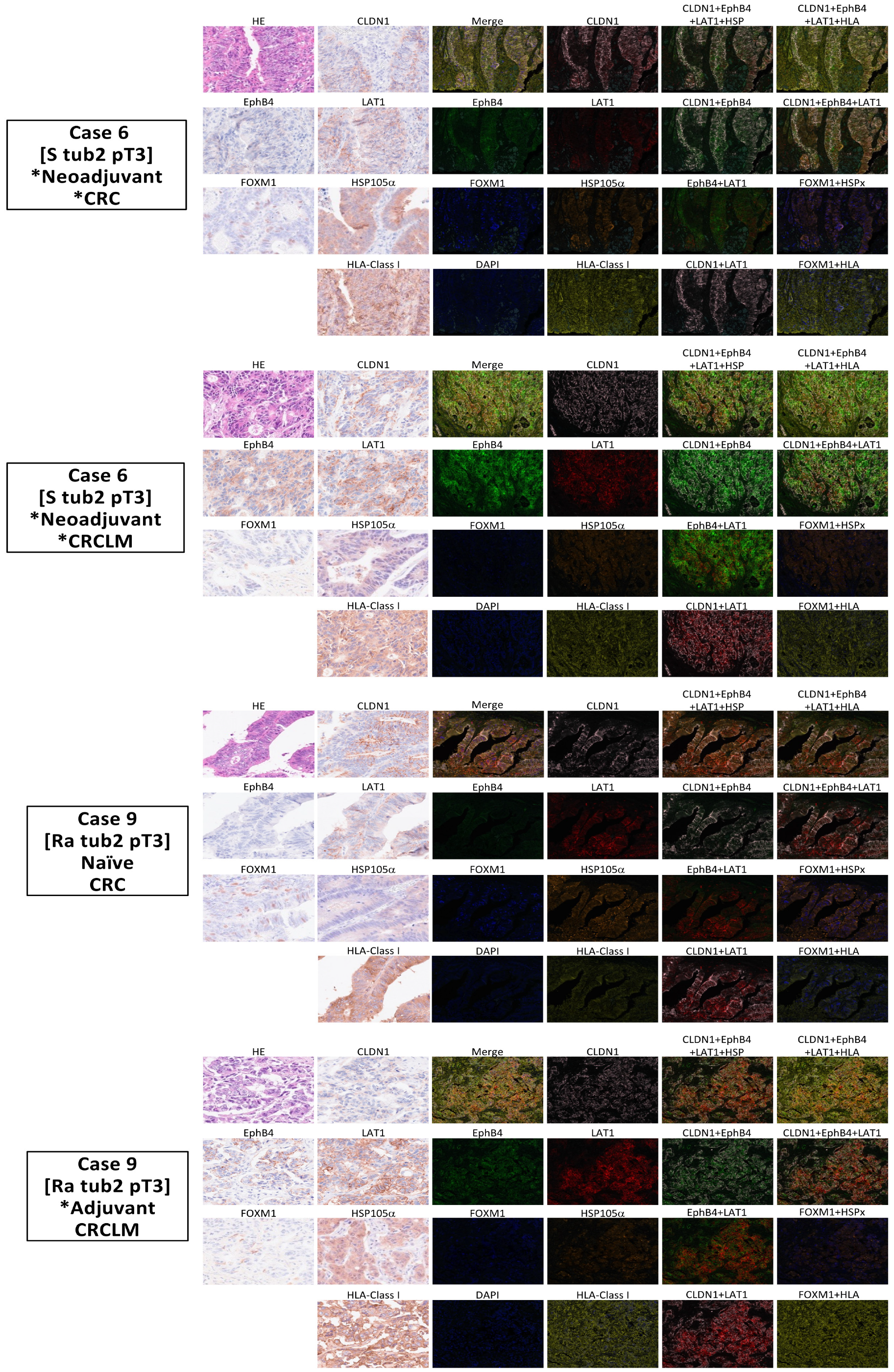
2.4. The Development of a Multiplex Fluorescence Immunohistochemical Staining System for Common Cancer Antigens and HLA Class I Detection
3. Discussion
4. Materials and Methods
4.1. Clinical Samples
4.2. Immunohistochemical Analysis of Common Cancer Antigens and HLA Class I
4.3. Multiplex Fluorescence Immunohistochemical Staining and Analysis of Cancer Antigens and HLA Class I
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAF | Cancer-associated fibroblast |
| CAR-T | Chimeric antigen receptor-T |
| CLDN1 | Claudin 1 |
| CRCLM | Colorectal cancer liver metastases |
| EphB4 | Ephrin type-B receptor 4 |
| FOXM1 | Forkhead box M1 |
| HLA | Human leukocyte antigen |
| HSP105α | Heat shock protein 105α |
| IHC | Immunohistochemical staining |
| LAT1 | L-type amino acid transporter 1 |
| MFIH | Multiplex fluorescence immunohistochemistry |
| MMR | Mismatch repair |
| NCCN | National Comprehensive Cancer Network |
| ROBO1 | Roundabout homolog-1 |
| ROI | Region of interest |
| SPARC | Secreted protein acidic and rich in cysteine |
| TCR-T | T cell receptor-T |
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA: A Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, F.; Tsukamoto, S.; Kato, T.; Nagata, H.; Takamizawa, Y.; Moritani, K.; Kinugasa, Y.; Esaki, M.; Kanemitsu, Y.; Igarashi, A. Cost-effectiveness analysis of postoperative surveillance for stage IV colorectal cancer in Japan: An economic modeling study. Ann Gastroenterol Surg. 2025, 9, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; de Gramont, A.; Figueras, J.; Kokudo, N.; Kunstlinger, F.; Loyer, E.; Poston, G.; Rougier, P.; Rubbia-Brandt, L.; Sobrero, A.; et al. Managing synchronous liver metastases from colorectal cancer: A multidisciplinary international consensus. Cancer Treat. Rev. 2015, 41, 729–741. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Adam, M.; Chang, G.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.A.; Cooper, H.S.; Deming, D.; Garrido-Laguna, I.; et al. Colon Cancer, Version 3.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2024, 22, e240029. [Google Scholar] [CrossRef] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Colon Cancer Version 5.2024. Available online: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf (accessed on 22 August 2024).
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Rectal Cancer Version 4.2024. Available online: https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf (accessed on 22 August 2024).
- Chalabi, M.; Fanchi, L.F.; Dijkstra, K.K.; Van den Berg, J.G.; Aalbers, A.G.; Sikorska, K.; Lopez-Yurda, M.; Grootscholten, C.; Beets, G.L.; Snaebjornsson, P.; et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat. Med. 2020, 26, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Chalabi, M.; Verschoor, Y.L.; van den Berg, J.; Sikorska, K.; Beets, G.; Lent, A.V.; Grootscholten, M.C.; Aalbers, A.; Buller, N.; Marsman, H.; et al. LBA7 neoadjuvant immune checkpoint inhibition in locally advanced MMR-deficient colon cancer: The NICHE-2 study. Ann. Oncol. 2022, 33, S1389. [Google Scholar] [CrossRef]
- Hu, H.; Kang, L.; Zhang, J.; Wu, Z.; Wang, H.; Huang, M.; Lan, P.; Wu, X.; Wang, C.; Cao, W.; et al. Neoadjuvant PD-1 blockade with toripalimab, with or without celecoxib, in mismatch repair-deficient or microsatellite instability-high, locally advanced, colorectal cancer (PICC): A single-centre, parallel-group, non-comparative, randomised, phase 2 trial. Lancet Gastroenterol. Hepatol. 2022, 7, 38–48. [Google Scholar] [CrossRef]
- Li, Y.; Liang, F.; Li, Z.; Zhang, X.; Wu, A. Neoadjuvant immunotherapy for patients with microsatellite instability-high or POLE-mutated locally advanced colorectal cancer with bulky tumors: New optimization strategy. Clin. Color. Cancer 2025, 24, 18–31.e2. [Google Scholar] [CrossRef]
- Cook, A.D.; Single, R.; McCahill, L.E. Surgical resection of primary tumors in patients who present with stage IV colorectal cancer: An analysis of surveillance, epidemiology, and end results data, 1988 to 2000. Ann. Surg. Oncol. 2005, 12, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Nakatsura, T.; Yoshitake, Y.; Senju, S.; Monji, M.; Komori, H.; Motomura, Y.; Hosaka, S.; Beppu, T.; Ishiko, T.; Kamohara, H.; et al. Glypican-3, overexpressed specifically in human hepatocellular carcinoma, is a novel tumor marker. Biochem. Biophys. Res. Commun. 2003, 306, 16–25. [Google Scholar] [CrossRef]
- Tsuchiya, N.; Yoshikawa, T.; Fujinami, N.; Saito, K.; Mizuno, S.; Sawada, Y.; Endo, I.; Nakatsura, T. Immunological efficacy of glypican-3 peptide vaccine in patients with advanced hepatocellular carcinoma. Oncoimmunology 2017, 6, e1346764. [Google Scholar] [CrossRef] [PubMed]
- Sayem, M.A.; Tomita, Y.; Yuno, A.; Hirayama, M.; Irie, A.; Tsukamoto, H.; Senju, S.; Yuba, E.; Yoshikawa, T.; Kono, K.; et al. Identification of glypican-3-derived long peptides activating both CD8+ and CD4+ T cells; prolonged overall survival in cancer patients with Th cell response. Oncoimmunology 2016, 5, e1062209. [Google Scholar] [CrossRef]
- Sawada, Y.; Yoshikawa, T.; Ofuji, K.; Yoshimura, M.; Tsuchiya, N.; Takahashi, M.; Nobuoka, D.; Gotohda, N.; Takahashi, S.; Kato, Y.; et al. Phase II study of the GPC3-derived peptide vaccine as an adjuvant therapy for hepatocellular carcinoma patients. Oncoimmunology 2016, 5, e1129483. [Google Scholar] [CrossRef]
- Shimizu, Y.; Mizuno, S.; Fujinami, N.; Suzuki, T.; Saito, K.; Konishi, M.; Takahashi, S.; Gotohda, N.; Tada, T.; Toyoda, H.; et al. Plasma and tumoral glypican-3 levels are correlated in patients with hepatitis C virus-related hepatocellular carcinoma. Cancer Sci. 2020, 111, 334–342. [Google Scholar] [CrossRef]
- Taniguchi, M.; Mizuno, S.; Yoshikawa, T.; Fujinami, N.; Sugimoto, M.; Kobayashi, S.; Takahashi, S.; Konishi, M.; Gotohda, N.; Nakatsura, T. Peptide vaccine as an adjuvant therapy for glypican-3-positive hepatocellular carcinoma induces peptide-specific CTLs and improves long prognosis. Cancer Sci. 2020, 111, 2747–2759. [Google Scholar] [CrossRef]
- Ukai, M.; Yokoi, A.; Yoshida, K.; Suzuki, S.; Shibata, K.; Kikkawa, F.; Nakatsura, T.; Kajiyama, H. Extracellular miRNAs as predictive biomarkers for glypican-3-derived peptide vaccine therapy response in ovarian clear cell carcinoma. Cancers 2021, 13, 550. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Sakata, J.; Utsumi, F.; Sekiya, R.; Kajiyama, H.; Shibata, K.; Kikkawa, F.; Nakatsura, T. Efficacy of glypican-3-derived peptide vaccine therapy on the survival of patients with refractory ovarian clear cell carcinoma. Oncoimmunology 2016, 5, e1238542. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Yoshikawa, T.; Kojima, T.; Shoda, K.; Nosaka, K.; Mizuno, S.; Wada, S.; Fujimoto, Y.; Sasada, T.; Kohashi, K.; et al. Heat shock protein 105 peptide vaccine could induce antitumor immune reactions in a phase I clinical trial. Cancer Sci. 2019, 110, 3049–3060. [Google Scholar] [CrossRef]
- Sawada, Y.; Komori, H.; Tsunoda, Y.; Shimomura, M.; Takahashi, M.; Baba, H.; Ito, M.; Saito, N.; Kuwano, H.; Endo, I.; et al. Identification of HLA-A2 or HLA-A24-restricted CTL epitopes for potential HSP105-targeted immunotherapy in colorectal cancer. Oncol. Rep. 2014, 31, 1051–1058. [Google Scholar] [CrossRef]
- Miyazaki, M.; Nakatsura, T.; Yokomine, K.; Senju, S.; Monji, M.; Hosaka, S.; Komori, H.; Yoshitake, Y.; Motomura, Y.; Minohara, M.; et al. DNA vaccination of HSP105 leads to tumor rejection of colorectal cancer and melanoma in mice through activation of both CD4+ T cells and CD8+ T cells. Cancer Sci. 2005, 96, 695–705. [Google Scholar] [CrossRef]
- Kinoshita, H.; Takenouchi, K.; Tsukamoto, N.; Ohnuki, K.; Suzuki, T.; Nakatsura, T. Identification of 68 HLA-A24 and -A2-restricted cytotoxic T lymphocyte-inducing peptides derived from 10 common cancer-specific antigens frequently expressed in various solid cancers. Neoplasia 2025, 61, 101135. [Google Scholar] [CrossRef]
- Xia, G.; Kumar, S.R.; Masood, R.; Zhu, S.; Reddy, R.; Krasnoperov, V.; Quinn, D.I.; Henshall, S.M.; Sutherland, R.L.; Pinski, J.K.; et al. EphB4 expression and biological significance in prostate cancer. Cancer Res. 2005, 65, 4623–4632. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.R.; Singh, J.; Xia, G.; Krasnoperov, V.; Hassanieh, L.; Ley, E.J.; Scehnet, J.; Kumar, N.G.; Hawes, D.; Press, M.F.; et al. Receptor tyrosine kinase EphB4 is a survival factor in breast cancer. Am. J. Pathol. 2006, 169, 279–293. [Google Scholar] [CrossRef]
- Masood, R.; Kumar, S.R.; Sinha, U.K.; Crowe, D.L.; Krasnoperov, V.; Reddy, R.K.; Zozulya, S.; Singh, J.; Xia, G.; Broek, D.; et al. EphB4 provides survival advantage to squamous cell carcinoma of the head and neck. Int. J. Cancer 2006, 119, 1236–1248. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.R.; Scehnet, J.S.; Ley, E.J.; Singh, J.; Krasnoperov, V.; Liu, R.; Manchanda, P.K.; Ladner, R.D.; Hawes, D.; Weaver, F.A.; et al. Preferential induction of EphB4 over EphB2 and its implication in colorectal cancer progression. Cancer Res. 2009, 69, 3736–3745. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Tao, Z.; Shen, Z.; Wang, X.; Hua, F. Down-regulation of EphB4 phosphorylation is necessary for esophageal squamous cell carcinoma tumorigenecity. Tumor Biol. 2014, 35, 7225–7232. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.Y.; Pasquale, E.B.; Owen, L.B.; Ethell, I.M. The EphB4 receptor-tyrosine kinase promotes the migration of melanoma cells through Rho-mediated actin cytoskeleton reorganization. J. Biol. Chem. 2006, 281, 32574–32586. [Google Scholar] [CrossRef]
- Li, M.; Zhao, J.; Qiao, J.; Song, C.; Zhao, Z. EphB4 regulates the growth and migration of pancreatic cancer cells. Tumor Biol. 2014, 35, 6855–6859. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B.D.; Liu, R.; Rolle, C.E.; Tan, Y.C.; Krasnoperov, V.; Kanteti, R.; Tretiakova, M.S.; Cervantes, G.M.; Hasina, R.; Hseu, R.D.; et al. The EphB4 receptor tyrosine kinase promotes lung cancer growth: A potential novel therapeutic target. PLoS ONE 2013, 8, e67668. [Google Scholar] [CrossRef] [PubMed]
- Visco, Z.R.; Sfakianos, G.; Grenier, C.; Boudreau, M.-H.; Simpson, S.; Rodriguez, I.; Whitaker, R.; Yao, D.Y.; Berchuck, A.; Murphy, S.K.; et al. Epigenetic regulation of claudin-1 in the development of ovarian cancer recurrence and drug resistance. Front. Oncol. 2021, 11, 620873. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, J.; Jiang, Y.; Xu, W.; Li, X.; Jing, W. CLDN1 increases drug resistance of non-small cell lung cancer by activating autophagy via up-regulation of ULK1 phosphorylation. Med. Sci. Monit. 2017, 23, 2906–2916. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tong, H.; Li, T.; Qiu, W.; Zhu, Z. Claudin-1 silencing increases sensitivity of liver cancer HepG2 cells to 5-fluorouracil by inhibiting autophagy. Oncol. Lett. 2019, 18, 5709–5716. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, P.; Singh, A.B.; Deane, N.G.; No, Y.; Shiou, S.R.; Schmidt, C.; Neff, J.; Washington, M.K.; Beauchamp, R.D. Claudin-1 regulates cellular transformation and metastatic behavior in colon cancer. J. Clin. Investig. 2005, 115, 1765–1776. [Google Scholar] [CrossRef]
- Oku, N.; Sasabe, E.; Ueta, E.; Yamamoto, T.; Osaki, T. Tight junction protein claudin-1 enhances the invasive activity of oral squamous cell carcinoma cells by promoting cleavage of laminin-5 gamma2 chain via matrix metalloproteinase (MMP)-2 and membrane-type MMP-1. Cancer Res. 2006, 66, 5251–5257. [Google Scholar] [CrossRef]
- Leotlela, P.D.; Wade, M.S.; Duray, P.H.; Rhode, M.J.; Brown, H.F.; Rosenthal, D.T.; Dissanayake, S.K.; Earley, R.; Indig, F.E.; Nickoloff, B.J.; et al. Claudin-1 overexpression in melanoma is regulated by pkc and contributes to melanoma cell motility. Oncogene 2007, 26, 3846–3856. [Google Scholar] [CrossRef]
- Wang, Q.; Holst, J. L-Type amino acid transporter and cancer: Targeting the mTORC1 pathway to inhibit neoplasia. Am. J. Cancer Res. 2015, 5, 1281–1294. [Google Scholar]
- Yuan, M.; Guo, H.; Li, J.; Sui, C.; Qin, Y.; Wang, J.; Khan, Y.H.; Ye, L.; Xie, F.; Wang, H.; et al. Slit2 and Robo1 induce opposing effects on metastasis of hepatocellular carcinoma Sk-hep-1 cells. Int. J. Oncol. 2016, 49, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Funahashi, S.; Yamauchi, N.; Shibahara, J.; Midorikawa, Y.; Kawai, S.; Kinoshita, Y.; Watanabe, A.; Hippo, Y.; Ohtomo, T.; et al. Identification of ROBO1 as a novel hepatocellular carcinoma antigen and a potential therapeutic and diagnostic target. Clin. Cancer Res. 2006, 12, 3257–3264. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, A.; Erdogan, Z.M.; Kim, Y.J.; Choi, Y.L.; Lu, H.; Katzenellenbogen, B.S. The forkhead transcription factor FOXM1 promotes endocrine resistance and invasiveness in estrogen receptor-positive breast cancer by expansion of stem-like cancer cells. Breast Cancer Res. 2014, 16, 436. [Google Scholar] [CrossRef]
- Hu, G.; Yan, Z.; Zhang, C.; Cheng, M.; Yan, Y.; Wang, Y.; Deng, L.; Lu, Q.; Luo, S. FOXM1 promotes hepatocellular carcinoma progression by regulating KIF4A expression. J. Exp. Clin. Cancer Res. 2019, 38, 188. [Google Scholar] [CrossRef]
- Lien, H.C.; Hsiao, Y.H.; Lin, Y.S.; Yao, Y.T.; Juan, H.F.; Kuo, W.H.; Hung, M.-C.; Chang, K.J.; Hsieh, F.J. Molecular signatures of metaplastic carcinoma of the breast by large-scale transcriptional profiling: Identification of genes potentially related to epithelial-mesenchymal transition. Oncogene 2007, 26, 7859–7871. [Google Scholar] [CrossRef]
- Rempel, S.A.; Golembieski, W.A.; Ge, S.; Lemke, N.; Elisevich, K.; Mikkelsen, T.; Gutiérrez, J.A. SPARC: A signal of astrocytic neoplastic transformation and reactive response in human primary and xenograft gliomas. J. Neuropathol. Exp. Neurol. 1998, 57, 1112–1121. [Google Scholar] [CrossRef][Green Version]
- Ledda, M.F.; Adris, S.; Bravo, A.I.; Kairiyama, C.; Bover, L.; Chernajovsky, Y.; Mordoh, J.; Podhajcer, O.L. Suppression of SPARC expression by antisense RNA abrogates the tumorigenicity of human melanoma cells. Nat. Med. 1997, 3, 171–176. [Google Scholar] [CrossRef]
- Alvarez, M.J.; Prada, F.; Salvatierra, E.; Bravo, A.I.; Lutzky, V.P.; Carbone, C.; Pitossi, F.J.; Chuluyan, H.; Podhajcer, O.L. Secreted protein acidic and rich in cysteine produced by human melanoma cells modulates polymorphonuclear leukocyte recruitment and antitumor cytotoxic capacity. Cancer Res. 2005, 65, 5123–5132. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Senju, S.; Hirata, S.; Ikuta, Y.; Hayashida, Y.; Irie, A.; Harao, M.; Imai, K.; Tomita, Y.; Tsunoda, T.; et al. Identification of SPARC as a candidate target antigen for immunotherapy of various cancers. Int. J. Cancer 2010, 127, 1393–1403. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, X.; Liao, Z.; He, C.; Hu, X. TGFBI protein high expression predicts poor prognosis in colorectal cancer patients. Int. J. Clin. Exp. Pathol. 2015, 8, 702–710. [Google Scholar]
- Infante, J.R.; Matsubayashi, H.; Sato, N.; Tonascia, J.; Klein, A.P.; Riall, T.A.; Yeo, C.; Iacobuzio-Donahue, C.; Goggins, M. Peritumoral fibroblast SPARC expression and patient outcome with resectable pancreatic adenocarcinoma. J. Clin. Oncol. 2007, 25, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Porte, H.; Chastre, E.; Prevot, S.; Nordlinger, B.; Empereur, S.; Basset, P.; Chambon, P.; Gespach, C. Neoplastic progression of human colorectal cancer is associated with overexpression of the stromelysin-3 and BM-40/SPARC genes. Int. J. Cancer 1995, 64, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Chiavarina, B.; Costanza, B.; Ronca, R.; Blomme, A.; Rezzola, S.; Chiodelli, P.; Giguelay, A.; Belthier, G.; Doumont, G.; Van Simaeys, G.; et al. Metastatic colorectal cancer cells maintain the TGFβ program and use TGFBI to fuel angiogenesis. Theranostics 2021, 11, 1626–1640. [Google Scholar] [CrossRef]
- Seki, M.; Watanabe, A.; Enomoto, S.; Kawamura, T.; Ito, H.; Kodama, T.; Hamakubo, T.; Aburatani, H. Human ROBO1 is cleaved by metalloproteinases and γ-secretase and migrates to the nucleus in cancer cells. FEBS Lett. 2010, 584, 2909–2915. [Google Scholar] [CrossRef]
- Ballard, M.S.; Hinck, L. A roundabout way to cancer. Adv. Cancer Res. 2012, 114, 187–235. [Google Scholar] [CrossRef] [PubMed]
- Nakatsura, T.; Takenouchi, K.; Kataoka, J.; Ito, Y.; Kikuchi, S.; Kinoshita, H.; Ohnuki, K.; Suzuki, T.; Tsukamoto, N. Expression Profiles of Five Common Cancer Membrane Protein Antigens Collected for the Development of Cocktail CAR-T Cell Therapies Applicable to Most Solid Cancer Patients. Int. J. Mol. Sci. 2025, 26, 2145. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.J.; Svoboda, J.; Chong, E.A.; Nasta, S.D.; Mato, A.R.; Anak, Ö.; Brogdon, J.L.; Pruteanu-Malinici, I.; Bhoj, V.; Landsburg, D.; et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N. Engl. J. Med. 2017, 377, 2545–2554. [Google Scholar] [CrossRef]
- Ghosh, A.; Smith, M.; James, S.E.; Davila, M.L.; Velardi, E.; Argyropoulos, K.V.; Gunset, G.; Perna, F.; Kreines, F.M.; Levy, E.R.; et al. Donor CD19 CAR T cells exert potent graft-versus-lymphoma activity with diminished graft-versus-host activity. Nat. Med. 2017, 23, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.A.; Grover, N.S.; Beaven, A.W.; Lulla, P.D.; Wu, M.F.; Ivanova, A.; Wang, T.; Shea, T.C.; Rooney, C.M.; Dittus, C.; et al. Anti-CD30 CAR-T cell therapy in relapsed and refractory Hodgkin lymphoma. J. Clin. Oncol. 2020, 38, 3794–3804. [Google Scholar] [CrossRef] [PubMed]
- Garrido, F.; Ruiz-Cabello, F.; Cabrera, T.; Pérez-Villar, J.J.; López-Botet, M.; Duggan-Keen, M.; Stern, P.L. Implications for immunosurveillance of altered HLA class I phenotypes in human tumours. Immunol. Today. 1997, 18, 89–95. [Google Scholar] [CrossRef]
- Garrido, F.; Algarra, I. MHC antigens and tumor escape from immune surveillance. Adv. Cancer Res. 2001, 83, 117–158. [Google Scholar] [CrossRef]
- Dhatchinamoorthy, K.; Colbert, J.D.; Rock, K.L. Cancer immune evasion through loss of MHC class I antigen presentation. Front. Immunol. 2021, 12, 636568. [Google Scholar] [CrossRef]
- Cabrera, T.; Collado, A.; Fernandez, M.A.; Ferron, A.; Sancho, J.; Ruiz-Cabello, F.; Garrido, F. High frequency of altered HLA class I phenotypes in invasive colorectal carcinomas. Tissue Antigens 1998, 52, 114–123. [Google Scholar] [CrossRef]
- Swets, M.; König, M.H.; Zaalberg, A.; Dekker-Ensink, N.G.; Gelderblom, H.; van de Velde, C.J.; van den Elsen, P.J.; Kuppen, P.J. HLA-G and classical HLA class I expression in primary colorectal cancer and associated liver metastases. Hum. Immunol. 2016, 77, 773–779. [Google Scholar] [CrossRef]
- Watson, N.F.; Ramage, J.M.; Madjd, Z.; Spendlove, I.; Ellis, I.O.; Scholefield, J.H.; Durrant, L.G. Immunosurveillance is active in colorectal cancer as downregulation but not complete loss of MHC class I expression correlates with a poor prognosis. Int. J. Cancer 2006, 118, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Turcotte, S.; Katz, S.C.; Shia, J.; Jarnagin, W.R.; Kingham, T.P.; Allen, P.J.; Fong, Y.; D’Angelica, M.I.; DeMatteo, R.P. Tumor MHC class I expression improves the prognostic value of T-cell density in resected colorectal liver metastases. Cancer Immunol. Res. 2014, 2, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Aptsiauri, N.; Garrido, F. The challenges of HLA class I loss in cancer immunotherapy: Facts and hopes. Clin. Cancer Res. 2022, 28, 5021–5029. [Google Scholar] [CrossRef] [PubMed]
- Kloor, M.; Michel, S.; von Knebel Doeberitz, M. Immune evasion of microsatellite unstable colorectal cancers. Int. J. Cancer 2010, 127, 1001–1010. [Google Scholar] [CrossRef]
- Ericsson, C.; Seregard, S.; Bartolazzi, A.; Levitskaya, E.; Ferrone, S.; Kiessling, R.; Larsson, O. Association of HLA class I and class II antigen expression and mortality in uveal melanoma. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2153–2156. [Google Scholar]
- Wei, T.; Zeng, C.; Li, Q.; Xiao, Z.; Zhang, L.; Zhang, Q.; Ren, L. FOXM1/DEPDC1 feedback loop promotes hepatocarcinogenesis and represents promising targets for cancer therapy. Cancer Sci. 2024, 115, 3041–3053. [Google Scholar] [CrossRef]
- Zheng, Y.; Guo, J.; Zhou, J.; Lu, J.; Chen, Q.; Zhang, C.; Qing, C.; Koeffler, H.P.; Tong, Y. FoxM1 transactivates PTTG1 and promotes colorectal cancer cell migration and invasion. BMC Med. Genom. 2015, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Michelakos, T.; Kontos, F.; Kurokawa, T.; Cai, L.; Sadagopan, A.; Krijgsman, D.; Weichert, W.; Durrant, L.G.; Kuppen, P.J.; Ferrone, C.R.; et al. Differential role of HLA-A and HLA-B,C expression levels as prognostic markers in colon and rectal cancer. J. Immunother. Cancer 2022, 10, e004115. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, J.; Wang, X.; Zhang, C.; Yu, Z.; Liu, J.; Tai, Z.; Luo, Z.; Yi, X.; Zhong, Z. Whole exome and transcriptome sequencing reveal clonal evolution and exhibit immune-related features in metastatic colorectal tumors. Cell Death Discov. 2021, 7, 222. [Google Scholar] [CrossRef]
- Akazawa, Y.; Nobuoka, D.; Takahashi, M.; Yoshikawa, T.; Shimomura, M.; Mizuno, S.; Fujiwara, T.; Nakamoto, Y.; Nakatsura, T. Higher human lymphocyte antigen class I expression in early-stage cancer cells leads to high sensitivity for cytotoxic T lymphocytes. Cancer Sci. 2019, 110, 1842–1852. [Google Scholar] [CrossRef]
- Xiao, J.; Yu, X.; Meng, F.; Zhang, Y.; Zhou, W.; Ren, Y.; Li, J.; Sun, Y.; Sun, H.; Chen, G.; et al. Integrating spatial and single-cell transcriptomics reveals tumor heterogeneity and intercellular networks in colorectal cancer. Cell Death Dis. 2024, 15, 326. [Google Scholar] [CrossRef]
- Chao, S.; Zhang, F.; Yan, H.; Wang, L.; Zhang, L.; Wang, Z.; Xue, R.; Wang, L.; Wu, Z.; Jiang, B.; et al. Targeting intratumor heterogeneity suppresses colorectal cancer chemoresistance and metastasis. EMBO Rep. 2023, 24, e56416. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.R.; Simeone, D.M.; Smith, C.J.; Welling, T.H.; Kirtane, K.; Grierson, P.; Morelli, M.P.; Hecht, J.R.; Patel, S.P.; Locke, F.L.; et al. EVEREST-1: A seamless phase 1/2 study of A2B530, a carcinoembryonic antigen (CEA) logic-gated Tmod CAR T-cell therapy, in patients with solid tumors associated with CEA expression also exhibiting human leukocyte antigen (HLA)-A*02 loss of heterozygosity (LOH). J. Clin. Oncol. 2024, 42 (Suppl. 16), TPS2698. [Google Scholar]
- Ouladen, S.; Oruji, E. Chimeric Antigen Receptor-T Cells in Colorectal Cancer: Pioneering New Avenues in Solid Tumor Immunotherapy. J. Clin. Oncol. 2025, 43, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Morishita, Y.; Takenouchi, K.; Sakashita, S.; Matsuura, K.; Hayashi, R.; Nakatsura, T. Immunohistochemical analysis of common cancer antigens in head and neck squamous cell carcinoma. Anticancer. Res. 2022, 42, 5751–5761. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]


| Primary Colorectal Cancer | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cancer Antigens * | CLDN1 | EphB4 | LAT1 | FOXM1 | HSP105α | ROBO1 | SPARC | HLA class I |
| Score: Median [range] | ||||||||
| All cases (n = 25) | 5.3 [0–6.0] | 5.0 [0–6.0] | 5.3 [0.7–6.0] | 5.0 [0–6.0] | 6.0 [3.0–6.0] | 4.7 [0.7–6.0] | 5.7 [1.7–6.0] | 4.7 [0–5.3] |
| Cases of expression > 3 | ||||||||
| All cases (n = 25) | 23 (92.0%) | 24 (96.0%) | 24 (96.0%) | 22 (88.0%) | 24 (96.0%) | 23 (92.0%) | 24 (96.0%) | 20 (80.0%) |
| Colorectal cancer liver metastases | ||||||||
| Cancer Antigens * | CLDN1 | EphB4 | LAT1 | FOXM1 | HSP105α | ROBO1 | SPARC | HLA class I |
| Score: Median [range] | ||||||||
| All cases (n = 60) | 4.3 [0–6.0] | 4.3 [0–6.0] | 3.7 [0–6.0] | 2.7 [0–6.0] | 5.0 [0–6.0] | 4.0 [0–6.0] | 2.7 [0–5.0] | 3.7 [0–6.0] |
| Naïve (n = 12) | 4.3 [3.3–6.0] | 5.0 [0–6.0] | 3.7 [1.3–5.7] | 2.5 [0–5.0] | 4.7 [3.3–6.0] | 4.2 [0–6.0] | 2.3 [1.0–5.0] | 3.7 [0–5.0] |
| Chemotherapy (n = 48) | 4.3 [0–6.0] | 4.0 [0–6.0] | 3.7 [0–6.0] | 2.7 [0–6.0] | 5.0 [0–6.0] | 3.7 [0–6.0] | 2.7 [1.0–4.0] | 3.7 [0–6.0] |
| Cases of expression > 3 | ||||||||
| All cases (n = 60) | 53 (88.3%) | 44 (76.6%) | 36 (60.0%) | 23 (38.3%) | 57 (95.0%) | 38 (63.3%) | 20 (33.3%) | 37 (61.7%) |
| Naïve (n = 12) | 12 (100.0%) | 8 (66.7%) | 7 (58.3%) | 2 (16.7%) | 12 (100.0%) | 8 (66.7%) | 2 (16.7%) | 10 (83.3%) |
| Chemotherapy (n = 48) | 41 (85.4%) | 36 (75.0%) | 29 (60.4%) | 21 (43.8%) | 45 (93.8%) | 30 (62.5%) | 18 (37.5%) | 27 (56.2%) |
| Primary Colorectal Cancer | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cancer Antigens * | CLDN1 | EphB4 | LAT1 | FOXM1 | HSP105α | ROBO1 | SPARC | HLA class I |
| Score: Median [range] | ||||||||
| All cases (n = 14) | 5.7 [0–6.0] | 5.0 [0–6.0] | 5.3 [3.0–6.0] | 4.5 [2.7–6.0] | 5.0 [0–6.0] | 5.0 [0.7–6.0] | 5.7 [1.7–6.0] | 4.0 [0–5.3] |
| Naïve (n = 11) | 5.7 [0–6.0] | 5.0 [5.0–6.0] | 5.3 [3.0–6.0] | 4.7 [2.7–6.0] | 5.0 [0–6.0] | 4.7 [0.7–6.0] | 5.7 [1.7–6.0] | 4.0 [0–5.3] |
| Chemotherapy (n = 3) | 5.7 [4.0–6.0] | 4.3 [2.3–6.0] | 5.3 [5.0–5.7] | 4.3 [3.0–4.7] | 5.20 [4.3–6.0] | 5.0 [5.0–6.0] | 6.0 [5.0–6.0] | 3.3 [1.3–5.3] |
| Cases of expression > 3 | ||||||||
| All cases (n = 14) | 13 (92.9%) | 12 (85.7%) | 13 (92.9%) | 11 (78.6%) | 13 (92.9%) | 12 (85.7%) | 13 (92.9%) | 9 (64.2%) |
| Naïve (n = 11) | 10 (90.9%) | 10 (90.9%) | 10 (90.9%) | 9 (81.8%) | 10 (90.9%) | 9 (81.8%) | 10 (90.9%) | 7 (63.6%) |
| Chemotherapy (n = 3) | 3 (100.0%) | 2 (66.7%) | 3 (100.0%) | 2 (66.7%) | 3 (100.0%) | 3 (100.0%) | 3 (100.0%) | 2 (66.7%) |
| Colorectal cancer liver metastases | ||||||||
| Cancer Antigens * | CLDN1 | EphB4 | LAT1 | FOXM1 | HSP105α | ROBO1 | SPARC | HLA class I |
| Score: Median [range] | ||||||||
| All cases (n = 14) | 5.5 [1.7–6.0] | 4.3 [0–6.0] | 5.2 [0–6.0] | 2.3 [0–3.3] | 5.0 [3.3–6.0] | 5.0 [4.0–6.0] | 2.7 [0–5.0] | 5.0 [1.7–6.0] |
| Naïve (n = 5) | 5.0 [1.7–5.7] | 5.0 [2.3–5.3] | 5.0 [0.3–6.0] | 3.0 [2.3–3.3] | 5.3 [4.7–5.7] | 5.0 [4.0–5.7] | 0.0 [0–5.0] | 5.0 [1.7–5.7] |
| Chemotherapy (n = 9) | 5.0 [1.7–6.0] | 4.0 [0–6.0] | 5.3 [0–6.0] | 1.3 [0.3–3.3] | 5.0 [3.3–6.0] | 5.0 [2.7–6.0] | 2.7 [0–4.3] | 5.0 [2.7–5.7] |
| Cases of expression > 3 | ||||||||
| All cases (n = 14) | 13 (92.9%) | 9 (64.2%) | 11 (78.6%) | 4 (28.6%) | 14 (100.0%) | 12 (85.7%) | 5 (35.7%) | 11 (78.6%) |
| Naïve (n = 5) | 5 (100.0%) | 3 (60.0%) | 3 (60.0%) | 3 (60.0%) | 5 (100.0%) | 4 (80.0%) | 2 (40.0%) | 4 (80.0%) |
| Chemotherapy (n = 9) | 8 (88.9%) | 6 (66.7%) | 8 (88.9%) | 1 (11.1%) | 9 (100.0%) | 8 (88.9%) | 3 (33.3%) | 7 (77.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kataoka, J.; Takenouchi, K.; Suzuki, T.; Ohnuki, K.; Tsukada, Y.; Gotohda, N.; Ito, M.; Nakatsura, T. Identification of Common Cancer Antigens Useful for Specific Immunotherapies to Colorectal Cancer and Liver Metastases. Int. J. Mol. Sci. 2025, 26, 7402. https://doi.org/10.3390/ijms26157402
Kataoka J, Takenouchi K, Suzuki T, Ohnuki K, Tsukada Y, Gotohda N, Ito M, Nakatsura T. Identification of Common Cancer Antigens Useful for Specific Immunotherapies to Colorectal Cancer and Liver Metastases. International Journal of Molecular Sciences. 2025; 26(15):7402. https://doi.org/10.3390/ijms26157402
Chicago/Turabian StyleKataoka, Jun, Kazumasa Takenouchi, Toshihiro Suzuki, Kazunobu Ohnuki, Yuichiro Tsukada, Naoto Gotohda, Masaaki Ito, and Tetsuya Nakatsura. 2025. "Identification of Common Cancer Antigens Useful for Specific Immunotherapies to Colorectal Cancer and Liver Metastases" International Journal of Molecular Sciences 26, no. 15: 7402. https://doi.org/10.3390/ijms26157402
APA StyleKataoka, J., Takenouchi, K., Suzuki, T., Ohnuki, K., Tsukada, Y., Gotohda, N., Ito, M., & Nakatsura, T. (2025). Identification of Common Cancer Antigens Useful for Specific Immunotherapies to Colorectal Cancer and Liver Metastases. International Journal of Molecular Sciences, 26(15), 7402. https://doi.org/10.3390/ijms26157402








